Abstract
Aim
The effects of neighbor’s species density and nutrient availability in the sediment are essential to understand the structuring rules of emergent macrophyte communities. The objective of this paper was to investigate how the density of Polygonum ferrugineum and the availability of nutrients in the sediment influence the establishment of Alternanthera philoxeroides.
Methods
After collection, we sectioned the stems of each species so that each propagule obtained was composed of two nodes. These propagules were planted in trays with moist sediment for rooting and development of new leaves for 24 days, and only then were transferred to the experimental mesocosms. Our experimental design had an A. philoxeroides propagule submitted to the following treatments: I – control (planted alone); II – associated with three P. ferrugineum propagules; III – associated with five P. ferrugineum propagules. There were 36 mesocosms arranged inside the greenhouse, with half of them representing conditions of low nutrient availability and half representing conditions of high nutrient availability (with 12.5 g of NPK in the sediment). The experiment lasted 60 days, and the following response variables were measured: aerial length, root length, aerial, root and total dry biomass. The response variables related to biomass were obtained after the plants were dried in an oven at 60°C. We also calculated the relative interaction index (RII) for each treatment, in order to analyze the interactions between the species. Each response variable was analyzed using a two-way ANOVA.
Results
Among the main results obtained, we can highlight the lower accumulation of biomass in A. philoxeroides under conditions of low nutrient availability and high density of P. ferrugineum.
Conclusions
These results indicate the negative effects of P. ferrugineum density on the establishment of A. philoxeroides, contributing to the understanding of the dynamics and structuring rules of the emergent macrophyte community.
Keywords:
interspecific competition; neighbors’ species; emergent macrophytes; experiment in a greenhouse; relative interaction index
Resumo
Objetivo
Os efeitos da densidade de espécies vizinhas e da disponibilidade de nutrientes no sedimento são essenciais para compreender as regras de estruturação de comunidades de macrófitas emergentes. O objetivo deste trabalho foi investigar como a densidade de Polygonum ferrugineum e a disponibilidade de nutrientes no sedimento influenciam o estabelecimento de Alternanthera philoxeroides.
Métodos
Após a coleta, seccionamos os caules de cada espécie de modo que cada propágulo obtido fosse composto de dois nós. Esses propágulos foram plantados em bandejas com sedimento úmido para o enraizamento e desenvolvimento de novas folhas por 24 dias e, só então foram transferidos para os mesocosmos experimentais. Nosso delineamento experimental contou com um propágulo de A. philoxeroides submetido aos seguintes tratamentos: I – controle (plantado sozinho); II – associado com três propágulos de P. ferrugineum; III – associado a cinco propágulos de P. ferrugineum. Houve 36 mesocosmos dispostos dentro da casa de vegetação, com metades deles representando condições de baixa disponibilidade de nutrientes e metade representando condições de alta disponibilidade de nutrientes (com 12,5 g de NPK no sedimento). O experimento durou 60 dias, sendo medido as seguintes variáveis respostas: comprimento aéreo, comprimento da raiz e biomassa seca da parte aérea, raiz e total. As variáveis respostas relacionadas a biomassa foram obtidas após as plantas secarem em estufa a 60°C. Também calculamos o índice de interação relativa (RII) para cada tratamento, com o intuito de analisar as interações entre as espécies. Cada variável resposta foi analisada através de uma Anova two-way.
Resultados
Entre os principais resultados obtidos, pode-se destacar o menor acumulo de biomassa em A. philoxeroides em condições de baixa disponibilidade de nutrientes e de alta densidade de P. ferrugineum.
Conclusões
Esses resultados indicam os efeitos negativos da densidade de P. ferrugineum sobre o estabelecimento de A. philoxeroides, contribuindo para a compreensão da dinâmica e das regras de estruturação da comunidade de macrófitas emergentes.
Palavras-chave:
competição interespecífica; espécies vizinhas; macrófitas emergentes; experimento em casa de vegetação; índice de interação relativa
1. Introduction
Freshwater ecosystems such as floodplains can be divided into different compartments according to their biotic and abiotic characteristics (Esteves & Caliman, 2011Esteves, F.A., & Caliman, A., 2011. Águas continentais: características do meio, compartimentos e suas comunidades. In: Esteves, F.A., ed. Fundamentos de limnologia. Rio de Janeiro: Interciência, 113-118, 3 ed.). The coastal region of a lake, for example, is characterized by being the transition area between the bodies of water and the terrestrial environment, presenting a high diversity of species due to the complexity of trophic levels that it supports (Almeida et al., 2006Almeida, V.L., Larrazábal, M.E., Moura, A.N., & Melo Júnior, M., 2006. Rotífera das zonas limnética e litorânea do reservatório de Tapacurá, Pernambuco, Brasil. Iheringia Ser. Zool. 96(4), 445-451. http://dx.doi.org/10.1590/S0073-47212006000400009.
http://dx.doi.org/10.1590/S0073-47212006...
; Esteves & Caliman, 2011Esteves, F.A., & Caliman, A., 2011. Águas continentais: características do meio, compartimentos e suas comunidades. In: Esteves, F.A., ed. Fundamentos de limnologia. Rio de Janeiro: Interciência, 113-118, 3 ed.). Among the biotic elements present in the coastal region, the emergent aquatic macrophytes have several morphological, anatomical, and physiological characteristics that allow them to survive in flooding conditions, differently from the surrounding terrestrial and strictly aquatic species (Du & Wang, 2014Du, Z.Y., & Wang, Q.F., 2014. Correlations of life form, pollination mode and sexual system in aquatic angiosperms. PLoS One 9(12), e115653. PMid:25525810. http://dx.doi.org/10.1371/journal.pone.0115653.
http://dx.doi.org/10.1371/journal.pone.0...
).
Because they occupy a very variable environment and are subject to changes caused by periodic floods (Wang et al., 2008Wang, J.W., Yu, D., Xiong, W., & Han, Y.Q., 2008. Above- and belowground competition between two submersed macrophytes. Hydrobiologia 607(1), 113-122. http://dx.doi.org/10.1007/s10750-008-9371-7.
http://dx.doi.org/10.1007/s10750-008-937...
; Kobayashi et al., 2009Kobayashi, T., Ryder, D.S., Gordon, G., Shannon, I., Ingleton, T., Carpenter, M., & Jacobs, S.J., 2009. Short-term response of nutrients, carbon and planktonic microbial communities to floodplain wetland inundation. Aquat. Ecol. 43(4), 843-858. http://dx.doi.org/10.1007/s10452-008-9219-2.
http://dx.doi.org/10.1007/s10452-008-921...
; Peršić et al., 2009Peršić, V., Horvatic, J., Has-Schon, E., & Bogut, I., 2009. Changes in N and P limitation induced by water level fluctuations in Nature Park Kopački Rit (Croatia): nutrient enrichment bioassay. Aquat. Ecol. 43(1), 27-36. http://dx.doi.org/10.1007/s10452-007-9156-5.
http://dx.doi.org/10.1007/s10452-007-915...
), factors such as the availability of nutrients in the sediment can influence the dynamics and composition of macrophyte communities (Rolon et al., 2008Rolon, A.S., Lacerda, T., Maltchik, L., & Guadagnin, D.L., 2008. Influence of area, habitat and water chemistry on richness and composition of macrophyte assemblages in southern Brazilian wetlands. J. Veg. Sci. 19(2), 221-228. http://dx.doi.org/10.3170/2008-8-18359.
http://dx.doi.org/10.3170/2008-8-18359...
). Among emergent macrophytes, those whose roots are fixed in the sediment and the shoot remains outside the water column (Sculthorpe, 1967Sculthorpe, C.D. 1967. The biology of Aquatic Vascular Plants. London: Edward Arnold Publishers.; Padial et al., 2009Padial, A.A., Carvalho, P., Thomaz, S.M., Boschilia, S.M., Rodrigues, R.B., & Kobayashi, J.T., 2009. The role of an extreme flood disturbance on macrophyte assemblages in a Neotropical floodplain. Aquat. Sci. 71(4), 389-398. http://dx.doi.org/10.1007/s00027-009-0109-z.
http://dx.doi.org/10.1007/s00027-009-010...
), conditions of low nutrient availability are more limiting when a species coexists with a high density of individuals of neighbor species, which can trigger strong competition. The increase in competition between macrophytes, as reported in the literature, can lead to decreases in some morphophysiological characters such as shoot, leaf area, stem diameter and even a reduction in plant biomass (Brahim et al., 1998Brahim, K., Ray, D.T., & Dierig, D.A., 1998. Growth and yield characteristics of Lesquerella fendleri as a function of plant density. Ind. Crops Prod. 9(1), 63-71. http://dx.doi.org/10.1016/S0926-6690(98)00015-6.
http://dx.doi.org/10.1016/S0926-6690(98)...
; Muller et al., 2000Muller, I., Schmid, B., & Weiner, J., 2000. The effect of nutrient availability on biomass allocation Patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 3(2), 115-127. http://dx.doi.org/10.1078/1433-8319-00007.
http://dx.doi.org/10.1078/1433-8319-0000...
; Wang et al., 2008Wang, J.W., Yu, D., Xiong, W., & Han, Y.Q., 2008. Above- and belowground competition between two submersed macrophytes. Hydrobiologia 607(1), 113-122. http://dx.doi.org/10.1007/s10750-008-9371-7.
http://dx.doi.org/10.1007/s10750-008-937...
).
Investigations on the effects of interspecific competition and the nutrients available in the sediment are essential for understanding the rules of structuring the community of emergent macrophytes, since these interactions interfere with the performance of species, allowing or not the coexistence of individuals (Xie et al., 2006Xie, Y.H., An, S., Wu, B., & Wang, W., 2006. Density-dependent root morphology and root distribution in the submerged plant Vallisneria natans. Environ. Exp. Bot. 57(1-2), 195-200. http://dx.doi.org/10.1016/j.envexpbot.2005.06.001.
http://dx.doi.org/10.1016/j.envexpbot.20...
; Silveira et al., 2018Silveira, M.J., Alves, D.C., & Thomaz, S.M., 2018. Effects of the density of the invasive macrophyte Hydrilla verticillata and root competition on growth of one native macrophyte in different sediment fertilities. Ecol. Res. 33(5), 927-934. http://dx.doi.org/10.1007/s11284-018-1602-4.
http://dx.doi.org/10.1007/s11284-018-160...
). One way to evaluate the effects of increasing density and nutrient availability experimentally is through the additive model, in which there is a target species, in which density effects are measured, and a neighbor’s species, which interacts with a species. -target and within the experiment can have its density manipulated (Gibson et al., 1999Gibson, D.J., Connolly, J., Hartnett, D.C., & Weidenhamer, J.D., 1999. Designs for greenhouse studies of interactions between plants. J. Ecol. 87(1), 1-16. http://dx.doi.org/10.1046/j.1365-2745.1999.00321.x.
http://dx.doi.org/10.1046/j.1365-2745.19...
).
Inventories of aquatic flora carried out in the Upper Paraná River floodplain registered six species of macrophytes of the genus Polygonum (Polygonaceae), being Polygonum ferrugineum Weed. a dominant species in these environments (Ferreira et al., 2011Ferreira, F.A., Mormul, R.P., Thomaz, S.M., Pott, A., & Pott, V.J., 2011. Macrophytes in the Upper Paraná River Floodplain: checklist and comparison with other large South American Wetlands. Rev. Biol. Trop. 59(2), 541-556. PMid:21717850.; Souza et al., 2017Souza, D.C., Cunha, E.R., Murillo, A.R., Silveira, M.J., Pulzatto, M.M., Dainez-Filho, M.S., Lolis, L.A., & Thomaz, S.M., 2017. Species inventory of aquatic macrophytes in the last undammed stretch of the Upper Paraná River, Brazil. Acta Limnol. Bras. 29(0), e115. http://dx.doi.org/10.1590/s2179-975x6017.
http://dx.doi.org/10.1590/s2179-975x6017...
). Through measurements carried out in the field with the aid of a 0.25 m2 metal square, similar to that performed by Murillo et al. 2019, we visually evaluated the identity and density of species that co-occur with P. ferrugineum. We observed that in banks dominated by this species, there is rarely the presence of Alternanthera philoxeroides (Marthius) Grisebach (Amaranthaceae). This observation allows us to question whether, under natural conditions, a high density of P. ferrugineum individuals limits the establishment of A. philoxeroides.
It should be noted that these two macrophytes are emergent (Thomaz et al., 2002Thomaz, S.M., Pagioro, T.A., Bini, L.M., & Souza, D.C., 2002. Macrófitas aquáticas da planície de Inundação do Alto rio Paraná: listagem de espécies e padrões de diversidade em ampla escala [online]. PELD, Relatório Anual 2002. Retrieved in 2020, May 30, from http://www.peld.uem.br/Relat2002/pdf/comp_biotico_macrofitas.pdf.
http://www.peld.uem.br/Relat2002/pdf/com...
) and native to aquatic environments in South America (Sainty et al., 1998Sainty, G., McCorkelle, G., & Julien, M., 1998. Control and spread of Alligator Wedd Alternanthera philoxeroides (Mart.) Griseb. in Australia: lessons for others regions. Wetlands Ecol. Manage. 5(3), 195-201. http://dx.doi.org/10.1023/A:1008248921849.
http://dx.doi.org/10.1023/A:100824892184...
; Costa et al., 2005Costa, N.V., Cardoso, L.A., Marchi, S.R., Domingos, V.D., & Martins, D., 2005. Controle químico de plantas daninhas aquáticas: Alternanthera philoxeroides, Enhydra anagallis e Pycreus decumbens. Planta Daninha 23(2), 335-342. http://dx.doi.org/10.1590/S0100-83582005000200022.
http://dx.doi.org/10.1590/S0100-83582005...
; Ferreira et al., 2011Ferreira, F.A., Mormul, R.P., Thomaz, S.M., Pott, A., & Pott, V.J., 2011. Macrophytes in the Upper Paraná River Floodplain: checklist and comparison with other large South American Wetlands. Rev. Biol. Trop. 59(2), 541-556. PMid:21717850.), and that A. philoxeroides becomes invasive in parts of the world such as China and the United States (Yu et al., 2007Yu, L.Q., Fujiii, Y., Zhou, Y., Zhang, J., Lu, Y., & Xuan, S. 2007. Response of exotic invasive weed Alternanthera philoxeroides to environmental factors and its competition with rice. Rice Sci. 14(1), 49-55. http://dx.doi.org/10.1016/S1672-6308(07)60008-0.
http://dx.doi.org/10.1016/S1672-6308(07)...
; Julien et al., 1995Julien, M.H., Skarratt, B., & Maywald, G.F., 1995. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manage. 33, 55-60.). Previous studies carried out with this species in environments that are considered invasive have shown its greater tolerance and productivity in soils with different nutrient availability (Chen et al., 2013Chen, Y., Zhou, Y., Yin, T.F., Liu, C.X., & Luo, F.L., 2013. The invasive wetland plant Alternanthera philoxeroides shows a higher tolerance to waterlogging than its native congener Alternanthera sessilis. PLoS One 8(11), e81456. PMid:24303048. http://dx.doi.org/10.1371/journal.pone.0081456.
http://dx.doi.org/10.1371/journal.pone.0...
). Sun et al. (2020)Sun, J., Javed, Q., Azeem, A., Ullah, M.S., Rasool, G., & Du, D., 2020. Addition of Phosphorus and nitrogen support the invasiveness of Alternanthera philoxeroides under water stress. Clean Soil Air Water 48(9), 2000059. http://dx.doi.org/10.1002/clen.202000059.
http://dx.doi.org/10.1002/clen.202000059...
, for example, observed better growth performance and biomass production in A. philoxeroides planted in soils with combined nitrogen and phosphorus addition, despite stressful water conditions. Despite this, it is necessary to observe the responses of this species to different nutrient availability and in interaction with other macrophyte species in environments where it is native.
The aim of this study was to investigate how the density of P. ferrugineum and the availability of nutrients in the sediment influence the establishment of A. philoxeroides. The experiment was carried out in a greenhouse to test the following hypotheses: i) The establishment of A. philoxeroides is negatively affected when this species grow together with high densities of P. ferrugineum. We predict that A. philoxeroides will present reductions in values of morphological characters when submitted to high densities of P. ferrugineum in comparison with the control treatment. The hypotheses ii) is that the establishment of A. philoxeroides is positively influenced when this species grow in conditions with high availability of nutrients, regardless of the density of P. ferrugineum. We predict that A. philoxeroides will not show a reduction in the values of morphological traits in conditions of high nutrient concentration (e.g. nitrogen and phosphorus) of sediment because greater avaliables of these nutrients of sediment inhibits the impact of high densities of Polygonum ferrugineum.
2. Material and Methods
2.1. Plant materials
To investigate the effects of the density of P. ferrugineum and the nutrient available in the sediment on the development of A. philoxeroides, both species were collected in Lagoa das Garças, located in Upper Paraná River floodplain (22 ° 45'S, 53 ° 15'W and 22 ° 45'S, 53 ° 30'W) in October 2018. In the field, vegetative stem branches located in the median portion of the stem axis of these individuals were collected, which consisted of well-developed structures, excluding, therefore, apical and basal portions of P. ferrugineum and A. philoxeroides.
These plants were taken to the aquatic macrophyte laboratory at the Universidade Estadual de Maringá (NUPÉLIA-UEM), where they were carefully washed with tap water to remove the adhered material (periphyton, algae, and invertebrates, etc.). Leaves and roots that the stem branches already had were also removed and the stems were sectioned so that each fragment obtained was composed of two nodes and a region of the internode (approximately 10 cm). After these fragments were put in the plastic recipient (43.5 cm x 29.3 cm x 7.7 cm) containing a 4 cm layer of sediment kept moist with a 2 cm water slide inside each recipient. We shower each of these plastic recipients every two days to avoid possible evaporation-related water loss. The stem fragments, inserted in this plastic recipient, were accommodated in an experimental area subjected to these conditions for 24 days, the time necessary for the development of new leaves, shoots, and roots.
For the experiment, after the acclimatization interval, the newly developed propagules were randomly transplanted into 36 experimental plastic buckets of 80 L, considered mesocosms, containing 5 kg of wet sediment each. Due to similar ages and sizes, all fragments used in this experiment were considered equivalent. In 18 mesocosms arranged in the experimental area, 12.5 g of mineral fertilizer NPK Vitaplan (4-8-4) were added at the beginning of the experiment. This addition of fertilizer was manipulated to make the sediment rich in nutrients, considered a high nutrient (H nutrient) treatment. For the other 18 mesocosms, which did not receive fertilizers, we considered the treatment as poor in nutrients (L nutrient). At the beginning of the experiment, two liters of tap water were added to each experimental bucket to keep the sediment moist. To avoid possible losses related to evaporation, the experimental buckets were monitored throughout the experiment, with water being replaced whenever necessary.
2.2. Experimental design
The experiment was conducted in an experimental area at the Universidade Estadual de Maringá (UEM) from November 2018 to January 2019, comprising an interval of 60 days. The experimental design employed in this study was the additive model to test competition, revised by Gibson et al. (1999)Gibson, D.J., Connolly, J., Hartnett, D.C., & Weidenhamer, J.D., 1999. Designs for greenhouse studies of interactions between plants. J. Ecol. 87(1), 1-16. http://dx.doi.org/10.1046/j.1365-2745.1999.00321.x.
http://dx.doi.org/10.1046/j.1365-2745.19...
, in which the density of the target species is kept constant (in this case one individual) and the density of the neighbor species varies (two or five individuals, in our experiment). Such a model, in this way, can be used to investigate the competitive interactions between macrophytes that occur in natural environments, and to examine whether the density of a neighbor species can influence the development or establishment of a target species.
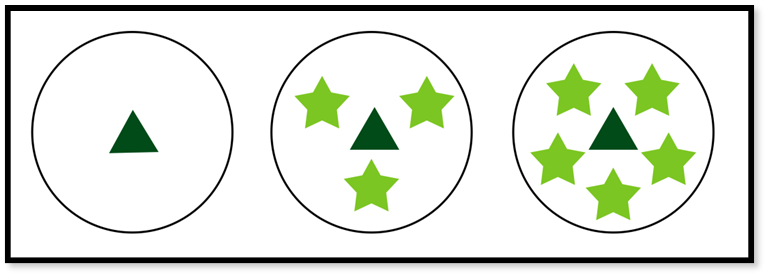
Our experimental design had the following arrangement: i) control treatment: a fragment of A. philoxeroides, considered our target species, planted alone (under L nutrient and H nutrient conditions); ii) low density treatment: an A. philoxeroides propagule in the presence of three propagules of the neighbor species P. ferrugineum (under L nutrient and H nutrient conditions) and iii) high density treatment: an A. philoxeroides propagule was planted in the presence of five P. ferrugineum propagules (both under L nutrient and H nutrient conditions) (Figure 1).
Experimental design of the control treatment, in high and low density of Polygonum ferrugineum to which Alternanthera philoxeroides was submitted. Each of these treatments had 12 replicates (half in high nutrient availability - H Nutrient; half in low nutrient availability - L Nutrient), totaling 36 experimental units. Alternanthera philoxeroides (▲); Polygonum ferrugineum (★).
Each of these treatments had six replicates for each nutrient concentration, high (12.5 g of nutrients added to the sediment) or low (no nutrients added to the sediment), totaling 36 experimental plastic recipients randomly placed inside the greenhouse. The use of three and five P. ferrugineum propagules to represent the two densities of neighbor species that we used in our experimental design was based on the results obtained in situ. For this, we used a 0.25 m2 metal square to evaluate the density of P. ferrugineum in macrophyte banks in Upper Paraná River floodplain in which it co-occurred with at least one individual of A. philoxeroides, according to the approach used by Murillo et al. (2019)Murillo, R.A., Alves, D.C., Machado, R.S., Silveira, M.J., Rodrigues, K.F., & Thomaz, S.M., 2019. Responses of two macrophytes of the genus Polygonum to water level fluctuations and interspecific competition. Aquat. Bot. 157, 10-16. http://dx.doi.org/10.1016/j.aquabot.2019.05.003.
http://dx.doi.org/10.1016/j.aquabot.2019...
.
After 60 days, the plants were removed from the treatments, washed to remove the sediment adhered to the roots and the values of the height above the sediment and the length of the main root were measured with a tape measure. Subsequently, the shoot and the roots were separated with scissors, packed in newspaper, and taken to dry in an oven at a continuous temperature of 60 ° C until they obtained constant biomass. The plant material was then removed from the greenhouse and the aboveground and root dry weight biomass were measured with one precision digital scale.
2.3. Statistical analyses
To statistically analyze the values recorded for A. philoxeroides after the period in which competition for density and at two different nutritional levels was submitted, the following response variables were considered: root and shoot biomass, total biomass, shoot height and, length root. For each of these variables, the assumptions of normality were examined with the Shapiro-Wilk (W) test and the homoscedasticity of the data with the Levene test, both with a significance level > 0.05. After, a two-way ANOVA (P < 0.05) was performed in the Statistica 7.0 software to test the statistical difference of the effects on nutrients treatments, neighbors species density and their interactions on parameter variation. Tukey HSD test were performed to examine the differences among the treatment. The values of all response variables, except root length, were transformed with log (x + 1) to fit the assumptions of normality and homoscedasticity of the data required to perform a two-way analysis of variance (ANOVA).
2.4. Index of ecological interactions between species
For the purpose of measure and classify the ecological interactions that occurred in the different treatments used in our experiment, we calculated the relative interaction index (RII) proposed by Armas et al. (2004)Armas, C., Ordiales, R., & Pugnaire, F.I., 2004. Measuring plant interactions: a new comparative index. Ecology 85(10), 2682-2686. http://dx.doi.org/10.1890/03-0650.
http://dx.doi.org/10.1890/03-0650...
on the total biomass response variable. For this, we use the formula (Equation 1):
where Bp + N is the value of the total biomass of the target plant in the presence of neighbors and Bp – N is the value of the total biomass of the target plant in the absence of neighbors, as used in Souza et al. (2020)Souza, S.N.G., Piedade, M.T.F., Demarchi, L.O., & Lopes, A., 2020. Implications of global changes for the development and ecological interactions between two key Amazonian aquatic macrophytes. Acta Bot. Bras. 35(1), 111-121. http://dx.doi.org/10.1590/0102-33062020abb0138.
http://dx.doi.org/10.1590/0102-33062020a...
. The RII is an index with values ranging from -1 to 1, where negative and positive values indicate competition and facilitation ecological interactions, respectively. Values close to 0, on the other hand, imply neutral relationships. Finally, we ran a two-way ANOVA on the values obtained for the RII, in order to evaluate the effect of nutrient concentration, neighbor species densities and their interactions on the ecological relationships developed between A. philoxeroidesand P. ferrugineum.
3. Results
The results obtained from our greenhouse study indicated the strong effects of the density of the neighbor species P. ferrugineum and the concentration of nutrients in the sediment on the establishment of A. philoxeroides (Table 1).
Anova two-way results testing the effects of nutrients and densities, and their interactions with morphological traits of Alternanthera philoxeroides.
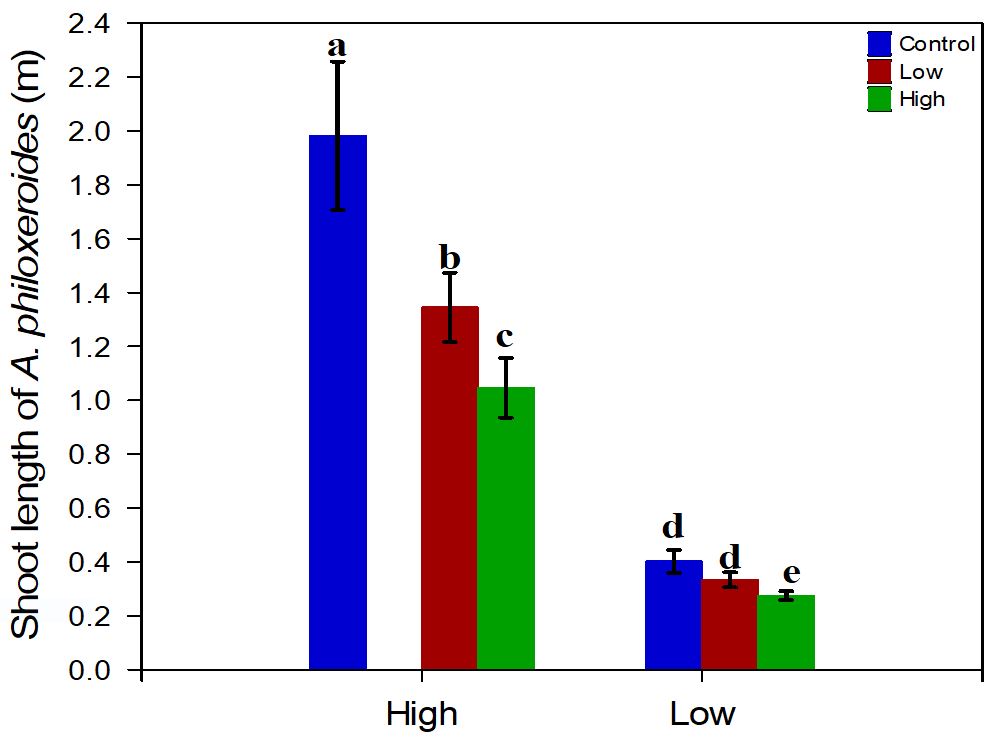
Two-way ANOVA revealed that P. ferrugineum density significantly decreases the shoot length of A. philoxeroides (P> 0.000). The effects of nutrient availability in the sediment (P < 0.000), on the other hand, indicated that there was a significantly greater growth of the shoot length of the target species in the H nutrient treatments in relation to the control treatment (Figure 2), a fact also confirmed by the Tukey's post-hoc test (Table 2). Furthermore, there was no significant interaction between the two factors tested for this response variable (P>0.385).
Shoot length of Alternanthera philoxeroides according to the density of neighbor specie (H density or L density), the nutrient level (H nutrient or L nutrient) and treatment control.
Results of Tukey test carried a posteriori of ANOVA two way to verify the difference of density of P. ferrugineum and nutrient on shoot length of A. philoxeroides.
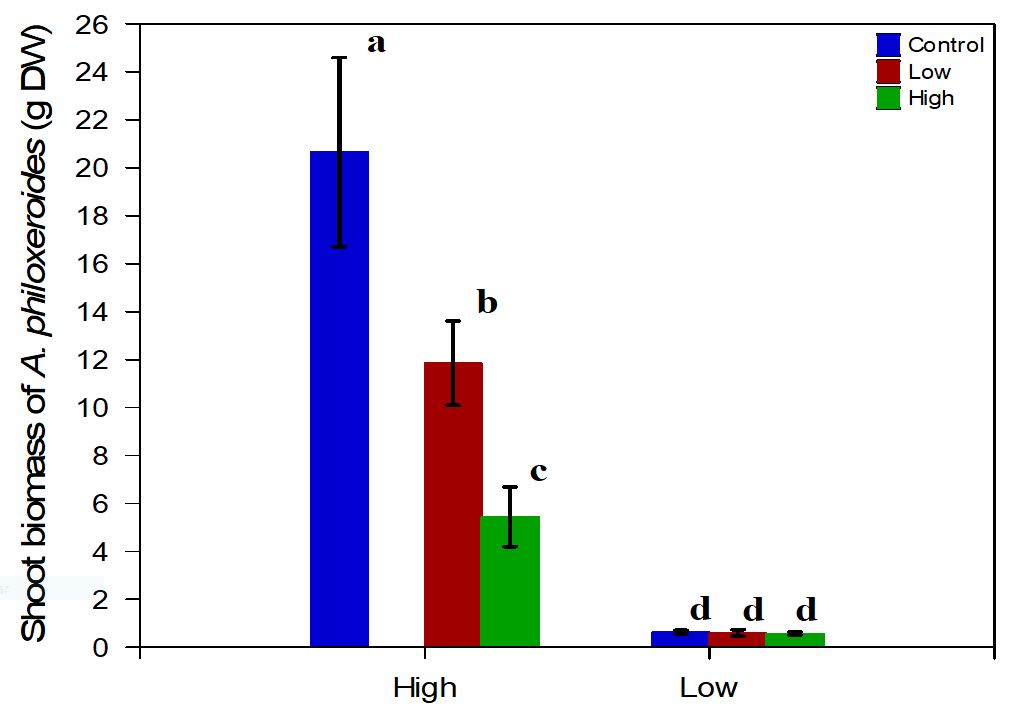
The density of neighbor species (P>0.009), the availability of nutrients in the sediment (P<0.000) and the interaction between these two predictors (P>0.020) had significant effects on the shoot biomass of the target specie (Table 1). There was a significant increase in the values recorded for this response variable according to the concentration of nutrients, and the shoot biomass of A. philoxeroides was 16.36 g in the H nutrient X L density treatment, approximately 52.77 times greater than that of the H nutrient X L density treatment. lowest value recorded in the L nutrient X H density treatment (Figure 3). Another trend observed was the decrease in shoot biomass with increasing density, which ranged from 0.31 g to 16.36 g, with the highest value recorded for the H nutrient X L density treatment.
Shoot biomass of Alternanthera philoxeroides according to the density of neighbor specie (H density or L density), the level of nutrients (H nutrient or Lnutrient) and the treatment control.
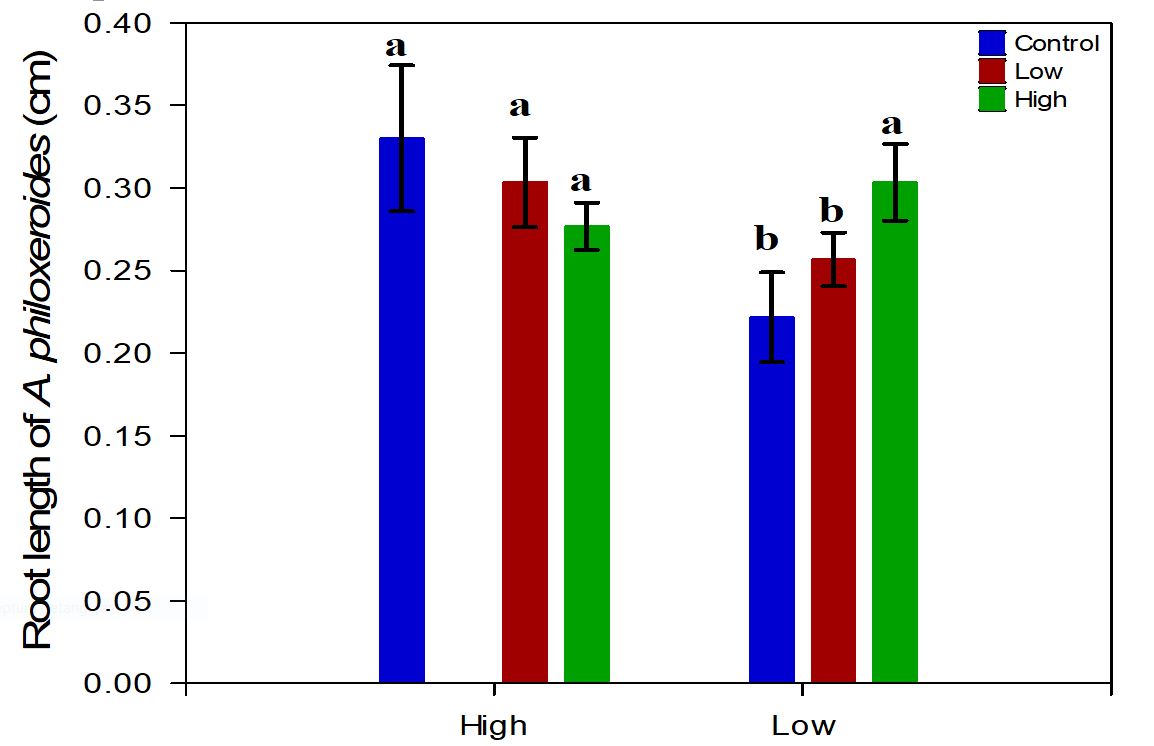
Neighbor density (P>0.855), nutrient availability (P>0.052) and the interaction between these factors (P>0.073) had no significant effects on root length (Table 1). Although not significant, with the increase in the availability of nutrients in the sediment, the length of the roots of the target species showed an opposite trend in relation to the density of neighbors. Thus, A. philoxeroides had greater root growth in the L nutrient X H density treatment, to the detriment of the lower root growth presented in the H nutrient X L density treatment (Figure 4).
Root length of Alternanthera philoxeroides according to the density of neighbor specie (H density or Ldensity), the level of nutrients (H nutrient or Lnutrient) and the treatment control.
A largely negative effect of nutrient availability on root biomass was shown by bidirectional ANOVA (P < 0.000). The Tukey test revealed that nutrient availability in the sediment was a considerable factor: all replicas of target species subjected to treatments with low nutrient availability, whether in high or low P. ferrugineum density, suffered root biomass loss (Table 3). The effects of density (P> 0.048) and interaction between density and nutrients (P> 0.043) were also significant (Table 1), with A. philoxeroides accumulating on average 4.02 g more root biomass in the H nutrient + L density treatments. than in the H nutrient + H density treatment (Figure 5).
Results of Tukey test carried a posteriori of ANOVA two way to verify the difference of density of P. ferrugineum and nutrient on root biomass of A. philoxeroides.
Root biomass of Alternanthera philoxeroides according to the density of neighbor specie (H density or L density), the level of nutrients (H nutrient or L nutrient) and the treatments controls.
As expected, total biomass was significantly affected by density (P>0.013), nutrient availability in the sediment (P<0.000) and by the interaction between these two factors (P>0.015) (Table 1). Generally, the values obtained for the total biomass increased with the greater availability of nutrients in the sediment and decreased with the increase in the density of neighbor specie (Figure 6). The highest values of total biomass were obtained in the treatment H nutrient + L density and the lowest in the treatments L nutrient + H density and L nutrient + L density (Table 4). Comparatively, the A. philoxeroides propagules submitted to the H nutrient + L density treatment had a total biomass 20.05 times greater than the propagules of the L nutrient + H density treatments, for example.
Total biomass of Alternanthera philoxeroides according to the density of neighbor specie (H density or Ldensity), the level of nutrient (Hnutrient or Lnutrient) and the treatment control.
Results of Tukey test carried a posteriori of ANOVA two way to verify the difference of density of P. ferrugineum and nutrient on total biomass of A. philoxeroides.
The RII index was statistically significant between the different nutrient concentrations (P > 0.008), but it was not significant for the density of the neighbor specie (P > 0.229) nor for the interaction between these two predictors (P > 0.290). According to the RII, the ecological interactions developed between A. philoxeroides and P. ferrugineum in the H nutrient + L density treatment varied greatly between competitive, facilitation and neutral. For the L nutrient + L density treatment, the interactions were strongly competitive. In the H nutrient + H density treatment, the ecological relationships alternated between competitive and facilitation. Finally, in the L nutrient + H density treatment, A. philoxeroides showed competitive disadvantages in all experimental units, except for one in which the RII results indicated a neutral relationship (Figure 7).
RII index demonstrating the ecological relationships between Alternanthera philoxeroides in relation to the density of the neighbor specie Polygonum ferrugineum and the availability of nutrients. Values: 0 indicates neutrality, < 0 indicates competition, and > 0 facilitation.
4. Discussion
The results obtained with our experimental study demonstrated the effects of the density of neighbor specie and of nutrient concentration dissolved in the sediment on the establishment of A. philoxeroides. The growth of this species was suppressed when it was competing with high densities of the neighbor specie P. ferrugineum (a condition also verified through field observations) mainly in soils characterized as low nutrients by our experimental design. Thus we can corroborate the two hypotheses where the establishment of A. philoxeroides would be negatively affected when this species grow together with high densities of P. ferrugineum and that the establishment of A. philoxeroideswould be positively influenced when this species grow in conditions with high availability of nutrients, regardless of the density of P. ferrugineum.
Our results were in agreement with those found in other studies with A. philoxeroides. When comparing the abundance and performance of this species with the native Alternanthera sessilis in different microhabitats of the riparian zone of the Puyang River (China), Pan et al. (2006)Pan, X., Geng, Y., Zhang, W., Li, B., & Chen, J., 2006. The influence of abiotic stress and phenotypic plastic on the distribution of invasive Alternanthera philoxeroides along a riparian zone. Acta Oecol. 30(3), 333-341. http://dx.doi.org/10.1016/j.actao.2006.03.003.
http://dx.doi.org/10.1016/j.actao.2006.0...
observed that the performance of A. philoxeroides was better in more productive habitats, contrary to what occurred in less productive areas where the native A. sessilis had a greater relative coverage. Despite these results, the authors found that A. philoxeroides had a good ability to establish itself in less productive places with a high density of A. sessilis, standing out in relation to other native species such as Polygonum hydropiper, which occurred in these microhabitats.
These findings indicate tolerance and a certain dominance in relation to other neighbors species, unlike what we observed for A. philoxeroides in Paraná River habitats, where they rarely co-occur in environments with a high density of species such as P. ferrugineum. Regarding the performance of A. philoxeroides in relation to the fluctuation of the level of nutrients in the soil, Chen et al. (2013)Chen, Y., Zhou, Y., Yin, T.F., Liu, C.X., & Luo, F.L., 2013. The invasive wetland plant Alternanthera philoxeroides shows a higher tolerance to waterlogging than its native congener Alternanthera sessilis. PLoS One 8(11), e81456. PMid:24303048. http://dx.doi.org/10.1371/journal.pone.0081456.
http://dx.doi.org/10.1371/journal.pone.0...
demonstrated that this species had a greater ability to obtain resources in environments with low nutrient availability than A. sessilis, which is also contradictory to our findings. These divergences found in relation to the literature are understandable since A. philoxeroides becomes invasive in several countries (Julien et al., 1995Julien, M.H., Skarratt, B., & Maywald, G.F., 1995. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manage. 33, 55-60.; Geng et al., 2006Geng, Y.P., Pan, X.Y., Xu, C.Y., Zhang, W.J., Li, B., & Chen, J.K., 2006. Phenotypic plasticity of invasive Alternanthera philoxeroides in relation to different water availability compared to its native congener. Acta Oecol. 30(3), 380-385. http://dx.doi.org/10.1016/j.actao.2006.07.002.
http://dx.doi.org/10.1016/j.actao.2006.0...
; Schooler et al., 2007Schooler, S.S., Yeates, A., Wilson, J.R., & Julien, M.H., 2007. Herbivory, mowing, and herbicides differently affect production and nutrient allocation of Alternanthera philoxeroides. Aquat. Bot. 86(1), 62-68. http://dx.doi.org/10.1016/j.aquabot.2006.09.004.
http://dx.doi.org/10.1016/j.aquabot.2006...
), presenting advantages in the competition with native species. In aquatic environments in South America, where this species comes from, a lower competitive capacity may help to explain its low frequency of occurrence in banks dominated by P. ferrugineum.
In general terms, we found that mesocosms with high availability of nutrients allowed greater length and shoot biomass in A. philoxeroides, which indicates that there was a greater investment in the development of the photosynthetic organ in accordance with the increase of nutrients in the sediment. However, even with high nutrient availability, A. philoxeroides accumulated less shoot biomass in treatments with high density of P. ferrugineum than in treatments with low density of the neighbor specie, which shows the strong effects of density. When analyzing the effects of density and nutrient addition on Spartina alterniflora, Zhao et al. (2010)Zhao, Y.J., Qing, H., Zhao, C.J., Zhou, C.F., Zhang, W.G., Xiao, Y., & An, S.Q., 2010. Phenotypic plasticity of Spartina alterniflora and Phragmites australis in response to nitrogen addition and intraspecific competition. Hydrobiologia 637(1), 143-155. http://dx.doi.org/10.1007/s10750-009-9992-5.
http://dx.doi.org/10.1007/s10750-009-999...
observed a lower growth of shoot length when this species grew in environments with high density of neighbors species and a higher rate of shoot biomass in treatments with high availability of nutrients, with results similar to ours.
We also found that the root length of A. philoxeroides did not show statistically significant differences in relation to the density and nutrient conditions tested in our experiment. A graphical analysis of this response variable, however, allows us to identify that there was a slight tendency towards greater growth of A. philoxeroides root in treatments with low nutrient availability and high density of the neighbor specie, with the result of the two-way Anova for the nutrients (p> 0.052) being very close to p <0.05. This greater growth of A. philoxeroides root, when associated with high densities of P. ferrugineum and low nutrient availability, may be a direct response to the stress caused by increased competition.
In fact, plants with longer root lengths can acquire more nutrients in soils with low availability of this resource (Gioria & Osborne, 2014Gioria, M., & Osborne, B.A., 2014. Resource competition in plant invasions: emerging patterns and research needs. Front. Plant Sci. 5, 501. PMid:25324851. http://dx.doi.org/10.3389/fpls.2014.00501.
http://dx.doi.org/10.3389/fpls.2014.0050...
), which is an expected morphological response for macrophytes in a competitive state (Kankanamge & Kodithuwakku, 2017Kankanamge, C.E., & Kodithuwakku, H., 2017. Effect of interspecific competition on the growth and nutrient uptake of three macrophytes in nutrient-rich water. Aquat. Ecol. 51(4), 625-634. http://dx.doi.org/10.1007/s10452-017-9640-5.
http://dx.doi.org/10.1007/s10452-017-964...
; Craine & Dybzinski, 2013Craine, J.M., & Dybzinski, R., 2013. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 27(4), 833-840. http://dx.doi.org/10.1111/1365-2435.12081.
http://dx.doi.org/10.1111/1365-2435.1208...
). When examining the vegetative growth patterns of three annual species, McConnaughay & Coleman (1999)McConnaughay, K.D.M., & Coleman, J.S., 1999. Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80(8), 2581-2593. http://dx.doi.org/10.1890/0012-9658(1999)080[2581:BAIPOO]2.0.CO;2.
http://dx.doi.org/10.1890/0012-9658(1999...
found that there was greater investment in the growth of Polygonum pensylvanicum roots under conditions characterized as oligotrophic, results similar to ours.
The root biomass response variable, on the other hand, showed a significant increase in treatments with high nutrient availability, especially at low densities of neighbor specie. This reaction may be evidence that A. philoxeroides has its ability to allocate biomass to the roots negatively influenced when it coexists with P. ferrugineum in environments with low nutrient availability. The information available in the ecological literature of A. philoxeroides indicates that stressful conditions can, in fact, alter the rates of biomass allocation to the root of this species (Geng et al., 2006Geng, Y.P., Pan, X.Y., Xu, C.Y., Zhang, W.J., Li, B., & Chen, J.K., 2006. Phenotypic plasticity of invasive Alternanthera philoxeroides in relation to different water availability compared to its native congener. Acta Oecol. 30(3), 380-385. http://dx.doi.org/10.1016/j.actao.2006.07.002.
http://dx.doi.org/10.1016/j.actao.2006.0...
).
According to our analyses, treatments with high nutrient availability can positively influence the total biomass of A. philoxeroides, especially when the density of P. ferrugineum was low. Under these conditions, competition for nutrients tends to be lower, favoring the acquisition of these resources by our target species, which was potentiated in mesocosms with high availability of nutrients. These results are strongly supported by other experimental studies. When investigating the effects of nutrient addition on three emergent macrophytes, Deegan et al. (2012)Deegan, B., White, S., & Ganf, G., 2012. Nutrients and water levels fluctuations: a study of three aquatic plants. River Res. Appl. 28(3), 359-368. http://dx.doi.org/10.1002/rra.1461.
http://dx.doi.org/10.1002/rra.1461...
noticed a significant difference in the total biomass of Cyperus gymnocaulos at higher nutrient concentrations, while there were no differences for this variable response in Triglochin procerum and Typha. domingensis. Macek & Rejmánková (2007)Macek, P., & Rejmánková, E., 2007. Response of emergent macrophytes to experimental nutrient and salinity addition. Funct. Ecol. 21(3), 478-488. http://dx.doi.org/10.1111/j.1365-2435.2007.01266.x.
http://dx.doi.org/10.1111/j.1365-2435.20...
observed that the total biomass of Typha domingensis was positively correlated with nitrogen and phosphorus, and only with nitrogen in Eleocharis cellulosa.
The RII index showed that there are changes in the type and intensity of ecological interactions developed between A. philoxeroides and P. ferrugineum, depending mainly on the level of nutrients available in the sediment. According to the results of this index for our experiment, it is possible to affirm that the effect of competition by A. philoxeroides was more severe the higher the density of P. ferrugineum and the lower the availability of nutrients within each treatment. This finding may be related to the fact that coexistence with several plants can induce a given species to compete as neighbors acquire limiting nutrients from the soil (Craine & Dybzinski, 2013Craine, J.M., & Dybzinski, R., 2013. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 27(4), 833-840. http://dx.doi.org/10.1111/1365-2435.12081.
http://dx.doi.org/10.1111/1365-2435.1208...
). In other words, the resource-gathering capacity of A. philoxeroides was altered under these conditions, which in natural environments may limit the establishment of our target species.
In conclusion, the interspecific competition investigated here demonstrates that the negative effects found for A. philoxeroides were mediated by P. ferrugineum density and nutritional levels. According to Stoll & Weiner (2000)Stoll, P., & Weiner, J., 2000. A neighborhood view of interactions among individual plants. In: Dieckmann, U., Law, R. & Metz, J.A.J., eds. The geometry of ecological interactions: simplifying spatial complexity. Cambridge:Cambridge University Press. http://dx.doi.org/10.1017/CBO9780511525537.003.
http://dx.doi.org/10.1017/CBO97805115255...
, the result of competition in experiments that follow the neighbor-target species approach is determined by the ability of neighbor species to limit resources and the tolerance of target species to these restrictions, which can occur via plasticity phenotypic.
Inferences made from the morphological characters of A. philoxeroides, however, must be observed with caution. Our experimental design does not allow us to extrapolate the results obtained to situations where the density of P. ferrugineum is greater than that applied in this work, nor in scenarios with the establishment of propagules at different stages of development, as can happen in nature. In summary, our findings are important for understanding the rules of structuring and dynamics of emergent macrophyte communities, as well as helping to understand the ecological characteristics of A. philoxeroides in its natural range.
Acknowledgements
A.J.S. Vicente and L.F. Cândido thank Araucária Foundation for granting the scientific initiation scholarship. We also thank Itaipu Binacional for funding this research through the project (13,144 14): Studies of Aquatic Macrophytes.
References
- Almeida, V.L., Larrazábal, M.E., Moura, A.N., & Melo Júnior, M., 2006. Rotífera das zonas limnética e litorânea do reservatório de Tapacurá, Pernambuco, Brasil. Iheringia Ser. Zool. 96(4), 445-451. http://dx.doi.org/10.1590/S0073-47212006000400009
» http://dx.doi.org/10.1590/S0073-47212006000400009 - Armas, C., Ordiales, R., & Pugnaire, F.I., 2004. Measuring plant interactions: a new comparative index. Ecology 85(10), 2682-2686. http://dx.doi.org/10.1890/03-0650
» http://dx.doi.org/10.1890/03-0650 - Brahim, K., Ray, D.T., & Dierig, D.A., 1998. Growth and yield characteristics of Lesquerella fendleri as a function of plant density. Ind. Crops Prod. 9(1), 63-71. http://dx.doi.org/10.1016/S0926-6690(98)00015-6
» http://dx.doi.org/10.1016/S0926-6690(98)00015-6 - Chen, Y., Zhou, Y., Yin, T.F., Liu, C.X., & Luo, F.L., 2013. The invasive wetland plant Alternanthera philoxeroides shows a higher tolerance to waterlogging than its native congener Alternanthera sessilis. PLoS One 8(11), e81456. PMid:24303048. http://dx.doi.org/10.1371/journal.pone.0081456
» http://dx.doi.org/10.1371/journal.pone.0081456 - Costa, N.V., Cardoso, L.A., Marchi, S.R., Domingos, V.D., & Martins, D., 2005. Controle químico de plantas daninhas aquáticas: Alternanthera philoxeroides, Enhydra anagallis e Pycreus decumbens. Planta Daninha 23(2), 335-342. http://dx.doi.org/10.1590/S0100-83582005000200022
» http://dx.doi.org/10.1590/S0100-83582005000200022 - Craine, J.M., & Dybzinski, R., 2013. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 27(4), 833-840. http://dx.doi.org/10.1111/1365-2435.12081
» http://dx.doi.org/10.1111/1365-2435.12081 - Deegan, B., White, S., & Ganf, G., 2012. Nutrients and water levels fluctuations: a study of three aquatic plants. River Res. Appl. 28(3), 359-368. http://dx.doi.org/10.1002/rra.1461
» http://dx.doi.org/10.1002/rra.1461 - Du, Z.Y., & Wang, Q.F., 2014. Correlations of life form, pollination mode and sexual system in aquatic angiosperms. PLoS One 9(12), e115653. PMid:25525810. http://dx.doi.org/10.1371/journal.pone.0115653
» http://dx.doi.org/10.1371/journal.pone.0115653 - Esteves, F.A., & Caliman, A., 2011. Águas continentais: características do meio, compartimentos e suas comunidades. In: Esteves, F.A., ed. Fundamentos de limnologia. Rio de Janeiro: Interciência, 113-118, 3 ed.
- Ferreira, F.A., Mormul, R.P., Thomaz, S.M., Pott, A., & Pott, V.J., 2011. Macrophytes in the Upper Paraná River Floodplain: checklist and comparison with other large South American Wetlands. Rev. Biol. Trop. 59(2), 541-556. PMid:21717850.
- Geng, Y.P., Pan, X.Y., Xu, C.Y., Zhang, W.J., Li, B., & Chen, J.K., 2006. Phenotypic plasticity of invasive Alternanthera philoxeroides in relation to different water availability compared to its native congener. Acta Oecol. 30(3), 380-385. http://dx.doi.org/10.1016/j.actao.2006.07.002
» http://dx.doi.org/10.1016/j.actao.2006.07.002 - Gibson, D.J., Connolly, J., Hartnett, D.C., & Weidenhamer, J.D., 1999. Designs for greenhouse studies of interactions between plants. J. Ecol. 87(1), 1-16. http://dx.doi.org/10.1046/j.1365-2745.1999.00321.x
» http://dx.doi.org/10.1046/j.1365-2745.1999.00321.x - Gioria, M., & Osborne, B.A., 2014. Resource competition in plant invasions: emerging patterns and research needs. Front. Plant Sci. 5, 501. PMid:25324851. http://dx.doi.org/10.3389/fpls.2014.00501
» http://dx.doi.org/10.3389/fpls.2014.00501 - Julien, M.H., Skarratt, B., & Maywald, G.F., 1995. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manage. 33, 55-60.
- Kankanamge, C.E., & Kodithuwakku, H., 2017. Effect of interspecific competition on the growth and nutrient uptake of three macrophytes in nutrient-rich water. Aquat. Ecol. 51(4), 625-634. http://dx.doi.org/10.1007/s10452-017-9640-5
» http://dx.doi.org/10.1007/s10452-017-9640-5 - Kobayashi, T., Ryder, D.S., Gordon, G., Shannon, I., Ingleton, T., Carpenter, M., & Jacobs, S.J., 2009. Short-term response of nutrients, carbon and planktonic microbial communities to floodplain wetland inundation. Aquat. Ecol. 43(4), 843-858. http://dx.doi.org/10.1007/s10452-008-9219-2
» http://dx.doi.org/10.1007/s10452-008-9219-2 - Macek, P., & Rejmánková, E., 2007. Response of emergent macrophytes to experimental nutrient and salinity addition. Funct. Ecol. 21(3), 478-488. http://dx.doi.org/10.1111/j.1365-2435.2007.01266.x
» http://dx.doi.org/10.1111/j.1365-2435.2007.01266.x - McConnaughay, K.D.M., & Coleman, J.S., 1999. Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80(8), 2581-2593. http://dx.doi.org/10.1890/0012-9658(1999)080[2581:BAIPOO]2.0.CO;2
» http://dx.doi.org/10.1890/0012-9658(1999)080[2581:BAIPOO]2.0.CO;2 - Muller, I., Schmid, B., & Weiner, J., 2000. The effect of nutrient availability on biomass allocation Patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 3(2), 115-127. http://dx.doi.org/10.1078/1433-8319-00007
» http://dx.doi.org/10.1078/1433-8319-00007 - Murillo, R.A., Alves, D.C., Machado, R.S., Silveira, M.J., Rodrigues, K.F., & Thomaz, S.M., 2019. Responses of two macrophytes of the genus Polygonum to water level fluctuations and interspecific competition. Aquat. Bot. 157, 10-16. http://dx.doi.org/10.1016/j.aquabot.2019.05.003
» http://dx.doi.org/10.1016/j.aquabot.2019.05.003 - Padial, A.A., Carvalho, P., Thomaz, S.M., Boschilia, S.M., Rodrigues, R.B., & Kobayashi, J.T., 2009. The role of an extreme flood disturbance on macrophyte assemblages in a Neotropical floodplain. Aquat. Sci. 71(4), 389-398. http://dx.doi.org/10.1007/s00027-009-0109-z
» http://dx.doi.org/10.1007/s00027-009-0109-z - Pan, X., Geng, Y., Zhang, W., Li, B., & Chen, J., 2006. The influence of abiotic stress and phenotypic plastic on the distribution of invasive Alternanthera philoxeroides along a riparian zone. Acta Oecol. 30(3), 333-341. http://dx.doi.org/10.1016/j.actao.2006.03.003
» http://dx.doi.org/10.1016/j.actao.2006.03.003 - Peršić, V., Horvatic, J., Has-Schon, E., & Bogut, I., 2009. Changes in N and P limitation induced by water level fluctuations in Nature Park Kopački Rit (Croatia): nutrient enrichment bioassay. Aquat. Ecol. 43(1), 27-36. http://dx.doi.org/10.1007/s10452-007-9156-5
» http://dx.doi.org/10.1007/s10452-007-9156-5 - Rolon, A.S., Lacerda, T., Maltchik, L., & Guadagnin, D.L., 2008. Influence of area, habitat and water chemistry on richness and composition of macrophyte assemblages in southern Brazilian wetlands. J. Veg. Sci. 19(2), 221-228. http://dx.doi.org/10.3170/2008-8-18359
» http://dx.doi.org/10.3170/2008-8-18359 - Sainty, G., McCorkelle, G., & Julien, M., 1998. Control and spread of Alligator Wedd Alternanthera philoxeroides (Mart.) Griseb. in Australia: lessons for others regions. Wetlands Ecol. Manage. 5(3), 195-201. http://dx.doi.org/10.1023/A:1008248921849
» http://dx.doi.org/10.1023/A:1008248921849 - Schooler, S.S., Yeates, A., Wilson, J.R., & Julien, M.H., 2007. Herbivory, mowing, and herbicides differently affect production and nutrient allocation of Alternanthera philoxeroides. Aquat. Bot. 86(1), 62-68. http://dx.doi.org/10.1016/j.aquabot.2006.09.004
» http://dx.doi.org/10.1016/j.aquabot.2006.09.004 - Sculthorpe, C.D. 1967. The biology of Aquatic Vascular Plants. London: Edward Arnold Publishers.
- Silveira, M.J., Alves, D.C., & Thomaz, S.M., 2018. Effects of the density of the invasive macrophyte Hydrilla verticillata and root competition on growth of one native macrophyte in different sediment fertilities. Ecol. Res. 33(5), 927-934. http://dx.doi.org/10.1007/s11284-018-1602-4
» http://dx.doi.org/10.1007/s11284-018-1602-4 - Souza, D.C., Cunha, E.R., Murillo, A.R., Silveira, M.J., Pulzatto, M.M., Dainez-Filho, M.S., Lolis, L.A., & Thomaz, S.M., 2017. Species inventory of aquatic macrophytes in the last undammed stretch of the Upper Paraná River, Brazil. Acta Limnol. Bras. 29(0), e115. http://dx.doi.org/10.1590/s2179-975x6017
» http://dx.doi.org/10.1590/s2179-975x6017 - Souza, S.N.G., Piedade, M.T.F., Demarchi, L.O., & Lopes, A., 2020. Implications of global changes for the development and ecological interactions between two key Amazonian aquatic macrophytes. Acta Bot. Bras. 35(1), 111-121. http://dx.doi.org/10.1590/0102-33062020abb0138
» http://dx.doi.org/10.1590/0102-33062020abb0138 - Stoll, P., & Weiner, J., 2000. A neighborhood view of interactions among individual plants. In: Dieckmann, U., Law, R. & Metz, J.A.J., eds. The geometry of ecological interactions: simplifying spatial complexity. Cambridge:Cambridge University Press. http://dx.doi.org/10.1017/CBO9780511525537.003
» http://dx.doi.org/10.1017/CBO9780511525537.003 - Sun, J., Javed, Q., Azeem, A., Ullah, M.S., Rasool, G., & Du, D., 2020. Addition of Phosphorus and nitrogen support the invasiveness of Alternanthera philoxeroides under water stress. Clean Soil Air Water 48(9), 2000059. http://dx.doi.org/10.1002/clen.202000059
» http://dx.doi.org/10.1002/clen.202000059 - Thomaz, S.M., Pagioro, T.A., Bini, L.M., & Souza, D.C., 2002. Macrófitas aquáticas da planície de Inundação do Alto rio Paraná: listagem de espécies e padrões de diversidade em ampla escala [online]. PELD, Relatório Anual 2002. Retrieved in 2020, May 30, from http://www.peld.uem.br/Relat2002/pdf/comp_biotico_macrofitas.pdf
» http://www.peld.uem.br/Relat2002/pdf/comp_biotico_macrofitas.pdf - Wang, J.W., Yu, D., Xiong, W., & Han, Y.Q., 2008. Above- and belowground competition between two submersed macrophytes. Hydrobiologia 607(1), 113-122. http://dx.doi.org/10.1007/s10750-008-9371-7
» http://dx.doi.org/10.1007/s10750-008-9371-7 - Xie, Y.H., An, S., Wu, B., & Wang, W., 2006. Density-dependent root morphology and root distribution in the submerged plant Vallisneria natans. Environ. Exp. Bot. 57(1-2), 195-200. http://dx.doi.org/10.1016/j.envexpbot.2005.06.001
» http://dx.doi.org/10.1016/j.envexpbot.2005.06.001 - Yu, L.Q., Fujiii, Y., Zhou, Y., Zhang, J., Lu, Y., & Xuan, S. 2007. Response of exotic invasive weed Alternanthera philoxeroides to environmental factors and its competition with rice. Rice Sci. 14(1), 49-55. http://dx.doi.org/10.1016/S1672-6308(07)60008-0
» http://dx.doi.org/10.1016/S1672-6308(07)60008-0 - Zhao, Y.J., Qing, H., Zhao, C.J., Zhou, C.F., Zhang, W.G., Xiao, Y., & An, S.Q., 2010. Phenotypic plasticity of Spartina alterniflora and Phragmites australis in response to nitrogen addition and intraspecific competition. Hydrobiologia 637(1), 143-155. http://dx.doi.org/10.1007/s10750-009-9992-5
» http://dx.doi.org/10.1007/s10750-009-9992-5
Edited by
Publication Dates
-
Publication in this collection
28 Mar 2022 -
Date of issue
2022
History
-
Received
06 Nov 2021 -
Accepted
02 Feb 2022