ABSTRACT
The macroscopic anatomy and the microscopic and ultrastructural features of the Harderian gland (HG), lacrimal gland (LG) and superficial gland of the third eyelid (SGTE) of the adult European bison are described. In addition, morphometric studies were conducted and were followed by statistical analysis of the results. Tissue sections were stained with hematoxylin and eosin, methyl green-pyronin Y, periodic acid-Schiff, Alcian blue pH 2.5, aldehyde fuchsin and Hale's dialysed iron. Analysis of the staining showed that the HG has a multilobular tubuloalveolar structure with mixed secretion. The LG and the SGTE have a multilobar tubuloacinar structure with serous secretion in the LG and mucoserous in the SGTE. The TEM study demonstrates that the secretory cells of the HG, LG and SGTE have similar ultrastructural appearance, with two types of secretory vesicles in the cytoplasm of all studied glands. The histochemical staining methods and the TEM study revealed the secretory activity in the HG, LG and SGTE ducts. The structural studies can be important for establishing relations between morphological structure and functions of these glands. It can have clinical implications especially when taking into consideration the protective mechanisms of the eye.
KEY WORDS.
Accessory organs of the eye; European bison; morphology
The orbital glands, mainly lacrimal and Harderian glands (LG and HG), are important for normal eye physiology. Additionally, mammals with a third eyelid (TE) have the superficial gland of the third eyelid (SGTE). The HG is also known as the deep gland of the third eyelid (Nomina Anatomica Veterinaria 2012Nomina Anatomica Veterinaria (2012) Nomina Anatomica Veterinaria. Hannover, World Association of Veterinary Anatomists, 5th ed., p. 149-150. Available online at:Available online at:http://www.wava-amav.org/nav_nev.htm
[Accessed: 30/09/2015]
http://www.wava-amav.org/nav_nev.htm...
). Studies of the orbital glands were previously conducted in many species of animals (Aldana Marcos et al. 2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.,Djeridane 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.,Gargulio et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.,Johnston et al. 1983Johnston HS, Mcgadey J, Thompson GG, Moore MR, Payne AP (1983) The Harderian gland, its secretory and porphyrin content in the Mongolian gerbil (Meriones unguiculatus). Journal of Anatomy 137(3): 615-630.,1985Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.,Martin et al. 1988Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.,Pradidarcheep et al. 2003Pradidarcheep W, Asavapongpatana S, Mingsakul T, Poonkhum R, Nilbu-Nga S, Somana R (2003) Microscopic anatomy of the orbital Harderian gland in the common tree shrew (Tupaia glis). Journal of Morphology 255(3): 328-336. doi: 10.1002/jmor.10066
https://doi.org/10.1002/jmor.10066...
,Weaker 1981Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.). The HG and the SGTE are situated in the inner canthus, while the LG is located in the outer canthus of the orbit. The SGTE is a cluster of glandular tissue within the TE, while the LG is part of the lacrimal apparatus and is responsible for the production of the aqueous layer of the tear film (Payne 1994Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.,Shadkhast et al. 2008Sahadkhast M, Bigham AS, Vhadkhast N, Bigham AS, Vajdi N, Shafiei Z (2008) Lacrimal apparatus system in goat (Capra aegagrus hircus): Anatomical and radiological study. Asian Journal of Animal Veterinary Advance 3: 457-460. doi: 10.3923/ajava.2008.457.460
https://doi.org/10.3923/ajava.2008.457.4...
). The tear film is composed of three layers, where the aqueous layer is produced in most of animals by both LG and SGTE (Davidson & Kuonen 2004Davidson HJ, Kuonen VJ (2004) The tear film and ocular mucins. Veterinary Ophthalmology 7(2): 71-77. doi: 10.1111/j.1463-5224.2004.00325.x
https://doi.org/10.1111/j.1463-5224.2004...
). Furthermore, in some animals such as cattle, rodents and birds, the HG is the source of the aqueous layer of the tear film (Davidson & Kuonen 2004Davidson HJ, Kuonen VJ (2004) The tear film and ocular mucins. Veterinary Ophthalmology 7(2): 71-77. doi: 10.1111/j.1463-5224.2004.00325.x
https://doi.org/10.1111/j.1463-5224.2004...
). The aqueous layer, which is responsible for moisturizing and nurturing the cornea, is the major component of the tear film. The antibacterial factors in it, including immunoglobulins and soluble mucins, help to maintain corneal health (Iwata 1973Iwata S (1973) Chemical composition of the aqueous phase. International Ophthalmology Clinics 13(1): 29-46.).
The HG is the largest eye gland in many mammals and has been described for most terrestrial vertebrates (Payne 1994Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.,Rehorek et al. 2000Rehorek SJ, Firth BT, Hutchinson MN (2000) Can an orbital gland function in the vomeronasal sense? A study of the pygopodid Harderian gland. Canadian Journal of Zoology 78(4): 648-654. doi: 10.1139/cjz-78-4-648
https://doi.org/10.1139/cjz-78-4-648...
). Most of the research on it, however, concern rodents (Payne 1994Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.). The HG is mostly horseshoe-shaped with a small (upper) and a large (lower) lobe. The shape of this lobe varies in different species of animals (Payne 1994Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.). The HG of mammals is a relatively large, intraorbital gland situated on the anterior surface of the orbit. However, the anatomical location of this gland within the orbit, as well as its size and its morphological details, vary across mammalian species (Aldana Marcos & Affanni 2005Aldana Marcos HJ, Affanni JM (2005) Anatomy, histology, histochemistry and fine structure of the Harderian gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Anatomy & Embryology 209(5): 409-424.,Rehorek et al. 2007Rehorek SJ, Hillenius WJ, Sanjur J Chapman NG (2007) One gland, two lobes: organogenesis of the Harderian and "nictitans" gland of the Chinese muntjac (Muntiacus reevesi) and fallow deer (Dama dama). Annals of Anatomy 189(5): 434-446. doi: 10.1016/j.aanat.2006.10.007
https://doi.org/10.1016/j.aanat.2006.10....
). The HG can be classified based on histochemical properties into the following categories: serous, mucous, mixed and lipid gland (W.J. Paule unpubl. data). The HG may be of a compound tubular type or compound tubuloalveolar type surrounded by a network of myoepithelial cells (Payne 1994Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.,Sakai 1981Sakai T (1981) The mammalian Harderian gland: morphology, biochemistry, function and physiology. Archives of Histology Japan 44(4): 299-333.,1992Sakai T (1992) Comparative anatomy of the mammalian Harderian glands. Berlin, Springer Verlag, p. 7-23.). The secretory products of the HG are important to its functioning (Buzzell 1996Buzzell GR (1996) The Harderian gland: perspectives. Microscopy Research and Technique 34(1): 2-5.). The main function of the HG is to lubricate the eye, whereas other functions vary among species. The HG is a production site for immunoglobulins, which are delivered to the conjunctival sac in birds (Fix & Arp 1991Fix AS, Arp LH (1991) Quantification of particle uptake by Conjunctiva-Associated Lymphoid Tissue (CALT) in chickens. Avian Diseases 35(1): 174-179.,Van Ginkel et al. 2012Van Ginkel FW, Gulley SL, Lammers A, Hoer FJ, Gurjar R, Toro H (2012) Conjunctiva-associated lymphoid tissue in avian mucosal immunity. Developmental & Comparative Immunology 36(2): 289-297. doi: 10.1016/j.dci.2011.04.012
https://doi.org/10.1016/j.dci.2011.04.01...
). In rodents the HG plays a photoprotective role and is a part of a photoreceptive pineal-related axis. It is also the source of pheromones and growth factors. In mammals the HG lubricates the eye and the TE (Chieffi et al. 1996Chieffi G, Baccari GC, Di Mateo L, D'Istria M, Minucci S, Varriale B (1996) Cell biology of the Harderian gland. International Review of Cytology 168: 1-80.).
Another important gland of the eye is the LG. The main structure of this gland consists of lobes, in which the tubular secretory epithelium drains into ducts and finally onto the ocular surface (Aldana Marcos et al. 2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.). In mammals the LG synthesizes and excretes different chemical substances, including proteins and mucosubstances, which are a part of the tear film (Pinard et al. 2003aPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003a) Evaluation of lysozyme and lactoferrin in lacrimal and other ocular glands of bison and cattle and in tears of bison. American Journal of Veterinary Research 64(1): 104-108.). The LG contains both B and T cells of the immune system, as well as plasma cells, which are scattered throughout the interstitium of the gland (Walcott 1998Walcott B (1998) The lacrimal gland and its veil of tears. Physiology 13: 97-103.). In general, the LG and the SGTE have tubuloacinar structures (Pinard et al. 2003aPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003a) Evaluation of lysozyme and lactoferrin in lacrimal and other ocular glands of bison and cattle and in tears of bison. American Journal of Veterinary Research 64(1): 104-108.,Shadkhast & Bigham 2010Shadkhast M, Bigham AS (2010) A histo-anatomical study of dorsal lacrimal gland in Iranian river buffalo. Vet Scan 5: 1-5.). The LG can produce serous or mucoserous secretions (Mohammadpour 2008Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
https://doi.org/10.3923/jbs.2008.1104.11...
) and according to its product it is classified as serous gland, as in goats (Kuhnel 1968bKühnel W (1968b) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. II. Ziege. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 86: 430-443.); or mucoserous gland as in cattle (Kuhnel 1968aKühnel W (1968a) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. V. Rind. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 87: 504-525.). The mucoserous secretion was described in pigs and horses, while mucous secretion is produced by the canine lacrimal gland (Martin et al. 1988Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.); and serous secretion by rats (Lober 1989Lorber M (1989) Elastic fibers in the rat extraorbital lacrimal gland duct system. Investigative Ophthalmology & Visual Science 30: 2002-2011.). In sheep and goats the LGs are mixed type glands consisting of serous and mucous acini (Gargulio et al. 2000Gargiulo AM, Dall'Aglio C, Coliolo P, Ceccarelli P, Pedini V (2000) Complex carbohydrate histochemistry and ultracytochemistry of the sheep lacrimal gland. Anatomia Histologia Embryologia 29(1): 19-23. doi: 10.1046/j.1439-0264.2000.00229.x
https://doi.org/10.1046/j.1439-0264.2000...
).
The SGTE has a similar structure to the LG and is also a compound tubuloacinar gland, which produces a mucoserous secretion by merocrine process and supplies the aqueous fraction of the tear film. The aqueous layer of the tear film has an active role in the lubrication and protection of the ocular surface (conjunctiva and cornea). It contains antibacterial factors, including immunoglobulins, and is involved in the regeneration of the damaged cornea (Klećkowska-Nawrot et al. 2013Klećkowska-Nawrot J, Marycz K, Czogała J, Kujawa K, Janeczek M, Chrószcz A, Brudnicki W (2013) Morphology of the lacrimal gland and superficial gland of the third eyelid of Roe deer (Capreolus capreolus L.). Pakistan Veterinary Journal 33(2): 139-144.).
In the literature, the morphology of the HG, LG and SGTE glands was described for many animals (Alsafy 2010Alsafy MAM (2010) Comparative morphological studies on the lacrimal apparatus of one humped camel, goat, and donkey. Journal of Biological Sciences 10(3): 224-230. doi: 10.3923/jbs.2010.224.230
https://doi.org/10.3923/jbs.2010.224.230...
,Cabral et al. 2005Cabral VP, Laus JL, Zaidan ML, Dagli ML, Pereira GT, Talieri IC, Monteiro ER, Villela Mamede F (2005) Canine lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciencia Rural 35(2): 391-397. doi: 10.1590/S0103-84782005000200023
https://doi.org/10.1590/S0103-8478200500...
,Fahmy et al. 1971Fahmy MFA, Arnautovic I, Abdalla O (1971) The morphology of the tarsal glands and the glands of the third eyelid in the one-humped camel (Camelus dromedarius). Acta Anatomica 78(1): 40-46.,Gargulio et al. 2000Gargiulo AM, Dall'Aglio C, Coliolo P, Ceccarelli P, Pedini V (2000) Complex carbohydrate histochemistry and ultracytochemistry of the sheep lacrimal gland. Anatomia Histologia Embryologia 29(1): 19-23. doi: 10.1046/j.1439-0264.2000.00229.x
https://doi.org/10.1046/j.1439-0264.2000...
,Henker et al. 2013Henker R, Scholz M, Gaffling S, Asano N, Hampel U, Garries F, Horngger J, Paulsen F (2013) Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland Xenografting. Plos One 8(9): e74046. doi: 10.1371/journal.pone.0074046
https://doi.org/10.1371/journal.pone.007...
,Sakai 1989Sakai T (1989) Major ocular glands (Harderian gland and lacrimal gland) of the musk shrew (Suncus murinus) with a review on the comparative anatomy and histology of the mamma lian lacrimal glands. Journal of Morphology 201(1): 39-57.) including the American bison (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). The European bison is classified as an endangered species. Although the European bison seems to be similar to the American bison, there are many differences between them. The orbital glands of the American bison were described and compared with those of cattle. Information about the morphology and histology of these glands in the European bison, on the other hand, is lacking, and consequently, the orbital glands of the American and European bisons have not been compared. We describe the normal anatomical, histological and fine structures of the orbital glands of female and male European bisons to determine the histochemical profile of their HG, LG and SGTE glands. Structural studies of these glands can be important to ascertain the relationships between morphological structures and functions. Furthermore, detailed information about these glands broadens the comparative anatomy of domestic animals and provides basis for further research.
MATERIAL AND METHODS
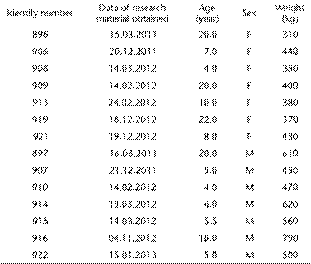
The study was conducted on seven males (4-20 years old, 430-790 kg of body weight) and seven females (4-22 years old, 310-440 kg of body weight) of the European bison, Bison bonasus bonasus (Linnaeus, 1758) (Table 1). The research material was collected during years 2011-2013 and came from a herd that inhabited Bialowieza Forest (Bialowieza National Park, Poland). The research subjects, which qualified for selective hunting on the basis of a decision of the General Director of Environmental Protection (DOP-OZGIZ. 6401.6.2011.ls.2 January 27th, 2011; DOP-OZGIZ. 6401.06.7.2012.ls. February 3rd, 2012; DOP-OZGIZ. 6401.06.7.2012.1s.1 the December 4th, 2012), were shot and killed by us. The morphometric studies were performed on the HG, LG and SGTE collected from each male and female. The samples of glands were collected immediately after death and fixed in 4% buffered formaldehyde for 72 hours (for light microscopy) and in 2.5% glutaraldehyde dissolved in 0.1 M phosphate buffer of pH 7.4 for two weeks for ultrastructural studies (electron microscopy).
Macroscopic study
The glands were first examined by the naked eye and then examined under the stereoscopic Zeiss Stemi 2000-C microscope (Carl Zeiss, Jena, Germany). A 0.5-4% solution of acetic acid, and 70% ethyl alcohol were used for a clear presentation of the structures of the HG, LG and SGTE. Methods characteristic of topographic anatomy - holotopy and syntopy - were used for describing the morphology of the HG, LG, SGTE and TE. Morphometric measurements of glands were conducted in all collected glands using an electronic slide caliper with the accuracy of 0.1 mm. The statistical analysis was conducted with Student t-test.
Light microscopy examination
Histological study
The research material was directly fixed in 4% buffered formaldehyde for 72 hours, rinsed in running water for 24 hours, then it was processed in a vacuum tissue processor - ETP (RVG3, INTELSINT, Italy), embedded in paraffin and cut on sliding microtome Slide 2003 (Pfm A.g., Germany) into 3-4 µm sections. Then samples were stained with haematoxylin and eosin (H&E) to demonstrate the general structure of glands. The Masson-Goldner and H&E staining were performed in the TE. The H&E and Masson-Goldner staining scoring system was based on a standard protocol described previously (Burck 1975Burck NC (1975) Technika histologiczna. Warszawa, PZWL.). In addition, the methyl green-pyronin Y method (MGP Y) (Kurnick 1955Kurnick NB (1955) Pyronin Y in the methyl-green-pyronin histological stain. Stain Technique 30(5): 213-230.) was used for the demonstration of plasma cells.
Histomorphometric study in H&E stained sections
For histomorphometric study of capsule thickness, diameters of alveoli, acini and tubules and interlobares septa thickness, the method described byEl-Fadaly et al. (2014El-Fadaly AB, El-Shaarawy EAA, Rizk AA, Nasralla MM, Shuaib DMA (2014) Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Annals of Anatomy 196(5): 336-351. doi: 10.1016/j.aanat.2014.06.005
https://doi.org/10.1016/j.aanat.2014.06....
) was used. Measurement was performed on the HG, LG and SGTE.
Capsule thickness and interlobares septa thickness. Capsule and interlobares septa thickness measurement was performed on 60 randomly chosen parts from each sex at 50x magnification.
Alveolar/acinar and tubular diameters. The alveolar/acinar and tubular diameters were measured in central fields at 400x magnification. Measurements were made on the widest diameter of the transversely cut alveoli/acini and tubules of 60 randomly chosen alveoli/acini and tubules from each sex.
Histological measurements of gland structures were made with the help of Axio Vision 4.8 (Carl Zeiss MicroImaging GmbH, Jena, Germany). The statistical analysis was conducted with the Student t-test, at the significance level was P<0.05.
Histochemical analysis
Histochemical analysis of the three glands was conducted in order to identify the presence of neutral or weakly acidic glycoproteins or glycogen with periodic acid-Schiff staining (PAS); acidic sulfated or sialylated mucosubstances with Alcian blue pH 2.5 (AB pH 2.5); sulfated acid mucosubstances (SAM) and carboxylated acid mucosubstances (CAM) with Hale's dialysed iron staining (HDI); sulfated acid mucosubstances and elastic fibers with aldehyde fuchsin staining (AF). All obtained slides were examined using Zeiss Axio Scope A1 light microscope (Carl Zeiss, Jena, Germany) for histological and histochemical description. PAS, AB pH 2.5, AF and HDI staining scoring system was based on a standard protocol described previously (Spicer & Henson 1967Spicer SC, Henson JG (1967) Methods for localizing mucosubstances in epithelial and connective tissue, p. 78-112. In: Bajusz E, Jamin F (Eds.) Series on Methods and Achievements in Experimental Pathology. Basal, S. Karger Press, vol. 2.).
Electron microscopy examination
The collected material was fixed in 2.5% glutaraldehyde dissolved in 0.1 M phosphate buffer at pH 7.4 for two weeks, and rinsed in a phosphate buffer. The material was then post-fixed in 4% OsO4 for 2 hours. After re-rinsing in a phosphate buffer, the fragments of the glands were dehydrated in an acetone series (from 30 to 100%). The dehydrated material was immersed in Epon 812 epoxide resin. Blocks were cut with a diamond knife into 70 nm sections using a Leica Reichert Ultra Cut microtome (Leica Microsystem Wetzlar GmbH, Germany). Preparations were observed through the Zeiss EM 900 (Carl Zeiss, Jena, Germany) transmission electron microscope operating at 80 kV. The study was performed in Electron Microscopy Laboratory of Wroclaw University.
RESULTS
Gross anatomy
Harderian gland. The HG was located on the ventral straight muscle in the medioventral part of the periorbita. This gland had dorsal and ventral borders as well as anterior and posterior ends. The posterior end was directed to the cone of the periorbita and contacted the ventral border of the SGTE and the ventral oblique muscle. The gross appearance of the gland was uniform, undivided and oval, and light pink, (Fig. 2). On the medial surface, a single duct was observed, which conveyed the secretions in the internal side of the TE. The mean size (length x width x thickness with SD) of the HG was 51.88 mm (±1.3) x 27.23 mm (±1.9) x 8.25 mm (±0.7) in females and 52.39 mm (±1.4) x 28.15 mm (±1.8) x 8.46 mm (±0.8) in males (Fig. 5). There was no effect of sex on the morphometric results of the examined HG.
Lacrimal gland. The LG was situated in the dorsolateral angle of the periorbita, between the dorsal rectus and the lateral rectus muscles of the eyeball. It was oval and light pink with a convex periorbital surface and a concave intraorbital surface. The gross appearance the gland was uniform and undivided (Fig. 3). The excretion of the LG went into the conjunctival sac by small excretory ductules which opened into the lower conjunctival fornix. The mean size (length x width x thickness with SD) of the LG was 48.68 mm (±1.5) x 16.02 mm (±1.2) x 7.21 mm (±0.3) in females and 49.51 mm (±1.6) x 17.36 mm (±1.2) x 7.9 mm (±0.3) in males (Fig. 2). There was no effect of sex on the morphometric results of the examined LG.
Superficial gland of the third eyelid. The SGTE was located between the medial rectus muscle and the ventral rectus muscle and was partially covered by the ventral oblique muscle of the eyeball. The SGTE was bipartite with an accessory lobe and was brightly colored (Figs. 2 and 4). The mean size (length x width x thickness ± SD) of the SGTE was 36.06 mm (±1.4) x 21.65 mm (±2.0) x 7.34 mm (±0.3) in females and 35.68 mm (±1.4) x 20.39 mm (±1.9) x 7.46 mm (±0.3) in males (Fig. 5). There was no effect of sex on the morphometric results of the examined SGTE.
Third eyelid. The marginal part of the TE was thin and pigmented (Figs. 1, 2 and 4). The shape of the TE resembled an anchor and it was composed of cartilage which consisted of a lower and an upper branch and a crossbar (Fig. 2). The lower and upper branches of the TE cartilage stiffened the fold of the TE while the crosswise part extended towards the periorbital cone. The crosswise part of the TE was surrounded by the SGTE (Fig. 4).
Macrograph of the HG, LG and SGTE with TE in European bison: (1) third eyelid. Bar = 1.0 cm; (2) Harderian gland; (3) lacrimal gland. Bar = 1.0 cm; (4) superficial gland of the third eyelid with third eyelid. (SE) Superior eyelid, (IE) inferior eyelid, (TE) third eyelid, (HG) Harderian gland, (LG) lacrimal gland, (SGTE) superficial gland of the third eyelid, (AL) accessory lobe. Scale bars: 1.0 cm.
Morphometric parameters (mm) of the HG, LG and SGTE in European bison. (HG) Harderian gland, (LG) lacrimal gland, (SGTE) superficial gland of the third eyelid. Values are expressed as mean ± standard deviations.
Morphometric parameters (µm) of alveoli/acini, tubules, interlobular septa and capsule in the HG (6), LG (7) and SGTE (8) of European bison with respect males and females. Values are expressed as mean ± standard deviations.
Light microscopic study
Histological study
Harderian gland. The HG was a multilobar tubuloalveolar gland with a predominance of alveoli. It was covered by a thick connective tissue capsule, with collagen and elastic fibers which were detected in the aldehyde fuchsin staining method (Figs. 9 and 18). The connective tissue capsule also contained blood vessels. The average thickness of the capsule was 241.25 µm (±38.22) in females and 218.27 µm (±20.82) in males (Fig. 6). There were no significant differences in the thickness of the capsule between males and females. The connective tissue penetrated from the capsule into the glandular tissue and formed numerous thick and sparse thin septa, which divided the gland into small and big lobes (Fig. 10 and 11). The average thickness of the interlobares septa was 189.38 µm (±74.7) in females and 148.86 µm (±49.7) in males (Fig. 6). There were no significant differences between males and females in the thickness of the interlobares septa. Within the interlobares septa, numerous adipocytes and blood vessels were observed (Fig. 10). The glandular parenchyma contained the alveoli with irregular, wide lumen, which were composed of tall conical cells with round nuclei. The alveoli were surrounded by basal myoepithelial cells (Fig. 13). The mean outer diameter of the glandular alveoli was 43.15 µm (±2.74) in females and 33.53 µm (±2.97) in males (Fig. 6). There were no significant differences in the diameter of the alveoli between males and females. The secretory cell nuclei were big and oval and were located in their basal portion. These cells had eosinophilic granular cytoplasm (Figs. 12 and 13). The tubules with wide lumen were composed of one layer of cubic cells. These cells had oval nuclei located in the basal area of each cell, and eosinophilic cytoplasm (Figs. 12 and 13). The mean outer diameter of the tubules was 61.08 µm (±1.94) in females and 52.53 µm (±2.8) in males (Fig. 6). There were no significant differences in the diameter of the tubules of males and females. The MGP Y staining showed many of plasma cells and lymphocytes located between the plasma cells in the interstitium of the gland. The plasma cells had a characteristic dark-pink nucleus and pink cytoplasm (Figs. 14 and 15).
Light micrograph of HG in European bison: (9) H&E stain. Bar = 100 µm; (10) H&E stain. Bar = 200 µm; (11) H&E stain. Bar = 200 µm; (12) H&E stain. Bar = 10 µm; (13) H&E stain. Bar = 5 µm; (14) MGP Y stain. Bar = 10 µm; (15) MGP Y stain. Bar = 2 µm; (16) PAS stain. Bar = 100 µm; (17) PAS stain. Bar = 5 µm; (18) AF stain. Bar = 100 µm; (19) AB pH 2.5 stain. Bar = 100 µm; (20) AB pH 2.5 stain. Bar = 20 µm; (21) HDI stain. Bar = 100 µm; (22) HDI stain. Bar = 10 µm. (C) Capsule, (CT) connective tissue, (L) lobus, (AT) adipose tissue, (BV) blood vessels, (T) trabeculae, (A) alveoli, (Tu) tubules, (black asterisk) strong reaction, (red asterisk) weakly reaction, (Mc) myoepithelial cells, (PC) plasma cells.
Lacrimal gland. The LG was a multilobar tubuloacinar gland with predominance of acini over tubules. It was covered by a thick connective tissue capsule, consisting of collagen and elastic fibers, which were detected by the aldehyde fuchsin staining method (Figs. 23 and 30). The average thickness of the capsule was 395.5 µm (±33.8) in females and 316.91 µm (±75.35) in males (Fig. 7). There were no significant differences in the thickness of the capsule between male and female. The capsular connective tissue penetrated into the glandular parenchyma forming septa, which divided the gland into small and big lobes (Fig. 23). The average thickness of the interlobares septa was 169.22 µm (±88.9) in females and 138.82 µm (±82.1) in males (Fig. 7). There were no significant differences in the thickness of interlobares septa between male and female. Within interlobares septa sparse adipocytes and blood vessels were present (Fig. 23). The acini of irregular lumen were composed of tall conical cells surrounded by basal myoepithelial cells (Fig. 25). The mean outer diameter of the glandular acini was 34.36 µm (±4.9) in females and 27.98 µm (±3.92) in males (Fig. 7). There were no significant differences in acini diameter between male and female. The big and round secretory cell nuclei were located in their basal part. These cells had basophilic granular and vacuolated cytoplasm (Figs. 24 and 25). The tubules with large lumen were composed of one layer of cubic cells with round nuclei, located in the basal areas of the cells (Figs. 24 and 25). The mean outer diameter of tubules was 46.72 µm (±4.47) in females and 42.89 µm (±6.01) in males (Fig. 7). There were no significant differences in diameter of tubules between male and female. The MGP Y staining demonstrated numerous plasma cells (with a characteristic dark-pink nucleus and pink cytoplasm) and sparse lymphocytes, both of which were localized in the glandular interstitium (Fig. 26and 27).
Light micrograph of LG in European bison: (23) H&E stain. Bar = 50 µm; (24) H&E stain. Bar = 20 µm; (25) H&E stain. Bar = 5 µm; (26) MGP Y stain. Bar = 10 µm; (27) MGP Y stain. Bar = 2 µm; ; (28) PAS stain. Bar = 100 µm; (29) PAS stain. Bar = 10 µm; (30) AF stain. Bar = 100 µm; (31) AB pH 2.5 stain. Bar = 100 µm; (32) AB pH 2.5 stain. Bar = 10 µm; (33) HDI stain. Bar = 100 µm; (34) HDI stain. Bar = 10 µm. (C) Capsule, (CT) connective tissue, (L) lobus, (BV) blood vessels, (T) trabeculae, (A) acini, (Tu) tubules, (black asterisk) strong reaction, (red asterisk) weakly reaction, (Mc) myoepithelial cells, (PC) plasma cells, (Lc) lymphocyte.
Superficial gland of the third eyelid. The SGTE was surrounded by a connective tissue capsule, which divided the gland structure into clearly visible lobes. The average thickness of the capsule was 352.72 µm (±36.21) in females and 405.94 µm (±80.49) in males (Fig. 8). There were no significant differences in the thickness of the capsule between male and female. Numerous single adipocytes or small or big aggregations of adipocytes were visible within interlobares septa (Figs. 35 and 36). Additionally, sparse blood vessels were observed. The average thickness of the interlobares septa was 245.9 µm (±54.15) in females and 243.84 µm (±90.1) in males (Fig. 8). There were no significant differences in the thickness of interlobares septa between male and female. The SGTE had a multilobar tubuloacinar structure. The mean outer diameter of the glandular acini was 43.86 µm (±7.25) in females and 35.42 µm (±5.14) in males (Fig. 8). There were no significant differences between male and female. The acini had small lumen and were composed of tall conical secretory cells with eosinophilic granular and vacuolated cytoplasm. The rounded nuclei of acini cells were located close to the base of the cells (Fig. 37). The tubules had cubic epithelium with eosinophilic cytoplasm. The nuclei of tubular cells were oval and located in the central part of these cells (Fig. 37). The mean outer diameter of tubules was 57.03 µm (±5.01) in females and 49.62 µm (±5.6) in males (Fig. 8). There were no significant differences between male and female. The MGP Y staining method in the SGTE demonstrated rare plasma cells without lymphocytes (Fig. 38).
Light micrograph of SGTE in European bison: (35) H&E stain. Bar = 200 µm; (36) H&E stain. Bar = 100 µm; (37) H&E stain. Bar = 5 µm; (38) MGP Y stain. Bar = 50 µm; (39) PAS stain. Bar = 100 µm; (40) PAS stain. Bar = 10 µm; (41) AF stain. Bar = 100 µm; (42) AB pH 2.5 stain. Bar = 100 µm; (43) AB pH 2.5 stain. Bar = 10 µm; (44) HDI stain. Bar = 100 µm; (45) HDI stain. Bar = 10 µm. (C) Capsule, (CTE) cartilage of the third eyelid, (CF) collagenous fibers, (CT) connective tissue, (L) lobus, (BV) blood vessels, (T) trabeculae, (AT) adipose tissue, (A) acini, (Tu) tubules, (*) strong reaction, (Mc) myoepithelial cells, (PC) plasma cells.
Third eyelid. The TE cartilage was surrounded by a thick layer of elastic and collagen fibers abundant in blood vessels (Figs. 46 and 47). The cartilage of the TE was composed of hyaline tissue with numerous chondrocytes and a small amount of intercellular substance. Chondrocytes which were located deeper in the cartilage had oval shape, and were mostly single or they sometimes formed an isogenic group composed of 2-3 cells (Fig. 48). The conjunctival surface of the TE had non-cornified multilayer epithelium with numerous melanocytes (Fig. 49). Many blood vessels were observed in the basal part of the TE. Furthermore, connective tissue consisted of numerous blood vessels, fibrocytes, elastic and collagen fibers demonstrated in Masson-Goldner staining method (Fig. 47). The ocular surface of the TE was covered by non-cornified multilayer epithelium too (Fig. 51) possessing numerous goblet cells with a high number of secretory granules (Fig. 51). In the ocular surface of the TE, numerous aggregations of lymphocytes organized in lymph nodules were observed (Fig. 50).
Light micrograph of TE in European bison: (46) H&E stain. Bar = 100 µm; (47) Masson-Goldner stain. Bar = 200 µm; (48) H&E stain. Bar = 100 µm; (49) H&E stain. Bar = 20 µm; (50) H&E stain. Bar = 20 µm; (51) H&E stain. Bar = 20 µm. (CT) Connective tissue, (CTE) cartilage of the third eyelid, (BV) blood vessels, (CF) collagenous fibers, (OS) ocular surface, (CS) conjunctival surface, (SGTE) superficial gland of the third eyelid, (MC) melanocytes, (FM) free margin, (GC) goblet cells, (Lc) lymphocytes.
Histochemical study
Harderian gland. The PAS staining method demonstrated the presence of secretory cells with a very weak reaction evaluated as (+/-) (Figs. 16, 17andTable 2). The AF staining showed a negative reaction (-) in alveoli and tubules (Fig. 18andTable 2). The histochemical analysis with AB pH 2.5 indicated the two types of reaction in glandular cells: a slightly positive reaction (+/-, red asterisk) in sparse alveoli and tubules and an weak positive reaction (+, black asterisk) in single alveoli (Figs. 19, 20andTable 2). The HDI method showed numerous alveoli and tubules cells with an average positive reaction (++, red asterisk) and sparse alveoli with a strong reaction (+++, black asterisk) (Figs. 21, 22andTable 2). Histochemical studies using PAS, AB pH 2.5, AF and HDI showed that the HG in the European bison had a mixed character of secretion.
Histochemical analysis of the HG, LG and SGTE in European Bison. (HG) Harderian gland, (LG) lacrimal gland, (SGTE) superficial gland of the third eyelid; Staining: (PAS) periodic acid- Schiff, (AB pH 2.5) Alcian blue pH 2.5, (HDI) Hale's dialysed iron, (AF) aldehyde fuchsin, (SAM) sulfated acid mucosubstances, (CAM) carboxylated acid mucosubstances.
Lacrimal gland. The PAS staining showed acini and tubules cells with a slightly positive reaction (+/-, black asterisk) (Figs. 28, 29andTable 2). In AF staining a negative reaction (-) both in acini and tubules was observed (Fig. 30andTable 2). A weak positive reaction (+/-) of AB pH 2.5 staining was demonstrated in sparse acini and tubules in marginal and central parts of lobes (Figs. 31, 32andTable 2). Furthermore, the HDI method showed a larger number of acini and tubules cells with a positive reaction (+, red asterisk) or with a average positive reaction (++, black asterisk) in some parts of the gland (Figs. 33, 34andTable 2). Histochemical studies using PAS, AB pH 2.5, AF and HDI showed that the LG in the European bison had a serous secretion but some of acini had a slightly mucous nature.
Superficial gland of the third eyelid. The PAS method demonstrated the presence of secretory cells with a positive reaction (+) (Figs. 39, 40andTable 2). The AF staining showed a negative reaction (-) (Fig. 41andTable 2). The staining with AB pH 2.5 method detected the presence of a weak positive reaction (+) in acini and tubules (Figs. 42, 43andTable 2). The HDI method showed a weak positive reaction (+) in the majority parts of the gland (Figs. 44, 45andTable 2). Histochemical studies using PAS, AB pH 2.5, AF and HDI showed that the SGTE in the European bison had a mucoserous character of secretion.
Electron microscopic study
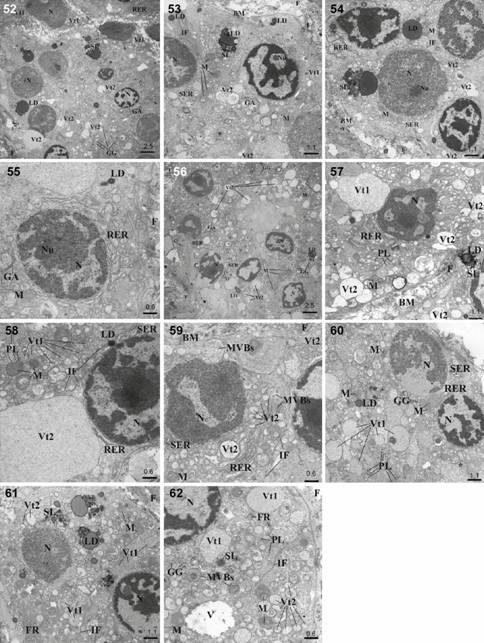
The TEM study showed that the secretory cells of the HG, LG and SGTE in the European bison had a similar ultrastructural appearance. The nuclei in all alveoli/acini were large, spherical and basally located and they contained visible nucleoli and considerable amounts of heterochromatin (Figs. 52, 58 and 61) whereas the nuclei of tubules were oval (Figs. 53, 56, 60). These gland cells were frequently mononucleate, however sparse cells with a dual nucleus were also present. The nuclear envelope of two distinct membranes around the perinuclear cisterna was clearly seen (Figs. 58 and 61). The plasma cells visible in the HG had an ovoid basally located nucleus with condensed heterochromatin arranged in clumps (Fig. 55). The rough endoplasmic reticulum (RER) was present in all glands, surrounding the nucleus and often showing continuity with the nuclear envelope (Figs. 54, 57 and 60). The RER in the LG was also observed as an accumulation between secretory vesicles (Fig. 59). The smooth endoplasmic reticulum (SER) appeared as small fenestrated cisternae which formed concentric layers located in the supranuclear region of secretory cells (Figs. 53, 57 and 60). The numerous mitochondria, large and oval, with frequent transverse cristae and the dense matrix were observed in the cell cytoplasm within all glands. Numerous mitochondria were associated with cisternae of RER and were located near the secretory vesicles (Figs. 54, 58 and 62). In the secretory cells big lipid droplets were located in a tight contact with the secondary lysosomes or secretory granules. Secondary lysosomes were characterized by a weakly visible membrane and contained electron-dense, small and oval granules. The lipid droplets were mostly located near the secretory cell nuclei and secretory vesicles (Figs. 52 and 61). The lipid droplets and the secondary lysosomes were more numerous in the HG and SGTE than in the LG (Fig. 56). The Golgi apparatus was located near the nucleus and was composed of low and flat cistern layers and narrow vesicles and vacuoles (Figs. 53 and 56). Within the cytoplasm the small primary lysosomes and rare glycogen granules were present in the HG, LG and SGTE secretory cells (Figs. 52 and 62). The sparse extensive membrane structures arranged in concentric lamellae were observed in the HG and LG cells (Figs. 53, 56 and 60). Within the cytoplasm in the HG, LG and SGTE intermediate filaments were also present (Figs. 54, 58 and 61). Two types of secretory vesicles were detected in the cytoplasm of these glands, both in alveoli/acini (Fig. 52) and tubules (Fig. 53). Type 1 of vesicles had high electron-density and contained varying amount of homogenous material. In the LG and SGTE, type 1 vesicles were bigger than in the HG (Figs. 57, 58, 60 and 62). Type 2 vesicles had lower electron-density with fine-grained contents. Numerous oval granules of varying sizes were scattered within the cytoplasm of the secretory cells. Sparse multivesicular bodies (MVBs), also referred to as late endosomes, were observed in the cytoplasm of the LG and SGTE cells. Late endosomes were corpuscles with a clear limited membrane with densely packed vesicles (Figs. 59 and 62). In the secretory cells of the SGTE, sparse single vacuoles were demonstrated (Fig. 62). The vacuoles were bounded by a single membrane.
Survey electron micrograph of the HG, LG and SGTE cells in European bison. (52-55) HG cells: (52) Note of the secretory cells. Bar = 2.5 µm; (53) Note secretory cells with vesicles type 1 and 2. Bar = 1.1 µm; (54) Note secretory cells with vesicles type 1 and 2 and big lipid drops. Bar = 1.1 µm; (55) Visible part of plasma cell. Bar = 0.6 µm; (56-59) LG cells: (56) Note of the secretory cells. Bar = 2.5 µm; (57) Note secretory cells with vesicles type 1 and 2. Bar = 1.1 µm; (58) Note secretory cells with vesicles type 1 and 2. Bar = 0.6 µm; (59) Clearly visible multivesicular bodies (MVBs). Bar = 0.6 µm; (60-62) SGTE cells: (60) Note of the secretory cells with vesicles type 1 and 2. Bar = 1.1 µm; (61) Note secretory cells with lipid drops and secondary lysosomes. Bar = 1.1 µm; (62) Note secretory cells with vesicles type 1 and 2 and multivesicular bodies (MVBs). Bar = 0.6 µm. (N) Nucleus of the cell, (Nu) nucleolus, (RER) rough endoplasmic reticulum, (M) mitochondria, (SER) smooth endoplasmic reticulum, (GG) glycogen granules, (LD) lipid drops, (SL) secondary lysosomes, (BM) basement membrane, (F) filaments, (GA) Golgi apparatus, (IF) intermediate filaments, , (PL) primary lysosomes, (MVBs) multivesicular bodies, (Vt1) vesicles type 1, (Vt2) vesicle type 2, (FR) free ribosomes, (V) vacuola, (*) extensive membrane structures arranged in concentric lamellae.
DISCUSSION
The structures of the HG, LG and SGTE glands of the European bison are similar to other animals (Aldana Marcos et al. 2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.,Djeridane 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.,Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.,Johnston et al. 1983Johnston HS, Mcgadey J, Thompson GG, Moore MR, Payne AP (1983) The Harderian gland, its secretory and porphyrin content in the Mongolian gerbil (Meriones unguiculatus). Journal of Anatomy 137(3): 615-630.,Johnston et al. 1985Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.,Martin et al. 1988Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.,Pradidarcheep et al. 2003Pradidarcheep W, Asavapongpatana S, Mingsakul T, Poonkhum R, Nilbu-Nga S, Somana R (2003) Microscopic anatomy of the orbital Harderian gland in the common tree shrew (Tupaia glis). Journal of Morphology 255(3): 328-336. doi: 10.1002/jmor.10066
https://doi.org/10.1002/jmor.10066...
,Weaker 1981Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.). However, in the present study, some new features were discovered in the macroscopic and microscopic structures of the ocular glands of the European bison. The HG of the European bison is located distally from the SGTE (as in cattle), and it histologically similar to the HG of other ruminants (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). Nevertheless, the HG is oval in the European bison, whereas in the American bison and cattle this gland is elongated-triangular with a cobble-stoned proximal part and a distal portion that appears smooth (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). In addition to differences in the macroscopic structure of the HG, histological differences were also noticed. The HG of the European bison has a multilobar tubuloalveolar structure with a predominance of alveoli. In the European bison the alveoli and tubules of the HG are composed of a single layer of cells. In rodents, as in the Plain mouse, tubules and alveoli are also formed by a single layer of cells (Johnston et al. 1985Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.). The secretory cells of the European bison have eosinophilic cytoplasm with lipid vacuoles. These lipid vacuoles are also observed in rodents (Djeridane 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.). Furthermore, the HG of rodents also contains porphyrin stored as solid intraluminal accretions (Johnston et al. 1985Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.), which does not occur in the HG of the European bison (Johnston et al. 1985Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.,Shirama et al. 1981Shirama K, Furuya T, Takeo Y, Shimuzu K, Maekawa K (1981) Influences of some endocrine glands and of hormone replacement on the porphyrins of the Harderian gland of mice. Journal of Endocrinology 91(2): 305-311.).
The histochemical analysis of the HG in the European bison revealed that it is a mixed gland. The PAS staining demonstrated the presence of secretory cells with a slightly positive reaction, which proves the presence of neutral or weakly acidic glycoproteins. Another reaction is observed in the large alveoli of the American bison and cattle HG, where the PAS staining gives a negative reaction but a positive reaction in smaller alveoli (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). We observed, in the European bison, a very weak positive reaction in the alveoli and in the tubules. Stronger and weaker reactions were described for the American bison and cattle HGs. The AB pH 2.5 staining demonstrates the presence of sialytated and sulfated mucosubstances in the European bison with two types of reaction: slightly positive or weak. Similar differences in the AB pH 2.5 staining reaction are also observed in cattle and the American bison (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). The HDI staining indicates numerous alveoli and tubules with an average positive reaction and rare ones with a strong reaction in the European bison whereas in cattle and the American bison it demonstrates alveoli with a mixture of positive and negative reactions and tubules with a HDI-negative reaction (Pinard et al. 2003b).
In general, the lacrimal gland of many species has been described as a compound tubuloacinar gland as in European bison (Pinard et al. 2003aPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003a) Evaluation of lysozyme and lactoferrin in lacrimal and other ocular glands of bison and cattle and in tears of bison. American Journal of Veterinary Research 64(1): 104-108., b). In animals the LG may vary both in shape and color (Cabral et al. 2005Cabral VP, Laus JL, Zaidan ML, Dagli ML, Pereira GT, Talieri IC, Monteiro ER, Villela Mamede F (2005) Canine lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciencia Rural 35(2): 391-397. doi: 10.1590/S0103-84782005000200023
https://doi.org/10.1590/S0103-8478200500...
). The LG of the European bison and other ruminants is basically similar in appearance, distinctly lobulated and located on the dorsolateral surface of the eyeball (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
,Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.,Maala et al. 2007Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.). One exception is cattle, which have an accessory lobe (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). The European bison does not possess a bipartite LG as seen in cattle. In the European bison the LG is oval and light pink, while in sheep it is red, flattened and oval (Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.), except for Lori sheep, where the LG is pale brown and irregular oval to spherical (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
). In camels the LG appears as a flattened, elongated and irregular structure (Mohammadpour 2008Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
https://doi.org/10.3923/jbs.2008.1104.11...
). The Philippine water buffalo also has a flattened LG but pink to red (Maala et al. 2007Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.). In the majority of animal species, as well as in the European bison, the dorsal surface of the gland is convex while the ventral surface is concave, which results from the location of the gland between the orbit and the eyeball (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
,Mohammadpour 2008Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
https://doi.org/10.3923/jbs.2008.1104.11...
). The size of the LG in the European bison and in camel are similar (Mohammadpour 2008Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
https://doi.org/10.3923/jbs.2008.1104.11...
). The analysis of the length-to-width ratio of the LG reveals similar results for the American bison and cattle (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). ThoughCornell-Bell et al. 1985Cornell-Bell AH, Sullivan DA, Allansmith MR (1985) Gender-related differences in the morphology of the lacrimal gland. Investigative Ophthalmology & Visual Science 26(8): 1170-1175.documented sex differences in the LG, no sex influences on the anatomical characteristics of the LG have been observed in the European bison, similarly as in cattle (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). In contrast such differences are reported in rats, mice, rabbits and humans (Djeradine 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.,Jensen et al. 1969Jensen OA, Falbe-Hansejn I, Jacobsen T, Michelsen A (1969) Mucosubstances of the acini of the human lacrimal gland (Orbital part). Acta Ophthalmology 47(3): 605-619. doi: 10.1111/j.1755-3768.1969.tb08147.x
https://doi.org/10.1111/j.1755-3768.1969...
). Additionally, in the camel, the left LG is significantly larger that the right one (Mohammadpour 2008Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
https://doi.org/10.3923/jbs.2008.1104.11...
). No significant differences have been reported in the European bison between the left and right glands, but the gland is heavier and larger in males than in their female counterparts. This sexual dimorphism has also been observed in Lori sheep (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
) and dogs (Cabral et al. 2005Cabral VP, Laus JL, Zaidan ML, Dagli ML, Pereira GT, Talieri IC, Monteiro ER, Villela Mamede F (2005) Canine lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciencia Rural 35(2): 391-397. doi: 10.1590/S0103-84782005000200023
https://doi.org/10.1590/S0103-8478200500...
). As in other animals, the fluid produced by the LG of the European bison drains through ducts into the conjunctival space beneath the upper eyelid (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
). In all described species as well as in the European bison this gland is lobar in appearance and is surrounded by the connective tissue, which consists mainly of collagen fibers. However, in both the European bison and the Lori sheep, a portion of the LG is surrounded by adipose tissue (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
).Martin et al. (1988Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.) indicated the presence of abundant clustered or individual fat cells through the glandular interstitium, which is also characteristic of the European bison. Each lobule in the LG of the European bison is made up of several acini with round to oval shaped secretory cells, whereas in cattle the acini cells are flat, low and cuboidal (Kühnel 1968aKühnel W (1968a) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. V. Rind. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 87: 504-525.). The cytoplasm of acini cells in the LG in the European bison and the Lori sheep gives an eosinophilic reaction (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
). A few basally located myoepithelial cells are observed in the European bison as well as in other species (Maala et al. 2007Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.).
The histochemical and electron microscopy study shows that the secretory cells of the LG consist of three cell types: mucous, mucoserous or serous (Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.). The secretory components of the LG, such as mucosubstances, can be revealed by applying histochemical methods. In samples taken from the LG of the European bison, the gland is characterized as a serous type different than in other mammals, including ruminants (Abbasi et al. 2014Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
https://doi.org/10.4172/2157-7579.100015...
,Kühnel 1968aKühnel W (1968a) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. V. Rind. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 87: 504-525.), pigs, goats and rodents. Since rodents have also additional lacrimal gland, it is difficult to compare their LG with the LG of ruminants and rodents (Djeradine 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.,1994Djeridane Y (1994) The Harderian gland and its excretory duct in the Wistar rat. A histological and ultrastructural study. Journal of Anatomy 184(3): 553-566.). The mucosubstances in the glandular acini of the LG od the European bison are acidic. The LG cell of the American bison and cattle contain neutral and acid, as well as sialytated and sulphated mucosubstances (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
), whereas in European bison they are carboxylated. The LG cells in humans are strongly PAS-positive and acini have a high amount of sialic acid and small amounts of weakly acid sulfated mucopolysaccharides in peripherally located glandular cells (Jensen et al. 1969Jensen OA, Falbe-Hansejn I, Jacobsen T, Michelsen A (1969) Mucosubstances of the acini of the human lacrimal gland (Orbital part). Acta Ophthalmology 47(3): 605-619. doi: 10.1111/j.1755-3768.1969.tb08147.x
https://doi.org/10.1111/j.1755-3768.1969...
). Instead, the secretory cells in European bison are characterized by slightly positive reaction in PAS and AB pH 2.5 staining. In cattle all the gland cells contain PAS-positive granules with neutral mucopolysaccharides and some of the cells are rich in AB pH 2.5 staining reactive material - acid mucopolysaccharides (Kühnel 1968aKühnel W (1968a) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. V. Rind. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 87: 504-525.). In Philippine water buffalo the majority of the acini cells demonstrate a strong reaction in PAS and AB pH 2.5 staining (Maala et al. 2007Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.). The secretory acini of the female Philippine water buffalo also show a stronger reaction than those of the male (Maala et al. 2007Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.), which is not observed in the European bison.
The LG and SGTE have a similar histological structure (Aldana Marcos et al. 2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.). The LG and SGTE of the European bison, the American bison and cattle are also similar in histochemical analyses (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). But while the SGTE of the American bison and cattle resemble each other (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
), an accessory lobe is present in the SGTE of the European bison. Differences are also observed in the size of the SGTE. Despite reported similarities, the SGTE is longer in the American bison than in cattle (Pinard et al. 2003bPinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
https://doi.org/10.1046/j.1439-0264.2003...
). Sexual differences can also be present in the LG and SGTE, as described in dogs (Cabral et al. 2005Cabral VP, Laus JL, Zaidan ML, Dagli ML, Pereira GT, Talieri IC, Monteiro ER, Villela Mamede F (2005) Canine lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciencia Rural 35(2): 391-397. doi: 10.1590/S0103-84782005000200023
https://doi.org/10.1590/S0103-8478200500...
). However, statistically significant sexual differences in the SGTE of the European bison have not been found. In this research, the SGTE of the European bison was elongated and brightly colored, unlike in camel, whose SGTE is oval and irregular in outline (Fahmy et al. 1971Fahmy MFA, Arnautovic I, Abdalla O (1971) The morphology of the tarsal glands and the glands of the third eyelid in the one-humped camel (Camelus dromedarius). Acta Anatomica 78(1): 40-46.). The secretory cells of the SGTE in the European bison are mucoserous, while they have a typical appearance of serous cells in the camel (Fahmy et al. 1971Fahmy MFA, Arnautovic I, Abdalla O (1971) The morphology of the tarsal glands and the glands of the third eyelid in the one-humped camel (Camelus dromedarius). Acta Anatomica 78(1): 40-46.).
The fine structure of the HG has been described in many rodents (Djeridane 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.), but information about the ultrastructural appearance of this gland in mammals is scarce. The fine structure of the HG of the European bison in some aspects resembles those of other animals. The two types of cells in this gland, which the authors observed in the European bison's HG, have also been described byDjeridane (1994Djeridane Y (1994) The Harderian gland and its excretory duct in the Wistar rat. A histological and ultrastructural study. Journal of Anatomy 184(3): 553-566.) in Wistar rats. The more numerous A type contain large lipid vacuoles. The numerous lipid vacuoles and two types of cells are also indicated byDjeridane (1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.),Lopez et al. (1992Lopez JM, Tolivia J, Alvarez-Uria M (1992) Postnatal development of the Harderian gland in the Syrian golden hamster (Mesocricetus auratus): A light and electron microscopic study. Anatomical Record 233(4): 597-616. doi: 10.1002/ar.1092330414
https://doi.org/10.1002/ar.1092330414...
), andWeaker (1981Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.). The nuclei of both types of cells in the European bison are spherical, large and are located basally, with one or two prominent nucleoli as in rodents (Djeridane 1992Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.). These secretory cells in the HG of the European bison have rough endoplasmic reticulum scattered throughout the cytoplasm, but are mainly filled with extraordinarily well developed smooth endoplasmic reticulum and infrequently occurring Golgi apparatus. Similar results were found byDjeridane (1994Djeridane Y (1994) The Harderian gland and its excretory duct in the Wistar rat. A histological and ultrastructural study. Journal of Anatomy 184(3): 553-566.). AlsoWeaker (1981Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.) described an expansive network of smooth endoplasmic reticulum in the secretory cells. The cytoplasm of the armadillo's secretory cells contains varying amounts of microtubules and microfilaments (Weaker 1981Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.). The tubules have also been observed byBucana & Nadakavukaren (1973Bucana CD, Nadakavukaren MJ (1973) Ultrastructural investigation of the postnatal development of the hamster Harderian gland. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 142(1): 1-12.) in the HG of hamsters and in the present study.
In the European bison, as in sheep, each acinus of the LG is composed of pyramidal or columnar cells, and most cells are filled with morphologically heterogeneous granules. In senile rats, numerous lipid droplets are located in the cytoplasm of the LG acini (El-Fadaly et al. 2014El-Fadaly AB, El-Shaarawy EAA, Rizk AA, Nasralla MM, Shuaib DMA (2014) Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Annals of Anatomy 196(5): 336-351. doi: 10.1016/j.aanat.2014.06.005
https://doi.org/10.1016/j.aanat.2014.06....
), as in the European bison. AlsoMartin et al. (1988Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.) observed lipid droplets in the canine LG. In the study conducted byGargiulo et al. (1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.), mucus cells in the LG of sheep are filled with mucous droplets of homogenous matrix. Mucus granules of these cells appear uniformly electron-lucent, whereas the serous granules of serous cells are uniformly electron-dense (Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.). Similar results have been demonstrated for the LG of the European bison. The mucoserous granules have a dense core embedded in matrix, which was previously described byGargiulo et al. (1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.).El-Fadaly et al. (2014El-Fadaly AB, El-Shaarawy EAA, Rizk AA, Nasralla MM, Shuaib DMA (2014) Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Annals of Anatomy 196(5): 336-351. doi: 10.1016/j.aanat.2014.06.005
https://doi.org/10.1016/j.aanat.2014.06....
) &Aldana Marcos et al. (2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.) demonstrated the presence of electron-dense and moderately electron-dense secretory granules in acinar cells of the LG. The nuclei of these cells have a widely dispersed chromatin and prominent nucleoli. In the study ofAldana Marcos et al. (2002Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.), the basal region of mucus acinar cells contains flattened cisternae of RER. In the LG of senile rats the decrease in protein secretory granules is associated with changes in the organization of the RER, including dilatation of cisternae. At the ultrastructural level, the serous cells of LG are protein-secreting cells. In contrast to the mucous cells, they have an extensive RER made up of numerous parallel cisternae and a well-developed Golgi apparatus (Gargiulo et al. 1999Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.).
ACKNOWLEDGEMENTS
We are grateful to the staff of Bialowieza National Park (Poland) for supplying the research material. This study was supported by the statutory research and development activity funds assigned to the Faculty of Veterinary Medicine at Wroclaw University of Environmental and Life Sciences and by the statutory research and development activity funds assigned to the Faculty of Veterinary Medicine at Warsaw University of Life Sciences. Publication supported by Wrocław Center of Biotechnology, program Leading National Research Center (KNOW) for the years 2014-2018.
LITERATURE CITED
- Abbasi M, Karimi H, Gharzi A (2014) Preliminary anatomical and histological study of lacrimal gland in Lori sheep. Journal of Veterinary Science & Technology 5(1): 1-4. doi: 10.4172/2157-7579.1000154
» https://doi.org/10.4172/2157-7579.1000154 - Aldana Marcos HJ, Ferrari C, Cervino C, Affanni JM (2002) Histology, histochemistry and fine structure of the lacrimal and nictitans gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Experimental Eye Research 75(6): 731-744.
- Aldana Marcos HJ, Affanni JM (2005) Anatomy, histology, histochemistry and fine structure of the Harderian gland in the South American armadillo Chaetophractus villosus (Xenarthra, Mammalia). Anatomy & Embryology 209(5): 409-424.
- Alsafy MAM (2010) Comparative morphological studies on the lacrimal apparatus of one humped camel, goat, and donkey. Journal of Biological Sciences 10(3): 224-230. doi: 10.3923/jbs.2010.224.230
» https://doi.org/10.3923/jbs.2010.224.230 - Bucana CD, Nadakavukaren MJ (1973) Ultrastructural investigation of the postnatal development of the hamster Harderian gland. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 142(1): 1-12.
- Burck NC (1975) Technika histologiczna. Warszawa, PZWL.
- Buzzell GR (1996) The Harderian gland: perspectives. Microscopy Research and Technique 34(1): 2-5.
- Cabral VP, Laus JL, Zaidan ML, Dagli ML, Pereira GT, Talieri IC, Monteiro ER, Villela Mamede F (2005) Canine lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciencia Rural 35(2): 391-397. doi: 10.1590/S0103-84782005000200023
» https://doi.org/10.1590/S0103-84782005000200023 - Chieffi G, Baccari GC, Di Mateo L, D'Istria M, Minucci S, Varriale B (1996) Cell biology of the Harderian gland. International Review of Cytology 168: 1-80.
- Cornell-Bell AH, Sullivan DA, Allansmith MR (1985) Gender-related differences in the morphology of the lacrimal gland. Investigative Ophthalmology & Visual Science 26(8): 1170-1175.
- Davidson HJ, Kuonen VJ (2004) The tear film and ocular mucins. Veterinary Ophthalmology 7(2): 71-77. doi: 10.1111/j.1463-5224.2004.00325.x
» https://doi.org/10.1111/j.1463-5224.2004.00325.x - Djeridane Y (1992) The Harderian gland of desert rodents: a histological and ultrastructural study. Journal of Anatomy 180(3): 465-480.
- Djeridane Y (1994) The Harderian gland and its excretory duct in the Wistar rat. A histological and ultrastructural study. Journal of Anatomy 184(3): 553-566.
- El-Fadaly AB, El-Shaarawy EAA, Rizk AA, Nasralla MM, Shuaib DMA (2014) Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Annals of Anatomy 196(5): 336-351. doi: 10.1016/j.aanat.2014.06.005
» https://doi.org/10.1016/j.aanat.2014.06.005 - Fahmy MFA, Arnautovic I, Abdalla O (1971) The morphology of the tarsal glands and the glands of the third eyelid in the one-humped camel (Camelus dromedarius). Acta Anatomica 78(1): 40-46.
- Fix AS, Arp LH (1991) Quantification of particle uptake by Conjunctiva-Associated Lymphoid Tissue (CALT) in chickens. Avian Diseases 35(1): 174-179.
- Gargiulo AM, Coliolo P, Ceccarelli P, Pedini V (1999) Ultrastructural study of sheep lacrimal gland. Veterinary Research 30(4): 345-351.
- Gargiulo AM, Dall'Aglio C, Coliolo P, Ceccarelli P, Pedini V (2000) Complex carbohydrate histochemistry and ultracytochemistry of the sheep lacrimal gland. Anatomia Histologia Embryologia 29(1): 19-23. doi: 10.1046/j.1439-0264.2000.00229.x
» https://doi.org/10.1046/j.1439-0264.2000.00229.x - Henker R, Scholz M, Gaffling S, Asano N, Hampel U, Garries F, Horngger J, Paulsen F (2013) Morphological features of the porcine lacrimal gland and its compatibility for human lacrimal gland Xenografting. Plos One 8(9): e74046. doi: 10.1371/journal.pone.0074046
» https://doi.org/10.1371/journal.pone.0074046 - Iwata S (1973) Chemical composition of the aqueous phase. International Ophthalmology Clinics 13(1): 29-46.
- Jensen OA, Falbe-Hansejn I, Jacobsen T, Michelsen A (1969) Mucosubstances of the acini of the human lacrimal gland (Orbital part). Acta Ophthalmology 47(3): 605-619. doi: 10.1111/j.1755-3768.1969.tb08147.x
» https://doi.org/10.1111/j.1755-3768.1969.tb08147.x - Johnston HS, Mcgadey J, Thompson GG, Moore MR, Payne AP (1983) The Harderian gland, its secretory and porphyrin content in the Mongolian gerbil (Meriones unguiculatus). Journal of Anatomy 137(3): 615-630.
- Johnston HS, Mcgadey J, Thompson GG, Moore MR, Breed WG, Payne AP (1985) The Harderian gland, its secretory and porphyrin content in the Plain mouse (Pseudomys australis). Journal of Anatomy 140(2): 337-350.
- Klećkowska-Nawrot J, Marycz K, Czogała J, Kujawa K, Janeczek M, Chrószcz A, Brudnicki W (2013) Morphology of the lacrimal gland and superficial gland of the third eyelid of Roe deer (Capreolus capreolus L). Pakistan Veterinary Journal 33(2): 139-144.
- Kurnick NB (1955) Pyronin Y in the methyl-green-pyronin histological stain. Stain Technique 30(5): 213-230.
- Kühnel W (1968a) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. V. Rind. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 87: 504-525.
- Kühnel W (1968b) Vergleichende histologische, histochemische und electronen-mikroskopische Untersuchungen an Tränendrüsen. II. Ziege. Zeitschrift für Zellforschung Mikroskopicsche und Anatomie 86: 430-443.
- Lopez JM, Tolivia J, Alvarez-Uria M (1992) Postnatal development of the Harderian gland in the Syrian golden hamster (Mesocricetus auratus): A light and electron microscopic study. Anatomical Record 233(4): 597-616. doi: 10.1002/ar.1092330414
» https://doi.org/10.1002/ar.1092330414 - Lorber M (1989) Elastic fibers in the rat extraorbital lacrimal gland duct system. Investigative Ophthalmology & Visual Science 30: 2002-2011.
- Maala CP, Cartagena RA, De Ocampo GD (2007) Macroscopic Histological and Histochemical Characterization of the Lacrimal Gland of the Philippine Water Buffalo (Bubalus bubalus). Philippine Journal of Veterinary Medicine 44(2): 69-75.
- Martin CL, Munnell J, Kawsan R (1988) Normal ultrastructure and histochemical characteristics of canine lacrimal glands. American Journal of Veterinary Research 49(9): 1566-1572.
- Mohammadpour AA (2008) Anatomical characteristics of dorsal lacrimal gland in one humped camel (Camelus dromedarius). Journal of Biological Sciences 8(6): 1104-1106. doi: 10.3923/jbs.2008.1104.1106
» https://doi.org/10.3923/jbs.2008.1104.1106 - Nomina Anatomica Veterinaria (2012) Nomina Anatomica Veterinaria. Hannover, World Association of Veterinary Anatomists, 5th ed., p. 149-150. Available online at:Available online at:http://www.wava-amav.org/nav_nev.htm [Accessed: 30/09/2015]
» http://www.wava-amav.org/nav_nev.htm - Payne AP (1994) The Harderian gland: A tercentennial review. Journal of Anatomy 185(1): 1-49.
- Pinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003a) Evaluation of lysozyme and lactoferrin in lacrimal and other ocular glands of bison and cattle and in tears of bison. American Journal of Veterinary Research 64(1): 104-108.
- Pinard CL, Weiss ML, Brightman AH, Fenwick BW, Davidson HJ (2003b) Normal anatomical and histochemical characteristics of the lacrimal glands in the American bison and cattle. Anatomia Histologia Embryologia 32(5): 257-262. doi: 10.1046/j.1439-0264.2003.00460.x
» https://doi.org/10.1046/j.1439-0264.2003.00460.x - Pradidarcheep W, Asavapongpatana S, Mingsakul T, Poonkhum R, Nilbu-Nga S, Somana R (2003) Microscopic anatomy of the orbital Harderian gland in the common tree shrew (Tupaia glis). Journal of Morphology 255(3): 328-336. doi: 10.1002/jmor.10066
» https://doi.org/10.1002/jmor.10066 - Rehorek SJ, Firth BT, Hutchinson MN (2000) Can an orbital gland function in the vomeronasal sense? A study of the pygopodid Harderian gland. Canadian Journal of Zoology 78(4): 648-654. doi: 10.1139/cjz-78-4-648
» https://doi.org/10.1139/cjz-78-4-648 - Rehorek SJ, Hillenius WJ, Sanjur J Chapman NG (2007) One gland, two lobes: organogenesis of the Harderian and "nictitans" gland of the Chinese muntjac (Muntiacus reevesi) and fallow deer (Dama dama). Annals of Anatomy 189(5): 434-446. doi: 10.1016/j.aanat.2006.10.007
» https://doi.org/10.1016/j.aanat.2006.10.007 - Sakai T (1981) The mammalian Harderian gland: morphology, biochemistry, function and physiology. Archives of Histology Japan 44(4): 299-333.
- Sakai T (1989) Major ocular glands (Harderian gland and lacrimal gland) of the musk shrew (Suncus murinus) with a review on the comparative anatomy and histology of the mamma lian lacrimal glands. Journal of Morphology 201(1): 39-57.
- Sakai T (1992) Comparative anatomy of the mammalian Harderian glands. Berlin, Springer Verlag, p. 7-23.
- Shadkhast M, Bigham AS (2010) A histo-anatomical study of dorsal lacrimal gland in Iranian river buffalo. Vet Scan 5: 1-5.
- Sahadkhast M, Bigham AS, Vhadkhast N, Bigham AS, Vajdi N, Shafiei Z (2008) Lacrimal apparatus system in goat (Capra aegagrus hircus): Anatomical and radiological study. Asian Journal of Animal Veterinary Advance 3: 457-460. doi: 10.3923/ajava.2008.457.460
» https://doi.org/10.3923/ajava.2008.457.460 - Shirama K, Furuya T, Takeo Y, Shimuzu K, Maekawa K (1981) Influences of some endocrine glands and of hormone replacement on the porphyrins of the Harderian gland of mice. Journal of Endocrinology 91(2): 305-311.
- Spicer SC, Henson JG (1967) Methods for localizing mucosubstances in epithelial and connective tissue, p. 78-112. In: Bajusz E, Jamin F (Eds.) Series on Methods and Achievements in Experimental Pathology. Basal, S. Karger Press, vol. 2.
- Walcott B (1998) The lacrimal gland and its veil of tears. Physiology 13: 97-103.
- Weaker RJ (1981) Light microscopic and ultrastructural features of the Harderian gland of the nine-banded armadillo. Journal of Anatomy 133(1): 49-65.
- Van Ginkel FW, Gulley SL, Lammers A, Hoer FJ, Gurjar R, Toro H (2012) Conjunctiva-associated lymphoid tissue in avian mucosal immunity. Developmental & Comparative Immunology 36(2): 289-297. doi: 10.1016/j.dci.2011.04.012
» https://doi.org/10.1016/j.dci.2011.04.012
Publication Dates
-
Publication in this collection
31 Oct 2015
History
-
Received
13 Mar 2015 -
Reviewed
19 June 2015 -
Accepted
19 July 2015