Abstract
We present here a taxonomic study of Apocynaceae from the Atlantic Forest remnants in the state of Rio Grande do Norte (RN), Northeast Brazil. Twenty-four species in 18 genera, distributed in Apocynoid grade (3 genera/5 species), Asclepiadoideae (8 genera/10 spp.), and Rauvolfioid grade (7 genera/9 spp.) were recorded. The most species-rich genera were Aspidosperma, Ditassa, and Mandevilla with three species each. The other genera were represented by one species each. Five genera and seven species were recorded in Rio Grande do Norte for the first time. Descriptions, identification key and comments on distribution and taxonomy of both genera and species are presented, as well as illustrations and figures.
Key words
new records; Northeastern Brazil; Restinga; semideciduous forest; taxonomy
Resumo
Apresentamos aqui o estudo taxonômico de Apocynaceae em remanescentes de Mata Atlântica no estado do Rio Grande do Norte, Brasil. Foram encontradas 24 espécies em 18 gêneros distribuídos no grado Apocynoid (3 gêneros/5 espécies), em Asclepidoideae (8 gêneros/10 spp.), e grado Rauvolfioid (7 gêneros/9 spp.). Os gêneros com maior riqueza de espécies foram Aspidosperma, Ditassa e Mandevilla com três espécies cada. Os outros gêneros estão representados por uma espécie cada. Cinco gêneros e sete espécies são novas ocorrências para o Rio Grande do Norte e são citados aqui pela primeira vez. Descrições, chave de identificação, comentários taxonômicos e sobre distribuição para gêneros e espécies são apresentados, além de ilustrações e fotos.
Palavras-chave
novas ocorrências; nordeste do Brasil; Restinga; floresta semidecídua; taxonomia
Introduction
Apocynaceae has a cosmopolitan distribution with almost 357 genera and ca. 5,100 species and is one of the 10 largest families among angiosperms (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169., 2012; Nazar et al. 2013Nazar N, Goyder DJ, Clarkson JJ, Mahmood T & Chase MW (2013) The taxonomy and systematics of Apocynaceae: where we stand in 2012. Botanical Journal of the Linnean Society 171: 482-490.). In Brazil, it is one the most representative family, with 782 species in 78 genera (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction).
At first, Apocynaceae was considered as only one family, Apocineae, by Jussieu (1789)Jussieu AL (1789) Genera plantarum. Viduam Herissant, Paris. 498p., but was later divided into Asclepiadeae and Apocineae sensu stricto by Brown (1810)Brown R (1810) On the Asclepiadeae, a natural order of plants separated from the Apocyneae of Jussieu. Memoirs of the Wernerian Natural History Society 1: 12-78.. Afterwards, Schlechter (1905)Schlechter R (1905) Periplocaceae e Asclepiadaceae. In: Schumann K & Lauterbach K (eds.) Flora der Deutschen Schutzgebiete in der Südsee. Bornträeger, Leipzig. Pp. 351-369. divided Asclepiadeae into the two families Periplocaceae and Asclepiadaceae. Based on advanced phylogenetic studies and morphological and molecular data, Asclepiadaceae and Periplocaceae are not recognized as families anymore, because this would make Apocynaceae s.s. paraphyletic (Sennblad & Bremer 1996Sennblad B & Bremer B (1996) The familial and subfamilial relationships of Apocynaceae and Asclepiadaceae evaluated with rbcLdata. Plant Systematics and Evolution 202: 153-176.; Endress & Stevens 2002Endress M & Stevens W (2002) The renaissance of the Apocynaceae s.l.: recent advances in systematics, phylogeny, and evolution: introduction. Annals of the Missouri Botanical Garden 88: 517-522.; Livshultz et al. 2007Livshultz T, Middleton DJ, Endress ME & Williams JK (2007) Phylogeny of Apocynoideae and the APSA Clade (Apocynaceae s.l.). Annals of the Missouri Botanical Garden 94: 324-359.).
Thus, Asclepiadaceae has become a subfamily within Apocynaceae under the name Asclepiadoideae, along with the other four subfamilies Apocynoideae, Periplocoideae, Rauvolfioideae, and Secamonoideae (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l. The Botanical Review 66: 1-56.; Endress et al. 2014Endress M, Liede-Schumann S & Meve U (2014) An updated classification for Apocynaceae. Phytotaxa 159: 175-194.); thus, Apocynaceae has become a monophyletic group (Simões et al. 2007Simões AO, Livshultz T, Conti E & Endress ME (2007) Phylogeny and systematics of the Rauvolfioideae (Apocynaceae) based on molecular and morphological evidence. Annals of the Missouri Botanical Garden 94: 268-297.). As Rauvolfioideae and Apocynoideae form paraphyletic groups (Endress et al. 2007Endress ME, Liede-Schumann S & Meve U (2007) Advances in Apocynaceae: the enlightenment, an introduction. Annals of the Missouri Botanical Garden 94: 259-267.), Simões et al. (2016)Simões AO, Kinoshita LS, Koch I, Silva MJ & Endress ME (2016) Systematics and character evolution of Vinceae (Apocynaceae). Taxon 65: 99-122. proposed the disuse of the taxonomic categories Rauvolfioideae and Apocynoideae and the adoption of the informal terms Rauvolfioid and Apocynoid grades for these groups.
In Brazil, the family is represented by Rauvolfioid and Apocynoid grades and the subfamily Asclepiadoideae (Rapini 2012Rapini A (2012) Taxonomy “under construction”: advances in the systematics of Apocynaceae, with emphasis on the brazilian Asclepiadoideae. Rodriguésia 63: 075-088.), distributed in all regions and phytogeographic domains of the country. Its center of diversity is in the Atlantic Rainforest with 367 species (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.).
The family is characterized by the presence of latex, a bicarpellary and hemisyncarpic to syncarpic ovary, a fused distal part of style (style-head), often opposite leaves, in some cases alternate or verticillate leaves, colleters usually present in nodes and leaf blades and in inflorescences and flowers, at the base of pedicels, bracts and calyx lobes (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l. The Botanical Review 66: 1-56.; Gomes et al. 2008Gomes SM, Kinoshita LS & Castro MM (2008) Hemisincarpia e nectário apendicular enfocados através de ontogênese floral em Mandevilla velame (A. St.-Hil.) Pichon, Apocynoideae. Brazilian Journal of Botany 31: 81-93.; Judd et al. 2009Judd WS, Campbell CS, Kellogg EA, Stevens PF & Donoghue MJ (2009) Sistemática vegetal: um enfoque filogenético. 3ª ed. Artmed, Porto Alegre. 632p.; Rapini 2012Rapini A (2012) Taxonomy “under construction”: advances in the systematics of Apocynaceae, with emphasis on the brazilian Asclepiadoideae. Rodriguésia 63: 075-088.). Due to the presence of large and showy flowers, many of its species are used as ornamentals, as species from genus (e.g., Allamanda L. and Mandevilla Lindl.) (Sakane & Shepherd 1986Sakane M & Shepherd GJ (1986) Revisão do gênero Allamanda L. (Apocynaceae). Revista Brasileira de Botânica 9: 125-149.; Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ). The family is also known for its chemical compounds with pharmaceutical potential and for its high-quality wood (e.g., Aspidosperma Mart. & Zucc. and Rauvolfia L.) (Rao 1956Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.; Marcondes-Ferreira 1988Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p. ). Special mention to Catharanthus roseus (L.) G.Don, one of the most important species with pharmaceutical potential due to its various chemical compounds, including used in cancer treatment (Barkat et al. 2017Barkat MA, Abul H & Rahman MA (2017) Agricultural, pharmaceutical and therapeutic interior of Catharanthus roseus (L.) G.Don. In: Naeem M, Aftab T & Khan M (eds.) Catharanthus roseus. Springer, Cham. 426p.; Mishra & Verma 2017Mishra JN & Verma NK (2017) A brief study on Catharanthus Roseus: a review. International Journal of Research in Pharmacy and Pharmaceutical Sciences 2: 20-23.; Paarakh et al. 2019Paarakh MP, Swathi S, Taj T, Tejashwini V & Tejashwini B (2019) Catharanthus Roseus Linn - a review. Acta Scientific Pharmaceutical Sciences 3: 19-24.).
Among the most important works on Apocynaceae for Brazil, the Flora brasiliensis by Müller (1860)Müller JA (1860) Apocynaceae. In: Martius CFP & Eichler AW (eds.) Flora brasiliensis. Frid. Fleischer, Lipsiae. Vol. 6, pars 1. pp. 1-196. stands out, in addition to the recent taxonomic studies mostly conducted in the southeastern region of the country (e.g., Vasconcellos & Gouvea 1993Vasconcellos MB & Gouvea LSK (1993) As Apocynaceae da região de Poços de Caldas, Minas Gerais, Brasil. Acta Botanica Brasilica 7: 107-127.; Fontella-Pereira et al. 1995Fontella-Pereira J, Valente MC & Marquete NFS (1995) Flora da Serra do Cipó, Minas Gerais: Asclepiadaceae. Boletim de Botânica da Universidade de São Paulo 14: 131-179.; Koch & Kinoshita 1999Koch I & Kinoshita LS (1999) As Apocynaceae s. str. da região de Bauru, São Paulo, Brasil. Acta Botanica Brasilica 13: 61-86.; Simões & Kinoshita 2002Simões AO & Kinoshita LS (2002) The Apocynaceae s.str. of the Carrancas region, Minas Gerais, Brazil. Darwiniana 40: 127-169.; Konno 2005Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.; Souza-Silva 2008Souza-Silva RF (2008) Estudos taxonômicos em Apocynaceae, com ênfase na Flora da Bahia. Trabalho de conclusão do curso de Bacharel em Ciências Biológicas. Universidade Estadual de Feira de Santana, Feira de Santana. 149p. ; Watanabe et al. 2009Watanabe MCT, Roque N & Rapini A (2009) Apocynaceae sensu strictum no Parque Municipal de Mucugê, Bahia, Brasil, incluindo a publicação válida de dois nomes em Mandevilla Lindl. Iheringia 64: 63-75.; Morokawa et al. 2013Morokawa R, Simões AO & Kinoshita LS (2013) Apocynaceae s. str. do Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Rodriguésia 64: 179-199.). For northeast Brazil, we can mention the “Flora do Pico das Almas”, Bahia state (Sales 1995Sales MF (1995) Apocynaceae. In: Stannard B (ed.) Flora of the Pico das Almas, Chapada Diamantina, Bahia, Brazil. Royal Botanic Gardens, Kew. Pp. 128-135. ), “Checklist das Plantas do Nordeste Brasileiro...” (Barbosa et al. 2006Barbosa MR, Sothers C, Mayo S, Gamarra-Rojas CFL & Mesquita AC (2006) Checklist das plantas do nordeste brasileiro: Angiospermas e Gymnospermas. Ministério da Ciência e Tecnologia, Brasília. 156p.), “Flora de Sergipe” (Prata et al. 2013Prata APN, Amaral MCE, Farias MCV & Alves MV (2013) Flora de Sergipe. Vol. 1. Gráfica e Editora Triunfo, Aracaju. 592p.) and “Flora da Usina São José”, Pernambuco state (Coutinho & Louzada 2018Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.). For Rio Grande do Norte state, only some floristic surveys were performed, in which some species of the family were listed (e.g., Freire 1990Freire MSB (1990) Levantamento florístico do Parque das Dunas do Natal. Acta Botanica Brasilica 4: 41-59.; Oliveira et al. 2012aOliveira ACP, Penha AS, Souza RF & Loiola MIB (2012a) Composição florística de uma comunidade savânica no Rio Grande do Norte, nordeste do Brasil. Acta Botanica Brasilica 26: 559-569., 2012bOliveira ACP, Mota ML & Loiola MIB (2012b) Diversidade florísta e chave de identificação de trepadeiras em uma floresta estacional semidecidual em Parnamirim, RN, Brasil. Revista Caatinga 25: 153-158., 2013Oliveira RC, Silva AS, Ribeiro ARO, Araújo JE, Oliveira OF & Camacho RGV (2013) List of Angiosperm Species of the Riparian Vegetation of the Apodi-Mossoró River, Rio Grande do Norte, Brazil. Check List 9: 740-751.).
Thus, taxonomic studies on Apocynaceae in the state are lacking, which is also the case for the flora in general. However, over the past few years, several floristic and taxonomic studies have been published, increasing the knowledge about the flora of Rio Grande do Norte (RN) (e.g., Ferreira et al. 2009Ferreira CGT, Oliveira RC, Valls JM & Loiola MIB (2009) Poaceae da Estação Ecológica do Seridó, Rio Grande do Norte, Brasil. Hoehnea 36: 679-707.; Oliveira et al. 2012aOliveira ACP, Penha AS, Souza RF & Loiola MIB (2012a) Composição florística de uma comunidade savânica no Rio Grande do Norte, nordeste do Brasil. Acta Botanica Brasilica 26: 559-569., 2012b; São-Mateus et al. 2013São-Mateus WMB, Cardoso D, Jardim JG & Queiroz LP (2013) Papilionoideae (Leguminosae) na Mata Atlântica do Rio Grande do Norte, Brasil. Biota Neotropica 13: 315-362.; Versieux et al. 2013aVersieux LM, Magalhães R & Calvente A (2013a) Extension of the Cryptanthus range in Northeastern Brazil with new findings in the phenotypic variation including changes in the trichome’s distribution, thus enhancing the understanding of the Cryptanthus zonatus complex (Bromeliaceae). Phytotaxa 109: 54-60., 2013bVersieux LM, Tomaz EC & Jardim JG (2013b) New genus and species records of Bromeliaceae in the Caatinga of Rio Grande do Norte state, northeastern Brazil: Orthophytum disjunctum L.B. Sm. (Bromelioideae) and Tillandsia paraibensis R.A. Pontes (Tillandsioideae). Check List 9: 663- 665.; Colombo et al. 2016Colombo BR, Kaehler M & Calvente A (2016) An inventory of the Bignoniaceae from the Brazilian state of Rio Grande do Norte highlights the importance of small herbaria to biodiversity studies. Phytotaxa 278: 019-028.; Soares Neto & Jardim 2015Soares Neto RL & Jardim JG (2015) Capparaceae no Rio Grande do Norte, Brasil. Rodriguésia 66: 847-857.; Soares et al. 2017Soares AS, Pastore JFB & Jardim JG (2017) New records, conservation assessments and distribution of Lamiaceae in Rio Grande do Norte, northeastern, Brazil. Phytotaxa 311: 043-056.; Moura et al. 2018Moura EO, Sousa VF, Soares AS & Versieux LM (2018) Private environmental consultancy reveals five genera and ten species of angiosperms new to Rio Grande do Norte state, northeastern Brazil. Check List 14: 439-451.; Soares et al. 2019Soares AS, Pastore JFB & Jardim JG (2019) Lamiaceae no Rio Grande do Norte, Brasil. Rodriguesia 70: 02-17. ). According to the BFG (2015)BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113., RN’s flora has increased by 72.8% in number of species since the first edition of the Brazilian Flora in 2010 (Forzza et al. 2010Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa A, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Martinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de Plantas e Fungos do Brasil. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 874p.).
Besides the local need for studies about Apocynaceae, it is also relevant to highlight the importance of works made in the RN Atlantic Rainforest. In general, the Atlantic Rainforest is highly fragmented and at risk (MMA 2010MMA - Ministério do Meio Ambiente (2010) Mata Atlântica: patrimônio nacional dos brasileiros. In: Campanili M & Schaffer WB (orgs.) Biodiversidade 34. Secretaria de Biodiversidade e Florestas, Brasília. 408p.), and considering the local context, its fragments are the least monitored and with the highest information scarcity among the northeastern states (Maciel 2011Maciel LVB (2011) Análise dos remanescentes de Mata Atlântica no estado do Rio Grande do Norte: uma perspectiva em alta resolução. Dissertação de Mestrado. Universidade Federal do Rio Grande do Norte, Natal. 47p. ). Moreover, its Permanent Preservation Areas (APPs) and Reserve Areas are highly degraded due to little incentive for scientific research, public policies, and conservation actions (Maciel 2011Maciel LVB (2011) Análise dos remanescentes de Mata Atlântica no estado do Rio Grande do Norte: uma perspectiva em alta resolução. Dissertação de Mestrado. Universidade Federal do Rio Grande do Norte, Natal. 47p. ).
Therefore, this work aimed to conduct a taxonomic study of Apocynaceae in Rio Grande do Norte Atlantic Coastal Forest, with the presentation of identification keys, descriptions, taxonomic and geographic distribution comments, and illustrations and photos of the species. It contributes to the generation of subsidies for studies and conservation actions for the species of Apocynaceae and the Atlantic Forest remnants of Rio Grande do Norte state.
Materials and Methods
Area of study
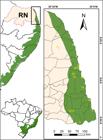
Rio Grande do Norte state (RN) covers an area of 52,811 km2 (IBGE 2014IBGE - Instituto Brasileiro de Geografia e Estatística (2014) Estados: Rio Grande do Norte. Available at <https://cidades.ibge.gov.br/brasil/rn/panorama. Access on 10 August 2021.
https://cidades.ibge.gov.br/brasil/rn/pa...
) and is located in the northeast of Brazil (Fig. 1a,b). The climate is tropical rainy climate with dry summers on the east and along the coast; the western part is semi-arid (Alvares et al. 2013Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM & Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.; Guerra et al. 2013Guerra AG, Maia A & Spinelli ACGO (2013) Perfil do Rio Grande do Norte, Secretaria de Estado do Planejamento e das Finanças. Instituto de Desenvolvimento Econômico e Meio Ambiente, Natal. 191p.). The area of the Atlantic Forest in the state, located mostly on the eastern coast, has extension 350,994 hectares (7% of the state area) (SOS Mata Atlântica & INPE 2018SOS Mata Atlântica & INPE (2018) Atlas dos remanescentes florestais da Mata Atlântica, Período 2016-2017, relatório técnico. São Paulo. Available at <http://mapas.sosma.org.br/site_media/download/Atlas_Mata_Atlantica_2016-2017_relatorio_tecnico_2018_final.pdf> . Access on 8 May 2020.
http://mapas.sosma.org.br/site_media/dow...
). The Atlantic Forest strip consists of semideciduous seasonal and lowland deciduous forest, including Restinga and mangroves, the Cerrado-Caatinga transition area (ecotone and enclaves), as well as the northeastern refuges or “brejos de altitude”, in the far west of the state (Oliveira-Filho & Fontes 2000Oliveira-Filho AT & Fontes MAL (2000) Patterns of Floristic Differentiation among Atlantic Forests in Southeastern Brazil and the Influence of Climate. Biotropica 32: 793-810.; Cestaro & Soares 2004Cestaro LA & Soares JJ (2004) Variações florística e estrutural e relações fitogeográficas de um fragmento de floresta decidual no Rio Grande do Norte, Brasil. Acta Botanica Brasilica 18: 203-218.; Ribeiro et al. 2009Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ & Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142: 1141-1153.; IBGE 2012IBGE - Instituto Brasileiro de Geografia e Estatística (2012) Manual Técnico da Vegetação Brasileira. 2ª ed. IBGE, Rio de Janeiro. 270p.; Oliveira et al. 2012aOliveira ACP, Penha AS, Souza RF & Loiola MIB (2012a) Composição florística de uma comunidade savânica no Rio Grande do Norte, nordeste do Brasil. Acta Botanica Brasilica 26: 559-569.). The Restinga is an associated ecosystem located along the Brazilian coast (Rizzini 1997Rizzini CT (1997) Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. 2ª ed. Âmbito Cultural, Rio de Janeiro. 748p.), the Cerrado is a Brazilian savanna-like phytogeographic domain, characteristic of Central Brazil (Oliveira & Marquis 2002Oliveira P & Marquis R (2002) Preface. In: Oliveira P & Marquis R (eds.) The Cerrados of Brazil: ecology and natural history of a Neotropical Savanna. Columbia University Press, New York. 424p.), the Caatinga is a Brazilian semi-arid phytogeographic domain, in northeast Brazil (Prado 2003Prado DE (2003) As Caatingas da América do Sul. In: Leal IR, Tabarelli M & Silva JMC (eds.) Ecologia e conservação da Caatinga. Editora Universitária-UFPE, Recife. 822p.) and the “brejos de altitude” are rainforest “islands” in highlands and plateaus surrounded by Caatinga in northeast Brazil (Tabarelli & Santos 2004Tabarelli M & Santos AMM (2004) Uma breve descrição sobre a história natural dos brejos nordestinos. In: Porto KC, Cabral JJP & Tabarelli M (orgs.) Brejos de altitude em Pernambuco e Paraíba: história natural, ecologia e conservação. Série Biodiversidade, 9. Cap. 2. Ministério do Meio Ambiente, Brasília. Pp. 17-24.).
a. Map of Rio Grande do Norte, highlighting the study area (Atlantic Forest range in green); b. study area including the Atlantic Forest range (green) in RN. Numbers represent the localities sampled, as detailed in Table 1.
Data collection
Data were collected in areas with remnants of Atlantic Rainforest, in Protected Areas (UCs) and in private areas (Fig. 1a,b; Tab. 1), between July and December 2014 and between May and July 2015 by active searches along the trails and forests edges (Fig. 1a,b). Fruit and flower samples were preserved in 70% alcohol for later laboratory analysis, and the voucher specimen was stored in the UFRN herbarium collection. Duplicates were donated to HUEFS, MOSS and RB herbaria, acronyms according to Thiers (continuously updated), and HST, acronym according to “Rede Brasileira de Herbários” (2019Rede Brasileira de Herbários (2019) Available at <https://www.botanica.org.br/catalogo-da-rede-brasileirade-herbarios/>. Access on 09 August 2021.
https://www.botanica.org.br/catalogo-da-...
).
Localities visited in the remnants of the Atlantic Forest of the state Rio Grande do Norte, organized by municipalities. * = Vegetation typology according to the IBGE (2012)IBGE - Instituto Brasileiro de Geografia e Estatística (2012) Manual Técnico da Vegetação Brasileira. 2ª ed. IBGE, Rio de Janeiro. 270p..
Specimens were consulted in CEPEC, HUEFS, MOSS and UFRN herbaria, acronyms according to Thiers (continuously updated), and HST, acronym according to “Rede Brasileira de Herbários” (2019), as well as samples available online in EAC, HCDAL, HST, IPA and SP on INCT-Virtual Herbarium of Flora and Fungi/speciesLink (INCT 2019INCT - Herbário Virtual da Flora e dos Fungos (2019) speciesLink. Available at <http://inct.splink.org.br>. Access on 30 april 2019.
http://inct.splink.org.br...
). Materials from outside the study area, including from other states, were used to complement the specimen descriptions (see additional material). Data from the following studies were also consulted and mentioned in the comments of the descriptions: Rao (1956)Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354., Rapini (2001)Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169., Viana et al. (2017)Viana SS, Santos JUM & Simões AO (2017) Taxonomic diversity of Apocynaceae at the Marajó Island, PA, Brazil. Rodriguesia 68: 623-652., Coutinho & Louzada (2018)Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714. and Fernandes et al. (2018)Fernandes GEA, Mota NFO & Simões AO (2018) Flora das cangas da Serra dos Carajás, Pará, Brasil: Apocynaceae. Rodriguésia 69: 3-23..
Morphological analysis to identify specimens was performed by a specialized literature search (e.g., Rao 1956Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.; Sakane 1981Sakane M (1981) Revisão do gênero Allamanda L. (Apocynaceae) no Brasil. Dissertação de Mestrado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 107p. ; Marcondes-Ferreira 1988Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p. ; Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ; Leeuwenberg 1994Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.; Morales 1999aMorales JF (1999a) A new species of Macoubea (Apocynaceae) from Mesoamerica. Novon 9: 86-88.,b; Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.; Spina 2004Spina AP (2004) Estudos taxonômico, micro-morfológico e filogenético do gênero Himatanthus Willd. ex Schult. (Apocynaceae: Rauvolfioideae - Plumerieae). Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 191p. ; Konno 2005Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.; Morales 2005aMorales JF (2005a) Estudios en las Apocynaceae neotropicales XIX: la familia Apocynaceae (Rauvolfioideae, Apocynoideae) de Costa Rica. Darwiniana 43: 90-191.,b; Rapini & Farinaccio 2008Rapini A & Farinaccio MA (2008) Two taxonomic changes in Asclepiadoideae (Apocynaceae) from Brazil. Neodiversity 3: 19-21.; Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). For morphological description, the terms adopted by Radford et al. (1974)Radford AE, Dickson WC, Massey JR & Bell CR (1974) Vascular plant systematics. Harper Collins & Row, New York. 891p. and Gonçalves & Lorenzi (2011)Gonçalves EG & Lorenzi H (2011) Morfologia vegetal: organografia e dicionário ilustrado de morfologia das plantas vasculares. 2ª ed. Instituto Plantarum de Estudos da Flora, São Paulo. 544p. were used. In this work the genera and species will be presented following the proposal of Simões et al. (2016)Simões AO, Kinoshita LS, Koch I, Silva MJ & Endress ME (2016) Systematics and character evolution of Vinceae (Apocynaceae). Taxon 65: 99-122.. Exotic cultivated species were not included in the taxonomic treatment but cited in the Results and Discussion and illustrated on clipboards with photos for the interested public.
Points 4 and 5 (Fig. 1b) are outside the Atlantic Rainforest area because of Law 11.428 (Brasil 2006Brasil (2006) Lei n. 11.428, de 22 de dezembro de 2006. Presidência da República, Casa Civil, Subchefia para Assuntos Jurídicos, Brasília. Available at <http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/lei/l11428.htm> . Access on 5 April 2019.
http://www.planalto.gov.br/ccivil_03/_at...
), which regulates the official boundaries for the domain in Brazil, did not include these areas. However, local studies suggest the inclusion of these areas in the Atlantic Forest domain, based on their floristic and phytophysiognomic relationships (Cestaro & Soares 2004Cestaro LA & Soares JJ (2004) Variações florística e estrutural e relações fitogeográficas de um fragmento de floresta decidual no Rio Grande do Norte, Brasil. Acta Botanica Brasilica 18: 203-218.; Maciel 2011Maciel LVB (2011) Análise dos remanescentes de Mata Atlântica no estado do Rio Grande do Norte: uma perspectiva em alta resolução. Dissertação de Mestrado. Universidade Federal do Rio Grande do Norte, Natal. 47p. ). For the definition of the new Apocynaceae records, the studies carried out in the Rio Grande do Norte state and which included the family were consulted (e.g., Freire 1990Freire MSB (1990) Levantamento florístico do Parque das Dunas do Natal. Acta Botanica Brasilica 4: 41-59.; Oliveira et al. 2012aOliveira ACP, Penha AS, Souza RF & Loiola MIB (2012a) Composição florística de uma comunidade savânica no Rio Grande do Norte, nordeste do Brasil. Acta Botanica Brasilica 26: 559-569., 2012b, 2013), and consultation of the Flora do Brasil (2020, under construction).
Results and Discussion
We registered 24 species in 18 genera distributed in Apocynoid (3 genera/5 species), Asclepiadoideae (8 gen/10 spp.), and Rauvolfioid (7 gen./9 spp.)(Figs. 3-9). The genera more representative were Aspidosperma Mart. & Zucc., Ditassa R.Br. and Mandevilla Lindl. with three species each, while the other genera were represented by one species each. Most of the species registered were climbers, totaling 13 spp. (54%), whereas seven species (29%) were arboreal, three (13%) were shrubby, and one (4%) was herbaceous.
Seven new occurrences of Apocynaceae in RN are represented here: Cynanchum roulinioides (E. Fourn.) Rapini, Ditassa hispida (Vell.) Fontella, Macoubea guianensis Aubl., Mandevilla microphylla (Stadelm.) M.F. Sales & Kin.-Gouv., Odontadenia lutea (Vell.) Markgr., Tabernaemontana flavicans Willd. ex Roem. & Schult., and Temnadenia odorifera (Vell.) J.F. Morales. Five genera from the new records are registered for this state for the first time: Cynanchum L., Macoubea Aubl., Odontadenia Benth., Tabernaemontana L., and Temnadenia Miers.
Two species (Aspidosperma sp. and Ditassa sp.), it was not possible to identify at the species level. However, the characteristics found distinguish these taxons from the other species in this study, as evidenced in the identification key and in the comments.
Besides the mentioned species, nine species were observed only as plants grown in gardens and flowerbeds: Allamanda cathartica L., Catharanthus roseus (L.) G.Don, Cryptostegia grandiflora R.Br., Nerium oleander L., Plumeria pudica Jacq., P. rubra L., Tabernaemontana divaricata (L.) R.Br. ex Roem. & Schult., T. pandacaqui Poir. and Thevetia peruviana (Pers.) K.Schum (Fig. 2).
a. Allamanda cathartica – flowering branch and flowers in anthesis (lateral view). b. Catharanthus roseus – flowers (front view). c. Cryptostegia grandiflora – flowers (front view). d. Nerium oleander – flowering branch and flowers in anthesis. e. Plumeria pudica – flowering branch. f. Plumeria rubra – flowering branch and flowers in anthesis. g. Tabernaemontana divaricata – flowering branch and flowers in anthesis (front view). h. Tabernaemontana pandacaqui – flowering branch and flowers in anthesis (front view). i. Thevetia peruviana – flower (lateral view). Photos: a-g. Jaerton C. Sousa Júnior; h, i. Jomar G. Jardim.
Identification key for species of Apocynaceae in the Atlantic Forest of Rio Grande do Norte
-
1. Herbs erect; corona with cornicle falcate, arcuate over the gynostegium...................15. Asclepias curassavica
-
1’. Shrubs, trees, or climbers; corona absent or, if present, with cornicle absent...................2
-
2. Shrubs or trees...................3
-
3. Leaves sessile; follicles spiny...................1. Allamanda blanchetii
-
3’. Leaves petiolate; follicles, berries or drupes smooth, striate, muricate, or rugose...................4
-
4. Branches and leaves covered with a greyish-white wax; gynostegium present; follicles ventricose...................17. Calotropis procera
-
4’. Branches and leaves lacking a greyish-white wax; gynostegium absent; follicles, berries or drupes never ventricose...................5
-
5. Leaves verticillate; drupes 0.7 × 0.6 cm...................8. Rauvolfia ligustrina
-
5’. Leaves opposite or alternate; follicles or berries > 0.7 × 0.6 cm...................6
-
6. Branches hollow; follicles cylindric, elongated, 18.6–27.8 cm long.........................................................6. Himatanthus bracteatus
-
6’. Branches full; follicles dolabriform, ellipsoid, or berries globoses, < 18 cm long...................7
-
7. Follicle ellipsoid...................9. Tabernaemontana flavicans
-
7’. Follicles dolabriform or berries globoses...................8
-
8. Leaves opposite distichous; blade with 2.9–5 × 1.2–2.1 cm; berry globose (1.7–3 × 1.1–2.5 cm)...................5. Hancornia speciosa
-
8’. Leaves alternate or opposite decussate; blades > 2.9–5 × 1.2–2.1 cm; follicles dolabriform or berry globose (5.5 × 3.5 cm)...................9
-
9. Leaves opposite decussate; berry globose.........................................................7. Macoubea guianensis
-
9’. Leaves alternate; follicles dolabriform...................10
-
10. Trunk sulcate; tertiary veins inconspicuous.........................................................2. Aspidosperma brasiliense
-
10’. Trunk smooth; tertiary veins conspicuous...................11
-
11. Branches with cataphylls on apical buds, nodes congested, like “scratches” perpendicular to the branch, latex white...................4. Aspidosperma sp.
-
11’. Branches lacking cataphylls on apical buds, nodes lax, latex red...................3. Aspidosperma aff. limae
-
-
-
-
-
-
-
-
-
-
2’. Climbers herbaceous or woody...................12
-
12. Corolla salverform or infundibuliform; corona absent; pollen dispersed in monads...................13
-
13. Calyx lobes 12–13 × 8.5 mm, unequal; corolla light yellow to greenish.........................................................13. Odontadenia lutea
-
13’. Calyx lobes < 12–13 × 8.5 mm, equal; corolla yellow, light pink to white, or purple.........................................................14
-
14. Latex transparent; corolla salverform, lilac-pink; follicles 24 cm long.........................................................14. Temnadenia odorifera
-
14’. Latex white; corolla infundibuliform, yellow or light pink to white; follicles < 24 cm long...................15
-
15. Leaf blade densely pubescent to velutinous on abaxial face; upper tube of corolla 2.8‒3.4 cm long, externally pilose, faux red......................................12. Mandevilla scabra
-
15’. Leaf blade glabrous, or hirsute only on the base of midrib on abaxial face; upper tube of corolla < 2.8–3.4 cm long, externally glabrous, faux yellow or pink to dark pink...................16
-
16. Corolla light pink to white, faux pink, tube lacking fleshy projections on the internal base; follicles 9.4–11.8 cm long.........................................................11. Mandevilla moricandiana
-
16’. Corolla and faux yellow, tube with five fleshy projections on the internal base; follicles 18 cm long...................10. Mandevilla microphylla
-
-
-
-
-
12’. Corolla campanulate, rotate or urceolate; corona present; pollen dispersed in pollinarium...................17
-
17. Corona simple...................18
-
18. Corolla campanulate; corona segments alternate to anthers...................18. Cynanchum roulinioides
-
18’. Corolla rotate or urceolate; corona segments opposite to anthers...................19
-
19. Leaf blades with base rounded; pollinia 0.03 × 0.02 mm, obdeltoid.........................................................16. Blepharodon pictum
-
19’. Leaf blades with base cordate; pollinia 0.5–0.6 × 0.2–0.4 mm, obovoid to ellipsoid.........................................................20
-
20. Corolla rotate, lobes with apex light green and base purple on both faces; follicles 4.9 cm long, tuberculate...................23. Ibatia ganglinosa
-
20’. Corolla urceolate, lobes vinaceous adaxially and green abaxially; follicles 19.2 cm long, striate to slightly lamellate...................24. Marsdenia altissima
-
-
-
-
17’. Corona doubled...................21
-
21. Corona with internal segments shaped as vesicular bags, globose.........................................................22. Funastrum clausum
-
21’. Corona with internal segments narrowly deltate or subulate...................22
-
22. Branches and leaf blades hirsute; corona with external and internal segments subulate.........................................................20. Ditassa hispida
-
22’. Branches and leaf blades pilose to glabrous; corona with external segments narrowly deltate or ovate to spatulate and internal ones narrowly deltate...................23
-
23. Branches glabrous; corona with external segments 0.6–0.7 mm long, ovate to spatulate, internal segments 0.2–0.5 mm long, apex not touching the dorsal surface of anthers...................19. Ditassa crassifolia
-
23’. Branches pilose bilaterally; corona with external segments 1.9–2 mm long, narrowly deltate, internal segments 0.7 mm long, apex touching the dorsal surface of anthers......................................21. Ditassa sp.
-
-
-
-
-
-
Taxonomic treatment I. Rauvolfioid grade
This grade is composed of 11 tribes and 79 genera (Endress et al. 2014Endress M, Liede-Schumann S & Meve U (2014) An updated classification for Apocynaceae. Phytotaxa 159: 175-194.), with distribution in the Americas, Africa, Europe, and Asia, in tropical and temperate regions, more rarely in subtropical regions. It also occurs in the Pacific Bay (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l. The Botanical Review 66: 1-56.). Rauvolfioid is recognized by flowers mainly with sinistrorse aestivation, more rarely dextrorse, by the corona absent, by the anthers free from the style-head, without gynostegium, and the fruit types follicle, berry and drupe.
Allamanda L., Mant. 146: 214. 1771.
This genus is native to the Neotropical region and can be found from Mexico to Argentina (Sakane & Shepherd 1986Sakane M & Shepherd GJ (1986) Revisão do gênero Allamanda L. (Apocynaceae). Revista Brasileira de Botânica 9: 125-149.), with 14 species (Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). It has a wide distribution in Brazil, where it is represented by 13 species, 11 of which are endemic. Nine species occur in the northeast, with three being reported for Rio Grande do Norte (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). Some species are commonly known for their ornamental value, such as A. blanchetii and A. cathartica (Sakane & Shepherd 1986Sakane M & Shepherd GJ (1986) Revisão do gênero Allamanda L. (Apocynaceae). Revista Brasileira de Botânica 9: 125-149.).
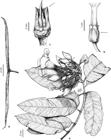
1. Allamanda blanchetii A. DC., Prodr. 8: 319. 1844. Fig. 3a
a. Allamanda blanchetii – immature fruit. b-c. Aspidosperma brasiliense – b. sulcate trunk; c. old fruits. d-e. Aspidosperma sp. – d. habit (flowering specimen); e. immature fruits. f-g. Himatanthus bracteatus – f. detail of flower (front view); g. immature fruit. h. Macoubea guianensis – branch (apical part). i. Mandevilla microphylla – detail of inflorescence with flower in anthesis (lateral view). Photos: a-c; f, g, i. Jaerton C. Sousa Júnior; d, e, h. Jomar G. Jardim.
Shrub, ca. 2 m tall, branches cylindrical, full, olive-green, slightly striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, interpetiolar; pilose to glabrous. Leaves verticillate, 4–5 per node, sessile; blade 3–5.7 × 1.8–3.4 cm, ovate, apex acuminate, base attenuate, margin entire, slightly revolute; venation brochidodromous, 12 secondary veins, tertiary veins little distinct; discolorous, chartaceous, colleters 5–7, intrapetiolar; pilose on both faces. Cyme terminal, bostryx, 2-flowered; peduncle 0.5 cm long, pilose; bracts 2.6–3 × 0.7–1.3 mm, deltate, colleters absent; pilose to glabrous on both faces. Flowers with pedicel 0.3–0.5 cm long, pilose; calyx lobes 5.7–8 × 1.8–5 mm, ovate to lanceolate, equal, apex acute, margin ciliate, colleters absent; pilose on both faces; corolla infundibuliform, aestivation sinistrorse, vinaceous, faux vinaceous, lobes 1.8–1.9 × 2–2.1 cm, orbicular, vinaceous on both faces, margin glabrous, glabrous on both faces; upper tube 2.2 × 2 cm, glabrous on both faces; lower tube 2 × 0.5 cm, glabrous on both faces, lanuginose around the anthers; anthers 5.5 cm long, lanceolate to narrowly deltate, apex acute, base auriculate; free from the style-head; pollen dispersed in monads; nectar disk five-lobuled, concrescent with the ovary; ovary 2 mm long, ovoid, superior; style-head 2.5 mm long, cylindrical, apex bicuspidate. Follicles 6.3–6.4 × 3.3–3.9 cm, ellipsoid, laterally compressed, symmetrical, brown, spiny, glabrous; seeds 22 × 19 mm, orbicular, brown, glabrous.
Examined material: Ceará-Mirim, entrada na RN-309 para Pureza, c. 4,3 km da entrada, c. 3 km a leste seguindo pelo canavial, 05°34’38”S, 35°26’45”W, elev. 63 m, 14.XII.2011, fl. and fr., J.G. Jardim et al. 6152 (UFRN); entrada na RN-309 para Pureza, c. 4,3 km da entrada, c. 3 km a leste seguindo pelo canavial, 05°34’38”S, 35°26’45”W, elev. 63 m, 9.VIII.2014, fl. and fr., J.C. Sousa Jr & J.G. Jardim 46 (UFRN); Fazenda Santa Maria, 05°38’04”S, 35°25’32”W, elev. 33 m, 26.IX.1998, fl., W.R. Mizael 38 (MOSS). Parnamirim, Hidrominas Santa Maria, 13.X.2005, fl., A. Ribeiro & J. Silva 174 (UFRN).
Additional material: BRAZIL. RIO GRANDE DO NORTE: Acari, Sítio Talhado, proximidades do km 17, BR-427, vicinal c. de 3 km da rodovia, Serra após casas do sítio, 06°19’53”S, 36°37’29”W, elev. 378 m, 26.II.2011, fl., A.A. Roque et al. 897 (UFRN); Serra Negra do Norte, Estação Ecológica do Seridó, entrada do lajedo, 06°34’22”S, 37°15’29”W, elev. 219 m, 4.II.2018, fl. and fr., A.C. Davi et al. 26 (UFRN).
This species is endemic to Brazil and distributed throughout all northeastern states and Goiás, occurring mainly in the Caatinga (Sakane 1981Sakane M (1981) Revisão do gênero Allamanda L. (Apocynaceae) no Brasil. Dissertação de Mestrado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 107p. ; Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In the study area, it was found on edges of deciduous and semideciduous seasonal forests in enclaves of the Cerrado. This is the species’ first report of occurrence in the Atlantic Forest, since it has previously been registered only in the Caatinga. Collected with flowers and fruits from August to December.
It is easily recognized by the vinaceous corolla, a unique feature in the genus (Sakane 1981Sakane M (1981) Revisão do gênero Allamanda L. (Apocynaceae) no Brasil. Dissertação de Mestrado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 107p. ), the verticillate, pilose leaves with three to five leaves per node, the five-lobuled nectar disk and the laterally compressed and spiny fruits.
Aspidosperma Mart. & Zucc., Flora 7(Beil. 4): 135. 1924, nom. cons.
This genus is distributed along the Neotropics, from Mexico to Argentina, with the exception of Chile, including some islands in Central America (Marcondes-Ferreira 1988Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p. ), and is composed of 64 species (Pereira et al. 2019Pereira ASS, Devides-Castello AC, Simões AO & Koch I (2019) Reestablishment, new records, and a key for the species of Aspidosperma (Apocynaceae) from the Brazilian Amazon. Acta Botanica Brasilica 33: 1-20.). It has a wide distribution in Brazil, where 56 species are reported, 23 of which are endemic (Pereira et al. 2019Pereira ASS, Devides-Castello AC, Simões AO & Koch I (2019) Reestablishment, new records, and a key for the species of Aspidosperma (Apocynaceae) from the Brazilian Amazon. Acta Botanica Brasilica 33: 1-20.). The species might occur in woodlands as well as in the Cerrado, Caatinga, Campos de altitude (high mountain grasslands), Chaco, and Restinga (Marcondes-Ferreira 1988Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p. ). Of all species occurring in Brazil, 20 are found in the northeastern region and four in Rio Grande do Norte (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). Species from this genus are known for providing good-quality wood and alkaloids used in pharmacology and traditional medicine (Marcondes-Ferreira 1988Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p. ; Pereira et al. 2019Pereira ASS, Devides-Castello AC, Simões AO & Koch I (2019) Reestablishment, new records, and a key for the species of Aspidosperma (Apocynaceae) from the Brazilian Amazon. Acta Botanica Brasilica 33: 1-20.).
Represented by large trees or shrubs, with a trunk generally longitudinally sulcate, generally alternate leaves, as well as dolabriforms follicles.
2. Aspidosperma brasiliense A. S. S. Pereira & A. C. D. Castello, Phytotaxa 326(4): 236. 2017. Fig. 3b-c
Tree, ca. 20 m tall, trunk sulcate, branches cylindrical, full, dark to light brown, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters absent; glabrous. Leaves alternate, spiraled; petiole 7–8 mm long, cylindrical, ventrally sulcate, colleters absent; striate, glabrous; blade 5.7–11.2 × 3.6–4.4 cm, elliptic to oblong, apex acute, slightly acuminate and retuse, base acute to cuneate, slightly attenuate; margin entire, slightly revolute, flat at the base; venation brochidodromous, 14–16 secondary veins, little evident, tertiary veins inconspicuous; discolorous, chartaceous, colleters absent; glabrous on both faces, slightly incanous on midrib in both faces. Corymbiform, ca. 23-flowered; peduncle ca. 7 mm long, pubescent; bracts elliptic (Fernandes et al. 2018Fernandes GEA, Mota NFO & Simões AO (2018) Flora das cangas da Serra dos Carajás, Pará, Brasil: Apocynaceae. Rodriguésia 69: 3-23.). Flowers with pedicel 0.1–0.3 cm long, pilose; calyx lobes 1.5–2.5 × 1.3–1.5 mm, ovate, equal, apex obtuse to acute, margin pilose, colleters absent; pilose on both faces; corolla salverform, aestivation sinistrorse, green to yellowish, lobes 0.7–1.2 × 0.2–0.3 cm, lanceolate, white on both faces, margins ciliate, pilosity concentrated at the apex; glabrous or pilose adaxially, pilose abaxially; tube 2.8–3.2 × 0.1–0.2 cm, glabrous on both faces; anthers 2–2.2 mm long, narrowly deltate to lanceolate, apex acute to apiculate, base cordate; free from the style-head; pollen dispersed in monads; nectaries not seen; ovary 1.3 mm long, terete, superior; style-head 1.7 mm long, fusiform, apex bicuspidate. Follicles 5.9 × 4.4 cm, slightly dolabriform, divergent, dark to light brown, slightly muricate, glabrous; seeds 4,5–5 × 4–4.5 cm, oblate, light brown, glabrous.
Examined material: Baía Formosa, Mata Estrela, 06º22’32”S, 35º01’18”W, 7.XII.2014, fr., J.C. Sousa Jr et al. 63 (UFRN).
Additional material: BRAZIL. BAHIA: Lençóis, ca. 5 km da estrada de Lençóis por BR-242, 22.XII.1981, fl., A.M.V. Carvalho et al. 1084 (CEPEC); Lençóis/Wagner, Assentamento Rio Bonito, 12º20’24”S, 41º10’48”W, 3.VI.2001, fr., D.M. Loureiro et al. 186 (CEPEC, ALCB).
This species is endemic to Brazil, where it occurs in the Amazon, Caatinga, Cerrado, and Atlantic Forest. It is found throughout Brazil in states of the northern, northeastern, central-western, and southeastern regions (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In the study area, this species was found in well-preserved areas of semideciduous seasonal forest. Collected with old fruits in December.
It can be recognized by the dolabriform and muricate follicles and differs from other species in this genus by having a sulcate trunk.
3. Aspidosperma aff. limae Woodson, Ann. Missouri Bot. Gard. 47: 74. 1960.
Tree, ca. 8 m tall, trunk smooth; branches cylindrical, full, light green, striate, nodes lax, lacking cataphylls on apical buds, latex red, nodal colleters absent; incanous to glabrous. Leaves alternate, distichous; petiole 10–17 mm long, cylindrical to compressed, ventrally sulcate, colleters absent, rugose, incanous to glabrous; leaf blade 14.1–20.6 × 3.8–6.5 cm, elliptic to oblong, apex acuminate, base acute to attenuate; margin entire to slightly repand, revolute, flat at the base; venation brochidodromous, 27–34 secondary veins, tertiary veins conspicuous; concolorous, chartaceous, colleters absent, glabrous adaxially, incanous to glabrous abaxially. Inflorescence not seen. Flowers not seen. Follicles 9.2 × 6.4 cm, dolabriform, divergent, dark to light brown, lenticelate, striate, pubescent to velutinous; seeds not seen.
Examined material: Tibau do Sul, Parque Estadual Mata de Pipa, 06°15’13”S, 35°03’11”W, elev. 45‒60 m, 14.VIII.2012, fr., J.G. Jardim & L.A. Cestaro 6371 (UFRN).
In the study area, it was found in preserved areas of semideciduous seasonal forest. A. limae is endemic to Brazil, with occurrence restricted to the northeastern region, in the states of Alagoas, Ceará, and Pernambuco (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). This taxon is treated here as affinis because even though it matches the morphology and distribution of Aspidosperma limae, it was not collected with flowers and, therefore, its identity could not be confirmed. Collected with fruits in August.
Aspidosperma aff. limae differs from other species of Aspidosperma in this study by the branches lacking cataphylls on the apical buds, lax nodes, and reddish latex.
4. Aspidosperma sp. Figs. 3d-e; 4
a-c. Aspidosperma sp. – a. branch with fruits; b. apical gem, detail of cataphylls (arrow) in the apical gem; c. detail of the stem showing the congested nodes, like “stretch marks” (arrow) perpendicular to the branch. (a-c. J. G. Jardim et al. 6657).
Tree, ca. 7 m tall, trunk smooth; branches cylindrical, full, dark brown, striate, nodes congested, like scratches perpendicular to the branch; with cataphylls on apical buds, latex white, nodal colleters absent; pubescent to glabrous. Leaves alternate spiraled; petiole 12–17 mm long, compressed, slightly ventrally sulcate, colleters absent, striate, glabrous; blade 6.3–10.3 × 2.8–4.2 cm, elliptic to oblong, apex acuminate, base acute to cuneate, slightly attenuate; margin entire to slightly repand, revolute, flat at the base; venation brochidodromous, 11–17 secondary veins, tertiary veins conspicuous; discolorous, chartaceous, colleters absent, glabrous adaxially to slightly incanous on midrib, glabrous abaxially. Inflorescence not seen. Flowers not seen. Follicles 6.6–8.2 × 2.9–3.3 cm, dolabriform, divergent, dark brown, rugose, pubescent; seeds not seen.
Examined material: Tibau do Sul, trilha a leste do Parque Estadual Mata de Pipa, 06º14’45”S, 35º03’20”W, 2.VIII.2012, fr., J.G. Jardim et al. 6368 (UFRN); Parque Estadual Mata de Pipa, 06º14’45”S, 35º03’20”W, 2.VII.2014, fr., J.G. Jardim et al. 6657 (UFRN).
In the study area, it occurs in semideciduous seasonal forest and in arboreal Restinga, on dunes. Collected with fruits in July and August.
Aspidosperma sp. differs from other species of the genus by the branches with congested nodes, like scratches perpendicular to the branch, and by the presence of cataphylls on the apical buds.
Hancornia Gomes, Observ. Bot.-Med. Nonnullis Bras. Pl. 2: 1, t. 1. 1803.
This is a monospecific genus with a broad distribution in Brazil, except for Santa Catarina and Rio Grande do Sul, and in northern Paraguay, occurring mainly in areas of the Cerrado (Koch & Kinoshita 1999Koch I & Kinoshita LS (1999) As Apocynaceae s. str. da região de Bauru, São Paulo, Brasil. Acta Botanica Brasilica 13: 61-86.; BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.).
5. Hancornia speciosa Gomes, Mem. Math. Phis. Acad. Real Sci. Lisboa 2: 1, pl. 1. 1803.
Tree, ca. 2–3 m tall, trunk smooth; branches cylindrical, full, brown to reddish, smooth to slightly rugose, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters numerous, mainly intrapetiolar; glabrous. Leaves opposite distichous; petiole 6.5–10.4 cm long, cylindric, ventrally sulcate, colleters absent; smooth, glabrous; blade 2.9–5 × 1.2–2.1 cm, oblong, apex acuminate, base obtuse; margin entire, slightly revolute; venation craspedodromous, ca. 50 secondary veins, tertiary veins inconspicuous; discolorous, chartaceous, collects absent, glabrous on both faces. Cyme terminal, dichasium, 3-flowered; peduncle 0.3–0.6 cm long, glabrous; bracts 0.6–1.8 × 0.1–0.4 mm, deltate, colleters absent, slightly pilose to glabrous on both faces. Flowers with pedicel 0.5–0.9 cm long, pilose to glabrous; calyx lobes 1.3–1.5 × 0.8–0.9 mm, ovate, equal, apex acute, margin glabrous, colleters absent; pilose adaxially, glabrous abaxially; corolla salverform, aestivation sinistrorse, reddish, faux yellowish, lobes 0.7–1.2 × 0.2–0.3 cm, lanceolate, white on both faces, margins ciliate to glabrous, pilosity concentrated at the apex; pilose to glabrous adaxially, pilose abaxially; tube 2.8–3.2 × 0.1–0.2 cm, glabrous on both faces; anthers 2–2.2 mm long, narrowly deltate to lanceolate, apex acute to apiculate, base cordate; free from the style-head; pollen dispersed in monads; nectaries not seen; ovary 1.3 mm long, terete, superior; style-head 1.7 mm long, fusiform, apex bicuspidate. Berry 1.7–3 × 1.1–2.5 cm, globose, symmetrical, reddish yellow, smooth, glabrous; seeds 11 × 2 mm, elliptic, brown, glabous.
Examined material: Maxaranguape, comunidade Caraúbas, 05°27’88”S, 35°17’14”W, elev. 37 m, 27.VII.2010, fl., D.F. Torres & E.S. Oliveira 95 (UFRN). Natal, Mata dos Saguis, 05°50’34”S, 35°12’46”W, 16.VIII.2012, fr., L.M. Versieux 534 (UFRN). Nísia Floresta, chácara de Julie e Werner, 05°57’30”S, 35°10’30”W, elev. 20 m, 12.X.1997, fl., L.A. Cestaro 125 (UFRN). Parnamirim, Centro de Lançamento da Barreira do Inferno, 20.XI.2008, fl., A.M.G. Costa 1 (UFRN). Rio do Fogo, distrito de Barra do Punaú, 05°20’43”S, 35°24’60”W, 3.XII.2013, fl. and fr., M.A.O. Bitencourt 3 (UFRN).
This species is widely distributed in Brazil, except for the states of Acre, Rio Grande do Sul, Roraima, and Santa Catarina (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction), and the study area. It generally occurs in open and sunny areas of Cerrado enclaves associated to Restinga and to the semideciduous seasonal forest. Collected with fruits and flowers from July to December.
Commonly known, H. speciosa stands out by opposite distichous and oblong leaves and by the secondary vein patterns, parallel to each other. It can also be distinguished by the corolla with white lobes and by the globose fruits, yellow with reddish striae, with a sweet flavor and used to make juices, ice creams and jellies.
Himatanthus Willd. ex. Schult. in Roem. & Schult., Syst. Veg. 5: 221 (1819).
This genus is restricted to the Neotropical region (Spina 2004Spina AP (2004) Estudos taxonômico, micro-morfológico e filogenético do gênero Himatanthus Willd. ex Schult. (Apocynaceae: Rauvolfioideae - Plumerieae). Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 191p. ) and occurs in almost all Brazilian regions, except for the south (Flora of Brasil 2020). All nine species of the genus are registered in Brazil, and two of which are endemic (H. bracteatus and H. drasticus). In addition, two species are also distributed in Rio Grande do Norte (Flora of Brasil 2020).
6. Himatanthus bracteatus (A. DC.) Woodson, Ann. Missouri Bot. Gard. 25: 200. 1937. Fig. 3f-g
Tree, ca. 6–10 m tall, trunk smooth; branches cylindric, hollow, light to dark brown, striate, scaly, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters numerous, intrapetiolar; glabrous. Leaves alternate, spiraled; petiole 4.2–26 mm long, cylindric, ventrally sulcate to slightly sulcate, colleters absent, striate, glabrous; blade 6.8–21.3 × 2.7–8.1 cm, obovate to oblanceolate, apex obtuse to round, cuspidate, slightly retuse, base attenuate; margin entire, slightly revolute; venation brochidodromous, 15–22 secondary veins, tertiary veins little evident; discolorous, chartaceous, colleters absent, glabrous adaxially, slightly pilose to glabrous abaxially. Cyme apical, cincinnus, dichotomous with a reduced axis, 10–39-flowered; peduncle 3–10.4 cm long, glabrous; bracts 22–36 × 8–12 mm, navicular; with colleters numerous on the adaxial base; glabrous on both faces. Flowers with pedicel 0.6–0.9 cm long, glabrous; calyx lobes 0.4–0.9 × 0.5–1.5 mm, deltate, unequal, apex acute, margin glabrous, colleters absent; glabrous on both faces; corolla salverform, aestivation sinistrorse, yellowish, faux yellow, lobes 3.4–5.5 × 0.7–1.4 cm, oblong to obovate, white on both faces, margin glabrous, glabrous on both faces; tube 2–2.6 × 0.1–0.3 cm, externally glabrous, internally pilose, from the faux to above the anthers and below them; anthers 1.9–2.4 mm long, lanceolate, apex acute, base slightly cordate; free from the style-head; pollen dispersed in monads; nectaries absent; ovary 2–2.4 mm long, ellipsoid, half-inferior; style-head 1.7–1.9 mm long, cylindric, apex widened. Follicles 18.6–27.8 × 2.3–2.8 cm, cylindric, elongated, parallel, brown, striate, glabrous; seeds 32–46 × 15–28 mm, orbicular to oblong, brown, glabrous.
Examined material: Canguaretama, Rod. BR-101, c. 1,5 km N da cidade, 19.XII.2010, J.G. Jardim et al. 5865 (UFRN); vicinal entre os canavais, c. 7,5 km da BR-101, 06°31’29”S, 35°10’07”W, elev. 109 m, 2.VIII.2014, fr., J.C. Sousa Jr et al. 41 (UFRN). Espírito Santo, c. 8,2 km ao S da sede municipal, no caminho para a barragem do Rio Piquiri, 06°23’05”S, 35°17’31”W, elev. 192 m, 15.V.2015, fl. and fr., J.C. Sousa Jr et al. 65 (UFRN). Goianinha, Fazenda Nossa Senhora do Carmo, 06°19’11”S, 35°13’10”W, elev. 120 m, 12.II.2011, fl. and fr., A.A. Roque et al. 1121 (UFRN).
This species is endemic to Brazil and to the Atlantic Forest, distributed from Rio Grande do Norte to Rio de Janeiro and in Minas Gerais (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In the study area, it was found in well-preserved or deteriorated areas of semideciduous seasonal forest. Collected with flowers from December to May and fruits from May to August.
It is recognized by the large and alternate spiraled leaves, the navicular bracts, the cylindric style-head, and the cylindric and elongated follicles with winged seeds.
Macoubea Aubl., Hist. Pl. Guiane Suppl.: 17, t. 378. 1775.
Neotropical genus composed of three species, one (M. mesoamericana J.F.Morales) endemic to Costa Rica and Panamá and two [M. guianensis Aubl. and M. sprucei (Müll.Arg.) Markgr.] distributed mainly in the Amazon (Morales 1999aMorales JF (1999a) A new species of Macoubea (Apocynaceae) from Mesoamerica. Novon 9: 86-88., 2005a). In Brazil, it occurs mainly in some states of the northern and northeastern regions, besides Mato Grosso and Espírito Santo (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). It represents a new genus occurrence for Rio Grande do Norte.
7. Macoubea guianensis Aubl., Hist. Pl. Guiane 2 (Suppl.): 18, t. 378. 1775. Fig. 3h
Tree, ca. 8–16 m tall, trunk smooth; branches cylindrical, full, light brown, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters not seen; incanous to glabrescent. Leaves opposite, decussate; petiole 12–26 mm long, cylindric, ventrally slightly sulcate, colleters absent; striate, incanous to glabrous; blade 11.2–15.4 × 6–7.2 cm, obovate to oblanceolate, apex cuspidate to slightly retuse, base cuneate to obtuse; margin repand, flat; venation brochidodromous, 9 secondary veins, tertiary veins conspicuous; discolorous, chartaceous, colleters absent, incanous to glabrous adaxially, slightly pilose to glabrous abaxially. Cyme apical, corymbiform, 12-flowered; peduncle 3.5–4.4 cm long, pubescent to glabrous; bracts 1–2 × 1–1.5 mm, deltate to obovate; colleters not seen; pubescent on both faces. Flowers with pedicel 1.5–2.9 cm long, glabrous; calyx lobes 2 × 1 mm, obovate to deltate, equal, apex acute, margin pubescent, colleters at the base of the lobes; pubescent to glabrous on both faces; corolla salverform, white, white faux; upper tube 4.4–5.8 × 3–3.8 mm, cylindrical; lower tube 2–3 × 2.5–3 mm; anthers 4–5 mm long; nectaries absent; ovary 2–2.3 mm long, tetragonal, superior; style-head 1.8–2 mm long, conic-elongated (Viana et al. 2017Viana SS, Santos JUM & Simões AO (2017) Taxonomic diversity of Apocynaceae at the Marajó Island, PA, Brazil. Rodriguesia 68: 623-652.). Berry 5.5–6.5 × 3.5–5 cm, globose, symetrical, brown to ferruginous, rugose, glabrous; seeds 5.5–6 × 1–1.5 mm, cylindric to norrowly ellipitic, brown, rugose, glabrous.
Examined material: Canguaretama, APP próxima à vicinal entre cultivos de cana-de-açúcar, 06°31’52”S, 35°10’33”W, elev. 69 m, 20.IX.2014, fr., J.G. Jardim et al. 6741 (UFRN).
Additional material: BRAZIL. BAHIA: Alagoinhas, Campus II/ UNEB, 12°08’00”S, 38°26’00”W, elev. 120‒150 m, 5.XII.2000, fr., N.G. Jesus 441 (HUEFS). Vera Cruz, Fazenda Moponga, 13°05’16”S, 38°47’23”W, 29.IX.2011, E. Matos et al. 2650 (HUEFS). Santa Cruz Cabrália, entre os km 45–56 da Rod. Eunápolis/Porto Seguro, 22.X.1978, fl., S.A. Mori et al. 10954 (CEPEC); Reserva Biológica de Una, 15°09’00”S, 39°05’00”W. 30.III.1994, fr., A.M.A. Amorim et al. 1631 (CEPEC). PERNAMBUCO: Recife, Dois Irmãos, mata, próximo à jaula das onças pintadas, 08°03’14”S, 34°52’52”W, 24.IX.1954, fl. and fr., D. Andrade-Lima 54-1896 (IPA).
This species is distributed in Brazil throughout the north (Acre, Amazonas, Pará, Rondônia), in the central-west only in Mato Grosso, in the northeast (Bahia, Maranhão, Pernambuco), and in the southeast only in Espírito Santo (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). This is a new occurrence for Rio Grande do Norte, where it was found in seasonal semideciduous forest and riparian forest. Collected with fruits in September.
It is characterized by the opposite decussate leaves, generally broad, large in relation to the other species in the study area and the fruit, a globose berry, large.
Rauvolfia L., Sp. Pl. 1: 208. 1753.
Rauvolfia has 70 species, with pantropical distribution (except for Australia), with 35 species occurring in the Neotropics (Rao 1956Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.; Koch 2002Koch I (2002) Estudo das espécies neotropicais de Rauvolfia L. (Apocynaceae). Tese de Doutorado. Universidade Estadual de Campinas, Campinas. 292p.; Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). It is distributed throughout Brazil (except for Alagoas, Espírito Santo, Mato Grosso, Pernambuco, and Tocantins), where 20 species have been registered, 12 of which are endemic (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). Only one species has been registered in Rio Grande do Norte (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). Some Rauvolfia species produce chemical compounds of clinical and pharmacological use, others are sources of wood, and some are used as ornamental plants (Rao 1956Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.).
8. Rauvolfia ligustrina Willd. ex Roem. & Schult., Syst. Veg. (ed. 15 bis) 4: 805. 1819.
Shrub, ca. 1.2 m tall; branches cylindric, full, whitish to brown, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters numerous, lateral to the petiole and intrapetiolar; pilose. Leaves verticillate, 3 per node; petiole 3–4 mm long, cylindric, ventrally sulcate, colleters not seen; striate, pilose; blade 5.2–5.3 × 2.4–2.7 cm, ovate to lanceolate, apex acuminate, base cuneate; margin entire, slightly revolute; venation brochidodromous, 14–16 secondary veins, tertiary veins conspicuous; discolorous, chartaceous, colleters absent; glabrous on both faces, pilose on the base of midrib in both faces. Cyme apical or subaxillary, a dichasium, 16–27-flowered; peduncle 2.6 cm long, pilose; bracts 1–1.1 × 0.1–0.2, narrowly deltate; colleters 2–4, on the adaxial base, alternate; pilose on both faces. Flowers with pedicel 0.08–0.12 cm long, pubescent to incanous; calyx lobes 1 × 1 mm, deltate, equal, apex acute, margins glabrous, colleters absent; slightly pubescent to glabrous on both faces; corolla urceolate, aestivation sinistrorse, white, faux white, lobes 0.1 × 0.1–0.2 cm, ovate to deltate, white on both faces, margins slightly pubescent to glabrous, slightly pubescent to glabrous,; tube 0.3 × 0.2 cm, externally slightly pubescent to glabrous; anthers ca. 1 mm long, ovate; free from the style-head; ovary ca. 1.5 mm long, subspherical, superior; style-head ca. 1 mm long, calyptriform (Rao 1956Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.). Drupes 0.7 × 0.6 cm, subglobose, symetrical, reddish, rugose, indumentum not seen; seeds 4–6 × 3–4 mm, obconic, rugose.
Examined material: Parnamirim, Pium, 20.IX.1987, fl., A. Dantas 42 (UFRN).
Additional material: BRAZIL. BAHIA: Cachoeira, vale dos Rios Paraguaçu e Jacuípe, 19°39’S, 39°05’W, fl. and fr., Grupo Pedra do Cavalo 358 (CEPEC). CEARÁ: Sobral, EMBRAPA Caprinos, 03°42’00”S, 40°21’00”W, elev. 83 m, 15.I.2002, fl. and fr., M. Mamede 5 (EAC). PARAÍBA: Serra da Onça, Areia, 06°57’48”S, 35°41’30.1”W, 17.III.1992, fl. and fr., L.P. Félix 4733 (HST).
Rauvolfia ligustrina is distributed throughout all northeastern and central-western regions, besides the states of Amazonas, Pará, and Rio de Janeiro (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In the study area, the species was found in semideciduous seasonal forest. Collected with flowers in September.
It is characterized by the verticillate leaves, with small ovate to lanceolate blades, urceolate corolla with white lobes, and subglobose and reddish fruits.
Tabernaemontana L., Sp. Pl. 1: 210. 1753.
The genus is pantropical, with about 100 species, 45 of which are restricted to the Neotropical region (Leeuwenberg 1994Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.; Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). In Brazil, it is widely distributed with 30 species, nine of which are endemic (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). This is a new genus occurrence for Rio Grande do Norte. A few species produce chemical compounds of medicinal use and have edible fruits (Leeuwenberg 1994Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.).
9. Tabernaemontana flavicans Willd. ex Roem. & Schult., Syst. Veg. (ed. 15 bis) 4: 797. 1819.
Shrub, ca. 1.5 m tall; branches cylindric, full, brown to light green, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters absent; glabrous. Leaves opposite decussate; petiole 5–7 mm long, cylindric, sulcate ventrally, colleters absent; smooth, glabrous; blade 8.9–9 × 3.1–5 mm, oblong to obovate, apex acuminate, base obtuse; margin entire, slightly revolute; venation brochidodromous, 7–14 secondary veins, tertiary veins little evident; slightly discolorous, chartaceous, colleters absent; glabrous on both faces. Raceme axillary, corymbiform, 3–6 flowered; peduncle 0.1–0.6 cm long, glabrous; bracts 1.7–2.2 × 1.4–3 mm, deltate, colleters 2–4, on the adaxial base, opposite; glabrous on both faces. Flowers with pedicel 0.9–1.3 cm long, glabrous; calyx lobes 2.5–3.4 × 2–2.5 mm, deltate to ovate, equal, apex acute, margin glabrous, colleters 5–8, on the middle of the adaxial base, opposite; glabrous on both faces; corolla salverform, aestivation sinistrorse, white, faux yellowish, lobe 1.8 × 0.8 cm, oblong, white on both faces, margin glabrous, glabrous on both faces; upper tube 0.7 × 0.4 cm, glabrous; lower tube ca. 1 × 0.3 cm, externally glabrous, internally barbellate to lanuginose below the anthers; anthers 5.3 mm long, narrowly deltate, apex acute, base sagittate; free from the style-head; pollen dispersed in monads; nectaries absent; ovary 1.5 × 2 mm; style-head 1 mm long, cylindric, apex acute to truncate. Follicles 3.9 × 2.3 cm, ellipsoid, divergent, green, smooth, glabrous; seeds 5–7 × 3–4 mm, ellipisoid, striate.
Examined material: Baía Formosa, Mata Estrela, 06º24’06”S, 35º00’15”W, 10.IX.2011, fl., J.L. Costa-Lima & W.M.B. São-Mateus 567 (UFRN).
Additional material: BRAZIL. BAHIA: Lamarão do Passé,12°31’S, 38°24’W, 21.IX.1984, fl., L.R. Noblick & Lemos 3388 (CEPEC, HUEFS). Una, km 6 Rod. Una/Canavieras (BA001), ramal à direita, 01.IV.1980, fr., L. A. Mattos Silva et al. 730 (CEPEC); Reserva Biológica de Una, 15°09’00”S, 39°05’00”W, 10.XI.1993, fl., A. M.A. Amorim et al. 1447 (CEPEC).
This species is found in Venezuela and Peru; in Brazil, is distributed in some states of the northern (Amazonas, Pará and Rondônia), northeastern (Alagoas, Bahia, Maranhão and Pernambuco), central-western (Mato Grosso), and southeastern (Espírito Santo, Minas Gerais e Rio de Janeiro) regions (Leeuwenberg 1994Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.; Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). It occurs in wet or dry forests, especially in the understory or even in low vegetation (Leeuwenberg 1994Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.). This is a new occurrence for Rio Grande do Norte, found in well-conserved semideciduous seasonal forests. Collected with flowers in September.
It can be characterized by the opposite decussate leaves, with a large, oblong to obovate blade, and apex acuminate, white corolla, and ellipsoid and divergent follicles.
II. Grade Apocynoid
This grade is composed of nine tribes and 82 genera (Endress et al. 2014Endress M, Liede-Schumann S & Meve U (2014) An updated classification for Apocynaceae. Phytotaxa 159: 175-194.), distributed in the Americas, Africa, Europe, and Asia, throughout tropical and subtropical regions and more rarely in temperate regions (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l. The Botanical Review 66: 1-56.). Apocynoid has corona absent, anthers fused to the style-head forming a gynostegium, pollen usually dispersed in monads, and disc-shaped nectaries on the ovary base, sometimes absent.
Mandevilla Lindl., Edwards’s Bot. Reg. 3: t. 7. 1840.
This genus is distributed from Mexico and the Antilles to Argentina and has about 170 species (Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ; Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). It occurs throughout Brazil, where 66 species are found, of which 40 are endemic; four species are listed for Rio Grande do Norte (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). These are species with ornamental potential and of pharmacological use in traditional medicine (Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ; Cordeiro et al. 2014Cordeiro SZ, Simas NK, Henriques AB & Sato A (2014) Micropropagation and callogenesis in Mandevilla guanabarica (Apocynaceae), an endemic plant from Brazil. Crop Breeding and Applied Biotechnology 14: 108-115.).
The species are generally climbers, but some can be shrubs or subshrubs, and they have interpetiolar node appendages, which are small or developed as a ring and wrapping the node.
10. Mandevilla microphylla (Stadelm.) M. F. Sales & Kin.-Gouv., Iheringia, Bot. 64(1): 68. 2009. Fig. 3i
Woody climber, ca. 2 m tall, branches cylindric, full, brown to reddish-brown, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, interpetiolar; glabrous. Leaves opposite decussate; petiole 3.5–12 mm long, cylindric, abaxially sulcate, colleters absent, smooth; hirsute to glabrous; blade 3.2–5.9 × 2–4 cm, ovate, apex acuminate, base obtuse to cordate; margin entire, revolute; venation brochidodromous, 8–11 secondary veins, tertiary veins conspicuous; discolorous, slightly coriaceous, colleter 1, on the adaxial base of midrib; glabrous on both faces, hirsute abaxially on the base of midrib. Raceme axillary, 4–7-flowered; peduncle 0.4–4.4 cm long, pubescent to incanous; bracts 0.5–1 × 0.9–1.3 mm, deltate; colleters absent; incanous to pubescent on both faces. Flowers with pedicel 0.8–1.1 cm long, glabrous; calyx lobes 0.4–1.1 × 1–1.5 mm, deltate, equal, apex cuneate, margin glabrous, colleters absent; glabrous on both faces; corolla infundibuliform, aestivation dextrorse, yellow, faux yellow, lobes 3.4–3.6 × 3–3.2 cm, obovate, yellow on both faces; margin pubescent to pilose, glabrous on both faces, the base incanous to pubescent on both faces; upper tube 1.4 × 1.8 cm, glabrous on both faces; lower tube 3 × 0.5 cm, externally glabrous, internally pubescent to velutinous around the anthers and on the filaments, with 5 fleshy projections on the internal base; anthers 7.5 mm long, narrowly elliptic to lanceolate, apex acute, base cordate; fused to the style-head; pollen dispersed in monads; nectar disk 5-lobed, concrescent to the ovary; ovary 1.6 mm long, ellipsoid to ovoid, superior; style-head 1.2 mm long, umbraculiform, apex apiculate. Follicles 18 × 0.5 cm, cylindric, parallel, brown, striate, glabrous; seeds 8 × 1 mm, linear, brown, glabrous.
Examined material: Baía Formosa, RPPN Mata Estrela, 06°22’54”S, 35°00’48”W, elev. 54 m, 7.XII.2014, fl., J.C. Sousa Jr et al. 64 (UFRN).
Additional material: BRAZIL. BAHIA: Jeremoabo, Estação Ecológica do Raso da Catarina, 09°20’00”S, 38°29’00”W, 8.VII.1983, fl. and fr., L.P. Queiroz 733 (HUEFS).
This species is endemic to Brazil, with occurrence restricted to the northeastern region, in the states of Bahia, Paraíba, and Pernambuco (Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ; BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). This is a new occurrence for Rio Grande do Norte, found in semideciduous seasonal forest and open areas in arboreal Restinga. Collected with flowers in December.
It is characterized by a yellow corolla and faux and differs from the other species found in the study by the five fleshy projections on the inner base of the corolla tube.
11. Mandevilla moricandiana (A. DC.) Woodson, Ann. Missouri Bot. Gard. 20(4): 705. 1933. Fig. 5a
a. Mandevilla moricandiana – mature fruit showing the comose seeds. b. Mandevilla scabra – flower (front view). c. Odontadenia lutea – flowering branch. d. Temnadenia odorifera – flowering branch, with flowers in anthesis and immature fruit (lateral view; upside down). e. Cynanchum roulinioides – flowering branch. f. Ditassa crassifolia – immature fruit. g. Ditassa sp. – pollinarium. h. Ibatia ganglinosa – detail of inflorescence. i. Marsdenia altissima – detail of inflorescence. Photos: a, b, f, g, i. Jaerton C. Sousa Júnior; c-e; h. Jomar G. Jardim.
Woody climber, ca. 1.5‒3 m tall, branches cylindric to slightly compressed, light gray to brown, smooth to slightly rugose, nodes congested, lacking cataphylls on apical buds, latex white, nodal colleters 2–5, interpetiolar; slightly pilose to glabrous. Leaves opposite decussate; petiole 2.4–5.2 mm long, cylindric, ventrally sulcate, colleters absent, smooth, pilose; blade 1.6–2.6 × 1.1–1.6 cm, obovate, apex cuspidate, base cuneate to acute, margin entire to slightly repand, slightly revolute; venation brochidodromous, 7–10 secondary veins, tertiary veins conspicuous; discolorous, coriaceous, colleters 2, on the adaxial base of midrib; glabrous on both faces. Raceme axillary, 1–6-flowered; peduncle 0.3–2.4 cm long, glabrous; bracts 0.8–1.9 × 0.5–1.1 mm, deltate to narrowly deltate, colleters absent; glabrous on both faces. Flowers with pedicel 0.9–1.2 cm long, glabrous; calyx lobes 5.4–5.5 × 1.4–2 mm, lanceolate, equal, apex acuminate, margin glabrous, colleters 2–4, on the adaxial base, alternate; glabrous on both faces; corolla infundibuliform, aestivation dextrorse, light pink to white, faux pink to dark pink, lobes 1.8–2.3 × 1.8–2.5 cm, obovate, white on both faces, margin slightly pilose, glabrous on both faces, slightly pilose to glabrous on the faux; upper tube 1.1–1.4 × 0.3–0.5 cm, glabrous on both faces; lower tube 1.5–1.9 × 0.2 cm, externally glabrous, internally velutinous to tomentose below the anthers, lacking fleshy projections on the internal base; anthers 6–7.3 mm long, linear, apex acute to cuspidate, base sagittate; fused to style-head; pollen dispersed in monads; nectaries ovoid, 2, concrescent with the ovary; ovary 1.5–1.6 mm long, ovoid to ellipsoid, superior; style-head 1.8–2 mm long, umbraculiform, apex acute. Follicle 9.4–11.8 × 0.4–0.6 cm, cylindric, parallel, light castaneous to brown, striate, glabrous; seeds 0.6 × 0.1 mm, linear, brown, pubescent to incanous.
Examined material: Ceará-Mirim, Praia de Muriú, 05°33’05”S, 35°15’30”W, elev. 30 m, 4.VII.2014, fl., J.C. Sousa Jr & L.A. Cestaro 33 (UFRN). Extremoz, APA Jenipabú, 05°42’32”S, 35°12’21”W, elev. 30 m, 27.X.2012, fr., D.O. Batista et al. (UFRN). Goianinha, Mata do violão, 06°18’81”S, 35°12’62”W, 2.VIII.2014, fl., J.C. Sousa Jr et al. 42 (UFRN). Nísia Floresta, APA Bonfim-Guaraíra, 05°98’44”S, 35°11’22”W, 3.XI.2013, fl. and fr., A.K. Morais 22 (UFRN); APA Bonfim-Guaraíra, 06°05’25”S, 35°08’01”W, elev. 9 m, 1.XI.2014, fl., E.O. Moura et al. 247 (UFRN). Parnamirim, Praia de Cotovelo, 05°57’55”S, 35°08’32”W, elev. 11 m, 22.XI.2014, fl. and fr., J.C. Sousa Jr et al. 61 (UFRN).
Mandevilla moricandiana is endemic to Brazil, occurring in the northeastern region, from Ceará to Bahia, and in Rio de Janeiro (Sales 1993Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. ; BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). In the study area, it was found in Restinga, in congruence with Sales (1993)Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p. . Collected with flowers from July to November and with fruits in October and November.
This species can be recognized by the obovate leaves and differs from other species of the genus found here by the light pink or white corolla with faux pink to dark pink.
12. Mandevilla scabra (Hoffmanns. ex Roem. & Schult.) K. Schum., Nat. Pflanzenfam. 4(2): 171. 1895. Fig. 5b
Herbaceous climber, ca. 2 m tall, branches cylindric, full, light green to dark brown, slightly striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 1–7, lateral to the petiole and interpetiolar; pilose to glabrous. Leaves opposite decussate; petiole 2.2–6.3 mm long, cylindric, ventrally sulcate, colleters absent, smooth; pilose to glabrous; blade 3.9–8.1 × 1.6–4.7 cm, lanceolate to oblong, apex cuspidate to rostrate, base cordate, margin entire, slightly revolute; venation brochidodromous, 8–10 secondary veins, tertiary veins conspicuous; discolorous, chartaceous, colleters 2–21, adaxially along the midrib; slightly pilose to glabrous adaxially, pilosity concentrated on the midrib, densely pubescent to velutinous abaxially, base pilose abaxially. Raceme axillary, 15–43-flowered; peduncle 0.5–2.9 cm long, pilose to glabrous; bracts 2.4–3.9 × 0.6–1.3 mm, narrowly deltate to deltate, colleters absent; pubescent to glabrous on both faces. Flowers with pedicel 0.1–0.4 cm long, pubescent to incanous; calyx lobes 2–2.6 × 1–1.6 mm, deltate, equal, apex acuminate, margins ciliate to glabrous, colleter 1, on the adaxial base, opposite; incanous to glabrous on both faces; corolla infundibuliform, aestivation dextrose, yellow, faux red, lobes 1.5–1.7 × 1.6–1.8 cm, obovate, yellow on both faces, margin glabrous, glabrous adaxially, pubescent to pilose abaxially; upper tube 2.8–3.4 × 1.7–3.2 cm, externally pilose, internal indumentum not seen, lower tube 2.2–2.9 × 0.2–0.4 cm, externally pilose, internal indumentum not seen, internal base lacking fleshy projections; anthers 5.5–5.8 mm long, fused to the style-head; nectar disk 5–lobed; ovary 1.1–1.5 mm long, ovoid; style-head 2–3 mm long, umbraculiform [Coutinho & Louzada (2018)Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.]. Follicles 7.2–13.6 × 0.1–0.4 cm, cylindrical, elongated, parallel, brown, striate, slightly incanous to glabrous; seeds 9 × 2 mm, slightly oblong to elliptic, brown to slightly golden, slightly pubescent to velutinous.
Examined material: Natal, Parque da Cidade, 05°50’39”S, 35°14’30”W, 27.VI.2007, fl., V.R.R. Sena et al. 187 (UFRN). Pium, 05°57’30”S, 35°09’40”W, elev. 20 m, 30.IX.1980, fl., O.F. Oliveira 1348 (MOSS). Parnamirim, vale do Rio Pitimbu, 05°54’00”S, 35°12’00”W, elev. 20 m, 24.VIII.1997, fl. and fr., L.A. Cestaro 38 (UFRN); praia de Cotovelo, 05°54’56”S, 35°15’46”W, 22.III.2007, fl. and fr., M.I.B. Loiola et al. 1171 (UFRN). Nova Parnamirim, next to the Jiqui Country Club, 05°54’56”S, 35°15’46.1”W, 22.V.2012, fl., B.F. Caroline et al. (UFRN). Rio do Fogo, distrito de Punaú, 05°16’22”S, 35°22’59”W, 25.V.2009, fl., A.C.P. de Oliveira & J.L.Costa-Lima 965 (UFRN).
Mandevilla scabra is widely distributed in Brazil (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In the study area, it was found on edges of semideciduous seasonal forest, in riparian forests, and in Restinga. Collected with flowers and fruits from March to September.
This species stands out within the genus by the leaves with 2 to 21 colleters distributed adaxially along the midrib, by the pilose to glabrous inflorescence, the yellow corolla with a red faux, and the oblong to elliptic seeds.
Odontadenia Benth., J. Bot. (Hooker) 3: 242. 1841.
This genus is found from Mexico and the Antilles to Brazil and Bolivia, with 20 species (Morales 2009Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.). In Brazil, there are 16 species, two of which are endemic, occurring along the entire northern and central-western regions, in most of the southeast, and in some states of the northeast (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). This is a new genus occurrence for Rio Grande do Norte.
13. Odontadenia lutea (Vell.) Markgr., Repert. Spec. Nov. Regni Veg. 20: 24. 1924. Figs. 5c; 6
a-d. Odontadenia lutea – a. flowering branch; b. detail of unequal calyx lobes (arrow); c. detail of the ovary showing the nectariferous disk (arrow) sectioned; d. immature fruit. (a-c. J. G. Jardim et al. 6759; d. R. L. Soares Neto & J. G. Jardim 75).
Woody climber, ca. 5–10 m tall, branches cylindrical, full, light brown, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, interpetiolar, stiples; incanous to pilose. Leaves opposite decussate; petiole 14–16 mm long, cylindric, ventrally sulcate, colleters numerous, intrapetiolar, slightly striate, incanous; blade 13.7–19 × 6.1–6.3 cm, elliptic to lanceolate, apex acuminate, base obtuse to round, margin entire, slightly repand, revolute; venation brochidodromous, with 12–14 secondary veins, tertiary veins conspicuous; discolorous, chartaceous, colleters absent; glabrous adaxially, pubescent abaxially, incanous on the midrib abaxially. Cyme apical, tirsiform, 7–34-flowered; peduncle 0.4 cm long, incanous; bracts 4–6 × 4.5–4.8 mm, deltate to narrowly deltate, colleters absent; incanous adaxially, mainly longitudinally on the middle portion, incanous abaxially. Flowers with pedicel 2.5–2.7 cm long, incanous; calyx lobes 12–13 × 8.5 mm, ovate, unequal, apex rounded to cuspidate, margins incanous, colleters 3, on the adaxial base, alternate; glabrous on both faces; corolla infundibuliform, aestivation dextrorse, light yellow to greenish, faux green, lobes 2.6–2.7 × 2.5–2.6 cm, obovate, white on both faces, margin incanous, glabrous on both faces; upper tube 1.7–1.9 × 2.2–2.3 cm, glabrous on both faces; lower tube 2.6–2.7 × 0.6–0.7 cm, externally glabrous, internally barbellate, mainly below the anthers; anthers 0.8–0.9 mm long, narrowly deltate, apex acute, base sagittate; fused to style-head; pollen dispersed in monads; nectar disk 5-lobulate, concrescent with the ovary; ovary 2 mm long, ovoid to ellipsoid, superior; style-head 3 mm long, oblong, apex capitate. Follicles 9.4 × 0.6 cm, cylindrical, elongated, divaricate, green to vine, striate, glabrous; seeds not seen.
Examined material: São José de Mipibu, 06°04’29”S, 35°24’18”W, 3.XII.2013, fr., R.L. Soares Neto & J.G Jardim 75 (UFRN); c. 6.5 km ao sul da sede municipal, 06°31’27”S, 35°07’16”W, elev. 38 m, 20.IX.2014, fl., J.G. Jardim et al. 6759 (UFRN).
Distributed in the northern (Amazonas, Pará and Tocantins), northeastern (Bahia), central-western (Distrito Federal, Goiás, Mato Grosso do Sul and Mato Grosso), and southeastern (Minas Gerais, Rio de Janeiro and São Paulo) Brazilians regions (BGF 2015), also in Bolivia and Peru (Morales 1999bMorales JF (1999b) A synopsis of the genus Odontadenia: series of revisions of Apocynaceae XLV. Bulletin du Jardin Botanique National de Belgique 67: 381-477.). This is a new occurrence for Rio Grande do Norte, found in well-conserved semideciduous seasonal forests. Collected with flowers in September and fruits in December.
It can be recognized by the many-flowered inflorescence, the unequal calyx lobes, the light yellow to greenish corolla, and the 5-lobulate nectar disk, forming a wall around the ovary.
Temnadenia Miers, On the Apocynaceae of South America 207. 1878.
Genus restricted to South America, with center of diversity in Brazil, composed of four species, two of which are endemic to Brazil, one to Colombia, and the other with a wider occurrence in Bolivia, Brazil, and Peru (Morales 2005bMorales JF (2005b) Estudios en las Apocynaceae Neotropicales XIII: revisión del género Temnadenia (Apocynoideae, Echiteae). Candollea 60: 207-231.). The three species occurring in Brazil are found in the southeastern region and in some states of the northern, central-western, and northeastern regions (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). It is a new genus occurrence for Rio Grande do Norte.
14. Temnadenia odorifera (Vell.) J. F. Morales, Novon 9(2): 240. 1999. Fig. 5d
Woody climber, ca. 2.5 m tall, branches cylindric, full, brown, slightly striate, nodes lax, lacking cataphylls on apical buds, latex tranparent, nodal colleters numerous, interpetiolar; pilose. Leaves opposite decussate; petiole 3.3–4.6 mm long, cylindric, ventrally sulcate, colleters numerous, intrapetiolar; slightly striate; velutinous; blade 6.5–7.7 × 5.1 cm, ovate, apex acuminate, base rounded to subcordate, margin entire, revolute; venation brochidodromous, 6 secondary veins, tertiary veins little evident; discolorous, chartaceous, colleters absent; pubescent on both faces. Raceme axillary, ca. 24-flowered; peduncle 2.9 cm long, pubescent; bracts 1.3 × 0.1 mm, filiform, colleters not seen, pilose on both faces. Flowers with pedicel 0.5–0.9 cm long, pubescent; calyx lobes 5–5.6 × 2–2.5 mm, deltate, equal, apex acute, margin slightly pilose to glabrous, colleters absent; pilose to glabrescent on both faces; corolla salverform, aestivation dextrose, lilac-pink, faux yellow, lobes 1.6–1.7 × 0.9–1.2 cm, oblong, pink on both faces, margin glabrous, glabrous on both faces; tube 1.9–2.4 × 0.4 cm, glabrous on both faces; anthers ca. 8 mm long, fused to style-head; nectar disk 5-lobed; ovary 1.8 mm long, ovoid; style-head 1.5–2 mm long, cylindrical (Coutinho & Louzada 2018Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.). Follicles 24 × 0.8 cm, cylindric, elongated, parallel, green to red, striate, glabrous; seeds 16 × 2 mm, linear, green to wine, glabrous.
Examined material: Canguaretama, entrada na BR-101 após a divisa do RN com a PB, c. 1 km após o posto fiscal, vicinal entre cultivos de cana-de-açúcar, 06°31’52”S, 35°10’33”W, elev. 69 m, 20.IX.2014, fr., J.G. Jardim et al. 6752 (UFRN). Extremoz, APA Jenipabu, entorno do ECOPOSTO, 05°42’20”S, 35°12’43”W, elev. 20–60 m, 7.V. 2011, fl., J.G. Jardim 5977 (UFRN). Tibau do Sul, Parque Estadual Mata de Pipa, trilha com acesso pela E.T. da CAERN, 06°14’20”S, 35°03’53”W, elev. 50–60 m, 2.VIII.2012, fl. and fr., J.G. Jardim et al. 6332 (UFRN). Parnamirim, Hidrominas Santa Maria, 05°56’13.9”S, 35°15’04.3”W, elev. 32 m, 23.X.2004, fl., A. Ribeiro & R.T. Queiroz 61 (UFRN).
Additional material: BRAZIL. PERNAMBUCO: Exú, Chapada do Araripe, 07°44’10.2”S, 40°14’26.4”W, 16.II.1984, fl., G. Fotius & I.B. Sá 3791 (HST). SÃO PAULO: Cubatão, em cultivo no Horto Botânico de Cubatão, 23°53’24”S, 46°25’12”W, 29.V.1901, fl., CGG5813 (SP).
This species is endemic to Brazil and to the Atlantic Forest, with occurrence in the northeastern (Alagoas, Bahia, Ceará and Pernambuco), southeastern (Espírito Santo, Rio de Janeiro and São Paulo), and southern (Paraná and Santa Catarina) regions (Morales 2005bMorales JF (2005b) Estudios en las Apocynaceae Neotropicales XIII: revisión del género Temnadenia (Apocynoideae, Echiteae). Candollea 60: 207-231.; Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). It represents a new occurrence for Rio Grande do Norte and was found in semideciduous seasonal forests and in arboreal Restinga. Collected with flowers and fruits from May to October.
It is characterized by the transparent latex, the salverform lilac-pink corolla with oblong lobes, pink on both faces, and by the elongated follicles.
III. Subfamily Asclepiadoideae
This subfamily has five tribes and 164 genera (Endress et al. 2014Endress M, Liede-Schumann S & Meve U (2014) An updated classification for Apocynaceae. Phytotaxa 159: 175-194.), with cosmopolitan distribution, from the tropics to temperate regions (Endress & Bruyns 2000Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l. The Botanical Review 66: 1-56.) and in dry areas, especially in Africa (Stevens 2001Stevens PF (2001 onwards) Angiosperm phylogeny website version 12, July 2012 [and more or less continuously updated since]. Available at <http://www.mobot.org/MOBOT/research/APweb/welcome.html>. Access on 6 February 2019.
http://www.mobot.org/MOBOT/research/APwe...
onwards). Asclepiadoideae is characterized by the corona present, by the two-locular anthers, pollen dispersed in a pollinarium and transported by translators composed of retinacle and caudicle.
Asclepias L., Species Plantarum 1: 214. 1753.
This genus only occurs on the American continent, mainly in North America, and is composed of approximately 120 species (Morillo 1997 apud Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). It has a wide distribution in Brazil, where six species are listed, four of which are endemic. Among the species found in Brazil, one has been registered in Rio Grande do Norte (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.).
15. Asclepias curassavica L., Sp. Pl. 1: 215. 1753.
Herb erect, ca. 0.4–1 m tall, branches cylindric, full, light green, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2–5, lateral to the petiole; slightly pubescent to glabrescent. Leaves opposite decussate; petiole 8–11 mm long, cylindric, ventrally sulcate, colleters absent; striate, slightly pubescent to glabrescent; blade 14.1–15.7 × 2.3–3.1 cm, elliptic to obovate, apex acute to acuminate, base attenuate, margin entire, flat; venation brochidodromous, 17–19 secondary veins, tertiary veins conspicuous; concolorous, membranaceous, colleters absent; slightly pubescent to glabrescent on both faces, mainly on the midrib. Cyme apical, corymbiform, 7–9-flowered; peduncle 5–6.1 cm long, slightly pubescent; bracts 4.2–5.4 × 0.2–0.8 mm, narrowly deltate, colleters 2, on the base adaxial, alternate; slightly pubescent to glabrescent adaxially, pubescent to pilose abaxially. Flowers with pedicel 1.4–1.6 cm long, pubescent; calyx lobes 4–4.4 × 1–1.3 mm, lanceolate, equal, apex acute, margin slightly ciliate, colleters 4, on the base adaxial, alternate; glabrous adaxially, pubescent abaxially; corolla reflexed exposing the corona, aestivation valvar, red, lobes 0.7–1.2 × 0.3 cm, elliptic, red, margins glabrous, papillose to glabrous adaxially, glabrous abaxially; tube inconspicuous; corona simple, orange; segments longer than gynostegium, opposite to anthers, segments 3.2 × 1.4–1.8 mm, cucullate, apex obtuse to rounded; united to each other at the base; inserted on the anthers base; corniculum falcate adaxially, arcuate over the gynostemium; anthers 2.1–2.4 mm long, rectangular, apex acute, base cordate, wings longer than dorsal surface, dorsally flat, membranaceous apex suborbicular, flat; fused to the style-head; pollen dispersed in pollinarium. Retinacle 0.3 × 0.18 mm, deltate to oblong, brown, smaller than pollinia; caudicles 0.46–0.5 × 0.11 mm, linear, descendant, lateral reticulate membrane present, inserted laterally on the middle of the retinacle, inserted on the upper portion of the pollinia apices; pollinia 0.88 × 0.33‒0.35 mm, ellipsoid; nectary absent; ovary 1.7 mm long, ovoid to ellipsoid, superior; gynostegium 2.7 mm long, cylindric, style-head with apex flat. Follicle 7.3 × 1 cm, fusiform, parallel, colour not seen, striate, glabrous; seeds 6.5 × 3.5 mm, ovate to elliptic, brown, glabrous.
Examined material: Canguaretama, localizada entre os Rios Espinho e Curimataú, 06°24’20”S, 35°08’00”W, elev. 60 m, 9.XI.1980, fl., O.F. Oliveira 1465 (MOSS). Macaíba, próximo à Fazenda Lindóia, 05°51’30”S, 35°21’30”W, 1.X.1980, fl., O.F. Oliveira 1406 (MOSS). Parnamirim, Riacho Águas Vermelhas, 11.III.2006, fl., A. Ribeiro & J. Silva 240 (UFRN). Vila Flor, Cunhaú, 06°19’00”S, 35°02’30”W, 25.II.1981, fl. and fr., O.F. Oliveira 1695 (MOSS).
Additional material: BRAZIL. CEARÁ: Crato, Lameiro (Rosto), braço do Rio Batateira, 07°14’03”S, 39°24’33.8”W, 27.IX.2005, fl., M.A.P. Silva et al. (HCDAL). PERNAMBUCO: Buíque, brejo de São José, 08°37’23”S, 37°09’20.9”W, 4.X.1983, fl., F. Gallindo et al. CFPE695 (IPA). Maraial, Engenho Curtume, 08°46’57”S, 35°48’32”W, 5.IX.1997, fl., J.E. Gomes de Lima et al. 247 (HST). RIO GRANDE DO NORTE: Venha-Ver, açude público, 06°19’37”S, 38°29’11”W, elev. 564 m, 4.VIII.2010, fl. and fr., A.A. Roque 860 (UFRN).
This species is distributed worldwide, with predominance in tropical regions (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). In Brazil, it has a wide distribution, occurring in all states (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). In Rio Grande do Norte, it was found in semideciduous seasonal forests, generally near water courses. Collected with flowers and fruits from October to March.
It is characterized by the flowers with red petals and by the orange corona, exposed, with a falcate cornicle, arcuate over the gynostegium.
Blepharodon Decne, in DC. & A.DC., Prodr. 8: 603. 1844.
This genus is exclusive of the American continent, composed of around 20 species, most of them concentrated in the north of South America (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). It occurs all over Brazil, with 13 species, seven of which are endemic. A single species is listed for Rio Grande do Norte (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.).
16. Blepharodon pictum (Vahl) W. D. Stevens, Novon 10(3): 242. 2000.
Herbaceous climber, ca. 1 m tall, branches cylindric, full, light green, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2–6, interpetiolar; glabrous. Leaves opposite distichous; petiole 6–6.5 mm long, cylindric, ventrally sulcate, colleters absent, slightly striate to smooth, glabrous; blades 3.9–5.2 × 1.7–1.8 cm, oblong to slightly lanceolate, apex acuminate, base round, margin entire, slightly revolute; venation brochidodromous, 16–19 secondary veins, little evident, tertiary veins little evident; discolorous, chartaceous, colleters 2, on the adaxial base of midrib; glabrous adaxially, slightly pilose on the midrib, glabrous abaxially. Cyme subaxillary, corymbiform, 3-flowered; peduncle 0.2–0.3 cm long, glabrous; bracts 1.4 × 0.1–0.2 mm, narrowly deltate, colleters absent; glabrous on both faces. Flowers with pedicel 1.1–1.5 cm long, glabrous; calyx lobes 1.5–2 × 1–1.5 mm, ovate, equal, imbricate, apex acute, margin glabrous, colleters 2, on the adaxial base, alternate; glabrous on both faces; corolla subrotate, aestivation valvar, green to purple, lobes 0.8 × 0.3 cm, lanceolate, green adaxially, apex green and base purple abaxially, margin pilose, glabrous adaxially, pilose abaxially; tube inconspicuous; corona simple; segments as high as the gynostegium, opposite to the anthers, segments 3 × 2.5 mm, cotyliform, apex acute, united with the corolla; inserted on the anthers base; anthers ca. 3 mm long, rectangular, apex not seen, base not seen; wings longer than dorsal surface, dorsal shape not seen, membranaceous appendage suborbicular (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.), fused to style-head; pollen dispersed in pollinarium. Retinacle 0.03 × 0.02 mm, ovate to elliptic, brown, as large as pollinia; caudicles 0.01 × 0.01 mm, linear, horizontal, reticulate membrane not seen, inserted laterally on the retinacle base, inserted laterally on the middle of the pollinia; pollinia 0.03 × 0.02 mm, obdeltoid; ovary 1.2–1.6 mm long, ovoid; nectaries absent (Coutinho & Louzada 2018Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.); gynostegium, 3 mm long, cylindric, style-head with apex convex. Follicle 5.1 × 2 mm, fusiform, parallel, green, striate, glabrous; seeds ca. 0.5 × 0.2 cm (Coutinho & Louzada 2018Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.).
Examined material: Macaíba, Escola Agrícola de Jundiaí, Mata do Bebo, 05°53’31.6”S, 35°21’11.5”W, 27.VIII.2011, fl., E.O. Moura et al. 22 (UFRN). Additional material: BRAZIL. BAHIA: Santa Terezinha, Serra da Jibóia, Reserva Jequetibá, 12°52’17”S, 39°28’48”W, elev. 506 m, 16.V.2011, fl., E. Melo 9602 (HUEFS). RIO GRANDE DO NORTE: Coronel João Pessoa, locality Mata Redonda, 06°16’00”S, 38°23’00”W, elev. 750 m, 25.V.1981, fl., O.F. Oliveira 1834 (MOSS).
This species is widely distributed in Brazil, with the exception of the states of Piauí, Santa Catarina, and Rio Grande do Sul. It has been reported in many vegetation types of the Amazon, Atlantic, Caatinga, and Cerrado domains (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). It is found in disturbed areas, in Cerrado, and along forest edges (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). In Rio Grande do Norte, it was found in semideciduous seasonal forests. Collected with flowers in August.
It is characterized by the obdeltoid pollinia, with similar dimensions of length and width. It can also be distinguished by the glabrous branches, the leaf blades with a rounded base, with 16 to 19 secondary veins, and by the green and purple corolla.
Calotropis R. Br., Asclepiadeae: 28. 1810.
Distributed in tropical and subtropical Africa and Asia, with three species, one of which has been introduced into Central and South America (Rahman & Wilcock 1991Rahman MA & Wilcock CC (1991) A taxonomic revision of Calotropis (Asclepiadaceae). Nordic Journal of Botany 11: 301-308.) and already naturalized in Brazil (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). The species in this genus provide natural fiber and apparently have potential to be used in traditional medicine (Rahman & Wilcock 1991Rahman MA & Wilcock CC (1991) A taxonomic revision of Calotropis (Asclepiadaceae). Nordic Journal of Botany 11: 301-308.).
17. Calotropis procera (Aiton) W. T. Aiton, Hort. Kew. 2(2): 78. 1811.
Shrub, ca. 1.5–2.5 m tall, branches cylindric, full, light green, slightly striate, covered by greyish-white wax, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, interpetiolar; slightly pubescent to glabrescent. Leaves opposite decussate, subsessile; petiole 1–3 mm long, cylindric, ventrally sulcate, colleters 2, intrapetiolar; slightly striate, slightly pubescent to glabrescent; blade 16.5–19 × 8.5–9.5 cm, obovate to elliptic, apex cuspidate, base cordate, margin entire, flat; venation brochidodromous, 7–10 secondary veins, tertiary veins somewhat conspicuous; concolorous, chartaceous, covered by a greyish-white wax, colleters numerous, on the adaxial base; glabrous on both faces. Cyme submaxillary, racemiform, 9-flowered; peduncle 3.4–4.6 cm long, slightly pubescent to glabrescent; bracts 7.4 × 1.4 mm, lanceolate, colleters absent. Flowers with pedicel 3.2–3.3 cm long, pubescent; calyx lobes 4.1–5 × 1.7–2.2 mm, elliptic to ovate, equal, apex acute, margins glabrous, colleters 4–5, on the adaxial base, opposite; glabrous on both faces; corolla campanulate, aestivation valvar, white, lobes 1.3–1.4 × 0.7 cm, deltate, apex purple and base green adaxially, white to purple abaxially; margins glabrous, slightly pubescent to glabrescent on both faces; tube 0.1 × 0.2 cm, glabrous on both faces; corona simple, apex purple, base white; segments just below the gynostegium, opposite to anthers, segments 4.3–4.7 × 3.3–3.5 mm, sigmoid, compressed laterally, apex rounded, united to each other at the base, inserted on the anthers base; anthers 1.4–2 long, rectangular, apex truncate, base slightly cordate, wings longer than dorsal surface, dorsal shape not seen; membranaceous appendage depressed ovate, inflexed; fused to style-head; pollen dispersed in pollinarium. Retinacle 0.3–0.35 × 0.1 mm, oblong, brown, smaller than pollinia; caudicles 0.3 mm long, slightly rectangular, descending, basal reticulate membrane present, inserted laterally on the lower third of the retinacle, inserted laterally on the pollinia apices; pollinia 1.2–1.3 × 0.4 mm, ovoid to oblong; nectaries absent; ovary 1.8–2 mm long, ellipsoid, superior; gynostegium 6 mm long, cylindric, style-head with apex slightly convex. Follicles 6.3–7.1 × 4.5–4.6 cm, ellipsoid to globose, divergent colour not seen, rugose, ventricose, slightly pubescent to glabrescent; seeds 5–6 × 4–5 mm, ellipsoid, brown.
Examined material: Natal, Dunas de Mãe Luiza, Farol, 05°47’42”S, 35°12’33.8”W, 27.VII.1952, fl., S. Tavares 111 (IPA). Neópolis, 20.I.2009, fl. and fr., T.R. Ferreira 02 (UFRN).
Additional material: BRAZIL. RIO GRANDE DO NORTE: Acari, próximo ao sangradouro da Barragem de Gargalheiras, 06°25’28”S, 36°36’14”W, elev. 310 m, 24.III.2012, fl., A.A. Roque 1340 (UFRN).
This species is naturalized in Brazil, found mainly in the northeastern and southeastern regions, except for Alagoas and Rio de Janeiro, respectively, besides Distrito Federal, Mato Grosso do Sul, and some states of the northern region (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). In the study area, it was found in anthropized places in Restinga, in road margins, and along forest edges. Collected with flowers and fruits from January to July.
The species has a white campanulate corolla, with lobe purple and green, and fruits rugose and ventricose. It can also be differentiated by the subsessile leaves, by the plant being completely greyish-white and sericeous, and by the corona with sigmoid and laterally compressed segments.
Cynanchum L., Species Plantarum 1: 212. 1753.
Worldwide distribution, with about 400 species (Liede 1997Liede S (1997) American Cynanchum (Asclepiadaceae) - a preliminary infrageneric classification. Novon 7: 172-181.). In Brazil, seven species are recognized, two of which are endemic, and distributed almost everywhere in the country, except for some states of the northern, southern, southeastern, and northeastern regions (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). This is a new genus occurrence for Rio Grande do Norte.
18. Cynanchum roulinioides (E. Fourn.) Rapini, Bol. Bot. Univ. São Paulo 21(2): 278. 2003. Figs. 5e; 7
a-c. Cynanchum roulinioides – a. habit; b. flower detail showing corona segments (arrow) alternate to anthers; c. detail of the anther and the membranous appendix. (a-c. J. G. Jardim et al. 6060).
Woody climber, ca. 3–5 m tall, branches cylindric, full, light green, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, lateral to the petiole; pubescent. Leaves opposite distichous; petiole 1.6–3 mm long, cylindric, ventrally sulcate, colleters absent; smooth to striate, glabrescent; blade 4.9–6.6 × 2.9–3.7 cm, ovate, apex acuminate, base cordate, margin entire, slightly repand, flat; venation brochidodromous, 6–8 secondary veins, tertiary veins conspicuous; discolorous, membranaceous, colleters 4–5, on the adaxial base of midrib; slightly pubescent to glabrous adaxially, glabrous abaxially. Cyme subaxillary, racemiform, 13-flowered; peduncle 0.6–1.5 cm long, pubescent; bracts 0.6 × 0.3 mm, ovate to deltate, colleters absent; glabrous on both faces. Flowers with pedicel 0.2 cm long, slightly pubescent to glabrous; calyx lobes 1.3 × 0.6–0.7 mm, ovate, equal, apex acute, margin glabrous, colleters 3, between the lobes; glabrous on both faces; corolla campanulate, aestivation valvar, yellow and purple, lobes 1.3 × 0.8 cm, deltate, yellow adaxially, apex yellowish and base purple abaxially, margin glabrous, pilose adaxially, mainly on the middle, glabrous abaxially; tube 0.05 × 0.06 cm, glabrous on both faces; corona simple, yellowish; segments almost as high as the gynostegium, alternate to anthers, segments 1.06 × 0.3 mm, oblong, apex acute to cuspidate, united to each other at the base, and to the corolla and gynostegium; insert on the gynostegium base; anthers 0.51 mm long, vertically subglobose, apex truncate, base rounded, wings shorter than dorsal surface, dorsally convex, membranaceous appendage deltate, inflexed, fused to style-head; pollen dispersed in pollinarium. Retinacle 0.16 × 0.06–0.07 mm, slightly rhombic, brown, smaller than pollinia; caudicles 0.17–0.19 × 0.03 mm, filiform, descending, basal reticulate membrane present, inserted laterally on the retinacle base, inserted laterally on the pollinia apices; pollinia 0.37 × 0.11–0.12 mm, oblong; nectaries absent; ovary 0.7 mm long, ovoid to oblong, superior; gynostegium 1.18 mm long, cylindric, style-head with apex mamillate, Follicles 6 × 2.5 cm, fusiform, parallel, colour not seen, striate, glabrous; seeds not seen.
Examined material: Ceará-Mirim, distrito de Castelo, Faz. Diamante, 05°35’39”S, 35°25’50”W, elev. 55 m, 18.VIII.2011, fl., J.G. Jardim et al. 6060 (UFRN). Additional material: BRAZIL. BAHIA: Rio de Contas, Estrada Real, na parte baixa, 13°36’48”S, 41°48’25”W, 17.I.2003, fl., R.M. Harley & A.M. Giulietti 54559 (HUEFS); Estrada Real, 13°36’00”S, 41°48’00”W, 20.IV.2003, fr., A. Rapini 1070 (HUEFS).
This species is distributed in Brazil in the northeastern (Bahia, Ceará, Pernambuco and Piauí), central-western (Goiás, Mato Grosso do Sul and Mato Grosso), and southeastern (Minas Gerais, Rio de Janeiro and São Paulo) regions (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction), besides Paraguay (Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). This is a new occurrence for Rio Grande do Norte, found in semideciduous seasonal forest. Collected with flowers in August.
It is characterized by the campanulate corolla and by the corona segments, which are alternated in relation to the anthers and opposite in relation to the corolla lobes.
Ditassa R. Br., On the Asclepiadeae 38. 1810.
This genus has about 100 species (Konno & Fontella-Pereira 2004Konno TUP & Fontella-Pereira J (2004) Some nomenclatural and taxonomic notes on Brazilian Ditassa (Apocynaceae: Asclepiadoideae). Kew Bulletin 59: 297-300.) and is native to the Neotropical region, occurring in all Latin America, except for Chile, and with the centers of diversity in Brazil and on the Guiana Plateau, mainly in Venezuela (Konno 2005Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.). It is found throughout Brazil, except for the state of Amapá, represented by 60 species, 49 of which are endemic. In Rio Grande do Norte, it is represented by four species (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction).
The species are characterized as climbers with leaves never cordate, the umbelliform cymes, and by rotate corolla.
19. Ditassa crassifolia Decne., Prodr. 8: 576. 1844. Fig. 5f
Woody climber, ca. 1–2.5 m tall, branches cylindric, full, dark brown to light castaneous, striate, nodes congested, lacking cataphylls on apical buds, latex white, nodal colleters 2–4, interpetiolar; glabrous. Leaves opposite decussate; petiole 2–3.5 mm, cylindric, ventrally sulcate, colleters absent; striate, glabrous; blade 0.5–2.4 × 0.4–1.1 cm, obovate to elliptic, apex cuspidate, base acute, margin entire, revolute; venation brochidodromous, 8–10 secondary veins, tertiary veins inconspicuous; discolorous, chartaceous, colleters 2–3, on the adaxial base of midrib; glabrous on both faces, pilose on the midrib on both faces. Cyme subaxillary, umbelliform, 8–13-flowered; peduncle 0.1 cm long, slightly pilose to glabrous; bracts 0.04–0.7 × 0.06–0.4 mm, ovate, colleters absent; glabrous on both faces. Flowers with pedicel 0.3–1 long, glabrous; calyx lobes 0.8–1 × 0.5–0.8 mm, ovate, to deltate, equal, apex acute, margins glabrous, colleters 2, on the adaxial base, alternate; glabrous on both faces; corolla rotate, aestivation not seen, white to light yellow, lobes 0.2 × 0.1 cm, lanceolate, white on both faces, margins glabrous, velutinous to pubescent adaxially, glabrous abaxially; tube inconspicuous; corona doubled, white to slightly yellow; segments just below the gynostegium, opposite to anthers, external segments 0.6–0.7 × 0.3 mm, ovate to spatulate, apex acute, united to each other at the base (Konno 2005Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.); internal segments 0.2–0.5 × 0.1–0.3 mm, narrowly deltate, apex acute, apex not touching the dorsal surface of anthers; inserted on the anthers base; anthers 0.8–0.9 mm long, rectangular, apex truncate, base cordate; wings longer than the dorsal surface, dorsally convex, membranaceous appendage suborbicular, inflexed, fused to style-head; pollen dispersed in pollinarium. Retinacle 0.18–0.19 mm long, oblong to ovate, brown to castaneous, larger than pollinia; caudicles 0.04–0.08 mm long, narrowly deltate, horizontal, basal reticulate membrane present, inserted laterally on the retinacle base, inserted laterally on the pollinia apices; pollinia 0.17–0.18 × 0.08–0.11 mm, obovoid to ellipsoid; nectaries absent; ovary 0.6–0.7 mm long, ovoid, superior; gynostegium 0.6 mm long, cylindric, style-head with apex capitate. Follicles 1.7–3.6 × 0.1–0.8 cm, fusiform, divergent, colour not seen, striate, glabrous; seeds 5–6 × 1–2 mm, obovate to oblong, brown, glabrous.
Examined material: Ceará Mirim, estrada para Jacumã, estrada de barro adjacente, 05°34’52.2”S, 35°14’58”W, 18.V.2007, fl. and fr., A.M. Marinho & R.A. Costa 22 (UFRN); Praia de Muriú, 05°33’05”S, 35°15’30”W, 4.VII.2014, fl., J.C. Sousa Jr & L.A. Cestaro 30 (UFRN); RN-309 em direção à Pureza, entrada c. 4.3 km da RN-064, 05°34’38”S, 35°26’45”W, 9.VIII.2014, fl. and fr., J.C. Sousa Jr & J.G. Jardim 47 (UFRN). Extremoz, APA Jenipabú, 05°42’20”S, 35°12’43”W, 7.V.2011, fl., J.G. Jardim 5968 (UFRN). Natal, Parque da Cidade Dom Nivaldo Monte, 05°50’55”S, 35°13’45”W, 13.VIII.14, fl., J.C. Sousa Jr & A. Ribeiro 51 (UFRN). Nísia Floresta, dunas de Búzios, 30.III.2008, fl., F.S.R. Souza 10 (UFRN).
Additional material: BRAZIL. PARAÍBA: Mamanguape, 9.VII.2004, fl. and fr., S.M.C. Barbeiro 2521 (UFRN).
Ditassa crassifolia is endemic to Brazil and distributed in the states of Alagoas, Bahia, Paraíba, Pernambuco, Rio Grande do Norte, Sergipe, and Espírito Santo (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In Rio Grande do Norte, it was found associated with Restinga. Collected with flowers and fruits from March to August.
It is recognized by the glabrous leaves and differs from other species of the genus found in this study by the glabrous branches and the external, ovate to spatulate corona segments.
20. Ditassa hispida (Vell.) Fontella, Bradea 3(2): 5. 1979.
Woody climber, height not seen, branches cylindric, full, light brown, striate, nodes congested, lacking cataphylls on apical buds, latex not seen, nodal colleters 2, lateral to the petiole; hirsute. Leaves opposite, decussate; petiole 4–5 mm long, cylindric, ventrally sulcate, colleters absent; striate, pilose to hirsute; blade 2.9–3.4 × 1.3–1.5 cm, oblong to lanceolate, apex acuminate, base rounded, margin entire, slightly revolute; venation brochidodromous, 5–6 secondary veins, tertiary veins inconspicuous; discolorous, membranaceous, colleters 2, on the adaxial base of midrib; hirsute on both faces. Cyme subaxillary, umbelliform, 8–10-flowered; peduncle 0.06–0.15 cm long, glabrous; bracts 0.6–0.7 × 0.3–0.4 mm, ovate, colleters absent; glabrous adaxially, hirsute to pilose abaxially. Flowers with pedicel 0.5–0.7 cm long, glabrous; calyx lobes 0.9–1 × 0.6–0.7 mm, ovate to lanceolate, equal, white, apex acute, margins glabrous, colleters 2, on the adaxial base, alternate; glabrous adaxially, pilose to hirsute abaxially; corolla rotate, aestivation not seen, white, lobes 0.3–0.4 × 0.05–0.1 cm, lanceolate, white on both faces, margins glabrous, pubescent adaxially, glabrous abaxially; tube inconspicuous; corona doubled, white; external segments longer and the internal ones slightly above the gynostegium, opposite to anthers, external segments 2.23–2.43 × 0.2 mm, subulate, apex acute, long acuminate, united to each other at the base; internal segments 0.71–0.81 × 0.1 mm, subulate, apex acute, long acuminate, apex not touching the dorsal surface of anthers; inserted on the anthers base; anthers 0.56‒0.57 mm long, rectangular, apex truncate, base cordate; wings longer than the dorsal surface, dorsally convex, membranaceous appendage suborbicular, inflexed, fused to style-head; pollen dispersed in pollinarium. Retinacle 0.15 × 0.1 mm, oblong, brown, smaller than pollinia; caudicles 0.07–0.08 × 0.03–0.04 mm, deltate to narrowly deltate, horizontal, basal reticulate membrane present, inserted laterally on the retinacle base, inserted laterally on the pollinia apices; pollinia 0.25–0.27 × 0.1–0.11 mm, oblong; nectaries absent; ovary 0.5 mm long, ovoid, superior; gynostegium 1.14 mm long, cylindric, style-head with apex capitate. Follicles 2.7 × 0.3 cm, fusiform, divergent, green, striate, hirsute; seeds not seen.
Examined material: Ceará-Mirim, Fazenda Diamante, 05°35’25”S, 35°25’51”W, elev. 54 m, 2.V.2012, fl., A.A. Roque & W.M.B. São-Mateus 1356 (UFRN).
Additional material: BRAZIL. BAHIA: Jeremoabo, Fazenda Natureza, 10°01’40”S, 38°26’20”W, elev. 427 m, 12.VIII.2005, fr., A. Rapini et al. 1243 (HUEFS).
In Brazil, it occurs in the northeastern (Alagoas, Bahia, Ceará, Maranhão, Paraíba, Pernambuco and Sergipe) and southeastern regions (Espírito Santo, Minas Gerais, Rio de Janeiro and São Paulo), besides the states of Pará and Paraná (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). It is also distributed in Guiana, French Guiana, and Argentina (Konno 2005Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.). It is a new occurrence for Rio Grande do Norte and was found in semideciduous seasonal forest. Collected with flowers and fruits in May.
This species can be identified by the pilose to hirsute branches, leaves, bracts, and calyx, and the hirsute follicle. It differs from other species of the genus found in this study by the corona with external and internal segments subulate.
a-c. Ditassa sp. – a. flowering branch; b. flower detail in anthesis; c. detail of the hairy branch bilaterally. (a-c. J. G. Jardim & J. C. Sousa Jr. 6672).
Woody climber, ca. 1 m tall, branches cylindric, full, light brown, striate, nodes congested, lacking cataphylls on apical buds, latex not seen, nodal colleters 2, interpetiolar; pilose bilaterally. Leaves opposite, decussate; petiole 2.2–4 mm long, cylindric, ventrally sulcate, canaliculate, colleters absent; striate, pilose to glabrous; blade 1.5–2.4 × 1–1.7 cm, oblong to elliptic, apex cuspidate, base rounded to slightly obtuse, margin entire, slightly revolute; venation brochidodromous, 3–4 secondary veins, tertiary veins little evident; discolorous, chartaceous, colleters 2, on the adaxial base of midrib; slightly pilose adaxially, pilosity concentrated on the midrib, glabrescent abaxially. Cyme subaxillary, umbelliform, 5–7-flowered; peduncle 0.1 cm long, glabrescent to glabrous; bracts 1–1.2 × 0.7–0.8 mm, ovate, colleters absent; glabrous on both faces. Flowers with pedicel 0.5–0.6 cm long, glabrous; calyx lobes 1 × 0.7 mm, ovate to deltate, equal, apex acute, margin glabrous, colleters 2, on the adaxial base, alternate; glabrous on both faces; corolla rotate, aestivation not seen, white, lobes 0.2 × 0.1 cm, linear to narrowly oblong, white on both faces, margins glabrous, pubescent adaxially, glabrous abaxially; tube inconspicuous; corona doubled, white to transparent; external segments longer and the internal ones shorter than gynostegium, opposite to anthers, external segments 1.9–2 × 0.3–0.4 mm, narrowly deltate, apex acute, united to each other at the base; internal segments 0.7 × 0.1 mm, narrowly deltate, apex acute, apex touching the dorsal surface of anthers; inserted on the anthers base; anthers 0.6–0.7 mm long, rectangular, apex cuneate, base cordate, wings longer than dorsal surface, dorsally convex, membranaceous appendage suborbicular, inflexed, fused to style-head; pollen dispersed in pollinarium. Retinacle 0.2 × 0.08 mm, elliptic, brown, smaller or as large as pollinia; caudicles 0.07 × 0.02–0.03 mm, deltate, horizontal to slightly descending, basal reticulate membrane present, inserted laterally on the retinacle base, inserted laterally on the upper third of pollinia; pollinia 0.24–0.26 × 0.09 mm, obovoid; nectaries absent; ovary 0.4–0.5 mm long, ovoid to slightly oblong, superior; gynostegium 1–1.1 mm long, cylindric, style-head with apex capitate. Follicles not seen; seeds not seen.
Examined material: Tibau do Sul, Parque Estadual Mata de Pipa, 06°14’45”S, 35°03’20”W, elev. 45–60 m, 2.VII.2014, fl., J.G. Jardim & J.C. Sousa Jr. 6672 (UFRN).
It was found at only one locality, associated with edges of semideciduous seasonal forest. Collected with flowers in June.
It can be characterized by the bilaterally pilose branches and internal segments of the corona, with the apex touching the dorsal surface of anthers. Ditassa sp. is similar to Ditassa arianeae Fontella & E.A.Schwarz, but these taxa differ because the external segments of the corona in Ditassa sp. are narrowly deltate and continuous, while in D. arianeae, they are narrowly deltate, but abruptly narrowed at the middle.
Funastrum E. Fourn., Annales des Sciences Naturelles, Botanique, 4(14): 388. 1882.
This genus is composed of 19 species, occurring in the Americas from the South of the United States to Argentina (Holm 1950Holm RW (1950) The American Species of Sarcostemma R. Br. (Asclepiadaceae). Annals of the Missouri Botanical Garden 37: 477-560.; Stevens 2009Stevens WD (2009) Funastrum. In: Davidse G, Sánchez MS, Knapp S & Cabrera FC (eds.) Flora Mesoamericana - Cucurbitaceae a Polemoniaceae 4(1), i-xvi. Missouri Botanical Garden, Saint Louis. Pp. 1-855.). In Brazil, there are only two widely distributed species, absent in the states of Sergipe, Rondônia, and Santa Catarina (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.).
22. Funastrum clausum (Jacq.) Schltr., Repert. Spec. Nov. Regni Veg. 13: 283. 1914.
Herbaceous climber, height not seen, branches cylindric, full, light green, striate, nodes lax, lacking cataphylls on apical buds, latex white, nodal colleters 2, lateral to the petiole; velutinous to glabrescent. Leaves opposite distichous; petiole 2.7–4 mm long, cylindric, sulcus absent, colleters absent, slightly striate, velutinous; blade 3.2–3.8 × 1.9–2.4 cm, elliptic, apex cuspidate, base subcordate, margin entire to repand, slightly revolute; venation brochidodromous, 8–11 secondary veins, tertiary veins slightly evident; discolorous, membranaceous, colleters 3–4, on the adaxial base of midrib; pubescent to glabrescent adaxially, velutinous abaxially, mainly on the base. Cyme axillary, umbelliform, 3–13-flowered; peduncle 0.05–0.1 cm long, velutinous; bracts not seen. Flowers with pedicel 1.4–1.8 cm long, velutinous; calyx lobes 3–3.1 × 1.1–1.5 mm, ovate to lanceolate, equal, apex acute, margins velutinous, colleters 2, on the adaxial base, alternate; slightly pilose to glabrous adaxially, velutinous abaxially; corolla rotate, aestivation not seen, white to light-green, lobes 0.7 × 0.3–0.4 cm, ovate to lanceolate, white adaxially, white to light-green abaxially, margins velutinous, slightly pubescent to glabrous adaxially, velutinous to glabrous abaxially; tube inconspicuous; corona doubled; segments slightly longer than gynostegium, opposite to anthers, external segments 0.7 × 1.1 mm, suborbicular, reduced to small appendages, apex rounded to obtuse, united to each other at the base; internal segments 1.4–1.9 × 1–1.3 mm, shaped as vesicular bags, globose, apex obtuse, apex not touching the dorsal surface of anthers; inserted on the anthers base; anthers 1.4–1.7 mm long, rectangular, apex cuneate, base sagittate, wings longer than dorsal surface, dorsally flat; membranaceous appendage orbicular, inflexed; fused to style-head; pollen dispersed in pollinarium. Retinacle 0.3–0.31 × 0.23–0.25 mm, trullate, brown, smaller than pollinia; caudicle 0.17–0.21 × 0.03 mm, narrowly deltate, horizontal, basal reticulate membrane present, inserted laterally on the retinacle base, inserted laterally on the pollinia apices; pollinia 0.83–0.89 × 0.18–0.2 mm, oblong; nectaries absent; ovary 1.5 mm long, ovoid to ellipsoid, superior; gynostegium 2–3.2 mm long, umbraculiform, style-head mamillated. Follicles 5.5–5.8 × 1.7–1.8 cm, fusiform, disposition not seen, colour not seen, striate, velutinous; seeds 4.6–5 × 2.9–3.2 mm, deltate, brown, glabrous.
Examined material: Ceará-Mirim, Rod. RN-064 sentido Ceará-Mirim/Ielmo Marinho, 05°39’09”S, 35°27’46”W, 12.VIII.2012, fl. and fr., A.A. Roque & E.O. Moura 1456 (UFRN).
Addtional material: BRAZIL. RIO GRANDE DO NORTE: Acari, margens da barragem Gargalheiras, 06°24’25”S, 36°36’01”W, 20.III.2011, fl. and fr., A.A. Roque & J.L. Farias 912 (UFRN); trilha aos poços da barragem, 06°25’35”S, 36°36’09”W, 8.IX.2012, fl., L.M. Versieux et al. 606 (UFRN). Currais Novos, região dos Apertados, 06°19’47”S, 36°30’29”W, 27.V.2010, fl. and fr., J.G. Jardim et al. 5735 (UFRN). Pau dos Ferros, Sítio Aroeira, 05°57’45”S, 38°07’45”W, elev. 150 m, 15.VI.1980, fl., O.F. Oliveira 1052 (MOSS). Serra Negra do Norte, Estação Ecológica do Seridó, 06°34’68”S, 37°15’39”W, 21.V.2005, fr., R.T. Queiroz 396 (UFRN).
Widely distributed in Brazil, with the exception of the states of Sergipe, Rondônia, Santa Catarina, and Rio Grande do Sul (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In Rio Grande do Norte, it was found in deciduous seasonal forest generally near water courses. Collected with flowers and fruits in August.
Characterized by the velutinous branches, umbellate inflorescence with long pedicels, rotate corolla, white to light-green and lobes with a velutinous margin, and corona with internal segments globose shaped as vesicular bags.
Ibatia Decne., in DC. & A.DC., Prodr. 8: 599. 1844.
This genus is restricted to the American continent, especially in the Neotropical region (Morillo 2012Morillo GN (2012) Aportes al conocimiento de las Gonolobinae (Apocynaceae - Asclepiadoideae). Pittieria 36: 13-57.), with 300 accepted species (Morillo 1997 apud Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). It is distributed all over Brazil, where 46 species are recognized, 30 of which are endemic, and four species are found in Rio Grande do Norte (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.), however, only one species was found in the study area, the others [I. endressiae Fontella & Goes, I. harleyi (Fontella & Morillo) Morillo and I. nigra (Decne.) Morillo] occur in the Caatinga.
23. Ibatia ganglinosa (Vell.) Morillo, Pittieria 36: 23. 2012. Figs. 5h; 9
a-b. Ibatia ganglinosa – a. branch with flowers and fruit; b. detail of the gynostegium, showing its apex, anthers (continuous arrows) and a pollinarium (dashed arrow). (a-b. M. B. Sousa 143; A. A. Roque 1038).
Woody climber, ca. 1.5–3 m tall, branches cylindric, full, brown to light green, striate, nodes lax, apical stems lacking cataphylls, latex white, nodal colleters absent; pubescent. Leaves opposite distichous; petiole 20–46 mm long, cylindric, ventrally sulcate, colleters absent; striate, pubescent to pilose; blade 6–7.2 × 3.1–5.6 cm, ovate, apex acute to acuminate, base cordate, margin entire, slightly revolute; venation brochidodromous, 6‒7 secondary veins, tertiary veins little evident; discolorous, membranaceous, colleters 4, on the adaxial base of midrib; pubescent to pilose adaxially, velutinous abaxially. Cyme subaxillary, type not seen, 2–6-flowered; peduncle 0.2 cm long, pubescent to velutinous; bracts 0.3–1 mm long, narrowly deltate, colleters absent; glabrous adaxially, pubescent to pilose abaxially. Flowers with pedicel 0.2–0.4 cm long, pilose; calyx lobes 2.3–2.5 × 0.5–1 mm, narrowly oblong to ovate, equal, apex acute, margin glabrous, colleters 2, on the adaxial base, alternate; glabrous on both faces, pilose abaxially along the longitudinal axis; corolla rotate, aestivation not seen, light green to purple, lobes 0.3 × 0.2 cm, deltate, apex light green and base purple on both faces, margins glabrous; pubescent to pilose adaxially, pubescent abaxially; tube 0.05 × 0.15 cm, glabrous on both faces; corona simple, apex green, base purple; segments approximately or almost as high as the gynostegium, opposite to anthers, segments 1.8 × 1.7 mm, shaped as a 5-lobed ring, rhomboid, apex deltate, united to each other from the base to half of its length, inserted on the anthers base; anthers 0.6 mm long, rectangular, apex truncate, base not seen, wings absent, dorsally convex, membranaceous appendage absent, fused to the style-head; pollen dispersed in pollinarium. Retinacle 0.25 × 0.09 mm, narrowly obovate to narrowly oblong, dark brown, smaller than pollinia; caudicles 0.08–0.09 mm, linear, horizontal, basal reticulate membrane present, inserted laterally on the middle of the pollinia; pollinia 0.55–0.57 × 0.41–0.43 mm, obovoid to ellipsoid; nectaries absent; ovary 1–1.2 mm long, ovoid, superior; gynostegium 0.7–0.8 mm long, cylindric, style-head with apex flat. Follicles 4.9 × 2.5–4.9 cm, ovoid, disposition not seen, light green to greyish, tuberculate, pubescent to velutinous; seeds 5.3–5.5 × 2.7–3.5 mm, ovate to slightly oblong, brown, glabrous.
Examined material: Natal, dunas em frente ao D.O.L. - Setor A, 05°48’00”S, 35°13’00”W, 26.VIII.1980, fl. and fr., Projeto Parque das Dunas 44 (MOSS); Parque das Dunas, 25.VII.2009, fl., M.B. Sousa 143 (UFRN); trilha da Geologia, Pq. das Dunas, 05°49’30”S, 35°11’00”W, 4.IX.1997, fr., L.A. Cestaro 67 (UFRN); 05°49’30”S, 35°11’00”W, 9.VI.1999, fl. and fr., L.A. Cestaro 115 (UFRN); Pq. das Dunas, 19.VII.2005, fl. and fr., UFRN 2174 (UFRN). Nísia Floresta, Praia de Tabatinga, tabuleiros litorâneos, 06°05’00”S, 35°12’00”W, 27.IX.1984, fl., A. Dantas et al. 96 (IPA). Parnamirim, EMPARN, Mata do Jiqui, 29.XI.2007, fr., J.E.D. Barbosa 10 (UFRN).
Additional material: BRAZIL. RIO GRANDE DO NORTE: Campo Redondo, Fazenda Giromão, 5.VIII.2009, fr., A.A. Roque 1038 (UFRN).
Endemic to Brazil and distributed in the northeastern region, with the exception of Piauí, and in the southeast, except for São Paulo, mainly in Restingas from Ceará to Rio de Janeiro (Rapini & Farinaccio 2008Rapini A & Farinaccio MA (2008) Two taxonomic changes in Asclepiadoideae (Apocynaceae) from Brazil. Neodiversity 3: 19-21.). It also occurs in the Caatinga, on forest edges and disturbed vegetation, and on sandy soil and rocky outcrops (Rapini & Farinaccio 2008Rapini A & Farinaccio MA (2008) Two taxonomic changes in Asclepiadoideae (Apocynaceae) from Brazil. Neodiversity 3: 19-21.). In Rio Grande do Norte, it was found in Restinga, corroborating with the data on its occurrence mentioned above. Collected with flowers from June to September and with fruits from June to November.
Ibatia ganglinosa is generally mistaken for I. maritima (Jacq.) Decne. in herbaria; however, the first can be easily recognized and differentiated from the second by having the gynostegium with apex flat, while I. maritima (≡ Matalea maritima (Jacq.) Woodson] has the gynostegium with apex rostrate (Rapini & Farinaccio 2008Rapini A & Farinaccio MA (2008) Two taxonomic changes in Asclepiadoideae (Apocynaceae) from Brazil. Neodiversity 3: 19-21.). Besides the difference in the gynostegium apex, I. glanglinosa occurs mainly in the northeastern region, while I. maritima only occurs in the northern region (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.). I. glanglinosa can also be recognized by the corona segments shaped as a 5-lobed ring and by the obovoid to ellipsoid pollinia.
Marsdenia R. Br., Prodromus Florae Novae Hollandiae 460. 1810, nom. cons. (1810).
This genus is distributed in warm and wet regions of the world, with approximately 300 species (Morillo 1997 apud Rapini 2001Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.). Expect for the state of Acre, it has a wide range of occurrence in Brazil, where 34 species occur, 25 of which are endemic; two species are found in Rio Grande do Norte (BFG 2015BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.) but only one species was found in the study area. The second (M. megalantha Goyder & Morillo) has been found only in the Caatinga so far.
24. Marsdenia altissima (Jacq.) Dugand, Mutisia 9(1). 1952. Fig. 5i
Woody climber, ca. 2–7 m tall, branches cylindric, full, light green, striate, nodes congested, lacking cataphylls on apical buds, latex white, nodal colleters 1–3, lateral to the petiole; velutinous. Leaves opposite decussate; petiole 3.2–4.3 mm long, cylindric, ventrally sulcate, colleters 2–3, intrapetiolar; slightly striate, velutinous; blade 9.7–11.3 × 6.3–10.6 cm, ovate, apex acuminate, base cordate, margin entire, slightly revolute; venation brochidodromous, 5–6 secondary veins, tertiary veins conspicuous; discolorous, membranaceous, colleters 5–11, on the adaxial base of midrib; pubescent adaxially, pubescent to velutinous abaxially, mainly on the veins. Cyme axillary, glomeruliform to umbelliform, 13–14-flowered; peduncle 1 cm long, velutinous; bracts 2.2‒3.3 × 0.6–0.7 mm, narrowly deltate to linear, colleters not seen; velutinous on both faces. Flowers with pedicel 0.4–0.5 cm long, velutinous; calyx lobes 3–3.8 × 2–2.5 mm, ovate, equal, apex acute, margin velutinous, colleters 2, on the adaxial base, alternate; glabrous adaxially, velutinous abaxially; corolla urceolate, aestivation not seen, green, lobes 3.4–3.9 × 2.6–3.8 cm, ovate to elliptic, vinaceous adaxially, green abaxially, margins pubescent to velutinous; glabrous adaxially, pubescent to velutinous abaxially; tube 4.5–5 × 3.5 cm, velutinous to glabrous on both faces; corona simple; segments lower than gynostegium, opposite to anthers, segments 1.5 × 0.7 mm, ovate, apex rounded to truncate, free from each other at the base, inserted on the dorsal surface of anthers; anthers 2.4–2.5 mm long, rectangular to deltate, apex deltate, base truncate, wings shorter than dorsal surface, dorsal shape not seen, membranaceous appendage deltate, inflexed; fused to style-head; pollen dispersed in pollinarium. Retinacle 0.05–0.1 mm long, elliptic, light brown, smaller than pollinia; caudicles 0.4–0.6 mm long, filiform, horizontal, basal reticulate membrane absent, inserted laterally on the lower third of the retinacle, inserted laterally on the pollinia base; pollinia 0.5–0.6 × 0.2 mm, obovoid; nectaries absent; ovary 1.8–2 mm long, ellipsoid, superior; gynostegium 4 mm long, cylindric, style-head with apex stipitate. Follicles 19.2 × 5.1 cm, ovoid to lanceolate, disposition not seen, colour not seen, striate to slightly lamelate, velutinous to tomentose; seeds 12–16 × 8–12 mm, ovate to elliptic, brown, sericeous to glabrous.
Examined material: Extremoz, APA Jenipabu, 05°42’20”S, 35°12’43”W, elev. 61 m, 25.VIII.2010, fl., A.M. Marinho & E.O. Moura 163 (UFRN); 05°42’28”S, 35°12’38”W, elev. 31 m, 2.II.2011, fl., J.L. Costa-Lima et al. 320 (UFRN).
Additional material: BRAZIL. BAHIA: Campo Formoso, povoado de Buraco, 10°09’07”S, 40°51’07”W, elev. 544 m, 20.IX.2006, fr., J.A. Siqueira-Filho et al. 1742 (UFRN). CEARÁ: Quixadá, próximo à Fazenda São Francisco, 04°57’15”S, 38°57’53”W, elev. 171 m, 12.III.2014, fl., J.G. Jardim et al. 6537 (UFRN).
Marsdenia altissima is distributed in all states of the northeastern, central-western, and southeastern regions, except in Espírito Santo, besides the states of Pará, Rondônia, and Roraima (Flora do Brasil 2020Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
http://floradobrasil.jbrj.gov.br/...
, under construction). In Rio Grande do Norte, it was found associated with arboreal Restinga. Collected with flowers in February to August.
Its main features are the velutinous leaves, many-flowered and congested inflorescences, vinaceous to green corolla lobes, filiform caudicles inserted on the pollinia bases, and the large, lanceolate, and velutinous follicles.
Acknowledgements
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education), for providing a Master’s degree scholarship to the first author; to the curators of the visited herbaria; to Pâmela Lavor, Mayara Alves, Tianisa Boeira, and André Viana, for helping us with map construction; to the team of the Laboratório de Botânica Sistemática - UFRN; to Rhudson Cruz, for the illustration; to Arthur Soares, for his suggestions; to Eduardo Tomaz, for the photo clipboards and for the translation; and the anonymous reviewers and the publisher Marli Morim, for the contributions that helped to improve this study. JGJ is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for its Productivity Research fellowships.
References
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM & Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22: 711-728.
- Barbosa MR, Sothers C, Mayo S, Gamarra-Rojas CFL & Mesquita AC (2006) Checklist das plantas do nordeste brasileiro: Angiospermas e Gymnospermas. Ministério da Ciência e Tecnologia, Brasília. 156p.
- Barkat MA, Abul H & Rahman MA (2017) Agricultural, pharmaceutical and therapeutic interior of Catharanthus roseus (L.) G.Don. In: Naeem M, Aftab T & Khan M (eds.) Catharanthus roseus. Springer, Cham. 426p.
- BFG - The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085-1113.
- Brasil (2006) Lei n. 11.428, de 22 de dezembro de 2006. Presidência da República, Casa Civil, Subchefia para Assuntos Jurídicos, Brasília. Available at <http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/lei/l11428.htm> . Access on 5 April 2019.
» http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/lei/l11428.htm - Brown R (1810) On the Asclepiadeae, a natural order of plants separated from the Apocyneae of Jussieu. Memoirs of the Wernerian Natural History Society 1: 12-78.
- Cestaro LA & Soares JJ (2004) Variações florística e estrutural e relações fitogeográficas de um fragmento de floresta decidual no Rio Grande do Norte, Brasil. Acta Botanica Brasilica 18: 203-218.
- Colombo BR, Kaehler M & Calvente A (2016) An inventory of the Bignoniaceae from the Brazilian state of Rio Grande do Norte highlights the importance of small herbaria to biodiversity studies. Phytotaxa 278: 019-028.
- Cordeiro SZ, Simas NK, Henriques AB & Sato A (2014) Micropropagation and callogenesis in Mandevilla guanabarica (Apocynaceae), an endemic plant from Brazil. Crop Breeding and Applied Biotechnology 14: 108-115.
- Coutinho TS & Louzada RB (2018) Flora da Usina São José, Igarassu, Pernambuco: Apocynaceae. Rodriguésia 69: 699-714.
- Endress ME & Bruyns PV (2000) A revised classification of Apocynaceae s.l The Botanical Review 66: 1-56.
- Endress M & Stevens W (2002) The renaissance of the Apocynaceae s.l: recent advances in systematics, phylogeny, and evolution: introduction. Annals of the Missouri Botanical Garden 88: 517-522.
- Endress ME, Liede-Schumann S & Meve U (2007) Advances in Apocynaceae: the enlightenment, an introduction. Annals of the Missouri Botanical Garden 94: 259-267.
- Endress M, Liede-Schumann S & Meve U (2014) An updated classification for Apocynaceae. Phytotaxa 159: 175-194.
- Fernandes GEA, Mota NFO & Simões AO (2018) Flora das cangas da Serra dos Carajás, Pará, Brasil: Apocynaceae. Rodriguésia 69: 3-23.
- Ferreira CGT, Oliveira RC, Valls JM & Loiola MIB (2009) Poaceae da Estação Ecológica do Seridó, Rio Grande do Norte, Brasil. Hoehnea 36: 679-707.
- Flora do Brasil (2020, em construção) Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Available at <http://floradobrasil.jbrj.gov.br/>. Access on 5 May 2019.
» http://floradobrasil.jbrj.gov.br/ - Fontella-Pereira J, Valente MC & Marquete NFS (1995) Flora da Serra do Cipó, Minas Gerais: Asclepiadaceae. Boletim de Botânica da Universidade de São Paulo 14: 131-179.
- Forzza RC, Baumgratz JFA, Bicudo CEM, Carvalho Jr. AA, Costa A, Costa DP, Hopkins M, Leitman PM, Lohmann LG, Maia LC, Martinelli G, Menezes M, Morim MP, Coelho MAN, Peixoto AL, Pirani JR, Prado J, Queiroz LP, Souza VC, Stehmann JR, Sylvestre LS, Walter BMT & Zappi D (2010) Catálogo de Plantas e Fungos do Brasil. Instituto de Pesquisas Jardim Botânico do Rio de Janeiro. Vol. 1. Andrea Jakobsson Estúdio, Rio de Janeiro. 874p.
- Freire MSB (1990) Levantamento florístico do Parque das Dunas do Natal. Acta Botanica Brasilica 4: 41-59.
- Gomes SM, Kinoshita LS & Castro MM (2008) Hemisincarpia e nectário apendicular enfocados através de ontogênese floral em Mandevilla velame (A. St.-Hil.) Pichon, Apocynoideae. Brazilian Journal of Botany 31: 81-93.
- Gonçalves EG & Lorenzi H (2011) Morfologia vegetal: organografia e dicionário ilustrado de morfologia das plantas vasculares. 2ª ed. Instituto Plantarum de Estudos da Flora, São Paulo. 544p.
- Guerra AG, Maia A & Spinelli ACGO (2013) Perfil do Rio Grande do Norte, Secretaria de Estado do Planejamento e das Finanças. Instituto de Desenvolvimento Econômico e Meio Ambiente, Natal. 191p.
- Holm RW (1950) The American Species of Sarcostemma R. Br. (Asclepiadaceae). Annals of the Missouri Botanical Garden 37: 477-560.
- INCT - Herbário Virtual da Flora e dos Fungos (2019) speciesLink. Available at <http://inct.splink.org.br>. Access on 30 april 2019.
» http://inct.splink.org.br - IBGE - Instituto Brasileiro de Geografia e Estatística (2012) Manual Técnico da Vegetação Brasileira. 2ª ed. IBGE, Rio de Janeiro. 270p.
- IBGE - Instituto Brasileiro de Geografia e Estatística (2014) Estados: Rio Grande do Norte. Available at <https://cidades.ibge.gov.br/brasil/rn/panorama Access on 10 August 2021.
» https://cidades.ibge.gov.br/brasil/rn/panorama - Judd WS, Campbell CS, Kellogg EA, Stevens PF & Donoghue MJ (2009) Sistemática vegetal: um enfoque filogenético. 3ª ed. Artmed, Porto Alegre. 632p.
- Jussieu AL (1789) Genera plantarum. Viduam Herissant, Paris. 498p.
- Koch I & Kinoshita LS (1999) As Apocynaceae s. str da região de Bauru, São Paulo, Brasil. Acta Botanica Brasilica 13: 61-86.
- Koch I (2002) Estudo das espécies neotropicais de Rauvolfia L. (Apocynaceae). Tese de Doutorado. Universidade Estadual de Campinas, Campinas. 292p.
- Konno TUP & Fontella-Pereira J (2004) Some nomenclatural and taxonomic notes on Brazilian Ditassa (Apocynaceae: Asclepiadoideae). Kew Bulletin 59: 297-300.
- Konno TUP (2005) Ditassa R.Br. (Asclepiadoideae: Apocynaceae) no Brasil. Tese de Doutorado. Universidade de São Paulo, São Paulo. 238p.
- Leeuwenberg AJM (1994) A revision of Tabernaemontana two. The new world species and Stemnadenia. Series of revisions of Apocynaceae: XXXVI. Royal Botanic Gardens, Kew. 450p.
- Liede S (1997) American Cynanchum (Asclepiadaceae) - a preliminary infrageneric classification. Novon 7: 172-181.
- Livshultz T, Middleton DJ, Endress ME & Williams JK (2007) Phylogeny of Apocynoideae and the APSA Clade (Apocynaceae s.l). Annals of the Missouri Botanical Garden 94: 324-359.
- Maciel LVB (2011) Análise dos remanescentes de Mata Atlântica no estado do Rio Grande do Norte: uma perspectiva em alta resolução. Dissertação de Mestrado. Universidade Federal do Rio Grande do Norte, Natal. 47p.
- Marcondes-Ferreira W (1988) Aspidosperma Mart., nom. cons. (Apocynaceae): estudos taxonômicos. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 442p.
- Mishra JN & Verma NK (2017) A brief study on Catharanthus Roseus: a review. International Journal of Research in Pharmacy and Pharmaceutical Sciences 2: 20-23.
- MMA - Ministério do Meio Ambiente (2010) Mata Atlântica: patrimônio nacional dos brasileiros. In: Campanili M & Schaffer WB (orgs.) Biodiversidade 34. Secretaria de Biodiversidade e Florestas, Brasília. 408p.
- Morales JF (1999a) A new species of Macoubea (Apocynaceae) from Mesoamerica. Novon 9: 86-88.
- Morales JF (1999b) A synopsis of the genus Odontadenia: series of revisions of Apocynaceae XLV. Bulletin du Jardin Botanique National de Belgique 67: 381-477.
- Morales JF (2005a) Estudios en las Apocynaceae neotropicales XIX: la familia Apocynaceae (Rauvolfioideae, Apocynoideae) de Costa Rica. Darwiniana 43: 90-191.
- Morales JF (2005b) Estudios en las Apocynaceae Neotropicales XIII: revisión del género Temnadenia (Apocynoideae, Echiteae). Candollea 60: 207-231.
- Morales JF (2009) La Famila Apocynaceae (Apocynoideae, Rauvolfioideae) en Guatemala. Darwiniana 47: 140-184.
- Morillo GN (2012) Aportes al conocimiento de las Gonolobinae (Apocynaceae - Asclepiadoideae). Pittieria 36: 13-57.
- Morokawa R, Simões AO & Kinoshita LS (2013) Apocynaceae s. str do Parque Nacional da Serra da Canastra, Minas Gerais, Brasil. Rodriguésia 64: 179-199.
- Moura EO, Sousa VF, Soares AS & Versieux LM (2018) Private environmental consultancy reveals five genera and ten species of angiosperms new to Rio Grande do Norte state, northeastern Brazil. Check List 14: 439-451.
- Müller JA (1860) Apocynaceae. In: Martius CFP & Eichler AW (eds.) Flora brasiliensis Frid. Fleischer, Lipsiae. Vol. 6, pars 1. pp. 1-196.
- Nazar N, Goyder DJ, Clarkson JJ, Mahmood T & Chase MW (2013) The taxonomy and systematics of Apocynaceae: where we stand in 2012. Botanical Journal of the Linnean Society 171: 482-490.
- Oliveira P & Marquis R (2002) Preface. In: Oliveira P & Marquis R (eds.) The Cerrados of Brazil: ecology and natural history of a Neotropical Savanna. Columbia University Press, New York. 424p.
- Oliveira ACP, Penha AS, Souza RF & Loiola MIB (2012a) Composição florística de uma comunidade savânica no Rio Grande do Norte, nordeste do Brasil. Acta Botanica Brasilica 26: 559-569.
- Oliveira ACP, Mota ML & Loiola MIB (2012b) Diversidade florísta e chave de identificação de trepadeiras em uma floresta estacional semidecidual em Parnamirim, RN, Brasil. Revista Caatinga 25: 153-158.
- Oliveira RC, Silva AS, Ribeiro ARO, Araújo JE, Oliveira OF & Camacho RGV (2013) List of Angiosperm Species of the Riparian Vegetation of the Apodi-Mossoró River, Rio Grande do Norte, Brazil. Check List 9: 740-751.
- Oliveira-Filho AT & Fontes MAL (2000) Patterns of Floristic Differentiation among Atlantic Forests in Southeastern Brazil and the Influence of Climate. Biotropica 32: 793-810.
- Paarakh MP, Swathi S, Taj T, Tejashwini V & Tejashwini B (2019) Catharanthus Roseus Linn - a review. Acta Scientific Pharmaceutical Sciences 3: 19-24.
- Pereira ASS, Devides-Castello AC, Simões AO & Koch I (2019) Reestablishment, new records, and a key for the species of Aspidosperma (Apocynaceae) from the Brazilian Amazon. Acta Botanica Brasilica 33: 1-20.
- Prado DE (2003) As Caatingas da América do Sul. In: Leal IR, Tabarelli M & Silva JMC (eds.) Ecologia e conservação da Caatinga. Editora Universitária-UFPE, Recife. 822p.
- Prata APN, Amaral MCE, Farias MCV & Alves MV (2013) Flora de Sergipe. Vol. 1. Gráfica e Editora Triunfo, Aracaju. 592p.
- Radford AE, Dickson WC, Massey JR & Bell CR (1974) Vascular plant systematics. Harper Collins & Row, New York. 891p.
- Rahman MA & Wilcock CC (1991) A taxonomic revision of Calotropis (Asclepiadaceae). Nordic Journal of Botany 11: 301-308.
- Rao AS (1956) A revision of Rauvolfia with particular reference to the american species. Annals of the Missouri Botanical Garden 43: 253-354.
- Rapini A (2001) Asclepiadoideae (Apocynaceae) da Cadeia do Espinhaço de Minas Gerais, Brasil. Boletim de Botânica 19: 55-169.
- Rapini A & Farinaccio MA (2008) Two taxonomic changes in Asclepiadoideae (Apocynaceae) from Brazil. Neodiversity 3: 19-21.
- Rapini A (2012) Taxonomy “under construction”: advances in the systematics of Apocynaceae, with emphasis on the brazilian Asclepiadoideae. Rodriguésia 63: 075-088.
- Rede Brasileira de Herbários (2019) Available at <https://www.botanica.org.br/catalogo-da-rede-brasileirade-herbarios/>. Access on 09 August 2021.
» https://www.botanica.org.br/catalogo-da-rede-brasileirade-herbarios/ - Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ & Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142: 1141-1153.
- Rizzini CT (1997) Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. 2ª ed. Âmbito Cultural, Rio de Janeiro. 748p.
- Sakane M (1981) Revisão do gênero Allamanda L. (Apocynaceae) no Brasil. Dissertação de Mestrado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 107p.
- Sakane M & Shepherd GJ (1986) Revisão do gênero Allamanda L. (Apocynaceae). Revista Brasileira de Botânica 9: 125-149.
- Sales MF (1993) Estudos taxonômicos de Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 413p.
- Sales MF (1995) Apocynaceae. In: Stannard B (ed.) Flora of the Pico das Almas, Chapada Diamantina, Bahia, Brazil. Royal Botanic Gardens, Kew. Pp. 128-135.
- São-Mateus WMB, Cardoso D, Jardim JG & Queiroz LP (2013) Papilionoideae (Leguminosae) na Mata Atlântica do Rio Grande do Norte, Brasil. Biota Neotropica 13: 315-362.
- Schlechter R (1905) Periplocaceae e Asclepiadaceae. In: Schumann K & Lauterbach K (eds.) Flora der Deutschen Schutzgebiete in der Südsee. Bornträeger, Leipzig. Pp. 351-369.
- Sennblad B & Bremer B (1996) The familial and subfamilial relationships of Apocynaceae and Asclepiadaceae evaluated with rbcLdata. Plant Systematics and Evolution 202: 153-176.
- Simões AO & Kinoshita LS (2002) The Apocynaceae s.str of the Carrancas region, Minas Gerais, Brazil. Darwiniana 40: 127-169.
- Simões AO, Livshultz T, Conti E & Endress ME (2007) Phylogeny and systematics of the Rauvolfioideae (Apocynaceae) based on molecular and morphological evidence. Annals of the Missouri Botanical Garden 94: 268-297.
- Simões AO, Kinoshita LS, Koch I, Silva MJ & Endress ME (2016) Systematics and character evolution of Vinceae (Apocynaceae). Taxon 65: 99-122.
- Soares Neto RL & Jardim JG (2015) Capparaceae no Rio Grande do Norte, Brasil. Rodriguésia 66: 847-857.
- Soares AS, Pastore JFB & Jardim JG (2017) New records, conservation assessments and distribution of Lamiaceae in Rio Grande do Norte, northeastern, Brazil. Phytotaxa 311: 043-056.
- Soares AS, Pastore JFB & Jardim JG (2019) Lamiaceae no Rio Grande do Norte, Brasil. Rodriguesia 70: 02-17.
- SOS Mata Atlântica & INPE (2018) Atlas dos remanescentes florestais da Mata Atlântica, Período 2016-2017, relatório técnico. São Paulo. Available at <http://mapas.sosma.org.br/site_media/download/Atlas_Mata_Atlantica_2016-2017_relatorio_tecnico_2018_final.pdf> . Access on 8 May 2020.
» http://mapas.sosma.org.br/site_media/download/Atlas_Mata_Atlantica_2016-2017_relatorio_tecnico_2018_final.pdf - Souza-Silva RF (2008) Estudos taxonômicos em Apocynaceae, com ênfase na Flora da Bahia. Trabalho de conclusão do curso de Bacharel em Ciências Biológicas. Universidade Estadual de Feira de Santana, Feira de Santana. 149p.
- Spina AP (2004) Estudos taxonômico, micro-morfológico e filogenético do gênero Himatanthus Willd. ex Schult. (Apocynaceae: Rauvolfioideae - Plumerieae). Tese de Doutorado. Instituto de Biologia. Universidade Estadual de Campinas, Campinas. 191p.
- Stevens PF (2001 onwards) Angiosperm phylogeny website version 12, July 2012 [and more or less continuously updated since]. Available at <http://www.mobot.org/MOBOT/research/APweb/welcome.html>. Access on 6 February 2019.
» http://www.mobot.org/MOBOT/research/APweb/welcome.html - Stevens WD (2009) Funastrum. In: Davidse G, Sánchez MS, Knapp S & Cabrera FC (eds.) Flora Mesoamericana - Cucurbitaceae a Polemoniaceae 4(1), i-xvi. Missouri Botanical Garden, Saint Louis. Pp. 1-855.
- Tabarelli M & Santos AMM (2004) Uma breve descrição sobre a história natural dos brejos nordestinos. In: Porto KC, Cabral JJP & Tabarelli M (orgs.) Brejos de altitude em Pernambuco e Paraíba: história natural, ecologia e conservação. Série Biodiversidade, 9. Cap. 2. Ministério do Meio Ambiente, Brasília. Pp. 17-24.
- Thiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at <http://sweetgum.nybg.org/science/ih/> . Access on 7 april 2019.
» http://sweetgum.nybg.org/science/ih/ - Vasconcellos MB & Gouvea LSK (1993) As Apocynaceae da região de Poços de Caldas, Minas Gerais, Brasil. Acta Botanica Brasilica 7: 107-127.
- Versieux LM, Magalhães R & Calvente A (2013a) Extension of the Cryptanthus range in Northeastern Brazil with new findings in the phenotypic variation including changes in the trichome’s distribution, thus enhancing the understanding of the Cryptanthus zonatus complex (Bromeliaceae). Phytotaxa 109: 54-60.
- Versieux LM, Tomaz EC & Jardim JG (2013b) New genus and species records of Bromeliaceae in the Caatinga of Rio Grande do Norte state, northeastern Brazil: Orthophytum disjunctum L.B. Sm. (Bromelioideae) and Tillandsia paraibensis R.A. Pontes (Tillandsioideae). Check List 9: 663- 665.
- Viana SS, Santos JUM & Simões AO (2017) Taxonomic diversity of Apocynaceae at the Marajó Island, PA, Brazil. Rodriguesia 68: 623-652.
- Watanabe MCT, Roque N & Rapini A (2009) Apocynaceae sensu strictum no Parque Municipal de Mucugê, Bahia, Brasil, incluindo a publicação válida de dois nomes em Mandevilla Lindl. Iheringia 64: 63-75.
List of exsiccatae
Amorim AMA 1631 (7), 1447 (9). Andrade-Lima D 54-1896 (7). Barbeiro SMC 2521 (19). Barbosa JED 10 (24). Batista DO UFRN 15825 (11). Bitencourt MAO 3 (5). Caroline BF UFRN 17525 (12). Carvalho AMV 1084 (2). Cestaro LA 125 (5), 38 (12), 67 (24), 115 (24). Costa AMG 1 (5). Costa-Lima JL 567 (9), 320 (23). Dantas A 42 (8), 96 (24). Davi AC 26 (1). Félix LP 4733 (8). Ferreira TR 2 (17). Fotius G 3791 (14). Gallindo F CFPE695 (15). Gomes de Lima JE 247 (15). Grupo Pedra do Cavalo 358 (8). Harley RM 54559 (18). Jardim JG 6152 (1), 6371 (3), 6368 (4), 6657 (4), 5865 (6), 6741 (7), 6759 (13), 6752 (14), 5977 (14), 6332 (14), 6060 (18), 5968 (19), 6672 (21), 5735 (22), 6537 (23). Jesus NG 441 (7). Loiola MIB 1171 (12). Loureiro DM 186 (2). Mamede M 5 (8). Marinho AM 22 (19), 163 (23). Matos E 2650 (7). Mattos Silva LA 730 (9). Melo E 9602 (16). Mizael WR 38 (1). Morais AK 22 (11). Mori SA 10954 (7). Moura EO 247 (11), 22 (16). Noblick LR 3388 (9). Oliveira ACP 965 (12). Oliveira OF 1348 (12), 1465 (15), 1406 (15), 1695 (15), 1834 (16), 1052 (22). Projeto Parque das Dunas 44 (24). Queiroz LP 733 (10). Queiroz RT 396 (22). Rapini A 1070 (18), 1243 (20). Ribeiro A 174 (1), 61 (14), 240 (15). Roque AA 897 (1), 1121 (6), 860 (15), 1340 (17), 1356 (20), 1456 (22), 912 (22), 1038 (24). CGG5813 SP (14). UFRN2174 UFRN (24). Sena VRR 187 (12). Silva MAP HCDAL 1633 (15). Siqueira-Filho JA 1742 (23). Soares Neto RL 75 (13). Sousa Jr JC 46 (1), 63 (2), 41 (6), 65 (6), 64 (10), 33 (11), 42 (11), 61 (11), 30 (19), 47 (19), 51 (19). Sousa MB 143 (24). Souza FSR 10 (19). Tavares S 111 (17). Torres DF 95 (5). Versieux LM 534 (5), 606 (22).
Edited by
Publication Dates
-
Publication in this collection
27 Sept 2021 -
Date of issue
2021
History
-
Received
07 Oct 2019 -
Accepted
28 July 2020