Abstract
Moringa oleifera is an arboreal plant belonging to the family Moringaceae distributed in tropical areas and has gained enormous attention in the last decades. This research is a review on the association between aqueous extracts of M. oleifera leaves and diabetes mellitus and understanding its pharmacological functions and underlying mechanisms. The research refinement demonstrated the pharmaceutical potential of M. oleifera and its phytochemicals, given its antidiabetic effect. The prospective analysis showed the amount of application within IPC A61K in health area. The secondary metabolites present in M. oleifera, glucosinolates, flavonoids, and phenolic compounds may be responsible, in part, for the disease control hypoglycemic actions. Glucosinolates, when metabolized by salivary enzymes, give rise to sulforaphanes that act in preventing type 2 diabetes and in reducing insulin resistance. Flavonoids interact with intestinal enzymes by modifying carbohydrate metabolism by regulating glycemic levels, in addition to increasing insulin sensitivity. Phenolic compounds increase the expression of glucose transporters (GLUT4) and reduce the synthesis of fatty acids and cholesterol, contributing to the reduction of glucose resistance and blood sugar control. Moringa oleifera can be used as complementary therapy of the type-2 diabetes.
Keywords:
diabetes; hypoglycemic activity;
Moringa oleifera
; phytochemicals; technological prospecting
Resumo
Moringa oleífera Lam. é uma planta arbórea pertencente à família Moringaceae distribuída em áreas tropicais e que tem ganhado enorme atenção nas últimas décadas. Esta pesquisa é uma revisão sobre a associação entre extratos aquosos de folhas de M. oleifera e diabetes mellitus e compreender suas funções farmacológicas e mecanismos subjacentes. O refinamento da pesquisa demonstrou o potencial farmacêutico da M. oleifera e seus fitoquímicos, dado seu efeito antidiabético. A análise prospectiva mostrou a quantidade de aplicação dentro do IPC A61K na área da saúde. Os metabólitos secundários presentes em M. oleifera, glucosinolatos, flavonóides e compostos fenólicos podem ser responsáveis, em parte, pelas ações hipoglicemiantes de controle da doença. Os glucosinolatos, quando metabolizados por enzimas salivares, dão origem a sulforafanos que atuam na prevenção do diabetes tipo 2 e na redução da resistência à insulina. Os flavonóides interagem com as enzimas intestinais modificando o metabolismo dos carboidratos, regulando os níveis glicêmicos, além de aumentar a sensibilidade à insulina. Os compostos fenólicos aumentam a expressão dos transportadores de glicose (GLUT4) e reduzem a síntese de ácidos graxos e colesterol, contribuindo para a redução da resistência à glicose e controle da glicemia. Moringa oleifera pode ser usada como terapia complementar do diabetes tipo 2.
Palavras-chave:
diabetes; atividade hipoglicêmica;
Moringa oleifera
; fitoquímicos; prospecção tecnológica
Introduction
Plant species used as medicines usually occupy a predominant position in the results of botanical and ethnobotanical investigations of a determined region or ethnic group ( Pasa et al.2010 Pasa MC, Silva GG, Souza SS & Gonçalves KG (2010) Abordagem etnobotânica de Moringa oleifera Lam.: do cultivo ao uso da espécie em Rondonópolis. Boletim FLOVET 1: 1-18. Available at < https://periodicoscientificos.ufmt.br/ojs/index.php/flovet/article/view/648>. Access on 15 October 2020.
https://periodicoscientificos.ufmt.br/oj...
). The last two decades, chemical and pharmacological studies on bioactive substances derived from plants have had a major boost. The chemical and pharmacological researches aim to obtain new compounds with therapeutic and/or nutritional properties ( Araújo-Leonídio et al. 2019 Araújo-Leonídio AR, Almeira ADES, Freire Filha LG & Andrade MA (2019) Atividade antimicrobiana de Moringa oleifera Lam. Revista Gestão & Tecnologia 1: 4-15.).
Moringa oleifera Lam. is an arboreal plant distributed in tropical areas ( Faizi et al. 1994 Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Khalid Aftab K & Gilani AH (1994) Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. Journal of Natural Products 57: 1256-1261.; Bennett et al. 2003 Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L & Kroon PA (2003) Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. Journal of Agriculture and Food Chemistry 51: 3546-3553.), stands out due to its wide diversity of features. This plant is grown worldwide, mainly in places with dry tropical climates ( Estrada-Hernández et al. 2016 Estrada-Hernández O, Hernández-Rodríguez OA & Guerro-Prietro VM (2016) Múltiples formas de aprovechar los beneficios de moringa (Moringa oleifera Lam.). Tecnociencia Chihuahua X: 101-108.). The species adapts to different climatic conditions ( Falowo et al. 2018 Falowo AB, Mukumbo FE, Idamokoro EM, Lorenzo JM, Afolayan AJ & Muchenjev V (2018) Multi-functional application of Moringa oleifera Lam. in nutritionand animal food products: a review. Food research international 106: 317-334.) and to semiarid soils ( Lorenzi & Matos 2008Lorenzi H & Matos FJ (2008) Plantas medicinais no Brasil: nativas e exóticas cultivadas. 2ª ed. Ed. Plantarum, Nova Odessa. 576p.; Olson & Fahey 2011Olson ME & Fahey JW (2011) Moringa oleifera: un árbol multiusos para las zonas tropicales secas. Revista Mexicana de Biodiversidad 82: 1071-1082.), typical, for example, of the northeastern hinterland in semi-arid area from Brazil ( Bakke et al. 2010 Bakke IA, Souto JS, Souto PC & Bakke AO (2010) Características de crescimento e valor forrageiro da moringa (Moringa oleifera lam.) Submetida a diferentes adubos orgânicos e intervalos de corte. Engenharia Ambiental: Pesquisa e Tecnologia 7: 133-144.; Gualberto et al. 2014 Gualberto AF, Ferrari GM, Ferrari KMDEA, Preto BDEL & Ferrari JL (2014) Características, propriedades e potencialidades da moringa (Moringa oleifera Lam.): aspectos agroecológicos. Revista verde 9: 19-25.).
Moringa oleifera has been extensively studied due to its chemical and biological properties. Hence, there is a strong appeal for its cultivation, use and rational consumption ( Gualberto et al. 2014 Gualberto AF, Ferrari GM, Ferrari KMDEA, Preto BDEL & Ferrari JL (2014) Características, propriedades e potencialidades da moringa (Moringa oleifera Lam.): aspectos agroecológicos. Revista verde 9: 19-25.; Tshabalala et al. 2019 Tshabalala T, Ncube B, Madala NE, Nyakudya TT, Moyo HP, Sibanda M & Ndhlala AR (2019) Scribbling the cat: a case of the “miracle” plant, Moringa oleifera. Plants 8: 510.).
This plant is fast growing, with varied applications in agriculture, medicine, livestock, and in biological systems including the human body ( Ndubuaku et al. 2015 Ndubuaku UM, Uchenna NV, Baiyeri KP & Ukonze J (2015) Anti-nutrient, vitamin and other phytochemical compositions of oldand succulent Moringa (Moringa oleifera Lam.) leaves as influenced by poultry manure application. African Journal of Biotechnology 14: 2502-2509.). Its leaves, flowers, pods, and seeds have nutritional value, and all parts of the plant have medicinal value ( Santos 2014Santos B, Santos E, Gualbert N, Barretto L, Santos J & Silva G (2014) Formulação de chá gelado a base de flor de moringa (Moringa oleifera Lam.): estúdio de aceptabilidade. V Encontro Nacional de Moringa. Universidade Estadual de Maringá, Maringá. 9p. Available at < https://www.academia.edu/13019069/FORMULA%C3%87%C3%83O_DE_CH%C3%81_GELADO_A_BASE_DE_FLOR_DE_MORINGA_MORINGA_OLE%C3%8DFERA_LAM_ESTUDO_DE_ACEITABILIDADE>. Access on 15 October 2020.
https://www.academia.edu/13019069/FORMUL...
). These parts are used in the treatment of diseases and in the production of drugs against bacteria, fungi, viruses, and other pathogens in humans ( Falowo et al. 2018 Falowo AB, Mukumbo FE, Idamokoro EM, Lorenzo JM, Afolayan AJ & Muchenjev V (2018) Multi-functional application of Moringa oleifera Lam. in nutritionand animal food products: a review. Food research international 106: 317-334.). Regarding nutrient composition, recent studies have shown that its leaves, seeds, and stems are rich in proteins, essential amino acids, minerals, vitamins, and other bioactive compounds ( Moyo et al. 2012a Moyo B, Masika PJ & Muchenje V (2012a) Antimicrobial activities of Moringa oleifera Lam leaf extracts. African Journal of Biotechnology 11: 2797-2802., bMoyo B, Masika PJ & Muchenje V (2012b) Effect of supplementing cross bred X hosalop-earedgoatcastrates with Moringa oleifera leaves on growth performance, carcassand non-carcasscharacteristics. Tropical Animal Health and Production 44: 801-809.; Valdez-Solana et al. 2015 Valdez-Solana MA, Mejía-García VY, Téllez-Valencia A, García-Aarenas G, Salas-Pacheco J, Alba-Romero JJ & Sierra-Campos E (2015) Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. Journal of Chemistry 1: 9.).
When studying this species, Ruiz et al. (2012 Ruiz RB, Odio RMR & Carrion MEB (2012) Moringa oleifera: una opción saludable para el bienestar; Moringa oleifera: a healthyoption for thewell-being. Medisan 16: 1596-1599. Available at < https://pesquisa.bvsalud.org/portal/resource/pt/cum-51901>. Access on 15 October 2020.
https://pesquisa.bvsalud.org/portal/reso...
) highlighted the following non-clinical and clinical pharmacological evidences: cardiac and circulatory stimulant, antitumor, antipyretic, antiepileptic, antispasmodic, diuretic, hepatoprotective. Other studies show improved vision, mental alertness, and bone strength. It has potential benefits in malnutrition and general weakness, also improving the health of lactating mothers, and menopause, depression, and osteoporosis outcomes ( Kuete 2017Kuete V (2017) Medicinal spices and vegetables from Africa: therapeutic potential against metabolic, inflammatory, infectious and systemic diseases. Academic Press. Pp. 485-496.). It is worth noting that its leaves were used to combat malnutrition, especially among babies ( Moyo et al. 2011 Moyo B, Masika PJ, Hugo A & Muchenje V (2011) Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology 10: 12925-12933.).
Studies of glucosinolates and isothiocyanates isolated M. oleifera show in vitro and preclinical studies antibacterial activity, antioxidant and anti- inflammatory response ( Fahey et al. 2018 Fahey JW, Olson ME, Stephenson KK, Wade KL, Chodur GM, Odee D, Nouman W, Massiah M, Alt J, Egner PA & Hubbard WC (2018) The diversity of chemoprotective glucosinolates in Moringaceae (Moringa spp.). Scientific Reports 8: 7994.; Jaja-Chimedza et al. 2017 Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF & Raskin I (2017) Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. Plos One 12: e0182658.; Kim et al. 2017 Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP & Raskin I (2017) Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. Plos One 12(9): e0184709.; Waterman et al. 2014 Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, Lila MA & Raskin I (2014) Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 103: 114-122.). Other scientific studies using M. oleifera have also been shown to be an alternative in chronic diseases such as arthritis, cardiovascular disease, anti-hyperlipidemic, anti-ulcer, regulating blood glucose levels, anti-hyperglicemic, and type-2 diabetes ( Kim et al. 2018 Kim Y, Jaja-Chimedza A, Merrill D, Mendes O & Raskin I (2018) A 14-day repeated-dose oral toxicological evaluation of an isothiocyanate-enriched hydro-alcoholic extract from Moringa oleifera Lam. seeds in rats. Toxicology Reports 5: 418-426.; Kou et al. 2018 Kou X, Li B, Olayanju JB, Drake JM & Chen N (2018) Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 10: 343.; Gupta et al. 2012 Gupta R, Manthur M, Bajaj VK, Katariya P, Yadav S, Kamal R & Gupta RS (2012) Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. Journal of Diabetes 4: 164-171.; John & Chellappa 2005John S & Chellappa AR (2005) Hypoglycemic effect of Moringa oleifera (drumstick) leaves on human diabetic subjects and albino rats. The Indian Journal of Nutrition and Dietetics 42: 22-29.). Many of these properties are related to antioxidant compounds such as flavonoids, phenolic compounds, carotenoids, tocopherols, glucosinolates and isothiocyanates which play an important role in controlling oxidative stress in chronic diseases ( Saucedo-Pompa et al. 2018 Saucedo-Pompa S, Torres-Castillo JA, Castro-López C, Rojas R, Sánchez-Alejo EJ, Ngangyo-Heya M & Martínez-Ávila GCG (2018) Moringa plants: bioactive compounds and promising applications in food products. Food Research International 111: 438-450.; Fahey et al. 2018 Fahey JW, Olson ME, Stephenson KK, Wade KL, Chodur GM, Odee D, Nouman W, Massiah M, Alt J, Egner PA & Hubbard WC (2018) The diversity of chemoprotective glucosinolates in Moringaceae (Moringa spp.). Scientific Reports 8: 7994.; Jaja-Chimedza et al. 2017 Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF & Raskin I (2017) Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. Plos One 12: e0182658.; Maizuwo et al. 2017 Maizuwo AI, Hassan AS, Momoh H & Muhammad JA (2017) Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): a review. Journal of Drug Design and Medicinal Chemistry 3: 60-66.).
In view of all the positive qualities mentioned above, M. oleifera has shown great prominence due to its numerous benefits. Therefore, it has attracted interest for research on its use, mainly on its efficiency for health. This article is a technological, phytochemical, and pharmacological survey on the hypoglycemic activity of aqueous extracts of M. oleifera.
Material and Methods
This is a prospective study conducted in patents and scientific databases from July to September 2020. First, the searches were carried out in follows patent databases: a) WIPO PatentScope; b) National Institute of Industrial Property (INPI); c) United States Patent and Trademark Office (USPTO); d) The Lens - Free & Open Patent and Scholarly Search; e) Espacenet; and f) Questel Orbit Intelligence. In each patent database, the term “ Moringa oleifera” was used in combination with one of the following descriptors: antioxidant, antimicrobial, antibacterial, anti- inflammatory, anticancer, antifungal, hypertension, neuroprotective, antiepileptic, coronavirus, zebrafish, alkaloids, flavonoids, terpenoids, Alzheimer, diabetes, isothiocyanate, glucosinolates, oil and extraction methods. A thorough and detailed analysis of the patent documents “ Moringa oleifera” AND “Diabetes” was carried out.
The temporal and quantitative search was refined and limited to the following fields: title, summary, and claims, using the Boolean operator AND to combine the terms. In the INPI patent database, the descriptors in Portuguese were used ( Tab. 1).
The searches for scientific articles were conducted in the following databases: Science direct, Scielo, Nature, Springer, BMC, Wiley and Pubmed. These searches considered articles that had the descriptors in the title, abstract, and keywords fields, being published from 2000 to 2020. The same terms and Boolean operated used to perform patent searches were also used to perform scientific article searches. The temporal and quantitative search was refined and limited to the following fields: title and abstract. All quantitative research results were analyzed and no filters were performed to prioritize extract type, solvent type or particular type of plant part.
Results and Discussion
Prospecting resulted in 3,594 patents distributed in the search databases WIPO (610 patents), INPI (5 patents), USPTO (447 patents), The Lens (700 patents), Espacenet (765 patents), and Questel Orbit Intelligence (1,067 patents). The Brazilian database INPI presented only five patents, demonstrating that technology in the use of M. oleifera is not a priority in the country. The Questel Orbit Intelligence data platform provided 1,067 patents involving all the cited descriptors, and in all descriptors searched showing the greater number of results in relation the others. This validates the choice of this database for the analysis of studies. Correlating M. oleifera with diabetes resulted in 79 patents.
China is the country that produces the most patents ( Fig. 1) of products with M. oleifera, which demonstrates the advance of research and the use of technology involving this plant, in addition to a wide knowledge based on Traditional Chinese Medicine and the culture of a people to make use of plants and other natural products to treat diseases. This stems from their traditional medicinal culture, which dates back to around 2,500 BC and is based on the use of plants with curative powers to treat diseases that affect society over time ( Schenkel et al. 2003 Schenkel EP, Gosman G & Petrovick PR (2003) Produtos de origem vegetal e o desenvolvimento de medicamentos. In: Simões CMO (eds.) Farmacognosia: da planta ao medicamento. 5a. ed. Ed. UFSC, Porto Alegre. 833p.).
Patent publications by countries per year between 2000 and 2020. Abbreviations by country. CN = China; WO = World; US = United States of America; IN = India; EP = Europe; BR = Brazil; AU = Austria; CA = Canada; JP = Japan; KR = Korea; PH = Phillipines; FR = France; MX = Mexico; TW = Taiwan; CL = Chile; CO = Colombia; ES = Spain; IL = Israel; OA = African Intellectual Property Organization; UY = Uruguay. Source: Questel Orbit Intelligence 2020Questel. Orbit Intelligence. In: Orbit Intelligence. 10 sep. 2020. Available at < https://www.questel.com/ip-intelligence-software/orbit-intelligence/>. Access on 9 nov. 2022
https://www.questel.com/ip-intelligence-... .
The chart shows an increase in publications between the years 2013 and 2018, with 108 documents published. However, this number decreased in the subsequent year, with only 4 publications. The year 2020, until the time of this research, presented 1 document. This fact may be due to the new COVID-19 pandemic, where attention is focused on the discovery of a vaccine. This chart helps in identifying possible target markets for this technology, and highlights the need for investments in research on M. oleifera in Brazil to conduct a double blind and randomized clinical study to prove its effectiveness and safety and then make its pharmacological properties better known for a possible commercialization of the plant in the country.
The search for diabetes and Moringa oleifera ( Fig. 2) in the Questel Orbit® database resulted in 79 patents, among which 15 patents were assigned to A61K36 (medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g., traditional herbal medicines).
Concepts grouped for the search term “ Moringa oleifera and diabetes”. Source: Questel Orbit Intelligence 2020Questel. Orbit Intelligence. In: Orbit Intelligence. 10 sep. 2020. Available at < https://www.questel.com/ip-intelligence-software/orbit-intelligence/>. Access on 9 nov. 2022
https://www.questel.com/ip-intelligence-... .
The 15 patents mentioned were: a functional food based on M. oleifera and chaya ( Cnidoscolus aconitifolius), with biological properties, used as an aid in the control of diabetes and its complications, as well as for other types of circulatory and gastric disorders (MX2018004530); a composition containing Moringa oleifera for the treatment of hyperglycemia and hyperlipidemia (CN105833109); a technological method for oil extraction of Moringa oleifera seed (CN110856518); powders from Anacardium occidentale, Moringa oleifera, Sclerocarya birrea, and Prosopis africana extracts for the treatment of diabetes-related diseases (WO 02/94299); a pharmaceutical product obtained from M. oleifera for the treatment of diabetes-related diseases; a herbal medicine for the treatment of diabetes- related diseases (OA11761); a herbal formulation with high concentrations of polyphenols, having antioxidant, anti-inflammatory, antiviral, and microbial properties, for the control of diabetes and cholesterol and for the regulation of the immune system (WO2018/220428); synergistic herbal compositions combining extracts, fractions or phytochemicals, or mixtures derived from Moringa oleifera, Murraya koenigii, and Curcuma longa for the purpose of increasing lean body mass and brown adipose tissue (accelerating its conversion from white adipose tissue), and inhibiting excessive generation of advanced glycation products and related pathological conditions (WO2019/ 077629); a dietary supplement comprising 65–85 parts of a M. oleifera extract, 5–12 parts of hydroxytyrosol, 5–12 parts of oleuropein, and 5–11 parts of piperine, which may include a nutraceutical carrier with antioxidant and anti- inflammatory properties and can be used in the preparation of medicines and health products (CN107518399); a dietary supplement comprising 80–90 parts of M. oleifera extract and 10–20 parts of piperine, which may also comprise carriers and/ or nutraceutical and/or pharmaceutical adjuvants to compose oral dosage forms, the supplement having excellent antioxidant and anti-inflammatory effects and possibility of use in the preparation of medicines and health products (CN107456470); a dietary supplement consisting of hydroxytyrosol, oleuropein, piperine, and moringa oil extracts, with antioxidant and anti-inflammatory effects, which can be used in the manufacture of medicines (CN107518399); a formulation based on medicinal herbs, comprising M. oleifera, (malunggay), Capsicum frutescens, (cayenne pepper), Siling labuyo (kolikot), and Azadirachta indica (neem), used to control diabetes, decrease hypertension, balance heart and prostate conditions, eliminate infection conditions, and improve the immune system (PH12013000032); a nutritional powder for the adjuvant treatment of diabetes, hypertension, cardiovascular disease, obesity, low immunity, and neoplasia, prepared from the leaves, flowers, fruits, seeds, and branches of M. oleifera through their respective spraying and mixing (CN1903232); a traditional Chinese medicine and a health product to balance metabolism to radically treat early type II diabetes (CN107802770).
Prospecting showed that the search for patents with the term diabetes was concentrated in the pharmaceutical area, followed by the areas of food chemistry, fine organic chemistry, polymers, biotechnology, analysis of biological materials, and chemical analysis, measurement and chemistry of materials. These data suggest that the genus M. oleifera can be applied in the medical-therapeutic area as a nutraceutical in complementary and alternative medicine. The number of Chinese patents demonstrated its potential use. However, in Brazil, ANVISA Resolution No. 1478, of June 3, 2019, prohibits the marketing, distribution, manufacturing, import and advertising of all foods containing M. oleifera due to the lack of scientific studies to prove the safety of Moringa oleifera in foods with therapeutic claims to treat cancer, depression, epilepsy, treatment of diabetes and cardiovascular disease. Nevertheless, data from the technological and scientific databases presented in this study demonstrate that M. oleifera has potential biological activities as an antioxidant and antidiabetic.
Therefore, it is also necessary to meet the requirements of ANVISA’s public notice 07/2019, which establishes an exhaustive search to collect data and information on the safety of use of M. oleifera in food, including data on evidence of adverse reactions, safety of use based on randomized controlled clinical trials conducted from clinical trials on M. oleifera. Other evidence of the history of use should be demonstrated from the combination of scientific evidence, historical records, official commercial information on production and sales during a given period. A history of use based on research data on food acquisition or consumption and documents published by international authorities, which attest to the consumption of food, by a given population, for two or more generations, under the proposed conditions.
Regarding patent status, the majority (64.1%) of the patents granted are active, which denotes their relative importance. This shows that there are possible practical applications for these products, including the involvement of large companies and universities that enable their maintenance.
The incidence of diabetes is increasing in the developing world, with an increase in the number of diabetes patients in younger age groups. Therapeutic management of diabetes without side effects remains a challenge. In response, there is a growing interest in evaluating herbal remedies, which are seen as less toxic ( Gupta et al. 2012 Gupta R, Manthur M, Bajaj VK, Katariya P, Yadav S, Kamal R & Gupta RS (2012) Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. Journal of Diabetes 4: 164-171.), but like as medicines, the herbal medicines also have side effects and possible interaction with drugs when intake in combinations, then its necessary clinical trials to proven the efficacy, safety and low risks of side effects during combined treatments.
Diabetes mellitus (DM) is a chronic metabolic disease, in which insulin production ceases, increasing blood glucose levels (hyperglycemia). This condition remains for long periods and can affect organs, nerves, and blood vessels ( Diabetes Brazilian Society 2019Diabetes Brazilian Society (2019) O que é diabetes? Mecanismo. Available at < https://www.diabetes.org.br>. Access on 09 October 2022.
https://www.diabetes.org.br...
). Studies show that M. oleifera leaves oil are a rich source of secondary metabolites, revealing various therapeutic or medicinal properties, with great antidiabetic potential. This explains the strong appeal for its cultivation, use, and rational consumption ( Gualberto et al. 2014 Gualberto AF, Ferrari GM, Ferrari KMDEA, Preto BDEL & Ferrari JL (2014) Características, propriedades e potencialidades da moringa (Moringa oleifera Lam.): aspectos agroecológicos. Revista verde 9: 19-25.). Lack of insulin at the metabolic level causes derangement of carbohydrates, fats, and proteins, eventually leading to a series of long-term microvascular and macrovascular complications ( Divi et al. 2012 Divi SM, Bellamkonda R & Dasireddy SK (2012) Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose fed insulin resistant and STZ induced diabetic wistar rats: a comparative study. Asian Journal of Pharmaceutical Clinical Research 5: 67-72.).
In view of the rich phytochemical profile of M. oleifera, advances in biotechnological techniques have enabled the generation of new paths aimed at improving the overall commercial value of this plant ( Gupta et al. 2018 Gupta S, Jain R, Kachhwaha S & Kothari SL (2018) Nutritionaland medicinal applicationsof Moringa oleifera Lam - review of current status and future possibilities. Journal of Herbal Medicine 11: 1-11.). Furthermore, the secondary metabolites contained therein are of high interest because of their medicinal value ( Saralaya et al. 2010 Saralaya MG, Patel P, Roy MPSP & Patel AN (2010) Research article antidiarrheal activity of methanolic extractof Moringa oleifera Lam roots in experimental animal models. International Journal of Pharmaceutical Research 2: 25-29.). The nutritional value of the plant stems from these numerous compounds being present in all its parts ( Mansour et al. 2019 Mansour M, Mohamed MF, Elhalwagi A, El-ITriby HA, Shawki HH & Abdelhamid IA (2019) Moringa peregrina leaves extracts induce apoptosis and cell cycle arresto the patocellular carcinoma. BioMed research international 2019: 13p.). Researchers have identified more than 200 compounds in its leaves, stem, roots, and seeds, which can be classified in groups as hydrocarbon ketones, fatty acids, alcohols, aldehydes, terpenes, and others ( Falowo et al. 2018 Falowo AB, Mukumbo FE, Idamokoro EM, Lorenzo JM, Afolayan AJ & Muchenjev V (2018) Multi-functional application of Moringa oleifera Lam. in nutritionand animal food products: a review. Food research international 106: 317-334.). Research on phytochemical sources, which demonstrates a variety of secondary metabolites in the chemical composition of various parts of M. oleifera by gas chromatography coupled with mass spectrometry, identified the following constituents: carotenoids, polyphenols, alkaloids, isothiocyanates, tannins, saponins, and oxalates. Secondary metabolites were also detected: phenolic acids, glucosinolates, and flavonoids ( Maghu et al. 2017 Maghu TK, Sharma A & Younis K (2017) Effect of drumstick leaves (Moringa oleifera) incorporation on quality of Khakhra. In: Ui-Islam S, plant-based natural products: derivatives and applications. Ed. Willey, New Jersey. Pp. 129-144.). Stohs & Hartman (2015Stohs SJ & Hartman MJ (2015) Review of the safety and efficacy of Moringa oleifera. Phytotherapy Research 29: 796-804.) also identified prominent phytochemicals: chlorogenic acid, rutin, kaempferol, myricetin, benzylamine, phenolic acids derivatives (gallic acid, 4-Isopropylbenzoic acid, and caffeic acid); nitriles glycosides: niaziminin and niazinin, while Faizi et al. (1994 Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Khalid Aftab K & Gilani AH (1994) Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. Journal of Natural Products 57: 1256-1261.) identified thiocarbamate glycosides: niaziminin A and B with hypotensive activity.
Phytochemical investigations of M. oleifera revealed the presence of 4-o-acetyl-α-1-rhamnopyranosyloxy) benzyl isothiocyanate, 4-(-l-rhamnopyranosyloxy) benzyl isothiocyanate, niazimicin, pterygospermin, isothiocyanate α-benzyl, and 4-(rhamnopyranosyloxy) benzyl glucosinolates in different part of plant including leaves, flowers, seeds, pods (drumsticks), roots, bark, gum and oil (from seeds) ( Fahey 2005Fahey JW (2005) Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. J Trees for Life Journal 1: 5. Available at < https://www.tfljournal.org/article.php/20051201124931586>. Access on 15 October 2020.
https://www.tfljournal.org/article.php/2...
). Bennett et al. (2003 Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L & Kroon PA (2003) Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. Journal of Agriculture and Food Chemistry 51: 3546-3553.) carried out an exhaustive study investigating the phytochemical composition of M. oleifera and M. stenopetala using samples from Africa and Central America, using different plant parts (leaves, seeds, branches, stem bark, roots) and with identification by LC-MS and with confirmations by analytical standard techniques. This phytochemical study demonstrated the presence of different phytochemicals such as glucosinolates [4-(RL-rhamnopyranosyloxy)-benzylglucosinolate, benzyl glucosinolate, monoacetyl isomers of this glucosinolate], flavonoids glycoside (quercetin-3-O-glucoside, quercetin-3-O-(6’-malonyl-glucoside), kaempferol- 3-O-glucoside, kaempferol-3-O-(6’-malonyl-glucoside), quercetin 3-O-rhamnoglucoside), chlorogenic acid derivatives (3-caffeoylquinic acid and 5-caffeoylquinic acid) and phenolics (flavonoids, anthocyanins, proanthocyanidins, and cinnamates). The extensive literature documenting the diversity of glucosinolate applications in biomedicine indicates a promising potential for use in future areas of research in: 1. Antibiotics, antifungal, and antiviral agents; 2. Prevention of biofilms in medical implants, catheters, and industrial equipment; 3. Nutritional additives with anticancer properties; 4. Advanced food packaging technology to improve the shelf life of food products ( Melrose 2019Melrose J (2019) The Glucosinolates: a sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 7: 62.).
Considering these functionalities, the bioactivity of M. oleifera has gained enormous attention in the last decade, which has led to the increasing exploration and understanding of its pharmacological functions and underlying mechanisms ( Biswas et al. 2012 Biswas SK, Chowdhury A, Das J, Roy A & Hosen SMZ (2012) Pharmacological potentials of Moringa Oleifera Lam.: a review. International Journal of Pharmaceutical Science and Research 3: 305-310.). When using Moringa parts to treat different human diseases. Sileshi et al. (2014 Sileshi T, Makonnen E, Debella A & Tesfaye B (2014) Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. Journal Coastal Life Medicine 2: 214-221.) demonstrated in vivo pharmacological evidences using animal model for 70% ethanol extracts of Moringa stenopetala leaves. The 70% crude ethanol extract and ethanol liquid-liquid subfraction presented antihyperglycemic effect at a dose of 500 mg/kg with significant reduction of blood glucose levels at 2h (53.44%) and 4.5h (46.34%) in diabetic mice.
Vásquez-León et al. (2017 Vásquez-León LA, Páramo-Calderón DE, Robles-Olvera VJ, Valdés-Rodríguez OA, Pérez-Vázquez A, García-Alvarado MA & Rodríguez-Jimenes GC (2017) Variation in bioactive compounds and antiradical activity of Moringa oleifera leaves: influence of climatic factors, tree age, and soil parameters. European Food Research and Technology 243: 1593-1608.) show that phytochemicals; glucosinolates, flavonoids, and phenolic acids; stand out with hypoglycemic effects, and there may be variations in the chemical composition of M. oleifera depending on the part of the plant, climate, and soil parameters.
Some phytochemical constituents found in the genus M. oleifera are considered responsible for hypoglycemic, delipidemic, antioxidant and anti-inflammatory activities, namely: flavonoids (quercetin, rutin, apigenin, kaempferol, similarin, apigetrin), phenolic acids (vanillic acid, gallic acid, salicylic acid), cinnamic acids (caffeic acid, ferulic acid, cinnamic acid), hydroxycinnamic acids (p-coumaric acid), hydroxycinnamic esters (chlorogenic acid), benzylamine-type alkaloids, moringine, in addition to glucosinolates and their active metabolites isothiocyanates, thiocyanates and nitriles ( Coskun et al. 2005 Coskun O, Kanter M, Korkmaz A & Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research 51: 117-123.; Bour et al. 2005 Bour S, Visentin V, Prevot D, Daviaud D, Saulnier-Blache JS, Guigne C, Valet P & Carpene C (2005) Effects of oral administration of benzylamine on glucose tolerance and lipid metabolism in rats. Journal of Physiology and Biochemistry 61: 371-379.; Prabhakar & Doble 2011aPrabhakar PK & Doble M (2011a) Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3T3-L1 adipocytes. Journal of Agricultural and Food Chemistry 59: 9844.; Amara et al. 2021 Amara I, Ontario ML, Scuto M, Lo Dico GM, Sciuto S, Greco V, Abid-Essefi S, Signorile A, Salinaro AT & Calabrese V (2021) Moringa oleifera protects SH-SY5Ycells from DEHP-induced endoplasmic reticulum stress and apoptosis. Antioxidants 10: 532.; Faizi et al. 1994 Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Khalid Aftab K & Gilani AH (1994) Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. Journal of Natural Products 57: 1256-1261.; Fuentes et al. 2015 Fuentes F, Paredes-Gonzalez X & Kong ANT (2015) Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Current Pharmacology Reports 1: 179-196.; Wang et al. 2017 Wang N, Yi WJ, Tan L, Zhang JH, Xu J, Chen Y, Qin M, Yu S, Guan J & Zhang R (2017) Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cellular & Developmental Biology - Animal 53: 554-563.; Ghosh et al. 1935 Ghosh S, Chopra NR & Dutt A (1935) Chemical examination of bark of Moringa pterygosperma. Indian Journal of Medical Research 22: 789.; Iffiú-Soltész et al. 2010 Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, Bour S, Woodley J, Milagro FI, Martinez JA, Valet P & Christian Carpéné C (2010) Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacological Research 61: 355-63.). The aurantiamide acetate is an unusual dipeptide derivative inhibited the secretion TNFα and IL-2 from lipopolysaccharide-stimulated peripheral blood lymphocytes in culture ( Sashidhara et al. 2009 Sashidhara KV, Rosaiah JN, Tyagi E, Shukla R, Raghubir R & Rajendran SM (2009) Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents. European Journal of Medicinal Chemistry 44: 432-436.). Tab. S1 (available on supplementary material < https://doi.org/10.6084/m9.figshare.21350460.v1>) shows some of these substances and their pharmacological activities in in vitro or preclinical studies.
Glucosinolates are a diverse group of secondary plant metabolites that are particularly abundant in cruciferous vegetables as Brassicaceae and are a heterogeneous group of sulfur and nitrogen containing glycosidic secondary metabolites and water-soluble found in M. oleifera ( Bennett et al. 2003 Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L & Kroon PA (2003) Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. Journal of Agriculture and Food Chemistry 51: 3546-3553.; Tshabalala et al. 2019 Tshabalala T, Ncube B, Madala NE, Nyakudya TT, Moyo HP, Sibanda M & Ndhlala AR (2019) Scribbling the cat: a case of the “miracle” plant, Moringa oleifera. Plants 8: 510.). However, these compounds are broken down during metabolism by plant enzymes known as myrosinases, or salivary enzymes that transform glucosinolates into isothiocyanates (ITCS), among them sulforaphane (SFN) (-)-[1-isothiocyanato-4-(methylsulfinyl)- butane], a natural compound that has antioxidant and anti-inflammatory properties, renoprotective and modulates the risk of type 2 diabetes (TD2) ( Fig. 3) ( Guerrero-Beltrán et al. 2010 Guerrero-Beltrán CE, Calderón-Oliver M, Tapia E, Medina Campos ON, Sa’nchez-Gonzalez DJ, Martinez-Martinez CM, Ortiz-Vega KM, Franco M & Pedraza-Chaverri J (2010) Sulforaphane protects against cisplatin-induced nephrotoxicity. Journal of Toxicologic Pathology 64: 503-508.).
The findings of preclinical studies and in vitro studies in Tab. S1 (available on supplementary material < https://doi.org/10.6084/m9.figshare.21350460.v1>) show that the aqueous extracts of Moringa oleifera and active constituents present in the aqueous extracts show that Moringa oleifera are considered safe for nutritional or therapeutic consumption at doses ≤ 1,000 mg/ kg/day ( Awodele et al. 2012 Awodele O, Oreagba IA, Odoma S, Silva JAT & Osunkalu VO (2012) Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). Journal of Ethnopharmacology 139: 330-336.; Asare et al. 2012 Asare GA, Gyan B, Bugyei K, Adjei S, Mahama R, Addo P, Otu-Nyarko L, Wiredu EK & Nyarko A (2012) Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. Journal of Ethnopharmacology 139: 265-272.; Adedapo et al. 2009 Adedapo AA, Mogbojuri OM & Emikpe BO (2009) Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. Journal of Medicinal Plants Research 3: 586-591.; Isitua & Ibeh 2013Isitua CC & Ibeh IN (2013) Toxicological assessment of aqueous extract of Moringa oleifera and Caulis Bambusae leaves in rabbits. Journal of Clinical Toxicology S12: 003.; Villarruel-López et al. 2018 Villarruel-López A, López-de la Mora DA, Vázquez-Paulino OD, Puebla-Mora AG, Torres-Vitela MR, Guerrero-Quiroz LA & Nuño K (2018) Effect of Moringa oleifera consumption on diabetic rats. BMC Complementary and Alternative Medicine 18: 127.).
Moringa oleifera and its secondary metabolites can act in the control of hypoglycemia, diabetes mellitus and metabolic syndrome through some pharmacological pathways, which were cited below in the next paragraphs.
Moringa oleifera aqueous extract and polyphenolic compounds (Phenolic acids, hydroxycinnamic acids, hydroxycinnamic esters and flavonoids: silymarin, quercetin, apigenin, apigetrin) can exert antioxidant action on hepatocytes and pancreatic cells by scavenging free radicals, decreasing oxidative stress by reactive oxygen species (ROS). Alteration of the cellular antioxidant defense system occurs by increasing the activity of antioxidant enzymes (SOD, CAT, GST, GSHPx) and decreasing lipid peroxidation by reducing levels of (LPO, MDA, NO), resulting in β-cell protection of the pancreas against oxidative stress and decreasing the hyperglycemia generated by ROS ( Ndong et al. 2007 Ndong M, Uhera M, Katsumata S & Suzuku K (2007) Effects of oral administration of Moringa oleifera Lam. on glucose tolerance in Goto-Kakizaki and wistar rats. Journal of Clinical Biochemistry Nutrition 40: 229-233.; Jaiswal et al. 2009 Jaiswal D, Kumar RAIP, Kumar A, Mehta S & Watal G (2009) Effect of Moringa oleifera Lam leaves aqueous extract therapy on hyperglycemic rats. Journal of Ethnopharmacology 123: 392-396., 2013Jaiswal D, Raia PK, Mehta S, Chatterji S, Shukla S, Rai DK, Sharma G, Sharma B, Khair S & Watal G (2013) Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pacific Journal of Tropical Medicine 6: 426-432.; Coskun et al. 2005 Coskun O, Kanter M, Korkmaz A & Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research 51: 117-123.; Sharma et al. 2008 Sharma M, Anwer T, Pillai K, Haque SE, Najmi A & Sultana Y (2008) Silymarin, a flavonoid antioxidant, protects streptozotocin-induced lipid peroxidation and β-Cell damage in rat pancreas. Oriental Pharmacy and Experimental Medicine 8: 146-153.; Wang et al. 2017 Wang N, Yi WJ, Tan L, Zhang JH, Xu J, Chen Y, Qin M, Yu S, Guan J & Zhang R (2017) Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cellular & Developmental Biology - Animal 53: 554-563.; Zhang et al. 2020 Zhang R, Shi J, Wang T, Qiu X, Liu R, Li Y, Gao Q & Wang N (2020) Apigetrin ameliorates streptozotocin-induced pancreatic β-cell damages via attenuating endoplasmic reticulum stress. In Vitro Cellular & Developmental Biology - Animal 56: 622-634.).
Moringa oleifera extract enriched with isothiocyanates and isothiocyanates significantly reduce inflammatory response which involves different signaling elements as cytokines (interleukins 1β and 6, IL-1β and IL-6, and TNF-α), nitric oxide (NO) produced by nitric oxide synthase (iNOS) during chronic inflammatory diseases. In addition, isothiocyanates, sufurophane and sufurophene upregulate the nuclear factor (erythroid-derived 2)-like 2 (NrF2), an important transcription factors, which activates multiple antioxidant and chemoprotective genes, including phase II detoxification enzymes such as NAD(P) H:quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO1) and glutathione S-transferase (GST) inhibiting inflammatory signaling ( Waterman et al. 2014 Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, Lila MA & Raskin I (2014) Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 103: 114-122., 2015Waterman C, Rojas-Silva P, Tumer TB, Kuhn P, Richard AJ, Wicks S, Sthephens JM, Wang Z, Mynatt R, Cefalu W & Raskin I (2015) Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Molecular Nutrition & Food Research 59: 1013-1024. < https://doi.org/10.1002/mnfr.201400679>
https://doi.org/10.1002/mnfr.201400679...
; Chen et al. 2018 Chen J, Bao C, Kim JT, Cho JS, Qiu S & Lee HJ (2018) Sulforaphene inhibition of adipogenesis via hedgehog signaling in 3T3-L1 adipocytes. Journal of Agriculture and Food Chemistry 66: 11926-11934. < https://doi.org/10.1021/acs.jafc.8b04330>
https://doi.org/10.1021/acs.jafc.8b04330...
; Jaja-Chimedza et al. 2017 Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF & Raskin I (2017) Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. Plos One 12: e0182658.; Cheng et al. 2019 Cheng D, Gao L, Su S, Sargsyan D, Wu R, Raskin I & Kong A-h N-g (2019) Moringa isothiocyanate activates Nrf2: potential role in diabetic nephropathy. The American Association of Pharmaceutical Scientistis Journal 21: 31. < https://doi.org/10.1208/s12248-019-0301-6>
https://doi.org/10.1208/s12248-019-0301-...
). The SFN mechanism ( Fig. 4) protects cells against oxidative damage, inducing phase 2 detoxification enzymes. Its mechanism of action is by activation of the nuclear factor E2 mediated by the transcription factor nuclear erythroid type 2 (Nrf2) ( Song et al. 2009 Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB & Park BH (2009) Sulforaphane protects against cytokineand streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicology and applied pharmacology 235: 57-67.; Cheng et al. 2019 Cheng D, Gao L, Su S, Sargsyan D, Wu R, Raskin I & Kong A-h N-g (2019) Moringa isothiocyanate activates Nrf2: potential role in diabetic nephropathy. The American Association of Pharmaceutical Scientistis Journal 21: 31. < https://doi.org/10.1208/s12248-019-0301-6>
https://doi.org/10.1208/s12248-019-0301-...
), which through breaks down from the complex forming together with protein 1 associated with Kelch type ECH (keap 1). Breaking out of the complex; SFN acts on the cell nucleus via the Antioxidant Response Element (ARE), modulating the gene expression of the antioxidant enzymes NADPH quinone oxidoreductase (NQO1), hemeoxygenase-1 (HO-1), and g-glutamylcysteine ligase (YGCL), also interacting with the mitogen-activated protein kinase (MAPK) pathway.
Sulfurophane also activates the transcription factor NrF2 during alcohol-induced hepatic steatosis and subsequently activate the heme oxygenase-1 promoting lowered oxidant stress by the decline in lipid peroxidation and decreased the accumulation of lipid in liver cells cultured in presence of ethanol ( Zhou et al. 2014 Zhou R, Lin J & Wu D (2014) Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochimica et Biophysica Acta 1840: 209-218.). Another important mechanism of isothiocyanates, sulfurophanes and sulfurophenes is related to anti-diabetic, anti-obesity activity, in the inhibition of adipogenesis. Isothiocyanates promote the reduction of insulin, leptin, resistin, cholesterol and hepatic glucose 6-phosphate ( Waterman et al. 2015 Waterman C, Rojas-Silva P, Tumer TB, Kuhn P, Richard AJ, Wicks S, Sthephens JM, Wang Z, Mynatt R, Cefalu W & Raskin I (2015) Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Molecular Nutrition & Food Research 59: 1013-1024. < https://doi.org/10.1002/mnfr.201400679>
https://doi.org/10.1002/mnfr.201400679...
).
Reports by Choi et al. (2014 Choi K-M, Lee Y-S, Kim W, Kim SJ, Kyong-Oh Shin K-O, Yu J-Y, Lee MK, Lee Y-M, Hong JT, Yun Y-P & Yoo H-S (2014) Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. Journal of Nutritional Biochemistry 25: 201-207.) demonstrated sulfurophanes decreased the expression of peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα) and leptin in the adipose tissue mice, in addition of increase adiponectin expression and subsequent attenuation of visceral adiposity, adipocyte hypertrophy, fat accumulation and triglyceride in the liver and serum total cholesterol.
The report by Eseberri et al. (2015 Eseberri I, Miranda J, Lasa A, Churruca I & Portillo MP (2015) Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxidative Medicine and Cellular Longevity 2015: 1-11. < http://dx.doi.org/10.1155/2015/480943>
http://dx.doi.org/10.1155/2015/480943...
) showed quercetin (0.5–10 µM) is able to inhibit the differentiation of preadipocytes into adipocytes by reducing C/EBPβ gene expression, SREBP1 mature protein levels, and PPARγ gene expression and reducing triacylglycerol (TGA), but it is concentrations greater than 10 µM are required to reduce TGA in the maturation phase of adipocytes. Report by Peng et al. (2018 Peng S-G, Pang Y-L, Zhu Q, Kang J-H, Liu M-X & Wang Z (2018) Chlorogenic acid functions as a novel agonist of PPAR-2 during the differentiation of mouse 3T3-L1 preadipocytes. BioMed Research International 2018: 1-14. < https://doi.org/10.1155/2018/8594767>
https://doi.org/10.1155/2018/8594767...
) has demonstrated that phenolic compounds, Chlorogenic acid (CGA), act strongly regulating the phase of differentiation of pre-adipocytes into adipocytes, by acting in the upregulated expression of the differentiation PPARγ2 and decreased intracellular triacylglycerol synthesis. Chlorogenic acid also acts in the upregulated expression of the lipolysis but cannot act in the lipid synthesis. Report by Chen et al. 2018 Chen J, Bao C, Kim JT, Cho JS, Qiu S & Lee HJ (2018) Sulforaphene inhibition of adipogenesis via hedgehog signaling in 3T3-L1 adipocytes. Journal of Agriculture and Food Chemistry 66: 11926-11934. < https://doi.org/10.1021/acs.jafc.8b04330>
https://doi.org/10.1021/acs.jafc.8b04330...
demonstrates that Sulfurophene (SE) is more effective than sulfurophane (SA) inhibiting adipogenesis in 3T3-L1 adipocytes by suppressing the PPARγ and CCAAT/enhancer-binding protein α (C/EBPα) reducing fat accumulation in 3T3-L1 adipocytes. SE inhibits adipocyte differentiation and may be an effective natural agent for preventing adipocyte hyperplasia and obesity. Sakuma et al. (2022 Sakuma S, Yasuda K, Kitahara R, Tsujimoto K, Yamashita K, Hoshino N, Fujimoto Y & Okuhira K (2022) Comparative effects of sulforaphane and allyl isothiocyanate on 3T3-L1 adipogenesis. Journal of Nutrition and Metabolism 2022: 1-8. < https://doi.org/10.1155/2022/8705163>
https://doi.org/10.1155/2022/8705163...
) by the same mechanism demonstrated that sulfurophane may be a more potent adipocyte differentiation inhibitor than allyl isothiocyanate demonstrating a possible strategy for the prevention of obesity and metabolic syndrome.
Flavonoids are of interest because of their importance in carbohydrate metabolism and glycemic homeostasis. They act by interacting with intestinal enzymes, inhibiting α-glycosidase, whose function is to degrade starches and carbohydrates, giving rise to glucose. They also act on α-amylase, inhibiting starch metabolism ( Cazarolli et al. 2008 Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MSRB, Folador P, Damazio RG, Pizzolatti MG & Silva FRMB (2008) Flavonoids: cellular and molecular mechanism of action in glucose homeostasis. Mini-Review in Medicinal Chemistry 8: 1032-1038.). According to Williamson (2013Williamson G (2013) Possible effects of dietary polyphenols on sugar absorption and digestion. Molecular nutrition & food research 57: 48-57.), the inhibition of Na+-dependent glucose transporters (SGLT-1) impairs glucose transport. Valle (2016Valle IFA (2016) Análise do efeito dos flavonoides na resposta glicêmica e insulinêmica: uma revisão de literatura. Trabalho de conclusão de curso. Universidade de Brasília, Brasília. 37p.) compiled data from studies on flavonoids and diabetes. The author observed that this secondary metabolite increases insulin sensitivity, evidencing the role of flavonoids in glycemic control since deregulation of postprandial sugar levels is an important indicator of glycemic disorders involving carbohydrate metabolism. The study also showed that the flavonoid quercetin and the drug glibenclamide have similar action, reinforcing the possible hypoglycemic effect ( Rodriguez de Sotillo & Hadley 2002Rodriguez de Sotillo DV & Hadley M (2002) Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. The Journal of Nutritional Biochemistry 13: 717-726.).
There are two important ways of activating the GLUT4 glucose transporter, which are PI3K and PPARγ. The PPARγ pathway includes Grb, SOS, Ras, Raf, and MAP kinase. In turn, the PI3K pathway comprises PDK, Akb, protein kinase B, and other mediators. Some commercial drugs act by increasing the expression of PPARγ and GLUT4. Activation of PPARγ by its agonist or by PI3K increases glucose uptake in 3T3-L1 adipocytes. PI3K is a signaling molecule in the insulin cascade which induces glucose uptake by the cell via the activation of GLUT4 translocation ( Prabhakar & Doble 2011bPrabhakar PK & Doble M (2011b) Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Advances in Endocrinology and Metabolism 2: 103114. DOI: 10.1177/2042018811411356.
https://doi.org/10.1177/2042018811411356...
).
Phenolic acids significantly increase the expression of GLUT4 and PI3K, whereas chlorogenic and cinnamic acids significantly affect the expression of the PPARγ gene. These hydroxycinnamic acid derivatives ( Fig. 5) act differently in the expression of the negative regulators of the insulin cascade. Thus, it can be assumed that hydroxycinnamic acid derivatives increase the uptake of 2-deoxy-d-glucose (2DG) mediated by PI3K-dependent translocation of GLUT4, while chlorogenic acid and cinnamic acid increase 2DG uptake through the translocation of GLUT4 via PPARγ. These secondary metabolites also significantly reduce the expression of fatty acid synthase (an enzyme that regulates the synthesis of fatty acids) and HMG-CoA reductase, which is a cholesterol-synthesis limiting enzyme. A reduction in the synthesis of fatty acids and cholesterol can reduce resistance to insulin, therefore being a strong contributor in the prevention of type 2 diabetes ( Hemmerle et al. 1997 Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, Paulus E & Herling AW (1997) Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. Journal of Medicinal Chemistry 40: 137-145.; Karthikesan et al. 2010 Karthikesan K, Pari L & Menon VP (2010) Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chemico-Biological Interactions 188: 643-650.; Prabhakar & Doble 2011bPrabhakar PK & Doble M (2011b) Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Advances in Endocrinology and Metabolism 2: 103114. DOI: 10.1177/2042018811411356.
https://doi.org/10.1177/2042018811411356...
).
Mechanism of action of hydroxycinnamic acid derivatives and commercial drugs. A = ferulic acid, eugenol, p-coumaric acid, cinnamic acid, caffeic acid and chlorogenic acid; B = chlorogenic acid, cinnamic acid; and C = thiazolidinedione; (+) denotes an increase and (-) denotes a decrease.
Phenolic acids increase glucose uptake by activating PI3K or PPARγ. Caffeic, chlorogenic, and cinnamic acids reduce the expression of the enzymes HMGCoA reductase and FAS, which play an important role in complications secondary to hypoglycemia ( Prabhakar & Doble 2011bPrabhakar PK & Doble M (2011b) Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Advances in Endocrinology and Metabolism 2: 103114. DOI: 10.1177/2042018811411356.
https://doi.org/10.1177/2042018811411356...
).
The alkaloid moringinine (a benzylamine) was initially purified from M. oleifera root bark ( Ghosh et al. 1935 Ghosh S, Chopra NR & Dutt A (1935) Chemical examination of bark of Moringa pterygosperma. Indian Journal of Medical Research 22: 789.). The early study using an intraperitoneal treatment with M. oleifera extracts which contain the alkaloid moringinine has been shown to prevent hyperglycemia response in alloxan-induced diabetic rats. Benzylamine treatment resulted in a decrease in plasma free fatty acids in both fed and fasted conditions. Benzylamine treatment improved glucose tolerance as shown by the reduction of hyperglycemic response to intra-peritoneal glucose load ( Bour et al. 2005 Bour S, Visentin V, Prevot D, Daviaud D, Saulnier-Blache JS, Guigne C, Valet P & Carpene C (2005) Effects of oral administration of benzylamine on glucose tolerance and lipid metabolism in rats. Journal of Physiology and Biochemistry 61: 371-379.). Preclinical study in mice rendered insulin-resistant when fed a High-Fat-Diet (HFD) and receiving or not benzylamine in their drinking water showed lower body weight gain and fasting blood glucose, lower total plasma cholesterol and hyperglycemic response to glucose load when compared to HFD control. In adipocytes, insulin-induced activation of glucose transport and inhibition of lipolysis remained unchanged ( Iffiú- Soltész et al. 2010 Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, Bour S, Woodley J, Milagro FI, Martinez JA, Valet P & Christian Carpéné C (2010) Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacological Research 61: 355-63.).
The controlled study with untreated diabetes type-2 patients examined the effect of M. oleifera addition to a standardized meal, taken after an overnight fast, affected the 1-h and 2-h post-prandial glucose levels (PPG), in relation to the standard meal alone oral glucose load. Only the M. oleifera leaf-supplemented meal elicited a lower response (-21%, P <0.01). Plasma insulin AUCs did not differ significantly between the two meals, suggesting that the hypoglycemic effect of M. oleifera leaf supplementation was not due to increased insulin secretion ( William et al. 1993 William F, Lakshminarayanan S & Chegu H (1993) Effect of some Indian vegetables on the glucose and insulin response in diabetic subjects. International Journal of Food Sciemce and Nutrition 44: 191-196.) (Tab. S2, available on supplementary material < https://doi.org/10.6084/m9.figshare.21350460.v1>).
A clinical study with a group of 60 diabetes mellitus type 2 patients. Patients in the experimental group were prescribed two M. oleifera leaf tablets/ day, one after breakfast, the other after dinner for 90 days. In the control group, Blood glycated haemoglobin (HbA1c) and post-prandial glucose levels (PPG) progressed downwardly with time, but the change was not significant. In the experimental group, in contrast, HbA1c decreased by 0.4% point (from 7.8 ± 0.5 to 7.4 ± 0.6; P<0.01). PPG in the experimental group progressively decreased with treatment duration, by 9% after 30 days, 17% after 60 days, and 29% after 90 days (P<0.01), indicating that M. oleifera medication can induce with time better glucose tolerance ( Ghiridhari et al. 2011 Ghiridhari VVA, Malhati D & Geetha K (2011) Anti-diabetic properties of drumstick (Moringa oleifera) leaf tablets. International Journal of Health & Nutrition 2: 1-5.) (Tab. S2, available on supplementary material < https://doi.org/10.6084/m9.figshare.21350460.v1>).
The hypoglycemic effect of M. oleifera leaf dietary consumption over a 40-day period in diabetes mellitus type 2 patients. The fasting plasma glucose levels and post-prandial glucose levels at the end of the protocol (final) were compared to baseline levels. They were significantly reduced in the experimental group (Fasting plasma glucose: FPG: -28%, P < 0.01; Post-prandial glucose, PPG: -26%, P < 0.05) ( Kumari 2010Kumari DJ (2010) Hypoglycemic effect of Moringa oleifera and Azadirachta indica in type-2 diabetes. Bioscan 5: 211-214.) (Tab. S2, available on supplementary material < https://doi.org/10.6084/m9.figshare.21350460.v1>).
The combination of herbal medicine that have phenolic acids with hypoglycemic properties with thiazolidinedione and metformin allows an increase in the hypoglycemic effects of these commercialized drugs. This increase, in turn, allows a reduction in their dose and, consequently, in their side and adverse effects. The action of phenolic acids in 2DG uptake is time and dose-dependent ( Prabhakar & Doble 2011bPrabhakar PK & Doble M (2011b) Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Advances in Endocrinology and Metabolism 2: 103114. DOI: 10.1177/2042018811411356.
https://doi.org/10.1177/2042018811411356...
).
The pharmacological properties of M. oleifera are studied worldwide, resulting in a vast patent production. This was reinforced by this technological prospecting that led to numerous patents, enabling the identification of applications within IPC A61K (medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g., traditional herbal medicines), with the most varied biological functions. Noteworthy, 79 of these patents correlated with diabetes. The study demonstrated the application of the plant mainly in the pharmaceutical area and secondly in food chemistry.
Among the secondary metabolites present, glucosinolates, flavonoids, and phenolic compounds have antidiabetic potential and can control hyperglycemic. Glucosinolates, when metabolized by salivary enzymes, give rise to sulforaphanes that act in preventing type 2 diabetes and in reducing insulin resistance. Flavonoids interact with intestinal enzymes by modifying carbohydrate metabolism by regulating glycemic levels, in addition to increasing insulin sensitivity. Phenolic compounds increase the expression of glucose transporters (GLUT4) and reduce the synthesis of fatty acids and cholesterol, contributing to the reduction of glucose resistance and blood sugar control.
Moringa oleifera has a significant antihyperglycemic action and can control the overweight. The preclinical and clinical data presented in the scientific literature using extracts and powder of the M. oleifera plant has shown a reduction in hyperglycemia and type-2 diabetes mellitus. The M. oleifera has shown to be a promising plant for the preparation of extracts, teas and herbal products for health, especially to be used as complementary therapy for the treatment of type-2 diabetes, combined or not with oral antihyperglycemic drugs and since that advised by doctors and other health professionals.
Acknowledgments
The authors thank CNPq, CAPES, FAPEAL, and UFAL, for the technical and financial support. The authors thank CEAPA and the Production and Value Chain in medicinal plants and herbal from Alagoas; and family farming cooperatives from INHAPI-AL.
- Adedapo AA, Mogbojuri OM & Emikpe BO (2009) Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. Journal of Medicinal Plants Research 3: 586-591.
- Amara I, Ontario ML, Scuto M, Lo Dico GM, Sciuto S, Greco V, Abid-Essefi S, Signorile A, Salinaro AT & Calabrese V (2021) Moringa oleifera protects SH-SY5Ycells from DEHP-induced endoplasmic reticulum stress and apoptosis. Antioxidants 10: 532.
- Araújo-Leonídio AR, Almeira ADES, Freire Filha LG & Andrade MA (2019) Atividade antimicrobiana de Moringa oleifera Lam. Revista Gestão & Tecnologia 1: 4-15.
- Asare GA, Gyan B, Bugyei K, Adjei S, Mahama R, Addo P, Otu-Nyarko L, Wiredu EK & Nyarko A (2012) Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. Journal of Ethnopharmacology 139: 265-272.
- Awodele O, Oreagba IA, Odoma S, Silva JAT & Osunkalu VO (2012) Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). Journal of Ethnopharmacology 139: 330-336.
- Bakke IA, Souto JS, Souto PC & Bakke AO (2010) Características de crescimento e valor forrageiro da moringa (Moringa oleifera lam.) Submetida a diferentes adubos orgânicos e intervalos de corte. Engenharia Ambiental: Pesquisa e Tecnologia 7: 133-144.
- Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L & Kroon PA (2003) Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. Journal of Agriculture and Food Chemistry 51: 3546-3553.
- Biswas SK, Chowdhury A, Das J, Roy A & Hosen SMZ (2012) Pharmacological potentials of Moringa Oleifera Lam.: a review. International Journal of Pharmaceutical Science and Research 3: 305-310.
- Bour S, Visentin V, Prevot D, Daviaud D, Saulnier-Blache JS, Guigne C, Valet P & Carpene C (2005) Effects of oral administration of benzylamine on glucose tolerance and lipid metabolism in rats. Journal of Physiology and Biochemistry 61: 371-379.
- Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MSRB, Folador P, Damazio RG, Pizzolatti MG & Silva FRMB (2008) Flavonoids: cellular and molecular mechanism of action in glucose homeostasis. Mini-Review in Medicinal Chemistry 8: 1032-1038.
- Chen J, Bao C, Kim JT, Cho JS, Qiu S & Lee HJ (2018) Sulforaphene inhibition of adipogenesis via hedgehog signaling in 3T3-L1 adipocytes. Journal of Agriculture and Food Chemistry 66: 11926-11934. < https://doi.org/10.1021/acs.jafc.8b04330>
» https://doi.org/10.1021/acs.jafc.8b04330 - Cheng D, Gao L, Su S, Sargsyan D, Wu R, Raskin I & Kong A-h N-g (2019) Moringa isothiocyanate activates Nrf2: potential role in diabetic nephropathy. The American Association of Pharmaceutical Scientistis Journal 21: 31. < https://doi.org/10.1208/s12248-019-0301-6>
» https://doi.org/10.1208/s12248-019-0301-6 - Choi K-M, Lee Y-S, Kim W, Kim SJ, Kyong-Oh Shin K-O, Yu J-Y, Lee MK, Lee Y-M, Hong JT, Yun Y-P & Yoo H-S (2014) Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. Journal of Nutritional Biochemistry 25: 201-207.
- Coskun O, Kanter M, Korkmaz A & Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research 51: 117-123.
- Diabetes Brazilian Society (2019) O que é diabetes? Mecanismo. Available at < https://www.diabetes.org.br>. Access on 09 October 2022.
» https://www.diabetes.org.br - Divi SM, Bellamkonda R & Dasireddy SK (2012) Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose fed insulin resistant and STZ induced diabetic wistar rats: a comparative study. Asian Journal of Pharmaceutical Clinical Research 5: 67-72.
- Eseberri I, Miranda J, Lasa A, Churruca I & Portillo MP (2015) Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxidative Medicine and Cellular Longevity 2015: 1-11. < http://dx.doi.org/10.1155/2015/480943>
» https://doi.org/10.1155/2015/480943 - Estrada-Hernández O, Hernández-Rodríguez OA & Guerro-Prietro VM (2016) Múltiples formas de aprovechar los beneficios de moringa (Moringa oleifera Lam.). Tecnociencia Chihuahua X: 101-108.
- Fahey JW (2005) Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. J Trees for Life Journal 1: 5. Available at < https://www.tfljournal.org/article.php/20051201124931586>. Access on 15 October 2020.
» https://www.tfljournal.org/article.php/20051201124931586 - Fahey JW, Olson ME, Stephenson KK, Wade KL, Chodur GM, Odee D, Nouman W, Massiah M, Alt J, Egner PA & Hubbard WC (2018) The diversity of chemoprotective glucosinolates in Moringaceae (Moringa spp.). Scientific Reports 8: 7994.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Khalid Aftab K & Gilani AH (1994) Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. Journal of Natural Products 57: 1256-1261.
- Falowo AB, Mukumbo FE, Idamokoro EM, Lorenzo JM, Afolayan AJ & Muchenjev V (2018) Multi-functional application of Moringa oleifera Lam. in nutritionand animal food products: a review. Food research international 106: 317-334.
- Fuentes F, Paredes-Gonzalez X & Kong ANT (2015) Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Current Pharmacology Reports 1: 179-196.
- Ghiridhari VVA, Malhati D & Geetha K (2011) Anti-diabetic properties of drumstick (Moringa oleifera) leaf tablets. International Journal of Health & Nutrition 2: 1-5.
- Ghosh S, Chopra NR & Dutt A (1935) Chemical examination of bark of Moringa pterygosperma. Indian Journal of Medical Research 22: 789.
- Gualberto AF, Ferrari GM, Ferrari KMDEA, Preto BDEL & Ferrari JL (2014) Características, propriedades e potencialidades da moringa (Moringa oleifera Lam.): aspectos agroecológicos. Revista verde 9: 19-25.
- Guerrero-Beltrán CE, Calderón-Oliver M, Tapia E, Medina Campos ON, Sa’nchez-Gonzalez DJ, Martinez-Martinez CM, Ortiz-Vega KM, Franco M & Pedraza-Chaverri J (2010) Sulforaphane protects against cisplatin-induced nephrotoxicity. Journal of Toxicologic Pathology 64: 503-508.
- Gupta R, Manthur M, Bajaj VK, Katariya P, Yadav S, Kamal R & Gupta RS (2012) Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. Journal of Diabetes 4: 164-171.
- Gupta S, Jain R, Kachhwaha S & Kothari SL (2018) Nutritionaland medicinal applicationsof Moringa oleifera Lam - review of current status and future possibilities. Journal of Herbal Medicine 11: 1-11.
- Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, Paulus E & Herling AW (1997) Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. Journal of Medicinal Chemistry 40: 137-145.
- Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, Bour S, Woodley J, Milagro FI, Martinez JA, Valet P & Christian Carpéné C (2010) Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacological Research 61: 355-63.
- Isitua CC & Ibeh IN (2013) Toxicological assessment of aqueous extract of Moringa oleifera and Caulis Bambusae leaves in rabbits. Journal of Clinical Toxicology S12: 003.
- Jaiswal D, Kumar RAIP, Kumar A, Mehta S & Watal G (2009) Effect of Moringa oleifera Lam leaves aqueous extract therapy on hyperglycemic rats. Journal of Ethnopharmacology 123: 392-396.
- Jaiswal D, Raia PK, Mehta S, Chatterji S, Shukla S, Rai DK, Sharma G, Sharma B, Khair S & Watal G (2013) Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pacific Journal of Tropical Medicine 6: 426-432.
- Jaja-Chimedza A, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF & Raskin I (2017) Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. Plos One 12: e0182658.
- John S & Chellappa AR (2005) Hypoglycemic effect of Moringa oleifera (drumstick) leaves on human diabetic subjects and albino rats. The Indian Journal of Nutrition and Dietetics 42: 22-29.
- Karthikesan K, Pari L & Menon VP (2010) Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chemico-Biological Interactions 188: 643-650.
- Kim Y, Jaja-Chimedza A, Merrill D, Mendes O & Raskin I (2018) A 14-day repeated-dose oral toxicological evaluation of an isothiocyanate-enriched hydro-alcoholic extract from Moringa oleifera Lam. seeds in rats. Toxicology Reports 5: 418-426.
- Kim Y, Wu AG, Jaja-Chimedza A, Graf BL, Waterman C, Verzi MP & Raskin I (2017) Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. Plos One 12(9): e0184709.
- Kou X, Li B, Olayanju JB, Drake JM & Chen N (2018) Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 10: 343.
- Kuete V (2017) Medicinal spices and vegetables from Africa: therapeutic potential against metabolic, inflammatory, infectious and systemic diseases. Academic Press. Pp. 485-496.
- Kumari DJ (2010) Hypoglycemic effect of Moringa oleifera and Azadirachta indica in type-2 diabetes. Bioscan 5: 211-214.
- Lorenzi H & Matos FJ (2008) Plantas medicinais no Brasil: nativas e exóticas cultivadas. 2ª ed. Ed. Plantarum, Nova Odessa. 576p.
- Maghu TK, Sharma A & Younis K (2017) Effect of drumstick leaves (Moringa oleifera) incorporation on quality of Khakhra. In: Ui-Islam S, plant-based natural products: derivatives and applications. Ed. Willey, New Jersey. Pp. 129-144.
- Maizuwo AI, Hassan AS, Momoh H & Muhammad JA (2017) Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): a review. Journal of Drug Design and Medicinal Chemistry 3: 60-66.
- Mansour M, Mohamed MF, Elhalwagi A, El-ITriby HA, Shawki HH & Abdelhamid IA (2019) Moringa peregrina leaves extracts induce apoptosis and cell cycle arresto the patocellular carcinoma. BioMed research international 2019: 13p.
- Melrose J (2019) The Glucosinolates: a sulphur glucoside family of mustard anti-tumour and antimicrobial phytochemicals of potential therapeutic application. Biomedicines 7: 62.
- Moyo B, Masika PJ & Muchenje V (2012a) Antimicrobial activities of Moringa oleifera Lam leaf extracts. African Journal of Biotechnology 11: 2797-2802.
- Moyo B, Masika PJ & Muchenje V (2012b) Effect of supplementing cross bred X hosalop-earedgoatcastrates with Moringa oleifera leaves on growth performance, carcassand non-carcasscharacteristics. Tropical Animal Health and Production 44: 801-809.
- Moyo B, Masika PJ, Hugo A & Muchenje V (2011) Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology 10: 12925-12933.
- Ndong M, Uhera M, Katsumata S & Suzuku K (2007) Effects of oral administration of Moringa oleifera Lam. on glucose tolerance in Goto-Kakizaki and wistar rats. Journal of Clinical Biochemistry Nutrition 40: 229-233.
- Ndubuaku UM, Uchenna NV, Baiyeri KP & Ukonze J (2015) Anti-nutrient, vitamin and other phytochemical compositions of oldand succulent Moringa (Moringa oleifera Lam.) leaves as influenced by poultry manure application. African Journal of Biotechnology 14: 2502-2509.
- Olson ME & Fahey JW (2011) Moringa oleifera: un árbol multiusos para las zonas tropicales secas. Revista Mexicana de Biodiversidad 82: 1071-1082.
- Pasa MC, Silva GG, Souza SS & Gonçalves KG (2010) Abordagem etnobotânica de Moringa oleifera Lam.: do cultivo ao uso da espécie em Rondonópolis. Boletim FLOVET 1: 1-18. Available at < https://periodicoscientificos.ufmt.br/ojs/index.php/flovet/article/view/648>. Access on 15 October 2020.
» https://periodicoscientificos.ufmt.br/ojs/index.php/flovet/article/view/648 - Peng S-G, Pang Y-L, Zhu Q, Kang J-H, Liu M-X & Wang Z (2018) Chlorogenic acid functions as a novel agonist of PPAR-2 during the differentiation of mouse 3T3-L1 preadipocytes. BioMed Research International 2018: 1-14. < https://doi.org/10.1155/2018/8594767>
» https://doi.org/10.1155/2018/8594767 - Prabhakar PK & Doble M (2011a) Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3T3-L1 adipocytes. Journal of Agricultural and Food Chemistry 59: 9844.
- Prabhakar PK & Doble M (2011b) Effect of natural products on commercial oral antidiabetic drugs in enhancing 2-deoxyglucose uptake by 3T3-L1 adipocytes. Therapeutic Advances in Endocrinology and Metabolism 2: 103114. DOI: 10.1177/2042018811411356.
» https://doi.org/10.1177/2042018811411356 - Questel. Orbit Intelligence. In: Orbit Intelligence. 10 sep. 2020. Available at < https://www.questel.com/ip-intelligence-software/orbit-intelligence/>. Access on 9 nov. 2022
» https://www.questel.com/ip-intelligence-software/orbit-intelligence/ - Rodriguez de Sotillo DV & Hadley M (2002) Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. The Journal of Nutritional Biochemistry 13: 717-726.
- Ruiz RB, Odio RMR & Carrion MEB (2012) Moringa oleifera: una opción saludable para el bienestar; Moringa oleifera: a healthyoption for thewell-being. Medisan 16: 1596-1599. Available at < https://pesquisa.bvsalud.org/portal/resource/pt/cum-51901>. Access on 15 October 2020.
» https://pesquisa.bvsalud.org/portal/resource/pt/cum-51901 - Sakuma S, Yasuda K, Kitahara R, Tsujimoto K, Yamashita K, Hoshino N, Fujimoto Y & Okuhira K (2022) Comparative effects of sulforaphane and allyl isothiocyanate on 3T3-L1 adipogenesis. Journal of Nutrition and Metabolism 2022: 1-8. < https://doi.org/10.1155/2022/8705163>
» https://doi.org/10.1155/2022/8705163 - Santos B, Santos E, Gualbert N, Barretto L, Santos J & Silva G (2014) Formulação de chá gelado a base de flor de moringa (Moringa oleifera Lam.): estúdio de aceptabilidade. V Encontro Nacional de Moringa. Universidade Estadual de Maringá, Maringá. 9p. Available at < https://www.academia.edu/13019069/FORMULA%C3%87%C3%83O_DE_CH%C3%81_GELADO_A_BASE_DE_FLOR_DE_MORINGA_MORINGA_OLE%C3%8DFERA_LAM_ESTUDO_DE_ACEITABILIDADE>. Access on 15 October 2020.
» https://www.academia.edu/13019069/FORMULA%C3%87%C3%83O_DE_CH%C3%81_GELADO_A_BASE_DE_FLOR_DE_MORINGA_MORINGA_OLE%C3%8DFERA_LAM_ESTUDO_DE_ACEITABILIDADE - Saralaya MG, Patel P, Roy MPSP & Patel AN (2010) Research article antidiarrheal activity of methanolic extractof Moringa oleifera Lam roots in experimental animal models. International Journal of Pharmaceutical Research 2: 25-29.
- Sashidhara KV, Rosaiah JN, Tyagi E, Shukla R, Raghubir R & Rajendran SM (2009) Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents. European Journal of Medicinal Chemistry 44: 432-436.
- Saucedo-Pompa S, Torres-Castillo JA, Castro-López C, Rojas R, Sánchez-Alejo EJ, Ngangyo-Heya M & Martínez-Ávila GCG (2018) Moringa plants: bioactive compounds and promising applications in food products. Food Research International 111: 438-450.
- Schenkel EP, Gosman G & Petrovick PR (2003) Produtos de origem vegetal e o desenvolvimento de medicamentos. In: Simões CMO (eds.) Farmacognosia: da planta ao medicamento. 5a. ed. Ed. UFSC, Porto Alegre. 833p.
- Sharma M, Anwer T, Pillai K, Haque SE, Najmi A & Sultana Y (2008) Silymarin, a flavonoid antioxidant, protects streptozotocin-induced lipid peroxidation and β-Cell damage in rat pancreas. Oriental Pharmacy and Experimental Medicine 8: 146-153.
- Sileshi T, Makonnen E, Debella A & Tesfaye B (2014) Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. Journal Coastal Life Medicine 2: 214-221.
- Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS, Park R, Kwon KB & Park BH (2009) Sulforaphane protects against cytokineand streptozotocin-induced beta-cell damage by suppressing the NF-kappaB pathway. Toxicology and applied pharmacology 235: 57-67.
- Stohs SJ & Hartman MJ (2015) Review of the safety and efficacy of Moringa oleifera. Phytotherapy Research 29: 796-804.
- Tshabalala T, Ncube B, Madala NE, Nyakudya TT, Moyo HP, Sibanda M & Ndhlala AR (2019) Scribbling the cat: a case of the “miracle” plant, Moringa oleifera. Plants 8: 510.
- Valdez-Solana MA, Mejía-García VY, Téllez-Valencia A, García-Aarenas G, Salas-Pacheco J, Alba-Romero JJ & Sierra-Campos E (2015) Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. Journal of Chemistry 1: 9.
- Valle IFA (2016) Análise do efeito dos flavonoides na resposta glicêmica e insulinêmica: uma revisão de literatura. Trabalho de conclusão de curso. Universidade de Brasília, Brasília. 37p.
- Vásquez-León LA, Páramo-Calderón DE, Robles-Olvera VJ, Valdés-Rodríguez OA, Pérez-Vázquez A, García-Alvarado MA & Rodríguez-Jimenes GC (2017) Variation in bioactive compounds and antiradical activity of Moringa oleifera leaves: influence of climatic factors, tree age, and soil parameters. European Food Research and Technology 243: 1593-1608.
- Villarruel-López A, López-de la Mora DA, Vázquez-Paulino OD, Puebla-Mora AG, Torres-Vitela MR, Guerrero-Quiroz LA & Nuño K (2018) Effect of Moringa oleifera consumption on diabetic rats. BMC Complementary and Alternative Medicine 18: 127.
- Wang N, Yi WJ, Tan L, Zhang JH, Xu J, Chen Y, Qin M, Yu S, Guan J & Zhang R (2017) Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cellular & Developmental Biology - Animal 53: 554-563.
- Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, Lila MA & Raskin I (2014) Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 103: 114-122.
- Waterman C, Rojas-Silva P, Tumer TB, Kuhn P, Richard AJ, Wicks S, Sthephens JM, Wang Z, Mynatt R, Cefalu W & Raskin I (2015) Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Molecular Nutrition & Food Research 59: 1013-1024. < https://doi.org/10.1002/mnfr.201400679>
» https://doi.org/10.1002/mnfr.201400679 - William F, Lakshminarayanan S & Chegu H (1993) Effect of some Indian vegetables on the glucose and insulin response in diabetic subjects. International Journal of Food Sciemce and Nutrition 44: 191-196.
- Williamson G (2013) Possible effects of dietary polyphenols on sugar absorption and digestion. Molecular nutrition & food research 57: 48-57.
- Zhang R, Shi J, Wang T, Qiu X, Liu R, Li Y, Gao Q & Wang N (2020) Apigetrin ameliorates streptozotocin-induced pancreatic β-cell damages via attenuating endoplasmic reticulum stress. In Vitro Cellular & Developmental Biology - Animal 56: 622-634.
- Zhou R, Lin J & Wu D (2014) Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochimica et Biophysica Acta 1840: 209-218.
Edited by
Area Editor:
Publication Dates
-
Publication in this collection
21 Nov 2022 -
Date of issue
2022
History
-
Received
02 Aug 2021 -
Accepted
25 Feb 2022







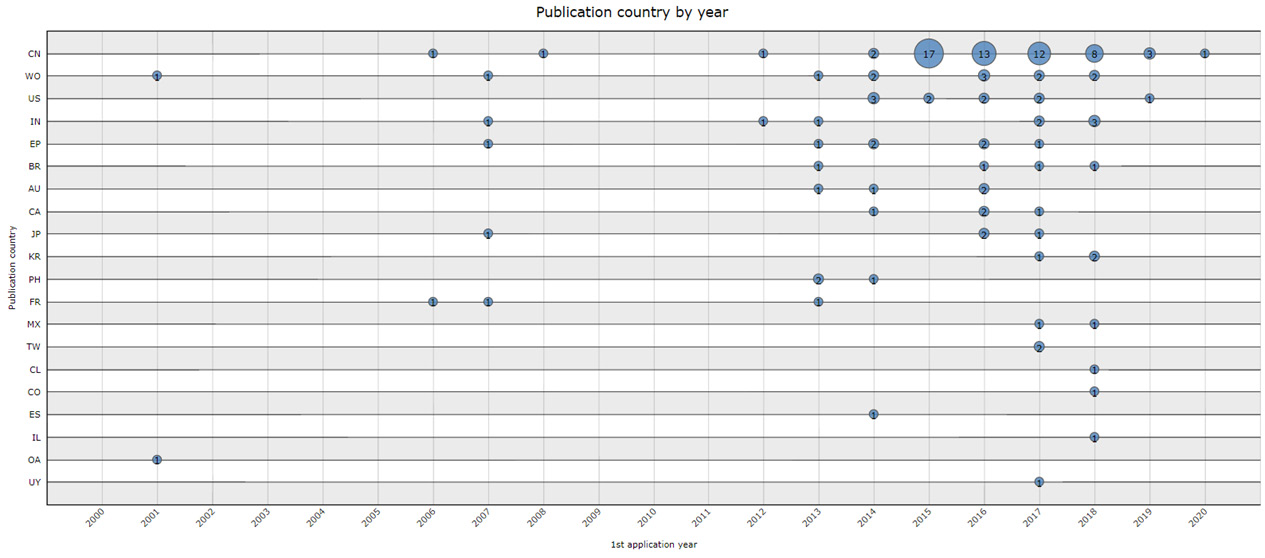

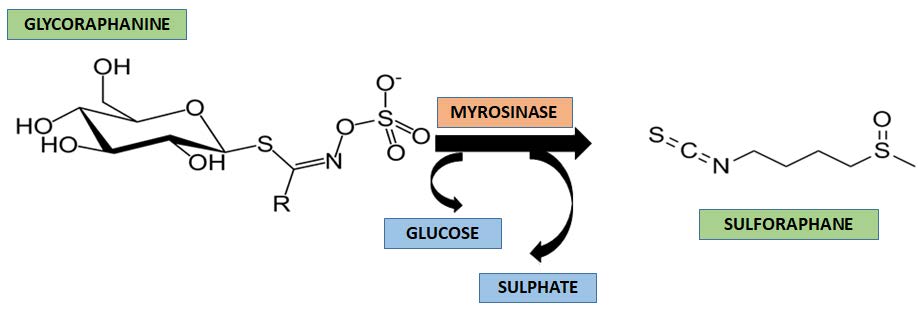 Source: Adapted from Melrose 2019.
Source: Adapted from Melrose 2019.
 Source: Adapted from Fuentes et al. 2015.
Source: Adapted from Fuentes et al. 2015.
 Source: Adapted from Prabhakar & Doble 2011b.
Source: Adapted from Prabhakar & Doble 2011b.