Abstract
Several possible correlations between dioecy and eco-morphological features have been suggested to infer the conditions that would favor this sexual system. Dioecy has been associated either with specialized or generalized pollination systems. The genus Simarouba has six dioecious species that seem to have generalized pollinators. In this study, we examined the reproductive biology aspects of two Simarouba species to evaluate which of their eco-morphological attributes may favor dioecy. Our data suggest that S. amara and S. versicolor have small, inconspicuous, and abundant flowers of nocturnal anthesis and are only pollinated by nocturnal moths. They have a stable sexual system with flower, inflorescence, and flowering phenology dimorphism that is part of their pollination system strategies. Male plants of both species produce more flowers per individual than do female plants. A larger floral display may have an essential role in increasing male reproductive success. However, both species showed low reproductive efficacy, especially S. versicolor. The low reproductive efficacy may be due to the lack of pollinators or to the inefficacy of the pollinators available locally.
Key words
Cerrado; dioecy; moth pollination; Simarouba; Simaroubaceae
Resumo
Várias correlações entre dioicia e características ecomorfológicas têm sido sugeridas para inferir as condições que favorecem este sistema sexual. A dioicia tem sido associada a sistemas de polinização especializados ou generalistas. O gênero Simarouba possui seis espécies dióicas que parecem ter polinizadores generalistas. Neste trabalho, examinamos aspectos da biologia reprodutiva de duas espécies desse gênero para investigar quais de seus atributos ecomorfológicos reprodutivos podem favorecer a dioicia. Nossos dados sugerem que S. amara e S. versicolor têm flores pequenas, abertas, abundantes, com antese noturna que são polinizadas apenas por mariposas também noturnas. Elas possuem um sistema sexual estável com dimorfismo na flor, inflorescência e fenologia de floração, que faz parte de suas estratégias de polinização. As plantas masculinas de ambas as espécies produzem mais flores por indivíduo do que as femininas. Esta alta produção de flores tem um papel importante no aumento do sucesso reprodutivo masculino. Ambas as espécies apresentaram baixa eficácia reprodutiva, especialmente S. versicolor. A baixa eficácia reprodutiva pode ser devido à falta de polinizadores ou à ineficácia dos polinizadores disponíveis localmente.
Palavras-chave
Cerrado; dioicia; polinização por mariposa; Simarouba; Simaroubaceae
Introduction
The flower is an organ that has several complex structures and is adapted to sexual reproduction (Endress 1995Endress PK (1995) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambrigde. 311p.). Most plant species have hermaphroditic flowers, and about 5–6% of Angiosperms are dioecious with staminate and pistillate flowers on separate plants (Renner 2014Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an up-dated online database. American Journal of Botany 101: 1588-1596. doi: 10.3732/ajb.1400196). Dioecy has probably evolved several times within angiosperms from hermaphroditism (Ross 1982Ross MD (1982) Five evolutionary pathways to subdioecy. The American Naturalist 119: 297-318.), via gynodioecy (Charlesworth & Charlesworth 1978Charlesworth B & Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. The American Naturalist 112: 975-997.), monoecy (Dorken & Barret 2004Dorken ME & Barrett SC (2004) Sex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proceedings of the Royal Society B Biological Sciences 271: 213-219. doi: 10.1098/rspb.2003.2580), or heterostyly (Darwin 1877Darwin CR (1877) The different forms of flowers on plants of the same species. John Murray, London. ; Lloyd 1979Lloyd DG (1979) Evolution toward dioecy in heterostylous populations. Plant Systematics and Evolution 131: 71-80.). Selective forces for the evolution of this sexual system involve genetic and ecological mechanisms (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311; Givnish 1982Givnish TJ (1982) Outcrossing versus ecological constraints in the evolution of dioecy. The American Naturalist 119: 849-865.; Thomson & Barret 1981Thomson JD & Barrett SCH (1981) Selection for outcrossing, sexual selection and the evolution of dioecy in plants. The American Naturalist 118: 443-449.). The sex separation between male and female plants requires cross-breeding between different individuals and can bring genetic advantages to the population (Charlesworth & Charlesworth 1978Charlesworth B & Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. The American Naturalist 112: 975-997.; Freeman et al. 1997Freeman DC, Doust JL, El-Keblawi A, Miglia KJ & McArthur ED (1997) Sexual specialization and inbreeding avoidance in the evolution of dioecy. The Botanical Review 63: 65-92.) and improve individuals’ reproductive fitness (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311; Thomson & Barret 1981). On the other hand, these plants become dependent on pollination vectors (Oliveira & Maruyama 2014Oliveira PE & Maruyama PK (2014) Sistemas reprodutivos. In: Rech AR, Agostini K, Oliveira PE & Machado IC (org.) Biologia da polinização. Projeto Cultural, Rio de Janeiro. Pp. 71-93.). As a consequence of this sexual separation, staminate and pistillate flowers have secondary sexual characteristics, such as differences in size and attractiveness, which are considered adaptive strategies that allow the directional flow of pollen to co-specific stigmas (Grant 1995Grant V (1995) Sexual selection in plants: pros and cons. Proceedings of the National Academy of Sciences of the United States of America 92: 1247-1250. doi: 10.1073/pnas.92.5.1247; Lloyd & Webb 1977Lloyd DG & Webb CJ (1977) Secondary sex characters in plants. The Botanical Review 43: 177-216. doi: 10.1007/BF02860717).
Some authors argued that ecological constraints are more important than selection for outcrossing in dioecy evolution (Givnish 1982Givnish TJ (1982) Outcrossing versus ecological constraints in the evolution of dioecy. The American Naturalist 119: 849-865.; Bawa 1980bBawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467-474. doi: 10.2307/2408216; Willson 1994Willson MF (1994) Sexual selection in plants: perspectives and overview. The American Naturalist 144: 13-39.). Sexual specialization would optimize the resource allocation for reproduction because male and female functions would demand different resources in distinguished ways (Moore & Pannell 2011Moore JC & Pannell JR (2011) Sexual selection in plants. Current Biology 21: R176-R182.; Tonnabel et al. 2017Tonnabel J, Patrice D & Pannell JR (2017) Sex-specific strategies of resource allocation in response to competition for light in a dioecious plant. Oecologia 185: 675-686. doi: 10.1007/s00442-017-3966-5). The male function would be involved mainly with the production and dispersion of pollen to increase the availability of mating partners, and the female function would invest in ovule and fruit production, optimizing the quality of seeds (Janzen 1977Janzen DH (1977) A note on optimal mate selection by plants. The American Naturalist 111: 365-371.; Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311; Lloyd 1982Lloyd DG (1982) Selection of combined versus separate sexes in seed plants. The American Naturalist 120: 571-585.).
Several possible correlations between dioecy and various eco-morphological features have been suggested to infer the conditions that would favor this sexual system (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311; Renner & Feil 1993Renner SS & Feil JP (1993) Pollinators of tropical dioecious angiosperms. American Journal of Botany 80: 1100-1107. doi: 10.2307/2445757; Renner & Ricklefs 1995Renner SS & Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. American Journal of Botany 82: 596-606. doi: 10.2307/2445418; Renner 2014Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an up-dated online database. American Journal of Botany 101: 1588-1596. doi: 10.3732/ajb.1400196). According to Bawa (1980a)Bawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311, flowers of dioecious species are generally small and unspecialized, have light and inconspicuous colors, and are produced in more significant numbers in male than in female plants. This sexual dimorphism is interpreted as one of the consequences of sexual selection to increase an individual’s sexual display and promote male reproductive success (Bawa & Opler 1975Bawa KS & Opler PA (1975) Dioecism in tropical forest trees. Evolution 29: 167-179. doi: 10.1111/j.1558-5646. 1975.tb00824.x; Bawa 1980bBawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467-474. doi: 10.2307/2408216). Some other authors supported the ecological association of dioecy with small insect-pollinated flowers (Givnish 1982Givnish TJ (1982) Outcrossing versus ecological constraints in the evolution of dioecy. The American Naturalist 119: 849-865.; Thomson & Brunet 1990Thomson JD & Brunet J (1990) Hypotheses for the evolution of dioecy in plants. Trends in Ecology & Evolution 5: 11-16.). However, these correlations pointed out by Bawa (1980a)Bawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311 were contested by Renner & Feil (1993)Renner SS & Feil JP (1993) Pollinators of tropical dioecious angiosperms. American Journal of Botany 80: 1100-1107. doi: 10.2307/2445757, who considered the occurrence of dioecy to be more common in plants with more specialized flowers. Few field observations about sexual systems in natural populations show which eco-morphological attributes favor dioecy (Renner 2014Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an up-dated online database. American Journal of Botany 101: 1588-1596. doi: 10.3732/ajb.1400196).
The genus Simarouba Aubl. has six tree or shrub species, all of which are dioecious, occurring in Cerrado, Amazonian Forest, and Dry forest of Central America and Caribbean Island (Franceschinelli et al. 1999Franceschinelli EV, Yamoto K & Shepherd GJ (1999) Distinctions among three Simarouba species. Systematic Botany 23: 479-488. doi: 488 10.2307/2419379). All genus species are truly dioecious, but other closely related genera, such as Simaba Aubl., particularly section Tenuiflorae, also show signs of dicliny (Alves et al. 2017Alves GGN, El Ottra JGL, Devecchi MF, Demarco D & Pirani JR (2017) Structures of the flower of Simaba (Simaroubaceae) and its anatomical novelties. Botanical Journal Linnean Society 183: 162-176. doi: 10.1111/boj.12486). The six species of Simarouba have distribution ranges restricted to the Neotropical region (Clayton 2011Clayton JW (2011) Simaroubaceae. In: Kubitzki K (ed.) The families and genera of vascular plants. Flowering plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin. Pp. 408-423.), with two of them, S. amara Aubl. and S. versicolor A. St.-Hil, occurring in Brazil (Devecchi & Pirani 2016Devecchi MF & Pirani JR (2016) Flora of the cangas of the Serra dos Carajás, Pará, Brazil: Simaroubaceae. Rodriguesia 67: 1471-1476. doi: 10.1590/2175-7860201667551). Some studies emphasized the phenology and reproductive biology of S. amara (Pinto et al. 2005Pinto AM, Ribeiro RJ, Alencar JC & Barbosa AP (2005) Fenologia de Simarouba amara Aubl. na reserva florestal Adolpho Ducke, Manaus, AM. Acta Amazonica 35: 347-352. doi: 10.1590/S0044-59672005000300007). Dioecy was reported as a sexual system for this species, with pollination performed by several small insects (Bawa 1994Bawa KS (1994) Pollinators of tropical dioecious angiosperms: A reassessment? No, not yet. American Journal of Botany 81: 456-460. https://doi.org/10.2307/2445495.; Hardesty et al. 2005Hardesty BD, Dick CW, Kremer A, Hubbell S & Bermingham E (2005) Spatial genetic structure of Simarouba amara Aubl. (Simaroubaceae), a dioecious, animal-dispersed neotropical tree, in Barro Colorado Island, Panama. Heredity 95: 290-297.). Pollination by generalist bees has been suggested for S. versicolor (Reis et al. 2012Reis SM, Mohr A, Gomes L, Silva ACS, Abreu MF & Lenza E (2012) Síndromes de polinização e dispersão de espécies lenhosas em um fragmento de Cerrado sentido restrito na transição Cerrado - Floresta Amazônica. Heringeriana 6: 28-41.). These works reinforce the theory that dioecious species generally have more generalized flowers and pollinators.
Simarouba amara Aubl. has a broad geographical distribution, occurring from Central America to Southeast Brazil. Simarouba versicolor A. St.-Hil. occurs from North to Southeast Brazil and in Bolivia (Pirani & Thomas 2015Pirani JR & Thomas WW (2015) Simaroubaceae. In: Jorgensen P, Nee M & Bech S (eds.) Catálogo de las plantas vasculares de Bolivia. Missouri Botanical Garden Press, Saint-Louis. Pp. 1199-1200.). Both species can co-occur and co-flower in the Cerrado biome. However, regardless of the wide-ranging area of occurrence, data on these species’ reproductive systems are still scarce or inconclusive.
In this context, the objective of this work was to expand knowledge about the sexual system and pollination of Simarouba species that occur in Brazil. We intended to examine both floral and pollinating attributes related to dioecy. Specifically, we evaluate whether these species’ pollination is performed by small and diverse insects, considering Bawa’s (1980a) premises, or if their flowers are specialized to be pollinated by a specific group of pollinators as suggested by Renner & Feil (1993)Renner SS & Feil JP (1993) Pollinators of tropical dioecious angiosperms. American Journal of Botany 80: 1100-1107. doi: 10.2307/2445757 for most dioecious species. We expect that both species have small and generalist flowers pollinated by diverse insects, such as suggested by other authors (Bawa 1994Bawa KS (1994) Pollinators of tropical dioecious angiosperms: A reassessment? No, not yet. American Journal of Botany 81: 456-460. https://doi.org/10.2307/2445495.; Hardesty et al. 2005Hardesty BD, Dick CW, Kremer A, Hubbell S & Bermingham E (2005) Spatial genetic structure of Simarouba amara Aubl. (Simaroubaceae), a dioecious, animal-dispersed neotropical tree, in Barro Colorado Island, Panama. Heredity 95: 290-297.; Reis et al. 2012Reis SM, Mohr A, Gomes L, Silva ACS, Abreu MF & Lenza E (2012) Síndromes de polinização e dispersão de espécies lenhosas em um fragmento de Cerrado sentido restrito na transição Cerrado - Floresta Amazônica. Heringeriana 6: 28-41.). We also expect that both Simarouba species have low reproductive efficacy, and the staminate flowers are produced in a larger amount than pistillate ones, as suggested by Bawa (1980a)Bawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311 for dioecious species.
Material and Methods
We conducted the study from July to October 2015 and 2016 for S. amara Aubl., and June to November 2016 for S. versicolor St.- Hil in the Santuário de Vida Silvestre Vagafogo, in the municipality of Pirenópolis, state of Goiás, Brazil (15°49’00’’ S, 48°59’30’’W). The average altitude of the locality is 750 m above sea level, and the local vegetation is characterized as a fragment of seasonal semideciduous forest and cerradão (Venturoli 2008Venturoli F (2008) Manejo de Floresta Estacional Semidecídua Secundária em Pirenópolis, Goiás. Tese de Doutorado. Universidade de Brasília, Brasília. 203p.).
Study species
Simarouba amara is a tree species (Fig. 1a) that can reach up to 35 m in height in the Amazon Forest’s terra firme forests, where it can be quite common species. This species also inhabits the Cerrado biome at the southern end of its geographical distribution (Raw & Hay 1985Raw A & Hay J (1985) Fire and other factors affecting a population of Simarouba amara in “cerradão” near Brasília, Brazil. Revista Brasileira de Botanica 8: 101-107.; Franceschinelli et al. 1999Franceschinelli EV, Yamoto K & Shepherd GJ (1999) Distinctions among three Simarouba species. Systematic Botany 23: 479-488. doi: 488 10.2307/2419379). It is also a common species in different phytophysiognomies of Cerrado, especially in Cerradão and Cerrado sensu strito of its Central Brazil distribution (Raw & Hay 1985Raw A & Hay J (1985) Fire and other factors affecting a population of Simarouba amara in “cerradão” near Brasília, Brazil. Revista Brasileira de Botanica 8: 101-107.; Franceschinelli et al. 1999Franceschinelli EV, Yamoto K & Shepherd GJ (1999) Distinctions among three Simarouba species. Systematic Botany 23: 479-488. doi: 488 10.2307/2419379). Simarouba versicolor is also a tree species (Fig. 2a), but it inhabits mainly Cerrado (sensu lato) and the Caatinga biomes (Pirani & Thomas 2015Pirani JR & Thomas WW (2015) Simaroubaceae. In: Jorgensen P, Nee M & Bech S (eds.) Catálogo de las plantas vasculares de Bolivia. Missouri Botanical Garden Press, Saint-Louis. Pp. 1199-1200.). These species were reported to be of pharmacological, commercial, and ecological importance (Souza 1997Souza MH (1997) Madeiras tropicais brasileiras. IBAMA/LPF, Brasília. 151p.; Trevisan & Macedo 2003Trevisan MTS & Macedo FV (2003) Seleção de plantas com atividade anticolinasterase para tratamento da doença de Alzheimer. Química Nova 26: 301-304.; Cortes 2012Cortes JM (2012) Desenvolvimento de espécies nativas do Cerrado a partir do plantio de mudas e da regeneração natural em uma área em processo de recuperação, Planaltina-DF. Dissertação de Mestrado. Universidade de Brasília, Brasília. 89p.).
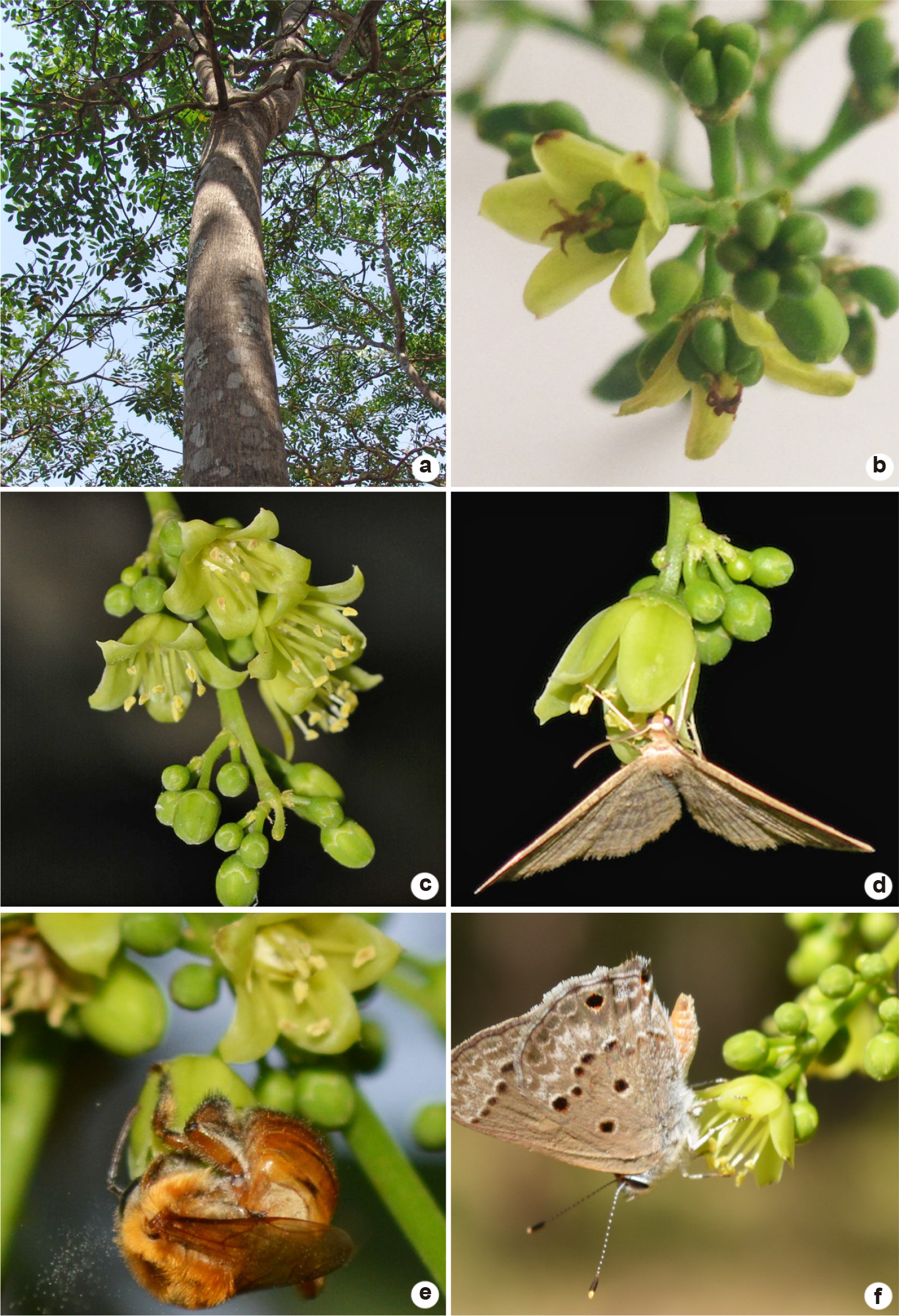
a-f. Simarouba amara in Santuário de Vida Silvestre Vagafogo, Pirenópolis, GO, Brazil – a. tree of S. amara, 14 m tall; b. pistillate flowers; c. staminate flowers; d. Pleuroprucha insularia visiting a staminate flower of S. amara; e. Melipona rufiventris taking pollen out of a staminate flower in pre-anthesis; f. the butterfly Echynargus isola visiting a staminate flower of S. amara in the morning.
a-d. Simarouba versicolor in Santuário de Vida Silvestre Vagafogo, Pirenópolis, GO, Brazil – a. tree of S. versicolor, 8m tall; b. pistillate flower; c. staminate flowers; d. Noctuidae in a staminate flower of S. versicolor.
Flowering phenology, floral morphology, and biology
We described the morphology of flowers and inflorescences using ten pistillate and ten staminate plants of both species (five inflorescences and five flowers per analyzed plant). For each species, we registered the opening time, the emission of odor, pollen grain release in 10 staminate and 10 pistillate flowers of 10 individuals of each sex. We evaluated stigmatic receptivity in the field by adding a few drops of 3% H2O2 solution to the stigma of flowers (Kearns & Inouye 1993Kearns CA & Inouye D (1993) Techniques for pollinations biologists. Colorado, University Press of Colorado, Niwot. 583p.). We estimated male fertility by analyzing pollen viability. We collected five flowers in pre-anthesis from five individuals and fixed them in 50% FAA for 24h, transferring them to 70% alcohol after that. We prepared slides by staining pollen grains with 2% acetic carmine. We visualized all pollen grains we found on the slides using an optical microscope at 10% magnification and counted with the aid of a manual counter.
We performed phenological analyses of S. amara and S. versicolor using 20 individuals of each species (ten males and ten females), which we marked and identified during the flowering period. We made weekly observations of flower production during the reproductive period of each species. We quantified the flowering phenophase by assigning points that designated an approximate value to its intensity (Fournier 1974Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24: 422-423.). We also calculated the flowering phenophase activity index between male and female flowers and between the studied species (Bencke & Morellato 2002Bencke CS & Morellato LPC (2002). Comparação de dois métodos de avaliação da fenologia de plantas, sua interpretação e representação. Brazilian Journal of Botany, 25: 269-275.).
Sex ratio and display of staminate and pistillate flowers
We determined the sex ratio from the number of male and female individuals that were reproductive in one study population per species. We used a Chi-square test to assess the ratio (1:1) of male and female individuals using R (R Core Team 2015R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Satistical Computing, Vienna. Available at <https://www.gbif.org/pt/tool/81287/r-a-language-and-environment-for-statistical-computing>. Acess on 9 August 2016.
https://www.gbif.org/pt/tool/81287/r-a-l...
). We evaluated the species’ sexual pattern during their reproductive period of 2015–2016 for S. amara, and 2016–2017 for S. versicolor, and we observed whether the plants would remain dioecious and with the same sex from one reproductive period to the other.
We counted the number of flowers of four branches located on the plant’s opposite side from five male and five female individuals to analyze flower production investment in plants from both sexes. Using a digital caliper with 0.01 mm accuracy, we also performed a morphometric analysis of staminate and pistillate flower petals of 100 flowers (50 staminate flowers and 50 pistillate flowers) from five plants of each sex for S. amara. We performed the same analysis for S. versicolor using 60 flowers (30 staminate flowers and 30 pistillate flowers) from five plants of each sex. Finally, we used an independent t-test to determine any differences in the average flower production and the average size of staminate and pistillate petals and inflorescences.
Floral visitors
We observed the behavior of the floral visitors for both species at different times of the day and night during six non-consecutive days of the flowering peak, under low air humidity (20 to 35%), warm to mild temperature (34 to 17 °C), and mild wind (lower than 3 km/h). We observed during the whole day and night, totaling 84 hours of observation for each species. We collected data on the frequency of pollinators’ visits in three male and three female plants of one population for each species through focal observation for two hours (number of pollinators visits / 2h) of observation during three nights. We considered pollinators those animals that touched the stigmas of the pistillate flowers and the anthers of the staminate flowers when visiting them to collect the floral resources (Alves-dos-Santos et al. 2016Alves-dos-Santos I, Silva CI, Pinheiro M & Kleinert AMP (2016) Quando um visitante floral é um polinizador? Rodriguésia 67: 295-307. doi: 10.1590/2175-7860201667202). We analyzed the body of each insect with the aid of a stereomicroscope to assess the presence of pollen grains. We compared blades with pollen grains found on the insects with the pollen grains of S. amara and S. versicolor flowers under an optical microscope. We identified the insects and deposited them in the Coleção Entomológica of Laboratório de Biologia Reprodutiva Vegetal of Universidade Federal de Goiás.
Reproductive system and efficacy
We performed the following experiments: manual cross-pollination (when we carried pollen from the anthers of staminate flowers to the stigmas of pistillate flowers from previously bagged inflorescences); apomixis (when we marked and bagged pistillate flowers in pre-anthesis); open pollination (when we marked pistillate flowers but did not bag them); and a test to check for wind pollination (when we bagged pre-anthesis pistillate flowers using a tissue with a large pore size that allowed the pollen grains to get into the bag). We performed each procedure on a minimum of 30 flowers from five different plants per treatment.
We used organza tissue bags measuring 10×10×14 cm in the manual cross-pollination and apomixis experiments. This material was closed enough to prevent visitors’ entrance and allow ventilation for flower’s and fruit’s development. In the wind pollination treatment, we used micro-perforated fabric bags to prevent insect visitation but permit the passage (entry) of pollen grains into the bags. We compared fruit yield between open pollination (OP, control) and manual cross-pollination (CP) to determine reproductive efficacy (RE) (modified from Freitas 2013Freitas L (2013) Concepts of pollinator performance: is a simple approach necessary to achieve a standardized terminology? Brazilian Journal of Botany 36: 3-8.):
We multiplied the number of flowers by five because each flower has five apocarpous carpels that can develop up to five drupoid fruits.
Results
Flowering phenology, floral morphology, and biology
Simarouba amara and S. versicolor have greenish-yellow unisexual flowers arranged in terminal or axillary panicles. The terminal or axillary panicles of S. versicolor are pendulous on male plants and erect on female plants. In S. amara, the male and female panicles are erect, making it easy to distinguish the male plants of both species during the flowering period. Both species have actinomorphic, pentameric, gamosepalous, and dialypetalous flowers. The pistillate flowers (Figs. 1b; 2b) have a superior, pentacarpellate, and apocarpous ovary with the presence of a nectariferous gynophore and ten rudimentary stamens composed of pilose filaments and reduced non-functional anthers. The style is gynobasic, and the stigma is pentalobate. The staminate flowers (Figs. 1c; 2c) also have a nectariferous gynophore with reduced carpels and are diplostemonous. The anthers are basifixed com with longitudinal dehiscence. The pistillate flowers can produce up to five independent drupes measuring 0.3–1.5 × 0.2–1.0 cm for S. amara and 2.0–2.5 × 1.5–2.0 cm for S. versicolor. They are very dark in color, close to black, and take around 50 days to mature.
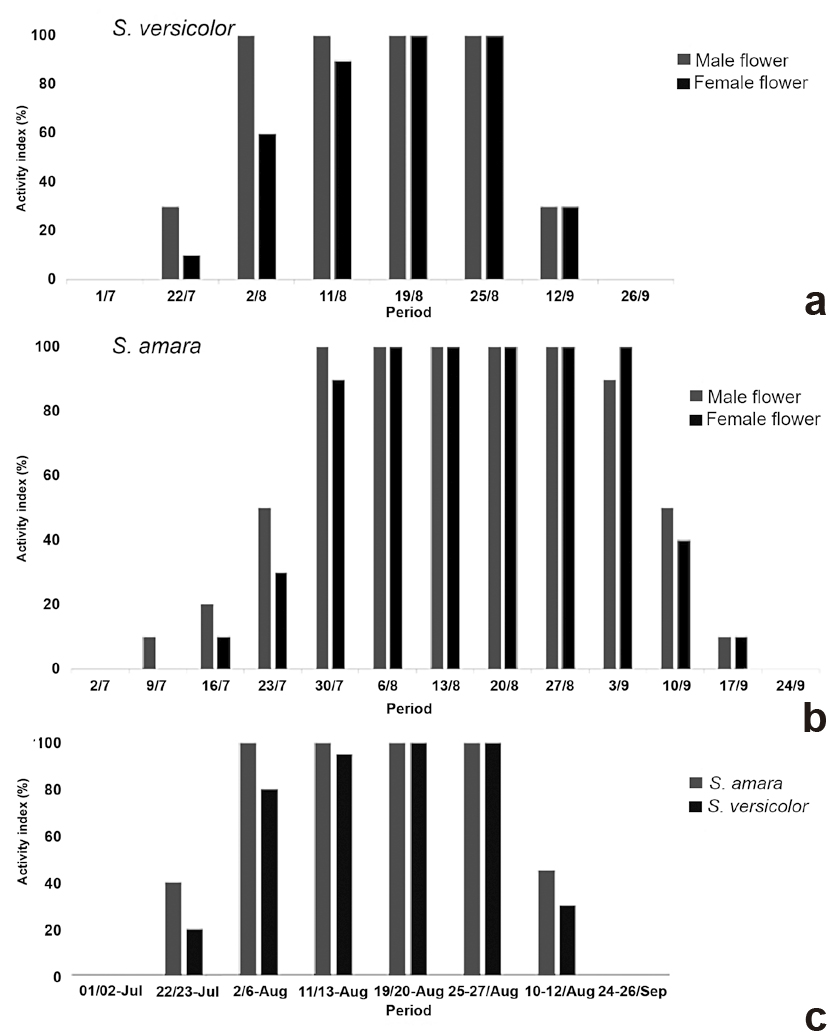
In both species, flowering started in July and ended in September (Fig.3), showing simultaneous flowering (Fig. 3c), although S. amara started flowering a week earlier and finished a week later than S. versicolor. The period of greatest flowering intensity occurred in middle August for S. amara, remaining in flower until the end of September. For S. versicolor flowering peak was at the end of August and beginning of September, ending abruptly in the middle of September. There was more significant overlap between the male and female flowering period in S. versicolor than in S. amara, in which staminate flowers start open in abundance a week or ten days before pistillate flowers (Fig. 3a-b).
a-c. Activity index (%) of the flowering phenology – a. Simarouba amara; b. S. versicolor; c. between both species.
For both species, staminate flowers opened in the late afternoon, between 17:15h and 18:00h. Pistillate flowers open between 18:00 to 20:00 for S. amara and 19:30 to 21:00h for S. versicolor. However, on very hot days (36 °C or more), the opening of staminate flowers began around 16:30h.
The pistillate flowers of S. amara and S. versicolor and the staminate flowers of S. amara remained open for nearly two days while the staminate flowers of S. versicolor expired and dropped within 24 hours. For both S. amara and S. versicolor, open pistillate flowers presented stigmas receptive with a clear and bright appearance for 12 hours. After that, stigmas were slightly dark and did not bubble in the 3% H2O2 solution, indicating that they were no longer receptive. Therefore, the stigma seems to be receptive during the night and in the following early morning only.
Pollen started to be available in staminate flowers one to two hours after the buds began to open and remained functional during the night. In the morning, the amount of pollen on both species’ anthers is very low or null. Ninety percent of the pollen grains analysis from pre-anthesis flowers of S. amara and S. versicolor had their cytoplasm stained with acetic carmine and considered viable on the same day of anthesis. Flowers of S. amara and S. versicolor produced a mild odour that we could sense only in the early evening when the flowers were in the anthesis process.
Sex ratio and display of staminate and pistillate flowers
The proportion of female (n = 41) and male (n = 42) flowering plants was 1:1 (χ² = 0.31; p = 0.58) for S. amara and for S. versicolor (female, n = 14 and male n = 17, χ² = 0.29; p = 0.59). There was no change in the sex of individuals for during the study period for both species.
The average number of staminate flowers/inflorescence for S. amara (X = 4048.30 ± 3223.12) was 10.8 times higher than that of pistillate flowers (X = 392.20 ± 138.04; t = 2.53, p = 0.03). The results were similar for S. versicolor, with the number of staminate flowers being 10.3 times higher (X = 1836.75 ± 288.64) than that of the pistillate flowers (X = 169.25 ± 20.61; t = 16.29, p < 0.01). Besides, petals of staminate flowers of S. amara (X = 4.26 ± 0.5272 mm) were larger than those of pistillate flowers (X = 4.06 ± 0.2399 mm; t = 4.80; p < 0.01). We observed the opposite for S. versicolor, with pistillate flower petals (X = 3.78 ± 0.31 mm) being larger than those of staminate flowers (X = 2.87 ± 0.42mm; t = 6.97; p < 0.01).
Floral visitors
We observed Noctuidae, Crambidae, Geometridae, and Uraniidae moths on both pistillate and staminate flowers of S. amara during the night, mainly between 18:00–23:00h (Tab. 1; Figs. 1d; 2d). We observed Crambidae and Geometridae moths visiting flowers of S. versicolor mostly between 19:00 to 23:00h (Tab. 1; Fig. 1d). All observed moths touched the anthers of staminate flowers and the stigma of pistillate flowers during their visits to both species’ flowers. We found pollen grains on the ventral part, the antennae, and the proboscis of all moths (N = 9 for S. amara and N = 7 for S. versicolor) collected both in pistillate and staminate flowers. These pollen grains were collected and observed on slides under de microscopy.
The nocturnal pollinators and diurnal visitors of flowers of Simarouba amara and Simarouba versicolor (Pirenópolis GO, Brazil).
The visits of the moths to staminate flowers of both species started at nightfall and became more intense between 21:00 and 22:00h. To pistillate flowers, the visits occurred mainly between 21:00 to 23:00h and were rarer than for male plants for both species, especially for S. versicolor. In S. amara, we saw 1–3 moths/hour visiting around 10–15 male flowers/plant and two moths/hour visiting 5–7 female flowers/plant. In S. versicolor, 0.5–1 moths/hour visiting around 6–12 male flowers/plant and 0.5 moth/hour visiting 3–5 female flowers/plant.
Diurnal insects were rarely seen visiting pistillate flowers of both Simarouba species and were not considered effective pollinators. At the end of the afternoon, we observed bees visiting the staminate flowers mainly for pollen collection in both species (Tab. 1; Fig. 1e). They started visiting flowers of male individuals by 17:00h, remaining until nightfall. They got the rest of the pollen from the S. amara and S. versicolor old staminate flowers and, sometimes, nectar from S. amara extrafloral nectaries. Eventually, we observed Apis mellifera Linnaeus 1758 and Melipona rufiventris Lepeletier, 1836, forcing anther opening of pre-anthesis flowers to get pollen.
Early in the mornings, bees of Trigona sp., butterflies of Echynargus isola (Lycaenidae) (Fig. 1f), and more rarely wasps visited staminate flowers of both species to collect nectar and the rest of pollen left on anthers at the end of the floral anthesis of both Simarouba species. We also observed ants and bees, such as Tetragonisca angustula Latreille 1811 (Apidae: Meliponini), getting nectar at the leaf extrafloral nectaries of male plants of S. amara.
Reproductive system and efficacy
The reproductive efficacy was low for both species. The flowers subjected to the cross-pollination treatment had a higher fruit production rate than did marked open flowers for both species (Tab. 2). However, flowers of S. amara subjected to the cross-pollination and control treatments had higher rates of fruit production than did flowers of S. versicolor subjected to the same treatments. Therefore, the reproductive efficacy (RE) was higher for S. amara than for S. versicolor (Tab. 2). In the apomixis experiment, few fruits were produced in both species (Tab. 2). We did not verify anemophilous fruit production for both species.
Reproductive efficacy and the fruit set (number of fruits / number of flowers) in open pollination, cross-pollination, wind pollination, and apomixis treatments for Simarouba amara and Simarouba versicolor (Pirenópolis GO, Brazil). (N) = number of flowers per treatment.
Discussion
Simarouba amara and S. versicolor have small, inconspicuous, and abundant flowers, which are common characteristics among tropical dioecious species and were associated with the attraction of small social bees, wasps, and flies pollinators (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311; Beach 1981Beach JH (1981) Pollinator foraging and the evolution of dioecy. The American Naturalist 118: 572-577.). However, some floral characteristics of Simarouba, particularly pale open flowers with nocturnal anthesis and mild nocturnal odor, restrict its pollination to nocturnal visitors, such as small moths. These floral features may be related to phalaenophily syndrome (Faegri & Van der Pijl 1979Faegri K & Van der Pijl L (1979) Principles of pollination ecology. Pergamon Press, Oxford. 247p.). The small moths touch the anthers and stigmas of the staminate and pistillate flowers, respectively, while visiting flowers for nectar, when the stigmas are receptive, the anthers are properly open and full of pollen. These results corroborate the theory of Renner & Feil (1993)Renner SS & Feil JP (1993) Pollinators of tropical dioecious angiosperms. American Journal of Botany 80: 1100-1107. doi: 10.2307/2445757 that tropical dioecious species’ pollination involves a more specialized pollination system.
The floral characteristics of Simarouba do not preclude the visit of other insects such as small bees in the daytime. However, diurnal insects were not effective pollinators because few pollen grains were available in the anthers of staminate flowers, and the visits to pistillate flowers were null or rarely observed in the daytime. Some bees sought pollen out of pre-anthesis staminate flowers that still have their anthers closed in the afternoon, visiting almost exclusively male plants for pollen. Besides, the female flowers did not have their stigma receptive in the afternoon, only in the night.
As suggested by Bawa (1980a)Bawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311 for dioecious species, the study species present low reproductive efficacy (RE = 18.7% for S. amara and 6.5% for S. versicolor), and male plants produce more flowers than female ones. These characteristics were previously reported for other dioecious species, including Cerrado species (Oliveira 1996Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191: 235-243.; Freeman et al. 1997Freeman DC, Doust JL, El-Keblawi A, Miglia KJ & McArthur ED (1997) Sexual specialization and inbreeding avoidance in the evolution of dioecy. The Botanical Review 63: 65-92.; Amorim & Oliveira 2006Amorim FW & Oliveira PE (2006) Estrutura sexual e ecologia reprodutiva de Amaioua guianensis Aubl. (Rubiaceae), uma espécie dióica de formações florestais de cerrado. Brazilian Journal of Botany 29: 353-362.). A larger male floral display plays an important role in increasing the pollen amount in the system and the male reproductive success (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311). For S. amara, staminate flowers also presented larger petals, as previously observed for other dioecious species (Amorim et al. 2011Amorim FW, Mendes-Rodrigues C, Maruyama PK & Oliveira PE (2011) Sexual ratio and floral biology of the dioecious Neea theifera Oerst. (Nyctaginaceae) in a cerrado rupestre of central Brazil. Acta Botanica Brasilica 25: 785-792. doi: 10.590/S0102-33062011000400006; Franceschinelli et al. 2015aFranceschinelli EV, Carmo RM, Silva Neto CM & Mesquita Neto JN (2015a) Functional dioecy and moth pollination in Cabralea canjerana subsp. canjerana (Meliaceae). Darwiniana 3: 96-107. doi: 10.14522/darwiniana.2015.31.599). Larger staminate flowers may occur when pollinator attraction confers a more significant gain in male than female reproductive success (Delph et al. 1996Delph LF, Galloway LF & Stanton ML (1996) Sexual dimorphism in flower size. The American Naturalist 148: 299-320.). In this case, male reproductive success would be conditioned by high flower production and pollen release, whereas female reproductive success would be conditioned by high fruit and seed production and maturation (Freeman et al. 1997Freeman DC, Doust JL, El-Keblawi A, Miglia KJ & McArthur ED (1997) Sexual specialization and inbreeding avoidance in the evolution of dioecy. The Botanical Review 63: 65-92.). The female function is especially energy-intensive when the fruits are fleshy, and the seeds are large (Bawa 1980aBawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311), such as for S. amara and S. versicolor. Furthermore, the high cost of reproduction for females may incur late flowering (Cipollini & Whigham 1994Cipollini LM & Whigham DF (1994) Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae). American Journal of Botany 81: 65-75.) and low pistillate flower production (Thomas & La Frankie 1993Thomas SC & La Frankie JV (1993) Sex, size, and inter year variation in flowering among dioecious trees of the Malayan rain forest. Ecology 74: 1529-1537.), as observed for the study species.
The staminate flowers of S. versicolor are smaller than the pistillate flowers. Perhaps, larger pistillate flowers are essential for increasing female floral display, as the number of flowers in female inflorescences of S. versicolor is lower than the inflorescences of S. amara. Pistillate flowers of larger petals may enhance female reproductive efficacy in S. versicolor, which is relatively low relative to S. amara and other dioecious species of the Cerrado (Fuzeto et al. 2001Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003; Lenza & Oliveira 2005Lenza E & Oliveira PE (2005) Biologia reprodutiva de Tapirira guianensis Aubl. (Anarcadinaceae), uma espécie dióica em mata de galeria do Triangulo Mineiro, Brasil. Brazilian Journal of Botany 28: 179-190. doi: 10.1590/S0100-84042005000100015; Mendes et al. 2011Mendes FN, Rêgo MMC & Albuquerque PM (2011) Fenologia e biologia reprodutiva de duas espécies de Byrsonima Rich. (Malpighiaceae) em área de Cerrado no Nordeste do Brasil. Biota Neotropica 11: 103-115. doi: 10.1590/S1676-06032011000400011).
The temporal separation of flowering between the sexes, such as observed mainly in S. versicolor is brief and should not cause damage to the pollination system. Early flowering of male plants should, at first, allow the initial attraction of pollinators and the recognition of flowers of this species as important resource donors (nectar). The available pollen grains should later be delivered to pistillate flowers due to the common morphological similarities between the flowers of different sexes of dioecious species (Bawa 1980bBawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467-474. doi: 10.2307/2408216; Lenza & Oliveira 2006Lenza E & Oliveira PE (2006) Biologia reprodutiva e fenologia de Virola sebifera Aubl. (Myristicaceae) em mata mesofítica de Uberlândia, MG, Brasil. Brazilian Journal of Botany 29: 443-451. doi: 10.1590/S0100-84042006000300011; Franceschinelli et al. 2015aFranceschinelli EV, Carmo RM, Silva Neto CM & Mesquita Neto JN (2015a) Functional dioecy and moth pollination in Cabralea canjerana subsp. canjerana (Meliaceae). Darwiniana 3: 96-107. doi: 10.14522/darwiniana.2015.31.599). After a few days, when the pistillate flowers open, the pollinators recognize the available resource and the simultaneous flowering of plants of different sexes can provide greater pollen flow to female plants (Bawa 1980bBawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467-474. doi: 10.2307/2408216; Bullock & Bawa 1981Bullock SH & Bawa KS (1981) Sexual dimorphism and the annual flowering pattern in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae) in a Costa Rican rainforest. Ecology 61: 1494-1504.; Piratelli et al. 1998Piratelli AJ, Piña-Rodrigues FCM, Gandara FB, Santos EMG & Costa LGS (1998) Biologia da polinização de Jacaratia spinosa (Aubl.) ADC. (Caricaceae) em mata residual do Sudeste brasileiro. Revista Brasileira de Biologia 58: 671-679.; Fuzeto et al. 2001Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003; Lenza & Oliveira 2006Lenza E & Oliveira PE (2006) Biologia reprodutiva e fenologia de Virola sebifera Aubl. (Myristicaceae) em mata mesofítica de Uberlândia, MG, Brasil. Brazilian Journal of Botany 29: 443-451. doi: 10.1590/S0100-84042006000300011).
Despite the specialized pollination system, a low reproductive efficacy was observed for both studied species, especially for S. versicolor. Some factors were cited as limiting pollinator efficiencies in the open areas of the Cerrado, such as seasonal drought, high temperatures, and distance between plants of the same species (Oliveira 1996Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191: 235-243.). The population density of S. versicolor was about a third of the density of S. amara (EV. Franceschienlli, unpublished data), which may restrict the genetic exchange between co-specific plants, especially if dioecious. The fruit set (7.97%) for S. amara from natural pollination was similar to the fruit set for Virola sebifera (5.8%) (Lenza & Oliveira 2006Lenza E & Oliveira PE (2006) Biologia reprodutiva e fenologia de Virola sebifera Aubl. (Myristicaceae) em mata mesofítica de Uberlândia, MG, Brasil. Brazilian Journal of Botany 29: 443-451. doi: 10.1590/S0100-84042006000300011), lower than for Tapirira guianensis (23.5%) (Lenza & Oliveira 2005Lenza E & Oliveira PE (2005) Biologia reprodutiva de Tapirira guianensis Aubl. (Anarcadinaceae), uma espécie dióica em mata de galeria do Triangulo Mineiro, Brasil. Brazilian Journal of Botany 28: 179-190. doi: 10.1590/S0100-84042005000100015) and Cabralea canjerana subsp. polytricha (32%) (Fuzeto et al. 2001Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003), but a little higher than for a few other dioecious species of Cerrado (Oliveira 1996Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191: 235-243.).
The low reproductive efficacy of these species may be due to the low frequency of the visits of the moths to pistillate and staminate flowers, in particular for S. versicolor. However, other dioecious Cerrado species are also pollinated by moths, such as C. canjerana subsp. polytricha (Fuzeto et al. 2001Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003) and Amaioua guianensis Aubl (Amorim & Oliveira 2006Amorim FW & Oliveira PE (2006) Estrutura sexual e ecologia reprodutiva de Amaioua guianensis Aubl. (Rubiaceae), uma espécie dióica de formações florestais de cerrado. Brazilian Journal of Botany 29: 353-362.) have higher reproductive efficiencies than S. amara, although they also have low pollinator’s visitation rates. Changes in the reproductive system of species may be common at the extremes of their distributions, where the primary pollinators may not be present and genetic diversity within populations may be smaller, increasing inbreeding and disrupting crossbreeding strategies (Barrett 1996Barrett SCH (1996) The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society B351: 725-733.; Reich et al. 2021Reich AR, Ollerton J, Dalsgaard B, Jorge LR, Sandel B, Svenning JC, Baronio GJ & Sazima M (2021) Population-level plant pollination mode is influenced by Quaternary climate and pollinators. Biotropica 53: 632-642. <https://doi.org/10.1111/btp.12905>). Also, we conducted the present study in a 5 km2 Cerrado vegetation fragment with areas in different conservation states, which may also contribute to the low visitation rate of the pollinators to the studied flowers.
The data showed here demonstrate that the studied species are dioecious with various characteristics consistent with this sexual system, such as small, inconspicuous flowers and staminate plants producing a more considerable amount of flowers. The pollination system of S. amara and S. versicolor is performed specifically by nocturnal moths, but the populations studied revealed low reproductive efficacy. This may be due to the lack of pollinators in the area or the inefficiency of the available pollinators. Instead, small nocturnal moths may also be another group of pollinators that inefficiently pollinated dioecious species, such as Meliaceae species (Bawa 1990Bawa KS (1990) Plant-pollinator interactions in tropical rain forests. Annual Reviews of Ecology and Systematics 21: 399-422.; Fuzeto et al. 2001Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003; Mabberley 2010Mabberley DJ (2010) Meliaceae. In: Kubitzki K (ed.) Flowering plants. Eudicots. The families and genera of vascular plants. Vol. 10. Springer, Berlin, Heidelberg. Pp. 185-211.; Franceschinelli et al. 2015bFranceschinelli EV, Carmo RM, Silva Neto CM, Mesquita Neto JN, Gonçalves BG & Bergamini LL (2015b) Reproductive success of Cabralea canjerana (Meliaceae) in Atlantic forest fragments, Brazil. Revista de Biologia Tropical 63: 515-524.). Future studies with both species in other better-conserved areas of Cerrado and the Amazon Forest (S. amara) could assess whether these observed characteristics and reproductive efficacy are consistent with their occurrence areas.
Acknowledgements
This research was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) agency, which provided a scholarship grant for INMF. DPS (CNPq-Proc. Number: 304494/2019-4) and EVF (CNPq-Proc. Number: 312325/2015-0) were supported by productivity grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico. We thank to Dr. Carolina M. dos Santos, for the Lepidopterans identification, and to Dr. Fernando A. Silveira, for the bee’s identification. We also thank to two anonymous reviewers, for their constructive comments, which improved the manuscript.
References
- Alves-dos-Santos I, Silva CI, Pinheiro M & Kleinert AMP (2016) Quando um visitante floral é um polinizador? Rodriguésia 67: 295-307. doi: 10.1590/2175-7860201667202
- Alves GGN, El Ottra JGL, Devecchi MF, Demarco D & Pirani JR (2017) Structures of the flower of Simaba (Simaroubaceae) and its anatomical novelties. Botanical Journal Linnean Society 183: 162-176. doi: 10.1111/boj.12486
- Amorim FW & Oliveira PE (2006) Estrutura sexual e ecologia reprodutiva de Amaioua guianensis Aubl. (Rubiaceae), uma espécie dióica de formações florestais de cerrado. Brazilian Journal of Botany 29: 353-362.
- Amorim FW, Mendes-Rodrigues C, Maruyama PK & Oliveira PE (2011) Sexual ratio and floral biology of the dioecious Neea theifera Oerst. (Nyctaginaceae) in a cerrado rupestre of central Brazil. Acta Botanica Brasilica 25: 785-792. doi: 10.590/S0102-33062011000400006
- Barrett SCH (1996) The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society B351: 725-733.
- Bawa KS (1980a) Evolution of dioecy in flowering plants. Annual Reviews of Ecology and Systematics 11: 15-39. doi: 10.1146/annurev.es.11.110180.000311
- Bawa KS (1980b) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34: 467-474. doi: 10.2307/2408216
- Bawa KS (1990) Plant-pollinator interactions in tropical rain forests. Annual Reviews of Ecology and Systematics 21: 399-422.
- Bawa KS (1994) Pollinators of tropical dioecious angiosperms: A reassessment? No, not yet. American Journal of Botany 81: 456-460. https://doi.org/10.2307/2445495.
- Bawa KS & Opler PA (1975) Dioecism in tropical forest trees. Evolution 29: 167-179. doi: 10.1111/j.1558-5646. 1975.tb00824.x
- Beach JH (1981) Pollinator foraging and the evolution of dioecy. The American Naturalist 118: 572-577.
- Bencke CS & Morellato LPC (2002). Comparação de dois métodos de avaliação da fenologia de plantas, sua interpretação e representação. Brazilian Journal of Botany, 25: 269-275.
- Bullock SH & Bawa KS (1981) Sexual dimorphism and the annual flowering pattern in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae) in a Costa Rican rainforest. Ecology 61: 1494-1504.
- Charlesworth B & Charlesworth D (1978) A model for the evolution of dioecy and gynodioecy. The American Naturalist 112: 975-997.
- Cipollini LM & Whigham DF (1994) Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae). American Journal of Botany 81: 65-75.
- Clayton JW (2011) Simaroubaceae. In: Kubitzki K (ed.) The families and genera of vascular plants. Flowering plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae. Springer, Berlin. Pp. 408-423.
- Cortes JM (2012) Desenvolvimento de espécies nativas do Cerrado a partir do plantio de mudas e da regeneração natural em uma área em processo de recuperação, Planaltina-DF. Dissertação de Mestrado. Universidade de Brasília, Brasília. 89p.
- Darwin CR (1877) The different forms of flowers on plants of the same species. John Murray, London.
- Delph LF, Galloway LF & Stanton ML (1996) Sexual dimorphism in flower size. The American Naturalist 148: 299-320.
- Devecchi MF & Pirani JR (2016) Flora of the cangas of the Serra dos Carajás, Pará, Brazil: Simaroubaceae. Rodriguesia 67: 1471-1476. doi: 10.1590/2175-7860201667551
- Dorken ME & Barrett SC (2004) Sex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proceedings of the Royal Society B Biological Sciences 271: 213-219. doi: 10.1098/rspb.2003.2580
- Endress PK (1995) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambrigde. 311p.
- Faegri K & Van der Pijl L (1979) Principles of pollination ecology. Pergamon Press, Oxford. 247p.
- Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24: 422-423.
- Franceschinelli EV, Yamoto K & Shepherd GJ (1999) Distinctions among three Simarouba species. Systematic Botany 23: 479-488. doi: 488 10.2307/2419379
- Franceschinelli EV, Carmo RM, Silva Neto CM & Mesquita Neto JN (2015a) Functional dioecy and moth pollination in Cabralea canjerana subsp. canjerana (Meliaceae). Darwiniana 3: 96-107. doi: 10.14522/darwiniana.2015.31.599
- Franceschinelli EV, Carmo RM, Silva Neto CM, Mesquita Neto JN, Gonçalves BG & Bergamini LL (2015b) Reproductive success of Cabralea canjerana (Meliaceae) in Atlantic forest fragments, Brazil. Revista de Biologia Tropical 63: 515-524.
- Freeman DC, Doust JL, El-Keblawi A, Miglia KJ & McArthur ED (1997) Sexual specialization and inbreeding avoidance in the evolution of dioecy. The Botanical Review 63: 65-92.
- Freitas L (2013) Concepts of pollinator performance: is a simple approach necessary to achieve a standardized terminology? Brazilian Journal of Botany 36: 3-8.
- Fuzeto AP, Barbosa AAA & Lomonaco C (2001) Cabralea canjerana subsp. polytricha (A. Juss.) Penn. (Meliaceae), uma espécie dióica. Acta Botanica Brasilica 15: 162-175. doi: 10.1590/S0102-33062001000200003
- Givnish TJ (1982) Outcrossing versus ecological constraints in the evolution of dioecy. The American Naturalist 119: 849-865.
- Grant V (1995) Sexual selection in plants: pros and cons. Proceedings of the National Academy of Sciences of the United States of America 92: 1247-1250. doi: 10.1073/pnas.92.5.1247
- Hardesty BD, Dick CW, Kremer A, Hubbell S & Bermingham E (2005) Spatial genetic structure of Simarouba amara Aubl. (Simaroubaceae), a dioecious, animal-dispersed neotropical tree, in Barro Colorado Island, Panama. Heredity 95: 290-297.
- Janzen DH (1977) A note on optimal mate selection by plants. The American Naturalist 111: 365-371.
- Kearns CA & Inouye D (1993) Techniques for pollinations biologists. Colorado, University Press of Colorado, Niwot. 583p.
- Lenza E & Oliveira PE (2005) Biologia reprodutiva de Tapirira guianensis Aubl. (Anarcadinaceae), uma espécie dióica em mata de galeria do Triangulo Mineiro, Brasil. Brazilian Journal of Botany 28: 179-190. doi: 10.1590/S0100-84042005000100015
- Lenza E & Oliveira PE (2006) Biologia reprodutiva e fenologia de Virola sebifera Aubl. (Myristicaceae) em mata mesofítica de Uberlândia, MG, Brasil. Brazilian Journal of Botany 29: 443-451. doi: 10.1590/S0100-84042006000300011
- Lloyd DG (1979) Evolution toward dioecy in heterostylous populations. Plant Systematics and Evolution 131: 71-80.
- Lloyd DG (1982) Selection of combined versus separate sexes in seed plants. The American Naturalist 120: 571-585.
- Lloyd DG & Webb CJ (1977) Secondary sex characters in plants. The Botanical Review 43: 177-216. doi: 10.1007/BF02860717
- Mabberley DJ (2010) Meliaceae. In: Kubitzki K (ed.) Flowering plants. Eudicots. The families and genera of vascular plants. Vol. 10. Springer, Berlin, Heidelberg. Pp. 185-211.
- Mendes FN, Rêgo MMC & Albuquerque PM (2011) Fenologia e biologia reprodutiva de duas espécies de Byrsonima Rich. (Malpighiaceae) em área de Cerrado no Nordeste do Brasil. Biota Neotropica 11: 103-115. doi: 10.1590/S1676-06032011000400011
- Moore JC & Pannell JR (2011) Sexual selection in plants. Current Biology 21: R176-R182.
- Oliveira PE (1996) Dioecy in the Cerrado vegetation of Central Brazil. Flora 191: 235-243.
- Oliveira PE & Maruyama PK (2014) Sistemas reprodutivos. In: Rech AR, Agostini K, Oliveira PE & Machado IC (org.) Biologia da polinização. Projeto Cultural, Rio de Janeiro. Pp. 71-93.
- Pinto AM, Ribeiro RJ, Alencar JC & Barbosa AP (2005) Fenologia de Simarouba amara Aubl. na reserva florestal Adolpho Ducke, Manaus, AM. Acta Amazonica 35: 347-352. doi: 10.1590/S0044-59672005000300007
- Pirani JR & Thomas WW (2015) Simaroubaceae. In: Jorgensen P, Nee M & Bech S (eds.) Catálogo de las plantas vasculares de Bolivia. Missouri Botanical Garden Press, Saint-Louis. Pp. 1199-1200.
- Piratelli AJ, Piña-Rodrigues FCM, Gandara FB, Santos EMG & Costa LGS (1998) Biologia da polinização de Jacaratia spinosa (Aubl.) ADC. (Caricaceae) em mata residual do Sudeste brasileiro. Revista Brasileira de Biologia 58: 671-679.
- R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Satistical Computing, Vienna. Available at <https://www.gbif.org/pt/tool/81287/r-a-language-and-environment-for-statistical-computing>. Acess on 9 August 2016.
» https://www.gbif.org/pt/tool/81287/r-a-language-and-environment-for-statistical-computing - Raw A & Hay J (1985) Fire and other factors affecting a population of Simarouba amara in “cerradão” near Brasília, Brazil. Revista Brasileira de Botanica 8: 101-107.
- Reich AR, Ollerton J, Dalsgaard B, Jorge LR, Sandel B, Svenning JC, Baronio GJ & Sazima M (2021) Population-level plant pollination mode is influenced by Quaternary climate and pollinators. Biotropica 53: 632-642. <https://doi.org/10.1111/btp.12905>
- Reis SM, Mohr A, Gomes L, Silva ACS, Abreu MF & Lenza E (2012) Síndromes de polinização e dispersão de espécies lenhosas em um fragmento de Cerrado sentido restrito na transição Cerrado - Floresta Amazônica. Heringeriana 6: 28-41.
- Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an up-dated online database. American Journal of Botany 101: 1588-1596. doi: 10.3732/ajb.1400196
- Renner SS & Feil JP (1993) Pollinators of tropical dioecious angiosperms. American Journal of Botany 80: 1100-1107. doi: 10.2307/2445757
- Renner SS & Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. American Journal of Botany 82: 596-606. doi: 10.2307/2445418
- Ross MD (1982) Five evolutionary pathways to subdioecy. The American Naturalist 119: 297-318.
- Souza MH (1997) Madeiras tropicais brasileiras. IBAMA/LPF, Brasília. 151p.
- Thomas SC & La Frankie JV (1993) Sex, size, and inter year variation in flowering among dioecious trees of the Malayan rain forest. Ecology 74: 1529-1537.
- Thomson JD & Barrett SCH (1981) Selection for outcrossing, sexual selection and the evolution of dioecy in plants. The American Naturalist 118: 443-449.
- Thomson JD & Brunet J (1990) Hypotheses for the evolution of dioecy in plants. Trends in Ecology & Evolution 5: 11-16.
- Tonnabel J, Patrice D & Pannell JR (2017) Sex-specific strategies of resource allocation in response to competition for light in a dioecious plant. Oecologia 185: 675-686. doi: 10.1007/s00442-017-3966-5
- Trevisan MTS & Macedo FV (2003) Seleção de plantas com atividade anticolinasterase para tratamento da doença de Alzheimer. Química Nova 26: 301-304.
- Venturoli F (2008) Manejo de Floresta Estacional Semidecídua Secundária em Pirenópolis, Goiás. Tese de Doutorado. Universidade de Brasília, Brasília. 203p.
- Willson MF (1994) Sexual selection in plants: perspectives and overview. The American Naturalist 144: 13-39.
Edited by
Publication Dates
-
Publication in this collection
01 Apr 2022 -
Date of issue
2022
History
-
Received
29 Oct 2020 -
Accepted
22 Apr 2021