RECOMMENDATIONS OF THE BRAZILIAN SOCIETY OF RHEUMATOLOGY
Recommendations for the management and treatment of ankylosing spondylitis* * Study with the seal of the Brazilian Society of Rheumatology.
Percival Degrava Sampaio-BarrosI,II,** ** Corresponding author. E-mail: pdsampaiobarros@uol.com.br (P.D. Sampaio-Barros). ; Mauro KeisermanIII; Eduardo de Souza MeirellesIV; Marcelo de Medeiros PinheiroV; Antonio Carlos XimenesVI; Valderílio Feijó AzevedoVII; Rubens BonfiglioliVIII; Sueli CarneiroIX,X; Roberto RanzaXI; Wanderley BernardoXII; Célio Roberto GonçalvesI,XIII
IDivision of Rheumatology, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil
IIPresident of the Spondyloarthritis Commision of the Brazilian Society of Rheumatology (2006-2012), São Paulo, SP, Brazil
IIIHospital São Lucas, Pontifícia Universidade Católica de Porto Alegre, Porto Alegre, RS, Brazil
IVUnit of Rheumatology, Service, Instituto de Ortopedia e Traumatologia, FMUSP, São Paulo, SP, Brazil
VUniversidade Federal de São Paulo, São Paulo, SP, Brazil
VIHospital Geral de Goiânia, Goiânia, GO, Brazil
VIIUniversidade Federal do Paraná, Curitiba, PR, Brazil
VIIIPontifícia Universidade Católica de Campinas, Campinas, SP, Brazil
IXUniversidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
XUniversidade Estadual do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
XIUniversidade Federal de Uberlândia, Uberlândia, MG, Brazil
XIICoordinator of the Projeto Diretrizes, Associação Médica Brasileira, São Paulo, SP, Brazil
XIIIPresident of the Spondyloarthritis Commision of the Brazilian Society of Rheumatology (2012-2014), São Paulo, SP, Brazil
Introduction
The concept of seronegative spondyloarthropathies was established in 1974, when British researchers Moll and Wright proposed the grouping of certain diseases that had been, until then, considered completely different from each other but that in fact had several common features. Such characteristics encompassed clinical aspects (inflammatory axial pain associated with arthritis, predominant in the large joints of the lower limbs, and peripheral enthesopathy), radiological aspects (sacroiliitis), and laboratory aspects (seronegativity for rheumatoid factor because, until the 70's, some researchers considered ankylosing spondylitis (AS) to be an axial component of rheumatoid arthritis) in individuals with a genetic predisposition (linked to the histocompatibility antigen HLA-B27). This set consisted of AS, psoriatic arthritis, reactive arthritis, and enteropathic arthropathies (associated with inflammatory bowel disease).1
In 2009, specialists from the Assessment on Spondyloarthritis International Society (ASAS) proposed changing the name of the group to spondyloarthritis (SpA), emphasising the axial ("spondylo") and peripheral ("arthritis") components of the diseases in this group. At the same time, the classification criteria were proposed for axial SpA2,3 and, subsequently, peripheral SpA.4 The changes also included a description of the criteria for the diagnosis of sacroiliitis by magnetic resonance (MR).5 These new diagnostic criteria and classifications contributed to a better characterisation of the broad spectrum of diseases in this group.
Among the diseases in the SpA group, without a doubt, AS is the most common and best represents the set of classical manifestations of SpA. A significant number of patients with an undifferentiated SpA diagnosis, which could initially be termed non-radiographic axial SpA or peripheral SpA, evolve to AS if followed long-term.6 Therefore, knowledge regarding the classification criteria for SpA and AS is important in the diagnosis and monitoring of patients in this group.With the advent of new treatments for AS, early diagnosis and the institution of a specific treatment is necessary to improve the quality of life of these patients, who are usually young adults in the prime of their productive lives.
1. What are the best criteria for an individual to be considered affected by a spondyloarthritis?
Axial spondyloarthritis
The ASAS group conducted a Delphi study with the participation of its members to select the possible variables that should be assessed in patients with axial SpA. These variables were evaluated in a prospective study that included 647 patients with back pain lasting over three months without apparent cause or known diagnosis, with or without peripheral symptoms, whose symptoms started before the age of 45 years, followed up in 25 universities of 16 countries.
After statistical assessment, the criteria proposed were based on two main variables: sacroiliitis by imaging (hip radiograph or MRI) and the histocompatibility antigen HLA-B27. The presence of a main variable (sacroiliitis by imaging or HLA-B27 positive) and of one (when sacroiliitis by imaging) or two (when HLA-B27 positive) criteria that are characteristic of SpA [inflammatory back pain, peripheral arthritis, enthesitis, dactylitis, cutaneous psoriasis, Crohn's disease or ulcerative colitis, good response to nonsteroidal anti-inflammatory drugs (NSAID), family history of SpA, HLA-B27 positive, elevated C-reactive protein] are crucial for the patient to be classified with axial SpA. The sensitivity of this criteria group is 82.9%, and the specificity is 84.4%2,3(B).
Peripheral spondyloarthritis
The criteria from the ASAS group for peripheral SpA are as follows: peripheral joint manifestations (arthritis, enthesitis, dactylitis) associated with one or more variables (psoriasis, inflammatory bowel disease, previous infection, HLA-B27, uveitis, sacroiliitis imaging) or two or more parameters (arthritis, enthesitis, dactylitis, inflammatory low back pain in the past, family history of spondyloarthritis) in patients with peripheral manifestations starting prior to 45 years of age. The sensitivity of these criteria is 79.5%, and the specificity is 83.3%. Thus, in a clinical setting with a high prevalence of peripheral SpA (66.2%), the use of these diagnostic criteria increases the probability of a diagnostic certainty to 90%4(B).
Recommendation 1
Currently, the best criteria that allows for the classification of a patient as having axial or peripheral SpA is the one proposed by the ASAS.
2. What is the role of MRI in the initial evaluation of axial spondyloarthritis?
Diagnosis
The use of MRI, through components such as oedema, erosion, fat infiltration, and ankylosis, allows for the diagnosis of axial SpA with sensitivity and specificity of 90% and 97%, respectively, conferring a positive likelihood ratio of 30 and, therefore, a 97% diagnostic certainty when positive and 91% when negative7(A).
The diagnosis of more than five fatty Romanus lesions (high signal on T1-weighted MRI) is associated with an axial SpA diagnosis in patients with low back pain, with an 86% certainty (likelihood ratio: 12.6)8(B).
Prognosis
The combination of severe sacroiliitis, diagnosed by MRI, with a positive HLA-B27 predicts the development of future AS (eightyear follow-up) with a sensitivity and specificity of 62% and 92%, respectively, with a positive post-test probability of 80% and a negative post-test probability of 83%. Significant sacroiliitis, isolated, predicts the diagnosis with a positive posttest probability of 50% and a negative post-test probability of 84%9(A).
The persistence of active inflammation in the shiny corners, diagnosed by MRI in AS patients receiving anti-TNF α treatment during two years of follow-up, predicts a 14.9% increase (number needed to harm, NNH: 7) in the risk of developing new syndesmophytes. In cases where the inflammation has been treated with anti-TNFα, this risk increases by 11.4% (NNH: 8)10(A).
A two- to seven-year follow-up of patients with axial SpA and the evaluation of sacroiliac changes (Danish score - erosion, oedema, and fatty infiltration) by MRI showed that chronic changes increase the risk in AS patients. Activity scores > 2, chronic > 1, erosion > 1, and of fatty infiltration > 4 at the beginning of follow-up are associated with the chronicity of the sacroiliac changes with a diagnostic accuracy of 74%, 77%, 79%, and 68%, respectively11(A).
Recommendation 2
In patients with axial SpA, magnetic resonance has diagnostic and prognostic importance.
3. When should HLA-B27 be requested in a patient with axial spondyloarthritis?
Axial spondyloarthritis
The prevalence of positive HLA-B27 in patients with axial SpA is increased by 38.2% compared with patients without axial SpA. HLA-B27 is one of the classification criteria required, and, when associated with other variables (such as images and clinical criteria), it allows for the classification of a patient as having axial SpA with a sensitivity and specificity of 83.7% and 83.3%, respectively, leading to a diagnostic certainty of 83% when positive and negative3(B).
Ankylosing spondylitis
In patients with AS, the prevalence of positive HLA-B27 can be 90.2%. Compared with negative HLA-B27 patients, the patients with AS have a longer disease duration, a 23.6% (NNH: 4) increase in previous or current use of NSAIDs, and an 18.9% (NNH: 4) increase in the risk of biological indicators (ASAS criteria). HLA-B27-positive patients may have a more severe disease, with an increase in ocular (38.9% vs. 12.5%), pulmonary (4.2% vs. 0%), and cardiac (4.3% vs. 0%) comorbidities associated with higher values of the functional index (Bath Ankylosing Spondylitis Functional Index, BASfi) and disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI)12(B).
The analysis of predictive factors of AS in patients with inflammatory low back pain shows that sacroiliitis diagnosed by MRI, when associated with positive HLA-B27, increases the specificity and sensitivity of the diagnosis compared with imaging alone from 84% to 94% and 33% to 62%, respectively. A positive HLA-B27 alone is capable of predicting the disease with a 48% probability and can rule out the disease when negative with a probability of 88%13(A).
In the evaluation of AS patients sorted by age at disease onset (< 20 years of age, 21-30 years of age, 31-40 years of age, and > 40 years of age), a positive HLA-B27 is found in 94.6%, 90.2%, 74.1%, and 61.2% of the patients, respectively. Thus, positive HLA-B27 is associated with a younger age of onset14(B).
The chance of the presence of the HLA-B27 gene in patients with familial AS is 344% higher than in patients with sporadic ankylosing spondylitis15(B).
Recommendation 3
HLA-B27 is particularly useful in determining prognosis, especially in AS patients and with regard to time of onset.
4. What is the evidence for the use of physical rehabilitation in patients with ankylosing spondylitis?
The treatment of AS patients based on postural rehabilitation and the rehabilitation of the flexor and extensor musculature, according to the Global Postural Reeducation (GPR) method, or by means of 20 exercises (cervical, thoracic and lumbar mobility and flexibility, stretching of flexor musculature and strengthening of extensor musculature, and exercises for thoracic expansion) provides significant improvement compared with pre-treatment, as measured using the Bath Ankylosing Spondylitis Metrologic Index (BASMI, which includes the Schöber modified test, cervical rotations, lumbar flexion, and intermalleolar distance), BASDAI, and BASfi. A comparison between the two treatments showed better results with postural rehabilitation using GPR within a year of follow-up16,17(A).
AS patients undergoing regular rehabilitations programs for four weeks were subjected to evaluation after 28 weeks regarding their health (patient global assessment, pain, morning rigidity, BASfi, BASDAI, and fatigue) and the ASAS-IC (Assessments in Ankylosing Spondylitis working group's Improvement Criteria) criteria. The programs offer personalised evaluation of physical therapy, group exercises, passive therapy, relaxation, and patient education, with differences in two components - resistance versus mobility. After 16 weeks, both forms of rehabilitation (resistance and mobility) showed significant improvements in several variables (except BASDAI); however, the rehabilitation centred on mobility led to an increase in the proportion of patients achieving ASAS20 and ASAS40 to 27% (number needed to treat, NNT: 4) and 19% (NNT: 5), respectively, compared with resistance. In 28 weeks of follow-up, the results were greater in patients with dominance in the mobility component. At 16 weeks, mobility increased the benefits as measured using the Schöber test (20% and 40%) in 18% and 19% of patients, respectively, and with respect to lateral flexion (20% and 40%) in 37% and 36% of patients, respectively18(A).
Recommendation 4
Rehabilitation programs benefit AS patients, especially during the period in which the patients are undergoing rehabilitation.
Specific programs that focus on mobility improvement have shown superior results.
5. What is the evidence for the use of corticosteroids in patients with ankylosing spondylitis?
The comparison of two methylprednisone doses (375 mg vs. 1 g intravenous for three days) in the treatment of AS patients not responding to NSAIDs demonstrated that thoracic and lumbar mobility, pain, and morning rigidity improved at both doses with no differences between the doses. Adverse events occurred in both groups, with the main events being dizziness, dry mouth, sleep disturbances, irritability, impotence, and weight gain19(A). Methylprednisone has not been used in clinical practice in the last decade due to its side effects and the emergence of more advanced therapeutic modalities.
The treatment of patients with AS and low back pain for more than three months with an injection of 40 mg of triamcinolone acetate in the sacroiliac joint guided by computed tomography (CT) demonstrated, after six months of follow up, a decrease in pain intensity (assessed by the visual analogue scale, VAS), and in sacroiliac pain (assessed by MRI) with evidence of a 72% increased response (NNT: 1). There was also a significant reduction of 31% in the use of NSAIDs and in Mennell's sign (NNT: 3)20,21(B).
Recommendation 5
An intra-articular injection with triamcinolone acetate in the sacroiliac joint may provide short- and medium-term benefits, and it is a therapeutic option for cases that are non-responsive to NSAIDs and exhibiting isolated sacroiliac pain. There is no evidence that allows for the evaluation of low doses of prednisone (or equivalent corticosteroid) in ankylosing spondylitis.
6. In which situations should continuous NSAID use be recommended for patients with ankylosing spondylitis?
The comparison of the use of 20 mg/day piroxicam, 15 mg/day meloxicam, or 22.5 mg/day meloxicam in patients with AS for 52 weeks showed a reduction in pain intensity in 27%, 28%, and 27% of patients, respectively. The increase in percentage response (Ankylosing Spondylitis Functional Index, ASfi) was similar after 6 weeks and 12 months of follow up after treatment with piroxicam, 15 mg meloxicam, and 22.5 mg meloxicam, being 20%, 33%, and 26%, respectively. A small percentage (16%; NNT: 6) of patients using 22.5 mg meloxicam had to discontinue treatment for 12 months compared with all other treatments. In 52 weeks, there were signs of increased gastrointestinal adverse events of 19%, 7%, and 5% after treatment with piroxicam, 15 mg meloxicam, and 22.5 mg meloxicam, respectively22(A).
Patients with AS and pain between 40 mm and 100 mm (VAS), treated with etoricoxib at 90 mg/day, etoricoxib at 120 mg/ day, or naproxen at 500 mg 2x/day showed significant improvement in back pain (100 mm, VAS) and disease activity according to the patient global assessment (100 mm, VAS) and functional assessment (BASfi) after six weeks of follow-up. Comparing medications, etoricoxib had results superior to naproxen, while the different doses of etoricoxib had similar results. There was an increase in the proportion of patients achieving the criteria for partial remission with 90 mg etoricoxib (NNT: 9), 120 mg etoricoxib (NNT: 7), and naproxen (NNT: 16).There was no difference between adverse events; the most common adverse events were as follows: headache; diarrhoea; heartburn; respiratory infection; and gastrointestinal and cardiovascular events23(A).
In patients requiring daily NSAID treatment and exhibiting a pain intensity greater than 50 mm (VAS), a comparison of celecoxib at 200 mg/day, celecoxib at 400 mg/day, or naproxen at 500 mg 2x/day showed, at 12 weeks, that the three treatment regimens produced improvements and benefits with regard to pain intensity, disease activity (patient global assessment, VAS), functional improvement (BASfi), and adverse events.The regimen with the lowest effect was 200 mg celecoxib.
The most common adverse event was gastrointestinal disorders, the most common of which was dyspepsia. With naproxen, there were severe events (ulcers and haemorrhage)24(A). A comparison of celecoxib at 200 mg or 400 mg with diclofenac at 72 mg 2x/day showed a 13% (NNH:8) increase in gastrointestinal adverse events with diclofenac25(A).
As per the analgesic action in AS patients, the NNT in six weeks of follow-up of 90 mg etoricoxib, 120 mg etoricoxib, and 1 g naproxen, relative to back pain improvement (> 30%), was 2, 2, and 3, respectively; relative to an improvement > 30% in the BASDAI score, the NNT was 2, 2, and 3, respectively26(A).
Using NSAIDs (celecoxib at 100 mg or 200 mg 2x/day) continuously or on demand over two years in AS patients can lead to benefits related to the signs and symptoms and radiological progression of the lesion (Stoke Ankylosing Spondylitis Spine Score) or damage related to adverse events. The signs and symptoms after 24 months of follow up were similar between the two regimens; however, the radiological progression was three times higher in the on-demand regimen than in the continuous regimen. Although there were more adverse events in the continuous regimen, the difference was not significant.The most common adverse events were hypertension, abdominal pain, and dyspepsia27(A).
Recommendation 6
The continuous use of NSAIDs is more effective then on-demand use. In patients with moderate to severe pain, the prescription of COX-2 inhibitors is a long-term treatment option.
7. What is the evidence for the use of conventional drugs (methotrexate and sulfasalazine, among others) in patients with ankylosing spondylitis?
Methotrexate
There is evidence that the use of methotrexate at 10 mg/week for 24 weeks in patients with AS produces no difference in disease activity (as measured by BASDAI) and mobility (as measured by BASMI) compared to patients without methotrexate treatment28(B).
A response (compound index) to treatment of AS patients with methotrexate at 7.5 mg/week for 24 weeks is considered when a result > 20% is obtained in at least five of the following scales: a) intensity of morning rigidity (VAS); b) physical wellbeing (VAS); c) disease activity (BASDAI); d) function (BASfi); e) function (Health Assessment Questionnaire for Spondyloarthropathies, HAQ-S); f) disease activity (Physician's global assessment, VAS); and g) disease activity (How do you describe the current level of disease activity?). When comparing patients with receiving methotrexate or not, we observed a 42% increase (NNT: 2) in response (compound index) at 24 weeks and a 32% increase (NNT: 3) in response (BASDAI). Adverse events did not differ among patients who did or did not use methotrexate29(A).
Sulfasalazine
There was no difference between the beginning of treatment with sulfasalazine (2 to 3 g/day) and after 3-36 months of treatment with regard to physical function, pain, spine mobility, peripheral arthritis, and patient global assessment. However, when comparing the outcomes of response among patients treated or not with sulpha, there was a significant difference in favour of treatment. Regarding spine rigidity, sulfasalazine treatment reduced the score (VAS), although there was no difference in outcome regarding morning rigidity. There was an increase of
0.47 in the risk of loss of adherence to treatment due to adverse events in patients treated with sulpha (relative risk reduction), with reports of serious adverse reactions (pruritic erythematous rash, with nausea, anorexia, and insomnia)30(A).
In patients with axial SpA, remission (ASAS criteria and MRI) at 48 weeks was greater in patients treated with etanercept (33%) compared with sulphasalazine (11%). However, after a year of follow up, there was no difference in the response between the two treatments31(B).
Leflunomide
In AS patients, the number of responders according to the ASAS20 criteria with the use of leflunomide (27%) was similar to patients not undergoing treatment (20%). After 24 months of treatment, there was no significant difference in disease activity (BASG), the index of disease activity (BASDAI), the functional index (BASfi), pain, mobility (BASMI), or joint oedema.There was a 20% increase (NNT: 5) in the risk of adverse events: gastrointestinal disorders; respiratory infections; dermatitis and pruritus; fatigue; venous thrombosis; and elevated hepatic enzymes32(A).
Recommendation 7
Methotrexate and sulfasalazine represent therapeutic options for ankylosing spondylitis.
8. What are the indications for the use of biological agents that block tumour necrosis factor (anti-TNF) in ankylosing spondylitis?
Infliximab
The treatment of patients with active or severe AS (BASDAI > 4 and back pain > 4 mm, VAS) by means of an intravenous infusion of infliximab (5 mg/kg) on weeks 0, 2, and 6 can produce benefits related to a reduction in disease activity by 50% as measured by the criteria of disease activity (BASDAI), functional index (BASfi), and mobility (BASMI).
Infliximab is effective with regard to all criteria: a 44% (NNT: 2) increase in the percentage of patients with an improved BASDAI (50% improvement) at 12 weeks, with a 38% improvement in the score relative to the previous week at the start of the treatment; a 27% (NNT:4) and 17% (NNT:6) reduction in the percentage of arthritis and enthesitis after 12 weeks, respectively; a 27% (NNT:3) reduction in the use of NSAIDs (50%); and a 28% (NNT:4) reduction in the number of patients using NSAIDs during the period. The most frequent adverse events were respiratory infection, ganglionic tuberculosis, fever, and leukopenia33(A).
Maintenance of treatment with infliximab (5 mg/kg every 6 weeks) after the initial phase (weeks 0, 2, and 6) and after 54 weeks showed that 47% of the patients (NNT: 2) had a reduced BASDAI (50% reduction). Medication use was reduced by 70%, with a 33.3% reduction in the indices of peripheral arthritis and enthesitis (NNT: 3) and a 31% reduction in the number of hospitalisations (NNT: 3)34,35(B).
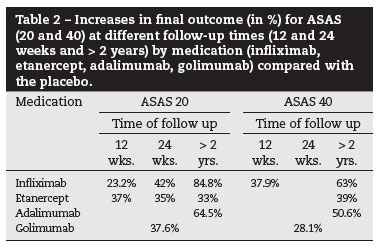
Over two years, 58% of patients achieved at least 50% reduction in the BASDAI score, which is similar to the response level after a year. Of the treated patients, 94% and 85% reached at least a 20% response on BASDAI in weeks 54 and 102, respectively. Changes in the BASfiand BASMI score were significant compared with the beginning of the treatment, and they were similar between years one and two of follow up36(A). During the second year of follow up, 90% of patients reported adverse events, with the most frequent being respiratory infection, rhinitis, herpes, osteoporosis, syncope, pancreatitis, and metrorrhagia37(B). After three years (156 weeks) of follow up, response to treatment measures by the ASAS40 was 50%38(B). After five years of follow-up, the ASAS20 and ASAS40 responses were 84% and 63%, respectively39(B).
At the end of eight years of follow up, a state of lower disease activity (BASDAI < 3) was achieved in 63.6% of patients, and this result was similar to that obtained at three months of follow-up. Furthermore, 71.4% remained with BASDAI values < 3. The ASAS20 response was maintained at 84.8%, and BAS-DAI50 was present in 57.6% of the cases. The adverse events were tuberculosis, allergic granulomatosis, pancreatitis, and an overall decrease in general health. There was a reduction in enthesitis by 30.3% (NNT: 3), in peripheral arthritis in 21.2% (NNT: 5), and uveitis in 36% (NNT: 3)40(B).
The ASAS20 criteria as a measure of response to treatment of AS patients is composed of at least a 20% improvement after treatment, with absolute improvement of at least one unit (scale 0 to 10) in at least three of the following areas without deterioration of the initial condition: patient global assessment; back pain; function (BASfi); and morning rigidity (questions five and six of BASDAI). AS patients with BASDAI > 4 and back pain > 4 mm (VAS) treated with 5 mg/kg infliximab on weeks 0, 2, 6, 12, and 18 show an increase of 42% (NNT: 2) in the ASAS20 response and of 21.1% (NNT: 5) in partial ASAS response after 24 weeks of follow up. The BASDAI response increases in 40.3% (NNT: 2) and the BASfiresponse in 34.2% (NNT: 3) of patients. Serious adverse events occur such as dizziness, cholecystitis, arthritis, leukocytosis, pneumonia, hemiparesis, low back pain, fever, and ganglioneuroma36(A).
In patients with AS and a BASDAI score > 4, treatment with infliximab at 3 mg/kg on weeks 0, 2, and 6 led to, at 12 weeks of follow up, increases in the percentage of patients who reach ASAS20 by 23.2% (NNT: 4) and those who reach ASAS40 by 37.9% (NNT: 3). At up to one year of follow up, the increased dose of 5 mg/kg maintained the clinical response. The most common adverse events were nasopharyngitis, respiratory infection, itching, nausea, dizziness, headache, and fever41(A).
Etanercept
Patients with active AS, defined by inflammatory back pain, morning rigidity for at least 45 minutes and moderate disease activity, who underwent treatment with etanercept at 25 mg SC 2x/week for four months, increased the treatment response by 50% (NNT: 2), defined as > 20% improvement in three of five measures of disease activity (ASAS, BASfi, patient global assessment, and joint swelling). Adverse events were mild infection, diarrhoea, tinnitus, and headache42(A).
In patients with active AS (BASDAI > 4 and with back pain > 4 mm,VAS), treatment with etanercept at 25 mg SC 2x/week for six months had the following benefits: a 51% increase in BASDAI50 score (NNT: 2); a 53.6% increase in ASAS20 score (NNT: 2); and a 55% increase in the discontinuation of the use of NSAIDs (NNT: 2). In the three-month follow-up, 75% of the patients showed reoccurrence, and the most common adverse event was respiratory infection43(A).
The treatment of patients with active AS (a score of 30 mm for morning rigidity measured by VAS and a score of 30 mm for two of three parameters - patient global assessment, low back pain measured by VAS, and BASfiscore) with etanercept at 25 mg SC 2x/week for 24 weeks resulted in an increase of 31% (NNT: 3) in ASAS20 at 12 weeks, an increase of 35% (NNT: 3) in ASAS20 at 24 weeks, and an increase in the BASDAI score and in mobility. Regarding adverse events, the most common were as follows: lymphadenopathy; cellulitis; respiratory infection; ulcerative colitis; intestinal obstruction; and bone fractures44(A). The proportions of patients who, after 192 weeks of treatment, achieved ASAS20 and ASAS40 responses were 81% and 69%, respectively, with increases of 33% and 39% compared with the scores after two weeks of treatment45(B).
Considering AS activity with an intensity > 30 mm (VAS) measured in four domains [spinal inflammation (score), low back pain, global disease activity assessment, and physical function], the prescription of etanercept (25 mg, 2x/week) for 12 weeks resulted in a 37% increase (NNT: 3) in the ASAS20 response, a 50% increase in the ASAS50 response, a 43% improvement in spinal inflammation and low back pain, a 37% reduction in the patient global assessment, a 35% improvement in the functional index (BASfi), and a 62% improvement in the disease activity index (BASDAI). The most common adverse events were as follows: allergic reaction or bleeding at the site of injection; headaches; nausea; asthenia; dizziness; diarrhoea; rash; abdominal pain; and paresthesia46(A).
AS patients showing one of the criteria that defines severe spinal ankylosis (i.e., two intervertebral adjacent bridges and/ or lumbar spine fusion; three intervertebral adjacent bridges and/or thoracic spine fusion; or two intervertebral adjacent bridges and/or cervical spine fusion) can be treated with etanercept at 50 mg/week for 12 weeks. This treatment led to improvement in the following parameters: a 34% increase (NNT: 3) in the percentage of patients who achieve ASAS20; a 23% increase (NNT: 5) in patients who achieve BASDAI50; a 25% increase (NNT: 4) in the number of patients with improvements through Minimum Clinically Important Improvement (MCII); and improvement of parameters of pulmonary function (vital capacity, VC), forced VC, and the FEV1/VC ratio. The most common adverse events were infusion reactions and neutropenia47(A).
AS with inflammatory activity (BASDAI > 4 and back pain > 4) maintained for more than 12 weeks that is non-responsive to treatment of less than two NSAIDs for at least three months can be treated with etanercept at 50 or 100 mg/week for 12 weeks with the following results: there was no difference between the two treatments with respect to ASA20,ASAS40, partial remission, or BASDAI. Adverse events in both treatment regimens did not differ, the main such events being infection, gastrointestinal disorders, and infusion reactions48(A).
Adalimumab
The treatment of AS patients non-responsive to NSAIDs with 40 mg adalimumab every other week for 24 weeks reduces pain [measured by total back pain scores (VAS), night pain (VAS), Medical Outcomes Study Short Form-36 Health Survey (SF-36)], improvement of fatigue and of disease activity (measured by BASDAI), and improvement in morning rigidity (BASfi)49(B).
AS patients treated with 40 mg adalimumab every other week for 24 weeks showed improvement in activities related to the disease (BASDAI), function (BASfi), and quality of life [measured byAS Quality of Life Questionnaire (ASQoL)].There was a 27.2% increase (NNT: 4) in the Minimum Clinically Important Difference (MCID) score. The responses/improvements measured by the ASA20 and ASAS40 scores were 64.5% and 50.6%, respectively, and these were maintained for two years of treatment. The follow up of patients for three and five years of treatment showed the stability of the response measured by various scores, including SF-3650-52(A).
Golimumab
AS patients with a BASDAI score > 4, a back pain score > 4 (VAS), and an inadequate response to previous use of NSAIDs or drugs that alter the disease course, when treated with golimumab at 50 mg or 100 mg every four weeks for 24 weeks, showed the following results: 37.6% and 38.2% increases in the ASAS20 responses for the 50 mg and 100 mg doses, respectively; 28.1% and 38.9% increases in the ASAS40 responses for the 50 mg and 100 mg doses, respectively; and a 36.1% increase in the percentage of patients who achieve a BASDAI50 score (NNT: 3). Up to the 24th week, the proportion of patients who experienced at least one adverse event was 79.9%, regardless of dose, but with an increase in patients who did not use active drugs. The adverse events included the following: nasopharyngitis; respiratory infections; fatigue; headache; diarrhoea; erythema at the injection site; and increased liver enzymes. The proportion of patients with a serious adverse event was 3.6% with the 50 mg dose and 6.4% with the 100 mg dose. The adverse events included the following: myocardial infarction; fatigue; depression; hypertension; chest pain; blepharitis; nausea; vomiting; hepatitis; influenza-like symptoms; pain in the extremities; and weight gain53(A). There were signs of benefits with respect to sleep quality [measured by the Jenkins Sleep Evaluation Questionnaire (JSEQ)]54(A). The follow up of these patients for two years showed good maintenance of this good response according to the different scores.55
Recommendation 8
Patients with active and severe AS, clinically defined as BAS-DAI > 4 and back pain > 4 mm (VAS), and with no response for three months with NSAIDs and/or drugs that can alter the disease course can be treated as follows: with infliximab at 3-5 mg/kg every six to eight weeks for up to eight years of follow up; with etanercept at 50 mg per week, for up to four years of follow up; with adalimumab at 40 mg every other week for up to five years of follow up; or with golimumab at 50 mg every four weeks, for up to two years.
9. Is there a difference in efficacy among anti-TNF drugs in patients with ankylosing spondylitis?
The main results of individual efficacy of infliximab, etanercept, adalimumab and golimumab in BASDAI, ASAS, discontinuation of NSAIDs, hospitalisation, arthritis, and enthesitis are described below.
Infliximab
Infliximab is effective with the following criteria: a 44% increase (NNT: 2) in the percentage of patients with an improved BASDAI50 at 12 weeks, with a 38% improvement in the score compared to the week prior to treatment; a 27% (NNT: 4) and a 17% (NNT: 6) reduction in the percentage of patients experiencing arthritis and enthesitis, respectively, after 12 weeks; a 37% reduction (NNT: 3) in the use of NSAIDs (50%); and a 28% increase (NNT: 4) in the number of patients not needing NSAIDs in that period33(A).
After 54 weeks, 47% of the patients (NNT: 2) had a 50% reduction in BASDAI; the use of other medications also decreased by 70%.There was a 33.3% (NNT: 3) reduction in the prevalence of peripheral arthritis and enthesitis and a 31% reduction in the number of hospitalisations (NNT:3)34,35(B).
In the two-year follow-up, 58% of the patients reached at least a 50% reduction in BASDAI, which is a level similar to the one-year response. Of the patients treated, 94% and 85% achieved at least 20% BASDAI response at weeks 54 and 102, respectively37(B).
The efficacy measured by ASAS40 is 50%37(B). After five years of follow up, the ASAS20 and ASAS40 responses were 84% and 63%, respectively39(B). At the end of the eight years of follow up, a lower activity state of disease (BASDAI < 3) was present in 63.6% of patients - results similar to those obtained in three months of follow up. Furthermore, 71.4% of patients remained with BASDAI values < 3; the ASAS20 response is maintained at 84.4%, and BASDAI50 is present in 57.56% of all cases40(B).
After 24 weeks of follow up, there was a 42% increase (NNT: 2) in ASAS20 response and 21.1% increase (NNT: 5) in partial ASAS response. The BASDAI response increases in 40.3% (NNT: 2) and the BASfiresponse in 34.2% (NNT: 3)40(A). In 12 weeks of follow up, there was an increase of 23.2% (NNT: 4) in the percentage of patients who achieved ASAS20 and of 37.9% (NNT: 3) in patients who achieved ASAS4040(A).
Etanercept
Etanercept use has benefits for treated patients; it increased the BASDAI50 response by 51% (NNT: 2), the ASAS20 score by 53.6% (NNT: 2), and discontinuation of NSAID use by 55% (NNT: 2)40(A). Furthermore, it resulted in a 31% increase (NNT: 3) in ASAS20 after 12 weeks, a 35% increase (NNT: 3) in ASAS20 after 24 weeks, and improvements in BASDAI score and mobility44(A).
The proportions of patients who reach ASAS20 and ASAS40 responses after 192 weeks of treatment are 81% and 69%, respectively, with increases of 33% and 39% compared with the score two weeks prior to treatment45(B).
The use of etanercept resulted in a 37% increase (NNT: 3) in ASAS20 response, a 50% increase in the ASAS50 response, a 43% improvement in spine inflammation and low back pain, a 37% reduction in the overall assessment of the patients, and 35% and 62% improvements in BASfiand BASDAI, respectively46(A).
Adalimumab
The responses/improvements measured by the ASAS20 and ASAS40 score were 64.5% and 50.6%, respectively, and were maintained over two years of treatment50,51(A).
Golimumab
At 24 weeks, Golimumab showed the following results: increases of 37.6% and 38.2% in ASAS20 for the 50 mg and 100 mg doses, respectively; increases of 28.1% and 38.9% in ASAS40 with the 50 mg and 100 mg doses, respectively; and a 36.1% increase (NNT: 3) in the percentage of patients who achieved BASDAI5053,54(A).
Synthesis of the results by outcome
Tables 1, 2, and 3 show the synthesis of the BASDAI and ASAS outcomes, and reduction of NSAID use, respectively.
Arthritis and enthesitis
Infliximab
A reduction of 27% (NNT: 4) and of 17% (NNT: 6) at 12 weeks. A reduction of 33% (NNT: 3) at 54 weeks.
Hospitalisation
Infliximab
A reduction of 31% (NNT: 3) at 54 weeks.
The common outcome among the medications allows for indirect comparison by calculation of NNT for the main outcomes: BASDAI 20 and 50, ASAS 20 and 40, and a reduction in NSAID use (50%) (Table 4).
Recommendation 9
The anti-TNF biological agents (infliximab, etanercept, adalimumab, and golimumab) showed benefits in the treatment of AS patients with regard to the BASDAI and ASAS criteria and NSAID use. None of the drugs was more effective than the others.
10. Is there a difference in the safety among the anti-TNF drugs in patients with ankylosing spondylitis?
There is heterogeneous information with regard to adverse events among the four medications being used, likely due to factors such as follow-up time, the number of consistent studies available, the multiplicity of events that occur, and partial information retrieved. Nevertheless, several adverse events are common to all forms of treatment.
Infliximab
The most common adverse event was respiratory infection, which can lead to ganglionar tuberculosis, pulmonary granulomatosis, or leukopenia. Discontinuity of treatment due to severe adverse events occured in 12% of patients33(A).
A high proportion of patients (82.2%) showed more than one adverse event. The majority of adverse events were moderate; however, 3.5% of patients showed serious adverse events (dizziness, cholecystitis, arthritis, leukocytosis, pneumonia, hemiparesis, low back pain, fever, and ganglioneuroma), and 2.7% of patients discontinued treatment. Other adverse events were pharyngitis, rhinitis, a transient elevation of liver enzymes, and nausea36(A).
Severe adverse events occurred at a rate of 12%, and the discontinuation rate was 6%. During the second year of follow up, 90% of patients reported adverse events, and the most frequent were the following: respiratory infection, rhinitis, herpes, myalgia, pancreatitis, and infusion reactions37(B).
Adverse events occurred 90.8% of the time; most were moderate, but 18.4% were severe, 9.2% required hospitalisation, and 2.6% led to discontinuation of treatment. The most common adverse events were nasopharyngitis, respiratory infection, itching, nausea, dizziness, headache, and fever41(A).
Of the patients who discontinued treatment, 55% did so due to the adverse events, which most frequently were increased liver enzymes, infusion reaction, and a loss of efficacy. Other adverse events were tuberculosis, allergic granulomatosis, pancreatitis, and a worsening of the general condition. There has been shown a 30.3% reduction (NNT: 3) in enthesitis, 21.2% (NNT: 5) in peripheral arthritis, and 36% (NNT: 3) in anterior uveitis40(B).
Etanercept
The most common adverse events were mild infections, injection site reactions, diarrhoea, tinnitus, orbicular oculi fasciculation, headache and respiratory infections, nausea, asthenia, dizziness, abdominal pain, and paresthesias42,43,46(A).
There may be a 5% discontinuity in the treatment with etanercept due to adverse events, with the most common being the following: lymphadenopathy; cellulitis; respiratory infection; ulcerative colitis; intestinal obstruction; and bone fractures44(A).
Severe adverse events (lung cancer and neutropenia, 5%) also led to discontinuation of treatment. The most common adverse events (62% of cases) were infusion reaction and neutropenia47(A).
Fifty percent of patients experienced adverse events, and 5% of these were severe (diarrhoea with abdominal pain and distension). The main adverse events were infections, gastrointestinal disorders, and infusion reactions48(A).
Adalimumab
During two years of adalimumab use, adverse events were moderate and the most common (5%) were nasopharyngitis, respiratory infection and headache, and Crohn's disease; 10% were severe adverse events, 4.5% led to discontinuation of the medication, 1.3% were neoplasias, and 3.9% were uveitis50(A).
Golimumab
Up to the 24th week, the proportion of patients who experienced at least one adverse event was 79.9%, with no difference with respect to dosing but with an increase in patients not using the active drug. These adverse events included the following: nasopharyngitis; respiratory infections; fatigue; headache; diarrhoea; erythema at the injection site; increased liver enzymes; and at least one infection (48.6%).The proportion of patients with one severe adverse event was 3.6% with 50 mg and 6.4% with 100 mg; among them were the following: myocardial infarction; fatigue; depression; hypertension; chest pain; blepharitis; nausea; vomiting; hepatitis; influenza-like symptoms; extremity pain; and weight gain. Due to these effects, 2.9% of patients discontinued treatment53(A).
Recommendation 10
The moderate and severe adverse events and treatment discontinuation show similar indices and types in all four treatment regimens (infliximab, etanercept, adalimumab, and golimumab). No drug is safer than the others.
11. Is the use of anti-TNF treatment capable of reducing structural damage in patients with ankylosing spondylitis?
Infliximab
The treatment of AS patients with infliximab at 5 mg/kg reduced the number of bone lesions (by MRI) in 30 weeks of follow up. This result was superior when combined with methotrexate compared with treatment with methotrexate alone. A significant increase in bone mineral density occurred in patients treated with infliximab, especially in the femur, pelvis, and spine56(A).
AS patients treated with infliximab at 5 mg/kg every six weeks for three years can be evaluated using the modified Stokes Ankylosing Spondylitis Spinal Score (mSASSS), considering as a lesion the presence of at least one syndesmophytes (mSASSS > 2) and, by radiological progression, defined as the change from 0 to 1 for syndesmophytes or ankylosis (mSASSS > 2). In this period, there was an increase in mSASSS score and in the number of patients (increase in 11.3% - NNH: 8) with radiological lesions, with the development of new lesions being common in the first two years of treatment, slowing in the following period57(B).
The use of infliximab at 5 mg/kg for 96 weeks in AS patients led to an mSASSS score increase, although 34% of patients showed a decrease of one or more points. Furthermore, some patients show a decrease of two or more (19.9%), three or more (14.7%), and four or more (10.9%)58 points in the score(B).
Etanercept
The changes in radiographic score of bone lesions (mSASSS) in the cervical and lumbar spine after 96 months of treatment with etanercept were similar to the ones observed in patients with no treatment, with a worsening in the scores compared with the beginning of treatment59(B).
Recommendation 11
The use of anti-TNF drugs (infliximab and etanercept) produces no reduction in the structural damage in patients with ankylosing spondylitis.
12. Is there evidence for the efficacy and safety of anti-TNF drugs in extra-articular manifestations in patients with ankylosing spondylitis?
Uveitis
Among the AS patients treated with infliximab, the incidence of anterior uveitis was 3.4 per 100 patients/year compared with patients treated with etanercept, with an incidence of 7.9 per 100 patients/year. In non-treated patients, the incidence of anterior uveitis was 15.6 per 100 patients/year, a significant difference compared with those receiving anti-TNF drugs60(B).
The use of 40 mg adalimumab every other week for 12 weeks in AS patients reduced the incidence of anterior uveitis by 51% (NNT: 2), reduced in 58% of patients (NNT: 2) the incidence of anterior uveitis in patients with a history of uveitis, in 68% (NNT: 2) of patients with a recent history, in 50% (NNT: 2) of patients with symptomatic anterior uveitis, in 45% (NNT: 2) of patients with chronic uveitis, and reduces the occurrence of uveitis in 58% of patients (NNT: 2)61(B).
Inflammatory bowel disease
In AS patients, it is estimated that inflammatory bowel diseases (Crohn's disease and ulcerative colitis) affects 1.3 per 100 patients/year. The treatment of these patients with anti-TNF for a period of 14 to 156 weeks has distinct results depending on the drug used: infliximab 0.2 patients/year, etanercept 2.2 patients/year, and adalimumab 2.3 patients/year, with this difference being favourable and significant for the use of infliximab. Infliximab treatment (0.04) reduced the risk of inflammatory bowel disease in 42% (NNT: 2) of patients compared with etanercept and in 12% (NNT: 8) of patients compared with adalimumab62(B).
Psoriasis
The use of adalimumab, etanercept, and infliximab in AS patients may be associated with the development of psoriasis [psoriasis vulgaris (psoriasis plaque) or palmoplantar pustular] after an average of four months. However, in approximately 40% of patients with psoriasis, the anti-TNF use resolved the lesions63(C).
Osteoporosis
The use of anti-TNF agents for two years in AS patients can result in increased bone mineral density in the spine and femur, regardless of the presence of lumbar syndesmophytes. The changes in bone density in the lumbar spine correlated, in two years of follow up, with changes in BASDAI and BASfiscores. There were no differences between the results obtained with infliximab and etanercept64(C).
Recommendation 12
With regard to the extra-articular manifestations, anti-TNF drugs reduce the incidence of anterior uveitis in patients with ankylosing spondylitis, can reduce the incidence of inflammatory bowel disease (especially infliximab), show controversial results as to benefit or damage with regard to the induction of psoriasis, and cause an increase in bone mineral density aligned with the clinical response in ankylosing spondylitis.
13. What is the evidence that supports the switch of anti-TNF agents in patients with ankylosing spondylitis?
Of the AS patients who discontinued infliximab treatment (5 mg/kg every six to eight weeks, during the last two years) due to clinical failure (absence of BASDAI score reduction), 70% responded to a switch to etanercept, with an average reduction in BASDAI of 7.1 (± 3.6) to 4.1 (± 7.3) in ten months of follow-up65(C).
AS patients treated with 5 mg/kg of infliximab every eight weeks with a failure to maintain a clinical response of 20% according to the ASAS20 can be treated with 50 mg of etanercept for 24 to 54 weeks, without showing severe adverse events (infusion reaction, dizziness, headache) but with the following benefits: 78% ASAS20 response, 52% ASAS50 response, and 39% ASAS70 response, in 24 weeks; and 74% ASAS20 response, 61% ASAS50 response, and 39% ASAS70 response, in 54 weeks; changes in BASDAI score from 6.9 (± 1.3) to 3.1 (± 3.1) in the 24th week and to 2.9 (± 1.7) on the 54th week66(B).
When defining the response to anti-TNF treatment in AS patients as a 50% response in the BASDAI score, a clinical response was obtained in 75% of patients who switched form infliximab to etanercept and in 57.1% of those who switched from etanercept to adalimumab. The patients who switched anti-TNF agents due to adverse events or inadequate efficacy presented with a similar clinical response (70% and 61.5%, respectively). The patients who switched from infliximab to etanercept showed a response after three months of 83.3% (NNT: 1)67(B).
The use of anti-TNF medication (infliximab, etanercept, adalimumab) in the treatment of AS patients demonstrated after 12 weeks an 88% clinical response (measured by BASDAI and BASfiscores). Of the patients who did not respond, or of those who exhibited severe adverse reactions during treatment despite having a good response, 13% switched anti-TNF agents; of those, 93% showed a clinical response after the switch68(C).
The patients with severe AS in treatment with infliximab, etanercept, or adalimumab may need to switch anti-TNF agents (17% of cases) due to inefficiency (67%) or adverse events (28%). Of these patients, 67% and 86% maintained a response (measured by the reduction of 50% in BASDAI) at 6 to 12 months, respectively. The average value of BASDAI (IQR) before the switch was 6.92, and after the switch, at 3, 6, and 12 months, it was 3.98, 370, and 2.92, respectively69(B).
Of the 38% of AS patients who did not achieve a clinical response after treatment with anti-TNF agents in three to four months, approximately 24% and 11%, respectively, required a second or third different anti-TNF agent. Of the patients treated with a second anti-TNF agent, 46% showed an adequate response. Of the patients requiring a third anti-TNF agent, 100% obtained a complete response70(B).
The patients who switched anti-TNF medication (16%) were treated with their first medication (etanercept, infliximab, or adalimumab) for an average of 294 days and started the second anti-TNF agent 32 days, on average, after discontinuing the first medication. Etanercept was administered subcutaneously at a dose of 25 mg 2x/week or 50 mg 1x/ week. Infliximab was prescribed intravenously on weeks 0, 2, and 6 and every six to eight weeks, with an average dose of 4-5 mg/kg. Adalimumab was administered subcuteaneously at 40 mg every 15 days. After three months of the first anti-TNF switch, the clinical response as measured by BASDAI50, ASAS20 and ASAS40 was 25%, 47%, and 30%, respectively. After three months of treatment with the second anti-TNF, the response as measured by BASDAI50, ASAS20, and ASAS40 was 28%, 40%, and 30%, respectively. There was no difference in response between the first and the second switch71(B).
The switch of anti-TNF agents due to insufficient response or adverse events increased in three months from 14% to 21% in the BASDAI 50 and ASAS 40, respectively, in the first and second switch71(B).
Recommendation 13
The switch of anti-TNF agent represents a therapeutic strategic option when there is inadequate clinical response or adverse events.
14. How long should anti-TNF drugs be used in the follow up of a patient with ankylosing spondylitis?
Treatment of AS for 102 weeks with infliximab (5 mg/kg) resulted in a 42% increase in the clinical response (ASAS20) obtained in the 24th week, and it was maintained in the second period. The clinical response measured by ASAS20 in the two weeks of treatment increased from 43% to 89%. Although in the 102nd week, 97.5% of patients showed adverse events. During the treatment, there was no difference in the occurrence of adverse events between the 24th and 102nd week. The increase regarding patients not treated with infliximab was 22% (NNH: 5).The most common adverse event was upper respiratory tract infection (48.7%)72(A).
In the following two years, 58% of the patients treated with infliximab reached at least a 50% reduction in BASDAI, which was similar to the response level at year one. Of the treated patients, 94% and 85% reached at least 20% in the BASDAI response in weeks 54 and 102, respectively. Adverse events in the second year (90% of patients) of treatment occurred in a proportion similar to the first year37(B).
Partial remission, defined as a score < 2 in each of the four ASAS domains (PatGA, NRS-P, BASfi, and BASDAI), was reached in 34.2% of the AS patients in five years of treatment with infliximab compared with 36.8% in three years of treatment. After five years of treatment, the efficacy of infliximab remained stable compared with the first three years, with an average BASDAI score of 2.5 and 2.5, a BASfiof 3.0 and 2.9, a PatGA of 2.7 and 2.6, and a BASMI 2.8 and 2.6, respectively. There was no difference in adverse events between the two periods (94%)39(B).
After eight year of follow up, a state of low disease activity (BASDAI < 3) was achieved in 63.3% of patients treated with infliximab, a result similar to that obtained in three months of follow-up. Furthermore, 71.4% of patients continued to have BASDAI values < 3. ASAS20 response was maintained in 84.8% of patients, and BASDAI50 was present in 57.6% of cases. Of the patients who discontinued treatment, 55% do so due to adverse events, of which, the most frequent were an increase in hepatic enzymes, infusion reaction, and loss of efficacy40(B).
During five years of follow up, there was a 50% increase (NNT: 2) in adherence to treatment with infliximab due to the reduction of symptom reoccurrence73(B).
After 192 weeks of treatment with etanercept, 81% of patients reached an ASAS20 response, and 69% reached an ASAS40 response, with increases of 33% and 39%, respectively, compared with the scores after two weeks of treatment. There was a 14.1% increase in adverse events and a 4.7% increase in discontinuity compared with patients that were not treated with etanercept45(B).
The response/improvement with adalimumab treatment, measured by ASAS20 and ASAS40 scores, was maintained during the three years of treatment and were 64.5% and 50.6%, respectively. Furthermore, the benefits measured by BASDAI, BASfi, and SF-36 score obtained in the 24th week were maintained up to the 156th week. Adverse events lead to discontinuity in 4.5% of cases in two years of treatment with adalimumab and 30% in three years50(A).
Recommendation 14
The long-term use of anti-TNF drugs in ankylosing spondylitis patients maintains the clinical response without an increase in adverse events. Currently, it is estimated that its use should be for an indefinite period of time.
15. Is there evidence for the use of biological agents with other mechanisms of action in ankylosing spondylitis?
Rituximab
The treatment of patients with active AS (BASDAI > 4) without the previous use of an anti-TNF with 1000 mg of rituximab during 24 weeks produced a clinical response according to ASAS20,ASAS40, and BASDAI50 of 30%, 10%, and no response, respectively. In patients with a history of anti-TNF failure, the ASAS20,ASAS40, and BASDAI50 responses were 40%, 30%, and 50%, respectively74(B).
Tocilizumab
The patients with an AS diagnosis and Crohn's disease who were not responsive to treatment with three biological agents (infliximab, abatacept, and certolizumab) and were treated for 11 months with tocilizumab (8 mg/kg every 15 weeks) showed a stable BASfi(6.0), improvement in BASDAI from 6.1 to 4.3, and a reduction of oedema and morning rigidity75(C). The AS patients who were unresponsive to infliximab, etanercept, and adalimumab and were treated with tocilizumab 8 mg/kg every four weeks showed an improvement in BASDAI from 6.1 to 3.6 and a reduction in ASDAS (Ankylosing Spondylitis Disease Activity Score) from 5.8 to 1.6 after 26 weeks, although the MRI showed a persistence of inflammatory signs76(C). The patients with AS treatment failure after five years of treatment with anti-TNF drugs (infliximab, etanercept, and adalimumab) who were treated with tocilizumab 8 mg/kg showed a reduction in BASDAI from 3 to 0.9, BASfifrom 6 to 1.5, and ASDAS 2.2 to 1.3 after four weeks (after 12 months, the reduction of ASDAS was 0.9)77(C).
Abatacept
In patients with an AS diagnosis and active disease (BASDAI scores and low back pain > 4), treatment with abatacept during 24 weeks led to ASAS40 response in 13% of patients without a previous history of anti-TNF use and in 0% of non-responsive patients, regardless of previous treatment with at least two NSAIDs.The same was true for ASAS20, with indices of 27% and 20%, respectively. There were no signs of response in the BAS-DAI and ASDAS scores in the patients, neither for those without prior anti-TNF use nor for those without response78(B).
Recommendation 15
The use of rituximab and abatacept is not effective and does not justify its use in patients with ankylosing spondylitis. The available evidence for tocilizumab does not allow for it to be recommended.
Conflicts of interest
Sampaio-Barros PD: Participation in the boards of the Abbott, Merck, and Pfizer Laboratories; received payment for lectures and participated in conferences and symposia from the Abbott, Actelion, Jansenn, MSD, Pfizer, and Roche Laboratories; Principal Investigator in clinical trials performed in Brazil by Roche.
Keiserman M: Consultant for Abbott, Merck, and Pfizer Laboratories; received payment for lectures and participation in conferences and symposia from Abbott, Actelion, Janssen, Merck, Pfizer, and Roche Laboratories; Investigator in clinical trials performed in Brazil by the Bristol-Myers-Squibb, Merck, and Roche laboratories.
Meirelles ES: Participation in the boards of Pfizer and Janssen laboratories; received payment for lectures and participation in conferences and symposia from Abbott, AstraZeneca, Janssen, Lilly, MSD, Pfizer, Roche, Sanofi-Aventis, and Servier Laboratories; Principal Investigator in clinical trials performed by Novartis and Roche laboratories in Brazil.
Pinheiro MM:Participation in the MSD board; received payment for lectures and participation in conferences and symposia from the Abbott, Janssen, Novartis, Merck, Pfizer, and Roche Laboratories; Principal Investigator in clinical trials performed in Brazil by Roche.
Ximenes AC: Participation in boards of the Bristol, Merck, and Pfizer Laboratories; received payment for lectures and participation in conferences and symposia from the Abbott, Aché, Janssen, Pfizer, and Roche Laboratories; Principal Investigator in clinical trials performed in Brazil by MSD, Pfizer, Roche, and UCB laboratories.
Azevedo VF: Consultant for Abbott, Janssen, Pfizer, and Roche Laboratories; received payment for lectures and participation in conferences and symposia from Abbott, BristolMyers-Squibb, Janssens, MSD, and Roche Laboratories; Investigator in clinical trials performed in Brazil by BMS, Galen Research, Roche, and UCB laboratories.
Bonfiglioli R: Participation in boards of Abbott, Merck, and Pfizer Laboratories; received payment for lectures and participation in conferences and symposia from Abbott, Actelion, Janssen, Merck, Pfizer, and Roche Laboratories; Principal Investigator and/or subinvestigator in clinical trials performed in Brazil by Bristol-Myers-Squibb, Merck, and Roche.
Carneiro S: Participation in the MSD board; received payment for lectures and participation in conferences and symposia from Abbott, Janssen, MSD, and Pfizer Laboratories.
Ranza R: Participation in boards of the Abbott, Merck, and Pfizer Laboratories; received payment for lectures and participation in conferences and symposia from Abbott, Janssen, Merck, Pfizer, and Roche Laboratories; Principal Investigator in clinical trials performed in Brazil by Roche.
Bernardo W: declares no conflicts of interest.
Gonçalves CR: Participation in boards of the Abbott and Merck Laboratories; received payment for lectures and participation in conferences and symposia from Abbott, Aché, Aventis, Janssen, MSD, and Pfizer Laboratories; Investigator in clinical trials performed in Brazil by Roche.
REFERENCES
1. Moll JMH, Haslock I, MacRae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter's disease, the intestinal arthropathies, and Behcet's syndrome. Medicine 1974;53:343-64.
2. Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classifi cation criteria for axial spondyloarthritis (part I): classifi cation of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770-6.
3. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classifi cation criteria for axial spondyloarthritis (part II): validation and fi nal selection. Ann Rheum Dis 2009;68:777-83.
4. Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classifi cation criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25-31.
5. Rudwaleit M, Jurik AG, Hermann KG, et al Landewe R, van der Heijde D, Baraliakos X, et al. Defi ning active sacroiliitis on magnetic resonance imaging (MRI) for classifi cation of axial spondyloarthritis: a consensual approach for the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520-7.
6. Sampaio-Barros PD, Bortoluzzo AB, Conde RA, Costallat LT, Samara AM, Bertolo MB. Undifferentiated spondyloarthritis: a long-term followup. J.Rheumatol 2010;37(6):1195-9.
7. Weber U, Lambert RG, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 2010;62:3048-58.
8. Bennett AN, Rehman A, Hensor EM, Marzo-Ortega H, Emery P, McGonagle D. The fatty Romanus lesion: aMoll JMH, Haslock I, MacRae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter's disease, the intestinal arthropathies, and Behcet's syndrome. Medicine 1974;53:343-64.non-inflammatory spinal MRI lesion specifi c for axial spondyloarthropathy. Ann Rheum Dis 2010;69:891-4.
9. Bennett AN, McGonagle D, O'Connor P, Hensor EM, Sivera F, Coates LC, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413-8.
10. Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009;60:93-102.
11. Madsen KB, Schiøttz-Christensen B, Jurik AG. Prognostic signifi cance of magnetic resonance imaging changes of the sacroiliac joints in spondyloarthritis--a followup study. J Rheumatol 2010;37:1718-27.
12. Freeston J, Barkham N, Hensor E, Emery P, Fraser A. Ankylosing spondylitis, HLA-B27 positivity and the need for biologic therapies. Joint Bone Spine 2007;74:140-3.
13. Bennett AN, McGonagle D, O'Connor P, Hensor EM, Sivera F, Coates LC, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413-8.
14. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker- Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717-27.
15. Joshi R, Reveille JD, Brown MA, Weisman MH, Ward MM, Gensler LS, et al. Is there a higher genetic load of susceptibility loci in familial ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:780-4.
16. Fernández-de-Las-Peñas C, Alonso-Blanco C, Morales- Cabezas M, Miangolarra-Page JC. Two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2005;84:407-19.
17. Fernández-de-Las-Peñas C, Alonso-Blanco C, Alguacil- Diego IM, Miangolarra-Page JC. One-year follow-up of two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2006;85:559-67.
18. Staalesen Strumse YA, Nordvåg BY, Stanghelle JK, Røisland M, Winther A, Pajunen PA, et al. Effi cacy of rehabilitation for patients with ankylosing spondylitis: comparison of a four-week rehabilitation programme in a Mediterranean and a Norwegian setting. J Rehabil Med 2011;43:534-42.
19. Peters ND, Ejstrup L. Intravenous methylprednisolone pulse therapy in ankylosing spondylitis. Scand J Rheumatol 1992;21:134-8.
20. Braun J, Bollow M, Seyrekbasan F, Häberle HJ, Eggens U, Mertz A, et al. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and followup by dynamic magnetic resonance imaging. J Rheumatol 1996;23:659-64.
21. Bollow M, Braun J, Taupitz M, Häberle J, Reibhauer BH, Paris S, et al. CT-guided intraarticular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow-up with contrast-enhanced MRI. J Comput Assist Tomogr 1996;20:512-21.
22. Dougados M, Gueguen A, Nakache JP, Velicitat P, Veys EM, Zeidler H, et al. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford) 1999;38:235-44.
23. van der Heijde D, Baraf HS, Ramos-Remus C, Calin A, Weaver AL, Schiff M, et al. Evaluation of the effi cacy of etoricoxib in ankylosing spondylitis: results of a fi ftytwo- week, randomized, controlled study. Arthritis Rheum 2005;52:1205-15.
24. Barkhuizen A, Steinfeld S, Robbins J, West C, Coombs J, Zwillich S. Celecoxib is effi cacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 2006;33:1805-12.
25. Sieper J, Klopsch T, Richter M, Kapelle A, Rudwaleit M, Schwank S, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 2008;67:323-9.
26. Peloso PM, Gammaitoni A, Smugar SS, Wang H, Moore AR. Longitudinal numbers-needed-to-treat (NNT) for achieving various levels of analgesic response and improvement with etoricoxib, naproxen, and placebo in ankylosing spondylitis. BMC Musculoskelet Disord 2011;12:165.
27. Wanders A, van der Heijde D, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756-65.
28. Roychowdhury B, Bintley-Bagot S, Bulgen DY, Thompson RN, Tunn EJ, Moots RJ. Is methotrexate effective in ankylosing spondylitis? Rheumatology (Oxford) 2002;41:1330-2.
29. Gonzalez-Lopez L, Garcia-Gonzalez A, Vazquez-Del-Mercado M, Muñoz-Valle JF, Gomez-Nava JI. Effi cacy of methotrexate in ankylosing spondylitis: a randomized, double blind, placebo controlled trial. J Rheumatol 2004;31:1568-74.
30. Chen J, Liu C. Is sulfasalazine effective in ankylosing spondylitis? A systematic review of randomized controlled trials. J Rheumatol 2006;33:722-31.
31. Song IH, Althoff CE, Haibel H, Hermann KG, Poddubnyy D, Listing J, et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012;71:1212-5.
32. van Denderen JC, van der Paardt M, Nurmohamed MT, de Ryck YM, Dijkmans BA, van der Horst-Bruinsma IE. Double blind, randomised, placebo controlled study of leflunomide in the treatment of active ankylosing spondylitis. Ann Rheum Dis 2005;64:1761-4.
33. Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicentre trial. Lancet 2002;359:1187-93.
34. Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Long-term effi cacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 2003;48:2224-33.
35. Listing J, Brandt J, Rudwaleit M, Zink A, Sieper J, Braun J. Impact of anti-tumour necrosis factor alpha treatment on admissions to hospital and days of sick leave in patients with ankylosing spondylitis. Ann Rheum Dis 2004;63:1670-2.
36. van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Effi cacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91.
37. Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Two year maintenance of effi cacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 2005;64:229-34.
38. Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, et al. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford) 2005;44:670-6.
39. Braun J, Baraliakos X, Listing J, Fritz C, Alten R, Burmester G, et al. Persistent clinical effi cacy and safety of antitumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis 2008;67:340-5.
40. Baraliakos X, Listing J, Fritz C, Haibel H, Alten R, Burmester GR, et al. Persistent clinical effi cacy and safety of infliximab in ankylosing spondylitis after 8 years - early clinical response predicts long-term outcome. Rheumatology (Oxford) 2011;50:1690-9.
41. Inman RD, Maksymowych WP; CANDLE Study Group. A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010;37:1203-10.
42. Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 2002;346:1349-56.
43. Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003;48:1667-75.
44. Davis JC Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230-6.
45. Davis JC Jr, van der Heijde DM, Braun J, Dougados M, Clegg DO, Kivitz AJ, et al. Effi cacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis 2008;67:346-52.
46. Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-600.
47. Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Effi cacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE). Ann Rheum Dis 2011;70:799-804.
48. Navarro-Sarabia F, Fernández-Sueiro JL, Torre-Alonso JC, Gratacos J, Queiro R, Gonzalez C, Loza E, et al. High-dose etanercept in ankylosing spondylitis: results of a 12-week randomized, double blind, controlled multicentre study (LOADET study). Rheumatology (Oxford) 2011;50:1828-37.
49. Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis JC Jr, et al. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and effi cacy for ankylosing spondylitis (ATLAS). J Rheumatol 2008;35:1346-53.
50. van der Heijde DM, Revicki DA, Gooch KL, Wong RL, Kupper H, Harnam N, et al. Physical function, disease activity, and health-related quality-of-life outcomes after 3 years of adalimumab treatment in patients with ankylosing spondylitis. Arthritis Res Ther 2009;11:R124.
51. van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis 2009;68:922-9.
52. Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie S, Pangan AL. Early response to adalimumab predicts longterm remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:700-6.
53. Inman RD, Davis JC Jr, van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Effi cacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402-12.
54. Deodhar A, Braun J, Inman RD, Mack M, Parasuraman S, Buchanan J, et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266-71.
55. Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S, Hsu B. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis 2012;71:661-7.
56. Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O'connor P, Tan AL, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis 2005;64:1568-75.
57. Baraliakos X, Listing J, Brandt J, Haibel H, Rudwaleit M, Sieper J, et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatology (Oxford) 2007;46:1450-3.
58. van der Heijde D, Landewé R, Baraliakos X, Houben H, van Tubergen A, Williamson P et al. Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Radiographic fi ndings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063-70.
59. van der Heijde D, Landewé R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324-31.
60. Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 2005;52:2447-51.
61. Rudwaleit M, Rødevand E, Holck P, Vanhoof J, Kron M, Kary S, et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 2009;68:696-701.
62. Braun J, Baraliakos X, Listing J, Davis J, van der Heijde D, Haibel H, et al. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum 2007;57:639-47.
63. Wendling D, Balblanc JC, Briançon D, Brousse A, Lohse A, Deprez P, et al. Onset or exacerbation of cutaneous psoriasis during TNFalpha antagonist therapy. Joint Bone Spine 2008;75:315-8.
64. Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol 2008;35:855-61.
65. Delaunay C, Farrenq V, Marini-Portugal A, Cohen JD, Chevalier X, Claudepierre P. Infliximab to etanercept switch in patients with spondyloarthropathies and psoriatic arthritis: preliminary data. J Rheumatol 2005;32:2183-5.
66. Cantini F, Niccoli L, Benucci M, Chindamo D, Nannini C, Olivieri I, et al. Switching from infliximab to once-weekly administration of 50 mg etanercept in resistant or intolerant patients with ankylosing spondylitis: results of a fi fty-fourweek study. Arthritis Rheum 2006;55:812-6.
67. Conti F, Ceccarelli F, Marocchi E, Magrini L, Spinelli FR, Spadaro A, et al. Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis 2007;66:1393-7.
68. Coates LC, Cawkwell LS, Ng NW, Bennett AN, Bryer DJ, Fraser AD, et al. Real life experience confi rms sustained response to long-term biologics and switching in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:897-900.
69. Pradeep DJ, Keat AC, Gaffney K, Brooksby A, Leeder J, Harris C. Switching anti-TNF therapy in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:1726-7.
70. Haberhauer G, Strehblow C, Fasching P. Observational study of switching anti-TNF agents in ankylosing spondylitis and psoriatic arthritis versus rheumatoid arthritis. Wien Med Wochenschr 2010;160:220-4.
71. Lie E, van der Heijde D, Uhlig T, Mikkelsen K, Rødevand E, Koldingsnes W, et al. Effectiveness of switching between TNF inhibitors in ankylosing spondylitis: data from the NOR-DMARD register. Ann Rheum Dis 2011;70:157-63.
72. Braun J, Deodhar A, Dijkmans B, Geusens P, Sieper J, Williamson P, et al. Effi cacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum 2008;59:1270-8.
73. Heldmann F, Brandt J, van der Horst-Bruinsma IE, Landewe R, Sieper J, Burmester GR, et al. The European ankylosing spondylitis infliximab cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximab. Clin Exp Rheumatol 2011;29:672-80.
74. Song IH, Heldmann F, Rudwaleit M, Listing J, Appel H, Braun J, et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum 2010;62:1290-7.
75. Brulhart L, Nissen MJ, Chevallier P, Gabay C. Tocilizumab in a patient with ankylosing spondylitis and Crohn's disease refractory to TNF antagonists. Joint Bone Spine 2010;77:625-6.
76. Henes JC, Horger M, Guenaydin I, Kanz L, Koetter I. Mixed response to tocilizumab for ankylosing spondylitis. Ann Rheum Dis 2010;69:2217-8.
77. Cohen JD, Ferreira R, Jorgensen C. Ankylosing spondylitis refractory to tumor necrosis factor blockade responds to tocilizumab. J Rheumatol 2011;38:1527.
78. Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis 2011;70:1108-10.
Final elaboration
December 2012
Description of the method used for evidence preparation
The members of the Comissão de Espondiloartrites da Sociedade Brasileira de Reumatologia (Commission on Spondyloarthritis of the Brazilian Society of Rheumatology, SBR) 2010-2012 took part in the Evidence Preparation Course given by the Associação Médica Brasileira (Brazilian Medical Association, AMB) in São Paulo in the first semester of 2011. The questions were finally concluded at a meeting of the Commission on Spondyloarthritis held on 15 October 2011 in Florianópolis (SC, Brazil), during the 18th Southern Cone Rheumatology Meeting and were later approved by all the coordinators of the Brazilian Spondyloarthritis Registry. The 15 clinical questions considered to be relevant were structured using the P.I.C.O. method (patient; intervention or indicator; comparison; outcome). The literature search was conducted by searching the databases MEDLINE, EMBASE, SciElo/Lilacs, and the Cochrane Library through February, 2012 (Appendix). Critical assessment of the evidence in the selected articles was performed using the Jadad score. Next, the answers to the questions included in the Recommendations were elaborated, and all the selected references exhibit the corresponding grade of recommendation and strength of scientific evidence.The references were updated through August, 2012, entered into a single file by the coordinator, and sent to the co-authors in two successive rounds for preparation of the final version.
Grades of recommendation and strength of evidence
A: Most consistent experimental and observational studies.
B: Less consistent experimental and observational studies.
C: Case reports (uncontrolled studies).
D: Opinion that is not substantiated by critical evaluation, based on consensus, physiological studies or animal models.
Objective
To establish recommendations for the management (classification and evaluation criteria by magnetic resonance and genetics) of spondyloarthritis and for the treatment of ankylosing spondyloarthritis.
Appendix
Question 1
What are the clinical criteria for an individual to be considered affected by a spondyloarthritis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND specificity [Title/Abstract]
Question 2
What is the role of MRI in the initial evaluation of axial spondyloarthritis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND Magnetic Resonance Imaging AND (specificity[Title/Abstract] OR (prognos*[Title/Abstract] OR (first[Title/Abstract] AND episode[Title/Abstract]) OR cohort[Title/Abstract]))
Question 3
When should HLA-B27 be requested in a patient with axial spondyloarthritis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND HLA-B27 AND (specificity [Title/ Abstract] OR (prognos*[Title/Abstract] OR (first[Title/Abstract] AND episode[Title/Abstract]) OR cohort[Title/Abstract]))
Question 4
What is the evidence for the use of physical rehabilitation in patients with ankylosing spondylitis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND HLA-B27 AND (specificity [Title/Abstract] OR (prognos*[Title/Abstract] OR (first [Title/ Abstract] AND episode [Title/Abstract]) OR cohort [Title/Abstract]))
Question 5
What is the evidence for the use of corticosteroids in patients with ankylosing spondylitis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND (Steroids OR Androstanes OR Androstanols OR Androstenes OR Cardanolides OR Cardenolides OR Cardiac Glycosides OR Sterols OR Cyclosteroids OR Estranes OR Estrenes OR Gonanes OR Homosteroids OR Testolactone OR Hydroxysteroids OR Ketosteroids OR 17-Ketosteroids OR Norsteroids OR Norandrostanes OR Norpregnanes OR Pregnanes OR Pregnadienes OR Pregnanediol OR Pregnanediones OR Pregnanetriol OR Pregnanolone OR Pregnatrienes OR Pregnenes OR Tetrahydrocortisol OR Sapogenins OR Secosteroids OR Beclomethasone OR Chlormadinone OR Cyproterone OR Fluorinated OR Betamethasone OR Dexamethasone OR Flumethasone OR Fluocinolone OR Fluocortolone OR Fluorometholone OR Fluoxymesterone OR Fluprednisolone OR Flurandrenolone OR Flurogestone OR Paramethasone OR Triamcinolone OR Prednisolone OR Hydrocortisone OR corticosteroids OR Mineralocorticoids OR Glucocorticoids OR Hydroxycorticosteroids) AND ((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading])
Question 6
In which situations should continuous NSAID use be recommended for patients with ankylosing spondylitis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND (Anti-Inflammatory Agents OR Cyclooxygenase 2 OR COX-2 OR rofecoxib OR Ibuprofen OR celecoxib OR Naproxen OR Acetaminophen OR NSAID OR paracetamol OR parecoxib OR diclofenac OR aspirin OR meloxicam OR acetylsalicylic OR piroxicam) AND (randomized controlled trial[Publication Type] OR (randomised[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract]))
Question 7
What is the evidence for the use of conventional drugs (methotrexate and sulfasalazine, among others) in patients with ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (methotrexate OR leflunomide OR sulfasalazine OR gold sodium OR hydroxychloroquine OR ciclosporin)
Question 8
What are the indications for the use of biological agents that block tumour necrosis factor (anti-TNF) in ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept)
Question 9
Is there a difference in efficacy among anti-TNF drugs in patients with ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept)
Question 10
Is there a difference in the safety among the anti-TNF drugs in patients with ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept)
Question 11
Is the use of anti-TNF treatment capable of reducing structural damage in patients with ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept)
Question 12
Is there evidence for the efficacy and safety of anti-TNF drugs in extra-articular manifestations in patients with ankylosing spondylitis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept) AND (Uveitis OR Iridocyclitis OR Cardiovascular Diseases OR Pulmonary Fibrosis OR Lung diseases OR Extra-articular OR Extra articular OR Inflammatory Bowel Diseases OR psoriatic arthritis OR Psoriasis OR Crohn Disease OR bone)
Question 13
What is the evidence that supports the switch of anti-TNF agents in patients with ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND switch* AND (randomised controlled trial[Publication Type] OR (randomised[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract]))
Question 14
How long should anti-TNF drugs be used in the follow up of a patient with ankylosing spondylitis?
(Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease) AND (Tumor Necrosis Factor-alpha OR golimumab OR infliximab OR adalimumab OR etanercept)
Question 15
Is there evidence for the use of biological agents with other mechanisms of action in ankylosing spondylitis?
Spondylitis, Ankylosing OR Bechterew's Disease OR Ankylosing Spondyloarthritis OR Rheumatoid Spondylitis OR Marie-Struempell Disease AND (rituximab OR tocilizumab OR abatacept OR Antibodies, Monoclonal)
-
1Moll JMH, Haslock I, MacRae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter's disease, the intestinal arthropathies, and Behcet's syndrome. Medicine 1974;53:343-64.
-
2Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classifi cation criteria for axial spondyloarthritis (part I): classifi cation of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770-6.
-
3Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classifi cation criteria for axial spondyloarthritis (part II): validation and fi nal selection. Ann Rheum Dis 2009;68:777-83.
-
4Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classifi cation criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25-31.
-
5Rudwaleit M, Jurik AG, Hermann KG, et al Landewe R, van der Heijde D, Baraliakos X, et al. Defi ning active sacroiliitis on magnetic resonance imaging (MRI) for classifi cation of axial spondyloarthritis: a consensual approach for the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520-7.
-
6Sampaio-Barros PD, Bortoluzzo AB, Conde RA, Costallat LT, Samara AM, Bertolo MB. Undifferentiated spondyloarthritis: a long-term followup. J.Rheumatol 2010;37(6):1195-9.
-
7Weber U, Lambert RG, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 2010;62:3048-58.
-
9Bennett AN, McGonagle D, O'Connor P, Hensor EM, Sivera F, Coates LC, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413-8.
-
10Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum 2009;60:93-102.
-
11Madsen KB, Schiøttz-Christensen B, Jurik AG. Prognostic signifi cance of magnetic resonance imaging changes of the sacroiliac joints in spondyloarthritis--a followup study. J Rheumatol 2010;37:1718-27.
-
12Freeston J, Barkham N, Hensor E, Emery P, Fraser A. Ankylosing spondylitis, HLA-B27 positivity and the need for biologic therapies. Joint Bone Spine 2007;74:140-3.
-
13Bennett AN, McGonagle D, O'Connor P, Hensor EM, Sivera F, Coates LC, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413-8.
-
14Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker- Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717-27.
-
15Joshi R, Reveille JD, Brown MA, Weisman MH, Ward MM, Gensler LS, et al. Is there a higher genetic load of susceptibility loci in familial ankylosing spondylitis? Arthritis Care Res (Hoboken) 2012;64:780-4.
-
16Fernández-de-Las-Peñas C, Alonso-Blanco C, Morales- Cabezas M, Miangolarra-Page JC. Two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2005;84:407-19.
-
17Fernández-de-Las-Peñas C, Alonso-Blanco C, Alguacil- Diego IM, Miangolarra-Page JC. One-year follow-up of two exercise interventions for the management of patients with ankylosing spondylitis: a randomized controlled trial. Am J Phys Med Rehabil 2006;85:559-67.
-
18Staalesen Strumse YA, Nordvåg BY, Stanghelle JK, Røisland M, Winther A, Pajunen PA, et al. Effi cacy of rehabilitation for patients with ankylosing spondylitis: comparison of a four-week rehabilitation programme in a Mediterranean and a Norwegian setting. J Rehabil Med 2011;43:534-42.
-
19Peters ND, Ejstrup L. Intravenous methylprednisolone pulse therapy in ankylosing spondylitis. Scand J Rheumatol 1992;21:134-8.
-
20Braun J, Bollow M, Seyrekbasan F, Häberle HJ, Eggens U, Mertz A, et al. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and followup by dynamic magnetic resonance imaging. J Rheumatol 1996;23:659-64.
-
21Bollow M, Braun J, Taupitz M, Häberle J, Reibhauer BH, Paris S, et al. CT-guided intraarticular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow-up with contrast-enhanced MRI. J Comput Assist Tomogr 1996;20:512-21.
-
22Dougados M, Gueguen A, Nakache JP, Velicitat P, Veys EM, Zeidler H, et al. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford) 1999;38:235-44.
-
23van der Heijde D, Baraf HS, Ramos-Remus C, Calin A, Weaver AL, Schiff M, et al. Evaluation of the effi cacy of etoricoxib in ankylosing spondylitis: results of a fi ftytwo- week, randomized, controlled study. Arthritis Rheum 2005;52:1205-15.
-
24Barkhuizen A, Steinfeld S, Robbins J, West C, Coombs J, Zwillich S. Celecoxib is effi cacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 2006;33:1805-12.
-
25Sieper J, Klopsch T, Richter M, Kapelle A, Rudwaleit M, Schwank S, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 2008;67:323-9.
-
26Peloso PM, Gammaitoni A, Smugar SS, Wang H, Moore AR. Longitudinal numbers-needed-to-treat (NNT) for achieving various levels of analgesic response and improvement with etoricoxib, naproxen, and placebo in ankylosing spondylitis. BMC Musculoskelet Disord 2011;12:165.
-
27Wanders A, van der Heijde D, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756-65.
-
28Roychowdhury B, Bintley-Bagot S, Bulgen DY, Thompson RN, Tunn EJ, Moots RJ. Is methotrexate effective in ankylosing spondylitis? Rheumatology (Oxford) 2002;41:1330-2.
-
29Gonzalez-Lopez L, Garcia-Gonzalez A, Vazquez-Del-Mercado M, Muñoz-Valle JF, Gomez-Nava JI. Effi cacy of methotrexate in ankylosing spondylitis: a randomized, double blind, placebo controlled trial. J Rheumatol 2004;31:1568-74.
-
30Chen J, Liu C. Is sulfasalazine effective in ankylosing spondylitis? A systematic review of randomized controlled trials. J Rheumatol 2006;33:722-31.
-
31Song IH, Althoff CE, Haibel H, Hermann KG, Poddubnyy D, Listing J, et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012;71:1212-5.
-
32van Denderen JC, van der Paardt M, Nurmohamed MT, de Ryck YM, Dijkmans BA, van der Horst-Bruinsma IE. Double blind, randomised, placebo controlled study of leflunomide in the treatment of active ankylosing spondylitis. Ann Rheum Dis 2005;64:1761-4.
-
33Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicentre trial. Lancet 2002;359:1187-93.
-
34Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Long-term effi cacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum 2003;48:2224-33.
-
35Listing J, Brandt J, Rudwaleit M, Zink A, Sieper J, Braun J. Impact of anti-tumour necrosis factor alpha treatment on admissions to hospital and days of sick leave in patients with ankylosing spondylitis. Ann Rheum Dis 2004;63:1670-2.
-
36van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Effi cacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91.
-
37Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, et al. Two year maintenance of effi cacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 2005;64:229-34.
-
38Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, et al. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford) 2005;44:670-6.
-
39Braun J, Baraliakos X, Listing J, Fritz C, Alten R, Burmester G, et al. Persistent clinical effi cacy and safety of antitumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis 2008;67:340-5.
-
40Baraliakos X, Listing J, Fritz C, Haibel H, Alten R, Burmester GR, et al. Persistent clinical effi cacy and safety of infliximab in ankylosing spondylitis after 8 years - early clinical response predicts long-term outcome. Rheumatology (Oxford) 2011;50:1690-9.
-
41Inman RD, Maksymowych WP; CANDLE Study Group. A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010;37:1203-10.
-
42Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 2002;346:1349-56.
-
43Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum 2003;48:1667-75.
-
44Davis JC Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230-6.
-
45Davis JC Jr, van der Heijde DM, Braun J, Dougados M, Clegg DO, Kivitz AJ, et al. Effi cacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis 2008;67:346-52.
-
46Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594-600.
-
47Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Effi cacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE). Ann Rheum Dis 2011;70:799-804.
-
48Navarro-Sarabia F, Fernández-Sueiro JL, Torre-Alonso JC, Gratacos J, Queiro R, Gonzalez C, Loza E, et al. High-dose etanercept in ankylosing spondylitis: results of a 12-week randomized, double blind, controlled multicentre study (LOADET study). Rheumatology (Oxford) 2011;50:1828-37.
-
49Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis JC Jr, et al. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and effi cacy for ankylosing spondylitis (ATLAS). J Rheumatol 2008;35:1346-53.
-
50van der Heijde DM, Revicki DA, Gooch KL, Wong RL, Kupper H, Harnam N, et al. Physical function, disease activity, and health-related quality-of-life outcomes after 3 years of adalimumab treatment in patients with ankylosing spondylitis. Arthritis Res Ther 2009;11:R124.
-
51van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis 2009;68:922-9.
-
52Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie S, Pangan AL. Early response to adalimumab predicts longterm remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:700-6.
-
53Inman RD, Davis JC Jr, van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Effi cacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402-12.
-
54Deodhar A, Braun J, Inman RD, Mack M, Parasuraman S, Buchanan J, et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266-71.
-
55Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S, Hsu B. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis 2012;71:661-7.
-
56Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O'connor P, Tan AL, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis 2005;64:1568-75.
-
57Baraliakos X, Listing J, Brandt J, Haibel H, Rudwaleit M, Sieper J, et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatology (Oxford) 2007;46:1450-3.
-
58van der Heijde D, Landewé R, Baraliakos X, Houben H, van Tubergen A, Williamson P et al. Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Radiographic fi ndings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063-70.
-
59van der Heijde D, Landewé R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324-31.
-
60Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 2005;52:2447-51.
-
61Rudwaleit M, Rødevand E, Holck P, Vanhoof J, Kron M, Kary S, et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 2009;68:696-701.
-
62Braun J, Baraliakos X, Listing J, Davis J, van der Heijde D, Haibel H, et al. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum 2007;57:639-47.
-
63Wendling D, Balblanc JC, Briançon D, Brousse A, Lohse A, Deprez P, et al. Onset or exacerbation of cutaneous psoriasis during TNFalpha antagonist therapy. Joint Bone Spine 2008;75:315-8.
-
64Briot K, Gossec L, Kolta S, Dougados M, Roux C. Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol 2008;35:855-61.
-
65Delaunay C, Farrenq V, Marini-Portugal A, Cohen JD, Chevalier X, Claudepierre P. Infliximab to etanercept switch in patients with spondyloarthropathies and psoriatic arthritis: preliminary data. J Rheumatol 2005;32:2183-5.
-
66Cantini F, Niccoli L, Benucci M, Chindamo D, Nannini C, Olivieri I, et al. Switching from infliximab to once-weekly administration of 50 mg etanercept in resistant or intolerant patients with ankylosing spondylitis: results of a fi fty-fourweek study. Arthritis Rheum 2006;55:812-6.
-
67Conti F, Ceccarelli F, Marocchi E, Magrini L, Spinelli FR, Spadaro A, et al. Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis 2007;66:1393-7.
-
68Coates LC, Cawkwell LS, Ng NW, Bennett AN, Bryer DJ, Fraser AD, et al. Real life experience confi rms sustained response to long-term biologics and switching in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:897-900.
-
69Pradeep DJ, Keat AC, Gaffney K, Brooksby A, Leeder J, Harris C. Switching anti-TNF therapy in ankylosing spondylitis. Rheumatology (Oxford) 2008;47:1726-7.
-
70Haberhauer G, Strehblow C, Fasching P. Observational study of switching anti-TNF agents in ankylosing spondylitis and psoriatic arthritis versus rheumatoid arthritis. Wien Med Wochenschr 2010;160:220-4.
-
71Lie E, van der Heijde D, Uhlig T, Mikkelsen K, Rødevand E, Koldingsnes W, et al. Effectiveness of switching between TNF inhibitors in ankylosing spondylitis: data from the NOR-DMARD register. Ann Rheum Dis 2011;70:157-63.
-
72Braun J, Deodhar A, Dijkmans B, Geusens P, Sieper J, Williamson P, et al. Effi cacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum 2008;59:1270-8.
-
73Heldmann F, Brandt J, van der Horst-Bruinsma IE, Landewe R, Sieper J, Burmester GR, et al. The European ankylosing spondylitis infliximab cohort (EASIC): a European multicentre study of long term outcomes in patients with ankylosing spondylitis treated with infliximab. Clin Exp Rheumatol 2011;29:672-80.
-
74Song IH, Heldmann F, Rudwaleit M, Listing J, Appel H, Braun J, et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum 2010;62:1290-7.
-
75Brulhart L, Nissen MJ, Chevallier P, Gabay C. Tocilizumab in a patient with ankylosing spondylitis and Crohn's disease refractory to TNF antagonists. Joint Bone Spine 2010;77:625-6.
-
76Henes JC, Horger M, Guenaydin I, Kanz L, Koetter I. Mixed response to tocilizumab for ankylosing spondylitis. Ann Rheum Dis 2010;69:2217-8.
-
77Cohen JD, Ferreira R, Jorgensen C. Ankylosing spondylitis refractory to tumor necrosis factor blockade responds to tocilizumab. J Rheumatol 2011;38:1527.
-
78Song IH, Heldmann F, Rudwaleit M, Haibel H, Weiss A, Braun J, et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann Rheum Dis 2011;70:1108-10.
Publication Dates
-
Publication in this collection
16 Sept 2013 -
Date of issue
June 2013