Abstracts
Gastrointestinal manifestations in systemic lupus erythematosus (SLE) are not uncommon. Non specific symptoms are often observed, such as abdominal pain, nausea, vomiting and diarrhea. On the other hand, pneumatosis cystoides intestinalis, which is characterized by multiple gas-filled cysts located throughout the intestinal wall, is a rare condition in SLE. We describe a case of a 20-year-old man who was admitted with fever, weight loss, headache and arthralgia and had a diagnosis of systemic lupus erythematosus. During his hospital stay, he developed abdominal symptoms that suggested intestinal vasculitis. The computed tomography of the abdomen showed the double halo sign, or target sign and pneumatosis cystoides intestinalis. The patient presented complete recovery after conservative treatment, with intestinal rest and total parenteral nutrition.
systemic lupus erythematosus; vasculitis; pneumatosis cystoides intestinalis; gastrointestinal tract
As manifestações gastrointestinais no lúpus eritematoso sistêmico (LES) não são incomuns. Frequentemente são encontrados sintomas inespecíficos, como dor abdominal, náuseas, vômitos e diarreia. Por outro lado, a pneumatose intestinal, caracterizada por múltiplos cistos preenchidos por ar na parede intestinal, é uma condição raramente associada ao LES. Descreve-se a seguir o caso de um homem de 20 anos que foi internado por febre, perda ponderal, cefaleia e artrite, cuja investigação mostrou tratar-se de LES. Na evolução, apresentou quadro abdominal sugestivo de vasculite intestinal, com tomografia computadorizada de abdome revelando sinal do duplo halo ou do alvo e pneumatose intestinal. Realizado tratamento conservador com antibioticoterapia endovenosa, repouso intestinal e nutrição parenteral total, com resolução do quadro abdominal.
lúpus eritematoso sistêmico; vasculite; pneumatose cistoide intestinal; trato gastrointestinal
CASE REPORT
ISpecialization in Rheumatology at Hospital de Clínicas - UFPR (ongoing)
IIInternal Medicine Resident Physician at Hospital de Clínicas - UFPR
IIIProfessor of the Discipline of Rheumatology at Hospital de Clínicas - UFPR
IVResident Physician in Rheumatology at Hospital de Clínicas - UFPR
Correspondence to
ABSTRACT
Gastrointestinal manifestations in systemic lupus erythematosus (SLE) are not uncommon. Non specific symptoms are often observed, such as abdominal pain, nausea, vomiting and diarrhea. On the other hand, pneumatosis cystoides intestinalis, which is characterized by multiple gas-filled cysts located throughout the intestinal wall, is a rare condition in SLE. We describe a case of a 20-year-old man who was admitted with fever, weight loss, headache and arthralgia and had a diagnosis of systemic lupus erythematosus. During his hospital stay, he developed abdominal symptoms that suggested intestinal vasculitis. The computed tomography of the abdomen showed the double halo sign, or target sign and pneumatosis cystoides intestinalis. The patient presented complete recovery after conservative treatment, with intestinal rest and total parenteral nutrition.
Keywords: systemic lupus erythematosus, vasculitis, pneumatosis cystoides intestinalis, gastrointestinal tract.
INTRODUCTION
Pneumatosis intestinalis (PI) is an unusual condition, characterized by multiple cysts filled with gas in the intestinal mucosa, submucosa or subserosa. The first description dates from 1730, but Meyer was the first to use the term.1 There are several associated conditions and among the conjunctive tissue diseases, the most common is systemic sclerosis.1 It rarely occurs in systemic lupus erythematosus (SLE), with only 14 cases described to date in the literature. We describe herein a rare case of PI in a 20-year-old man with a recent diagnosis of SLE.
CASE REPORT
A previously healthy twenty-year-old male patient, presented fever for 10 days, accompanied by weight loss, severe headache and history of two painful oral ulcers.
At admission, he presented regular general status, dehydration and looked pale. He was conscious, oriented and febrile. Cervical lymphadenomegaly was observed, without oral ulcers. Chest examination was normal. The abdomen was flat, flaccid and hydro-aerial noises were present. The patient presented pain in the right hypochondriac region; the liver was palpated 4 cm below the right costal border and the spleen could not be palpated. The patient presented knee and elbow arthritis. The neurological assessment was normal.
Laboratory assessment showed: anemia (Hemoglobin: 11.0 g/dL); lymphopenia (331/mm3); thrombocytopenia (63.000/mm3); Coombs reagent; reticulocytes: 0.3%; normal liver function tests; ESR 44 mm/h; creatinine 1.4 mg/dL; urea 64 mg/dL; partial urinalysis with protein +++, 10,000 leukocytes, 40,000 erythrocytes, > 10 hyaline cylinders; negative blood cultures.
Due to the presence of fever and headache, a cerebrospinal fluid sample was collected, which showed the presence of asseptic meningitis - 11.6 leukocytes; 61% monomorphonuclear and 29% polymorphonuclear; protein 72.9 mg/dL; glucose 43 mg/dL; VDRL, gram, culture, Ziehl, serological test for herpes and direct mycological examination were negative.
The serological tests for hepatitis B and C, HIV and toxoplasmosis were negative; for CMV and EBV, IgG was positive and IgM was negative, respectively. The Antinuclear Antibody (ANA) test was positive, with a titer higher than 1:640, homogenous nuclear pattern; C3 and C4 were decreased (52 and 8 mg/dL, respectively); ENA profile was non-reagent and Anti-DNA was > 1:640.
The echocardiogram showed an ejection fraction of 74% (> 58%), small pericardial effusion, with no vegetation. Systemic lupus erythematosus (SLE) was diagnosed (arthritis, serositis, hematological alterations, nephritis, anti-DNA and ANA). The patient started to present mental confusion, generalized tonic-clonic seizures, abdominal pain and nausea. A skull computed tomography (CT) with contrast showed diffuse hypodensities in the brain hemispheres, which could not rule out vasculitis. Pulse therapy with 1g/day of methylprednisolone IV was started due to the severe Central Nervous System involvement, followed by 1g of cyclophosphamide IV on the fourth day, which was immediately followed by complete neurological recovery and worsening of the abdominal picture, with gas and stool retention. The abdomen was slightly distended and hypertympanic; hydro-aerial noise was absent and the patient presented diffuse pain on palpation, with rebound tenderness (Blumberg sign).
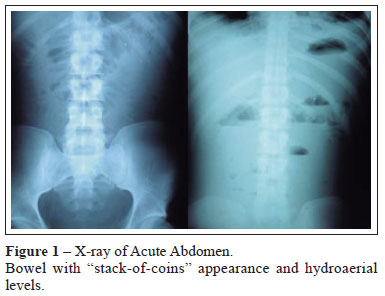
The acute abdomen X-ray showed hydro-aerial levels and small bowel dilatation, with "stack-of-coins" appearance (Figure 1).
The ultrasonography (US) showed enlarged kidneys and loss of cortico-medullary differentiation; moderate amount of free fluid in the peritoneal cavity, edema and thickening of the small bowel loop, with increased echogenicity of the mesenteric fat around it. The abdominal CT showed, in addition to the US findings, liquid distension and thickening of the small bowel loop wall, associated to PI (Figure 2).
The probable diagnosis of vasculitis of the small vessels of the intestinal wall was attained through the CT, which justified the diffuse enteric involvement, loop edema and PI.
The surgical approach was chosen together with the conservative treatment - antibiotic therapy by IV route, bowel rest and total parenteral nutrition (TPN) - while waiting for the response to the pulse therapy. As there was free fluid in the cavity, a paracentesis was performed and bacterial peritonitis was ruled out.
There was improvement in abdominal pain. After 8 days, the patient started to have bowel movements. Four days later, an oral diet was re-introduced and was well tolerated. A new abdominal CT performed 10 days later demonstrated significant improvement in the intestinal loop distension and wall edema, with disappearance of the PI.
The patient was discharged with oral prednisone (1 mg/kg/d), in addition to planned monthly pulses of cyclophosphamide IV. During the follow-up, he did not present new exacerbations of the intestinal picture.
DISCUSSION
The incidence of vasculitis of the gastrointestinal tract varies between 0.2% and 53% in lupus, with a close correlation with disease activity. There have been 14 cases of PI associated with SLE described in the literature. Underlying vasculitis was demonstrated in 50% of them.2,3,4,5,6
The vasculitis usually affects small vessels of the intestinal wall. The histological assessment demonstrates atrophy and degeneration of the media, fibrinoid necrosis, thrombosis and lymphocytic infiltrate in lamina propria; the immunohistochemical analysis shows deposit of immune complexes, complement and fibrinogen.4,7 PI is supposedly caused by an increase in the intraluminal pressure, mucosal injury and gas production by bacteria in the mucosa.1,6,8The clinical picture is variable. It can manifest as unspecific abdominal discomfort, diarrhea, abdominal distension, to acute abdomen with massive bleeding. The use of high-dose immunosuppressive drugs and corticoids can mask peritonitis. The absence of hydro-aerial noise and abdominal defense are late signs. Gastrointestinal tract vasculitis is almost always accompanied by activity in other organs, such as skin, kidneys and CNS.2,3,4,5 PI is usually asymptomatic; however, the cysts can rupture and cause pneumoperitoneum.1,9,10There seems to be an association between new leukopenia and thrombocytopenia or worsening of the pre-existing leukopenia with intestinal vasculitis.4,7
The X-ray is usually normal at first, but can demonstrate signs of paralytic ileus, loop edema and pneumoperitoneum. X-rays with contrast medium can demonstrate sign of "thumb printing' (edema or hemorrhage of submucosa, highly specific for intestinal ischemia) and escape of contrast medium in intestinal perforation.4,5,7 The echography can demonstrate wall thickening and the presence of free fluid.4,11
The "gold standard" assessment is the computed tomography (CT). The most frequent findings are loop edema, wall thickening and loop enhancement by the contrast (target sign or double halo sign, highly suggestive of vasculitis). It is also sensitive to detect collections of intramural gas, such as PI.4,8,9,10,11,12,13
Arteriography is not useful, as the disease usually affect small vessels. Endoscopy and colonoscopy are useful to locate ulcers and perform biopsies.4
The differential diagnosis is a challenge due to the multiple causes of abdominal pain in the patient with SLE.14
When there is no evidence of intestinal perforation, ischemia is potentially reversible and a conservative treatment is indicated. That includes high-dose corticoids (prednisone 1-2 mg/kg/day) or pulse therapy with methylprednisolone 1 g/day, for 3 days. Intestinal rest must be associated (TPN). Prokinetic agents can improve the peristaltism and reduce intraluminal pressure. Intravenous antibiotic therapy is used as adjuvant in the presence of IP; it aims at reducing bacterial overgrowth and gas production by anaerobic bacteria.3,4,5,15 Cyclophosphamide pulse therapy, 0.75-1 g/m, is indicated in refractory cases.2,4,13
There have been reports on the use of inhaled oxygen or hyperbaric chamber in an attempt to remove the gas from the cysts and of octreotide to improve motility and reduce bacterial overgrowth.10
Prognosis is poorer when there is surgical indication, which can be improved when the procedure is performed early.4,5,7 Early clinical suspicion and treatment are essential for a good evolution. Factors associated with recurrence are thickening of the intestinal wall > 9 cm at the tomography and lower cumulative dose of immunosuppressive agents.15
Although gastrointestinal tract vasculitis and IP are rare manifestations of SLE, the present case report describes a possible association between the two conditions.
REFERENCES
-
1Yamaguchi Y, Ohno S, Yamazaki S, Ideguchi H, Shirai A, Takeno M et al A case of systemic lupus erithematosus complicated with pneumatosis cystoides intestinalis. Mod Rheumatol 2005; 15:440-4.
-
2Machado WM, Freire BFA, Rocha OM, Azambuja CAP, Oliveira MEC. Proposta de questionário para caracterização da prevalência de sintomas digestivos nas doenças difusas do tecido conjuntivo. Arq. Gastroenterol 2004; 1:64-70.
-
3Grimbacher B, Huber M, Kempis J, Kalden P, Uhl M, Khöler G et al Successful treatment of gastrointestinal vasculitis due to systemic erythematosus lupus with intravenous pulse cyclophosphamide: a clinical case report and review of the literature. B J Rheum 1998; 37:1023-8.
-
4Sultan SM, Ioannou Y, Isenberg DA. A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology 1999; 38:917-32.
-
5Hallequa DS, Wallace DJ. Gastrointestinal manifestations of systemic lupus erythematosus. Curr Opin Rheumatol 2000; 12:379-85.
-
6Mizogushi F, Nanki T, Miyasaka N. Pneumatosis cystoides intestinalis following lupus enteritis and peritonitis. Inter Med 2008; 47:1267-71.
-
7Lee CK, Ahn MS, Lee EY, Shin JH, Cho YS, Ha HK et al Acute abdominal pain in systemic lupus erythematosus: focus on lupus enteritis (gastrointestinal vasculitis). Ann Rheum Dis 2002; 61:547-50.
-
8Freiman D, Chon H, Bilaniuk L. Pneumatosis intestinalis in systemic lupus erythematosus. Radiology 1975; 116:563-4.
-
9Hiraishi T, Tokuda M, Mitsunaka H, Dobashi H, Takahara J. Assymptomatic pneumatosis cystoides intestinalis in a patient with systemic lupus erythematosus. Ryumachi 1999; 39:580-5.
-
10Atsumi T, Sagawa A, Watanabe I, Amasaki Y, Katsumata K, Nakabayashi T et al Pneumoperitoneum without perforation of the gastrointestinal tract in a patient with systemic lupus erythematosus. Ryumachi 1991; 31:398-404.
-
11Caiado AHM, Menezes MR, Ishikawa WY, Gattas G, Sampaio ML, Yamashiro E et al. Qual o seu diagnóstico? Radiol Bras 2003; 36:V-VII.
-
12Byun JY, Ha HK, Yu SY, Min KJ, Park SH, Kim HY et al CT Features of systemic lupus erythematosus in patients with acute abdominal pain: emphasis on ischemic bowel disease. Radiology 1999; 211:203-9.
-
13Laing TJ. Gastrointestinal vasculitis and pneumatosis intestinalis due to systemic lupus erythematosus: successful treatment with pulse intravenous cyclophosphamide. Am J Med 1988; 85:555-8.
-
14Osler W. On the visceral complications of erythema exudativum multiforme. Am J Med Sci 1895; 110:629-46.
-
15Kim YG, Ha HK, Nah SS, Lee CK, Moon HB, Yoo B. Acute abdominal pain in systemic lupus erythematosus: factors contributing to recurrence of lupus enteritis. Ann Rheum Dis 2006; 65:1537-8.
Systemic lupus erythematosus complicated by intestinal vasculitis and pneumatosis intestinalis
Publication Dates
-
Publication in this collection
12 Nov 2010 -
Date of issue
Oct 2010
History
-
Accepted
26 Aug 2010 -
Received
25 Jan 2010