Abstract
The aim of this study was to incorporate tucuma oil (Astrocaryum vulgare) into PolyCaprolactone (PCL) electrospun fibers and evaluate its physicochemical properties and cell viability. FTIR and DRX confirmed that tucuma oil (TO) does not affect the chemical properties of PCL and that the oil was loaded into the PCL microstructure, while TGA analysis showed that the oil increased the thermal stability of the polymeric fibers. SEM showed that the addition of the oil modified fibers structure by reducing the average fiber size from 5.5 μm to 1.7 μm for TO loaded samples. Cell viability assay demonstrated an increment on cell proliferation from 80% of pure PCL to 100% for samples containing TO. Therefore, it can be concluded that tucuma oil can be incorporated into PCL to form fibers by electrospinning, without meaningful changes in its physicochemical properties and increasing its biocompatibility.
Keywords:
cytotoxicity; vegetal oil; fibers
1. Introduction
There are several studies focusing on the manufacture of polymeric fibers and one of the most used processes is electrospinning[11 Ambekar, R. S., & Kandasubramanian, B. (2019). Advancements in nanofibers for wound dressing: A review. European Polymer Journal, 117, 304-336. http://dx.doi.org/10.1016/j.eurpolymj.2019.05.020.
http://dx.doi.org/10.1016/j.eurpolymj.20...
2 Heseltine, P. L., Hosken, J., Agboh, C., Farrar, D., Homer-Vanniasinkam, S., & Edirisinghe, M. (2019). Fiber formation from silk fibroin using pressurized gyration. Macromolecular Materials and Engineering, 304(1), 1800577. http://dx.doi.org/10.1002/mame.201800577.
http://dx.doi.org/10.1002/mame.201800577...
3 Padron, S., Fuentes, A., Caruntu, D., & Lozano, K. (2013). Experimental study of nanofiber production through forcespinning. Journal of Applied Physics, 113(2), 024318. http://dx.doi.org/10.1063/1.4769886.
http://dx.doi.org/10.1063/1.4769886...
4 Hou, T., Li, X., Lu, Y., & Yang, B. (2017). Highly porous fibers prepared by centrifugal spinning. Materials & Design, 114, 303-311. http://dx.doi.org/10.1016/j.matdes.2016.11.019.
http://dx.doi.org/10.1016/j.matdes.2016....
5 Lee, H., Watanabe, K., Kim, M., Gopiraman, M., Song, K.-H., Lee, J. S., & Kim, I. S. (2016). Handspinning Enabled Highly Concentrated Carbon Nanotubes with Controlled Orientation in Nanofibers. Scientific Reports, 6(1), 37590. http://dx.doi.org/10.1038/srep37590. PMid:27876892.
http://dx.doi.org/10.1038/srep37590...
6 Cipitria, A., Skelton, A., Dargaville, T. R., Dalton, P. D., & Hutmacher, D. W. (2011). Design, fabrication and characterization of PCL electrospun scaffolds - A review. Journal of Materials Chemistry, 21(26), 9419-9453. http://dx.doi.org/10.1039/c0jm04502k.
http://dx.doi.org/10.1039/c0jm04502k...
7 Machado-Paula, M. M., Corat, M. A. F., Lancellotti, M., Mi, G., Marciano, F. R., Vega, M. L., Hidalgo, A. A., Webster, T. J., & Lobo, A. O. (2020). A comparison between electrospinning and rotary-jet spinning to produce PCL fibers with low bacteria colonization. Materials Science and Engineering C, 111, 110706. http://dx.doi.org/10.1016/j.msec.2020.110706. PMid:32279777.
http://dx.doi.org/10.1016/j.msec.2020.11...
-88 Pedram Rad, Z., Mokhtari, J., & Abbasi, M. (2018). Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Materials Science and Engineering C, 93, 356-366. http://dx.doi.org/10.1016/j.msec.2018.08.010. PMid:30274067.
http://dx.doi.org/10.1016/j.msec.2018.08...
]. This technique has such as being able to produce scaffolds with a controlled fiber diameter, high surface area, and porous structure[11 Ambekar, R. S., & Kandasubramanian, B. (2019). Advancements in nanofibers for wound dressing: A review. European Polymer Journal, 117, 304-336. http://dx.doi.org/10.1016/j.eurpolymj.2019.05.020.
http://dx.doi.org/10.1016/j.eurpolymj.20...
,99 Oliveira, P. S., Rodrigues, L. F., Jr., Volkmer, T. M., Gerra, N. B., & Santos, L. A. L. (2019). Obtaining bioabsorable scaffolds from PLGA and IR blend with the addition of hydroxyap-atite. Revista Materia, 24(3). http://dx.doi.org/10.1590/s1517-707620190003.0705.
http://dx.doi.org/10.1590/s1517-70762019...
10 Rodríguez-Tobías, H., Morales, G., & Grande, D. (2019). Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Materials Science and Engineering C, 101, 306-322. http://dx.doi.org/10.1016/j.msec.2019.03.099. PMid:31029324.
http://dx.doi.org/10.1016/j.msec.2019.03...
11 Koepsell, L., Remund, T., Bao, J., Neufeld, D., Fong, H., & Deng, Y. (2011). Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. Journal of Biomedical Materials Research. Part A, 99A(4), 564-575. http://dx.doi.org/10.1002/jbm.a.33216. PMid:21936046.
http://dx.doi.org/10.1002/jbm.a.33216...
-1212 Aghajanpoor, M., Hashemi-Najafabadi, S., Baghaban-Eslaminejad, M., Bagheri, F., Mohammad Mousavi, S., & Azam Sayyahpour, F. (2017). The effect of increasing the pore size of nanofibrous scaffolds on the osteogenic cell culture using a combination of sacrificial agent electrospinning and ultrasonication. Journal of Biomedical Materials Research. Part A, 105(7), 1887-1899. http://dx.doi.org/10.1002/jbm.a.36052. PMid:28256792.
http://dx.doi.org/10.1002/jbm.a.36052...
]. The ability to reproduce and manipulate the electrospinning process in vitro on a spatiotemporal scale similar to that of native tissue provides a great potential of clinical success[66 Cipitria, A., Skelton, A., Dargaville, T. R., Dalton, P. D., & Hutmacher, D. W. (2011). Design, fabrication and characterization of PCL electrospun scaffolds - A review. Journal of Materials Chemistry, 21(26), 9419-9453. http://dx.doi.org/10.1039/c0jm04502k.
http://dx.doi.org/10.1039/c0jm04502k...
,1313 Sharma, D., & Satapathy, B. K. (2019). Performance evaluation of electrospun nanofibrous mats of polylactic acid (PLA)/poly (ε-caprolactone) (PCL) blends. Materials Today: Proceedings, 19(Pt 2), 188-195. http://dx.doi.org/10.1016/j.matpr.2019.06.698.
http://dx.doi.org/10.1016/j.matpr.2019.0...
,1414 Tan, H.-L., Kai, D., Pasbakhsh, P., Teow, S.-Y., Lim, Y.-Y., & Pushpamalar, J. (2020). Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: a potential scaffold for tissue engineering. Colloids and Surfaces. B, Biointerfaces, 188, 110713. http://dx.doi.org/10.1016/j.colsurfb.2019.110713. PMid:31884080.
http://dx.doi.org/10.1016/j.colsurfb.201...
]. The wide range of commercially available biomaterials, as well as the strategies adopted in tissue engineering and regenerative medicine, favor the search for new products and methodologies to obtain these[1515 Marques, D. R., Volkmer, T. M., & Santos, L. A. (2015). Natural polymers: tissue engineering scaffolds. In M. Mishra (Ed.), Encyclopedia of biomedical polymers and polymeric biomaterials (pp. 5648-5657). Boca Raton: CRC Press. http://dx.doi.org/10.1201/9781351237970.
http://dx.doi.org/10.1201/9781351237970...
16 Zhang, B., He, J., Shi, M., Liang, Y., & Guo, B. (2020). Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chemical Engineering Journal, 400, 125994. http://dx.doi.org/10.1016/j.cej.2020.125994.
http://dx.doi.org/10.1016/j.cej.2020.125...
-1717 Liang, Y., He, J., & Guo, B. (2021). Functional hydrogels as wound dressing to enhance wound healing. ACS Nano, 15(8), 12687-12722. http://dx.doi.org/10.1021/acsnano.1c04206. PMid:34374515.
http://dx.doi.org/10.1021/acsnano.1c0420...
].
In line with this approach, the manufacture of scaffolds from absorbable, hydrolytically degradable polymers belonging to the aliphatic polyester class is being widely investigated for the use in tissue engineering[1818 Sartore, L., Inverardi, N., Pandini, S., Bignotti, F., & Chiellini, F. (2019). PLA/PCL-based foams as scaffolds for tissue engineering applications. Materials Today: Proceedings, 7(Pt 1), 410-417. http://dx.doi.org/10.1016/j.matpr.2018.11.103.
http://dx.doi.org/10.1016/j.matpr.2018.1...
19 Shao, H., Yu, X., Lin, T., Peng, J., Wang, A., Zhang, Z., Zhang, Y., Liu, S., & Zhao, M. (2020). Effect of PCL concentration on PCL/CaSiO3 porous composite scaffolds for bone engineering. Ceramics International, 46(9), 13082-13087. http://dx.doi.org/10.1016/j.ceramint.2020.02.079.
http://dx.doi.org/10.1016/j.ceramint.202...
20 Patrício, T., Domingos, M., Gloria, A., & Bártolo, P. (2013). Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP, 5, 110-114. http://dx.doi.org/10.1016/j.procir.2013.01.022.
http://dx.doi.org/10.1016/j.procir.2013....
21 Yao, Q., Cosme, J. G. L., Xu, T., Miszuk, J. M., Picciani, P. H. S., Fong, H., & Sun, H. (2017). Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials, 115, 115-127. http://dx.doi.org/10.1016/j.biomaterials.2016.11.018. PMid:27886552.
http://dx.doi.org/10.1016/j.biomaterials...
22 Coverdale, B. D. M., Gough, J. E., Sampson, W. W., & Hoyland, J. A. (2017). Use of lecithin to control fiber morphology in electrospun poly (ɛ-caprolactone) scaffolds for improved tissue engineering applications. Journal of Biomedical Materials Research. Part A, 105(10), 2865-2874. http://dx.doi.org/10.1002/jbm.a.36139. PMid:28608414.
http://dx.doi.org/10.1002/jbm.a.36139...
23 Wan, X., Liu, P., Jin, X., Xin, X., Li, P., Yuan, J., & Shen, J. (2018). Electrospun PCL/keratin/AuNPs mats with the catalytic generation of nitric oxide for potential of vascular tissue engineering. Journal of Biomedical Materials Research. Part A, 106(12), 3239-3247. http://dx.doi.org/10.1002/jbm.a.36521. PMid:30289598.
http://dx.doi.org/10.1002/jbm.a.36521...
-2424 Yongcong, F., Zhang, T., Liverani, L., Boccaccini, A. R., & Sun, W. (2019). Novel biomimetic fiber incorporated scaffolds for tissue engineering. Journal of Biomedical Materials Research. Part A, 107(12), 2694-2705. http://dx.doi.org/10.1002/jbm.a.36773. PMid:31390481.
http://dx.doi.org/10.1002/jbm.a.36773...
]. Their inherent biocompatibility properties and the possibility of undergoing hydrolysis in the body make these biomaterials suitable for the tissue reconstruction process. In addition to these, one can list its ease of processing and modulation of degradation rate, mechanical and visco-elastic properties[2525 Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (Eds.). (2013). Biomaterials science an introduction to materials in medicine. San Diego: Elsevier Academic Press. http://dx.doi.org/10.1016/C2009-0-02433-7.
http://dx.doi.org/10.1016/C2009-0-02433-...
].
Electrospun fibers are extremely attractive in the biomaterials field due to their large surface area to volume ratio[2626 Ding, J., Zhang, J., Li, J., Li, D., Xiao, C., Xiao, H., Yang, H., Zhuang, X., & Chen, X. (2019). Electrospun polymer biomaterials. Progress in Polymer Science, 90, 1-34. http://dx.doi.org/10.1016/j.progpolymsci.2019.01.002.
http://dx.doi.org/10.1016/j.progpolymsci...
]. Its potential applications include tissue engineering scaffolds, drug delivery media, wound healing, filtration media, composites[88 Pedram Rad, Z., Mokhtari, J., & Abbasi, M. (2018). Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Materials Science and Engineering C, 93, 356-366. http://dx.doi.org/10.1016/j.msec.2018.08.010. PMid:30274067.
http://dx.doi.org/10.1016/j.msec.2018.08...
,2727 Ibrahim, H. M., & Klingner, A. (2020). A review on electrospun polymeric nanofibers: production parameters and potential applications. Polymer Testing, 90, 106647. http://dx.doi.org/10.1016/j.polymertesting.2020.106647.
http://dx.doi.org/10.1016/j.polymertesti...
,2828 Juncos Bombin, A. D., Dunne, N. J., & McCarthy, H. O. (2020). Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Materials Science and Engineering C, 114, 110994. http://dx.doi.org/10.1016/j.msec.2020.110994. PMid:32993991.
http://dx.doi.org/10.1016/j.msec.2020.11...
], among others. It is known that the properties and internal molecular structure of polymers are strongly affected by their processing conditions. Thus, understanding the processing - structure - property relationship is of great importance for the development of polymeric fibers that meet the demands of the desired application[2929 Inai, R., Kotaki, M., & Ramakrishna, S. (2005). Structure and properties of electrospun PLLA single nanofibres. Nanotechnology, 16(2), 208-213. http://dx.doi.org/10.1088/0957-4484/16/2/005. PMid:21727424.
http://dx.doi.org/10.1088/0957-4484/16/2...
].
Several studies have been seeking viable alternatives for the use of absorbable polymers containing natural oils as those oils may enhance the material properties without significantly alter the structure of the polymeric matrix. In this idea, the tucuma oil shows great application possibilities, due to the small toxicity presented and efficiency in the controlled drug delivery[3030 Matos, K. A. N., Lima, D. P., Barbosa, A. P. P., Mercadante, A. Z., & Chisté, R. C. (2019). Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chemistry, 272, 216-221. http://dx.doi.org/10.1016/j.foodchem.2018.08.053. PMid:30309535.
http://dx.doi.org/10.1016/j.foodchem.201...
].
The tucuma palm (Astrocaryum vulgare), which is found in the Amazon Rain forest, is considered a pioneer of expressive growth, fire resistant with ability to sprout after burning and mainly inhabits the poultry and pastures. The kernel of the palm tree is externally covered with an oily orange canopy from which the oil is extracted[3131 Baldissera, M. D., Souza, C. F., Grando, T. H., Sagrillo, M. R., da Silva, A. S., Stefani, L. M., & Monteiro, S. G. (2017). The use of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice: effects on behavior, oxidant/antioxidant status, and enzymes involved in brain neurotransmission. Molecular and Cellular Biochemistry, 436(1-2), 159-166. http://dx.doi.org/10.1007/s11010-017-3087-9. PMid:28577189.
http://dx.doi.org/10.1007/s11010-017-308...
]. Among tropical seeds, tucuma palm is an economical source of vegetal oils[3232 Lalouckova, K., Skrivanova, E., Rondevaldova, J., Frankova, A., Soukup, J., & Kokoska, L. (2021). In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus. Scientific Reports, 11(1), 177. http://dx.doi.org/10.1038/s41598-020-80481-0. PMid:33420288.
http://dx.doi.org/10.1038/s41598-020-804...
] and it is also abundant in the northwest, north, and central-west regions of Brazil[3333 Costa, B. E. T., Santos, O. V., Corrêa, N. C. F., & França, L. F. (2016). Comparative study on the quality of oil extracted from two Tucumã varieties using supercritical carbon dioxide. Food Science and Technology (Campinas), 36(2), 322-328. http://dx.doi.org/10.1590/1678-457X.0094.
http://dx.doi.org/10.1590/1678-457X.0094...
]. Besides that, the simplicity of the extraction process to obtain the oil, which is mainly based on mechanical cold-pressing[3434 Silva, C. N., Hyacienth, D. C., Ferreira, A. M., Vilhena, J. C., Florentino, A. C., Cruz, R. A., Bereau, D., Robinson, J.-C., Carvalho, J. C., & Fernandes, C. P. (2015). Development of nanoemulsions with Tucumã (Astrocaryum vulgare) fruits oil. Journal of Nanomedicine Research, 2(2), 00024. http://dx.doi.org/10.15406/jnmr.2015.02.00024.
http://dx.doi.org/10.15406/jnmr.2015.02....
], also turns it into a promising biotechnological resource. In this view, tucuma oil has been already used in the biomedical field due to the high fatty acids content and the fact it has several carotenoids as bioactive compounds[3535 Cordenonsi, L. M., Santer, A., Sponchiado, R. M., Wingert, N. R., Raffin, R. P., & Schapoval, E. E. S. (2019). Amazonia products in novel lipid nanoparticles for fucoxanthin encapsulation. AAPS PharmSciTech, 21(1), 32. http://dx.doi.org/10.1208/s12249-019-1601-y. PMid:31863211.
http://dx.doi.org/10.1208/s12249-019-160...
]. Based on the aforementioned, its incorporation into electrospun biomaterials trends to improve biocompatibility in addition to physical-chemical properties[3636 Ghosal, K., Manakhov, A., Zajíčková, L., & Thomas, S. (2017). Structural and surface compatibility study of modified electrospun poly(ε-caprolactone) (PCL) composites for skin tissue engineering. AAPS PharmSciTech, 18(1), 72-81. http://dx.doi.org/10.1208/s12249-016-0500-8. PMid:26883261.
http://dx.doi.org/10.1208/s12249-016-050...
37 Ghosal, K., Thomas, S., Kalarikkal, N., & Gnanamani, A. (2014). Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: preliminary physico-chemical studies for skin substitute. Journal of Polymer Research, 21(5), 410. http://dx.doi.org/10.1007/s10965-014-0410-y.
http://dx.doi.org/10.1007/s10965-014-041...
38 Ghosal, K., Agatemor, C., Špitálsky, Z., Thomas, S., & Kny, E. (2019). Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chemical Engineering Journal, 358, 1262-1278. http://dx.doi.org/10.1016/j.cej.2018.10.117.
http://dx.doi.org/10.1016/j.cej.2018.10....
39 Ghosal, K., Chandra, A., Praveen, G., Snigdha, S., Roy, S., Agatemor, C., Thomas, S., & Provaznik, I. (2018). Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Scientific Reports, 8(1), 5058. http://dx.doi.org/10.1038/s41598-018-23378-3. PMid:29568048.
http://dx.doi.org/10.1038/s41598-018-233...
-4040 Ghosal, K., Kováčová, M., Humpolíček, P., Vajďák, J., Bodík, M., & Špitalský, Z. (2021). Antibacterial photodynamic activity of hydrophobic carbon quantum dots and polycaprolactone based nanocomposite processed via both electrospinning and solvent casting method. Photodiagnosis and Photodynamic Therapy, 35, 102455. http://dx.doi.org/10.1016/j.pdpdt.2021.102455. PMid:34311091.
http://dx.doi.org/10.1016/j.pdpdt.2021.1...
].
In this work, a study for obtaining PCL fibers with the addition of tucuma oil by the electrospinning process was carried out. The proposal aimed to evaluate whether the tucuma could be electrospun together with the PCL and whether this product would present good biocompatibility for its application as scaffolds or dressings for pressure injuries. Different amounts of tucuma oil were added to the PCL and its impact on morphology, physical and chemical properties, and its cytotoxic effect in cell media were studied.
2. Materials and Methods
2.1 Electrospinning methodology
For the preparation of a homogeneous polymer solution, 15% (w/v) PCL was dissolved in acetone at 50 °C using a hot plate and it was magnetic stirred until the polymer was completely solubilized. This solution was transferred to a 5 mL syringe with an 18 gauge needle. After some tests, the experimental parameters were optimized to 10 kV of potential difference and 10 cm distance between the tip of the needle containing the polymeric solution and the target. The tucuma oil was added into polymeric PCL solution before the electrospun process on the concentrations describe in Table 1.
2.2 Characterization
2.2.1 X-ray diffraction (XRD)
The characterization of the crystalline planes of the samples was performed using a Bruker D2 PHASER diffractometer. Couple Two Theta / Theta scan with 1482 steps of 1 second, 2θ from 5° to 70° and increment of 0.05° per second. The search was done at the International Center for Diffraction Data (ICDD) Database, file PDF2014 - PDF-2 Release 2014 RDB.
2.2.2 Fourier-transform infrared spectroscopy (FTIR)
The FTIR technique was used to verify the presence of functional groups in the PCL matrix. The assay was performed on a Spectrometer FTIR/NIR, model FRONTIER, brand PERKINELMER, with ATR methodology and resolution of 8 cm-1 and scanning from 4000 to 650 cm-1.
2.2.3 Thermogravimetric analysis (TGA)
Thermal behavior of samples was evaluated under N2 atmosphere with flow rate of 20 mL/min and heating rate of 10 °C/min. The testing was performed from 30 to 600 °C using one Perkin Elmer instrument, model TGA 400.
2.2.4 Scanning electron microscopy (SEM)
The morphology and size of the fibers were characterized by scanning electron microscopy (SEM), using a Hitachi, model tm3000 (secondary electron – SE). The software ImageJ was used to measure the diameter of the fibers. Fifty measurements were performed per sample
2.2.5 Cell viability (MTT)
The MTT assay for cell viability was used to verify the cytotoxicity of the obtained scaffolds by using an experimental protocol similar to that described by Wilms et al.[4141 Wilms, L. C., Hollman, P. C. H., Boots, A. W., & Kleinjans, J. C. S. (2005). Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutation Research, 582(1-2), 155-162. http://dx.doi.org/10.1016/j.mrgentox.2005.01.006. PMid:15781220.
http://dx.doi.org/10.1016/j.mrgentox.200...
].
Blood Collection for toxicological tests: Peripheral blood samples were obtained from three discard samples from the Clinical Analysis Laboratory of the Franciscan University, under the approval of the Institution's Human Ethics Committee (CAAE: 31211214.4.0000.5306) with no identification data. Samples were obtained by venipuncture using Vacutainer®-type heparin tubes, which were used to separate the Peripheral Blood Mononuclear Cell (PBMCs).
Treatments: A culture medium containing only the cells was used as a negative control, while the PCL, PCL100, PCL250, and PCL500 samples were incubated with PBMCs in an environment with 5% CO2 at 37 °C for 24h. Moreover, the results were represented as a function of optical density and then, the cell viability was calculated through spectrophotometry at the 570 nm wavelength, according to Equation 1:
Where:
OD570e: mean value for optical density of 100% of the extract of the test samples.
OD570b: mean value for optical density of the blanks.
2.3 Statistical analysis
For the analysis of the SEM images, the IMAGE J software was used, where 50 measurements were taken from each group. Subsequently, the data were entered at the software OriginPro 8.1. The one-way ANOVA test was used to see if there was a significant difference between the measurements where p would have to be less than 0.05. Also, for the MTT assay for cell viability, the treatments were compared to the negative control through one-way ANOVA to check for statistical differences between the groups and Dunnett’s post-hoc test to compare their means. The 95% confidence interval was used and p<0.05 were considered significant.
3. Results and Discussions
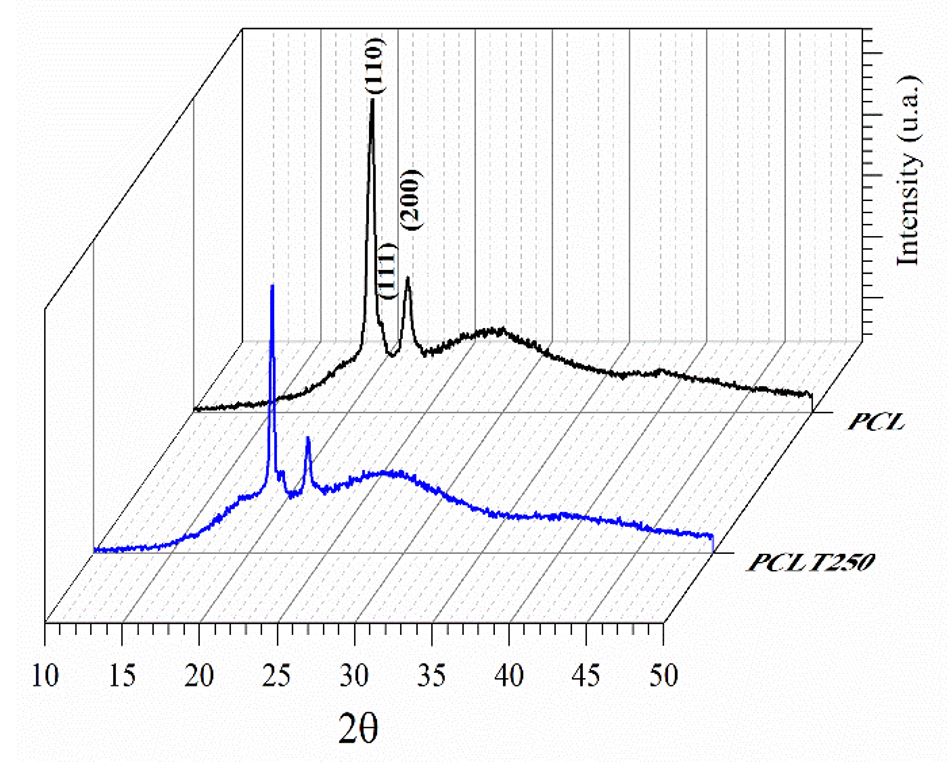
Figure 1 shows the diffractograms selected for PCL and the PCL mixed with tucuma oil at 250 µg/mL and it shows it has the same structural characteristics. When the PCL/tucuma is analyzed at different concentrations, a crystal structure is obtained[3030 Matos, K. A. N., Lima, D. P., Barbosa, A. P. P., Mercadante, A. Z., & Chisté, R. C. (2019). Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chemistry, 272, 216-221. http://dx.doi.org/10.1016/j.foodchem.2018.08.053. PMid:30309535.
http://dx.doi.org/10.1016/j.foodchem.201...
]. Diffractogram peaks present between 2Ɵ ranging from 21° to 24° are typical of PCL.
The FTIR-ATR spectrum of tucuma oil, PCL and PCL + tucuma oil are shown in Figure 2 and it contains characteristics bands of a vegetable oil[4242 Leonardi, B., Arauz, L. J., & Baruque-Ramos, J. (2019). Chemical characterization of amazonian non-polar vegetal extracts (buriti, Tucumã, Brazil Nut, Cupuaçu, and Cocoa) by Infrared Spectroscopy (FTIR) and Gas Chromatography (GC-FID). Infarma - Ciências Farmacêuticas, 31(3), 163-176. http://dx.doi.org/10.14450/2318-9312.v31.e3.a2019.pp163-176.
http://dx.doi.org/10.14450/2318-9312.v31...
43 Shimamoto, G. G., Favaro, M. M. A., & Tubino, M. (2015). Simple methods via Mid-IR or 1 H NMR spectroscopy for the determination of the iodine value of vegetable oils. Journal of the Brazilian Chemical Society, 26(7), 1431-1437. http://dx.doi.org/10.5935/0103-5053.20150111.
http://dx.doi.org/10.5935/0103-5053.2015...
44 Ali, M. E., Nina Naquiah, A. N., Mustafa, S., & Hamid, S. B. A. (2015). Differentiation of frog fats from vegetable and marine oils by Fourier Transform Infrared Spectroscopy andchemometric analysis. Croatian Journal of Food Science and Technology, 7(1), 1-8. http://dx.doi.org/10.17508/CJFST.2015.7.1.03.
http://dx.doi.org/10.17508/CJFST.2015.7....
-4545 Gomez, N. A., Abonia, R., Cadavid, H., & Vargas, I. H. (2011). Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. Journal of the Brazilian Chemical Society, 22(12), 2292-2303. http://dx.doi.org/10.1590/S0103-50532011001200009.
http://dx.doi.org/10.1590/S0103-50532011...
]. These bands are described in Table 2, according to the findings of Leonardi et al.[4242 Leonardi, B., Arauz, L. J., & Baruque-Ramos, J. (2019). Chemical characterization of amazonian non-polar vegetal extracts (buriti, Tucumã, Brazil Nut, Cupuaçu, and Cocoa) by Infrared Spectroscopy (FTIR) and Gas Chromatography (GC-FID). Infarma - Ciências Farmacêuticas, 31(3), 163-176. http://dx.doi.org/10.14450/2318-9312.v31.e3.a2019.pp163-176.
http://dx.doi.org/10.14450/2318-9312.v31...
], Ali et al.[4444 Ali, M. E., Nina Naquiah, A. N., Mustafa, S., & Hamid, S. B. A. (2015). Differentiation of frog fats from vegetable and marine oils by Fourier Transform Infrared Spectroscopy andchemometric analysis. Croatian Journal of Food Science and Technology, 7(1), 1-8. http://dx.doi.org/10.17508/CJFST.2015.7.1.03.
http://dx.doi.org/10.17508/CJFST.2015.7....
] and Gomez et al.[4545 Gomez, N. A., Abonia, R., Cadavid, H., & Vargas, I. H. (2011). Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. Journal of the Brazilian Chemical Society, 22(12), 2292-2303. http://dx.doi.org/10.1590/S0103-50532011001200009.
http://dx.doi.org/10.1590/S0103-50532011...
]. Bands in the region between 2980 and 2830 cm-1 are characteristic of the asymmetric and symmetric stretching modes of the C-H methylene group. This molecule is present both in PCL and in vegetable oils, such as tucuma. The carbonyl C=O aliphatic stretching was observed in 1733 cm-1 and 1744 cm-1 to PCL and PCl + tucama oil, respectively.
Infrared spectroscopy of PCL and PCL samples with 100 µg/mL, 250 µg/mL and 500 µg/mL of tucuman oil.
ATR-FTIR spectrum analysis for all samples[3535 Cordenonsi, L. M., Santer, A., Sponchiado, R. M., Wingert, N. R., Raffin, R. P., & Schapoval, E. E. S. (2019). Amazonia products in novel lipid nanoparticles for fucoxanthin encapsulation. AAPS PharmSciTech, 21(1), 32. http://dx.doi.org/10.1208/s12249-019-1601-y. PMid:31863211.
http://dx.doi.org/10.1208/s12249-019-160... ,3737 Ghosal, K., Thomas, S., Kalarikkal, N., & Gnanamani, A. (2014). Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: preliminary physico-chemical studies for skin substitute. Journal of Polymer Research, 21(5), 410. http://dx.doi.org/10.1007/s10965-014-0410-y.
http://dx.doi.org/10.1007/s10965-014-041... ,3838 Ghosal, K., Agatemor, C., Špitálsky, Z., Thomas, S., & Kny, E. (2019). Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chemical Engineering Journal, 358, 1262-1278. http://dx.doi.org/10.1016/j.cej.2018.10.117.
http://dx.doi.org/10.1016/j.cej.2018.10.... ].
It was observed that the incorporation of tucuma oil in PCL resulted in an increase in the intensity of the bands related to the methylene group, since all samples of PCL + tucuma presented greater intensity in these bands when compared to pure PCL. Nevertheless, it is not possible to see a significant difference in these intensities when the PCL + tucuma oil spectra are compared between them. Thus, the FTIR results suggest that the quantity of 100 µg/mL is sufficient to promote alterations in PCL properties.
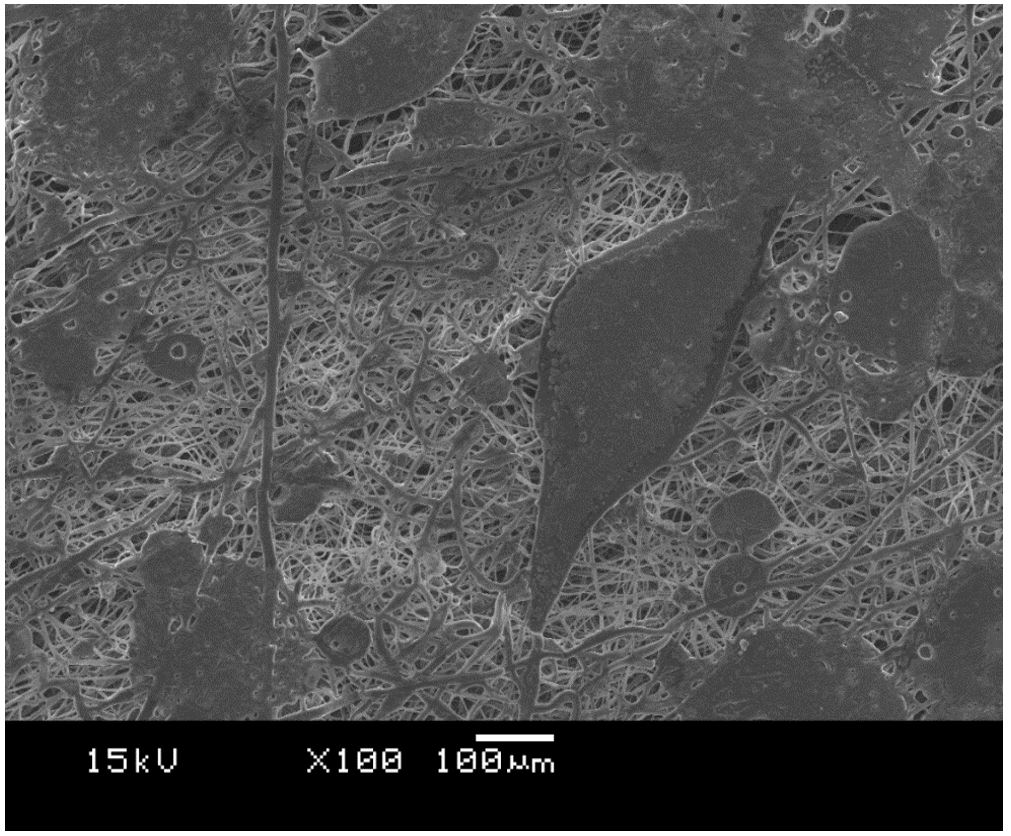
Figure 3 shows the SEM images from PCL and PCL with tucuma oil. The medium diameter from PCL fibers was the 5.3 ± 3.7 µm and the PCL fibers with tucuma oil were 1.72 ± 0.90 µm, 2.6 ± 1.3 µm, 2.9 ± 1.2 µm, to samples with 100 µg/mL, 250 µg/mL and 500 µg/mL, respectively. The ANOVA test showed a significant difference among PCL fibers diameter and all samples with tucuma oil. The same result was observed between and PCL plus 100 µg/mL and PCL plus 500 µg/mL (p < 0.05), despite of difference between fibers diameter from PCL plus 100 µg/mL and PCL plus 250 µg/mL the ANOVA test did not find a significant difference when comparing them (p = 0.29). These results were different from the work from Felgueiras et al.[4646 Felgueiras, H. P., Homem, N. C., Teixeira, M. A., Ribeiro, A. R. M., Antunes, J. C., & Amorim, M. T. P. (2020). Physical, Thermal, and antibacterial effects of active essential oils with potential for biomedical applications loaded onto cellulose acetate/polycaprolactone wet-spun microfibers. Biomolecules, 10(8), 1129. http://dx.doi.org/10.3390/biom10081129. PMid:32751893.
http://dx.doi.org/10.3390/biom10081129...
], where there was not statistical change in fiber diameters when an essential oil was added to polymer fibers. However, them are in accord with the showed by Tampau et al.[4747 Tampau, A., González-Martínez, C., & Chiralt, A. (2018). Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocolloids, 79, 158-169. http://dx.doi.org/10.1016/j.foodhyd.2017.12.021.
http://dx.doi.org/10.1016/j.foodhyd.2017...
] and Hasanpour Ardekani-Zadeh and Hosseini[4848 Hasanpour Ardekani-Zadeh, A., & Hosseini, S. F. (2019). Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydrate Polymers, 223, 115108. http://dx.doi.org/10.1016/j.carbpol.2019.115108. PMid:31426968.
http://dx.doi.org/10.1016/j.carbpol.2019...
], where the addition of oil furthered the diameter fibers reduction, when compared to polymer without oil. This comportment could be explained by change of physical-chemical characteristics of the polymer solution by tucuma oil that modifying the chain entanglements, responsible for fiber formation[4949 Kanani, A. G., & Bahrami, S. H. (2011). Effect of changing solvents on poly(ε-caprolactone) nanofibrous webs morphology. Journal of Nanomaterials, 2011, 724153. http://dx.doi.org/10.1155/2011/724153.
http://dx.doi.org/10.1155/2011/724153...
]. Figure 4 shows the SEM image to PCL plus 500 µg/mL tucuma oil where can be see a region in the sample that polymeric fibers collapsed. This could be justified by the insufficient evaporation of solvent due the large quantity of tucuma oil[4949 Kanani, A. G., & Bahrami, S. H. (2011). Effect of changing solvents on poly(ε-caprolactone) nanofibrous webs morphology. Journal of Nanomaterials, 2011, 724153. http://dx.doi.org/10.1155/2011/724153.
http://dx.doi.org/10.1155/2011/724153...
]. This result could explain the increased of diameter fibers from samples loaded with more tucuma oil, where minus tucuma oil has finner fibers and great quantity of tucuma oil has thicker fibers (1.72 ± 0.90 µm to PCL plus 100 µg/mL and 2.9 ± 1.2 µm to PCL plus 500 µg/mL).
SEM of samples with different concentrations of oil and pure PCL, (A) 100 µg/mL, (B) 250 µg/mL, (C) 500 µg/mL and (D) PCL.
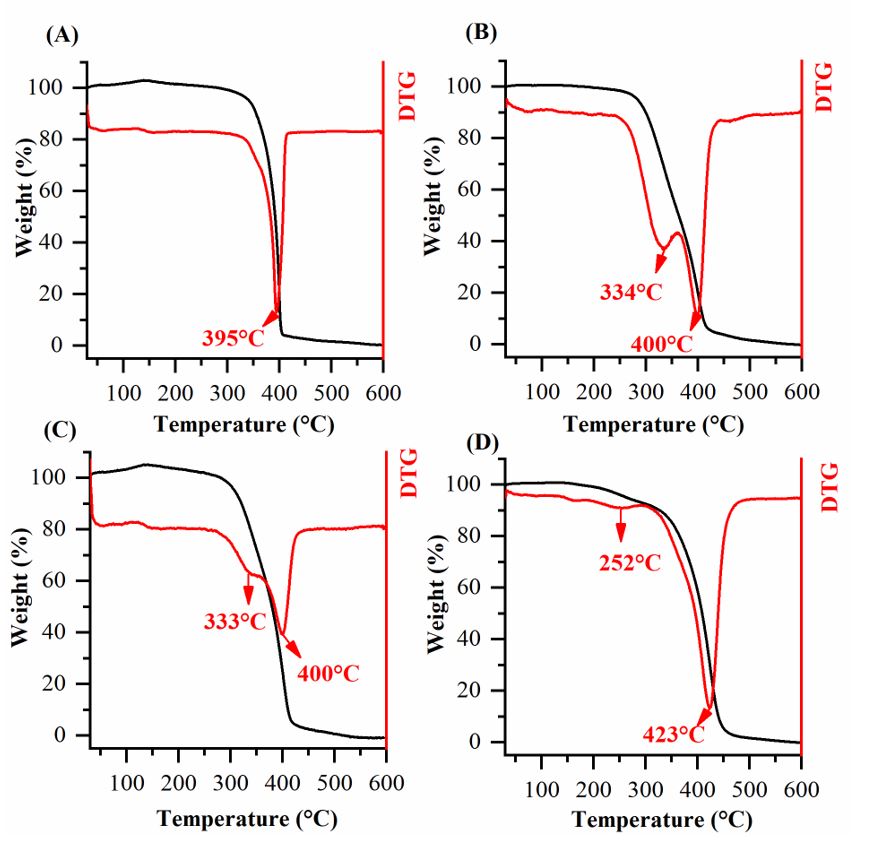
Thermogravimetric analysis (Figure 5) showed that the tucuma oil incorporation (100-500 µg/mL) in PCL leads to a higher thermal stability. Moreover, the events in the region around 330 °C in Figure 5B, Figure 5C and Figure 5D may be occurring together with another thermal event, resulting in a wide endothermic peak around 423 °C. To verify this, the DTG curve for sample PC500 (Figure 6) was analyzed by least squares fit of gaussian functions and deconvolution revealed two peaks (392 °C and 424 °C). This result is consistent with the other samples (PCL100 and PCL250), however, it is noted that the endothermic peaks were moved to higher temperatures. This is due to the greater amount of tucuma oil added to the PCL.
The in vitro effect of tucuma-loaded fiber scaffolds on PBMCs donated by volunteers was investigated by MTT (Figure 7). Test results showed no cytotoxicity behavior in any of the evaluated samples. Pure PCL nanofibers showed a cell viability around 95.7% against PBMC cells, however, this result is significantly smaller when compared to negative control and to all tucuma-loaded PCL samples (p≤0.05). On the other hand, there was no significant difference in cell viability with all tucuma-loaded samples and negative control (p≤0.05). The results exhibited that tucuma oil could induce cell proliferation on PCL fiber mats. In agreement with the results obtained on this paper, Ongaratto et al.[5050 Ongaratto, F., Bonadiman, B. S. R., Marafon, F., Kosvoski, G. C., Chaves, C. C., Chaves, C. M., Cruz, I. B. M., & Bagatini, M. D. (2020). Efeito in vitro do extrato de Tucumã (astrocaryum aculeatum) em células mononucleares de sangue periférico. Brazilian Journal of Health Review, 3(3), 5055-5062. http://dx.doi.org/10.34119/bjhrv3n3-087.
http://dx.doi.org/10.34119/bjhrv3n3-087...
] studied the cellular viability of different tucuma extract concentrations (5, 10, 50, 100 e 500 μg/mL) on PBMCs. The viability of the cells seeded on the pure PCL fibers mats was significantly lower than that of cells cultured on the control well, this result can be credited to the PCL fibers hydrophobic nature[5151 Bui, H. T., Chung, O. H., Dela Cruz, J., & Park, J. S. (2014). Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromolecular Research, 22(12), 1288-1296. http://dx.doi.org/10.1007/s13233-014-2179-6.
http://dx.doi.org/10.1007/s13233-014-217...
]. The incorporation of tucuman oil into PCL fiber mats leaded to an improvement on cell viability, which suggests that it possess in its chemical composition molecules such as the β-carotene[5252 Nascimento, K., Copetti, P. M., Fernandes, A., Klein, B., Fogaça, A., Zepka, L. Q., Wagner, R., Ourique, A. F., Sagrillo, M. R., & da Silva, J. E. P. (2021). Phytochemical analysis and evaluation of the antioxidant and antiproliferative effects of Tucumã oil nanocapsules in breast adenocarcinoma cells (MCF-7). Natural Product Research, 35(12), 2060-2065. http://dx.doi.org/10.1080/14786419.2019.1648460. PMid:34096432.
http://dx.doi.org/10.1080/14786419.2019....
] that can regulate the expression of genes responsible for cell proliferation and differentiation by controlling ROS production and lipid peroxidation[5353 Sagrillo, M. R., Garcia, L. F., de Souza, O. C., Fo., Duarte, M. M., Ribeiro, E. E., Cadoná, F. C., & da Cruz, I. B. (2015). Tucumã fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chemistry, 173, 741-748. http://dx.doi.org/10.1016/j.foodchem.2014.10.067. PMid:25466084.
http://dx.doi.org/10.1016/j.foodchem.201...
54 Lomenick, B., Shi, H., Huang, J., & Chen, C. (2015). Identification and characterization of β-sitosterol target proteins. Bioorganic & Medicinal Chemistry Letters, 25(21), 4976-4979. http://dx.doi.org/10.1016/j.bmcl.2015.03.007. PMid:25804720.
http://dx.doi.org/10.1016/j.bmcl.2015.03...
-5555 Elliott, R. (2005). Mechanisms of genomic and non-genomic actions of carotenoids. Biochimica et Biophysica Acta. 1740(2), 147-154. http://dx.doi.org/10.1016/j.bbadis.2004.12.009. PMid:15949681.
http://dx.doi.org/10.1016/j.bbadis.2004....
].
Cell viability assay of Peripheral Blood Monocytes (PBMC) Lymphocytes on PCL, 100, 250 and 500 μg/mL Tucuma-loaded PCL nanofibers after 24 hours (p≤0.05). Values are expressed as mean ± S.D. of three parallel measurements. (* - significant difference; NS - nonsignificant difference).
4. Conclusions
PCL fibers incorporated with tucuma oil were successfully electrospun and were evaluated with respect to their chemical, physical and morphological properties as well as cytotoxicity. The incorporation of tucuma oil did not affected the PCL chemical structure, which was confirmed by XRD and FTIR. Also, the SEM analysis confirmed the fibrous network of PCL and the addition of tucuma oil into this microstructure. In respect to the morphologic properties, it was noted that the mean diameter of PCL fibers decreased with the addition of tucuma oil. Besides that, the incorporation of tucuma oil into the PCL matrix led to a higher thermal stability compared to pristine PCL. Regarding cytotoxicity, the incorporation of tucuma oil showed enhanced biocompatibility, once it increased the cell viability evaluated by an MTT assay and did not present cytotoxicity against PBMCs. Thus, PCL can be electrospun with tucuma oil to achieve a fibrous biomaterial with increased biocompatibility and interesting physical and morphological properties, without altering the PCL chemical structure.
5. Acknowledgements
Universidade Franciscana for providing the main materials and infrastructure required to develop this research. To the Instituto Federal de Educação, Ciência e Tecnologia do Amapá, the Universidade Federal de Pelotas, and the Universidade Federal do Rio Grande do Sul. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
-
How to cite: Bressa, N. R., Oviedo, V. R., Machado, A. M. B., Almeida, W. L., Volkmer, T. M., Santos, L. A. L., Sagrillo, M. R., & Rodrigues Junior, L. F. (2021). Incorporation of astrocaryum vulgare (tucuma) oil into PCL electrospun fibers. Polímeros: Ciência e Tecnologia, 31(3), e2021036. https://doi.org/10.1590/0104-1428.20210056
6. References
-
1Ambekar, R. S., & Kandasubramanian, B. (2019). Advancements in nanofibers for wound dressing: A review. European Polymer Journal, 117, 304-336. http://dx.doi.org/10.1016/j.eurpolymj.2019.05.020
» http://dx.doi.org/10.1016/j.eurpolymj.2019.05.020 -
2Heseltine, P. L., Hosken, J., Agboh, C., Farrar, D., Homer-Vanniasinkam, S., & Edirisinghe, M. (2019). Fiber formation from silk fibroin using pressurized gyration. Macromolecular Materials and Engineering, 304(1), 1800577. http://dx.doi.org/10.1002/mame.201800577
» http://dx.doi.org/10.1002/mame.201800577 -
3Padron, S., Fuentes, A., Caruntu, D., & Lozano, K. (2013). Experimental study of nanofiber production through forcespinning. Journal of Applied Physics, 113(2), 024318. http://dx.doi.org/10.1063/1.4769886
» http://dx.doi.org/10.1063/1.4769886 -
4Hou, T., Li, X., Lu, Y., & Yang, B. (2017). Highly porous fibers prepared by centrifugal spinning. Materials & Design, 114, 303-311. http://dx.doi.org/10.1016/j.matdes.2016.11.019
» http://dx.doi.org/10.1016/j.matdes.2016.11.019 -
5Lee, H., Watanabe, K., Kim, M., Gopiraman, M., Song, K.-H., Lee, J. S., & Kim, I. S. (2016). Handspinning Enabled Highly Concentrated Carbon Nanotubes with Controlled Orientation in Nanofibers. Scientific Reports, 6(1), 37590. http://dx.doi.org/10.1038/srep37590 PMid:27876892.
» http://dx.doi.org/10.1038/srep37590 -
6Cipitria, A., Skelton, A., Dargaville, T. R., Dalton, P. D., & Hutmacher, D. W. (2011). Design, fabrication and characterization of PCL electrospun scaffolds - A review. Journal of Materials Chemistry, 21(26), 9419-9453. http://dx.doi.org/10.1039/c0jm04502k
» http://dx.doi.org/10.1039/c0jm04502k -
7Machado-Paula, M. M., Corat, M. A. F., Lancellotti, M., Mi, G., Marciano, F. R., Vega, M. L., Hidalgo, A. A., Webster, T. J., & Lobo, A. O. (2020). A comparison between electrospinning and rotary-jet spinning to produce PCL fibers with low bacteria colonization. Materials Science and Engineering C, 111, 110706. http://dx.doi.org/10.1016/j.msec.2020.110706 PMid:32279777.
» http://dx.doi.org/10.1016/j.msec.2020.110706 -
8Pedram Rad, Z., Mokhtari, J., & Abbasi, M. (2018). Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Materials Science and Engineering C, 93, 356-366. http://dx.doi.org/10.1016/j.msec.2018.08.010 PMid:30274067.
» http://dx.doi.org/10.1016/j.msec.2018.08.010 -
9Oliveira, P. S., Rodrigues, L. F., Jr., Volkmer, T. M., Gerra, N. B., & Santos, L. A. L. (2019). Obtaining bioabsorable scaffolds from PLGA and IR blend with the addition of hydroxyap-atite. Revista Materia, 24(3). http://dx.doi.org/10.1590/s1517-707620190003.0705
» http://dx.doi.org/10.1590/s1517-707620190003.0705 -
10Rodríguez-Tobías, H., Morales, G., & Grande, D. (2019). Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Materials Science and Engineering C, 101, 306-322. http://dx.doi.org/10.1016/j.msec.2019.03.099 PMid:31029324.
» http://dx.doi.org/10.1016/j.msec.2019.03.099 -
11Koepsell, L., Remund, T., Bao, J., Neufeld, D., Fong, H., & Deng, Y. (2011). Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. Journal of Biomedical Materials Research. Part A, 99A(4), 564-575. http://dx.doi.org/10.1002/jbm.a.33216 PMid:21936046.
» http://dx.doi.org/10.1002/jbm.a.33216 -
12Aghajanpoor, M., Hashemi-Najafabadi, S., Baghaban-Eslaminejad, M., Bagheri, F., Mohammad Mousavi, S., & Azam Sayyahpour, F. (2017). The effect of increasing the pore size of nanofibrous scaffolds on the osteogenic cell culture using a combination of sacrificial agent electrospinning and ultrasonication. Journal of Biomedical Materials Research. Part A, 105(7), 1887-1899. http://dx.doi.org/10.1002/jbm.a.36052 PMid:28256792.
» http://dx.doi.org/10.1002/jbm.a.36052 -
13Sharma, D., & Satapathy, B. K. (2019). Performance evaluation of electrospun nanofibrous mats of polylactic acid (PLA)/poly (ε-caprolactone) (PCL) blends. Materials Today: Proceedings, 19(Pt 2), 188-195. http://dx.doi.org/10.1016/j.matpr.2019.06.698
» http://dx.doi.org/10.1016/j.matpr.2019.06.698 -
14Tan, H.-L., Kai, D., Pasbakhsh, P., Teow, S.-Y., Lim, Y.-Y., & Pushpamalar, J. (2020). Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: a potential scaffold for tissue engineering. Colloids and Surfaces. B, Biointerfaces, 188, 110713. http://dx.doi.org/10.1016/j.colsurfb.2019.110713 PMid:31884080.
» http://dx.doi.org/10.1016/j.colsurfb.2019.110713 -
15Marques, D. R., Volkmer, T. M., & Santos, L. A. (2015). Natural polymers: tissue engineering scaffolds. In M. Mishra (Ed.), Encyclopedia of biomedical polymers and polymeric biomaterials (pp. 5648-5657). Boca Raton: CRC Press. http://dx.doi.org/10.1201/9781351237970
» http://dx.doi.org/10.1201/9781351237970 -
16Zhang, B., He, J., Shi, M., Liang, Y., & Guo, B. (2020). Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chemical Engineering Journal, 400, 125994. http://dx.doi.org/10.1016/j.cej.2020.125994
» http://dx.doi.org/10.1016/j.cej.2020.125994 -
17Liang, Y., He, J., & Guo, B. (2021). Functional hydrogels as wound dressing to enhance wound healing. ACS Nano, 15(8), 12687-12722. http://dx.doi.org/10.1021/acsnano.1c04206 PMid:34374515.
» http://dx.doi.org/10.1021/acsnano.1c04206 -
18Sartore, L., Inverardi, N., Pandini, S., Bignotti, F., & Chiellini, F. (2019). PLA/PCL-based foams as scaffolds for tissue engineering applications. Materials Today: Proceedings, 7(Pt 1), 410-417. http://dx.doi.org/10.1016/j.matpr.2018.11.103
» http://dx.doi.org/10.1016/j.matpr.2018.11.103 -
19Shao, H., Yu, X., Lin, T., Peng, J., Wang, A., Zhang, Z., Zhang, Y., Liu, S., & Zhao, M. (2020). Effect of PCL concentration on PCL/CaSiO3 porous composite scaffolds for bone engineering. Ceramics International, 46(9), 13082-13087. http://dx.doi.org/10.1016/j.ceramint.2020.02.079
» http://dx.doi.org/10.1016/j.ceramint.2020.02.079 -
20Patrício, T., Domingos, M., Gloria, A., & Bártolo, P. (2013). Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP, 5, 110-114. http://dx.doi.org/10.1016/j.procir.2013.01.022
» http://dx.doi.org/10.1016/j.procir.2013.01.022 -
21Yao, Q., Cosme, J. G. L., Xu, T., Miszuk, J. M., Picciani, P. H. S., Fong, H., & Sun, H. (2017). Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials, 115, 115-127. http://dx.doi.org/10.1016/j.biomaterials.2016.11.018 PMid:27886552.
» http://dx.doi.org/10.1016/j.biomaterials.2016.11.018 -
22Coverdale, B. D. M., Gough, J. E., Sampson, W. W., & Hoyland, J. A. (2017). Use of lecithin to control fiber morphology in electrospun poly (ɛ-caprolactone) scaffolds for improved tissue engineering applications. Journal of Biomedical Materials Research. Part A, 105(10), 2865-2874. http://dx.doi.org/10.1002/jbm.a.36139 PMid:28608414.
» http://dx.doi.org/10.1002/jbm.a.36139 -
23Wan, X., Liu, P., Jin, X., Xin, X., Li, P., Yuan, J., & Shen, J. (2018). Electrospun PCL/keratin/AuNPs mats with the catalytic generation of nitric oxide for potential of vascular tissue engineering. Journal of Biomedical Materials Research. Part A, 106(12), 3239-3247. http://dx.doi.org/10.1002/jbm.a.36521 PMid:30289598.
» http://dx.doi.org/10.1002/jbm.a.36521 -
24Yongcong, F., Zhang, T., Liverani, L., Boccaccini, A. R., & Sun, W. (2019). Novel biomimetic fiber incorporated scaffolds for tissue engineering. Journal of Biomedical Materials Research. Part A, 107(12), 2694-2705. http://dx.doi.org/10.1002/jbm.a.36773 PMid:31390481.
» http://dx.doi.org/10.1002/jbm.a.36773 -
25Ratner, B. D., Hoffman, A. S., Schoen, F. J., & Lemons, J. E. (Eds.). (2013). Biomaterials science an introduction to materials in medicine San Diego: Elsevier Academic Press. http://dx.doi.org/10.1016/C2009-0-02433-7
» http://dx.doi.org/10.1016/C2009-0-02433-7 -
26Ding, J., Zhang, J., Li, J., Li, D., Xiao, C., Xiao, H., Yang, H., Zhuang, X., & Chen, X. (2019). Electrospun polymer biomaterials. Progress in Polymer Science, 90, 1-34. http://dx.doi.org/10.1016/j.progpolymsci.2019.01.002
» http://dx.doi.org/10.1016/j.progpolymsci.2019.01.002 -
27Ibrahim, H. M., & Klingner, A. (2020). A review on electrospun polymeric nanofibers: production parameters and potential applications. Polymer Testing, 90, 106647. http://dx.doi.org/10.1016/j.polymertesting.2020.106647
» http://dx.doi.org/10.1016/j.polymertesting.2020.106647 -
28Juncos Bombin, A. D., Dunne, N. J., & McCarthy, H. O. (2020). Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Materials Science and Engineering C, 114, 110994. http://dx.doi.org/10.1016/j.msec.2020.110994 PMid:32993991.
» http://dx.doi.org/10.1016/j.msec.2020.110994 -
29Inai, R., Kotaki, M., & Ramakrishna, S. (2005). Structure and properties of electrospun PLLA single nanofibres. Nanotechnology, 16(2), 208-213. http://dx.doi.org/10.1088/0957-4484/16/2/005 PMid:21727424.
» http://dx.doi.org/10.1088/0957-4484/16/2/005 -
30Matos, K. A. N., Lima, D. P., Barbosa, A. P. P., Mercadante, A. Z., & Chisté, R. C. (2019). Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chemistry, 272, 216-221. http://dx.doi.org/10.1016/j.foodchem.2018.08.053 PMid:30309535.
» http://dx.doi.org/10.1016/j.foodchem.2018.08.053 -
31Baldissera, M. D., Souza, C. F., Grando, T. H., Sagrillo, M. R., da Silva, A. S., Stefani, L. M., & Monteiro, S. G. (2017). The use of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice: effects on behavior, oxidant/antioxidant status, and enzymes involved in brain neurotransmission. Molecular and Cellular Biochemistry, 436(1-2), 159-166. http://dx.doi.org/10.1007/s11010-017-3087-9 PMid:28577189.
» http://dx.doi.org/10.1007/s11010-017-3087-9 -
32Lalouckova, K., Skrivanova, E., Rondevaldova, J., Frankova, A., Soukup, J., & Kokoska, L. (2021). In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent, lauric acid, with oxacillin in Staphylococcus aureus. Scientific Reports, 11(1), 177. http://dx.doi.org/10.1038/s41598-020-80481-0 PMid:33420288.
» http://dx.doi.org/10.1038/s41598-020-80481-0 -
33Costa, B. E. T., Santos, O. V., Corrêa, N. C. F., & França, L. F. (2016). Comparative study on the quality of oil extracted from two Tucumã varieties using supercritical carbon dioxide. Food Science and Technology (Campinas), 36(2), 322-328. http://dx.doi.org/10.1590/1678-457X.0094
» http://dx.doi.org/10.1590/1678-457X.0094 -
34Silva, C. N., Hyacienth, D. C., Ferreira, A. M., Vilhena, J. C., Florentino, A. C., Cruz, R. A., Bereau, D., Robinson, J.-C., Carvalho, J. C., & Fernandes, C. P. (2015). Development of nanoemulsions with Tucumã (Astrocaryum vulgare) fruits oil. Journal of Nanomedicine Research, 2(2), 00024. http://dx.doi.org/10.15406/jnmr.2015.02.00024
» http://dx.doi.org/10.15406/jnmr.2015.02.00024 -
35Cordenonsi, L. M., Santer, A., Sponchiado, R. M., Wingert, N. R., Raffin, R. P., & Schapoval, E. E. S. (2019). Amazonia products in novel lipid nanoparticles for fucoxanthin encapsulation. AAPS PharmSciTech, 21(1), 32. http://dx.doi.org/10.1208/s12249-019-1601-y PMid:31863211.
» http://dx.doi.org/10.1208/s12249-019-1601-y -
36Ghosal, K., Manakhov, A., Zajíčková, L., & Thomas, S. (2017). Structural and surface compatibility study of modified electrospun poly(ε-caprolactone) (PCL) composites for skin tissue engineering. AAPS PharmSciTech, 18(1), 72-81. http://dx.doi.org/10.1208/s12249-016-0500-8 PMid:26883261.
» http://dx.doi.org/10.1208/s12249-016-0500-8 -
37Ghosal, K., Thomas, S., Kalarikkal, N., & Gnanamani, A. (2014). Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: preliminary physico-chemical studies for skin substitute. Journal of Polymer Research, 21(5), 410. http://dx.doi.org/10.1007/s10965-014-0410-y
» http://dx.doi.org/10.1007/s10965-014-0410-y -
38Ghosal, K., Agatemor, C., Špitálsky, Z., Thomas, S., & Kny, E. (2019). Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chemical Engineering Journal, 358, 1262-1278. http://dx.doi.org/10.1016/j.cej.2018.10.117
» http://dx.doi.org/10.1016/j.cej.2018.10.117 -
39Ghosal, K., Chandra, A., Praveen, G., Snigdha, S., Roy, S., Agatemor, C., Thomas, S., & Provaznik, I. (2018). Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Scientific Reports, 8(1), 5058. http://dx.doi.org/10.1038/s41598-018-23378-3 PMid:29568048.
» http://dx.doi.org/10.1038/s41598-018-23378-3 -
40Ghosal, K., Kováčová, M., Humpolíček, P., Vajďák, J., Bodík, M., & Špitalský, Z. (2021). Antibacterial photodynamic activity of hydrophobic carbon quantum dots and polycaprolactone based nanocomposite processed via both electrospinning and solvent casting method. Photodiagnosis and Photodynamic Therapy, 35, 102455. http://dx.doi.org/10.1016/j.pdpdt.2021.102455 PMid:34311091.
» http://dx.doi.org/10.1016/j.pdpdt.2021.102455 -
41Wilms, L. C., Hollman, P. C. H., Boots, A. W., & Kleinjans, J. C. S. (2005). Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutation Research, 582(1-2), 155-162. http://dx.doi.org/10.1016/j.mrgentox.2005.01.006 PMid:15781220.
» http://dx.doi.org/10.1016/j.mrgentox.2005.01.006 -
42Leonardi, B., Arauz, L. J., & Baruque-Ramos, J. (2019). Chemical characterization of amazonian non-polar vegetal extracts (buriti, Tucumã, Brazil Nut, Cupuaçu, and Cocoa) by Infrared Spectroscopy (FTIR) and Gas Chromatography (GC-FID). Infarma - Ciências Farmacêuticas, 31(3), 163-176. http://dx.doi.org/10.14450/2318-9312.v31.e3.a2019.pp163-176
» http://dx.doi.org/10.14450/2318-9312.v31.e3.a2019.pp163-176 -
43Shimamoto, G. G., Favaro, M. M. A., & Tubino, M. (2015). Simple methods via Mid-IR or 1 H NMR spectroscopy for the determination of the iodine value of vegetable oils. Journal of the Brazilian Chemical Society, 26(7), 1431-1437. http://dx.doi.org/10.5935/0103-5053.20150111
» http://dx.doi.org/10.5935/0103-5053.20150111 -
44Ali, M. E., Nina Naquiah, A. N., Mustafa, S., & Hamid, S. B. A. (2015). Differentiation of frog fats from vegetable and marine oils by Fourier Transform Infrared Spectroscopy andchemometric analysis. Croatian Journal of Food Science and Technology, 7(1), 1-8. http://dx.doi.org/10.17508/CJFST.2015.7.1.03
» http://dx.doi.org/10.17508/CJFST.2015.7.1.03 -
45Gomez, N. A., Abonia, R., Cadavid, H., & Vargas, I. H. (2011). Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. Journal of the Brazilian Chemical Society, 22(12), 2292-2303. http://dx.doi.org/10.1590/S0103-50532011001200009
» http://dx.doi.org/10.1590/S0103-50532011001200009 -
46Felgueiras, H. P., Homem, N. C., Teixeira, M. A., Ribeiro, A. R. M., Antunes, J. C., & Amorim, M. T. P. (2020). Physical, Thermal, and antibacterial effects of active essential oils with potential for biomedical applications loaded onto cellulose acetate/polycaprolactone wet-spun microfibers. Biomolecules, 10(8), 1129. http://dx.doi.org/10.3390/biom10081129 PMid:32751893.
» http://dx.doi.org/10.3390/biom10081129 -
47Tampau, A., González-Martínez, C., & Chiralt, A. (2018). Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocolloids, 79, 158-169. http://dx.doi.org/10.1016/j.foodhyd.2017.12.021
» http://dx.doi.org/10.1016/j.foodhyd.2017.12.021 -
48Hasanpour Ardekani-Zadeh, A., & Hosseini, S. F. (2019). Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydrate Polymers, 223, 115108. http://dx.doi.org/10.1016/j.carbpol.2019.115108 PMid:31426968.
» http://dx.doi.org/10.1016/j.carbpol.2019.115108 -
49Kanani, A. G., & Bahrami, S. H. (2011). Effect of changing solvents on poly(ε-caprolactone) nanofibrous webs morphology. Journal of Nanomaterials, 2011, 724153. http://dx.doi.org/10.1155/2011/724153
» http://dx.doi.org/10.1155/2011/724153 -
50Ongaratto, F., Bonadiman, B. S. R., Marafon, F., Kosvoski, G. C., Chaves, C. C., Chaves, C. M., Cruz, I. B. M., & Bagatini, M. D. (2020). Efeito in vitro do extrato de Tucumã (astrocaryum aculeatum) em células mononucleares de sangue periférico. Brazilian Journal of Health Review, 3(3), 5055-5062. http://dx.doi.org/10.34119/bjhrv3n3-087
» http://dx.doi.org/10.34119/bjhrv3n3-087 -
51Bui, H. T., Chung, O. H., Dela Cruz, J., & Park, J. S. (2014). Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromolecular Research, 22(12), 1288-1296. http://dx.doi.org/10.1007/s13233-014-2179-6
» http://dx.doi.org/10.1007/s13233-014-2179-6 -
52Nascimento, K., Copetti, P. M., Fernandes, A., Klein, B., Fogaça, A., Zepka, L. Q., Wagner, R., Ourique, A. F., Sagrillo, M. R., & da Silva, J. E. P. (2021). Phytochemical analysis and evaluation of the antioxidant and antiproliferative effects of Tucumã oil nanocapsules in breast adenocarcinoma cells (MCF-7). Natural Product Research, 35(12), 2060-2065. http://dx.doi.org/10.1080/14786419.2019.1648460 PMid:34096432.
» http://dx.doi.org/10.1080/14786419.2019.1648460 -
53Sagrillo, M. R., Garcia, L. F., de Souza, O. C., Fo., Duarte, M. M., Ribeiro, E. E., Cadoná, F. C., & da Cruz, I. B. (2015). Tucumã fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chemistry, 173, 741-748. http://dx.doi.org/10.1016/j.foodchem.2014.10.067 PMid:25466084.
» http://dx.doi.org/10.1016/j.foodchem.2014.10.067 -
54Lomenick, B., Shi, H., Huang, J., & Chen, C. (2015). Identification and characterization of β-sitosterol target proteins. Bioorganic & Medicinal Chemistry Letters, 25(21), 4976-4979. http://dx.doi.org/10.1016/j.bmcl.2015.03.007 PMid:25804720.
» http://dx.doi.org/10.1016/j.bmcl.2015.03.007 -
55Elliott, R. (2005). Mechanisms of genomic and non-genomic actions of carotenoids. Biochimica et Biophysica Acta 1740(2), 147-154. http://dx.doi.org/10.1016/j.bbadis.2004.12.009 PMid:15949681.
» http://dx.doi.org/10.1016/j.bbadis.2004.12.009
Publication Dates
-
Publication in this collection
07 Jan 2022 -
Date of issue
2021
History
-
Received
01 Aug 2021 -
Reviewed
28 Nov 2021 -
Accepted
30 Nov 2021