Abstract
A new species of miniature fish of the characid genus Priocharax is described from a small lake near the rio Ipixuna, rio Purus drainage, Amazonas State, Brazil. It is distinguished from all congeners except P. pygmaeus by the lower number of teeth on the maxilla and dentary. It differs from P. pygmaeus by the presence of two postcleithra and 22–27 branched anal-fin rays (vs absence and 19–22). The new species is further distinguished from other species of Priocharax by a combination of characters involving the number of pelvic-fin rays and branched anal-fin rays, the number of postcleithra, the shape of postcleithrum 3, and the absence of the claustrum. Molecular evidence based on COI sequences of all valid species of Priocharax also corroborates the validity of this new species.
Keywords:
Biodiversity; Miniaturization; Ontogenetic truncation; Osteology; Taxonomy
Resumo
Uma nova espécie de peixe miniatura do gênero de caracídeo Priocharax é descrita de um pequeno lago próximo ao rio Ipixuna, drenagem do rio Purus, estados do Amazonas, Brasil. Ela difere de todas as congêneres, exceto P. pygmaeus pelo menor número de dentes no maxilar e dentário. Distingue-se de P. pygmaeus pela presença de dois pós-cleitros e 22–27 raios ramificados na nadadeira anal (vs ausência e 19–22). A nova espécie também difere de outras congêneres por uma combinação de caracteres como número de raios das nadadeiras pélvica e anal, número de pós-cleitros e formato do pós-cleitro 3, e ausência de claustrum. Evidências moleculares com base em sequências do gene COI de todas as espécies válidas também corrobora a validade da nova espécie.
Palavras-chave:
Biodiversidade; Miniaturização; Osteologia; Taxonomia; Truncamento ontogenético
INTRODUCTION
PriocharaxWeitzman & Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52. is a genus of characid fishes from the Amazon and Orinoco basins that currently includes four species, all of them miniature. Priocharax ariel Weitzman & Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52. and P. pygmaeusWeitzman & Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52. were both described with the genus from the upper reaches of the ríos Orinoco and Negro in Venezuela and from the upper río Amazonas in Letícia, Colombia, respectively (Weitzman, Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52.). Almost 30 years later, Priocharax nanusToledo-Piza, Mattox & Britz, 2014Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. http://dx.doi.org/10.1590/1982-0224-20130171
http://dx.doi.org/10.1590/1982-0224-2013...
was described from the surroundings of Santa Isabel do Rio Negro in the middle rio Negro, Brazil. Recently, Priocharax variiMattox, Souza, Toledo-Piza, Britz & Oliveira, 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1...
was described from the rio Jamari, a tributary of the Madeira system, also in Brazil.
All four species share the conspicuous larval form of the pectoral fin in adults (i.e., a rayless fin with a soft flap of cartilage without endoskeletal ossifications) which is interpreted as a terminal ontogenetic truncation in Priocharax (Mattox et al., 2016Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
). In addition, the four species also share other characters useful to distinguish Priocharax from most characids such as small conical teeth along the premaxilla, maxilla (which is fully toothed) and dentary, a triangular pseudotympanum anterior to the rib of the fifth vertebra, a diminutive body size, a translucid colour pattern and the presence of 5–6 branched pelvic-fin rays. In a recent expedition to the rio Ipixuna, an affluent of the Purus drainage, specimens of a new species were sampled which is described herein.
MATERIAL AND METHODS
Morphological analysis. Counts and measurements follow Fink, Weitzman, (1974)Fink WL, Weitzman SH. The so-called cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithsonian Contrib Zool. 1974; 172:1–46. https://doi.org/10.5479/si.00810282.172
https://doi.org/10.5479/si.00810282.172...
, Weitzman, Vari, (1987)Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52., and Menezes, Weitzman, (1990)Menezes NA, Weitzman SH. Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proc Biol Soc Wash. 1990; 103(2):380–426. and were taken on the left side of each specimen whenever possible. All measurements other than standard length (SL) are expressed as percentages of SL, except for subunits of the head which are expressed as percentages of head length (HL). Caudal-peduncle depth is also expressed as a percentage of caudal-peduncle length (Weitzman, Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52.) and snout length is also expressed as a percentage of orbital diameter. Measurements were taken point to point with a precision of 0.1 mm from digital photographs of specimens taken under a Zeiss Discovery V20 stereomicroscope. In text and Tabs., SD is used for standard deviation. Counts of vertebrae, supraneurals, teeth, gill-rakers, procurrent caudal-fin rays, and information about osteological characters were obtained from six specimens cleared and double stained for cartilage and bone following the protocol of Taylor, Van Dyke, (1985)Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–109. Available from: https://sfi-cybium.fr/en/node/2423
https://sfi-cybium.fr/en/node/2423...
. Total vertebral number includes the four vertebrae of the Weberian apparatus as separate elements. The compound ural centrum was counted as a single vertebra. The gill raker at the junction of the ceratobranchial and epibranchial is considered as the posteriormost gill raker on the lower part of the gill arch. Information on meristic and morphometric data of Priocharax ariel, P. pygmaeus, P. nanus, and P. varii were taken from Weitzman, Vari, (1987)Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52., Toledo-Piza et al., (2014)Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. http://dx.doi.org/10.1590/1982-0224-20130171
http://dx.doi.org/10.1590/1982-0224-2013...
, and Mattox et al. (2020)Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1...
, except for percentage of snout length in relation to orbital diameter which were not available directly from those studies.
Photographs were made with a Zeiss Discovery V20 stereomicroscope using a Zeiss Axiocam digital camera attached. Osteological terminology follows Weitzman, (1962)Weitzman SH. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol Bull. 1962; 8(1):1–77. with updates summarized in Mattox et al. (2014)Mattox GMT, Britz R, Toledo-Piza M. Skeletal development and ossification sequence of the characiform Salminus brasiliensis (Teleostei: Ostariophysi: Characidae). Ichthyol Explor Freshw. 2014; 25(2):103–58.. In the description, the frequency of each count is provided in parentheses after the respective count, with the count of the holotype indicated by an asterisk. Specimens examined are deposited in the Coleção de Peixes da Universidade Federal de Rondônia (UFRO–I), Instituto Nacional de Pesquisas da Amazônia (INPA), Laboratório de Biologia e Genética de Peixes, Universidade Estadual Paulista, Botucatu (LBP), Museu de Zoologia da Universidade de São Paulo (MZUSP), and United States National Museum of Natural History – Smithsonian (USNM). Sampling for this study was authorized by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) through permit number SISBIO/MMA 45429 and it is in accordance with the National Council for the Control of Animal Experimentation (CONCEA) approved by UNESP Ethics Committee on Use of Animals (CEUA), protocol number 1058.
Taxon sampling for molecular analysis. Specimens for the molecular study are deposited and preserved in 95% ethanol in the collection of the LBP. Twenty sequences of specimens of Priocharax were used (from Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1...
) plus one sequence of the outgroup taxon Galeocharax humeralis (Valenciennes, 1834) deposited in the GenBank database by Díaz et al. (2016)Díaz J, Villanova GV, Brancolini F, del Pazo F, Posner VM, Grimberg A, Arranz SE. First DNA barcode reference library for the identification of South American freshwater fish from the lower Paraná River. PloS ONE. 2016; 11(7):e0157419. https://doi.org/10.1371/journal.pone.0157419
https://doi.org/10.1371/journal.pone.015...
. For this study, five sequences of the new species were generated. Voucher data are summarized in Tab. 1.
Species, lots, vouchers, basins, locality information, and GenBank accession numbers of samples used in this study. In the locality column, Brazilian states are abbreviated as follows: AM = Amazonas, RO = Roraima.
DNA extraction and sequencing. DNA extraction followed Ivanova et al., (2006)Ivanova NV, Dewaard JR, Hebert PD. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes. 2006; 6(4):998–1002. https://doi.org/10.1111/j.1471-8286.2006.01428.x
https://doi.org/10.1111/j.1471-8286.2006...
, and partial sequences of the cytochrome c oxidase subunit I (COI gene) were amplified by polymerase chain reaction (PCR) with primers FishF1, FishR1 and FishF2, FishR2 (Ward et al., 2005Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia’s fish species. Philos Trasn R Soc Lond B Biol Sci. 2005; 360(1462):1847–57. https://doi.org/10.1098/rstb.2005.1716
https://doi.org/10.1098/rstb.2005.1716...
) or L6252-Asn, H7271–COXI (Melo et al., 2011Melo BF, Benine RC, Mariguela TC, Oliveira C. A new species of Tetragonopterus Cuvier, 1816 (Characiformes: Characidae: Tetragonopterinae) from the rio Jari, Amapá, northern Brazil. Neotrop Ichthyol. 2011; 9(1):49–56. http://dx.doi.org/10.1590/S1679-62252011000100002
http://dx.doi.org/10.1590/S1679-62252011...
). Amplifications were performed in a total volume of 12.5 µl with 1.25 µl of 10X buffer, 0.25 μl of MgCl2 (50 mM), 0.2 μl dNTPs (2 mM), 0.5 μl of each primer (5 mM), 0.1 μl of PHT Taq DNA polymerase (Phoneutria), 1.0 μl of genomic DNA (200 ng), and 8.7 μl ddH2O. The thermocycling were processed in a thermocycler (Applied Byosystems, Veriti), with initial denaturation (94°C for 5 min), followed by 30 cycles of chain denaturation (94°C for 40s), primer hybridization (FishF1 – FishR1 = 52°C (30s); FishF2 – FishR2 = 50ºC (30s); L6252-Asn – H7271-COXI = 54ºC (30s)) and nucleotide extension (68°C for 1 min), plus a final extension (68°C for 8 min). All PCR products were visually checked on 1% agarose gels and then purified with ExoSap–IT (USB Corporation) following the manufacturer’s instructions. The purified PCR products were submitted to sequencing reactions using BigDye Terminator v 3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems), purified again through ethanol precipitation, and loaded onto an ABI 3130 DNA Analyzer automatic sequencer (Applied Biosystems). All sequences produced in this study were deposited in GenBank.
Molecular data analysis. Sequences were assembled to consensus using Geneious v7.1.9 (Kearse et al., 2012Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28(12):1647–49. https://doi.org/10.1093/bioinformatics/bts199
https://doi.org/10.1093/bioinformatics/b...
) and aligned using the MUSCLE algorithm (Edgar, 2004Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004; 5(113):1–19. http://www.biomedcentral.com/1471-2105/5/113
http://www.biomedcentral.com/1471-2105/5...
) under default parameters. The method of Xia et al., (2003)Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evol. 2003; 26(1):1–07. https://doi.org/10.1016/S1055-7903(02)00326-3
https://doi.org/10.1016/S1055-7903(02)00...
in DAMBE v5.3.38 (Xia, 2013Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol. 2013; 30(7):1720–28. https://doi.org/10.1093/molbev/mst064
https://doi.org/10.1093/molbev/mst064...
) was used to evaluate the index of substitution saturation (Iss). Nucleotide composition and substitution patterns were estimated in MEGA X (Kumar et al., 2018Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018; 35(6):1547–49. https://doi.org/10.1093/molbev/msy096
https://doi.org/10.1093/molbev/msy096...
). A maximum likelihood (ML) analysis was conducted under RAxML HPC-PTHREADS-SSE3 (Stamatakis, 2006Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinform. 2006; 22(21):2688–90. https://doi.org/10.1093/bioinformatics/btl446
https://doi.org/10.1093/bioinformatics/b...
) with the GTRGAMMA model using five random parsimony starting trees (Stamatakis et al., 2008Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008; 57(5):758–71. https://doi.org/10.1080/10635150802429642
https://doi.org/10.1080/1063515080242964...
) on 2x 40 CPU 128GB Brycon server at LBP/UNESP. Automatic Barcode Gap Discovery analysis (ABGD; Puillandre et al., 2012Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012; 21(8):1864–77. https://doi.org/10.1111/j.1365-294X.2011.05239.x
https://doi.org/10.1111/j.1365-294X.2011...
) and Poisson Tree Processes (PTP; Zhang et al., 2013Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013; 29(22):2869–76. https://doi.org/10.1093/bioinformatics/btt499
https://doi.org/10.1093/bioinformatics/b...
) were used in the molecular identification of species. ABGD was performed using the webserver (http://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html), under the default parameters of Pmin = 0.001 to Pmax = 0.1, steps = 10, X (relative gap width) = 1.5, Nb bins (for distance distribution) = 20, and the Kimura (K80) molecular model. PTP were generated using the ML tree and other parameters at default in the PTP webserver (http://species.h-its.org/ptp/). ABGD and PTP were performed excluding the outgroup taxa Galeocharax humeralis. Overall and pairwise genetic distances were estimated based on the Kimura 2-parameter model (K2P) + Gamma using MEGA X and the order of groups was based on the ABGD and PTP results.
RESULTS
Priocharax britzi, new species
urn:lsid:zoobank.org:act:A2309042-25EE-4849-8488-77B2A5FDE955
Holotype. MZUSP 125822, 13.7 mm SL, Brazil, Amazonas, Canutama, small lake approximately 400 m from the rio Ipixuna, after bridge crossing this river, on the right side of the Transamazônica Road (BR–230) going from Humaitá to Lábrea, rio Ipixuna drainage, upper Purus basin, 07°31’11.46’’S 63°20’59.58’’W, 3 Sep 2018, G. M. T. Mattox & S. Souza.
Paratypes. All collected with holotype. LBP 28493, 5, 11.7–12.7 mm SL. MZUSP 125823, 43, 9.3–14.1 mm SL (6 c&s, 11.0–12.9 mm SL). UFRO-ICT 27662, 5, 10.2–12.0 mm SL.
Diagnosis.Priocharax britzi is distinguished from all congeners except P. pygmaeus by the lower number of teeth in the maxilla (21–30 vs 32–58) and dentary (26–29 vs 33–55). It differs from P. pygmaeus in the presence of two postcleithra (vs absence) and by having 22–27 (modes 25 and 26) branched anal-fin rays (vs 19–22, mode 19). The new species is further distinguished from P. nanus and P. varii by having i,5 pelvic-fin rays (vs i,6) and a slender and sinuous postcleithrum 3 (vs postcleithrum 3 relatively thick and straight), from P. ariel by the presence of 22–27 branched anal-fin rays (vs 16–22) and the presence of two postcleithra (vs absence). Priocharax britzi is further distinguished from P. varii by the absence of the adipose fin (vs presence) and from P. nanus by the absence of the claustrum (vs presence). Complementarily, Priocharax britzi has a shorter snout relative to the orbital diameter when compared to all congeners except P. nanus. This difference is reflected in the range of the proportion of snout length in relation to orbital diameter and its mean which is 45–59 (mean = 53.6; SD = 3.8) in P. britzi vs 60–76 (mean = 65.5; SD = 4.9) in P. pygmaeus, 54–81 (mean = 67.0; SD = 5.1) in P. varii and 54–84 (mean = 68.4; SD = 6.3) in P. ariel. The molecular analyses provide additional support towards the recognition of the new species.
Priocharax britzi, A. Holotype, male, MZUSP 125822, 13.7 mm SL; Brazil, Amazonas, Canutama, small lake approximately 400 m from the rio Ipixuna, after bridge crossing this river, on the right side of the Transamazônica Road (BR–230) going from Humaitá to Lábrea, rio Ipixuna drainage. B. Live paratype photographed immediately after capture.
Morphometric data of Priocharax britzi. N = number of specimens; SD = standard deviation. Range includes the holotype. [Note: In the recent description of Priocharax variiMattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1... :423, table 2), data of anal-fin length is incorrect. The correct information is as follows: Holotype = 22, range = 20–24, mean = 21.8, SD = 0.9, with n = 48 including the holotype].
Description. For overall appearance, see Fig. 1. Morphometric data are presented in Tab. 2. Body laterally compressed and elongated, greatest depth at vertical through dorsal-fin origin. Dorsal-fin origin approximately at midbody, at vertical slightly anterior to anal-fin origin. Pectoral-fin bud at vertical through anterior portion of pseudotympanum. Pelvic-fin origin approximately midway between posterior margin of opercle and anal-fin origin. Dorsal profile of head and body slightly convex from tip of snout to dorsal-fin origin. Dorsal profile of body along dorsal-fin base nearly straight, gently sloping posteroventrally; sloping more conspicuous from latter point to caudal peduncle. Dorsal profile of caudal peduncle slightly concave to base of dorsal procurrent rays. Ventral profile of head and body slightly convex from symphysis of lower jaw to vertical through pectoral-fin origin; straight to slightly convex from latter point to pelvic-fin origin. Ventral profile of body posteroventrally sloping from pelvic-fin to anal-fin origin; straight and posterodorsally rising along anterior one-half of anal-fin base, gently concave from latter point to base of ventral procurrent rays. Caudal peduncle short. Pseudotympanum located anterior to rib of fifth vertebra.
Snout round in lateral view. Eye about one-third of head length. Infraorbitals 2 and 3 present but not fully developed in three specimens, absent in the others. Infraorbitals 1, 4 to 6 and supraorbital absent in all specimens. Antorbital present but poorly developed in five specimens (Fig. 2), absent in one specimen. Mouth terminal with lower jaw slightly shorter than upper jaw. Tip of maxilla elongate, posterior border reaching vertical through posterior border of pupil. Premaxillary teeth in single series, premaxilla with 20(3), 22(1), or 23(2) teeth. Maxilla with 21(1), 22(1), 23(1), 25(1), 26(1), or 30(1) teeth. Dentary with 26(1), 27(3), 28(1), or 29(1) teeth. Dentary teeth in single series, with few anterior teeth slightly displaced anteriorly. A conspicuous elongate foramen at the anterior portion of the dentary. All jaw teeth small, conical and lingually curved to a moderate extent (Fig. 3).
Priocharax britzi, paratypes, MZUSP 125823, c&s; anterior region of head in lateral view detailing the orbital region. A. 12.5 mm SL. B. 12.9 mm SL, image flipped. Antorbital and infraorbitals delimited by black line for clarity. Ant = antorbital; IO2 – 3 = infraorbitals 2 – 3. Scale bar = 0.2 mm.
Priocharax britzi, paratype, MZUSP 125823, 12.9 mm SL, c&s; jaws in lateral view. Ana = anguloarticular; Cm = coronomeckelian; De = dentary; MC = Meckel’s cartilage; Mx = maxilla; Pmx = premaxilla; Ra = retroarticular. Scale bar = 0.2 mm.
Dorsal-fin rays ii,9*(43). Endoskeletal part of pectoral fin and some thin exoskeletal bones showing larval structure (Fig. 4). Cartilaginous pectoral-radial plate with incomplete longitudinal middle fissure leaving upper and lower halves connected at base and tip; base articulating with vertically elongated scapulocoracoid cartilage and round distal margin with larval-like pectoral-fin fold supported solely by actinotrichia. Pectoral-fin rays absent. All bones of endoskeletal pectoral girdle absent, exoskeletal part with posttemporal, supracleithrum, cleithrum and two postcleithra. Postcleithrum 3 poorly developed, slender and sinuous. Cleithrum with posteriorly directed, curved process immediately below ventral tip of supracleithrum. Pelvic-fin rays i,5*(47). Posterior tip of pelvic fin falling short of origin of anal fin but extending slightly beyond vent. Anal-fin rays ii, 22(2), 23(8), 24(7), 25*(13), 26(13), or 27(2). Anal-fin margin concave with anterior elongate lobe formed by elongated fin rays and posterior section of short rays. Caudal-fin rays i,9,7,i (1), or i,9,8,i*(37), dorsal procurrent rays 8(2) or 9(4), ventral procurrent rays 6(3), 7(2), or 8(1). Caudal fin forked. Adipose fin absent.
Squamation present in almost all specimens, but scales highly deciduous and easily lost during handling. Scales cycloid, very thin, with no obvious circuli or radii. Scales in midlateral row 23(2), 24(6), 25(12), 26*(17), 27(4), or 28(3); no canal bearing lateral-line scales on body. Scale rows between dorsal-fin origin and pelvic-fin origin 8(1), 9(7), or 10*(2). Scale rows around caudal peduncle 8(7), 9(1), or 10*(1). Predorsal scales typically absent with one or two scales just anterior to dorsal fin in few specimens. Scales restricted to base of caudal-fin rays, not covering caudal-fin lobes.
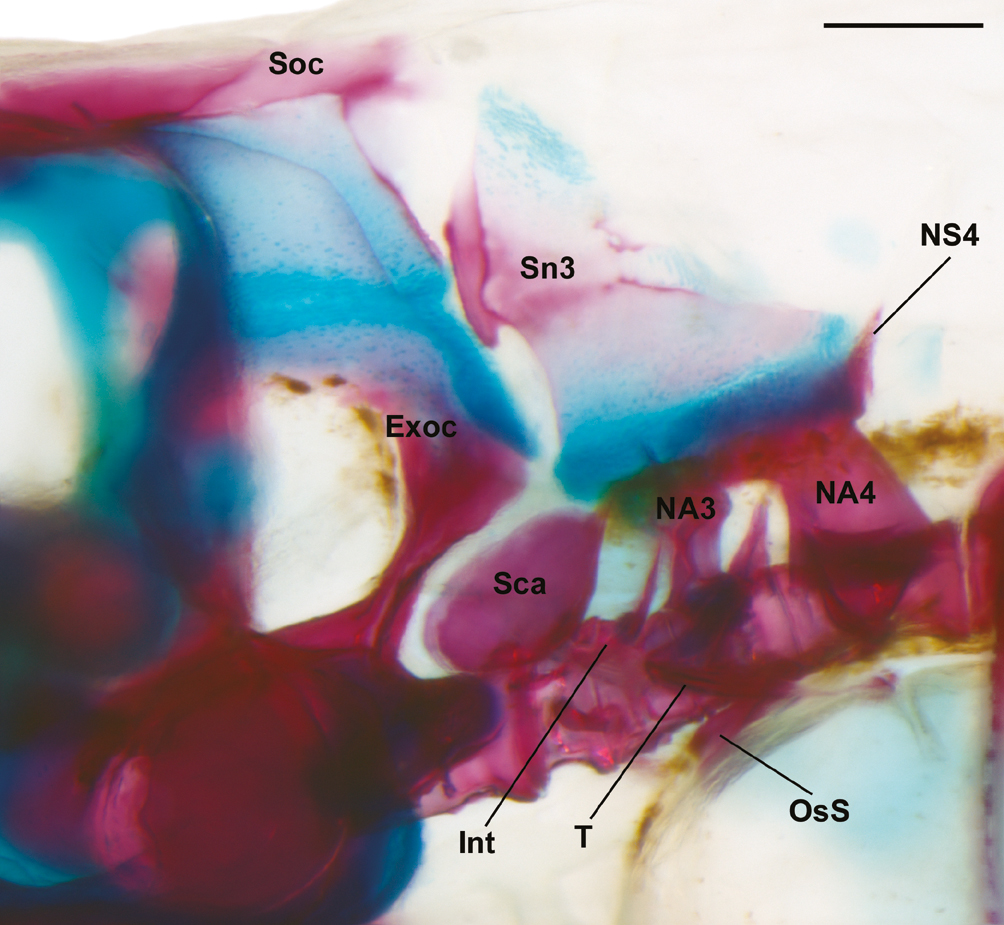
Total vertebrae 32(1) or 33(5); abdominal vertebrae 14(6); caudal vertebrae 18(1) or 19(5). Total number of gill-rakers on first branchial arch 9(1) or 11(5), upper limb gill-rakers 1(1) or 3(5), lower limb gill-rakers 8(6). Weberian apparatus well-developed, all components ossified except for claustrum (Fig. 5). Large gap between neural arches 3 and 4, with gap partially filled by dorsally projecting pointed process from vertebral centrum 3. Inner arm of suspensorium large, projecting forward to vertical through middle of second centrum. Supraneurals 5(5) or 6(1).
Coloration in alcohol. Overall ground coloration pale yellow (Fig. 1A). Scattered melanophores on dorsal portion of head in a few specimens. Guanine present in eye of most specimens, with melanophores on dorsal surface of eye. Scattered melanophores on bases of posteriormost anal-fin rays forming irregular line along posterior half of fin. Another irregular dark line extending along ventral margin of hypaxial myomeres posteriorly from vertical through seventh branched anal-fin ray. Both lines separated anteriorly but approaching each other posteriorly. A few scattered melanophores on bases of dorsalmost and ventralmost caudal-fin rays. All fins hyaline.
Coloration in life. Body mostly translucent (Fig. 1B). A few melanophores and xanthophores scattered along dorsal surface of vertebral column and on dorsal surface of swim bladder. Xanthophores on dorsal surface of head from vertical through middle of eye to posterior margin of skull. A thin line of chromatophores along anal-fin base, from vertical through 6th branched ray to terminus of anal-fin base. Two faint blotches of xanthophores on bases of caudal-fin lobes, remaining caudal fin and other fins hyaline. Eye silvery, dorsal half predominantly dark.
Shoulder girdle in lateral view of A. Priocharax britzi, paratype, MZUSP 125823, 12.9 mm SL, c&s. Image flipped. B. Priocharax cf. ariel, UFRO 15521, 11.8 mm SL, c&s. Cl = cleithrum; PecRdC = pectoral-fin radial cartilage; Pcl = unidentified postcleithrum; Pcl3 = postcleithrum 3; Pt = posttemporal; ScCoC = scapulocoracoid cartilage; Sucl = supracleithrum. Arrows point to middle fissure on pectoral-fin radial cartilage. Pcl3 slightly displaced ventrally from original position in A. Scale bars = 0.5 mm.
Priocharax britzi, paratype, MZUSP 125823, 12.9 mm SL, c&s; Weberian apparatus in lateral view. Exoc = exoccipital; Int = intercalarium; NA3 – 4 = neural arches 3 – 4; NS4 = neural spine 4; OsS = os suspensorium; Sca = scaphium; Sn3 = supraneural 3; Soc = supraoccipital; T = tripus. Scale bar = 0.2 mm.
Sexual dimorphism. Two of 48 analysed specimens with hooks on anal-fin rays (12.4–13.7 mm SL), the larger specimen also with hooks on pelvic-fin rays (Fig. 6). Hooks on anal fin located on posterior margins of posterior unbranched and three anterior most branched rays, typically one hook per segment. Five, seven, seven, and four hooks respectively on each anal-fin ray of larger specimen, two, four, two, one hooks on anal-fin rays of smaller one. Hooks of larger specimen well developed, especially along middle portion of each ray. Hooks of smaller specimen less developed. Hooks on pelvic fin of larger specimen not as developed and resembling bumps along branched rays 1–3 of contralateral fins, more developed on more lateral branched ray. Hooks on pelvic fin always along medial edge of rays. Smaller specimen without hooks on pelvic fin.
Molecular analysis. The final matrix comprised 26 terminals with 642 bp and 213 variable sites (33.2%). The nucleotide composition was 23.4% adenine, 17.8% guanine, 32.4% thymine, and 26.2% cytosine. The Iss values were lower than Iss.c values, indicating the absence of saturation. The maximum likelihood (ML) tree showed high bootstrap values supporting each of the analysed species (Fig. 7). The convergence of initial and recursive partitions of the ABGD and ML solution of the PTP analysis delimited five species of Priocharax: P. varii, P. ariel, P. pygmaeus, P. nanus, and P. britzi (Fig. 7; S1 and S2). The overall mean of genetic distances (K2P) among Priocharax species was 0.194±0.018. Intraspecific genetic distances ranged from 0.000 within P. pygmaeus to 0.002±0.001 within P. ariel and P. varii. The values of interspecific distances ranged from 0.179±0.021 between P. varii and P. nanus to 0.249±0.025 between P. pygmaeus and P. ariel (Tab. 3).
Priocharax britzi, holotype, MZUSP 125822, 13.7 mm SL. A. Left lateral view of anal fin showing bony hooks. B. Ventral view of pelvic fin showing bony hooks. Scale bars = 0.5 mm.
Maximum likelihood tree of five species of Priocharax, based on the COI gene (642 bp). Bars represent the number of species obtained by the ABGD and bPTP analyses. Numbers near nodes represent bootstrap values.
Pairwise K2P genetic distances (and standard deviation) within and among species of Priocharax. Intraspecific genetic distances are highlighted in bold. Values are presented in percentages followed by standard deviation. Number of sequences: P. ariel = 6, P. britzi = 5, P. nanus = 1, P. pygmaeus = 9 and P. varii = 4.
Geographical distribution.Priocharaxbritzi is known only from its type locality in a marginal lake approximately 400 m from the rio Ipixuna, a tributary of the rio Purus (Fig. 8). Specimens from a close locality in the main channel of the rio Ipixuna (UFRO 15521) were examined morphologically and did not match the diagnostic characters of P. britzi (see discussion below). The Fig. 8 also shows the known distribution of all congeners.
Ecological notes. Specimens of Priocharax britzi were collected between 3 pm and 5 pm in a small lake approximately 15 m wide and 35 m long, apparently isolated from the main channel of the rio Ipixuna (Fig. 9). The water level was low as sampling occurred during the dry season (September), so it is possible that this lake connects to the main channel in the wet season. The specimens were collected near the shore at depths varying from 0.5–1.0 m. Vegetation around the lake was composed mainly of dead logs and branches. At the time of sampling, the bottom was muddy with patches of leaf litter. Priocharax britzi was collected with the characiforms Carnegiella marthae Myers, 1927, Hemigrammus sp., Hemigrammus cf. gracilis (Lütken, 1875), Hyphessobrycon rosaceus Durbin, 1909, Iguanodectes spilurus (Günther, 1864), Microcharacidium sp., Nannostomus digrammus (Fowler, 1913), Nannostomus eques Steindachner, 1867, the catfish Amblydoras affinis (Kner, 1855), the knifefishes Brachyhypopomus beebei (Schultz, 1944), Microsternarchus bilineatus Fernández-Yépez, 1968, the cichlids Apistogramma cf. pulchra Kullander, 1980, Biotoecus opercularis (Steindachner, 1875), and Crenicichla cyanotus Cope, 1870.
Map of South America with a close-up of the upper and central portions of the Amazon basin, Brazil, illustrating the distribution of the five species of Priocharax: P. britzi described herein from the rio Purus basin (green losangle), P. varii (red dot), P. ariel (black dots), P. nanus (blue dot), and P. pygmaeus (yellow dots). Colors are the same as in the Fig. 7. Some dots may represent more than one lot.
Small lake approximately 400 m from the rio Ipixuna, Canutama, Amazonas, Brazil, the type locality of Priocharax britzi.
Etymology.Priocharax britzi is named after Dr. Ralf Britz, noteworthy ichthyologist and a dear friend. Dr. Britz has mastered the world of small fishes and has described more than 20 miniature species, including two species of Priocharax. A noun in the genitive case.
Conservation status.Priocharax britzi was found in a single location, an isolated small lake near the road (Fig. 8). The environmental conditions of the lake seem degraded, with depauperate riparian vegetation and silted substrate. Efforts to find specimens of the new species in the nearby rio Ipixuna and other close locations were not successful. However, the type locality is near a protected area (Floresta Nacional de Balata-Tufari), which was not sampled. Future studies should better investigate the possibility that P. britzi occuring in other localities within the protected area and along portions of the rio Ipixuna that may connect to the lake during the wet season. Furthermore, there are no known imminent threats that would put the species at risk of extinction. Hence, we suggest that P. britzi should be classified as Least Concern (LC) according to International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019IUCN Standards and Petitions Committee. Guidelines for using theIUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. 2019. Available at: https://www.iucnredlist.org/documents/ RedListGuidelines.pdf
https://www.iucnredlist.org/documents/...
) pending further information on its real distribution.
DISCUSSION
Priocharax britzi was sampled in a small lake approximately 400 m from the main channel of the rio Ipixuna. Additional specimens of Priocharax (UFRO 15521, n = 11) originating from the main channel of the rio Ipixuna approximately 500 m from the type locality of P. britzi are morphologically more similar to P. ariel than to P. britzi; they are herein identified as P. cf. ariel. Those specimens were collected in 2012 and no tissue samples were available to perform molecular analyses. Several attempts to sample more specimens of Priocharax cf. ariel from the main channel of the rio Ipixuna in September 2018 were unsuccessful. Osteologically, P. britzi differs clearly from P. cf. ariel by the presence of postcleithrum 3 (vs absence) (Fig. 4) and in the lower number of supraneurals [5(5) or 6(1) vs 7(3) or 8(1) in P. cf. ariel]. Furthermore, P. britzi has more branched anal-fin rays (22–27; modes 25 and 26 vs 19–22; mode=19 in P. cf. ariel) with a consequently longer anal-fin base (30–37% of SL, mean = 34.3%, SD = 1.3% vs 25–29% of SL, mean = 27.5%, SD = 1.3%).
Meristic characters of specimens of Priocharax cf. ariel from the main channel of the rio Ipixuna are within the range of variation of Priocharax ariel with the exception of the number of maxillary (25–30) and dentary (27–35) teeth which are considerably lower from the values reported by Weitzman, Vari, (1987)Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52. for P. ariel (38–58 and 38–55, respectively). Though these lower tooth counts suggest that P. cf. ariel may represent an additional undescribed species, the lack of molecular data for P. cf. ariel from the main channel of the rio Ipixuna currently makes it impossible to test their conspecificity with P. ariel.
Specimens of Priocharax britzi smaller than 11.0 mm SL lack the antorbital. In specimens with 11.6 mm SL and larger, the antorbital is present and gradually more developed. Weitzman, Vari, (1987)Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52. noted a similar pattern in P. ariel and P. pygmaeus in which the antorbital is only apparent in specimens larger than 13.5 mm SL and 12.2 mm SL, respectively. In those two species the antorbital is the only ossified bone of the infraorbital series, a condition also shared with Priocharax nanus and P. varii (Toledo-Piza et al., 2014Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. http://dx.doi.org/10.1590/1982-0224-20130171
http://dx.doi.org/10.1590/1982-0224-2013...
; Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1...
). Conversely, in P. britzi infraorbital 3 is present at 12.5 mm SL and infraorbital 2 is present at 12.9 mm SL (Fig. 2). Mattox et al., (2016)Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
reported the presence of three infraorbitals in 18 specimens of Priocharax sp. from the rio Negro, all larger than 12.7 mm SL. The taxonomic significance of the more extensive ossification of the infraorbital series in specimens from that drainage still needs further study.
Two out of 48 specimens of Priocharax britzi have hooks on the anal fin, a classic trait of sexual dimorphism in characids usually present only in mature males (Fig. 6) (e.g., Malabarba, Weitzman, 2003Malabarba LR, Weitzman SH. Description of a new genus with six new species from Southern Brazil, Uruguay and Argentina, with a discussion of a putative characid clade (Teleostei: Characiformes: Characidae). Comun Mus Ciênc Tecnol PUCRS, Sér Zool, Porto Alegre. 2003; 16(1):67–151.; Camelier, Zanata, 2014Camelier P, Zanata AM. A new species of Astyanax Baird & Girard (Characiformes: Characidae) from the Rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil, with comments on bony hooks on all fins. J Fish Biol. 2014; 84(2):475–90. https://doi.org/10.1111/jfb.12295
https://doi.org/10.1111/jfb.12295...
). Among congeners, hooks on the anal fin were previously reported only for males of Priocharax ariel (Weitzman, Vari, 1987Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52.:645). More recently, Mattox et al., (2016)Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
reported that in addition of hooks on the anal fin, Priocharax sp. from the rio Negro also had hooks on the pelvic-fin rays. Of the two specimens of Priocharax britzi with hooks reported herein, one has hooks on both the anal and pelvic fins and the other has hooks only on the anal fin. This apparent intraspecific variation may rather reflect different stages of maturity among specimens, as the larger specimen (13.7 mm SL) has more hooks on both fins, with the hooks on the anal fin more developed, while the smaller specimen (12.4 mm SL) has fewer hooks only on the anal fin, with those hooks less developed. Gonads were not checked for maturity because such an examination is a destructive process and specimens of this species are still rare.
Knowledge about the diversity of species of Priocharax remained stable for approximately 27 years, from Weitzman, Vari’s (1987) proposition of the genus and description of P. ariel and P. pygmaeus until 2014, when efforts to collect and study new specimens from the rio Negro basin resulted in the description of P. nanus by Toledo-Piza et al., (2014)Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. http://dx.doi.org/10.1590/1982-0224-20130171
http://dx.doi.org/10.1590/1982-0224-2013...
. More recent efforts to collect and study specimens from other Amazon subdrainages have resulted in two new species descriptions: P. varii (Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
http://dx.doi.org/10.26049/VZ70-3-2020-1...
) and P. britzi (herein). Those efforts combined with the examination of material deposited in collections have also shown that the genus is more widespread than previously recorded and revealed that the taxonomy of Priocharax is more complex, as exemplified by the specimens collected in the main channel of the rio Ipixuna discussed above. Those detailed studies focusing on Priocharax have revealed new aspects about the diversity of this genus and reinforce that there is still much to be discovered regarding the Neotropical freshwater ichthyofauna, especially its miniature species.
Comparative material examined.Priocharax ariel: Brazil, Amazonas, rio Negro basin: MZUSP 39778, 4, 13.5–14.6 mm SL; MZUSP 55099, 8, 12.4–14.2 mm SL; MZUSP 55097, 4 of 6, 12.2–12.7 mm SL; MZUSP 62230, 2 of 4, 15.1–15.2 mm SL. Rio Madeira basin: UFRO-ICT 5761, 1, 14.5 mm SL; UFRO-ICT 19660, 1, 10.9 mm SL; UFRO-ICT 20954, 8 of 10, 10.1–11.8 mm SL. Rondônia, rio Madeira basin: UFRO-ICT 2151, 11 of 18, 13.7–16.1 mm SL (4 c&s, 13.7–16.1 mm SL); UFRO-ICT 5744, 3, 12.1–12.5 mm SL; UFRO-ICT 5745, 3, 11.6–12.9 mm SL; UFRO-ICT 8449, 1, 17.1 mm SL; UFRO-ICT 13825, 1, 14.2 mm SL; UFRO-ICT 17412, 2 of 3, 11.6–12.4 mm SL; UFRO-ICT 20075, 1, 17.4 mm SL. Venezuela, Territorio Federal Amazonas, río Orinoco basin: MZUSP 36497, 50, 11.8–15.2 mm SL, paratypes; MZUSP 55142, 12, 12.0–14.7 mm SL (5 c&s, 12.0–14.0 mm SL), paratypes. Priocharax cf. ariel: Brazil, Amazonas, rio Purus basin: UFRO-ICT 15521, 11 of 17, 10.0–13.6 mm (4 c&s, 11.3–12.7 mm SL). Priocharax nanus: Brazil, Amazonas, rio Negro basin: INPA 39891, 4, 12.5–13.9 mm SL, paratypes; MZUSP 114014, 13.8 mm SL, holotype; MZUSP 114015, 9, 12.1–15.3 mm SL (3 c&s, 14.1–15.3 mm SL), paratypes; MZUSP 114016, 5, 12.6–14.6 mm SL (2 c&s, 13.4–13.8 mm SL), paratypes; MZUSP 114017, 3, 13.5–14.6 mm SL (1 c&s, 14.6 mm SL), paratypes; MZUSP 114018, 11, 11.1–15.4 mm SL (5 c&s, 12.0–14.0 mm SL), paratypes; USNM 427007, 4, 12.1–13.3 mm SL, paratypes. Priocharax pygmaeus: Colombia, Departamento Amazonas, rio Amazonas basin: MZUSP 36498, 5, 10.2–10.7 mm SL, paratypes. Peru, Loreto, río Ucayali basin: MZUSP 85644, 1, 16.5 mm SL. río Ampiyacu basin: MZUSP 121212, 10, 11.5–12.7 mm SL. Priocharax varii: Brazil, Rondônia, rio Madeira basin: LBP 28495, 5, 12.5–12.7 mm SL, paratypes. MZUSP 125786, 12.2 mm SL, holotype. MZUSP 125787, 44, 11.8–14.0 mm SL (5 c&s, 11.8–13.7 mm SL), paratypes. UFRO-ICT 27656, 5, 12.2–13.3 mm SL, paratypes.
ACKNOWLEDGEMENTS
Most of this study was conducted at the Departamento de Biologia, UFSCar – Universidade Federal de São Carlos, which provided space and access to facilities. The molecular analyses were conducted at the Departamento de Biologia Estrutural e Funcional, UNESP Botucatu. The authors are thankful for Silas Souza (UFSCar) who provided valuable help during fieldwork. William Ohara (UFRO) provided information for the fieldwork and discussed some aspects of other localities in the upper rio Ipixuna. Carolina Doria (UFRO) sent specimens on loan and provided formalin and alcohol for the expedition, to whom the authors are grateful. Michel Gianeti and Osvaldo Oyakawa (MZUSP) and Aline Andriolo (UFRO) provided curatorial assistance. Beatriz Corazza, Caio Dallevo-Gomes and Karolina Reis aided in species identification. This manuscript benefitted from various inputs by Kevin Conway (Texas A&M University), Brian Sidlauskas (Oregon State University), and a discussion on the conservation status of the new species with Carla Polaz (ICMBio). GMTM acknowledges FAPESP Proc 2017/01970–4; CS acknowledges FAPESP Proc 2017/06551–0; CO acknowledges FAPESP Proc 2018/20610–1, 2016/09204–6, 2014/26508–3 and CNPq Proc 306054/2006–0.
REFERENCES
- Camelier P, Zanata AM. A new species of Astyanax Baird & Girard (Characiformes: Characidae) from the Rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil, with comments on bony hooks on all fins. J Fish Biol. 2014; 84(2):475–90. https://doi.org/10.1111/jfb.12295
» https://doi.org/10.1111/jfb.12295 - Díaz J, Villanova GV, Brancolini F, del Pazo F, Posner VM, Grimberg A, Arranz SE. First DNA barcode reference library for the identification of South American freshwater fish from the lower Paraná River. PloS ONE. 2016; 11(7):e0157419. https://doi.org/10.1371/journal.pone.0157419
» https://doi.org/10.1371/journal.pone.0157419 - Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004; 5(113):1–19. http://www.biomedcentral.com/1471-2105/5/113
» http://www.biomedcentral.com/1471-2105/5/113 - Fink WL, Weitzman SH. The so-called cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithsonian Contrib Zool. 1974; 172:1–46. https://doi.org/10.5479/si.00810282.172
» https://doi.org/10.5479/si.00810282.172 - IUCN Standards and Petitions Committee. Guidelines for using theIUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. 2019. Available at: https://www.iucnredlist.org/documents/ RedListGuidelines.pdf
» https://www.iucnredlist.org/documents/ - Ivanova NV, Dewaard JR, Hebert PD. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes. 2006; 6(4):998–1002. https://doi.org/10.1111/j.1471-8286.2006.01428.x
» https://doi.org/10.1111/j.1471-8286.2006.01428.x - Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28(12):1647–49. https://doi.org/10.1093/bioinformatics/bts199
» https://doi.org/10.1093/bioinformatics/bts199 - Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018; 35(6):1547–49. https://doi.org/10.1093/molbev/msy096
» https://doi.org/10.1093/molbev/msy096 - Malabarba LR, Weitzman SH. Description of a new genus with six new species from Southern Brazil, Uruguay and Argentina, with a discussion of a putative characid clade (Teleostei: Characiformes: Characidae). Comun Mus Ciênc Tecnol PUCRS, Sér Zool, Porto Alegre. 2003; 16(1):67–151.
- Mattox GMT, Britz R, Toledo-Piza M. Skeletal development and ossification sequence of the characiform Salminus brasiliensis (Teleostei: Ostariophysi: Characidae). Ichthyol Explor Freshw. 2014; 25(2):103–58.
- Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
» https://doi.org/10.1002/jmor.20477 - Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Vertebr Zool. 2020; 70(3):417–33. http://dx.doi.org/10.26049/VZ70-3-2020-11
» http://dx.doi.org/10.26049/VZ70-3-2020-11 - Melo BF, Benine RC, Mariguela TC, Oliveira C. A new species of Tetragonopterus Cuvier, 1816 (Characiformes: Characidae: Tetragonopterinae) from the rio Jari, Amapá, northern Brazil. Neotrop Ichthyol. 2011; 9(1):49–56. http://dx.doi.org/10.1590/S1679-62252011000100002
» http://dx.doi.org/10.1590/S1679-62252011000100002 - Menezes NA, Weitzman SH. Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proc Biol Soc Wash. 1990; 103(2):380–426.
- Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012; 21(8):1864–77. https://doi.org/10.1111/j.1365-294X.2011.05239.x
» https://doi.org/10.1111/j.1365-294X.2011.05239.x - Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinform. 2006; 22(21):2688–90. https://doi.org/10.1093/bioinformatics/btl446
» https://doi.org/10.1093/bioinformatics/btl446 - Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008; 57(5):758–71. https://doi.org/10.1080/10635150802429642
» https://doi.org/10.1080/10635150802429642 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–109. Available from: https://sfi-cybium.fr/en/node/2423
» https://sfi-cybium.fr/en/node/2423 - Toledo-Piza M, Mattox GMT, Britz R Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. http://dx.doi.org/10.1590/1982-0224-20130171
» http://dx.doi.org/10.1590/1982-0224-20130171 - Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia’s fish species. Philos Trasn R Soc Lond B Biol Sci. 2005; 360(1462):1847–57. https://doi.org/10.1098/rstb.2005.1716
» https://doi.org/10.1098/rstb.2005.1716 - Weitzman SH. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol Bull. 1962; 8(1):1–77.
- Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from Northern South America. Proc Biol Soc Wash. 1987; 100(3):640–52.
- Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol. 2013; 30(7):1720–28. https://doi.org/10.1093/molbev/mst064
» https://doi.org/10.1093/molbev/mst064 - Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evol. 2003; 26(1):1–07. https://doi.org/10.1016/S1055-7903(02)00326-3
» https://doi.org/10.1016/S1055-7903(02)00326-3 - Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013; 29(22):2869–76. https://doi.org/10.1093/bioinformatics/btt499
» https://doi.org/10.1093/bioinformatics/btt499
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Mattox GMT, Souza CS, Toledo-Piza M, Oliveira C. A new miniature species of Priocharax (Characiformes: Characidae) from the upper rio Ipixuna, Purus drainage, Brazil. Neotrop Ichthyol. 2021; 19(2):e210048. https://doi.org/10.1590/1982-0224-2021-0048
Edited-by
Publication Dates
-
Publication in this collection
09 July 2021 -
Date of issue
2021
History
-
Received
17 Feb 2021 -
Accepted
17 June 2021