ABSTRACT
The reproductive biology of thresher shark species of the Ecuadorian Pacific was analysed based on 1236 specimens of Alopias pelagicus (711 females and 525 males) and 354 of A. superciliosus (164 females and 190 males) landed in “Playita Mía”, from January to December of 2019. The length of A. pelagicus females ranged between 67.2 and 184 cm PCL (precaudal length) and the males between 69.0 and 178.4 cm PCL, A. superciliosus registered a minimum and maximum size of 76.0 and 202.2 cm PCL for females and 94.0 and 204.8 cm PCL for males. The most frequently captured size class for A. pelagicus was 147.2−157.2 cm PCL and for A. superciliosus was 156.0-166.0 cm PCL. The sex ratio (F:M) for A. pelagicus and A. superciliosus was 1.35F:1M and 0.86F:1M respectively. For A. pelagicus males the inflection point of the clasper length adjustment, was 134.2 cm PCL and size at first sexual maturity (L 50 ) was estimated at 136.0 cm PCL. For A. superciliosus males the inflection point of the clasper length adjustment, was 136.8 cm PCL, and the first sexual maturity (L 50 ) was estimated at 138.7 cm PCL.
Keywords:
Ecuador; Conservation; Lengths; South Pacific; Shark
RESUMO
A biologia reprodutiva de espécies de tubarão debulhador do Pacífico Equador foi analisada com base em 1236 exemplares de Alopias pelagicus (711 fêmeas e 525 machos) e 354 de A. superciliosus (164 fêmeas e 190 machos) desembarcados em “Playita Mía”, entre janeiro e dezembro de 2019. O comprimento das fêmeas de A. pelagicus variou entre 67,2 e 184 cm PCL (comprimento pré-corte) e os machos entre 69,0 e 178,4 cm PCL, A. superciliosus registrou um tamanho mínimo e máximo de 76,0 e 202,2 cm PCL para as fêmeas e 94,0 e 204,8 cm PCL para machos. A classe de tamanho mais frequentemente capturada para A. pelagicus foi 147,2−157,2 cm PCL e para A. superciliosus foi 156,0-166,0 cm PCL. A proporção sexual (F:M) para A. pelagicus e A. superciliosus foi de 1,35F:1M e 0,86F:1M, respectivamente. Para machos de A. pelagicus, o ponto de inflexão do ajuste do comprimento do grampo foi de 134,2 cm PCL e o tamanho na primeira maturidade sexual (L 50 ) foi estimado em 136,0 cm PCL. Para machos de A. superciliosus o ponto de inflexão do ajuste do comprimento do grampo foi de 136,8 cm PCL, e a primeira maturidade sexual (L 50 ) foi estimada em 138,7 cm PCL.
Palavras-chave:
Ecuador; Conservação; Comprimentos; Pacífico Sul; Tubarão
INTRODUCTION
The analysis of the reproductive variables linked to the life history of sharks, helps to gather information of great importance to improve fisheries management (Fischer et al., 2009Fischer AF, Hazin FHV, Carvalho F, Viana DL, Rêgo MG, Wor C. Biological aspects of sharks caught off the Coast of Pernambuco, Northeast Brazil. Braz J Biol. 2009; 69(4):1173-81. https://doi.org/10.1590/S1519-69842009000500023
https://doi.org/10.1590/S1519-6984200900...
; Cotton et al., 2011Cotton CF, Grubbs RD, Daly-Engel TS, Lynch PD, Musick JA. Age, growth and reproduction of a common deep-water shark, shortspine spurdog (Squalus cf. mitsukurii), from Hawaiian waters. Mar Freshw Res. 2011; 62(7):811-22. https://doi.org/10.1071/MF10307
https://doi.org/10.1071/MF10307...
). The estimation of sexual maturity at a certain average length, is a key element to estimate the productivity of species (Corro-Espinosa et al., 2011Corro-Espinosa D, Márquez-Farías JF, Muhlia-Melo A. Talla de madurez del tiburón bironche Rhizoprionodon longurio en el Golfo de California, México. Cienc Mar. 2011; 37(2):201-14. Available from: http://www.scielo.org.mx/pdf/ciemar/v37n2/v37n2a7.pdf
http://www.scielo.org.mx/pdf/ciemar/v37n...
; Liu et al., 2015Liu K-M, Chin C-P, Chen C-H, Chang J-H. Estimating finite rate of population increase for sharks based on vital parameters. PLoS ONE . 2015; 10(11):e0143008. https://doi.org/10.1371/journal.pone.0143008
https://doi.org/10.1371/journal.pone.014...
). Similarly, the estimation of the sex ratio in fish makes it possible to reduce the presence of structural biases in the stock assessment models, improving the diagnosis of the biological reference parameters (Morson et al., 2015Morson JM, Bochenek EA, Powell EN, Hasbrouck EC, Gius JE, Cotton CF, Gerbino K, Froehlich T. Estimating the Sex Composition of the summer flounder catch using fishery-independent data. Mar Coast Fish. 2015; 7(1):393-408. https://doi.org/10.1080/19425120.2015.1067261
https://doi.org/10.1080/19425120.2015.10...
). There are currently three species of thresher sharks descripted, the common thresher Alopias vulpinus (Bonnaterre, 1788); the pelagic thresher Alopias pelagicus Nakamura, 1935, and the bigeye thresher Alopias superciliosus (Lowe, 1841), all of them from the family Alopiidae (Compagno, 1984Compagno LJV. Sharks of the world (Vol. 4): an annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. Rome: Food and Agriculture Organization of the United Nations; 1984. Available from: http://www.fao.org/3/ad123e/ad123e00.htm
http://www.fao.org/3/ad123e/ad123e00.htm...
; Sepulveda et al., 2005Sepulveda CA, Wegner NC, Bernal D, Graham JB. The red muscle morphology of the thresher sharks (family Alopiidae). J Exp Biol. 2005; 208(22):4255-61. https://doi.org/10.1242/jeb.01898
https://doi.org/10.1242/jeb.01898...
; Dharmadi, Wiadnyana, 2013Dharmadi F, Wiadnyana NN. Biological aspects and catch fluctuation of the pelagic thresher shark, Alopias pelagicus from the Indian Ocean. Proceedings of the Design Symposium on Conservation of Ecosystem. 2013; 3:77-83. https://doi.org/10.14989/176185
https://doi.org/10.14989/176185...
), with all three species reported in Ecuador (Aguilar et al., 2005Aguilar F, Chalén X, Villón C. Plan de acción nacional de tiburones. Proceso de investigación recursos bioacuáticos y ambiente. Quito: Instituto Nacional de Pesca; 2005. Available from: http://www.fao.org/fishery/docs/DOCUMENT/IPOAS/national/ecuador/PlandeAccionTiburonesPAT-Ec.pdf
http://www.fao.org/fishery/docs/DOCUMENT...
). The thresher sharks are distinguished from other shark species, because of their long scythe-like caudal fins, and are part of the Lamniformes order. They form part of worldwide fisheries with big mortality rates, because of the high fishing pressure on them, since their meat and fins are very appreciated for human consumption (Compagno, 1984Compagno LJV. Sharks of the world (Vol. 4): an annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. Rome: Food and Agriculture Organization of the United Nations; 1984. Available from: http://www.fao.org/3/ad123e/ad123e00.htm
http://www.fao.org/3/ad123e/ad123e00.htm...
; Smith et al., 2008Smith SE, Rasmussen RC, Ramon DA, Cailliet GM. The biology and ecology of thresher sharks (Alopiidae). In: Pikitch EK, Camhi MD, Babcock EA, editors. Sharks of the open ocean: biology, fisheries and conservation. Oxford: Blackwell Publishing; 2008. p.60-68.; Nelson et al., 2016Nelson JS, Grande TC, Wilson MVH. Fishes of the World. Hoboken: John Wiley & Sons; 2016.; Young et al., 2016Young CN, Carlson JK, Hutchinson M, Kobayashi D, McCandless C, Miller MH, Teo SLH, Warren T. Status review report: common thresher (Alopias vulpinus) and bigeye thresher (Alopias superciliosus) sharks. Final Report to National Marine Fisheries Service; 2016.).
Alopias pelagicus is an epipelagic and relative large (up to 330 cm TL) shark species, which has a limited distribution to the Indian and Pacific oceans (Cardeñosa et al., 2014Cardeñosa D, Hyde J, Caballero S. Genetic diversity and population structure of the pelagic thresher shark (Alopias pelagicus) in the Pacific Ocean: evidence for two evolutionarily significant units. PloS ONE. 2014; 9(10):e110193. https://doi.org/10.1371/journal.pone.0110193
https://doi.org/10.1371/journal.pone.011...
). This species presents embryonic oophagy as a reproductive strategy, which is a form of matrotrophic viviparity where, after initial yolk-sac nutrition, growing embryos ingest unfertilized eggs to support further development (Musick et al., 2005Musick JA, Ellis JK, Hamlett W. Reproductive evolution of chondrichthyans. In: Hamlett WC, editor. Reproductive biology and phylogeny of chondrichthyes: sharks, batoids and chimaeras. Enfield: Science Pulishers; 2005. p.45-71.). This species also presents a low fecundity, with gestations of only 1-2 pups after an unknown reproductive period, which is presumed to have a duration of a year or less. It is one of the most abundant pelagic shark species in the Eastern Tropical Pacific (ETP) and the Western Pacific (WP), it is a cosmopolite species that lives in tropical and subtropical waters, in which the pelagic thresher is exposed to the overexploitation produced by commercial, artisanal and also illegal fisheries from these regions (Liu et al., 1999Liu K-M, Chen C-T, Liao T-H, Joung S-J. Age, growth, and reproduction of the pelagic thresher shark, Alopias pelagicus in the Northwestern Pacific. Copeia. 1999; 1999(1):68-74. https://doi.org/10.2307/1447386
https://doi.org/10.2307/1447386...
; Tsai et al., 2010Tsai W-P, Liu K-M, Joung S-J. Demographic analysis of the pelagic thresher shark, Alopias pelagicus, in the north-western Pacific using a stochastic stage-based model. Mar Freshw Res . 2010; 61(9):1056-66. http://dx.doi.org/10.1071/Mf09303
http://dx.doi.org/10.1071/Mf09303...
; Drew et al., 2015Drew M, White WT, Harry AV, Huveneers C. Age, growth and maturity of the pelagic thresher Alopias pelagicus and the scalloped hammerhead Sphyrna lewini. J Fish Biol. 2015; 86(1):333-54. https://doi.org/10.1111/jfb.12586
https://doi.org/10.1111/jfb.12586...
).
Alopias superciliosus is a highly migratory species, with a circumtropical and subtropical distribution, from coastal to oceanic waters and also tempered zones, it can be found at depths of 750 m (Liu et al., 1998Liu K-M, Chiang P-J, Chen C-T. Age and growth estimates of the bigeye thresher shark, Alopias superciliosus, in northeastern Taiwan waters. Fish Bull (Wash D C) . 1998; 96(3):482-91.; Camhi et al., 2009Camhi MD, Valenti SV, Fordham SV, Fowler SL, Gibson C, editors. The conservation status of pelagic sharks and rays: report of the IUCN shark specialist group. Pelagic shark red list workshop. Newbury: IUCN Species Survival Commission Shark Specialist Group; 2009.; Gökoğlu et al., 2017Gökoğlu M, Teker S, Julian D. First report of thresher sharks (Alopiidae) in the Gulf of Antalya. Iran J Fish Sci. 2017; 16(3):1108-13. Available from: http://hdl.handle.net/1834/12263
http://hdl.handle.net/1834/12263...
). It is easily distinguished from the other species of their family, because of the unique shape of its head, since it has a dorsal crest that gives them a peculiar ‘helmeted’ appearance, this is because of the deep grooves that this species presents, which extends from the superior part of the eyes to the superior region of the gill openings; it has large and oval eyes, with an interorbital space that is almost flat (Weng, Block, 2004Weng KC, Block BA. Diel vertical migration of the bigeye thresher shark (Alopias superciliosus), a species possessing orbital retia mirabilia. Fish Bull . 2004; 102(1):221-29.; Navia, Mejía-Falla, 2011Navia AF, Mejía-Falla PA. Guía para la identificación de especies de tiburones y rayas comercializadas en el Pacífico colombiano. Santiago de Cali: Fundación Colombiana para la Investigación y Conservación de tiburones y rayas, SQUALUS; 2011.; Mas et al., 2014Mas F, Forselledo R, Domingo A. Length-length relationships for six pelagic shark species commonly caught in the southwestern Atlantic Ocean. Collect Vol Sci Pap. 2014; 70:2441-50.; Farrag, 2017Farrag MMS. New record of the bigeye thresher shark, Alopias superciliosus Lowe, 1841 (Family: Alopiidae) from the eastern Mediterranean Sea, Egypt. Int J Fish Aquat Stud. 2017; 5(2):316-18.). Its reproductive ontogeny is also achieved by embryonic oophagy, where unfertilized eggs are produced by the pregnant female throughout most of their pregnancy, which the developing embryos ingest and store in a large bulging yolk-stomach, this being their only energy supply until birth (Snelson et al., 2008Snelson FF Jr, Burgess GH, Roman BL. The reproductive biology of pelagic elasmobranchs. In: Pikitch EK, Camhi MD, Babcock EA, editors. Sharks of the open ocean: biology, fisheries and conservation . Oxford: Blackwell Publishing ; 2008. p.24-45.).
In virtue of the current capture volumes, both species have recently shifted from the “Vulnerable” (VU) to the “Endangered” (EN) category of the International Union for Conservation of Nature (IUCN) (Camhi et al., 2009Camhi MD, Valenti SV, Fordham SV, Fowler SL, Gibson C, editors. The conservation status of pelagic sharks and rays: report of the IUCN shark specialist group. Pelagic shark red list workshop. Newbury: IUCN Species Survival Commission Shark Specialist Group; 2009.; Rigby et al., 2019Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Herman K, Jabado RW, Liu KM, Marshall A, Pacoreau N, Romanov E, Sherley RB, Winker H. Alopias pelagicus [Internet]. The IUCN Red List of Threatened Species 2019; 2019. https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T161597A68607857.en
https://dx.doi.org/10.2305/IUCN.UK.2019-...
). This study has the main objective to gain updated information about some of the reproductive aspects of the thresher sharks of Ecuador, examining elements such as the sex ratio, the frequency and lengths distribution, and the size at first sexual maturity (L 50 ) , with the aim of providing data that help in the efforts for those species conservation.
MATERIAL AND METHODS
The samplings were carried out at the landing stage of Tarqui beach, located in the canton of Manta (00°56’59”S 80°42’34”W), Ecuadorian Pacific, throughout the months of January to December of 2019. The landed organisms were sexed and measured in centimetres (cm) with a graduated measuring tape, the taken measures were: precaudal length (PCL) and the interdorsal length (IDL), this was because the landed individuals lacked the superior lobe of their caudal fin, which difficulted the recording of the total length (TL). In males, the exterior clasper length (CL) was registered in centimetres (cm), and other characteristics such as the rotation, calcification (null, partial or total), the riphiodon aperture, and the presence or absence of sperm (Mejía‐Falla et al., 2012Mejía-Falla PA, Navia AF, Cortés E. Reproductive variables of Urotrygon rogersi (Batoidea: Urotrygonidae): a species with a triannual reproductive cycle in the eastern tropical Pacific Ocean. J Fish Biol . 2012; 80(5):1246-66. http://dx.doi.org/10.1111/j.1095-8649.2012.03237.x
http://dx.doi.org/10.1111/j.1095-8649.20...
). The males that presented a non-calcified clasper, were classified as juveniles or immatures; and those individuals that presented calcification, rotation and an easy aperture of their riphiodon, were considered as adults or mature (Clark, von Schmidt, 1965Clark E, von Schmidt K. Sharks of the central Gulf coast of Florida. Bull Mar Sci. 1965; 15(1):13-83.; Aguilar et al., 2007Aguilar F, Revelo W, Coello D, Cajas J, Ruíz W, Díaz M, Moreno J. Desembarques artesanales de tiburones y rayas en los principales puertos pesqueros del Ecuador durante 2006. Guayaquil: Informe Interno, Instituto Nacional de Pesca; 2007.; Martínez-Ortíz, 2012Martínez-Ortíz J. Tiburones del Océano Pacifico oriental. Estudio de casos. Manta: Ministerio de Agricultura, Ganadería, Acuacultura y Pesca; 2012;; Romero‐Caicedo, Carrera‐Fernández, 2015Romero-Caicedo AF, Carrera-Fernández M. Reproduction of the whitesnout guitarfish Rhinobatos leucorhynchus in the Ecuadorian Pacific Ocean. J Fish Biol . 2015; 87(6):1434-48. https://doi.org/10.1111/jfb.12794
https://doi.org/10.1111/jfb.12794...
).
The length composition was represented with frequency histograms of combined sexes from both species, via a null hypothesis based on a 1:1 proportion, using the Chi-square test. Normality tests were applied, as well as tests of homogeneity of variance and tests of hypothesis to determine the presence of significant differences between sexes (Milton, 1964Milton RC. An extended table of critical values for the Mann-Whitney (Wilcoxon) two-sample statistic. J Am Stat Assoc. 1964; 59(307):925-34. https://doi.org/10.2307/2283111
https://doi.org/10.2307/2283111...
; MacFarland, Yates, 2016MacFarland TW, Yates JM. Introduction to nonparametric statistics for the biological sciences using R. Switzerland: Springer; 2016.). The Pearson’s correlation coefficient between IDL and PCL, and an analysis of variables (ANOVA) to determine the existence of significant differences between the lengths of the sampled months.
Normally, the fishermen cut the superior lobe of the caudal fin. For that reason a simple linear regression analysis between IDL and PCL was made, in order to acquire reliable data based on extrapolations extracted from the equation of the line (Romero-Caicedo et al., 2014Romero-Caicedo AF, Galvan-Magaña F, Martínez-Ortiz J. Reproduction of the pelagic thresher shark Alopias pelagicus in the equatorial Pacific. J Mar Biol Assoc U K. 2014; 94(7):1501-07. https://doi.org/10.1017/S0025315414000927
https://doi.org/10.1017/S002531541400092...
).
In males, the sexual maturity was based on a logistic curve, which was modified with raw data, as a way to obtain the inflection point of the clasper length, the following equation was used where a is the inflection point, LC min and LC max are the values of the clasper length, being respectively the minimum and the maximum value (Piner et al., 2005Piner KR, Hamel OS, Menkel JL, Wallace JR, Hutchinson CE. Age validation of canary rockfish (Sebastes pinniger) from off the Oregon coast (USA) using the bomb radiocarbon method. Can J Fish Aquat Sci. 2005; 62(5):1060-66. https://doi.org/10.1139/f05-082
https://doi.org/10.1139/f05-082...
). For the adjustment of a logistic model for the binominal data of maturity (0 immatures; 1 matures), these were grouped in the category of 0, non-calcified; and 1, calcified, this category was calculated from the equation in order to estimate the size at first sexual maturity (L 50 ) (Smart et al., 2016Smart JJ, Chin A, Tobin AJ, Simpfendorfer CA. Multimodel approaches in shark and ray growth studies: strengths, weaknesses and the future. Fish Fish. 2016; 17(4):955-71. https://doi.org/10.1111/faf.12154
https://doi.org/10.1111/faf.12154...
). All the statistical analyses carried, were performed using the software R Studio Development Core Team 2016 package, AquaticLifeHistory in R (Smart et al., 2016Smart JJ, Chin A, Tobin AJ, Simpfendorfer CA. Multimodel approaches in shark and ray growth studies: strengths, weaknesses and the future. Fish Fish. 2016; 17(4):955-71. https://doi.org/10.1111/faf.12154
https://doi.org/10.1111/faf.12154...
; Smart, 2019Smart J. AquaticLifeHistory: Fisheries life history analysis using contemporary methods [Internet]. R package; 2019. Available from: https://github.com/jonathansmart/AquaticLifeHistory
https://github.com/jonathansmart/Aquatic...
).
RESULTS
During the sampling period, 1236 individuals of A. pelagicus were registered, with 711 (57.52%) being females, and 525 (42.48%) being males. The sex ratio was 1.35F:1M (x 2 , P < 0.05) in which significant differences were found, specifically in the months of January, February, April, and June (Tab. 1A). The minimum and maximum length of females were respectively 67.2 and 184.0 (average = 141.1 ± 0.8) cm PCL, and in the case of males, their sizes ranged from 69.0-178.4 (average = 142.0 ± 0.9) cm PCL. The maximum length frequency interval, for both males and females, was registered at 147.2-157.2 cm PCL (Fig. 1A). The females presented larger lengths than males, but there were no significant differences between sexes (U Mann-Whitney, P > 0.05; K-S, P > 0.05). A positive correlation between PCL and IDL was found (R2 = 0.93) (Fig. 2A) and no differences between sexes were registered (ANCOVA = 0.73, P > 0.05). Samples were obtained during all months of 2019, June was the month that presented the highest frequency (n = 259, 20.95%; 113 (9.13%) males and 146 (11.81%) females), whilst the lowest frequency was registered in October (n = 30, 2.43%; 11 (0.89%) males and 19 (1.54%) females), significant differences were notified between the sampled months (ANOVA K-W, P < 0.05).
| Composition of sizes of male and female thresher sharks Alopias pelagicus (A) and A. superciliosus (B) landed in the town of Playita Mia, Manta Ecuador.
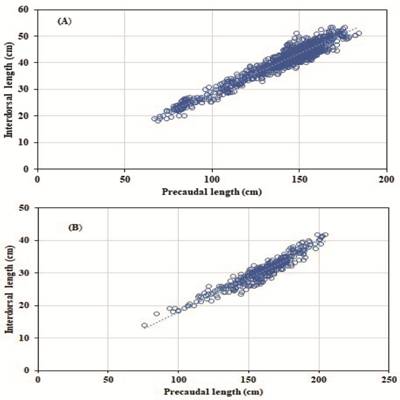
| Relationship between interdorsal and length precaudal of the thresher sharks Alopias pelagicus (A) and A. superciliosus (B). A linear regression, coefficient of determination (R2 = 0.93 A, R2 = 0.91, B).
|Monthly distribution of sex ratio and chi-square (x 2 ) of Alopias pelagicus (A) and A. superciliosus (B). *Present significant differences.
Alopias superciliosus registered a total of 354 individuals, which were distributed between 164 females (46.33%), and 190 males (53.67%), displaying a sexual proportion of 0.86F:1M (x 2 , P > 0.05) (Tab. 1B). The length of the males oscillated from 94.0-204.8 (average = 160.30 ± 1.39) cm PCL, while the females ranged from 76.0-202.2 (average = 159.03 ± 1.84) cm PCL, for both males and females. The highest frequency interval was registered between 156-166 (Fig. 1B). The males showed larger lengths than the females, but there were no significant differences between sexes (U Mann-Whitney, P > 0.05; K-S, P > 0.05). Between IDL and PCL, a positive correlation was found (R2 = 0.91) (Fig. 2A) and no differences between sexes were detected (ANCOVA = 0.87, P > 0.05). The month with the highest frequency was June (n = 41, 11.58%; 20 (5.65%) females and 21 (5.93%) males), and the month that presented the lowest frequency was August (n = 13, 3.67%; 6 (1.69%) females and 7 (1.98%) males), between the sampled months there were no significant differences registered (ANOVA, P > 0.05).
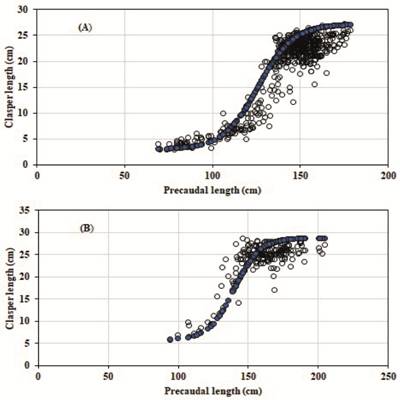
The males of the pelagic thresher shark registered 161 (30.73%) specimens with non-calcified claspers, while 363 (69.27%) presented total calcification (Fig. 3A). The males with immature claspers (non-calcified), had lengths that ranged from 69-155 cm PCL with a clasper length (CL) of 3-20.2 cm, but in the case of the males with mature claspers (calcified) exhibited lengths of 130.6-178.4 cm PCL, and 16.6-27 cm of CL. The inflection point of the clasper was estimated at 134.2 cm PCL, while the average size at first maturity for males was estimated at 136.03 cm PCL (Fig. 4A).
| Relationship between the length of the clasper and the length precaudal of the thresher sharks Alopias pelagicus (A) and A. superciliosus (B).
| Mean length (CI 95%) at maturity of the thresher sharks Alopias pelagicus (A) and A. superciliosus (B).
Alopias superciliosus registered 20 (10.53%) individuals with non-calcified claspers, and 170 (89.47%) with totally calcified claspers (Fig. 3B). The males with immature claspers (non-calcified) displayed lengths of 94-140 cm PCL with a clasper length (CL) that ranged from 5.8-21.2 cm, while the males with mature claspers (calcified) exhibited lengths of 134-204.8 cm PCL and with a CL that went between 17-27.8 cm. The inflection point of the clasper was estimated at 136.8 cm PCL, while the average size at first maturity size of males was estimated at 138.73 cm PCL (Fig. 4A).
DISCUSSION
The evaluation of the quantitative relations between the length, maturity and reproductive aspects scientifically reinforces the management plans designed for the future sustainability of the resources (Harry et al., 2013Harry AV, Tobin AJ, Simpfendorfer CA. Age, growth and reproductive biology of the spot-tail shark, Carcharhinus sorrah, and the Australian blacktip shark, C. tilstoni, from the Great Barrier Reef World Heritage Area, north-eastern Australia. Mar Freshw Res . 2013; 64(4):277-93. http://dx.doi.org/10.1071/MF12142
http://dx.doi.org/10.1071/MF12142...
). At the Ecuadorian level, for A. pelagicus, Martínez-Ortíz et al. (2007Martínez-Ortíz J, Galván-Magaña F, Carrera-Fernández M, Mendoza-Intriago D, Estupiñán-Montaño C, Cedeño-Figueroa L. Abundancia estacional de tiburones desembarcados en Manta-Ecuador. In: Martínez-Ortíz J, Galván-Mangaña F, editors. Tiburones en el Ecuador: Casos de estudio. Manta: EPESPO-PMRC; 2007. p.9-27.) reported that the range of the precaudal length (PCL) had values between 48 and 284 cm for females, and 50-210 cm (n = 3685) for males; significant value differences up to 19.2 cm with respect to the minimum length for both sexes, and 100 cm with the maximum length of the females reported in this study (PCL females: 67.2-184 cm and PCL males: 69-178.4 cm) (n = 1236). This variability of lengths may indicate that during the time between studies, the fishing pressure could have decreased the populations of immature individuals of this species (Musyl et al., 2011Musyl MK, Brill RW, Curran DS, Fragoso NM, McNaughton LM, Nielsen A, Kikkawa BS, Moyes CD. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish Bull. 2011; 109(4):341-68.; Oliver et al., 2015Oliver S, Braccini M, Newman SJ, Harvey ES. Global patterns in the bycatch of sharks and rays. Mar Policy . 2015; 54:86-97. https://doi.org/10.1016/j.marpol.2014.12.017
https://doi.org/10.1016/j.marpol.2014.12...
). The actual fishing efforts is tending towards the capture of individuals whose lengths are superior to the average age of maturity (unimodal distribution: 147.2-157.2 cm); something that, combined to the slow biological recuperation of this species, could lead to overfishing (Smith et al., 2008Smith SE, Rasmussen RC, Ramon DA, Cailliet GM. The biology and ecology of thresher sharks (Alopiidae). In: Pikitch EK, Camhi MD, Babcock EA, editors. Sharks of the open ocean: biology, fisheries and conservation. Oxford: Blackwell Publishing; 2008. p.60-68.; Drew et al., 2015Drew M, White WT, Harry AV, Huveneers C. Age, growth and maturity of the pelagic thresher Alopias pelagicus and the scalloped hammerhead Sphyrna lewini. J Fish Biol. 2015; 86(1):333-54. https://doi.org/10.1111/jfb.12586
https://doi.org/10.1111/jfb.12586...
). It has to be taken into account that A. pelagicus is the species with the highest capture rate of all elasmobranchs in Ecuador (Herrera et al., 2012Herrera M, Coello D, Cajas J. Desembarques y aspectos biológicos de Elasmobranquios en las pesquerías artesanales del Ecuador durante 2011. Boletín científico y técnico. 2012; 22:1-08.; Martínez-Ortiz, García-Domínguez, 2013Martínez-Ortiz J, García-Domínguez M. Guía de campo. Condrictios del Ecuador. Quimeras, tiburones y rayas. Manta: Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP)/ViceMinisterio de Acuacultura y Pesca (VMAP)/Subsecretaria de Recursos Pesqueros (SRP); 2013.; Coello, Herrera, 2018Coello D, Herrera M. Desembarque de tiburones en las pesquerías artesanales del Ecuador durante el 2012. Rev Cient Cien Nat Ambien. 2018; 12(1):1-08.). According to Martínez-Ortiz et al. (2015Martínez-Ortiz J, Aires-da-Silva AM, Lennert-Cody CE, Maunder MN. The Ecuadorian artisanal fishery for large pelagics: species composition and spatio-temporal dynamics. PLoS ONE . 2015; 10(8):e0135136. https://doi.org/10.1371/journal.pone.0135136
https://doi.org/10.1371/journal.pone.013...
), shark catches from longlines in Ecuador are largely dominated by thresher sharks (Alopiidae), mainly A. pelagicus with 96.8% caught (27380.5 mt per month) and 3.1% (88.68 mt per month) for A. superciliosus, data from January 2008 to December 2012. For comparative purpose with the bibliography that is in TL (total length), a data conversion with linear regression of the relation between (TL) and (PCL) was made. It is important to emphasise that before reaching the actual pronounced progression exposed in this study, the measurements of Coello and Herrera (2018) (TL females: 110-338 cm and TL males: 108-341 cm) recollected during the course of 2012, chronologically show a pattern of the increase of minimum lengths captures of A. pelagicus in the country. However, in Ecuador, there are no reports of lengths larger than those maximum values described for this species by Martínez-Ortiz and García-Domínguez (2013Martínez-Ortiz J, García-Domínguez M. Guía de campo. Condrictios del Ecuador. Quimeras, tiburones y rayas. Manta: Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP)/ViceMinisterio de Acuacultura y Pesca (VMAP)/Subsecretaria de Recursos Pesqueros (SRP); 2013.), which are: TL for females ranging from 96-375 cm, and TL for males 91.4-353 cm. The work of Romero-Caicedo et al. (2014Romero-Caicedo AF, Galvan-Magaña F, Martínez-Ortiz J. Reproduction of the pelagic thresher shark Alopias pelagicus in the equatorial Pacific. J Mar Biol Assoc U K. 2014; 94(7):1501-07. https://doi.org/10.1017/S0025315414000927
https://doi.org/10.1017/S002531541400092...
), carried out between 2005 and 2006, is the only previous study that reports similar PCL measurements with this present study, which oscillate between 70 and 180 cm in the case of females, and from 68-183 cm for the males, presenting the highest frequency of individuals at lengths of 135-144 cm (n = 241). Regarding other regions, Smith et al. (2008) presented a length composition of the Gulf of California in Mexico collected from 1998 to 1999, in which the females and males of A. pelagicus presented an average precaudal length (PCL) of 134.3 ± 8.3 cm and 130.4 ± 8.6 cm respectively, clearly those are lower measurements than the ones reported in this investigation (PCL average females = 141.05 ± 0.82 and PCL average males = 142.02 ± 0.95) with a shorter length interval (109-162 cm) and significant differences of the PCL in both sexes. In the Indian Ocean, the measurements fluctuate from 96-350 cm of TL, which might reflect the existence of nurseries and reproduction zones of this species along some coasts (e.g., Arabian Sea, India or Indonesia) (Najmudeen et al., 2019Najmudeen TM, Zacharia PU, Seetha PK, Sunil KTS, Radhakrishnan M, Akhildev S, Sipson A. Length-weight relationships of three species of pelagic sharks from southeastern Arabian Sea. Reg Stud Mar Sci. 2019; 29:100647. https://doi.org/10.1016/j.rsma.2019.100647
https://doi.org/10.1016/j.rsma.2019.1006...
; Ichsan et al., 2020Ichsan M, Ula S, Simeon B, Muttaqin E, Booth H. Thresher sharks (Alopiidae) catch in the pelagic fisheries of Western Indonesia. Earth Eviron Sci. 2020; 420:012013. http://doi.org/10.1088/1755-1315/420/1/012013
http://doi.org/10.1088/1755-1315/420/1/0...
). Additionally, in Indonesia it was noted that immature individuals are dominating the elasmobranch fisheries (Winter et al., 2020Winter ST, Fahmi, Rudianto D, Laglbauer BJL, Ender I, Simpfendorfer CA. Immature individuals dominate elasmobranch fisheries of the Bali Strait. Mar Freshw Res . 2020; 71(11):1488-1500. https://doi.org/10.1071/MF19300
https://doi.org/10.1071/MF19300...
).
In regard of the sex ratio, a greater presence of females was registered (1.35F:1M) differing significantly from the expected (1F:1M), in a similar relation that was estimated by Romero-Caicedo et al. (2014Romero-Caicedo AF, Galvan-Magaña F, Martínez-Ortiz J. Reproduction of the pelagic thresher shark Alopias pelagicus in the equatorial Pacific. J Mar Biol Assoc U K. 2014; 94(7):1501-07. https://doi.org/10.1017/S0025315414000927
https://doi.org/10.1017/S002531541400092...
); however, the Ecuadorian coast has notified even more biased proportional magnitudes of females in captures, Coello, Herrera (2018Coello D, Herrera M. Desembarque de tiburones en las pesquerías artesanales del Ecuador durante el 2012. Rev Cient Cien Nat Ambien. 2018; 12(1):1-08.) presented a sex ratio of 2.5F:1M; White et al. (2020White WT, Baje L, Appleyard SA, Chin A, Smart JJ, Simpfendorfer CA. Shark longline fishery of Papua New Guinea: size and species composition and spatial variation of the catches. Mar Freshw Res . 2020; 71(6):627-40. https://doi.org/10.1071/MF19191
https://doi.org/10.1071/MF19191...
) also reported a sex ratio with a clear prevalence of females (2.6F:1M) for Papua New Guinea in the Pacific Ocean. That higher occurrence of females, could be related with sexual segregation (Klimley, 1987Klimley AP. The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini. Environ Biol Fish. 1987; 18:27-40. https://doi.org/10.1007/BF00002325
https://doi.org/10.1007/BF00002325...
; Sims, 2006Sims DW. Differences in habitat selection and reproductive strategies of male and female sharks. In: Ruckstuhl K, Neuhaus P, editors. Sexual segregation in vertebrates. Cambridge: Cambridge University Press; 2006. p.127-47. https://doi.org/10.1017/CBO9780511525629.009
https://doi.org/10.1017/CBO9780511525629...
), in which females have a preference for warmer and more coastal waters in order to improve the fecundity of their thermic niches (Wearmouth, Sims, 2008Wearmouth VJ, Sims DW. Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv Mar Biol. 2008; 54:107-70. https://doi.org/10.1016/s0065-2881(08)00002-3
https://doi.org/10.1016/s0065-2881(08)00...
), which could entail a biased exploitation pattern of the resource (Hazin et al., 2006Hazin FHV, Fischer AF, Broadhurst MK, Veras D, Oliveira PG, Burgess GH. Notes on the reproduction of Squalus megalops off northeastern Brazil. Fish Res. 2006; 79(3):251-57. https://doi.org/10.1016/j.fishres.2006.04.006
https://doi.org/10.1016/j.fishres.2006.0...
; Mucientes et al., 2009Mucientes GR, Queiroz N, Sousa LL, Tarroso P, Sims DW. Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol Lett. 2009; 5(2):156-59. https://doi.org/10.1098/rsbl.2008.0761
https://doi.org/10.1098/rsbl.2008.0761...
).
This study found a positive correlation between the interdorsal length (IDL) and the precaudal length (PCL), which shows that both values are strongly and directly correlated (Oshitani et al., 2003Oshitani S, Nakano H, Tanaka S. Age and growth of the silky shark Carcharhinus falciformis from the Pacific Ocean. Fish Sci. 2003; 69(3):456-64. http://dx.doi.org/10.1046/j.1444-2906.2003.00645.x
http://dx.doi.org/10.1046/j.1444-2906.20...
; Polo‐Silva et al., 2018Polo-Silva C, Acevedo G, Siu S, Carvajal JM, Ixquiac M, Bessudo S, Suarez AM, Puentes V. Morphometric relationships for some species of elasmobranch from tropical eastern Pacific. J Appl Ichthyol. 2018; 34(1):157-61. https://doi.org/10.1111/jai.13460
https://doi.org/10.1111/jai.13460...
). This equation can now be used to estimate values of PCL, from measurements of IDL in individuals that have suffered any cephalic mutilation or the removal of the caudal fin, as long as it is measured with very good accuracy (Mas et al., 2014Mas F, Forselledo R, Domingo A. Length-length relationships for six pelagic shark species commonly caught in the southwestern Atlantic Ocean. Collect Vol Sci Pap. 2014; 70:2441-50.; Santana-Hernández et al., 2014Santana-Hernández H, Tovar-Ávila J, Valdéz-Flores JJ. Estimation of the total, fork and precaudal lengths for the silky shark, Carcharhinus falciformis (Carcharhiniformes: Carcharhinidae), from the interdorsal length. Hidrobiologica. 2014; 24(2):159-62.; Briones-Mendoza et al., 2018Briones-Mendoza J, Pincay-Espinoza JE, Palma-Chávez J, Romero-Caicedo A. Notas sobre la biología del tiburón mamona Mustelus lunulatus (Carcharhiniformes: Triakidae) en el Pacífico Central ecuatoriano. Rev Biol Mar Oceanogr. 2018; 53(2):279-84. http://dx.doi.org/10.22370/rbmo.2018.53.2.1301
http://dx.doi.org/10.22370/rbmo.2018.53....
).
The first sexual maturity length (L50 ) for males was estimated at 136.03 cm PCL (254.8 TL) from the inflection point of the ogive from the sigmoidal model that ties the clasper length (CL) and the precaudal length (PCL) when the binominal data of the biological condition of the clasper (calcified or non-calcified) are combined; a model that has been already used to estimate the maturity of chondrichthyans (Briones-Mendoza et al., 2016Briones-Mendoza J, Pincay-Espinoza J, Palma-Chávez J, Romero-Caicedo A. Notas sobre la biología del tiburón azul Prionace glauca (Carcharhiniformes: Carcharhinidae) en aguas ecuatorianas. Rev Mex Biodivers. 2016; 87(4):1387-90. https://doi.org/10.1016/j.rmb.2016.09.007
https://doi.org/10.1016/j.rmb.2016.09.00...
), Lamniformes (Jensen et al., 2002Jensen CF, Natanson LJ, Pratt HL Jr, Kohler N, Campana SE. The reproductive biology of the porbeage shark (Lamna nasus) in the western north Atlantic Ocean. Fish Bull (Wash D C). 2002; 100(4):727-38.) and even in species of the family Alopiidae (Natanson, Gervelis, 2013Natanson LJ, Gervelis BJ. The reproductive biology of the common thresher shark in the western north Atlantic Ocean. Trans Am Fish Soc. 2013; 142(6):1546-62. http://dx.doi.org/10.1080/00028487.2013.811099
http://dx.doi.org/10.1080/00028487.2013....
). Almost 70% of mature individuals were registered, of which the immature male that had the longest precaudal length was 155 cm PCL and the mature male that presented the lowest length was 130.6 cm of PCL. For the Ecuadorian coast and employing the same methodology, Romero-Caicedo et al. (2014Romero-Caicedo AF, Galvan-Magaña F, Martínez-Ortiz J. Reproduction of the pelagic thresher shark Alopias pelagicus in the equatorial Pacific. J Mar Biol Assoc U K. 2014; 94(7):1501-07. https://doi.org/10.1017/S0025315414000927
https://doi.org/10.1017/S002531541400092...
) differed in the calculation of the male L50 which was estimated to be at 144 cm of PCL; with clasper length averages of 4.8 cm (immatures) and 22.9 cm (matures), whose numbers are in the range of the clasper dimensions found in this study; similar findings of the biggest immature male (158 cm PCL) and the smallest mature male (138 cm PCL) were emitted. Coello et al. (2010Coello D, Herrera M, Calle M, Castro R, Medina C, Chalén X. Incidencia de tiburones, rayas, aves, tortugas y maniferos marinos en la pesqueria artesanal con enmalle de superficie en la caleta pesquera de Santa Rosa (Provincia de Santa Elena). Ecuador: Instituto Nacional de Pesca del Ecuador (Boletin Especial Ano 2); 2010.) mention that the length for males in which the 50% of the population presents specific sexual characteristics for reproduction is 259 cm TL. Other estimates of L50 occur in other regions, such as the published by Reardon et al. (2013Reardon MB, Márquez-Farías F, Trejo T, Clarke SC. Alopias pelagicus [Internet]. IUCN red list of threatened species; 2013. Available from: https://www.fish.gov.au/docs/SharkReport/FRDC_Alopias_pelagicus.pdf
https://www.fish.gov.au/docs/SharkReport...
) which they estimate at 250 cm TL for Australia; Ichsan et al. (2020Ichsan M, Ula S, Simeon B, Muttaqin E, Booth H. Thresher sharks (Alopiidae) catch in the pelagic fisheries of Western Indonesia. Earth Eviron Sci. 2020; 420:012013. http://doi.org/10.1088/1755-1315/420/1/012013
http://doi.org/10.1088/1755-1315/420/1/0...
) with 232 cm TL for Indonesia; or Liu et al. (1999Liu K-M, Chen C-T, Liao T-H, Joung S-J. Age, growth, and reproduction of the pelagic thresher shark, Alopias pelagicus in the Northwestern Pacific. Copeia. 1999; 1999(1):68-74. https://doi.org/10.2307/1447386
https://doi.org/10.2307/1447386...
) and Liu et al. (2006Liu K-M, Chang Y-T, Ni I-H, Jin C-B. Spawning per recruit analysis of the pelagic thresher shark, Alopias pelagicus, in the eastern Taiwan waters. Fish Res . 2006; 82(1-3):56-64. https://doi.org/10.1016/j.fishres.2006.08.013
https://doi.org/10.1016/j.fishres.2006.0...
) in the range of sizes from 145-150 cm PCL (282-292 cm TL) for Taiwan. The reason why there is a tendency for the diminution of the average sizes reported in this investigation, can be attributed to many factors: amongst them, it is proposed that this species could be responding with a strategy of reducing their average size as a tactic to autoregulate the populations that are exposed to a severe exploitation, something that has already been reported before in pelagic sharks (Baum, Myers, 2004Baum JK, Myers RA. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol Lett. 2004; 7(2):135-45. https://doi.org/10.1111/j.1461-0248.2003.00564.x
https://doi.org/10.1111/j.1461-0248.2003...
; Frisk et al., 2005Frisk MG, Miller TJ, Dulvy NK. Life histories and vulnerability to exploitation of elasmobranchs: inferences from elasticity, perturbation and phylogenetic analyses. J Northwest Atl Fish Sci. 2005; 37:27-45. http://dx.doi.org/10.2960/J.v35.m514
http://dx.doi.org/10.2960/J.v35.m514...
). However, it is important to also note that environmental factors according to latitudinal gradients and aquatic conditions from different regions (Dharmadi, Wiadnyana, 2013Dharmadi F, Wiadnyana NN. Biological aspects and catch fluctuation of the pelagic thresher shark, Alopias pelagicus from the Indian Ocean. Proceedings of the Design Symposium on Conservation of Ecosystem. 2013; 3:77-83. https://doi.org/10.14989/176185
https://doi.org/10.14989/176185...
; Alejo-Plata et al., 2016Alejo-Plata MC, Ahumada-Sempoal MÁ, Gómez-Márquez JL, González-Acosta A. Estructura poblacional y aspectos reproductivos del tiburón piloto Carcharhinus falciformis (Müller & Henle, 1839) (Carcharhiniformes: Carcharhinidae) en la costa de Oaxaca, México. Lat Am J Aquat Res. 2016; 44(3):513-24. https://doi.org/10.3856/vol44-issue3-fulltext-10
https://doi.org/10.3856/vol44-issue3-ful...
) also influence in the variability of the parameter measurements registered in other places of the world.
Alopias superciliosus, by contrast, is a species that has been poorly studied with regards of its biological aspects, because of its low abundance in Ecuadorian waters (Bustamante, Lamilla, 2006Bustamante C, Lamilla J. Estado actual de la pesquería y biología de los condricthyes en Chile. In: Bustamante C, Lamilla J, editors. Realidades en la pesquería de tiburones de la costa del Pacífico latinoamericano. II taller de cooperación internacional. Tiburones de Pacífico: ¿Manejo local o conjunto? Apéndices y memórias. Valdívia: Universidad Austral de Chile; 2006. p.56-66.). In this context, the work of Martínez-Ortíz et al. (2007Martínez-Ortíz J, Galván-Magaña F, Carrera-Fernández M, Mendoza-Intriago D, Estupiñán-Montaño C, Cedeño-Figueroa L. Abundancia estacional de tiburones desembarcados en Manta-Ecuador. In: Martínez-Ortíz J, Galván-Mangaña F, editors. Tiburones en el Ecuador: Casos de estudio. Manta: EPESPO-PMRC; 2007. p.9-27.) reported substantial differences in the male PCL in their investigation, these ones being an average of 20.2 cm smaller than the evaluated in this present study, whilst the females stay in a similar length range; this data may suggests that the populations of juvenile males has been diminishing over the years, and that the capture incidence is currently biasing towards adult organisms whose overfishing is putting this species biological sustainability in danger. Varghese et al. (2015Varghese SP, Vijayakumaran K, Tiburtius A, Mhatre VD. Diversity, abundance and size structure of pelagic sharks caught in tuna longline survey in the Indian seas. Indian J Geomarine Sci. 2015; 44(1):26-36.) specified an average PCL for the Indian Ocean, whose lengths are lower than the ones from this present study, concretely the specified lengths were 152.19 ± 21.48 cm. In this study, the sex ratio was in favour of the males, similar to what was found by Coello and Herrera (2018Coello D, Herrera M. Desembarque de tiburones en las pesquerías artesanales del Ecuador durante el 2012. Rev Cient Cien Nat Ambien. 2018; 12(1):1-08.). By the contrary, Carr et al. (2013Carr LA, Stier AC, Fietz K, Montero I, Gallagher AJ, Bruno JF. Illegal shark fishing in the Galápagos Marine Reserve. Mar Policy. 2013; 39:317-21. https://doi.org/10.1016/j.marpol.2012.12.005
https://doi.org/10.1016/j.marpol.2012.12...
) indicated an inverse proportion, in which the females were more abundant (2.27H:1M), documented in the captures of an illegal shark fishing boat in the Galápagos Marine Reserve. The calculation of the sex ratio for other regions tends to have a higher male incidence in coastal waters, such as the notified by Carvalho (2015Carvalho JF. Population dynamics and fisheries assessment of the bigeye thresher (Alopias superciliosus) in the Atlantic: a comparison between North Atlantic and South Atlantic stocks. [PhD Thesis]. Algarve: Universidade do Algarve; 2015.), Varghese et al. (2015Varghese SP, Vijayakumaran K, Tiburtius A, Mhatre VD. Diversity, abundance and size structure of pelagic sharks caught in tuna longline survey in the Indian seas. Indian J Geomarine Sci. 2015; 44(1):26-36., 2016Varghese SP, Unnikrishnan N, Gulati DK, Ayoob AE. Size, sex and reproductive biology of seven pelagic sharks in the eastern Arabian Sea. J Mar Biol Assoc U K . 2016; 97(1):181-96. http://dx.doi.org/10.1017/S0025315416000217
http://dx.doi.org/10.1017/S0025315416000...
), and Hacohen-Domené et al. (2020Hacohen-Domené A, Polanco-Vásquez F, Estupiñan-Montaño C, Graham RT. Description and characterization of the artisanal elasmobranch fishery on Guatemala’s Caribbean coast. PLoS ONE. 2020; 15(1):e0227797. https://doi.org/10.1371/journal.pone.0227797
https://doi.org/10.1371/journal.pone.022...
) for the Arabian Sea, Guatemala and the Atlantic coasts. However, Carvalho (2015) suggests that it may be some hints around some islands of the Atlantic ocean, such as the Cape Verde archipelago, where the sex ratio is biased towards the presence of females, in accordance with what was found by Carr et al. (2013) for the Galápagos archipelago in the Pacific ocean; which indicates the preference of A. superciliosus females for this type of open ocean geological formations as zones for pupping zones and nurseries, these particular kind of well delimited areas naturally decrease the risk of predation of neonate individuals by other pelagic shark species, and have a high prey availability (Springer, 1967Springer S. Social organization of shark population. In: Gilbert PW, Matheswon RF, Rall DP, editors. Sharks, skate and rays. Baltimore: Johns Hopkins University Press; 1967. p.149-74.).
De-Wysiecki, Braccini (2017De Wysiecki AM, Braccini JM. Shark length-length relationships: Studying morphology allows the detection of bias in routine fisheries sampling. Reg Stud Mar Sci . 2017; 16:290-93. https://doi.org/10.1016/j.rsma.2017.10.005
https://doi.org/10.1016/j.rsma.2017.10.0...
), encourage the use of the morphometry to standardise and improve the usage of the available data, as well as the compilation of biological data based on the length relations of sharks, as long as the statistical analysis is reliable. About 90% of the male sharks that were examined in this study were evaluated as totally mature, and the first sexual maturity length (L50 ) that was calculated, who determines it at 180.8 cm PCL (+42.07 cm) based on the inspection of the clasper external features, the researcher also informs of a higher margin of the clasper lengths (CL) (4.4-31 cm) for the same region. Varghese et al. (2016Varghese SP, Unnikrishnan N, Gulati DK, Ayoob AE. Size, sex and reproductive biology of seven pelagic sharks in the eastern Arabian Sea. J Mar Biol Assoc U K . 2016; 97(1):181-96. http://dx.doi.org/10.1017/S0025315416000217
http://dx.doi.org/10.1017/S0025315416000...
) by the same token measured CL in organisms from the Arabian Sea and they found a lower interval (3.2-22.4 cm), as well as individuals whose immature lengths were up to 171 cm PCL (+31 cm) and the mature specimens presented the same minimal length as it was reported here. Liu et al. (1999Liu K-M, Chen C-T, Liao T-H, Joung S-J. Age, growth, and reproduction of the pelagic thresher shark, Alopias pelagicus in the Northwestern Pacific. Copeia. 1999; 1999(1):68-74. https://doi.org/10.2307/1447386
https://doi.org/10.2307/1447386...
) also estimated a L 50 which is above the calculations, being situated in the class of 150-155 cm PCL (+16.27 cm), this case being for the Taiwanese coastline. Verifying these last values, it is noteworthy that the pattern of the length declining in the valuations of the L 50 it is present, as is the case of A. pelagicus and the causes could be analogous in both species. Additionally, Moreno, Morón (1992Moreno J, Morón J. Reproductive biology of the Bigeye Thresher Shark, Alopias superciliosus (Lowe, 1939). Mar Freshw Res . 1992; 43(1):77-86. https://doi.org/10.1071/MF9920077
https://doi.org/10.1071/MF9920077...
) believe that the male maturity length should not be determined by the size and the calcification status of their claspers, but also by the fold level of the deferent ducts when these store sperm, as a clear signal of sexual maturity, adding another argument that could explain the fluctuant model of the results of the diverse studies. The results suggest that the month of June is the most important for the landing of captured individuals of both species, because it presented the highest frequency. It was also noted the existence of a prevalence of adults over juveniles in captures. Also, the sexual proportion observed in both species could indicate a defined sexual segregation. This information is useful to study the impacts of the fisheries on both species, particularly knowing that these species are poorly studied in the Ecuadorian Pacific.
ACKNOWLEDGMENTS
The authors thank José S. Villamar and all the fishing port merchants for their help in the data collection. And a special thanks to the memory of Dr. David V. de la Torre, a great researcher, teacher, friend and extraordinary human being.
REFERENCES
- Aguilar F, Chalén X, Villón C. Plan de acción nacional de tiburones. Proceso de investigación recursos bioacuáticos y ambiente. Quito: Instituto Nacional de Pesca; 2005. Available from: http://www.fao.org/fishery/docs/DOCUMENT/IPOAS/national/ecuador/PlandeAccionTiburonesPAT-Ec.pdf
» http://www.fao.org/fishery/docs/DOCUMENT/IPOAS/national/ecuador/PlandeAccionTiburonesPAT-Ec.pdf - Aguilar F, Revelo W, Coello D, Cajas J, Ruíz W, Díaz M, Moreno J. Desembarques artesanales de tiburones y rayas en los principales puertos pesqueros del Ecuador durante 2006. Guayaquil: Informe Interno, Instituto Nacional de Pesca; 2007.
- Alejo-Plata MC, Ahumada-Sempoal MÁ, Gómez-Márquez JL, González-Acosta A. Estructura poblacional y aspectos reproductivos del tiburón piloto Carcharhinus falciformis (Müller & Henle, 1839) (Carcharhiniformes: Carcharhinidae) en la costa de Oaxaca, México. Lat Am J Aquat Res. 2016; 44(3):513-24. https://doi.org/10.3856/vol44-issue3-fulltext-10
» https://doi.org/10.3856/vol44-issue3-fulltext-10 - Baum JK, Myers RA. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol Lett. 2004; 7(2):135-45. https://doi.org/10.1111/j.1461-0248.2003.00564.x
» https://doi.org/10.1111/j.1461-0248.2003.00564.x - Briones-Mendoza J, Pincay-Espinoza J, Palma-Chávez J, Romero-Caicedo A. Notas sobre la biología del tiburón azul Prionace glauca (Carcharhiniformes: Carcharhinidae) en aguas ecuatorianas. Rev Mex Biodivers. 2016; 87(4):1387-90. https://doi.org/10.1016/j.rmb.2016.09.007
» https://doi.org/10.1016/j.rmb.2016.09.007 - Briones-Mendoza J, Pincay-Espinoza JE, Palma-Chávez J, Romero-Caicedo A. Notas sobre la biología del tiburón mamona Mustelus lunulatus (Carcharhiniformes: Triakidae) en el Pacífico Central ecuatoriano. Rev Biol Mar Oceanogr. 2018; 53(2):279-84. http://dx.doi.org/10.22370/rbmo.2018.53.2.1301
» http://dx.doi.org/10.22370/rbmo.2018.53.2.1301 - Bustamante C, Lamilla J. Estado actual de la pesquería y biología de los condricthyes en Chile. In: Bustamante C, Lamilla J, editors. Realidades en la pesquería de tiburones de la costa del Pacífico latinoamericano. II taller de cooperación internacional. Tiburones de Pacífico: ¿Manejo local o conjunto? Apéndices y memórias. Valdívia: Universidad Austral de Chile; 2006. p.56-66.
- Camhi MD, Valenti SV, Fordham SV, Fowler SL, Gibson C, editors. The conservation status of pelagic sharks and rays: report of the IUCN shark specialist group. Pelagic shark red list workshop. Newbury: IUCN Species Survival Commission Shark Specialist Group; 2009.
- Cardeñosa D, Hyde J, Caballero S. Genetic diversity and population structure of the pelagic thresher shark (Alopias pelagicus) in the Pacific Ocean: evidence for two evolutionarily significant units. PloS ONE. 2014; 9(10):e110193. https://doi.org/10.1371/journal.pone.0110193
» https://doi.org/10.1371/journal.pone.0110193 - Carr LA, Stier AC, Fietz K, Montero I, Gallagher AJ, Bruno JF. Illegal shark fishing in the Galápagos Marine Reserve. Mar Policy. 2013; 39:317-21. https://doi.org/10.1016/j.marpol.2012.12.005
» https://doi.org/10.1016/j.marpol.2012.12.005 - Carvalho JF. Population dynamics and fisheries assessment of the bigeye thresher (Alopias superciliosus) in the Atlantic: a comparison between North Atlantic and South Atlantic stocks. [PhD Thesis]. Algarve: Universidade do Algarve; 2015.
- Clark E, von Schmidt K. Sharks of the central Gulf coast of Florida. Bull Mar Sci. 1965; 15(1):13-83.
- Coello D, Herrera M. Desembarque de tiburones en las pesquerías artesanales del Ecuador durante el 2012. Rev Cient Cien Nat Ambien. 2018; 12(1):1-08.
- Coello D, Herrera M, Calle M, Castro R, Medina C, Chalén X. Incidencia de tiburones, rayas, aves, tortugas y maniferos marinos en la pesqueria artesanal con enmalle de superficie en la caleta pesquera de Santa Rosa (Provincia de Santa Elena). Ecuador: Instituto Nacional de Pesca del Ecuador (Boletin Especial Ano 2); 2010.
- Compagno LJV. Sharks of the world (Vol. 4): an annotated and illustrated catalogue of shark species known to date. Part 2. Carcharhiniformes. Rome: Food and Agriculture Organization of the United Nations; 1984. Available from: http://www.fao.org/3/ad123e/ad123e00.htm
» http://www.fao.org/3/ad123e/ad123e00.htm - Corro-Espinosa D, Márquez-Farías JF, Muhlia-Melo A. Talla de madurez del tiburón bironche Rhizoprionodon longurio en el Golfo de California, México. Cienc Mar. 2011; 37(2):201-14. Available from: http://www.scielo.org.mx/pdf/ciemar/v37n2/v37n2a7.pdf
» http://www.scielo.org.mx/pdf/ciemar/v37n2/v37n2a7.pdf - Cotton CF, Grubbs RD, Daly-Engel TS, Lynch PD, Musick JA. Age, growth and reproduction of a common deep-water shark, shortspine spurdog (Squalus cf. mitsukurii), from Hawaiian waters. Mar Freshw Res. 2011; 62(7):811-22. https://doi.org/10.1071/MF10307
» https://doi.org/10.1071/MF10307 - Dharmadi F, Wiadnyana NN. Biological aspects and catch fluctuation of the pelagic thresher shark, Alopias pelagicus from the Indian Ocean. Proceedings of the Design Symposium on Conservation of Ecosystem. 2013; 3:77-83. https://doi.org/10.14989/176185
» https://doi.org/10.14989/176185 - Drew M, White WT, Harry AV, Huveneers C. Age, growth and maturity of the pelagic thresher Alopias pelagicus and the scalloped hammerhead Sphyrna lewini J Fish Biol. 2015; 86(1):333-54. https://doi.org/10.1111/jfb.12586
» https://doi.org/10.1111/jfb.12586 - Farrag MMS. New record of the bigeye thresher shark, Alopias superciliosus Lowe, 1841 (Family: Alopiidae) from the eastern Mediterranean Sea, Egypt. Int J Fish Aquat Stud. 2017; 5(2):316-18.
- Fischer AF, Hazin FHV, Carvalho F, Viana DL, Rêgo MG, Wor C. Biological aspects of sharks caught off the Coast of Pernambuco, Northeast Brazil. Braz J Biol. 2009; 69(4):1173-81. https://doi.org/10.1590/S1519-69842009000500023
» https://doi.org/10.1590/S1519-69842009000500023 - Frisk MG, Miller TJ, Dulvy NK. Life histories and vulnerability to exploitation of elasmobranchs: inferences from elasticity, perturbation and phylogenetic analyses. J Northwest Atl Fish Sci. 2005; 37:27-45. http://dx.doi.org/10.2960/J.v35.m514
» http://dx.doi.org/10.2960/J.v35.m514 - Gökoğlu M, Teker S, Julian D. First report of thresher sharks (Alopiidae) in the Gulf of Antalya. Iran J Fish Sci. 2017; 16(3):1108-13. Available from: http://hdl.handle.net/1834/12263
» http://hdl.handle.net/1834/12263 - Hacohen-Domené A, Polanco-Vásquez F, Estupiñan-Montaño C, Graham RT. Description and characterization of the artisanal elasmobranch fishery on Guatemala’s Caribbean coast. PLoS ONE. 2020; 15(1):e0227797. https://doi.org/10.1371/journal.pone.0227797
» https://doi.org/10.1371/journal.pone.0227797 - Harry AV, Tobin AJ, Simpfendorfer CA. Age, growth and reproductive biology of the spot-tail shark, Carcharhinus sorrah, and the Australian blacktip shark, C. tilstoni, from the Great Barrier Reef World Heritage Area, north-eastern Australia. Mar Freshw Res . 2013; 64(4):277-93. http://dx.doi.org/10.1071/MF12142
» http://dx.doi.org/10.1071/MF12142 - Hazin FHV, Fischer AF, Broadhurst MK, Veras D, Oliveira PG, Burgess GH. Notes on the reproduction of Squalus megalops off northeastern Brazil. Fish Res. 2006; 79(3):251-57. https://doi.org/10.1016/j.fishres.2006.04.006
» https://doi.org/10.1016/j.fishres.2006.04.006 - Herrera M, Coello D, Cajas J. Desembarques y aspectos biológicos de Elasmobranquios en las pesquerías artesanales del Ecuador durante 2011. Boletín científico y técnico. 2012; 22:1-08.
- Ichsan M, Ula S, Simeon B, Muttaqin E, Booth H. Thresher sharks (Alopiidae) catch in the pelagic fisheries of Western Indonesia. Earth Eviron Sci. 2020; 420:012013. http://doi.org/10.1088/1755-1315/420/1/012013
» http://doi.org/10.1088/1755-1315/420/1/012013 - Jensen CF, Natanson LJ, Pratt HL Jr, Kohler N, Campana SE. The reproductive biology of the porbeage shark (Lamna nasus) in the western north Atlantic Ocean. Fish Bull (Wash D C). 2002; 100(4):727-38.
- Klimley AP. The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini Environ Biol Fish. 1987; 18:27-40. https://doi.org/10.1007/BF00002325
» https://doi.org/10.1007/BF00002325 - Liu K-M, Chang Y-T, Ni I-H, Jin C-B. Spawning per recruit analysis of the pelagic thresher shark, Alopias pelagicus, in the eastern Taiwan waters. Fish Res . 2006; 82(1-3):56-64. https://doi.org/10.1016/j.fishres.2006.08.013
» https://doi.org/10.1016/j.fishres.2006.08.013 - Liu K-M, Chen C-T, Liao T-H, Joung S-J. Age, growth, and reproduction of the pelagic thresher shark, Alopias pelagicus in the Northwestern Pacific. Copeia. 1999; 1999(1):68-74. https://doi.org/10.2307/1447386
» https://doi.org/10.2307/1447386 - Liu K-M, Chiang P-J, Chen C-T. Age and growth estimates of the bigeye thresher shark, Alopias superciliosus, in northeastern Taiwan waters. Fish Bull (Wash D C) . 1998; 96(3):482-91.
- Liu K-M, Chin C-P, Chen C-H, Chang J-H. Estimating finite rate of population increase for sharks based on vital parameters. PLoS ONE . 2015; 10(11):e0143008. https://doi.org/10.1371/journal.pone.0143008
» https://doi.org/10.1371/journal.pone.0143008 - MacFarland TW, Yates JM. Introduction to nonparametric statistics for the biological sciences using R. Switzerland: Springer; 2016.
- Martínez-Ortíz J. Tiburones del Océano Pacifico oriental. Estudio de casos. Manta: Ministerio de Agricultura, Ganadería, Acuacultura y Pesca; 2012;
- Martínez-Ortiz J, Aires-da-Silva AM, Lennert-Cody CE, Maunder MN. The Ecuadorian artisanal fishery for large pelagics: species composition and spatio-temporal dynamics. PLoS ONE . 2015; 10(8):e0135136. https://doi.org/10.1371/journal.pone.0135136
» https://doi.org/10.1371/journal.pone.0135136 - Martínez-Ortíz J, Galván-Magaña F, Carrera-Fernández M, Mendoza-Intriago D, Estupiñán-Montaño C, Cedeño-Figueroa L. Abundancia estacional de tiburones desembarcados en Manta-Ecuador. In: Martínez-Ortíz J, Galván-Mangaña F, editors. Tiburones en el Ecuador: Casos de estudio. Manta: EPESPO-PMRC; 2007. p.9-27.
- Martínez-Ortiz J, García-Domínguez M. Guía de campo. Condrictios del Ecuador. Quimeras, tiburones y rayas. Manta: Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP)/ViceMinisterio de Acuacultura y Pesca (VMAP)/Subsecretaria de Recursos Pesqueros (SRP); 2013.
- Mas F, Forselledo R, Domingo A. Length-length relationships for six pelagic shark species commonly caught in the southwestern Atlantic Ocean. Collect Vol Sci Pap. 2014; 70:2441-50.
- Mejía-Falla PA, Navia AF, Cortés E. Reproductive variables of Urotrygon rogersi (Batoidea: Urotrygonidae): a species with a triannual reproductive cycle in the eastern tropical Pacific Ocean. J Fish Biol . 2012; 80(5):1246-66. http://dx.doi.org/10.1111/j.1095-8649.2012.03237.x
» http://dx.doi.org/10.1111/j.1095-8649.2012.03237.x - Milton RC. An extended table of critical values for the Mann-Whitney (Wilcoxon) two-sample statistic. J Am Stat Assoc. 1964; 59(307):925-34. https://doi.org/10.2307/2283111
» https://doi.org/10.2307/2283111 - Moreno J, Morón J. Reproductive biology of the Bigeye Thresher Shark, Alopias superciliosus (Lowe, 1939). Mar Freshw Res . 1992; 43(1):77-86. https://doi.org/10.1071/MF9920077
» https://doi.org/10.1071/MF9920077 - Morson JM, Bochenek EA, Powell EN, Hasbrouck EC, Gius JE, Cotton CF, Gerbino K, Froehlich T. Estimating the Sex Composition of the summer flounder catch using fishery-independent data. Mar Coast Fish. 2015; 7(1):393-408. https://doi.org/10.1080/19425120.2015.1067261
» https://doi.org/10.1080/19425120.2015.1067261 - Mucientes GR, Queiroz N, Sousa LL, Tarroso P, Sims DW. Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol Lett. 2009; 5(2):156-59. https://doi.org/10.1098/rsbl.2008.0761
» https://doi.org/10.1098/rsbl.2008.0761 - Musick JA, Ellis JK, Hamlett W. Reproductive evolution of chondrichthyans. In: Hamlett WC, editor. Reproductive biology and phylogeny of chondrichthyes: sharks, batoids and chimaeras. Enfield: Science Pulishers; 2005. p.45-71.
- Musyl MK, Brill RW, Curran DS, Fragoso NM, McNaughton LM, Nielsen A, Kikkawa BS, Moyes CD. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fish Bull. 2011; 109(4):341-68.
- Najmudeen TM, Zacharia PU, Seetha PK, Sunil KTS, Radhakrishnan M, Akhildev S, Sipson A. Length-weight relationships of three species of pelagic sharks from southeastern Arabian Sea. Reg Stud Mar Sci. 2019; 29:100647. https://doi.org/10.1016/j.rsma.2019.100647
» https://doi.org/10.1016/j.rsma.2019.100647 - Natanson LJ, Gervelis BJ. The reproductive biology of the common thresher shark in the western north Atlantic Ocean. Trans Am Fish Soc. 2013; 142(6):1546-62. http://dx.doi.org/10.1080/00028487.2013.811099
» http://dx.doi.org/10.1080/00028487.2013.811099 - Navia AF, Mejía-Falla PA. Guía para la identificación de especies de tiburones y rayas comercializadas en el Pacífico colombiano. Santiago de Cali: Fundación Colombiana para la Investigación y Conservación de tiburones y rayas, SQUALUS; 2011.
- Nelson JS, Grande TC, Wilson MVH. Fishes of the World. Hoboken: John Wiley & Sons; 2016.
- Oliver S, Braccini M, Newman SJ, Harvey ES. Global patterns in the bycatch of sharks and rays. Mar Policy . 2015; 54:86-97. https://doi.org/10.1016/j.marpol.2014.12.017
» https://doi.org/10.1016/j.marpol.2014.12.017 - Oshitani S, Nakano H, Tanaka S. Age and growth of the silky shark Carcharhinus falciformis from the Pacific Ocean. Fish Sci. 2003; 69(3):456-64. http://dx.doi.org/10.1046/j.1444-2906.2003.00645.x
» http://dx.doi.org/10.1046/j.1444-2906.2003.00645.x - Piner KR, Hamel OS, Menkel JL, Wallace JR, Hutchinson CE. Age validation of canary rockfish (Sebastes pinniger) from off the Oregon coast (USA) using the bomb radiocarbon method. Can J Fish Aquat Sci. 2005; 62(5):1060-66. https://doi.org/10.1139/f05-082
» https://doi.org/10.1139/f05-082 - Polo-Silva C, Acevedo G, Siu S, Carvajal JM, Ixquiac M, Bessudo S, Suarez AM, Puentes V. Morphometric relationships for some species of elasmobranch from tropical eastern Pacific. J Appl Ichthyol. 2018; 34(1):157-61. https://doi.org/10.1111/jai.13460
» https://doi.org/10.1111/jai.13460 - Reardon MB, Márquez-Farías F, Trejo T, Clarke SC. Alopias pelagicus [Internet]. IUCN red list of threatened species; 2013. Available from: https://www.fish.gov.au/docs/SharkReport/FRDC_Alopias_pelagicus.pdf
» https://www.fish.gov.au/docs/SharkReport/FRDC_Alopias_pelagicus.pdf - Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Herman K, Jabado RW, Liu KM, Marshall A, Pacoreau N, Romanov E, Sherley RB, Winker H. Alopias pelagicus [Internet]. The IUCN Red List of Threatened Species 2019; 2019. https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T161597A68607857.en
» https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T161597A68607857.en - Romero-Caicedo AF, Carrera-Fernández M. Reproduction of the whitesnout guitarfish Rhinobatos leucorhynchus in the Ecuadorian Pacific Ocean. J Fish Biol . 2015; 87(6):1434-48. https://doi.org/10.1111/jfb.12794
» https://doi.org/10.1111/jfb.12794 - Romero-Caicedo AF, Galvan-Magaña F, Martínez-Ortiz J. Reproduction of the pelagic thresher shark Alopias pelagicus in the equatorial Pacific. J Mar Biol Assoc U K. 2014; 94(7):1501-07. https://doi.org/10.1017/S0025315414000927
» https://doi.org/10.1017/S0025315414000927 - Santana-Hernández H, Tovar-Ávila J, Valdéz-Flores JJ. Estimation of the total, fork and precaudal lengths for the silky shark, Carcharhinus falciformis (Carcharhiniformes: Carcharhinidae), from the interdorsal length. Hidrobiologica. 2014; 24(2):159-62.
- Sepulveda CA, Wegner NC, Bernal D, Graham JB. The red muscle morphology of the thresher sharks (family Alopiidae). J Exp Biol. 2005; 208(22):4255-61. https://doi.org/10.1242/jeb.01898
» https://doi.org/10.1242/jeb.01898 - Sims DW. Differences in habitat selection and reproductive strategies of male and female sharks. In: Ruckstuhl K, Neuhaus P, editors. Sexual segregation in vertebrates. Cambridge: Cambridge University Press; 2006. p.127-47. https://doi.org/10.1017/CBO9780511525629.009
» https://doi.org/10.1017/CBO9780511525629.009 - Smart J. AquaticLifeHistory: Fisheries life history analysis using contemporary methods [Internet]. R package; 2019. Available from: https://github.com/jonathansmart/AquaticLifeHistory
» https://github.com/jonathansmart/AquaticLifeHistory - Smart JJ, Chin A, Tobin AJ, Simpfendorfer CA. Multimodel approaches in shark and ray growth studies: strengths, weaknesses and the future. Fish Fish. 2016; 17(4):955-71. https://doi.org/10.1111/faf.12154
» https://doi.org/10.1111/faf.12154 - Smith SE, Rasmussen RC, Ramon DA, Cailliet GM. The biology and ecology of thresher sharks (Alopiidae). In: Pikitch EK, Camhi MD, Babcock EA, editors. Sharks of the open ocean: biology, fisheries and conservation. Oxford: Blackwell Publishing; 2008. p.60-68.
- Snelson FF Jr, Burgess GH, Roman BL. The reproductive biology of pelagic elasmobranchs. In: Pikitch EK, Camhi MD, Babcock EA, editors. Sharks of the open ocean: biology, fisheries and conservation . Oxford: Blackwell Publishing ; 2008. p.24-45.
- Springer S. Social organization of shark population. In: Gilbert PW, Matheswon RF, Rall DP, editors. Sharks, skate and rays. Baltimore: Johns Hopkins University Press; 1967. p.149-74.
- Tsai W-P, Liu K-M, Joung S-J. Demographic analysis of the pelagic thresher shark, Alopias pelagicus, in the north-western Pacific using a stochastic stage-based model. Mar Freshw Res . 2010; 61(9):1056-66. http://dx.doi.org/10.1071/Mf09303
» http://dx.doi.org/10.1071/Mf09303 - Varghese SP, Unnikrishnan N, Gulati DK, Ayoob AE. Size, sex and reproductive biology of seven pelagic sharks in the eastern Arabian Sea. J Mar Biol Assoc U K . 2016; 97(1):181-96. http://dx.doi.org/10.1017/S0025315416000217
» http://dx.doi.org/10.1017/S0025315416000217 - Varghese SP, Vijayakumaran K, Tiburtius A, Mhatre VD. Diversity, abundance and size structure of pelagic sharks caught in tuna longline survey in the Indian seas. Indian J Geomarine Sci. 2015; 44(1):26-36.
- Wearmouth VJ, Sims DW. Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv Mar Biol. 2008; 54:107-70. https://doi.org/10.1016/s0065-2881(08)00002-3
» https://doi.org/10.1016/s0065-2881(08)00002-3 - Weng KC, Block BA. Diel vertical migration of the bigeye thresher shark (Alopias superciliosus), a species possessing orbital retia mirabilia. Fish Bull . 2004; 102(1):221-29.
- White WT, Baje L, Appleyard SA, Chin A, Smart JJ, Simpfendorfer CA. Shark longline fishery of Papua New Guinea: size and species composition and spatial variation of the catches. Mar Freshw Res . 2020; 71(6):627-40. https://doi.org/10.1071/MF19191
» https://doi.org/10.1071/MF19191 - Winter ST, Fahmi, Rudianto D, Laglbauer BJL, Ender I, Simpfendorfer CA. Immature individuals dominate elasmobranch fisheries of the Bali Strait. Mar Freshw Res . 2020; 71(11):1488-1500. https://doi.org/10.1071/MF19300
» https://doi.org/10.1071/MF19300 - De Wysiecki AM, Braccini JM. Shark length-length relationships: Studying morphology allows the detection of bias in routine fisheries sampling. Reg Stud Mar Sci . 2017; 16:290-93. https://doi.org/10.1016/j.rsma.2017.10.005
» https://doi.org/10.1016/j.rsma.2017.10.005 - Young CN, Carlson JK, Hutchinson M, Kobayashi D, McCandless C, Miller MH, Teo SLH, Warren T. Status review report: common thresher (Alopias vulpinus) and bigeye thresher (Alopias superciliosus) sharks. Final Report to National Marine Fisheries Service; 2016.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Briones-Mendoza J, Carrasco-Puig P, Toala-Franco D. Reproductive biology aspects of Alopias pelagicus and A. superciliosus (Lamniformes: Alopiidae) in the Ecuadorian Pacific. Neotrop Ichthyol. 2021; 19(4):e210015. https://doi.org/10.1590/1982-0224-2021-0015
Edited by
Publication Dates
-
Publication in this collection
13 Dec 2021 -
Date of issue
2021
History
-
Received
13 Jan 2021 -
Accepted
02 July 2021