Abstract
Diet and morphology of Serrapinnus notomelas and Serrapinnus sp.1 were investigated across ontogeny, as a way to elucidate the key elements linked to the resource partitioning (a main driver for species coexistence). Fish sampling was conducted monthly between October 2010 and March 2012. Individuals were captured, identified, and classified into juvenile or adult. Our results show ontogenetic and interspecific differences in feeding abilities and morphological traits. Differences in body shape (relative area of the dorsal fin, length of head, height of the caudal peduncle, the aspect ratio of the pectoral and pelvic fin) favored divergent swimming performances (more maneuverability in S. notomelas and continuous swimming to Serrapinnus sp.1). We also observed divergences in trophic apparatus traits and correlations with different diets. In this context, it is highlighted that understanding the relationship between morphology and diet can assist in elucidating the processes that permeate the coexistence between sympatric species, and between ontogenetic periods. Besides, the relevant contribution of the measures of the trophic apparatus (gill raker length, the number of teeth cuspids, and intestinal coefficient) in trophic segregation seems to be a strong evidence in favor of the proposed discriminatory and predictive capacities of these traits.
Keywords:
Diet; Ecomorphology; Interspecific variability; Ontogeny; Trophic apparatus
Resumo
Dieta e morfologia de Serrapinnus notomelas e Serrapinnus sp.1 foram investigadas ao longo da ontogenia, como forma de elucidar os principais elementos ligados à partição de recursos (principal fator para a coexistência entre espécies). Foram realizadas coletas de peixes mensalmente entre outubro de 2010 e março de 2012. Os indivíduos foram capturados, identificados e classificados em juvenis ou adultos. Nossos resultados mostram diferenças ontogenéticas e interespecíficas na alimentação e características morfológicas. Diferenças na forma corporal (área da nadadeira dorsal, comprimento da cabeça, altura do pedúnculo caudal, proporção das nadadeiras peitorais e pélvicas) favoreceram desempenhos de natação divergentes (maior manobrabilidade para S. notomelas e natação contínua para Serrapinnus sp.1). Também observamos divergências nas características do aparato trófico e correlações com diferentes dietas. Nesse contexto, destaca-se que compreender a relação entre morfologia e dieta pode auxiliar na elucidação dos processos que permeiam a coexistência entre espécies simpátricas e entre períodos ontogenéticos. Além disso, a relevante contribuição das medidas do aparato trófico (número de cúspide nos dentes, rastros branquiais e coeficiente intestinal) na segregação trófica parece ser uma forte evidência a favor das propostas de capacidades discriminatórias e preditivas dessas características.
Palavras-chave:
Aparato trófico; Dieta; Ecomorfologia; Ontogenia; Variabilidade interespecífica
INTRODUCTION
Macrophyte stands in floodplain areas have been suggested as essential for the biological diversity maintenance, since they provide structurally complex habitats (Agostinho et al., 2007Agostinho AA, Thomaz SM, Gomes LC, Baltar SLSMA. Influence of the macrophyte Eichhornia azurea on fish assemblage of the Upper Paraná River floodplain (Brazil). Aquat Ecol. 2007; 41(4):611–19. https://doi.org/10.1007/s10452-007-9122-2
https://doi.org/10.1007/s10452-007-9122-...
; Dibble, Thomaz, 2009Dibble ED, Thomaz SM. Use of fractal dimension to assess habitat complexity and its influence on dominant invertebrates inhabiting tropical and temperate macrophytes. J Freshw Ecol. 2009; 24(1):93–102. https://doi.org/10.1080/02705060.2009.9664269
https://doi.org/10.1080/02705060.2009.96...
; Yofukuji et al., 2021Yofukuji KY, Cardozo ALP, Quirino BA, Aleixo MHF, Fugi R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia. 2021; 848:913–27. https://doi.org/10.1007/s10750-020-04501-w
https://doi.org/10.1007/s10750-020-04501...
). These environments offer favorable abiotic conditions such as lower light and temperature, acting as food sources and refuge against predators (Colares et al., 2013Colares MAM, Bonecker CC, Simões NR, Alves GM, Lansac-Tôha FA. Structure of the zooplankton communities in macrophytes stand of a Neotropical floodplain (the Paraná River, Brazil). Int Rev Hydrobiol. 2013; 98(2):89–103. https://doi.org/10.1002/iroh.201301471
https://doi.org/10.1002/iroh.201301471...
; Cunha et al., 2019Cunha ER, Winemiller KO, Silva JCB, Lopes TM, Gomes LC, Thomaz SM et al. α and β diversity of fishes in relation to a gradient of habitat structural complexity supports the role of environmental filtering in community assembly. Aquat Sci. 2019; 81(38):1–12. https://doi.org/10.1007/s00027-019-0634-3
https://doi.org/10.1007/s00027-019-0634-...
; Yofukuji et al., 2021Yofukuji KY, Cardozo ALP, Quirino BA, Aleixo MHF, Fugi R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia. 2021; 848:913–27. https://doi.org/10.1007/s10750-020-04501-w
https://doi.org/10.1007/s10750-020-04501...
). Besides, they offer substrates that are suitable for spawning and foraging, thus supporting a greater diversity of organisms (Delariva et al., 1994Delariva RL, Agostinho AA, Nakatani K, Baumgartner G. Ichthyofauna associated to aquatic macrophytes in the upper Parana River floodplain. Rev Unimar. 1994; 16(Supl. 3):41–60.; Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
; Yofukuji et al., 2021Yofukuji KY, Cardozo ALP, Quirino BA, Aleixo MHF, Fugi R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia. 2021; 848:913–27. https://doi.org/10.1007/s10750-020-04501-w
https://doi.org/10.1007/s10750-020-04501...
). In addition to species that temporarily use macrophyte stands, such as larvae and juveniles of large species, smaller-sized species with a short life cycle live in these environments (Prado et al., 2016Prado AVR, Goulart E, Pagotto JPA. Ecomorphology and use of food resources: inter- and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop Ichthyol. 2016; 14(4):e150140. https://doi.org/10.1590/1982-0224-20150140
https://doi.org/10.1590/1982-0224-201501...
). These smaller-sized species that inhabit the macrophyte stands are residents, dependent and influenced by the dynamics provided by the macrophyte stands, including possible interspecific interactions (i.e., competition for trophic resource) (Cunha et al., 2019Cunha ER, Winemiller KO, Silva JCB, Lopes TM, Gomes LC, Thomaz SM et al. α and β diversity of fishes in relation to a gradient of habitat structural complexity supports the role of environmental filtering in community assembly. Aquat Sci. 2019; 81(38):1–12. https://doi.org/10.1007/s00027-019-0634-3
https://doi.org/10.1007/s00027-019-0634-...
; Yofukuji et al., 2021Yofukuji KY, Cardozo ALP, Quirino BA, Aleixo MHF, Fugi R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia. 2021; 848:913–27. https://doi.org/10.1007/s10750-020-04501-w
https://doi.org/10.1007/s10750-020-04501...
). In this regard, the complexity of the conditions and resources noted in macrophytes acts as robust environmental filters influencing the selection of traits adjusted to the demands of food and shelter over time (Prado et al., 2016Prado AVR, Goulart E, Pagotto JPA. Ecomorphology and use of food resources: inter- and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop Ichthyol. 2016; 14(4):e150140. https://doi.org/10.1590/1982-0224-20150140
https://doi.org/10.1590/1982-0224-201501...
; Cunha et al., 2019Cunha ER, Winemiller KO, Silva JCB, Lopes TM, Gomes LC, Thomaz SM et al. α and β diversity of fishes in relation to a gradient of habitat structural complexity supports the role of environmental filtering in community assembly. Aquat Sci. 2019; 81(38):1–12. https://doi.org/10.1007/s00027-019-0634-3
https://doi.org/10.1007/s00027-019-0634-...
; Quirino et al., 2021Quirino BA, Lansac-Tôha FM, Thomaz SM, Heino J, Fugi R. Macrophyte stand complexity explains the functional α and β diversity of fish in a tropical river-floodplain. Aquat Sci. 2021; 83(12):1–14. https://doi.org/10.1007/s00027-020-00768-2
https://doi.org/10.1007/s00027-020-00768...
).
According to the classical niche theory, the effects of competition can be reduced and the coexistence can be favored when the differentiation occurs in, at least, one dimension of the niche (Hutchinson, 1961Hutchinson GE. The paradox of the plankton. Am Nat. 1961; 95(882):137–45. https://doi.org/10.1086/282171
https://doi.org/10.1086/282171...
; Leray et al., 2019Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ et al. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol. 2019; 28(10):2694–710. https://doi.org/10.1111/mec.15090
https://doi.org/10.1111/mec.15090...
). Spatial, temporal, and trophic dimensions are considered predominant factors in the structure of the communities, with food being the most important for fish, since it modulates basal activity levels (Schoener, 1974Schoener TW. Competition and the form of habitat shift. Theor Popul Biol. 1974; 6(3):265–307. https://doi.org/10.1016/0040-5809(74)90013-6
https://doi.org/10.1016/0040-5809(74)900...
; Ross, 1986Ross ST. Resource partitioning in fish assemblages: a review of field studies. Copeia. 1986; 1986(2):352–88. https://doi.org/10.2307/1444996
https://doi.org/10.2307/1444996...
; Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.; Dehnhard et al., 2020Dehnhard N, Achurch H, Clarke J, Michel LN, Southwell C, Sumner MD et al. High inter - and intraspecific niche overlap among three sympatrically breeding, closely related seabird species: Generalist foraging as an adaptation to a highly variable environment? J Anim Ecol. 2020; 89(1):104–19. https://doi.org/10.1111/1365-2656.13078
https://doi.org/10.1111/1365-2656.13078...
). Thereby, partitioning food resources is seen as a mediator process that decreases the probability of local extinction of the inferior competitor while enables long-term species’ coexistence (Moncayo-Estrada et al., 2011Moncayo-Estrada R, Lind OT, Escalera-Gallardo C. Trophic interactions among sympatric zooplanktivorous fish species in volume change conditions in a large, shallow, tropical lake. Neotrop Ichthyol. 2011; 9(1):169–76. https://doi.org/10.1590/S1679-62252011005000003
https://doi.org/10.1590/S1679-6225201100...
; Walker et al., 2013Walker RH, Kluender ER, Inebnit TE, Adams SR. Differences in diet and feeding ecology of similar-sized spotted (Lepisosteus oculatus) and shortnose (Lepisosteus platostomus) gars during flooding of a south-eastern US river. Ecol Freshw Fish. 2013; 22(4):617–25. https://doi.org/10.1111/eff.12066
https://doi.org/10.1111/eff.12066...
).
Resource partitioning is shaped by distinct evolutionary processes. The morphology and ecological opportunities are interpolated and underlying pathways (Alexander, 1967Alexander RM. Functional design in fishes. London: Hutchinson University; 1967.; Chase, Leibold, 2003Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.; Silva et al., 2016Silva NCS, Costa AJL, Louvise J, Soares BE, Reis VCS, Albrecht MP et al. Resource partitioning and ecomorphological variation in two syntopic species of Lebiasinidae (Characiformes) in an Amazonian stream. Acta Amaz. 2016; 46(1):25–36. https://doi.org/10.1590/1809-4392201501024
https://doi.org/10.1590/1809-43922015010...
; Neves et al., 2021Neves MP, Kratina P, Delariva RL, Jones JI, Fialho CB. Seasonal feeding plasticity can facilitate coexistence of dominant omnivores in Neotropical streams. Rev Fish Biol Fish. 2021; 31:417–32. https://doi.org/10.1007/s11160-021-09648-w
https://doi.org/10.1007/s11160-021-09648...
). More conservative intrinsic aspects, such as morphological traits, shape and constrain an organism’s ability to perform tasks related to resource use (Carroll, 2001Carroll SB. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001; 409:1102–09. https://doi.org/10.1038/35059227
https://doi.org/10.1038/35059227...
). The body shape reveals a lot about the locomotion performance, which, in a way, is the basis of how fishes can access food resources (Gatz, 1979Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane stud zool bot. 1979; 21(2): 91–124.; Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.). Some studies have indicated that the body morphology reflects differences in swimming performances, such as continuous swimming, acceleration, and maneuverability (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
; Casatti, Castro, 2006Casatti L, Castro RMC. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotrop Ichthyol. 2006; 4(2):203–14. https://doi.org/10.1590/S1679-62252006000200006
https://doi.org/10.1590/S1679-6225200600...
). It also reflects the net energy obtained from an incorporated food source as well as the best performance in the capture, cost and efficiency in the selection, digestion, and absorption (Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.). In this sense, aspects of the trophic apparatus - such as the number of teeth cuspids, number and length of gill rakers, and intestine length - can be excellent indicators in elucidating dietary preferences (Bonato et al., 2017Bonato KO, Burress ED, Fialho CB. Dietary differentiation in relation to mouth and tooth morphology of a neotropical characid fish community. Zool Anz. 2017; 267:31–40. https://doi.org/10.1016/j.jcz.2017.01.003
https://doi.org/10.1016/j.jcz.2017.01.00...
; Ohara et al., 2017; Delariva, Neves, 2020Delariva RL, Neves MP. Morphological traits correlated with resource partitioning among small characin fish species coexisting in a Neotropical river. Ecol Freshw Fish. 2020; 29(4):640–53. https://doi.org/10.1111/eff.12540
https://doi.org/10.1111/eff.12540...
; Nascimento et al., 2020Nascimento CP, Santos NCL, Dal Vesco BM, Gomes LC. Trophic morphology features allow Astyanax endemic species coexistence in a Neotropical river system. J Fish Biol. 2020; 97(3):776–84. https://doi.org/10.1111/jfb.14433
https://doi.org/10.1111/jfb.14433...
). Despite such a strong theoretical background (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
; Carroll, 2001Carroll SB. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001; 409:1102–09. https://doi.org/10.1038/35059227
https://doi.org/10.1038/35059227...
; Casatti, Castro, 2006Casatti L, Castro RMC. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotrop Ichthyol. 2006; 4(2):203–14. https://doi.org/10.1590/S1679-62252006000200006
https://doi.org/10.1590/S1679-6225200600...
), studies showing a direct link between the diet divergence and specific morphological adaptations are still scarce. Then, the combined use of morphological traits can be a robust proxy for determining potential divergent uses of habitat and obtaining of food in structured habitats, such as macrophyte stands.
Differentiation in the foraging niche can also occur at the expense of changes in morphology during the ontogenetic development, especially when it is derived from allometry (Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.). In fact, morphological traits can shape the diet through their influence on the feeding capability of a fish (Lukoschek, McCormick, 2001Lukoschek V, McCormick MI. Ontogeny of diet changes in a tropical benthic carnivorous fish, Parupeneus barberinus (Mullidae): relationship between foraging behaviour, habitat use, jaw size, and prey selection. Mar Biol. 2001; 138:1099–113. https://doi.org/10.1007/s002270000530
https://doi.org/10.1007/s002270000530...
; Sánchez-Hernández, Cobo, 2016Sánchez-Hernández J, Cobo F. Ontogenetic shifts in terrestrial reliance of stream-dwelling brown trout. J Limnol. 2016; 75(2):409–14. https://doi.org/10.4081/jlimnol.2016.1322
https://doi.org/10.4081/jlimnol.2016.132...
). Such relationships have been largely documented for most fish clades (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
; Willis et al., 2005Willis SC, Winemiller KO, Lopez-Fernandez H. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia. 2005; 142:284–95. https://doi.org/10.1007/s00442-004-1723-z
https://doi.org/10.1007/s00442-004-1723-...
). For example, larger head and mouth and longer fins allow the consumption of larger food items and foraging over long distances, respectively (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
; Willis et al., 2005Willis SC, Winemiller KO, Lopez-Fernandez H. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia. 2005; 142:284–95. https://doi.org/10.1007/s00442-004-1723-z
https://doi.org/10.1007/s00442-004-1723-...
). Smaller food items, such as phytoplankton, zooplankton, and insects, are consumed in the early stages of life cycles or by smaller size classes (Neves et al., 2015Neves MP, Delariva RL, Guimarães ATB, Sanches PV. Carnivory during ontogeny of the Plagioscion squamosissimus: a successful non-native fish in a lentic environment of the Upper Paraná River basin. PLoS One. 2015; 10(11):e0141651. https://doi.org/10.1371/journal.pone.0141651
https://doi.org/10.1371/journal.pone.014...
; Kliemann et al., 2019Kliemann BCK, Baldasso MC, Pini SFR, Makrakis MC, Makrakis S, Delariva RL. Assessing the diet and trophic niche breadth of an omnivorous fish (Glanidium ribeiroi) in subtropical lotic environments: intraspecific and ontogenic responses to spatial variations. Mar Freshw Res. 2019; 70(8):1116–18. https://doi.org/10.1071/MF18149
https://doi.org/10.1071/MF18149...
; Sánchez-Hernández et al., 2019Sánchez-Hernández J, Nunn AD, Adams CE, Amundsen PA. Causes and consequences of ontogenetic dietary shifts: a global synthesis using fish models. Biol Rev Camb Philos Soc. 2019; 94(2):539–54. https://doi.org/10.1111/brv.12468
https://doi.org/10.1111/brv.12468...
). Besides, it has been empirically well established that different impositions of energy requirements between juveniles and adults can determine changes in the diet throughout the development (Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.; Keppeler et al., 2015Keppeler FW, Lanés LEK, Rolon AS, Stenert C, Lehmann P, Reichard M et al. The morphology-diet relationship and its role in the coexistence of two species of annual fishes. Ecol Freshw Fish. 2015; 24(1):77–90. https://doi.org/10.1111/eff.12127
https://doi.org/10.1111/eff.12127...
). Therefore, changes in the foraging capacity and consequent food selectivity occur in a way that corresponds to functional expectations throughout the life cycle (Keppeler et al., 2015Keppeler FW, Lanés LEK, Rolon AS, Stenert C, Lehmann P, Reichard M et al. The morphology-diet relationship and its role in the coexistence of two species of annual fishes. Ecol Freshw Fish. 2015; 24(1):77–90. https://doi.org/10.1111/eff.12127
https://doi.org/10.1111/eff.12127...
).
In order to provide support for the niche divergence hypothesis, studying the ontogenetic differentiation of sympatric and congeneric species is especially useful. Serrapinnus notomelas (Eigenmann, 1915) and Serrapinnus sp.1 are the most abundant species in isolated lakes of the upper Paraná River (Delariva et al., 1994Delariva RL, Agostinho AA, Nakatani K, Baumgartner G. Ichthyofauna associated to aquatic macrophytes in the upper Parana River floodplain. Rev Unimar. 1994; 16(Supl. 3):41–60.; Piana et al., 2006Piana PA, Gomes LC, Cortez EM. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná River floodplain lagoons. Neotrop Ichthyol. 2006; 4(1):81–86. https://doi.org/10.1590/S1679-62252006000100008
https://doi.org/10.1590/S1679-6225200600...
). Furthermore, these small fishes are residents of the macrophyte stands (Pelicice et al., 2005Pelicice FM, Agostinho AA, Thomaz SM. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecol (Montrouge). 2005; 27(1):9–16. https://doi.org/10.1016/j.actao.2004.08.004
https://doi.org/10.1016/j.actao.2004.08....
; Quirino et al., 2018Quirino BA, Carniatto N, Thomaz SM, Fugi R. Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish. 2018; 28(1):97–109. https://doi.org/10.1111/eff.12434
https://doi.org/10.1111/eff.12434...
). Despite several studies on diet and types of preferential macrophytes used by Serrapinnus species in the upper Paraná River floodplain (Pelicice et al., 2005Pelicice FM, Agostinho AA, Thomaz SM. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecol (Montrouge). 2005; 27(1):9–16. https://doi.org/10.1016/j.actao.2004.08.004
https://doi.org/10.1016/j.actao.2004.08....
; Piana et al., 2006Piana PA, Gomes LC, Cortez EM. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná River floodplain lagoons. Neotrop Ichthyol. 2006; 4(1):81–86. https://doi.org/10.1590/S1679-62252006000100008
https://doi.org/10.1590/S1679-6225200600...
; Alves et al., 2011Alves GHZ, Tófoli RM, Novakowski GC, Hahn NS. Food partitioning between sympatric species of Serrapinnus (Osteichthyes, Cheirodontinae) in a tropical stream. Acta Sci Biol Sci. 2011; 33(2):153–59. https://doi.org/10.4025/actascibiolsci.v33i2.7593
https://doi.org/10.4025/actascibiolsci.v...
; Carniatto et al., 2012Carniatto N, Fugi R, Cantanhêde G, Gubiani ÉA, Hahn NS. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol Bras. 2012; 24(4):363–72. https://doi.org/10.1590/S2179-975X2013005000007
https://doi.org/10.1590/S2179-975X201300...
; Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
, 2018Quirino BA, Carniatto N, Thomaz SM, Fugi R. Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish. 2018; 28(1):97–109. https://doi.org/10.1111/eff.12434
https://doi.org/10.1111/eff.12434...
), information on the morphological traits and their implications for the swimming performance (sensuCasatti, Castro, 2006Casatti L, Castro RMC. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotrop Ichthyol. 2006; 4(2):203–14. https://doi.org/10.1590/S1679-62252006000200006
https://doi.org/10.1590/S1679-6225200600...
) and the consequent shaping of the genus feeding is lacking.
Here, we focus on the diet and morphology of S. notomelas and Serrapinnus sp.1 variation across ontogeny, to elucidate the key elements linked to the resource partitioning and, therefore, understand the main driver of the coexistence of these species in macrophyte stands. Specifically, we tested whether: i) There are differences in the diet and in the trophic niche breadth between species and ontogeny; ii) Differences in ecomorphological indices related to swimming occur between species and ontogeny; and iii) Trophic apparatus (gill raker length, the number of teeth cuspids, and intestinal coefficient) differs between species. Since according to the classic niche theory, the variation in a niche dimension can avoid competition and favor coexistence Hutchinson, 1961Hutchinson GE. The paradox of the plankton. Am Nat. 1961; 95(882):137–45. https://doi.org/10.1086/282171
https://doi.org/10.1086/282171...
), we predicted that these species should differ in both morphology and diet during ontogeny. We expect to find differences in ecomorphological indices related to fin areas, body height and peduncle. When divergent, these morphological traits reflect in a greater efficiency in continuous swimming or maneuverability. They are also key indicators for the access of the fish to prey in macrophyte stands. We also hope to find differences in the trophic apparatus, especially those traits related to the acquisition (number of teeth cuspids and gill rakers) and the absorption of food (intestine length). Correlations between combined morphological traits and the diet can primarily reveal the microhabitat occupation by individuals (juveniles and adults) and species.
MATERIAL AND METHODS
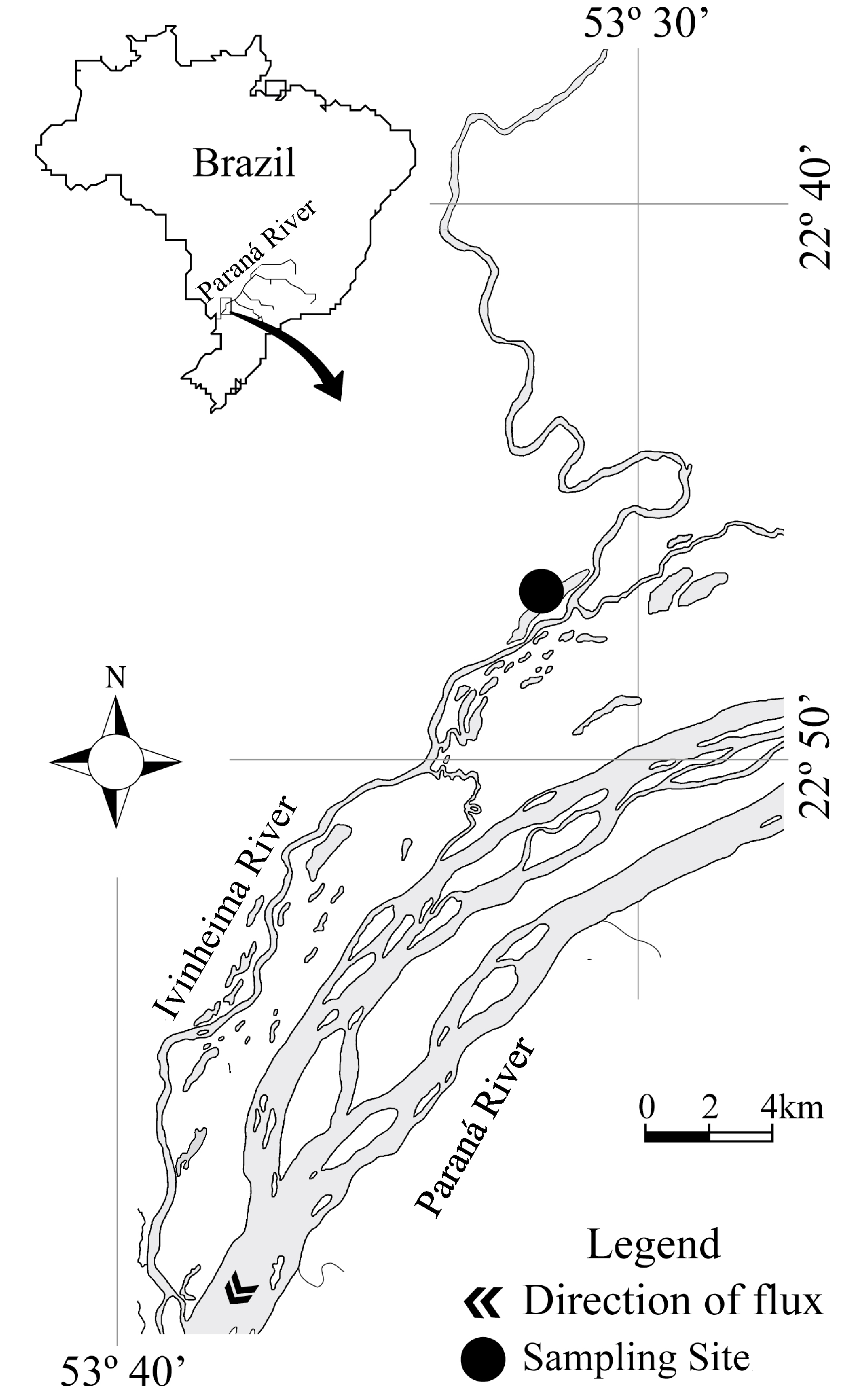
Study area. This study was conducted in one lake permanently connected to Ivinheima River, a tributary of the upper Paraná River floodplain (Fig. 1). The Finado Raimundo Lake (22°47’40”S 53°32’14”W) is shallow (3 m on average) and has approximately 3,700 m length and 400 m width. The lake presents extensive macrophyte stands colonizing its littoral region. Eichhornia azurea, a floating aquatic macrophyte, is the predominant species, and Eichhornia crassipes, less abundant, is a free-floating species like the first concerning morphology. Polygonum spp., an emergent macrophyte also presents a great abundance.
The connectivity of the lake with the main river channel promotes environments with fluctuations in the water level, which favors the establishment of macrophytes with high and intermediate complexity (Thomaz et al., 2009Thomaz SM, Carvalho P, Padial AA, Kobayashi JT. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Braz J Biol. 2009; 69(2 suppl):617–25. https://doi.org/10.1590/s1519-69842009000300016
https://doi.org/10.1590/s1519-6984200900...
). These, in turn, are more stable habitats and present higher density and richness of fish species, including species of the genus Serrapinnus (Dias et al., 2017aDias RM, Ortega JCG, Gomes LC, Agostinho AA. Trophic relationships in fish assemblages of Neotropical floodplain lakes: selectivity and feeding overlap mediated by food availability. Iheringia Ser Zool. 2017a; 107:e2017035. https://doi.org/10.1590/1678-4766e2017035
https://doi.org/10.1590/1678-4766e201703...
).
Sampling. Fish sampling was conducted monthly between October 2010 and March 2012, however only in July, August, and September 2011 (dry season, Quirino et al., 2018Quirino BA, Carniatto N, Thomaz SM, Fugi R. Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish. 2018; 28(1):97–109. https://doi.org/10.1111/eff.12434
https://doi.org/10.1111/eff.12434...
) was possible to observe the co-occurrence of the two species of Serrapinnus selected for this study in the macrophyte stands, with a similar number of individuals for each group (juveniles and adults) (Fig. 2).
Study area: location of sampling site in the upper Paraná River floodplain, Mato Grosso do Sul State, Brazil.
Number of individuals from Serrapinnus notomelas and Serrapinnus sp.1 sampled concerning precipitation (mm) between October/2010 and March/2012 in a lake in the upper Paraná River floodplain, Brazil. SnA = S. notomelas Adult; SnJ = S. notomelas juvenile; Sp1A = Serrapinnus sp.1 adult; Sp1J = Serrapinnus sp.1 juvenile.
In the Finado Raimundo Lake, three macrophyte stands about two meters long were selected randomly after visual inspection of the coastal region. For each stand, a slow and cautious approach was performed so as not to scare off fish (active sampling) and using a sieve (dimensions = 1.5 mx 1.0 m; mesh size = 0.5 mm; Nakatani et al., 2001Nakatani K, Agostinho AA, Baumgartner G, Bialetzki A, Sanches PV, Makrakis MC et al. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá: EDUEM; 2001.). It was pushed under the macrophyte stands and swiftly lifted to the surface to trap fish, resulting in three samples/month (one each stand). The juveniles and adults caught (total number/month) were euthanized with clove oil and fixed in 4% formaldehyde buffered with calcium carbonate. The project was approved by the Committee for Animal Ethics and Experimental at Universidade Estadual de Maringá (UEM) under protocol 123/2010. License for collection nº 09/2005 (IMAP - Instituto de Meio Ambiente Pantanal, N° 23/304974/2002).
Laboratory procedures. The individuals were identified according to Ota et al., (2018)Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: Revised, annotated and updated. Neotrop Ichthyol. 2018; 16(2):e170094. https://doi.org/10.1590/1982-0224-20170094
https://doi.org/10.1590/1982-0224-201700...
and the confirmation of the identity of the specimens collected was made by a specialist in the group (Dr. Carla Simone Pavanelli, Nupélia/UEM), including the distinction of Serrapinnus sp.1 from the other species. The individuals were classified in juvenile or adult (Fuiman, Werner, 2002Fuiman LA, Werner RG, editors. Fishery science: the unique contributions of early life stages. Oxford: Blackwell Science; 2002.) according to the size of the first maturation, and this value was obtained inVazzoler, 1996Vazzoler AEAM. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá: EDUEM;1996.. Voucher specimens were deposited in the fish collection Nupélia/UEM (S. notomelas, NUP 11249 and NUP 4173; Serrapinnus sp.1, NUP 7571).
Diet. The stomach contents of 121 individuals were analyzed, under a stereomicroscope and optical microscope, to identify food items consumed by individuals. The food items were quantified using the volumetric method (displacement of each food item from the stomach contents that is measured, usually on some type of graduated measuring device) (Hyslop, 1980Hyslop EJ. Stomach contents analysis – a review of methods and their application. J Fish Biol. 1980; 17(4):411–29. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
https://doi.org/10.1111/j.1095-8649.1980...
). In a gridded Petri dish, the food items were compressed with glass slides until 1 mm height. The number of quadrants occupied by each food item on the dish was multiplied by 0.001 to obtain volumes in milliliters as proposed by Hellawell, Abel, (1971)Hellawell JM, Abel R. A rapid volumetric method for the analysis of the food of fishes. J Fish Biol. 1971; 3(1):29–37. https://doi.org/10.1111/j.1095-8649.1971.tb05903.x
https://doi.org/10.1111/j.1095-8649.1971...
. Food items were identified to the lowest possible taxonomic level according to the literature (Bicudo, Bicudo, 1970Bicudo CEM, Bicudo RMT. Algas de águas continentais brasileiras. São Paulo: Fundação Brasileira para o Desenvolvimento do Ensino de Ciências; 1970.; Mugnai et al., 2010Mugnai R, Nessimian JL, Baptista DF. Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro. Rio de Janeiro: Technical books; 2010.).
Morphology. Thirty individuals of each species and development period (juvenile and adult) were used for morphology, except when the total number of individuals was less (i.e., when the total number of individuals was less than 30, we used the number of individuals available). To evaluate the body morphology, we performed 27 linear morphometric measurements and areas related to the trunk, fin, head, eye, and mouth. Morphological variables were measured, whenever possible, on the left side of specimens. Linear morphometric measurements were obtained with the aid of a digital caliper (precision of 0.01 mm) while the areas were calculated in the AutoCAD 2015 software. The fins and eyes were drawn in plastic material, digitized, and inserted into the program (https://www.autodesk.com/education/free-software/featured, accessed February 27, 2019).
From the linear morphological measurements and the measured areas, 16 ecomorphological indices were calculated. These indices indicate the habitat use, locomotion, and foraging and are relevant because they minimize the effect of body size, focusing mainly on body shape and structures (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
). Additionally, we measured traits related to the trophic apparatus, whose characteristics can be considered a proxy for the performance of food capture and processing: number of teeth cuspids, gill rakers, and intestine length. The number of teeth cuspids was counted for each specimen with the assistance of a stereoscopic microscope. The first left gill arch was carefully extracted from everyone and the length of the gill rakers was measured with the support of an optical microscope. The digestive tract was dissected, and the intestinal length was measured using a digital caliper (accuracy: 0.01 mm).
Morphological traits (ecomorphological indices and trophic apparatus) information is provided in S1.
Data analysis. The volume (ml) matrix of the food items consumed was used for the analysis of diet data. To verify differences in diet between species and ontogeny (juvenile and adult) was used the Permutational Multivariate Analysis of Variance (PERMANOVA). This analysis using the Bray-Curtis dissimilarity index, with 9,999 random permutations (Anderson, 2001Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001; 26(1):32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
https://doi.org/10.1111/j.1442-9993.2001...
). Additionally, the Similarity Percentage Method (SIMPER) was used to verify which food items contributed to the difference observed between species (S. notomelas and Serrapinnus sp.1) and ontogeny (juvenile and adult). All possible pairs of samples were compared using the Bray-Curtis measure (Clarke, 1993Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993; 18(1):117–43. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
https://doi.org/10.1111/j.1442-9993.1993...
).
To test for differences in the trophic niche between ontogeny (juvenile and adult) and between the species (S. notomelas and Serrapinnus sp.1), the trophic niche breadth was calculated using the Permutational Analysis of the Multivariate Dispersion (PERMDISP; Anderson, 2006Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006; 62(1):245–53. https://doi.org/10.1111/j.1541-0420.2005.00440.x
https://doi.org/10.1111/j.1541-0420.2005...
). Moreover, through this analysis, the variation in the diet among individuals of a population is observed since the increase in niche breadth in a population can occur if all individuals increase the niche breadth or if the variation in diet among individuals of the population increases (Abbey-Lee et al., 2013Abbey-Lee RN, Gaiser EE, Trexler JC. Relative roles of dispersal dynamics and competition in determining the isotopic niche breadth of a wetland fish. Freshw Biol. 2013; 58(4):780–92. https://doi.org/10.1111/fwb.12084
https://doi.org/10.1111/fwb.12084...
). In this way, the PERMDISP shows differences in the diet breadth by measuring the spatial dispersion of the diets of the population, using a matrix of similarity of diet (Correa, Winemiller, 2014Correa SB, Winemiller KO. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology. 2014; 95(1):210–24. https://doi.org/10.1890/13-0393.1
https://doi.org/10.1890/13-0393.1...
). By PERMDISP, the distance to the centroid of a group defined a priori is calculated, in this case the species–ontogeny, through a Principal Coordinate Analysis (PCoA). The calculation of the centroid of the group was performed using the dissimilarity measure of Bray-Curtis, allowing the comparison of the average dissimilarity in n individual observations within the group. Here, distance to the centroid (D) corresponds to trophic niche breadth. The assumption was that differences in the distance between species indicate that some species have more restricted or broader diets than others (Correa, Winemiller, 2014Correa SB, Winemiller KO. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology. 2014; 95(1):210–24. https://doi.org/10.1890/13-0393.1
https://doi.org/10.1890/13-0393.1...
; Silva et al., 2017Silva JC, Gubiani ÉA, Neves MP, Delariva RL. Coexisting small fish species in lotic neotropical environments: evidence of trophic niche differentiation. Aquat Ecol. 2017; 51:275–88. https://doi.org/10.1007/s10452-017-9616-5
https://doi.org/10.1007/s10452-017-9616-...
). To test the null hypothesis that the trophic niche breadth did not differ among the groups (species/ontogeny: juvenile and adult), an F statist was calculated to compare the average distance of each sample to the median of the group. Subsequently, the p-value was obtained through 9,999 permutations of the residuals of the least-squares (Anderson, 2006Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006; 62(1):245–53. https://doi.org/10.1111/j.1541-0420.2005.00440.x
https://doi.org/10.1111/j.1541-0420.2005...
). Post hoc pairwise comparisons were made by Tukey’s honest significant difference method.
To verify possible morphological divergences in body shape and trophic traits, we used the Canonical Variate Analysis (CVA). For this analysis the morphological data for 94 individuals was used, with a similar proportion of individuals of each period of development (26 juveniles and 20 adults of S. notomelas, 28 juveniles and 20 adults of Serrapinnus sp.1). Nineteen morphological traits were included in the CVA. To equal weight each trait, the data were standardized using a z-transformation; thus, the mean of each measure was equal to 0, and its standard deviation was equal to 1 (Villéger et al., 2010Villéger S, Miranda JR, Hernández DF, Mouillot D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol Appl. 2010; 20(6):1512–22. https://doi.org/10.1890/09-1310.1
https://doi.org/10.1890/09-1310.1...
). Canonical Variate Analysis (CVA) is used to describe differences among the group means (Zelditch et al., 2004Zelditch ML, Swiderski DL, Sheets HD. Geometric morphometrics for biologists: a primer. London: Academic Press; 2004.), and allows us to find the number of significant dimensions of the group space. In this way, the first axes account for all relevant information, and the other axes represent only noise (Mardia et al., 1979Mardia KV, Kent TJ, Bibby JM. Multivariate analysis. San Diego: Academic Press; 1979.). To verify if the morphological differences were correlated with diet, Spearman’s Correlation Coefficients were estimated for all morphological traits and main food items displayed in the SIMPER.
The level of statistical significance for all analyses was p < 0.05. The PERMANOVA, SIMPER, and CVA were performed in the PAST program (ver. 2.08, Paleontological Statistics Software, https://palaeo-electronica.org/2001 _1/past/issue1_01.htm accessed February 19, 2019). PERMDISP and Spearman’s correlation coefficients were run using the R Programming Environment (R Foundation for Statistical Computing, Vienna, Austria) with the help of the Vegan (Oksanen, 2015Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial [Internet]. R package; 2015. Available from: https://www.mooreecology.com/uploads/2/4/2/1/24213970/vegantutor.pdf
https://www.mooreecology.com/uploads/2/4...
), Corrplot (Wei, Simko, 2017Wei T, Simko V. “corrplot”: Visualization of a Correlation Matrix [Internet]. R package; 2017. Available from https://github.com/taiyun/corrplot
https://github.com/taiyun/corrplot...
), and Hmisc package (Harrell Jr, 2020Harrell Jr FE. Package “Hmisc”[Internet]. R package; 2020. Available from https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf
https://cran.r-project.org/web/packages/...
).
RESULTS
The stomach contents of 121 individuals belonging to two species, S. notomelas and Serrapinnus sp.1, were analyzed (Tab. 1). From the stomach content analysis, different feeding patterns was observed, with S. notomelas individuals consuming algae from several taxa, and Serrapinnus sp.1 (mostly juveniles) exhibited higher proportions of animal origin items, such as Copepoda and other zooplankton (Tab. 1).
Food items consumed by juveniles and adults of Serrapinnus notomelas and Serrapinnus sp.1 in a lake in the upper Paraná River floodplain, Brazil. In parenthesis there are the number of analyzed stomachs. Values are based on the percentage data of the volume of food items. *Represents values less than 0.01. The most consumed food items are in bold.
Significant differences were observed in the diet between S. notomelas and Serrapinnus sp.1 (PERMANOVA two way, F = 7.67, p = 0.0001) and between juveniles and adults of both species (PERMANOVA two way, F = 4.88, p = 0.0001). Among the food items that composed the diet of species, Cyanophyta (algae) and zooplankton were the most relevant important items that contributed to the observed differences between species and ontogeny (juvenile and adult). Juveniles and adults of S. notomelas and adults of Serrapinnus sp.1 mainly consumed Cyanophyta (algae), whereas juveniles of Serrapinnus sp.1 mainly consumed other zooplankton (Tab. 2).
Significant differences were observed in the average niche breadth (distance to centroid) between species and ontogeny (juvenile and adult) (PERMDISP, F = 22.918, p < 0.01). Among the species, a greater average niche breadth was observed for Serrapinnus sp.1. However, considering the ontogeny for each species, average niche breadth was higher for juveniles of S. notomelas and adults of Serrapinnus sp.1 (Fig. 3).
Results of the dissimilarity analysis (SIMPER) for the proportion of food items of Serrapinnus notomelas and Serrapinnus sp.1 between juveniles and adults in a lake in the upper Paraná River floodplain, Brazil.
Variation in the diet breadth of Serrapinnus notomelas and Serrapinnus sp.1 using PERMDISP, for the juveniles and adults in a lake in the upper Paraná River floodplain, Brazil. Boxes represent the 25th and 75th quartiles and demonstrate the individual variation of the trophic niche. The horizontal bars in each box represent the average niche breadth. Whiskers indicate the range and individual symbols indicate outliers. J=juveniles; A= adults.
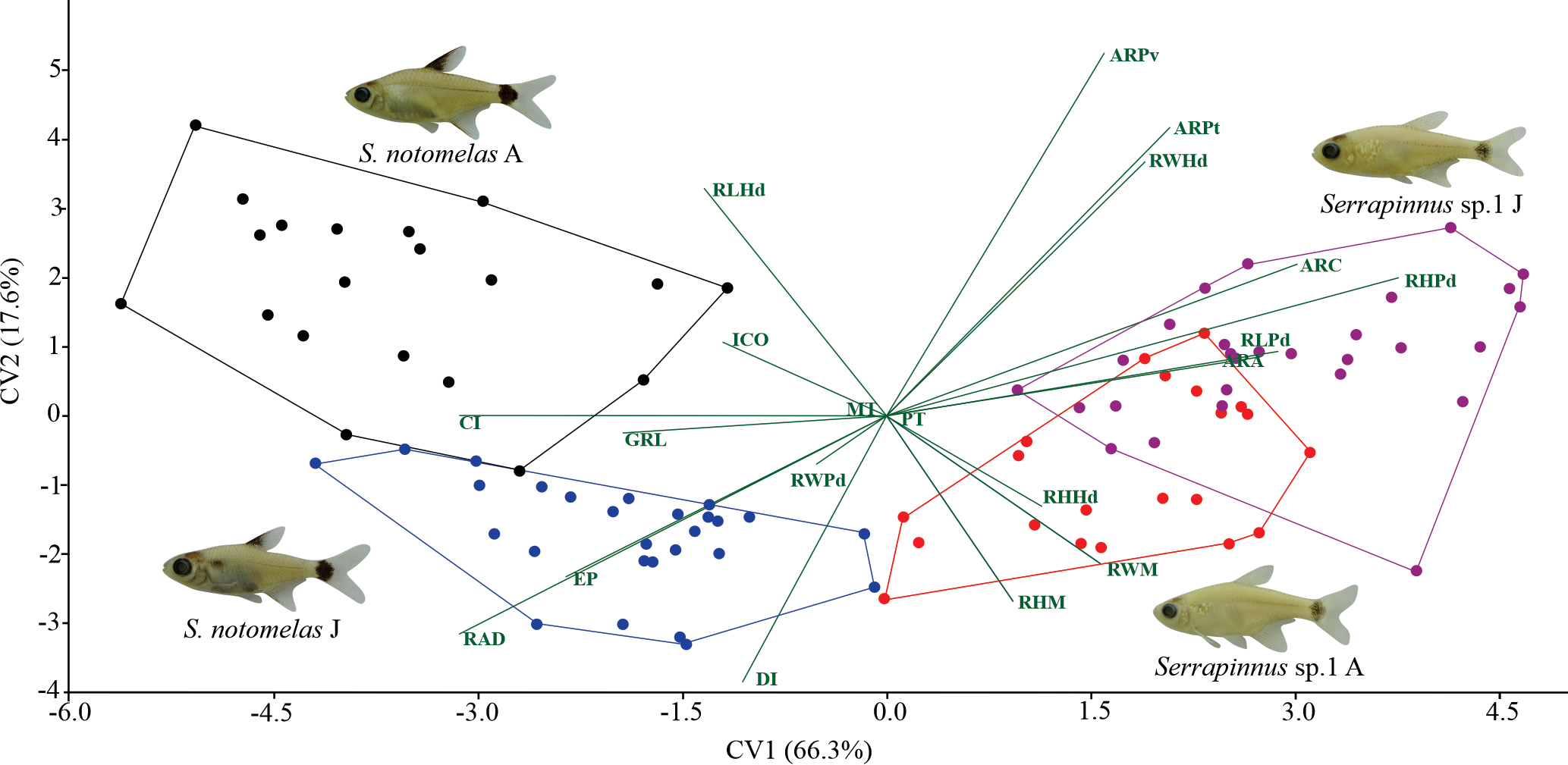
Canonical Variate Analysis of morphological traits demonstrated inter-intraspecific differences. The negative CV1 (66.3%) scores segregated S. notomelas due to the intestinal coefficient, the relative area of the dorsal fin, relative length of head, and gill raker length. In contrast, the positive scores segregated Serrapinnus sp.1 due to the relative height of the caudal peduncle, the aspect ratio of the pectoral and pelvic fin. The CV2 (17.6%) scores segregated adults and juveniles, mainly for S. notomelas. Negative scores related to the S. notomelas juveniles with relative area of the dorsal fin, and relative position of eye; and Serrapinnus sp.1 adult with a relative height of mouth and relative height of head. Positive scores related to the intestinal coefficient with S. notomelas adult; and the aspect ratio of the pelvic fin, the relative width of the head and relative height of the peduncle with to Serrapinnus sp.1 juvenile (Tab. 3; Fig. 4).
Results of the Canonical Variate Analysis (CVA) of the morphological traits for Serrapinnus notomelas and Serrapinnus sp.1 in a lake in the upper Paraná River floodplain, Brazil. The eigenvalues for each axis and the percentage of the variance explained by each of CV1 and CV2 are shown. The five highest positive and negative values of the morphological traits were highlighted in bold and selected for interpretation.
Spearman correlation coefficients between food items and morphological traits showed significant negative and positive correlations (Fig. 5). Algae presented a negative correlation with aspect ratio of the pelvic fin (r = -0.22; p = 0.03); and positive correlation with the relative area of dorsal fin (r = 0.28; p = 0.006). In turn, zooplankton was correlated negatively with compression index (r = -0.25; p = 0.01), the relative area of dorsal fin (r = -0.25; p = 0.01), multicuspid teeth (r = -0.34; p = 0.0007), and gill raker length (r = -0.26; p = 0.01); and positively correlated with relative height of caudal peduncle (r = 0.27; p = 0.006), aspect ratio of the anal fin (r = 0.24; p = 0.02), aspect ratio of the pectoral fin (r = 0.38; p = 0.0001), relative width of the head (r = 0.25; p = 0.01) and pentacuspid teeth (r = 0.34; p = 0.0007) (Fig. 5).
Canonical variate analysis illustrating differences in morphological traits for the Serrapinnus notomelas and Serrapinnus sp.1 in a lake in the upper Paraná River floodplain, Brazil. CI – Compression index; DI – Depression index; RLPd – Relative lenght of caudal peduncule; RHPd – Relative height of caudal peduncule; RWPd –Relative width of caudal peduncule; RAD – Relative area of dorsal fin; ARC – Aspect ratio of caudal fin; ARA – Aspect ratio of anal fin; ARPt – Aspect ratio of pectoral fin; ARPv – Aspect ratio of pelvic fin; RLHd – Relative length of head; RHHd – Relative height of head; RWHd – Relative width of head; RHM – Relative height of mouth; RWM – Relative width of mouth; EP – Relative position of eye; MT – multicuspid teeth; PT – pentacuspid teeth; ICO – Intestinal coefficient; GRL – Gill raker length.
Graph of Spearman’s correlation coefficient calculated between the morphological traits indicated by CVA and main food items consumed by Serrapinnus notomelas and Serrapinnus sp.1 in a lake in the upper Paraná River floodplain, Brazil. Values of r and p indicate the correlation and statistical significance, respectively. Positive correlations are represented by blue color and negative correlations by red color. ALG – algae; ZOO – zooplankton; DI – Depression index; CI – Compression index; RLPd – Relative lenght of caudal peduncule; RHPd – Relative height of caudal peduncule; RWPd – Relative width of caudal peduncule; RAD – Relative area of dorsal fin; ARC – Aspect ratio of caudal fin; ARA – Aspect ratio of anal fin; ARPt – Aspect ratio of pectoral fin; ARPv – Aspect ratio of pelvic fin; RLHd – Relative length of head; RHHd – Relative height of head; RWHd – Relative width of head; RHM – Relative height of mouth; RWM – Relative width of mouth; EP – Relative position of eye; MT – multicuspid teeth; PT – pentacuspid teeth; ICO – Intestinal coefficient; GRL – Gill raker length.
DISCUSSION
Inter and intraspecific (juveniles and adults) differences in diet, trophic niche breadth, and morphological traits confirm the differentiation of trophic niche between the sympatric and congeneric species that we evaluated here. In this way, despite the morphological similarity (phylogenetic conservatism; characids with compressed bodies, lateral eyes, and lateral pectoral fins), inter and intraspecific differences in body shape favor different swimming abilities (more efficient continuous swimming or maneuverability) with implications for the segregation of microhabitat and food use within the macrophyte stands. Moreover, we observed relevant relationships between the diet and the trophic apparatus morphology (i.e., gill raker length, the number of teeth cuspids, and intestinal coefficient). Overall, our results reveal that the species studied exhibited morphological differences that resulted in food resource partitioning, corroborating the predictions of the niche theory (Hutchinson, 1961Hutchinson GE. The paradox of the plankton. Am Nat. 1961; 95(882):137–45. https://doi.org/10.1086/282171
https://doi.org/10.1086/282171...
; Leray et al., 2019Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ et al. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol. 2019; 28(10):2694–710. https://doi.org/10.1111/mec.15090
https://doi.org/10.1111/mec.15090...
). Since the natural selection acts upon species that can maintain themselves in an environment while they exploit it (Chase, Leibold, 2003Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.), the observed segregation and high seasonality in the availability of resources in these macrophyte stands (Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
) should permeate the dynamics of competitors (intra and interspecific) and favor the coexistence of species.
Serrapinnus notomelas and Serrapinnus sp.1 consumed mostly algae and zooplankton, respectively. Adults and juveniles of S. notomelas and adults of Serrapinnus sp.1 mainly consumed algae, while juveniles of Serrapinnus sp.1 mainly consumed zooplankton. Algae and zooplankton are abundant in macrophyte stands (Colares et al., 2013Colares MAM, Bonecker CC, Simões NR, Alves GM, Lansac-Tôha FA. Structure of the zooplankton communities in macrophytes stand of a Neotropical floodplain (the Paraná River, Brazil). Int Rev Hydrobiol. 2013; 98(2):89–103. https://doi.org/10.1002/iroh.201301471
https://doi.org/10.1002/iroh.201301471...
; Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
). Algae proliferate in the periphyton that is formed in the roots and stems of aquatic macrophytes (Biolo, Rodrigues, 2013Biolo S, Rodrigues L. Structure of the periphytic algae associated with a floating macrophyte in an open lake on the upper Paraná River floodplain, Brazil. Acta Sci Biol Sci. 2013; 35(4):513–19. https://doi.org/10.4025/actascibiolsci.v35i4.18663
https://doi.org/10.4025/actascibiolsci.v...
; Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
), whereas microcrustaceans use roots and stems as shelter against predation (Colares et al., 2013Colares MAM, Bonecker CC, Simões NR, Alves GM, Lansac-Tôha FA. Structure of the zooplankton communities in macrophytes stand of a Neotropical floodplain (the Paraná River, Brazil). Int Rev Hydrobiol. 2013; 98(2):89–103. https://doi.org/10.1002/iroh.201301471
https://doi.org/10.1002/iroh.201301471...
; Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
). Therefore, S. notomelas and Serrapinnus sp.1 individuals explored resources that are typically available in macrophytes in a different way, thus differing in foraging habits. Variations in the main food consumption have been reported for this genus in other studies conducted in the same region. For example, for S. notomelas, seasonal variations in the diet were observed with the predominant consumption of invertebrates (Quirino et al., 2015Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
https://doi.org/10.1007/s10452-015-9535-...
) and zooplankton (Quirino et al., 2018Quirino BA, Carniatto N, Thomaz SM, Fugi R. Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish. 2018; 28(1):97–109. https://doi.org/10.1111/eff.12434
https://doi.org/10.1111/eff.12434...
).
The average trophic niche breadth differed between species and ontogeny. The higher average niche breadth of Serrapinnus sp.1 revealed that individuals, mostly adults, consumed a greater variety of food. These findings, in addition to the higher consumption of animal items, indicate more trophic flexibility and omnivorous habit for Serrapinnus sp.1. In contrast, S. notomelas seems to be more specialized on plants, since the diet has low variability; besides, intra-population variation was low too, in accordance to what is expected for this trophic guild (Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.). The broad trophic niche and high trophic flexibility may favor the coexistence between species (Chase, Leibold, 2003Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.; Neves et al., 2021Neves MP, Kratina P, Delariva RL, Jones JI, Fialho CB. Seasonal feeding plasticity can facilitate coexistence of dominant omnivores in Neotropical streams. Rev Fish Biol Fish. 2021; 31:417–32. https://doi.org/10.1007/s11160-021-09648-w
https://doi.org/10.1007/s11160-021-09648...
). The flexibility of omnivorous fishes in changing their diet when faced with environmental variation, allows the trophic niche differentiation (Chase, Leibold, 2003Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.). The expansion or contraction of the trophic niche favors resource partitioning, thus allowing the coexistence and avoiding/decreasing the effects of competition, especially when it comes to congeners species (Van Valen, 1965Van Valen L. Morphological variation and width of ecological niche. Am Nat. 1965; 99(908):377–90.; Bolnick et al., 2010Bolnick DI, Ingram T, Stutz WE, Snowberg LK, Lau OL, Paull JS. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc R Soc Lond B Biol Sci. 2010; 277(1689):1789–97. https://doi.org/10.1098/rspb.2010.0018
https://doi.org/10.1098/rspb.2010.0018...
; Neves et al., 2021Neves MP, Kratina P, Delariva RL, Jones JI, Fialho CB. Seasonal feeding plasticity can facilitate coexistence of dominant omnivores in Neotropical streams. Rev Fish Biol Fish. 2021; 31:417–32. https://doi.org/10.1007/s11160-021-09648-w
https://doi.org/10.1007/s11160-021-09648...
). In this perspective, we suggest that the greatest trophic niche breadth observed for Serrapinnus sp.1 can be a partitioning resource mechanism with S. notomelas. Thereby, considering the morphological limitations, the trophic niche was expanded to supply energy demands and avoid trophic competition with S. notomelas.
A relationship between the diet and the morphological attributes, since the morphology influences foraging strategies and tactics, has been demonstrated by several studies in fishes (Bonato et al., 2017Bonato KO, Burress ED, Fialho CB. Dietary differentiation in relation to mouth and tooth morphology of a neotropical characid fish community. Zool Anz. 2017; 267:31–40. https://doi.org/10.1016/j.jcz.2017.01.003
https://doi.org/10.1016/j.jcz.2017.01.00...
; Portella et al., 2017Portella T, Lobón-Cerviá J, Manna LR, Bergallo HG, Mazzoni R. Eco-morphological attributes and feeding habits in coexisting characins. J Fish Biol. 2017; 90(1):129–46. https://doi.org/10.1111/jfb.13162
https://doi.org/10.1111/jfb.13162...
; Ornelas-García et al., 2018Ornelas-García CP, Córdova-Tapia F, Zambrano L, Bermúdez-González MP, Mercado-Silva N, Mendoza-Garfias B et al. Trophic specialization and morphological divergence between two sympatric species in Lake Catemaco, Mexico. Ecol Evol. 2018; 8(10):4867–75. https://doi.org/10.1002/ece3.4042
https://doi.org/10.1002/ece3.4042...
; Kliemann et al., 2019Kliemann BCK, Baldasso MC, Pini SFR, Makrakis MC, Makrakis S, Delariva RL. Assessing the diet and trophic niche breadth of an omnivorous fish (Glanidium ribeiroi) in subtropical lotic environments: intraspecific and ontogenic responses to spatial variations. Mar Freshw Res. 2019; 70(8):1116–18. https://doi.org/10.1071/MF18149
https://doi.org/10.1071/MF18149...
; Delariva, Neves, 2020Delariva RL, Neves MP. Morphological traits correlated with resource partitioning among small characin fish species coexisting in a Neotropical river. Ecol Freshw Fish. 2020; 29(4):640–53. https://doi.org/10.1111/eff.12540
https://doi.org/10.1111/eff.12540...
). According to Alexander, (1967)Alexander RM. Functional design in fishes. London: Hutchinson University; 1967., the morphological mechanisms used by a fish to obtain food represent adaptations that allow them to take advantage of the specific prey. Interspecific differences in morphological traits for the species under study, as well as the significant correlation between the diet and the morphological traits, demonstrate that dietary divergences were favored by different performances in the locomotion and in the feeding abilities (prey capture, selection, and absorption).
Serrapinnus notomelas exhibited a larger dorsal fin, which involves a better stabilization capacity in deflections, thus providing lower resistance to perform maneuvers such as dorsoventral or lateral movements (Gosline, 1971Gosline WA. Functional morphology and classification of teleostean fishes. Honolulu: University of Hawaii Press; 1971. https://doi.org/10.1515/9780824885311
https://doi.org/10.1515/9780824885311...
). Besides, it allows the exploration of structurally complex habitats, as is the case of macrophyte stands in lentic waters (Esguícero, Arcifa, 2010Esguícero ALH, Arcifa MS. Which is the best environment for the development of the early life stages of fish during the dry season? Acta Limnol Bras. 2010; 22(3):267–75. https://doi.org/10.4322/actalb.02203003
https://doi.org/10.4322/actalb.02203003...
; Prado et al., 2016Prado AVR, Goulart E, Pagotto JPA. Ecomorphology and use of food resources: inter- and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop Ichthyol. 2016; 14(4):e150140. https://doi.org/10.1590/1982-0224-20150140
https://doi.org/10.1590/1982-0224-201501...
). Thus, it can be inferred that the ability to stabilize deflections and greater maneuverability facilitates the consumption of algae that are associated with the stems and roots of macrophytes. This inference is corroborated by the high consumption of algae exhibited by this species and the positive correlation of this item with the relative area of the dorsal fin. By contrast, Serrapinnus sp.1 had higher peduncles and larger pectoral and pelvic areas than S. notomelas. The high peduncle implies less is maneuverability, and broad pectoral and pelvic fins favor continuous swimming at higher speeds and provide a greater ability to balance (Gatz, 1979Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane stud zool bot. 1979; 21(2): 91–124.; Wainwright et al., 2002Wainwright PC, Bellwood DR, Westneat MW. Ecomorphology of locomotion in labrid fishes. Environ Biol Fishes. 2002; 65:47–62. https://doi.org/10.1023/A:1019671131001
https://doi.org/10.1023/A:1019671131001...
). So, since microcrustaceans extensively move between the roots and stems of macrophytes (Choi et al., 2015Choi JY, Kim SK, Jeong KS, Joo GJ. Distribution pattern of epiphytic microcrustaceans in relation to different macrophyte microhabitats in a shallow wetland (Upo wetlands, South Korea). Oceanol Hydrobiol Stud. 2015; 44(2):151–63.), broad pectoral fins increase the swimming speed and enhance foraging efficiency in this habitat. Hence, the swimming performance of Serrapinnus sp.1 favors the capture of these organisms. The correlation between zooplankton and the anal fin aspect ratio, as well as the relative height of the caudal peduncle, reinforces these findings.
Associated with the swimming performance, differences in morphological traits related to the food capability (that is, obtaining, selecting, and processing food in the digestive tract; sensuGerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.) may additionally explain the dietary segregation here reported. The peculiarities of the trophic apparatus (gill raker length and intestinal coefficient) were the main traits that differed between the species. Longer gill rakers, as observed for S. notomelas, were correlated with the higher consumption of algae. More specialized structures, such as elongated intestine have been listed as adaptive responses to extract nutrients from plants (poorer in readily assimilable energy) Gerking, 1994Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.). Likewise, the long and more juxtaposed gill rakers give advantages in filtering algae (Gatz, 1979Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane stud zool bot. 1979; 21(2): 91–124.; Kramer, Bryant, 1995Kramer DL, Bryant MJ. Intestine length in the fishes of a tropical stream: 2. Relationships to diet - the long and short of a convoluted issue. Environ Biol Fishes. 1995; 42:129–41. https://doi.org/10.1007/BF00001991
https://doi.org/10.1007/BF00001991...
; Villéger et al., 2010Villéger S, Miranda JR, Hernández DF, Mouillot D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol Appl. 2010; 20(6):1512–22. https://doi.org/10.1890/09-1310.1
https://doi.org/10.1890/09-1310.1...
), an abundant resource in macrophyte stands.
Another important morphological characteristic that was correlated with the diet was the number of teeth cuspids. Algivorous species tend to have multicuspid teeth for cutting or tearing (Gibson, 2015Gibson SZ. Evidence of a specialized feeding niche in a Late Triassic ray-finned fish: evolution of multidenticulate teeth and benthic scraping in †Hemicalypterus. Sci Nat. 2015; 102(10):1–07. https://doi.org/10.1007/s00114-015-1262-y
https://doi.org/10.1007/s00114-015-1262-...
; Ohara et al., 2017Ohara WM, Abrahão VP, Espíndola VC. Hyphessobrycon platyodus (Teleostei: Characiformes), a new species from the Rio Madeira basin, Brazil, with comments on how multicuspid teeth relate to feeding habits in Characidae. J Fish Biol. 2017; 91(3):835–50. https://doi.org/10.1111/jfb.13383
https://doi.org/10.1111/jfb.13383...
), which are in accordance to observed for S. notomelas. In contrast, species with few cusps, often between three or five, tend to be omnivorous (Winemiller, 1991Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
https://doi.org/10.2307/2937046...
), as in the case of Serrapinnus sp.1.
We also observed differences in diet and morphology throughout ontogeny, corroborating with several authors who reported these variations and related them to the energy requirement during development (see Neves et al., 2015Neves MP, Delariva RL, Guimarães ATB, Sanches PV. Carnivory during ontogeny of the Plagioscion squamosissimus: a successful non-native fish in a lentic environment of the Upper Paraná River basin. PLoS One. 2015; 10(11):e0141651. https://doi.org/10.1371/journal.pone.0141651
https://doi.org/10.1371/journal.pone.014...
; Dias et al., 2017bDias RM, Silva JCB, Gomes LC, Agostinho AA. Effects of macrophyte complexity and hydrometric level on fish assemblages in a Neotropical floodplain. Environ Biol Fishes. 2017b; 100:703–16. https://doi.org/10.1007/s10641-017-0597-y
https://doi.org/10.1007/s10641-017-0597-...
; Schilling et al., 2017Schilling HT, Hughes JM, Smith JA, Everett JD, Stewart J, Suthers IM. Latitudinal and ontogenetic variation in the diet of a pelagic mesopredator (Pomatomus saltatrix), assessed with a classification tree analysis. Mar Biol. 2017; 164(75):1–10. https://doi.org/10.1007/s00227-017-3105-1
https://doi.org/10.1007/s00227-017-3105-...
; Kliemann et al., 2019Kliemann BCK, Baldasso MC, Pini SFR, Makrakis MC, Makrakis S, Delariva RL. Assessing the diet and trophic niche breadth of an omnivorous fish (Glanidium ribeiroi) in subtropical lotic environments: intraspecific and ontogenic responses to spatial variations. Mar Freshw Res. 2019; 70(8):1116–18. https://doi.org/10.1071/MF18149
https://doi.org/10.1071/MF18149...
). Body size and the demand for energy increase with the growth of the fish. Thus, morphological changes may occur that allow the consumption of a greater range of resources, whether in size or quantity of food, which meet energy demand (Werner, Gilliam, 1984Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst. 1984; 15:393–425. https://doi.org/10.1146/annurev.es.15.110184.002141
https://doi.org/10.1146/annurev.es.15.11...
; Scharf et al., 2000Scharf FS, Juanes F, Rountree RA. Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar Ecol Prog Ser. 2000; 208:229–48. https://doi.org/10.3354/meps208229
https://doi.org/10.3354/meps208229...
; Marsh et al., 2017Marsh JM, Mueter FJ, Iken K, Danielson S. Ontogenetic, spatial and temporal variation in trophic level and diet of Chukchi Sea fishes. Deep Sea Res 2 Top Stud Oceanogr. 2017; 135:78–94. http://dx.doi.org/10.1016/j.dsr2.2016.07.010
http://dx.doi.org/10.1016/j.dsr2.2016.07...
). Diet and morphology adjustments between adults and juveniles were verified mainly for Serrapinnus sp.1. We observed a wider head and mouth and the consumption of Diptera larvae, Bryozoa, and Porifera (more energy-efficient resources) in adults. Additionally, we found a greater average trophic niche breadth for adults, reinforcing that there is the exploration of a wide variety of habitats and the consumption of items of larger sizes as the fish grows (Chase, Leibold, 2003Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.; Ward et al., 2006Ward AJW, Webster MM, Hart PJB. Intraspecific food competition in fishes. Fish Fish (Oxf). 2006; 7(4):231–61. https://doi.org/10.1111/j.1467-2979.2006.00224.x
https://doi.org/10.1111/j.1467-2979.2006...
; Tupinambás et al., 2015Tupinambás TH, Pompeu PS, Gandini CV, Hughes RM, Callisto M. Fish stomach contents in benthic macroinvertebrate assemblage assessments. Braz J Biol. 2015; 75(1):157–64. https://doi.org/10.1590/1519-6984.09913
https://doi.org/10.1590/1519-6984.09913...
; Schilling et al., 2017Schilling HT, Hughes JM, Smith JA, Everett JD, Stewart J, Suthers IM. Latitudinal and ontogenetic variation in the diet of a pelagic mesopredator (Pomatomus saltatrix), assessed with a classification tree analysis. Mar Biol. 2017; 164(75):1–10. https://doi.org/10.1007/s00227-017-3105-1
https://doi.org/10.1007/s00227-017-3105-...
).
When compared to adults, juveniles of S. notomelas had larger dorsal fins and juveniles of Serrapinnus sp.1 had larger pelvic fins. Moreover, juveniles of S. notomelas had dorsally positioned eyes when compared to adults. These morphological traits are related to the ability to balance, stabilize in deflections, and capture prey in the water column (Gosline, 1971Gosline WA. Functional morphology and classification of teleostean fishes. Honolulu: University of Hawaii Press; 1971. https://doi.org/10.1515/9780824885311
https://doi.org/10.1515/9780824885311...
; Gatz, 1979Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane stud zool bot. 1979; 21(2): 91–124.; Pouilly et al., 2003Pouilly M, Lino F, Bretenoux JG, Rosales C. Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. J Fish Biol. 2003; 62(5):1137–58. https://doi.org/10.1046/j.1095-8649.2003.00108.x
https://doi.org/10.1046/j.1095-8649.2003...
). This set of divergences suggests ontogenetic segregation in the habitat use, which can be a strategy to avoid intra-interspecific competition (Hutchinson, 1961Hutchinson GE. The paradox of the plankton. Am Nat. 1961; 95(882):137–45. https://doi.org/10.1086/282171
https://doi.org/10.1086/282171...
; Leray et al., 2019Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ et al. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol. 2019; 28(10):2694–710. https://doi.org/10.1111/mec.15090
https://doi.org/10.1111/mec.15090...
). It is inferred those adults and juveniles of both species explored different habitats, with the water column mainly inhabited by juveniles. These findings are important from the perspective of conservation. Considering that macrophyte stands are strongly dependent on the water level and seasonal dynamics (Thomaz et al., 2009Thomaz SM, Carvalho P, Padial AA, Kobayashi JT. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Braz J Biol. 2009; 69(2 suppl):617–25. https://doi.org/10.1590/s1519-69842009000300016
https://doi.org/10.1590/s1519-6984200900...
), atypical changes in these dynamics can affect juveniles and adults of one or another species in a divergent way. Furthermore, this segregation in the habitat use implies differentiation of the trophic niche, reinforcing the mechanisms for inter-intraspecific coexistence predicted by the niche theory.
In summary, our results show differences in morphological traits and correlations with different feeding habits. We infer that the correlation between the locomotor and feeding abilities may result from interactions of the individuals, with their preys and habitats. These associations increase opportunities for independent diversification, and it may explain the species coexistence in macrophyte stands, corroborating with the niche theory. Moreover, the ontogenetic differences in diet and morphology observed for the two species, indicate a segregation in the habitat use. The information from the trophic apparatus (intestinal coefficient, gill raker length, and the number of teeth cuspids) is valuable to demonstrate the trophic segregation. In our analysis, the combined use of morphological traits is an excellent way to objectively identify cases of adaptive and evolutionary divergence between phylogenetically closely related species.
Finally, we encourage future studies to evaluate other niche dimensions (space, time) that can help favoring the coexistence (Leray et al., 2019Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ et al. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol. 2019; 28(10):2694–710. https://doi.org/10.1111/mec.15090
https://doi.org/10.1111/mec.15090...
) between pairs of congeners species. In addition, there is a lack of studies considering factors unevaluated here, such as seasonality, spatiality, resources availability, and influence of predators, which can interfere in the behavior of individuals (Hart et al., 2016Hart SP, Schreiber SJ, Levine JM. How variation between individuals affects species coexistence. Ecol Lett. 2016; 19(8):825–38. https://doi.org/10.1111/ele.12618
https://doi.org/10.1111/ele.12618...
). Also, the isotopic niche approach is also valuable because it can reveal the assimilation of food resources and the metabolic state (Post, 2002Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002; 83(3):703-18. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
https://doi.org/10.1890/0012-9658(2002)0...
; Carvalho et al., 2017Carvalho DR, Castro DMP, Callisto M, Moreira MZ, Pompeu PS. The trophic structure of fish communities from streams in the Brazilian Cerrado under different land uses: an approach using stable isotopes. Hydrobiologia. 2017; 795:199–217. https://doi.org/10.1007/s10750-017-3130-6
https://doi.org/10.1007/s10750-017-3130-...
).
ACKNOWLEDGEMENTS
Funding: this work was supported by programs PIE/PELD/CNPq (Process number 558118/2009–7) and CNPq (Process number 480804/2010–9), and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001”. We would like to thank the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia/UEM) for logistical support, and the people from the Laboratório de Ictioplâncton (Nupélia/UEM) who helped in field collection, sorting, and identification. We would also like to thank Dr. Carla S. Pavanelli (Nupélia) for confirming the identity of the collected specimens.
REFERENCES
- Abbey-Lee RN, Gaiser EE, Trexler JC. Relative roles of dispersal dynamics and competition in determining the isotopic niche breadth of a wetland fish. Freshw Biol. 2013; 58(4):780–92. https://doi.org/10.1111/fwb.12084
» https://doi.org/10.1111/fwb.12084 - Agostinho AA, Thomaz SM, Gomes LC, Baltar SLSMA. Influence of the macrophyte Eichhornia azurea on fish assemblage of the Upper Paraná River floodplain (Brazil). Aquat Ecol. 2007; 41(4):611–19. https://doi.org/10.1007/s10452-007-9122-2
» https://doi.org/10.1007/s10452-007-9122-2 - Alexander RM. Functional design in fishes. London: Hutchinson University; 1967.
- Alves GHZ, Tófoli RM, Novakowski GC, Hahn NS. Food partitioning between sympatric species of Serrapinnus (Osteichthyes, Cheirodontinae) in a tropical stream. Acta Sci Biol Sci. 2011; 33(2):153–59. https://doi.org/10.4025/actascibiolsci.v33i2.7593
» https://doi.org/10.4025/actascibiolsci.v33i2.7593 - Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001; 26(1):32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
» https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x - Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006; 62(1):245–53. https://doi.org/10.1111/j.1541-0420.2005.00440.x
» https://doi.org/10.1111/j.1541-0420.2005.00440.x - Bicudo CEM, Bicudo RMT Algas de águas continentais brasileiras. São Paulo: Fundação Brasileira para o Desenvolvimento do Ensino de Ciências; 1970.
- Biolo S, Rodrigues L. Structure of the periphytic algae associated with a floating macrophyte in an open lake on the upper Paraná River floodplain, Brazil. Acta Sci Biol Sci. 2013; 35(4):513–19. https://doi.org/10.4025/actascibiolsci.v35i4.18663
» https://doi.org/10.4025/actascibiolsci.v35i4.18663 - Bolnick DI, Ingram T, Stutz WE, Snowberg LK, Lau OL, Paull JS. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc R Soc Lond B Biol Sci. 2010; 277(1689):1789–97. https://doi.org/10.1098/rspb.2010.0018
» https://doi.org/10.1098/rspb.2010.0018 - Bonato KO, Burress ED, Fialho CB. Dietary differentiation in relation to mouth and tooth morphology of a neotropical characid fish community. Zool Anz. 2017; 267:31–40. https://doi.org/10.1016/j.jcz.2017.01.003
» https://doi.org/10.1016/j.jcz.2017.01.003 - Carniatto N, Fugi R, Cantanhêde G, Gubiani ÉA, Hahn NS. Effects of flooding regime and diel cycle on diet of a small sized fish associated to macrophytes. Acta Limnol Bras. 2012; 24(4):363–72. https://doi.org/10.1590/S2179-975X2013005000007
» https://doi.org/10.1590/S2179-975X2013005000007 - Carroll SB. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001; 409:1102–09. https://doi.org/10.1038/35059227
» https://doi.org/10.1038/35059227 - Carvalho DR, Castro DMP, Callisto M, Moreira MZ, Pompeu PS. The trophic structure of fish communities from streams in the Brazilian Cerrado under different land uses: an approach using stable isotopes. Hydrobiologia. 2017; 795:199–217. https://doi.org/10.1007/s10750-017-3130-6
» https://doi.org/10.1007/s10750-017-3130-6 - Casatti L, Castro RMC. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotrop Ichthyol. 2006; 4(2):203–14. https://doi.org/10.1590/S1679-62252006000200006
» https://doi.org/10.1590/S1679-62252006000200006 - Chase JM, Leibold MA. Ecological niches: linking classical and contemporary approaches. Chicago: University of Chicago Press; 2003.
- Choi JY, Kim SK, Jeong KS, Joo GJ. Distribution pattern of epiphytic microcrustaceans in relation to different macrophyte microhabitats in a shallow wetland (Upo wetlands, South Korea). Oceanol Hydrobiol Stud. 2015; 44(2):151–63.
- Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993; 18(1):117–43. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
» https://doi.org/10.1111/j.1442-9993.1993.tb00438.x - Colares MAM, Bonecker CC, Simões NR, Alves GM, Lansac-Tôha FA. Structure of the zooplankton communities in macrophytes stand of a Neotropical floodplain (the Paraná River, Brazil). Int Rev Hydrobiol. 2013; 98(2):89–103. https://doi.org/10.1002/iroh.201301471
» https://doi.org/10.1002/iroh.201301471 - Correa SB, Winemiller KO. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology. 2014; 95(1):210–24. https://doi.org/10.1890/13-0393.1
» https://doi.org/10.1890/13-0393.1 - Cunha ER, Winemiller KO, Silva JCB, Lopes TM, Gomes LC, Thomaz SM et al α and β diversity of fishes in relation to a gradient of habitat structural complexity supports the role of environmental filtering in community assembly. Aquat Sci. 2019; 81(38):1–12. https://doi.org/10.1007/s00027-019-0634-3
» https://doi.org/10.1007/s00027-019-0634-3 - Dehnhard N, Achurch H, Clarke J, Michel LN, Southwell C, Sumner MD et al High inter - and intraspecific niche overlap among three sympatrically breeding, closely related seabird species: Generalist foraging as an adaptation to a highly variable environment? J Anim Ecol. 2020; 89(1):104–19. https://doi.org/10.1111/1365-2656.13078
» https://doi.org/10.1111/1365-2656.13078 - Delariva RL, Agostinho AA, Nakatani K, Baumgartner G. Ichthyofauna associated to aquatic macrophytes in the upper Parana River floodplain. Rev Unimar. 1994; 16(Supl. 3):41–60.
- Delariva RL, Neves MP. Morphological traits correlated with resource partitioning among small characin fish species coexisting in a Neotropical river. Ecol Freshw Fish. 2020; 29(4):640–53. https://doi.org/10.1111/eff.12540
» https://doi.org/10.1111/eff.12540 - Dias RM, Ortega JCG, Gomes LC, Agostinho AA. Trophic relationships in fish assemblages of Neotropical floodplain lakes: selectivity and feeding overlap mediated by food availability. Iheringia Ser Zool. 2017a; 107:e2017035. https://doi.org/10.1590/1678-4766e2017035
» https://doi.org/10.1590/1678-4766e2017035 - Dias RM, Silva JCB, Gomes LC, Agostinho AA. Effects of macrophyte complexity and hydrometric level on fish assemblages in a Neotropical floodplain. Environ Biol Fishes. 2017b; 100:703–16. https://doi.org/10.1007/s10641-017-0597-y
» https://doi.org/10.1007/s10641-017-0597-y - Dibble ED, Thomaz SM. Use of fractal dimension to assess habitat complexity and its influence on dominant invertebrates inhabiting tropical and temperate macrophytes. J Freshw Ecol. 2009; 24(1):93–102. https://doi.org/10.1080/02705060.2009.9664269
» https://doi.org/10.1080/02705060.2009.9664269 - Esguícero ALH, Arcifa MS. Which is the best environment for the development of the early life stages of fish during the dry season? Acta Limnol Bras. 2010; 22(3):267–75. https://doi.org/10.4322/actalb.02203003
» https://doi.org/10.4322/actalb.02203003 - Fuiman LA, Werner RG, editors. Fishery science: the unique contributions of early life stages. Oxford: Blackwell Science; 2002.
- Gatz AJ. Ecological morphology of freshwater stream fishes. Tulane stud zool bot. 1979; 21(2): 91–124.
- Gerking SD. Larval feeding. In: Gerking SD. Feeding Ecology of Fish. San Diego: Academic Press; 1994. p.139–70.
- Gibson SZ. Evidence of a specialized feeding niche in a Late Triassic ray-finned fish: evolution of multidenticulate teeth and benthic scraping in †Hemicalypterus Sci Nat. 2015; 102(10):1–07. https://doi.org/10.1007/s00114-015-1262-y
» https://doi.org/10.1007/s00114-015-1262-y - Gosline WA. Functional morphology and classification of teleostean fishes. Honolulu: University of Hawaii Press; 1971. https://doi.org/10.1515/9780824885311
» https://doi.org/10.1515/9780824885311 - Harrell Jr FE. Package “Hmisc”[Internet]. R package; 2020. Available from https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf
» https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf - Hart SP, Schreiber SJ, Levine JM. How variation between individuals affects species coexistence. Ecol Lett. 2016; 19(8):825–38. https://doi.org/10.1111/ele.12618
» https://doi.org/10.1111/ele.12618 - Hellawell JM, Abel R. A rapid volumetric method for the analysis of the food of fishes. J Fish Biol. 1971; 3(1):29–37. https://doi.org/10.1111/j.1095-8649.1971.tb05903.x
» https://doi.org/10.1111/j.1095-8649.1971.tb05903.x - Hutchinson GE. The paradox of the plankton. Am Nat. 1961; 95(882):137–45. https://doi.org/10.1086/282171
» https://doi.org/10.1086/282171 - Hyslop EJ. Stomach contents analysis – a review of methods and their application. J Fish Biol. 1980; 17(4):411–29. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
» https://doi.org/10.1111/j.1095-8649.1980.tb02775.x - Keppeler FW, Lanés LEK, Rolon AS, Stenert C, Lehmann P, Reichard M et al The morphology-diet relationship and its role in the coexistence of two species of annual fishes. Ecol Freshw Fish. 2015; 24(1):77–90. https://doi.org/10.1111/eff.12127
» https://doi.org/10.1111/eff.12127 - Kliemann BCK, Baldasso MC, Pini SFR, Makrakis MC, Makrakis S, Delariva RL. Assessing the diet and trophic niche breadth of an omnivorous fish (Glanidium ribeiroi) in subtropical lotic environments: intraspecific and ontogenic responses to spatial variations. Mar Freshw Res. 2019; 70(8):1116–18. https://doi.org/10.1071/MF18149
» https://doi.org/10.1071/MF18149 - Kramer DL, Bryant MJ. Intestine length in the fishes of a tropical stream: 2. Relationships to diet - the long and short of a convoluted issue. Environ Biol Fishes. 1995; 42:129–41. https://doi.org/10.1007/BF00001991
» https://doi.org/10.1007/BF00001991 - Leray M, Alldredge AL, Yang JY, Meyer CP, Holbrook SJ, Schmitt RJ et al. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol Ecol. 2019; 28(10):2694–710. https://doi.org/10.1111/mec.15090
» https://doi.org/10.1111/mec.15090 - Lukoschek V, McCormick MI. Ontogeny of diet changes in a tropical benthic carnivorous fish, Parupeneus barberinus (Mullidae): relationship between foraging behaviour, habitat use, jaw size, and prey selection. Mar Biol. 2001; 138:1099–113. https://doi.org/10.1007/s002270000530
» https://doi.org/10.1007/s002270000530 - Mardia KV, Kent TJ, Bibby JM. Multivariate analysis. San Diego: Academic Press; 1979.
- Marsh JM, Mueter FJ, Iken K, Danielson S. Ontogenetic, spatial and temporal variation in trophic level and diet of Chukchi Sea fishes. Deep Sea Res 2 Top Stud Oceanogr. 2017; 135:78–94. http://dx.doi.org/10.1016/j.dsr2.2016.07.010
» http://dx.doi.org/10.1016/j.dsr2.2016.07.010 - Moncayo-Estrada R, Lind OT, Escalera-Gallardo C. Trophic interactions among sympatric zooplanktivorous fish species in volume change conditions in a large, shallow, tropical lake. Neotrop Ichthyol. 2011; 9(1):169–76. https://doi.org/10.1590/S1679-62252011005000003
» https://doi.org/10.1590/S1679-62252011005000003 - Mugnai R, Nessimian JL, Baptista DF. Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro. Rio de Janeiro: Technical books; 2010.
- Nakatani K, Agostinho AA, Baumgartner G, Bialetzki A, Sanches PV, Makrakis MC et al. Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação. Maringá: EDUEM; 2001.
- Nascimento CP, Santos NCL, Dal Vesco BM, Gomes LC. Trophic morphology features allow Astyanax endemic species coexistence in a Neotropical river system. J Fish Biol. 2020; 97(3):776–84. https://doi.org/10.1111/jfb.14433
» https://doi.org/10.1111/jfb.14433 - Neves MP, Delariva RL, Guimarães ATB, Sanches PV. Carnivory during ontogeny of the Plagioscion squamosissimus: a successful non-native fish in a lentic environment of the Upper Paraná River basin. PLoS One. 2015; 10(11):e0141651. https://doi.org/10.1371/journal.pone.0141651
» https://doi.org/10.1371/journal.pone.0141651 - Neves MP, Kratina P, Delariva RL, Jones JI, Fialho CB. Seasonal feeding plasticity can facilitate coexistence of dominant omnivores in Neotropical streams. Rev Fish Biol Fish. 2021; 31:417–32. https://doi.org/10.1007/s11160-021-09648-w
» https://doi.org/10.1007/s11160-021-09648-w - Ohara WM, Abrahão VP, Espíndola VC Hyphessobrycon platyodus (Teleostei: Characiformes), a new species from the Rio Madeira basin, Brazil, with comments on how multicuspid teeth relate to feeding habits in Characidae. J Fish Biol. 2017; 91(3):835–50. https://doi.org/10.1111/jfb.13383
» https://doi.org/10.1111/jfb.13383 - Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial [Internet]. R package; 2015. Available from: https://www.mooreecology.com/uploads/2/4/2/1/24213970/vegantutor.pdf
» https://www.mooreecology.com/uploads/2/4/2/1/24213970/vegantutor.pdf - Ornelas-García CP, Córdova-Tapia F, Zambrano L, Bermúdez-González MP, Mercado-Silva N, Mendoza-Garfias B et al Trophic specialization and morphological divergence between two sympatric species in Lake Catemaco, Mexico. Ecol Evol. 2018; 8(10):4867–75. https://doi.org/10.1002/ece3.4042
» https://doi.org/10.1002/ece3.4042 - Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: Revised, annotated and updated. Neotrop Ichthyol. 2018; 16(2):e170094. https://doi.org/10.1590/1982-0224-20170094
» https://doi.org/10.1590/1982-0224-20170094 - Pelicice FM, Agostinho AA, Thomaz SM. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effects of plant biomass and diel period. Acta Oecol (Montrouge). 2005; 27(1):9–16. https://doi.org/10.1016/j.actao.2004.08.004
» https://doi.org/10.1016/j.actao.2004.08.004 - Piana PA, Gomes LC, Cortez EM. Factors influencing Serrapinnus notomelas (Characiformes: Characidae) populations in upper Paraná River floodplain lagoons. Neotrop Ichthyol. 2006; 4(1):81–86. https://doi.org/10.1590/S1679-62252006000100008
» https://doi.org/10.1590/S1679-62252006000100008 - Portella T, Lobón-Cerviá J, Manna LR, Bergallo HG, Mazzoni R. Eco-morphological attributes and feeding habits in coexisting characins. J Fish Biol. 2017; 90(1):129–46. https://doi.org/10.1111/jfb.13162
» https://doi.org/10.1111/jfb.13162 - Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002; 83(3):703-18. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
» https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2 - Pouilly M, Lino F, Bretenoux JG, Rosales C. Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. J Fish Biol. 2003; 62(5):1137–58. https://doi.org/10.1046/j.1095-8649.2003.00108.x
» https://doi.org/10.1046/j.1095-8649.2003.00108.x - Prado AVR, Goulart E, Pagotto JPA. Ecomorphology and use of food resources: inter- and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop Ichthyol. 2016; 14(4):e150140. https://doi.org/10.1590/1982-0224-20150140
» https://doi.org/10.1590/1982-0224-20150140 - Quirino BA, Carniatto N, Gaiotto JV, Fugi R. Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol. 2015; 49:431–40. https://doi.org/10.1007/s10452-015-9535-2
» https://doi.org/10.1007/s10452-015-9535-2 - Quirino BA, Carniatto N, Thomaz SM, Fugi R. Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish. 2018; 28(1):97–109. https://doi.org/10.1111/eff.12434
» https://doi.org/10.1111/eff.12434 - Quirino BA, Lansac-Tôha FM, Thomaz SM, Heino J, Fugi R. Macrophyte stand complexity explains the functional α and β diversity of fish in a tropical river-floodplain. Aquat Sci. 2021; 83(12):1–14. https://doi.org/10.1007/s00027-020-00768-2
» https://doi.org/10.1007/s00027-020-00768-2 - Ross ST. Resource partitioning in fish assemblages: a review of field studies. Copeia. 1986; 1986(2):352–88. https://doi.org/10.2307/1444996
» https://doi.org/10.2307/1444996 - Sánchez-Hernández J, Cobo F. Ontogenetic shifts in terrestrial reliance of stream-dwelling brown trout. J Limnol. 2016; 75(2):409–14. https://doi.org/10.4081/jlimnol.2016.1322
» https://doi.org/10.4081/jlimnol.2016.1322 - Sánchez-Hernández J, Nunn AD, Adams CE, Amundsen PA. Causes and consequences of ontogenetic dietary shifts: a global synthesis using fish models. Biol Rev Camb Philos Soc. 2019; 94(2):539–54. https://doi.org/10.1111/brv.12468
» https://doi.org/10.1111/brv.12468 - Scharf FS, Juanes F, Rountree RA. Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar Ecol Prog Ser. 2000; 208:229–48. https://doi.org/10.3354/meps208229
» https://doi.org/10.3354/meps208229 - Schilling HT, Hughes JM, Smith JA, Everett JD, Stewart J, Suthers IM. Latitudinal and ontogenetic variation in the diet of a pelagic mesopredator (Pomatomus saltatrix), assessed with a classification tree analysis. Mar Biol. 2017; 164(75):1–10. https://doi.org/10.1007/s00227-017-3105-1
» https://doi.org/10.1007/s00227-017-3105-1 - Schoener TW. Competition and the form of habitat shift. Theor Popul Biol. 1974; 6(3):265–307. https://doi.org/10.1016/0040-5809(74)90013-6
» https://doi.org/10.1016/0040-5809(74)90013-6 - Silva JC, Gubiani ÉA, Neves MP, Delariva RL. Coexisting small fish species in lotic neotropical environments: evidence of trophic niche differentiation. Aquat Ecol. 2017; 51:275–88. https://doi.org/10.1007/s10452-017-9616-5
» https://doi.org/10.1007/s10452-017-9616-5 - Silva NCS, Costa AJL, Louvise J, Soares BE, Reis VCS, Albrecht MP et al. Resource partitioning and ecomorphological variation in two syntopic species of Lebiasinidae (Characiformes) in an Amazonian stream. Acta Amaz. 2016; 46(1):25–36. https://doi.org/10.1590/1809-4392201501024
» https://doi.org/10.1590/1809-4392201501024 - Thomaz SM, Carvalho P, Padial AA, Kobayashi JT. Temporal and spatial patterns of aquatic macrophyte diversity in the Upper Paraná River floodplain. Braz J Biol. 2009; 69(2 suppl):617–25. https://doi.org/10.1590/s1519-69842009000300016
» https://doi.org/10.1590/s1519-69842009000300016 - Tupinambás TH, Pompeu PS, Gandini CV, Hughes RM, Callisto M. Fish stomach contents in benthic macroinvertebrate assemblage assessments. Braz J Biol. 2015; 75(1):157–64. https://doi.org/10.1590/1519-6984.09913
» https://doi.org/10.1590/1519-6984.09913 - Van Valen L. Morphological variation and width of ecological niche. Am Nat. 1965; 99(908):377–90.
- Vazzoler AEAM. Biologia da reprodução de peixes teleósteos: teoria e prática. Maringá: EDUEM;1996.
- Villéger S, Miranda JR, Hernández DF, Mouillot D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol Appl. 2010; 20(6):1512–22. https://doi.org/10.1890/09-1310.1
» https://doi.org/10.1890/09-1310.1 - Wainwright PC, Bellwood DR, Westneat MW. Ecomorphology of locomotion in labrid fishes. Environ Biol Fishes. 2002; 65:47–62. https://doi.org/10.1023/A:1019671131001
» https://doi.org/10.1023/A:1019671131001 - Walker RH, Kluender ER, Inebnit TE, Adams SR. Differences in diet and feeding ecology of similar-sized spotted (Lepisosteus oculatus) and shortnose (Lepisosteus platostomus) gars during flooding of a south-eastern US river. Ecol Freshw Fish. 2013; 22(4):617–25. https://doi.org/10.1111/eff.12066
» https://doi.org/10.1111/eff.12066 - Ward AJW, Webster MM, Hart PJB. Intraspecific food competition in fishes. Fish Fish (Oxf). 2006; 7(4):231–61. https://doi.org/10.1111/j.1467-2979.2006.00224.x
» https://doi.org/10.1111/j.1467-2979.2006.00224.x - Wei T, Simko V. “corrplot”: Visualization of a Correlation Matrix [Internet]. R package; 2017. Available from https://github.com/taiyun/corrplot
» https://github.com/taiyun/corrplot - Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst. 1984; 15:393–425. https://doi.org/10.1146/annurev.es.15.110184.002141
» https://doi.org/10.1146/annurev.es.15.110184.002141 - Willis SC, Winemiller KO, Lopez-Fernandez H. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia. 2005; 142:284–95. https://doi.org/10.1007/s00442-004-1723-z
» https://doi.org/10.1007/s00442-004-1723-z - Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. https://doi.org/10.2307/2937046
» https://doi.org/10.2307/2937046 - Yofukuji KY, Cardozo ALP, Quirino BA, Aleixo MHF, Fugi R. Macrophyte diversity alters invertebrate community and fish diet. Hydrobiologia. 2021; 848:913–27. https://doi.org/10.1007/s10750-020-04501-w
» https://doi.org/10.1007/s10750-020-04501-w - Zelditch ML, Swiderski DL, Sheets HD. Geometric morphometrics for biologists: a primer. London: Academic Press; 2004.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Kliemann BCK, Galdioli EM, Bialetzki A, Delariva RL. Morphological divergences as drivers of diet segregation between two sympatric species of Serrapinnus (Characidae: Cheirodontinae) in macrophyte stands in a neotropical floodplain lake. Neotrop Ichthyol. 2021; 19(2):e200139. https://doi.org/10.1590/1982-0224-2020-0139
Edited-by
Publication Dates
-
Publication in this collection
09 July 2021 -
Date of issue
2021
History
-
Received
1 Apr 2020 -
Accepted
11 June 2021