Abstract
The objective of this study was to investigate some life history traits of the palaemonid shrimp Typton carneus collected from a reef in northeastern Brazil. Samples of the sponges Amphimedon compressa, A. viridis, Desmapsamma anchorata, Dysidea etheria, Haliclona implexiformis and Tedania ignis were analyzed and shrimps were removed from them. A total of 41 individuals were found in Te. ignis, three in H. implexiformis and one in D. etheria; the latter two sponges are new records of sponge hosts for Ty. carneus. Of the specimens associated with Te. ignis, 24 were males, 10 ovigerous females, six non-ovigerous females and one juvenile male. Fecundity varied between 19 to 56 eggs (37 ± 14) per female, and the mean egg volume was 0.033 ± 0.010 mm³. Eleven heterosexual pairs were obtained. Characteristics of the pairs suggest a monogamous mating system for the studied population, such as: the absence of sexual dimorphism in weaponry and body size and presence of paired non-brooding and brooding females carrying eggs in different development stages; and a sex ratio that does not differ from the expected 1:1. However, the lack of size-assortative pairing as well as the low proportion of pairs, compared to solitary individuals, have been observed in polygamous mating systems.
Keywords:
Caridean shrimps; heterosexual pairing; Porifera; symbiosis;
Tedania ignis
INTRODUCTION
A wide range of caridean shrimps, those of the families Alpheidae Rafinesque, 1815Rafinesque, C.S. 1815 Analyse de la nature ou tableau de l’Univers et des corps organisés. Palerme, L’Imprimerie de Jean Barravecchia, 224p., Anchistioididae Borradaile, 1915Borradaile, L.A. 1915. Notes on Carides. The Annals and Magazine of Natural History, 15: 205-213. , Hippolytidae C.S. Bate, 1888Bate, C.S. 1888. Report on the Crustacea Macrura collected by the Challenger during the years 1873-76. In: J. Murray (ed), Report on the Scientific Results of the Voyage of H.M.S. ”Challenger” during the years 1873-76 Under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N. C. Wyville Thomson and J. Murray (series eds), Zoology. Vol. 24. i-xc, 1-942. pls., 1-157. Edinburgh, Neill and Company., Lysmatidae Dana, 1852Dana, J.D. 1852. United States Exploring Expedition during the years 1838, 1839, 1840, 1841, 1842, under the Command of Charles Wilkes, U.S.N. Vol. 13. Crustacea. Part I. C. Sherman, Philadelphia, 685p. and Palaemonidae Rafinesque, 1815Rafinesque, C.S. 1815 Analyse de la nature ou tableau de l’Univers et des corps organisés. Palerme, L’Imprimerie de Jean Barravecchia, 224p., for example, have been reported in association with sponges (Bruce, 1976Bruce, A.J. 1976. Coral Reef Caridea and “Commensalism”. Micronesica, 12: 83-98. ; Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). However, for most species, the exact type of association is still unknown (Hultgren, 2014Hultgren, K.M. 2014. Variable effects of symbiotic snapping shrimps on their sponge hosts. Marine Biology, 161: 1217-1227.). Shrimps associated with other invertebrates exhibit specialized morphological characteristics; however, their adaptive value still needs to be better understood (Bauer, 2004Bauer, R.T. 2004. Remarkable shrimps: adaptations and natural history of the carideans. Norman, University of Oklahoma Press, 282p. ; Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). For example, palaemonid shrimps of the genus OnychocarisNobili, 1904Nobili, G. 1904. Diagnoses préliminaires de vingt-huit espèces nouvelles de stomatopodes et decápodes macroures de la Mer Rouge. Bulletin du Muséum d’Histoire Naturelle, 10: 228-238. and TyptonCosta, 1844Costa, O.G. 1844. Su due nuovi generi di Crostacei decapodi macrouri. Annali delle Accademia degli Aspiranti Naturalisti, Napoli, 2: 285-292. have a vermiform body, a relatively short cephalothorax and an elongated pleon, which presumes adaptations to life in tubular sponge channels (Bruce, 1976Bruce, A.J. 1976. Coral Reef Caridea and “Commensalism”. Micronesica, 12: 83-98. ). The interior architecture of many sponges, with a large network of channels of different diameters, provides a suitable habitat, not only for these shrimps, but for several other groups as well (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ).
Species of Typton are usually smaller than 10 mm and are characterized by a laterally compressed rostrum with no teeth, mandible without palp, strongly asymmetrical second pair of pereopods and second pair of pleopods without appendix masculine, among other morphological features (Holthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ; Vieira et al., 2012Vieira, R.R.R.; Ferreira, R.S. and D’Incao, F. 2012. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa, 3149: 1-38.). These shrimps are known as sponge symbionts, where they are found alone or in heterosexual pairs (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). Overlapping generations, with juveniles remaining, at least initially, in the natal sponge, have been observed in some sponge-dwelling shrimps (Duffy, 2003Duffy, J.E. 2003. The ecology and evolution of eusociality in sponge-dwelling shrimp. p. 1-38. In: T. Kikuchi (ed), Genes, Behavior, and Evolution in Social Insects. Sapporo, Japan, University of Hokkaido Press. ). However, cases in which larvae are released into the water column, where they remain for days or weeks before settling into the benthos, have also been recorded (Duffy, 2003Duffy, J.E. 2003. The ecology and evolution of eusociality in sponge-dwelling shrimp. p. 1-38. In: T. Kikuchi (ed), Genes, Behavior, and Evolution in Social Insects. Sapporo, Japan, University of Hokkaido Press. ). When embryonic development is abbreviated or direct, larger eggs and high yolk amounts are expected (Bauer, 1991Bauer, R.T. 1991. Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. p. 181-191. In: A. Wenner and A. Kuris (eds), Crustacean egg production. Crustacean Issues 7. Rotterdam, Balkema Press.; Wehrtmann and Albornoz, 2002Wehrtmann, I.S. and Albornoz, L. 2002. Evidence of different reproductive traits in the transisthmian sister species, Alpheus saxidomus and A. simus (Decapoda, Caridea, Alpheidae): description of the first postembryonic stage. Marine Biology, 140: 605-612.). If Typton shrimps experience such development, they are expected to have large embryos.
The genus currently includes 20 species distributed worldwide (De Grave and Fransen, 2011De Grave, S. and Fransen, C.H.J.M. 2011. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zoologische Mededelingen, 85: 195-588. ; Almeida et al., 2014Almeida, A.O.; Anker, A. and Mantelatto, F.L. 2014. A new snapping species of the shrimp genus Typton Costa, 1844 (Decapoda: Palaemonidae) from the coast of São Paulo, southeastern Brazil. Zootaxa, 3835: 110-120.; Ayón-Parente et al., 2015Ayón-Parente, M.; Hendrickx, E.M. and Galvan-Villa, C.M. 2015. A new species of the genus Typton Costa (Crustacea: Decapoda: Palaemonidae: Pontoniinae) from the eastern tropical Pacific. Zootaxa, 3926: 430-438.; Neves, 2020Neves, K. 2020. A new species of the shrimp genus Typton Costa, 1844 (Malacostraca, Decapoda, Palaemonidae) from the Cabo Verde Archipelago. Zootaxa, 4768: 264-270.), of which seven have been recorded in Brazil: Typton carneusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. , Typton distinctus Chace, 1972, Typton fapespae Almeida, Anker and Mantelatto, 2014, Typton gnathophylloides, Holthuis, 1951, Typton prionurus Holthuis, 1951, Typton tortugae McClendon, 1911 and Typton vulcanus Holthuis, 1951 (Coelho et al., 2006Coelho, P.A.; Almeida, A.O.; Souza-Filho, J.F.; Bezzera, L.E.A. and Giraldes, B.W. 2006. Diversity and distribution of the marine and estuarine shrimps (Dendobranchiata, Stenopodidea and Caridea) from North and Northeast Brazil. Zootaxa, 1221: 41-62.; Vieira et al., 2012Vieira, R.R.R.; Ferreira, R.S. and D’Incao, F. 2012. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa, 3149: 1-38.; Almeida et al., 2014Almeida, A.O.; Anker, A. and Mantelatto, F.L. 2014. A new snapping species of the shrimp genus Typton Costa, 1844 (Decapoda: Palaemonidae) from the coast of São Paulo, southeastern Brazil. Zootaxa, 3835: 110-120.; Pachelle et al., 2015Pachelle, P.P.G.; Anker, A. and Tavares, M. 2015. New and additional records of the sponge shrimp genus Typton Costa, 1844 (Decapoda: Palaemonidae) from the Brazilian coast. Papéis Avulsos de Zoologia, 55: 317-322. ; 2016Pachelle, P.P.G.; Anker, A.; Mendes, C.B. and Bezerra, L.E.A. 2016. Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa, 4131: 1-63.; Soledade et al., 2017Soledade, G.O.; Santos, G.G.; Pinheiro, U. and Almeida, A.O. 2017. New records of association between caridean shrimps (Decapoda) and sponges (Porifera) in Abrolhos Archipelago, northeastern Brazil. Nauplius, 25: e2017027.).
Typton carneus is distributed in the western Atlantic in western Florida, Bahamas, Barbuda, Cuba (Los Arroyos), Mexico (Bahia de la Ascensión), Haiti (Tortuga), Panama, Tobago, and Brazil (Fernando de Noronha, Ceará, Paraíba, and Pernambuco States), from 0 - 73 m depth (Holthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ; Chace, 1972Chace Jr, F.A. 1972. The shrimps of the Smithsonian-Bredin Caribbean Expeditions with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithsonian Contributions to Zoology, 98: 1-179.; Coelho et al., 2006Coelho, P.A.; Almeida, A.O.; Souza-Filho, J.F.; Bezzera, L.E.A. and Giraldes, B.W. 2006. Diversity and distribution of the marine and estuarine shrimps (Dendobranchiata, Stenopodidea and Caridea) from North and Northeast Brazil. Zootaxa, 1221: 41-62.; Román-Contreras and Martínez-Mayén, 2010Román-Contreras, R. and Martínez-Mayén, M. 2010. Palaemonidae (Crustacea: Decapoda: Caridea) from the shallow waters from Quintana Roo, Mexican Caribbean coast. Revista Mexicana de Biodiversidad, 81: 43-51.; Vieira et al., 2012Vieira, R.R.R.; Ferreira, R.S. and D’Incao, F. 2012. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa, 3149: 1-38.; Pachelle et al., 2016Pachelle, P.P.G.; Anker, A.; Mendes, C.B. and Bezerra, L.E.A. 2016. Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa, 4131: 1-63.; De Grave and Anker, 2017De Grave, S. and Anker, A. 2017. An annotated checklist of marine caridean and stenopodidean shrimps (Malacostraca: Decapoda) of the Caribbean coast of Panama. Nauplius, 25: e2017015.). However, information about its biological aspects, such as interactions with its hosts and reproduction, is still wanting, and data is mostly limited to occurrences and associations with sponges (Holthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ; Chace, 1972Chace Jr, F.A. 1972. The shrimps of the Smithsonian-Bredin Caribbean Expeditions with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithsonian Contributions to Zoology, 98: 1-179.; Ramos-Porto and Coelho, 1998Ramos-Porto, M. and Coelho, P.A. 1998. Malacostraca. Eucarida. Caridea (Alpheoidea excluded). p. 325-350. In: P.S. Young (ed), Catalogue of Crustacea of Brazil. Museu Nacional, Rio de Janeiro. (Série Livros 6).; Vieira et al., 2012Vieira, R.R.R.; Ferreira, R.S. and D’Incao, F. 2012. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa, 3149: 1-38.; Pachelle et al., 2016Pachelle, P.P.G.; Anker, A.; Mendes, C.B. and Bezerra, L.E.A. 2016. Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa, 4131: 1-63.; De Grave and Anker, 2017De Grave, S. and Anker, A. 2017. An annotated checklist of marine caridean and stenopodidean shrimps (Malacostraca: Decapoda) of the Caribbean coast of Panama. Nauplius, 25: e2017015.). So far, the only specific study about this shrimp species analyzed its stomach contents, suggesting a possible parasitic habit on their sponge hosts (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). The objective of the present study was to address certain aspects of the life history of Ty. carneus, such as association with sponge hosts, breeding biology and heterosexual pairing (pairing frequency, sexual dimorphism between paired males and females, presence of size-assortative pairing) in a reef area in northeastern Brazil.
MATERIAL AND METHODS
Three specimens of the sponges Amphimedon compressa Duchassaing and Michelotti, 1864, Amphimedon viridis Duchassaing and Michelotti, 1864, Desmapsamma anchorataCarter, 1882Carter, H.J. 1882. Some sponges from the West Indies and Acapulco in the Liverpool Free Museum described, with general and classificatory remarks. Annals and Magazine of Natural History, 9: 266-301., Dysidea etheriaLaubenfels, 1936Laubenfels, M.W. 1936. A discussion of the sponge fauna of the Dry Tortugas in particular and the West Indies in general, with material for a revision of the families and orders of the Porifera. Papers from the Tortugas Laboratory of the Carnegie Institution of Washington, 467: 1-225., Haliclona implexiformisHechtel, 1965Hechtel, G.J. 1965. A systematic study of the Demospongiae of Port Royal, Jamaica. Bulletin of the Peabody Museum of Natural History, 20: 1-103. and Tedania ignis Duchassaing and Michelotti, 1864 were collected monthly from reefs off Praia de Pontas de Pedra, Goiana Municipality, Pernambuco, northeastern Brazil (07°37’00”S 34°48’51”W) (Fig. 1) from September 2014 to February 2016. Collections were carried out during periods of spring low tide, totaling 54 specimens analyzed for each species. The sponges were collected by free diving in the shallow subtidal zone at a maximum depth of one meter. The sponge specimens were sampled with a minimum distance of five meters between each sponge and were removed from the substrate with knives and spatulas. Afterwards, each specimen was separately packed in a plastic bag and preserved in 70 % ethanol. Size, volume and weight of the living sponge specimens were not recorded. In the laboratory, shrimps were removed from the sponge channels with tweezers and preserved in 70 % ethanol after entire host fragmentation. Individuals retrieved from the same channel were considered paired. Voucher specimens were deposited in the crustacean collection at the Museu de Oceanografia Professor Petrônio Alves Coelho of the Universidade Federal de Pernambuco, Recife, Brazil (MOUFPE 19327 - 19416).
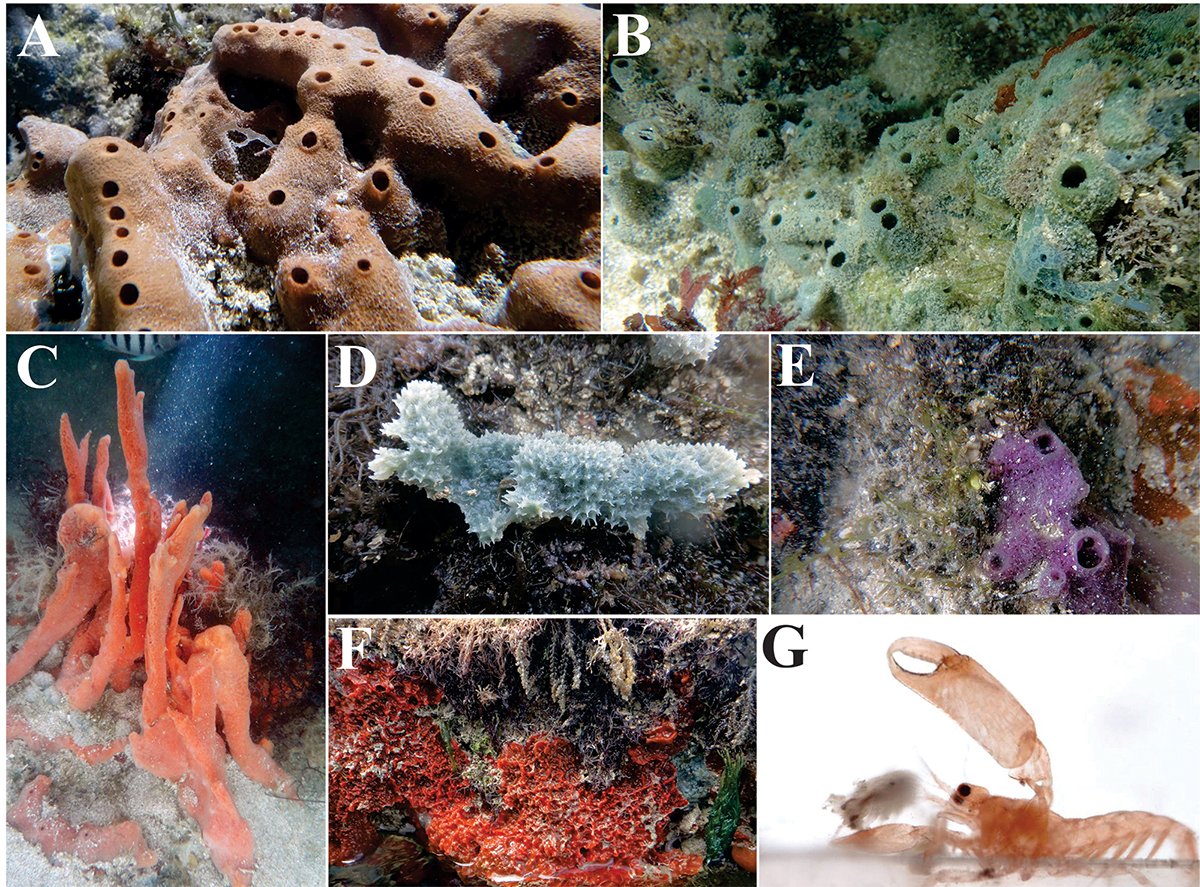
(A-F) Sponges sampled from Ponta de Pedras, Pernambuco, northeastern Brazil. A, Amphimedon compressaDuchassaing and Michelotti, 1864Duchassaing De Fonbressin, P. and Michelotti, G. 1864. Spongiaires de la Mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem, 21: 1-124.; B, Amphimedon viridisDuchassaing and Michelotti, 1864Duchassaing De Fonbressin, P. and Michelotti, G. 1864. Spongiaires de la Mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem, 21: 1-124.; C, Desmapsamma anchorataCarter, 1882Carter, H.J. 1882. Some sponges from the West Indies and Acapulco in the Liverpool Free Museum described, with general and classificatory remarks. Annals and Magazine of Natural History, 9: 266-301.; D, Dysidea etheriaLaubenfels, 1936Laubenfels, M.W. 1936. A discussion of the sponge fauna of the Dry Tortugas in particular and the West Indies in general, with material for a revision of the families and orders of the Porifera. Papers from the Tortugas Laboratory of the Carnegie Institution of Washington, 467: 1-225.; E, Haliclona implexiformisHechtel, 1965Hechtel, G.J. 1965. A systematic study of the Demospongiae of Port Royal, Jamaica. Bulletin of the Peabody Museum of Natural History, 20: 1-103.; F, Tedania ignisDuchassaing and Michelotti, 1864Duchassaing De Fonbressin, P. and Michelotti, G. 1864. Spongiaires de la Mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem, 21: 1-124.; G, Living individual of Typton carneusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. just retrieved from its sponge host, lateral view (Photographs: A-F, T.E.R. Cavalcanti; G, F. Barletta).
Shrimp were identified using identification keys (e.g., Chace, 1972; Abele and Kim, 1986Abele, L.G. and Kim, W. 1986. An illustrated guide to the marine decapod crustaceans of Florida. Florida Department of Environmental Regulation, Technical Series, 8: 1-225.) and by consulting the original species descriptions (Holthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ). Sex of caridean shrimps is mostly determined by the presence (males) or absence (females) of the appendix masculina on the endopod of the second pleopod (Bauer, 2004Bauer, R.T. 2004. Remarkable shrimps: adaptations and natural history of the carideans. Norman, University of Oklahoma Press, 282p. ). However, as such a structure is absent in males of Typton, we analyzed characters such as the presence/absence (females/males) of eggs underneath the pleon and the height of the pleura of the pleonal segments (lower in males than in females) for sex recognition. Individuals with a CL larger than the smallest ovigerous female were considered adults. Due to the small number (four) of shrimps found in the other sponge species, the breeding biology and heterosexual pairing analyzes were conducted based on those sampled in Te. ignis only.
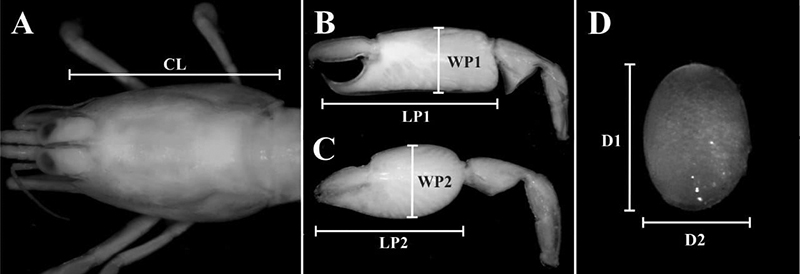
Subsequently, each individual was photographed under a stereomicroscope with an image capture system (Leica EZ4). The same procedure was adopted for the eggs, which were removed from the pleon of the ovigerous females with brushes, and egg development stages were classified according to Mossolin et al. (2006Mossolin, E.C.; Shimizu, R.M. and Bueno, S.L.S. 2006. Population structure of Alpheus armillatus (Decapoda, Alpheidae) in São Sebastião and Ilhabela, southeastern Brazil. Journal of Crustacean Biology, 26: 48-54.): initial (I - embryos with no visible eyes; yolk occupying more than 75 % of the embryonic volume), intermediate (II - embryos with small, elongated eyes; yolk occupying 50-75 % of the embryo volume) and final (III - embryos with well-developed eyes; yolk occupying 25-50 % of the embryo volume). The longest (d1) and shortest (d2) egg diameter were measured in the ImageJ software (1.45s) (Schneider et al., 2012Schneider, C.A., Rasband, W.S. and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9: 671-675. ) to calculate the volume using the formula: v = 1/6 × π × (d1)² × d2 (Bauer, 1991Bauer, R.T. 1991. Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. p. 181-191. In: A. Wenner and A. Kuris (eds), Crustacean egg production. Crustacean Issues 7. Rotterdam, Balkema Press.) (Fig. 2). Only females carrying eggs in stage I were used (n = 5), (1.09-2.16 mm in CL) due to the low number of females with eggs in stage III. Mean egg volume was measured from the same five ovigerous females, all of which carried well-preserved eggs.
Morphometric dimensions obtained for the sponge-dwelling shrimp Typton carneusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. from Ponta de Pedras, Pernambuco, northeastern Brazil, sampled between September 2014 and February 2016. A, Carapace, dorsal view; B, major chela of the second pereiopod, lateral view; C, minor chela of the second pereiopod, lateral view; D, egg. (CL) Carapace length; (LP1 and WP1) propodus length and width of the major chela, respectively; (LP2 and WP2) propodus length and width of the minor chela, respectively; (D1 and D2) longest and shortest egg diameter, respectively.
Afterwards, the following body parts were measured using ImageJ software: carapace length (CL) (from the tip of the rostrum to the posterior margin of the carapace) and the length and width of propodus of the second pair of pereopods (major and minor chelae). All data were tested for normality, non-parametric tests were used whenever possible and the significance level of 5 % was adopted for all statistical analyses. Differences in mean CL and other body parts (length and width of propodus of the second pair of pereopods) between the sexes were verified by an analysis of covariance (ANCOVA) with Kruskal-Wallis test a posteriori. The CL was used as the independent variable in all analyzes. The presence of CL correlations between paired males and females (i.e., size-assortative pairing) was calculated by the Spearman correlation coefficient. A Chi-Square test with Equal Expected Proportions with a Yates Correction a posteriori was used to determine whether the sex ratio followed the expected ratio of 1:1. Statistical analyzes were performed in the Paleontological Statistic program (Past), version 2.17c and in the BioEstat, version 5.3.
RESULTS
A total of 45 individuals were obtained, of which 41 (91.1 %) were found in Te. ignis, three (6.7 %) in H. implexiformis and only one (2.2 %) in D. etheria. In Te. ignis, shrimps were present in 25 of the 54 analyzed sponges (prevalence of 46 %).
A total of 24 males and 16 females (10 ovigerous) were considered adults (CL > 1.10 mm, which was the CL of the smallest ovigerous females) and one was considered juvenile (CL = 0.95 mm). The majority of ovigerous females (9) carried stage I eggs, while stage III eggs were observed in only one female. Ovigerous females were observed in six of the 18 sampling months, both in dry (September to March) and rainy (April to August) seasons in the study area (Fig. 3).
Monthly distribution of ovigerous females of the sponge-dwelling shrimp Typton carneusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. from Pontas de Pedras, Pernambuco, northeastern Brazil, sampled between September 2014 and February 2016. Dry season: from September to February; Rainy season: from March to August.
Fecundity of females carrying stage I eggs varied from 19 to 56 eggs (37 ± 14, mean ± standard deviation) with a mean volume of 0.033 ± 0.010 mm³. The female with stage III eggs (1.75 mm in CL) was carrying 22 eggs. The CL of non-ovigerous females and ovigerous females varied from 1.28 to 2.15 mm (1.72 ± 0.31 mm) and 1.10 to 2.16 mm (1.87 ± 0.31 mm), respectively. The CL of males ranged from 1.46 to 2.14 mm (1.78 ± 0.19 mm). Males and ovigerous females differed in mean CL size (p < 0.05). No statistical differences in CL were found among the other classes (males vs. non-ovigerous females, males vs. total females, and non-ovigerous females vs. ovigerous females) (p > 0.05).
The mean length of the propodus of the major chela did not differ between the sexes (males 2.52 ± 0.83 mm and females 2.54 ± 0.68 mm). On the other hand, the mean length of the propodus of the minor chela was slightly larger in females (1.60 ± 0.33 mm) when compared to males (1.48 ± 0.31 mm). In relation to the mean width of the major and minor chelae, slightly larger values were observed in females (major chela, 1.01 ± 0.24 mm; minor chela 0.74 ± 0.15 mm) than in males (major chela, 0.99 ± 0.28 mm; minor chela, 0.68 ± 0.13 mm). However, such mean values did not differ statistically between the sexes (p > 0.05).
Of the 41 specimens obtained in Te. ignis, 12 were solitary and 12 were paired (one pair of males and 11 heterosexual pairs) in 22.2 % of the sponge samples. Additionally, a group of five individuals (4 % of the individuals) was found in one sponge sample (1.9 % of Te. ignis samples). In 53.7 % of the analyzed samples no shrimp were observed. Within paired individuals, eight females (72.7 %) were ovigerous, seven of them were carrying stage I eggs and one was carrying stage III eggs. Paired females have similar lengths as males (1.89 ± 0.21 mm vs. 1.89 ± 0.18 mm, respectively), with no significant difference between the sexes (p > 0.05). No CL size correlation (size-assortative pairing) was observed in the heterosexual pairs (r = -0.10023; p = 0.77) (Fig. 4). The sex ratio was 1:0.64 (M:F) and did not differ statistically from the expected ratio of 1:1 (p = 0.21).
Correlation between the carapace lengths of heterosexual pairs of sponge-dwelling shrimp Typton carneusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. from Pontas de Pedras, Pernambuco, northeastern Brazil, sampled between September 2014 and February 2016.
DISCUSSION
Distribution of Typton carneus in sponge hosts
The number of Ty. carneus individuals found indicates a tight symbiotic fit regarding sponges, especially with Te. ignis. In fact, some association records of Typton spp. with TedaniaGray, 1867Gray, J.E. 1867. Notes on the arrangement of sponges, with the descriptions of some new genera. Proceedings of the Zoological Society of London, 1867: 492-558. sponges (Tedania klausiWulff, 2006Wulff, J.L. 2006. Sponge systematics by starfish: predators distinguish cryptic sympatric species of Caribbean fire sponges, Tedania ignis and Tedania klausi n.sp. (Demospongiae, Poecilosclerida). Biological Bulletin, 211: 83-94. and Te. ignis) have been documented (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ; Santana-Moreno et al., 2013Santana-Moreno, L.D.; De Grave, S. and Simões, N. 2013. New records of caridean shrimps (Decapoda: Caridea) from shallow water along the northern Yucatan peninsula coasts of México. Nauplius, 21: 225- 238.; De Grave and Anker, 2017De Grave, S. and Anker, A. 2017. An annotated checklist of marine caridean and stenopodidean shrimps (Malacostraca: Decapoda) of the Caribbean coast of Panama. Nauplius, 25: e2017015.), probably since these sponges have suitable morphological and chemical characteristics, and are potentially used as a food source, according to Ďuriš’ et al. (2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ) (see below). Herein, we report the occurrence of Ty. carneus in D. etheria and H. implexiformis for the first time, increasing knowledge about sponge hosts of this shrimp. This indicates that Ty. carneus has some type of specialization to live in Te. ignis in the study area but it can explore other sponge hosts. Along with the sponges analyzed in this study, Ty. carneus has been recorded to inhabit others (Chace, 1972Chace Jr, F.A. 1972. The shrimps of the Smithsonian-Bredin Caribbean Expeditions with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithsonian Contributions to Zoology, 98: 1-179.; Ramos-Porto and Coelho, 1998Ramos-Porto, M. and Coelho, P.A. 1998. Malacostraca. Eucarida. Caridea (Alpheoidea excluded). p. 325-350. In: P.S. Young (ed), Catalogue of Crustacea of Brazil. Museu Nacional, Rio de Janeiro. (Série Livros 6).; Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ) (see Tab. 1for a summary of previous association records between species of Typton and sponges).
Species of palaemonid shrimps of the genus TyptonCosta, 1844Costa, O.G. 1844. Su due nuovi generi di Crostacei decapodi macrouri. Annali delle Accademia degli Aspiranti Naturalisti, Napoli, 2: 285-292. and their hosts/habitats.
A few species of Typton have not been recorded in association with sponges. Typton ascensionisManning and Chace, 1990Manning, R.B. and Chace, Jr. F.A. 1990. Decapod and stomatopod Crustacea from Ascension Island, South Atlantic Ocean. Smithsonian Contributions to Zoology, 503: 1-91. and Typton holthuisiDe Grave, 2010De Grave, S. 2010. A new species of the genus Typton Costa (Decapoda, Palaemonidae, Pontoniinae) from Ascension Island. Crustaceana Monographs, 14: 209-218. have both been found in association with algae (Manning and Chace, 1990Manning, R.B. and Chace, Jr. F.A. 1990. Decapod and stomatopod Crustacea from Ascension Island, South Atlantic Ocean. Smithsonian Contributions to Zoology, 503: 1-91.; De Grave, 2010De Grave, S. 2010. A new species of the genus Typton Costa (Decapoda, Palaemonidae, Pontoniinae) from Ascension Island. Crustaceana Monographs, 14: 209-218.), whereas Typton granulosusAyón-Parente, Hendrickx and Galvan-Villa, 2015Ayón-Parente, M.; Hendrickx, E.M. and Galvan-Villa, C.M. 2015. A new species of the genus Typton Costa (Crustacea: Decapoda: Palaemonidae: Pontoniinae) from the eastern tropical Pacific. Zootaxa, 3926: 430-438., was associated with the sea-cucumber Holothuria (Halodeima) inornataSemper, 1868Semper, C. 1868. Reisen im Archipel der Philippinen. Zweiter Theil. Wissenschaftliche Resultate. Erster Band. Holothurien. Hefte iv. and v: Unpaginated. (Ayón-Parente et al., 2015Ayón-Parente, M.; Hendrickx, E.M. and Galvan-Villa, C.M. 2015. A new species of the genus Typton Costa (Crustacea: Decapoda: Palaemonidae: Pontoniinae) from the eastern tropical Pacific. Zootaxa, 3926: 430-438.). Further studies regarding such relationships should be carried out to provide more information about the biological aspects of these caridean shrimps. Such refinement is important for a broader understanding of the relationships between these organisms.
Shrimp abundance by host may be explained by a set of factors, including morphological differences between them and chemical influence of their biological compounds, among others. Tedania ignis and H. implexiformis present high surface rugosity and an abundance of internal spaces, as well as a large body volume which could favor colonization of their internal spaces by symbiotic organisms such as shrimps. The occurrence of only one individual in D. etheria suggests that this sponge is not as suitable as Te. ignis and H. implexiformis for Ty. carneus colonization. On the other hand, although A. compressa and A. viridis have internal spaces suitable for shrimp colonization, they did not harbor any Ty. carneus individuals. This may have been caused by factors besides morphology. Amphimedon compressa makes an amphitoxin, which is a chemical defense against predation by reef fish (Albrizio et al., 1995Albrizio, S.; Cimeniello, P.; Fattorusso, E.; Magno, S. and Pawlik, J.R. 1995. Amphitoxin, a new high molecular weight antifeedant pyridinium salt from the Caribbean sponge Amphimedon compressa. Journal of Natural Products, 58: 647-652. ), which could explain the absence of Ty. carneus. Furthermore, A. viridis produces a halitoxin, which is moderately lethal to rats (Berlink et al., 1996Berlink, R.G.S.; Ogawa, C.A.; Almeida, A.M.P.; Sanchez, M.A.A.; Malpezzi, E.L.A.; Costa, L.V.; Hadju, E. and Freitas, J.C. 1996. Chemical and pharmacological characterization of halitoxin from Amphimedon viridis (Porifera) from the southeastern Brazilian coast. Comparative Biochemistry and Physiology, 115: 155-163.) and, in another experiment, may have killed all hydrozoan Zyzzyzus warreniCalder, 1988Calder, D.R. 1988. Shallow-water hydroids of Bermuda. The Athecatae. Royal Ontario Museum Life Sciences Contributions, 148: 1-107. larvae that settled on this sponge (Campos et al., 2012Campos, C.J.A.; Migotto, A.E.; Pinheiro, U. and Marques, A.C. 2012. Sponges as substrata and early life history of the tubulariid Zyzzyzus warreni (Cnidaria: Hydrozoa) in the São Sebastião Channel, Brazil. Marine Biology Research, 8: 573-583.). However, Ty. gnathophylloides has a previous association record with such a sponge (Soledade et al., 2017Soledade, G.O.; Santos, G.G.; Pinheiro, U. and Almeida, A.O. 2017. New records of association between caridean shrimps (Decapoda) and sponges (Porifera) in Abrolhos Archipelago, northeastern Brazil. Nauplius, 25: e2017027.), indicating that this shrimp may tolerate the toxins produced by this host. Chemical defenses were also experimentally observed in D. etheria, which shows low predation by starfish (Waddell and Pawlik, 2000Waddell, B. and Pawlik, J.R. 2000. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Marine Ecology Progress Series, 195: 133-144.). Information provided herein could be used to test hypotheses about sponge toxicity and Typton shrimp host distribution in the future.
Despite having some toxicity, an expressed Tedanolide, which is found in Te. ignis, did not prevent the establishment or development of Z. warreni larvae under experimental conditions (Schmitz et al., 1984Schmitz, F.J.; Gunasekera, S.P.; Yalamanchili, G.; Hossain, M.B. and Van Der Helm, D. 1984. Tedanolide: a potent cytotoxic macrolide from the Caribbean sponge Tedania ignis. Journal of the American Chemical Society, 106: 7251-7252.), nor did it prevent a high predation rate by the starfish Echinaster echinophorusLamarck, 1816Lamarck, J.B.P. 1816. Histoire naturelle des animaux sans vertèbres. Tome second. Paris, Verdière, 568p. and Echinaster sentusSay, 1825Say, T. 1825. On the species of the Linnaean genus Asterias inhabiting the coast of the U.S. Journal of the Academy of Natural Sciences of Philadelphia, 5: 141-154. (Waddell and Pawlik, 2000Waddell, B. and Pawlik, J.R. 2000. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Marine Ecology Progress Series, 195: 133-144.). This suggests that this compound may not repel other organisms (Waddell and Pawlik, 2000Waddell, B. and Pawlik, J.R. 2000. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Marine Ecology Progress Series, 195: 133-144.), making it possible to harbor ecto- and endobionts (Rützler, 2004Rützler, K. 2004. Sponges on coral reefs: a community shaped by competitive cooperation. Bolletino dei Musei e Degli Istituti Biologici dell’Univiversità di Genova, 68: 85-148. ). Thus, the high prevalence of Ty. carneus in Te. ignis in the present study suggests a possible lack of toxicity of this sponge to this shrimp.
The orange color of Ty. carneus (Fig. 1G) resembles Te. ignis coloration (Fig. 1F). Such cryptic coloration could also explain the higher occurrence of this shrimp in Te. ignis when compared to the other sponges analyzed here. However, the shrimps were observed in the internal portion, inhabiting the largest and deepest chambers of the host, where cryptic coloration may not be relevant. Such coloration could actually be caused by the sequestration of pigments from its host’s tissues, which has already been recorded for other shrimp species that are symbionts of sponges and echinoderms (Periclimenes sororNobili, 1904Nobili, G. 1904. Diagnoses préliminaires de vingt-huit espèces nouvelles de stomatopodes et decápodes macroures de la Mer Rouge. Bulletin du Muséum d’Histoire Naturelle, 10: 228-238. , Tuleariocaris holthuisiHipeau-Jacquotte, 1971Hipeau-Jacquotte, R. 1971. Notes de faunistique et de biologie marines de Madagascar, V. Platypontonia hyotis nov. sp. (Decapoda Natantia, Pontoniinae). Crustaceana, 20: 125-140. , Gnathophylloides mineriSchmitt, 1933Schmitt, W.L. 1933. Four new species of decapod crustaceans from Porto Rico. American Museum Novitates, 662: 1-9. , and Periclimenaeus caraibicusHolthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ) (Castro, 1971Castro, P. 1971. The natantian shrimps (Crustacea, Decapoda) associated with invertebrates in Hawaii. Pacific Science, 25: 395-403. ; Patton et al., 1985 Patton, W.K.; Patton, R.J. and Barnes, A. 1985. On the biology of Gnathophylloides mineri, a shrimp inhabiting the sea urchin Tripneustes ventricosus. Journal of Crustacean Biology, 5: 616-626. ). However, pigment sequestration in Ty. carneus needs to be further investigated. Information about the color of shrimps collected in H. implexiformis, a typically purple sponge (Fig. 1E), would shed some light on possible pigment sequestration by Ty. carneus. However, such information was not recorded in this study.
Although the actual relationship between Ty. carneus and its hosts are still unclear, there are morphological indications that suggest a parasitic habit of the species regarding its host. These include, for example, morphology of the fingers of the major chelae on the second pair of pereopods with scissors-like closure, cutting edge of these fingers with grooves caused by intense mechanical abrasion, and a series of small teeth on these cutting edges (see Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). Another characteristic is a large subtriangular lobe at the proximal end of the cutting edge of the propodus, present on the chelae of Ty. carneus and other shrimps living on host sponges; possibly conducting and controlling the movement of the dactyl of the fixed finger (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). There is also evidence of parasitism based on the presence of sponge spicules in the stomach contents of this shrimp, as well as for congenerics Ty. distinctus and Typton spongicolaCosta, 1844Costa, O.G. 1844. Su due nuovi generi di Crostacei decapodi macrouri. Annali delle Accademia degli Aspiranti Naturalisti, Napoli, 2: 285-292. (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ). However, this parasitic feeding habit does not necessarily mean that the damage caused by the shrimp is not offset by some benefit to the host and, any kind of benefit is still unknown in this relationship (Ďuriš et al., 2011Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987. ).
Breeding biology
Herein, we provide initial information about the breeding biology of Ty. carneus. Most females associated with Te. ignis were ovigerous (62.5 %), indicating the importance of the host as a reproduction site. The frequency of ovigerous females was low (in six of the 18 sampling months); however, their occurrence in both dry and rainy seasons in the study area suggests continuous reproduction for Ty. carneus, which has been observed for other tropical caridean shrimps (Bauer, 1989Bauer, R.T. 1989. Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127: 175-187.; Terossi and Mantellato, 2010Terossi, M. and Mantelatto, F.L. 2010. Sexual ratio, reproductive period and seasonal variation of the gonochoric shrimp Hippolyte obliquimanus (Caridea: Hippolytidae). Marine Biology Research, 6: 213-219.; Almeida et al., 2011Almeida, A.C.; Fransozo, V.; Teixeira, G.M.; Furlan, M.; Hiroki, K.A.N. and Fransozo, A. 2011. Population structure and reproductive period of whitebelly prawn Nematopalaemon schmitti (Holthuis, 1950) (Decapoda: Caridea: Palaemonidae) on the southeastern coast of Brazil.Invertebrate Reproduction and Development, 55: 30-39.).
Only two other studies (original descriptions of two other Typton species) have recorded the number of eggs carried by breeding females. Bruce (1973Bruce, A.J. 1973. Typton australis sp. nov., a new pontoniid shrimp from the Great Barrier Reef, Australia. Records of the Australian Museum, 28: 253-263.; 2000Bruce, A.J. 2000. Typton manningi and T. capricorniae, new species, new pontoniine shrimps from northern Queensland, with a review of the Indo-West Pacific species of Typton Costa (Decapoda: Palaemonidae). Journal of Crustacean Biology, 20: 87-100.) reported ovigerous females of Typton australis Bruce, 1973 and Typton manningi Bruce, 2000 carrying approximately 10 and 150 eggs, respectively. When redescribing Ty. spongicola, Bruce (2009Bruce, A.J. 2009. A re-description of Typton spongicola Costa, 1844, the type species of the genus Typton Costa, 1844 (Crustacea: Decapoda: Pontoniinae). Cahiers de Biologie Marine, 50: 383-394. ) mentioned that this shrimp produces many small eggs, which suggests higher fecundity for this species. However, comparisons with Ty. carneus fecundity are not reliable because Bruce (1973Bruce, A.J. 1973. Typton australis sp. nov., a new pontoniid shrimp from the Great Barrier Reef, Australia. Records of the Australian Museum, 28: 253-263.; 2000Bruce, A.J. 2000. Typton manningi and T. capricorniae, new species, new pontoniine shrimps from northern Queensland, with a review of the Indo-West Pacific species of Typton Costa (Decapoda: Palaemonidae). Journal of Crustacean Biology, 20: 87-100.) only examined the fecundity of one ovigerous female. Different factors may influence fecundity in caridean shrimps, including female and egg sizes (Sastry, 1983Sastry, A.N. 1983. Ecological aspects of reproduction. p. 179-270. In: F.J. Vernberg and W.B. Vernberg (eds), The biology of Crustacea. Environmental adaptations, Vol 8. New York, Academic Press.; Hartnoll, 1985Hartnoll, R.G. 1985. Growth, sexual maturity and reproductive output. p. 101-128. In: A. Wenner (ed), Factors in Adult Growth. Crustacean Issues, Vol. 3. Rotterdam/Boston, A.A. Balkema Publishers.). The apparent low fecundity in Ty. carneus may be related to the small size of the shrimp, which is directly related to the size of the body cavity for oocytes to grow in, as well as the space available for egg incubation in the pleon (Bauer, 1991Bauer, R.T. 1991. Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. p. 181-191. In: A. Wenner and A. Kuris (eds), Crustacean egg production. Crustacean Issues 7. Rotterdam, Balkema Press.; Corey and Reid, 1991Corey, S. and Reid, D.M. 1991. Comparative fecundity of decapod crustaceans. I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana, 60: 270-294.).
On the other hand, fecundity may be related to an embryonic development strategy. In some crustaceans, the duration of embryonic development is directly proportional to egg size, due to the amount of yolk needed to supply embryonic development. Thus, species with abbreviated or direct development tend to produce larger eggs with more yolk (Bauer, 1991Bauer, R.T. 1991. Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. p. 181-191. In: A. Wenner and A. Kuris (eds), Crustacean egg production. Crustacean Issues 7. Rotterdam, Balkema Press.; Wehrtmann and Albornoz, 2002Wehrtmann, I.S. and Albornoz, L. 2002. Evidence of different reproductive traits in the transisthmian sister species, Alpheus saxidomus and A. simus (Decapoda, Caridea, Alpheidae): description of the first postembryonic stage. Marine Biology, 140: 605-612.). Furthermore, the low fecundity combined with the large egg size in Ty. carneus - up to 0.7 mm in diameter and larger than those of other Typton species (e.g., Ty. granulosus: 0.4 mm; Ty. prionurus: 0.5 mm) (Holthuis, 1951Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332. ; Ayón-Parente et al., 2015Ayón-Parente, M.; Hendrickx, E.M. and Galvan-Villa, C.M. 2015. A new species of the genus Typton Costa (Crustacea: Decapoda: Palaemonidae: Pontoniinae) from the eastern tropical Pacific. Zootaxa, 3926: 430-438.) - may indicate abbreviated or direct development, which could be an adaptation for living inside sponges. A similar example is the direct development presented by some sponge-dwelling and eusocial species of the genus Synalpheus C.S. Bate, 1888Bate, C.S. 1888. Report on the Crustacea Macrura collected by the Challenger during the years 1873-76. In: J. Murray (ed), Report on the Scientific Results of the Voyage of H.M.S. ”Challenger” during the years 1873-76 Under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N. C. Wyville Thomson and J. Murray (series eds), Zoology. Vol. 24. i-xc, 1-942. pls., 1-157. Edinburgh, Neill and Company. (Corey and Reid, 1991Corey, S. and Reid, D.M. 1991. Comparative fecundity of decapod crustaceans. I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana, 60: 270-294.; Duffy, 1996Duffy, J.E. 1996. Eusociality in a coral-reef shrimp. Nature, 381: 512-514.). However, the production of small eggs and apparently higher fecundity in Ty. spongicola, and the scarce evidence from their larvae, suggest extended larval development in the plankton, at least for this species (Lebour, 1925Lebour, M.V. 1925. The eggs and newly hatched larva of Typton spongicola OG Costa. Journal of the Marine Biological Association of the United Kingdom, 13: 848-853.; 1949Lebour, M.V. 1949. The last larva and post-larva of Typton spongicola from Plymouth (Crustacea Decapoda). Journal of the Marine Biological Association of the United Kingdom, 28: 667-672.; Fincham and Williamson, 1978Fincham, A.A. and Williamson, D.I. 1978. Crustacea Decapoda: larvae, IV: Caridea, families: Palaemonidae and Processidae. p. 1-8. In: J.H. Fraser (ed), Fiches d’Identification du Zooplancton, Fiche No. 159/160. Denmark.). Finally, our data suggest an absence of overlapping generations of Ty. carneus (only one juvenile observed) in the sponge host, which has also been observed in some sponge-dwelling shrimp species with direct development (e.g., some eusocial Synalpheus species) (Duffy, 2003Duffy, J.E. 2003. The ecology and evolution of eusociality in sponge-dwelling shrimp. p. 1-38. In: T. Kikuchi (ed), Genes, Behavior, and Evolution in Social Insects. Sapporo, Japan, University of Hokkaido Press. ). Future studies about Typton species could elucidate if large eggs and low fecundity are consequences of the small size of these shrimps and/or due to any type of developmental abbreviation.
Heterosexual pairing
Characteristics regarding paired individuals, such as the absence of sexual dimorphism in weaponry and body size, presence of paired non-brooding and brooding females carrying eggs in different development stages and a sex ratio that did not differ from the expected 1:1, suggest that the population presents a monogamous mating system (see Bauer, 2004Bauer, R.T. 2004. Remarkable shrimps: adaptations and natural history of the carideans. Norman, University of Oklahoma Press, 282p. ; Baeza et al., 2016Baeza, J.A.; Simpson, L.; Ambrosio, L.J.; Guéron, R. and Mora, N. 2016. Monogamy in a hyper-symbiotic shrimp. PLoS ONE, 11: e0149797. ; Correa and Thiel, 2003Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187-203.); as documented for some caridean shrimps like alpheids (Knowlton, 1980Knowlton, N. 1980. Sexual selection and dimorphism in two demes of a symbiotic, pair-bonding snapping shrimp. Evolution, 34: 161-173.; Correa and Thiel, 2003Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187-203.; Baeza, 2008Baeza, J.A. 2008. Social monogamy in the shrimp Pontonia margarita, a symbiont of Pinctada mazatlanica, off the Pacific coast of Panama. Marine Biology, 153: 387-395. ; Pescinelli et al., 2017Pescinelli, R.A.; Davanso, T.M. and Costa, R.C. 2017. Social monogamy and egg production in the snapping shrimp Alpheus brasileiro (Caridea: Alpheidae) from the south-eastern coast of Brazil. Journal of the Marine Biological Association of the United Kingdom, 97: 1519-1526. ; Soledade et al., 2018Soledade, G.O.; Santos, P.S.; Araújo, M.S.L.C. , Mantelatto, F.L. and Almeida, A.O. 2018. Heterosexual pairing in three Alpheus (Crustacea: Alpheidae) snapping shrimps from northeastern Brazil. Vie et Milieu, 68: 109-117.). However, the lack of size-assortative pairing, as well as the low proportion of heterosexual pairs (37.93 %), compared to solitary individuals (48 %), does not support monogamy when considering that monogamous populations should present paired shrimps more often than solitary ones (Baeza et al., 2016Baeza, J.A.; Simpson, L.; Ambrosio, L.J.; Guéron, R. and Mora, N. 2016. Monogamy in a hyper-symbiotic shrimp. PLoS ONE, 11: e0149797. ). Such features have been observed in polygamous mating systems of caridean shrimps (see Correa and Thiel, 2003Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187-203.). Our controversial findings highlight the need for broader studies, with larger sample sizes, that assess demographic data, such as frequency of solitary, paired or more sets of individuals in the environment to address the mating system of Ty. carneus populations.
Our results provide further insights into some life history traits of Ty. carneus. The frequency of Ty. carneus collected in Te. ignis indicates that this sponge is an important host for these shrimps in the study area. The observation of heterosexual pairing, as well as the high percentage of ovigerous females in our sample, indicate the importance of the host as a reproduction site for this symbiont shrimp. Furthermore, the occurrence of ovigerous females in both dry and rainy seasons suggests continuous reproduction in the study area. The fecundity of the studied population was low, which may be related to small body-size and/or to the existence of some type of abbreviated development. The results regarding population features of the mating system are controversial with some of the population features assessed herein suggesting a monogamous mating system and other features supporting a polygamous system. Thus, the mating system of Ty. carneus remains unknown and should be addressed in future studies based on larger sample sizes and include specific demographic information.
ACKNOWLEDGMENTS
We thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, PROTAX 562320/2010-5 and Universal 454908/2014-8 to UP) and FACEPE (Fundação de Amparo à Ciência e Tecnologia de Pernambuco, APQ-0839-2.04/08 to UP and APQ-0572-2.04/15 to AOA), for providing grants. PHP and TC thank FACEPE for providing master’s and doctoral scholarships, respectively. UP and AOA thank CNPq for the Research Scholarship Support (PQ # 309078/2017-2 and 304235/2019-9, respectively). The authors also thank M.Sc. Rodrigo Guéron for his help with some statistical analyses. We also thank the anonymous reviewers for their contributions that improved this paper.
REFERENCES
- Abele, L.G. and Kim, W. 1986. An illustrated guide to the marine decapod crustaceans of Florida. Florida Department of Environmental Regulation, Technical Series, 8: 1-225.
- Albrizio, S.; Cimeniello, P.; Fattorusso, E.; Magno, S. and Pawlik, J.R. 1995. Amphitoxin, a new high molecular weight antifeedant pyridinium salt from the Caribbean sponge Amphimedon compressa Journal of Natural Products, 58: 647-652.
- Almeida, A.O.; Anker, A. and Mantelatto, F.L. 2014. A new snapping species of the shrimp genus Typton Costa, 1844 (Decapoda: Palaemonidae) from the coast of São Paulo, southeastern Brazil. Zootaxa, 3835: 110-120.
- Almeida, A.C.; Fransozo, V.; Teixeira, G.M.; Furlan, M.; Hiroki, K.A.N. and Fransozo, A. 2011. Population structure and reproductive period of whitebelly prawn Nematopalaemon schmitti (Holthuis, 1950) (Decapoda: Caridea: Palaemonidae) on the southeastern coast of Brazil.Invertebrate Reproduction and Development, 55: 30-39.
- Ayón-Parente, M.; Hendrickx, E.M. and Galvan-Villa, C.M. 2015. A new species of the genus Typton Costa (Crustacea: Decapoda: Palaemonidae: Pontoniinae) from the eastern tropical Pacific. Zootaxa, 3926: 430-438.
- Baeza, J.A. 2008. Social monogamy in the shrimp Pontonia margarita, a symbiont of Pinctada mazatlanica, off the Pacific coast of Panama. Marine Biology, 153: 387-395.
- Baeza, J.A.; Simpson, L.; Ambrosio, L.J.; Guéron, R. and Mora, N. 2016. Monogamy in a hyper-symbiotic shrimp. PLoS ONE, 11: e0149797.
- Bate, C.S. 1888. Report on the Crustacea Macrura collected by the Challenger during the years 1873-76. In: J. Murray (ed), Report on the Scientific Results of the Voyage of H.M.S. ”Challenger” during the years 1873-76 Under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N. C. Wyville Thomson and J. Murray (series eds), Zoology. Vol. 24. i-xc, 1-942. pls., 1-157. Edinburgh, Neill and Company.
- Bauer, R.T. 1989. Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127: 175-187.
- Bauer, R.T. 1991. Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. p. 181-191. In: A. Wenner and A. Kuris (eds), Crustacean egg production. Crustacean Issues 7. Rotterdam, Balkema Press.
- Bauer, R.T. 2004. Remarkable shrimps: adaptations and natural history of the carideans. Norman, University of Oklahoma Press, 282p.
- Berlink, R.G.S.; Ogawa, C.A.; Almeida, A.M.P.; Sanchez, M.A.A.; Malpezzi, E.L.A.; Costa, L.V.; Hadju, E. and Freitas, J.C. 1996. Chemical and pharmacological characterization of halitoxin from Amphimedon viridis (Porifera) from the southeastern Brazilian coast. Comparative Biochemistry and Physiology, 115: 155-163.
- Borradaile, L.A. 1915. Notes on Carides. The Annals and Magazine of Natural History, 15: 205-213.
- Bowerbank, J.S. 1875. Contributions to a general history of the Spongidae. Part VII. Proceedings of the Zoological Society of London, 1875: 281-296.
- Bruce, A.J. 1972. Notes on some Indo-Pacific Pontoniinae, XXI. Typton bawii sp. nov., the first occurrence of the genus Typton Costa in the Indian Ocean (Decapoda Natantia, Palaemonidae). Crustaceana, 23: 243-254.
- Bruce, A.J. 1973. Typton australis sp. nov., a new pontoniid shrimp from the Great Barrier Reef, Australia. Records of the Australian Museum, 28: 253-263.
- Bruce, A.J. 1976. Coral Reef Caridea and “Commensalism”. Micronesica, 12: 83-98.
- Bruce, A.J. 1977. Notes on some Indo-Pacific Pontoniinae, XXVIII. Typton wasini sp. nov., from Wasin Island, Kenya. Crustaceana, 32: 272-285.
- Bruce, A.J. 1978. Typton crosslandi sp. nov., a new pontoniine shrimp, from the Galapagos Islands. Crustaceana, 35: 294-300.
- Bruce, A.J. 1984. The pontoniine shrimp fauna of Australia. Australian Museum Memoir, 18: 195-218.
- Bruce, A.J. 2000. Typton manningi and T. capricorniae, new species, new pontoniine shrimps from northern Queensland, with a review of the Indo-West Pacific species of Typton Costa (Decapoda: Palaemonidae). Journal of Crustacean Biology, 20: 87-100.
- Bruce, A.J. 2009. A re-description of Typton spongicola Costa, 1844, the type species of the genus Typton Costa, 1844 (Crustacea: Decapoda: Pontoniinae). Cahiers de Biologie Marine, 50: 383-394.
- Bruce, A.J. 2010. Pontoniine Shrimps (Crustacea: Decapoda: Palaemonidae) from the CReefs 2009 Heron Island Expedition, with a review of the Heron Island pontoniine fauna. Zootaxa, 2541: 50-68.
- Calder, D.R. 1988. Shallow-water hydroids of Bermuda. The Athecatae. Royal Ontario Museum Life Sciences Contributions, 148: 1-107.
- Campos, C.J.A.; Migotto, A.E.; Pinheiro, U. and Marques, A.C. 2012. Sponges as substrata and early life history of the tubulariid Zyzzyzus warreni (Cnidaria: Hydrozoa) in the São Sebastião Channel, Brazil. Marine Biology Research, 8: 573-583.
- Carter, H.J. 1882. Some sponges from the West Indies and Acapulco in the Liverpool Free Museum described, with general and classificatory remarks. Annals and Magazine of Natural History, 9: 266-301.
- Castro, P. 1971. The natantian shrimps (Crustacea, Decapoda) associated with invertebrates in Hawaii. Pacific Science, 25: 395-403.
- Chace Jr, F.A. 1972. The shrimps of the Smithsonian-Bredin Caribbean Expeditions with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithsonian Contributions to Zoology, 98: 1-179.
- Coelho, P.A.; Almeida, A.O.; Souza-Filho, J.F.; Bezzera, L.E.A. and Giraldes, B.W. 2006. Diversity and distribution of the marine and estuarine shrimps (Dendobranchiata, Stenopodidea and Caridea) from North and Northeast Brazil. Zootaxa, 1221: 41-62.
- Corey, S. and Reid, D.M. 1991. Comparative fecundity of decapod crustaceans. I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana, 60: 270-294.
- Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187-203.
- Costa, O.G. 1844. Su due nuovi generi di Crostacei decapodi macrouri. Annali delle Accademia degli Aspiranti Naturalisti, Napoli, 2: 285-292.
- Dana, J.D. 1852. United States Exploring Expedition during the years 1838, 1839, 1840, 1841, 1842, under the Command of Charles Wilkes, U.S.N. Vol. 13. Crustacea. Part I. C. Sherman, Philadelphia, 685p.
- De Grave, S. 2010. A new species of the genus Typton Costa (Decapoda, Palaemonidae, Pontoniinae) from Ascension Island. Crustaceana Monographs, 14: 209-218.
- De Grave, S. and Anker, A. 2017. An annotated checklist of marine caridean and stenopodidean shrimps (Malacostraca: Decapoda) of the Caribbean coast of Panama. Nauplius, 25: e2017015.
- De Grave, S. and Fransen, C.H.J.M. 2011. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zoologische Mededelingen, 85: 195-588.
- Duarte, L.F.L. and Nalesso, R.C. 1996. The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuarine, Coastal and Shelf Science, 42: 139-151.
- Duchassaing De Fonbressin, P. and Michelotti, G. 1864. Spongiaires de la Mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem, 21: 1-124.
- Duffy, J.E. 1996. Eusociality in a coral-reef shrimp. Nature, 381: 512-514.
- Duffy, J.E. 2003. The ecology and evolution of eusociality in sponge-dwelling shrimp. p. 1-38. In: T. Kikuchi (ed), Genes, Behavior, and Evolution in Social Insects. Sapporo, Japan, University of Hokkaido Press.
- Ďuriš, Z.; Horká, I.; Juračka, P.J.; Petrusek, A. and Sandford, F. 2011. These squatters are not innocent: The evidence of parasitism in sponge-inhabiting shrimps. PLoS ONE, 6: e21987.
- Fincham, A.A. and Williamson, D.I. 1978. Crustacea Decapoda: larvae, IV: Caridea, families: Palaemonidae and Processidae. p. 1-8. In: J.H. Fraser (ed), Fiches d’Identification du Zooplancton, Fiche No. 159/160. Denmark.
- Fransen, C.H.J.M.; Holthuis, L.B. and Adema, J.P.H.M. 1997. Type-catalogue of the Decapod Crustacea in the collections of the Nationaal Natuurhistorisch Museum, with appendices of pre-1900 collectors and material. Zoologische Verhandelingen, 311: 1-344.
- Gray, J.E. 1867. Notes on the arrangement of sponges, with the descriptions of some new genera. Proceedings of the Zoological Society of London, 1867: 492-558.
- Hartnoll, R.G. 1985. Growth, sexual maturity and reproductive output. p. 101-128. In: A. Wenner (ed), Factors in Adult Growth. Crustacean Issues, Vol. 3. Rotterdam/Boston, A.A. Balkema Publishers.
- Hechtel, G.J. 1965. A systematic study of the Demospongiae of Port Royal, Jamaica. Bulletin of the Peabody Museum of Natural History, 20: 1-103.
- Hipeau-Jacquotte, R. 1971. Notes de faunistique et de biologie marines de Madagascar, V. Platypontonia hyotis nov. sp. (Decapoda Natantia, Pontoniinae). Crustaceana, 20: 125-140.
- Holthuis, L.B. 1951. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. I. The subfamilies Euryrhynchidae and Pontoniinae. Allan Hancock Foundation Publications of the University of Southern California, Occasional Paper, 11: 1-332.
- Hultgren, K.M. 2014. Variable effects of symbiotic snapping shrimps on their sponge hosts. Marine Biology, 161: 1217-1227.
- Hunt, O.D. 1925. The food of the bottom fauna of the Plymouth fishing grounds. Journal of the Marine Biological Association of the United Kingdom, 13: 560-599.
- Knowlton, N. 1980. Sexual selection and dimorphism in two demes of a symbiotic, pair-bonding snapping shrimp. Evolution, 34: 161-173.
- Lamarck, J.B.P. 1815. Suite des polypiers empâtés. Mémoires du Muséum d’Histoire Naturelle, Paris, 1: 69-80.
- Lamarck, J.B.P. 1816. Histoire naturelle des animaux sans vertèbres. Tome second. Paris, Verdière, 568p.
- Laubenfels, M.W. 1936. A discussion of the sponge fauna of the Dry Tortugas in particular and the West Indies in general, with material for a revision of the families and orders of the Porifera. Papers from the Tortugas Laboratory of the Carnegie Institution of Washington, 467: 1-225.
- Laubier, L. 1966. Le coralligene des Alberes: monographie biocenotique. Annales de l’Institut Océanographique de Monaco, 43: 139-316.
- Lebour, M.V. 1925. The eggs and newly hatched larva of Typton spongicola OG Costa. Journal of the Marine Biological Association of the United Kingdom, 13: 848-853.
- Lebour, M.V. 1949. The last larva and post-larva of Typton spongicola from Plymouth (Crustacea Decapoda). Journal of the Marine Biological Association of the United Kingdom, 28: 667-672.
- Manning, R.B. and Chace, Jr. F.A. 1990. Decapod and stomatopod Crustacea from Ascension Island, South Atlantic Ocean. Smithsonian Contributions to Zoology, 503: 1-91.
- Mcclendon, J.F. 1911. On adaptations in structure and habits of some marine animals of Tortugas, Florida. Papers from the Tortugas Laboratory of the Carnegie Institution of Washington, 3: 57-62.
- Montagu, G. 1814. An essay on sponges, with descriptions of all the species that have been discovered on the Coast of Great Britain. Memoirs of the Wernerian Natural History Society, 2: 67-122.
- Moraes, I.R.; Bergamasco, G.R.; Santos, R.C.; Antunes, M.; Soledade, G.O.; Costa, R.C. and Castilho, A.L. 2020. New record of the sponge-dwelling shrimp Typton distinctus Chace, 1972 (Decapoda: Caridea: Palaemonidae) in São Paulo State, Brazil. Zootaxa, 4763: 444- 446.
- Mossolin, E.C.; Shimizu, R.M. and Bueno, S.L.S. 2006. Population structure of Alpheus armillatus (Decapoda, Alpheidae) in São Sebastião and Ilhabela, southeastern Brazil. Journal of Crustacean Biology, 26: 48-54.
- Neves, K. 2020. A new species of the shrimp genus Typton Costa, 1844 (Malacostraca, Decapoda, Palaemonidae) from the Cabo Verde Archipelago. Zootaxa, 4768: 264-270.
- Nobili, G. 1904. Diagnoses préliminaires de vingt-huit espèces nouvelles de stomatopodes et decápodes macroures de la Mer Rouge. Bulletin du Muséum d’Histoire Naturelle, 10: 228-238.
- Pachelle, P.P.G.; Anker, A. and Tavares, M. 2015. New and additional records of the sponge shrimp genus Typton Costa, 1844 (Decapoda: Palaemonidae) from the Brazilian coast. Papéis Avulsos de Zoologia, 55: 317-322.
- Pachelle, P.P.G.; Anker, A.; Mendes, C.B. and Bezerra, L.E.A. 2016. Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa, 4131: 1-63.
- Patton, W.K.; Patton, R.J. and Barnes, A. 1985. On the biology of Gnathophylloides mineri, a shrimp inhabiting the sea urchin Tripneustes ventricosus Journal of Crustacean Biology, 5: 616-626.
- Pescinelli, R.A.; Davanso, T.M. and Costa, R.C. 2017. Social monogamy and egg production in the snapping shrimp Alpheus brasileiro (Caridea: Alpheidae) from the south-eastern coast of Brazil. Journal of the Marine Biological Association of the United Kingdom, 97: 1519-1526.
- Rafinesque, C.S. 1815 Analyse de la nature ou tableau de l’Univers et des corps organisés. Palerme, L’Imprimerie de Jean Barravecchia, 224p.
- Ramos-Porto, M. and Coelho, P.A. 1998. Malacostraca. Eucarida. Caridea (Alpheoidea excluded). p. 325-350. In: P.S. Young (ed), Catalogue of Crustacea of Brazil. Museu Nacional, Rio de Janeiro. (Série Livros 6).
- Ridley, S.O. and Dendy, A. 1886. Preliminary report on the Monaxonida collected by H.M.S. Challenger. Annals and Magazine of Natural History, 18: 325-351.
- Román-Contreras, R. and Martínez-Mayén, M. 2010. Palaemonidae (Crustacea: Decapoda: Caridea) from the shallow waters from Quintana Roo, Mexican Caribbean coast. Revista Mexicana de Biodiversidad, 81: 43-51.
- Rützler, K. 2004. Sponges on coral reefs: a community shaped by competitive cooperation. Bolletino dei Musei e Degli Istituti Biologici dell’Univiversità di Genova, 68: 85-148.
- Santana-Moreno, L.D.; De Grave, S. and Simões, N. 2013. New records of caridean shrimps (Decapoda: Caridea) from shallow water along the northern Yucatan peninsula coasts of México. Nauplius, 21: 225- 238.
- Sastry, A.N. 1983. Ecological aspects of reproduction. p. 179-270. In: F.J. Vernberg and W.B. Vernberg (eds), The biology of Crustacea. Environmental adaptations, Vol 8. New York, Academic Press.
- Say, T. 1825. On the species of the Linnaean genus Asterias inhabiting the coast of the U.S. Journal of the Academy of Natural Sciences of Philadelphia, 5: 141-154.
- Schmidt, O. 1862. Die Spongien des adriatischen Meeres. Leipzig, Wilhelm Engelmann, i-viii, 1-88.
- Schmitt, W.L. 1933. Four new species of decapod crustaceans from Porto Rico. American Museum Novitates, 662: 1-9.
- Schmitz, F.J.; Gunasekera, S.P.; Yalamanchili, G.; Hossain, M.B. and Van Der Helm, D. 1984. Tedanolide: a potent cytotoxic macrolide from the Caribbean sponge Tedania ignis Journal of the American Chemical Society, 106: 7251-7252.
- Schneider, C.A., Rasband, W.S. and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9: 671-675.
- Semper, C. 1868. Reisen im Archipel der Philippinen. Zweiter Theil. Wissenschaftliche Resultate. Erster Band. Holothurien. Hefte iv. and v: Unpaginated.
- Soledade, G.O.; Santos, G.G.; Pinheiro, U. and Almeida, A.O. 2017. New records of association between caridean shrimps (Decapoda) and sponges (Porifera) in Abrolhos Archipelago, northeastern Brazil. Nauplius, 25: e2017027.
- Soledade, G.O.; Santos, P.S.; Araújo, M.S.L.C. , Mantelatto, F.L. and Almeida, A.O. 2018. Heterosexual pairing in three Alpheus (Crustacea: Alpheidae) snapping shrimps from northeastern Brazil. Vie et Milieu, 68: 109-117.
- Terossi, M. and Mantelatto, F.L. 2010. Sexual ratio, reproductive period and seasonal variation of the gonochoric shrimp Hippolyte obliquimanus (Caridea: Hippolytidae). Marine Biology Research, 6: 213-219.
- Van Soest, R.W.M. 1984. Marine sponges from Curaçao and other Caribbean localities. Part III. Poecilosclerida. Studies on the Fauna of Curaçao and other Caribbean Islands, 66: 1-173.
- Vieira, R.R.R.; Ferreira, R.S. and D’Incao, F. 2012. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa, 3149: 1-38.
- Voultsiadou-Koukoura, E. and Koukouras, A. 1989. Remarks on sponge-decapod associations in the North Aegean Sea. Scientific Annals of the School of Biology, 1: 251-256.
- Waddell, B. and Pawlik, J.R. 2000. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Marine Ecology Progress Series, 195: 133-144.
- Wehrtmann, I.S. and Albornoz, L. 2002. Evidence of different reproductive traits in the transisthmian sister species, Alpheus saxidomus and A. simus (Decapoda, Caridea, Alpheidae): description of the first postembryonic stage. Marine Biology, 140: 605-612.
- Wulff, J.L. 2006. Sponge systematics by starfish: predators distinguish cryptic sympatric species of Caribbean fire sponges, Tedania ignis and Tedania klausi n.sp. (Demospongiae, Poecilosclerida). Biological Bulletin, 211: 83-94.
Publication Dates
-
Publication in this collection
11 June 2021 -
Date of issue
2021
History
-
Received
04 May 2020 -
Accepted
28 Jan 2021