Abstracts
OBJECTIVE: To assess use of stress ulcer prophylaxis in patients admitted to five pediatric intensive care units (PICUs) in Porto Alegre, Brazil. METHODS: This was a multicenter, prospective, cross-sectional observational study. PICUs were visited on randomly defined days between April 2006 and February 2007, and the medical records of admitted patients were reviewed. Patients whose records had been previously assessed were excluded, as were those with upper gastrointestinal bleeding on admission. Data were collected on age, gender, admission diagnosis, severity of illness, administration of stress ulcer prophylaxis, rationale for prophylaxis, and first-line prophylactic agent of choice. Variables were described as absolute and relative frequencies, mean and standard deviation, or median and interquartile range as appropriate. Pearson's chi-square test for linear trend or Fisher's exact test were used to assess possible associations. The level of significance was set at 5% (p < 0.05). RESULTS: 398 patients (57% male) were assessed [median age, 16 months (IQR 4-65); median length of PICU stay, 4 days (IQR 1-9)]. Respiratory illness was the main reason for admission (32.7%). Most patients received stress ulcer prophylaxis (77.5%; range, 66-91%). Mechanical ventilation (22.3%) was the most common rationale provided, followed by informal routine use of prophylaxis (21.4%). Only one of the participating PICUs had a specific care protocol for use of stress ulcer prophylaxis. Ranitidine was the most commonly used drug (84.5% of cases). Evidence of minor gastrointestinal bleeding was found in 3% of patients; none had clinically significant bleeds. CONCLUSIONS: Administration of stress ulcer prophylaxis is a common practice in the participating PICUs, with ranitidine the most commonly used drug. Among the various rationales provided, mechanical ventilation and informal routine use were the most prevalent.
Gastroduodenal ulcer; prophylaxis; children; intensive care; gastrointestinal bleeding
OBJETIVO: Avaliar a utilização de profilaxia para úlcera de estresse (UE), em pacientes internados, de cinco unidades de terapia intensiva pediátrica (UTIP) de Porto Alegre (RS). MÉTODOS: Estudo multicêntrico, prospectivo, transversal, observacional. Foram avaliados os prontuários dos pacientes internados em dia definido para visitação, entre abril de 2006 e fevereiro de 2007, excluindo os avaliados em visitas anteriores e aqueles com hemorragia digestiva alta na admissão. Foram avaliados a idade, o gênero, o diagnóstico na admissão, a gravidade da doença, o uso de profilaxia para UE, a sua justificativa e o medicamento profilático utilizado como primeira escolha. As variáveis foram descritas como frequências absoluta e relativa, ou média e desvio padrão/mediana, e intervalo interquartil (IQ). Os testes qui-quadrado de Pearson, de tendência linear, ou exato de Fisher foram utilizados para avaliar as associações. O nível de significância adotado foi de 5%, sendo estatisticamente significativo p < 0,05. RESULTADOS: Foram avaliados 398 pacientes, sendo 57% do gênero masculino. A mediana de idade foi de 16 meses (IQ4-65) e mediana de permanência em UTIP foi de 4 dias (IQ1-9). O principal motivo de internação foi doença respiratória (32,7%). Usaram profilaxia 77,5% dos pacientes, variando de 66 a 91%; a ventilação mecânica (22,3%) foi a justificativa mais prevalente, seguida de rotina informal do serviço (21,4%). Apenas uma das UTIP tinha protocolo assistencial para profilaxia de UE. A ranitidina foi o medicamento mais empregado (84,5%). CONCLUSÕES: O uso de profilaxia para UE foi prática frequente nas UTIP avaliadas, sendo a ranitidina a droga de escolha. Entre as justificativas, a ventilação mecânica e o uso baseado em rotinas institucionais foram as mais prevalentes.
Úlcera gastroduodenal; profilaxia; crianças; terapia intensiva; sangramento gastrointestinal
ORIGINAL ARTICLE
Stress ulcer prophylaxis in pediatric intensive care units
Taisa E. AraujoI; Sandra M. G. VieiraII; Paulo R. A. CarvalhoIII
IMestre, Saúde da Criança e do Adolescente. Programa de Pós-Graduação em Saúde da Criança e do Adolescente, Faculdade de Medicina (FAMED), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
IIDoutora, Gastroenterologia. UFRGS, Porto Alegre, RS, Brazil. Unidade de Gastroenterologia Pediátrica, Serviço de Pediatria, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil
IIIDoutor, Pediatria. Departamento de Pediatria e Puericultura, FAMED, UFRGS, Porto Alegre, RS, Brazil. Unidade de Tratamento Intensivo Pediátrica, Serviço de Pediatria, HCPA, Porto Alegre, RS, Brazil
ABSTRACT
OBJECTIVE: To assess use of stress ulcer prophylaxis in patients admitted to five pediatric intensive care units (PICUs) in Porto Alegre, Brazil.
METHODS: This was a multicenter, prospective, cross-sectional observational study. PICUs were visited on randomly defined days between April 2006 and February 2007, and the medical records of admitted patients were reviewed. Patients whose records had been previously assessed were excluded, as were those with upper gastrointestinal bleeding on admission. Data were collected on age, gender, admission diagnosis, severity of illness, administration of stress ulcer prophylaxis, rationale for prophylaxis, and first-line prophylactic agent of choice. Variables were described as absolute and relative frequencies, mean and standard deviation, or median and interquartile range as appropriate. Pearsons chi-square test for linear trend or Fishers exact test were used to assess possible associations. The level of significance was set at 5% (p < 0.05).
RESULTS: 398 patients (57% male) were assessed [median age, 16 months (IQR 4-65); median length of PICU stay, 4 days (IQR 1-9)]. Respiratory illness was the main reason for admission (32.7%). Most patients received stress ulcer prophylaxis (77.5%; range, 66-91%). Mechanical ventilation (22.3%) was the most common rationale provided, followed by informal routine use of prophylaxis (21.4%). Only one of the participating PICUs had a specific care protocol for use of stress ulcer prophylaxis. Ranitidine was the most commonly used drug (84.5% of cases). Evidence of minor gastrointestinal bleeding was found in 3% of patients; none had clinically significant bleeds.
CONCLUSIONS: Administration of stress ulcer prophylaxis is a common practice in the participating PICUs, with ranitidine the most commonly used drug. Among the various rationales provided, mechanical ventilation and informal routine use were the most prevalent.
Keywords: Gastroduodenal ulcer, prophylaxis, children, intensive care, gastrointestinal bleeding.
Introduction
Critically ill patients are at risk of stress-related mucosal disease (SRMD), which leads to increased morbidity and mortality in the intensive care unit (ICU) setting. In pediatric patients, the prevalence of stress ulcer-related gastrointestinal bleeding ranges from 6 to 43%, with major bleeding rates as high as 1.6 to 5.3%.1
Although the pathophysiology of SRMD has yet to be fully elucidated, factors involved in its etiology include decreased gastric pH, increased permeability of the gastric mucosa, and ischemia.2 When gastric pH rises above 3.5-4.0, the frequency of SRMD and upper gastrointestinal bleeding declines significantly.3
Several studies have suggested that mechanical ventilation (MV) is one of the most significant of various probable risk factors for this condition, both in adults and children admitted to intensive care.4-6 A study by Chaïbou et al. found three independent risk factors for major upper gastrointestinal bleeding in critically ill children: respiratory failure [odds ratio (OR) = 10.2], coagulopathy (OR = 9.3), and a pediatric risk of mortality (PRISM) score > 10 (OR = 4.0).6 The PRISM score assesses several clinical and laboratory parameters and is used to predict mortality in the pediatric intensive care unit (PICU) environment.
Drugs commonly used for stress ulcer (SU) prophylaxis include antacids, sucralfate, H2 receptor antagonists, and proton pump inhibitors,7 and their use should be restricted to patients with risk factors for stress ulcer.
Few studies have assessed the prevalence of SRMD/SU prophylaxis in pediatric patients. Most studies date back to the 1990s, and none have been conducted in Brazil.5,6 SU prophylaxis has been associated with major complications, such as hospital-acquired pneumonia, particularly in critically ill patients.8 Establishing a profile of SU prophylaxis administration in a certain population is therefore an important part of any thorough review of the appropriateness of this intervention.
The objective of the present study was to assess the prevalence of SU chemoprophylaxis in patients admitted to ICUs in the city of Porto Alegre, state of Rio Grande do Sul, Brazil, and identify the main rationales provided for prophylaxis and the drug most commonly used for this purpose.
Methods
This was a prospective, cross-sectional, observational study conducted at five mixed medical/surgical PICUs in the city of Porto Alegre, including one located at a trauma center and one at a center of excellence in cardiac surgery. These five PICUs had a total of 64 beds, which are available to patients covered by the Brazilian Unified Health System (UHS) as well as private uninsured patients and those with health insurance. All PICUs were located at teaching hospitals. The study was approved by the Research Ethics Committees of all participating hospitals. As this is a purely observational study with a chart review design, nearly all ethics committees agreed that informed consent would not be required, with the authors expressing a commitment to signing a patient confidentiality form (covering identifying information and other data) instead. One participating institution, however, believed informed consent was in order; consent was therefore obtained from all patients enrolled from this hospital.
A review was conducted of the charts of all patients receiving care at the participating PICUs on the day of visitation. PICUs were visited on randomly defined days between April 2006 and February 2007.
The exclusion criteria were: age > 18 years; evidence of prior upper gastrointestinal bleeding; current history of epistaxis, facial trauma, or other injuries or factors that could be mistaken for gastrointestinal bleeding; diagnosis of brain death on admission; assessment on prior occasions; long-term hospital stay (> 1 year); use of stress ulcer prophylaxis prior to PICU admission; and refusal of informed consent (in the hospital where it was required).
Data collected included age, gender, diagnosis on admission, disease severity, length of PICU stay at the time of assessment, use of stress ulcer prophylaxis and/or treatment for upper gastrointestinal bleeding, switching of drugs used for either purpose, and presence of gastrointestinal bleeding during PICU stay up to the time of assessment. Data were collected from patient records; in the event of conflicting, unclear, or missing data, attending physicians were contacted for clarification. Visits were performed at random, without prearrangement with any treating physicians and with no interference on the choice to administer stress ulcer prophylaxis.
Due to a substantial difference in number of beds, one of the participating PICUs was subdivided into two virtual units for ease of data analysis and to avoid identification. The ICU with a distinctively greater number of beds was therefore analyzed as two separate units for the purposes of this study; accordingly, results are reported for six, not five, PICUs.
Upper gastrointestinal bleeding was defined as any episode of hematemesis, melena, or hematochezia; aspiration of any volume of blood through a nasogastric (NG) tube; or drainage of bloody or coffee-ground stomach contents from an NG tube.5,9 Upper gastrointestinal bleeding was defined as clinically important if hypotension, death, or a need for blood transfusion developed within 24 hours of bleeding.5 Patient severity was assessed according to multiple organ dysfunction syndrome (MODS) criteria as defined by Prouxl et al.10 Mortality risk scores, such as the pediatric index of mortality (PIM) or PRISM, were not taken into account when classifying patients by severity, as use and choice of risk scores was not standardized among the participating institutions. It would thus be impossible to compare the disease severity of patients admitted to different PICUs using these scores as parameters.
The sample size for an estimated proportion of 50% of patients receiving SRMD prophylaxis was calculated as 385 patients, with a 95% confidence interval and a margin of error of 5%. Data were entered into a Microsoft Office Excel spreadsheet and statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) 14.0 software package. Categorical variables were expressed as absolute and relative frequencies and quantitative variables as mean and standard deviation (for apparently symmetric distributions) or median and interquartile range (IQR) (for asymmetric distributions). Pearsons chi-square test for linear trend or Fishers exact test were used as appropriate to evaluate associations between variables. The significance level was set as 5% (p < 0.05).
Results
Over the course of the study period, 561 patients were admitted to and treated in the participating PICUs; 122 of these were removed from the sample as they met one or more of the aforementioned exclusion criteria. This left 398 patients, 57% of whom were male. Median age was 16 months (IQR, 465 months) and median length of PICU stay at the time of assessment (that is, time elapsed between PICU admission and assessment) was 4 days (IQR, 19 days).
The most common diagnoses at admission were respiratory illness (32.7%), postoperative recovery (30.9%), trauma (12.8%), and heart disease (6.8%). Other diagnoses accounted for less than 5% of cases each.
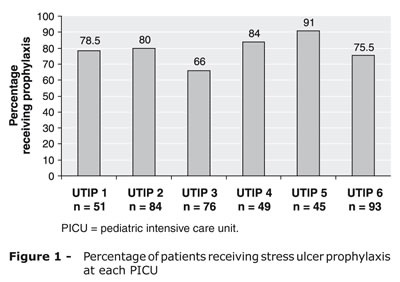
The overall prevalence of SU prophylaxis was 77.5% (n = 309; range, 66 to 91%). Figure 1 shows the distribution of SU prophylaxis across the participating PICUs. Overall, at the time of review, 68% of patients (n = 271) were receiving some prophylactic agent, whereas 9.5% (n = 38) had previously received prophylaxis at some point over the course of their ICU stay.
The main rationales for prophylaxis were mechanical ventilation (22.3%) and routine use (21.4%). Other rationales are listed in Table 1. The term "severe condition" was used by the physician on call to justify SU prophylaxis when no specific rationale was listed on the patients chart but the physician believed the patient had multiple factors to warrant administration of prophylaxis. For the purposes of this study, "routine use" refers to informal practice alone, not guideline- or protocol-based use, and generally included rationales such as respiratory stress, corticosteroid use, or some postoperative settings.
Ranitidine was used most commonly for prophylaxis (84.5% of cases), followed by omeprazole as the sole alternative.
Classification of patients by severity showed that respiratory failure (61%), cardiac dysfunction (38.2%), and coagulopathy (16.3%) were most prevalent. Table 2 presents a breakdown of patients by severity and PICU, showing that patients in PICU 2 were more severely ill (p < 0.001). Among patients with respiratory failure, 85.2% received prophylaxis; conversely, 10% had been given no prophylactic drugs whatsoever as of the time of assessment, even though prophylaxis was indicated. Prophylaxis was given to 86.2% of patients with coagulopathy, whereas 4.6% of those in this group did not receive it.
Analysis of severity also showed that use of prophylaxis increased with the number of organ systems affected, as shown in Figure 2. Among patients with multiple organ dysfunction affecting four or more systems (n = 10), two were no longer receiving prophylaxis, but treatment (due to evidence of upper gastrointestinal bleeding) instead. It is important to note that the number of patients in each group declined as the number of affected organ systems increased. Choice to administer SU prophylaxis was directly associated with increased number of organ systems with altered function (chi-square test for linear trend = 36.2; p < 0.001).
Discussion
The overall profile of patients included in our sample was similar to that of other studies conducted in the PICU setting.11-15 Use of SU prophylaxis was highly prevalent in all participating institutions, with 77.5% of patients receiving prophylaxis at some point during their PICU stay. Our review of then literature showed that only two prior studies provided information on the prevalence of SU prophylaxis in children; these reported prevalence rates of 11 and 14.5% respectively, with use of prophylaxis restricted to high-risk patients.5,6 No pediatric studies on SRMD prophylaxis have been conducted in Brazil. The only investigation on the subject conducted in the country was carried out in an adult ICU, and reported that 73.5% of patients received SU prophylaxisa rate very similar to that found in the present study.16
The prevalence of prophylaxis administration in our sample of PICUs ranged from 66 to 91%. The unit in which the highest rate was found considers SU prophylaxis a routine intervention to be used in nearly all patients admitted to intensive care. Interestingly, the unit in which prophylaxis was least prevalent was also the only one that had a formal protocol in place for stress ulcer prophylaxis. Even so, prophylaxis was prescribed often.
According to the current literature, the most prevalent risk factors for upper gastrointestinal bleeding in critically ill patients are respiratory failure/mechanical ventilation and coagulation disorders.2,5,6,17 The incidence of clinically important (major) upper gastrointestinal bleeding is so low in patients with one or no risk factors (0.1%) that SU prophylaxis would be entirely unwarranted in this group; it has been suggested that prophylaxis be indicated only in patients with two out of these three risk factors.6
Mechanical ventilation was clearly regarded as a major indication for prophylaxis by the physicians in our sample; it was the main rationale provided (22.3%), alongside routine use (21.4%). The very presence of a "routine use" category, representing purely habitual practice unrelated to any formal institutional care protocols, suggests that standardized, evidence-based management is absent. Other, less representative indications included administration of prophylaxis for no identifiable reason (7%) and other reasons (also 7%; these included epigastric pain, gastroesophageal reflux, vomiting, absence of enteral feeding, general postoperative care, and discretionary use by the attending physician).
In the present study, coagulopathy was provided as the rationale for SU prophylaxis in only two patients (0.6%). This may be considered an underestimate, as most patients had more than one reason for prophylaxis in the assessment of the ICU physician; these patients were included in the "severe condition" category. Alternately, some may have been placed in the "post cardiac surgery" category and given anticoagulant therapy. Other reasons for prophylaxis mentioned in this study were head trauma, major burns, status post neurosurgical intervention, and organ transplantation; these have also been mentioned as reasons in other studies.18
A lack of precise criteria for indicating prophylaxis in adults has also been established: one study showed that 25.7% of high-risk patients were not given SU prophylaxis even as 71.4% of low-risk patients received it.16
Studies have shown that critical care specialists are aware of the risk factors associated with SU in ICU patients. There is, however, a disconnect between this awareness and the choice to administer prophylaxis, as shown by the fact that, even after correct identification of at-risk patients, some physicians recommend prophylaxis for all those admitted to intensive care.2,19
In this study, ranitidine was the main drug used for SU prophylaxis: it was the first choice of therapy in 84.5% of cases. This predominance of ranitidine use has been reported elsewhere in the literature.15,19,20 Some authors claim that elevation of stomach pH levels above a threshold value is an appropriate intervention for prevention of upper gastrointestinal bleeding, although not all clinically important bleeds can be prevented by gastric pH control.21 The primary objective of acid suppression therapy is to prevent SRMD by keeping gastric pH above 4.0. Increasing pH to this level, however, proves inadequate in patients with acute upper gastrointestinal bleeding or those at risk of rebleeding after successful hemostasis. The level of stomach pH control provided by PPIs has proved superior to that achieved by H2 blockers, which produce early tolerance and loss of antisecretory effect (usually as early as the second or third day of administration), and may therefore be inadequate for critically ill patients.22
In addition to being the first choice of medication for SU prophylaxis in the participating institutions, ranitidine was and is administered for long periods, usually for the duration of the patients PICU stay, with no consideration given to stomach pH management or development of tolerance to the drug.21
In all participating PICUs, use of prophylaxis was found to increase with the number of organ systems with altered function. All patients with multiple organ dysfunction syndrome (affecting four or more systems) were administered prophylaxis. The 20% such patients who were not actively receiving prophylaxis (Figure 1) had an established diagnosis of upper gastrointestinal bleeding and had been switched to treatment instead. It is extremely important to stress that 47.4% of patients receiving prophylaxis had no degree of organ system dysfunction whatsoever.
The present study confirmed the hypothesis that SU prophylaxis is highly prevalent in Brazil, and could, according to the available literature, be considered inappropriate in many situations. Prophylaxis should be administered to patients with the above-described risk factors and maintained until these factors have resolved; physicians should bear in mind that the use of certain prophylactic agents may lead to drug tolerance, drug-drug interactions, and infectious complications. We believe the use of a formal care protocol containing well-established criteria and indications for SU prophylaxis could substantially reduce its prevalence and, consequently, cut costs and reduce the potential for associated complications.
We are aware of the limitations of this study; its cross-sectional design provides an idea of how prophylaxis is being used in the study environment alone. Therefore, we suggest that further studies be undertaken to identify risk factors associated with the need for SU prophylaxis and determine the best choice of medication for SU prophylaxis in critically ill pediatric patients.
Acknowledgements
The authors would like to thank the staff and Chiefs of the five participating pediatric intensive care units for their assistance: Dr. Eliana de Andrade Trotta of the Hospital de Clínicas de Porto Alegre (HCPA), Universidade Federal do Rio Grande do Sul (UFRGS); Dr. Cláudia Pires Ricachinevsky of the Hospital da Criança Santo Antônio, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA); Dr. Policarpo Blanco Lopez of the Hospital de Pronto Socorro Municipal de Porto Alegre; Dr. Maria Helena Lovato Dellazzana of the Hospital Materno Infantil Presidente Vargas; and Dr. Pedro Celiny Ramos Garcia of Hospital São Lucas, Pontifícia Universidade do Rio Grande do Sul (PUCRS). We also extend our gratitude to the patients included in this study and to their families.
References
- 1. Deerojanawong J, Peongsujarit D, Vivatvakin B, Prapphal N. Incidence and risk factors of upper gastrointestinal bleeding in mechanically ventilated children. Pediatr Crit Care Med. 2009;10:91-5.
- 2. Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Crit Care Med. Canadian Critical Care Trials Group. 1999;27:2812-7.
- 3. Lugo RA, Harrison AM, Cash J, Sweeley J, Vernon DD. Pharmacokinetics and pharmacodynamics of ranitidine in critically ill children. Crit Care Med. 2001:29:759-64.
- 4. Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, et at. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377-81.
- 5. Lacroix J, Nadeau D, Laberge S, Gauthier M, Lapierre G, Farrel CA. Frequency of upper gastrointestinal bleeding in a pediatric intensive care unit. Crit Care Med. 1992;20:35-42.
- 6. Chaïbou M, Tucci M, Dugas MA, Farrel CA, Proulx F, Lacroix J. Clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit: a prospective study. Pediatrics. 1998;102:933-8.
- 7. Gurgueira GL, Carvalho WB. Hemorragia digestiva alta: aspectos pediátricos. http://brazilpednews.org.br/deze2001/bnp113p.pdf Acesso: 27/08/2003.
- 8. Beaulie M, Williamson D, Sirois C, Lachaine J. Do proton-pump inhibitors increase the risk for nosocomial pneumonia in a medical intensive care unit? J Crit Care. 2008;23:513-8.
- 9. Chawla S, Seth D, Mahajan P, Kamat D. Upper gastrointestinal bleeding in children. Clin Pediatr (Phila). 2007;46:16-21.
- 10. Proulx F, Fayon M, Farrel CA, Lacroix J, Gauthier M. Epidemiology of sepsis an multiple organ dysfunction syndrome in children. Chest. 1996;109:1033-7.
- 11. Alievi PT, Carvalho PR, Trotta EA, Mombelli Filho R. The impact of admission to a pediatric intensive care unit assessed by means of global and cognitive performance scales. J Pediatr (Rio J). 2007;83:505-11.
- 12. Alves MJ, Alves MV, Bastos HD. Validaçäo do uso de escores preditivos em uma unidade de terapia intensiva pediatrica do Brasil. Rev Bras Ter Intensiva. 2000;12:36-43.
- 13. Parra ME, González AF, Ochoa WC, Vélez AQ. Morbimortalidad en la unidad de cuidados intensivos pediátricos del Hospital Universitário San Vicente de Paúl, Medellín, Colombia, 2001-2005. IATREIA. 2008;21:33-40.
- 14. Einloft PR, Garcia PC, Piva JP, Bruno F, Kipper DJ, Fiori RM. Perfil epidemiológico de dezesseis anos de uma unidade de terapia intensiva pediátrica. Rev Saude Publica. 2002;36:728-33.
- 15. Carvalho PR, Feldens L, Seitz EE, Rocha TS, Soledade MA, Trotta EA. Prevalência das síndromes inflamatórias sistêmicas em uma unidade de tratamento intensivo pediátrica terciária. J Pediatr (Rio J). 2005;81:143-8.
- 16. Machado AS, Teixeira C, Furlanetto L, Tonietto T, Balzano PC, Vieira SR, et al. Profilaxia para úlcera de estresse nas unidades de terapia intensiva: estudo observacional multicêntrico. Rev Bras Ter Intens. 2006;18:229-33.
- 17. Cochran EB, Phelps SJ, Tolley EA, Stidham GL. Prevalence of, and risk factors for, upper gastrointestinal tract bleeding in critically ill pediatric patients. Crit Care Med. 1992;20:1519-23.
- 18. Mutlu GM, Mutlu EA, Factor P. GI Complications in Patients Receiving Mechanical Ventilation. Chest. 2001;119:1222-41.
- 19. Daley RJ, Rebuck JA, Welage LS, Rogers FB. Prevention of stress ulceration: current trends in critical care. Crit Care Med. 2004;32:2008-13.
- 20. Nithiwathanapong C, Reungrongrat S, Ukarapol N. Prevalence and risk factors of stress-induced gastrointestinal bleeding in critically ill children. World J Gastroenterol. 2005;11:6839-42.
- 21. Noble DW. Proton pump inhibitors and stress ulcer prophylaxis: pause for thought? Crit Care Med. 2002;30:1175-6.
- 22. Fennerty MB. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: rationale for the therapeutic benefits of acid suppression. Crit Care Med. 2002;30:S351-5.
Publication Dates
-
Publication in this collection
17 Jan 2011 -
Date of issue
Dec 2010
History
-
Received
26 July 2010 -
Accepted
22 Sept 2010