Abstracts
OBJECTIVES: To evaluate the dietary intake of children and adolescents with juvenile idiopathic arthritis (JIA) and juvenile systemic lupus erythematosus (JSLE) using a 24-hour diet recall and relating it to the patients clinical and anthropometric characteristics and to the drugs used in their treatment. METHODS: By means of a cross-sectional study, we assessed the 24-hour diet recalls of outpatients. Their nutritional status was classified according to the CDC (2000). The computer program NutWin UNIFESP-EPM was used for food intake calculation. The Recommended Dietary Allowances and the Brazilian food pyramid were used for quantitative and qualitative analysis. RESULTS: Median age was 12 years for JIA patients and 16.5 years for JSLE patients. Among the JIA patients, 37.5% had active disease, and among the JSLE patients, 68.2% showed Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) > 4. Malnutrition was found in 8.3 and 4.5% of the JIA and JSLE patients, respectively, and obesity was present in 16.7 and 18.2%. For JIA patients, the excessive intake of energy, protein, and lipids was 12.5, 75, and 31.3%, respectively. For JSLE patients, the excessive intake of energy, protein, and lipids was 13.6, 86.4, and 36.4%, respectively. Low intake of iron, zinc, and vitamin A was found in 29.2 and 50, 87.5 and 86.4, and 87.5 and 95.2% of the JIA and JSLE patients, respectively. There was not a significant association between intake, disease activity, and nutritional status. CONCLUSION: Patients with rheumatic diseases have inadequate dietary intake. There is excessive intake of lipids and proteins and low intake of micronutrients.
Rheumatic diseases; dietary intake; child; adolescent
OBJETIVOS: Avaliar o consumo alimentar de crianças e adolescentes com artrite idiopática juvenil (AIJ) e lúpus eritematoso sistêmico (LES) por recordatório de 24 horas e relacioná-lo com características clínicas e antropométricas e com os medicamentos empregados. MÉTODOS: Em estudo transversal, avaliamos os recordatórios de 24 horas de pacientes ambulatoriais. O estado nutricional foi classificado pelo CDC, 2000. Para o cálculo da ingestão, utilizamos o software NutWin UNIFESP-EPM. Para a análise quantitativa e qualitativa, adotamos as Recommended Dietary Allowances e a pirâmide alimentar brasileira. RESULTADOS: A mediana de idade foi 12 na AIJ e 16,5 anos no LES. Na AIJ, 37,5% dos pacientes estavam em atividade de doença, e, no LES, 68,2% tinham Systemic Lupus Erythematosus Disease Activity Index > 4. Foi encontrada desnutrição em 8,3 e 4,5% dos pacientes com AIJ e com LES, respectivamente, e obesidade, em 16,7 e 18,2%. Na AIJ, o consumo excessivo de energia, proteína e lipídios foi de 12,5, 75 e 31,3%, respectivamente. No LES, o consumo excessivo de energia, proteína e lipídios foi de 13,6, 86,4 e 36,4%, respectivamente. Consumo deficiente de ferro, zinco e vitamina A foi observado em 29,2 e 50, 87,5 e 86,4 e 87,5 e 95,2% dos pacientes com AIJ e LES, respectivamente. Não houve relação significante entre consumo, atividade da doença e estado nutricional. CONCLUSÃO: Pacientes com doenças reumáticas apresentam inadequação do consumo alimentar. Ressaltamos a ingestão excessiva de lipídios e proteínas e a ingestão insuficiente de micronutrientes.
Doenças reumáticas; ingestão de alimentos; criança; adolescente
ORIGINAL ARTICLE
Inadequate dietary intake of children and adolescents with juvenile idiopathic arthritis and systemic lupus erythematosus
Michelle C. CaetanoI;Thaís T. OrtizI;Maria Teresa S. L. R. A. TerreriII;Roseli O. S. SarniIII;Simone G. L. SilvaI;Fabíola I. S. SouzaIV;Maria Odete E. HilárioV
IMestranda, Ciências Aplicadas à Pediatria, Departamento de Pediatria, Universidade Federal de São Paulo Escola Paulista de Medicina (UNIFESP-EPM), São Paulo, SP, Brazil
IIProfessora afiliada, Disciplina de Alergia, Imunologia Clínica e Reumatologia, Departamento de Pediatria, UNIFESP-EPM, São Paulo, SP, Brazil
IIIDoutora, Medicina, UNIFESP-EPM, São Paulo, SP, Brazil
IVMestre, Ciências Aplicadas à Pediatria, UNIFESP-EPM, São Paulo, SP, Brazil
VProfessora associada, Chefe, Setor de Reumatologia, Disciplina de Alergia, Imunologia Clínica e Reumatologia, Departamento de Pediatria, UNIFESP-EPM, São Paulo, SP, Brazil
Correspondence Correspondence: Maria Teresa Terreri Rua Loefgreen, 2381/141 CEP 04035-970 - São Paulo, SP - Brazil Tel.: +55 (11) 5579.1590 Fax: +55 (11) 5579.1590 E-mail: teterreri@terra.com.br
ABSTRACT
OBJECTIVES: To evaluate the dietary intake of children and adolescents with juvenile idiopathic arthritis (JIA) and juvenile systemic lupus erythematosus (JSLE) using a 24-hour diet recall and relating it to the patients clinical and anthropometric characteristics and to the drugs used in their treatment.
METHODS: By means of a cross-sectional study, we assessed the 24-hour diet recalls of outpatients. Their nutritional status was classified according to the CDC (2000). The computer program NutWin UNIFESP-EPM was used for food intake calculation. The Recommended Dietary Allowances and the Brazilian food pyramid were used for quantitative and qualitative analysis.
RESULTS: Median age was 12 years for JIA patients and 16.5 years for JSLE patients. Among the JIA patients, 37.5% had active disease, and among the JSLE patients, 68.2% showed Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) > 4. Malnutrition was found in 8.3 and 4.5% of the JIA and JSLE patients, respectively, and obesity was present in 16.7 and 18.2%. For JIA patients, the excessive intake of energy, protein, and lipids was 12.5, 75, and 31.3%, respectively. For JSLE patients, the excessive intake of energy, protein, and lipids was 13.6, 86.4, and 36.4%, respectively. Low intake of iron, zinc, and vitamin A was found in 29.2 and 50, 87.5 and 86.4, and 87.5 and 95.2% of the JIA and JSLE patients, respectively. There was not a significant association between intake, disease activity, and nutritional status.
CONCLUSION: Patients with rheumatic diseases have inadequate dietary intake. There is excessive intake of lipids and proteins and low intake of micronutrients.
Keywords: Rheumatic diseases, dietary intake, child, adolescent
Introduction
1,231,3Juvenile systemic lupus erythematosus (JSLE) is a rare disease with varied clinical manifestations depending on the organ or system affected, and it may have an abrupt or insidious onset.4 Children and adolescents with JSLE usually have more severe disease onset and course when compared with adult patients.5 Cardiovascular complications of SLE should be carefully treated since they are the third most frequent cause of death among SLE patients, with renal and infectious complications being the two most frequent causes of death.4 Atherosclerotic lesions have their onset during early childhood, and healthy diet and lifestyle are essential factors for the prevention of cardiovascular diseases, especially for JSLE patients.5
During the past decade, the advances in terms of diagnosis and treatment of rheumatic diseases enabled patients to achieve longer survival. With the improvement of survival rates, some events that used to be less frequent became part of the disease progression.6,7 Thus, chronic complications related to SLE, inadequate eating habits and lifestyle, such as atherosclerosis, obesity, and osteoporosis, may worsen the patients prognosis and should be prevented.8
The evaluation of dietary intake using recalls is an important tool for the diagnosis of inadequate intake related to unhealthy eating habits aimed at providing appropriate nutritional counseling.9,10 There are few studies available in the literature assessing the dietary intake of JIA and JSLE patients. Among the different questionnaires available, the 24-hour diet recall can be considered the most frequently used for the evaluation of dietary and nutrient intake in individuals from different population groups.10 This questionnaire assesses the current diet and estimates absolute and relative values of energy and nutrient intake widely distributed in the total amount of food consumed by the individual with a high level of specificity.10
A population questionnaire assessing the nutritional status of healthy Brazilian children and adolescents suggested that there is an increasing trend in the prevalence of overweight (approximately 20%).11 This fact, combined with the higher risk for the development of chronic diseases, such as cardiovascular diseases,12 in individuals with rheumatic diseases and with the shortage of recent studies assessing the dietary intake in this condition, motivated us to conduct the present study.
The objective of this study was to assess the dietary intake of children and adolescents with JIA and JSLE using the 24-hour diet recall and relating it to the patients clinical and anthropometric characteristics and to the drugs used in their treatment.
Methods
We assessed, by means of a retrospective cross-sectional study, the dietary intake of JIA patients, who were classified according to the criteria of the International League of Associations for Rheumatology,13 and JSLE patients, according to the Hochberg criteria,14 who were being treated at the outpatient clinic of pediatric rheumatology of Universidade Federal de São Paulo Escola Paulista de Medicina (UNIFESP-EPM), São Paulo, Brazil, which provides health care to patients of the Brazilian public Unified Health System (Sistema Único de Saúde, SUS).
The sample comprised all patients who attended the outpatient clinic for medical and nutritional visits from May 2007 to August 2008. Those patients who did not accept to participate in the study, who did not appropriately answer the diet recall, or who had another associated disease different from the collagenosis under investigation were excluded.
This study was approved by the Research Ethics Committee of UNIFESP-EPM.
Demographic, clinical and treatment data were obtained from medical records. We used clinical and anthropometric data of the most recent medical visit, considering the date the 24-hour diet recall was answered.
For the dietary intake assessment, we used the 24-hour diet recall, an instrument that identifies all foods and beverages, as well as their amounts, consumed during the day previous to the interview. All questionnaires were administered by two nutritionists (M.C. and T.O.). Based on the interview, a quantitative analysis using the computer program NutWin UNIFESP-EPM was carried out. NutWin uses the database of the Recommended Dietary Allowances (RDA). With the purpose of analyzing the percentage of macronutrients consumed compared to the total amount of calories, we considered 50 and 60% as the appropriate percentage of calories from carbohydrates, between 10 and 15% the percentage related to proteins, and between 25 and 30% the percentage of lipids. We also performed a qualitative analysis of the questionnaire using the portions suggested by the food pyramid adapted by Philippi et al.15
Anthropometric assessment
Weight and height were measured according to the recommendations of the World Health Organization (WHO). For weight measurement, the patient was instructed to take off his/her shoes, dressing accessories and warm clothes, keeping only a t-shirt and a pair of light pants. Body weight was measured using a digital scale Welmy W200 with capacity of 200 kg and 100-gram divisions. For height measurement, we used a wall-mounted stadiometer. Anthropometric measures were taken according to the recommendations by Jellife.16
In order to identify the patients nutritional status, we calculated the body mass index (BMI) using the 2000 CDC Growth Charts17 as reference.
Assessment of disease activity
For JIA, the following parameters were considered: number of active articulations, articular movement limitation, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), presence of uveitis, and systemic signs/symptoms.
For assessment of SLE activity, the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) was used considering a score higher than 4 as active disease.18
Mean daily dose of corticoid, use of nonsteroidal anti-inflammatory drug, gastric protector, and methotrexate were calculated.
Statistical analysis
Relative and absolute frequency tables were used to identify the characteristics of the population studied, and we defined the measurements of central tendency according to the normality or abnormality characteristics of the variables investigated. The chi-square test and Fishers exact test were used to compare our results with the 24-hour diet recall, anthropometric measures, disease activity, and use of medication. Significance level was set at 5%.
Results
Seventy patients were assessed; 48 of them had JIA and 22 had JSLE. Their demographic and clinical characteristics are described in Table 1. Distribution according to sex shows prevalence of female patients in both groups. The anthropometric assessment demonstrated that 61.4% of the individuals were eutrophic.
With regard to macronutrient intake, we found that the JIA group had an excessive intake of energy, protein, and lipids in 12.5, 75, and 31.3% of the cases, respectively. The percentage of the JSLE group that consumed higher amounts of energy, protein, and lipids than the values recommended was 13.6, 86.4, and 36.4%, respectively.
Regarding low intake of macronutrients, we found that 41.7, 8.3, and 31.3% of the JIA patients consumed low amounts of energy, protein, and lipids, respectively. For the JSLE group, the percentage of consumption lower than the recommended value was 45.5% in terms of energy and 36.4% with regard to lipids. None of the patients with JSLE consumed lower amounts of protein than the recommended values.
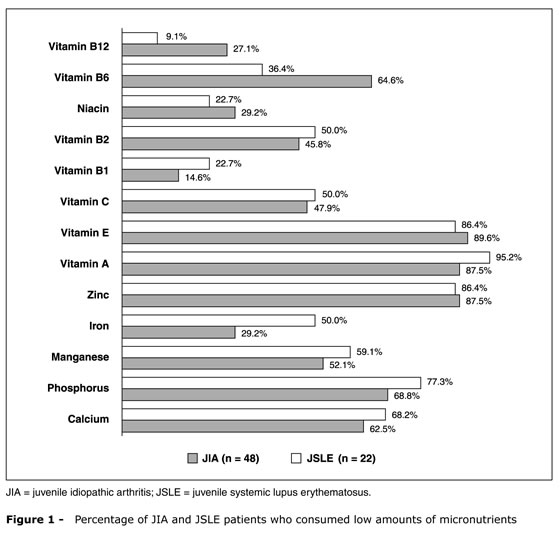
The percentage of patients with JIA and JSLE who consumed low amounts of micronutrients (vitamins and minerals), according to the qualitative analysis of the 24-hour diet recall, is shown in Figure 1.
The JIA group did not show a significant association between the intake of energy, macro and micronutrients and age, BMI, disease activity, disease subtype (oligoarticular, polyarticular, systemic), use of methotrexate, use of oral corticoid, or pulse therapy with methylprednisolone.
In the JSLE group, we also did not find a significant association between the intake of energy, macro and micronutrients and age, BMI, disease activity (SLEDAI), use of oral corticoid, or pulse therapy with methylprednisolone.
The qualitative analysis of the dietary intake showed low consumption of milk and derivatives, as well as fruits and vegetables and excessive consumption of oils and fats, as well as sugar and sweets (Table 2).
Figure 2 shows the consumption of coffee and certain foods rich in sugar, lipid, and sodium.
Discussion
The present study demonstrated that children and adolescents with JIA and JSLE have qualitative and quantitative inadequate dietary intake. Despite the intrinsic limitations of the 24-hour diet recall, since it does not analyze daily variations and relies on the interviewees memory, other studies have demonstrated that it is a useful method to estimate dietary intake because the mean intake does not have significant daily changes.9,10
Population studies assessing the dietary intake of this age group are rare, and it is not possible to compare them because they use different methods.
With regard to the comparison of our results with the specific literature, it is also quite limited, since there are not studies assessing the dietary intake of children and adolescents with JSLE so far. In terms of JIA, there is a large number of studies on growth deficiency; however, the studies assessing the dietary intake of children and adolescents used different method.1-3,19-23
Regarding the low energy intake we found (41.7% in JIA patients and 45.5% in JSLE patients), our data are similar to those of studies involving healthy Brazilian subjects belonging to the same age group, with results ranging from 10.7 to 50%.9,24,25
Some studies conducted with JIA patients have shown divergent results with regard to the energy intake, demonstrating low intake,5,20 high intake,21 or no difference between the values2 when compared to healthy controls.
In addition, we found that other studies have not shown a statistically significant association between dietary intake, age, sex, disease activity, and use of medication in JIA patients.1,2 In the study by Cleary et al.,20 involving 123 patients and using multiple regression, the authors found a significant association between malnutrition and the number of articulations affected (more than five) and younger age. There was not a significant association between energy intake and malnutrition, but it was associated with disease subtype. In our study, the smaller number of JIA patients and the lower prevalence of malnutrition (8.3%) limited the stratification according to disease subtype.
So far, we could not find studies that can be compared to our results with regard to JSLE.
Our result regarding excessive protein intake in the JIA and JSLE groups was similar to that detected in a study involving healthy Brazilian subjects of the same age group.24 In a study that investigated Brazilian children and adolescents with JIA, Chaud et al.1 found excessive protein intake in 39% of the individuals assessed.
When the disease is active, there is higher need of protein intake due to muscle proteolysis and increased nitrogen urinary excretion. On the other hand, excessive protein intake could lead to weight loss, mainly of muscle mass, since non-protein calories are used for the incorporation of the excessive amount of protein consumed.1-3,26
Lean mass and bone mineral mass are directly related. Studies have shown low bone mineral mass in JIA and JSLE in other countries.22,23 Such deficit is multifactorial and may be caused by chronic inflammatory process, low calcium intake, and low sun exposure, among other factors. We can assume that an additional factor is the progressive and continuous loss of lean mass, a process worsened by high and constant protein intake according to our findings.
High lipid intake around 30 to 40% was found both in our sample and among healthy Brazilian children and adolescents.9,24 Alterations in the lipid profile are frequent, mainly in individuals with JSLE, and may contribute to the future development of cardiovascular complications.27
Reduced micronutrient intake (vitamins and minerals) has also been found in studies involving healthy children and adolescents.25,28 Some authors2,5,21 have reported that there is not a significant difference in terms of micronutrient intake between JIA patients and controls. A study conducted with SLE adult patients revealed significant impairment of the antioxidant defense compared to healthy controls related to the impairment of the nutritional status assessed by biomarkers of micronutrients such as retinol and beta-carotene.5
Oxidative stress is considered an important pathophysiologic mechanism for the development of chronic diseases such as obesity, dyslipidemias, and cardiovascular diseases.2
The qualitative analysis of intake based on the food groups of the food pyramid adapted by Philippi et al.15 shows low intake of fruits and vegetables, which are the main sources of exogenous antioxidants, in our patients with JIA and JSLE.
Similarly to our findings, other authors have highlighted the low intake of fruits and vegetables of healthy children and adolescents.29 There are not specific recommendations for patients with collagen disease and, therefore, we used the amounts recommended for the healthy population in order to assess the adequate intake.
Our study showed results similar to those of other Brazilian studies about dietary intake recently conducted by Kazapi et al.24 and Albuquerque & Monteiro25 using 24-hour diet recall for the assessment of healthy children and adolescents. Such fact emphasizes the importance of implementing a wide program of nutritional education aimed at establishing healthy eating habits and the consequent decrease in the risk of developing chronic diseases in the future.
The consequences of bad eating habits are even more important for children and adolescents with chronic rheumatic diseases. Chronic diseases are characterized by pro-oxidative effects that could be minimized by healthy lifestyle and eating habits. The opposite can also be assumed: inadequate eating habits, such as excessive intake of proteins and lipids combined with low micronutrient intake, could favor risk factors for cardiovascular diseases like dyslipidemia and increased homocysteine, which has been demonstrated in our population in previous studies.6,7,30
Despite the methodological limitations of the present study (sample size, cross-sectional and non-controlled design), the identification of inadequate eating habits is important, since it enables early intervention and effective reduction of risk factors for chronic diseases.
Conclusion
References
1. Chaud DM, Hilário MO, Yanaguibashi G, Amâncio OM. Avaliações dietética e antropométrica em pacientes com artrite reumatóide juvenil. Rev Assoc Med Bras. 2003;49:181-4.
2. Amâncio OM, Chaud DM, Yanaguibashi G, Hilário MO. Copper and zinc intake and serum levels in patients with juvenile rheumatoid arthritis. Eur J Clin Nutr. 2003;57:706-12.
3. Bisotto LS, Xavier RM, Machado SH, Bredemeier M, Brenol JC. Impacto da atividade inflamatória e uso de glicocorticóide nas variáveis nutricionais da artrite idiopática juvenil. Rev Bras Reumatol. 2005;45:291-300.
4. Brown AC. Lupus erythematosus and nutrition: a review of the literature. J Ren Nutr. 2000;10:170-83.
5. Bae SC, Kim SJ, Sung MK. Impaired antioxidant status and decreased dietary intake of antioxidants in patients with systemic lupus erythematosus. Rheumatol Int. 2002;22:238-43.
6. Nascif AK, Hilário MO, Terreri MT, Ajzen S, DAlmeida V, Plavnik FL et al. Endothelial function analysis and atherosclerotic risk factors in adolescents with systemic lupus erythematosus. Int J Adolesc Med Health. 2007;19:497-505.
7. do Prado R, DAlmeida VM, Guerra-Shinohara EG, Galdieri LC, Terreri MT, Hilario MO. Increased concentration of plasma homocysteine in children with Systemic Lupus Erythematosus. Clin Exp Rheumatol. 2006;24:594-8.
8. Freire BF, da Silva RC, Fabro AT, dos Santos DC. Lúpus eritematoso sistêmico: novo fator de risco para aterosclerose? Arq Bras Cardiol. 2006;87:300-6.
9. Carmos MB, Toral N, Silva MV, Slater B. Consumo de doces, refrigerantes e bebidas com adição de açúcar entre adolescentes da rede pública de ensino de Piracicaba, São Paulo. Rev Bras Epidemiol. 2006;9:121-30.
10. Cavalcante AA, Priore SE, Franceschini SC. Estudos de consumo alimentar: aspectos metodológicos gerais e o seu emprego na avaliação de crianças e adolescentes. Rev Bras Saude Matern Infant. 2004;4:229-40.
11. Ministério do Planejamento Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística. Pesquisa de orçamentos familiares 2002/2003 análise da disponibilidade domiciliar de alimentos e do estado nutricional no Brasil. Rio de Janeiro, RJ: IBGE; 2004.
12. Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198-200.
13. Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-4.
14. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum.1997;40:1725.
15. Philippi ST, Latterza AR, Cruz AT, Ribeiro LC. Pirâmide alimentar adaptada: guia para escolha dos alimentos. Rev Nutr. 1999;1265-80.
16. Jellife DB. The assessment of the nutritional status of the community. Geneva: WHO; 1966.
17. Centers for Disease Control and Prevention. National Center Health Statistics. Growth Charts. Atlanta, GA: CDC; 2000.
18. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity índex for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630-40.
19. Shaw KL, Southwood TR, McDonagh JE; British Society of Paediatric and Adolescent Rheumatology. Growing up and moving on in rheumatology: a multicentre cohort of adolescent with juvenile idiopathic arthritis. Rheumatology (Oxford). 2005;44:806-12.
20. Cleary AG, Lancaster AG, Annan F, Sills JA, Davidson JE. Nutritional impairment in juvenile idiopathic arthritis. Rheumatology (Oxford). 2004;43:1569-73.
21. Gómez-Vaquero C, Nolla JM, Fiter J, Ramon JM, Concustell R, Valverde J, et al. Nutritional status in patients with rheumatoid arthritis. Joint Bone Spine. 2001;68:403-9.
22. Mul D, Van Suijlekom-Smit LWA, ten Cate R, Bekkering WP, de Muinck Keizer-Schrama SM. Bone mineral density and body composition and influencing factors in children with rheumatic disease treated with corticosteroids. J Ped Endocrinol Metabolism. 2002;15:187-92.
23. Head AJ, Myers LK, Watsky MA, Greenwell MW, Barrow KD, Michelson JA, et al. Bone mineral density and turnover in non-corticosteroid treated African American children with juvenile rheumatoid arthritis. J Rheumatol. 2006;33:1001-3.
24. Kazapi IM, Di Pietro PF, Avancini SR, Freitas SF, Tramonte VL. Consumo de energia e macronutrientes por adolescentes de escolas públicas e privadas. Rev Nutr. 2001;14:27-33.
25. Albuquerque M, Monteiro AM. Ingestão de alimentos e adequação de nutrientes no final da infância. Rev Nutr. 2002;15:291-9.
26. Hilário MO, Terreri MT, Len CA. Nonsteroidal anti-inflammatory drugs: cyclooxygenase 2 inhibitors. J Pediatr (Rio J). 2006;82:S206-12.
27. Tyrrell P, Beyene J, Benseler S, Sarkissian T, Silverman, E. Predictors of lipid abnormalities in children with new-onset systemic lupus erythematosus. J Rheumatol. 2007;34:2112-9.
28. Santos JS, Costa CO, Sobrinho CL, Silva MC, Souza KE, Melo BO. Perfil antropométrico e consumo alimentar de adolescentes de Teixeira de Freitas Bahia. Rev Nutr. 2005;18:623-32.
29. Torral N, Slater B, Cintra IP, Fisberg M. Comportamento alimentar de adolescentes em relação ao consumo de frutas e verduras. Rev Nutr. 2006;19:331-40.
30. Terreri MT, Sarni RO, Prado R, Nascif ANS, DAlmeida V, Hilário MO. Hiperhomocisteinemia em crianças e adolescentes com Lúpus Eritematoso Sistêmico: avaliação evolutiva. Acta Reumatol Port. 2008;33:57-62.
Manuscript submitted Jun 5 2009, accepted for publication Aug 12 2009
This study was conducted at Universidade Federal de São Paulo Escola Paulista de Medicina (UNIFESP-EPM), São Paulo, SP, Brazil.
No conflicts of interest declared concerning the publication of this article.
Suggested citation: Caetano MC, Ortiz TT, Terreri MT, Sarni RO, Silva SG, Souza FI, et al. Inadequate dietary intake of children and adolescents with juvenile idiopathic arthritis and systemic lupus erythematosus. J Pediatr (Rio J). 2009;85(6):509-515.
- 1. Chaud DM, Hilário MO, Yanaguibashi G, Amâncio OM. Avaliações dietética e antropométrica em pacientes com artrite reumatóide juvenil. Rev Assoc Med Bras. 2003;49:181-4.
- 2. Amâncio OM, Chaud DM, Yanaguibashi G, Hilário MO. Copper and zinc intake and serum levels in patients with juvenile rheumatoid arthritis. Eur J Clin Nutr. 2003;57:706-12.
- 3. Bisotto LS, Xavier RM, Machado SH, Bredemeier M, Brenol JC. Impacto da atividade inflamatória e uso de glicocorticóide nas variáveis nutricionais da artrite idiopática juvenil. Rev Bras Reumatol. 2005;45:291-300.
- 4. Brown AC. Lupus erythematosus and nutrition: a review of the literature. J Ren Nutr. 2000;10:170-83.
- 5. Bae SC, Kim SJ, Sung MK. Impaired antioxidant status and decreased dietary intake of antioxidants in patients with systemic lupus erythematosus. Rheumatol Int. 2002;22:238-43.
- 6. Nascif AK, Hilário MO, Terreri MT, Ajzen S, DAlmeida V, Plavnik FL et al. Endothelial function analysis and atherosclerotic risk factors in adolescents with systemic lupus erythematosus. Int J Adolesc Med Health. 2007;19:497-505.
- 7. do Prado R, DAlmeida VM, Guerra-Shinohara EG, Galdieri LC, Terreri MT, Hilario MO. Increased concentration of plasma homocysteine in children with Systemic Lupus Erythematosus. Clin Exp Rheumatol. 2006;24:594-8.
- 8. Freire BF, da Silva RC, Fabro AT, dos Santos DC. Lúpus eritematoso sistêmico: novo fator de risco para aterosclerose? Arq Bras Cardiol. 2006;87:300-6.
- 9. Carmos MB, Toral N, Silva MV, Slater B. Consumo de doces, refrigerantes e bebidas com adição de açúcar entre adolescentes da rede pública de ensino de Piracicaba, São Paulo. Rev Bras Epidemiol. 2006;9:121-30.
- 10. Cavalcante AA, Priore SE, Franceschini SC. Estudos de consumo alimentar: aspectos metodológicos gerais e o seu emprego na avaliação de crianças e adolescentes. Rev Bras Saude Matern Infant. 2004;4:229-40.
-
11Ministério do Planejamento Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística. Pesquisa de orçamentos familiares 2002/2003 análise da disponibilidade domiciliar de alimentos e do estado nutricional no Brasil. Rio de Janeiro, RJ: IBGE; 2004.
- 12. Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198-200.
- 13. Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-4.
- 14. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum.1997;40:1725.
- 15. Philippi ST, Latterza AR, Cruz AT, Ribeiro LC. Pirâmide alimentar adaptada: guia para escolha dos alimentos. Rev Nutr. 1999;1265-80.
- 16. Jellife DB. The assessment of the nutritional status of the community. Geneva: WHO; 1966.
-
17Centers for Disease Control and Prevention. National Center Health Statistics. Growth Charts. Atlanta, GA: CDC; 2000.
- 18. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity índex for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630-40.
- 19. Shaw KL, Southwood TR, McDonagh JE; British Society of Paediatric and Adolescent Rheumatology. Growing up and moving on in rheumatology: a multicentre cohort of adolescent with juvenile idiopathic arthritis. Rheumatology (Oxford). 2005;44:806-12.
- 20. Cleary AG, Lancaster AG, Annan F, Sills JA, Davidson JE. Nutritional impairment in juvenile idiopathic arthritis. Rheumatology (Oxford). 2004;43:1569-73.
- 21. Gómez-Vaquero C, Nolla JM, Fiter J, Ramon JM, Concustell R, Valverde J, et al. Nutritional status in patients with rheumatoid arthritis. Joint Bone Spine. 2001;68:403-9.
- 22. Mul D, Van Suijlekom-Smit LWA, ten Cate R, Bekkering WP, de Muinck Keizer-Schrama SM. Bone mineral density and body composition and influencing factors in children with rheumatic disease treated with corticosteroids. J Ped Endocrinol Metabolism. 2002;15:187-92.
- 23. Head AJ, Myers LK, Watsky MA, Greenwell MW, Barrow KD, Michelson JA, et al. Bone mineral density and turnover in non-corticosteroid treated African American children with juvenile rheumatoid arthritis. J Rheumatol. 2006;33:1001-3.
- 24. Kazapi IM, Di Pietro PF, Avancini SR, Freitas SF, Tramonte VL. Consumo de energia e macronutrientes por adolescentes de escolas públicas e privadas. Rev Nutr. 2001;14:27-33.
- 25. Albuquerque M, Monteiro AM. Ingestão de alimentos e adequação de nutrientes no final da infância. Rev Nutr. 2002;15:291-9.
- 26. Hilário MO, Terreri MT, Len CA. Nonsteroidal anti-inflammatory drugs: cyclooxygenase 2 inhibitors. J Pediatr (Rio J). 2006;82:S206-12.
- 27. Tyrrell P, Beyene J, Benseler S, Sarkissian T, Silverman, E. Predictors of lipid abnormalities in children with new-onset systemic lupus erythematosus. J Rheumatol. 2007;34:2112-9.
- 28. Santos JS, Costa CO, Sobrinho CL, Silva MC, Souza KE, Melo BO. Perfil antropométrico e consumo alimentar de adolescentes de Teixeira de Freitas Bahia. Rev Nutr. 2005;18:623-32.
- 29. Torral N, Slater B, Cintra IP, Fisberg M. Comportamento alimentar de adolescentes em relação ao consumo de frutas e verduras. Rev Nutr. 2006;19:331-40.
- 30. Terreri MT, Sarni RO, Prado R, Nascif ANS, DAlmeida V, Hilário MO. Hiperhomocisteinemia em crianças e adolescentes com Lúpus Eritematoso Sistêmico: avaliação evolutiva. Acta Reumatol Port. 2008;33:57-62.
Correspondence:
Publication Dates
-
Publication in this collection
12 Jan 2010 -
Date of issue
Dec 2009
History
-
Accepted
12 Aug 2009 -
Received
05 June 2009