ABSTRACT:
The heating rate used during semen thawing plays an important role in reducing structural and functional damage to spermatozoa. In this study, we evaluated the influence of thawing temperature on semen quality, reactive oxygen species (ROS) production, and mitochondrial activity of cryopreserved bovine semen. A total of 195 straws of 0.5 mL from five Holstein Friesian bulls were used (39 straws per bull). Samples underwent 8 to 22 years of storage; they were processed under a standard protocol with tris-egg yolk and stored in liquid nitrogen. Samples were thawed for 30 seconds in a water bath at T1: 36 °C, T2: 38 °C or T3: 40 °C. Sperm motility and kinematics, morphology, structural membrane integrity (SMI), functional membrane integrity (FMI), acrosome integrity (AI), ROS, and mitochondrial membrane potential (ΔΨM) of post-thawing bovine sperm were evaluated. Generalized linear models were fitted to the data. Each model included the effects of bull, storage time, and treatment. The Shapiro-Wilk test was used to assess data normality, and means were compared using the Tukey test. T2 and T3 showed better results for sperm motility and kinematic parameters, SMI (%) (T1 41.9 ± 2.3; T2 45.7 ± 1.9; T3 47.4 ± 2.8), ROS (RFU/min) (T1 0.026 ± 0.007; T2 0.032 ± 0.001; T3 0.031 ± 0.001) and high-ΔΨM (RFU x 103) (67.1± 0,4; 71.3 ± 0.4; 74.2 ± 0.4) (P < 0.05). However, T1 had higher FMI (39.3 ± 2.3) than T2 (34.0 ± 1.9) (P < 0.05), though not significantly (P > 0.05) different from T3 (38.4 ± 2.2). Thawing temperatures of 38 °C and 40 °C increases motility, kinetics, membrane integrity, mitochondrial activity and ROS of cryopreserved bovine semen, compared with more conventional thawing at 36 °C.
Key words:
heating rate; bulls; sperm quality; oxidative stress
RESUMO:
A taxa de aquecimento usada durante o descongelamento do sêmen desempenha um papel importante na redução dos danos estruturais e funcionais nos espermatozóides. O objetivo desta pesquisa foi avaliar a influência da temperatura de descongelamento na qualidade do sêmen, produção de espécies reativas de oxigênio (ROS) e atividade mitocondrial do sêmen bovino criopreservado. Foram utilizados 195 palhetas de 0,5 mL de cinco touros Holstein Friesian (39 palhetas por touro). As amostras passaram por oito a 22 anos de armazenamento e foram processadas sob protocolo padrão com Tris-gema de ovo e armazenadas em nitrogênio líquido. As temperaturas de descongelamento foram T1: 36 °C, T2: 38 °C, T3: 40 °C, cada uma por 30 segundos em banho-maria. Pós-descongelamento, a motilidade e cinética dos espermatozoides, morfologia, integridade estrutural da membrana (SMI), integridade funcional da membrana (FMI), integridade acrossomal (AI), ROS e potencial de membrana mitocondrial (ΔΨM) foram avaliados. Modelos lineares generalizados foram ajustados. Cada modelo incluiu os efeitos de touro, tempo de armazenamento e tratamento. A normalidade dos dados foi avaliada pelo teste de Shapiro-Wilk e as médias comparadas pelo teste de Tukey. T2 e T3 apresentaram resultados mais elevados para a maioria dos parâmetros de motilidade e cinemática espermática, SMI (%) (T1 41,9 ± 2,3; T2 45,7 ± 1,9; T3 47,4 ± 2,8), ROS (RFU/min) (T1 0,026 ± 0,007; T2 0,032 ± 0,001; T3 0,031 ± 0,001) e alto ΔΨM (RFU x 103) (67,1 ± 0,4; 71,3 ± 0,4; 74,2 ± 0,4) (P < 0,05). No entanto, T1 apresentou maior FMI (%) (39,3 ± 2,3) em comparação a T2 (34,0 ± 1,9) (P < 0,05), mas não foi diferente do T3 (38,4 ± 2,2) (P > 0,05). Conclui-se que as temperaturas de descongelamento de 38 °C e 40 °C produzem um aumento na motilidade, cinética, integridade de membrana, atividade mitocondrial e ROS do sêmen bovino criopreservado, em comparação com o uso mais convencional de uma temperatura de descongelamento de 36 °C.
Palavras-chave:
taxa de aquecimento; touros; qualidade espermática; estresse oxidativo
INTRODUCTION:
Sperm cryopreservation allows the long-term maintenance of male gametes and is a fundamental process in assisted reproduction. Despite its optimization, cryopreservation has induced deleterious changes in sperm structure and function (TREULEN et al., 2018TREULEN, F. et al. Cryopreservation induces mitochondrial permeability transition in a bovine sperm model. Cryobiology, v.83, p.65-74, 2018. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0011224018301469 >. Accessed: Feb. 24, 2021. doi: 10.1016/j.cryobiol.2018.06.001.
https://linkinghub.elsevier.com/retrieve...
; WATSON, 2000WATSON, P. The causes of reduced fertility with cryopreserved semen. Animal Reproduction Science, v.60-61, p.481-492, 2000. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378432000000993 >. Accessed: Jul. 05, 2021. doi: 10.1016/s0378-4320(00)00099-3.
https://linkinghub.elsevier.com/retrieve...
). Cryopreservation involves thermal stress due to temperature changes during cooling, freezing and thawing, and osmotic pressure caused by adding high concentrations of cryoprotective agents and crystallization (BINIOVÁ et al., 2018BINIOVÁ, Z. et al. Effect of thawing method on bull sperm survival in ejaculates frozen in 4 mL and 8 mL volumes. Czech Journal of Animal Science, v.63, n.10, p.399-407, 2018. Available from: <Available from: https://www.agriculturejournals.cz/web/cjas.htm?type=article&id=117_2018-CJAS >. Accessed: May, 13, 2021. doi: 10.17221/117/2018-CJAS. doi: 10.17221/117/2018-CJAS.
https://www.agriculturejournals.cz/web/c...
; KHALIL et al., 2018KHALIL, W. A. et al. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. International Journal of Veterinary Science and Medicine, v.6, n.sup1, p.S49-S56, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 >. Accessed: Sept. 06, 2021. doi: 10.1016/j.ijvsm.2017.11.001.
https://www.tandfonline.com/doi/full/10....
). A high percentage of the surviving spermatozoa carry sublethal damage (FERRUSOLA et al., 2010FERRUSOLA, C. O. et al. Inhibition of the mitochondrial permeability transition pore reduces “apoptosis like” changes during cryopreservation of stallion spermatozoa. Theriogenology, v.74, n.3, p.458-465, 2010. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X10001445 >. Accessed: May, 07, 2021. doi: 10.1016/j.theriogenology.2010.02.029.
https://linkinghub.elsevier.com/retrieve...
).
Thawing frozen semen activates it physiologically; it is hence essential that thawing be done carefully at an optimal temperature with sufficient time to minimize the loss of semen quality (BORAH et al., 2015BORAH, B. K. D. et al. Effect of thawing methods on frozen semen quality of yak (Poephagus grunniens L.) bulls. Veterinary World, v.8, n.7, p.831-834, 2015. Available from: <Available from: http://www.veterinaryworld.org/Vol.8/July-2015/3.html >. Accessed: May, 09, 2021. doi: 10.14202/vetworld.2015.831-834.
http://www.veterinaryworld.org/Vol.8/Jul...
). Research on thawing protocols has shown that the thawing rate significantly affects the motility and functional traits of frozen-thawed sperm in conventional insemination doses (RASTEGARNIA et al., 2013RASTEGARNIA, A. et al. Effect of different thawing rates on post-thaw viability, Kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell Journal, v.14, n.4, p.306-313, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23577311/ >. Accessed: Apr. 10, 2021.
https://pubmed.ncbi.nlm.nih.gov/23577311...
).
Multiple studies have evaluated the effect of heating rate on sperm function. It has been reported that thawing at high temperatures increases the motility and kinematic characteristics of the sperm (RASTEGARNIA et al., 2013RASTEGARNIA, A. et al. Effect of different thawing rates on post-thaw viability, Kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell Journal, v.14, n.4, p.306-313, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23577311/ >. Accessed: Apr. 10, 2021.
https://pubmed.ncbi.nlm.nih.gov/23577311...
). Rapid thawing at high temperatures decreased sperm motility and the characteristics of the semen when evaluated after 2 hours of incubation, indicating that the rapid straw thawing method is not the most appropriate for artificial insemination (DOLEŽALOVÁ et al., 2017DOLEŽALOVÁ, M. et al. Effect of different hawing methods on bull’s semen characteristics. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis, v.65, n.3, p.815-822, 2017. Available from: <Available from: http://acta.mendelu.cz/doi/10.11118/actaun201765030815.html >. Accessed: Feb. 11, 2021. doi: 10.11118/actaun201765030815.
http://acta.mendelu.cz/doi/10.11118/acta...
).
Several studies have focused on the impact of bovine semen thawing temperature on seminal quality and fertilizing capacity (AL-BADRY, 2012AL-BADRY, K. I. Effect of various thawing times and temperatures on frozen semen quality of Friesian bulls in Iraq. International Journal of Animal and Veterinary Advances, v.4, n.6, p.384-388, 2012. Available from: <Available from: https://www.researchgate.net/publication/332465334_Effect_of_Various_Thawing_Times_and_Temperatures_on_Frozen_semen_Quality_of_Friesian_Bulls_in_Iraq >. Accessed: Jun. 21, 2022.
https://www.researchgate.net/publication...
; LYASHENKO, 2015LYASHENKO, A. Effect of different thawing procedures on the quality and fertility of the bull spermatozoa. Asian Pacific Journal of Reproduction, v.4, n.1, p.17-21, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S2305050014600518 >. Accessed: Jan. 21, 2021. doi: 10.1016/S2305-0500(14)60051-8.
https://www.sciencedirect.com/science/ar...
). However, it is difficult to locate studies where the effect of heating rate on mitochondrial activity and redox status of frozen-thawed bovine semen have been reported. Therefore, this study assessed the influence of three thawing temperatures on sperm quality, ROS production, and mitochondrial activity of frozen bovine semen.
MATERIALS AND METHODS:
Biological samples and treatments
Straws were selected from the semen bank at the Animal Reproduction Laboratory of the Universidad Nacional de Colombia, Medellin headquarters. A total of 195 straws of 0.5 mL from five Holstein Friesian bulls was used (39 straws per bull). Semen samples underwent 8 to 22 years of storage and were processed under a standard protocol with a tris-yolk extender at 60 × 106 spermatozoa/mL. Straws were cooled at 5 °C for three hours, frozen by exposure to liquid nitrogen vapor, and stored in a liquid nitrogen tank. Thawing temperatures (treatments) were T1: 36 °C, T2: 38 °C, T3: 40 °C, each for 30 s in a water bath. T1 was considered the reference protocol (control). Straws from each storage time and each bull were evaluated using the three treatments.
Sperm quality assessment
Three straws from three storage times were used for semen quality evaluation for each bull and treatment. The motility and kinematics were assessed using the Sperm Class Analyzer System (SCA®, Microptic S.L, Barcelona, Spain), with a phase-contrast microscope (Eclipse E200, Nikon, Inc, Japan). The software was configured as follows: 20 × 20 mm glass slide, optics in a negative phase, 7 µL drop, thermal plate at 37 °C, a particle size of 20 to 72 µm, and capture velocity of 25 fps. A minimum of 500 spermatozoids were evaluated in five observation fields. The variables included total motility (TM), progressive motility (PM), straight-line velocity (VSL), curvilinear velocity (VCL), average path velocity (VAP), linearity (LIN), the amplitude of lateral head (ALH), and beat cross frequency (BCF).
The morphology and structural membrane integrity (SMI) was assessed using eosin-nigrosine staining (BRITO et al., 2011BRITO, L. F. C. et al. Effect of method and clinician on stallion sperm morphology evaluation. Theriogenology, v.76, n.4, p.745-750, 2011. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X11001671 >. Accessed: Sept. 22, 2020. doi: 10.1016/j.theriogenology.2011.04.007.
https://linkinghub.elsevier.com/retrieve...
). Eosin-nigrosin solution was prepared by mixing 0.67 g of eosin Y, 10 g of nigrosin (Sigma-Aldrich, Eugene, OR), and 100 mL of 0.9% sodium chloride in water. A droplet of semen and a droplet of eosin-nigrosine solution were placed on a microscope slide, mixed, smeared, and set on a warming plate at 37 °C. Subsequently, 200 spermatozoa were assessed individually using an Eclipse E200 phase-contrast microscope. Sperm with a structurally intact membrane would not show eosin staining of the cytoplasm. Morphological abnormalities of the sperm were classified according to their head, midpiece, or tail location.
The functional membrane integrity (FMI) of sperm was assessed using the hypo-osmotic swelling test (HOST) (ROTA et al., 2000ROTA, A. et al. Hypoosmotic swelling (HOS) as a screening assay for testing in vitro fertility of bovine spermatozoa. Theriogenology, v.53, n.7, p.1415-1420, 2000. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X00002843 >. Accessed: Mar. 22, 2021. doi: 10.1016/S0093-691X(00)00284-3.
https://linkinghub.elsevier.com/retrieve...
). To provide a 100 mOsmol/L concentration, 20 µL of semen were added to 200 µL of a hypo-osmotic 5.4% sucrose solution. This mixture was incubated at 38.5 °C for 30 min. Then, 200 spermatozoa were counted using an Eclipse E200 microscope. Functional membrane sperm reacted to the hypo-osmotic shock by rolling up the tail.
Acrosome integrity was assessed with a fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) probe (Sigma-Aldrich, St. Louis, USA) (RATHI et al., 2001RATHI, R. et al. Evaluation of in vitro capacitation of stallion spermatozoa. Biology of Reproduction, v.65, n.2, p.462-470, 2001. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/11466214/ >. Accessed: Mar. 26, 2021. doi: 10.1095/biolreprod65.2.462.
https://pubmed.ncbi.nlm.nih.gov/11466214...
). Sperm samples were smeared and fixed for 10 min with 95% ethanol, dried at room temperature, then placed in darkness for 30 min with 25 µL of FITC-PNA (5 µg/mL) in phosphate buffer solution (PBS). The smears were then washed with distilled water, and 200 spermatozoa were located and evaluated using the phase contrast optics and HBO (Mercury short-arc lamp) fluorescence with a G-2A filter of an Eclipse E200 microscope. Sperm with normal or slightly disordered acrosomes were considered intact (RAJABI-TOUSTANI et al., 2019RAJABI-TOUSTANI, R. et al. Methodological improvement of fluorescein isothiocyanate peanut agglutinin (FITC-PNA) acrosomal integrity staining for frozen-thawed Japanese Black bull spermatozoa. Journal of Veterinary Medical Science, v.81, n.5, p.694-702, 2019. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/30606905/ >. Accessed: Sept. 03, 2021. doi: 10.1292/jvms.18-0560.
https://pubmed.ncbi.nlm.nih.gov/30606905...
).
Assessment of mitochondrial membrane potential (ΔΨM)
For ΔΨM evaluation, two straws from two storage times were used for each treatment and bull. Sperm ΔΨM was assessed using the JC-1 cationic fluorescent probe. A stock of JC-1 was prepared at 1.53 mM in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Eugene, OR, USA), and a concentration of 2 µM was used in the semen sample. Readings were done in a fluorescence microplate reader (LS 55 spectrofluorometer, Perkin Elmer, Waltham) (GRAVANCE et al., 2000GRAVANCE, C. G. et al. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology, v.53, n.9, p.1691-1703, 2000. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/10968415/ >. Accessed: Jun. 25, 2021. doi: 10.1016/s0093-691x(00)00308-3.
https://pubmed.ncbi.nlm.nih.gov/10968415...
). Each sample was placed in three wells and incubated for 40 min in the dark at room temperature. Evaluations were performed using excitation/emission wavelengths for low and high internal mitochondrial membrane potentials of 514/529 nm and 585/590 nm, respectively. Readings for each straw were taken in triplicate (RESTREPO & ROJANO, 2018RESTREPO, G; ROJANO, B. Actividad antioxidante del isoespintanol y el timol en el semen equino criopreservado. Rev. Investig. Vet. del Perú, v.29, n.1, p.205, 2018, Available from: <Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-93542017000300149&nrm=iso/0122-9354/ >. Accessed: Jun. 10, 2021. doi: 10.19052/mv.4397.
http://www.scielo.org.co/scielo.php?scri...
). The results were expressed as relative fluorescence units (RFU).
Evaluation of reactive oxygen species
ROS production was conducted using the 2,7-dichlorodihydrofluorescein diacetate methodology (H2DCFDA, Intervet International BV, Boxmeer, Netherlands) (AITKEN et al., 2013AITKEN, R. J. et al. On methods for the detection of reactive oxygen species generation by human spermatozoa: analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology, v.1, n.2, p.192-205, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23316012/ >. Accessed: Jun. 25, 2021. doi: 10.1111/j.2047-2927.2012.00056.x.
https://pubmed.ncbi.nlm.nih.gov/23316012...
). Each sample consisted of 30 μL of semen, 240 μL of buffer solution (pH 7.4) and 30 μL (40 mM) of H2DCFDA solution. Additionally, Trolox® (Merck, Darmstadt, Germany) was used as a reference antioxidant. Readings for each straw were taken in triplicate, every 180 s for 17 cycles, using an LS 55 spectrofluorometer (Perkin Elmer, Waltham, MA). ROS production rate by the semen was calculated by the slope (m = Δy/Δx), based on the evaluation of the ROS production kinetics (RFU/min) (RESTREPO & ROJANO, 2018RESTREPO, G; ROJANO, B. Actividad antioxidante del isoespintanol y el timol en el semen equino criopreservado. Rev. Investig. Vet. del Perú, v.29, n.1, p.205, 2018, Available from: <Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-93542017000300149&nrm=iso/0122-9354/ >. Accessed: Jun. 10, 2021. doi: 10.19052/mv.4397.
http://www.scielo.org.co/scielo.php?scri...
).
Statistical analysis
Data normality was assessed with the Shapiro-Wilk test. Parametric analyses were used, which included a logarithmic transformation of the results for continuous variables and arcsine transformation for variables expressed as percentages. General linear models were adjusted for post-thawed semen traits. Each model included the fixed effects of bull, straw storage time, and treatment. Means were compared using the Tukey’s test (HSD). Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS AND DISCUSSION:
Rapid thawing of semen decreases the harmful effects of the recrystallization processes and hydration, preventing damage to the sperm membrane and cytoplasm (LYASHENKO, 2015LYASHENKO, A. Effect of different thawing procedures on the quality and fertility of the bull spermatozoa. Asian Pacific Journal of Reproduction, v.4, n.1, p.17-21, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S2305050014600518 >. Accessed: Jan. 21, 2021. doi: 10.1016/S2305-0500(14)60051-8.
https://www.sciencedirect.com/science/ar...
). Our results showed higher heating temperatures produced higher motility and kinematics in spermatozoa (Table 1). Other authors reported an increase in progressive motility, VCL and VSL of thawed buffalo semen; although, they used higher temperatures (37 °C for 30 s, 50 °C for 15 s, and 70 °C for 7 s) compared with our study (SHAH et al., 2016SHAH, S. A., et al. Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology, v.4, n.5, p.972-976, 2016. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27153390/ >. Accessed: Oct. 04, 2021. doi: 10.1111/andr.12214.
https://pubmed.ncbi.nlm.nih.gov/27153390...
). In addition, it was reported that higher proportions of bovine spermatozoa with rapid progressive movement were observed after 2 h of post-thaw incubation when the thawing was done at more rapid rates (MUIÑO et al., 2008MUIÑO, R. Effect of different thawing rates on post-thaw sperm viability, kinematic parameters and motile sperm subpopulations structure of bull semen. Animal Reproduction Science, v.109, n.1-4, p. 50-64, 2008. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378432007003946 >. Accessed: Jan. 26, 2021. doi: 10.1016/j.anireprosci.2007.11.028.
https://www.sciencedirect.com/science/ar...
). However, some authors found that; although, thawing of semen with high heating rates produced higher sperm motility, this relative advantage disappeared after two hours of incubation (RASTEGARNIA et al., 2013RASTEGARNIA, A. et al. Effect of different thawing rates on post-thaw viability, Kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell Journal, v.14, n.4, p.306-313, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23577311/ >. Accessed: Apr. 10, 2021.
https://pubmed.ncbi.nlm.nih.gov/23577311...
; SHAH et al., 2016). According to this, semen thermoresistance could be an essential parameter to ascertain the impact of the heating rate on semen quality. However, previous studies have not found any relationship between thermoresistance and the rate of freezing, the rate of heating, or even with the fertility of thawed semen (VIANNA et al., 2009VIANNA F. P., ET AL. Thermoresistance sperm tests are not predictive of potential fertility for cryopreserved bull semen. Animal Reproduction Science, v.113, n.1-4, p.279-282, 2009. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378432008003278 >. Accessed: Jan. 27, 2022. doi: 10.1016/j.anireprosci.2008.06.009.
https://www.sciencedirect.com/science/ar...
; PUGLIESI et al., 2014PUGLIESI, G., et al. Impact of using a fast-freezing technique and different thawing protocols on viability and fertility of frozen equine spermatozoa. Andrologia, v.46, p.1055-1062, 2014. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/and.12205 >. Accessed: Jan. 27, 2017. doi: https://doi.org/10.1111/and.12205.
https://onlinelibrary.wiley.com/doi/abs/...
).
Freezing and thawing of semen are associated with sublethal and/or lethal damage such as oxidative stress, DNA damage, loss of sperm viability, and the functional capacity of the surviving sperm population (VARGHESE et al., 2016VARGHESE, T. et al. Loss of heat shock protein 70 from apical region of buffalo (Bubalus bubalis) sperm head after freezing and thawing. Theriogenology, v.85, n.5, p.828-834, 2016. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X15005786 >. Accessed: Aug. 01, 2021. doi: 10.1016/j.theriogenology.2015.10.029.
https://linkinghub.elsevier.com/retrieve...
). Membranes are thought to be the primary site of cryopreservation injury, and cooling results in decreased membrane fluidity and domain formation (BROWN & LONDON, 2000BROWN D, A, & LONDON, E. Structure and function of sphingolipid-and cholesterolrich membrane rafts. Journal of Biological Chemistry, v.275, p.17221-17224, 2000. Available from: <Available from: https://www.jbc.org/article/S0021-9258(19)83229-5/fulltext >. Accessed: Jan. 26, 2020. doi: 10.1074/jbc.R000005200.
https://www.jbc.org/article/S0021-9258(1...
; SIEME et al., 2015SIEME, H., et al. Sperm membrane behaviour during cooling and cryopreservation. Reproduction in Domestic Animals, v.50, p.20-26, 2015. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.12594 >. Accessed: Oct. 01, 2021. doi: 10.1111/rda.12594.
https://onlinelibrary.wiley.com/doi/10.1...
). Dehydration occurs when low cooling rates are used for freezing. At the same time, during thawing, the reverse process takes place, and sperm are exposed to hypotonic conditions resulting in water uptake and swelling (SIEME et al., 2015SIEME, H., et al. Sperm membrane behaviour during cooling and cryopreservation. Reproduction in Domestic Animals, v.50, p.20-26, 2015. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.12594 >. Accessed: Oct. 01, 2021. doi: 10.1111/rda.12594.
https://onlinelibrary.wiley.com/doi/10.1...
). Therefore, the thawing of semen affects the plasma membrane structure and can cause an early acrosomal reaction, reducing sperm functionality and fertilization capacity (KHALIL et al., 2018KHALIL, W. A. et al. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. International Journal of Veterinary Science and Medicine, v.6, n.sup1, p.S49-S56, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 >. Accessed: Sept. 06, 2021. doi: 10.1016/j.ijvsm.2017.11.001.
https://www.tandfonline.com/doi/full/10....
; RAJORIYA et al., 2020RAJORIYA, J. S. et al. Exogenous cholesterol prevents cryocapacitation‐like changes, membrane fluidity, and enhances in vitro fertility in bubaline spermatozoa. Reproduction in Domestic Animals, v.55, n.6, p.726-736, 2020. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.13674 >. Accessed: Jun. 01, 2021. doi: 10.1111/rda.13674.
https://onlinelibrary.wiley.com/doi/10.1...
; SIEME et al., 2015SIEME, H., et al. Sperm membrane behaviour during cooling and cryopreservation. Reproduction in Domestic Animals, v.50, p.20-26, 2015. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.12594 >. Accessed: Oct. 01, 2021. doi: 10.1111/rda.12594.
https://onlinelibrary.wiley.com/doi/10.1...
). Our results showed that plasmatic membrane integrity was affected by heating temperature (Table 1). Whereas T1 and T2 had reduced SMI and FMI, respectively, T3 proved superior by showing the best results for both variables. Despite this, our investigation showed that none of the heating rates affected the integrity of the acrosomal membrane, which in all cases was greater than 90%. Other researchers evaluated three heating rates (35 °C for 60 s, 37 °C for 30 s, and 75 °C for 9 s) in frozen semen from yak bulls. They observed that only the highest heating rate increased total acrosomal changes, including swollen, separating, and entirely lost acrosomes (BORAH et al., 2015BORAH, B. K. D. et al. Effect of thawing methods on frozen semen quality of yak (Poephagus grunniens L.) bulls. Veterinary World, v.8, n.7, p.831-834, 2015. Available from: <Available from: http://www.veterinaryworld.org/Vol.8/July-2015/3.html >. Accessed: May, 09, 2021. doi: 10.14202/vetworld.2015.831-834.
http://www.veterinaryworld.org/Vol.8/Jul...
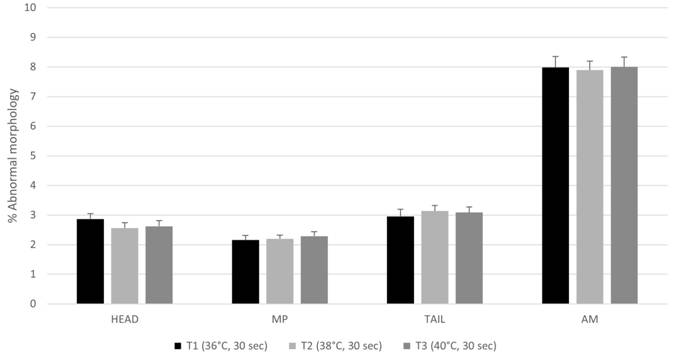
). In addition, less than 10% of spermatozoa had abnormal morphology (Figure 1). In another study in which three heating rates were evaluated in frozen bovine semen (37 °C for 30 s, 50 °C for 15 s and 70 °C for 5 s), similar results were observed for morphological and acrosome defects (NUR et al., 2003NUR, Z. Effect of different thawing procedures on the quality of bull semen. Revue de Médecine Vétérinaire, v.154, n.7, p.487-490, 2003. Available from: <Available from: https://www.researchgate.net/publication/288430281 >. Accessed: Jun. 26, 2022.
https://www.researchgate.net/publication...
).
- Morphology of cryopreserved bovine semen thawed at different temperatures. Results are expressed as mean ± standard error of mean (SEM). HEAD: Abnormal head morphology (%), MP: Abnormal midpiece morphology (%). TAIL: Abnormal tail morphology. TAM: Total abnormal morphology (%). No statistical differences were found (P > 0.05).
Sperm mitochondria must generate energy (ATP) to drive the flagellar movement to fertilization (AGARWAL et al., 2005AGARWAL, A.; GUPTA, S.; SHARMA, R. K. Role of oxidative stress in female reproduction. Reproductive Biology and Endocrinology, v.3, n.1, p.28, 2005. Available from: <Available from: https://rbej.biomedcentral.com/articles/10.1186/1477-7827-3-28 >. Accessed: Mar. 12, 2020. doi: 10.1186/1477-7827-3-28.
https://rbej.biomedcentral.com/articles/...
). In bull sperm, oxidative phosphorylation is the predominant pathway for energy generation (STOREY, 2008STOREY, B. T. Mammalian sperm metabolism: oxygen and sugar, friend and foe. The International Journal of Developmental Biology, v.52, n.5-6, p.427-437, 2008. Available from: <Available from:http://www.intjdevbiol.com/paper.php?doi=072522bs >. Accessed: May, 12, 2021. doi: Available from: 10.1387/ijdb.072522bs.
http://www.intjdevbiol.com/paper.php?doi...
). However, semen freezing and thawing processes can alter the sperm structure (KHALIL et al., 2018KHALIL, W. A. et al. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. International Journal of Veterinary Science and Medicine, v.6, n.sup1, p.S49-S56, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 >. Accessed: Sept. 06, 2021. doi: 10.1016/j.ijvsm.2017.11.001.
https://www.tandfonline.com/doi/full/10....
). Thawing of sperm triggers mitochondrial permeability transitions associated with increased intracellular calcium. This is accompanied by dissipation of the mitochondrial membrane potential (ΔΨM), a decrease of ATP levels, ROS production, and deterioration of plasma membrane integrity (TREULEN et al., 2018TREULEN, F. et al. Cryopreservation induces mitochondrial permeability transition in a bovine sperm model. Cryobiology, v.83, p.65-74, 2018. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0011224018301469 >. Accessed: Feb. 24, 2021. doi: 10.1016/j.cryobiol.2018.06.001.
https://linkinghub.elsevier.com/retrieve...
).
In our study, the increased motility and kinematics of thawed spermatozoa with higher temperatures could be related to mitochondrial function and the ability to produce enough ATP, which has been correlated with increased motility and hyperactivity of spermatozoa (KHALIL et al., 2018KHALIL, W. A. et al. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. International Journal of Veterinary Science and Medicine, v.6, n.sup1, p.S49-S56, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 >. Accessed: Sept. 06, 2021. doi: 10.1016/j.ijvsm.2017.11.001.
https://www.tandfonline.com/doi/full/10....
). However, in our results, none of the thawing treatments produced hyperactivation of spermatozoa. It is accepted that hyperactivation decreases LIN and VCL higher than 70 μm/s and ALH higher than 7 μm (NONGBUA et al., 2018NONGBUA, T. et al. Adding bovine seminal plasma prior to freezing improves post-thaw bull sperm kinematics but decreases mitochondrial activity. Systems Biology in Reproductive Medicine, v.64, n.3, p.183-190, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1080/19396368.2018.1455245 >. Accessed: Jul. 05, 2021. doi: 10.1080/19396368.2018.1455245.
https://www.tandfonline.com/doi/full/10....
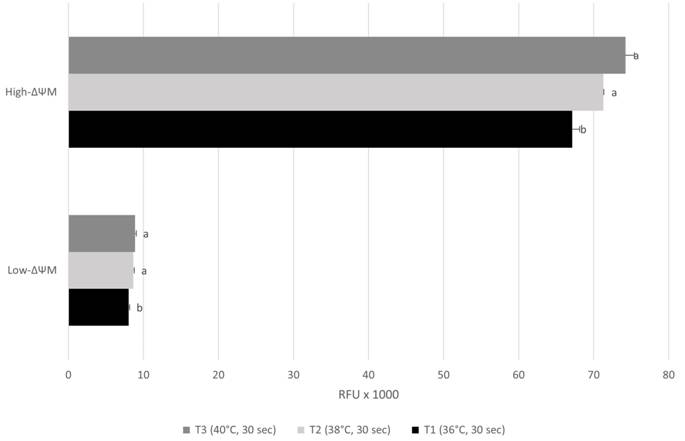
). In addition, we reported that the heating rate affected the mitochondrial membrane potential of thawed bovine semen (Figure 2). Better results were found for T3 and T2 than T1, for both high-ΔΨM and low-ΔΨM. (P < 0.05). Other authors have reported that high thawing rates increases the mitochondrial activity of sperm (SHAH et al., 2016SHAH, S. A., et al. Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology, v.4, n.5, p.972-976, 2016. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27153390/ >. Accessed: Oct. 04, 2021. doi: 10.1111/andr.12214.
https://pubmed.ncbi.nlm.nih.gov/27153390...
; SALIH et al., 2021SALIH, S., et al. Does ergothioneine and thawing temperatures improve rooster semen post-thawed quality? Poultry Science, v.100, n.10, p.101405, 2021. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0032579121004284 >. Accessed: Jan. 26, 2021. doi: 10.1016/j.psj.2021.101405.
https://www.sciencedirect.com/science/ar...
).
Mitochondrial membrane potential of cryopreserved bovine semen thawed at different temperatures.
Results are expressed as mean ± standard error of mean (SEM). High-ΔΨM: High internal mitochondrial membrane potential; Low-ΔΨM: Low internal mitochondrial membrane potential. RFU: Relative fluorescence units. Different letters for High-ΔΨM (A, B) or Low-ΔΨM (a, b) denote statistical differences (P < 0.05).
Mitochondrial osmotic stress results from mitochondrial permeability transition (ORTEGA-FERRUSOLA et al., 2017ORTEGA-FERRUSOLA, C. et al. Computational flow cytometry reveals that cryopreservation induces spermptosis but subpopulations of spermatozoa may experience capacitation-like changes. Reproduction, v.153, n.3, p.293-304, 2017. Available from: <Available from: https://rep.bioscientifica.com/view/journals/rep/153/3/293.xml >. Accessed: Feb. 02, 2021. doi: 10.1530/REP-16-0539.
https://rep.bioscientifica.com/view/jour...
). This leads to a blockage of the I-V complexes of the mitochondria, and ionic exchange stops, diminishing the ATP production. Therefore, spermatozoa are left without energy, decreasing motility (CASTRO POZO, 2019CASTRO POZO G, V. Evaluación de la producción de especies reactivas de oxigeno (ROS) en espermatozoides refrigerados con y sin dilutor. 2019. 22 p. Tesis, Universidad Científica del Sur. ). In addition, ROS inhibits the fructolytic and respiratory activity of sperm (JONES & MANN, 1977JONES, R; MANN, T. Damage to ram spermatozoa by peroxidation of endogenous phospholipids. Journal of Reproduction and Fertility, v.50, p.261-268, 1977. Available from: <Available from: https://doi.org/10.1530/jrf.0.0500261 >. Accessed: May, 09, 2022. doi: 10.1530/jrf.0.0500261.
https://doi.org/10.1530/jrf.0.0500261...
). Similarly, it has been suggested that reduced production of ΔΨM, ATP, and ROS occurs due to mitochondrial depolarization in frozen and thawed bovine spermatozoa (TREULEN et al., 2018TREULEN, F. et al. Cryopreservation induces mitochondrial permeability transition in a bovine sperm model. Cryobiology, v.83, p.65-74, 2018. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0011224018301469 >. Accessed: Feb. 24, 2021. doi: 10.1016/j.cryobiol.2018.06.001.
https://linkinghub.elsevier.com/retrieve...
). In addition, oxidative stress is created when the cellular generation of ROS overcomes the antioxidant defenses with irreversible oxidative damage (THOMSON et al., 2009THOMSON, L. K. et al. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Human Reproduction, v.24, n.9, p.2061-2070, 2009. Available from: <Available from: https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/dep214 >. Accessed: Jan. 22, 2021. doi: 10.1093/humrep/dep214.
https://academic.oup.com/humrep/article-...
). It may also result from the imbalance of the antioxidant mechanisms and the possible differences in the production of specific reactive species (KAR et al., 2015KAR, S.; DIVYASHREE, B. C.; ROY, S. C. Temporal leakage of Cu,Zn superoxide dismutase and loss of two low-molecular-weight forms of glutathione peroxidase-1 from buffalo (Bubalus bubalis) sperm after freezing and thawing. Theriogenology, v.83, n.4, p.512-519, 2015. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X14005597 >. Accessed: Oct. 06, 2021. doi: 10.1016/j.theriogenology.2014.10.014.
https://linkinghub.elsevier.com/retrieve...
). Antioxidant defenses decrease in bovine sperm after a freeze-thaw cycle when ROS detoxification is affected by enzymes such as catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD) (BILODEAU et al., 2000BILODEAU, J. F. et al. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Molecular Reproduction and Development, v.55, n.3, p.282-288, 2000. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1098-2795(200003)55:3%3C282::AID-MRD6%3E3.0.CO;2-7 >. Accessed: Oct. 04, 2021. doi: 10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7.
https://onlinelibrary.wiley.com/doi/abs/...
).
Our results showed that higher warming rates produced an increase in ROS production (P < 0.05) (Figure 3), that was potentially related to a rise in sperm metabolic rate. This agrees with a previous study that found that excess ROS can be produced when the demand for energy increases (VALLORANI et al., 2010VALLORANI, C. et al. Effects of antioxidants on boar spermatozoa during sorting and storage. Animal Reproduction Science, v.122, n.1-2, p.58-65, 2010. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/20709476/ >. Accessed: Oct. 04, 2021. doi: 10.1016/j.anireprosci.2010.07.007.
https://pubmed.ncbi.nlm.nih.gov/20709476...
). Furthermore, it is known H2O2 concentration may increase from reduced activity of glutathione peroxidase (GPx). This is often combined with an increase of ROS due to the lack of catalase after the cryopreservation of bovine spermatozoa (GÜRLER et al., 2015GÜRLER, H. et al. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology, v.86, n.2, p.562-571, 2015. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27039074/ >. Accessed: Oct. 04, 2021. doi: 10.1016/j.theriogenology.2016.02.007.
https://pubmed.ncbi.nlm.nih.gov/27039074...
). It has been proposed that more metabolically active spermatozoa are depleted faster due to the accumulation of metabolic products such as ROS and cytotoxic lipid aldehydes in a “live fast-die young paradigm (GIBB & AITKEN, 2016GIBB, Z.; AITKEN, R. J. The Impact of sperm metabolism during in vitro storage: The stallion as a model. BioMed Research International, v.2016, p.1-8, 2016. Available from: <Available from: http://www.hindawi.com/journals/bmri/2016/9380609/ >. Accessed: Jul. 05, 2022. doi: 10.1155/2016/9380609.
http://www.hindawi.com/journals/bmri/201...
).
Reactive oxygen species production of cryopreserved bovine semen thawed at different temperatures. The results are expressed as means ± standard error of mean (SEM). Δy/Δx: kinetics of ROS production expressed as relative fluorescence units per minute (RFU/min). Different letters denote statistical differences (P < 0.05).
This study showed that mitochondrial activity and oxidative stress should be considered when establishing heating rates for thawing cryopreserved semen. Thus, it may be possible to optimize and even develop new semen heating methods that improve post-thaw sperm quality and fertility.
CONCLUSION:
Thawing temperatures of 38 °C and 40 °C increased the motility, kinetics, membrane integrity, mitochondrial activity, and ROS of cryopreserved bovine semen compared with the more conventional thawing use at 36 °C. However, it is necessary to evaluate the effect of the heating rates used in this research on semen thermoresistance to better understand their impact on sperm structural and functional integrity.
ACKNOWLEDGMENTS
The authors would like to thank Universidad Nacional de Colombia for its financial support.
REFERENCES
- AGARWAL, A.; GUPTA, S.; SHARMA, R. K. Role of oxidative stress in female reproduction. Reproductive Biology and Endocrinology, v.3, n.1, p.28, 2005. Available from: <Available from: https://rbej.biomedcentral.com/articles/10.1186/1477-7827-3-28 >. Accessed: Mar. 12, 2020. doi: 10.1186/1477-7827-3-28.
» https://doi.org/10.1186/1477-7827-3-28.» https://rbej.biomedcentral.com/articles/10.1186/1477-7827-3-28 - AITKEN, R. J. et al. On methods for the detection of reactive oxygen species generation by human spermatozoa: analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology, v.1, n.2, p.192-205, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23316012/ >. Accessed: Jun. 25, 2021. doi: 10.1111/j.2047-2927.2012.00056.x.
» https://doi.org/10.1111/j.2047-2927.2012.00056.x.» https://pubmed.ncbi.nlm.nih.gov/23316012/ - AL-BADRY, K. I. Effect of various thawing times and temperatures on frozen semen quality of Friesian bulls in Iraq. International Journal of Animal and Veterinary Advances, v.4, n.6, p.384-388, 2012. Available from: <Available from: https://www.researchgate.net/publication/332465334_Effect_of_Various_Thawing_Times_and_Temperatures_on_Frozen_semen_Quality_of_Friesian_Bulls_in_Iraq >. Accessed: Jun. 21, 2022.
» https://www.researchgate.net/publication/332465334_Effect_of_Various_Thawing_Times_and_Temperatures_on_Frozen_semen_Quality_of_Friesian_Bulls_in_Iraq - BILODEAU, J. F. et al. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Molecular Reproduction and Development, v.55, n.3, p.282-288, 2000. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1098-2795(200003)55:3%3C282::AID-MRD6%3E3.0.CO;2-7 >. Accessed: Oct. 04, 2021. doi: 10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7.
» https://doi.org/10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7.» https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1098-2795(200003)55:3%3C282::AID-MRD6%3E3.0.CO;2-7 - BINIOVÁ, Z. et al. Effect of thawing method on bull sperm survival in ejaculates frozen in 4 mL and 8 mL volumes. Czech Journal of Animal Science, v.63, n.10, p.399-407, 2018. Available from: <Available from: https://www.agriculturejournals.cz/web/cjas.htm?type=article&id=117_2018-CJAS >. Accessed: May, 13, 2021. doi: 10.17221/117/2018-CJAS. doi: 10.17221/117/2018-CJAS.
» https://doi.org/10.17221/117/2018-CJAS. doi: 10.17221/117/2018-CJAS.» https://www.agriculturejournals.cz/web/cjas.htm?type=article&id=117_2018-CJAS - BORAH, B. K. D. et al. Effect of thawing methods on frozen semen quality of yak (Poephagus grunniens L.) bulls. Veterinary World, v.8, n.7, p.831-834, 2015. Available from: <Available from: http://www.veterinaryworld.org/Vol.8/July-2015/3.html >. Accessed: May, 09, 2021. doi: 10.14202/vetworld.2015.831-834.
» https://doi.org/10.14202/vetworld.2015.831-834.» http://www.veterinaryworld.org/Vol.8/July-2015/3.html - BRITO, L. F. C. et al. Effect of method and clinician on stallion sperm morphology evaluation. Theriogenology, v.76, n.4, p.745-750, 2011. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X11001671 >. Accessed: Sept. 22, 2020. doi: 10.1016/j.theriogenology.2011.04.007.
» https://doi.org/10.1016/j.theriogenology.2011.04.007.» https://linkinghub.elsevier.com/retrieve/pii/S0093691X11001671 - BROWN D, A, & LONDON, E. Structure and function of sphingolipid-and cholesterolrich membrane rafts. Journal of Biological Chemistry, v.275, p.17221-17224, 2000. Available from: <Available from: https://www.jbc.org/article/S0021-9258(19)83229-5/fulltext >. Accessed: Jan. 26, 2020. doi: 10.1074/jbc.R000005200.
» https://doi.org/10.1074/jbc.R000005200.» https://www.jbc.org/article/S0021-9258(19)83229-5/fulltext - CASTRO POZO G, V. Evaluación de la producción de especies reactivas de oxigeno (ROS) en espermatozoides refrigerados con y sin dilutor. 2019. 22 p. Tesis, Universidad Científica del Sur.
- DOLEŽALOVÁ, M. et al. Effect of different hawing methods on bull’s semen characteristics. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis, v.65, n.3, p.815-822, 2017. Available from: <Available from: http://acta.mendelu.cz/doi/10.11118/actaun201765030815.html >. Accessed: Feb. 11, 2021. doi: 10.11118/actaun201765030815.
» https://doi.org/10.11118/actaun201765030815.» http://acta.mendelu.cz/doi/10.11118/actaun201765030815.html - FERRUSOLA, C. O. et al. Inhibition of the mitochondrial permeability transition pore reduces “apoptosis like” changes during cryopreservation of stallion spermatozoa. Theriogenology, v.74, n.3, p.458-465, 2010. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X10001445 >. Accessed: May, 07, 2021. doi: 10.1016/j.theriogenology.2010.02.029.
» https://doi.org/10.1016/j.theriogenology.2010.02.029.» https://linkinghub.elsevier.com/retrieve/pii/S0093691X10001445 - GIBB, Z.; AITKEN, R. J. The Impact of sperm metabolism during in vitro storage: The stallion as a model. BioMed Research International, v.2016, p.1-8, 2016. Available from: <Available from: http://www.hindawi.com/journals/bmri/2016/9380609/ >. Accessed: Jul. 05, 2022. doi: 10.1155/2016/9380609.
» https://doi.org/10.1155/2016/9380609.» http://www.hindawi.com/journals/bmri/2016/9380609/ - GRAVANCE, C. G. et al. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology, v.53, n.9, p.1691-1703, 2000. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/10968415/ >. Accessed: Jun. 25, 2021. doi: 10.1016/s0093-691x(00)00308-3.
» https://doi.org/10.1016/s0093-691x(00)00308-3.» https://pubmed.ncbi.nlm.nih.gov/10968415/ - GÜRLER, H. et al. Effects of cryopreservation on sperm viability, synthesis of reactive oxygen species, and DNA damage of bovine sperm. Theriogenology, v.86, n.2, p.562-571, 2015. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27039074/ >. Accessed: Oct. 04, 2021. doi: 10.1016/j.theriogenology.2016.02.007.
» https://doi.org/10.1016/j.theriogenology.2016.02.007.» https://pubmed.ncbi.nlm.nih.gov/27039074/ - JONES, R; MANN, T. Damage to ram spermatozoa by peroxidation of endogenous phospholipids. Journal of Reproduction and Fertility, v.50, p.261-268, 1977. Available from: <Available from: https://doi.org/10.1530/jrf.0.0500261 >. Accessed: May, 09, 2022. doi: 10.1530/jrf.0.0500261.
» https://doi.org/10.1530/jrf.0.0500261.» https://doi.org/10.1530/jrf.0.0500261 - KAR, S.; DIVYASHREE, B. C.; ROY, S. C. Temporal leakage of Cu,Zn superoxide dismutase and loss of two low-molecular-weight forms of glutathione peroxidase-1 from buffalo (Bubalus bubalis) sperm after freezing and thawing. Theriogenology, v.83, n.4, p.512-519, 2015. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X14005597 >. Accessed: Oct. 06, 2021. doi: 10.1016/j.theriogenology.2014.10.014.
» https://doi.org/10.1016/j.theriogenology.2014.10.014.» https://linkinghub.elsevier.com/retrieve/pii/S0093691X14005597 - KHALIL, W. A. et al. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. International Journal of Veterinary Science and Medicine, v.6, n.sup1, p.S49-S56, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 >. Accessed: Sept. 06, 2021. doi: 10.1016/j.ijvsm.2017.11.001.
» https://doi.org/10.1016/j.ijvsm.2017.11.001.» https://www.tandfonline.com/doi/full/10.1016/j.ijvsm.2017.11.001 - LYASHENKO, A. Effect of different thawing procedures on the quality and fertility of the bull spermatozoa. Asian Pacific Journal of Reproduction, v.4, n.1, p.17-21, 2015. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S2305050014600518 >. Accessed: Jan. 21, 2021. doi: 10.1016/S2305-0500(14)60051-8.
» https://doi.org/10.1016/S2305-0500(14)60051-8.» https://www.sciencedirect.com/science/article/pii/S2305050014600518 - MUIÑO, R. Effect of different thawing rates on post-thaw sperm viability, kinematic parameters and motile sperm subpopulations structure of bull semen. Animal Reproduction Science, v.109, n.1-4, p. 50-64, 2008. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378432007003946 >. Accessed: Jan. 26, 2021. doi: 10.1016/j.anireprosci.2007.11.028.
» https://doi.org/10.1016/j.anireprosci.2007.11.028.» https://www.sciencedirect.com/science/article/pii/S0378432007003946 - NONGBUA, T. et al. Adding bovine seminal plasma prior to freezing improves post-thaw bull sperm kinematics but decreases mitochondrial activity. Systems Biology in Reproductive Medicine, v.64, n.3, p.183-190, 2018. Available from: <Available from: https://www.tandfonline.com/doi/full/10.1080/19396368.2018.1455245 >. Accessed: Jul. 05, 2021. doi: 10.1080/19396368.2018.1455245.
» https://doi.org/10.1080/19396368.2018.1455245.» https://www.tandfonline.com/doi/full/10.1080/19396368.2018.1455245 - NUR, Z. Effect of different thawing procedures on the quality of bull semen. Revue de Médecine Vétérinaire, v.154, n.7, p.487-490, 2003. Available from: <Available from: https://www.researchgate.net/publication/288430281 >. Accessed: Jun. 26, 2022.
» https://www.researchgate.net/publication/288430281 - ORTEGA-FERRUSOLA, C. et al. Computational flow cytometry reveals that cryopreservation induces spermptosis but subpopulations of spermatozoa may experience capacitation-like changes. Reproduction, v.153, n.3, p.293-304, 2017. Available from: <Available from: https://rep.bioscientifica.com/view/journals/rep/153/3/293.xml >. Accessed: Feb. 02, 2021. doi: 10.1530/REP-16-0539.
» https://doi.org/10.1530/REP-16-0539.» https://rep.bioscientifica.com/view/journals/rep/153/3/293.xml - PUGLIESI, G., et al. Impact of using a fast-freezing technique and different thawing protocols on viability and fertility of frozen equine spermatozoa. Andrologia, v.46, p.1055-1062, 2014. Available from: <Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/and.12205 >. Accessed: Jan. 27, 2017. doi: https://doi.org/10.1111/and.12205.
» https://doi.org/https://doi.org/10.1111/and.12205» https://onlinelibrary.wiley.com/doi/abs/10.1111/and.12205 - RAJABI-TOUSTANI, R. et al. Methodological improvement of fluorescein isothiocyanate peanut agglutinin (FITC-PNA) acrosomal integrity staining for frozen-thawed Japanese Black bull spermatozoa. Journal of Veterinary Medical Science, v.81, n.5, p.694-702, 2019. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/30606905/ >. Accessed: Sept. 03, 2021. doi: 10.1292/jvms.18-0560.
» https://doi.org/10.1292/jvms.18-0560.» https://pubmed.ncbi.nlm.nih.gov/30606905/ - RAJORIYA, J. S. et al. Exogenous cholesterol prevents cryocapacitation‐like changes, membrane fluidity, and enhances in vitro fertility in bubaline spermatozoa. Reproduction in Domestic Animals, v.55, n.6, p.726-736, 2020. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.13674 >. Accessed: Jun. 01, 2021. doi: 10.1111/rda.13674.
» https://doi.org/10.1111/rda.13674.» https://onlinelibrary.wiley.com/doi/10.1111/rda.13674 - RASTEGARNIA, A. et al. Effect of different thawing rates on post-thaw viability, Kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell Journal, v.14, n.4, p.306-313, 2013. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/23577311/ >. Accessed: Apr. 10, 2021.
» https://pubmed.ncbi.nlm.nih.gov/23577311/ - RATHI, R. et al. Evaluation of in vitro capacitation of stallion spermatozoa. Biology of Reproduction, v.65, n.2, p.462-470, 2001. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/11466214/ >. Accessed: Mar. 26, 2021. doi: 10.1095/biolreprod65.2.462.
» https://doi.org/10.1095/biolreprod65.2.462.» https://pubmed.ncbi.nlm.nih.gov/11466214/ - RESTREPO, G; ROJANO, B. Actividad antioxidante del isoespintanol y el timol en el semen equino criopreservado. Rev. Investig. Vet. del Perú, v.29, n.1, p.205, 2018, Available from: <Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-93542017000300149&nrm=iso/0122-9354/ >. Accessed: Jun. 10, 2021. doi: 10.19052/mv.4397.
» https://doi.org/10.19052/mv.4397.» http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-93542017000300149&nrm=iso/0122-9354/ - ROTA, A. et al. Hypoosmotic swelling (HOS) as a screening assay for testing in vitro fertility of bovine spermatozoa. Theriogenology, v.53, n.7, p.1415-1420, 2000. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X00002843 >. Accessed: Mar. 22, 2021. doi: 10.1016/S0093-691X(00)00284-3.
» https://doi.org/10.1016/S0093-691X(00)00284-3.» https://linkinghub.elsevier.com/retrieve/pii/S0093691X00002843 - SALIH, S., et al. Does ergothioneine and thawing temperatures improve rooster semen post-thawed quality? Poultry Science, v.100, n.10, p.101405, 2021. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0032579121004284 >. Accessed: Jan. 26, 2021. doi: 10.1016/j.psj.2021.101405.
» https://doi.org/10.1016/j.psj.2021.101405.» https://www.sciencedirect.com/science/article/pii/S0032579121004284 - SHAH, S. A., et al. Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology, v.4, n.5, p.972-976, 2016. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/27153390/ >. Accessed: Oct. 04, 2021. doi: 10.1111/andr.12214.
» https://doi.org/10.1111/andr.12214.» https://pubmed.ncbi.nlm.nih.gov/27153390/ - SIEME, H., et al. Sperm membrane behaviour during cooling and cryopreservation. Reproduction in Domestic Animals, v.50, p.20-26, 2015. Available from: <Available from: https://onlinelibrary.wiley.com/doi/10.1111/rda.12594 >. Accessed: Oct. 01, 2021. doi: 10.1111/rda.12594.
» https://doi.org/10.1111/rda.12594.» https://onlinelibrary.wiley.com/doi/10.1111/rda.12594 - STOREY, B. T. Mammalian sperm metabolism: oxygen and sugar, friend and foe. The International Journal of Developmental Biology, v.52, n.5-6, p.427-437, 2008. Available from: <Available from:http://www.intjdevbiol.com/paper.php?doi=072522bs >. Accessed: May, 12, 2021. doi: Available from: 10.1387/ijdb.072522bs.
» https://doi.org/10.1387/ijdb.072522bs.» http://www.intjdevbiol.com/paper.php?doi=072522bs - THOMSON, L. K. et al. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Human Reproduction, v.24, n.9, p.2061-2070, 2009. Available from: <Available from: https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/dep214 >. Accessed: Jan. 22, 2021. doi: 10.1093/humrep/dep214.
» https://doi.org/10.1093/humrep/dep214.» https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/dep214 - TREULEN, F. et al. Cryopreservation induces mitochondrial permeability transition in a bovine sperm model. Cryobiology, v.83, p.65-74, 2018. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0011224018301469 >. Accessed: Feb. 24, 2021. doi: 10.1016/j.cryobiol.2018.06.001.
» https://doi.org/10.1016/j.cryobiol.2018.06.001.» https://linkinghub.elsevier.com/retrieve/pii/S0011224018301469 - VALLORANI, C. et al. Effects of antioxidants on boar spermatozoa during sorting and storage. Animal Reproduction Science, v.122, n.1-2, p.58-65, 2010. Available from: <Available from: https://pubmed.ncbi.nlm.nih.gov/20709476/ >. Accessed: Oct. 04, 2021. doi: 10.1016/j.anireprosci.2010.07.007.
» https://doi.org/10.1016/j.anireprosci.2010.07.007.» https://pubmed.ncbi.nlm.nih.gov/20709476/ - VARGHESE, T. et al. Loss of heat shock protein 70 from apical region of buffalo (Bubalus bubalis) sperm head after freezing and thawing. Theriogenology, v.85, n.5, p.828-834, 2016. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093691X15005786 >. Accessed: Aug. 01, 2021. doi: 10.1016/j.theriogenology.2015.10.029.
» https://doi.org/10.1016/j.theriogenology.2015.10.029.» https://linkinghub.elsevier.com/retrieve/pii/S0093691X15005786 - VIANNA F. P., ET AL. Thermoresistance sperm tests are not predictive of potential fertility for cryopreserved bull semen. Animal Reproduction Science, v.113, n.1-4, p.279-282, 2009. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S0378432008003278 >. Accessed: Jan. 27, 2022. doi: 10.1016/j.anireprosci.2008.06.009.
» https://doi.org/10.1016/j.anireprosci.2008.06.009.» https://www.sciencedirect.com/science/article/pii/S0378432008003278 - WATSON, P. The causes of reduced fertility with cryopreserved semen. Animal Reproduction Science, v.60-61, p.481-492, 2000. Available from: <Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378432000000993 >. Accessed: Jul. 05, 2021. doi: 10.1016/s0378-4320(00)00099-3.
» https://doi.org/10.1016/s0378-4320(00)00099-3.» https://linkinghub.elsevier.com/retrieve/pii/S0378432000000993
-
0
CR-2021-0731.R2
BIOETHICS AND BIOSSECURITY COMMITTEE
-
The study complied with the Colombian legislation of ethics and experimentation with animals. While conducting this experiment, the authors fulfilled the Colombian Law 84 of December 1989, Chapter VI (Of the Use of Live Animals in Experiments and Research), Article 26 and Law 1774 of 2016, Article 1.
Edited by
Publication Dates
-
Publication in this collection
24 June 2022 -
Date of issue
2023
History
-
Received
12 Oct 2021 -
Accepted
17 Mar 2022 -
Reviewed
26 May 2022