Abstract
Most Candida infections are related to microbial biofilms often formed by the association of different species. The objective of this study was to evaluate the interactions between Candida albicans and non-albicans species in biofilms formed in vitro. The non-albicans species studied were:Candida tropicalis, Candida glabrata andCandida krusei. Single and mixed biofilms (formed by clinical isolates of C. albicans and non-albicans species) were developed from standardized suspensions of each strain (107 cells/mL), on flat-bottom 96-well microtiter plates for 48 hour. These biofilms were analyzed by counting colony-forming units (CFU/mL) in Candida HiChrome agar and by determining cell viability, using the XTT 2,3-bis (2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide colorimetric assay. The results for both the CFU/mL count and the XTT colorimetric assay showed that all the species studied were capable of forming high levels of in vitro biofilm. The number of CFU/mL and the metabolic activity of C. albicans were reduced in mixed biofilms with non-albicans species, as compared with a singleC. albicans biofilm. Among the species tested, C. krusei exerted the highest inhibitory action against C. albicans. In conclusion, C. albicans established antagonistic interactions with non-albicans Candida species in mixed biofilms.
Candida albicans; Candida glabrata; Candida tropicalis; Biofilms
Introduction
Yeasts of the Candida genus are opportunistic pathogens frequently encountered in humans, and can be isolated from 60% of the oral cavities of healthy adults.11. Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 2006;51(8):672-80. doi:10.1016/j.archoralbio.2006.02.005Candida species are responsible for the increasing frequency of infections in immunocompromised patient groups, such as HIV-infected individuals and those being treated with chemotherapy or broad-spectrum antibiotics.22. Jin Y, Samaranayake LP, Samaranayake YH, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49(10):789-98. doi:10.1016/j.archoralbio.2004.04.011C. albicans is considered the most prevalent and pathogenic species of this gender, and is responsible for most of the superficial and systemic fungal infections.11. Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 2006;51(8):672-80. doi:10.1016/j.archoralbio.2006.02.005Candida albicans is the fourth leading cause of bloodstream infections, and the third most commonly isolated organism from intravascular catheters; moreover, it is associated with the highest incidence of mortality.33. Harriot MM, Noverr MC. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19(11):557-63. doi:10.1016/j.tim.2011.07.004
Nevertheless, other Candida species have also emerged as clinically important opportunistic pathogens, such as Candida glabrata,Candida tropicalis and Candida krusei.44. Kirkpatrick WR, Lopez-Ribot JL, Mcatee RK, Patterson TF. Growth competition under broth an biofilms growing conditions. J Clin Microbiol. 2000;38(2):902-4. doi:10.1111/j.1567-1364.2006.00121.x For many years, C. glabrata was considered a relatively non-pathogenic saprophyte of the normal flora of healthy humans. However, C. glabrata can disseminate rapidly throughout the body, and infection caused by this species is associated with a high mortality rate.55. Silva S, Negri M, Henriques M, Oliveira R, Williams D, Azeredo J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011;19(5):241-7. doi:10.1016/j.tim.2011.02.003C. tropicalis is another non-albicans species considered an important opportunistic pathogen. It is most frequently isolated from candidiasis, mainly in patients confined to intensive care units, and has been associated with fluconazole resistance.66. Bizerra FC, Nakamura CV, Poersch CO, Svidzinski TI, Quesada RM, Goldenberg S, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8(3):442-50. doi:10.1111/j.1567-1364.2007.00347.xC. krusei is an emerging pathogen, described as a systemic pathogen in patients with compromised host resistance, such as those with acquired immunodeficiency syndrome (AIDS).77. Samaranayake LP, Samaranayake YH. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J Med Microbiol. 1994;41(5):295-310. doi:10.1099/00222615-41-5-295
A major attribute of the Candida genus virulence is its ability to form surface-attached microbial communities known as biofilms.88. Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med Sci. 2010;25(3):385-9. doi:10.1007/s10103-009-0706-z,99. Silva WJ, Seneviratne J, Parahitiyawa NB, Rosa EAR, Samaranayake LP, Del Bel Cury AA. Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz Dent J. 2008;19(4):364-9. doi:10.1590/S0103-64402008000400014 In general, biofilm development evolves according to four sequential steps: first, adhesion of microorganisms to a surface; second, discrete colony formation and organization of cells; third, secretion of extracellular polysaccharides (EPS) and maturation into a three-dimensional structure; and, fourth, dissemination of progeny biofilm cells.1010. Seneviratne CJ, Silva WJ, Jin LJ, Samaranayke LP. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54(11):2260-9. doi:10.1016/j.archoralbio.2009.08.002 The extracellular matrix of the biofilm can protect the cells from phagocytosis, maintain the integrity of the biofilm, and limit the diffusion of substances.1111. Costa AC, Rasteiro VM, Pereira CA, Hashimoto ES, Beltrame Jr M, Junqueira JC, et al. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol. 2011;56(11):1299-305. doi:10.1016/j.archoralbio.2011.05.013 It has been estimated that over 60% of microbial infections are involved with biofilm.11. Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 2006;51(8):672-80. doi:10.1016/j.archoralbio.2006.02.005 The high resistance of biofilms to antimicrobials (compared to their planktonic counterparts) is of major clinical relevance.1212. Thein ZM, Seneviratne CJ, Samaranayake YH, Samaranayake LP. Community lifestyle of Candida in mixed biofilms: a mini review. Mycoses. 2009;52(6):467-75. doi:10.1111/j.1439-0507.2009.01719.x
Biofilm infections may be caused by a single microbial species or by a mixture of different species.1313. Douglas LJ. Medical importance of biofilms in Candida infections. Rev Iberoam Micol. 2002;19:139-43. doi:10.1016/S0966-842X(02)00002-1 The complex structure of biofilms allows stratification into spatially organized populations of mixed-species communities.1414. El-Azizi MA, Starks SE, Khardori N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol. 2004;96(5):1067-73. doi:10.1111/j.1365-2672.2004.02213.xMicroorganisms have evolved complex mechanisms to promote their survival, defending themselves not only against adverse environmental and nutritional conditions, but also against competing organisms.1515. Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8(5):340-49. doi:10.1038/nrmicro2313 Polymicrobial biofilms are found in nearly every niche in the human body.1616. Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton W, Shirtliff ME. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59(3):493-503. doi:10.1111/j.1574-695X.2010.00710.x These mixtures of species make the therapeutic management of infectious diseases extremely difficult.1212. Thein ZM, Seneviratne CJ, Samaranayake YH, Samaranayake LP. Community lifestyle of Candida in mixed biofilms: a mini review. Mycoses. 2009;52(6):467-75. doi:10.1111/j.1439-0507.2009.01719.x The heterogeneity of species within mixed biofilms has made it difficult to assess the relevance and contribution of each individual species to pathogenesis and disease.33. Harriot MM, Noverr MC. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19(11):557-63. doi:10.1016/j.tim.2011.07.004
Bacteria are commonly associated with Candida in the polymicrobial biofilms found in medical devices.88. Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med Sci. 2010;25(3):385-9. doi:10.1007/s10103-009-0706-zHarriot and Noverr1717. Harriot MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53(9):3914-22. doi:10.1128/AAC.00657-09 studied the interactions in polymicrobial biofilms formed by C. albicans andStaphylococcus aureus in an in vitro model, and observed a synergistic relationship between the two species. On the other hand, Tampakakis et al.1818. Tampakakis E, Peleg AY, Mylonakis E. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica Serovar Typhimurium. Eukaryot Cell. 2009;8(5):732-7. doi:10.1128/EC.00016-09 analyzed anin vivo interaction between the species C. albicans and the Gram-negative Salmonella typhimurium. After observing that C. albicans filamentation was inhibited by the bacteria, they concluded that it was an antagonistic interaction.
However, most existing studies on mixed biofilm development involving Candida albicans have focused on bacterial-fungal infections, whereas there are only few that deal with interactions among fungi in mixed biofilms. Kirkpatrick et al.44. Kirkpatrick WR, Lopez-Ribot JL, Mcatee RK, Patterson TF. Growth competition under broth an biofilms growing conditions. J Clin Microbiol. 2000;38(2):902-4. doi:10.1111/j.1567-1364.2006.00121.x and Thein et al.1919. Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture surfaces. Arch Oral Biol. 2007;52(12):1200-8. doi:10.1016/j.archoralbio.2007.06.007 found that C. albicans established a competitive interaction with C. dubliniensis and C. krusei, respectively, in mixed biofilms formed in vitro. However, Pereira-Cenci et al.2020. Pereira-Cenci T, Deng DM, Kraneveld EA, Manders EM, Del Bel Cury AA, Ten Cate JM, et al. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch Oral Biol. 2008;53(18):755-64. doi:10.1016/j.archoralbio.2008.02.015 did not find competitive interactions in dual-species biofilms of C. albicans and C. glabrata grown on different dental materials. Recently, our laboratory developed an in vitro and in vivo study2121. Rossoni RD, Barbosa JO, Vilela SFG, Santos JD, Barros PP, Prata MCA, et al. Competitive interactions between C. albicans, C. glabrata and C. krusei during biofilm formation and development of experimental candidiasis. PLos One. 2015 Jul 6;10(7):e0131700. doi:10.1371/journal.pone.0131700 to evaluate the interactions ofC. albicans (ATCC 18804) with C. krusei (ATCC 6258) and C. glabrata (ATCC 9030). We demonstrated that C. albicans was able to establish competitive interactions with non-albicans species during biofilm formation and infection development processes in animal models.
Thus, the purpose of this study was to investigate the interactions betweenCandida albicans and non-albicans species in mixed biofilms formed in vitro, using clinical strains ofC. albicans, C. tropicalis, C. glabrata and C. krusei, isolated from oropharyngeal candidiasis lesions of HIV-positive patients.
Methodology
Microorganisms
This study was approved by the Human Research Ethics Committee of the Institute of Science and Technology of São José dos Campos, Universidade Estadual Paulista - UNESP (017/2011-PH/CEP).
Four clinical isolates were used in this study, including species ofCandida albicans, Candida glabrata, Candida tropicalis, andCandida krusei. These strains were provided by the Microbiology Laboratory of the Department of Biosciences and Oral Diagnosis (UNESP, São José dos Campos, SP, Brazil), after isolation from oropharyngeal candidiasis lesions of HIV-positive patients.2222. Junqueira JC, Vilela SFG, Rossoni RD, Barbosa JO, Costa ACBP, Rasteiro VMC, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo. 2012;54(1):17-24. doi:10.1590/S0036-46652012000100004
Preparation of Candidaspp. suspension
Standard suspensions containing 107 cells/mL were prepared for each strain. The strains were seeded on Sabouraud dextrose agar (Difco, Detroit, USA) and incubated at 37°C for 24 hour. Next, a loopful of growth was inoculated in Yeast Nitrogen Base (YNB) broth (Difco, Detroit, USA) supplemented with 100 mM glucose (Vetec, Rio de Janeiro, Brazil) and incubated at 37°C for 16 hour. The broth with the microbial growth was centrifuged at 2000 x g for 10 min (MPW Med. Instruments, Warsaw, Poland), after which the growth was washed twice with 5 mL of sterile physiological solution (PBS) (Laborclin, Pinhais, Brazil), resuspended in YNB medium supplemented with 100 mL glucose, and standardized to 107 cells/mL in a Neubauer counting chamber (Laboroptik GmbH, Bad Homburg, Germany).
In vitro formation of simple and mixed biofilms
The biofilm formation of Candida was performed as described by Seneviratne et al.,1010. Seneviratne CJ, Silva WJ, Jin LJ, Samaranayke LP. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54(11):2260-9. doi:10.1016/j.archoralbio.2009.08.002 with some modifications. Candida albicans biofilm was formed by adding 200 µL from a standardized suspension (107 cells/mL) to each well of flat-bottom 96-well microtiter plates (Corning Corporation, Corning, USA). The mixed biofilms (composed by C. albicans and non-albicans species) were formed by adding 100 µL aliquots of standardized suspensions of each species to each well of a flat-bottom 96-well microtiter plate. The plates were incubated for 1.5 h (adhesion phase) at 37°C at 75 rpm in an orbital shaker (Quimis, Diadema, Brazil). After the adhesion phase, the cell suspensions were aspirated, and each well was washed twice with 200 µL of phosphate buffered saline (PBS) to remove any planktonic cells. Then, 200 µL of YNB with 100 mM glucose was added to each washed well, and the plates were incubated for 48 h at 37°C at 75 rpm in an orbital shaker. The growth medium was replenished daily.
Analysis of biofilm by counting colony forming units (CFU/mL)
After formation of the biofilms, each well was washed twice with 200 µL of PBS to remove loosely adhered cells. Then, 200 µL of PBS was added to each well, and the biofilm cells were scraped off of the well using a sterile toothpick. A 100 µL aliquot was transferred to a Falcon tube (J Prolab, São José dos Pinhais, Brazil) containing 6 mL of sterile physiological solution, and an ultrasonic homogenizer (Sonoplus HD 2200, output power of 50 W) (Bandelin Electronic, Berlin, Germany) was used for 30 s to disrupt the biofilms. The homogenized solution was used for serial dilutions of the biofilm suspension, and 100 µL aliquots of each dilution were seeded on plates containingCandida HiChrome agar (Himedia, Mumbai, India) and incubated for 48 h at 37°C. After incubation, the CFU/mL values were determined.Candida species from the mixed biofilms were differentiated by the color of the colonies on Candida HiChrome agar: light green for C. albicans, blue for C. tropicalis, cream for C. glabrata and purple for C. krusei.
Analysis of biofilm cell viability using the XTT colorimetric assay
The biofilms formed were evaluated by a metabolic assay based on the reduction of XTT, a tetrazolium salt (Sigma–Aldrich, São Paulo, Brazil). The methodology described by Jin et al.22. Jin Y, Samaranayake LP, Samaranayake YH, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49(10):789-98. doi:10.1016/j.archoralbio.2004.04.011 was used for the XTT assay. In brief, XTT salt was dissolved in PBS at a final concentration of 1 mg/mL. The solution was filter-sterilized using a 0.22 µm-pore-size filter (MFS, Dublin, Ireland) and stored frozen at -80°C. Immediately before each assay, a menadione (Sigma–Aldrich, São Paulo, Brazil) solution was prepared at a final concentration of 0.4 mM and filter-sterilized. The XTT solution was thawed prior to each assay, and mixed with the menadione solution at a ratio of 20:1 (v:v).
Each well was washed four times with 200 µL of PBS to remove any non-adherent cells. Next, 158 µL of PBS, 40 µL of XTT and 2 µL of menadione were added to each of the pre-washed wells. The plates were incubated in the dark at 37°C for 3 hour. Afterwards, 100 µL of the solution was transferred to a new well, and any colorimetric change in the solution was measured using a microtiter plate reader (Tp Reader; Thermo Plate, Shenzhen, China) at 490 nm.
Statistical analysis
Ten assays were carried out per experimental group. For analysis purposes, CFU/mL values were transformed into logarithm values (log10). The CFU/mL count in monotypic and heterotypic biofilms was submitted to the Student ttest for each species studied. The results obtained from the XTT assay were evaluated by analysis of variance (ANOVA) and the Tukey test. A significance level of 5% (p < 0.05) was adopted (GraphPad Software - Inc., La Jolla, USA).
Results
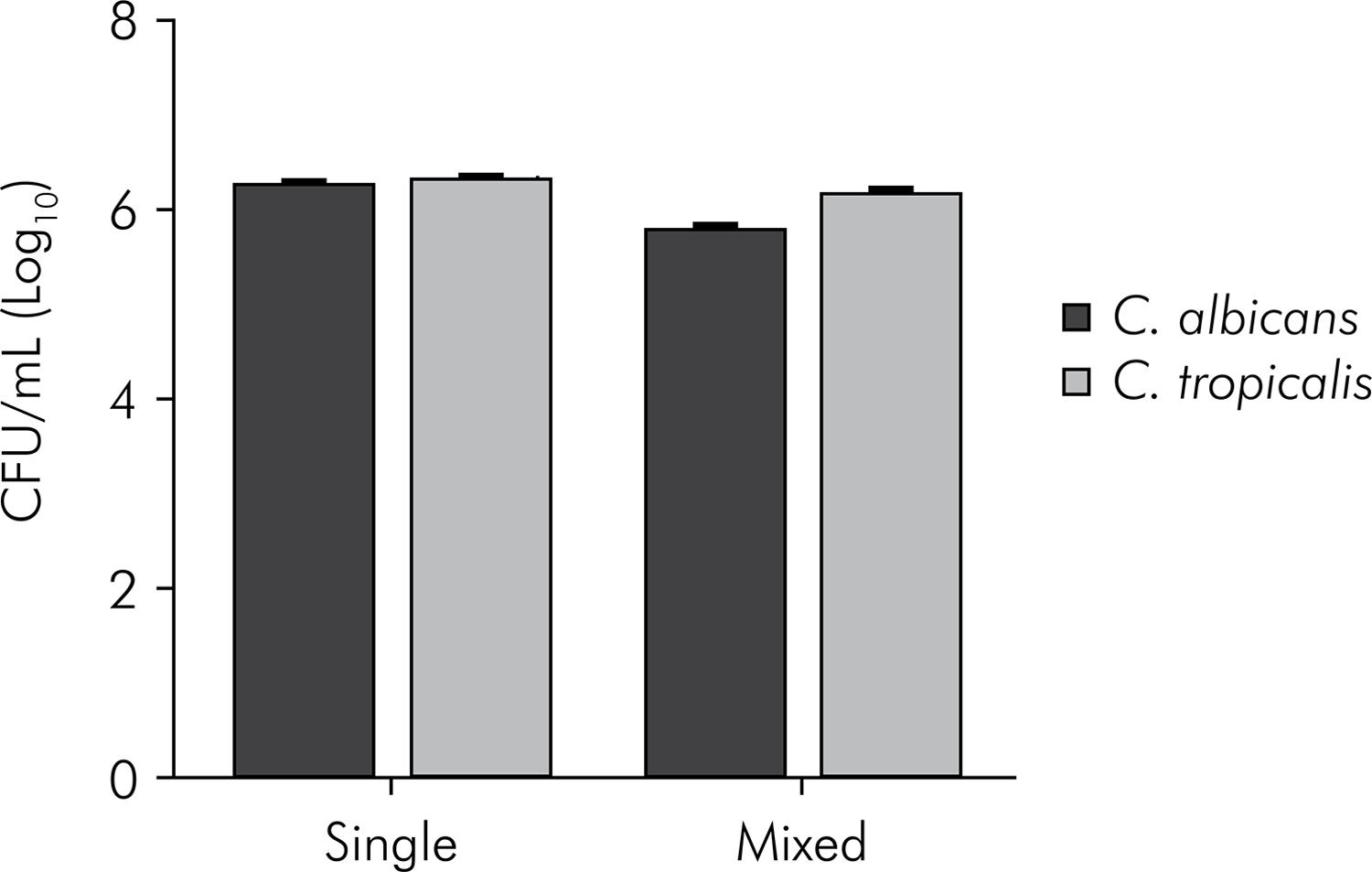
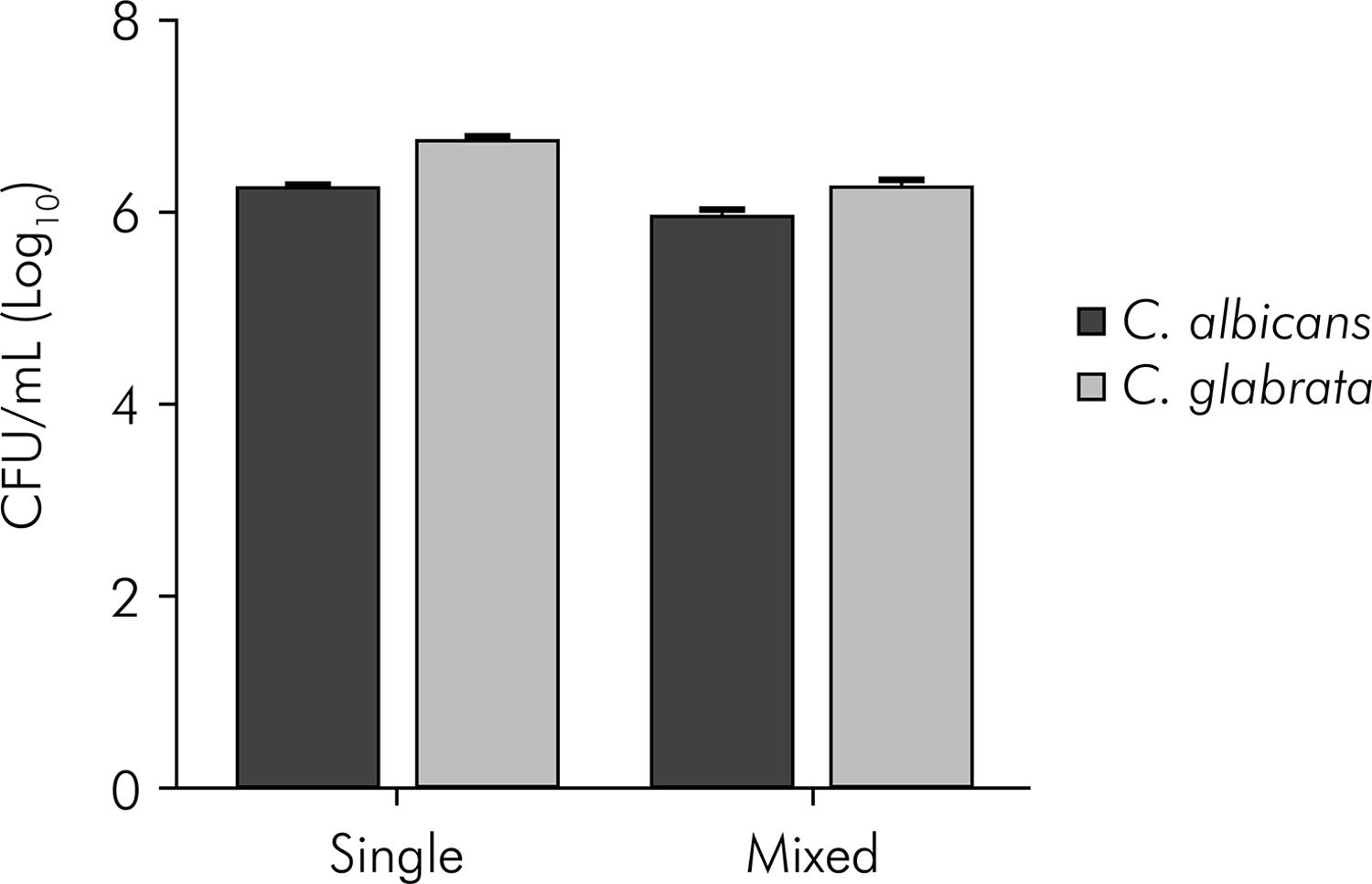
The mean and standard deviation values of the CFU/mL, obtained from the experiments described above, are shown in Figures 1, 2 and 3. All species were able to form in vitro biofilms, attaining 6.24 to 6.75 CFU/mL (log) in the single biofilms.
Mean values and standard deviation (n = 10) of CFU/mL (log) ofC. albicans and C. tropicalis, organized in single and mixed biofilms. Student t-test. C. albicans counts: statistically significant difference between biofilm formed by C. albicans and biofilm formed by C. albicans + C. tropicalis(p < 0.0001). C. tropicalis counts: statistically significant difference between biofilm formed by C. tropicalis and biofilm formed by C. albicans + C. tropicalis (p = 0.0023).
Mean values and standard deviation (n = 10) of CFU/mL (log10) ofC. albicans and C. glabrata, organized in single and mixed biofilms. Student t-test. C. albicans counts: statistically significant difference between biofilm formed by C. albicans and biofilm formed by C. albicans + C. glabrata (p < 0.0001). C. glabrata counts: statistically significant difference between biofilm formed by C. glabrata and biofilm formed by C. albicans+ C. glabrata (p < 0.0001).
Mean values and standard deviation (n = 10) of CFU/mL (log10) ofC. albicans and C. krusei, organized in single and mixed biofilms. Student t-test. C. albicans counts: statistically significant difference between biofilm formed by C. albicans and biofilm formed by C. albicans + C. krusei (p < 0.0001). C. krusei counts: statistically significant difference between biofilm formed by C. krusei and biofilm formed by C. albicans +C. krusei (p < 0.0001).
In the study of microbial interactions, C. albicans showed a significantly higher number of CFU/mL in the single biofilm as compared with the mixed biofilm, as regards the non-albicansCandida species. However, the most significant difference between the single and the mixed biofilm was observed for the interactions of C. albicans and C. krusei (p < 0.0001). In the single biofilm, C. albicans grew to 6.24 log (CFU/mL), but the result was 0.40 log when it was grown in the mixed biofilm with C. krusei. These data indicate that the presence of C. krusei in the biofilm inhibited the growth of C. albicans.
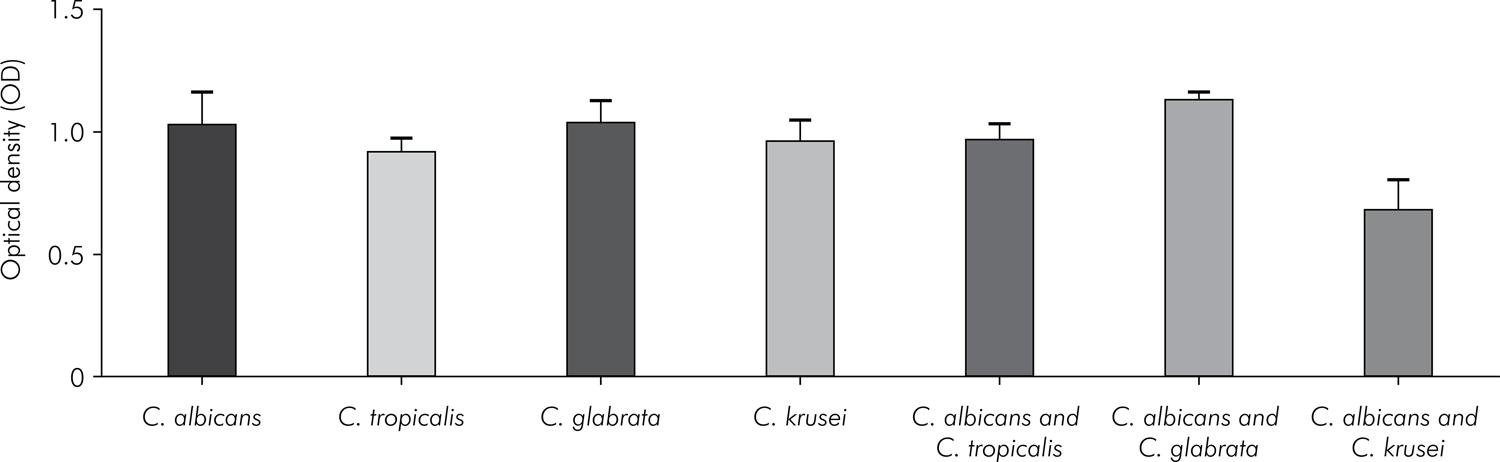
The results from the cell viability XTT colorimetric assay are shown in Figure 4. The metabolic activity observed in the single biofilms formed by C. albicans, C. tropicalis, C. glabrata, and C. krusei was similar for the species studied. The results for the interactions between C. albicans and non-albicansspecies show that the highest metabolic activity was observed for the interaction between C.albicans and C. glabrata, and the lowest, for the interaction between C. albicans andC.krusei. When the single biofilm formed by C. albicans was compared with mixed biofilms formed by the C. albicans and non-albicans species, a statistically significant difference was found only for the interaction with C. krusei (p < 0.0001).
Mean values and standard deviation (n = 10) of the metabolic activity (XTT analysis) for single biofilms formed by C. albicans, C. tropicalis, C. glabrata or C. Krusei, and for mixed biofilms formed by the interactions of C. albicans-C. tropicalis, C. albicans-C. glabrata or C. albicans-C. krusei. Analysis of variance (ANOVA): statistically significant differences among the groups (p < 0.0001). Tukey test: C. albicans vs. C. tropicalis (p = 0.0982), C. albicans vs. C. glabrata (p = 0.9999),C. albicans vs. C. krusei (p = 0.6625), C. tropicalis vs. C. glabrata (p = 0.0742), C. tropicalis vs. C. krusei (p = 0.9138), C. glabrata vs. C. krusei (p = 0.5859), C. albicans vs. C. albicans and C. tropicalis (p = 0.7269),C. albicans vs. C. albicans and C. glabrata (p = 0.2459), C. albicans vs. C. albicans and C. krusei (p < 0.0001),C. tropicalis vs. C. albicans and C. tropicalis (p = 0.8751), C. glabrata vs. C. albicans and C. glabrata (p = 0.3036),C. krusei vs. C. albicans and C. krusei (p < 0.0001).
Discussion
Recent studies have shown that biofilms in nature are formed by multiple microbial species that are closely associated and interact with each other.1616. Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton W, Shirtliff ME. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59(3):493-503. doi:10.1111/j.1574-695X.2010.00710.x Some microorganisms have evolved mutualistic or even synergistic interactions to facilitate cohabitation on epithelial surfaces and to utilize metabolic by-products efficiently, whereas others have developed competitive antagonistic approaches during colonization.2323. Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25(1):193-213. doi:10.1128/CMR.00013-11
Species of the genus Candida are the most prevalent fungal pathogens in the oral cavity, and their ability to form biofilms is associated with their virulence.2424. Thein ZM, Samaranayake YH, Samaranayake LP. In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch Oral Biol. 2007;52(8):761-7. doi:10.1016/j.archoralbio.2007.01.009Candida albicans is considered the most important species, and is found in approximately 70% of oral infections. Nonetheless, non-albicansspecies, such as Candida glabrata, Candida tropicalis and Candida krusei, are also frequently associated with the development of infections.2525. Dovigo LN, Pavarina AC, Ribeiro DG, Adriano CS, Bagnato VS. Photodynamic inactivation of four Candida species induced by photogem®. Braz J Microbiol. 2010;41(1):42-9. doi:10.1590/S1517-83822010000100009Candida albicans sometimes exists in a mixture with other microbial species in this environment.2626. Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299(1):1-8. doi:10.1111/j.1574-6968.2009.01668.x The virulence of C. albicans may be influenced not only by its own properties but also by its interactions with cohabitating oral microorganisms.2020. Pereira-Cenci T, Deng DM, Kraneveld EA, Manders EM, Del Bel Cury AA, Ten Cate JM, et al. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch Oral Biol. 2008;53(18):755-64. doi:10.1016/j.archoralbio.2008.02.015 Further research is required to understand the interactions that occur when different species of Candida coexist in a biofilm.
In the present study, the interactions between C. albicans and non-albicans species in mixed biofilms were evaluated by counting colony-forming units (CFU/mL) and by determining cell viability using the XTT colorimetric assay. The counting of CFU/mL was performed using a chromogenic medium (Candida HiChrome agar) that allows the colonies ofC. albicans, C. tropicalis, C. glabrata and C. krusei to be differentiated by color. Chromogenic media are widely used for presumptive identification ofCandida species. Rajkumari et al.2727. Rajkumari N, Mathur P, Xess I, Misra MC. Distribution of different yeasts isolates among trauma patients and comparison of accuracy in identification of yeasts by automated method versus conventional methods for better use in low resource countries. Indian J Med Microbiol. 2014;32(4):391-7. doi:10.4103/0255-0857.142243 compared the accuracy between the Automated VITEK 2 Compact system and a chromogenic medium (CHROMagar) for identification of 445 yeast isolates. VITEK 2 was able to correctly identify 354 (79.5%) isolates, whereas the chromogenic medium correctly identified 381 (85.6%) isolates.
The results obtained by counting CFU/mL show antagonistic interactions betweenC. albicans with C. tropicalis, C. glabrata, and C. krusei. El-Azizi et al.1414. El-Azizi MA, Starks SE, Khardori N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol. 2004;96(5):1067-73. doi:10.1111/j.1365-2672.2004.02213.x investigated the interactions between C. albicans and non-albicans species usingin vitro models. C. guilliermondii, C. krusei or C. lipolytica were added to a preformedC. albicans biofilm. An increase in the biofilm growth ofCandida spp. was observed in the in vitromodel, except for C. guilliermondii. Similar results were observed when C. albicans was added simultaneously with non-albicans species to form a mixed biofilm. However, the number of C. albicans cells in the biofilm was significantly reduced when the cells were added to the preformed C. guilliermondii, C. krusei or C. lipolytica biofilms, indicating a competition for adhesion sites.
Among the non-albicansCandida species, C. kruseiwas the species with the highest inhibitory activity against C. albicans. Its greater number of CFU/mL suggests that C. krusei prevailed in this interaction. Using a biofilm model similar to that of this study and Candida reference strains, Rossoni et al.2121. Rossoni RD, Barbosa JO, Vilela SFG, Santos JD, Barros PP, Prata MCA, et al. Competitive interactions between C. albicans, C. glabrata and C. krusei during biofilm formation and development of experimental candidiasis. PLos One. 2015 Jul 6;10(7):e0131700. doi:10.1371/journal.pone.0131700 verified that the inhibition of C. albicans was greater in the presence of C. krusei than in the presence of C. glabrata. Thein et al.1919. Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture surfaces. Arch Oral Biol. 2007;52(12):1200-8. doi:10.1016/j.archoralbio.2007.06.007 also studied the interaction between C. albicans and C. kruseiusing an in vitro biofilm model, and observed antagonism between the species; C. krusei suppressed approximately 85% of C. albicans growth. These results suggest that there may be competition for nutrients between these species or that C. krusei produces signaling molecules that inhibit the growth of C. albicans. Furthermore, it has been suggested that C. krusei has a higher initial rate of colonization, perhaps because of C. krusei’s very high cell surface hydrophobicity and resultant adherence to acrylic surfaces.77. Samaranayake LP, Samaranayake YH. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J Med Microbiol. 1994;41(5):295-310. doi:10.1099/00222615-41-5-295 In addition, biofilm formation and coaggregation effects allow competing microorganisms to maximize the colonization surface area in an environment that is highly competitive for space and nutrients.2323. Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25(1):193-213. doi:10.1128/CMR.00013-11
To date, most previous studies have focused on analyzing biofilm formation by counting CFU/mL; however, Seneviratne et al.1010. Seneviratne CJ, Silva WJ, Jin LJ, Samaranayke LP. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54(11):2260-9. doi:10.1016/j.archoralbio.2009.08.002 suggest that biofilm development results should be analyzed by more than one method. In this study, in addition to colony counting, we used an XTT reduction assay that permits measurement of the metabolic activity of the microbial eukaryotic population present in biofilms. The intracellular reduction of XTT releases a formazan compound that can be quantified by colorimetric estimation, thereby allowing characterization of the metabolic activity of cells.99. Silva WJ, Seneviratne J, Parahitiyawa NB, Rosa EAR, Samaranayake LP, Del Bel Cury AA. Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz Dent J. 2008;19(4):364-9. doi:10.1590/S0103-64402008000400014
The single biofilms formed by C. albicans, C. tropicalis, C. glabrata, and C. krusei, on flat-bottom 96-well microtiter plates for 48 h, showed similar metabolic activity, thus confirming the CFU counting method. Seneviratne et al.1010. Seneviratne CJ, Silva WJ, Jin LJ, Samaranayke LP. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54(11):2260-9. doi:10.1016/j.archoralbio.2009.08.002 evaluated the growth kinetics of Candida biofilms by counting CFU after 1.5, 24, 48 and 72 h of development. At an adherence time of 1.5 h, approximately 1-7 x 105 cells/mL had adhered to the well of the microtiter plate. After 48 h, the biofilm community reached a plateau of 0.3-2.2 x 108 cells/mL, and then declined by 72 hour. The spectrometric profile (520 nm) was also assessed and correlated with the number of CFU, showing maximum cell density at 48 h, declining thereafter.
In mixed biofilms, the results of the XTT colorimetric assay were compared with the single biofilm formed by C. albicans, because it is not possible to differentiate the cellular activity of the individual species, based only on the results of the XTT method. The results obtained in the single biofilms formed byC. albicans were similar to those of the biofilms mixed withC. tropicalis or C. glabrata. However, the presence of C. krusei in the mixed biofilm reduced cell viability, compared with the cell viability observed in the single biofilm formed by C. albicans. Pathak et al.2828. Pathak KA, Sharma S, Shrivastva P. Multi-species biofilm of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J Appl Oral Sci. 2012;20(1):70-5. doi:10.1590/S1678-77572012000100013 also studied the interactions among C. albicans, C. glabrata, C. tropicalisand C. krusei in vitro through optical density values using the crystal violet assay. C. tropicalis decreased the biofilm production of non-albicans species. However, C. krusei negatively impacted the biofilm formation of multiple species, and the presence of C. albicans biofilms increased in the layer of heterotypic biofilms, indicating that C. albicans may provide a substrate for non-albicans species in or on acrylic materials.
In this study, we used clinical isolates from oropharyngeal candidiasis lesions of HIV-positive patients; however, only 1 clinical strain of each species was tested. Previous studies demonstrated that the clinical isolates of Candidaexhibit intraspecies variability in relation to biofilm forming ability.2929. Sanches-Vargas LO, Estrada-Barraza D, Pozos-Guillen AJ, Rivas-Caceres R. Biofilm formation by oral clinical isolates of Candida species. Arch Oral Biol. 2013;58(10):1318-26. doi:10.1016/j.archoralbio.2013.06.006,3030. Hu L, Du X, Li T, Song Y, Zai S, Hu X, et al. Genetic and phenotypic characterization of Candida albicans strains isolated from infectious disease patients in Shanghai. J Med Microbiol. 2015 Jan;64(1):74-83. doi:10.1099/jmm.0.080200-0 Muadcheingka and Tantivitayakul3131. Muadcheingka T, Tantivitayakul P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch Oral Biol. 2015;60(6):894-901. doi:10.1016/j.archoralbio.2015.03.002 evaluated the cell surface hydrophobicity (CSH) of 250 clinical isolates from oral candidiasis. CSH is an intrinsic property of the external cell wall layer of Candida; it promotes strong binding and irreversible adherence to the mucosal membrane or other substrates, contributing to biofilm formation. These authors observed that the CSH values were strain dependent and not species-specific.
In brief, both the counting of CFU/mL and the XTT colorimetric assay results showed that C. krusei exerted strong inhibitory action on C. albicans during biofilm formation. This data confirms previous findings that the XTT assay yielded results similar to those of the CFU method, thereby making it a useful tool for studying biofilms formed in vitro. In addition, further studies are needed to elucidate the mechanisms of the antagonistic interaction between C. krusei and C. albicans,focusing especially on how C. krusei affects the quorum sensing-mediated molecules, the filamentation and the pathogenicity of C. albicans. This study should also be widened to include a larger number of clinical strains of C. albicans, C. tropicalis,C. glabrata and C. krusei.
Conclusion
Considering the clinical Candida strains analyzed in this study, we concluded that C. albicans established antagonistic interactions with non-albicans Candida species in mixed biofilms. C. krusei significantly inhibited the growth and the metabolic activity ofC. albicans in the mixed biofilm.
Acknowledgements
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP, Brazil, a state funding agency (grant 2011/10071-7).
References
-
1Thein ZM, Samaranayake YH, Samaranayake LP. Effect of oral bacteria on growth and survival of Candida albicans biofilms. Arch Oral Biol. 2006;51(8):672-80. doi:10.1016/j.archoralbio.2006.02.005
-
2Jin Y, Samaranayake LP, Samaranayake YH, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49(10):789-98. doi:10.1016/j.archoralbio.2004.04.011
-
3Harriot MM, Noverr MC. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19(11):557-63. doi:10.1016/j.tim.2011.07.004
-
4Kirkpatrick WR, Lopez-Ribot JL, Mcatee RK, Patterson TF. Growth competition under broth an biofilms growing conditions. J Clin Microbiol. 2000;38(2):902-4. doi:10.1111/j.1567-1364.2006.00121.x
-
5Silva S, Negri M, Henriques M, Oliveira R, Williams D, Azeredo J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011;19(5):241-7. doi:10.1016/j.tim.2011.02.003
-
6Bizerra FC, Nakamura CV, Poersch CO, Svidzinski TI, Quesada RM, Goldenberg S, et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8(3):442-50. doi:10.1111/j.1567-1364.2007.00347.x
-
7Samaranayake LP, Samaranayake YH. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J Med Microbiol. 1994;41(5):295-310. doi:10.1099/00222615-41-5-295
-
8Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med Sci. 2010;25(3):385-9. doi:10.1007/s10103-009-0706-z
-
9Silva WJ, Seneviratne J, Parahitiyawa NB, Rosa EAR, Samaranayake LP, Del Bel Cury AA. Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz Dent J. 2008;19(4):364-9. doi:10.1590/S0103-64402008000400014
-
10Seneviratne CJ, Silva WJ, Jin LJ, Samaranayke LP. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54(11):2260-9. doi:10.1016/j.archoralbio.2009.08.002
-
11Costa AC, Rasteiro VM, Pereira CA, Hashimoto ES, Beltrame Jr M, Junqueira JC, et al. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol. 2011;56(11):1299-305. doi:10.1016/j.archoralbio.2011.05.013
-
12Thein ZM, Seneviratne CJ, Samaranayake YH, Samaranayake LP. Community lifestyle of Candida in mixed biofilms: a mini review. Mycoses. 2009;52(6):467-75. doi:10.1111/j.1439-0507.2009.01719.x
-
13Douglas LJ. Medical importance of biofilms in Candida infections. Rev Iberoam Micol. 2002;19:139-43. doi:10.1016/S0966-842X(02)00002-1
-
14El-Azizi MA, Starks SE, Khardori N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol. 2004;96(5):1067-73. doi:10.1111/j.1365-2672.2004.02213.x
-
15Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8(5):340-49. doi:10.1038/nrmicro2313
-
16Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton W, Shirtliff ME. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59(3):493-503. doi:10.1111/j.1574-695X.2010.00710.x
-
17Harriot MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53(9):3914-22. doi:10.1128/AAC.00657-09
-
18Tampakakis E, Peleg AY, Mylonakis E. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica Serovar Typhimurium. Eukaryot Cell. 2009;8(5):732-7. doi:10.1128/EC.00016-09
-
19Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture surfaces. Arch Oral Biol. 2007;52(12):1200-8. doi:10.1016/j.archoralbio.2007.06.007
-
20Pereira-Cenci T, Deng DM, Kraneveld EA, Manders EM, Del Bel Cury AA, Ten Cate JM, et al. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch Oral Biol. 2008;53(18):755-64. doi:10.1016/j.archoralbio.2008.02.015
-
21Rossoni RD, Barbosa JO, Vilela SFG, Santos JD, Barros PP, Prata MCA, et al. Competitive interactions between C. albicans, C. glabrata and C. krusei during biofilm formation and development of experimental candidiasis. PLos One. 2015 Jul 6;10(7):e0131700. doi:10.1371/journal.pone.0131700
-
22Junqueira JC, Vilela SFG, Rossoni RD, Barbosa JO, Costa ACBP, Rasteiro VMC, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo. 2012;54(1):17-24. doi:10.1590/S0036-46652012000100004
-
23Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25(1):193-213. doi:10.1128/CMR.00013-11
-
24Thein ZM, Samaranayake YH, Samaranayake LP. In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch Oral Biol. 2007;52(8):761-7. doi:10.1016/j.archoralbio.2007.01.009
-
25Dovigo LN, Pavarina AC, Ribeiro DG, Adriano CS, Bagnato VS. Photodynamic inactivation of four Candida species induced by photogem® Braz J Microbiol. 2010;41(1):42-9. doi:10.1590/S1517-83822010000100009
-
26Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299(1):1-8. doi:10.1111/j.1574-6968.2009.01668.x
-
27Rajkumari N, Mathur P, Xess I, Misra MC. Distribution of different yeasts isolates among trauma patients and comparison of accuracy in identification of yeasts by automated method versus conventional methods for better use in low resource countries. Indian J Med Microbiol. 2014;32(4):391-7. doi:10.4103/0255-0857.142243
-
28Pathak KA, Sharma S, Shrivastva P. Multi-species biofilm of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J Appl Oral Sci. 2012;20(1):70-5. doi:10.1590/S1678-77572012000100013
-
29Sanches-Vargas LO, Estrada-Barraza D, Pozos-Guillen AJ, Rivas-Caceres R. Biofilm formation by oral clinical isolates of Candida species. Arch Oral Biol. 2013;58(10):1318-26. doi:10.1016/j.archoralbio.2013.06.006
-
30Hu L, Du X, Li T, Song Y, Zai S, Hu X, et al. Genetic and phenotypic characterization of Candida albicans strains isolated from infectious disease patients in Shanghai. J Med Microbiol. 2015 Jan;64(1):74-83. doi:10.1099/jmm.0.080200-0
-
31Muadcheingka T, Tantivitayakul P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch Oral Biol. 2015;60(6):894-901. doi:10.1016/j.archoralbio.2015.03.002
Publication Dates
-
Publication in this collection
2016
History
-
Received
25 Oct 2014 -
Reviewed
26 Aug 2015 -
Accepted
09 Nov 2015