Abstract
The unconscious use of pesticides causes various adverse effects on non-target organisms, including humans. Enzymes that control metabolism become the target of the pesticide and the organs are damaged due to toxic effects. Glutathione s-transferase (GST, EC 2.5.1.18), an important enzyme of the detoxification mechanism and antioxidant defense system, can be affected by such toxic substances. Therefore, the effect of fenarimol on GST enzyme activity was investigated in our study. For this, 200 mg/kg fenarimol was administered intraperitoneally to male and female rats at different periods (2, 4, 8, 16, 32, 64 and 72 hours). After application, GST enzyme activity was analysed in the liver, kidney, brain and small intestine tissues of the rats. According to our results, activation (liver, kidney, small intestine) or inhibition (brain) of the generally GST enzyme was observed in the tissues of rats exposed to fenarimol. It is thought that the increase and/or decrease in this enzyme activity may be the cause of the toxic effect of fenarimol.
Keywords:
pesticide; fungicide; fenarimol; glutathione s-transferase
HIGHLIGHTS
Changes in GST enzyme activity in rat tissues exposed to fenarimol were investigated.
GST activity was increased in liver, kidney and small intestine tissues to fenarimol exposure.
GST activity was decreased in brain intestine tissues to fenarimol exposure.
Fenarimol can cause a toxic effect on metabolism by causing changes in GST activity.
INTRODUCTION
Pesticides are defined as a toxic substance or mixture of substances that kill or eliminate unwanted organisms, prevent them from multiplying and feeding [11 Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res. 2003;543(3):251-72. doi:10.1016/S1383-5742(03)00015-2.
https://doi.org/10.1016/S1383-5742(03)00...
]. Unconsciously and intensely used pesticides pose a problem for various organisms and the environment, and during their use they can accumulate in food, soil, water and air, or undergo a dangerous conversion [22 Faria M, Prats E, Rosas Ramírez JR, Bellot M, Bedrossiantz J, Pagano M, et al. Androgenic activation, impairment of the monoaminergic system and altered behavior in zebrafish larvae exposed to environmental concentrations of fenitrothion. Sci Total Environ. 2021;775:145671. doi:10.1016/j.scitotenv.2021.145671.
https://doi.org/10.1016/j.scitotenv.2021...
3 Fiorino E, Sehonova P, Plhalova L, Blahova J, Svobodova Z, Faggio C, et al. Effect of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res Int. 2018;25(9):8542-9. doi:10.1007/s11356-017-1141-5.
https://doi.org/10.1007/s11356-017-1141-...
-44 Blahova J, Cocilovo C, Plhalova L, Svobodova Z, Faggio C. Embryotoxicity of atrazine and its degradation products to early life stages of zebrafish (Danio rerio). Environ Toxicol Pharmacol. 2020;77:103370. doi:10.1016/j.etap.2020.103370.
https://doi.org/10.1016/j.etap.2020.1033...
]. Therefore, they cause adverse effects (acute poisoning, hormone and nervous system disorders, cancer and diabetes) on non-target organisms and humans [55 Stara A, Kubec J, Zuskova E, Buric M, Faggio C, Kouba A, et al. Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procambarus virginalis). Chemospere. 2019;224:616-25. doi:10.1016/j.chemosphere.2019.02.187.
https://doi.org/10.1016/j.chemosphere.20...
6 Stara A, Bellinvia R, Velisek J, Strouhova A, Kouba A, Faggio C, et al. Acute exposure of common yabby (Cherax destructor) to the neonicotinoid pesticide. Sci Total Environ. 2019;665:718-23. doi:10.1016/j.scitotenv.2019.02.202.
https://doi.org/10.1016/j.scitotenv.2019...
7 Pagano M, Stara A, Aliko V, Faggio C. Impact of Neonicotinoids to Aquatic Invertebrates-In Vitro Studies on Mytilus galloprovincialis: A Review. J Mar Sci Eng. 2020;8(10):801. doi:10.3390/jmse8100801.
https://doi.org/10.3390/jmse8100801...
8 Stara A, Pagano M, Capillo G, Fabrello J, Sandova M, Albano M, et al. Acute effects of neonicotinoid insecticides on Mytilus galloprovincialis: a case study with the active compound thiacloprid and the commercial formulation Calypso 480 SC. Ecotoxicol Environ Saf. 2020;203(7):110980. doi:10.1016/j.ecoenv.2020.110980.
https://doi.org/10.1016/j.ecoenv.2020.11...
9 Stara A, Pagano M, Capillo G, Fabrello J, Sandova M, Vazzana I, et al. Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci Total Environ. 2020;700:134914. doi:10.1016/j.scitotenv.2019.134914.
https://doi.org/10.1016/j.scitotenv.2019...
10 Sula E, Aliko V, Barceló D, Faggio C. Combined effects of moderate hypoxia, pesticide and PCBs upon Crucian Carp fish, Carassius carassius, from a freshwater lake-in situ ecophysiological approach. Aquat Toxicol. 2020;228:105644. doi: 10.1016/j.aquatox.2020.105644.
https://doi.org/10.1016/j.aquatox.2020.1...
11 Horrigan L, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect. 2002;110(5):445-56. doi: 10.1289/ehp.02110445.
https://doi.org/10.1289/ehp.02110445...
12 Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628-33. doi:10.1002/ps.1406.
https://doi.org/10.1002/ps.1406...
-1313 Bronstein AC, Spyker DA, Cantilena LR, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila). 2012;50(10):911-1164. doi:10.3109/15563650.2012.746424.
https://doi.org/10.3109/15563650.2012.74...
]. Some pesticides such as dichlorvos, acetamipride, cyhalothrin, haloxyfop-p-methyl, 2,4-dichlorophenoxy acetic acid, cypermethrin, imidacloprid, fenoxaprop-p-ethyl, glyphosate isopropylamine salt have been shown to inhibit the GST enzyme at millimolar level in blueberry fruits [1414 Balcı N, Türkan F, Şakiroğlu H, Aygün A, Şen F. Purification and characterization of glutathione S-transferase from blueberry fruits (Vaccinium arctostaphylos L.) and investigated of some pesticide inhibition effects on enzyme activity. Heliyon. 2019;5(4),e01422. doi:10.1016/j.heliyon.2019.e01422.
https://doi.org/10.1016/j.heliyon.2019.e...
].
Pesticides that enter the body indirectly are removed from the body by detoxification reactions. Depending on the type of chemical change, these reactions can be divided into four groups: oxidation, reduction, hydrolysis (phase 1) and conjugation (phase 2). Pesticides are oxidized in phase 1 by cytochrome P450 monooxygenases in the liver and converted into polar compounds with short biological half-lives. In phase 2, the modified pesticides are conjugated with high polarity glucuronic acid or glutathione (GSH) in water. As a result of pesticide biotransformation, biologically activated pesticide metabolites increase their biological half-life by covalently binding to tissue macromolecules (DNA and protein). Binding of these pesticide metabolites to DNA or esterases causes a number of oncogenic or neurotoxic abnormalities to accelerate [1515 Kurutas EB, Kilinç M. Effects of Pesticides on Biological Systems. Arch Med Rev J. 2003;12(3):215-28.].
When pesticides with different classification criteria are grouped according to the target organism, fenarimol in the fungicide class is used for protection against fungal spores or fungi, especially in agriculture [1616 Zhang H, Wang X, Zhuang S, Qian M, Jiang K, Wang X, et al. Enantioselective separation and simultaneous determination of fenarimol and nuarimol in fruits, vegetables, and soil by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2012;404(6-7):1983-91. doi:10.1007/s00216-012-6325-8.
https://doi.org/10.1007/s00216-012-6325-...
,1717 Oh K, Matsumoto T, Yamagami A, Hoshi T, Nakano T, Yoshizawa Y. Fenarimol, a pyrimidine-type fungicide, inhibits brassinosteroid biosynthesis. Int J Mol Sci. 2015;16(8):17273-88. doi:10.3390/ijms160817273.
https://doi.org/10.3390/ijms160817273...
]. Members of this class with various active ingredients can affect different (single-site or multi-site) mechanisms. Those specific to the region (single region) show their effects only at one point of the metabolic pathway or on an enzyme and/or protein, depending on the needs of the target organism. Multicides, on the other hand, are effective in different metabolic pathways and target basic cellular processes that inhibit various biosynthesis in mushrooms [1818 Bolognesi C, Merlo FD. Pesticides: Human Health Effects. In: Encyclopedia of Environmental Health. Elsevier, 2019.118-32.]. In mammalian cells, fenarimol induces a variety of cytochrome P450 isoforms from all inducible families, including key enzymes involved in the biosynthesis and metabolism of steroids [1919 Andersen HR, Bonefeld-Jørgensen EC, Nielsen F, Jarfeldt K, Jayatissa MN, Vinggaard AM. Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett. 2006;163(2):142-52. doi:10.1016/j.toxlet.2005.10.004.
https://doi.org/10.1016/j.toxlet.2005.10...
]. Again, as a result of studies in experimental animals, fenarimol has been shown to have teratogenic and oncogenic effects on reproduction and inhibit aromatase activity in male rats [2020 de Castro VLSS, de Mello MA, Diniz C, Morita L, Zucchi T, Poli P. Neurodevelopmental effects of perinatal fenarimol exposure on rats. Reprod Toxicol. 2007;23(1):98-105. doi:10.1016/j.reprotox.2006.09.001.
https://doi.org/10.1016/j.reprotox.2006....
]. In addition, as a result of a previous study conducted by our group, it was shown that hepatic marker enzyme activities (ALP, ALT, AST and CK) and some biochemical parameters in serum were affected in fenarimol-induced rats [2121 Ari F, Dere E. Effect of the sterol demethylation-inhibiting fungicide fenarimol on selected biochemical parameters in rats. Acta Vet Beo. 2010;60(1):31-8. doi:10.2298/AVB1001031A.
https://doi.org/10.2298/AVB1001031A...
].
GSTs that conjugate various hydrophobic compounds containing electrophilic centres with endogen/exogenous substances are members of the Phase-II detoxification enzyme family. In this way, it catalyses their removal from the body and their conversion into less toxic metabolites [2222 Espinoza HM, Williams CR, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110-111:37-44. doi:10.1016/j.aquatox.2011.12.012.
https://doi.org/10.1016/j.aquatox.2011.1...
,2323 Ozaslan MS, Demir Y, Küfrevioglu OI, Çiftci M. Some metals inhibit the glutathione S-transferase from Van Lake fish gills. J Biochem Mol Toxicol. 2017;31(11):e21967. doi:10.1002/jbt.21967.
https://doi.org/10.1002/jbt.21967...
]. GSTs that catalyse different substrates such as carcinogenic compounds, drugs, environmental pollutants, are classified according to their presence in the mitochondrial, cytosolic and microsomal parts of the cell [2323 Ozaslan MS, Demir Y, Küfrevioglu OI, Çiftci M. Some metals inhibit the glutathione S-transferase from Van Lake fish gills. J Biochem Mol Toxicol. 2017;31(11):e21967. doi:10.1002/jbt.21967.
https://doi.org/10.1002/jbt.21967...
]. Although it is commonly found in animals and plants, it has also been reported to be found in various organisms (flies, snails, sharks, some bacteria). The main and most important function of GST is the detoxification of reactive intermediates formed in xenobiotic metabolism. In addition, it has various physiological functions such as binding/transport of different ligands, reduction of lipid hydroperoxides, tyrosine catabolism, hormone biosynthesis and modulation of signal pathways [2222 Espinoza HM, Williams CR, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110-111:37-44. doi:10.1016/j.aquatox.2011.12.012.
https://doi.org/10.1016/j.aquatox.2011.1...
,2424 Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980;191(1):1-10. doi:10.1042/bj1910001.
https://doi.org/10.1042/bj1910001...
,2525 Hayes JD, Flanagan JU, Jowsey IR. Glutathione Transferases. Annu Rev Pharmacol Toxicol. 2005;45(1):51-88. doi:10.1146/annurev.pharmtox.45.120403.095857.
https://doi.org/10.1146/annurev.pharmtox...
].
In our study, the effect of fenarimol, which is frequently used in agriculture, on GST enzyme activity, which has an important role in the detoxification mechanism, was investigated. Accordingly, after fenarimol exposure, a significant increase in GST enzyme activity was observed in all tissues of the male and female rats, except the brain (GST inhibition was observed in the brain tissue). This study will serve as a source since there is no information about the GST enzyme activity of fenarimol in the literature.
MATERIAL AND METHODS
Animals
The study was evaluated and approved by the Bursa Uludag University Animal Use and Care Committee (Decision No. 3-06. 2003/3). The Wistar-Albino rats used in our study weighed 200-250 g and were obtained from the Experimental Animals Breeding and Research Center of Bursa Uludag University Faculty of Medicine. Rats were maintained in an environment with established standard environmental conditions (temperature 23 ± 2 °C and 12 h light/dark cycle).
Animal Treatment
A total of 48 rats were divided into 4 experimental and 2 control groups. This step was performed in the same way for each experiment period. (Control group n=16, experimental group n=32). While the experimental groups were injected intraperitoneally with 200 mg/kg LD50 dose of fenarimol (Sigma-Aldrich, St. Louis, MO), the control groups were given corn oil. Rats were fasted for 24 h before injection. After the injection, the animals were regularly fed with food and water until the trial period was completed. Rats in the treatment and control groups were kept in special cages. The animals were euthanized by cervical dislocation at the end of each specified interval (2, 4, 8, 16, 32, 64 and 72 h after injection). The brain, kidney, liver and small intestine of the rats were removed and perfused in cold 0.15 M KCl. The resulting tissues were homogenized at 2000 rpm in a T-line laboratory mixer type (model No: 136-2) homogenizer. Each homogenate was centrifuged at Dupont Instruments Sorvall (RC-5 super-fast cooled centrifuge) at 48000 g for 30 minutes at 4 °C.
Protein determination
The protein concentration was determined by the Bradford method [2626 Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54. doi:10.1006/abio.1976.9999.
https://doi.org/10.1006/abio.1976.9999...
] and bovine serum albumin was used as the protein standard.
Glutathione s-transferase activity
GST enzyme activities were determined by monitoring the thioether bond formed between GST and 1-chloro-2,4-dinitrobenzene (CDNB) spectrophotometrically at 340nm wavelength for 5 minutes. In the test mixture, 10 mM potassium phosphate buffer (pH: 6.7), 5mM GSH prepared in potassium phosphate buffer, CDNB prepared in 99% ethyl alcohol and cytosolic fractions of tissues were used as enzyme source. ε: Extinction Coefficient was accepted as 9.6mMˉ1 cmˉ1 [2727 Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-9.]. The mean of inter- and intra-assay coefficients of variation of GST activity were calculated 15% and 10% respectively.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.4.0 (Demo Version; GraphPad, San Diego, CA) statistical package program. Independent sample t-test was applied to examine the effect of fenarimol between experimental and control groups. The significance was calculated using one-way analysis of variance (ANOVA) and Student's t-test. A value of p<0.05, p<0.01, and p< 0.001 was considered statistically significant.
RESULTS
Effect of fenarimol on GST activity in liver tissue
The liver GST findings obtained as a result of the experiments are shown in Figure 1. When liver GST activity is examined in both male and female rats in all experimental periods, it is seen that GST activity is higher than the control group. GST activity is significant in all experimental periods in males and females (p <0.001).
Effect of fenarimol on GST activity in liver tissue of rats. *Refers to statistically significant differences compared to control: *(p <0.05); **(p <0.01); ***(p <0.001). Data are presented as mean ± SD (n=3) and analyses were performed using GraphPad program. SD, Standard Deviation; GST, Glutathione S-Transferase.
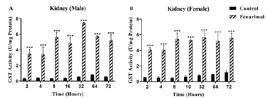
Effect of fenarimol on GST activity in kidney tissue
The kidney GST findings obtained as a result of the experiments are shown in Figure 2. When kidney GST activity is examined in both male and female rats in all experimental periods, it is seen that GST activity is higher than the control group. GST activity is significant in all experimental periods in males and females (p <0.001).
Effect of fenarimol on GST activity in the kidney tissue of rats. *Refers to statistically significant differences with respect to control: *(p <0.05); **(p <0.01); ***(p <0.001). Data are presented as mean ± SD (n=3) and analyses were performed using GraphPad program. SD, Standard Deviation; GST, Glutathione S-Transferase.
Effect of fenarimol on GST activity in brain tissue
Brain GST findings obtained as a result of experiments are shown in Figure 3. As a result of the statistical evaluation between the control and experimental groups, GST activity was observed to decrease. Inhibition of GST activity in male rats was statistically significant at all experimental hours (p <0.05 and p <0.001) and at 32nd and 64th h (p <0.01 and p <0.001) in female rats.
Effect of fenarimol on GST activity in the brain tissue of rats. *Refers to statistically significant differences with respect to control: *(p <0.05); **(p <0.01); ***(p <0.001). Data are presented as mean ± SD (n=3) and analyses were performed using GraphPad program. SD, Standard Deviation; GST, Glutathione S-Transferase.
Effect of fenarimol on GST activity in the small intestine tissue
The small intestine GST findings obtained as a result of the experiments are shown in Figure 4. When GST activity was examined in both male and female rats in all experimental periods, it was observed that GST activity was higher than the control group. GST activity is significant in all experimental hours except for the 64th and 72th h in male and female rats (p <0.001).
Effect of fenarimol on GST activity in the small intestine tissue of rats. *Refers to statistically significant differences with respect to control: *(p <0.05); **(p <0.01); ***(p <0.001). Data are presented as mean ± SD (n=3) and analyses were performed using GraphPad program. SD, Standard Deviation; GST, Glutathione S-Transferase.
DISCUSSION
To understand whether pesticides affect non-target organisms, enzyme activities in damaged tissues can be examined [2828 Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. Pesticide toxicity: A mechanistic approach. Exp Clin Sci J. 2018;17:1101-36. doi:10.17179/excli2018-1710.
https://doi.org/10.17179/excli2018-1710...
29 Gharaei A, Karimi M, Harijani JM, Miri M, Faggio C. Population growth of Brachionus calyciflorus affected by deltamethrin and imidacloprid insecticides. Iran J Fish Sci. 2020;19(2):588-6.
30 Petrovici A, Strungaru SA, Nicoara M, Robea MA, Solcan C, Faggio C. Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. J Mar Sci Eng. 2020;8(2):73. doi:10.3390/jmse8020073.
https://doi.org/10.3390/jmse8020073...
31 Plhalova L, Blahova J, Divisova L, Enevova V, Casuscelli Di Tocco F, Faggio C, et al. The effects of subchronic exposure to NeemAzal T/S on zebrafish (Danio rerio). Chem Ecol. 2018;34(3):199-210. doi:10.1080/02757540.2017.1420176.
https://doi.org/10.1080/02757540.2017.14...
-3232 Chromcova L, Blahova J, Zivna D, Plhalova L, Casuscelli Di Tocco F, Divisova L, et al. NeemAzal T/S - toxicity to early-life stages of common carp (Cyprinus carpio L.). Vet Med. 2015;60:(1):23-30. doi:10.17221/7922-VETMED.
https://doi.org/10.17221/7922-VETMED...
]. Although there are studies on the GST activity of pesticides in the literature [3333 Ari F, Dere E. Glutathione S-transferase activity in rats exposed to methyl parathion. Chem Ecol. 2008;24(3):213-9. doi:10.1080/02757540802119889.
https://doi.org/10.1080/0275754080211988...
34 Ari F, Dere E, Tosunoglu H, Alioglu I. The effects of fenarimol and methyl parathion on glucose 6-phosphate dehydrogenase enzyme activity in rats. Turk J Agric Res. 2017;4(3):275-80. doi:10.19159/tutad.310371.
https://doi.org/10.19159/tutad.310371...
35 Pavlidi N, Vontas J, Van Leeuwen T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Cur Opin Insect Sci. 2018;27:97-102. doi:10.1016/j.cois.2018.04.007.
https://doi.org/10.1016/j.cois.2018.04.0...
-3636 Wang H, Zhang X, Wang L, Zhu B, Guo W, Liu W, et al. Biochemical responses and DNA damage induced by herbicide QYR301 in earthworm (Eisenia fetida). Chemosphere. 2020;244:125512. doi:10.1016/j.chemosphere.2019.125512.
https://doi.org/10.1016/j.chemosphere.20...
], there is no study on the GST activity of fenarimol in rat tissues. Therefore, it is important to know the effect of fenarimol on rats. For example, in a study [3737 Güller P, Akkemik E, Kör S, Çitçi M. Investigation of Some Pesticides' Effects on Activities of Glutathione Reductase and Glutathione S-Transferase Purified from Turkey Liver under in Vitro Conditions. J Inst Sci Technol. 2018;8(3):211-7. doi:10.21597/jist.458632.
https://doi.org/10.21597/jist.458632...
] when they investigated the effects of lambda sihalotrin, cypermethrin, chlorpyrifos, dichlorvos, glyphosate isopropylamine pesticides on GST enzyme activity purified from turkey liver, found that these pesticides inhibit the GST enzyme in vitro. Therefore, they suggested that pesticides may pose a risk for living.
As a result of our studies, it was observed that GST activity in male and female rats treated with fenarimol increased significantly in all experimental hours (p <001). Since GST plays a role in the detoxification mechanism, an increase in its activity may have been observed to get rid of the toxic effect of fenarimol. When the results are evaluated, it is seen that GST activities in the liver and kidney are higher than other tissues (Figure 1 and Figure 2). The liver acts as the main organ in metabolism, and xenobiotic, antioxidant, various toxic substances are excreted through the kidneys after they are detoxified. Therefore, it can be concluded that GST activity is higher in these tissues after fenarimol toxicity. In a previous study of our group, after applying Dichlorvos, a different type of pesticide, it was observed that GST activity increased in all tissues investigated, but the highest effect was in the liver [3838 Ozdikicioglu F, Dere E, Tosunoglu H. The Effect of Dichlorvos on Glutathione S-Transferase Activity in Some Tissues of Rats. J Appl Biol Sci. 2008;2(1):35-8.]. A study with epoxyconazole, a triazole group fungicide, showed that concentrations of 8, 24, 40 mg/kg bw increase GST activation in liver and kidney tissues after 28 days of application to Wistar rats [3939 Hamdi H, Othmène YB, Ammar O, Klifi A, Hallara E, Ghali FB, et al. Oxidative stress, genotoxicity, biochemical and histopathological modifications induced by epoxiconazole in liver and kidney of Wistar rats. Environ Sci Pollut Res. 2019;26(17):17535-47. doi:10.1007/s11356-019-05022-3.
https://doi.org/10.1007/s11356-019-05022...
]. Carbofuran, a pesticide commonly used in [4040 Kaur M, Sandhir R. Comparative effects of acute and chronic carbofuran exposure on oxidative stress and drug-metabolizing enzymes in liver. Drug Chem Toxicol. 2006;29(4):415-21. doi:10.1080/01480540600837969.
https://doi.org/10.1080/0148054060083796...
] studies, has been shown to increase the GST enzyme activity by inducing the cytochrome P450 enzyme system in the rat liver. In another study, when the effect of in vitro methyl parathion on GST activity was examined, it was seen that GST activity was highest in the hepatic fractions of rats, mice and 10 adult people [4141 Bammler TK, Abel E, Eaton DL. Metabolism of methyl parathion by glutathione S-transferases in vitro. Chem Biol Interact. 2001;133(1):231-3.].
According to our results, it is seen that there is a significant decrease in GST activity in the brain tissue of both male and female rats (Figure 3). Accordingly, although the decrease in GST activity in female rats is significant at 32 and 64 h, male rats show this result in all experimental periods. This inhibition in GST enzyme activity may have occurred due to estrogen [4242 Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, Philips BJ, Hannink M, et al. In Vitro and in Vivo Regulation of Antioxidant Response Element-Dependent Gene Expression by Estrogens. Endocrinol. 2004;145(1):311-7. doi:10.1210/en.2003-0817.
https://doi.org/10.1210/en.2003-0817...
,4343 Fucic A, Gamulin M, Ferencic Z, Katic J, Krayer Von Krauss M, Bartonova A, et al. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ Heal A Glob Access Sci Source. 2012;11(1):1-9. doi:10.1186/1476-069X-11-S1-S8.
https://doi.org/10.1186/1476-069X-11-S1-...
]. Therefore, fenarimol, also known as an aromatase (estrogen synthase) inhibitor, can be interpreted as the cause of this decrease in the GST enzyme [1919 Andersen HR, Bonefeld-Jørgensen EC, Nielsen F, Jarfeldt K, Jayatissa MN, Vinggaard AM. Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett. 2006;163(2):142-52. doi:10.1016/j.toxlet.2005.10.004.
https://doi.org/10.1016/j.toxlet.2005.10...
,4444 Hirsch KS, Jones CD, Lindstrom TD, Stamm NB, Sutton GP, Taylor HM, et al. Discovery and development of a novel class of nonsteroidal aromatase inhibitors. Steroids. 1987;50(1-3):201-17. doi:10.1016/0039-128X(83)90072-7.
https://doi.org/10.1016/0039-128X(83)900...
45 Vinggaard AM, Breinholt V, Larsen JC. Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit Contam. 1999;16(12):533-42. doi:10.1080/026520399283678.
https://doi.org/10.1080/026520399283678...
46 Hinfray N, Porcher JM, Brion F. Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem Physiol C: Toxicol Pharmacol. 2006;144(3):252-62. doi:10.1016/j.cbpc.2006.09.002.
https://doi.org/10.1016/j.cbpc.2006.09.0...
-4747 Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, et al. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicol. 2006;228(1):98-108. doi:10.1016/j.tox.2006.08.021.
https://doi.org/10.1016/j.tox.2006.08.02...
]. This difference between male and female rats may be due to sex hormones affected by fenarimol. For example, chlorpyrifos, an organophosphate pesticide, is known to cause a change in hormonal balance, depending on gender [4848 Ventura C, Nieto M, Bourguignon N, Lux-Lantos V, Rodriguez H, Cao G, et al. Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J Steroid Biochem Mol Biol. 2016;156:1-9. doi:10.1016/j.jsbmb.2015.10.010.
https://doi.org/10.1016/j.jsbmb.2015.10....
]. In addition, atrazine has been observed to be an endocrine disrupter and inhibit testosterone production in male rats [4949 Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16(3):275-9. doi:10.1016/S0890-6238(02)00019-9.
https://doi.org/10.1016/S0890-6238(02)00...
]. In another study, propiconazole, a different type of fungicide, has been found to be effective on endocrine distribution and reproductive parameters in male rats [5050 Costa NO, Vieira ML, Sgarioni V, Pereira MRF, Montagnini BG, Mesquita SFP, et al. Evaluation of the reproductive toxicity of fungicide propiconazole in male rats. Toxicol. 2015;335:55-61. doi:10.1016/j.tox.2015.06.011.
https://doi.org/10.1016/j.tox.2015.06.01...
].
In conclusion, based on the experimental evidence obtained, we can suggest that GST can be useful in monitoring the effects of pesticide exposure on wildlife. However, more studies are needed to comprehensively examine the effects of fenarimol, which has been observed to cause a change in GST activity. In addition, fenarimol, which is widely used in agriculture, directly or indirectly affects the environment and living beings. Because this is critical to public health, caution should be exercised in the use of chemicals and consumers and/or manufacturers should be informed about potential hazards.
Acknowledgments
We thank Hakan Tosunoğlu for their technical assistance.
REFERENCES
-
1Bolognesi C. Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res. 2003;543(3):251-72. doi:10.1016/S1383-5742(03)00015-2.
» https://doi.org/10.1016/S1383-5742(03)00015-2 -
2Faria M, Prats E, Rosas Ramírez JR, Bellot M, Bedrossiantz J, Pagano M, et al. Androgenic activation, impairment of the monoaminergic system and altered behavior in zebrafish larvae exposed to environmental concentrations of fenitrothion. Sci Total Environ. 2021;775:145671. doi:10.1016/j.scitotenv.2021.145671.
» https://doi.org/10.1016/j.scitotenv.2021.145671 -
3Fiorino E, Sehonova P, Plhalova L, Blahova J, Svobodova Z, Faggio C, et al. Effect of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res Int. 2018;25(9):8542-9. doi:10.1007/s11356-017-1141-5.
» https://doi.org/10.1007/s11356-017-1141-5 -
4Blahova J, Cocilovo C, Plhalova L, Svobodova Z, Faggio C. Embryotoxicity of atrazine and its degradation products to early life stages of zebrafish (Danio rerio). Environ Toxicol Pharmacol. 2020;77:103370. doi:10.1016/j.etap.2020.103370.
» https://doi.org/10.1016/j.etap.2020.103370 -
5Stara A, Kubec J, Zuskova E, Buric M, Faggio C, Kouba A, et al. Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procambarus virginalis). Chemospere. 2019;224:616-25. doi:10.1016/j.chemosphere.2019.02.187.
» https://doi.org/10.1016/j.chemosphere.2019.02.187 -
6Stara A, Bellinvia R, Velisek J, Strouhova A, Kouba A, Faggio C, et al. Acute exposure of common yabby (Cherax destructor) to the neonicotinoid pesticide. Sci Total Environ. 2019;665:718-23. doi:10.1016/j.scitotenv.2019.02.202.
» https://doi.org/10.1016/j.scitotenv.2019.02.202 -
7Pagano M, Stara A, Aliko V, Faggio C. Impact of Neonicotinoids to Aquatic Invertebrates-In Vitro Studies on Mytilus galloprovincialis: A Review. J Mar Sci Eng. 2020;8(10):801. doi:10.3390/jmse8100801.
» https://doi.org/10.3390/jmse8100801 -
8Stara A, Pagano M, Capillo G, Fabrello J, Sandova M, Albano M, et al. Acute effects of neonicotinoid insecticides on Mytilus galloprovincialis: a case study with the active compound thiacloprid and the commercial formulation Calypso 480 SC. Ecotoxicol Environ Saf. 2020;203(7):110980. doi:10.1016/j.ecoenv.2020.110980.
» https://doi.org/10.1016/j.ecoenv.2020.110980 -
9Stara A, Pagano M, Capillo G, Fabrello J, Sandova M, Vazzana I, et al. Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci Total Environ. 2020;700:134914. doi:10.1016/j.scitotenv.2019.134914.
» https://doi.org/10.1016/j.scitotenv.2019.134914 -
10Sula E, Aliko V, Barceló D, Faggio C. Combined effects of moderate hypoxia, pesticide and PCBs upon Crucian Carp fish, Carassius carassius, from a freshwater lake-in situ ecophysiological approach. Aquat Toxicol. 2020;228:105644. doi: 10.1016/j.aquatox.2020.105644.
» https://doi.org/10.1016/j.aquatox.2020.105644 -
11Horrigan L, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect. 2002;110(5):445-56. doi: 10.1289/ehp.02110445.
» https://doi.org/10.1289/ehp.02110445 -
12Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628-33. doi:10.1002/ps.1406.
» https://doi.org/10.1002/ps.1406 -
13Bronstein AC, Spyker DA, Cantilena LR, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila). 2012;50(10):911-1164. doi:10.3109/15563650.2012.746424.
» https://doi.org/10.3109/15563650.2012.746424 -
14Balcı N, Türkan F, Şakiroğlu H, Aygün A, Şen F. Purification and characterization of glutathione S-transferase from blueberry fruits (Vaccinium arctostaphylos L.) and investigated of some pesticide inhibition effects on enzyme activity. Heliyon. 2019;5(4),e01422. doi:10.1016/j.heliyon.2019.e01422.
» https://doi.org/10.1016/j.heliyon.2019.e01422 -
15Kurutas EB, Kilinç M. Effects of Pesticides on Biological Systems. Arch Med Rev J. 2003;12(3):215-28.
-
16Zhang H, Wang X, Zhuang S, Qian M, Jiang K, Wang X, et al. Enantioselective separation and simultaneous determination of fenarimol and nuarimol in fruits, vegetables, and soil by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2012;404(6-7):1983-91. doi:10.1007/s00216-012-6325-8.
» https://doi.org/10.1007/s00216-012-6325-8 -
17Oh K, Matsumoto T, Yamagami A, Hoshi T, Nakano T, Yoshizawa Y. Fenarimol, a pyrimidine-type fungicide, inhibits brassinosteroid biosynthesis. Int J Mol Sci. 2015;16(8):17273-88. doi:10.3390/ijms160817273.
» https://doi.org/10.3390/ijms160817273 -
18Bolognesi C, Merlo FD. Pesticides: Human Health Effects. In: Encyclopedia of Environmental Health. Elsevier, 2019.118-32.
-
19Andersen HR, Bonefeld-Jørgensen EC, Nielsen F, Jarfeldt K, Jayatissa MN, Vinggaard AM. Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett. 2006;163(2):142-52. doi:10.1016/j.toxlet.2005.10.004.
» https://doi.org/10.1016/j.toxlet.2005.10.004 -
20de Castro VLSS, de Mello MA, Diniz C, Morita L, Zucchi T, Poli P. Neurodevelopmental effects of perinatal fenarimol exposure on rats. Reprod Toxicol. 2007;23(1):98-105. doi:10.1016/j.reprotox.2006.09.001.
» https://doi.org/10.1016/j.reprotox.2006.09.001 -
21Ari F, Dere E. Effect of the sterol demethylation-inhibiting fungicide fenarimol on selected biochemical parameters in rats. Acta Vet Beo. 2010;60(1):31-8. doi:10.2298/AVB1001031A.
» https://doi.org/10.2298/AVB1001031A -
22Espinoza HM, Williams CR, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110-111:37-44. doi:10.1016/j.aquatox.2011.12.012.
» https://doi.org/10.1016/j.aquatox.2011.12.012 -
23Ozaslan MS, Demir Y, Küfrevioglu OI, Çiftci M. Some metals inhibit the glutathione S-transferase from Van Lake fish gills. J Biochem Mol Toxicol. 2017;31(11):e21967. doi:10.1002/jbt.21967.
» https://doi.org/10.1002/jbt.21967 -
24Awasthi YC, Dao DD, Saneto RP. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980;191(1):1-10. doi:10.1042/bj1910001.
» https://doi.org/10.1042/bj1910001 -
25Hayes JD, Flanagan JU, Jowsey IR. Glutathione Transferases. Annu Rev Pharmacol Toxicol. 2005;45(1):51-88. doi:10.1146/annurev.pharmtox.45.120403.095857.
» https://doi.org/10.1146/annurev.pharmtox.45.120403.095857 -
26Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54. doi:10.1006/abio.1976.9999.
» https://doi.org/10.1006/abio.1976.9999 -
27Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-9.
-
28Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. Pesticide toxicity: A mechanistic approach. Exp Clin Sci J. 2018;17:1101-36. doi:10.17179/excli2018-1710.
» https://doi.org/10.17179/excli2018-1710 -
29Gharaei A, Karimi M, Harijani JM, Miri M, Faggio C. Population growth of Brachionus calyciflorus affected by deltamethrin and imidacloprid insecticides. Iran J Fish Sci. 2020;19(2):588-6.
-
30Petrovici A, Strungaru SA, Nicoara M, Robea MA, Solcan C, Faggio C. Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. J Mar Sci Eng. 2020;8(2):73. doi:10.3390/jmse8020073.
» https://doi.org/10.3390/jmse8020073 -
31Plhalova L, Blahova J, Divisova L, Enevova V, Casuscelli Di Tocco F, Faggio C, et al. The effects of subchronic exposure to NeemAzal T/S on zebrafish (Danio rerio). Chem Ecol. 2018;34(3):199-210. doi:10.1080/02757540.2017.1420176.
» https://doi.org/10.1080/02757540.2017.1420176 -
32Chromcova L, Blahova J, Zivna D, Plhalova L, Casuscelli Di Tocco F, Divisova L, et al. NeemAzal T/S - toxicity to early-life stages of common carp (Cyprinus carpio L.). Vet Med. 2015;60:(1):23-30. doi:10.17221/7922-VETMED.
» https://doi.org/10.17221/7922-VETMED -
33Ari F, Dere E. Glutathione S-transferase activity in rats exposed to methyl parathion. Chem Ecol. 2008;24(3):213-9. doi:10.1080/02757540802119889.
» https://doi.org/10.1080/02757540802119889 -
34Ari F, Dere E, Tosunoglu H, Alioglu I. The effects of fenarimol and methyl parathion on glucose 6-phosphate dehydrogenase enzyme activity in rats. Turk J Agric Res. 2017;4(3):275-80. doi:10.19159/tutad.310371.
» https://doi.org/10.19159/tutad.310371 -
35Pavlidi N, Vontas J, Van Leeuwen T. The role of glutathione S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Cur Opin Insect Sci. 2018;27:97-102. doi:10.1016/j.cois.2018.04.007.
» https://doi.org/10.1016/j.cois.2018.04.007 -
36Wang H, Zhang X, Wang L, Zhu B, Guo W, Liu W, et al. Biochemical responses and DNA damage induced by herbicide QYR301 in earthworm (Eisenia fetida). Chemosphere. 2020;244:125512. doi:10.1016/j.chemosphere.2019.125512.
» https://doi.org/10.1016/j.chemosphere.2019.125512 -
37Güller P, Akkemik E, Kör S, Çitçi M. Investigation of Some Pesticides' Effects on Activities of Glutathione Reductase and Glutathione S-Transferase Purified from Turkey Liver under in Vitro Conditions. J Inst Sci Technol. 2018;8(3):211-7. doi:10.21597/jist.458632.
» https://doi.org/10.21597/jist.458632 -
38Ozdikicioglu F, Dere E, Tosunoglu H. The Effect of Dichlorvos on Glutathione S-Transferase Activity in Some Tissues of Rats. J Appl Biol Sci. 2008;2(1):35-8.
-
39Hamdi H, Othmène YB, Ammar O, Klifi A, Hallara E, Ghali FB, et al. Oxidative stress, genotoxicity, biochemical and histopathological modifications induced by epoxiconazole in liver and kidney of Wistar rats. Environ Sci Pollut Res. 2019;26(17):17535-47. doi:10.1007/s11356-019-05022-3.
» https://doi.org/10.1007/s11356-019-05022-3 -
40Kaur M, Sandhir R. Comparative effects of acute and chronic carbofuran exposure on oxidative stress and drug-metabolizing enzymes in liver. Drug Chem Toxicol. 2006;29(4):415-21. doi:10.1080/01480540600837969.
» https://doi.org/10.1080/01480540600837969 -
41Bammler TK, Abel E, Eaton DL. Metabolism of methyl parathion by glutathione S-transferases in vitro. Chem Biol Interact. 2001;133(1):231-3.
-
42Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, Philips BJ, Hannink M, et al. In Vitro and in Vivo Regulation of Antioxidant Response Element-Dependent Gene Expression by Estrogens. Endocrinol. 2004;145(1):311-7. doi:10.1210/en.2003-0817.
» https://doi.org/10.1210/en.2003-0817 -
43Fucic A, Gamulin M, Ferencic Z, Katic J, Krayer Von Krauss M, Bartonova A, et al. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ Heal A Glob Access Sci Source. 2012;11(1):1-9. doi:10.1186/1476-069X-11-S1-S8.
» https://doi.org/10.1186/1476-069X-11-S1-S8 -
44Hirsch KS, Jones CD, Lindstrom TD, Stamm NB, Sutton GP, Taylor HM, et al. Discovery and development of a novel class of nonsteroidal aromatase inhibitors. Steroids. 1987;50(1-3):201-17. doi:10.1016/0039-128X(83)90072-7.
» https://doi.org/10.1016/0039-128X(83)90072-7 -
45Vinggaard AM, Breinholt V, Larsen JC. Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit Contam. 1999;16(12):533-42. doi:10.1080/026520399283678.
» https://doi.org/10.1080/026520399283678 -
46Hinfray N, Porcher JM, Brion F. Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem Physiol C: Toxicol Pharmacol. 2006;144(3):252-62. doi:10.1016/j.cbpc.2006.09.002.
» https://doi.org/10.1016/j.cbpc.2006.09.002 -
47Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, et al. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicol. 2006;228(1):98-108. doi:10.1016/j.tox.2006.08.021.
» https://doi.org/10.1016/j.tox.2006.08.021 -
48Ventura C, Nieto M, Bourguignon N, Lux-Lantos V, Rodriguez H, Cao G, et al. Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. J Steroid Biochem Mol Biol. 2016;156:1-9. doi:10.1016/j.jsbmb.2015.10.010.
» https://doi.org/10.1016/j.jsbmb.2015.10.010 -
49Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16(3):275-9. doi:10.1016/S0890-6238(02)00019-9.
» https://doi.org/10.1016/S0890-6238(02)00019-9 -
50Costa NO, Vieira ML, Sgarioni V, Pereira MRF, Montagnini BG, Mesquita SFP, et al. Evaluation of the reproductive toxicity of fungicide propiconazole in male rats. Toxicol. 2015;335:55-61. doi:10.1016/j.tox.2015.06.011.
» https://doi.org/10.1016/j.tox.2015.06.011
-
Funding:
This research was funded by Bursa Uludag University, grant number F(U)-2003/65.
Edited by
Editor-in-Chief:
Associate Editor:
Publication Dates
-
Publication in this collection
05 July 2021 -
Date of issue
2021
History
-
Received
28 Nov 2020 -
Accepted
22 Apr 2021