Abstract:
Aim
In this study, we examined the effects of non-native leaf litter on the functioning of an Atlantic Forest stream ecosystem.
Methods
Were tested two predictions: (i) Leaf litter from the native trees with high nutritional quality will have higher decomposer’s activity and faster litter decomposition; (ii) Given the presence of anti-grazing defenses, we also hypothesized that non-native leaf litter would be colonized by fewer invertebrates and that native species would be more species-rich. For this, in a forest stream (Florianópolis, SC, Brazil) we conduct the experiment to understand the decomposition and biological colonization of leaf litter among two non-native (Eucalyptus sp. and Pinus radiata D. Don) and two native trees (Ficus eximia Schott and Alchornea triplinervia (Spreng) Mull. Arg).
Results
Our predictions were partially corroborated. The percentage of dry mass remaining was lower for the native leaf litter. The invertebrate abundance and richness, and functional feeding groups vary between native and non-native leaf litter. Invertebrate abundance was higher in non-native Eucalyptus detritus, largely due to the high larval abundance of Chironomidae (Diptera).
Conclusions
Our results indicate that the presence of non-native riparian species can modify leaf decomposition and aquatic invertebrate communities in subtropical streams, with potential consequences for ecosystem functioning.
Keywords:
aquatic assemblages; detritus; exotic species; leaf breakdown; lotic ecosystem
Resumo:
Objetivo
Neste estudo, analisamos os efeitos da presença de detritos foliares de espécies não-nativas no funcionamento de um ecossistema de riacho presente na Mata Atlântica.
Métodos
Testamos duas predições (i) os detritos foliares de espécies nativas, provavelmente com maior qualidade nutricional, irão decompor de forma mais rápida se comparado aos detritos foliares das espécies não nativas (ii) devido a presença de defesas químicas nos detritos foliares de espécies vegetais não-nativas, estes serão menos colonizados e apresentarão uma menor riqueza taxonômica em comparação com os detritos foliares de espécies nativas. Para isso, em um riacho inserido em uma área de Mata Atlântica (Florianópolis, SC, Brasil) conduzimos um experimento para comparar a decomposição e colonização biológica de detritos foliares entre duas espécies vegetais não nativas (Eucalyptus sp. e Pinus radiata D. Don) e duas espécies vegetais nativas (Ficus eximia Schott e Alchornea triplinervia (Spreng) Mull. Arg).
Resultados
Especificamente, as nossas hipóteses foram parcialmente corroboradas. A porcentagem de massa seca remanescente foi menor para a serapilheira nativa. A abundância e riqueza de invertebrados e grupos funcionais de alimentação variam entre serrapilheira nativa e não nativa. A abundância de invertebrados foi maior em detritos de Eucalyptus sp, em grande parte devido à grande abundância de Chironomidae (Dípteros).
Conclusões
Os nossos resultados indicam que, a presença de espécies vegetais não nativas na zona ripária podem modificar a decomposição foliar e as comunidades de invertebrados aquáticos em riachos subtropicais, com potenciais consequências para o funcionamento do ecossistema.
Palavras-chave:
assembleias aquáticas; detritos foliares; espécies exóticas; decomposição foliar; ecossistemas lótico
1. Introduction
Riparian vegetation is an important part of riverine ecosystems and are essential for the structure and functioning of these ecosystems (Dufour et al., 2019Dufour, S., Rodríguez-González, P.M., & Laslier, M., 2019. Tracing the scientific trajectory of riparian vegetation studies: main topics, approaches and needs in a globally changing world. Sci. Total Environ. 653, 1168-1185. PMid:30759557. http://dx.doi.org/10.1016/j.scitotenv.2018.10.383.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Zelnik et al., 2020Zelnik, I., Mavrič Klenovšek, V., & Gaberščik, A., 2020. Complex undisturbed riparian zones are resistant to colonisation by invasive alien plant species. Water 12(2), 345. http://dx.doi.org/10.3390/w12020345.
http://dx.doi.org/10.3390/w12020345...
). However, despite the variety of ecosystem services provided by riparian vegetation, many riparian ecosystems have experienced widespread degradation, and the presence of non-native tree species is becoming a common worldwide concern (Dudgeon et al., 2006Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z., Knowler, D.J., Lévêque, C., Naiman, R.J., Prieur-Richard, A.H., Soto, D., Stiassny, M.L., & Sullivan, C.A., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 81(2), 163-182. PMid:16336747. http://dx.doi.org/10.1017/S1464793105006950.
http://dx.doi.org/10.1017/S1464793105006...
; Richardson et al., 2007Richardson, D.M., Holmes, P.M., Esler, K.J., Galatowitsch, S.M., Stromberg, J.C., Kirkman, S.P., Pyšek, P., & Hobbs, R.J., 2007. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 13(1), 126-139. http://dx.doi.org/10.1111/j.1366-9516.2006.00314.x.
http://dx.doi.org/10.1111/j.1366-9516.20...
; Castro-Díez & Alonso, 2017Castro-Díez, P., & Alonso, A., 2017. Effects of non-native riparian plants in riparian and fluvial ecosystems: a review for the Iberian Peninsula. Limnetica 36(2), 525-541. https://doi.org/10.23818/limn.36.19.
https://doi.org/10.23818/limn.36.19...
). The distribution of non-native tree species are expected to continue increasing in extent, with their spread facilitated by agriculture, urbanization and climatic changes, including increasing temperatures and altered precipitation patterns (Castro-Díez et al., 2019Castro-Díez, P., Vaz, A.S., Silva, J.S., van Loo, M., Alonso, Á., Aponte, C., Bayón, Á., Bellingham, P.J., Chiuffo, M.C., DiManno, N., Julian, K., Kandert, S., La Porta, N., Marchante, H., Maule, H.G., Mayfield, M.M., Metcalfe, D., Monteverdi, M.C., Núñez, M.A., Ostertag, R., Parker, I.M., Peltzer, D.A., Potgieter, L.J., Raymundo, M., Rayome, D., Reisman-Berman, O., Richardson, D.M., Roos, R.E., Saldaña, A., Shackleton, R.T., Torres, A., Trudgen, M., Urban, J., Vicente, J.R., Vilà, M., Ylioja, T., Zenni, R.D., & Godoy, O., 2019. Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. Camb. Philos. Soc. 94(4), 1477-1501. PMid:30974048. http://dx.doi.org/10.1111/brv.12511.
http://dx.doi.org/10.1111/brv.12511...
). The presence of non-native tree species can cause severe ecological, social and economic losses, and they are considered as one of the leading direct causes of biodiversity loss (Ferreira et al., 2016Ferreira, V., Koricheva, J., Pozo, J., & Graça, M.A.S., 2016. A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For. Ecol. Manage. 364, 27-38. http://dx.doi.org/10.1016/j.foreco.2016.01.002.
http://dx.doi.org/10.1016/j.foreco.2016....
; Cordero-Rivera et al., 2017Cordero–Rivera, A., Martínez-Álvarez, A., & Álvarez, M., 2017. Eucalypt plantations reduce the diversity of macroinvertebrates in small-forested streams. Anim Biodivers Conserv. 40(1), 87-97. https://doi.org/10.32800/abc.2017.40.0087.
https://doi.org/10.32800/abc.2017.40.008...
). On islands where space is a limiting factor, the presence of non-native trees may reduce the diversity of natural ecosystems by displacing species and displaying a high local dominance (Tershy et al., 2015Tershy, B.R., Shen, K.W., Newton, K.M., Holmes, N.D., & Croll, D.A., 2015. The importance of islands for the protection of biological and linguistic diversity. Bioscience 65(6), 592-597. http://dx.doi.org/10.1093/biosci/biv031.
http://dx.doi.org/10.1093/biosci/biv031...
). In the simplified ecosystems of islands, the native species are more vulnerable than the continental species, because they have smaller population sizes and less genetic diversity (Tershy et al., 2015Tershy, B.R., Shen, K.W., Newton, K.M., Holmes, N.D., & Croll, D.A., 2015. The importance of islands for the protection of biological and linguistic diversity. Bioscience 65(6), 592-597. http://dx.doi.org/10.1093/biosci/biv031.
http://dx.doi.org/10.1093/biosci/biv031...
).
Allochthonous litterfall supplied by riparian vegetation, which mainly comprises leaves, represents an important energy source for aquatic communities in shaded headwater streams (Webster & Benfield, 1986Webster, J.R., & Benfield, E.F., 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Evol. Syst. 17(1), 567-594. http://dx.doi.org/10.1146/annurev.es.17.110186.003031.
http://dx.doi.org/10.1146/annurev.es.17....
; Abelho, 2001Abelho, M., 2001. From litterfall to breakdown in streams: a review. Sci. World J. 1, 656-680. PMid:12805769. http://dx.doi.org/10.1100/tsw.2001.103.
http://dx.doi.org/10.1100/tsw.2001.103...
). Allochthonous litterfall can be retained on the streambed and used as food and habitat resource by aquatic microorganisms and invertebrates (Mathuriau et al., 2008Mathuriau, C., Thomas, A.G.B., & Chauvet, E., 2008. Seasonal dynamics of benthic detritus and associated macroinvertebrate communities in a neotropical stream. Fundam. Appl. Limnol. 171(4), 323-333. http://dx.doi.org/10.1127/1863-9135/2008/0171-0323.
http://dx.doi.org/10.1127/1863-9135/2008...
). Litterfall are decomposed through a combination of chemical, physical and biological in-stream processes that result in its fragmentation, transformation and incorporation into stream food webs in which both microorganisms (especially aquatic hyphomycetes) and detritivorous shredders have key roles (Hieber & Gessner, 2002Hieber, M., & Gessner, M.O., 2002. Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83(4), 1026-1038. http://dx.doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2.
http://dx.doi.org/10.1890/0012-9658(2002...
; Graça et al., 2015Graça, M.A.S., Ferreira, V., Canhoto, C., Encalada, A.C., Guerrero-Bolaño, F., Wantzen, K.M., & Boyero, L., 2015. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 100(1), 1-12. http://dx.doi.org/10.1002/iroh.201401757.
http://dx.doi.org/10.1002/iroh.201401757...
). Compared to native leaf litter, the non-native leaf litter, in some case, can show lower nitrogen and phosphorous concentrations and higher quantities of lignin, oils, tannins and other recalcitrant compounds, thus affecting the availability of palatable organic matter for in-stream decomposers (Baker et al., 2007Baker, A.C., Murray, B.R., & Hose, G.C., 2007. Relating pine-litter intrusion to plant-community structure in native eucalypt woodland adjacent to Pinus radiata (Pinaceae) plantations. Aust. J. Bot. 55(5), 521-532. http://dx.doi.org/10.1071/BT06135.
http://dx.doi.org/10.1071/BT06135...
; Cordero-Rivera et al., 2017Cordero–Rivera, A., Martínez-Álvarez, A., & Álvarez, M., 2017. Eucalypt plantations reduce the diversity of macroinvertebrates in small-forested streams. Anim Biodivers Conserv. 40(1), 87-97. https://doi.org/10.32800/abc.2017.40.0087.
https://doi.org/10.32800/abc.2017.40.008...
; Pereira & Ferreira, 2021Pereira, A., & Ferreira, V., 2021. Invasion of native riparian forests by acacia species affects in-stream litter decomposition and associated microbial decomposers. Microb Ecol 81(1), 14-25. PMid:32623497. https://doi.org/10.1007/s00248-020-01552-3.
https://doi.org/10.1007/s00248-020-01552...
). Aquatic invertebrates generally colonize soft leaf litter with high nutrient concentration and low structural and secondary compounds and decompose it faster than more recalcitrant litter (Canhoto & Graça, 1995Canhoto, C., & Graça, M.A.S., 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: tipula lateralis. Freshw. Biol. 34(2), 209-214. http://dx.doi.org/10.1111/j.1365-2427.1995.tb00881.x.
http://dx.doi.org/10.1111/j.1365-2427.19...
; Balibrea et al., 2017Balibrea, A., Ferreira, V., Gonçalves, V., & Raposeiro, P.M., 2017. Consumption, growth and survival of the endemic stream shredder Limnephilus atlanticus (Trichoptera, Limnephilidae) fed with distinct leaf species. Limnologica. 64, 31-37. https://doi.org/10.1016/j.limno.2017.04.002.
https://doi.org/10.1016/j.limno.2017.04....
). Aquatic invertebrates are sensitive to leaf litter quality, and they tends to be more diverse when riparian vegetation and litter inputs are high (Ferreira et al., 2015Ferreira, V., Larrañaga, A., Gulis, V., Basaguren, A., Elosegi, A., Graça, M.A.S., & Pozo, J., 2015. The effects of eucalypt plantations on plant litter decomposition and macroinvertebrate communities in Iberian streams. For Ecol Manag. 335, 129-38. http://dx.doi.org/10.1016/j.foreco.2014.09.013.
http://dx.doi.org/10.1016/j.foreco.2014....
).
Eucalyptus (Myrtaceae, a tree species originating especially from Australia) and Pinus (Pinaceae, a conifer species originating especially from Central America and North America) species have been introduced into several regions of the world (Valduga et al., 2016Valduga, M.O., Zenni, R.D., & Vitule, J.R.S., 2016. Ecological impacts of non-native tree species plantations are broad and heterogeneous: a review of Brazilian research. An. Acad. Bras. Cienc. 88(3, Suppl.), 1675-1688. PMid:27737335. http://dx.doi.org/10.1590/0001-3765201620150575.
http://dx.doi.org/10.1590/0001-376520162...
), mainly for use in the paper industry (Canhoto et al., 2004Canhoto, C., Abelho, M., & Graça, M.A.S., 2004. Efeitos das plantações de Eucalyptus globulus nos ribeiros de Portugal. Recur. Hidricos 25, 59-65.). Specific traits, such as having harder leaves, being a pyrophytic species and having a high capacity to adapt to different environmental conditions are responsible for their high invasiveness. In a meta-analysis, Ferreira et al. (2016)Ferreira, V., Koricheva, J., Pozo, J., & Graça, M.A.S., 2016. A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For. Ecol. Manage. 364, 27-38. http://dx.doi.org/10.1016/j.foreco.2016.01.002.
http://dx.doi.org/10.1016/j.foreco.2016....
suggested that Eucalyptus plantations can have stronger negative effects on leaf litter decomposition rates in streams naturally receiving high-quality litter inputs and where detritivore abundances are high. In the present study, we conducted an in situ experiment to (1) quantify differences in decomposition of leaf litter of two non-native and two native tree species, (2) determine how aquatic invertebrate communities respond to the presence of non-native leaf litter in a third-order subtropical island stream. We compared leaf-associated invertebrate colonization and leaf decomposition rates using leaf litter from two native tree species (Ficus eximia Schott and Alchornea triplinervia (Spreng) Mull. Arg) and two non-native tree species (Eucalyptus sp. and Pinus radiata D. Don) placed in a third-order subtropical island stream. Based on the previous research and differences in the leaf chemical among the native and non-native species we hypothesized that (i) leaf litter from the native species, probably with a high nutritional quality (e.g., soft and with high initial nitrogen concentration), will have higher decomposer activity and faster litter decomposition and; (ii) due the presence of anti-grazing defenses (Graça et al., 2002Graça, M.A., Pozo, J., Canhoto, C., & Elosegi, A., 2002. Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. Sci. World J. 2, 1173-1185. PMID: 12805976. http://dx.doi.org/10.1100/tsw.2002.193.
http://dx.doi.org/10.1100/tsw.2002.193...
), the non-native leaf litter would be colonized by fewer invertebrates and that native species would be more species-rich compared to the colonization of leaf litters from the non-native species.
2. Material and Methods
2.1. Study area
The study was conducted in a stretch of a third-order small stream located within a legally protected area with predominant dense Atlantic Rainforest in Florianópolis, Santa Catarina State, Southern Brazil (27° 44’ S and 48° 31’ W). We conducted the experiment in a forested stream to simulate possible ecological implications for the input of non-native riparian plants on associated invertebrate fauna and leaf litter decomposition. This nearly pristine stream drains an area of 1.66 km2 with primary forest in the higher regions close to the sources of the stream, and it is covered by dense mixed Atlantic Rainforest in an advanced stage of regeneration. The dominant plant families are Myrtaceae, Fabaceae, Rutaceae, Lauraceae, Meliaceae, Apocynaceae and Palmae (Lisboa et al., 2015Lisboa, L.K., Lemes-Silva, A.L., Siegloch, A.E., Gonçalves Junior, J.F., & Petrucio, M.M., 2015. Temporal dynamics of allochthonous coarse particulate organic matter in a subtropical Atlantic rainforest Brazilian stream. Mar. Freshw. Res. 66(8), 674-680. http://dx.doi.org/10.1071/MF14068.
http://dx.doi.org/10.1071/MF14068...
). The stream flows year-round, but the discharge can greatly increase in summer (December to March) due to a higher volume of austral rainfall. The climate in the area is typically subtropical, with rainfall well distributed throughout the year; although it is more intense in the spring and summer months (October–March) (Hennemann & Petrucio, 2011Hennemann, M.C., & Petrucio, M.M., 2011. Spatial and temporal dynamic of trophic relevant parameters in a subtropical coastal lagoon in Brazil. Environ. Monit. Assess. 181(1-4), 347-361. PMid:21190080. http://dx.doi.org/10.1007/s10661-010-1833-5.
http://dx.doi.org/10.1007/s10661-010-183...
; Lemes-Silva et al., 2016Lemes-Silva, A.L., Pires, J., Pagliosa, P.R., & Petrucio, M.M., 2016. Distribution of aquatic macroinvertebrate assemblages in a subtropical coastal lake: response to environmental parameters. Fundam. Appl. Limnol. 188(2), 113-127. http://dx.doi.org/10.1127/fal/2016/0786.
http://dx.doi.org/10.1127/fal/2016/0786...
).
2.2. Environmental parameters
At the occasion of installation (incubation), the electric conductivity, water temperature (°C), pH and dissolved oxygen concentration were measured in situ with portable optical probes WTW 350ii. The water velocity was estimated by measuring the time that a plastic float needed to travel 1 m of the stream (Couceiro et al., 2007Couceiro, S.R.M., Hamada, N., Luz, S.L.B., Forsberg, B.R., & Pimentel, T.P., 2007. Deforestation and sewage effects on aquatic macroinvertebrates in urban streams in Manaus, Amazonas, Brazil. Hydrobiologia 575(1), 271-284. http://dx.doi.org/10.1007/s10750-006-0373-z.
http://dx.doi.org/10.1007/s10750-006-037...
). The stream had a water temperature of 23.0 ± 2.3°C (mean ± standard deviation) and, and the water velocity of 0.15 ± 0.08 m s–1. Stream waters had a pH neutral (pH = 7.0 ± 0.3), with higher levels of dissolved oxygen (8.0 ± 2.3 mg L-1) and electrical conductivity (55.0 ± 2.3 mS cm-1). The mean rainfall during the studied period (see below the description of the study period) was 177.6 ± 81.0 mm, with the highest precipitation in March (292.2 mm) and the lowest precipitation in April (116.2 mm).
2.3. Data collection
The leaf decomposition experiment was conducted from February to May 2016. We evaluated the leaf litter of two native (A. triplinervia and F. eximia) and two non-native trees (Eucalyptus sp. and P. radiata). The non-native trees species were selected among the dominant riparian species to represent differences in terms of leaf litter quality and because they are found year-round in the watershed studied. Alchornea triplinervia and F. eximia are native species and occur frequently in the riparian zone of the studied stream (Lisboa et al., 2015Lisboa, L.K., Lemes-Silva, A.L., Siegloch, A.E., Gonçalves Junior, J.F., & Petrucio, M.M., 2015. Temporal dynamics of allochthonous coarse particulate organic matter in a subtropical Atlantic rainforest Brazilian stream. Mar. Freshw. Res. 66(8), 674-680. http://dx.doi.org/10.1071/MF14068.
http://dx.doi.org/10.1071/MF14068...
). The senescent leaf litter from A. triplinervia and F. eximia were collected from the floor immediately after natural abscission in the forest. Eucalyptus sp. leaves were directly collected from trees of similar age and vigour, and the leaf litter from P. radiata was collected from the floor during the season of peak litter fall. After the collection, the litter of each species was taken to the laboratory, oven-dried at 60 °C for 72 h and weighed to obtain the wet weight (g WW).. We weighed 3.0 ± 0.1 g WW of each species and enclosed individually in single-species litterbags of 15 × 20 cm (10 mm mesh size). The litterbags were incubated in five stretches of the stream, in low flow current areas in five similar reaches of the stream. At each reach, five iron bars with 16 litterbags (4 replicates by species) were anchored to the streambed ~ 0.3 m in depth on the stream. Four replicate samples of each leaf species were randomly withdrawn after 1, 7, 30 and 45 and 60 days. We prepared 80 litterbags (5 chain/segment × 4 bags × 4 leaf litter species, Figure 1).
Scheme illustrating of litter decomposition experiment with leaf litter from two native and two non-native trees realized in third-order small stream, Florianópolis, Santa Catarina, Brazil. This illustration was created in BioRender.com.
The retrieved litterbags were enclosed in individual plastic bags and taken to the laboratory. The litter remaining in bags was gently rinsed with distilled water into a 125 µm mesh sieve to remove small litter fragments and to retain the associated invertebrate fauna (see below). The remaining litter was placed in aluminium trays, oven-dried at 70°C for 72 hours and the leaf material was weighed to obtain dry biomass (DM). From dry biomass, we calculated the percentage of dry mass remaining (% DMR) for each replicate. Following that, we used %DMR to calculate the decay constant (k d−1) by first taking the natural log of %DMR for each collection day using the negative exponential model, where the remaining mass was expressed in grams, and time was expressed in days considering the following equation: k = − (ln [final mass/initial mass]/time). This model (Webster & Benfield, 1986Webster, J.R., & Benfield, E.F., 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Evol. Syst. 17(1), 567-594. http://dx.doi.org/10.1146/annurev.es.17.110186.003031.
http://dx.doi.org/10.1146/annurev.es.17....
) assumes that there is a constant and fractionated loss of material at any given moment.
2.4. Associated invertebrates
After rinsing the leaf material over 125 µm sieves the invertebrate fauna retained on the sieves were preserved in 70% ethanol until they were later counted and identified to family level using identification keys (Cummins et al., 2005Cummins, K.W., Merritt, R.W., & Andrade, P.C.N., 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 40(1), 69-89. http://dx.doi.org/10.1080/01650520400025720.
http://dx.doi.org/10.1080/01650520400025...
; Mugnai et al., 2010Mugnai, R., Nessimian, J.L., & Baptista, D.F., 2010. Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro. Rio de Janeiro: Technical Books.) and organisms were assigned to functional feeding groups as either as gathering-collector (CG), collector-filterer (CF), shredder (SD), scraper (SC) and predator (PD) (Cummins et al., 2005Cummins, K.W., Merritt, R.W., & Andrade, P.C.N., 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 40(1), 69-89. http://dx.doi.org/10.1080/01650520400025720.
http://dx.doi.org/10.1080/01650520400025...
). Chironomidae were classified as generalists in the functional feeding group classification because of the large number of generalist-feeding chironomid (Lemes-Silva et al., 2014Lemes-Silva, A.L., Pagliosa, P.R., & Petrucio, M.M., 2014. Inter- and intra-guild patterns of food resource utilization by chironomid larvae in a subtropical coastal lagoon. Limnology 15(1), 1-12. http://dx.doi.org/10.1007/s10201-013-0407-y.
http://dx.doi.org/10.1007/s10201-013-040...
). We measured invertebrate diversity within our colonized litterbags using species richness as the number of taxa present in each litterbag.
2.5. Data analysis
We analyzed our response variable % DMR and invertebrates’ colonization as a function of type of litter, if native or not. To do so, we ran a generalized linear mixed models (GLMM) with a Poisson (to aquatic invertebrates) and Gamma (% DMR) distributions. Gamma distribution is often used for nonnegative continuous variables and the Poisson distribution is often used to describe the distribution of a count variable. In our analysis the stream stretch was used as a random effect to account for the spatial autocorrelation of sampling points within streams and litter types (native and non-native detritus) as a fixed effect. To control temporal autocorrelation due to the repeated measures design, the period of exposure (1, 7, 30, 45 and 60) was included as a random effect (intercept and slope), and the residual diagnostics plotted from the ‘simulateResiduals’ function in the DHARMa package (Hartig, 2020Hartig, F., 2020. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed). Regression Models. R package version 0.3.3.0. Retrieved in 2021, Nov 15, from http://florianhartig.github.io/DHARMa/
http://florianhartig.github.io/DHARMa/...
) were used to examine the assumptions of each GLMM model. We used the glmmTMB function in R to conduct Tukey’s post hoc tests on our models, elucidating pairwise differences in the aquatic invertebrates, functional feeding groups and % DMR between native and non-native species. All generalized models were built using the packages in R.
Indicator value analyses (IndVal) were performed to identify which taxa of invertebrates were most important to the invertebrate community structure during the process of the colonisation of the leaf litter for each leaf litter species (Dufrêne & Legendre, 1997Dufrêne, M., & Legendre, P., 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67(3), 345-366. http://dx.doi.org/10.2307/2963459.
http://dx.doi.org/10.2307/2963459...
). The frequency and relative abundance of each group in the different leaf litter species were used in the analysis. The IndVal values were tested for significant differences among the native and non-native invasive plants using a Monte Carlo procedure (10,000 permutations). We graphically verified the normality and homogeneity of variance assumptions. When data transformation was necessary to approach a normal distribution and homogeneity of variance, the nature of the transformation is specifically indicated. All statistical analyses were performed using the open-source software R 4.0.3 (R Development Core Team, 2020R Development Core Team, 2020. R: A language and environment for statistical computing [Software]. Vienna: R Foundation for Statistical Computing.).
3. Results
3.1. Decomposition
We found a significant effect of detritus type (if native or not) and time of incubation on %DMR (z value = –5.274; p = 0.001, Figure 2). However, no difference was observed when the time and type of detritus was considered together (p = 0.244). A fast mass loss was observed for A. triplinervia (native species, k = 0.0973 ± 0.1215 d−1) followed by F. eximia (native species, k = 0.0884 ± 0.0130 d−1), P. radiata (non-native, k = 0.0633 ± 0.0579 d−1) and Eucaliptus sp. (non-native, k =0.0616 ± 0.0591d−1) during the days of incubation (~70% mass loss). However, there were no differences observed between two native species and two non-native during the incubation (GLMM p > 0.05). At day 1, 7, 30 and 45, A. triplinervia and F. eximia detritus had lost 20 to 50% more biomass than Eucalyptus sp. and P. radiata and significant differences was observed on the %DMR between the leaf litter (GLMM p < 0.05). By day 60, no significant difference was observed on the %DMR among the species. Unfortunately, there were no data for P. radiata at 60 days because the detritus were lost during the experiment.
Median percentage of dry biomass (g dry weight) remaining (% DMR) per species of leaf litter for each collection day (1, 7, 30, 45, 60). Letters indicate statistical differences between treatment combinations (p ≤ 0.05), which were evaluated across treatments per collection day using generalized linear models. Box and whisker plots represent the median and 25 and 75% quartiles; the lower and upper bars illustrate minimum and maximum values. Closed circles represent outliers. Legend: AT = A. triplinervia; FE = F. eximia; EU= Eucalyptus sp.; PR = P. radiata.
3.2. Invertebrates associated
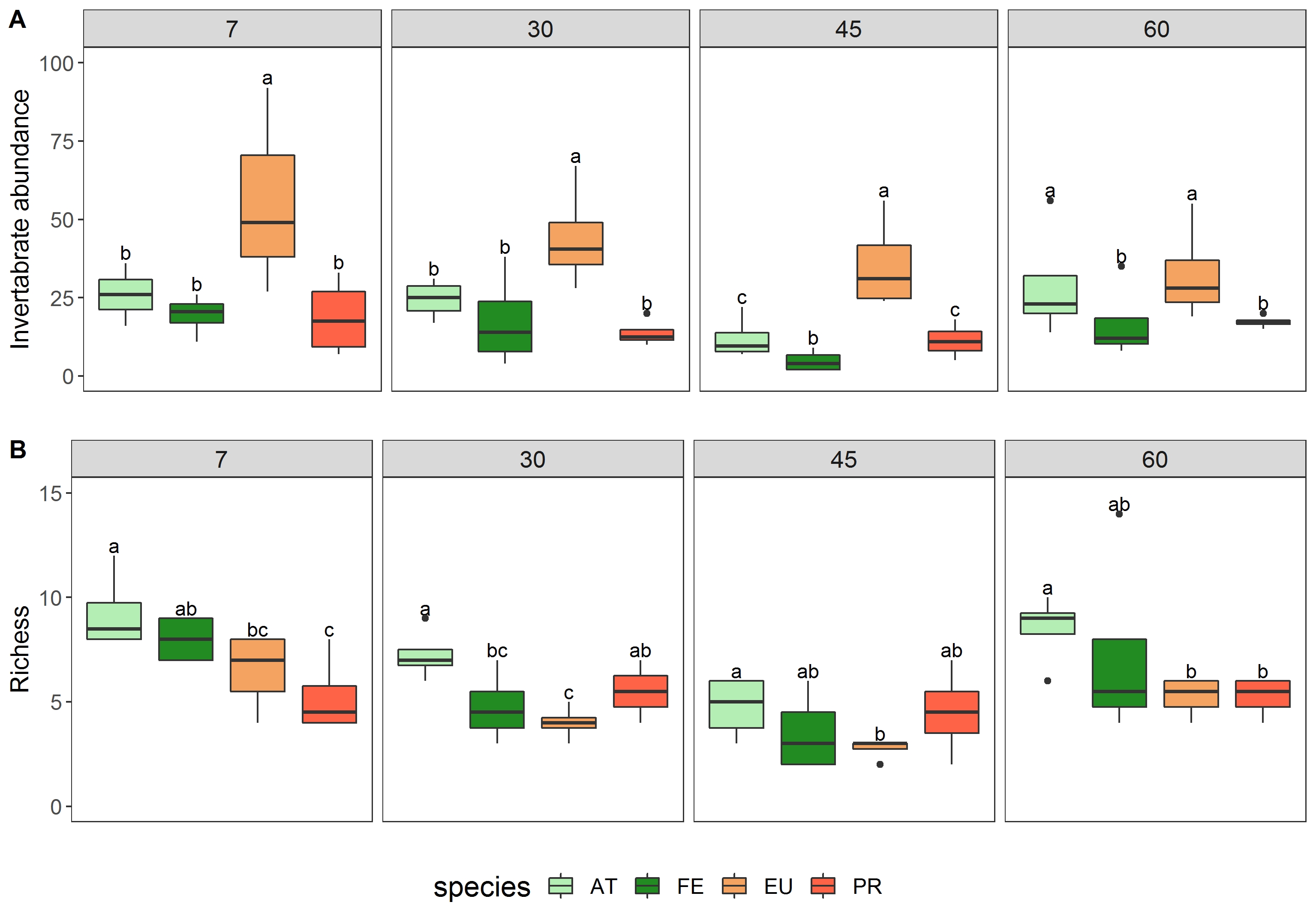
During the experiment, we recorded a total of 1, 561 invertebrates belonging to 18 families, with 600 invertebrates found in the native leaf litter (234 in F. eximia and 366 in A. triplinervia), and 961 invertebrates in the non-native leaf litter (721 in Eucalyptus sp. and 240 in P. radiata). Chironomidae (Diptera) and Leptophlebiidae (Ephemeroptera) were the predominant aquatic invertebrate taxa found on the leaf litter. The results of the indicator species analysis indicated Chironomidae (Indval = 50.38, p = 0.044) as the taxon associated with A. triplinervia and Leptophlebiidae (Indval = 45.07, p = 0.001) as the taxon exclusively associated with F. eximia. No indicator species were found on the Eucalyptus sp. and P. radiata leaf litters. The invertebrates abundance was different between the leaf litter from the native and non-native trees (F = 43.6, p = 0.0197, Figure 3A) and incubation time (p < 0.001). Total abundance was greater in Eucalyptus sp. on the day 7 and was significantly different between incubation periods, whereas the abundance on A. triplinervia, F. eximia and P. radiata were similar to each other. Taxon richness values were statistically different among litter types (GLMM, z value = 2. 955; p = 0.003) and between incubation periods (Figure 3B, p < 0.05). The leaf litter of A. triplinervia, F. eximia and P. radiata had a higher taxonomic richness of aquatic invertebrates than those of the Eucalyptus sp.
Median total invertebrate abundance (n.° ind./sample) and taxonomic richness (n.° ind./sample) per species and day collected in the subtropical streams in the Florianópolis Island, SC. Letters indicate statistical differences across treatments within each sampling day (p ≤ 0.05). See Figure 2 for definition of boxplots. Legend: AT = A. triplinervia; FE = F. eximia; EU= Eucalyptus sp., PR = P. radiata.
3.3. Functional feeding groups
The invertebrate community structure, in terms of the functional trophic groups, differed significantly among the types of leaf litter (if native or not) and sampling period (time). No significant interaction between these two factors were found. Shredders, the functional feeding group’ directly involved in litter decomposition, showed higher abundance in the native species (A. triplinervia = 15.0 ind.g–1 AFDM and F. eximia = 10.2 ind.g–1 AFDM). No shredder invertebrates were found in the Eucalyptus sp. The highest values were observed after 7 days, peaking on A. triplinervia on the 60 day of incubation. The most abundant functional feeding groups were generalists (here represented by the Chironomidae family), with the highest values observed on Eucalyptus sp. (Figure 4). The highest values were observed on the 7 days and the remaining sampling times were similar to each other. Abundances of gathering-collectors also differed, peaking on F. eximia. This functional feeding group showed the lowest densities after 45 and 60 days and the highest at 30 days. Predators varied in abundance and were most numerous on P. radiata and A. triplinervia. Predators reached their highest abundance after 45 days. Scrapers also differed in abundance with the lowest levels observed on the non- native detritus and the highest F. eximia. The lowest abundances were observed on the 7 and 45 days. Finally, the abundance of collector-filterer was significantly different between the detritus types (high abundance on the non-native leaf litter), although no difference was observed between the incubation periods.
Functional Feeding Groups of leaf-associated invertebrates (n.° ind./sample) with native and non-native species during the decomposition experiment showing the median values (the horizontal lines within the boxes) and the 25th and 75th percentiles (the top and bottom of the boxes), with the whiskers distinguishing the main body of the data from the outliers in Florianópolis Island, southeastern Brazil. Legend: AT = A. triplinervia; FE = F. eximia; EU= Eucalyptus sp., PR = P. radiata.
4. Discussion
The introduction of non-native invasive riparian species is deemed one of the major agents of global environmental changes (Pereira et al., 2012Pereira, H.M., Navarro, L.M.N., & Martins, I.S., 2012. Global biodiversity change: the bad, the good, and the unknown. Annu. Rev. Environ. Resour., 37, 25-50. http://dx.doi.org/10.1146/annurev-environ-042911-093511.
http://dx.doi.org/10.1146/annurev-enviro...
; Valduga et al., 2016Valduga, M.O., Zenni, R.D., & Vitule, J.R.S., 2016. Ecological impacts of non-native tree species plantations are broad and heterogeneous: a review of Brazilian research. An. Acad. Bras. Cienc. 88(3, Suppl.), 1675-1688. PMid:27737335. http://dx.doi.org/10.1590/0001-3765201620150575.
http://dx.doi.org/10.1590/0001-376520162...
), but information on the effects of invasive tree species on stream ecosystem functioning in an island ecosystem is still limited (Chauvet et al., 2016Chauvet, E., Ferreira, V., Giller, P.S., McKie, B.G., Tiegs, S.D., Woodward, G., Elosegi, A., Dobson, M., Fleituch, T., Graça, M.A.S., Gulis, V., Hladyz, S., Lacoursière, J.O., Lecerf, A., Pozo, J., Preda, E., Riipinen, M., Rîşnoveanu, G., Vadineanu, A., Vought, L.B.-M., & Gessner, M.O., 2016. Litter decomposition as an indicator of stream ecosystem functioning at local-to-continental scales: insights from the European RivFunction Project. Adv. Ecol. Res. 55, 99-182. http://dx.doi.org/10.1016/bs.aecr.2016.08.006.
http://dx.doi.org/10.1016/bs.aecr.2016.0...
; Ferreira et al., 2016Ferreira, V., Koricheva, J., Pozo, J., & Graça, M.A.S., 2016. A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For. Ecol. Manage. 364, 27-38. http://dx.doi.org/10.1016/j.foreco.2016.01.002.
http://dx.doi.org/10.1016/j.foreco.2016....
; Raposeiro et al., 2014Raposeiro, P.M., Martins, G.M., Moniz, I., Cunha, A., Costa, A.C., & Gonçalves, V., 2014. Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates vs. microbial decomposition of native vs. exotic plant species. Limnologica 45, 80-87. http://dx.doi.org/10.1016/j.limno.2013.10.006.
http://dx.doi.org/10.1016/j.limno.2013.1...
). As predicted, A. triplinervia and F. eximia decomposed much more rapidly than Eucalyptus sp and P. radiata, the two non-native tree species. Similarly slow decomposition of Eucalyptus (Graça et al., 2002Graça, M.A., Pozo, J., Canhoto, C., & Elosegi, A., 2002. Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. Sci. World J. 2, 1173-1185. PMID: 12805976. http://dx.doi.org/10.1100/tsw.2002.193.
http://dx.doi.org/10.1100/tsw.2002.193...
; Cizungu et al., 2014Cizungu, L., Staelens, J., Huygens, D., Walangululu, J., Muhindo, D., Cleemput, O.V., & Boeckx, P., 2014. Litterfall and leaf litter decomposition in a central African tropical mountain forest and Eucalyptus plantation. For. Ecol. Manag. 326(15), 109-116. https://doi.org/10.1016/j.foreco.2014.04.015.
https://doi.org/10.1016/j.foreco.2014.04...
) and P. radiata (Robson et al., 2009Robson, T., Baker, A., & Murray, B., 2009. Differences in leaf‐litter invertebrate assemblages between radiata pine plantations and neighbouring native eucalypt woodland. Austral Ecol. http://dx.doi.org/10.1111/j.1442-9993.2009.01936.x.
http://dx.doi.org/10.1111/j.1442-9993.20...
) has been observed in other forested streams (Graça et al., 2002Graça, M.A., Pozo, J., Canhoto, C., & Elosegi, A., 2002. Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. Sci. World J. 2, 1173-1185. PMID: 12805976. http://dx.doi.org/10.1100/tsw.2002.193.
http://dx.doi.org/10.1100/tsw.2002.193...
). However, other cases have shown a faster decomposition rate (Bachega et al., 2016Bachega, L.R., Bouillet, J.P., Piccolo, M.C., Saint-André, L., Bouvet, J.M., Nouvellon, Y., Gonçalves, J.L.M., Robin, A., & Laclau, J.P., 2016. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. For. Ecol. Manag. 359, 33-43. https://doi.org/10.1016/j.foreco.2015.09.026.
https://doi.org/10.1016/j.foreco.2015.09...
) or absence of differences (Guo & Sims, 1999Guo, L.B., & Sims, R.E.H., 1999. Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agric. Ecosyst. Environ. 75, 133-140. http://dx.doi.org/10.1016/S0167-8809(99)00069-9.
http://dx.doi.org/10.1016/S0167-8809(99)...
) in various parts of Brazil. For the genus Alchornea, similar rapid de composition has been reported in the savannah streams, with up to 80% biomass loss (~ 50 day of incubation) upon in coarse mesh bags (Tenkiano & Chauvet, 2018Tenkiano, N.S.D., & Chauvet, E., 2018. Leaf litter decomposition in Guinean savannah streams. Inland Waters 8(4), 413-421. http://dx.doi.org/10.1080/20442041.2018.1487175.
http://dx.doi.org/10.1080/20442041.2018....
).
In our study, as well as observed in previous studies in Spain and Portugal on the effects of Eucalyptus plantations in streams ecosystems (Abelho & Graça, 1996Abelho, M., & Graça, M.A.S., 1996. Effects of Eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in central Portugal. Hydrobiologia 324(3), 195-204. http://dx.doi.org/10.1007/BF00016391.
http://dx.doi.org/10.1007/BF00016391...
; Larrañaga et al., 2006Larrañaga, A., Larrañaga, S., Basaguren, A., Elosegi, A., & Pozo, J., 2006. Assessing impact of eucalyptus plantations on benthic macroinvertebrate communities by a litter exclusion experiment. – Ann. Limnol. –. Int. J. Limnol. 42(1), 1-8. http://dx.doi.org/10.1051/limn/2006002.
http://dx.doi.org/10.1051/limn/2006002...
), we found changes in invertebrate communities (mainly Shredders invertebrates) with consequences for the breakdown process. We believed that the difference observed in the leaf decomposition process can be explained by the low resource quality (e.g. lignin, cellulose and/or tannin content) of Eucalyptus sp. and P. radiata (Martínez et al., 2013Martínez, A., Larrañaga, A., Pérez, J., Basaguren, A., & Pozo, J., 2013. Leaf-litter quality effects on stream ecosystem functioning: a comparison among five species. Fundam. Appl. Limnol. 183(3), 239-248. http://dx.doi.org/10.1127/1863-9135/2013/0514.
http://dx.doi.org/10.1127/1863-9135/2013...
). Studies have demonstrated that aquatic invertebrates, mainly shredders, can detect leaves of higher nutritional quality and reject leaves of lower nutritional quality (Danger et al., 2012Danger, M., Cornut, J., Elger, A., & Chauvet, E., 2012. Effects of burial on leaf litter quality, microbial conditioning and palatability to three shredder taxa. Freshw. Biol. 57(5), 1017-1030. http://dx.doi.org/10.1111/j.1365-2427.2012.02762.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
). In fact, invertebrates prefer nitrogen-rich leaves with low amounts of secondary compounds (Cassoti et al., 2015). When exposed to these leaves, invertebrate shredders demonstrate high rates of consumption, and the high-quality leaves are processed more rapidly (Canhoto & Graça, 1995Canhoto, C., & Graça, M.A.S., 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: tipula lateralis. Freshw. Biol. 34(2), 209-214. http://dx.doi.org/10.1111/j.1365-2427.1995.tb00881.x.
http://dx.doi.org/10.1111/j.1365-2427.19...
). On the other hand, the presence of non-native leaf litter in the streambed can influence the feeding behaviour of the shredders, which can exhibit low consumption rates and high mortality (Abelho & Graça, 1996Abelho, M., & Graça, M.A.S., 1996. Effects of Eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in central Portugal. Hydrobiologia 324(3), 195-204. http://dx.doi.org/10.1007/BF00016391.
http://dx.doi.org/10.1007/BF00016391...
; Cassoti et al., 2015; Kiffer Junior et al., 2018). Here, we observed differences in the abundances of certain taxonomic groups, among which generalists (here as Chironomidae) and sensitive taxa such as the orders Ephemeroptera, Plecoptera and Trichoptera, including the shredders on leaves of non-native species.
The structural and biological composition of the forested riparian areas are important not only as determinants of the quality, quantity and seasonality of food sources to the heterotrophic aquatic food chains (Kominoski et al., 2013Kominoski, J.S., Shah, J.J.F., Canhoto, C., Fischer, D.G., Giling, D.P., González, E., Griffiths, N.A., Larrañaga, A., LeRoy, C.J., Mineau, M.M., McElarney, Y.R., Shirley, S.M., Swan, C.M., & Tiegs, S.D., 2013. Forecasting functional implications of global changes in riparian plant communities. Front. Ecol. Environ. 11(8), 423-432. http://dx.doi.org/10.1890/120056.
http://dx.doi.org/10.1890/120056...
) but also as stabilisers of the stream banks. Therefore, the quality of leaf litter entering freshwater ecosystems can alter aquatic communities (invertebrates and microbes), with potential breakdown rate effects (Barlocher & Graça, 2002Barlocher, F., & Graça, M.A.S., 2002. Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw. Biol. 47(6), 1123-1135. http://dx.doi.org/10.1046/j.1365-2427.2002.00836.x.
http://dx.doi.org/10.1046/j.1365-2427.20...
; Wright & Covich, 2005Wright, M.S., & Covich, A.P., 2005. The effect of macroinvertebrate exclusion on leaf breakdown rates in a tropical headwater stream. Biotropica 37(3), 403-408. http://dx.doi.org/10.1111/j.1744-7429.2005.00053.x.
http://dx.doi.org/10.1111/j.1744-7429.20...
). Litter decomposition is strongly related to the chemistry of leaf litter, and the contents of nitrogen and lignin are often used as proxies for litter quality (Schindler & Gessner, 2009Schindler, M.H., & Gessner, M.O., 2009. Functional leaf traits and biodiversity effects on litter decomposition in a stream. Ecology 90(6), 1641-1649. PMid:19569378. http://dx.doi.org/10.1890/08-1597.1.
http://dx.doi.org/10.1890/08-1597.1...
; Marcarelli et al., 2011Marcarelli, A.M., Baxter, C.V., Mineau, M.M., & Hall Junior, R.O., 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92(6), 1215-1225. PMid:21797150. http://dx.doi.org/10.1890/10-2240.1.
http://dx.doi.org/10.1890/10-2240.1...
). Phosphorous, carbon and leaf toughness have also been documented as good predictors of litter breakdown rates in streams (García et al., 2014García, L., Pardo, I., & Richardson, J.S., 2014. A cross‐continental comparison of stream invertebrate community assembly to assess convergence in forested headwater streams. Aquat. Sci. 76(1), 29-40. http://dx.doi.org/10.1007/s00027-013-0308-5.
http://dx.doi.org/10.1007/s00027-013-030...
; Frainer et al., 2015Frainer, A., Moretti, M.S., Xu, W., & Gessner, M.O., 2015. No evidence for leaf‐trait dissimilarity effects on litter decomposition, fungal decomposers, and nutrient dynamics. Ecology 96(2), 550-561. PMid:26240875. http://dx.doi.org/10.1890/14-1151.1.
http://dx.doi.org/10.1890/14-1151.1...
). As predicted, F. eximia and A. triplinervia leaves, the fastest-decomposing leaves, were associated with the highest taxonomic richness of invertebrate taxa throughout the entire experiment. This suggests that invertebrate communities, mainly shredders, aggregate on the litter with the highest quality, which in turn increases the rate of litter breakdown. Inversely, we observed that the leaf litter of non-native invasive species had lower taxa richness, which in turn decreased the rate of litter breakdown.
One aspect evaluated in the present study, the colonisation by invertebrates, supported the hypothesis that the invertebrates prefer eating the leaf litter from native species to non-native species, corroborating other studies (König et al., 2014König, R., Hepp, L.U., & Santos, S., 2014. Colonisation of low- and high-quality detritus by benthic macroinvertebrates during leaf breakdown in a subtropical stream. Limnologica 45, 61-68. http://dx.doi.org/10.1016/j.limno.2013.11.001.
http://dx.doi.org/10.1016/j.limno.2013.1...
). This preference was reflected mainly in the abundance of the shredders and collector-gatherers. The abundance of shredders was greater for A. triplinervia throughout the period in which the leaves of this species were incubated in the stream. This preference can be related to different leaf properties that determine leaf palatability (Graça, 2001Graça, M.A.S., 2001. The role of invertebrates on leaf litter decomposition in streams. Int. Rev. Hydrobiol. 86(4-5), 383-393. http://dx.doi.org/10.1002/1522-2632(200107)86:4/5<383::AID-IROH383>3.0.CO;2-D.
http://dx.doi.org/10.1002/1522-2632(2001...
). Lower levels of tannins and higher nitrogen contents in the A. triplinervia leaves may have favoured invertebrate colonisation, as tannins are one of the main secondary metabolites produced by plants and are generally negatively correlated with the feeding preferences of invertebrates (König et al., 2014König, R., Hepp, L.U., & Santos, S., 2014. Colonisation of low- and high-quality detritus by benthic macroinvertebrates during leaf breakdown in a subtropical stream. Limnologica 45, 61-68. http://dx.doi.org/10.1016/j.limno.2013.11.001.
http://dx.doi.org/10.1016/j.limno.2013.1...
).
The take-home message is clear: The replacement of native riparian vegetation, a major energy source for aquatic decomposers, by non-native species can alter the breakdown of leaf litter, which is an important ecosystem function. In conclusion, we believe that the evaluation of ecosystem-level processes in streams that have changing environmental conditions can be an alternative for a better understanding of the effects that different disturbances (here replacement of native vegetation by non-native species) have on aquatic invertebrates and stream ecosystem functioning. Therefore, understanding the relationship between riparian vegetation cover and stream ecosystem processes has become increasingly relevant to understanding the changes that have occurred in recent years in aquatic ecosystems. This knowledge will help to support current and future research in stream ecosystems, as the process of litter breakdown is considered a proxy for stream health and ecological integrity (Gessner & Chauvet, 2002Gessner, M.O., & Chauvet, E., 2002. A case for using litter breakdown to assess functional stream integrity. Ecol. Appl. 12(2), 498-510. http://dx.doi.org/10.1890/1051-0761(2002)012[0498:ACFULB]2.0.CO;2.
http://dx.doi.org/10.1890/1051-0761(2002...
; Clarke et al., 2008Clarke, A., Mac Nally, R., Bond, N., & Lake, P.S., 2008. Macroinvertebrate diversity in headwater streams: A review. Freshw. Biol. 53(9), 1707-1721. http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
; Kominoski et al., 2013Kominoski, J.S., Shah, J.J.F., Canhoto, C., Fischer, D.G., Giling, D.P., González, E., Griffiths, N.A., Larrañaga, A., LeRoy, C.J., Mineau, M.M., McElarney, Y.R., Shirley, S.M., Swan, C.M., & Tiegs, S.D., 2013. Forecasting functional implications of global changes in riparian plant communities. Front. Ecol. Environ. 11(8), 423-432. http://dx.doi.org/10.1890/120056.
http://dx.doi.org/10.1890/120056...
).
Acknowledgements
We are grateful to staff from Laboratory of Freshwater Ecology from Universidade Federal de Santa Catarina (UFSC, www.limnos.ufsc.br) for collaborative efforts related to the samplings. We thank the FLORAM (Fundação Municipal do Meio Ambiente de Florianópolis) and the PPGECO – UFSC (Programa de Pós-Graduação em Ecologia) for providing assistance for field and laboratory equipment. We also would like to thank Luis Carlos Pinto de Macedo Soares for the help with statistical analyses.
-
Cite as: Andriotti, J., Petrucio, M. M. and Silva, A. L. L. Exploring the impacts of non-native leaf litter on invertebrate community and leaf decomposition in a Atlantic Forest stream. Acta Limnologica Brasiliensia, 2022, vol. 34, e17
References
- Abelho, M., & Graça, M.A.S., 1996. Effects of Eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in central Portugal. Hydrobiologia 324(3), 195-204. http://dx.doi.org/10.1007/BF00016391
» http://dx.doi.org/10.1007/BF00016391 - Abelho, M., 2001. From litterfall to breakdown in streams: a review. Sci. World J. 1, 656-680. PMid:12805769. http://dx.doi.org/10.1100/tsw.2001.103
» http://dx.doi.org/10.1100/tsw.2001.103 - Bachega, L.R., Bouillet, J.P., Piccolo, M.C., Saint-André, L., Bouvet, J.M., Nouvellon, Y., Gonçalves, J.L.M., Robin, A., & Laclau, J.P., 2016. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. For. Ecol. Manag. 359, 33-43. https://doi.org/10.1016/j.foreco.2015.09.026
» https://doi.org/10.1016/j.foreco.2015.09.026 - Baker, A.C., Murray, B.R., & Hose, G.C., 2007. Relating pine-litter intrusion to plant-community structure in native eucalypt woodland adjacent to Pinus radiata (Pinaceae) plantations. Aust. J. Bot. 55(5), 521-532. http://dx.doi.org/10.1071/BT06135
» http://dx.doi.org/10.1071/BT06135 - Balibrea, A., Ferreira, V., Gonçalves, V., & Raposeiro, P.M., 2017. Consumption, growth and survival of the endemic stream shredder Limnephilus atlanticus (Trichoptera, Limnephilidae) fed with distinct leaf species. Limnologica. 64, 31-37. https://doi.org/10.1016/j.limno.2017.04.002
» https://doi.org/10.1016/j.limno.2017.04.002 - Barlocher, F., & Graça, M.A.S., 2002. Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw. Biol. 47(6), 1123-1135. http://dx.doi.org/10.1046/j.1365-2427.2002.00836.x
» http://dx.doi.org/10.1046/j.1365-2427.2002.00836.x - Canhoto, C., & Graça, M.A.S., 1995. Food value of introduced eucalypt leaves for a Mediterranean stream detritivore: tipula lateralis. Freshw. Biol. 34(2), 209-214. http://dx.doi.org/10.1111/j.1365-2427.1995.tb00881.x
» http://dx.doi.org/10.1111/j.1365-2427.1995.tb00881.x - Canhoto, C., Abelho, M., & Graça, M.A.S., 2004. Efeitos das plantações de Eucalyptus globulus nos ribeiros de Portugal. Recur. Hidricos 25, 59-65.
- Casotti, C.G., Kiffer Junior, W.P.J., Costa, L.C., Rangel, J.V., Casagrande, L.C., & Moretti, M.S., 2015. Assessing the importance of riparian zones conservation for leaf decomposition in streams. Nat. Conserv. Online 13(2), 178-182. http://dx.doi.org/10.1016/j.ncon.2015.11.011
» http://dx.doi.org/10.1016/j.ncon.2015.11.011 - Castro-Díez, P., & Alonso, A., 2017. Effects of non-native riparian plants in riparian and fluvial ecosystems: a review for the Iberian Peninsula. Limnetica 36(2), 525-541. https://doi.org/10.23818/limn.36.19
» https://doi.org/10.23818/limn.36.19 - Castro-Díez, P., Vaz, A.S., Silva, J.S., van Loo, M., Alonso, Á., Aponte, C., Bayón, Á., Bellingham, P.J., Chiuffo, M.C., DiManno, N., Julian, K., Kandert, S., La Porta, N., Marchante, H., Maule, H.G., Mayfield, M.M., Metcalfe, D., Monteverdi, M.C., Núñez, M.A., Ostertag, R., Parker, I.M., Peltzer, D.A., Potgieter, L.J., Raymundo, M., Rayome, D., Reisman-Berman, O., Richardson, D.M., Roos, R.E., Saldaña, A., Shackleton, R.T., Torres, A., Trudgen, M., Urban, J., Vicente, J.R., Vilà, M., Ylioja, T., Zenni, R.D., & Godoy, O., 2019. Global effects of non-native tree species on multiple ecosystem services. Biol. Rev. Camb. Philos. Soc. 94(4), 1477-1501. PMid:30974048. http://dx.doi.org/10.1111/brv.12511
» http://dx.doi.org/10.1111/brv.12511 - Cizungu, L., Staelens, J., Huygens, D., Walangululu, J., Muhindo, D., Cleemput, O.V., & Boeckx, P., 2014. Litterfall and leaf litter decomposition in a central African tropical mountain forest and Eucalyptus plantation. For. Ecol. Manag. 326(15), 109-116. https://doi.org/10.1016/j.foreco.2014.04.015
» https://doi.org/10.1016/j.foreco.2014.04.015 - Couceiro, S.R.M., Hamada, N., Luz, S.L.B., Forsberg, B.R., & Pimentel, T.P., 2007. Deforestation and sewage effects on aquatic macroinvertebrates in urban streams in Manaus, Amazonas, Brazil. Hydrobiologia 575(1), 271-284. http://dx.doi.org/10.1007/s10750-006-0373-z
» http://dx.doi.org/10.1007/s10750-006-0373-z - Cordero–Rivera, A., Martínez-Álvarez, A., & Álvarez, M., 2017. Eucalypt plantations reduce the diversity of macroinvertebrates in small-forested streams. Anim Biodivers Conserv. 40(1), 87-97. https://doi.org/10.32800/abc.2017.40.0087
» https://doi.org/10.32800/abc.2017.40.0087 - Cummins, K.W., Merritt, R.W., & Andrade, P.C.N., 2005. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 40(1), 69-89. http://dx.doi.org/10.1080/01650520400025720
» http://dx.doi.org/10.1080/01650520400025720 - Chauvet, E., Ferreira, V., Giller, P.S., McKie, B.G., Tiegs, S.D., Woodward, G., Elosegi, A., Dobson, M., Fleituch, T., Graça, M.A.S., Gulis, V., Hladyz, S., Lacoursière, J.O., Lecerf, A., Pozo, J., Preda, E., Riipinen, M., Rîşnoveanu, G., Vadineanu, A., Vought, L.B.-M., & Gessner, M.O., 2016. Litter decomposition as an indicator of stream ecosystem functioning at local-to-continental scales: insights from the European RivFunction Project. Adv. Ecol. Res. 55, 99-182. http://dx.doi.org/10.1016/bs.aecr.2016.08.006
» http://dx.doi.org/10.1016/bs.aecr.2016.08.006 - Clarke, A., Mac Nally, R., Bond, N., & Lake, P.S., 2008. Macroinvertebrate diversity in headwater streams: A review. Freshw. Biol. 53(9), 1707-1721. http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x
» http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x - Danger, M., Cornut, J., Elger, A., & Chauvet, E., 2012. Effects of burial on leaf litter quality, microbial conditioning and palatability to three shredder taxa. Freshw. Biol. 57(5), 1017-1030. http://dx.doi.org/10.1111/j.1365-2427.2012.02762.x
» http://dx.doi.org/10.1111/j.1365-2427.2012.02762.x - Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z., Knowler, D.J., Lévêque, C., Naiman, R.J., Prieur-Richard, A.H., Soto, D., Stiassny, M.L., & Sullivan, C.A., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 81(2), 163-182. PMid:16336747. http://dx.doi.org/10.1017/S1464793105006950
» http://dx.doi.org/10.1017/S1464793105006950 - Dufour, S., Rodríguez-González, P.M., & Laslier, M., 2019. Tracing the scientific trajectory of riparian vegetation studies: main topics, approaches and needs in a globally changing world. Sci. Total Environ. 653, 1168-1185. PMid:30759557. http://dx.doi.org/10.1016/j.scitotenv.2018.10.383
» http://dx.doi.org/10.1016/j.scitotenv.2018.10.383 - Dufrêne, M., & Legendre, P., 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67(3), 345-366. http://dx.doi.org/10.2307/2963459
» http://dx.doi.org/10.2307/2963459 - Ferreira, V., Larrañaga, A., Gulis, V., Basaguren, A., Elosegi, A., Graça, M.A.S., & Pozo, J., 2015. The effects of eucalypt plantations on plant litter decomposition and macroinvertebrate communities in Iberian streams. For Ecol Manag. 335, 129-38. http://dx.doi.org/10.1016/j.foreco.2014.09.013
» http://dx.doi.org/10.1016/j.foreco.2014.09.013 - Ferreira, V., Koricheva, J., Pozo, J., & Graça, M.A.S., 2016. A meta-analysis on the effects of changes in the composition of native forests on litter decomposition in streams. For. Ecol. Manage. 364, 27-38. http://dx.doi.org/10.1016/j.foreco.2016.01.002
» http://dx.doi.org/10.1016/j.foreco.2016.01.002 - Frainer, A., Moretti, M.S., Xu, W., & Gessner, M.O., 2015. No evidence for leaf‐trait dissimilarity effects on litter decomposition, fungal decomposers, and nutrient dynamics. Ecology 96(2), 550-561. PMid:26240875. http://dx.doi.org/10.1890/14-1151.1
» http://dx.doi.org/10.1890/14-1151.1 - García, L., Pardo, I., & Richardson, J.S., 2014. A cross‐continental comparison of stream invertebrate community assembly to assess convergence in forested headwater streams. Aquat. Sci. 76(1), 29-40. http://dx.doi.org/10.1007/s00027-013-0308-5
» http://dx.doi.org/10.1007/s00027-013-0308-5 - Gessner, M.O., & Chauvet, E., 2002. A case for using litter breakdown to assess functional stream integrity. Ecol. Appl. 12(2), 498-510. http://dx.doi.org/10.1890/1051-0761(2002)012[0498:ACFULB]2.0.CO;2
» http://dx.doi.org/10.1890/1051-0761(2002)012[0498:ACFULB]2.0.CO;2 - Guo, L.B., & Sims, R.E.H., 1999. Litter decomposition and nutrient release via litter decomposition in New Zealand eucalypt short rotation forests. Agric. Ecosyst. Environ. 75, 133-140. http://dx.doi.org/10.1016/S0167-8809(99)00069-9
» http://dx.doi.org/10.1016/S0167-8809(99)00069-9 - Graça, M.A., Pozo, J., Canhoto, C., & Elosegi, A., 2002. Effects of Eucalyptus plantations on detritus, decomposers, and detritivores in streams. Sci. World J. 2, 1173-1185. PMID: 12805976. http://dx.doi.org/10.1100/tsw.2002.193
» http://dx.doi.org/10.1100/tsw.2002.193 - Graça, M.A.S., 2001. The role of invertebrates on leaf litter decomposition in streams. Int. Rev. Hydrobiol. 86(4-5), 383-393. http://dx.doi.org/10.1002/1522-2632(200107)86:4/5<383::AID-IROH383>3.0.CO;2-D
» http://dx.doi.org/10.1002/1522-2632(200107)86:4/5<383::AID-IROH383>3.0.CO;2-D - Graça, M.A.S., Ferreira, V., Canhoto, C., Encalada, A.C., Guerrero-Bolaño, F., Wantzen, K.M., & Boyero, L., 2015. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 100(1), 1-12. http://dx.doi.org/10.1002/iroh.201401757
» http://dx.doi.org/10.1002/iroh.201401757 - Hartig, F., 2020. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed). Regression Models. R package version 0.3.3.0. Retrieved in 2021, Nov 15, from http://florianhartig.github.io/DHARMa/
» http://florianhartig.github.io/DHARMa/ - Hennemann, M.C., & Petrucio, M.M., 2011. Spatial and temporal dynamic of trophic relevant parameters in a subtropical coastal lagoon in Brazil. Environ. Monit. Assess. 181(1-4), 347-361. PMid:21190080. http://dx.doi.org/10.1007/s10661-010-1833-5
» http://dx.doi.org/10.1007/s10661-010-1833-5 - Hieber, M., & Gessner, M.O., 2002. Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83(4), 1026-1038. http://dx.doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2
» http://dx.doi.org/10.1890/0012-9658(2002)083[1026:COSDFA]2.0.CO;2 - Kominoski, J.S., Shah, J.J.F., Canhoto, C., Fischer, D.G., Giling, D.P., González, E., Griffiths, N.A., Larrañaga, A., LeRoy, C.J., Mineau, M.M., McElarney, Y.R., Shirley, S.M., Swan, C.M., & Tiegs, S.D., 2013. Forecasting functional implications of global changes in riparian plant communities. Front. Ecol. Environ. 11(8), 423-432. http://dx.doi.org/10.1890/120056
» http://dx.doi.org/10.1890/120056 - König, R., Hepp, L.U., & Santos, S., 2014. Colonisation of low- and high-quality detritus by benthic macroinvertebrates during leaf breakdown in a subtropical stream. Limnologica 45, 61-68. http://dx.doi.org/10.1016/j.limno.2013.11.001
» http://dx.doi.org/10.1016/j.limno.2013.11.001 - Larrañaga, A., Larrañaga, S., Basaguren, A., Elosegi, A., & Pozo, J., 2006. Assessing impact of eucalyptus plantations on benthic macroinvertebrate communities by a litter exclusion experiment. – Ann. Limnol. –. Int. J. Limnol. 42(1), 1-8. http://dx.doi.org/10.1051/limn/2006002
» http://dx.doi.org/10.1051/limn/2006002 - Lemes-Silva, A.L., Pagliosa, P.R., & Petrucio, M.M., 2014. Inter- and intra-guild patterns of food resource utilization by chironomid larvae in a subtropical coastal lagoon. Limnology 15(1), 1-12. http://dx.doi.org/10.1007/s10201-013-0407-y
» http://dx.doi.org/10.1007/s10201-013-0407-y - Lemes-Silva, A.L., Pires, J., Pagliosa, P.R., & Petrucio, M.M., 2016. Distribution of aquatic macroinvertebrate assemblages in a subtropical coastal lake: response to environmental parameters. Fundam. Appl. Limnol. 188(2), 113-127. http://dx.doi.org/10.1127/fal/2016/0786
» http://dx.doi.org/10.1127/fal/2016/0786 - Lisboa, L.K., Lemes-Silva, A.L., Siegloch, A.E., Gonçalves Junior, J.F., & Petrucio, M.M., 2015. Temporal dynamics of allochthonous coarse particulate organic matter in a subtropical Atlantic rainforest Brazilian stream. Mar. Freshw. Res. 66(8), 674-680. http://dx.doi.org/10.1071/MF14068
» http://dx.doi.org/10.1071/MF14068 - Marcarelli, A.M., Baxter, C.V., Mineau, M.M., & Hall Junior, R.O., 2011. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92(6), 1215-1225. PMid:21797150. http://dx.doi.org/10.1890/10-2240.1
» http://dx.doi.org/10.1890/10-2240.1 - Martínez, A., Larrañaga, A., Pérez, J., Basaguren, A., & Pozo, J., 2013. Leaf-litter quality effects on stream ecosystem functioning: a comparison among five species. Fundam. Appl. Limnol. 183(3), 239-248. http://dx.doi.org/10.1127/1863-9135/2013/0514
» http://dx.doi.org/10.1127/1863-9135/2013/0514 - Mathuriau, C., Thomas, A.G.B., & Chauvet, E., 2008. Seasonal dynamics of benthic detritus and associated macroinvertebrate communities in a neotropical stream. Fundam. Appl. Limnol. 171(4), 323-333. http://dx.doi.org/10.1127/1863-9135/2008/0171-0323
» http://dx.doi.org/10.1127/1863-9135/2008/0171-0323 - Mugnai, R., Nessimian, J.L., & Baptista, D.F., 2010. Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro. Rio de Janeiro: Technical Books.
- Pereira, A., & Ferreira, V., 2021. Invasion of native riparian forests by acacia species affects in-stream litter decomposition and associated microbial decomposers. Microb Ecol 81(1), 14-25. PMid:32623497. https://doi.org/10.1007/s00248-020-01552-3
» https://doi.org/10.1007/s00248-020-01552-3 - Pereira, H.M., Navarro, L.M.N., & Martins, I.S., 2012. Global biodiversity change: the bad, the good, and the unknown. Annu. Rev. Environ. Resour., 37, 25-50. http://dx.doi.org/10.1146/annurev-environ-042911-093511
» http://dx.doi.org/10.1146/annurev-environ-042911-093511 - R Development Core Team, 2020. R: A language and environment for statistical computing [Software]. Vienna: R Foundation for Statistical Computing.
- Raposeiro, P.M., Martins, G.M., Moniz, I., Cunha, A., Costa, A.C., & Gonçalves, V., 2014. Leaf litter decomposition in remote oceanic islands: the role of macroinvertebrates vs. microbial decomposition of native vs. exotic plant species. Limnologica 45, 80-87. http://dx.doi.org/10.1016/j.limno.2013.10.006
» http://dx.doi.org/10.1016/j.limno.2013.10.006 - Richardson, D.M., Holmes, P.M., Esler, K.J., Galatowitsch, S.M., Stromberg, J.C., Kirkman, S.P., Pyšek, P., & Hobbs, R.J., 2007. Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 13(1), 126-139. http://dx.doi.org/10.1111/j.1366-9516.2006.00314.x
» http://dx.doi.org/10.1111/j.1366-9516.2006.00314.x - Robson, T., Baker, A., & Murray, B., 2009. Differences in leaf‐litter invertebrate assemblages between radiata pine plantations and neighbouring native eucalypt woodland. Austral Ecol. http://dx.doi.org/10.1111/j.1442-9993.2009.01936.x
» http://dx.doi.org/10.1111/j.1442-9993.2009.01936.x - Schindler, M.H., & Gessner, M.O., 2009. Functional leaf traits and biodiversity effects on litter decomposition in a stream. Ecology 90(6), 1641-1649. PMid:19569378. http://dx.doi.org/10.1890/08-1597.1
» http://dx.doi.org/10.1890/08-1597.1 - Tenkiano, N.S.D., & Chauvet, E., 2018. Leaf litter decomposition in Guinean savannah streams. Inland Waters 8(4), 413-421. http://dx.doi.org/10.1080/20442041.2018.1487175
» http://dx.doi.org/10.1080/20442041.2018.1487175 - Tershy, B.R., Shen, K.W., Newton, K.M., Holmes, N.D., & Croll, D.A., 2015. The importance of islands for the protection of biological and linguistic diversity. Bioscience 65(6), 592-597. http://dx.doi.org/10.1093/biosci/biv031
» http://dx.doi.org/10.1093/biosci/biv031 - Valduga, M.O., Zenni, R.D., & Vitule, J.R.S., 2016. Ecological impacts of non-native tree species plantations are broad and heterogeneous: a review of Brazilian research. An. Acad. Bras. Cienc. 88(3, Suppl.), 1675-1688. PMid:27737335. http://dx.doi.org/10.1590/0001-3765201620150575
» http://dx.doi.org/10.1590/0001-3765201620150575 - Webster, J.R., & Benfield, E.F., 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Evol. Syst. 17(1), 567-594. http://dx.doi.org/10.1146/annurev.es.17.110186.003031
» http://dx.doi.org/10.1146/annurev.es.17.110186.003031 - Wright, M.S., & Covich, A.P., 2005. The effect of macroinvertebrate exclusion on leaf breakdown rates in a tropical headwater stream. Biotropica 37(3), 403-408. http://dx.doi.org/10.1111/j.1744-7429.2005.00053.x
» http://dx.doi.org/10.1111/j.1744-7429.2005.00053.x - Zelnik, I., Mavrič Klenovšek, V., & Gaberščik, A., 2020. Complex undisturbed riparian zones are resistant to colonisation by invasive alien plant species. Water 12(2), 345. http://dx.doi.org/10.3390/w12020345
» http://dx.doi.org/10.3390/w12020345
Edited by
Publication Dates
-
Publication in this collection
10 June 2022 -
Date of issue
2022
History
-
Received
15 Nov 2021 -
Accepted
24 May 2022