Abstract:
Aim
The variability in density and species diversity of decapod crustaceans was investigated on four islands with different degrees of anthropogenic disturbance around the city of Belém, State of Pará.
Methods
Samples were obtained from 15 creeks using artisanal traps, every three months between October 2013 and May 2014 on Combu, Onças, Cotijuba and Mosqueiro islands.
Results
Salinity and temperature little varied, which is common for a tropical Amazon estuary strongly influenced by freshwater inflow. A total of 8,367 decapods were captured, with one record of an exotic species Penaeus monodon. In all seasons, decapod density and richness tended to increase from Combu to Mosqueiro, with increasing proximity to the sea and higher salinity and pH. Except for Combu, species richness and Margalef diversity tended to be slightly greater in the wet season at all islands, especially Onças. Eveness and Shannon diversity did not vary greatly between seasons but were lowest at Onças in the dry season and highest at Combu, decreasing to Mosqueiro, in the wet season. In general, ecological indices are similar in the dry and transition dry to wet seasons, and in the wet season, dominance occurs at Mosqueiro Island. Macrobrachium acanthurus, C. bocourti and P. gracillis were associated with the wet season, whereas M. surinamicum prefers the dry season. M. amazonicum and Macrobrachium sp. have no well-defined seasonal pattern of occurrence at all the islands.
Conclusions
Despite anthropogenic disturbances and proximity to large human populations, especially on Mosqueiro Island, the density and diversity of decapod crustaceans appear to be reasonably unaffected for the moment.
Keywords:
Northern coast of Brazil; Macrobrachium amazonicum; Decapod, ecological index
Resumo:
Objetivo
A variabilidade na densidade e diversidade de espécies de crustáceos decápodes foi investigada em quatro ilhas com diferentes graus de perturbação antropogênica em torno da cidade de Belém, Estado do Pará.
Métodos
Foram obtidas amostras de 15 canais de maré utilizando armadilhas artesanais, a cada três meses, entre outubro de 2013 e maio de 2014 nas ilhas Combu, Onças, Cotijuba e Mosqueiro.
Resultados
A salinidade e a temperatura variaram pouco, o que é comum em um estuário amazônico tropical fortemente influenciado pelo influxo de água doce. Foram capturados um total de 8.367 decápodes, com um registro de uma espécie exótica, Penaeus monodon. Em todas as estações, a densidade e riqueza de decápodes aumentou de Combu para Mosqueiro, com a crescente proximidade do mar e maior salinidade e pH. Com exceção de Combu, a riqueza de espécies e diversidade de Margalef tendeu a ser ligeiramente maior na estação chuvosa em todas as ilhas, especialmente em Onças. A Equitabilidade e a diversidade de Shannon foram semelhantes entre as estações, mas eram mais baixas em Onças na estação seca e mais alta em Combu, diminuindo para Mosqueiro na estação chuvosa. Em geral, os índices ecológicos são semelhantes na estação seca e na transição da seca para a estação chuvosa, na estação chuvosa a dominância ocorre na Ilha de Mosqueiro. M. acanthurus, C. bocourti e P. gracillis foram associadas à estação das chuvas, enquanto que M. surinamicum prefere a estação seca. M. amazonicum e Macrobrachium sp. não têm um padrão de ocorrência sazonal definido em todas as ilhas.
Conclusões
Apesar das perturbações antropogênicas e da proximidade a grandes populações humanas, especialmente na Ilha do Mosqueiro, a densidade e diversidade de crustáceos decápodes parecem ser razoavelmente não afetadas no momento.
Palavras-chave:
Costa Norte do Brasil; Macrobrachium amazonicum; Decápodes; índices ecológicos
1. Introduction
Decapod crustaceans have been widely studied on the Amazon coast (Bentes et al., 2011Bentes, B.S., Martinelli, J.M., Souza, L.S., Cavalcante, D.V., Almeida, M.C., & Isaac, V.J., 2011. Spatial distribution of the amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará). Braz. J. Biol. 71(4), 925-935. http://dx.doi.org/10.1590/S1519-69842011000500013.
http://dx.doi.org/10.1590/S1519-69842011...
, Vergamini et al., 2011Vergamini, F.G., Pileggi, L.G., & Mantelatto, F.L., 2011. Genetic variability of the Amazon river prawn Macrobrachium amazonicum (Decapoda, Caridea, Palaemonidae). Contrib. Zool. 80(1), 67-83. http://dx.doi.org/10.1163/18759866-08001003.
http://dx.doi.org/10.1163/18759866-08001...
, Pantaleão et al., 2012Pantaleão, J.A.F., Hirose, G.L., & Costa, R.C.D., 2012. Relative growth, morphological sexual maturity, and size of Macrobrachium amazonicum (Heller 1862) (Crustacea, Decapoda, Palaemonidae) in a population with an entirely freshwater life cycle. Invertebr. Reprod. Dev. 56(3), 180-190. http://dx.doi.org/10.1080/07924259.2011.587276.
http://dx.doi.org/10.1080/07924259.2011....
, Oliveira et al., 2013Oliveira, D.B., Silva, D.C., & Martinelli-Lemos, J.M., 2013. Larval and adult density of the porcellanid crab Petrolisthes armatus (Anomura: Porcellanidae) in an Amazon estuary, northern Brazil. Zoologia 30(6), 592-600. http://dx.doi.org/10.1590/S1984-46702013005000002.
http://dx.doi.org/10.1590/S1984-46702013...
; Amaral et al., 2014Amaral, K.D.S., Vieira, I.M., Osório, F.M., Rocha, J., & Lima, J.D.F., 2014. Bioecology of the crab Ucides cordatus (Crustacea, Decapoda) in mangroves influenced by the Amazon River, Brazil. Acta Amazon. 44(2), 213-222. http://dx.doi.org/10.1590/S0044-59672014000200007.
http://dx.doi.org/10.1590/S0044-59672014...
; Lima et al., 2014Lima, J.D.F., Garcia, J.D.S., & Silva, T.C., 2014. Natural diet and feeding habits of a freshwater prawn (Macrobrachium carcinus: Crustacea, Decapoda) in the estuary of the Amazon River. Acta Amazon. 44(2), 235-244. http://dx.doi.org/10.1590/S0044-59672014000200009.
http://dx.doi.org/10.1590/S0044-59672014...
; de Oliveira et al., 2016de Oliveira, D.B., Martinelli-Lemos, J.M., de Souza, A.S., da Costa, J.R., & Abrunhosa, F.A., 2016. Does retention or exportation occur in the larvae of the mud shrimp Upogebia vasquezi (Decapoda, Gebiidea)? Implications for the reproductive strategy of the species on the Amazon coast. Hydrobiologia 773(1), 241-252. http://dx.doi.org/10.1007/s10750-016-2708-8.
http://dx.doi.org/10.1007/s10750-016-270...
; Freire et al., 2017Freire, J.L., Bentes, B., Fontes, V.B., & da Silva, E.M., 2017. Morphometric discrimination among three stocks of Macrobrachium amazonicum in the Brazilian Amazon. Limnologica 64, 1-10. http://dx.doi.org/10.1016/j.limno.2017.01.007.
http://dx.doi.org/10.1016/j.limno.2017.0...
) where they are extensively exploited by artisanal fisheries for subsistence and commercial purposes (Bentes et al., 2011Bentes, B.S., Martinelli, J.M., Souza, L.S., Cavalcante, D.V., Almeida, M.C., & Isaac, V.J., 2011. Spatial distribution of the amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará). Braz. J. Biol. 71(4), 925-935. http://dx.doi.org/10.1590/S1519-69842011000500013.
http://dx.doi.org/10.1590/S1519-69842011...
). As an example, Macrobrachium amazonicum populations invariably present signs of overfishing (Freire et al., 2012Freire, J.L., Marques, C.B., & Bentes, B., 2012. Estrutura populacional e biologia reprodutiva do camarão-da-amazônia Macrobrachium amazonicum (Heller,1862) (Decapoda: Palaemonidae) em um estuário da região nordeste do Pará, Brasil. Braz. J. Aquat. Sci. Tech. 16(2), 65-76. http://dx.doi.org/10.14210/bjast.v16n2.p65-76.
http://dx.doi.org/10.14210/bjast.v16n2.p...
), and are not self-renewing adequately due to the capture of immature shrimp, thus making their fishing unsustainable (Lucena-Frédou et al., 2010Lucena-Frédou, F., Rosa Filho, J.S., Silva, M.C.N., & Azevedo, E.F., 2010. Population dynamics of the river prawn, Macrobrachium amazonicum (Heller, 1862) (Decapoda, Palaemonidae) on Combu island (Amazon estuary). Crustaceana. 83(3), 277-290. http://dx.doi.org/10.1163/001121609X12596543952.
http://dx.doi.org/10.1163/001121609X1259...
).
The decapod genus Macrobrachium includes approximately 240 species (Wowor et al., 2009Wowor, D., Muthu, V., Meier, R., Balke, M., Cai, Y., & Ng, P.K.L., 2009. Evolution of life history traits in Asian freshwater prawns of the genus Macrobrachium (Crustacea: Decapoda: Palaemonidae) based on multilocus molecular phylogenetic analysis. Mol. Phylogenet. Evol. 52(2), 340-350. PMid:19489122. http://dx.doi.org/10.1016/j.ympev.2009.01.002.
http://dx.doi.org/10.1016/j.ympev.2009.0...
; De Grave & Fransen, 2011De Grave, S., & Fransen, C.H.J.M., 2011. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Netherlands: Zoologische Mededelingen Leiden.), and is found in tropical and subtropical fresh and brackish waters worldwide (Pereira et al., 2002Pereira, G., De Stefano, H., Staton, J., & Farrell, B., 2002. Phylogenetic relationships in some species of the genus Macrobrachium based on nucleotide sequences of the mitochondrial gene cytochrome oxidase. In: Escobar-Briones E., Alvarez F., ed. Modern Approaches to the Study of Crustacea. Boston: Springer, 319-322. http://dx.doi.org/10.1007/978-1-4615-0761-1_44.
http://dx.doi.org/10.1007/978-1-4615-076...
; Short, 2004Short, J.W., 2004. A revision of Australian river prawns, Macrobrachium (Crustacea: Decapoda: Palaemonidae). Hydrobiologia 525(1-3), 1-100. http://dx.doi.org/10.1023/B:HYDR.0000038871.50730.95.
http://dx.doi.org/10.1023/B:HYDR.0000038...
), as a result of its highly adaptive reproductive and life history strategy (Short, 2004Short, J.W., 2004. A revision of Australian river prawns, Macrobrachium (Crustacea: Decapoda: Palaemonidae). Hydrobiologia 525(1-3), 1-100. http://dx.doi.org/10.1023/B:HYDR.0000038871.50730.95.
http://dx.doi.org/10.1023/B:HYDR.0000038...
; Pileggi & Mantelatto, 2010Pileggi, L.G., & Mantelatto, F.L., 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae) with emphasis on the relationships among American species. Invertebr. Syst. 24(2), 194-208. http://dx.doi.org/10.1071/IS09043.
http://dx.doi.org/10.1071/IS09043...
). In South America, the genus has a wide distribution in the basins of the Orinoco, Amazonas and Paraguay rivers, being abundant in waters rich in sediment and dissolved salts in the central Amazon River basin (Odinetz-Collart & Moreira, 1993Odinetz-Collart, O., & Moreira, L.C., 1993. Potencial pesqueiro do camarão Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro). Amazoniana 12(3-4), 399-413.). In Brazil, there are 17 species of the genus (Pileggi & Mantelatto, 2012Pileggi, L.G., & Mantelatto, F.L., 2012. Taxonomic revision of doubtful Brazilian freshwater shrimp species of genus Macrobrachium (Decapoda, Palaemonidae). Iheringia Ser. Zool. 102(4), 426-437. http://dx.doi.org/10.1590/S0073-47212012005000012.
http://dx.doi.org/10.1590/S0073-47212012...
).
Shrimp and crabs inhabit marine, coastal and estuarine waters (Magalhães, 2003Magalhães, C., 2003. Brachyura: Pseudothelphusidae e Trichodactylidae. In: Melo, G.A.S. ed. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo: Loyola, 143-297.) and are important for connectivity among different trophic levels of the food web (Barros & Pimentel 2001Barros, M.P., & Pimentel, F.R., 2001. A fauna de Decapoda (Crustácea) do Estado do Pará, Brasil: lista preliminar de espécies. Bol. Mus. Para. Emilio Goeldi Zool. 17(1), 15-41.; Albertoni et al., 2003aAlbertoni, E.F., Palma-Silva, C., & Esteves, F.A., 2003a. Natural diet of three species of shrimp in a tropical coastal lagoon. Braz. Arch. Biol. Technol. 46(3), 395-403. http://dx.doi.org/10.1590/S1516-89132003000300011.
http://dx.doi.org/10.1590/S1516-89132003...
; 2003bAlbertoni, E.F., Palma-Silva, C., & Esteves, F.A., 2003b. Overlap of dietary niche and electivity of three shrimp species (Crustacea, Decapoda) in a tropical coastal lagoon (Rio de Janeiro, Brazil). Rev. Bras. Zool. 20(1), 135-140. http://dx.doi.org/10.1590/S0101-81752003000100017.
http://dx.doi.org/10.1590/S0101-81752003...
). With relatively short life cycles and high sensitivity to environmental pollutants, decapods are considered useful indicators of water quality (Rinderhagen et al., 2000Rinderhagen, M., Ritterhoff, J., & Zauke, G.P., 2000. Crustaceans as bioindicators. In: Gerhart, A., ed. Biomonitoring of Polluted Water - Reviews on Actual Topics. Zurich: Trans Tech Scitech Publications, 161-194.; Pârvulescu et al., 2011Pârvulescu, L., Pacioglu, O. & Hamchevici, C., 2011. The assessment of the habitat and water quality requirements of the stone crayfish (Austropotamobius torrentium) and noble crayfish (Astacus astacus) species in the rivers from the Anina Mountains (SW Romania). Knowl. Manag. Aquat. Ecosyst. 401, 03. https://doi.org/10.1051/kmae/2010036.
https://doi.org/10.1051/kmae/2010036...
; Kuklina et al., 2013Kuklina, I., Kouba, A., & Kozák, P., 2013. Real-time monitoring of water quality using fish and crayfish as bio-indicators: a review. Environ. Monit. Assess. 185(6), 5043-5053. PMid:23054288. http://dx.doi.org/10.1007/s10661-012-2924-2.
http://dx.doi.org/10.1007/s10661-012-292...
) as they respond rapidly to both natural and anthropogenic environmental impacts (Füreder & Reynolds 2003Füreder, L., & Reynolds, J.D., 2003. Is Austropotamobius pallipes a good bioindicator? Bull. Fr. Peche Piscic. 370-371(370-371), 157-163. http://dx.doi.org/10.1051/kmae:2003011.
http://dx.doi.org/10.1051/kmae:2003011...
; Alcorlo et al., 2006Alcorlo, P., Otero, M., Crehuet, M., Baltanás, A., & Montes, C., 2006. The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci. Total Environ. 366(1), 380-390. PMid:16546239. http://dx.doi.org/10.1016/j.scitotenv.2006.02.023.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Rak et al., 2011Rak, A.E., Said, I., Mohamed, M., & Abas, A., 2011. Effect of logging activities on water quality and benthic macroinvertebrate assemblages of Madek River Basin, Kluang, Johor, Malaysia. J. Appl. Sci. Environ. Manag. 15(2), 337-340. http://dx.doi.org/10.4314/jasem.v15i2.68518.
http://dx.doi.org/10.4314/jasem.v15i2.68...
).
Environmental, habitat and other factors, such as currents, temperature, salinity, nutrients, light, food availability and length of the larval life cycle influence the spatial and seasonal distribution of many decapods, and may also interfere in the composition, abundance and diversity of species, especially during the larval planktonic phase (Anger, 2001Anger, K., 2001. The biology of decapod crustacean larvae - Crustacean Issues. Netherlands: A. A. Balkema Publishers.; Koettker & Freire, 2006Koettker, A.G., & Freire, A.S., 2006. Spatial and temporal distribution of decapod larvae in the subtropical waters of the Arvoredo archipelago, SC, Brazil. Iheringia Ser. Zool. 96(1), 31-40. http://dx.doi.org/10.1590/S0073-47212006000100005.
http://dx.doi.org/10.1590/S0073-47212006...
; Landeira et al., 2010Landeira, J.M., Lozano-Soldevilla, F., Hernández-León, S., & Barton, E.D., 2010. Spatial variability of planktonic invertebrate larvae in the Canary Islands area. J. Mar. Biol. Assoc. U. K. 90(6), 1217-1225. http://dx.doi.org/10.1017/S0025315409990750.
http://dx.doi.org/10.1017/S0025315409990...
). Estuarine zones, located at the interface between river and sea, undergo wide spatial and seasonal variability in environmental conditions due to the influences of both continental riverine input and coastal waters (Bald et al., 2005Bald, J., Borja, A., Muxika, I., Franco, J., & Valencia, V., 2005. Assessing reference conditions and physico-chemical status according to the European Water Framework Directive: a case-study from the Basque Country (Northern Spain). Mar. Pollut. Bull. 50(12), 1508-1522. PMid:16038947. http://dx.doi.org/10.1016/j.marpolbul.2005.06.019.
http://dx.doi.org/10.1016/j.marpolbul.20...
).
Anthropogenic disturbances affect water quality and consequently aquatic biodiversity, and may interfere with trophic structure, spatio-temporal distribution of individuals and cause a decrease in species diversity (Eddy, 2005Eddy, F.B., 2005. Ammonia in estuaries and effects on fish. J. Fish Biol. 67(6), 1495-1513. http://dx.doi.org/10.1111/j.1095-8649.2005.00930.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
; Vieira & Shibatta, 2007Vieira, D.B., & Shibatta, O., 2007. Peixes como indicadores da qualidade ambiental do ribeirão Esperanca, município de Londrina, Paraná, Brasil. Biota Neotrop. 7(1), 57-65. http://dx.doi.org/10.1590/S1676-06032007000100008.
http://dx.doi.org/10.1590/S1676-06032007...
). Located to the west of Belém city (State capital of Pará, Brazil), and extending to Mosqueiro Island, Guajará Bay is formed by the convergence of the Guamá and Acará rivers. A group of islands and channels/creeks characterize the left bank of Guajará Bay. This region suffers from the effects of urban influences from Belém, such as the release of untreated sewage waste, fish processing industries and petrochemical residues, which are released directly into the Guajará Bay (Ribeiro, 2004Ribeiro, K.T.S., 2004. Água e saúde em Belém. Belém: Cejup.; Viana et al., 2010Viana, A.P., Lucena Frédou, F., Frédou, T., Torres, M.F., & Bordalo, A.O., 2010. Fish fauna as an indicator of environmental quality in an urbanised region of the Amazon estuary. J. Fish Biol. 76(3), 467-486. PMid:20666891. http://dx.doi.org/10.1111/j.1095-8649.2009.02487.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
; Wanderley, 2010Wanderley, C.M.S., 2010. Estudo da assembléia de larvas de peixes em relação à hidrodinâmica e à qualidade do ambiente aquático nas ilhas do Combu e Murucutu (Belém – Pará). Belém: Universidade Federal do Pará.).
The island of Mosqueiro is the largest, most urbanized and populous island around the city of Belém, with around 30 thousand inhabitants (IBGE, 2010Instituto Brasileiro de Geografia e Estatística – IBGE, 2010. Censo Brasileiro de 2010. Rio de Janeiro: IBGE. Retrieved in 2020, August 28, from https://www.ibge.gov.br/estatisticas/sociais/populacao/9662-censo-demografico-2010.html?=&t=resultados
https://www.ibge.gov.br/estatisticas/soc...
), has access both by river and by road and has a fishing port that supplies the markets of Belém and receives an intense flow of tourists to its 24 beaches (Ribeiro et al., 2013Ribeiro, O.W., Costa, M.A.F., & Costa Tavares, M.G., 2013. As práticas turísticas na orla oeste da Ilha de Mosqueiro, Região Metropolitana de Belém, PA. Rosa Ventos (Caxias Sul) 5(3), 528-544.). On the contrary, the smaller islands, Combu, Onças, and Cotijuba have little or no urbanization and are characterized by small, scattered riverine populations, which mainly carry out harvesting of fruit and small-scale artisanal fishing (Ribeiro, 2004Ribeiro, K.T.S., 2004. Água e saúde em Belém. Belém: Cejup.). In this context, the present study investigates how the spatio-temporal variation, abundance and diversity of decapod crustaceans are structured in relation to human activities in Guajará Bay and Mosqueiro Island, in the eastern Brazilian Amazon.
2. Material and Methods
2.1. Study area and sampling
Guajará Bay is formed by an island complex (Combu, Onças, and Cotijuba Islands) in the southern arm of the Amazon estuary, and further north is Mosqueiro Island, the largest of the four islands, which together, form a unique and dynamic aquatic environment characterized by high levels of fluvial and tidal energy and large amounts of suspended material (Dillenburg & Hesp, 2008Dillenburg, S.R., & Hesp, P.A., 2008. Geology and geomorphology of Holocene coastal barriers of Brazil. Berlin: Springer-Verlag.; Souza-Filho et al., 2011Souza-Filho, P.W.M., Paradella, W.R., Rodrigues, S.W., Costa, F.R., Mura, J.C., & Gonçalves, F.D., 2011. Discrimination of coastal wetland environments in the Amazon region based on multi-polarized L-band airborne Synthetic Aperture Radar imagery. Estuar. Coast. Shelf Sci. 95(1), 88-98. http://dx.doi.org/10.1016/j.ecss.2011.08.011.
http://dx.doi.org/10.1016/j.ecss.2011.08...
; Rosa-Filho et al., 2018), limiting the penetration of sunlight (Smith, 2002Smith, N.J., 2002. Amazon sweet sea: land, life, and water at the river’s mouth. Austin: University of Texas Press. http://dx.doi.org/10.7560/777705.
http://dx.doi.org/10.7560/777705...
; Gregório & Mendes, 2011Gregório, A.M.S., & Mendes, A.C., 2011. Batimetria e sedimentologia da baía de Guajará, Belém, Estado do Pará, Brasil. Amazônia 5: 53-72.). Semi-diurnal macrotides (up to 6 m in Pará) (DHN, 2010Diretoria de Hidrografia e Navegação – DHN, 2010. Tábuas de maré para o fundeadouro de Salinópolis (Estado do Pará). Retrieved in 2020, November 25, from http://www.dhn.mar.mil.br/chm/tabuasS
http://www.dhn.mar.mil.br/chm/tabuasS...
) flood channel banks in the region; significant saltwater entry only occurs during the driest months (July-December), when precipitation and river discharge are lower (Souza-Filho et al., 2011Souza-Filho, P.W.M., Paradella, W.R., Rodrigues, S.W., Costa, F.R., Mura, J.C., & Gonçalves, F.D., 2011. Discrimination of coastal wetland environments in the Amazon region based on multi-polarized L-band airborne Synthetic Aperture Radar imagery. Estuar. Coast. Shelf Sci. 95(1), 88-98. http://dx.doi.org/10.1016/j.ecss.2011.08.011.
http://dx.doi.org/10.1016/j.ecss.2011.08...
; Rosa-Filho et al., 2018).
Decapod sampling took place every three months between October 2013 and May 2014 at differing numbers of sites on three islands around Guajará Bay; Combu (n=2), Onças (n=4), and Cotijuba (n=2) and Mosqueiro Island (n=7) (Figure 1). To standardize the sampling locations, the number of creeks per island was decided as a function of the area of the respective island (km2). Sampling periods were categorized according to the prevailing season: Dry (October to November), Transition Dry to Wet or TDW (December to January), and Wet (February to May).
Sites at Combu Island (Combu, Piriquitaquara), Onças Island (Madre de Deus, Nazário, Piramanha Alto, Laranjeira), Cotijuba Island (Fazendinha, Novo Piri) in Guajará Bay and Mosqueiro Island (Baia do Sol, Barreiras, Mari Mari, Murubira, Pirajussara, Pratiquara, Sucurijuquara) sampled for decapod crustaceans between October 2013 and May 2014.
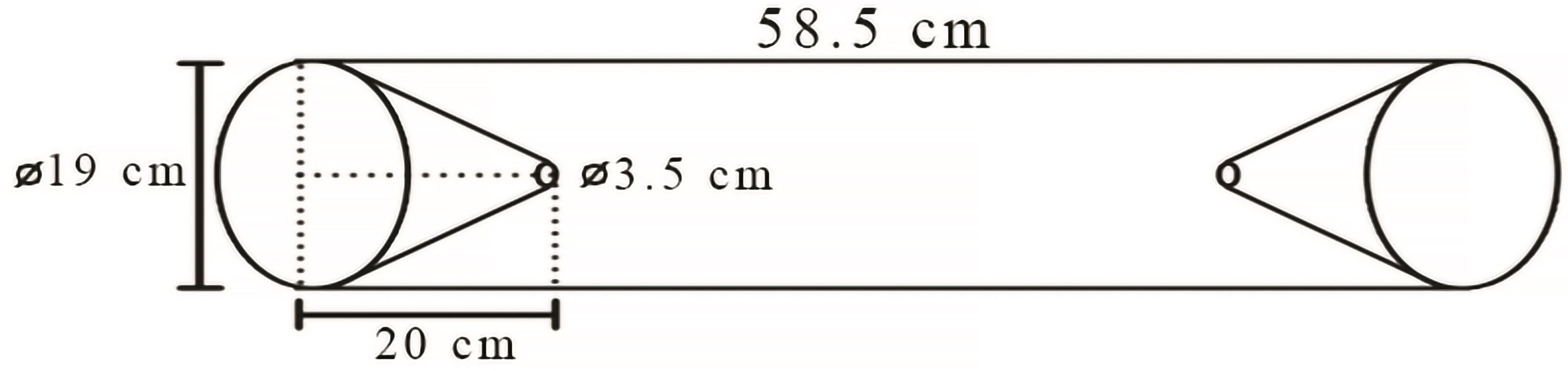
Specimens were collected with artisanal traps, known locally as matapis (Figure 2), which are made from thin rods of palm fronds (Astrocaryum spp. and Atrix spp.), baited with babaçu (Orbignya speciosa) flour. Five matapis were used at each site, arranged equidistant along a local stream, between its mouth and source. The traps were set two days before the new moon, on the last low tide of the day. On the first low tide of the following day (12 hours later), the traps were retrieved (Freire et al., 2012Freire, J.L., Marques, C.B., & Bentes, B., 2012. Estrutura populacional e biologia reprodutiva do camarão-da-amazônia Macrobrachium amazonicum (Heller,1862) (Decapoda: Palaemonidae) em um estuário da região nordeste do Pará, Brasil. Braz. J. Aquat. Sci. Tech. 16(2), 65-76. http://dx.doi.org/10.14210/bjast.v16n2.p65-76.
http://dx.doi.org/10.14210/bjast.v16n2.p...
). Due to sampling problems, data for Onças Island are not available for the transition period from dry to rainy (TDW).
Schematic diagram of an artisanal matapi trap for sampling decapod crustaceans between October 2013 and May 2014 in the Guajará Bay and Mosqueiro Island.
Specimens of shrimps and crabs collected in the traps were identified to the lowest possible taxonomic level. Salinity, temperature (°C), dissolved oxygen (% saturation), and pH were measured with a Hanna HI 9828 digital meter at both deployment and removal of traps, from which a mean value was calculated.
2.2. Data analysis
Density (N) was the sum per island of the individuals collected in the five matapis in each stream channel (site) in each season. Since the number of streams channel per island was decided based on their respective area, the total number of matapis per island was different at each station. Due to interference or loss of sampling units, faunal replicates were absent or low in certain seasons at some islands, and thus no attempt was made to carry out a statistical analysis on the ecological indices data. For each decapod sample, species richness was determined, and the Margalef (D), equitability (J') and Shannon diversity (H') indices were calculated in the Diverse routine in PRIMER 6.0. These ecological indices were chosen because they are commonly used in studies with abundance of decapod crustacean, responding in a robust way to the comparison of the fauna among the islands.
To check if decapod species richness was adequately sampled, species accumulation curves were generated for cumulative sampling effort on each island and the total cumulative sampling effort using the jackknife1 estimator, with sites added in random order, using the specaccum() function in the vegan package (Oksanen et al., 2020Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2020. Vegan: Community Ecology Package. R package version 2.5-7. Retrieved in 2021, January 12, from https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
) in GNU R 4.0.4 (R Core Team, 2021R Core Team, 2021. R: A Language and Environment for Statistical Computing. Retrieved in 2022, February 8, from https://www.R-project.org/
https://www.R-project.org/...
).
Ordination by canonical analysis of principal coordinates (CAP, Anderson & Willis, 2003Anderson, M.J., & Willis, T.J., 2003. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84(2), 511-525. http://dx.doi.org/10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2.
http://dx.doi.org/10.1890/0012-9658(2003...
) using the capscale() function in the vegan package (Oksanen et al., 2020Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2020. Vegan: Community Ecology Package. R package version 2.5-7. Retrieved in 2021, January 12, from https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
) on a Bray–Curtis dissimilarity matrix calculated from decapod crustacean species presence and absence data, was used to verify differences in species occurrence and composition among islands and seasons, as well as determine the degree of association with abiotic factors, also using the envfit() function in the vegan package.
Differences in the multivariate pattern of variation in environmental variables were formally checked using permutational multivariate analysis of variance (PERMANOVA) with the adonis() function in the vegan package (Oksanen et al., 2020Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2020. Vegan: Community Ecology Package. R package version 2.5-7. Retrieved in 2021, January 12, from https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
).
3. Results
3.1. Abiotic data
In the dry season, salinity varied between 0.01 and 1.78 among sites, being higher at Cotijuba and Mosqueiro, whereas temperature varied from 26.28 °C to 29.55 °C, being highest at Onças. In the dry to wet transition, salinity varied from 0.05 to 0.57, and temperature from 25.95 °C to 29.32 °C, tending to be highest at Mosqueiro. In the wet season, salinity was very low (0.01 to 0.25) and varied little among sites, but was relatively higher (0.25) at Mosqueiro sites. Temperature varied relatively more in the wet season (24.6 °C to 28.6 °C), being highest at Combu and decreasing to Mosqueiro.
Dissolved oxygen (% saturation), in general, showed moderate to high values in the dry and wet seasons, between 50 and 80, however, during the dry to wet transition, some Cotijuba and Mosqueiro measurements had markedly lower values around 10%. In the wet season, values were similar among islands. Values of pH were below 7 at all locations in the wet season, whereas in the dry season and in the dry to wet transition, pH varied more, with highest values of 8.56 and 8.77 occurring in the dry to wet transition (Figure 3). In general, pH tended to be higher at Cotijuba and Mosqueiro, and lower at Combu and Onças.
Seasonal variation in salinity, temperature (°C), dissolved oxygen (%) and pH at sites on Combu, Onças, and Cotijuba Islands in Guajará Bay and Mosqueiro Island in the Dry, Transition from Dry to Wet (TDW), and Wet seasons between October 2013 and May 2014.
Differences in environmental variables among islands and seasons were significant, the most important being seasonal differences, which explained 44.7% of the variation in the data (Table 1). However, interactions among islands, seasons and sites, that is, a lack of consistency in seasonal patterns in environmental variables among islands and the creeks sampled therein, were significant explaining 24.1%, and residual unexplained variability, a further 18.7% (Table 1).
Permutational multivariate analysis of variance (PERMANOVA) summary, testing for differences in decapod occurrence and composition sampled at sites on Combu, Onças, and Cotijuba Islands in Guajará Bay and Mosqueiro Island in the Dry, Transition from Dry to Wet (TDW), and Wet seasons between October 2013 and May 2014.
3.2. Biotic data
A total of 8,367 decapod crustaceans were captured, of which 7,371 were Macrobrachium amazonicum, the most common shrimp species (Table 2). Samples also included 36 freshwater crabs (including 30 Pachygrapsus gracilis) and two marine crabs, Callinectes bocourti. Considering the entire study period, the highest density of individuals (5,144), greatest species richness (10 species) and highest Margalef diversity (1.05) were recorded at Mosqueiro Island. Equitability was highest at Combu (0.28), and Shannon diversity (0.52) was highest at Onças Island.
Density (upper value) and relative frequency (%, lower value) of each decapod species captured at Combu, Onças, and Cotijuba Islands in Guajará Bay and Mosqueiro Island between October 2013 and May 2014, considering the entire study period.
In the dry season, density was higher in Cotijuba and Mosqueiro and species richness was generally similar among islands, but lower at Mosqueiro (Figure 4). Margalef diversity was similar among islands, being slightly lower at Mosqueiro. Eveness and Shannon diversity were similar among islands, except at Onças where values were lower. In the Transition from Dry to Wet (TDW), density was highest at Cotijuba, albeit a single observation, whereas richness, Margalef, Eveness and Shannon diversity were similar among Combu, Cotijuba and Mosqueiro islands (Figure 4). In the wet season, density increased from Combu to Mosqueiro. Species richness and Margalef diversity were highest at Onças and similar at the other islands. In contrast to density, Eveness and Shannon diversity decreased from Combu to Mosqueiro in the wet season (Figure 4). In general, ecological indices are similar in the dry and transition dry to wet seasons, and in the wet season, dominance occurs at Mosqueiro Island.
Ecological indices recorded for the decapod fauna at Combu, Onças, Cotijuba islands in Guajará Bay and Mosqueiro Island, in the Dry, Transition from Dry to Wet, and Wet seasons between October 2013 and May 2014. N = density, S = number of species, D = Margalef index, J’ = equitability, H’ = Shannon index. Data for Onças Island were not available for the Transition from Dry to Wet (TDW). Sample size varied from 1 to 7 (Dry), 0 to 6 (TDW) and 2 to 7 (Wet).
Onças Island appears to be well represented by sampling, reaching a well-defined plateau, as well as Combu and Cotijuba islands, which appear to be close to reaching a plateau. Mosqueiro Island, despite the higher sampling effort, is not close to the plateau, also reflected in the curve involving the total sampling effort at all the islands (Figure 5).
Species accumulation curves (Jackknife 1, random order of sites) for samples of decapods crustaceans in Guajará Bay and Mosqueiro Island between October 2013 and May 2014. Curves for each island and the total sampling effort.
Based on presence and absence data, the decapod fauna in Cotijuba was clearly distinct from that of Combu, whereas those of Mosqueiro and Onças overlapped considerably with the other islands (Figure 6A). Decapod occurrence and composition in the dry season was more distinct from that of the wet season and the dry to wet transition, the latter two overlapping entirely (Figure 6B). Macrobrachium sp., M. amazonicum and M. surinamicum were more associated with the dry season, when temperature, salinity and dissolved oxygen were higher, especially at Mosqueiro. Other species, especially, M. acanthurus, Pachygraulis gracillis, M. jelskii and others were associated with the wet season and the dry to wet transition, especially at Onças, Cotijuba and Combu islands. Of all the environmental variables, pH appeared to be least associated with the fauna by island or by season (Figure 6B).
Ordination of the first two axes resulting from the CAP analysis of decapod occurrence and species composition in samples trapped on islands Combu, Onças, Cotijuba islands in Guajará Bay and Mosqueiro Island in the Dry, Transition from Dry to Wet, and Wet seasons between October 2013 and May 2014 in relation to the abiotic variables salinity, temperature (°C), dissolved oxygen (% saturation) and pH. The ordination is overlaid with the ellipses of each island (A) and season (B).
The multivariate pattern in faunal occurrence was significantly, though weakly, associated (Envfit R2, P) with temperature (R2 = 0.064, P = 0.012) and salinity (R2 = 0.056, P = 0.034) but not with dissolved oxygen (R2 = 0.037, P = 0.098) and pH (R2 < 0.0001, P = 0.99).
4. Discussion
The Guajará bay can be considered as having the environmental characteristics of a river during almost all the year, due to discharge from the large Amazon and Tocantins/Araguia basin drainages, which effectively inhibit the advance of the oceanic saline wedge, especially during the rainy season. Guajará bay is most influenced by the mass of marine waters entering the mouth of the estuary in the dry season (Cavalcante et al., 2017Cavalcante, D.V., Bentes, B.S., & Martinelli-Lemos, J.M., 2017. Abundance and spatial-temporal distribution of Macrobrachium surinamicum Holthuis, 1948 (Palaemonidae) in the Amazon estuary, north of Brazil. Braz. J. Biol. 77(3), 594-601. PMid:27925014. http://dx.doi.org/10.1590/1519-6984.00316.
http://dx.doi.org/10.1590/1519-6984.0031...
).
Temperature showed relatively low variation in our data (24.63-29.55 °C). The constant water temperature is typical of the estuaries of the Amazon coast (Carvalho & Couto, 2011Carvalho, F.L., & Couto, E.C.G., 2011. Environmental variables influencing the Callinectes (Crustacea: Brachyura: Portunidae) species distribution in a tropical Estuary-Cachoeira River (Bahia, Brazil). J. Mar. Biol. Assoc. U. K. 91(4), 793-800. http://dx.doi.org/10.1017/S0025315410001700.
http://dx.doi.org/10.1017/S0025315410001...
) and confirms the thermal stability of equatorial coastal regions. Variation in water temperature among islands did not differ from that of other nearby locations, such as Vigia, 27.5 ºC and 28.0 ºC in the wet and dry seasons, respectively (Silva et al., 2002aSilva, K.C.A., Souza, R.A.L., & Cintra, I.H.A., 2002a. Camarão-cascudo Macrobrachium amazonicum (Heller, 1862) (Crustacea, Decapoda, Palaemonidae) no município de Vigia-Pará-Brasil. Bol. Tec.-Cient. CEPNOR 2(1), 41-73. http://dx.doi.org/10.32519/tjfas.v16i1.2135.
http://dx.doi.org/10.32519/tjfas.v16i1.2...
). Unlike temperate regions, where temperature is a seasonally limiting factor for decapod growth and distribution, in tropical regions, relatively constant favorable conditions allow for mating and larval development throughout the year (Costa & Soares-Gomes, 2009Costa, T., & Soares-Gomes, A., 2009. Population structure and reproductive biology of Uca rapax (Decapoda: Ocypodidae) in a tropical coastal lagoon, southeast Brazil. Zoologia 26(4), 647-657. http://dx.doi.org/10.1590/S1984-46702009000400009.
http://dx.doi.org/10.1590/S1984-46702009...
; Brandão et al., 2011Brandão, M.C., Stumpf, L., Macedo-Soares, L.C.P., & Freire, A.S., 2011. Spatial and temporal distribution of brachyuran crab larvae in Ibiraquera Lagoon, southern Brazil. Pan-Am. J. Aquat. Sci. 6(1), 16-27.).
The low variability in salinity (0.01-1.78) differs from that observed by Lima et al. (2019)Lima, F.A., Oliveira, T.F.D., & Martinelli-Lemos, J.M., 2019. Distribution of brachyuran larvae in an Amazonian estuary as evidence for retention and export. J. Crustac. Biol. 39(5), 602-612. http://dx.doi.org/10.1093/jcbiol/ruz051.
http://dx.doi.org/10.1093/jcbiol/ruz051...
who recorded salinity from 3 to 35 in the Marapanim, an Amazon estuary further east. However, our results confirm those of Rosário et al. (2016)Rosário, R.P., Borba, T.A., Santos, A.S., & Rollnic, M., 2016. Variability of salinity in Pará River Estuary: 2D analysis with flexible mesh model. J. Coast. Res. 75(10075), 128-132. http://dx.doi.org/10.2112/SI75-026.1.
http://dx.doi.org/10.2112/SI75-026.1...
in Guajará Bay, which show that this estuary does not present high salinity along its longitudinal profile, since there is rapid dilution of sea water by river discharge, thereby lowering salinity to between 0 and 5. Although in an estuarine region, our sampling sites were closer to the influence of large rivers, such as the Acará, Guamá, and Maguarí rivers, and thus our salinity values were low and invariable. Abrupt changes in sea level of up to six meters during the wet season are common in the region, due to a combination of macrotides and high freshwater discharge, resulting in oligohaline waters that keep the temperature and salinity constant in this estuarine region (Barros et al., 2011Barros, M.D.L.C., Sena, M.J.D.S., Mesquita, A.L.A., Blanco, C.J.C., & Secretan, Y., 2011. A water flow pattern analysis of Guajará Bay: Amazon Estuary-Brazil. J. Braz. Soc. Mech. Sci. Eng. 33(1), 79-85. http://dx.doi.org/10.1590/S1678-58782011000100012.
http://dx.doi.org/10.1590/S1678-58782011...
).
Intense mixing of waters of differing salinity allows coexistence of both marine and freshwater species according to seasonality (Bentes et al., 2011Bentes, B.S., Martinelli, J.M., Souza, L.S., Cavalcante, D.V., Almeida, M.C., & Isaac, V.J., 2011. Spatial distribution of the amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará). Braz. J. Biol. 71(4), 925-935. http://dx.doi.org/10.1590/S1519-69842011000500013.
http://dx.doi.org/10.1590/S1519-69842011...
). The results of the present study re-emphasize the influence of relatively low salinity levels on the distribution of the decapod species found in this region, which, according to Cavalcante et al. (2017)Cavalcante, D.V., Bentes, B.S., & Martinelli-Lemos, J.M., 2017. Abundance and spatial-temporal distribution of Macrobrachium surinamicum Holthuis, 1948 (Palaemonidae) in the Amazon estuary, north of Brazil. Braz. J. Biol. 77(3), 594-601. PMid:27925014. http://dx.doi.org/10.1590/1519-6984.00316.
http://dx.doi.org/10.1590/1519-6984.0031...
, would account for the occurrence of freshwater crab species - Sylviocarcinus devileii and Sylviocarcinus pictus, especially at Belém and Combu Island - where salinity was virtually zero throughout the year - as well as the presence of the marine-estuarine genera Uca and Callinectes on Mosqueiro Island. The wet season pH was slightly more acidic, as rainwater has a pH of around 5.5, and the influence of rainwater runoff may decrease pH. Variation in pH is the result of the interaction of several biogeochemical factors and is a primary indicator of water quality (Blume et al., 2010Blume, K.K., Macedo, J.C., Meneguzzi, A., Silva, L.B.D., Quevedo, D.M.D., & Rodrigues, M.A.S., 2010. Water quality assessment of the Sinos River, southern Brazil. Braz. J. Biol. 70(4, Suppl.), 1185-1193. PMid:21225160. http://dx.doi.org/10.1590/S1519-69842010000600008.
http://dx.doi.org/10.1590/S1519-69842010...
), however, regardless of the seasonal period, our values were close to those found by Montes et al. (2009)Montes, C.S., Ferreira, M.A.P., Santos, S.S.D., Von Ledebur, E.I.C.F., & Rocha, R.M., 2009. Branchial histopathological study of Brachyplatystoma rousseauxii (Castelnau, 1855) in the Guajará bay, Belém, Pará State, Brazil. Acta Sci. Biol. Sci. 32(1), 93-99. https://doi.org/10.4025/actascibiolsci.v32i1.4800.
https://doi.org/10.4025/actascibiolsci.v...
also in Guajará Bay, with a minimum of 5.10 and a maximum of 6.08, showing that anthropogenic factors did not appear associated with changes in pH in this period.
Relatively successful catches of species of the Macrobrachium genus seem to be a characteristic of Amazon estuary. However, the species M. amazonicum presented a higher density than the other species of the same genus, this difference may be related to the ecological flexibility of this species (Bentes et al., 2014Bentes, B., Martinelli-Lemos, J.M., Paes, E.T., Fernandes, S.C.P., Paula, J.D., & Isaac, V., 2014. Experimental study on the efficiency of different types of traps and baits for harvesting Macrobrachium amazonicum (Heller, 1862). Acta Sci. Biol. Sci. 36(4), 383-391. http://dx.doi.org/10.4025/actascibiolsci.v36i4.22852.
http://dx.doi.org/10.4025/actascibiolsci...
). High densities of M. amazonicum were recorded at all sites, reflecting the tolerance of the species to environmental variation, which occurs worldwide in tropical and subtropical fresh and brackish waters, inhabiting a variety of ecosystems (Pileggi & Mantelatto, 2010Pileggi, L.G., & Mantelatto, F.L., 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae) with emphasis on the relationships among American species. Invertebr. Syst. 24(2), 194-208. http://dx.doi.org/10.1071/IS09043.
http://dx.doi.org/10.1071/IS09043...
; Vergamini et al., 2011Vergamini, F.G., Pileggi, L.G., & Mantelatto, F.L., 2011. Genetic variability of the Amazon river prawn Macrobrachium amazonicum (Decapoda, Caridea, Palaemonidae). Contrib. Zool. 80(1), 67-83. http://dx.doi.org/10.1163/18759866-08001003.
http://dx.doi.org/10.1163/18759866-08001...
) from continental to coastal environments with different salinity gradients; (Short, 2004Short, J.W., 2004. A revision of Australian river prawns, Macrobrachium (Crustacea: Decapoda: Palaemonidae). Hydrobiologia 525(1-3), 1-100. http://dx.doi.org/10.1023/B:HYDR.0000038871.50730.95.
http://dx.doi.org/10.1023/B:HYDR.0000038...
; Freire et al., 2017Freire, J.L., Bentes, B., Fontes, V.B., & da Silva, E.M., 2017. Morphometric discrimination among three stocks of Macrobrachium amazonicum in the Brazilian Amazon. Limnologica 64, 1-10. http://dx.doi.org/10.1016/j.limno.2017.01.007.
http://dx.doi.org/10.1016/j.limno.2017.0...
).
The presence of the exotic species Penaeus monodon in the Brazilian Amazon has been previously reported. Silva et al. (2002b) Silva, K., Ramos-Porto, M., & Cintra, I.H.A., 2002b. Registro de Penaeus monodon Fabricius, 1798, na plataforma continental do estado do Amapá (Crustacea, Decapoda, Penaeidae). Bol. Tec.-Cient. CEPNOR 2(1), 75-80.registered the presence of two specimens from the continental shelf of the State of Amapá. Cintra et al. (2011)Cintra, I.H.A., Paiva, K.S., Botelho, M.N., & Silva, K.C.A., 2011. Presence of Penaeus monodon in the continental shelf of the State of Para, Northern Brazil (Crustacea, Decapoda, Penaeidae). Rev. Ciênc. Agrár. 54(3), 314-317. http://dx.doi.org/10.4322/rca.2012.028.
http://dx.doi.org/10.4322/rca.2012.028...
reported the capture of an individual by an industrial fishing vessel operating on the continental shelf of the State of Pará and Cintra et al. (2014)Cintra, I.H.A., Viana, C.S., Silva, B.B., & Silva, K.C.A., 2014. Novos registros de camarão-tigre-gigante Penaeus monodon Fabricius, 1798, na plataforma continental Amazônica (Crustacea, Decapoda, Penaeidae). Biota Amazôn. 4(2), 172-175. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v4n2p172-175.
http://dx.doi.org/10.18561/2179-5746/bio...
recorded the capture of three more individuals in 2013 also by an industrial fishing vessel operating on the Amazon continental shelf in the State of Pará. The species P. monodon, which may have been introduced into Brazilian waters by ballast water from ships (Farrapeira et al., 2007Farrapeira, C.M.R., Melo, A.V.O.M., Barbosa, D.F., & Silva, K.M.E.D., 2007. Ship hull fouling in the port of Recife, Pernambuco. Braz. J. Oceanogr. 55(3), 207-221. http://dx.doi.org/10.1590/S1679-87592007000300005.
http://dx.doi.org/10.1590/S1679-87592007...
), may be a threat to local Amazonian species, as the region seems to represent an ideal environment for their survival (Cintra et al., 2014Cintra, I.H.A., Viana, C.S., Silva, B.B., & Silva, K.C.A., 2014. Novos registros de camarão-tigre-gigante Penaeus monodon Fabricius, 1798, na plataforma continental Amazônica (Crustacea, Decapoda, Penaeidae). Biota Amazôn. 4(2), 172-175. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v4n2p172-175.
http://dx.doi.org/10.18561/2179-5746/bio...
, Lutz et al., 2015Lutz, Í., Nascimento, M., Isaac, V., Raiol, M., Silva, U., Mourão, K., Cintra, I., & Bentes, B., 2015. First record of giant-tiger-shrimp Penaeus monodon Fabricius, 1798, in an upper Amazon Estuary. Biota Amazôn. 5(3), 115-116. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v5n3p115-116.
http://dx.doi.org/10.18561/2179-5746/bio...
). The introduction of exotic species into different ecosystems may affect the survival of native species (Rodríguez, 2001Rodríguez, J.P., 2001. La amenaza de las especies exóticas para la conservación de la biodiversidad suramericana. Interciencia 26(10), 479-483.), which may lead to competition for resources, especially food, between species, generate changes in the balance of predator-prey relationships, and significantly impact trophic webs (Snyder & Evans, 2006Snyder, W.E., & Evans, E.W., 2006. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. Syst. 37(1), 95-122. http://dx.doi.org/10.1146/annurev.ecolsys.37.091305.110107.
http://dx.doi.org/10.1146/annurev.ecolsy...
; Baum & Worm, 2009Baum, J.K., & Worm, B., 2009. Cascading top‐down effects of changing oceanic predator abundances. J. Anim. Ecol. 78(4), 699-714. PMid:19298616. http://dx.doi.org/10.1111/j.1365-2656.2009.01531.x.
http://dx.doi.org/10.1111/j.1365-2656.20...
; Lord et al., 2019Lord, J.P., Harper, E.M., & Barry, J.P., 2019. Ocean acidification may alter predator-prey relationships and weaken nonlethal interactions between gastropods and crabs. Mar. Ecol. Prog. Ser. 616, 83-94. http://dx.doi.org/10.3354/meps12921.
http://dx.doi.org/10.3354/meps12921...
).
The type of trap used in the present study may account for the reduced proportion of crabs (marine and freshwater) in the samples, although this had been expected, given that local coastal communities traditionally use these traps for the harvesting of shrimp (Odinetz-Collart & Moreira, 1993Odinetz-Collart, O., & Moreira, L.C., 1993. Potencial pesqueiro do camarão Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro). Amazoniana 12(3-4), 399-413.). At higher salinities – closer to the sea – density and the number of species increased. Mosqueiro Island is closest to the sea and is located within an area of intense mixing of saline and fresh waters, resulting in a nutrient-rich environment (Smith, 2002Smith, N.J., 2002. Amazon sweet sea: land, life, and water at the river’s mouth. Austin: University of Texas Press. http://dx.doi.org/10.7560/777705.
http://dx.doi.org/10.7560/777705...
), which may favor the density of decapods.
The CAP analysis identified an association between M. acanthurus and the wet season, which is consistent with Díaz Herrera et al. (1998)Díaz Herrera, F., Sierra Uribe, E., Fernando Bückle Ramirez, L., & Garrido Mora, A., 1998. Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: palaemonidae). J. Therm. Biol. 23(6), 381-385. http://dx.doi.org/10.1016/S0306-4565(98)00029-1.
http://dx.doi.org/10.1016/S0306-4565(98)...
, who recorded the tolerance of the species for freshwater or low salinity, with adults capable of osmoregulation in fresh and brackish water at temperatures above 15 °C. However, during the larval stage, M. acanthurus has low tolerance to salinity (Choudhury, 1971Choudhury, P.C., 1971. Laboratory rearing of larvae of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836). Crustaceana 21(2), 113-126. http://dx.doi.org/10.1163/156854071X00300.
http://dx.doi.org/10.1163/156854071X0030...
).
The composition of the decapod fauna in the present study was variable and related to both location and season. The resulting temporal and spatial mosaic of salinity favors, besides shrimps, other crustaceans such as crabs. M. jelskii is classified as a species restricted to freshwater (Melo, 2003Melo, G.A.S., 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo: Loyola.), as evidenced by a greater association with the inner estuary in Onças and Combu islands that receive a greater riverine inflow and less influence from saline coastal waters. In addition, M. jelskii has abbreviated larval development (Magalhães, 2000Magalhães, C., 2000. Abbreviated development of Macrobrachium jelskii (Miers, 1877) (Crustacea: Decapoda: Palaemonidae) from the Rio Solimões foodplain, Brazil, reared in the laboratory. Nauplius 8(1), 1-15.), where females carry only a few large eggs (Anger, 2013Anger, K., 2013. Neotropical Macrobrachium (Caridea: Palaemonidae): on the biology, origin and radiation of freshwater-invading shrimp. J. Crustac. Biol. 33(2), 151-183. http://dx.doi.org/10.1163/1937240X-00002124.
http://dx.doi.org/10.1163/1937240X-00002...
). Palaemonidae species with abbreviated larval development occupy strictly freshwater habitats (Rabalais & Gore, 1985Rabalais, N.N., & Gore, R.H., 1985. Abbreviated development in decapods. In: Schram, F.R., ed. Crustacean issues: larval growth. Rotterdam: A.A. Balkema, 67-126, 2nd ed.).
The relative abundance of M. amazonicum during the dry season reflects the population dynamics of this species, which tends to be rare during the wet season (Bentes et al., 2011Bentes, B.S., Martinelli, J.M., Souza, L.S., Cavalcante, D.V., Almeida, M.C., & Isaac, V.J., 2011. Spatial distribution of the amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará). Braz. J. Biol. 71(4), 925-935. http://dx.doi.org/10.1590/S1519-69842011000500013.
http://dx.doi.org/10.1590/S1519-69842011...
). The increased volume of water during the wet season may facilitate the dispersal of many species of crustacean and fish (Odinetz-Collart & Moreira, 1993Odinetz-Collart, O., & Moreira, L.C., 1993. Potencial pesqueiro do camarão Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro). Amazoniana 12(3-4), 399-413.). Densities may thus be higher during the dry season, when river volume decreases and many individuals move out of the floodplain lakes, facilitating trapping. Variation in shrimp catches in the Amazon depends on the intensity of the floods, which interfere with survival and growth. In Amazon basin floodplain lakes, catches are higher during the dry season and lower during the wet season, due to the wide spatial dispersion of individuals with higher water volume and flow (Odinetz-Collart & Moreira, 1993Odinetz-Collart, O., & Moreira, L.C., 1993. Potencial pesqueiro do camarão Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro). Amazoniana 12(3-4), 399-413.). Although M. surinamicum is more associated with the dry period in our CAP analysis, Cavalcante et al. (2017)Cavalcante, D.V., Bentes, B.S., & Martinelli-Lemos, J.M., 2017. Abundance and spatial-temporal distribution of Macrobrachium surinamicum Holthuis, 1948 (Palaemonidae) in the Amazon estuary, north of Brazil. Braz. J. Biol. 77(3), 594-601. PMid:27925014. http://dx.doi.org/10.1590/1519-6984.00316.
http://dx.doi.org/10.1590/1519-6984.0031...
found that its distribution was not influenced by either salinity or temperature in the Guajará estuary, possibly since these factors did not change very much in the region, also evidenced in our results (salinity 0.01-1.78, temperature 24.63-29.55 °C).
Macrophyte wet season growth reduces shrimp intra-species competition, through increased microhabitat availability in the early stages of life (Nurminen et al., 2007Nurminen, L., Horppila, J., & Pekcan-Hekim, Z., 2007. Effect of light and predator abundance on the habitat choice of plant-attached zooplankton. Freshw. Biol. 52(3), 539-548. http://dx.doi.org/10.1111/j.1365-2427.2007.01724.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
) and introduction of suspended material increases productivity and energy flow (Paiva et al., 2006Paiva, R.S., Eskinazi-Leça, E., Passavante, J.Z.D.O., Da Silva-Cunha, M.G.G., & De Melo, N.F.A.C., 2006. Considerações ecológicas sobre o fitoplâncton da baía do Guajará e foz do rio Guamá, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi Ciênc. Nat. 1(2), 133-146. http://dx.doi.org/10.46357/bcnaturais.v1i2.748.
http://dx.doi.org/10.46357/bcnaturais.v1...
). Tides are the major natural disturbance in mangrove areas along the Pará coast, directly affecting abundance of zooplankton, crustaceans, and fish, as well as promoting the entry and exit of wastes and nutrients (Dittmar & Lara, 2001Dittmar, T., & Lara, R.J., 2001. Molecular evidence for lignin degradation in sulfate-reducing mangrove sediments (Amazonia, Brazil). Geochim. Cosmochim. Acta 65(9), 1417-1428. http://dx.doi.org/10.1016/S0016-7037(00)00619-0.
http://dx.doi.org/10.1016/S0016-7037(00)...
; Schories et al., 2003Schories, D., Barletta Bergan, A., Barletta, M., Krumme, U., Mehlig, U., & Rademaker, V., 2003. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecol. Manage. 11(4), 243-255. http://dx.doi.org/10.1023/A:1025011431984.
http://dx.doi.org/10.1023/A:102501143198...
; Krumme et al., 2004Krumme, U., Saint-Paul, U., & Rosenthal, H., 2004. Tidal and diel changes in the structure of a nekton assemblage in small intertidal mangrove creeks in northern Brazil. Aquat. Living Resour. 17(2), 215-229. http://dx.doi.org/10.1051/alr:2004019.
http://dx.doi.org/10.1051/alr:2004019...
; Krumme & Liang, 2004Krumme, U., & Liang, T.H., 2004. Tidal-induced changes in a copepod-dominated zooplankton community in a macrotidal mangrove channel in northern Brazil. Zool. Stud. 43(2), 404-414.).
The highest shrimp density was observed on Mosqueiro Island, which is the largest and most populous island studied in present work (IBGE, 2010Instituto Brasileiro de Geografia e Estatística – IBGE, 2010. Censo Brasileiro de 2010. Rio de Janeiro: IBGE. Retrieved in 2020, August 28, from https://www.ibge.gov.br/estatisticas/sociais/populacao/9662-censo-demografico-2010.html?=&t=resultados
https://www.ibge.gov.br/estatisticas/soc...
). Thus, a larger human population may be related to higher amounts of suspended organic matter that is a food source for shrimp. Eutrophication processes due to phosphorous and nitrogen-rich compounds from domestic and industrial waste cannot be recognized as a positive scenario (Viana et al., 2010Viana, A.P., Lucena Frédou, F., Frédou, T., Torres, M.F., & Bordalo, A.O., 2010. Fish fauna as an indicator of environmental quality in an urbanised region of the Amazon estuary. J. Fish Biol. 76(3), 467-486. PMid:20666891. http://dx.doi.org/10.1111/j.1095-8649.2009.02487.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
), and causes long term deleterious effects in the benthic fauna (Dorgham, 2014Dorgham, M., 2014. Effects of Eutrophication. In: Ansari, A., Gill, S., ed. Eutrophication: causes, consequences and control. Dordrecht: Springer, 29-44. http://dx.doi.org/10.1007/978-94-007-7814-6_3.
http://dx.doi.org/10.1007/978-94-007-781...
). Eutrophication of aquatic environments via sewage generates poor water quality, represented by a higher biochemical oxygen demand and consequently low dissolved oxygen concentrations due to the proliferation of microorganisms. Miranda et al. (2016)Miranda, M.V.T., Santos, M.L.S., Pereira, J.A.R, & Mesquita, K.F.C., 2016. Índices de qualidade da água da Ilha de Mosqueiro-PA. Rev. DAE 64(201), 74-81. http://dx.doi.org/10.4322/dae.2015.005.
http://dx.doi.org/10.4322/dae.2015.005...
observed values above the allowed (5mg/L) by CONAMA (Conselho Nacional do Meio Ambiente) Resolution No. 357/2005 (Brasil, 2005Brasil. Conselho Nacional do Meio Ambiente – CONAMA, 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências (Resolução CONAMA n°. 357, de 17 de março de 2005). Diário Oficial da União [da] República Federativa do Brasil, Poder Executivo, Brasília, DF.) for biochemical oxygen demand and low values of dissolved oxygen on Mosqueiro Island, relating the disposal of urban effluents and vessels such as pollution sources. However, our values of dissolved oxygen were similar between the islands, especially during the wet and dry seasons, which does not seem to be a limiting factor for both species diversity and abundance.
Studies of density and diversity of species between areas that receive different levels of environmental impacts are of vital importance for understanding how biotic and abiotic dynamics react to these factors of pollution of estuarine and coastal waters. Basic physical-chemical parameters and ecological indices can be especially valuable for the understanding of the current status of an ecological community, as well as future impacts (Mason, 1991Mason, C.F. 1991. Biology of freshwater pollution. London: Longman Scientific & Technical.). While ecological indices are often criticized due to their limitations for understanding community dynamics, they can provide a useful comparative tool for the analysis of impacts on community structure (Morris et al., 2014Morris, E.K., Caruso, T., Buscot, F., Fischer, M., Hancock, C., Maier, T.S., Meiners, T., Müller, C., Obermaier, E., Prati, D., Socher, S.A., Sonnemann, I., Wäschke, N., Wubet, T., Wurst, S., & Rillig, M.C., 2014. Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 4(18), 3514-3524. PMid:25478144. http://dx.doi.org/10.1002/ece3.1155.
http://dx.doi.org/10.1002/ece3.1155...
), especially in the long-term, given that impacts on species diversity may often be extremely subtle or masked by the dynamic restructuring of the community (Kay et al., 2018Kay, G.M., Tulloch, A., Barton, P.S., Cunningham, S.A., Driscoll, D.A., & Lindenmayer, D.B., 2018. Species co‐occurrence networks show reptile community reorganization under agricultural transformation. Ecography 41(1), 113-125. http://dx.doi.org/10.1111/ecog.03079.
http://dx.doi.org/10.1111/ecog.03079...
).
Aquatic organisms may be exposed to multiple stressors, such as the discharge of pollutants into the water, overfishing, habitat modifications and so on (Scholz et al., 2012Scholz, N.L., Fleishman, E., Brown, L., Werner, I., Johnson, M.L., Brooks, M.L., Mitchelmore, C.L., & Schlenk, D., 2012. A perspective on modern pesticides, pelagic fish declines, and unknown ecological resilience in highly managed ecosystems. Bioscience 62(4), 428-434. http://dx.doi.org/10.1525/bio.2012.62.4.13.
http://dx.doi.org/10.1525/bio.2012.62.4....
; Ward et al., 2013Ward, D.J., Simpson, S.L., & Jolley, D.F., 2013. Slow avoidance response to contaminated sediments elicits sublethal toxicity to benthic invertebrates. Environ. Sci. Technol. 47(11), 5947-5953. PMid:23634897. http://dx.doi.org/10.1021/es400152a.
http://dx.doi.org/10.1021/es400152a...
; Hook et al., 2014Hook, S.E., Gallagher, E.P., & Batley, G.E., 2014. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 10(3), 327-341. PMid:24574147. http://dx.doi.org/10.1002/ieam.1530.
http://dx.doi.org/10.1002/ieam.1530...
). Due to their short life cycle, presence of a larval phase, and their importance as components in trophic webs, crustaceans are often used as bioindicators by water quality monitoring programs (Ghisi et al., 2017Ghisi, N.C., Oliveira, E.C., Guiloski, I.C., de Lima, S.B., Silva de Assis, H.C., Longhi, S.J., & Prioli, A.J., 2017. Multivariate and integrative approach to analyze multiple biomarkers in ecotoxicology: a field study in Neotropical region. Sci. Total Environ. 609, 1208-1218. PMid:28787795. http://dx.doi.org/10.1016/j.scitotenv.2017.07.266.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Kumar et al., 2017Kumar, N., Krishnani, K.K., Meena, K.K., Gupta, S.K., & Singh, N.P., 2017. Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171, 265-274. PMid:28027471. http://dx.doi.org/10.1016/j.chemosphere.2016.12.066.
http://dx.doi.org/10.1016/j.chemosphere....
). The islands in Guajará Bay and Mosqueiro Island are heterogeneous environments, with shipping and ports, domestic and industrial effluents, and fishing and aquaculture operations affecting primarily its eastern margin (Mosqueiro Island and the islands around Belém). Our results show that although this region suffers from greater anthropogenic disturbances, mainly because it has a large human population, the current diversity and density of shrimp and crabs appear not to be greatly affected. However, the current species diversity found within the study area may change significantly over time, leading to ecological imbalances, which may ultimately affect the area’s traditional artisanal fisheries.
Acknowledgements
We are grateful to the Banco Nacional de Desenvolvimento Econônimo e Social (BNDES - Brazil).
-
Cite as: Lutz, Í et al. Spatio-temporal variation in the density and diversity of decapods captured with artisanal traps in an Amazon estuary. Acta Limnologica Brasiliensia, 2022, vol. 34, e9.
References
- Albertoni, E.F., Palma-Silva, C., & Esteves, F.A., 2003a. Natural diet of three species of shrimp in a tropical coastal lagoon. Braz. Arch. Biol. Technol. 46(3), 395-403. http://dx.doi.org/10.1590/S1516-89132003000300011
» http://dx.doi.org/10.1590/S1516-89132003000300011 - Albertoni, E.F., Palma-Silva, C., & Esteves, F.A., 2003b. Overlap of dietary niche and electivity of three shrimp species (Crustacea, Decapoda) in a tropical coastal lagoon (Rio de Janeiro, Brazil). Rev. Bras. Zool. 20(1), 135-140. http://dx.doi.org/10.1590/S0101-81752003000100017
» http://dx.doi.org/10.1590/S0101-81752003000100017 - Alcorlo, P., Otero, M., Crehuet, M., Baltanás, A., & Montes, C., 2006. The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci. Total Environ. 366(1), 380-390. PMid:16546239. http://dx.doi.org/10.1016/j.scitotenv.2006.02.023
» http://dx.doi.org/10.1016/j.scitotenv.2006.02.023 - Amaral, K.D.S., Vieira, I.M., Osório, F.M., Rocha, J., & Lima, J.D.F., 2014. Bioecology of the crab Ucides cordatus (Crustacea, Decapoda) in mangroves influenced by the Amazon River, Brazil. Acta Amazon. 44(2), 213-222. http://dx.doi.org/10.1590/S0044-59672014000200007
» http://dx.doi.org/10.1590/S0044-59672014000200007 - Anderson, M.J., & Willis, T.J., 2003. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84(2), 511-525. http://dx.doi.org/10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
» http://dx.doi.org/10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2 - Anger, K., 2001. The biology of decapod crustacean larvae - Crustacean Issues. Netherlands: A. A. Balkema Publishers.
- Anger, K., 2013. Neotropical Macrobrachium (Caridea: Palaemonidae): on the biology, origin and radiation of freshwater-invading shrimp. J. Crustac. Biol. 33(2), 151-183. http://dx.doi.org/10.1163/1937240X-00002124
» http://dx.doi.org/10.1163/1937240X-00002124 - Bald, J., Borja, A., Muxika, I., Franco, J., & Valencia, V., 2005. Assessing reference conditions and physico-chemical status according to the European Water Framework Directive: a case-study from the Basque Country (Northern Spain). Mar. Pollut. Bull. 50(12), 1508-1522. PMid:16038947. http://dx.doi.org/10.1016/j.marpolbul.2005.06.019
» http://dx.doi.org/10.1016/j.marpolbul.2005.06.019 - Barros, M.D.L.C., Sena, M.J.D.S., Mesquita, A.L.A., Blanco, C.J.C., & Secretan, Y., 2011. A water flow pattern analysis of Guajará Bay: Amazon Estuary-Brazil. J. Braz. Soc. Mech. Sci. Eng. 33(1), 79-85. http://dx.doi.org/10.1590/S1678-58782011000100012
» http://dx.doi.org/10.1590/S1678-58782011000100012 - Barros, M.P., & Pimentel, F.R., 2001. A fauna de Decapoda (Crustácea) do Estado do Pará, Brasil: lista preliminar de espécies. Bol. Mus. Para. Emilio Goeldi Zool. 17(1), 15-41.
- Baum, J.K., & Worm, B., 2009. Cascading top‐down effects of changing oceanic predator abundances. J. Anim. Ecol. 78(4), 699-714. PMid:19298616. http://dx.doi.org/10.1111/j.1365-2656.2009.01531.x
» http://dx.doi.org/10.1111/j.1365-2656.2009.01531.x - Bentes, B., Martinelli-Lemos, J.M., Paes, E.T., Fernandes, S.C.P., Paula, J.D., & Isaac, V., 2014. Experimental study on the efficiency of different types of traps and baits for harvesting Macrobrachium amazonicum (Heller, 1862). Acta Sci. Biol. Sci. 36(4), 383-391. http://dx.doi.org/10.4025/actascibiolsci.v36i4.22852
» http://dx.doi.org/10.4025/actascibiolsci.v36i4.22852 - Bentes, B.S., Martinelli, J.M., Souza, L.S., Cavalcante, D.V., Almeida, M.C., & Isaac, V.J., 2011. Spatial distribution of the amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Caridea, Palaemonidae) in two perennial creeks of an estuary on the northern coast of Brazil (Guajará Bay, Belém, Pará). Braz. J. Biol. 71(4), 925-935. http://dx.doi.org/10.1590/S1519-69842011000500013
» http://dx.doi.org/10.1590/S1519-69842011000500013 - Blume, K.K., Macedo, J.C., Meneguzzi, A., Silva, L.B.D., Quevedo, D.M.D., & Rodrigues, M.A.S., 2010. Water quality assessment of the Sinos River, southern Brazil. Braz. J. Biol. 70(4, Suppl.), 1185-1193. PMid:21225160. http://dx.doi.org/10.1590/S1519-69842010000600008
» http://dx.doi.org/10.1590/S1519-69842010000600008 - Brandão, M.C., Stumpf, L., Macedo-Soares, L.C.P., & Freire, A.S., 2011. Spatial and temporal distribution of brachyuran crab larvae in Ibiraquera Lagoon, southern Brazil. Pan-Am. J. Aquat. Sci. 6(1), 16-27.
- Brasil. Conselho Nacional do Meio Ambiente – CONAMA, 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências (Resolução CONAMA n°. 357, de 17 de março de 2005). Diário Oficial da União [da] República Federativa do Brasil, Poder Executivo, Brasília, DF.
- Carvalho, F.L., & Couto, E.C.G., 2011. Environmental variables influencing the Callinectes (Crustacea: Brachyura: Portunidae) species distribution in a tropical Estuary-Cachoeira River (Bahia, Brazil). J. Mar. Biol. Assoc. U. K. 91(4), 793-800. http://dx.doi.org/10.1017/S0025315410001700
» http://dx.doi.org/10.1017/S0025315410001700 - Cavalcante, D.V., Bentes, B.S., & Martinelli-Lemos, J.M., 2017. Abundance and spatial-temporal distribution of Macrobrachium surinamicum Holthuis, 1948 (Palaemonidae) in the Amazon estuary, north of Brazil. Braz. J. Biol. 77(3), 594-601. PMid:27925014. http://dx.doi.org/10.1590/1519-6984.00316
» http://dx.doi.org/10.1590/1519-6984.00316 - Choudhury, P.C., 1971. Laboratory rearing of larvae of the palaemonid shrimp Macrobrachium acanthurus (Wiegmann, 1836). Crustaceana 21(2), 113-126. http://dx.doi.org/10.1163/156854071X00300
» http://dx.doi.org/10.1163/156854071X00300 - Cintra, I.H.A., Paiva, K.S., Botelho, M.N., & Silva, K.C.A., 2011. Presence of Penaeus monodon in the continental shelf of the State of Para, Northern Brazil (Crustacea, Decapoda, Penaeidae). Rev. Ciênc. Agrár. 54(3), 314-317. http://dx.doi.org/10.4322/rca.2012.028
» http://dx.doi.org/10.4322/rca.2012.028 - Cintra, I.H.A., Viana, C.S., Silva, B.B., & Silva, K.C.A., 2014. Novos registros de camarão-tigre-gigante Penaeus monodon Fabricius, 1798, na plataforma continental Amazônica (Crustacea, Decapoda, Penaeidae). Biota Amazôn. 4(2), 172-175. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v4n2p172-175
» http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v4n2p172-175 - Costa, T., & Soares-Gomes, A., 2009. Population structure and reproductive biology of Uca rapax (Decapoda: Ocypodidae) in a tropical coastal lagoon, southeast Brazil. Zoologia 26(4), 647-657. http://dx.doi.org/10.1590/S1984-46702009000400009
» http://dx.doi.org/10.1590/S1984-46702009000400009 - De Grave, S., & Fransen, C.H.J.M., 2011. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Netherlands: Zoologische Mededelingen Leiden.
- de Oliveira, D.B., Martinelli-Lemos, J.M., de Souza, A.S., da Costa, J.R., & Abrunhosa, F.A., 2016. Does retention or exportation occur in the larvae of the mud shrimp Upogebia vasquezi (Decapoda, Gebiidea)? Implications for the reproductive strategy of the species on the Amazon coast. Hydrobiologia 773(1), 241-252. http://dx.doi.org/10.1007/s10750-016-2708-8
» http://dx.doi.org/10.1007/s10750-016-2708-8 - Díaz Herrera, F., Sierra Uribe, E., Fernando Bückle Ramirez, L., & Garrido Mora, A., 1998. Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: palaemonidae). J. Therm. Biol. 23(6), 381-385. http://dx.doi.org/10.1016/S0306-4565(98)00029-1
» http://dx.doi.org/10.1016/S0306-4565(98)00029-1 - Dillenburg, S.R., & Hesp, P.A., 2008. Geology and geomorphology of Holocene coastal barriers of Brazil. Berlin: Springer-Verlag.
- Diretoria de Hidrografia e Navegação – DHN, 2010. Tábuas de maré para o fundeadouro de Salinópolis (Estado do Pará). Retrieved in 2020, November 25, from http://www.dhn.mar.mil.br/chm/tabuasS
» http://www.dhn.mar.mil.br/chm/tabuasS - Dittmar, T., & Lara, R.J., 2001. Molecular evidence for lignin degradation in sulfate-reducing mangrove sediments (Amazonia, Brazil). Geochim. Cosmochim. Acta 65(9), 1417-1428. http://dx.doi.org/10.1016/S0016-7037(00)00619-0
» http://dx.doi.org/10.1016/S0016-7037(00)00619-0 - Dorgham, M., 2014. Effects of Eutrophication. In: Ansari, A., Gill, S., ed. Eutrophication: causes, consequences and control. Dordrecht: Springer, 29-44. http://dx.doi.org/10.1007/978-94-007-7814-6_3
» http://dx.doi.org/10.1007/978-94-007-7814-6_3 - Eddy, F.B., 2005. Ammonia in estuaries and effects on fish. J. Fish Biol. 67(6), 1495-1513. http://dx.doi.org/10.1111/j.1095-8649.2005.00930.x
» http://dx.doi.org/10.1111/j.1095-8649.2005.00930.x - Farrapeira, C.M.R., Melo, A.V.O.M., Barbosa, D.F., & Silva, K.M.E.D., 2007. Ship hull fouling in the port of Recife, Pernambuco. Braz. J. Oceanogr. 55(3), 207-221. http://dx.doi.org/10.1590/S1679-87592007000300005
» http://dx.doi.org/10.1590/S1679-87592007000300005 - Freire, J.L., Bentes, B., Fontes, V.B., & da Silva, E.M., 2017. Morphometric discrimination among three stocks of Macrobrachium amazonicum in the Brazilian Amazon. Limnologica 64, 1-10. http://dx.doi.org/10.1016/j.limno.2017.01.007
» http://dx.doi.org/10.1016/j.limno.2017.01.007 - Freire, J.L., Marques, C.B., & Bentes, B., 2012. Estrutura populacional e biologia reprodutiva do camarão-da-amazônia Macrobrachium amazonicum (Heller,1862) (Decapoda: Palaemonidae) em um estuário da região nordeste do Pará, Brasil. Braz. J. Aquat. Sci. Tech. 16(2), 65-76. http://dx.doi.org/10.14210/bjast.v16n2.p65-76
» http://dx.doi.org/10.14210/bjast.v16n2.p65-76 - Füreder, L., & Reynolds, J.D., 2003. Is Austropotamobius pallipes a good bioindicator? Bull. Fr. Peche Piscic. 370-371(370-371), 157-163. http://dx.doi.org/10.1051/kmae:2003011
» http://dx.doi.org/10.1051/kmae:2003011 - Ghisi, N.C., Oliveira, E.C., Guiloski, I.C., de Lima, S.B., Silva de Assis, H.C., Longhi, S.J., & Prioli, A.J., 2017. Multivariate and integrative approach to analyze multiple biomarkers in ecotoxicology: a field study in Neotropical region. Sci. Total Environ. 609, 1208-1218. PMid:28787795. http://dx.doi.org/10.1016/j.scitotenv.2017.07.266
» http://dx.doi.org/10.1016/j.scitotenv.2017.07.266 - Gregório, A.M.S., & Mendes, A.C., 2011. Batimetria e sedimentologia da baía de Guajará, Belém, Estado do Pará, Brasil. Amazônia 5: 53-72.
- Hook, S.E., Gallagher, E.P., & Batley, G.E., 2014. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 10(3), 327-341. PMid:24574147. http://dx.doi.org/10.1002/ieam.1530
» http://dx.doi.org/10.1002/ieam.1530 - Instituto Brasileiro de Geografia e Estatística – IBGE, 2010. Censo Brasileiro de 2010. Rio de Janeiro: IBGE. Retrieved in 2020, August 28, from https://www.ibge.gov.br/estatisticas/sociais/populacao/9662-censo-demografico-2010.html?=&t=resultados
» https://www.ibge.gov.br/estatisticas/sociais/populacao/9662-censo-demografico-2010.html?=&t=resultados - Kay, G.M., Tulloch, A., Barton, P.S., Cunningham, S.A., Driscoll, D.A., & Lindenmayer, D.B., 2018. Species co‐occurrence networks show reptile community reorganization under agricultural transformation. Ecography 41(1), 113-125. http://dx.doi.org/10.1111/ecog.03079
» http://dx.doi.org/10.1111/ecog.03079 - Koettker, A.G., & Freire, A.S., 2006. Spatial and temporal distribution of decapod larvae in the subtropical waters of the Arvoredo archipelago, SC, Brazil. Iheringia Ser. Zool. 96(1), 31-40. http://dx.doi.org/10.1590/S0073-47212006000100005
» http://dx.doi.org/10.1590/S0073-47212006000100005 - Krumme, U., & Liang, T.H., 2004. Tidal-induced changes in a copepod-dominated zooplankton community in a macrotidal mangrove channel in northern Brazil. Zool. Stud. 43(2), 404-414.
- Krumme, U., Saint-Paul, U., & Rosenthal, H., 2004. Tidal and diel changes in the structure of a nekton assemblage in small intertidal mangrove creeks in northern Brazil. Aquat. Living Resour. 17(2), 215-229. http://dx.doi.org/10.1051/alr:2004019
» http://dx.doi.org/10.1051/alr:2004019 - Kuklina, I., Kouba, A., & Kozák, P., 2013. Real-time monitoring of water quality using fish and crayfish as bio-indicators: a review. Environ. Monit. Assess. 185(6), 5043-5053. PMid:23054288. http://dx.doi.org/10.1007/s10661-012-2924-2
» http://dx.doi.org/10.1007/s10661-012-2924-2 - Kumar, N., Krishnani, K.K., Meena, K.K., Gupta, S.K., & Singh, N.P., 2017. Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171, 265-274. PMid:28027471. http://dx.doi.org/10.1016/j.chemosphere.2016.12.066
» http://dx.doi.org/10.1016/j.chemosphere.2016.12.066 - Landeira, J.M., Lozano-Soldevilla, F., Hernández-León, S., & Barton, E.D., 2010. Spatial variability of planktonic invertebrate larvae in the Canary Islands area. J. Mar. Biol. Assoc. U. K. 90(6), 1217-1225. http://dx.doi.org/10.1017/S0025315409990750
» http://dx.doi.org/10.1017/S0025315409990750 - Lima, F.A., Oliveira, T.F.D., & Martinelli-Lemos, J.M., 2019. Distribution of brachyuran larvae in an Amazonian estuary as evidence for retention and export. J. Crustac. Biol. 39(5), 602-612. http://dx.doi.org/10.1093/jcbiol/ruz051
» http://dx.doi.org/10.1093/jcbiol/ruz051 - Lima, J.D.F., Garcia, J.D.S., & Silva, T.C., 2014. Natural diet and feeding habits of a freshwater prawn (Macrobrachium carcinus: Crustacea, Decapoda) in the estuary of the Amazon River. Acta Amazon. 44(2), 235-244. http://dx.doi.org/10.1590/S0044-59672014000200009
» http://dx.doi.org/10.1590/S0044-59672014000200009 - Lord, J.P., Harper, E.M., & Barry, J.P., 2019. Ocean acidification may alter predator-prey relationships and weaken nonlethal interactions between gastropods and crabs. Mar. Ecol. Prog. Ser. 616, 83-94. http://dx.doi.org/10.3354/meps12921
» http://dx.doi.org/10.3354/meps12921 - Lucena-Frédou, F., Rosa Filho, J.S., Silva, M.C.N., & Azevedo, E.F., 2010. Population dynamics of the river prawn, Macrobrachium amazonicum (Heller, 1862) (Decapoda, Palaemonidae) on Combu island (Amazon estuary). Crustaceana. 83(3), 277-290. http://dx.doi.org/10.1163/001121609X12596543952
» http://dx.doi.org/10.1163/001121609X12596543952 - Lutz, Í., Nascimento, M., Isaac, V., Raiol, M., Silva, U., Mourão, K., Cintra, I., & Bentes, B., 2015. First record of giant-tiger-shrimp Penaeus monodon Fabricius, 1798, in an upper Amazon Estuary. Biota Amazôn. 5(3), 115-116. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v5n3p115-116
» http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v5n3p115-116 - Magalhães, C., 2000. Abbreviated development of Macrobrachium jelskii (Miers, 1877) (Crustacea: Decapoda: Palaemonidae) from the Rio Solimões foodplain, Brazil, reared in the laboratory. Nauplius 8(1), 1-15.
- Magalhães, C., 2003. Brachyura: Pseudothelphusidae e Trichodactylidae. In: Melo, G.A.S. ed. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo: Loyola, 143-297.
- Mason, C.F. 1991. Biology of freshwater pollution. London: Longman Scientific & Technical.
- Melo, G.A.S., 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo: Loyola.
- Miranda, M.V.T., Santos, M.L.S., Pereira, J.A.R, & Mesquita, K.F.C., 2016. Índices de qualidade da água da Ilha de Mosqueiro-PA. Rev. DAE 64(201), 74-81. http://dx.doi.org/10.4322/dae.2015.005
» http://dx.doi.org/10.4322/dae.2015.005 - Montes, C.S., Ferreira, M.A.P., Santos, S.S.D., Von Ledebur, E.I.C.F., & Rocha, R.M., 2009. Branchial histopathological study of Brachyplatystoma rousseauxii (Castelnau, 1855) in the Guajará bay, Belém, Pará State, Brazil. Acta Sci. Biol. Sci. 32(1), 93-99. https://doi.org/10.4025/actascibiolsci.v32i1.4800
» https://doi.org/10.4025/actascibiolsci.v32i1.4800 - Morris, E.K., Caruso, T., Buscot, F., Fischer, M., Hancock, C., Maier, T.S., Meiners, T., Müller, C., Obermaier, E., Prati, D., Socher, S.A., Sonnemann, I., Wäschke, N., Wubet, T., Wurst, S., & Rillig, M.C., 2014. Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 4(18), 3514-3524. PMid:25478144. http://dx.doi.org/10.1002/ece3.1155
» http://dx.doi.org/10.1002/ece3.1155 - Nurminen, L., Horppila, J., & Pekcan-Hekim, Z., 2007. Effect of light and predator abundance on the habitat choice of plant-attached zooplankton. Freshw. Biol. 52(3), 539-548. http://dx.doi.org/10.1111/j.1365-2427.2007.01724.x
» http://dx.doi.org/10.1111/j.1365-2427.2007.01724.x - Odinetz-Collart, O., & Moreira, L.C., 1993. Potencial pesqueiro do camarão Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro). Amazoniana 12(3-4), 399-413.
- Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Szoecs, E., & Wagner, H., 2020. Vegan: Community Ecology Package. R package version 2.5-7. Retrieved in 2021, January 12, from https://CRAN.R-project.org/package=vegan
» https://CRAN.R-project.org/package=vegan - Oliveira, D.B., Silva, D.C., & Martinelli-Lemos, J.M., 2013. Larval and adult density of the porcellanid crab Petrolisthes armatus (Anomura: Porcellanidae) in an Amazon estuary, northern Brazil. Zoologia 30(6), 592-600. http://dx.doi.org/10.1590/S1984-46702013005000002
» http://dx.doi.org/10.1590/S1984-46702013005000002 - Paiva, R.S., Eskinazi-Leça, E., Passavante, J.Z.D.O., Da Silva-Cunha, M.G.G., & De Melo, N.F.A.C., 2006. Considerações ecológicas sobre o fitoplâncton da baía do Guajará e foz do rio Guamá, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi Ciênc. Nat. 1(2), 133-146. http://dx.doi.org/10.46357/bcnaturais.v1i2.748
» http://dx.doi.org/10.46357/bcnaturais.v1i2.748 - Pantaleão, J.A.F., Hirose, G.L., & Costa, R.C.D., 2012. Relative growth, morphological sexual maturity, and size of Macrobrachium amazonicum (Heller 1862) (Crustacea, Decapoda, Palaemonidae) in a population with an entirely freshwater life cycle. Invertebr. Reprod. Dev. 56(3), 180-190. http://dx.doi.org/10.1080/07924259.2011.587276
» http://dx.doi.org/10.1080/07924259.2011.587276 - Pârvulescu, L., Pacioglu, O. & Hamchevici, C., 2011. The assessment of the habitat and water quality requirements of the stone crayfish (Austropotamobius torrentium) and noble crayfish (Astacus astacus) species in the rivers from the Anina Mountains (SW Romania). Knowl. Manag. Aquat. Ecosyst. 401, 03. https://doi.org/10.1051/kmae/2010036
» https://doi.org/10.1051/kmae/2010036 - Pereira, G., De Stefano, H., Staton, J., & Farrell, B., 2002. Phylogenetic relationships in some species of the genus Macrobrachium based on nucleotide sequences of the mitochondrial gene cytochrome oxidase. In: Escobar-Briones E., Alvarez F., ed. Modern Approaches to the Study of Crustacea. Boston: Springer, 319-322. http://dx.doi.org/10.1007/978-1-4615-0761-1_44
» http://dx.doi.org/10.1007/978-1-4615-0761-1_44 - Pileggi, L.G., & Mantelatto, F.L., 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae) with emphasis on the relationships among American species. Invertebr. Syst. 24(2), 194-208. http://dx.doi.org/10.1071/IS09043
» http://dx.doi.org/10.1071/IS09043 - Pileggi, L.G., & Mantelatto, F.L., 2012. Taxonomic revision of doubtful Brazilian freshwater shrimp species of genus Macrobrachium (Decapoda, Palaemonidae). Iheringia Ser. Zool. 102(4), 426-437. http://dx.doi.org/10.1590/S0073-47212012005000012
» http://dx.doi.org/10.1590/S0073-47212012005000012 - R Core Team, 2021. R: A Language and Environment for Statistical Computing. Retrieved in 2022, February 8, from https://www.R-project.org/
» https://www.R-project.org/ - Rabalais, N.N., & Gore, R.H., 1985. Abbreviated development in decapods. In: Schram, F.R., ed. Crustacean issues: larval growth. Rotterdam: A.A. Balkema, 67-126, 2nd ed.
- Rak, A.E., Said, I., Mohamed, M., & Abas, A., 2011. Effect of logging activities on water quality and benthic macroinvertebrate assemblages of Madek River Basin, Kluang, Johor, Malaysia. J. Appl. Sci. Environ. Manag. 15(2), 337-340. http://dx.doi.org/10.4314/jasem.v15i2.68518
» http://dx.doi.org/10.4314/jasem.v15i2.68518 - Ribeiro, K.T.S., 2004. Água e saúde em Belém. Belém: Cejup.
- Ribeiro, O.W., Costa, M.A.F., & Costa Tavares, M.G., 2013. As práticas turísticas na orla oeste da Ilha de Mosqueiro, Região Metropolitana de Belém, PA. Rosa Ventos (Caxias Sul) 5(3), 528-544.
- Rinderhagen, M., Ritterhoff, J., & Zauke, G.P., 2000. Crustaceans as bioindicators. In: Gerhart, A., ed. Biomonitoring of Polluted Water - Reviews on Actual Topics. Zurich: Trans Tech Scitech Publications, 161-194.
- Rodríguez, J.P., 2001. La amenaza de las especies exóticas para la conservación de la biodiversidad suramericana. Interciencia 26(10), 479-483.
- Rosa Filho, J.S., Pereira, L.C.C., Aviz, D., Braga, C.F., Monteiro, M.C., Da Costa, R.A.M., Asp, N.E., & Beasley, C.R., 2018. Benthic Estuarine Assemblages of the Brazilian North Coast (Amazonia Ecoregion). In: Lana P., Bernardino A., ed. Brazilian Estuaries. Cham: Springer, 39-74. http://dx.doi.org/10.1007/978-3-319-77779-5_2
» http://dx.doi.org/10.1007/978-3-319-77779-5_2 - Rosário, R.P., Borba, T.A., Santos, A.S., & Rollnic, M., 2016. Variability of salinity in Pará River Estuary: 2D analysis with flexible mesh model. J. Coast. Res. 75(10075), 128-132. http://dx.doi.org/10.2112/SI75-026.1
» http://dx.doi.org/10.2112/SI75-026.1 - Scholz, N.L., Fleishman, E., Brown, L., Werner, I., Johnson, M.L., Brooks, M.L., Mitchelmore, C.L., & Schlenk, D., 2012. A perspective on modern pesticides, pelagic fish declines, and unknown ecological resilience in highly managed ecosystems. Bioscience 62(4), 428-434. http://dx.doi.org/10.1525/bio.2012.62.4.13
» http://dx.doi.org/10.1525/bio.2012.62.4.13 - Schories, D., Barletta Bergan, A., Barletta, M., Krumme, U., Mehlig, U., & Rademaker, V., 2003. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecol. Manage. 11(4), 243-255. http://dx.doi.org/10.1023/A:1025011431984
» http://dx.doi.org/10.1023/A:1025011431984 - Short, J.W., 2004. A revision of Australian river prawns, Macrobrachium (Crustacea: Decapoda: Palaemonidae). Hydrobiologia 525(1-3), 1-100. http://dx.doi.org/10.1023/B:HYDR.0000038871.50730.95
» http://dx.doi.org/10.1023/B:HYDR.0000038871.50730.95 - Silva, K.C.A., Souza, R.A.L., & Cintra, I.H.A., 2002a. Camarão-cascudo Macrobrachium amazonicum (Heller, 1862) (Crustacea, Decapoda, Palaemonidae) no município de Vigia-Pará-Brasil. Bol. Tec.-Cient. CEPNOR 2(1), 41-73. http://dx.doi.org/10.32519/tjfas.v16i1.2135
» http://dx.doi.org/10.32519/tjfas.v16i1.2135 - Silva, K., Ramos-Porto, M., & Cintra, I.H.A., 2002b. Registro de Penaeus monodon Fabricius, 1798, na plataforma continental do estado do Amapá (Crustacea, Decapoda, Penaeidae). Bol. Tec.-Cient. CEPNOR 2(1), 75-80.
- Smith, N.J., 2002. Amazon sweet sea: land, life, and water at the river’s mouth. Austin: University of Texas Press. http://dx.doi.org/10.7560/777705
» http://dx.doi.org/10.7560/777705 - Snyder, W.E., & Evans, E.W., 2006. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. Syst. 37(1), 95-122. http://dx.doi.org/10.1146/annurev.ecolsys.37.091305.110107
» http://dx.doi.org/10.1146/annurev.ecolsys.37.091305.110107 - Souza-Filho, P.W.M., Paradella, W.R., Rodrigues, S.W., Costa, F.R., Mura, J.C., & Gonçalves, F.D., 2011. Discrimination of coastal wetland environments in the Amazon region based on multi-polarized L-band airborne Synthetic Aperture Radar imagery. Estuar. Coast. Shelf Sci. 95(1), 88-98. http://dx.doi.org/10.1016/j.ecss.2011.08.011
» http://dx.doi.org/10.1016/j.ecss.2011.08.011 - Vergamini, F.G., Pileggi, L.G., & Mantelatto, F.L., 2011. Genetic variability of the Amazon river prawn Macrobrachium amazonicum (Decapoda, Caridea, Palaemonidae). Contrib. Zool. 80(1), 67-83. http://dx.doi.org/10.1163/18759866-08001003
» http://dx.doi.org/10.1163/18759866-08001003 - Viana, A.P., Lucena Frédou, F., Frédou, T., Torres, M.F., & Bordalo, A.O., 2010. Fish fauna as an indicator of environmental quality in an urbanised region of the Amazon estuary. J. Fish Biol. 76(3), 467-486. PMid:20666891. http://dx.doi.org/10.1111/j.1095-8649.2009.02487.x
» http://dx.doi.org/10.1111/j.1095-8649.2009.02487.x - Vieira, D.B., & Shibatta, O., 2007. Peixes como indicadores da qualidade ambiental do ribeirão Esperanca, município de Londrina, Paraná, Brasil. Biota Neotrop. 7(1), 57-65. http://dx.doi.org/10.1590/S1676-06032007000100008
» http://dx.doi.org/10.1590/S1676-06032007000100008 - Wanderley, C.M.S., 2010. Estudo da assembléia de larvas de peixes em relação à hidrodinâmica e à qualidade do ambiente aquático nas ilhas do Combu e Murucutu (Belém – Pará). Belém: Universidade Federal do Pará.
- Ward, D.J., Simpson, S.L., & Jolley, D.F., 2013. Slow avoidance response to contaminated sediments elicits sublethal toxicity to benthic invertebrates. Environ. Sci. Technol. 47(11), 5947-5953. PMid:23634897. http://dx.doi.org/10.1021/es400152a
» http://dx.doi.org/10.1021/es400152a - Wowor, D., Muthu, V., Meier, R., Balke, M., Cai, Y., & Ng, P.K.L., 2009. Evolution of life history traits in Asian freshwater prawns of the genus Macrobrachium (Crustacea: Decapoda: Palaemonidae) based on multilocus molecular phylogenetic analysis. Mol. Phylogenet. Evol. 52(2), 340-350. PMid:19489122. http://dx.doi.org/10.1016/j.ympev.2009.01.002
» http://dx.doi.org/10.1016/j.ympev.2009.01.002
Edited by
Publication Dates
-
Publication in this collection
01 Apr 2022 -
Date of issue
2022
History
-
Received
22 Dec 2020 -
Accepted
24 Jan 2022