ABSTRACT
Objective:
Magnetic resonance spectroscopy (MRS) is a powerful tool for structural studies of chemical compounds and biomolecules and also documented promising findings as a potential imaging technology in thyroid oncology. This prospective study was to ascertain the clinical significance of 3 Tesla MRS in the evaluation of patients with thyroid nodules (TNs) as an ancillary diagnostic technique for thyroid carcinoma.
Materials and methods:
Magnetic resonance spectroscopy at 3T at echo- times (TEs) 136 and 270 ms was carried out on 15 patients with total number of 32 TNs larger than 1 cm3, which all were surgically resected. Choline (Chol) to creatine (Cr) ratio was assessed at 136 and 270 TEs on each nodule and a receiver operating characteristic (ROC) curve was used to determine optimal cut-off point. The findings were compared with histopathology of thyroid specimens.
Results:
There were 23 benign and 9 malignant lesions (7 papillary and 2 follicular thyroid carcinomas). The mean values of Chol/Cr at 136 and 270 TEs was 2.28 ± 3.65 and 1.52 ± 1.67 respectively and the difference between benign and malignant nodules was only significant at 136 TEs. The study revealed that Chol/ Cr ratio cut-off point of 2.5 best correlates with histopathology results (sensitivity = 75%; specificity = 100%; PPV = 100%; NPV= 92%).
Conclusion:
This preliminary study showed that 3T magnetic resonance spectroscopy might be a specific modality for the evaluation of thyroid nodules in differentiation of benign from malignant thyroid tissue. However, a larger series would give much greater confidence that this state-of-the-art technology will worth pursuing in clinical practice.
Keywords
Magnetic resonance spectroscopy; thyroid nodules; thyroid carcinoma; choline
INTRODUCTION
Thyroid nodules are quite common and estimated as being clinically presented in 4-7% of the population (11. Burman KD, Wartofsky L. Clinical Practice. Thyroid Nodules. N Engl J Med. 2015;373(24):2347-56.). Early and precise differentiation of malignant and benign nodules stays a remarkable diagnostic problem, but is critical to the patient's management (22. Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, et al. The natural history of benign thyroid nodules. JAMA. 2015;313(9):926-35.). Although there is not a consensus in the method of choice for initial evaluation, it is generally accepted that, adjunct with thyroid function tests, fine- needle aspiration cytology (FNAC), should be the first procedure to be done (33. Mandell DL, Genden EM, Mechanick JI, Bergman DA, Biller HF, Urken ML. Diagnostic accuracy of fine-needle aspiration and frozen section in nodular thyroid disease. Otolaryngol Head Neck Surg. 2001;124(5):531-6.). Nevertheless, with a reported sensitivity of 68-98% (mean 83%) and a specificity of 72-100% (mean 92%); FNAC does not seem to be a perfect diagnostic method for the assessment of thyroid nodules (44. Riazi A, Kalantarhormozi M, Nabipour I, Eghbali SS, Farzaneh M, Javadi H, et al. Technetium-99m methoxyisobutylisonitrile scintigraphy in the assessment of cold thyroid nodules: is it time to change the approach to the management of cold thyroid nodules? Nucl Med Commun. 2014;35(1):51-7.
5. Riazi A, Kalantarhormozi M, Nabipour I, Ostovar A, Javadi H, Assadi M. Comments on Wale et al.: Combined (99)mTc-methoxyisobutylisonitrile scintigraphy and fine-needle aspiration cytology offers an accurate and potentially cost-effective investigative strategy for the assessment of solitary or dominant thyroid nodules. Eur J Nucl Med Mol Imaging. 2014;41(3):575-6.-66. Nabipour I, Kalantarhormozi M, Assadi M. Thyroid nodule characterization using combined fine-needle aspiration and (99m)Tc-sestamibi scintigraphy strategy. AJR Am J Roentgenol. 2016;207(2):W21.).
The success of ultrasound as an inexpensive and noninvasive modality in discrimination benign from malignant nodules is presented in several reports (77. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology. 2018;287(1):29-36.,88. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL,Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587-95.). Although none of the sonographic properties have a reliable and sufficient sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in ascertaining malignancy of the nodules, the sonographic features of the nodules can assists in selecting the nodules for needle aspiration (77. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology. 2018;287(1):29-36.,99. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J. 2017;6(5):225-37.). Computed tomography of neck is often restricted by different artifacts (1010. Klinke T, Daboul A, Maron J, Gredes T, Puls R, Jaghsi A, et al.Artifacts in magnetic resonance imaging and computed tomography caused by dental materials. PLoS One. 2012;7(2):e31766.). Magnetic resonance spectroscopy (MRS) is the only non-invasive modality capable of measuring chemicals/metabolites within the body (1111. Minuto MN, Shintu L, Caldarelli S. Proteomics, and metabolomics: magnetic resonance spectroscopy for the presurgical screening of thyroid nodules. Curr Genomics. 2014;15(3):178-83.) as result of different magnetic frequency or chemical shifts (1111. Minuto MN, Shintu L, Caldarelli S. Proteomics, and metabolomics: magnetic resonance spectroscopy for the presurgical screening of thyroid nodules. Curr Genomics. 2014;15(3):178-83.). The concept that malignant cells develop a large number of proton nuclear magnetic resonance (1H NMR) visible molecules leads to potential role of MRS as a diagnostic modality in various cancers (1212. Noda Y, Kanematsu M, Goshima S, Kondo H, Watanabe H, Kawada H, et al. MRI of the thyroid for differential diagnosis of benign thyroid nodules and papillary carcinomas. AJR Am J Roentgenol. 2015;204(3):W332-5.). It is a powerful tool that has been used the nuclear magnetic resonance in NMR spectroscopy based upon the hydrogen-1 nuclei within the molecules of a matter, for the purpose of ascertaining the structure of its molecules (1313. Sugiki T, Kobayashi N, Fujiwara T Modern Technologies of Solution Nuclear Magnetic Resonance Spectroscopy for Threedimensional Structure Determination of Proteins Open Avenues for Life Scientists. Comput Struct Biotechnol J. 2017;15:328-39.).
Literature about MRS application especially using 3T for thyroid lesions is very limited. Most of the studies done initially were ex vivo, either MRS was performed on FNAC specimen or on tissue obtained at surgery (1414. Yoshioka Y, Sasaki J,Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and choline- containing lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13(7):377-83.
15. Jordan KW, Adkins CB, Cheng LL, Faquin WC. Application of magnetic-resonance-spectroscopy- based metabolomics to the fine-needle aspiration diagnosis of papillary thyroid carcinoma. Acta Cytol. 2011;55(6):584-9.-1616. Mackinnon WB, Delbridge L, Russell P, Lean CL, May GL, Doran S, et al. Two-dimensional proton magnetic resonance spectroscopy for tissue characterization of thyroid neoplasms. World J Surg. 1996;20(7):841-7.). Most few in vivo studies have been carried out with 1.5 T (1212. Noda Y, Kanematsu M, Goshima S, Kondo H, Watanabe H, Kawada H, et al. MRI of the thyroid for differential diagnosis of benign thyroid nodules and papillary carcinomas. AJR Am J Roentgenol. 2015;204(3):W332-5.,1515. Jordan KW, Adkins CB, Cheng LL, Faquin WC. Application of magnetic-resonance-spectroscopy- based metabolomics to the fine-needle aspiration diagnosis of papillary thyroid carcinoma. Acta Cytol. 2011;55(6):584-9.,1717. King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, et al. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005;54(1):112-7.).
The present study was carried out to assess the findings of MRS of thyroid nodules using a 3T MRI and its correlation with histopathology obtained at surgery.
MATERIALS AND METHODS
The present study was conducted in a university- affiliated tertiary referral Hospital from September 2010 to February 2013. A total number of 15 patients presenting with thyroid nodule larger than 1 cm3 were included in the study. Patients with previous thyroid surgery, known malignant disease or history of radiation were excluded. Moreover, patient's with contraindication for MRI such as claustrophobia and also being pace maker were excluded.
All MRI examinations were performed on a 3 Tesla MR unit (Magnetom Avanto; Siemens, Erlangen, Germany) with gradient strength of 33 mTs. Patients were positioned in supine position and were instructed not to swallow or move during the examination. Circularly polarized surface coil was placed over the neck. Fast scout scan in sagittal, axial and coronal planes was obtained. After localizing the lesion, the voxel was placed on the lesion and position checked in all three planes. The scan technique used was PRESS, single voxel technique. The sequence parameters employed with sampling numbers 512, averaging 16 and average scan time of 4.55 min. It was followed by water suppression pulses to be followed by data acquisition. Choline (Chol) to creatine (Cr) ratio was assessed at 136 and 270 TEs on each nodule. Image analyses were assessed by two radiologists and discrepant results were resolved by consensus and these findings were compared with the results of histopathological studies as gold standard test.
All patients had previously undergone ultrasound examination, FNAC and thyroid hormone measurements and also had previously been selected for surgery on the basis of their clinical and paraclinical results.
The study complies with the declaration of Helsinki and was approved by the institutional ethics committee of Tehran University of Medical Sciences, and all patients gave written informed consent.
Statistical analysis
The distribution of parameters was evaluated using probability plots and the Shapiro-Wilk test. Continuous variables are presented as mean ± SD, and categorical values as the absolute values and percentages. Fisher exact and chi-squared tests were applied to compare the qualitative variables and also T-test and Mann-Whitney U test between the quantitative variables in groups. The sensitivity, specificity, positive and negative predictive and also efficiency of values of each setting was acquired based on the definitive diagnosis established through histopathology. A receiver operating characteristic (ROC) curve analysis was done to determine optimal cut-off point for the discrimination of malignant from benign nodules. Cohen's kappa coefficient (κ) was applied inter-rater agreement of variables. Statistical analysis was performed using an IBM computer and PASW software, version 22.0 (SPSS, Inc., Chicago, USA).
RESULTS
A total of 15 patients (11 women, 4 men; mean age 39.5 ± 12.3 years, range 20-50) were included in this study. The total number of thyroid nodules was 32; 15 nodules were in the right lobe; 13 nodules in the left lobe and 4 nodules in the isthmus. Histological findings were malignant in 9 cases (seven cases of papillary carcinoma and two cases follicular carcinoma) and benign in 23 cases (Table 1). The volume of thyroid nodules was 11.67 cc; there was no significant difference in volume between malignant and benign nodules (p value > 0.05).
In term of metabolite profile semi quantitatively, significant choline peak was seen only in 7 cases (malignant = 7, benign = 0). The mean values of Chol/Cr at 136 and 270 TEs was 2.28 ± 3.65 and 1.52 ± 1.67 respectively and the difference between benign and malignant nodules was only significant at 136 TEs (p value < 0.05) (Table 2). Seven malignant cases showed significant choline peak at 3.22 ppm whereas 2 out of 9 malignant cases failed to show it. Among 23 benign lesions, none showed choline peak at 3.22 ppm.
Correlation of spectroscopic findings with pathologic diagnosis was presented in Table 3.
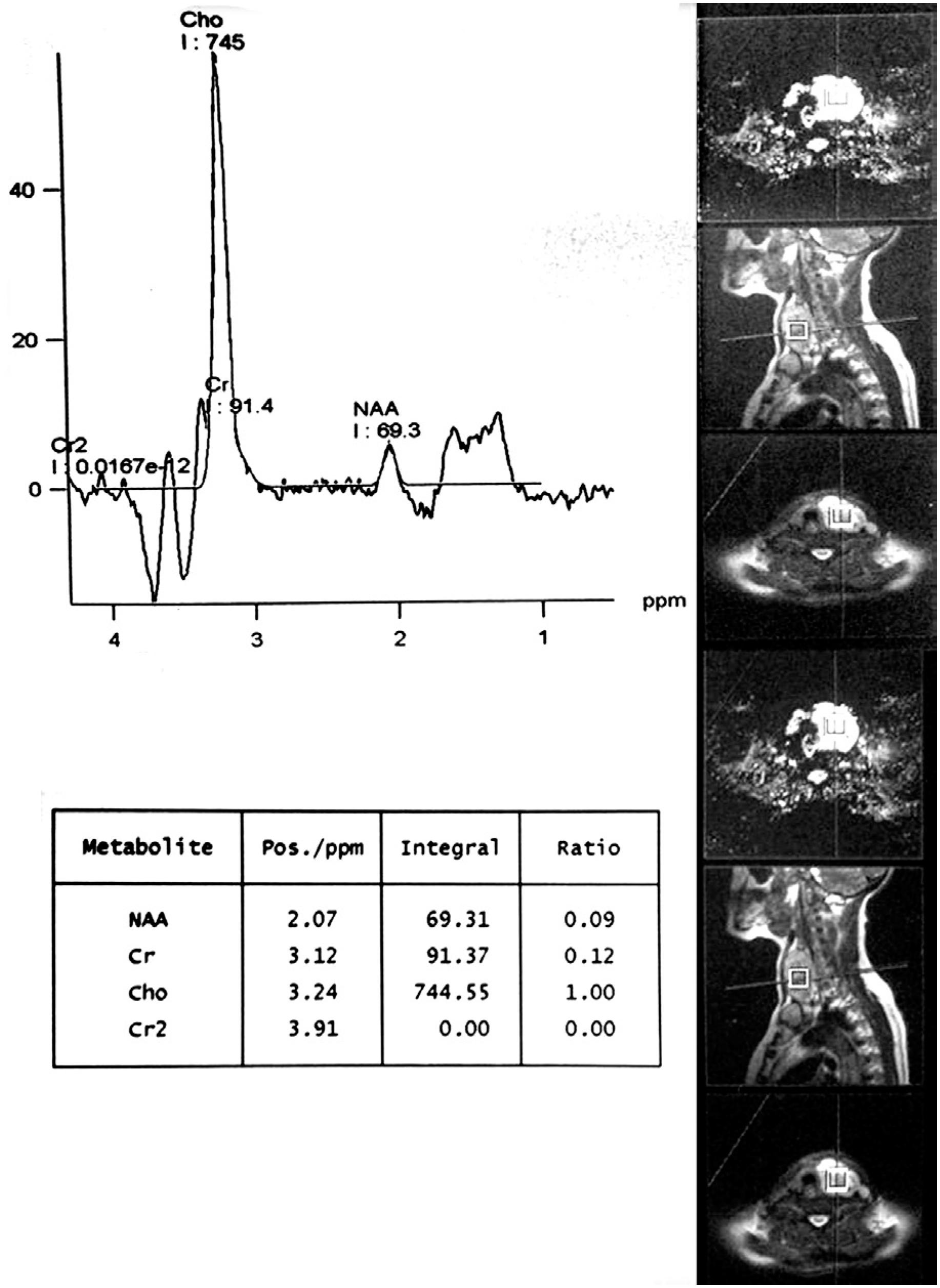
The area under the ROC curve (AUC) of Chol/Cr was significant at 136 TEs (0.86, p value = 0.00) while was not statistically at 270 TEs (0.78, p value = 0.68). Roc curve study revealed that Chol/Cr ratio at 136 TEs with a cut-off point of 2.5 best correlates with histopathology results (sensitivity = 75%; specificity = 100%; PPV = 100%; NPV= 92%) (Figures 1 and 2, Table 4).
Graph of a malignant thyroid nodule with a very high choline (Chol) to creatine (Cr) ratio on MRS.
Receiver operating characteristic curve analysis for the prediction of malignant thyroid nodules based on choline (Chol) to creatine (Cr) ratio at 136 TE.
DISCUSSION
The study depicted that the findings of 3T MRS of thyroid nodules correlated with histopathology obtained surgically.
In term of imaging, ultrasonography, as a main modality in the management of patients with thyroid nodules, has some very useful discriminatory values in distinguishing benign from malignant lesion (44. Riazi A, Kalantarhormozi M, Nabipour I, Eghbali SS, Farzaneh M, Javadi H, et al. Technetium-99m methoxyisobutylisonitrile scintigraphy in the assessment of cold thyroid nodules: is it time to change the approach to the management of cold thyroid nodules? Nucl Med Commun. 2014;35(1):51-7.,77. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology. 2018;287(1):29-36.,1818. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al.; AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis And Management OF Thyroid Nodules – 2016 Update. Endocr Pract. 2016;22(5):622-39.).
Though computed tomography and MR imaging studies allow a rapid and accurate assessment of the size of a goiter, its extension into the mediastinum, and its relationship to and impingement upon major structures with in the chest and neck, their accuracy is not high enough to different benign from malignant lesions (1919. Hoang JK, Branstetter BF 4th, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging. 2013;13:128-39.).
Parallel in anatomical and functional changes, in addition to histologic and cytologic alterations in malignancies, metabolic processes at the cellular level are also identified to trace changes. These metabolic changes can be discovered and quantified using a state of the art technique acknowledged as magnetic resonance spectroscopy (MRS) which has recently gained interest as a potential cancer imaging technology e.g. in thyroid cancer. Literature about MRS of in vivo evaluation of thyroid lesions is very limited (1717. King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, et al. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005;54(1):112-7.,2020. Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia. I: Discrimination between benign and malignant neoplasms. The American journal of medicine. 1994;96(4):383-8.
21. Gupta N, Goswami B, Chowdhury V, Ravishankar L, Kakar A. Evaluation of the role of magnetic resonance spectroscopy in the diagnosis of follicular malignancies of thyroid. Archives of surgery. 2011;146(2):179-82.-2222. Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. European journal of radiology. 2007;64(3):414-8.).
In a study, 1HMRS was carried out over tissue obtained at the time of surgery from 53 patients undergoing partial or total thyroidectomy for thyroid nodule (2020. Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia. I: Discrimination between benign and malignant neoplasms. The American journal of medicine. 1994;96(4):383-8.). When compared with histological diagnosis, 1HMRS distinguished normal thyroid tissue from invasive papillary, anaplastic and medullary carcinoma with P values of < 0.001 with negative predictive value 100% and specificity of 52% (2020. Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia. I: Discrimination between benign and malignant neoplasms. The American journal of medicine. 1994;96(4):383-8.).
Gupta and cols. studied 1.5T MRS in 25 patients with solitary thyroid nodule and they depicted a sensitivity and specificity of 100% and 94.11% respectively (2121. Gupta N, Goswami B, Chowdhury V, Ravishankar L, Kakar A. Evaluation of the role of magnetic resonance spectroscopy in the diagnosis of follicular malignancies of thyroid. Archives of surgery. 2011;146(2):179-82.). They also had worked previously on 26 patients using 1.5T MRS and demonstrated sensitivity of 100% and specificity of 88.88% (2222. Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. European journal of radiology. 2007;64(3):414-8.). King and cols. also worked 1HMRS in 13 patients of thyroid nodules with larger than 1 cm and demonstrated sensitivity of 87% and specificity of 100% (1717. King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, et al. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005;54(1):112-7.).
In the current study, 3T MRS was carried out on 32 thyroid nodules in which the Chol/Cr ratio cut-off point of 2.5 best correlates with histopathology results (sensitivity = 75%; specificity = 100%; PPV = 100%; NPV = 92%).
In this study, 2 out of 9 malignant cases failed to delineate the choline peak at 3.22 ppm which most probably because of small size of follicular cell carcinoma lesions.
In addition, 3 benign thyroid nodules showed Chol/Cr more than 1.5 but less than 2.5 which could be due to hyperplastic foci as result of increased hypercelluraity as shown in 2 hyperplastic nodules in prior study (2222. Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. European journal of radiology. 2007;64(3):414-8.).
In the current study, the sensitivity (75%) is less than two main prior studies (King and cols., 87%; Gupta and cols., 100%) while the specificity is comparable to two studies. It could be explained by two small follicular cancer lesions in our study.
In term of metabolite profile assessment, choline peak at 3.22 ppm is predominantly due to glycerophosphocholine and glycerophosphoethanolamine that form phospholipids of the cell membranes (2323. Bezabeh T, Ijare OB, Nikulin AE, Somorjai RL, Smith IC. MRS- based Metabolomics in Cancer Research. Magnetic resonance insights. 2014;7:1-14.). The choline content rises in malignancy because of rapid multiplication and proliferation of cells (2323. Bezabeh T, Ijare OB, Nikulin AE, Somorjai RL, Smith IC. MRS- based Metabolomics in Cancer Research. Magnetic resonance insights. 2014;7:1-14.). Height of choline peak depends on amount and nature of tissue under voxel. The creatinine peak indicates energy state of the cell (1414. Yoshioka Y, Sasaki J,Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and choline- containing lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13(7):377-83.,2424. Gujar SK, Maheshwari S, Bjorkman-Burtscher I, Sundgren PC. Magnetic resonance spectroscopy. Journal of neuroophthalmology: the official journal of the North American NeuroOphthalmology Society. 2005;25(3):217-26.).
In total, the numbers of cases especially cancers in this series is low and further studies with a larger series would give much greater confidence that the technique was worth pursuing in clinical practice.
In conclusion, magnetic resonance spectroscopy is a feasible option with promising results and it provided 100% specificity and 100% PPV in discrimination of benign from malignant thyroid nodules. Therefore, it can be complementary to other diagnostic techniques in patients with thyroid nodules; however, further exploration especially considering large number of patients with different thyroid nodules sizes is needed to validate its clinical role.
-
Disclosure: no potential conflict of interest relevant to this article was reported.
Acknowledgments: this study was the postgraduate thesis of Dr. Pirouz Pirouzi and was supported by Tehran University of Medical Sciences (grant no. 445678). We would like to express our sincere thank to colleagues at our centers for help in data acquisition.
REFERENCES
-
1Burman KD, Wartofsky L. Clinical Practice. Thyroid Nodules. N Engl J Med. 2015;373(24):2347-56.
-
2Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, et al. The natural history of benign thyroid nodules. JAMA. 2015;313(9):926-35.
-
3Mandell DL, Genden EM, Mechanick JI, Bergman DA, Biller HF, Urken ML. Diagnostic accuracy of fine-needle aspiration and frozen section in nodular thyroid disease. Otolaryngol Head Neck Surg. 2001;124(5):531-6.
-
4Riazi A, Kalantarhormozi M, Nabipour I, Eghbali SS, Farzaneh M, Javadi H, et al. Technetium-99m methoxyisobutylisonitrile scintigraphy in the assessment of cold thyroid nodules: is it time to change the approach to the management of cold thyroid nodules? Nucl Med Commun. 2014;35(1):51-7.
-
5Riazi A, Kalantarhormozi M, Nabipour I, Ostovar A, Javadi H, Assadi M. Comments on Wale et al.: Combined (99)mTc-methoxyisobutylisonitrile scintigraphy and fine-needle aspiration cytology offers an accurate and potentially cost-effective investigative strategy for the assessment of solitary or dominant thyroid nodules. Eur J Nucl Med Mol Imaging. 2014;41(3):575-6.
-
6Nabipour I, Kalantarhormozi M, Assadi M. Thyroid nodule characterization using combined fine-needle aspiration and (99m)Tc-sestamibi scintigraphy strategy. AJR Am J Roentgenol. 2016;207(2):W21.
-
7Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology. 2018;287(1):29-36.
-
8Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL,Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587-95.
-
9Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J. 2017;6(5):225-37.
-
10Klinke T, Daboul A, Maron J, Gredes T, Puls R, Jaghsi A, et al.Artifacts in magnetic resonance imaging and computed tomography caused by dental materials. PLoS One. 2012;7(2):e31766.
-
11Minuto MN, Shintu L, Caldarelli S. Proteomics, and metabolomics: magnetic resonance spectroscopy for the presurgical screening of thyroid nodules. Curr Genomics. 2014;15(3):178-83.
-
12Noda Y, Kanematsu M, Goshima S, Kondo H, Watanabe H, Kawada H, et al. MRI of the thyroid for differential diagnosis of benign thyroid nodules and papillary carcinomas. AJR Am J Roentgenol. 2015;204(3):W332-5.
-
13Sugiki T, Kobayashi N, Fujiwara T Modern Technologies of Solution Nuclear Magnetic Resonance Spectroscopy for Threedimensional Structure Determination of Proteins Open Avenues for Life Scientists. Comput Struct Biotechnol J. 2017;15:328-39.
-
14Yoshioka Y, Sasaki J,Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and choline- containing lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13(7):377-83.
-
15Jordan KW, Adkins CB, Cheng LL, Faquin WC. Application of magnetic-resonance-spectroscopy- based metabolomics to the fine-needle aspiration diagnosis of papillary thyroid carcinoma. Acta Cytol. 2011;55(6):584-9.
-
16Mackinnon WB, Delbridge L, Russell P, Lean CL, May GL, Doran S, et al. Two-dimensional proton magnetic resonance spectroscopy for tissue characterization of thyroid neoplasms. World J Surg. 1996;20(7):841-7.
-
17King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, et al. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005;54(1):112-7.
-
18Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al.; AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis And Management OF Thyroid Nodules – 2016 Update. Endocr Pract. 2016;22(5):622-39.
-
19Hoang JK, Branstetter BF 4th, Gafton AR, Lee WK, Glastonbury CM. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging. 2013;13:128-39.
-
20Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia. I: Discrimination between benign and malignant neoplasms. The American journal of medicine. 1994;96(4):383-8.
-
21Gupta N, Goswami B, Chowdhury V, Ravishankar L, Kakar A. Evaluation of the role of magnetic resonance spectroscopy in the diagnosis of follicular malignancies of thyroid. Archives of surgery. 2011;146(2):179-82.
-
22Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. European journal of radiology. 2007;64(3):414-8.
-
23Bezabeh T, Ijare OB, Nikulin AE, Somorjai RL, Smith IC. MRS- based Metabolomics in Cancer Research. Magnetic resonance insights. 2014;7:1-14.
-
24Gujar SK, Maheshwari S, Bjorkman-Burtscher I, Sundgren PC. Magnetic resonance spectroscopy. Journal of neuroophthalmology: the official journal of the North American NeuroOphthalmology Society. 2005;25(3):217-26.
Publication Dates
-
Publication in this collection
Oct 2018
History
-
Received
22 Apr 2018 -
Accepted
03 Aug 2018