Abstracts
PURPOSE: To determine the effects of oral L-glutamine (L-Gln) and the dipeptide l-alanyl-glutamine (L-Ala-Gln) upon the activity of the malate-aspartate shuttle in the rat distal small intestine following ischemia and reperfusion. METHODS: Seventy-two Wistar rats (350-400g), were randomized in 2 groups (n = 36): group S (Sham) and Group T (Treatment) and divided into 12 subgroups (n = 6): A-A6, and B1-B6. The subgroups A1-A3 were subjected to sham procedures at 30 and 60 minutes. Thirty minutes before the study, rats were treated with calcium caseinate, 0.5g/Kg (subgroups A1, A4, B1, B4), L-Gln, 0.5g / kg (subgroups A2, A5, B2 and B5) or L-Ala-Gln, 0.75g/Kg (subgroups A3, A6, B3, B6), administered by gavage. Ischemia was achieved by clamping the mesenteric vessels, delimiting a segment of bowel 5 cm long and 5 cm apart from the ileocecal valve. Samples were collected 30 and 60 minutes after start of the study for real-time PCR assay of malate dehydrogenases (MDH1-2) and aspartate-aminotransferases (GOT1-2) enzymes. RESULTS: Tissue MDH and GOT mRNA expression in intestinal samples from rats preconditioned with either L-Gln or L-Ala-Gln showed no significant differences both during ischemia and early reperfusion. CONCLUSION: Activation of the malate-aspartate shuttle system appears not to be the mechanism of glutamine-mediated elevation of glucose oxidation in rat intestine during ischemia/reperfusion injury.
Intestine; Ischemia; Reperfusion; Glutamine; Malate Dehydrogenase; RNA Messenger; Rats
OBJETIVO: Determinar os efeitos da administração oral de L-glutamina (L-Gln) e do dipeptídeo L-alanil-glutamina (L-Ala-Gln) sobre a atividade do ciclo malato-aspartato no intestino delgado distal de ratos após isquemia/reperfusão. MÉTODOS: Setenta e dois ratos Wistar (350-400g) foram randomizados em 2 grupos (n = 36): T grupo S (Sham) e grupo (Tratamento) e distribuídos em 12 subgrupos (n = 6): A-A6, e B1-B6. Os subgrupos A1-A3 foram submetidos a procedimentos "sham" aos 30 e 60 minutos. Trinta minutos antes do estudo, os ratos foram tratados com caseinato de cálcio, 0,5 g/kg (subgrupos A1, A4, B1 e B4), L-Gln, 0,5 g/kg (subgrupos A2, A5, B2 e B5) ou L-Ala -Gln, 0,75g/kg (subgrupos A3, A6, B3, B6), administrado por gavagem. A isquemia foi obtida por pinçamento dos vasos mesentéricos, delimitando um segmento do intestino cinco centímetros de comprimento e 5 cm da válvula ileocecal. Amostras foram coletadas aos 30-60 minutos para ensaio de PCR em tempo real das enzimas malato desidrogenases (MDH1-2), aspartato-aminotransferase (GOT1-2). RESULTADOS: A expressão de MDH e GOT mRNA nas amostras provenientes do intestino delgado de ratos pré-condicionados com L-Gln ou L-Ala-Gln não apresentou diferenças significativas, tanto durante a isquemia como na fase inicial de reperfusão. CONCLUSÃO: Ativação do ciclo malato-aspartato não parece ser o mecanismo de elevação glutamina-mediada da oxidação da glicose no intestino de ratos durante a isquemia / reperfusão.
Intestino Delgado; Isquemia; Reperfusão; Glutamina; Malato Desidrogenase; RNA Mensageiro; Ratos
6 - ORIGINAL ARTICLE
ISCHEMIA-REPERFUSION
Effect of glutamine on the mRNA level of key enzymes of malate-aspartate shuttle in the rat intestine subjected to ischemia reperfusion1 Correspondence: Sergio Botelho Guimarães Rua Professor Costa Mendes, 1608/3º andar, Bloco Didático 60430-140 Fortaleza - CE Brasil Tel.: (55-85)3366-8083 Fax: (55-85)3366-8064 sergiobotelho@terra.com.br
Efeito da glutamina sobre o nível de RNA Mensageiro das enzimas-chave do ciclo malato-aspartato no intestino de ratos submetidos à isquemia e reperfusão
Paulo Roberto Cavalcante de VasconcelosI; Claudio Duarte da Costa NetoII; Raquel Cavalcante de VasconcelosIII; Pedro Paulo Chaves de SouzaIV; Paulo Roberto Leitão VasconcelosV; Sérgio Botelho GuimarãesVI
IFellow Master degree, Department of Surgery, Postgraduate Program, UFC, Ceara, Brazil. Technical procedures, acquisition and interpretation of data, statistical analysis. The article is part of a master degree dissertation
IIProfessor, Department of Biochemistry and Cell Biology, USP, Ribeirao Preto-SP, Brazil. Technical procedures, critical revision and analysis of data
IIIGraduate student, UFC, Ceara, Brazil. Helped with technical procedures, acquisition of data
IVPhD, Department of Biochemistry and Cell Biology, USP, Ribeirao Preto-SP, Brazil. Technical procedures, critical revision and analysis of data
VPhD, Associate Professor, Coordinator, Postgraduate Program, Department of Surgery, UFC, Ceara, Brazil. Critical revision and analysis of data
VIPhD, Associate Professor, Department of Surgery, Head, LABCEX. UFC, Ceara, Brazil. Tutor, conception, design, intellectual and scientific content of the study, manuscript writing, statistical analysis
Correspondence Correspondence: Sergio Botelho Guimarães Rua Professor Costa Mendes, 1608/3º andar, Bloco Didático 60430-140 Fortaleza - CE Brasil Tel.: (55-85)3366-8083 Fax: (55-85)3366-8064 sergiobotelho@terra.com.br
ABSTRACT
PURPOSE: To determine the effects of oral L-glutamine (L-Gln) and the dipeptide l-alanyl-glutamine (L-Ala-Gln) upon the activity of the malate-aspartate shuttle in the rat distal small intestine following ischemia and reperfusion.
METHODS: Seventy-two Wistar rats (350-400g), were randomized in 2 groups (n = 36): group S (Sham) and Group T (Treatment) and divided into 12 subgroups (n = 6): A-A6, and B1-B6. The subgroups A1-A3 were subjected to sham procedures at 30 and 60 minutes. Thirty minutes before the study, rats were treated with calcium caseinate, 0.5g/Kg (subgroups A1, A4, B1, B4), L-Gln, 0.5g / kg (subgroups A2, A5, B2 and B5) or L-Ala-Gln, 0.75g/Kg (subgroups A3, A6, B3, B6), administered by gavage. Ischemia was achieved by clamping the mesenteric vessels, delimiting a segment of bowel 5 cm long and 5 cm apart from the ileocecal valve. Samples were collected 30 and 60 minutes after start of the study for real-time PCR assay of malate dehydrogenases (MDH1-2) and aspartate-aminotransferases (GOT1-2) enzymes.
RESULTS: Tissue MDH and GOT mRNA expression in intestinal samples from rats preconditioned with either L-Gln or L-Ala-Gln showed no significant differences both during ischemia and early reperfusion.
CONCLUSION: Activation of the malate-aspartate shuttle system appears not to be the mechanism of glutamine-mediated elevation of glucose oxidation in rat intestine during ischemia/reperfusion injury.
Keywords: Intestine, Small. Ischemia. Reperfusion. Glutamine. Malate Dehydrogenase. RNA Messenger. Rats.
RESUMO
OBJETIVO: Determinar os efeitos da administração oral de L-glutamina (L-Gln) e do dipeptídeo L-alanil-glutamina (L-Ala-Gln) sobre a atividade do ciclo malato-aspartato no intestino delgado distal de ratos após isquemia/reperfusão.
MÉTODOS: Setenta e dois ratos Wistar (350-400g) foram randomizados em 2 grupos (n = 36): T grupo S (Sham) e grupo (Tratamento) e distribuídos em 12 subgrupos (n = 6): A-A6, e B1-B6. Os subgrupos A1-A3 foram submetidos a procedimentos "sham" aos 30 e 60 minutos. Trinta minutos antes do estudo, os ratos foram tratados com caseinato de cálcio, 0,5 g/kg (subgrupos A1, A4, B1 e B4), L-Gln, 0,5 g/kg (subgrupos A2, A5, B2 e B5) ou L-Ala -Gln, 0,75g/kg (subgrupos A3, A6, B3, B6), administrado por gavagem. A isquemia foi obtida por pinçamento dos vasos mesentéricos, delimitando um segmento do intestino cinco centímetros de comprimento e 5 cm da válvula ileocecal. Amostras foram coletadas aos 30-60 minutos para ensaio de PCR em tempo real das enzimas malato desidrogenases (MDH1-2), aspartato-aminotransferase (GOT1-2).
RESULTADOS: A expressão de MDH e GOT mRNA nas amostras provenientes do intestino delgado de ratos pré-condicionados com L-Gln ou L-Ala-Gln não apresentou diferenças significativas, tanto durante a isquemia como na fase inicial de reperfusão.
CONCLUSÃO: Ativação do ciclo malato-aspartato não parece ser o mecanismo de elevação glutamina-mediada da oxidação da glicose no intestino de ratos durante a isquemia / reperfusão.
Descritores: Intestino Delgado. Isquemia. Reperfusão. Glutamina. Malato Desidrogenase. RNA Mensageiro. Ratos.
Introduction
Small bowel ischemia/reperfusion (IR) injury is a well known condition. Ischemia progressively damages the cell structures and, following the restoration of blood flow, lesions produced are further exacerbated1,2.
L-Glutamine (L-Gln), a five-carbon amino acid, formerly classified as a non-essential, is the most abundant amino acid in blood and tissue fluids and is considered to be essential during certain catabolic conditions3. Some studies have shown that L-Gln reduces atrophy of intestinal mucosa in animals subjected to total parenteral nutrition and prevents intestinal mucosal injury accompanying small bowel transplantation, chemotherapy and radiation4. Studies established that L-Gln is the main respiratory fuel of enterocytes. It was demonstrated that carbon derived from L-Gln contributed 46% of the carbon dioxide released from isolated perfused segments of rat jejunum5. In addition to its role as a fuel, L-Gln may be important to the small intestine because it is also a source of substrates for the synthesis of nucleic acids. Indeed, it has been argued that one reason for the high rate of L-Gln degradation in enterocytes and other cells, such as lymphocytes and macrophages, is to ensure regulation of the rate of nucleic acid synthesis6.
Experimental studies have demonstrated pro-glycolytic effect of L-Gln or dipeptide L-Alanyl-glutamine (L-Ala-Gln) when offered in nutraceutical doses7. L-Ala-Gln is a highly stable dipeptide and when infused intravenously is promptly hydrolyzed to L-Gln and alanine8.
The malate-aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the impermeable inner membrane of the mitochondria for oxidative phosphorylation in eukaryotes9. It is not known whether L-Gln supplementation may induce changes in the level or distribution of mRNA of enzymes of malate aspartate shuttle. Therefore the aim of this study was to examine the effects of L-Gln and L-Ala-Gln oral supplementation, on intestinal ischemia-reperfusion injury on the intestinal mucosa levels of the RNA Messenger (mRNA) of the key enzymes involved in the malate-aspartate shuttle, i.e., Malate dehydrogenases (malate dehydrogenase 1, NAD, soluble - MDH1 and malate dehydrogenase 2, NAD, mitochondrial - MDH2) and Aspartate-aminotransferases (glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1 - GOT1 and glutamic-oxaloacetic transaminase 2, mitochondrial (aspartate aminotransferase 2 - GOT2).
Methods
Approval (Protocol #06/07) for experimental use of laboratory animals was obtained from the Ethics Committee on Animal Research (CEPA) of the Federal University of Ceara, now Ethics Committee on the Use of Animals (CEUA), in view of the Federal Law No. 11794 of October 8, 2008, http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm and Decree No. 6689 of July 15, 2009 that regulated the Law 11794, available at: http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2009/Decreto/D6899.htm. The study was designed so as to minimize the number of animals required for the experiments.
Seventy-two healthy Wistar rats (350-400g) were randomized in 2 groups (n = 36): group S (Sham) and Group T (Treatment). The rats in each group were distributed in 12 subgroups (n = 6): A-A6, and B1-B6. The subgroups A1-A3 were subjected to sham procedures (laparotomy and handling of the bowel, without clamping or ischemia).This procedure was repeated at 30 and 60 minutes. Thirty minutes before the start of the study, rats were treated with calcium caseinate, 0.5 g/Kg (subgroups A1, A4, B1, B4), L-Gln, 0.5g / kg (subgroups A2, A5, B2 and B5) or L-Ala-Gln, 0.75g/Kg (subgroups A3, A6, B3, B6), administered by gavage.
Induction of ischemia and samples harvesting
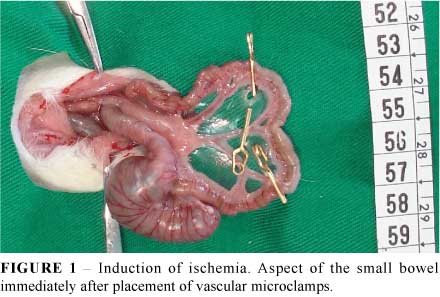
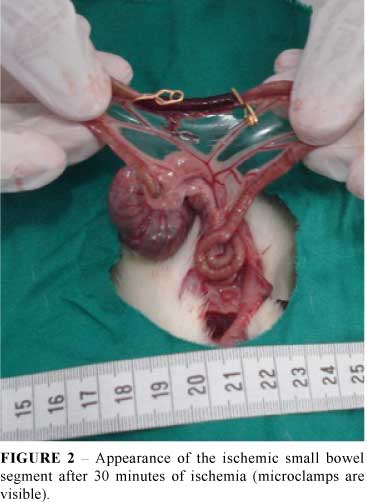
Before the induction of anesthesia, each rat was fasted for 12 h before IR injury but was allowed free access to water. The rats were anesthetized by an intra-peritoneal injection of xylazine (10 mg/kg) and ketamine (60 mg/kg). Ischemia was induced as described by Lindestrom et al.10. In brief, using sterile techniques, the abdomen was opened using a midline incision. After laparotomy, a segment of ileum 5 cm apart from the ileocecal valve was isolated. Ischemia was achieved by clamping the mesenteric vessels using three micro-clamps normally used for neurosurgery, delimiting a segment of bowel 5 cm long (Figure 1). During the period of ischemia the abdominal wall incision was closed with nylon 4-0 stitches to prevent fluid and heat loss. After 30 minutes (ischemic cycle) the abdominal cavity was reopened with the removal of stitches (Figure 2). In ischemia group the ischemic bowel segment was removed, blotted dry, weighed, and slit down the antimesenteric border for mucosal removal. The sample of intestinal mucosa was weighed and stored for the estimation of enzymes activity and mRNA concentration. In ischemia-reperfusion group the clamps were removed allowing the restoration of blood flow. Before closure of the abdominal cavity, the reperfusion was confirmed by the reappearance of pulsation in the mesentery arcade and the change in color of the loops of the small bowel. After another 30 minutes, the cavity was reopened and the ischemic segment was removed and treated as described above. Rats of Sham group (subgroups A1-A3) were subjected to laparotomy and handling of the bowel, without clamping or ischemia for 30 minutes. This procedure was repeated 30 minutes later for sample collection. The abdominal cavity was closed and reopened 30 minutes later for sample collection (subgroups A4-A6). Once completed all experimental procedures, all animals were killed by an overdose of anesthetics.
RNA extraction and cDNA synthesis
In order to demonstrate the possible changes in MDH1, MDH2 and GOT1, GOT2 (Table 1) in the rat intestine tissues, the mRNA levels for some of those enzymes were analyzed by real-time PCR. The small piece of these tissues were excised and homogenized in lysis buffer and the RNA was extracted using the kit RNAqueous-4PCR (Ambion®, Applied Biosystems do Brasil), following the manufacturer's recommendations. The amount of RNA was determined by absorbance at 260nm, whose purity was ascertained by the respective absorbance ratio at 260 and 280 nm. cDNA was synthetized using the reverse transcriptase Improm II (Promega®, Prodimol Biotecnologia S/A, Minas Gerais, Brazil) following the manufacturer´s recommendations. The amount of RNA used for the cDNA synthesis was 0.5µg. The primers used for amplification of genes encoding GOT 1 and 2, MDH 1 and 2, RPL13A were designed by Primer Express program (Applied Biosystems, USA) based on sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov). The sequences of primers and size of amplified fragments are listed below (Table 1).
Real-time PCR
Quantitative real-time PCR (qPCR) was performed using the SyBr Green PCR® master mix (Invitrogen Brasil Ltda, Sao Paulo, Brazil) in 96-well plates with 5µL volume per well and each sample analyzed in duplicate. The amplifications were performed on an ABI PRISM 7500 Sequence Detection System® and software (Applied Biosystems of Brazil, Sao Paulo, Brazil). To control for variability in amplification due to differences in starting RNA concentrations, RPL13A was used as internal standard. The controls were arbitrarily set to 100%.
Statistical analysis
Graphpad Prism 5.0 (GraphPad Software, San Diego,CA, USA, www.graphpad.com) was used for statistical analysis and graphics design. All data are expressed as mean ± standard deviation. Comparisons were made using one-way ANOVA followed by Bonferroni post test. A p value of < 0.05 was considered statistically significant
Results
No animal died during the experiment. Pretreatment with L-Ala-Gln or L-Gln did not change the mRNA levels of malate-aspartate shuttle key enzymes MDH1 (soluble), MDH2 (non soluble), GOT1 (soluble) and GOT2 (non soluble) in the rat intestine subjected to ischemia and or ischemia/reperfusion (Figures 3 to 10).
Discussion
Published studies showed that administration of L-Gln or L-Ala-Gln in various pathological conditions may enhance both anaerobic11-12 and aerobic glycolysis13. Other researchers have used the infusion of L-Ala-L-Gln associated with parenteral nutrition in Intensive Care Units patients14 and in patients with traumatic injury15. It was demonstrated that both L-Gln as well as L-AlaGln peptides have enhanced glucose utilization resulting in decreased glucose blood levels in those patients.
It was hypothesized in this study was that the offer of L-Gln or L-AlaGln by gavage, prior to ischemia/reperfusion, would provide greater availability to tissues rich in glutaminase, such as the small intestine promoting enhanced activation of this shuttle, leading to increased activity of the first phase of the glycolytic pathway in these tissues.
Therefore, in order to clarify the role of the malate-aspartate shuttle in the pro-glycolytic action of glutamine, we attempted to evaluate RT-PCR via the messenger RNA of key enzymes of the shuttle (RNA1 and RNA2 of GOT1, GOT2, MDH1 and MDH2). However, as demonstrated in our results, the offer of L-Gln or L- Ala-Gln to rats submitted to I/R did not induce changes in messenger RNA expression of these enzymes (Figures 3 to 10). The absence of similar studies in the medical literature did not allow a comparative analysis of these results. Other cellular mechanisms either via new formed proteins or through glutamate transporters, such as citrin or aralar-1, may elucidate further the glycolytic action of glutamine and its dipeptide l-alanyl-glutamine16-17.
Conclusion
Activation of the malate-aspartate shuttle system appears not to be the mechanism of glutamine-mediated elevation of glucose oxidation in rat intestine during ischemia/reperfusion injury.
Conflict of interest: none
Financial source: CNPq
1 Research performed at Experimental Surgery Laboratory (LABCEX), Faculty of Medicine, Federal University of Ceara (UFC), Fortaleza and Biochemistry and Cell Biology Laboratory, University of Sao Paulo (USP), Ribeirao Preto, Brazil.
- 1. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med.1985;17;312(3):159-63.
- 2. Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250(6 Pt 1):G749-53.
- 3. Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131(9):2543S-9.
- 4. Wu GH, Wang H, Zhang YW, Wu ZH, Wu ZG. Glutamine supplemented parenteral nutrition prevents intestinal ischemia-reperfusion injury in rats. World J Gastroenterol. 2004;10(17):2592-4.
- 5. Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980;255(1):107-12.
- 6. Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, Fürst P, Puchstein C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227(2):302-8.
- 7. Albers S, Wernerman J, Stehle P, Vinnars E, Fürst P. Availability of amino acid supplied by constant intravenous infusion of synthetic dipeptide in healthy man. Clin Sci. 1989;76(6):643-8.
- 8. Barbosa RC, Guimaraes SB, de Vasconcelos PR, Chaves CR. Metabolic effects of l-alanyl-glutamine in burned rats. Burns. 2006;32(6):721-7.
- 9. Kight CE, Fleming SE. Oxidation of glucose carbon entering the TCA cycle is reduced by glutamine in small intestine epithelial cells. Am J Physiol. 1995;268(6 Pt 1):G879-88.
- 10. Lindeström LM, Ekblad E. Structural and neuronal changes in rat ileum after ischemia with reperfusion. Dig Dis Sci. 2004;49(7-8):1212-22
- 11. Campos AB, Silva LFG, Vasconcelos PRL. Efeitos da L-Alanil -Glutamina sobre as concentrações in vivo de metabólitos durante a isquemia-reperfusão de intestino delgado em ratos Wistar. ABCD Arq Bras Cir Dig. 2002;15:63-6.
- 12. Bezerra Filho JE, Guimarães SB, Chaves CR Queiroz DAF, Vasconcelos, PRC de, Vasconcelos PRL de. Effects of L-alanyl-glutamine on in vivo kidney and blood concentrations of glucose, pyruvate and lactate in rats subjected to unilateral renal ischemia and reperfusion. Rev Bras Nutr Clin. 2002;17(4):122-5.
- 13. Alves MA, Guimarães SB, Dias DA, Vasconcelos PRC, Coelho VPM, Vasconcelos PRL. Effects of L-alanyl-glutamine upon the blood and kidney biochemical parameters in the rat hind limb model of ischemia/reperfusion. Acta Cir Bras. 2005;20(6):445-9.
- 14. Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, Merle V, Mazerolles M, Samba D, Guillou YM, Petit J, Mansoor O, Colas G, Cohendy R, Barnoud D, Czernichow P, Bleichner G. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34(3):598-604.
- 15.Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J, Andel M. Parenterally administered dipeptide alanyl-glutamine prevents worsening of insulin sensitivity in multiple-trauma patients. Crit Care Med. 2006;34(2):381-6.
- 16. Roesch K, Hynds PJ, Varga R, Tranebjaerg L, Koehler CM. The calcium-binding aspartate/glutamate carriers, citrin and aralar1, are new substrates for the DDP1/TIMM8a-TIMM13 complex. Hum Mol Genet. 2004;13(18):2101-11.
- 17. Wohlrab H. The human mitochondrial transport protein family: identification and protein regions significant for transport function and substrate specificity. Biochim Biophys Acta. 2005;1709(2):157-68.
Publication Dates
-
Publication in this collection
23 Sept 2011 -
Date of issue
2011