Abstract
Purpose:
To investigate the effect of mesenteric lymph drainage on the spleen injury and the expressions of inflammatory cytokines in splenic tissue in mice following hemorrhagic shock.
Methods:
Male C57 mice were randomly divided into the sham shock, shock and shock+drainage groups. The mice in both shock and shock+drainage groups suffered femoral artery bleeding, maintained mean arterial pressure (MAP) of 40±2 mmHg for 90 min, and were resuscitated. And mesenteric lymph drainage was performed in the shock+drainage group at the time of resuscitation. After three hours of resuscitation, the splenic tissues were harvested for the histological observation and protein and mRNA expression analysis of cytokines.
Results:
The spleen in the shock group revealed a significantly structural damage and increased mRNA expressions of MyD88 and TRAF6 and protein expressions of TIPE2, MyD88, TRIF and TRAF3 compared to the sham group. By contrast, the splenic pathological injury in the shock+drainage group was alleviated significantly, and the mRNA and protein expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 were significantly lower than those in the shock group.
Conclusion:
These results indicate that post-hemorrhagic shock mesenteric lymph drainage alleviates hemorrhagic shock-induced spleen injury and the expressions of inflammatory cytokines.
Key words:
Shock; Hemorrhagic; Spleen; Drainage; Inflammation; Mice
Introduction
Hemorrhagic shock is a common clinical intensive illness and is an important cause of trauma leading to death11. Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370-9. doi: 10.1056/NEJMra1705649.
https://doi.org/10.1056/NEJMra1705649...
–33. Langgartner D, Wachter U, Hartmann C, Groger M, Vogt J, Merz T, McCook O, Fink M, Kress S, Georgieff M, Kunze J F, Radermacher P L, Reber S O, Wepler M. Effects of psychosocial stress on subsequent hemorrhagic shock and resuscitation in male mice. Shock. 2019;51(6):725-30. doi: 10.1097/SHK.0000000000001204.
https://doi.org/10.1097/SHK.000000000000...
. The return of post-hemorrhagic shock mesenteric lymph (PHSML) is a key reason of multiple organ dysfunction caused by hemorrhagic shock. Therefore, mesenteric lymph drainage or mesenteric lymph duct ligation improve the multiple organ functions and reduce the tissue injury following hemorrhagic shock, such as lung, heart and kidney44. Du HB, Wang SH, Zhao ZG, Niu CY. Post-hemorrhagic shock mesenteric lymph is an important contributor to cardiac dysfunction following hemorrhagic shock. Acta Cir Bras. 2015;30(6):439-44. doi: 10.1590/S0102-865020150060000010.
https://doi.org/10.1590/S0102-8650201500...
–1010. Watkins AC, Caputo FJ, Badami C, Barlos D, Xu DZ, Lu Q, Feketeova E, Deitch E A. Mesenteric lymph duct ligation attenuates lung injury and neutrophil activation after intraperitoneal injection of endotoxin in rats. J Trauma. 2008;64(1):126-30. doi: 10.1097/TA.0b013e3181574a8a.
https://doi.org/10.1097/TA.0b013e3181574...
. Recently, the role of PHSML in the pathogenesis of hemorrhagic shock has attracted increasing attention1111. Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207 Suppl 1:E103-11. doi: 10.1111/j.1749-6632.2010.05713.x.
https://doi.org/10.1111/j.1749-6632.2010...
–1313. Moore EE. Mesenteric lymph: the critical bridge between dysfunctional gut and multiple organ failure. Shock. 1998;10(6):415-6. PMID: 9872680.. Hemorrhagic shock causes the immediate activation of immune system and rapid onset of inflammatory reaction, which leads to immune dysfunction and injury of immune organs1414. Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389(6):475-84. doi: 10.1007/s00423-004-0472-0.
https://doi.org/10.1007/s00423-004-0472-...
. Researches show that decreasing PHSML reflux is beneficial for reducing injury of splenic tissue after hemorrhagic shock1515. Liu H, Zhao ZG, Xing LQ, Zhang LM, Niu CY. Post-shock mesenteric lymph drainage ameliorates cellular immune function in rats following hemorrhagic shock. Inflammation. 2015;38(2):584-94. doi: 10.1007/s10753-014-9965-3.
https://doi.org/10.1007/s10753-014-9965-...
,1616. Tiesi G, Reino D, Mason L, Palange D, Tomaio JN, Deitch EA. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock. 2013;39(6):507-13. doi: 10.1097/SHK.0b013e318293d020.
https://doi.org/10.1097/SHK.0b013e318293...
, which is mediated by TLR4/TLR21616. Tiesi G, Reino D, Mason L, Palange D, Tomaio JN, Deitch EA. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock. 2013;39(6):507-13. doi: 10.1097/SHK.0b013e318293d020.
https://doi.org/10.1097/SHK.0b013e318293...
. Tumor necrosis factor-alpha-induced protein 8-like 2 (TIPE2) is required for maintaining immune homeostasis, and is preferentially expressed in lymphoid tissues1717. Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen Y H. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133(3):415-26. doi: 10.1016/j.cell.2008.03.026.
https://doi.org/10.1016/j.cell.2008.03.0...
, but the role of TIPE2 in the development of hemorrhagic shock-induced immune disorder remains unclear. In order to reveal the role of PHSML in hemorrhagic shock-induced spleen injury and inflammatory response, the present study observed the effects of PHSML drainage on the splenic tissue structure and the expressions of inflammatory cytokines, including TIPE2 and the downstream molecules of TLR4/TLR2.
Methods
Animals and the experimental group
Healthy and male C57 (8-10 weeks) mice purchased from Sibeifu (Beijing Biotechnology Co., Ltd.) were raised in the Animal Room and ate and drank freely. All animals used in this study were fasted for 8 hours and drank freely before the experiment. Eighteen mice were randomly divided into 3 groups (n=6 each group): sham group (anesthesia and operation, no bloodletting, sampling at the corresponding time point), shock group (established hemorrhagic shock model), shock + drainage group (established hemorrhagic shock model with drainage of mesenteric lymph). All animal experiment was performed according to the guideline and requirements of animal ethics.
Hemorrhagic shock model
Mice were anesthetized with isoflurane (RWD Life Science Co., 217180801) and intramuscular injection of 1% pentobarbital sodium (0.07 mL/kg, Merk, P11011). Bilateral femoral arteries were separated under stereoscopic microscope and inserted into bilateral femoral arteries according to the routine method in our lab77. Liu GQ, Zuo XH, Jiang LN, Zhang YP, Zhang LM, Zhao ZG, Niu C Y. Inhibitory effect of post-hemorrhagic shock mesenteric lymph drainage on the HMGB1 and RAGE in mouse kidney. Ren Fail. 2016;38(1):131-6. doi: 10.3109/0886022X.2015.1105026.
https://doi.org/10.3109/0886022X.2015.11...
. Mouse mean arterial pressure (MAP) was monitored in the left femoral artery using the PowerLab 15T four-channel multi-purpose data acquisition and analysis system (AD Instruments, Australia), and the right side was connected with 1 mL syringe and placed in NE-1000 programmed microinjection pump (American New Times Company) for bloodletting and liquid resuscitation. After 30 min-equilibrium period, bleeding was performed and MAP was maintained at the level of 40±2 mmHg for 90 min. During this period, close attention was paid to the MAP and the state of mice. After 90 min, the whole blood and equal ringer's solution were used to resuscitate at a uniform speed within 30 min. After resuscitation, all mice were placed on the operating table in supine position, and the status of MAP was closely observed for 3 hours. After 3 hours, mice were sacrificed by spinal cord dislocation method, and relevant samples were collected. After resuscitation, the mesenteric lymph was drained for 3 hours by one-time-using blood collection needle and blood vessels collection. The spleen samples were harvested from all mice for further study.
Histopathology observation
Small pieces of spleen were fixed in 4% paraformaldehyde. Specimens were then routinely dehydrated, wax immersed and embedded, etc., in continuous sections (3 μm), including exhibition piece and fishing piece, and sealed with neutral gum; then, the splenic morphology was observed. The degree of splenic injury was evaluated with a semiquantitative scoring system1818. da Silva AVA, Figueiredo FB, Menezes RC, Mendes-Junior AA, de Miranda LHM, Cupolillo E, Porrozzi R, Morgado F N. Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens. PLoS Negl Trop Dis. 2018;12(4):e0006445. doi: 10.1371/journal.pntd.0006445.
https://doi.org/10.1371/journal.pntd.000...
–2020. Giamarellos-Bourboulis EJ, Tziortzioti V, Koutoukas P, Baziaka F, Raftogiannis M, Antonopoulou A, Adamis T, Sabracos L, Giamarellou H. Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by pan-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2006;57(5):937-44. doi: 10.1093/jac/dkl084.
https://doi.org/10.1093/jac/dkl084...
. The details were as follows: 0, the morphology of spleen white pulp was obvious, which was characterized by obvious per arterial lymphoid sheath, gerontology center, capsule zone and marginal zone; 1, mild disorder of spleen white pulp, characterized by local hyperplasia; 2, moderate disorder of spleen white pulp, blurring of the boundary between white pulp and red pulp; 3, high disorder of spleen white pulp, almost no significant difference between white pulp and red pulp.
Real-time RT-PCR analysis
Total RNA was extracted from splenic tissue with Trizol reagent. RNA was reversely transcribed into cDNA using FastQuant RT Kit (Beijing Tiangen biology Co., Ltd.). RT-PCR was used to analyze the mRNA levels of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in splenic tissue with SYBR Green kit (Beijing Tiangen biology Co., Ltd.). PCR of GAPDH was used as an internal control with the same condition. The primers used in the experiment were synthesized by Shanghai Bioengineering Biology (Shanghai, China). The primer sequence of each gene is as follows in Table 1.
Western blot analysis
After the protein quantification with BCA kits, the protein levels of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in splenic tissue from different groups were analyzed by SDS-PAGE electrophoresis. The protein was then transferred to polyvinylidene fluoride membrane after electrophoresis. The membranes were blocked with 5% skim milk diluted in TBST, followed by an overnight incubation with primary antibodies against β-actin (1:2000 dilution; Applygen), TIPE2 (1:1000 dilution; protein tech), MyD88 (1:1000 dilution; cell signaling technology), TRIF (1:1000 dilution; Bioworld), TRAF3 (1:1000 dilution; Bioworld), TRAF6 (1:1000 dilution; Bioworld). The membranes were subsequently incubated with a horseradish peroxidase-conjugated secondary antibody (1:5000 dilution; Applygen). Finally, images were examined with an ImageQuant LAS 4000 imager, and strip density was analyzed by Quantity One software.
Statistical analysis
All data are present as means±standard deviation (SD) or means±standard error (SE). Difference between groups was analyzed using a one-way ANOVA with an LSD multiple-comparison test or a Student's t-test using SPSS software 22.0. P<0.05 was considered statistically significant.
Results
PHSML drainage alleviated the spleen injury induced by hemorrhagic shock
As shown in Figure 1, there were no significant pathological changes in splenic tissue from sham group, with the evidences of clearly lymphoid nodules and “germinal centers”. In contrast, there was serious spleen injury with the characterizations of thinner spleen cord and dilated spleen sinus in the shock group. However, there was slight spleen injury in the shock+drainage group. The results of semiquantitative score showed that hemorrhagic shock significantly increased the splenic histological score when compared to the sham group, and PHSML drainage significantly decreased the splenic histology score of the shocked mice (Fig. 1B).
Mesenteric lymph drainage reduced hemorrhagic shock-induced spleen injury. A. The changes of splenic morphology in different groups (HE staining, ×200). No histological alterations were observed in the spleen obtained from the sham mice. The structural looseness and local proliferation were observed in the spleen of the shock group, which was alleviated by mesenteric lymph drainage. B. The splenic histological score of different groups. Data are presented as mean ± SE (n=3). *P<0.05, vs. the sham group, # P<0.05, vs. the shock group.
PHSML drainage decreased the mRNA expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in spleen after hemorrhagic shock
RT-PCR method was used to detect the mRNA levels of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in spleen tissue of mice following hemorrhagic shock. The results showed that hemorrhagic shock significantly increased the mRNA levels of MyD88 and TRAF6 (P<0.05), and slightly increased the mRNA levels of TIPE2, TRIF and TRAF3 that were no statistically difference (Fig. 2). However, PHSML drainage significantly decreased the levels of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 compared with the shock group (P<0.05).
The mRNA expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in murine splenic tissue following hemorrhagic shock. Compared to the sham group, hemorrhagic shock increased the level of MyD88 and TRAF6 (B, E) and mesenteric lymph drainage reduced the mRNA expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 (A-E). Data are presented as mean ± SE (n=3). * P<0.05, vs. the sham group, # P<0.05, vs. the shock group.
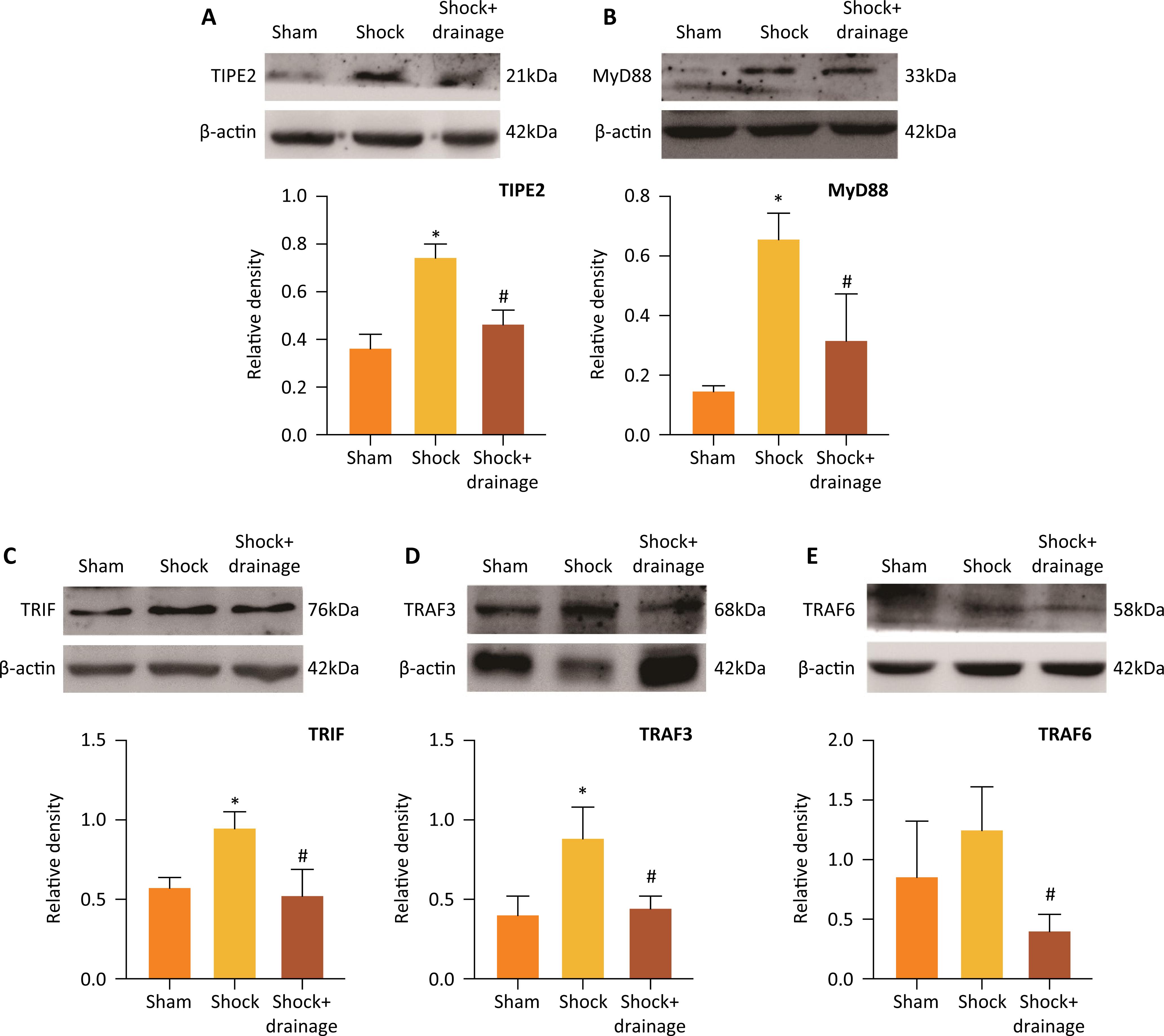
PHSML drainage reduced the protein expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in spleen after hemorrhagic shock
The protein expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in splenic tissue of mice following hemorrhagic shock were further detected by Western blot. As shown in Figure 3, hemorrhagic shock significantly increased the protein levels of TIPE2, MyD88, TRIF and TRAF3 than those in the sham group (P<0.05), and slightly increased the levels of TRAF6 with no statistical difference. Compared to shock group, drainage of PHSML significantly decreased the protein levels of TIPE2, MyD88, TRIF and TRAF3 (P<0.05).
The protein expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 in murine splenic tissue of mice following hemorrhagic shock. Compared to the sham group, hemorrhagic shock increased the expressions of TIPE2, MyD88, TRIF and TRAF3 (A-D), and mesenteric lymph drainage reduced the expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6 (A-E). Data are presented as mean ± SD (n=3). * P<0.05, vs. the sham group, # P<0.05, vs. the shock group.
Discussion
The present study found that hemorrhagic shock results in the spleen injury and enhances the protein or mRNA expressions of cytokines, such as TIPE2, TRIF, TRAF3, TRAF6 and MyD88 that are associated with inflammation and immune function. By contrast, PHSML drainage alleviates the tissue injury and decreases the expression of these cytokines in murine spleen following hemorrhagic shock.
Immune dysfunction plays a key role in the occurrence and development of multiple organ failure following hemorrhagic shock2121. Bortolotti P, Faure E, Kipnis E. Inflammasomes in tissue damages and immune disorders after trauma. Front Immunol. 2018;9:1900. doi: 10.3389/fimmu.2018.01900.
https://doi.org/10.3389/fimmu.2018.01900...
. The spleen is one of the most important immune organs playing a key role in the innate and adaptive immune responses, which are frequently affected in infectious diseases2222. Liu Z, Wu Y, Feng Y, Wu F, Liu RF, Wang LF, Liang J Y, Liu J H, Sun X, Wu Z D. Spleen atrophy related immune system changes attributed to infection of Angiostrongylus cantonensis in mouse model. Parasitol Res. 2017;116(2):577-87. doi: 10.1007/s00436-016-5322-9.
https://doi.org/10.1007/s00436-016-5322-...
, and spleen injury was involved in the pathophysiology of hemorrhagic shock-induced immune dysfunction2323. Warren M, Subramani K, Schwartz R, Raju R. Mitochondrial dysfunction in rat splenocytes following hemorrhagic shock. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2526-33. doi: 10.1016/j.bbadis.2017.08.024.
https://doi.org/10.1016/j.bbadis.2017.08...
. Therefore, the current study observed the change of splenic histopathology following hemorrhagic shock, and found that PHSML drainage reduced the spleen injury caused by hemorrhagic shock.
It is well known that TLRs mainly lead to two mainly signaling pathways, such as the MyD88-dependent and MyD88-independent pathways (also called TRIF-dependent pathways), to recognize the pathogen-associated patterns or danger-associated patterns. More and more studies reported that TLR plays an important role in inflammation induced by hemorrhagic shock and resuscitation2424. Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar T R. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202(3):407-17. doi: 10.1016/j.jamcollsurg.2005.11.021.
https://doi.org/10.1016/j.jamcollsurg.20...
. We found that hemorrhagic shock partly increased the mRNA or protein expressions of the downstream effectors of TLR2/TLR4 signaling pathways, such as MyD88, TRIF, TRAF3 and TRAF6. However, PHSML drainage followed by hemorrhagic shock significantly decreased the mRNA and protein levels of the above molecules. It is suggested that PHSML drainage remarkably reduces the splenic inflammatory response, which is involved in the beneficial effect of PHSML drainage, alleviating spleen injury following hemorrhagic shock.
TIPE2 is a research hot spot and important member of the TIPE family, and is a negative regulator in the process of innate immune response. TIPE2 is a critical regulator of T cell receptor (TCR) and TLR signaling1717. Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen Y H. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133(3):415-26. doi: 10.1016/j.cell.2008.03.026.
https://doi.org/10.1016/j.cell.2008.03.0...
. Its structure includes highly conserved TIPE2 homologous TH (TIPE homology) domain, which is composed of 7 α helix2525. Lou Y, Liu S. The TIPE (TNFAIP8) family in inflammation, immunity, and cancer. Mol Immunol. 2011;49(1-2):4-7. doi: 10.1016/j.molimm.2011.08.006.
https://doi.org/10.1016/j.molimm.2011.08...
,2626. Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, Sethi G, Fan L, Kunnumakkara A B. Novel tumor necrosis factor-alpha induced protein eight (TNFAIP8/TIPE) family: functions and downstream targets involved in cancer progression. Cancer Lett. 2018;432:260-71. doi: 10.1016/j.canlet.2018.06.017.
https://doi.org/10.1016/j.canlet.2018.06...
. It is reported that hepatitis C virus (HCV) could inhibit the expression of TIPE2 to enhance the TLR signaling pathway to promote the occurrence of chronic hepatitis in chronic hepatitis C infection2727. Kong L, Liu K, Zhang YZ, Jin M, Wu BR, Wang WZ, Li W, Nan YM, Chen YH. Downregulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol Int. 2013;7(3):844-9. doi: 10.1007/s12072-013-9435-2.
https://doi.org/10.1007/s12072-013-9435-...
. In addition, the overexpression of TIPE2 in macrophages can play a negative role in innate immunity by inhibiting TLR signal transduction in arthritis2828. Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y. Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol. 2013;191(9):4849-57. doi: 10.4049/jimmunol.1300053.
https://doi.org/10.4049/jimmunol.1300053...
. However, it is not clear how hemorrhagic shock affects the expression of TIPE2 and its effect on splenic tissue injury. Our results showed that hemorrhagic shock increased the protein expression of TIPE2, which was reversed by PHSML drainage. The results indicated that PHSML drainage decreased excessive anti-inflammatory response, thereby maintaining the inflammatory response balance. But its detailed molecular mechanism still remains unclear and needs to be further studied. Thus, in the future, we will use TLR2-/- and TLR4-/- mice to further explore the interaction between TIPE2 with the downstream effectors of TLR2/TLR4 signaling pathway, such as MyD88, TRIF, TRAF3 and TRAF6.
Conclusion
These current results indicate that PHSML drainage alleviates hemorrhagic shock-induced spleen injury and reduces the expressions of TIPE2, MyD88, TRIF, TRAF3 and TRAF6.
-
1
Research performed at Institute of Microcirculation, Hebei North University, Zhangjiakou Hebei, China.
-
Financial source: National Natural Science Foundation of China (No. 81701963)
Acknowledgement
To Chun-Yu Niu for support by the guidance of Research ideas and the cultivation of scientific research methods.
References
-
1Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370-9. doi: 10.1056/NEJMra1705649.
» https://doi.org/10.1056/NEJMra1705649 -
2Xu S, Zeng Z, Zhao M, Huang Q, Gao Y, Dai X, Lu J, Huang W, Zhao K. Evidence for SIRT1 mediated HMGB1 release from kidney cells in the early stages of hemorrhagic shock. Front Physiol. 2019;10:854. doi: 10.3389/fphys.2019.00854.
» https://doi.org/10.3389/fphys.2019.00854 -
3Langgartner D, Wachter U, Hartmann C, Groger M, Vogt J, Merz T, McCook O, Fink M, Kress S, Georgieff M, Kunze J F, Radermacher P L, Reber S O, Wepler M. Effects of psychosocial stress on subsequent hemorrhagic shock and resuscitation in male mice. Shock. 2019;51(6):725-30. doi: 10.1097/SHK.0000000000001204.
» https://doi.org/10.1097/SHK.0000000000001204 -
4Du HB, Wang SH, Zhao ZG, Niu CY. Post-hemorrhagic shock mesenteric lymph is an important contributor to cardiac dysfunction following hemorrhagic shock. Acta Cir Bras. 2015;30(6):439-44. doi: 10.1590/S0102-865020150060000010.
» https://doi.org/10.1590/S0102-865020150060000010 -
5Feinman R, Deitch EA, Aris V, Chu HB, Abungu B, Caputo FJ, Galante A, Xu D, Lu Q, Colorado I, Streck D, Dermody J, Soteropoulos P. Molecular signatures of trauma-hemorrhagic shock-induced lung injury: hemorrhage- and injury-associated genes. Shock. 2007;28(3):360-8. doi: 10.1097/shk.0b013e318048565b.
» https://doi.org/10.1097/shk.0b013e318048565b -
6Kojima M, Gimenes-Junior JA, Chan TW, Eliceiri BP, Baird A, Costantini TW, Coimbra R. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32(1):97-110. doi: 10.1096/fj.201700488R.
» https://doi.org/10.1096/fj.201700488R -
7Liu GQ, Zuo XH, Jiang LN, Zhang YP, Zhang LM, Zhao ZG, Niu C Y. Inhibitory effect of post-hemorrhagic shock mesenteric lymph drainage on the HMGB1 and RAGE in mouse kidney. Ren Fail. 2016;38(1):131-6. doi: 10.3109/0886022X.2015.1105026.
» https://doi.org/10.3109/0886022X.2015.1105026 -
8Sambol JT, Lee MA, Caputo FJ, Kawai K, Badami C, Kawai T, Deitch EA, Yatani A. Mesenteric lymph duct ligation prevents trauma/hemorrhage shock-induced cardiac contractile dysfunction. J Appl Physiol (1985). 2009;106(1):57-65. doi: 10.1152/japplphysiol.90937.2008.
» https://doi.org/10.1152/japplphysiol.90937.2008 -
9Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, Caputo F, Feinman R, and Deitch E A. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246(5):822-30. doi: 10.1097/SLA.0b013e3180caa3af.
» https://doi.org/10.1097/SLA.0b013e3180caa3af -
10Watkins AC, Caputo FJ, Badami C, Barlos D, Xu DZ, Lu Q, Feketeova E, Deitch E A. Mesenteric lymph duct ligation attenuates lung injury and neutrophil activation after intraperitoneal injection of endotoxin in rats. J Trauma. 2008;64(1):126-30. doi: 10.1097/TA.0b013e3181574a8a.
» https://doi.org/10.1097/TA.0b013e3181574a8a -
11Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207 Suppl 1:E103-11. doi: 10.1111/j.1749-6632.2010.05713.x.
» https://doi.org/10.1111/j.1749-6632.2010.05713.x -
12Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350-6. doi: 10.1016/j.surge.2012.03.003.
» https://doi.org/10.1016/j.surge.2012.03.003 -
13Moore EE. Mesenteric lymph: the critical bridge between dysfunctional gut and multiple organ failure. Shock. 1998;10(6):415-6. PMID: 9872680.
-
14Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389(6):475-84. doi: 10.1007/s00423-004-0472-0.
» https://doi.org/10.1007/s00423-004-0472-0 -
15Liu H, Zhao ZG, Xing LQ, Zhang LM, Niu CY. Post-shock mesenteric lymph drainage ameliorates cellular immune function in rats following hemorrhagic shock. Inflammation. 2015;38(2):584-94. doi: 10.1007/s10753-014-9965-3.
» https://doi.org/10.1007/s10753-014-9965-3 -
16Tiesi G, Reino D, Mason L, Palange D, Tomaio JN, Deitch EA. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock. 2013;39(6):507-13. doi: 10.1097/SHK.0b013e318293d020.
» https://doi.org/10.1097/SHK.0b013e318293d020 -
17Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen Y H. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133(3):415-26. doi: 10.1016/j.cell.2008.03.026.
» https://doi.org/10.1016/j.cell.2008.03.026 -
18da Silva AVA, Figueiredo FB, Menezes RC, Mendes-Junior AA, de Miranda LHM, Cupolillo E, Porrozzi R, Morgado F N. Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens. PLoS Negl Trop Dis. 2018;12(4):e0006445. doi: 10.1371/journal.pntd.0006445.
» https://doi.org/10.1371/journal.pntd.0006445 -
19Dkhil MA, Al-Quraishy S, Al-Khalifa MS. The effect of Babesia divergens infection on the spleen of Mongolian gerbils. Biomed Res Int. 2014:483854. doi: 10.1155/2014/483854.
» https://doi.org/10.1155/2014/483854 -
20Giamarellos-Bourboulis EJ, Tziortzioti V, Koutoukas P, Baziaka F, Raftogiannis M, Antonopoulou A, Adamis T, Sabracos L, Giamarellou H. Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by pan-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2006;57(5):937-44. doi: 10.1093/jac/dkl084.
» https://doi.org/10.1093/jac/dkl084 -
21Bortolotti P, Faure E, Kipnis E. Inflammasomes in tissue damages and immune disorders after trauma. Front Immunol. 2018;9:1900. doi: 10.3389/fimmu.2018.01900.
» https://doi.org/10.3389/fimmu.2018.01900 -
22Liu Z, Wu Y, Feng Y, Wu F, Liu RF, Wang LF, Liang J Y, Liu J H, Sun X, Wu Z D. Spleen atrophy related immune system changes attributed to infection of Angiostrongylus cantonensis in mouse model. Parasitol Res. 2017;116(2):577-87. doi: 10.1007/s00436-016-5322-9.
» https://doi.org/10.1007/s00436-016-5322-9 -
23Warren M, Subramani K, Schwartz R, Raju R. Mitochondrial dysfunction in rat splenocytes following hemorrhagic shock. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2526-33. doi: 10.1016/j.bbadis.2017.08.024.
» https://doi.org/10.1016/j.bbadis.2017.08.024 -
24Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar T R. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202(3):407-17. doi: 10.1016/j.jamcollsurg.2005.11.021.
» https://doi.org/10.1016/j.jamcollsurg.2005.11.021 -
25Lou Y, Liu S. The TIPE (TNFAIP8) family in inflammation, immunity, and cancer. Mol Immunol. 2011;49(1-2):4-7. doi: 10.1016/j.molimm.2011.08.006.
» https://doi.org/10.1016/j.molimm.2011.08.006 -
26Padmavathi G, Banik K, Monisha J, Bordoloi D, Shabnam B, Arfuso F, Sethi G, Fan L, Kunnumakkara A B. Novel tumor necrosis factor-alpha induced protein eight (TNFAIP8/TIPE) family: functions and downstream targets involved in cancer progression. Cancer Lett. 2018;432:260-71. doi: 10.1016/j.canlet.2018.06.017.
» https://doi.org/10.1016/j.canlet.2018.06.017 -
27Kong L, Liu K, Zhang YZ, Jin M, Wu BR, Wang WZ, Li W, Nan YM, Chen YH. Downregulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol Int. 2013;7(3):844-9. doi: 10.1007/s12072-013-9435-2.
» https://doi.org/10.1007/s12072-013-9435-2 -
28Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y. Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol. 2013;191(9):4849-57. doi: 10.4049/jimmunol.1300053.
» https://doi.org/10.4049/jimmunol.1300053
Publication Dates
-
Publication in this collection
25 Nov 2019 -
Date of issue
2019
History
-
Received
19 May 2019 -
Reviewed
21 July 2019 -
Accepted
18 Aug 2019