Abstracts
Objective
The objective is to present an update on the diagnosis and treatment of hypovitaminosis D, based on the most recent scientific evidence.
Materials and methods
The Department of Bone and Mineral Metabolism of the Brazilian Society of Endocrinology and Metabology (SBEM) was invited to generate a document following the rules of the Brazilian Medical Association (AMB) Guidelines Program. Data search was performed using PubMed, Lilacs and SciELO and the evidence was classified in recommendation levels, according to the scientific strength and study type.
Conclusion
A scientific update regarding hypovitaminosis D was presented to serve as the basis for the diagnosis and treatment of this condition in Brazil.
Vitamin D; cholecalciferol; PTH; osteoporosis; deficiency; insufficiency; diagnosis; treatment
Objetivo
Apresentar uma atualização sobre o diagnóstico e tratamento da hipovitaminose D baseada nas mais recentes evidências científicas.
Materiais e métodos
O Departamento de Metabolismo Ósseo e Mineral da Sociedade Brasileira de Endocrinologia e Metabologia (SBEM) foi convidado a conceber um documento seguindo as normas do Programa Diretrizes da Associação Médica Brasileira (AMB). A busca dos dados foi realizada por meio do PubMed, Lilacs e SciELO e foi feita uma classificação das evidências em níveis de recomendação, de acordo com a força científica por tipo de estudo.
Conclusão
Foi apresentada uma atualização científica a respeito da hipovitaminose D que servirá de base para o diagnóstico e tratamento dessa condição no Brasil.
Vitamina D; colecalciferol; PTH; osteoporose; deficiência; insuficiência; diagnóstico; tratamento
INTRODUCTION
Hypovitaminosis D is highly prevalent and represents a public health problem in the entire world. Studies show an elevated prevalence of this disease in several geographic regions, including Brazil. It can affect more than 90% of individuals, depending on the population studied (11 .Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al.; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807-20.).
Vitamin D is essential in functions related to bone metabolism, but it seems to be related in the pathophysiology of many diseases. In children, vitamin D deficiency leads to growth retardation and rickets. In adults, hypovitaminosis D leads to osteomalacia, to secondary hyperparathyroidism and consequently, to an increase in bone resorption, favoring bone mass loss and the development of osteopenia and osteoporosis. Muscle weakness can also happen, which further contributes to elevating the risk of fall and bone fractures among patients with low bone mass (22 .Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477-501.,33 .Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.).
The correct diagnosis of this condition and the identification of improvement or worsening factors can help the elaboration of more efficient strategies for the treatment of risk populations, such as the elderly and post-menopausal women.
This document represents the efforts of the Department of Bone Metabolism of the Brazilian Society of Endocrinology and Metabology (SBEM) for the development of recommendations based on evidence available in the scientific literature regarding the diagnosis and treatment of this condition. The objective of this document is to respond daily questions and to be a guideline for endocrinologists and clinicians in the Brazilian context.
MATERIALS AND METHODS
The elaboration of this guideline was motivated by the SBEM within its Practical Guidelines program. The model applied followed the Guidelines Program of the Brazilian Medical Association (AMB) and the Federal Council of Medicine (CFM). After the selection of collaborators, with a significant role and relevant publications in the area, clinical questions to be approached were elaborated.
The publication search was performed using MedLine-PubMed and SciELO-Lilacs. We
used the Oxford Classification, which evaluates the study design and considers
the best available evidence for each question, to categorize the recommendation
level or evidence strength of each article (44 .Levels of evidence and Grades of Recommendations – Oxford Centre
for Evidence-Based Medicine. Disponível em:
http://www.cebm.net/index.aspx?o=1025.
http://www.cebm.net/index.aspx?o=1025...
,55 .Programa Diretrizes. Associação Médica Brasileira. Disponível em:
http://www.projetodiretrizes.amb.org.br.
http://www.projetodiretrizes.amb.org.br...
).
The levels of recommendation and evidence strength were reported as:
-
experimental or observational studies with better consistency.
-
experimental or observational studies with less consistency.
-
case reports (non-controled studies).
-
opinion lacking critical evaluation, based on guidelines, physiological studies or animal models.
DEFINITION AND PHYSIOLOGY
1. What is vitamin D: a nutrient or a prohormone?
Although it is defined as a vitamin, conceptually it is a prohormone. In conjunction with the parathyroid hormone (PTH), it acts as an important regulator of calcium homeostasis and bone metabolism.
It can be obtained from food sources, such as cod liver oil and from other fat-rich fish (salmon, tuna, mackerel), or from endogenous cutaneous synthesis, which represents the most important source of this “vitamin” for the majority of human beings (22 .Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477-501.,33 .Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.,66 .Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034-45.,77 .Wacker M, Holick MF. Vitamin D – Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5(1):111-48.) (A). Table 1 shows some food sources of vitamin D (33 .Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.).
Vitamin D can be found in the form of ergocalciferol or vitamin D2 and cholecalciferol or vitamin D3 (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.). Vitamin D2 can be obtained from some yeast and plants, being produced for commercial use, through irradiation of the ergosterol present in some mushrooms (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.) (D).
In the skin, the precursor is the 7-dehydrocholesterol (7-DHC) (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.,99 .Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-6S.). During sun exposure, UVB photons (ultraviolet B, 290-315 nm) penetrate the epidermis and produce a photochemical fragmentation to originate pre-cholecalciferol. This intermediate is converted to vitamin D (or cholecalciferol) through a temperature-dependent isomerization (Figure 1).
Cholecalciferol is transported to the liver by DBP (vitamin D binding protein). In the liver, there is the hydroxylation of carbon 25 (CYP27B1), forming the 25-hydroxyvitamin D (25(OH)D), through a process which is not strictly regulated, since it happens without control, and depends on the combination of cutaneous and diet stocks of vitamin D (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.).
After the liver step, 25(OH)D is transported to the kidneys by DBP, where it is converted to calcitriol or 1,25-dihydroxyvitamin D [1,25(OH)2D] (Figure 1). This is the most active metabolite and it is responsible for stimulating intestinal calcium and phosphate absorption. The kidney hydroxylation is stimulated by PTH and suppressed by phosphate and FGF-23. Calcitriol production is strictly controlled by negative feedback, in a way to influence its own synthesis by the decrease of 1α-hydroxylase. It is responsible for accelerating its inactivation through the conversion of 25(OH)D to 24,25(OH)2D. This mechanism reflects a direct action of 1,25(OH)2D in the kidneys, however there is still an inhibitory action on PTH production in the parathyroids (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.,99 .Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-6S.). The 1α-hydroxylase can also be found in other cells and tissues, such as the skin, prostate, breast, intestine, lungs, pancreatic β cells, monocytes and parathyroid cells. The 1,25(OH)2D molecule can also be locally synthesized by these cells and tissues (88 .Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.,99 .Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-6S.) (D).
The vitamin D receptor (VDR) belongs to the superfamily of nuclear receptors regulating the transcription factors of the steroid hormones, retinoic acid, thyroid hormones and vitamin D. After binding 1,25(OH)2D to VDR, it interacts with the retinoic acid receptor, forming a heterodimeric complex (RXR-VDR), which then binds specific sequences of DNA, known as Vitamin D Responsive Elements (VDRE) (1010 .McDonnell DP, Pike JW, O’Malley BW. The vitamin D receptor: a primitive steroid receptor related to thyroid hormone receptor. J Steroid Biochem. 1988;30(1-6):41-6.,1111 .Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13(4):719-64.). The main target organs for 1,25(OH)2D are the intestine, bones, parathyroid glands and kidneys. However, its receptors have been found in several other tissues (1010 .McDonnell DP, Pike JW, O’Malley BW. The vitamin D receptor: a primitive steroid receptor related to thyroid hormone receptor. J Steroid Biochem. 1988;30(1-6):41-6.,1111 .Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13(4):719-64.) (D).
2. What are the effects on bone metabolism?
The best known and studied actions of vitamin D are related to bone metabolism, where it plays a crucial role. It participates in intestinal calcium absorption, muscle function, modulation of PTH secretion and bone cell function.
Parathyroid cells express the 1α-hydroxylase enzyme, and can synthetize the
active form of vitamin D (1,25(OH)2D) inside the cells using the
25(OH)D serum pool (1212 .Vieth R, Ladak Y, Walfish PG. Age-related changes in the
25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different
reason why older adults require more vitamin D. J Clin Endocrinol Metab.
2003;88(1):185-91.)
(B). In hypovitaminosis D, due to a minor intracellular
synthesis, there is a secondary hyperparathyroidism, which is associated to
an increase in bone resorption (22 .Lips P. Vitamin D deficiency and secondary hyperparathyroidism in
the elderly: consequences for bone loss and fractures and therapeutic
implications. Endocr Rev. 2001;22(4):477-501.,1313 .Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A,
Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor
null mice. Endocr Rev. 2008;29(6):726-76.
14 .Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal
Physiol. 2005;289(1):F8-28.
15 .Prestwood KM, Pannullo AM, Kenny AM, Pilbeam CC, Raisz LG. The
effect of a short course of calcium and vitamin D on bone turnover in older
women. Osteoporos Int. 1996;6:314-9.-1616 .Kamel S, Brazier M, Rogez JC, Vincent O, Maamer M, Desmet G, et al.
Different responses of free and peptide-bound cross-links to vitamin D and
calcium supplementation in elderly women with vitamin D insufficiency J Clin
Endocrinol Metab. 1996;81(10):3717-21.) (B), besides the fact
that the circulating levels of 1,25(OH)2D are, generally, normal.
There is an inverse correlation between PTH and 25(OH)D, described in
children (1717 .Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of
evidence-based review on vitamin D efficacy and safety in relation to bone
health. Am J Clin Nutr. 2008;88(2):513S-9S.) and the elderly (22 .Lips P. Vitamin D deficiency and secondary hyperparathyroidism in
the elderly: consequences for bone loss and fractures and therapeutic
implications. Endocr Rev. 2001;22(4):477-501.). Several cut off values for 25(OH)D
for the PTH normalization have been published, the majority, being around 28
and 40 ng/mL (70 to 100 nmol/L) (22 .Lips P. Vitamin D deficiency and secondary hyperparathyroidism in
the elderly: consequences for bone loss and fractures and therapeutic
implications. Endocr Rev. 2001;22(4):477-501.,1818 .McKenna MJ. Differences in vitamin D status between countries in
young adults and the elderly. Am J Med. 1992;93:69-77.
19 .van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J,
Moreiras O, et al. Serum vitamin D concentration among elderly people in Europe.
Lancet. 1995;346(8969):207-10.
20 .Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S,
et al. Combined calcium and vitamin D3 supplementation in elderly women:
confirmation of reversal of secondary hyperparathyroidism and hip fracture risk:
The Decalyos II Study. Osteroporos Int. 2002;13:257-64.
21 .Freaney R, McBrinn Y, McKenna MJ. Secondary hyperparathyroidism in
elderly people: combined effect of renal insufficiency and vitamin D deficiency.
Am J Clin Nutr. 1993;58:187-91.
22 .Souberbielle JC, Cormier C, Kindermans C, Gao P, Cantor T, Forette
F, et al. Vitamin D status and redefining serum parathyoid hormone reference
range in the elderly. J Clin Endocrinol Metab.
2001;86(7):3086-90.-2323 .McKenna MJ, Freaney R. Secondary hyperparathyroidism in the
elderly: means to defining hypovitaminosis D. Osteoporos Int. 1998;8 Suppl
2:S3-6.) (C). Other causes of
secondary hyperparathyroidism also have to be investigated, such as chronic
kidney disease (creatinine clearance below 60 mL/min), Paget’s disease,
hungry bone syndrome and the calcium and vitamin D malabsorption syndromes
(2424 .Cunningham J, Locatelli F, Rodriguez M. Secondary
hyperparathyroidism: pathogenesis, disease progression, and therapeutic options.
Clin J Am Soc Nephrol. 2011;6(4):913-21.).
Intestinal calcium absorption depends on the active vitamin D action in the duodenum, through a transcellular saturable process, whose stimulus leads to the synthesis of proteins such as calbindin-D9K (CaBP-9k) and the epithelial apical channel TRPV6 (1313 .Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-76.,1414 .Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28.) (D). However, there is evidence that the non-saturable transport, which happens with part of calcium absorption in the human ileum is also vitamin D sensitive (2525 .Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47(4):181-95.). According to Heaney and cols., individuals with 35 ng/mL of 25(OH)D presented higher absorption than those with 25 ng/mL (2626 .Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142-6.) (B). Increase of calcium absorption with increasing dose of vitamin D3 or serum 25(OH)D was recently observed, but there is no evidence of what the minimum value of 25(OH)D to ensure calcium absorption from the intestine in the range of 16-52 ng/mL evaluated in the study (2727 .Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Fazzari M, Ragolia L, et al. Vitamin D supplementation increases calcium absorption without a threshold effect. Am J Clin Nutr. 2014;99(3):624-31.).
Population studies correlated positively vitamin D concentration with bone
mass, mainly of the hip, but with 25(OH)D cut off points varying from 12 to
36 ng/mL (30-90 nmol/L) (2828 .Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive
association between 25-hydroxy vitamin D levels and bone mineral density: a
population-based study of younger and older adults. Am J Med.
2004;116(9):634-9.
29 .Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P.
Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum
parathyroid hormone and markers of bone turnover in older persons. J Clin
Endocrinol Metab. 2009;94(4):1244-50.-3030 .Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D
status, parathyroid function, bone turnover, and BMD in postmenopausal women
with osteoporosis: global perspective. J Bone Miner Res.
2009;24(4):693-701.) (C).
The muscle tissue expresses vitamin D receptors (1313 .Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-76.) and, clinically, muscle weakness and myopathy are observed in patients presenting severe vitamin D deficiency. Dhesi and cols., observed that the number of falls is higher among the elderly when they present the vitamin D deficiency (3131 .Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891-7.) (C). The administration of 800 IU of cholecalciferol for 12 weeks decreased in 49% the number of falls (3232 .Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-51.) (B). Cholecalciferol use is associated with the prevention of falls among the elderly with hypovitaminosis D, but not among those presenting normal serum values (3333 .Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611-5.) (B).
In a meta-analysis of the main osteoporosis intervention studies;
Bischoff-Ferrari and cols. indicated again 25(OH)D serum concentration above
30 ng/mL (75 nmol/L) to be the most beneficial for health in general
(A). Bone health, here represented by a better bone mineral
density (BMD), decreased risk of fall and femural and non-vertebral
osteoporotic fractures, seems to be benefited by 25(OH)D concentrations
equal to or higher than 30 ng/mL (75 nmol/L), concentrations around 36 ng/mL
(90 nmol/L) being suggested as the most advantageous (3434 .Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich
T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a
meta-analysis of randomized controlled trials. JAMA.
2005;293(18):2257-64.
35 .Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck
AE, Theiler R, et al. Fall prevention with supplemental and active forms of
vitamin D: a meta-analysis of randomised controlled trials. BMJ.
2009;339:b3692.-3636 .Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T,
Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin
D for multiple health outcomes. Am J Clin Nutr.
2006;84(1):18-28.). The same
25(OH)D values seem to benefit the muscle strength of lower limbs, which was
evaluated by the TUG (Time Up and Go) test, where the individual is
evaluated based on the time he needs to walk a distance equivalent to eight
steps. Individuals presenting 25(OH)D in the range of 36 to 40 ng/mL (90 and
100 nmol/L) seem to perform with higher speed. Evidence also suggests that
higher 25(OH)D values are associated with a lower risk for colorectal cancer
and periodontal disease (3636 .Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T,
Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin
D for multiple health outcomes. Am J Clin Nutr.
2006;84(1):18-28.)
(A).
Regarding the bone tissue, evidence suggests that 1,25(OH)2D stimulates mineralization, through an indirect process that happens with the increase in intestinal absorption of the minerals which are incorporated into the bone matrix. Physiological concentrations of calcitriol promote calcium mobilization to the bones, while the administration of large doses promotes excessive bone remodeling. Osteoblasts present 1,25(OH)2D receptor. This hormone modulates the gene expression of alkaline phosphatase and osteocalcin. Therefore, in the process of bone remodeling, 1,25(OH)2D is important for bone formation and reabsorption (3737 .Bikle DD. Vitamin D and bone. Curr Osteoporos Rep. 2012;10(2):151-9.).
Priemel and cols. evaluated 675 bone biopsies and correlated the histomorphometry findings with serum 25(OH)D concentration. The presence of bone mineralization defects was only found in individuals with concentrations below 30 ng/mL (75 nmol/L) (3838 .Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25(2):305-12.) (B).
The role of vitamin D in non-bone related endpoints, such as mortality, cardiovascular risk, cancer and autoimmune diseases is still controversial (3939 .de Paula FJA, Rosen CJ. Vitamin D safety and requirements. Arch Biochem Biophys. 2012; 523(1):64-72.,4040 .Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307-20.).
DIAGNOSIS
3. How to define hypovitaminosis D?
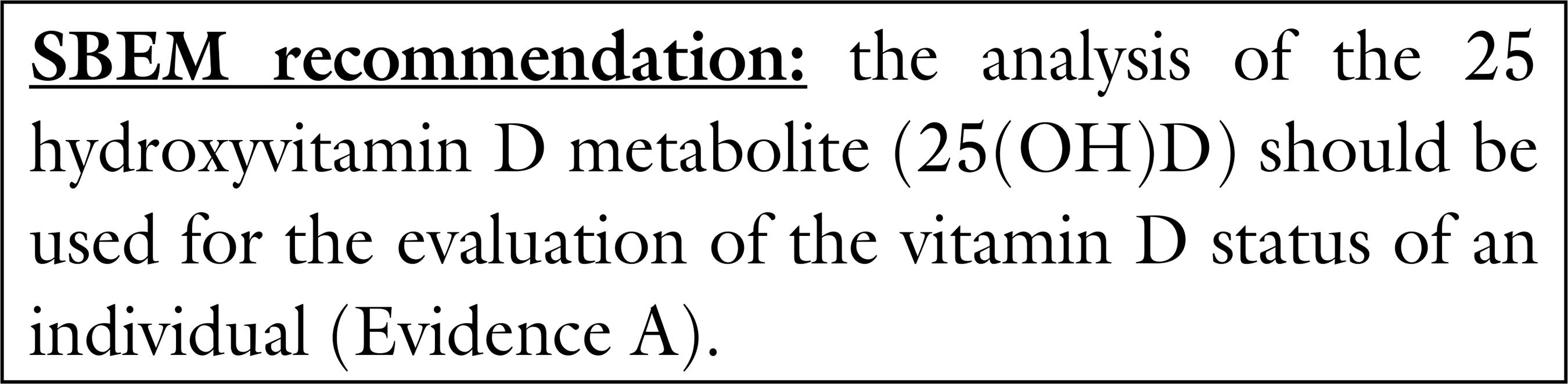
There is a consensus that 25(OH)D (calcidiol) is the most abundant metabolite and the best indicator for the evaluation of vitamin D status (A), the individuals being classified as: deficient, insufficient of sufficient in vitamin D (33 .Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.,66 .Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034-45.,4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.,4242 .Heaney RP. What is vitamin D insufficiency? And does it matter? Calcif Tissue Int. 2013;92(2):177-83.). On the other hand, there is no consensus regarding the cut off value for the definition of “vitamin D sufficiency” (66 .Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034-45.,4343 .Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26(3):455-7.,4444 .Chapuy M-C, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439-43.).
The values discussed in the medical literature, based on populational
studies, with emphasis on calcium homeostasis and bone health, vary from 20
to 32 ng/mL (50 to 80 nmol/L) (2626 .Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies
within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr.
2003;22(2):142-6.,4343 .Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are
deficient. J Bone Miner Res. 2011;26(3):455-7.
44 .Chapuy M-C, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et
al. Prevalence of vitamin D insufficiency in an adult normal population.
Osteoporos Int. 1997;7(5):439-43.
45 .Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of
vitamin D sufficiency: implications for establishing a new effective dietary
intake recommendation for vitamin D. J Nutr.
2005;135(2):317-22.
46 .Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R.
Estimates of optimal vitamin D status. Osteoporos Int.
2005;16(7):713-6.-4747 .Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS. Prevalence
of vitamin D deficiency and its correlation with PTH, biochemical bone turnover
markers and bone mineral density, among patients from ambulatories. Arq Bras
Endocrinol Metabol. 2008;52(3):482-8.). Several specialists agree that for
correction of secondary hyperparathyroidism, reduction of the risk of fall
and fractures and maximum calcium reabsorption, the best 25(OH)D cut off
value is 30 ng/mL (75 nmol/L) (66 .Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision
for the future. Exp Biol Med (Maywood). 2010;235(9):1034-45.,4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA,
Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of
vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2011;96(7):1911-30.,4646 .Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R.
Estimates of optimal vitamin D status. Osteoporos Int.
2005;16(7):713-6.). Thus, serum concentrations below 20 ng/mL (50 nmol/L) are
classified as deficiency; those ranging from 20 to 29 ng/mL (50 to 74
nmol/L) as insufficiency and between 30 and 100 ng/mL (75 and 250 nmol/L) as
sufficiency. Therefore, many consider 25(OH)D serum concentrations below 30
ng/mL as hypovitaminosis D (33 .Holick MF. Vitamin D deficiency. N Engl J Med.
2007;357(3):266-281.,4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA,
Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of
vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2011;96(7):1911-30.
42 .Heaney RP. What is vitamin D insufficiency? And does it matter?
Calcif Tissue Int. 2013;92(2):177-83.-4343 .Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are
deficient. J Bone Miner Res. 2011;26(3):455-7.,4848 .Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland
CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that
is effective. Am J Clin Nutr. 2007;85(3):649-50.,4949 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA,
Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency
and insufficiency revisited. J Clin Endocrinol Metab.
2012;97(4):1153-8.). These values were recognized by
the Endocrine Society guideline, although they differ from the ones accepted
(20 ng/mL) by the Institute of Medicine (IOM) (5050 .Institute of Medicine (IOM). Dietary Reference Intakes (DRIs) for
calcium and vitamin D. Report at a glance 2011. Disponível em:
http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx.
http://www.iom.edu/Reports/2010/Dietary-...
) (B). In general population, there is
no evidence of benefit in the measurement of 25(OH)D due to the high cost,
but according to the Endocrine Society, to maximize bone health;
supplementation is recommended for children up to 1 year with at least 400
IU/day; between 1 and 70 years, at least 600 IU/day while over 70 years old,
800 IU/day (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA,
Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of
vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2011;96(7):1911-30.).
4. What are the methodological implications for the plasma determinations of 25(OH)D?
Circulating 25(OH)D level is the best method to evaluate the individual vitamin D status. Nevertheless, there are controversies regarding the best method for 25(OH)D determination. Some factors should be considered when the levels of this vitamin are evaluated, such as the lack of a precise physiological regulatory control (feedback), the variability of methods and standards, the inclusion of contaminant metabolites in the analysis, among others. Radioimmunoassays (RIA) used in the past underestimated the levels of 25(OH)D when the dominant levels were 25(OH)D2. RIA have been replaced by automated chemiluminescent immunoassays, resulting in higher concentrations and by immunoenzymatic assays which measure total 25(OH)D, a combination of vitamin D2 (25(OH)D2) and vitamin D3 (25(OH)D3 (5151 .Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062-71.) (B).
The methods that do not employ direct immune detection are high performance liquid chromatography (HPLC) coupled to mass spectrometry (LC-MS), which can distinguish individual levels of 25(OH)D2 and 25(OH)D3. These are considered the gold standard for analysis and currently used as reference (5252 .El-Khoury JM, Reineks EZ, Wang S. Progress of liquid chromatography-mass spectrometry in measurement of vitamin D metabolites and analogues. Clin Biochem. 2011;44(1):66-76.) (B). Both 1,25(OH)2D and 25(OH)D circulate predominantly bound to proteins and their concentration can be determined. However, to evaluate the vitamin D status, 25(OH)D total serum level is used, including both D3 and D2 forms. The results can be reported in nanograms per milliliter (ng/mL) or nanomol per liter (nmol/L). For conversion, you just need to multiply the value obtained in ng/mL by 2.5, to obtain the value in nmol/L. Automated methods allow the use in clinical routines, they are fast and report 25(OH)D2 and 25(OH)D3 together, while LC-MS methods can distinguish between 25(OH)D2 and 25(OH)D3, being useful, then, for the evaluation of the effectiveness of D2 supplementation, versus endogenous D3 production. These chromatographic methods, although more precise, are more laborious and expensive (5353 .Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25 hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127-34.) (B).
The accuracy of the measurements varies widely between laboratories and between different assays, and even when testing identical samples, this variation can achieve 17 ng/mL (5353 .Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25 hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127-34.). The immunoassay requires the development of selective antibodies for 25(OH)D2 and 25(OH)D3, which preferentially do not show cross reaction with any other metabolite. Matrix effects can still occur, caused by endogenous components that modify the binding of the antibody to the material to be analyzed. Metabolites with lower physiological potential end up being included in the quantification, such as the 3-epimer of the 25(OH)D, which can correspond to up to 5% of the total 25(OH)D. As its molecular weight is identical to 25(OH)D, these are not separated by LC-MS. Finally, 24,25 dihydroxyvitamin D (24,25(OH)2D), considered an inactive metabolite, can correspond to up to 20% of the 25(OH)D measured, whereas some assays show 100% cross-reaction (5151 .Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062-71.,5454 .Binkley N, Wiebe D. Clinical controversies in vitamin D: 25(OH)D measurement, target concentration, and supplementation. J Clin Densitom. 2013;16(4):402-8.).
The use of a standard cut off value to evaluate vitamin D status is problematic if applied to all laboratories and all methods, considering there are still differences on vitamin D extraction from its binding protein, cross reaction between 25(OH)D2, 25(OH)D3 and other metabolites, besides the lack of standardization (5252 .El-Khoury JM, Reineks EZ, Wang S. Progress of liquid chromatography-mass spectrometry in measurement of vitamin D metabolites and analogues. Clin Biochem. 2011;44(1):66-76.,5353 .Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25 hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127-34.) and for this reason, quality control tools, such as DEQAS (International Vitamin D External Quality Assessment Scheme) were created, as an attempt to decrease the variation in data analysis (5555 .Carter GD, Berry JL, Gunter E, Jones G, Jones JC, Makin HL, et al. Proficiency testing of 25-hydroxyvitamin D (25-OHD) assays. J Steroid Biochem Mol Biol. 2010;121(1-2):176-9.).
The most used methods nowadays are competitive assays, based on specific antibodies and non-radioactive markers, the improvement in the comparison between results obtained from different methodologies being necessary. Whatever the method employed is a precise definition of the normality range is fundamental (5656 .Barake M, Daher RT, Salti I, Cortas NK, Al-Shaar L, Habib RH, et al. 25-hydroxyvitamin D assay variations and impact on clinical decision making. J Clin Endocrinol Metab. 2012;97(3):835-43.). It is also important to highlight that the intra-individual variability can vary from 12.1 to 40.3% (5757 .Singh DK, Farrington K, Twomey PJ. Analytical quality goals for 25-vitamin D based on biological variation. J Clin Lab Anal. 2011;25(2):130-3.).
The clinical conditions that interfere with 25(OH)D serum concentrations are highly dependent on environmental factors and lifestyle, particularly UVB sunlight exposure. Polymorphisms in CYP27B1, which codes for 1α-hydroxylase, showed strong correlation with variations in 25(OH)D level. The vitamin D binding protein (DBP) is the main transporter for vitamin D metabolites, its phenotype helping predict 25(OH)D serum concentrations. Certain polymorphic forms can be more efficient for vitamin D binding, activation and metabolism, interfering with circulating levels. Genetic polymorphisms greatly contribute to the heterogeneity of clinical manifestations of hypovitaminosis D, especially among ethnic groups (5151 .Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062-71.,5858 .Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880-1.) (B).
EPIDEMIOLOGY
5. Which are the risk populations for hypovitaminosis D? What is the prevalence in Brazil?
The Department of Bone and Mineral Metabolism from SBEM agrees with the guidelines published by the Endocrine Society, which does not recommend the 25(OH)D test for the general population considering the cost of this evaluation. The laboratory test is recommended for individuals under risk for hypovitaminosis D or for those with a relevant clinical condition. The candidates to be tested are the ones presenting the following conditions: rickets or osteomalacia, osteoporosis, history of falls and fractures in the elderly, obesity, pregnant and lactating women, patients with malabsorption syndromes (cystic fibrosis, inflammatory bowel disease, Chron’s disease, bariatric surgery), renal or liver insufficiency, hyperparathyroidism, medications interfering in vitamin D metabolism (anticonvulsants, glucocorticoids, antifungal, antiretroviral, cholestyramine, orlistat), granulomatous diseases and lymphomas (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.,5959 .McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814-22.) (A). It is also useful for the evaluation of hypothesis of vitamin D intoxication.
Besides that, it is important to highlight that all the conditions that limit
sunlight exposure can potentially cause hypovitaminosis D and can be added
to the list of individuals in photoprotection regimen (6060 .Reichrath J, Nürnberg B. Cutaneous vitamin D synthesis versus skin
cancer development: The Janus faces of solar UV-radiation. Dermatoendocrinol.
2009;1(5):253-61.) (D) and religious garment users (veil,
burqa, cassock and others) (6161 .Jamali Z, Asadikaram G, Mahmoodi M, Sayadi A, Jamalizadeh A,
Saleh-Moghadam M, et al. Vitamin D status in female students and its relation to
calcium metabolism markers, lifestyles, and polymorphism in vitamin D receptor.
Clin Lab. 2013;59(3-4):407-13.
62 .Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, El Kabbaj
S, et al. High prevalence of hypovitaminosis D in Morocco: relationship to
lifestyle, physical performance, bone markers, and bone mineral density. Semin
Arthritis Rheum. 2009;38(6):444-51.-6363 .Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D
in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res.
2000;15(9):1856-62.) (C).
Hypovitaminosis D is a world health problem and Brazil is part of this
scenario, also presenting an elevated prevalence of hypovitaminosis D in the
population (B). Table
2 presents some of the main Brazilian and international studies
that included Brazil, published in the last decade. In general, in several
regions of the country, the values indicate sub-optimum vitamin D
concentrations, verifying high prevalence of hypovitaminosis D in several
age groups. The majority of the studies included mainly the elderly and
post-menopausal women which are the populations at risk for osteoporosis
(C). However, three studies involving adolescents showed
high prevalence of hypovitaminosis D in this age group of the Brazilian
population (6464 .Peters BSE, dos Santos LC, Fisberg M, Wood RJ, Martini LA.
Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab.
2009;54(1):15-21.
65 .Oliveira RM, Novaes JF, Azeredo LM, Cândido AP, Leite IC.
Association of vitamin D insufficiency with adiposity and metabolic disorders in
Brazilian adolescents. Public Health Nutr. 2013;9:1-8.-6666 .Santos BR, Mascarenhas LP, Satler F, Boguszewski MC, Spritzer PM.
Vitamin D deficiency in girls from South Brazil: a cross-sectional study on
prevalence and association with vitamin D receptor gene variants. BMC Pediatr.
2012;12:62.). The factors that seem to favor the presence of
higher serum concentrations in our population are: younger age (6464 .Peters BSE, dos Santos LC, Fisberg M, Wood RJ, Martini LA.
Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab.
2009;54(1):15-21.,6767 .Unger MD, Cuppari L, Titan SM, Magalhães MC, Sassaki AL, dos Reis
LM, et al. Vitamin D status in a sunny country: where has the sun gone? Clin
Nutr. 2010;29(6):784-8.
68 .Maeda SS, Kunii IS, Hayashi L, Lazaretti-Castro M. The effect of
sun exposure on 25-hydroxyvitamin D concentrations in young healthy subjects
living in the city of São Paulo, Brazil. Braz J Med Biol Res.
2007;40(12):1653-9.-6969 .Maeda SS, Saraiva GL, Kunii IS, Hayashi LF, Cendoroglo MS, Ramos
LR, et al. Factors affecting vitamin D status in different populations in the
city of São Paulo, Brazil: the São PAulo vitamin D Evaluation Study (SPADES).
BMC Endocr Disord. 2013;13(1):14.), community life
(7070 .Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LM, Vieira JG, Maeda
SS, et al. Prevalence of vitamin D deficiency, insufficiency and secondary
hyperparathyroidism in the elderly inpatients and living in the community of the
city of São Paulo, Brazil. Arq Bras Endocrinol Metabol.
2007;51(3):437-42.), the practice of outdoors
physical activity (6464 .Peters BSE, dos Santos LC, Fisberg M, Wood RJ, Martini LA.
Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab.
2009;54(1):15-21.,7171 .Maeda SS, Kunii IS, Hayashi LF, Lazaretti-Castro M. Increases in
summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in
São Paulo, Brazil vary with age, gender and ethnicity. BMC Endocr Disord.
2010;10:12.), vitamin D oral supplementation
(7272 .Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS. Prevalence
of vitamin D deficiency and its correlation with PTH, biochemical bone turnover
markers and bone mineral density, among patients from ambulatories. Arq Bras
Endocrinol Metabol. 2008;52(3):482-8.), season of the year (spring,
summer) (6868 .Maeda SS, Kunii IS, Hayashi L, Lazaretti-Castro M. The effect of
sun exposure on 25-hydroxyvitamin D concentrations in young healthy subjects
living in the city of São Paulo, Brazil. Braz J Med Biol Res.
2007;40(12):1653-9.
69 .Maeda SS, Saraiva GL, Kunii IS, Hayashi LF, Cendoroglo MS, Ramos
LR, et al. Factors affecting vitamin D status in different populations in the
city of São Paulo, Brazil: the São PAulo vitamin D Evaluation Study (SPADES).
BMC Endocr Disord. 2013;13(1):14.
70 .Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LM, Vieira JG, Maeda
SS, et al. Prevalence of vitamin D deficiency, insufficiency and secondary
hyperparathyroidism in the elderly inpatients and living in the community of the
city of São Paulo, Brazil. Arq Bras Endocrinol Metabol.
2007;51(3):437-42.
71 .Maeda SS, Kunii IS, Hayashi LF, Lazaretti-Castro M. Increases in
summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in
São Paulo, Brazil vary with age, gender and ethnicity. BMC Endocr Disord.
2010;10:12.
72 .Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS. Prevalence
of vitamin D deficiency and its correlation with PTH, biochemical bone turnover
markers and bone mineral density, among patients from ambulatories. Arq Bras
Endocrinol Metabol. 2008;52(3):482-8.-7373 .Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LMQ, Vieira JGH, Kunii
I, et al. Influence of ultraviolet radiation on the production of 25
hydroxyvitamin D in the elderly population in the city of São Paulo (23° 34’S),
Brazil. Osteoporos Int. 2005;16(12):1649-54.), residence in sunny beach areas (7474 .Bandeira F, Griz L, Freese E, Lima DC, Thé AC, Diniz ET, et al.
Vitamin D deficiency and its relationship with bone mineral density among
postmenopausal women living in the tropics. Arq Bras Endocrinol Metabol.
2010;54(2):227-32.,7575 .Neves JP, Silva AS, Morais LC, Diniz Ada S, Costa MJ, Asciutti LS,
et al. 25-hydroxyvitamin D concentrations and blood pressure levels in
hypertensive elderly patients. Arq Bras Endocrinol Metabol.
2012;56(7):415-22.) and in lower latitudes (7676 .Arantes HP, Kulak CA, Fernandes CE, Zerbini C, Bandeira F, Barbosa
IC, et al. Correlation between 25-hydroxyvitamin D levels and latitude in
Brazilian postmenopausal women: from the Arzoxifene Generations Trial.
Osteoporos Int. 2013;24(10):2707-12.).
TREATMENT
6. How to treat hypovitaminosis D in patients who are at high risk for the deficiency?
Current evidence does not support the concept of general population supplementation (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.) (A). As the adequacy of vitamin D concentration in our population is closely related to the cutaneous production, secondary to sunlight exposure, individuals with low exposure represent the main population of deficient individuals. Therefore, a simple interview can bring important information on the probability of vitamin D deficiency in a specific individual.
The complementation of the daily needs, as well as the treatment of the deficiency should be performed for individuals with hypovitaminosis D risk (see Epidemiology section) or those to whom sunlight exposure is prohibited, due to skin cancer, transplants or systemic lupus erythematosus (A).
The most available vitamin D form for treatment and supplementation is cholecalciferol or vitamin D3 and this is the metabolite that has been shown to be the most effective one. Ergocalciferol or vitamin D2 can also be used as a supplement, however the studies show that, as its half-life is a little shorter than the one of D3, it should be used preferentially daily (8383 .Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981-8.). Besides that, some laboratory methods that test 25(OH)D recognize only 25(OH)D3, what can bring problems for the control of plasma levels when vitamin D2 is used for supplementation. Therefore, although supplementation and treatment can be done with both vitamin D metabolites, preference should be given to vitamin D3, due to the advantages on the maintenance of more stable concentrations.
The treatment doses vary according to the degree of deficiency and the target to be achieved. Apparently, 25(OH)D concentrations higher than 12 ng/mL would be sufficient to avoid rickets and osteomalacia, as well as to normalize intestinal absorption of calcium (8484 .Aloia JF, Chen DG, Yeh JK, Chen H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am Am J Clin Nutr. 2010;92(4):835-40.,8585 .Nordin BE. Evolution of the calcium paradigm: the relation between vitamin D, serum calcium and calcium absorption. Nutrients. 2010;2(9):997-1004.). However, to reduce fractures, concentrations above 24 ng/mL are necessary (8686 .Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40-9.), while to avoid the development of secondary hyperparathyroidism, concentrations above 30 ng/mL are desirable (6969 .Maeda SS, Saraiva GL, Kunii IS, Hayashi LF, Cendoroglo MS, Ramos LR, et al. Factors affecting vitamin D status in different populations in the city of São Paulo, Brazil: the São PAulo vitamin D Evaluation Study (SPADES). BMC Endocr Disord. 2013;13(1):14.). Therefore, especially during osteoporosis treatment, it is recommended that plasma 25(OH)D is kept above 30 ng/mL.
As a practical rule, one can predict that, for every 100 IU supplemented, an increase of 0.7 to 1.0 ng/mL is gained in the concentration of 25(OH)D (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.). However, other studies show that this dose-response curve is not linear.
In a study developed in Brazil, with an institutionalized population, showing high prevalence of hypovitaminosis D (40.4% with 25(OH)D < 20 ng/mL), supplementation with 7,000 IU/day, produced an average elevation of 7.5 ng/mL in 25(OH)D concentration after three months (8787 .Canto-Costa MH, Kunii I, Hauache OM. Body fat and cholecalciferol supplementation in elderly homebound individuals. Braz J Med Biol Res. 2006;39(1):91-8.) and this elevation achieved a plateau around six weeks. However, as it has already been recognized by other authors, this increase was more evident among those individuals with lower initial values (< 20 ng/mL), in whom the average increase was 10.3 ng/mL after three months of treatment, while those showing 25(OH)D concentration above 20 ng/mL, increased on average only 5.18 ng/mL. Besides that, 45% of the individuals still kept insufficient (30 ng/mL) and 10% still kept deficient (< 20 ng/mL) at the end of three months of supplementation. This demonstrates that, for values lower than 20 ng/mL, doses higher than 1,000 IU/day will be necessary if the target to be achieved is 30 ng/mL (B).
In a similar institutionalized population, Moreira-Pfrimer and cols. demonstrated, in a randomized, double blind prospective placebo controlled study, that an average dose of 3,700 IU/day of vitamin D3 for six months was able to take the treated group to average concentrations of 34.6 (variation from 20.9 to 48.4) ng/mL, while the placebo group kept in 20.7 (variation from 9.4 to 41.2) ng/mL (p < 0.0001). There was a significant increase in calcemia for the treated group, but no patient developed hypercalcemia (8888 .Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab. 2009;54(4):291-300.) (A).
Those institutionalized and bedridden are a population with elevated risk for deficiency. Mocanu and cols. evaluated the effect of the fortification of the bread roll with 320 mg of calcium and 5,000 IU of vitamin D on an institutionalized population for 12 months. It was possible to verify an effective increase in 25(OH)D (initial average 11.4 ng/mL and final average 50.0 ng/mL), with 92% of the individuals achieving concentrations higher than 30 ng/mL. No individual developed hypercalcemia ou hypercalciuria. PTH concentrations were reduced during treatment and there was a significant increase in BMD of the lumbar spine and proximal femur (8989 .Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, et al. Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D(3) per daily serving. Am J Clin Nutr. 2009;89(4):1132-7.). However, when reevaluated, this same population, three years after removal of supplementation, it was verified that the benefits gained with vitamin D supplementation had been lost (9090 .Mocanu V, Vieth R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 μg of vitamin D3. Nutr J. 2013;12:137.).
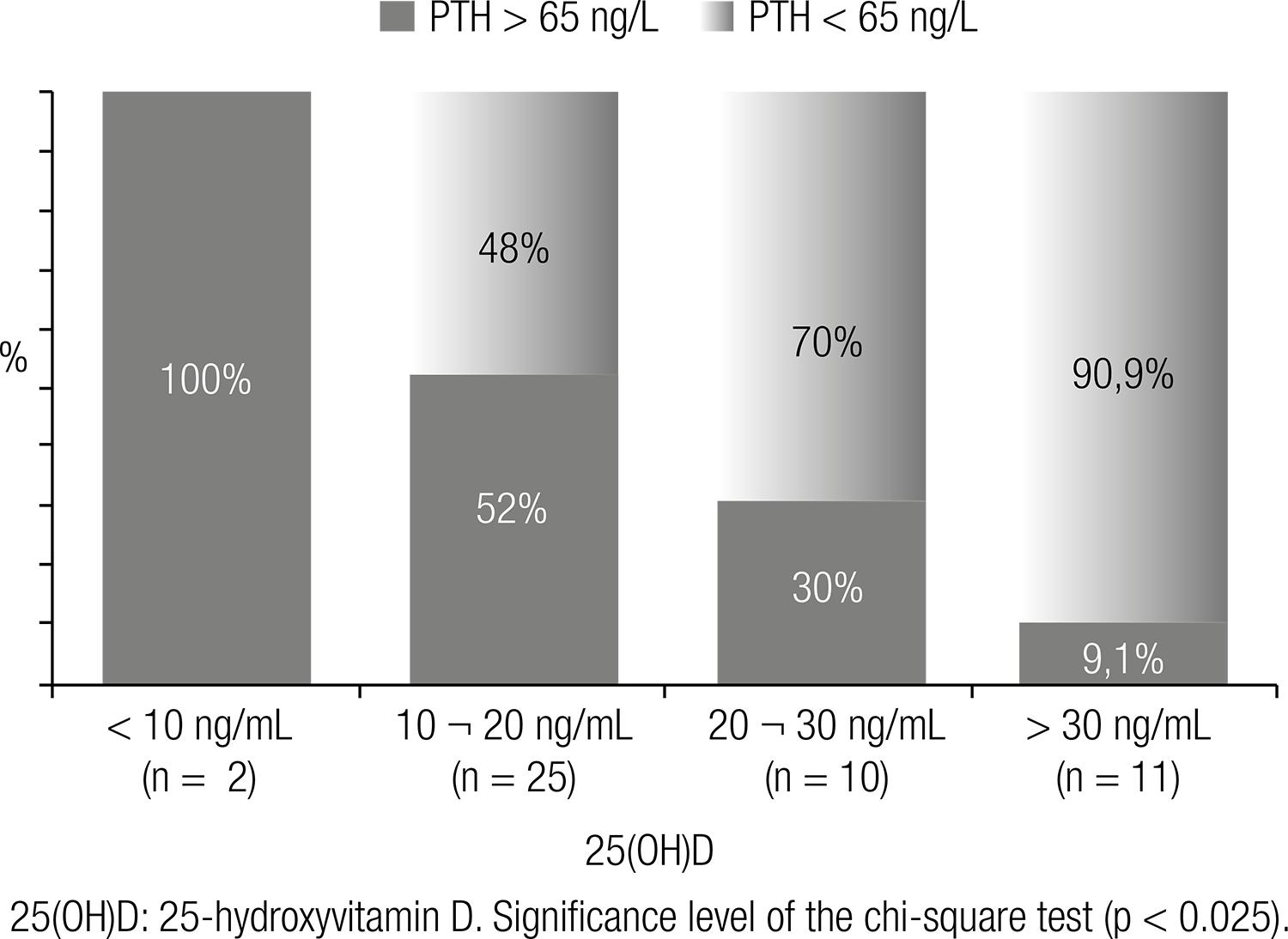
In a population of post-menopausal women undergoing treatment for osteoporosis, followed in a specific outpatient clinic, Camargo (9191 .Camargo MBR. Fatores determinantes do status de vitamina D em pacientes de um ambulatório especializado em osteoporose e sua interferência sobre a absorção de cálcio [tese]. São Paulo: Universidade Federal de São Paulo; 2013.) demonstrated that weekly doses higher than 7,000 IU (> 1,000 IU/day) are necessary to achieve vitamin D sufficiency (25(OH)D > 30 ng/mL), which is in accordance with the proposition of the Endocrine Society for the elderly at risk (Table 3). According to this Brazilian study, 73% of the patients, followed for at least three months in ambulatory directed to the treatment of osteoporosis were below the desired target concentration (> 30 ng/mL) (Figure 2). In the same study, a positive correlation was found between 25(OH)D concentrations and the femur BMD and a negative correlation with PTH (9191 .Camargo MBR. Fatores determinantes do status de vitamina D em pacientes de um ambulatório especializado em osteoporose e sua interferência sobre a absorção de cálcio [tese]. São Paulo: Universidade Federal de São Paulo; 2013.).
Percentage of non-adequacy of vitamin D according to the plasma concentrations of 25-hydroxyvitamin D (25(OH)D) in a population of individuals presenting osteoporosis at a medical school teaching ward (91).
In children and adolescents, the doses, apparently, do not vary much from the ones in the adults, with the exception of the first year of life (Table 3). Winzenberg and cols., in a meta-analysis involving six studies that evaluated vitamin D supplementation in healthy children, were able to group 343 participants that received placebo and 541 participants that received vitamin D and the analysis suggested a benefit of the supplementation on the in lumbar spine bone mineral density and total body bone mineral content of those children that were previously deficient (9292 .Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.). Therefore, as already observed in other studies, the positive endpoints of the supplementation are always much more evident when the populations studied were initially deficient (A).
Vieth in a study done in Canada, tested two vitamin D3 doses, compared to placebo. The first dose was 1,400 IU/week (or 200 IU/day), the same quantity recommended at current nutritional tables from the Ministry of Health in Brazil. The second dose was 14,000 IU/week, both groups being followed for 12 months. The group that received 1,400 IU/week did not present significant increase in 25(OH)D concentration, while the group receiving 14,000 IU obtained an increment of 15 to 30 ng/mL at the end of 12 months (9393 .Vieth R. Implications for 25-hydroxyvitamin D testing of public health policies about the benefits and risks of vitamin D fortification and supplementation. Scand J Clin Lab Invest Suppl. 2012;243:144-53.). Taking from that and similar studies, an alteration on the vitamin D daily recommendation tables was proposed in several countries.
In 2011, the Institute of Medicine, in the USA, an organ that regulates the
reference tables for daily recommended intake (DRI) for the general
population, increased the daily recommendation to 600 IU for individuals
between the ages of 1 and 70, and to 800 IU for those older than 70 (Table 3) (5050 .Institute of Medicine (IOM). Dietary Reference Intakes (DRIs) for
calcium and vitamin D. Report at a glance 2011. Disponível em:
http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx.
http://www.iom.edu/Reports/2010/Dietary-...
,9494 .Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et
al. The 2011 report on dietary reference intakes for calcium and vitamin D from
the Institute of Medicine: what clinicians need to know. J Clin Endocrinol
Metab. 2011;96(1):53-8.). However,
the Brazilian nutritional table remains with the daily recommended intake of
200 IU, although several national studies have demonstrated that the typical
meals of the Brazilian population are not a relevant vitamin D source (6464 .Peters BSE, dos Santos LC, Fisberg M, Wood RJ, Martini LA.
Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab.
2009;54(1):15-21.,9595 .Pinheiro MM, Schuch NJ, Genaro PS, Ciconelli RM, Ferraz MB, Martini
LA. Nutrient intakes related to osteoporotic fractures in men and women--the
Brazilian Osteoporosis Study (BRAZOS). Nutr J. 2009;8:6.), that we depend on cutaneous synthesis to obtain sufficiency
and that the deficiency is present in all age and populational groups,
especially among the elderly (7070 .Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LM, Vieira JG, Maeda
SS, et al. Prevalence of vitamin D deficiency, insufficiency and secondary
hyperparathyroidism in the elderly inpatients and living in the community of the
city of São Paulo, Brazil. Arq Bras Endocrinol Metabol.
2007;51(3):437-42.,7474 .Bandeira F, Griz L, Freese E, Lima DC, Thé AC, Diniz ET, et al.
Vitamin D deficiency and its relationship with bone mineral density among
postmenopausal women living in the tropics. Arq Bras Endocrinol Metabol.
2010;54(2):227-32.,7676 .Arantes HP, Kulak CA, Fernandes CE, Zerbini C, Bandeira F, Barbosa
IC, et al. Correlation between 25-hydroxyvitamin D levels and latitude in
Brazilian postmenopausal women: from the Arzoxifene Generations Trial.
Osteoporos Int. 2013;24(10):2707-12.,8080 .Lopes JB, Danilevicius CF, Takayama L, Caparbo VF, Scazufca M,
Bonfá E, et al. Vitamin D insufficiency: a risk factor to vertebral fractures in
community-dwelling elderly women. Maturitas. 2009;64(4):218-22.) (C).
Generally speaking, when 25(OH)D is lower than the target concentration (below 20 ng/mL), an attack dose is necessary to replenish the body stocks. The most used scheme currently is to administer 50,000 IU/week (or 7,000 IU/day) of vitamin D for six to eight weeks (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.). In case the desired concentration of 25(OH)D is not achieved, a new cycle can be proposed. As there can be some individual variation in the response to treatment, the reevaluation of the plasma values after each cycle is ideal, especially in the cases of more serious deficiencies, up to achieving the desired concentration. After this period, the maintenance dose should be defined and it varies according to the age group and concurrent conditions (Table 3). For the adults, maintenance doses vary between 400 and 2,000 IU, depending on the sunlight exposure and skin tone. For the elderly, the recommended doses vary from 1,000 to 2,000 IU/day or 7,000 to 14,000 IU/week. Obese individuals, presenting malabsorption conditions or in use of anticonvulsants might need doses that are twice or three times higer (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.) (A).
Due to the fact that it is a liposoluble substance, vitamin D is absorbed with fats and follows the enterohepatic circulation, meaning it is normally secreted through the gallbladder and reabsorbed in the small intestine. Thus, especially in cases of malabsorption, doses which are much higher than usual can be necessary in order to normalize 25(OH)D concentrations. Besides that, for supplementation studies and in the daily practice it is possible to notice some individual variation in blood concentrations of 25(OH)D reached in response to a same dose of vitamin D, suggesting individuals might present different competencies in intestinal absorption or its metabolization (9696 .Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012;97(8):2606-13.). It seems there is no difference considering vitamin D absorption in relation to fasting or meal type (9797 .Dawson-Hughes B, Harris SS, Palermo NJ, Ceglia L, Rasmussen H. Meal conditions affect the absorption of supplemental vitamin D3 but not the plasma 25-hydroxyvitamin D response to supplementation. J Bone Miner Res. 2013;28(8):1778-83.).
Vitamin D3, when administered as described above is very safe. Doses of up to 10,000 IU per day for five months did not induce signals of toxicity, which can be translated as hypercalcemia and hypercalciuria (9898 .Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-10. Erratum in: Am J Clin Nutr. 2003;78(5):1047.) (A). Toxic concentrations of 25(OH)D (> 90 ng/mL) are difficult to achieve with these routine doses (9999 .Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842-56.). In rare clinical situations, such as in some cases of granulomatous diseases (sarcoidosis, tuberculosis and chronic fungal infections) and some lymphoma, activated macrophages can locally produce 1,25(OH)2D in excess and induce hypercalcemia and hypercalciuria (B). Children with Williams syndrome are more predisposed to hypercalcemia. Therefore, under these conditions, supplementation should be more criterious and follow frequent monitoring of plasmatic and urinary calcium (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.).
7. What are the differences between vitamin D2 and D3?
Vitamin D sources are: sunlight exposure, diet and supplementation. The main difference between vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) is the source. In summary, vitamin D2 is the vitamin D from the plant sources, while the one from animal sources is in the form of vitamin D3. The D2 and D3 sources differ only due to the presence of an additional double bond and a methyl group incorporated to the long side chain of the biological form called D2 (100100 .Holick MF, Wacker M. Vitamin-D effects on skeletal and extraskeletal health and the need of supplementation. Nutrients. 2013;5(1):111-48.). The two forms present equivalent biological power and are activated in equally efficient ways by hydroxylases in humans. However, there is controversy on the bioequivalence of these formulations for supplementation. A meta-analysis evaluating only controlled and randomized studies that use vitamin D2 and D3 showed that vitamin D3 increased 25(OH)D levels more significantly when compared to vitamin D2 (p = 0.001), the single or in bolus dose of vitamin D3 being better than D2 (p = 0.0002). However, this advantage was lost in daily supplementation (101101 .Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357-64.) (A). Previous studies had already shown a small superiority of vitamin D3 when administered in single dose, to maintain 25(OH)D levels for longer time (102102 .Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694-7.).
When the two formulations were compared in daily use for 25 weeks, it was observed that those using vitamin D2 presented 25(OH)D average concentrations lower when compared to those that received D3, although, without altering PTH levels (103103 .Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr. 2013;109(6):1082-8.) (A). A recent study did not demonstrate difference in effectiveness when higher 25(OH)D levels were found and also in sustained serum concentration of 1,25(OH)2D3, after 11 weeks of supplementation with 1,000 IU of vitamin D2 or D3 per day (104104 .Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98(3):973-9.) (A).
The same was observed in the treatment of children with rickets and controls, where there was a similar increase in 25(OH)D and 1,25(OH)D levels with both formulations (105105 .Thacher TD, Fischer PR, Obadofin MO, Levine MA, Singh RJ, Pettifor JM. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J Bone Miner Res. 2010;25(9):1988-95.) (A). It is possible to conclude that both forms are equivalent in relation to daily supplementation and that vitamin D3 presents superiority in relation to the maintenance of the 25(OH)D levels for single dose supplementation.
8. What is the difference between vitamin D and calcitriol?
Calcitriol or 1,25(OH)2D is an active hormone, a final product of two vitamin D hydroxylations. Its endocrine action starts with renal production, finely controlled by the activity of the 1α-hydroxylase enzyme. This enzyme, present in the epithelial cells or the proximal convoluted tubules is stimulated mainly by the PTH and is inhibited by FGF-23, among other less important regulators. Circulating calcitriol itself also deviates its synthesis to an inactive product, the 24,25(OH)2D and, this way, protects the organism from its excess. The 1α-hydroxylase enzyme was identified in different tissues, what makes us believe there is some local production of calcitriol, with autocrine and paracrine actions. Opposite to renal cells, where calcitriol production is rigorously controlled, in these other tissues it is believed that production only depends on the presence of substrate (1414 .Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28.,4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.). While all the systems of the organism are fully functioning, the recommendation is for the supplementation and treatment of the deficiency to be done with vitamin D itself, allowing tissues to produce their necessities, controlled by local or hormonal mechanisms, in the case of renal tubular cells. The use of calcitriol should be reserved for special situations, such as in chronic kidney insufficiency, in type 1 and type 2 vitamin D dependent rickets and in hypophosphatemic rickets, or in cases of extreme malabsorption. The use of calcitriol presupposes a much more rigorous control of calcemia and calciuria, because hypercalcemia can frequently occur (1414 .Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28.,4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.) (A).
9. How to do supplementation in special cases?
a) In chronic kidney disease
The patient with chronic renal disease presents higher risk for vitamin D
deficiency. In renal disease, PTH concentrations also correlate with
circulating 25(OH)D levels (A). Therefore, it is believed
that vitamin D deficiency contributes to the development of secondary
hyperparathyroidism in chronic renal patients, independent on the renal
calcitriol production. Nowadays it is known that several tissues, such
as macrophages and osteoblasts have the capacity of producing active
vitamin D (calcitriol) and that this synthesis depends on the substrate,
therefore, it is not strictly regulated as the renal synthesis. Because
of that, the treatment of the deficiency and the adequacy of the
circulating levels of 25(OH)D is always recommended each time plasma
concentrations are lower than 30 ng/mL (106106 .Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work
Group. KDIGO clinical practice guideline for the diagnosis, evaluation,
prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder
(CKD-MBD). Kidney Int Suppl. 2009;(113):S1-130.
107 .Metzger M, Houillier P, Gauci C, Haymann JP, Flamant M, Thervet E,
et al.; NephroTest Study Group. Relation between circulating levels of 25(OH)
vitamin D and parathyroid hormone in chronic kidney disease: quest for a
threshold. J Clin Endocrinol Metab. 2013;98(7):2922-8.
108 .London G, Coyne D, Hruska K, Malluche HH, Martin KJ. The new kidney
disease: improving global outcomes (KDIGO) guidelines – expert clinical focus on
bone and vascular calcification. Clin Nephrol.
2010;74(6):423-32.-109109 .Cuppari L, Garcia Lopes MG, Kamimura MA. Vitamin D biology: from
the discovery to its significance in chronic kidney disease. J Ren Nutr.
2011;21(1):113-6.). According
to the opinion of the committee responsible for writing the treatment
guidelines for osteomineral disease in chronic renal patients in Brazil,
25(OH)D quantification is recommended at the end of each cycle of attack
doses, until the target concentration is achieved, and from then on,
every six months (110110 .Carvalho AB, Gueiros AP, Gueiros JE, Neves CL, Karohl C, Sampaio E,
et al. Guidelines on bone mineral disorder in chronic kidney disease--addendum
chapter 2. J Bras Nefrol. 2012;34(2):199-205.)
(D).
b) In the treatment of osteoporosis
A good part of vitamin D benefits on the risk of fractures observed in
the literature has been associated with the concomitant use of calcium.
Therefore, the adequacy of calcium intake, either through diet, or
through the use of calcium salts, is part of any protocol for
osteoporosis treatment. Recommended vitamin D doses are those capable of
taking and maintaining plasma concentration to 30 ng/mL or above,
avoiding, this way, the secondary hyperparathyroidism and the increase
in bone resorption (111111 .Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin
Densitom. 2003 Winter;6(4):307-14.
112 .Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE,
et al. Vitamin D status and response to treatment in post-menopausal
osteoporosis. Osteoporos Int. 2009;20(2):239-44.-113113 .Shab-Bidar S, Bours SP, Geusens PP, van der Velde RY, Janssen MJ,
van den Bergh JP. Suboptimal effect of different vitamin D3 supplementations and
doses adapted to baseline serum 25(OH)D on achieved 25(OH)D levels in patients
with a recent fracture: a prospective observational study. Eur J Endocrinol.
2013;169(5):597-604.) (A). The
non-adequacy of vitamin D concentration is considered one of the
potential failures in medicinal treatment of osteoporosis (significant
BMD loss and fractures).
c) In obesity and post-bariatric surgery
Obese patients present lower vitamin D concentration when compared to
non-obese and are considered a population at risk for the deficiency
(4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA,
Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of
vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin
Endocrinol Metab. 2011;96(7):1911-30.) (A).
Nowadays, bariatric surgery is a very used alternative to induce weight
loss in these individuals, possibly further aggravating this deficiency.
Therefore, it is advisable to correct this condition prior to surgery.
Santos and cols. demonstrated that women submitted to bariatric surgery
at least three years before, presented lower 25(OH)D values when
compared to normal paired controls, 77.1% of them presenting vitamin D
insufficiency/deficiency and 41.7% presenting secondary
hyperparathyroidism (Figure 3)
(114114 .Santos MT, Souza FI, Fonseca FL, Lazaretti-Castro M, Sarni RO.
Changes in bone metabolism markers in women after Roux-en-Y gastric bypass. Arq
Bras Endocrinol Metabol. 2012;56(6):376-82.). 25(OH)D
concentrations correlated inversely with PTH (r = -0,57, p < 0,05)
and directly with bone remodeling markers (CTX and osteocalcin) which,
together, can justify the increased risk for fracture observed in this
population by other researchers (C). Depending on the
surgical technique used and the degree of disabsorption promoted, some
individuals might have a lot of difficulty normalizing 25(OH)D and PTH
concentrations, being necessary to follow these parameters and to
consider a new attack dose or even higher maintenance doses that should
be individually adjusted (D). In some cases, the
orientation for frequent sunlight exposure and use of parenteral vitamin
D are resources that can be necessary (115115 .Viégas M, Vasconcelos RS, Neves AP, Diniz ET, Bandeira F. Bariatric
surgery and bone metabolism: a systematic review. Arq Bras Endocrinol Metabol.
2010;54(2):158-63.
116 .Nakamura KM, Haglind EG, Clowes JA, Achenbach SJ, Atkinson EJ,
Melton LJ 3rd, et al. Fracture risk following bariatric surgery: a
population-based study. Osteoporos Int. 2014;25(1):151-8.-117117 .Censani M, Stein EM, Shane E, Oberfield SE, McMahon DJ, Lerner S,
et al. Vitamin D Deficiency Is Prevalent in Morbidly Obese Adolescents Prior to
Bariatric Surgery. ISRN Obes. 2013;2013. pii: 284516.).
Prevalence of secondary hyperparathyroidism in a population of women submitted to bariatric surgery at least three years prior to the study enrollment. Different ranges of circulating 25(OH)D (114).
d) Pregnancy
It is a critical period, because women are oriented to avoid sunlight exposure. Vitamin D deficiency in pregnant women was associated to low birth weight of the newborn, besides some late endpoints, such as low bone mass and cardiovascular risk markers in school age children. In a recent meta-analysis, Aghajafari and cols. analyzed 31 studies, including 18,869 individuals and they concluded that the serum levels of 25(OH)D are related to gestational diabetes, preeclampsia, low birth weight newborn and bacterial vaginosis (118118 .Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169.). In another meta-analysis, vitamin D supplementation showed positive effect on the low birth weight reduction (119119 .Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26 Suppl 1:75-90.) (A). The doses recommended for supplementation in this period of life can be found in table 3.
When vitamin D deficiency is suspected, the treatment with higher doses is still indicated, but daily doses are preferable. The 25(OH)D concentration in the newborn shows high correlation with the one found in the mother. The placenta presents the 1α-hydroxylase enzyme and therefore, has the capacity of converting 25(OH)D to calcitriol. Apparently, this production is not strict controlled as that what happens in renal tubules, and depends only on the amount of substrate (4141 .Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.) (C). For this reason, the recommendation during pregnancy is to avoid weekly or monthly doses.
10. When should active vitamin D analogues be used?
Active vitamin D analogues are synthetic substances that directly bind the vitamin D receptor (VDR). They present different selectivity to the parathyroid cells, depending on its chemical structure, calcitriol (1,25(OH)2D3) shows smaller selectivity, promoting more side effects such as hypercalcemia, hyperphosphatemia and vascular calcifications. More selective compounds such as paricalcitol (19-nor-1α,25(OH)2D2), maxacalcitol (22-oxa-1α,25(OH)2D3) and doxercalciferol (1α(OH)D2) and eldecalcitol (1α,25(OH)2-2β-(3-hydroxypropyloxy)D3) and doxercalciferol (1α(OH)D2) and eldecalcitol (1α,25(OH)2-2β-(3-hydroxypropyloxy)D3) promote less adverse effects. Doxercalciferol and the alfacalcidol require liver 25-hydroxylation to become active (120120 .Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int. 2011;79(7):702-7.) (A).
a) Use in secondary hyperparathyroidism
The analogues are classically used to suppress PTH levels in patients with secondary hyperparathyroidism (SHPT) and chronic kidney disease (CKD). In CKD there is an increase in PTH levels, secondary to an alteration of the regulation of the fibroblast growth factor (FGF-23) in the PTH-vitamin D axis and the decrease of calcitriol production due to CKD itself. The suppression of PTH levels in patients in stages 3-4 of CKD is more than 40% in 90% of the patients (121121 .Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2):263-76.,122122 .Zangeneh F, Clarke BL, Hurley DL, Watts NB, Miller PD. Chronic Kidney Disease Mineral and Bone Disorders (CKD-MBD) What the Endocrinologist Needs to Know. Endocr Pract. 2013;10:1-46.) (A).
The dose used is variable depending on the CKD stage, if the patient is undergoing dialysis or not and also on the serum PTH concentration. In CKD stages 3-5, the ideal PTH levels are not defined yet, other PTH increasing factors having to be discarded. Hypocalcemia, vitamin D deficiency and hyperphosphatemia should be corrected initially. If PTH levels remain elevated and progressively increasing, the use of analogues such as calcitriol, should be considered. In CKD stage 5D with elevated and sustained PTH levels, the recommendation is to maintain PTH levels between two and nine times the upper limit of normality. There is no consensus on the doses of doxercalciferol and paricalcitol, some studies calculated the dose in relation to the initial PTH value dividing it by 80 to 120, to minimize the excessive suppression of PTH or hypercalcemia and hyperphosphatemia (106106 .Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1-130.) (B).
The use of vitamin D analogues minimizes bone loss in CKD due to the suppression of PTH levels and prevents bone remodeling reduction, due to the effect on the differentiation of normal osteoblasts and inhibition of osteoclastogenesis. However, the concern with the excessive suppression, which would lead to adynamic bone disease persists (122122 .Zangeneh F, Clarke BL, Hurley DL, Watts NB, Miller PD. Chronic Kidney Disease Mineral and Bone Disorders (CKD-MBD) What the Endocrinologist Needs to Know. Endocr Pract. 2013;10:1-46.) (B).
Several studies have demonstrated benefit on the survival of patients undergoing dialysis treated with calcitriol or vitamin D analogues. Besides that, there is a smaller risk of progression for terminal renal disease and an increase in the survival in patients with CKD stages 3-4 (123123 .Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(4):e61387.,124124 .Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37(3):239-48.) (B). There is doubt in relation to the benefit of vitamin D active analogues, compared to placebo, in relation to fractures, quality of life, hospitalizations, muscle function and fall in these patients (125125 .Hagino H, Takano T, Fukunaga M, Shiraki M, Nakamura T, Matsumoto T. Eldecalcitol reduces the risk of severe vertebral fractures and improves the health-related quality of life in patients with osteoporosis. J Bone Miner Metab. 2013;31(2):183-9.,126126 .Nakamura T, Takano T, Fukunaga M, Shiraki M, Matsumoto T. Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol. J Bone Miner Metab. 2013;31(4):417-22.) (A).
The most selective analogues, compared to calcitriol, demonstrate lower mortality, lower number of hospitalizations and lower duration of each hospitalization per year (124124 .Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37(3):239-48.) (B). Doxercalciferol, similar to paracalcitol, presents higher benefit in relation to survival, when compared to calcitriol. A dose-response was observed in the benefit when the levels of PTH were adjusted according to vitamin D (127127 .Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al.; Medical Directors of Dialysis Clinic Inc. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70(10):1858-65.) (B).
The use of analogues to avoid the evolution of nephropaty in diabetic patients is discussed, although the results are still conflicting, using microalbuminuria and albuminuria as markers. Results from VITAL study are awaited for a more definitive observation (120120 .Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int. 2011;79(7):702-7.,128128 .Bonakdaran S, Hami M, Hatefi A. The effects of calcitriol on albuminuria in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23(6):1215-20.,129129 .Jørgensen HS, Winther S, Povlsen JV, Ivarsen P. Effect of vitamin-D analogue on albuminuria in patients with non-dialysed chronic kidney disease stage 4-5: a retrospective single center study. BMC Nephrol. 2012;13:102.) (B).
b) Fracture prevention
The relationship between vitamin D levels with falls and fractures has been described, also as a significant linear predictor of major osteoporotic fractures in ten years (130130 .Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997-1006.) (B). A recent meta-analysis demonstrated a modest decrease in the prevention of fractures with the use of 1,25(OH)2D3 (calcitriol) and 1α-hydroxy-vitamin D3 (alfacalcidol), similar to that obtained using doses higher than 700 IU/day of vitamin D3 (3535 .Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692.) (A).
Eldecalcitol (1α,25-dihydroxy-2β-[3-hydroxypropiloxy] vitamin D3 is a new analogue of vitamin D active form, recently approved for the treatment of osteoporosis in Japan. Eldecalcitol presents a strong inhibitory effect on bone resorption and significantly increases bone mineral density. This drug showed a 26% decrease in the incidence of new vertebral fractures in three years and 71% decrease in the risk of wrist fracture, in comparison to alfacalcidol, but showed no benefit against hip fracture. An increase in serum calcium was observed, although hypercalcemia was only seen in 0.4% of patients (131) (A). Edelcalcitol compared to alfacalcidol promoted better quality of life and decreased severity of vertebral fractures (125125 .Hagino H, Takano T, Fukunaga M, Shiraki M, Nakamura T, Matsumoto T. Eldecalcitol reduces the risk of severe vertebral fractures and improves the health-related quality of life in patients with osteoporosis. J Bone Miner Metab. 2013;31(2):183-9.,126126 .Nakamura T, Takano T, Fukunaga M, Shiraki M, Matsumoto T. Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol. J Bone Miner Metab. 2013;31(4):417-22.) (A).
NON-BONE ACTIONS OF VITAMIN D
11. What is the evidence for extra-skeletal effects of vitamin D?
Traditionally, vitamin D was associated only with calcium metabolism functions. The possibility of existence of extra-skeletal effects occurred after the discovery of vitamin D receptor (VDR) in tissues not involved with calcium metabolism (e. g. skin, placenta, breast, prostate, and colon cancer cells) and the identification of the enzyme 1α-hydroxylase in extra-renal tissues. The question to be discussed is the real biological meaning of the presence of VDR and 1α-hydroxylase in different tissues (132132 .Christakos S, DeLuca H. Minireview: vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930-6.).
Nagpal and cols. (133133 .Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamina D receptor ligands. Endocr Rev. 2005;26(5):662-87.) reported that 1,25(OH)2D3 through its transcriptional activity was able to directly or indirectly regulate at least 200 genes. These genes are involved in the control of proliferation, apoptosis and angiogenesis in several tissues. The etiological connection between vitamin D deficiency and specific extra-skeletal diseases still needs to be identified in humans. Findings using animal models, regarding the beneficial effects of 1,25(OH)2D3 suggest mechanisms that involve similar human signaling pathways (134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17(4):602-10.,135135 .Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S-88S.) (B). The main non-skeletal effects studied in the literature will be described here.
a) Vitamin D and cardiovascular disease
Vitamin D deficiency was included as a new risk factor for cardiovascular diseases (CVD) based on observational studies that demonstrate a strong association between vitamin D deficiency and mortality due to cardiovascular disease, the mechanism being unclear in the literature (136136 .Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931-42.) (B). Potential hypothesis include the action in the regulation of genes involving renin production, proliferation of cardiac and vascular muscle cells, negative regulation of reactive C protein and other involvement in other proinflammatory processes. Fiscella and Franker (137137 .Fiscella K, Franker P. Vitamin D, race and cardiovascular mortality with findings from a national US sample. Ann Fam Med. 2010;8(1):11-8.) showed that black individuals, presenting calcitriol levels in the lower quartile presented 40% increase in the risk of death from coronary arterial disease (CAD) or stroke (B).
Serious vitamin D deficiency in patients with stabilized CVD is related
to 50% more deaths from stroke and three to five times more sudden death
(138138 .Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A,
Dimai HP, et al. Association of vitamin D deficiency with heart failure and
sudden cardiac death in a large cross-sectional study of patients referred for
coronary angiography. J Clin Endocrinol Metab.
2008;93(10):3927-35.). On the other hand,
another study, demonstrated that very high levels are associated with an
increase in the risk of ischemic myocardial disease (139139 .Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, et
al. Serum 25-hydroxivitamin D3 levels are elevated in South Indian patients with
ischemic heart disease. Eur J Epidemiol. 2001;17(6):567-71.). An increased risk of
systemic arterial hypertension and metabolic syndrome has been
demonstrated when sub-optimum vitamin D levels are detected (140140 .Christakos S, Hewison M, Gardner DG, Wagner CL, Sergeev IN, Rutten
E, et al. Vitamin D: beyond bone. Ann N Y Acad Sci.
2013;1287:45-58.). Observational and cohort
studies led to the potential vitamin D use as an anti-hypertensive
agent. Some studies demonstrated decreased levels of systolic blood
pressure upon supplementation. However, larger studies were not able to
prove these positive effects. Two prospective studies did not show
reduction in cardiovascular mortality upon vitamin D supplementation
(131131 .Noguchi Y, Kawate H, Nomura M, Takayanagi R. Eldecalcitol for the
treatment of osteoporosis. Clin Interv Aging. 2013;8:1313-21.). A meta-analysis
presented 8% reduction in the mortality from all causes with modest
doses (141141 .Motiwala SR, Wang TJ. Vitamin D and cardiovascular disease. Curr
Opin Nephrol Hypertens. 2011;20(4):345-53.). Nonetheless, a
recent meta-analysis including 51 studies, concluded that
supplementation did not have a significant effect in the mortality (RR
0.96), CAD incidence (RR 1.02) and stroke (RR 1.05) (136136 .Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA,
Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review
and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931-42.). So far, there is no strong
evidence for the screening of vitamin D deficiency in patients under
risk for CVD, as well as patients with previous CVD. Good prospective
studies are necessary for a better understanding of the efficacy of
supplementation in the risk reduction of cardiovascular disease (132132 .Christakos S, DeLuca H. Minireview: vitamin D: is there a role in
extraskeletal health? Endocrinology. 2011;152(8):2930-6.
133 .Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamina D
receptor ligands. Endocr Rev. 2005;26(5):662-87.-134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of
Vitamin D deficiency. Indian J Endocrinol Metab.
2013;17(4):602-10.) (B).
b) Vitamin D and diabetes
Epidemiological and observational studies demonstrate a potential involvement of vitamin D in the pathogenesis of the inflammatory process and in the prevention and control of both diabetes mellitus type 1 and type 2 (DM1 and DM2). Studies performed in animals and humans suggest that vitamin D can be a potential modifier of these diseases (142142 .Pittas AG, Harris SS, Stark PC, Dawson Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980-6.) (A).
Animal studies demonstrate that immunomodulatory and antiinflammatory actions of vitamin D reduce autoimmune insulinitis in DM1. It seems to suppress the antigen capacity of macrophages, inhibit the maturation of dendritic cells, modulate the development of CD4 lymphocytes and inhibit the production of cytokines such as interferon (IFN) and interleukin-2 (IL-2). These cytokines are known for activate macrophages and cytotoxic T cells, that lead to the destruction of pancreatic cells (143143 .Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35(1):11-7.).
In DM2, vitamin D acts reducing insulin resistance and increasing its secretion, through the modulation of the immune and inflammatory process. DM2 is associated to an increase in the levels of tumor necrosis factors α and β, C-reactive protein (CRP), plasminogen activating factor and interleukin-6 (142142 .Pittas AG, Harris SS, Stark PC, Dawson Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980-6.).
Epidemiological studies demonstrate that children with vitamin D deficiency present 2.4 times higher risk of developing DM1. In the EURODIAB study there was as a reduction in the risk of developing DM1 in 33% among supplemented children (144144 .Vitamin D supplement in early childhood and risk for Type 1 (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999;42(1):51-4.) (B). The same way the maternal supplementation demonstrates a protective effect to the newborn. A meta-analysis concluded that, childhood supplementation seems to be protective against the development of DM1 (130130 .Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997-1006.). In adults with the disease, a reduction in the insulin dose was seen with the calcitriol supplementation (145145 .Zipitis CS, Akobeng Ak. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512-7.) (B).
In rats with vitamin D deficiency, after supplementation there was improved insulin secretion (146146 .Pittas AG, Lau J, Hu B, Dawson Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-29.). Two large studies evidenced that the combined use of calcium and vitamin D reduced the risk of DM2. In a dose-response analysis, DM2 risk was reduced in 4% at each increment of 4 ng/mL in the concentration of 25(OH)D (147147 .Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422-8.) (B). In a meta-analysis, it was concluded that the insufficiency of calcium and vitamin D can negatively influence glycemia and that supplementation of both can be beneficial for the optimization of glucose metabolism (146146 .Pittas AG, Lau J, Hu B, Dawson Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-29.-147147 .Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422-8.) (B).
There is evidence suggesting that vitamin D has a role in the prevention
and treatment of DM1 and DM2, through its action on the immune system,
insulin secretion and resistance. However, further studies, using larger
populations, are necessary to better elucidate the mechanisms of action
and the doses necessary to present the best benefits (132132 .Christakos S, DeLuca H. Minireview: vitamin D: is there a role in
extraskeletal health? Endocrinology. 2011;152(8):2930-6.
133 .Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamina D
receptor ligands. Endocr Rev. 2005;26(5):662-87.-134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of
Vitamin D deficiency. Indian J Endocrinol Metab.
2013;17(4):602-10.,148148 .de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum
B, et al.; Women’s Health Initiative Investigators. Calcium plus vitamin D
supplementation and the risk of incident diabetes in the Women’s Health
Initiative. Diabetes Care. 2008;31(4):701-7.)
(A).
c) Vitamin D and cancer
Epidemiological studies demonstrated a correlation between sunlight exposure and mortality due to some types of cancer, as well as skin color seems to be related to an increase in the prevalence of colorectal, breast and prostate cancer (149149 .Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, et al. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576-87.) (B). The risk of development and death due to neoplasia is more elevated in places of higher latitude and this can be related to lower sunligh exposure. Women presenting vitamin D insufficiency show a higher risk of developing colorectal cancer when compared to women presenting sufficient vitamin D levels, although no benefit was observed in the use of vitamin D for prevention (150150 .Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311-36.,151151 .Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89.).
In tissues where 25(OH)D is available, there is paracrine production of 1,25(OH)2D3, which through binding to its receptor, VDR, regulates transcription of target genes, that act in the differentiation of normal and tumor cells. Epidemiological and pre-clinical studies suggest the action of vitamin D in cancer prevention and treatment. Polymorphisms in the VDR gene are associated with an increased risk for the development of neoplasias (150150 .Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311-36.). Local production of 1,25(OH)2D3 does not have any function in the control of calcium metabolism, but it presents autocrine and paracrine effects. In vitro, it is possible to observe a decrease in 1α-hydroxylase (CYP27B1) and in vitamin D receptor (VDR) as the tumor progresses, associated to an increase in 24-hydroxylase (CYP24A1), which is inactivating. In vitro and in vivo studies demonstrate the direct or indirect effect of 1,25(OH)2D3 and its analogues on proliferation, differentiation, apoptosis, angiogenesis, invasion and inflammation of malignant cells. Microarray studies show that 1,25(OH)2D3 influences the transcription of a great number of genes, mainly related to apoptosis control (149149 .Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, et al. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576-87.,152152 .Pike JW. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand Mol Cell Endocrinol. 2011;347(1-2):3-10.) (B).
Low vitamin D levels make the tissues more sensitive to pro-carcinogenic events. Vitamin D analogues are not able to eradicate tumor cells, however they can be used as adjuvants in cancer treatment. It is believed that high doses of these substances are necessary for a real benefit to be observed, although it increases adverse effects (153153 .Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20(2):R31-47.) (B).
A double-blind placebo controlled study determined that the use of 1,25(OH)2D3 in pre-leukemia showed promising results at the beginning, although it determined an increase in calcemia during the blastic crisis (154154 .Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, et al. 1 alpha,25-dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60). Biochem J. 1982;204(3):713-9.). In prostate cancer, the administration of 2,000 IU/day resulted in PSA levels reduction, though the presence of severe hypercalcemia determined the end of the study (134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17(4):602-10.).
Some non-hypercalcemic calcitriol analogues are associated with good prognosis in patients that present elevated VDR expression. However, the use of calcitriol and analogues for the treatment of cancer patients is so far uncertain. Most of the clinical studies were conducted in patients with prostate cancer and patients with advanced cancer that do not respond to traditional therapies. Laboratory evidence indicates that calcitriol generates a biological response that results in the inhibition of the neoplastic progress. However, large scale clinical studies are necessary to confirm the benefits of vitamin D use in neoplasias (155155 .Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88(2):296-307.,156156 .Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400, table of contents.) (B).
d) Vitamin D and autoimmune disease
Vitamin D action on the immune system seems to be mediated by B and T lymphocytes. VDR is present in these cells. The molecule 1,25(OH)2D3 inhibits the proliferation of T cells, suppresses the synthesis and proliferation of immunoglobulins, prevents the formation of IFN-γ (interferon-γ) and IL-2 (interleukin-2); besides increasing the activity of suppressor T cells (TH2). In humans, there is epidemiological evidence of the importance of vitamin D in the immune system (157157 .Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382-90.) (C).
Sunlight or vitamin D are environmental factors in the etiology of multiple sclerosis and can have a role together with class II MHC inherited factors. Epidemiological studies suggest that adults with elevated vitamin D levels present lower risk of developing multiple sclerosis. Women with high vitamin D intake have 42% less chance to develop this disease (134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17(4):602-10.,158158 .Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189(11):5155-64.) (B).
Preliminary studies suggest that activated vitamin D can be an efficacious treatment for rheumatoid arthritis. Treated mice showed decreased activity of the cell responsible for this disease. In humans, epidemiological studies also confirmed a negative association between the levels of vitamin D and the prevalence of the disease. Other autoimmune diseases that are being associated with vitamin D are: autoimmune encephalitis, systemic lupus erythematosus, intestinal inflammatory disease and autoimmune thyroiditis. Additional studies are necessary for the confirmation of data, and the use of vitamin D for the prevention and treatment of autoimmune diseases (155155 .Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88(2):296-307.,156156 .Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400, table of contents.).
e) Vitamin D and innate immunity
Recent studies suggest that vitamin D can modulate innate immunity. Hypovitaminosis D can present a negative impact in infectious diseases. It has been observed that 1,25(OH)2D3 has an antimicrobial activity, including Mycobacterium tuberculosis, through the stimulation of cathelicidin production (protein that acts on the destruction of pathological agents) (159159 .Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337-45.).
A study that used elevated vitamin D doses, 600,000 IU, in tuberculosis patients, demonstrated higher body weight increase and less residual disease in those who received the vitamin, in comparison to the controls. The patients that were deficient when they were enrolled in the study (25-hydroxyvitaminD < 20 ng/mL) showed a more significant increase in Mycobacterium tuberculosis induced IFN-γ (160160 .Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13:22.) (A).
A research with post-menopausal women who received 2,000 IU of vitamin D per day, showed a 90% reduction in upper respiratory tract infections, when compared to those who received 400 IU per day (B). Some studies also demonstrate that lower vitamin D levels can be a risk factor for sepsis. Studies for bacterial vaginosis, skin infection and of the oral cavity are being developed (155155 .Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88(2):296-307.).
f) Vitamin D and psoriasis
The active form of vitamin D is a powerful inhibitor of keratynocytes proliferation and can be safely used in non-malignant hyperproliferative diseases of the skin, such as psoriasis. Data from controlled randomized studies demonstrated that the active form is an effective and well-tolerated treatment in patients with chronic initial or moderate psoriasis plaques. The topic application of 1,25(OH)2D3 or its analogue calcipotriol can be used as a first line treatment against psoriasis (161161 .Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1,25-dihydroxyvitamin D3 and parathyroid hormone-related peptide in dermatology: a new perspective. J Investig Dermatol Symp Proc. 1996;1(1):1-9.).
g) Vitamin D and respiratory diseases
In children with asthma, the level of 25(OH)D seems to positively correlate with the control of the disease and the pulmonary function; and negatively with the use of corticoids. Few intervention studies, evaluating vitamin D supplementation with asthma exists in the literature (155155 .Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88(2):296-307.,156156 .Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400, table of contents.,162162 .Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermatoendocrinol. 2012;4(2):137-45.). One of them demonstrated that 1,200 IU per day in children was associated to 83% reduction in the risk of disease exacerbation. It is believed that the immunomodulating effects of vitamin D and the effects on the pulmonary function can be useful for the treatment of respiratory diseases (163163 .Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995-1000.).
h) Vitamin D and physical and cognitive function of the elderly
In large populational studies, low vitamin D levels are associated with mobility reduction, worsening in muscular function and this way, an increase in the risk of fall (132132 .Christakos S, DeLuca H. Minireview: vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930-6.,156156 .Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400, table of contents.,164164 .Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Mol Biol. 2004;89-90(1-5):497-501.). Vitamin D receptors present high concentrations in several areas of the central nervous system. Epidemiological studies demonstrated that low ingestion of vitamin D is associated with a cognitive decline, an increased risk for Alzheimer disease and depression. The mechanism suggested for this association includes the formation and aggregation of β-amiloid fibers, a deregulation of the gabaergic system and an increase in the calcium influx in the neurons (165165 .Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-41.).
Vitamin D seems to be involved in physiological and possibly pathologic changes that follow aging. If the supplementation can have a positive impact in the aging process is still uncertain and long-term intervention studies are necessary (133133 .Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamina D receptor ligands. Endocr Rev. 2005;26(5):662-87.,134134 .Visweswaran R, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17(4):602-10.).
i) Vitamin D and obesity
Obesity is associated with a higher prevalence of vitamin D deficiency,
interpreted as a sequestration by the adipose tissue. In fact, when
compared with non-obese individuals, the necessary dose for the
reposition of vitamin D is higher among the obese. Recent data suggest
that low concentrations of 25(OH)D could predict an acceleration in the
increase of fat mass and this way would be involved with an increase of
obesity incidence (135135 .Holick MF. Sunlight and vitamin D for bone health and prevention of
autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr.
2004;80(6 Suppl):1678S-88S.
136 .Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA,
Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review
and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931-42.-137137 .Fiscella K, Franker P. Vitamin D, race and cardiovascular mortality
with findings from a national US sample. Ann Fam Med.
2010;8(1):11-8.). Sergeev and cols., are
investigating the mechanism through which
1,25(OH)2D3 regulates the apoptosis of
adipocytes. Preliminary studies in rats suggest that the supplementation
with elevated doses of calcium and vitamin D reduce the weight and fat
mass in obese rats. Studies in humans are necessary for the evaluation
of the efficiency of vitamin D in the treatment of obesity (166166 .Song Q, Sergeev IN. Calcium and vitamin D in obesity. Nutr Res Rev.
2012;25(1):130-41.).
In recent meta-analyzes and systematic reviews, it was observed an association between 25-hydroxyvitamin D and several non-skeletal outcomes in observational studies, but that was not seen in randomized controlled trials (RCTs) (4040 .Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307-20.,151151 .Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89.). The effects of vitamin D in other tissues is still controversial.
CONCLUSIONS
Vitamin D food sources are scarce and humans depend mainly on cutaneous synthesis. Hypovitaminosis D is very frequent in our country. Laboratorial evaluation should be performed measuring 25(OH)D and the following should be considered individuals at risk for vitamin D deficiency: the elderly, osteoporosis patients, patients with history of falls and fractures, obese people, pregnant and lactating women, patients making use of medications that interfere with vitamin D metabolism (glucocorticoids, anticonvulsants, antifungal drugs), patients with malabsorption syndromes, primary hyperparathyroidism, renal or liver insufficiency, granulomatous diseases and lymphomas.
The most adequate normality value taking bone benefits into consideration is 30 ng/mL. The factors that seem to favor the presence of higher serum concentrations in our population are: younger age, community life, practice of outdoor physical exercises, oral vitamin D supplementation, season of the year (spring, summer), residence in sunny beach areas and in lower latitudes.
The most available vitamin D form for treatment and supplementation is cholecalciferol or vitamin D3. For patients with osteoporosis and increased risk of fractures it is recommended that 25(OH)D concentrations are kept above 30 ng/mL for full benefits on the prevention of secondary hyperparathyroidism, decreased risk of fall and better impact on BMD. For this purpose, maintenance doses between 1,000 and 2,000 IU are necessary. Vitamin D active forms, such as calcitriol or alfacalcidol should not be used when the objective is supplementation or in the treatment of vitamin D deficiency, because of their higher risk of adverse effects. Special considerations regarding pregnant and lactating women, patients with chronic kidney disease, obese people and those submitted to bariatric surgery.
Nowadays there is special interest in the research of extra-skeleton effects of vitamin D, due to observational studies that demonstrated association between low vitamin D concentration and several endpoints with mortality, cardiovascular complications, diabetes, cancer, autoimmune diseases, cognitive function, among others. However, in the moment it is still not possible to prove a cause-effect relationship.
REFERÊNCIAS
-
1Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al.; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807-20.
-
2Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477-501.
-
3Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
-
4Levels of evidence and Grades of Recommendations – Oxford Centre for Evidence-Based Medicine. Disponível em: http://www.cebm.net/index.aspx?o=1025.
» http://www.cebm.net/index.aspx?o=1025 -
5Programa Diretrizes. Associação Médica Brasileira. Disponível em: http://www.projetodiretrizes.amb.org.br.
» http://www.projetodiretrizes.amb.org.br -
6Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood). 2010;235(9):1034-45.
-
7Wacker M, Holick MF. Vitamin D – Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5(1):111-48.
-
8Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4-18.
-
9Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S-6S.
-
10McDonnell DP, Pike JW, O’Malley BW. The vitamin D receptor: a primitive steroid receptor related to thyroid hormone receptor. J Steroid Biochem. 1988;30(1-6):41-6.
-
11Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13(4):719-64.
-
12Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88(1):185-91.
-
13Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-76.
-
14Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8-28.
-
15Prestwood KM, Pannullo AM, Kenny AM, Pilbeam CC, Raisz LG. The effect of a short course of calcium and vitamin D on bone turnover in older women. Osteoporos Int. 1996;6:314-9.
-
16Kamel S, Brazier M, Rogez JC, Vincent O, Maamer M, Desmet G, et al. Different responses of free and peptide-bound cross-links to vitamin D and calcium supplementation in elderly women with vitamin D insufficiency J Clin Endocrinol Metab. 1996;81(10):3717-21.
-
17Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88(2):513S-9S.
-
18McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:69-77.
-
19van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, et al. Serum vitamin D concentration among elderly people in Europe. Lancet. 1995;346(8969):207-10.
-
20Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: The Decalyos II Study. Osteroporos Int. 2002;13:257-64.
-
21Freaney R, McBrinn Y, McKenna MJ. Secondary hyperparathyroidism in elderly people: combined effect of renal insufficiency and vitamin D deficiency. Am J Clin Nutr. 1993;58:187-91.
-
22Souberbielle JC, Cormier C, Kindermans C, Gao P, Cantor T, Forette F, et al. Vitamin D status and redefining serum parathyoid hormone reference range in the elderly. J Clin Endocrinol Metab. 2001;86(7):3086-90.
-
23McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int. 1998;8 Suppl 2:S3-6.
-
24Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913-21.
-
25Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47(4):181-95.
-
26Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142-6.
-
27Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Fazzari M, Ragolia L, et al. Vitamin D supplementation increases calcium absorption without a threshold effect. Am J Clin Nutr. 2014;99(3):624-31.
-
28Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634-9.
-
29Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244-50.
-
30Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24(4):693-701.
-
31Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891-7.
-
32Bischoff HA, Stähelin HB, Dick W, Akos R, Knecht M, Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343-51.
-
33Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611-5.
-
34Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257-64.
-
35Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692.
-
36Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
-
37Bikle DD. Vitamin D and bone. Curr Osteoporos Rep. 2012;10(2):151-9.
-
38Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25(2):305-12.
-
39de Paula FJA, Rosen CJ. Vitamin D safety and requirements. Arch Biochem Biophys. 2012; 523(1):64-72.
-
40Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307-20.
-
41Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30.
-
42Heaney RP. What is vitamin D insufficiency? And does it matter? Calcif Tissue Int. 2013;92(2):177-83.
-
43Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26(3):455-7.
-
44Chapuy M-C, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439-43.
-
45Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317-22.
-
46Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713-6.
-
47Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS. Prevalence of vitamin D deficiency and its correlation with PTH, biochemical bone turnover markers and bone mineral density, among patients from ambulatories. Arq Bras Endocrinol Metabol. 2008;52(3):482-8.
-
48Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649-50.
-
49Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97(4):1153-8.
-
50Institute of Medicine (IOM). Dietary Reference Intakes (DRIs) for calcium and vitamin D. Report at a glance 2011. Disponível em: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx.
» http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx -
51Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062-71.
-
52El-Khoury JM, Reineks EZ, Wang S. Progress of liquid chromatography-mass spectrometry in measurement of vitamin D metabolites and analogues. Clin Biochem. 2011;44(1):66-76.
-
53Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25 hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127-34.
-
54Binkley N, Wiebe D. Clinical controversies in vitamin D: 25(OH)D measurement, target concentration, and supplementation. J Clin Densitom. 2013;16(4):402-8.
-
55Carter GD, Berry JL, Gunter E, Jones G, Jones JC, Makin HL, et al. Proficiency testing of 25-hydroxyvitamin D (25-OHD) assays. J Steroid Biochem Mol Biol. 2010;121(1-2):176-9.
-
56Barake M, Daher RT, Salti I, Cortas NK, Al-Shaar L, Habib RH, et al. 25-hydroxyvitamin D assay variations and impact on clinical decision making. J Clin Endocrinol Metab. 2012;97(3):835-43.
-
57Singh DK, Farrington K, Twomey PJ. Analytical quality goals for 25-vitamin D based on biological variation. J Clin Lab Anal. 2011;25(2):130-3.
-
58Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880-1.
-
59McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814-22.
-
60Reichrath J, Nürnberg B. Cutaneous vitamin D synthesis versus skin cancer development: The Janus faces of solar UV-radiation. Dermatoendocrinol. 2009;1(5):253-61.
-
61Jamali Z, Asadikaram G, Mahmoodi M, Sayadi A, Jamalizadeh A, Saleh-Moghadam M, et al. Vitamin D status in female students and its relation to calcium metabolism markers, lifestyles, and polymorphism in vitamin D receptor. Clin Lab. 2013;59(3-4):407-13.
-
62Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, El Kabbaj S, et al. High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin Arthritis Rheum. 2009;38(6):444-51.
-
63Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15(9):1856-62.
-
64Peters BSE, dos Santos LC, Fisberg M, Wood RJ, Martini LA. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab. 2009;54(1):15-21.
-
65Oliveira RM, Novaes JF, Azeredo LM, Cândido AP, Leite IC. Association of vitamin D insufficiency with adiposity and metabolic disorders in Brazilian adolescents. Public Health Nutr. 2013;9:1-8.
-
66Santos BR, Mascarenhas LP, Satler F, Boguszewski MC, Spritzer PM. Vitamin D deficiency in girls from South Brazil: a cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr. 2012;12:62.
-
67Unger MD, Cuppari L, Titan SM, Magalhães MC, Sassaki AL, dos Reis LM, et al. Vitamin D status in a sunny country: where has the sun gone? Clin Nutr. 2010;29(6):784-8.
-
68Maeda SS, Kunii IS, Hayashi L, Lazaretti-Castro M. The effect of sun exposure on 25-hydroxyvitamin D concentrations in young healthy subjects living in the city of São Paulo, Brazil. Braz J Med Biol Res. 2007;40(12):1653-9.
-
69Maeda SS, Saraiva GL, Kunii IS, Hayashi LF, Cendoroglo MS, Ramos LR, et al. Factors affecting vitamin D status in different populations in the city of São Paulo, Brazil: the São PAulo vitamin D Evaluation Study (SPADES). BMC Endocr Disord. 2013;13(1):14.
-
70Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LM, Vieira JG, Maeda SS, et al. Prevalence of vitamin D deficiency, insufficiency and secondary hyperparathyroidism in the elderly inpatients and living in the community of the city of São Paulo, Brazil. Arq Bras Endocrinol Metabol. 2007;51(3):437-42.
-
71Maeda SS, Kunii IS, Hayashi LF, Lazaretti-Castro M. Increases in summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in São Paulo, Brazil vary with age, gender and ethnicity. BMC Endocr Disord. 2010;10:12.
-
72Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS. Prevalence of vitamin D deficiency and its correlation with PTH, biochemical bone turnover markers and bone mineral density, among patients from ambulatories. Arq Bras Endocrinol Metabol. 2008;52(3):482-8.
-
73Saraiva GL, Cendoroglo MS, Ramos LR, Araújo LMQ, Vieira JGH, Kunii I, et al. Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of São Paulo (23° 34’S), Brazil. Osteoporos Int. 2005;16(12):1649-54.
-
74Bandeira F, Griz L, Freese E, Lima DC, Thé AC, Diniz ET, et al. Vitamin D deficiency and its relationship with bone mineral density among postmenopausal women living in the tropics. Arq Bras Endocrinol Metabol. 2010;54(2):227-32.
-
75Neves JP, Silva AS, Morais LC, Diniz Ada S, Costa MJ, Asciutti LS, et al. 25-hydroxyvitamin D concentrations and blood pressure levels in hypertensive elderly patients. Arq Bras Endocrinol Metabol. 2012;56(7):415-22.
-
76Arantes HP, Kulak CA, Fernandes CE, Zerbini C, Bandeira F, Barbosa IC, et al. Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: from the Arzoxifene Generations Trial. Osteoporos Int. 2013;24(10):2707-12.
-
77Russo LA, Gregório LH, Lacativa PG, Marinheiro LP. Concentration of 25-hydroxyvitamin D in postmenopausal women with low bone mineral density. Arq Bras Endocrinol Metabol. 2009;53(9):1079-87.
-
78Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Int Med. 2006;260(3):245-54.
-
79Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Intern Med. 2006;260(3):245-54.
-
80Lopes JB, Danilevicius CF, Takayama L, Caparbo VF, Scazufca M, Bonfá E, et al. Vitamin D insufficiency: a risk factor to vertebral fractures in community-dwelling elderly women. Maturitas. 2009;64(4):218-22.
-
81Martini LA, Verly E Jr, Marchioni DM, Fisberg RM. Prevalence and correlates of calcium and vitamin D status adequacy in adolescents, adults, and elderly from the Health Survey-São Paulo. Nutrition. 2013;29(6):845-50.
-
82Cabral MA, Borges CN, Maia JM, Aires CA, Bandeira F. Prevalence of vitamin D deficiency during the summer and its relationship with sun exposure and skin phototype in elderly men living in the tropics. Clin Interv Aging. 2013;8:1347-51.
-
83Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981-8.
-
84Aloia JF, Chen DG, Yeh JK, Chen H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am Am J Clin Nutr. 2010;92(4):835-40.
-
85Nordin BE. Evolution of the calcium paradigm: the relation between vitamin D, serum calcium and calcium absorption. Nutrients. 2010;2(9):997-1004.
-
86Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40-9.
-
87Canto-Costa MH, Kunii I, Hauache OM. Body fat and cholecalciferol supplementation in elderly homebound individuals. Braz J Med Biol Res. 2006;39(1):91-8.
-
88Moreira-Pfrimer LD, Pedrosa MA, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab. 2009;54(4):291-300.
-
89Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, et al. Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D(3) per daily serving. Am J Clin Nutr. 2009;89(4):1132-7.
-
90Mocanu V, Vieth R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 μg of vitamin D3. Nutr J. 2013;12:137.
-
91Camargo MBR. Fatores determinantes do status de vitamina D em pacientes de um ambulatório especializado em osteoporose e sua interferência sobre a absorção de cálcio [tese]. São Paulo: Universidade Federal de São Paulo; 2013.
-
92Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
-
93Vieth R. Implications for 25-hydroxyvitamin D testing of public health policies about the benefits and risks of vitamin D fortification and supplementation. Scand J Clin Lab Invest Suppl. 2012;243:144-53.
-
94Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-8.
-
95Pinheiro MM, Schuch NJ, Genaro PS, Ciconelli RM, Ferraz MB, Martini LA. Nutrient intakes related to osteoporotic fractures in men and women--the Brazilian Osteoporosis Study (BRAZOS). Nutr J. 2009;8:6.
-
96Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012;97(8):2606-13.
-
97Dawson-Hughes B, Harris SS, Palermo NJ, Ceglia L, Rasmussen H. Meal conditions affect the absorption of supplemental vitamin D3 but not the plasma 25-hydroxyvitamin D response to supplementation. J Bone Miner Res. 2013;28(8):1778-83.
-
98Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204-10. Erratum in: Am J Clin Nutr. 2003;78(5):1047.
-
99Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842-56.
-
100Holick MF, Wacker M. Vitamin-D effects on skeletal and extraskeletal health and the need of supplementation. Nutrients. 2013;5(1):111-48.
-
101Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357-64.
-
102Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694-7.
-
103Logan VF, Gray AR, Peddie MC, Harper MJ, Houghton LA. Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr. 2013;109(6):1082-8.
-
104Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98(3):973-9.
-
105Thacher TD, Fischer PR, Obadofin MO, Levine MA, Singh RJ, Pettifor JM. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J Bone Miner Res. 2010;25(9):1988-95.
-
106Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1-130.
-
107Metzger M, Houillier P, Gauci C, Haymann JP, Flamant M, Thervet E, et al.; NephroTest Study Group. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: quest for a threshold. J Clin Endocrinol Metab. 2013;98(7):2922-8.
-
108London G, Coyne D, Hruska K, Malluche HH, Martin KJ. The new kidney disease: improving global outcomes (KDIGO) guidelines – expert clinical focus on bone and vascular calcification. Clin Nephrol. 2010;74(6):423-32.
-
109Cuppari L, Garcia Lopes MG, Kamimura MA. Vitamin D biology: from the discovery to its significance in chronic kidney disease. J Ren Nutr. 2011;21(1):113-6.
-
110Carvalho AB, Gueiros AP, Gueiros JE, Neves CL, Karohl C, Sampaio E, et al. Guidelines on bone mineral disorder in chronic kidney disease--addendum chapter 2. J Bras Nefrol. 2012;34(2):199-205.
-
111Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom. 2003 Winter;6(4):307-14.
-
112Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE, et al. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20(2):239-44.
-
113Shab-Bidar S, Bours SP, Geusens PP, van der Velde RY, Janssen MJ, van den Bergh JP. Suboptimal effect of different vitamin D3 supplementations and doses adapted to baseline serum 25(OH)D on achieved 25(OH)D levels in patients with a recent fracture: a prospective observational study. Eur J Endocrinol. 2013;169(5):597-604.
-
114Santos MT, Souza FI, Fonseca FL, Lazaretti-Castro M, Sarni RO. Changes in bone metabolism markers in women after Roux-en-Y gastric bypass. Arq Bras Endocrinol Metabol. 2012;56(6):376-82.
-
115Viégas M, Vasconcelos RS, Neves AP, Diniz ET, Bandeira F. Bariatric surgery and bone metabolism: a systematic review. Arq Bras Endocrinol Metabol. 2010;54(2):158-63.
-
116Nakamura KM, Haglind EG, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ 3rd, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25(1):151-8.
-
117Censani M, Stein EM, Shane E, Oberfield SE, McMahon DJ, Lerner S, et al. Vitamin D Deficiency Is Prevalent in Morbidly Obese Adolescents Prior to Bariatric Surgery. ISRN Obes. 2013;2013. pii: 284516.
-
118Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169.
-
119Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26 Suppl 1:75-90.
-
120Cunningham J, Zehnder D. New vitamin D analogs and changing therapeutic paradigms. Kidney Int. 2011;79(7):702-7.
-
121Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2):263-76.
-
122Zangeneh F, Clarke BL, Hurley DL, Watts NB, Miller PD. Chronic Kidney Disease Mineral and Bone Disorders (CKD-MBD) What the Endocrinologist Needs to Know. Endocr Pract. 2013;10:1-46.
-
123Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(4):e61387.
-
124Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37(3):239-48.
-
125Hagino H, Takano T, Fukunaga M, Shiraki M, Nakamura T, Matsumoto T. Eldecalcitol reduces the risk of severe vertebral fractures and improves the health-related quality of life in patients with osteoporosis. J Bone Miner Metab. 2013;31(2):183-9.
-
126Nakamura T, Takano T, Fukunaga M, Shiraki M, Matsumoto T. Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol. J Bone Miner Metab. 2013;31(4):417-22.
-
127Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al.; Medical Directors of Dialysis Clinic Inc. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70(10):1858-65.
-
128Bonakdaran S, Hami M, Hatefi A. The effects of calcitriol on albuminuria in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23(6):1215-20.
-
129Jørgensen HS, Winther S, Povlsen JV, Ivarsen P. Effect of vitamin-D analogue on albuminuria in patients with non-dialysed chronic kidney disease stage 4-5: a retrospective single center study. BMC Nephrol. 2012;13:102.
-
130Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997-1006.
-
131Noguchi Y, Kawate H, Nomura M, Takayanagi R. Eldecalcitol for the treatment of osteoporosis. Clin Interv Aging. 2013;8:1313-21.
-
132Christakos S, DeLuca H. Minireview: vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930-6.
-
133Nagpal S, Na S, Rathachalam R. Non calcemic actions of vitamina D receptor ligands. Endocr Rev. 2005;26(5):662-87.
-
134Visweswaran R, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17(4):602-10.
-
135Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S-88S.
-
136Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931-42.
-
137Fiscella K, Franker P. Vitamin D, race and cardiovascular mortality with findings from a national US sample. Ann Fam Med. 2010;8(1):11-8.
-
138Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927-35.
-
139Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, et al. Serum 25-hydroxivitamin D3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17(6):567-71.
-
140Christakos S, Hewison M, Gardner DG, Wagner CL, Sergeev IN, Rutten E, et al. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013;1287:45-58.
-
141Motiwala SR, Wang TJ. Vitamin D and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20(4):345-53.
-
142Pittas AG, Harris SS, Stark PC, Dawson Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980-6.
-
143Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35(1):11-7.
-
144Vitamin D supplement in early childhood and risk for Type 1 (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999;42(1):51-4.
-
145Zipitis CS, Akobeng Ak. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512-7.
-
146Pittas AG, Lau J, Hu B, Dawson Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017-29.
-
147Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422-8.
-
148de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al.; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31(4):701-7.
-
149Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, et al. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576-87.
-
150Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311-36.
-
151Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89.
-
152Pike JW. Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand Mol Cell Endocrinol. 2011;347(1-2):3-10.
-
153Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20(2):R31-47.
-
154Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishii Y, et al. 1 alpha,25-dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60). Biochem J. 1982;204(3):713-9.
-
155Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88(2):296-307.
-
156Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400, table of contents.
-
157Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382-90.
-
158Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189(11):5155-64.
-
159Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337-45.
-
160Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13:22.
-
161Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1,25-dihydroxyvitamin D3 and parathyroid hormone-related peptide in dermatology: a new perspective. J Investig Dermatol Symp Proc. 1996;1(1):1-9.
-
162Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermatoendocrinol. 2012;4(2):137-45.
-
163Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995-1000.
-
164Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Mol Biol. 2004;89-90(1-5):497-501.
-
165Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-41.
-
166Song Q, Sergeev IN. Calcium and vitamin D in obesity. Nutr Res Rev. 2012;25(1):130-41.
Publication Dates
-
Publication in this collection
July 2014
History
-
Received
31 Mar 2014 -
Accepted
18 June 2014