Abstracts
This article reports the case of a puerperal patient admitted with diagnosis of urinary tract infection and heart failure. This condition evolved with torsades de pointes ventricular arrhythmias, then, hypokalemia, and use of Ciprofloxacin. Ventricular arrhythmias did not present any improvement after potassium and magnesium replacement, but after implantation of temporary pacemaker, this condition showed signs of improvement. The patient was discharged with QTc at 490 ms, taking Propranolol.
Relatamos o caso de uma paciente puérpera, internada com diagnósticos de infecção do trato urinário e insuficiência cardíaca que evoluiu com arritmias ventriculares do tipo torsades de pointes, após hipopotassemia e uso de Ciprofloxacin. Não apresentou supressão das arritmias ventriculares após reposição de potássio e magnésio, mas após implante de marca-passo provisório. Recebeu alta hospitalar com QTc de 490 ms, em uso de Propranolol.
Arritmias cardíacas; Torsades de Pointes; insuficiência cardíaca; infecção urinária
Relatamos el caso de una paciente en puerperio, internada con diagnóstico de infección en el tracto urinario e insuficiencia cardíaca que evolucionó con arritmias ventriculares del tipo torsades de pointes, después de hipokalemia y uso de Ciprofloxacina. No presentó supresión de las arritmias ventriculares después de la reposición de potasio y magnesio, sino después de implante de marcapasos provisorio. Recibió alta hospitalaria con QTc de 490 ms, en uso de Propanolol.
Arritmias cardíacas; Torsades de Pointes; insuficiencia cardíaca; infección urinaria
CASE REPORT
Hospital São José do Avai, Itaperuna, Rio de Janeiro - Brazil
Mailing address
SUMMARY
This article reports the case of a puerperal patient admitted with diagnosis of urinary tract infection and heart failure. This condition evolved with torsades de pointes ventricular arrhythmias, then, hypokalemia, and use of Ciprofloxacin. Ventricular arrhythmias did not present any improvement after potassium and magnesium replacement, but after implantation of temporary pacemaker, this condition showed signs of improvement. The patient was discharged with QTc at 490 ms, taking Propranolol.
Key Words: Arrhythmias cardiac; Torsades de Pointes; heart failure; urinary infection.
Introduction
The long QT syndrome may have genetic or acquired origin. Regardless of etiology, patients with this diagnosis are at risk of sudden death as a consequence of malignant ventricular arrhythmias. The most frequently found one1 is polymorphic ventricular tachycardia, also known as torsades de pointes.
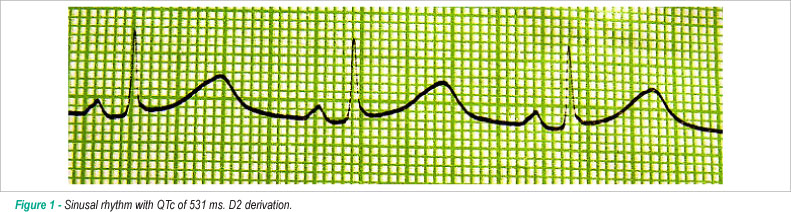
In this paper, we report the case of a young puerperal patient admitted with initial diagnosis of urinary infection and heart failure, who had suddenly begun to present pre-syncope and syncope episodes associated with tachycardia palpitations. An electrocardiogram made during the syncope episode revealed a prolonged QTc interval (531 ms) and torsades de pointes. The patient underwent potassium and magnesium replacement, implantation of temporary pacemaker and resolution of ventricular arrhythmias. A clinical improvement was observed. However, the QT interval did not come to normal values.
Case Report
The 23-year-old female patient was admitted with a history of fever, nausea, vomit and dyspnea at minimum efforts. She had an uneventful pregnancy, and was in her second month of postpartum care.
Upon admission, she was eupneic, with lower-limb edema (+/+4) and fever. Her blood pressure was at 120x80 and heart rate at 68 bpm. Heart auscultation revealed no alterations, but her lungs presented bibasal crackles. Her liver was palpable on the right costal edge.
She was initially admitted into a medical ward. Chest radiography revealed congested lungs. Her cardiac area was found normal. Laboratory tests: K+ 2.5, Mg ++ 1,8, leucogram 9200 (9/77), VHS 75 mm, urine with leukocyturia and hematuria, no cylinders. Proteinuria of 588 mg/24 h and positive urine culture for Escherichia coli. Treatment with Ciprofloxacin, Captopril, Aldactone, Furosemide and potassium replacement was started. Upon admission, an echocardiogram also revealed diffused hipokynesia, light left ventricular dysfunction (FE 53%), discreet pericardial effusion and normal-sized cardiac cavities.
On the fifth day of admission, the patient started to present pre-syncope and syncope episodes associated with palpitations. Firstly, an electrocardiogram revealed torsades de pointes ventricular arrhythmia and QTc of 531 ms (Figures 1 and 2). Upon transfer to the Intensive Care Unit, Ciprofloxacin was interrupted and potassium and magnesium replacement was intensified. Even with these ions at normal levels, the patient kept torsades de pointes episodes. Due to this reason, we decided to implant a temporary pacemaker, setting its frequency above her basal frequency, halting the ventricular arrhythmia episodes right after. The patient remained in the Intensive Care Unit for approximately one week, using temporary pacemaker, with no ventricular arrhythmias, clinical improvement, keeping the QTc interval around 582 ms and QT dispersion higher than 100 ms. In the ward, the temporary pacemaker was removed and 40 mg Propranolol was prescribed, three times a day. A new echocardiogram revealed regular left ventricular function.
The patient was discharged under use of Propranolol.
During the admission period, her first-degree relatives were submitted to electrocardiogram, but no prolonger QT interval was found.
One month after discharge, the patient brought the Holter test. The QTc was at 511 ms; however, the patient presented no symptoms.
Four months after hospital discharge, the patient presented another syncope episode with no prodromes. A new echocardiogram revealed QTc of 524 ms. Then, we decided to prescribe defibrillator implantation.
Discussion
The long QT syndrome (LQTS) is characterized by a prolongation in the QT interval in electrocardiogram and predisposition to malignant ventricular arrhythmias. Polymorphic ventricular tachycardia, also known as torsades de pointes, is a typical characteristic of this condition1.
Mutation in specific genes that codify the formation of subunits of ionic channels is responsible for genetic etiology. These mutant channels may present function gains or losses, and the unbalance of the ionic flow through these channels leads to the prolongation of the potential action that predisposes patients to ventricular arrhythmias.
Genetic inheritance may be recessive autosomal (Jervell and Lange-Neilsen Syndrome) or dominant (Romano-Ward Syndrome)2,3 and mutations in several human genes have been reported, namely: KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2 and CAV3.
The congenital forms are divided into 1 to 10. Types 1, 2 and 3 account for around 95% of all genetic LQTS cases. It is well-established that there are "trigger" factors for the development of ventricular arrhythmias.
The wide range of genetic long QT syndromes has led Swartz to define clinical criteria to diagnosis4.
Some patients, however, despite not suffering traditional genetic long QT syndrome may, upon the use of some drugs, develop a prolongation in QT intervals and be exposed to ventricular arrhythmias. Such cases were known as acquired long QT syndrome and we know that almost all drugs that prolong the QT interval by blocking hERG5 potassium channels.
Summing up, genetic or acquired factors that change the fine balance among the inlet and outlets of ionic channels significantly prolong the duration of the action potential and create a substratum for ventricular arrhythmias. The exact mechanism through which the action potential prolongation, in some ventricular myocardial areas, predisposes patients to malignant arrhythmias, is still a controversial issue5,6. Nevertheless, it is known that this ventricular repolarization heterogeneity may be demonstrated by the extent to which QT interval disperses, and values above 100 ms predict a worse prognosis in patients with genetic long QT7.
Hypokalemia combined with the use of Ciprofloxacin (a quinolone that may cause acquired long QT syndrome) made us suggest, at first, the hypothesis of acquired LQTS syndrome, once the patient, or even her close relatives did not present any previous history of syncope or arrhythmias. Nevertheless, keeping a long QT interval, a couple of weeks after serum potassium has come to normal levels and Ciprofloxacin interruption, made us consider it a probable genetic long QT syndrome. Then, defibrillator implantation of was suggested.
Symptoms and signs of congestive heart failure were quickly solved with the therapy prescribed. Fifteen days after admission, a new echocardiogram revealed regular left ventricular function. A myocarditis justified a picture of heart failure that could be quickly solved. We attributed proteinuria (non nephrotic) to the urinary tract infection.
Recent literature date point to the role of postpartum hormonal alterations (high levels of estrogen and progesterone), associated or not with fatigue, sleep deprivation, stress and noise (a newborn crying) as potential triggers to the appearance of torsades de pointes in puerperal women with genetic LQTS (notably type 2 - mutation on KCNH2 gene)8. A sensible approach would be the performance of an electrocardiogram every 30 days on these patients during pregnancy, as well as in the puerperal period. If a progressive QT increase or QTc above 500 ms was observed at any time, a closer clinical and cardiologic monitoring should have been made. After the nine month of pregnancy, the clinical follow-up could return to the pre-gestation standard, as the risk of ventricular arrhythmias becomes much smaller.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
References
- 1. Camanho LEM, Prata I, Ferreira FAC, Filho PJSM, Veronese FO, Saad EB. Electrocardiographic predictors of syncope and sudden death in patients with congenital long QT syndrome. Rev SOCERJ. 2007; 20: 91-6.
- 2. Romano C, Gemme G, Pongiglione R. Aritmie cardiache rare dell'etá pediatrica. II Acessi sincopali per fibrillazione ventricolare parossistica. Clin Pediatr (Bologna). 1963; 45: 656-83.
- 3. Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc. 1964; 54: 103-6.
- 4. Priori S, Schwartz P, Napolitano C, Bloise R, Ronchetti E, Griello M, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003; 348: 1866-74.
- 5. Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005; 115 (8): 2025-32.
- 6. Abriel H, Schläpfera J, Keller DI, Gavillet B, Buclin T, Biollaz J, et al. Molecular and clinical determinants of drug-induced long QT syndrome: an iatrogenic channelopathy. Swiss Med Wkly. 2004; 134: 685-94.
- 7. Day CP, McComb JM, Campbell RWF. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990; 63: 342-4.
- 8. Meregalli PG,Westendorp IC, Tan HL, Elsman P, Kok WE, Welde AA. Pregnancy and the risk of torsades de pointes in congenital long-QT syndrome. Neth Heart J. 2008; 16 (12): 422-5.
Postpartum torsades de pointes and long QT syndrome
Publication Dates
-
Publication in this collection
24 Nov 2009 -
Date of issue
Oct 2009
History
-
Received
10 Nov 2008 -
Reviewed
01 Apr 2009 -
Accepted
04 June 2009