| |
N° CT |
(Main ID / Secondary ID |
Primary registration base |
Public title |
Search |
Títle(s) of the identified scientific article(s) |
Letter AC |
N° of CT and letter of SA |
| Eryaspase |
1 |
NCT00723346/ GRASPALL 20S05-01 |
ClinicalTrials.gov |
Administration of Allogenic Red Blood Cells Loaded L-asparaginase in Cases of Relapse of Acute Lymphoblastic Leukaemia GRASPALL. |
I e II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002) |
a |
(1a) |
| L-asparaginase loaded red blood cells in…GRASPALL 2005-01 randomized trial (doi.org/10.1111/j.1365-2141.2011.08588.x). |
b |
(1b) |
| GRASPALL 2005.01 Clinical Study: L-Asparaginase Loaded into Red Blood Cells…Relapsed Acute Lymphoblastic Leukaemia (ALL) (doi.org/10.1182/blood.V112.11.306.306). |
c |
(1c) |
| L-Asparaginase Loaded Inside Red Cells Has An Acceptable Tolerability Profile On Bilirubin Value (doi.org/10.1182/blood.V122.21.2642.2642). |
d |
(1d) |
| 2 |
EUCTR2009-012584-34-BE/ GRASPALL 2009-06 |
EUCTR |
Clinical trial with GRASPA, Red Blood cells encapsulating L-Asparaginase, in patients affected by Acute Lymphoblastic leukemia at relapse. |
I e II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002). |
a |
(2a) |
| 3 |
NCT01523808/GRASPANC 2008-02 |
ClinicalTrials.gov |
Administration of GRASPA (Suspension of Erythrocytes Encapsulating L-asparaginase) in Patients With Pancreatic Cancer. |
I e II |
Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients With Pancreatic Adenocarcinoma (doi.org/10.1097/MPA.000000000 0000394). |
e |
(3e) |
| 4 |
NCT01810705/GRASPA-AML-2012-01 |
ClinicalTrials.gov |
GRASPA Treatment for Patients With Acute Myeloblastic Leukemia ENFORCE. |
I e II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002). |
a |
(4a) |
| GRASPA-AML 2012-01 study (NCT01810705): A multicenter,open, randomized phase 2b trialevaluating ERY001… for intensive chemotherapy (doi.org/10.1200/jco.2015.33.15_suppl.tps709). |
f |
(4f) |
| 5 |
NCT01910428/GRASPALL 2012-09 |
ClinicalTrials.gov |
L-asparaginase Encapsulated in Red Blood Cells (Eryaspase) for Treatment of Adult Patients With ALL or LBL |
I e II |
Not found |
|
|
| 6 |
EUCTR2012-002026-78-FI; GRASPA-AML-2012-01 |
EUCTR |
Clinical trial with GRASPA, Red Blood cells encapsulating L-Asparaginase, in patients affected by Acute Myeloid leukemia |
I e II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002). |
a |
(6a) |
| 7 |
EUCTR2013-004262-34-FR/ GRASPANC2013-03 |
EUCTR |
Clinical trial with L-asparaginase encapsulated in erythrcoytes in patients affected by metastatic pancreatic cancer after first line treatment |
I e II |
Not found |
|
|
| 8 |
NCT02195180/GRASPANC 2013-03 |
ClinicalTrials.gov |
Efficacy and Safety of L-asparaginase Encapsulated in RBC Combined With Gemcitabine or FOLFOX in 2nd Line for Progressive Metastatic Pancreatic Carcinoma |
I e II |
Circulating Tumor DNA is Prognostic and Potentially Predictive of Eryaspase Efficacy in Second-line in Patients with Advanced Pancreatic Adenocarcinoma (doi.org/10.1158/1078-0432.CCR-20-0950). |
g |
(8g) |
| Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial (doi.org/10.1016/j.ejca.2019.10.020). |
h |
(8h) |
| A Phase 2b of eryaspase in combination with gemcitabine or FOLFOX as second-line therapy in patients with metastatic pancreatic adenocarcinoma (NCT02195180) (doi.org/10.1093/annonc/mdx369.005). |
i |
(8i) |
| 9 |
EUCTR2018-002211-10-ES/ GRASPA-TNBC-2018-02; NCT03674242 |
EUCTR |
Study to determine whether the addition of eryaspase to gemcitabine and carboplatin will reduce the tumor burden and stabilizate the tumor progression |
I e II |
TRYbeCA-2: A randomized phase II/III study of eryaspase in combination with gemcitabine and carboplatin chemotherapy versus chemotherapy alone as first-line treatment in patients with metastatic or locally recurrent triple-negative breast cancer (doi.org/10.1093/annonc/mdz242.076). |
j |
(9j) |
| 10 |
NCT01518517/GRASPALL 2009-06 |
ClinicalTrials.gov |
GRASPA (Erythrocytes Encapsulating L-asparaginase) in Patients With Relapse of Acute Lymphoblastic Leukemia GRASPIVOTALL |
II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002). |
a |
(10a) |
| Pharmacokinetic and Pharmacodynamic Characterization of Graspa Versus Native L-Asparaginase in Combination with Cooprall Chemotherapy in a Phase 3 Randomized Trial for theTreatment of Patients with Relapsed Acute Lymphoblastic Leukemia (NCT01518517) (doi.org/10.1182/blood.V126.23.2492.2492). |
l |
(10l) |
| Pharmacodynamic characterization of eryaspase (L-asparaginase encapsulated in red blood cells) in combination with chemotherapy in a phase 2/3 trial in patients with relapsed acute lymphoblastic leukemia (NCT01518517) (doi.org/10.1200/JCO.2018.36.15_suppl.7049). |
m |
(10m) |
| Updated Clinical Activity of Graspa Versus Native l-Asparaginase in Combination with Cooprall Regimen in Phase 3 Randomized Trial in Patients with Relapsed Acute Lymphoblastic Leukemia (NCT01518517) (doi.org/10.1182/blood.V126.23.3723.3723). |
n |
(10n) |
| Evaluation of the Impact of the Presence of Neutralizing L-Asparaginase Antibodies on the Efficacy and Safety of Graspa in Phase 3 Randomized Trial Versus Native L-Asparaginase in Patients with Relapsed Acute Lymphoblastic Leukemia (NCT01518517) (doi.org/10.1182/blood.V126.23.3734.3734). |
o |
(10o) |
| Drug monitoring of ERY001 (erythrocyte encapsulated L-asparaginase) and native L-asparaginase (L-ASP) in combination with COOPRALL regimen in Phase 3 randomized trial in patients with relapsed acute lymphoblastic leukemia (doi.org/10.1200/jco.2015.33.15_suppl.e18036). |
p |
(10p) |
| Clinical activity of ERY001 (erythrocyte encapsulated l-asparaginase) and native l-asparaginase (L-ASP) in combination with COOPRALL regimen in phase III randomized trial in patients with relapsed acute lymphoblastic leukemia (ALL) (doi.org/10.1200/jco.2015.33.15_suppl.7004). |
q |
(10q) |
| 11 |
NCT01523782/ GRASPALL/GRAALLSA2-2008 |
ClinicalTrials.gov |
Administration of GRASPA (Suspension of Erythrocytes Encapsulating L-asparaginase) in Elderly Patients With First Line Acute Lymphoblastic Leukemia |
II |
Erythrocyte encapsulated l-asparaginase (GRASPA) in acute leukemia (doi.org/10.2217/ijh-2016-0002). |
a |
(11a) |
| A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study (doi.org/10.1002/ajh.24093). |
r |
(11r) |
| L-Asparaginase Loaded Inside Red Cells Has An Acceptable Tolerability Profile On Bilirubin Value (doi.org/10.1182/blood.V122.21.2642.2642). |
d |
(11d) |
| 12 |
NCT02197650/GRASPALL 2012-10-EAP |
ClinicalTrials.gov |
Expanded Access Program: Safety of Erythrocytes Encapsulating L-asparaginase (GRASPA®) in Combination With Polychemotherapy in Patients Under 55 Years Old With Acute Lymphoblastic Leukemia (ALL) at Risk to Receive Other Formulation of Asparaginase EAP |
II |
Expanded Access Program of Graspa for Treatment of Patients with Acute Lymphoblastic Leukemia Unable to Receive Other Form of L-Asparaginase - a Status Update (NCT02197650) (doi.org/10.1182/blood.V126. 23.4877.4877). |
s |
(12s) |
| L-Asparaginase Allergic Patients Treated with L-Asparaginase Loaded into Red Blood Cells in an Expanded Access Program. Report of Four Cases (doi.org/10.1182/blood.V124.21.937.937). |
t |
(12t) |
| 13 |
EUCTR2016-004451-70-DK; NOR-GRASPALL-2016 |
EUCTR |
Eryaspase treatment for children and young adults with leukemia and hypersensitivity to PEG-asparaginase. |
III |
Not found |
|
|
| 14 |
EUCTR2016-004451-70-NO; 2016-004451-70-DK NOR-GRASPALL-2016 |
EUCTR |
Eryaspase treatment for children and young adults with leukemia and hypersensitivity to PEG-asparaginase. |
III |
Not found |
|
|
| 15 |
NCT03267030/ NOR-GRASPALL-2016 |
ClinicalTrials.gov |
Asparaginase Encapsulated in Erythrocytes for Patients With ALL and Hypersensitivity to PEG-asparaginase |
III |
Not found |
|
|
| 16 |
NCT03665441; GRASPANC 2018-01 |
ClinicalTrials.gov |
Study of Eryaspase in Combination With Chemotherapy Versus Chemotherapy Alone as 2nd-Line Treatment in PAC Trybeca-1 |
III |
TRYbeCA-1: A randomized, phase 3 study of eryaspase in combination with chemotherapy versus chemotherapy alone as second-line treatment in patients with pancreatic adenocarcinoma (NCT03665441) (doi.org/10.1093/annonc/mdz155.097). |
u |
(16u) |
| 17 |
NCT04292743/ STUDY00002008 |
ClinicalTrials.gov |
Eryaspase With Modified FOLFIRINOX in Advanced Pancreatic Ductal Adenocarcinoma |
III |
A phase I dose escalation study of eryaspase in combination with modified FOLFIRINOX in locally advanced and metastatic pancreatic ductal adenocarcinoma (doi.org/10.1200/JCO.2021.39.3_suppl. TPS453). |
v |
(17v) |
| GSK318689 |
18 |
NCT03874234; 208436 |
ClinicalTrials.gov |
Safety, Tolerability and Pharmacokinetics (PKs) Investigation of GSK3186899 in Healthy Subjects |
Ia e IIa |
Not found |
|
|
| DNDI 0690 |
19 |
NCT03929016/ 2018-002021-35; DNDi-0690-01; QSC20093229016 |
ClinicalTrials.gov |
Single Oral Dose Escalation Study of DNDI-0690 in Healthy Subjects |
Ib e IIb |
Not found |
|
|
| 20 |
ISRCTN30122193; 2020-003963-24; DNDi-0690-02 / RD 777/34920 / IRAS 288914 |
ISRCTN |
A study to investigate the safety, tolerability and activity of multiple ascending doses of DNDI-0690 in healthy volunteers including assessment of heart and kidney function |
Ib e IIb |
Not found |
|
|
Total clinical studies = 20
Total scientific articles=22
Full list of clinical trials and their scientific articles = 27 (scientific article ‘a’ is a review and contains data from more than one clinical trial, and article ‘d’ includes data from two clinical trials). |

 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Note: A: State of completion of the clinical trial; B: Publication means of clinical trials results; C: Publication of clinical trails information (no results); The slice represented as Indeterminate means lack of information in the metadata indicating the completion of the study.Source: Elaborated by the authors (2021)
Note: A: State of completion of the clinical trial; B: Publication means of clinical trials results; C: Publication of clinical trails information (no results); The slice represented as Indeterminate means lack of information in the metadata indicating the completion of the study.Source: Elaborated by the authors (2021)
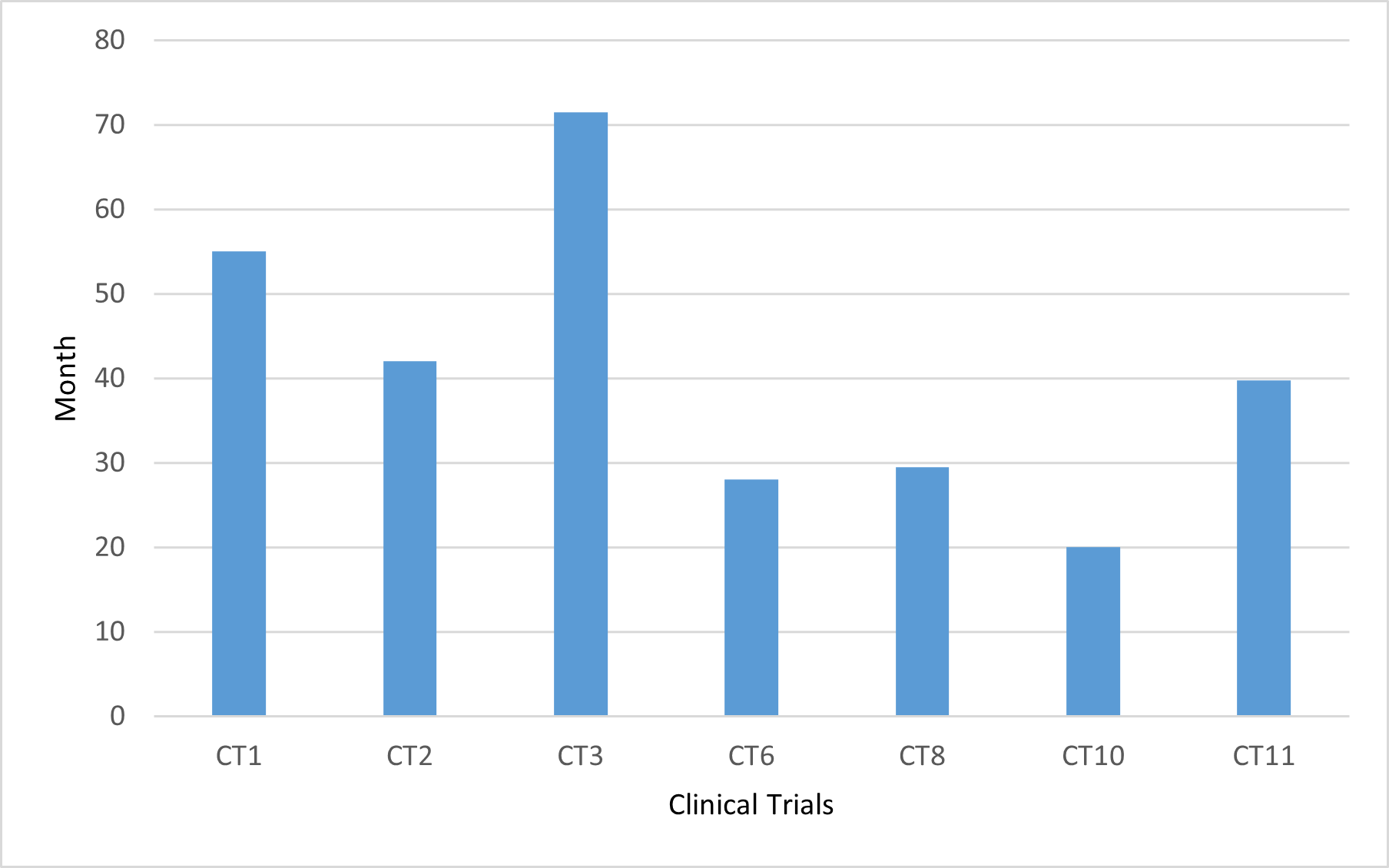 Source: Prepared by the authors (2021).
Source: Prepared by the authors (2021).