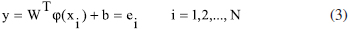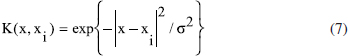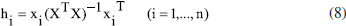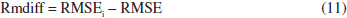In this study, molecular docking and molecular dynamics (MD) simulations were conducted to investigate both the binding site and interactions of 61 inhibitors with activin-like kinase-5 (ALK5) receptor. A MD simulation was performed on the receptor to obtain receptor conformation in a water environment. Docking analysis revealed that hydrophobic and hydrogen bonding interactions play important roles in ALK5-inhibitor complex. These interactions were confirmed by X-ray crystallography. Furthermore, to study receptor conformation stability, a second MD simulation on complex was performed in an aqueous environment. Radius of gyration for complex showed that the ALK5 conformation did not change in the presence of the inhibitor. 134 descriptors emerging from docking and molecular structure were calculated and the most feasible ones were used in quantitative structure -activity relationships (QSAR). The LS-SVR (least squares support vector regression) gave reliable model with Q² = 0.837 and R = 0.917. Finally, the types of interactions and properties of the descriptors were used to propose new inhibitors.
ALK5; docking; molecular dynamics simulation; least squares support vector regression; QSAR