Abstract:
Aim
The aim of this study was to determine the diversity of Trichoptera in subtropical streams and the effects of environmental variables and geographical position on alpha and beta diversity in natural and rural streams.
Methods
We collected Trichoptera with a Surber sampler in 12 small order subtropical streams (six streams with apparent absence of anthropic disturbance and six streams with rural activity in their drainage areas) and measured limnological variables. We evaluated the effects of environmental variability and geographical distance on the dissimilarity of the assemblage and calculated the contribution of alpha and beta diversity for each stream.
Results
We collected a total of 1,264 Trichoptera larvae distributed in 17 genera and 11 families. The genera Phylloicus and Smicridea were found in almost all streams. We observed a positive effect of environmental variability on biological variability but not of geographical distance. The environmental variability was basically generated by the influence of higher concentrations of dissolved organic carbon and nutrients. We observed the greatest contribution of the alpha diversity of the Trichoptera assemblages in natural streams and of beta diversity in the rural streams.
Conclusions
Our results demonstrate that the variability of Trichoptera is affected by environmental characteristics, but not by geographical position.
Keywords:
dissimilarity; taxonomic richness; ecological integrity; riparian vegetation; agricultural impact
Resumo:
Objetivo
O objetivo desse estudo foi determinar a diversidade de Trichoptera em riachos e o efeito das variáveis ambientais e posição geográfica na diversidade alfa e beta em riachos naturais e rurais.
Métodos
Coletamos larvas de Trichoptera com um amostrador Surber em 12 riachos subtropicais de pequena ordem (seis riachos com ausência aparente de distúrbios antrópicos e seis riachos com atividades rurais na área de drenagem) e quantificamos as variáveis limnológicas. Avaliamos os efeitos da variabilidade ambiental na dissimilaridade das assembleias e calculamos a contribuição da diversidade alfa e beta nos riachos.
Resultados
Coletamos um total de 1264 larvas de Trichoptera distribuidas em 17 gêneros e 11 famílias. Os gêneros Phylloicus e Smicridea foram encontramos em praticamente todos os riachos. Observamos um efeito positivo da variabilidade ambiental na variabilidade biológica, mas não da posição geográfica. A variabilidade ambiental foi gerada basicamente pela influência de maiores concentrações de carbono orgânico dissolvido e nutrientes. Observamos maior contribuição da diversidade alfa nas assembleias de Trichoptera nos riachos naturais, e da diversidade beta nos riachos rurais.
Conclusões
Nossos resultados demonstram que a variabilidade de Trichoptera é afetada por características ambientais, e não pela posição geográfica.
Palavras-chave:
dissimilaridade; riqueza taxonômica; integridade ecológica; vegetação ripária; impacto agrícola
1. Introduction
Riparian vegetation is important for the maintenance and regulation of the aquatic environment (Gonçalves Júnior et al., 2014GONÇALVES JÚNIOR, J.F., REZENDE, R.S., GREGÓRIO, R.S. and VALENTIN, G.C. Relationship between dynamics of litterfall and riparian plant species in a tropical stream. Limnologica-Ecology and Management of Inland Waters, 2014, 44, 40-48. http://dx.doi.org/10.1016/j.limno.2013.05.010.
http://dx.doi.org/10.1016/j.limno.2013.0...
), serving as a barrier to sediment supply and maintaining biological conditions. Shading provided by riparian vegetation assists in controlling the temperature of the streams and also increases the environmental complexity and availability of food (Naiman et al., 2010NAIMAN, R.J., DÉCAMPS, H. and MCCLAIN, M.E. Riparia: ecology, conservation, and management of streamside communities. Cambridge: Academic Press, 2010.). On landscape scale, riparian vegetation provides corridors for wildlife movement, which increases biodiversity on a spatial scale and contributes to the maintenance of water quality (Naiman et al., 2010NAIMAN, R.J., DÉCAMPS, H. and MCCLAIN, M.E. Riparia: ecology, conservation, and management of streamside communities. Cambridge: Academic Press, 2010.). However, freshwater environments are being impacted by a variety of anthropic activities (e.g. urbanization and agriculture) (Hepp et al., 2010HEPP, L.U., MILESI, S.V., BIASI, C. and RESTELLO, R.M. Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia, 2010, 27(1), 106-113. http://dx.doi.org/10.1590/S1984-46702010000100016.
http://dx.doi.org/10.1590/S1984-46702010...
; Effert-Fanta et al., 2019EFFERT-FANTA, E.L., FISCHER, R.U. and WAHL, D.H. Effects of riparian forest buffers and agricultural land use on macroinvertebrate and fish community structure. Hydrobiologia, 2019, 841(1), 45-64. http://dx.doi.org/10.1007/s10750-019-04006-1.
http://dx.doi.org/10.1007/s10750-019-040...
). Reducing riparian vegetation increase the temperature, nutrient concentration and sediment content within streams, which damages the ecological integrity of the aquatic ecosystem (Blevins et al., 2013BLEVINS, Z.W., EFFERT, E.L., WAHL, D.H. and SUSKI, C.D. Land use drives the physiological properties of a stream fish. Ecological Indicators, 2013, 24, 224-235. http://dx.doi.org/10.1016/j.ecolind.2012.06.016.
http://dx.doi.org/10.1016/j.ecolind.2012...
). Any changes generated by agriculture alter the physical and chemical structure of the streams, which can modify the composition of aquatic communities by taxa replacement (turnover) or changes in taxonomic richness, occurring gain and loss of taxa (nestedness) (Hepp & Santos, 2009HEPP, L.U. and SANTOS, S. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environmental Monitoring and Assessment, 2009, 157(1-4), 305-318. http://dx.doi.org/10.1007/s10661-008-0536-7. PMid:18843547.
http://dx.doi.org/10.1007/s10661-008-053...
; Hepp et al., 2010HEPP, L.U., MILESI, S.V., BIASI, C. and RESTELLO, R.M. Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia, 2010, 27(1), 106-113. http://dx.doi.org/10.1590/S1984-46702010000100016.
http://dx.doi.org/10.1590/S1984-46702010...
; Fugère et al., 2016FUGÈRE, V., KASANGAKI, A. and CHAPMAN, L.J. Land use changes in an afrotropical biodiversity hotspot affect stream alpha and beta diversity. Ecosphere, 2016, 7(6), 1-18. http://dx.doi.org/10.1002/ecs2.1355.
http://dx.doi.org/10.1002/ecs2.1355...
).
Understanding how environmental conditions influence the composition and distribution of biological communities locally and regionally is a major challenge for ecologists (Heino et al., 2015HEINO, J., MELO, A.S., BINI, L.M., ALTERMATT, F., AL-SHAMI, S.A., ANGELER, D.G., BONADA, N., BRAND, C., CALLISTO, M., COTTENIE, K., DANGLES, O., DUDGEON, D., ENCALADA, A., GÖTHE, E., GRÖNROOS, M., HAMADA, N., JACOBSEN, D., LANDEIRO, V.L., LIGEIRO, R., MARTINS, R.T., MISERENDINO, M.L., MD RAWI, C.S., RODRIGUES, M.E., ROQUE, F.O., SANDIN, L., SCHMERA, D., SGARBI, L.F., SIMAIKA, J.P., SIQUEIRA, T., THOMPSON, R.M. and TOWNSEND, C.R. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution, 2015, 5(6), 1235-1248. http://dx.doi.org/10.1002/ece3.1439. PMid:25859329.
http://dx.doi.org/10.1002/ece3.1439...
). Biological diversity can be defined as the variety and abundance of species in a particular unit of study (Magurran, 2013MAGURRAN, A.E. Measuring biological diversity. New York: John Wiley & Sons, 2013.). Whittaker (1960)WHITTAKER, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 1960, 30(3), 279-338. http://dx.doi.org/10.2307/1943563.
http://dx.doi.org/10.2307/1943563...
proposed the concepts of alpha, beta and gamma diversity, which characterize different levels of diversity. According to Whittaker (1960)WHITTAKER, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 1960, 30(3), 279-338. http://dx.doi.org/10.2307/1943563.
http://dx.doi.org/10.2307/1943563...
, the total biological diversity of a region (gamma diversity) can be partitioned into alpha diversity, which corresponds to community diversity in a given location, and beta diversity, which represents variation between communities in different locations.
Beta diversity can be caused by environmental or spatial factors. Spatially, the limitations of the dispersal ability of organisms, the differences in physical space and climate can generate high beta diversity among communities (Costa & Melo, 2008COSTA, S.S. and MELO, A.S. Beta diversity in stream macroinvertebrate assemblages: among-site and among-microhabitat components. Hydrobiologia, 2008, 598(1), 131-138. http://dx.doi.org/10.1007/s10750-007-9145-7.
http://dx.doi.org/10.1007/s10750-007-914...
; Roa-Fuentes et al., 2019ROA-FUENTES, C.A., HEINO, J., CIANCIARUSO, M.V., FERRAZ, S., ZENI, J.O. and CASATTI, L. Taxonomic, functional, and phylogenetic β‐diversity patterns of stream fish assemblages in tropical agroecosystems. Freshwater Biology, 2019, 64(3), 447-460. http://dx.doi.org/10.1111/fwb.13233.
http://dx.doi.org/10.1111/fwb.13233...
). Spatial features such as drainage basin geology can also influence biological variability (Schneck & Hepp, 2010SCHNECK, F. and HEPP, L.U. Fatores estruturadores de comunidades em riachos. Ciência e Ambiente, 2010, 41, 57-67.; Hawkins et al., 2015HAWKINS, C.P., MYKRÄ, H., OKSANEN, J. and VANDER LAAN, J.J. Environmental disturbance can increase beta diversity of stream macroinvertebrate assemblages. Global Ecology and Biogeography, 2015, 24(4), 483-494. http://dx.doi.org/10.1111/geb.12254.
http://dx.doi.org/10.1111/geb.12254...
). Among the environmental factors, stream velocity, creek depth, substrate type, temperature, light availability and nutrients play an important role in the structuring of aquatic communities (Allan & Castillo, 2007ALLAN, J.D. and CASTILLO, M.M. Stream ecology: structure and function of running waters. Dordrecht: Springer, 2007. http://dx.doi.org/10.1007/978-1-4020-5583-6.
http://dx.doi.org/10.1007/978-1-4020-558...
; Curry & Baird, 2015CURRY, C.J. and BAIRD, D.J. Habitat type and dispersal ability influence spatial structuring of larval Odonata and Trichoptera assemblages. Freshwater Biology, 2015, 60(10), 2142-2155. http://dx.doi.org/10.1111/fwb.12640.
http://dx.doi.org/10.1111/fwb.12640...
; Pitacco et al., 2019PITACCO, V., MISTRI, M., ALEFFI, I.F., LARDICCI, C., PRATO, S., TAGLIAPIETRA, D. and MUNARI, C. Spatial patterns of macrobenthic alpha and beta diversity at different scales in Italian transitional waters (central Mediterranean). Estuarine, Coastal and Shelf Science, 2019, 222, 126-138. http://dx.doi.org/10.1016/j.ecss.2019.04.026.
http://dx.doi.org/10.1016/j.ecss.2019.04...
).
The insects of the order Trichoptera are abundant and highly diverse in lotic ecosystems (Crisci-Bispo et al., 2007CRISCI-BISPO, V.L., BISPO, P.C. and FROEHLICH, C.G. Ephemeroptera, Plecoptera and Trichoptera assemblages in two Atlantic Rainforest streams, Southeastern Brazil. Revista Brasileira de Zoologia, 2007, 24(2), 312-318. http://dx.doi.org/10.1590/S0101-81752007000200007.
http://dx.doi.org/10.1590/S0101-81752007...
). They occur in clean and well-oxygenated waters, which makes them indicators of aquatic habitat quality and integrity (Rosenberg & Resh, 1993ROSENBERG, D.M. and RESH, V.H. Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman & Hall, 1993.; Breda et al., 2018BREDA, M., LAZARI, P.L., OLIVEIRA, M.B., MENEGAT, M.N., BERTOL, E.C., SILVA, G.S., DECIAN, V.S., RESTELLO, R.M. and HEPP, L.H. Composição e distribuição de Trichoptera (Insecta) em riachos subtropicais. Perspectiva, 2018, 42(157), 17-26.). These characteristics give Trichoptera potential to serve as a model for ecological studies on biological diversity patterns in aquatic environments. In this study, we collected Trichoptera larvae in streams located in a transitional region of the Atlantic Forest Biome. This region comprises the southernmost portion of this biome and is severely fragmented by rural activities.
This study had an exploratory character and we tried to answer the following question: how does the environmental variation generated by changes in land uses (e.g. agriculture) affect the alpha and beta components of the diversity of Trichoptera assemblages in streams? Thus, our objectives were: (i) to determine the diversity of Trichoptera in streams surrounded by riparian vegetation and agriculture; (ii) to determine the effects of environmental variables and geographical position on Trichoptera assemblages in natural and rural streams; and (iii) to determine the contribution of the alpha and beta components to the regional diversity of Trichoptera in the studied area.
2. Material and Methods
2.1. Study area
The region under study has a subtropical climate, with average annual temperatures of 18 °C and annual mean rainfall of 1,500 mm (Alvares et al., 2013ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., MORAES, G., LEONARDO, J. and SPAROVEK, G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 2013, 22(6), 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507.
http://dx.doi.org/10.1127/0941-2948/2013...
). About 20% of the region is covered by arboreal vegetation, the rest being occupied by rural and urban activities (Rovani et al., 2019ROVANI, I.L., SANTOS, J.E., DECIAN, V.S. and ZANIN, E.M. Assessing naturalness changes resulting from a historical land use in Brazil South Region: an analysis of the 1986-2016 period. Journal of Environmental Protection, 2019, 10(02), 149-163. http://dx.doi.org/10.4236/jep.2019.102010.
http://dx.doi.org/10.4236/jep.2019.10201...
). The vegetation is part of the Atlantic Forest biome, comprising a vegetative structure characterized by the presence of Araucaria angustifolia (Oliveira-Filho et al., 2015OLIVEIRA-FILHO, A.T., BUDKE, J.C., JARENKOW, J.A., EISENLOHR, P.V. and NEVES, D.R. Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. Journal of Plant Ecology, 2015, 8(3), 242-260. http://dx.doi.org/10.1093/jpe/rtt058.
http://dx.doi.org/10.1093/jpe/rtt058...
). We collected samples in 12 streams, six streams with apparent absence of anthropic disturbance (called “natural”) and six streams with rural activity in their drainage areas, comprised mainly by agriculture (called “rural”) according to direct observation of stream reach adjacent areas. The streams are located in southern Brazil, in the upper and middle portion of the Uruguay River (Figure 1). The maximum distance between a pair of streams was 120 km, while the minimum distance was 200 meters. The streams had a width of around 2 m, depth <30 cm and the bottom consisted of a predominant substrate of stones with leaf packs.
Study area in Southern Brazil. NatFW and NatEre = natural streams; AgriFW and AgriEre = rural streams.
2.2. Trichoptera sampling
We collected samples of aquatic insects from October to December 2016 in 12 streams of small order (≤ 2nd order). We collected the aquatic insects using a Surber sampler with an area of 0.09 m2 and a mesh of 250 μm. We collected three sub-samples on the stony substrate and three sub-samples on the leaf banks in each of the streams. We fixed the material collected in the field with 70% alcohol, packed it in plastic containers and transported it to the URI - Erechim Biomonitoring Laboratory. Subsequently, we sorted the samples in order to separate members of the Trichoptera from the other insect groups. We identified Trichoptera larvae up to genus level using Pes et al. (2005)PES, A.M.O., HAMADA, N. and NESSIMIAN, J. L. Chaves de Identificação de larvas para famílias e genêros de Trichoptera (Insecta) da Amazônia Central, Brasil. Revista Brasileira de Entomologia, 2005, 49(2), 181-204. http://dx.doi.org/10.1590/S0085-56262005000200002.
http://dx.doi.org/10.1590/S0085-56262005...
and Mugnai et al. (2010)MUGNAI, R., NESSIMIAN, J.L. and BAPTISTA, D.F. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro. 1. ed. Rio de Janeiro: Technical Books, 2010..
2.3. Limnological variables
Concomitant to insect collections, we measured the limnological variables in the field using a HORIBA® U50 Multiparameter Analyser in each of the streams sampled, as follows: water temperature, electrical conductivity, turbidity and dissolved oxygen. We collected water samples for laboratory analysis of total phosphorus and nitrite from spectrophotometric methods and total dissolved nitrogen (TDN) and dissolved organic carbon (DOC) using a Shimadzu® TOC analyser.
2.4. Data analysis
We evaluated the limnological differences between “natural” and “rural” streams using a t-test. We calculated a matrix of dissimilarity (Jaccard) using the data on presence and absence of the Trichoptera genera in the localities, aiming at evaluating beta diversity. For the environmental data we standardized the matrix according to the maximum value (using the “decostand” function in R) and calculated a matrix of Euclidean distance. We also calculated a matrix of Euclidean distance for geographic coordinate data (without standardization). We performed a linear regression with the distance matrices to evaluate the possible effects of environmental variability and geographic distance on biological dissimilarity. We performed a pMantel test in order to evaluate the relationship between biological variability (beta diversity) and environment variability and geographic distance. We used these matrices to calculate a Redundancy Analysis (RDA) in order to evaluate the effects of spatially structured environmental variable on the composition of the Trichoptera larvae.
Finally, we calculated the contribution of alpha and beta diversity within each stream to regional diversity, using “range” standardization method, wich standardize values into range 0 – 1. We used an additive partition method proposed by Lu et al. (2007)LU, H.P., WAGNER, H.H. and CHEN, X.Y. A contribution diversity approach to evaluate species diversity. Basic and Applied Ecology, 2007, 8(1), 1-12. http://dx.doi.org/10.1016/j.baae.2006.06.004.
http://dx.doi.org/10.1016/j.baae.2006.06...
, which determines the contribution percentage of each diversity scale (alpha and beta) to total diversity variation (gamma), using function ‘contribdiv’. This approach considers the average of unit diversity as alpha, the average of diversity that is not found in a unit as beta and gamma is the average amount of the diversity that each unit contribute do total diversity (γ = α + β). Additive diversity partitioning is based on diversity differentiation inside and between sample sites, allowing to calculate relative alpha and beta contribution at different scales. With this approach, is possible to compare alpha and beta diversities directly, since it treats both as proportional diversities means. We performed a t test to evaluate differences in contribution of alpha and beta diversity between natural and rural streams and a chi-squared test to verify differences in diversity proportions between stream types. We performed the analyses in the R (R Development Core Team, 2015R DEVELOPMENT CORE TEAM. R: a language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing, 2015 2018 [viewed 18 Mar. 2018]. Available from: http:// www.R-project.org) software, using functions of the “vegan” package (Oksanen et al., 2015OKSANEN, J., BLANCHET, F.G., KINDT, R., LEGENDRE, P., O’HARA, R.G., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H. and WAGNER, H. Vegan: Community Ecology Package. Vienna: R Foundation for Statistical Computing, 2015.).
3. Results
The limnological variables were similar among the studied streams (Table 1). The streams were well-oxygenated (> 5.5 mg L-1), with circumneutral pH (5 to 7.2) and values of electrical conductivity lower than 0.23 mS cm-1.
Limnological variables (mean ± SE) quantified in rural (n = 6) and natural (n = 6) streams located in southern Brazil.
We collected 1,264 Trichoptera larvae distributed in 17 genera and 11 families (Table 2). We collected on average 48.5 ± 20.3 (mean ± SE) individuals in natural streams and 162.1 ± 68.4 in rural streams. We observed on average 4.3 ± 0.8 genera of Trichoptera in the natural streams and 5.5 ± 1.2 genera in the rural streams. The genera Phylloicus and Smicridea were found in almost all streams (11 and 10 of the streams, respectively), whereas Austrotinodes, Cyrnellus, Helicopsyche, Hidroptila and Neotrichia were found in only one stream. Of the total genera, two were exclusive to natural streams, five were exclusive to rural streams and 11 genera were shared between both types of streams (Figure 2). The genera Cyrnellus and Helicopsyche were found exclusively in natural streams, whereas the genera Austrotinodes, Hydroptila, Neotrichia, Oxyethira and Triplectides were only found in rural streams.
Trichoptera genera identified in streams located in the North and Northwest of Rio Grande do Sul.
Venn diagram of the Trichoptera genera unique to natural and rural streams and shared between the two stream categories in Southern Brazil.
We observed that environmental variability increased biological variability (F(1, 64) = 13.3, p <0.001, R2 = 0.16; Figure 3A). However, geographical position between pairs of streams, had no effect on the variation of the Trichoptera communities (F(1, 64) = 3.3, p = 0.07, R2 = 0.04; Figure 3B). We observed that the genera of Trichoptera responded differently to some limnological variables. Thus, environmental variability was basically generated by the influence of higher concentrations of dissolved organic carbon on Chimarra and Wormaldia, while higher concentrations of nutrients (e.g. phosphorus, nitrite and total nitrogen) and dissolved oxygen influenced the genera Smicridea and Helicopsyche (Figure 4).
Linear regressions of the environment (A) and spatial (B) variation on the dissimilarity of Trichoptera genera.
Redundancy Analysis (RDA) indicating the relative importance of the environmental and spatial components in structuring the Trichoptera assemblages. Open square: natural streams, closed square: rural streams. DO: dissolved oxygen, TOC: total organic carbon, EC: electrical conductivity, TN: total nitrogen, WT: water temperature.
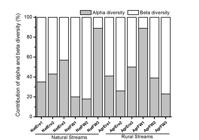
We observed the similar contribution of the Trichoptera assemblages alpha diversity in the natural streams (32.4%) and rural (26.7%) streams (t=0.6; df=10; p=0.35). In addition, we observed the same result to beta diversity (rural = 73.3%, natural = 67.6%; t=1.0; df=10; p=0.33) (Figure 5). However, the proportion of beta diversity contribution was higher than alpha in both stream types (natural: χ2=12.4, p<0.001; rural: χ2=21.7, p<0.001).
Contribution of alpha and beta diversity in the regional diversity for each natural and rural streams in Southern Brazil.
4. Discussion
Agricultural practices require a large amount of land, which may result in the removal of riparian vegetation (Rovani et al., 2019ROVANI, I.L., SANTOS, J.E., DECIAN, V.S. and ZANIN, E.M. Assessing naturalness changes resulting from a historical land use in Brazil South Region: an analysis of the 1986-2016 period. Journal of Environmental Protection, 2019, 10(02), 149-163. http://dx.doi.org/10.4236/jep.2019.102010.
http://dx.doi.org/10.4236/jep.2019.10201...
). Removal of vegetation causes numerous physical and chemical changes in freshwater environments, causing variations in biological communities (Hepp et al., 2010HEPP, L.U., MILESI, S.V., BIASI, C. and RESTELLO, R.M. Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia, 2010, 27(1), 106-113. http://dx.doi.org/10.1590/S1984-46702010000100016.
http://dx.doi.org/10.1590/S1984-46702010...
).
The diversity of Trichoptera observed in this study corresponds to only 2.7% of global diversity (Morse, 2018MORSE, J.C., ed. Trichoptera World Checklist [online]. 2018 [viewed 18 Mar. 2018]. Available from: http://entweb.clemson.edu/database/trichopt/index.htm
http://entweb.clemson.edu/database/trich...
) and 24.3% of Brazilian diversity (Santos et al., 2015SANTOS, A.P.M., DUMAS, L.L., JARDIM, G.A., SILVA, A.L.R. and NESSIMIAN, J.L. Brazilian caddisflies: checklists and bibliography [online]. 2015 [viewed 18 Mar. 2018]. Available from: https://sites.google.com/site/braziliancaddisflies
https://sites.google.com/site/brazilianc...
). Given that Trichoptera is one of the most diverse orders in aquatic environments, our study shows that the region under study is seriously threatened by the degradation of water resources. However, is possible that none region represents great part of Brazilian diversity of Trichoptera, showing a high endemism scenario. Trichoptera is considered an order that comprises demanding insects regarding the ecological integrity of the environment. These organisms have a preference for streams with good oxygenation and a low concentration of nutrients (Brasil et al., 2018BRASIL, L.S., SANTOS, D.C., VIEIRA, T.B., CABETTE, H.S.R., UMETSU, R.K. and GIEHL, N.F.S. Spatiotemporal dynamics in caddisfly (Insecta: Trichoptera) of a Cerrado stream, Brazil. Annales de Limnologie - Internation. Journal of Limnology, 2018, 54, 37-43. http://dx.doi.org/10.1051/limn/2018028.
http://dx.doi.org/10.1051/limn/2018028...
). The intense agricultural activity that takes place in the study region has had a negative effect on the quality of the streams. The main negative activity is the removal of riparian vegetation from small streams. The basins in general have about 80% of their area drained by small streams (<3rd order) (Allan & Castillo, 2007ALLAN, J.D. and CASTILLO, M.M. Stream ecology: structure and function of running waters. Dordrecht: Springer, 2007. http://dx.doi.org/10.1007/978-1-4020-5583-6.
http://dx.doi.org/10.1007/978-1-4020-558...
; Clarke et al., 2008CLARKE, A., MAC NALLY, R., BOND, N. and LAKE, P.S. Macroinvertebrate diversity in headwater streams: a review. Freshwater Biology, 2008, 53(90), 1707-1721. http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x.
http://dx.doi.org/10.1111/j.1365-2427.20...
). Thus, the removal of riparian vegetation is one of the main causes of environmental fragility in streams, reducing the quantity and quality of the water and, consequently, the aquatic diversity.
The genera Phylloicus and Smicridea were widely distributed among the studied streams. The occurrence of these two genera is associated with the availability of food resources in streams. Phylloicus larvae play an important trophic role in aquatic invertebrate assemblages, since they convert the particulate organic matter to fine particulate organic matter, serving as a food resource for other invertebrates, as well as contributing to leaf decomposition in streams (Graça et al., 2016GRAÇA, M.A.S., HYDE, K. and CHAUVET, E. Aquatic hyphomycetes and litter decomposition in tropical–subtropical low order streams. Fungal Ecology, 2016, 19, 182-189. http://dx.doi.org/10.1016/j.funeco.2015.08.001.
http://dx.doi.org/10.1016/j.funeco.2015....
). Smicridea has an alimentary filtering habit, collecting its food in the water column (Burneo, 2014BURNEO, A.E. Trophic variability of stream macroinvertebrates along an altitudinal gradient and among size groups in the Oyacachi River Basin [Bachelor's Thesis]. Quito: Universidad San Francisco de Quito, 2014.). The Philopotamidae family (genera Chimarra and Wormaldia) was associated with streams with higher concentrations of DOC, while the genera Smicridea and Helicopsyche were associated with higher concentrations of nutrients and oxygen. As verified in our study, Connolly et al. (2016)CONNOLLY, N.M., PEARSON, R.G. and PEARSON, B.A. Riparian vegetation and sediment gradients determine invertebrate diversity in streams draining an agricultural landscape. Agriculture, Ecosystems & Environment, 2016, 221, 163-173. http://dx.doi.org/10.1016/j.agee.2016.01.043.
http://dx.doi.org/10.1016/j.agee.2016.01...
found a relation between high primary productivity in the creeks and the distribution of larvae of the families Hydropsychidae and Philopotamidae, resulting from more open canopy and agricultural nutritional supplements.
The dissimilarity of Trichoptera assemblies was more sensitive to environmental than geographical position. Trichoptera assemblages usually are independent of environment variation, being structured mainly by space (Curry & Baird, 2015CURRY, C.J. and BAIRD, D.J. Habitat type and dispersal ability influence spatial structuring of larval Odonata and Trichoptera assemblages. Freshwater Biology, 2015, 60(10), 2142-2155. http://dx.doi.org/10.1111/fwb.12640.
http://dx.doi.org/10.1111/fwb.12640...
). The relationship observed with environment variability structured by space means that environmental variabilibility is important to Trichoptera beta diversity, but there is a slight spatial influence. This relationship can be due to ladscape structure of both regions, wich are part of a transition zone of Atlantic Forest, that posses diferent fitogeographic elements that can contribute differently in the input of allochtonous materials to the streams (specially the natural ones). The geographical position of the streams had no effect on the variability of the assemblies. Members of the order Trichoptera are known for their low dispersal ability (Hepp & Melo, 2013HEPP, L.U. and MELO, A.S. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia, 2013, 703(1), 239-246. http://dx.doi.org/10.1007/s10750-012-1367-7.
http://dx.doi.org/10.1007/s10750-012-136...
; Curry & Baird, 2015CURRY, C.J. and BAIRD, D.J. Habitat type and dispersal ability influence spatial structuring of larval Odonata and Trichoptera assemblages. Freshwater Biology, 2015, 60(10), 2142-2155. http://dx.doi.org/10.1111/fwb.12640.
http://dx.doi.org/10.1111/fwb.12640...
). Therefore, the distance between the streams could cause biological variability in the composition of the genera. We didn’t observe a clear effect of geographical position in beta diversity. Considering that the sample sites are characterized by a vegetative transition zone of Atlantic Forest and Pampa biomes, we believed that this is not a relevant factor to generate variation in beta diversity observed in both regions (Oliveira-Filho et al., 2015OLIVEIRA-FILHO, A.T., BUDKE, J.C., JARENKOW, J.A., EISENLOHR, P.V. and NEVES, D.R. Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. Journal of Plant Ecology, 2015, 8(3), 242-260. http://dx.doi.org/10.1093/jpe/rtt058.
http://dx.doi.org/10.1093/jpe/rtt058...
). Environmental variability between streams was related to increase on Trichoptera assemblage variability. This variability is certainly generated by the heterogeneity in the landscape observed in the region, in which a high percentage of area is planted with agricultural crops, making the landscape intensely fragmented.
The similar contribution in terms of alpha diversity in natural and rural streams (32.4% and 26.7%, respectively) demonstrated that local factors are important for the establishment of species. The heterogeneity of substrates observed in natural streams is fundamental for the constant establishment of new species and an increase in the alpha diversity of these sites (Hepp et al., 2012HEPP, L.U., LANDEIRO, V.L. and MELO, A.S. Experimental assessment of the effects of environmental factors and longitudinal position on alpha and beta diversities of aquatic insects in a Neotropical stream. International Review of Hydrobiology, 2012, 97(2), 157-167. http://dx.doi.org/10.1002/iroh.201111405.
http://dx.doi.org/10.1002/iroh.201111405...
; Ferreira et al., 2017FERREIRA, W.R., HEPP, L.U., LIGEIRO, R., MACEDO, D.R., HUGHES, R.M., KAUFMANN, P.R. and CALLISTO, M. Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecological Indicators, 2017, 72, 365-373. http://dx.doi.org/10.1016/j.ecolind.2016.08.042.
http://dx.doi.org/10.1016/j.ecolind.2016...
). Although the variability of substrata in the streams was not evaluated, it is known that natural environments with riparian vegetation on the banks naturally present a wide variety of habitats (Allan & Castillo, 2007ALLAN, J.D. and CASTILLO, M.M. Stream ecology: structure and function of running waters. Dordrecht: Springer, 2007. http://dx.doi.org/10.1007/978-1-4020-5583-6.
http://dx.doi.org/10.1007/978-1-4020-558...
). Substrate heterogeneity is an important factor in the partitioning of biological diversity into alpha and beta components (Hepp et al., 2012HEPP, L.U., LANDEIRO, V.L. and MELO, A.S. Experimental assessment of the effects of environmental factors and longitudinal position on alpha and beta diversities of aquatic insects in a Neotropical stream. International Review of Hydrobiology, 2012, 97(2), 157-167. http://dx.doi.org/10.1002/iroh.201111405.
http://dx.doi.org/10.1002/iroh.201111405...
; Milesi et al., 2016MILESI, S.V., DOLÉDEC, S. and MELO, A.S. Substrate heterogeneity influences the trait composition of stream insect communities: an experimental in situ study. Freshwater Science, 2016, 35(4), 1321-1329. http://dx.doi.org/10.1086/688706.
http://dx.doi.org/10.1086/688706...
). Furthermore, the way that Trichoptera adults lay eggs depends on environmental variability, since eggs are fixed on roots, leaves or submerged rocks and these kinds of substrates are available in abundance in natural streams, reinforce the greater contribution of alpha diversity in these streams (Pes et al., 2014PES, A.M.O., SANTOS, A.P.M., BARCELOS-SILVA, P. and CAMARGOS, L.M. Ordem Trichoptera. In: N. HAMADA, J.L. NESSIMIAN and R.B. QUERINO, eds. Insetos aquáticos na Amazônia brasileira: taxonomia, biologia e ecologia. Manaus: Embrapa Meio-Norte-Livros Científicos, 2014.; Lancaster & Glaister, 2018LANCASTER, J. and GLAISTER, A. Egg masses of some stream‐dwelling caddisflies (Trichoptera: Hydrobiosidae) from Victoria, Australia. Austral Entomology, 2018, 58(3), 561-568. http://dx.doi.org/10.1111/aen.12360.
http://dx.doi.org/10.1111/aen.12360...
).
The beta diversity represents the variation in the composition of the biological communities between different localities (Anderson et al., 2011ANDERSON, M.J., CRIST, T.O., CHASE, J.M., VELLEND, M., INOUYE, B.D., FREESTONE, A.L., SANDERS, N.J., CORNELL, H.V., COMITA, L.S., DAVIES, K.F., HARRISON, S.P., KRAFT, N.J.B., STEGEN, J.C. and SWENSON, N.G. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters, 2011, 14(1), 19-28. http://dx.doi.org/10.1111/j.1461-0248.2010.01552.x. PMid:21070562.
http://dx.doi.org/10.1111/j.1461-0248.20...
). Generally, variability in species composition can occur by environmental or spatial effects (Hepp & Melo, 2013HEPP, L.U. and MELO, A.S. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia, 2013, 703(1), 239-246. http://dx.doi.org/10.1007/s10750-012-1367-7.
http://dx.doi.org/10.1007/s10750-012-136...
). In this study, environmental variation was the main factor that caused dissimilarity in the Trichoptera assemblies, especially in the rural streams (73.3%). The ecological instability of streams located in rural landscapes may have caused homogeneity in the substrate, in addition to the light increasing nutrient concentrations and reducing dissolved oxygen concentrations. These characteristics affect the occurrence of certain genera of Trichoptera, leading to changes in the composition of the assemblages of these organisms. Ongaratto et al. (2018)ONGARATTO, R., LOUREIRO, R.C., RESTELLO, R.M. and HEPP, L.U. Effects of land use and limnological variables on the dissimilarity of common and rare aquatic insects in Atlantic Forest streams. Revista de Biología Tropical, 2018, 66(3), 1223-1231. http://dx.doi.org/10.15517/rbt.v66i3.30825.
http://dx.doi.org/10.15517/rbt.v66i3.308...
reported that electrical conductivity, pH and the presence of riparian vegetation were significantly correlated with the dissimilarity of insects of the orders Ephemeroptera, Plecoptera and Trichoptera.
In general, the contribution of both types of streams (natural and rural) was greater in terms of beta diversity. This is explained by the effect of environmental variability observed in all streams. While natural streams meet more appropriate conditions for the occurrence of rare species, rural streams meet adverse conditions that may result in changes in species composition. In conclusion, our results demonstrate that variability in Trichoptera assemblages was generated by the drastic changes in environmental characteristics that occurred among the studied streams. We observed differences between the contribution of beta diversity in streams, which was greater than stream alpha diversity. These results will help in determining appropriate measures for the conservation and restoration of aquatic ecosystems, such as the reestablishment and maintenance of riparian zones in different land use zones, and the increase of resources and sources of energy for streams.
Acknowledgements
We thank the PROSUP/CAPES for granting a scholarship to MB. We thank Irineu Jasan Dysarz for the help in the laboratory work. We thanks Fundação de Amparo à Pesquisa do Rio Grande do Sul for financial support (Edital Pró-Equipamentos 18/25510000374-3). RMR received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (409685/2016-0). LUH received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (421632/2016-0) and research productivity grants (305203/2017-7).
-
Cite as: Breda, M. et al. Alpha and beta diversities of Trichoptera (Insecta) assemblages in natural and rural subtropical streams. Acta Limnologica Brasiliensia, 2020, vol. 32, e14.
References
- ALLAN, J.D. and CASTILLO, M.M. Stream ecology: structure and function of running waters Dordrecht: Springer, 2007. http://dx.doi.org/10.1007/978-1-4020-5583-6
» http://dx.doi.org/10.1007/978-1-4020-5583-6 - ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., MORAES, G., LEONARDO, J. and SPAROVEK, G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 2013, 22(6), 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507
» http://dx.doi.org/10.1127/0941-2948/2013/0507 - ANDERSON, M.J., CRIST, T.O., CHASE, J.M., VELLEND, M., INOUYE, B.D., FREESTONE, A.L., SANDERS, N.J., CORNELL, H.V., COMITA, L.S., DAVIES, K.F., HARRISON, S.P., KRAFT, N.J.B., STEGEN, J.C. and SWENSON, N.G. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters, 2011, 14(1), 19-28. http://dx.doi.org/10.1111/j.1461-0248.2010.01552.x PMid:21070562.
» http://dx.doi.org/10.1111/j.1461-0248.2010.01552.x - BLEVINS, Z.W., EFFERT, E.L., WAHL, D.H. and SUSKI, C.D. Land use drives the physiological properties of a stream fish. Ecological Indicators, 2013, 24, 224-235. http://dx.doi.org/10.1016/j.ecolind.2012.06.016
» http://dx.doi.org/10.1016/j.ecolind.2012.06.016 - BRASIL, L.S., SANTOS, D.C., VIEIRA, T.B., CABETTE, H.S.R., UMETSU, R.K. and GIEHL, N.F.S. Spatiotemporal dynamics in caddisfly (Insecta: Trichoptera) of a Cerrado stream, Brazil. Annales de Limnologie - Internation. Journal of Limnology, 2018, 54, 37-43. http://dx.doi.org/10.1051/limn/2018028
» http://dx.doi.org/10.1051/limn/2018028 - BREDA, M., LAZARI, P.L., OLIVEIRA, M.B., MENEGAT, M.N., BERTOL, E.C., SILVA, G.S., DECIAN, V.S., RESTELLO, R.M. and HEPP, L.H. Composição e distribuição de Trichoptera (Insecta) em riachos subtropicais. Perspectiva, 2018, 42(157), 17-26.
- BURNEO, A.E. Trophic variability of stream macroinvertebrates along an altitudinal gradient and among size groups in the Oyacachi River Basin [Bachelor's Thesis]. Quito: Universidad San Francisco de Quito, 2014.
- CLARKE, A., MAC NALLY, R., BOND, N. and LAKE, P.S. Macroinvertebrate diversity in headwater streams: a review. Freshwater Biology, 2008, 53(90), 1707-1721. http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x
» http://dx.doi.org/10.1111/j.1365-2427.2008.02041.x - CONNOLLY, N.M., PEARSON, R.G. and PEARSON, B.A. Riparian vegetation and sediment gradients determine invertebrate diversity in streams draining an agricultural landscape. Agriculture, Ecosystems & Environment, 2016, 221, 163-173. http://dx.doi.org/10.1016/j.agee.2016.01.043
» http://dx.doi.org/10.1016/j.agee.2016.01.043 - COSTA, S.S. and MELO, A.S. Beta diversity in stream macroinvertebrate assemblages: among-site and among-microhabitat components. Hydrobiologia, 2008, 598(1), 131-138. http://dx.doi.org/10.1007/s10750-007-9145-7
» http://dx.doi.org/10.1007/s10750-007-9145-7 - CRISCI-BISPO, V.L., BISPO, P.C. and FROEHLICH, C.G. Ephemeroptera, Plecoptera and Trichoptera assemblages in two Atlantic Rainforest streams, Southeastern Brazil. Revista Brasileira de Zoologia, 2007, 24(2), 312-318. http://dx.doi.org/10.1590/S0101-81752007000200007
» http://dx.doi.org/10.1590/S0101-81752007000200007 - CURRY, C.J. and BAIRD, D.J. Habitat type and dispersal ability influence spatial structuring of larval Odonata and Trichoptera assemblages. Freshwater Biology, 2015, 60(10), 2142-2155. http://dx.doi.org/10.1111/fwb.12640
» http://dx.doi.org/10.1111/fwb.12640 - EFFERT-FANTA, E.L., FISCHER, R.U. and WAHL, D.H. Effects of riparian forest buffers and agricultural land use on macroinvertebrate and fish community structure. Hydrobiologia, 2019, 841(1), 45-64. http://dx.doi.org/10.1007/s10750-019-04006-1
» http://dx.doi.org/10.1007/s10750-019-04006-1 - FERREIRA, W.R., HEPP, L.U., LIGEIRO, R., MACEDO, D.R., HUGHES, R.M., KAUFMANN, P.R. and CALLISTO, M. Partitioning taxonomic diversity of aquatic insect assemblages and functional feeding groups in neotropical savanna headwater streams. Ecological Indicators, 2017, 72, 365-373. http://dx.doi.org/10.1016/j.ecolind.2016.08.042
» http://dx.doi.org/10.1016/j.ecolind.2016.08.042 - FUGÈRE, V., KASANGAKI, A. and CHAPMAN, L.J. Land use changes in an afrotropical biodiversity hotspot affect stream alpha and beta diversity. Ecosphere, 2016, 7(6), 1-18. http://dx.doi.org/10.1002/ecs2.1355
» http://dx.doi.org/10.1002/ecs2.1355 - GONÇALVES JÚNIOR, J.F., REZENDE, R.S., GREGÓRIO, R.S. and VALENTIN, G.C. Relationship between dynamics of litterfall and riparian plant species in a tropical stream. Limnologica-Ecology and Management of Inland Waters, 2014, 44, 40-48. http://dx.doi.org/10.1016/j.limno.2013.05.010
» http://dx.doi.org/10.1016/j.limno.2013.05.010 - GRAÇA, M.A.S., HYDE, K. and CHAUVET, E. Aquatic hyphomycetes and litter decomposition in tropical–subtropical low order streams. Fungal Ecology, 2016, 19, 182-189. http://dx.doi.org/10.1016/j.funeco.2015.08.001
» http://dx.doi.org/10.1016/j.funeco.2015.08.001 - HAWKINS, C.P., MYKRÄ, H., OKSANEN, J. and VANDER LAAN, J.J. Environmental disturbance can increase beta diversity of stream macroinvertebrate assemblages. Global Ecology and Biogeography, 2015, 24(4), 483-494. http://dx.doi.org/10.1111/geb.12254
» http://dx.doi.org/10.1111/geb.12254 - HEINO, J., MELO, A.S., BINI, L.M., ALTERMATT, F., AL-SHAMI, S.A., ANGELER, D.G., BONADA, N., BRAND, C., CALLISTO, M., COTTENIE, K., DANGLES, O., DUDGEON, D., ENCALADA, A., GÖTHE, E., GRÖNROOS, M., HAMADA, N., JACOBSEN, D., LANDEIRO, V.L., LIGEIRO, R., MARTINS, R.T., MISERENDINO, M.L., MD RAWI, C.S., RODRIGUES, M.E., ROQUE, F.O., SANDIN, L., SCHMERA, D., SGARBI, L.F., SIMAIKA, J.P., SIQUEIRA, T., THOMPSON, R.M. and TOWNSEND, C.R. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution, 2015, 5(6), 1235-1248. http://dx.doi.org/10.1002/ece3.1439 PMid:25859329.
» http://dx.doi.org/10.1002/ece3.1439 - HEPP, L.U. and MELO, A.S. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia, 2013, 703(1), 239-246. http://dx.doi.org/10.1007/s10750-012-1367-7
» http://dx.doi.org/10.1007/s10750-012-1367-7 - HEPP, L.U. and SANTOS, S. Benthic communities of streams related to different land uses in a hydrographic basin in southern Brazil. Environmental Monitoring and Assessment, 2009, 157(1-4), 305-318. http://dx.doi.org/10.1007/s10661-008-0536-7 PMid:18843547.
» http://dx.doi.org/10.1007/s10661-008-0536-7 - HEPP, L.U., LANDEIRO, V.L. and MELO, A.S. Experimental assessment of the effects of environmental factors and longitudinal position on alpha and beta diversities of aquatic insects in a Neotropical stream. International Review of Hydrobiology, 2012, 97(2), 157-167. http://dx.doi.org/10.1002/iroh.201111405
» http://dx.doi.org/10.1002/iroh.201111405 - HEPP, L.U., MILESI, S.V., BIASI, C. and RESTELLO, R.M. Effects of agricultural and urban impacts on macroinvertebrates assemblages in streams (Rio Grande do Sul, Brazil). Zoologia, 2010, 27(1), 106-113. http://dx.doi.org/10.1590/S1984-46702010000100016
» http://dx.doi.org/10.1590/S1984-46702010000100016 - LANCASTER, J. and GLAISTER, A. Egg masses of some stream‐dwelling caddisflies (Trichoptera: Hydrobiosidae) from Victoria, Australia. Austral Entomology, 2018, 58(3), 561-568. http://dx.doi.org/10.1111/aen.12360
» http://dx.doi.org/10.1111/aen.12360 - LU, H.P., WAGNER, H.H. and CHEN, X.Y. A contribution diversity approach to evaluate species diversity. Basic and Applied Ecology, 2007, 8(1), 1-12. http://dx.doi.org/10.1016/j.baae.2006.06.004
» http://dx.doi.org/10.1016/j.baae.2006.06.004 - MAGURRAN, A.E. Measuring biological diversity New York: John Wiley & Sons, 2013.
- MILESI, S.V., DOLÉDEC, S. and MELO, A.S. Substrate heterogeneity influences the trait composition of stream insect communities: an experimental in situ study. Freshwater Science, 2016, 35(4), 1321-1329. http://dx.doi.org/10.1086/688706
» http://dx.doi.org/10.1086/688706 - MORSE, J.C., ed. Trichoptera World Checklist [online]. 2018 [viewed 18 Mar. 2018]. Available from: http://entweb.clemson.edu/database/trichopt/index.htm
» http://entweb.clemson.edu/database/trichopt/index.htm - MUGNAI, R., NESSIMIAN, J.L. and BAPTISTA, D.F. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro 1. ed. Rio de Janeiro: Technical Books, 2010.
- NAIMAN, R.J., DÉCAMPS, H. and MCCLAIN, M.E. Riparia: ecology, conservation, and management of streamside communities Cambridge: Academic Press, 2010.
- OKSANEN, J., BLANCHET, F.G., KINDT, R., LEGENDRE, P., O’HARA, R.G., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H. and WAGNER, H. Vegan: Community Ecology Package Vienna: R Foundation for Statistical Computing, 2015.
- OLIVEIRA-FILHO, A.T., BUDKE, J.C., JARENKOW, J.A., EISENLOHR, P.V. and NEVES, D.R. Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. Journal of Plant Ecology, 2015, 8(3), 242-260. http://dx.doi.org/10.1093/jpe/rtt058
» http://dx.doi.org/10.1093/jpe/rtt058 - ONGARATTO, R., LOUREIRO, R.C., RESTELLO, R.M. and HEPP, L.U. Effects of land use and limnological variables on the dissimilarity of common and rare aquatic insects in Atlantic Forest streams. Revista de Biología Tropical, 2018, 66(3), 1223-1231. http://dx.doi.org/10.15517/rbt.v66i3.30825
» http://dx.doi.org/10.15517/rbt.v66i3.30825 - PES, A.M.O., HAMADA, N. and NESSIMIAN, J. L. Chaves de Identificação de larvas para famílias e genêros de Trichoptera (Insecta) da Amazônia Central, Brasil. Revista Brasileira de Entomologia, 2005, 49(2), 181-204. http://dx.doi.org/10.1590/S0085-56262005000200002
» http://dx.doi.org/10.1590/S0085-56262005000200002 - PES, A.M.O., SANTOS, A.P.M., BARCELOS-SILVA, P. and CAMARGOS, L.M. Ordem Trichoptera. In: N. HAMADA, J.L. NESSIMIAN and R.B. QUERINO, eds. Insetos aquáticos na Amazônia brasileira: taxonomia, biologia e ecologia Manaus: Embrapa Meio-Norte-Livros Científicos, 2014.
- PITACCO, V., MISTRI, M., ALEFFI, I.F., LARDICCI, C., PRATO, S., TAGLIAPIETRA, D. and MUNARI, C. Spatial patterns of macrobenthic alpha and beta diversity at different scales in Italian transitional waters (central Mediterranean). Estuarine, Coastal and Shelf Science, 2019, 222, 126-138. http://dx.doi.org/10.1016/j.ecss.2019.04.026
» http://dx.doi.org/10.1016/j.ecss.2019.04.026 - R DEVELOPMENT CORE TEAM. R: a language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing, 2015 2018 [viewed 18 Mar. 2018]. Available from: http:// www.R-project.org
- ROA-FUENTES, C.A., HEINO, J., CIANCIARUSO, M.V., FERRAZ, S., ZENI, J.O. and CASATTI, L. Taxonomic, functional, and phylogenetic β‐diversity patterns of stream fish assemblages in tropical agroecosystems. Freshwater Biology, 2019, 64(3), 447-460. http://dx.doi.org/10.1111/fwb.13233
» http://dx.doi.org/10.1111/fwb.13233 - ROSENBERG, D.M. and RESH, V.H. Freshwater biomonitoring and benthic macroinvertebrates New York: Chapman & Hall, 1993.
- ROVANI, I.L., SANTOS, J.E., DECIAN, V.S. and ZANIN, E.M. Assessing naturalness changes resulting from a historical land use in Brazil South Region: an analysis of the 1986-2016 period. Journal of Environmental Protection, 2019, 10(02), 149-163. http://dx.doi.org/10.4236/jep.2019.102010
» http://dx.doi.org/10.4236/jep.2019.102010 - SANTOS, A.P.M., DUMAS, L.L., JARDIM, G.A., SILVA, A.L.R. and NESSIMIAN, J.L. Brazilian caddisflies: checklists and bibliography [online]. 2015 [viewed 18 Mar. 2018]. Available from: https://sites.google.com/site/braziliancaddisflies
» https://sites.google.com/site/braziliancaddisflies - SCHNECK, F. and HEPP, L.U. Fatores estruturadores de comunidades em riachos. Ciência e Ambiente, 2010, 41, 57-67.
- WHITTAKER, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 1960, 30(3), 279-338. http://dx.doi.org/10.2307/1943563
» http://dx.doi.org/10.2307/1943563
Edited by
Publication Dates
-
Publication in this collection
01 July 2020 -
Date of issue
2020
History
-
Received
21 Mar 2019 -
Accepted
21 May 2020