Abstracts
Generation means was used to study the mode of inheritance of resistance to anthracnose stalk rot in tropical maize. Each population was comprised of six generations in two trials under a randomized block design. Inoculations were performed using a suspension of 10(5) conidia mL-1 applied into the stalk. Internal lesion length was directly measured by opening the stalk thirty days after inoculation. Results indicated contrasting modes of inheritance. In one population, dominant gene effects predominated. Besides, additive x dominant and additive x additive interactions were also found. Intermediate values of heritability indicated a complex resistance inheritance probably conditioned by several genes of small effects. An additive-dominant genetic model sufficed to explain the variation in the second population, where additive gene effects predominated. Few genes of major effects control disease resistance in this cross. Heterosis widely differed between populations, which can be attributed to the genetic background of the parental resistant lines.
generation means analysis; disease resistance; Zea mays; anthracnose; heterosis
Análise de médias foi usada para estudar o modo de herança da resistência a antracnose do colmo em milho tropical. Cada população foi composta de seis gerações que foram avaliadas para resistência em dois ensaios no delineamento de blocos ao acaso. As inoculações foram realizadas usando uma suspensão de 10(5) conídios mL-1 aplicados dentro do colmo. O comprimento interno de lesão foi medido diretamente após abertura do colmo 30 dias após a inoculação. Os resultados indicaram modos contrastantes de herança. Em uma população, os efeitos gênicos dominantes predominaram. Embora as interações, aditivo x dominante e aditivo x aditivo, também foram encontradas. Valores intermediários de herdabilidade indicaram herança da resistência complexa, provavelmente condicionada por vários genes de pequeno efeito. O modelo genético aditivo-dominante foi suficiente para explicar a variação na segunda população, onde os efeitos aditivos predominaram. Poucos genes de grande efeito controlam a resistência neste cruzamento. Heterose diferiu amplamente entre as populações o que pode ser explicado pelo background genético das linhagens genitoras resistentes.
análise de média de gerações; resistência a doenças; Zea mays; antracnose e heterose
ARTICLE
Inheritance of resistance to anthracnose stalk rot (Colletotrichum graminicola) in tropical maize inbred lines
Herança da resistência a antracnose do colmo (Colletotrichum graminicola) em linhagens endogâmicas de milho tropical
Rodrigo Rodrigues MatielloI,* * E-mail: rrmatiel@uepg.br ; Kátia Regiane BrunelliII; Maria Teresa Gomes LopesIII; Regina Mélo Sartori Coêlho MorelloIV; Herberte Pereira da SilvaIV; Luis Eduardo Aranha CamargoIV
IUniversidade Estadual de Ponta Grossa (UEPG), Departamento de Fitotecnia e Fitossanidade, 84.030-900, Ponta Grossa, PR, Brazil
IISakata Seed Sudamerica, Estrada da Bocaina, s/n, 12.906-840, Bragança Paulista, SP, Brazil
IIIUniversidade Federal do Amazonas (UFAM), Departamento de Produção Animal e Vegetal, 69.077-000, Manaus, AM, Brazil
IVUniversidade de São Paulo (USP/ESALQ), Departamento de Fitopatologia e Nematologia, 13.418-900, Piracicaba, SP, Brazil
ABSTRACT
Generation means was used to study the mode of inheritance of resistance to anthracnose stalk rot in tropical maize. Each population was comprised of six generations in two trials under a randomized block design. Inoculations were performed using a suspension of 105 conidia mL-1 applied into the stalk. Internal lesion length was directly measured by opening the stalk thirty days after inoculation. Results indicated contrasting modes of inheritance. In one population, dominant gene effects predominated. Besides, additive x dominant and additive x additive interactions were also found. Intermediate values of heritability indicated a complex resistance inheritance probably conditioned by several genes of small effects. An additive-dominant genetic model sufficed to explain the variation in the second population, where additive gene effects predominated. Few genes of major effects control disease resistance in this cross. Heterosis widely differed between populations, which can be attributed to the genetic background of the parental resistant lines.
Key words: generation means analysis, disease resistance, Zea mays, anthracnose, heterosis.
RESUMO
Análise de médias foi usada para estudar o modo de herança da resistência a antracnose do colmo em milho tropical. Cada população foi composta de seis gerações que foram avaliadas para resistência em dois ensaios no delineamento de blocos ao acaso. As inoculações foram realizadas usando uma suspensão de 105 conídios mL-1 aplicados dentro do colmo. O comprimento interno de lesão foi medido diretamente após abertura do colmo 30 dias após a inoculação. Os resultados indicaram modos contrastantes de herança. Em uma população, os efeitos gênicos dominantes predominaram. Embora as interações, aditivo x dominante e aditivo x aditivo, também foram encontradas. Valores intermediários de herdabilidade indicaram herança da resistência complexa, provavelmente condicionada por vários genes de pequeno efeito. O modelo genético aditivo-dominante foi suficiente para explicar a variação na segunda população, onde os efeitos aditivos predominaram. Poucos genes de grande efeito controlam a resistência neste cruzamento. Heterose diferiu amplamente entre as populações o que pode ser explicado pelo background genético das linhagens genitoras resistentes.
Palavras-chave: análise de média de gerações, resistência a doenças, Zea mays, antracnose e heterose.
INTRODUCTION
Several fungi cause stalk rot in maize, a disease of global importance for its negative effects on grain yield, stalk breakage and premature death of plants (Jarvis et al. 1984). In the U.S., the causal agents of stalk rot species include Gibberella zeae (Schwein) Petch, Colletotrichum graminicola (Ces.) Wils, Stenocarpella maydis (Berk.) Sutton and species of the genus Fusarium (F. verticillioides, F. proliferatum and F. subglutinans) (Gath and Munkvold 2002). Of these pathogens, Colletotrichum graminicola causes significant annual yield losses estimated at greater than one billion U.S. dollars in the Americas alone (Oestreich 2005). In Brazil, the highest frequency of stalk rot concerns C. graminicola, Stenocarpella maydis, S. macrospora, Fusarium graminearum and F. moniliforme (Pereira and Pereira 1976, Balmer and Pereira 1987, Reis and Casa 1996, Denti and Reis 2003, Costa et al. 2010).
During the past several years, an expansion of area sown with maize (Zea mays L.) over western Brazil and the widespread adoption of the no-tillage cropping system and of a second late cropping have caused a progressive increase in the severity of diseases that once were considered of secondary importance (Lim and White 1978, Coêlho et al. 2001, Denti and Reis 2001, Denti and Reis 2003). Anthracnose stalk rot caused by Colletotrichum graminicola (Ces.) Wil. is an example of such disease.
Symptoms of the disease generally appear on the rind as narrow and longitudinal lesions, initially with a brown-redish color that becomes dark brown and black with fruiting structures of the fungus (Dale 1963, Shurtleff 1980). Symptoms also include discoloration of pith and stalk breakage (Miles et al. 1980, Bergstrom and Nicholson 1999, Muimba-Kankolongo and Bergstrom 2011). Usually, these symptoms start immediately after flowering. The fungus also infects leaves, but there are indications that different genes control leaf and stalk anthracnose (Lim and White 1978, Zuber et al. 1981, Coêlho et al. 2001, Rezende et al. 2004). Studies concerning the mode of inheritance of genetic resistance to stalk rot indicated that it is conditioned by few loci mainly of additive genetic effects (Lim and White 1978, Weldekidan and Hawk 1993). Estimates of the number of genes also indicate oligogenic control with additive and dominant genetic effects (Pereira et al. 1989, Toman and White 1993). Callaway et al. (1990) found significant heterosis and general and specific combining abilities, indicating that either additive or dominant effects contribute to resistance. While most studies involving this pathosystem indicated a quantitative mode of inheritance of resistance, Badu-Apraku et al. (1987) reported a single dominant gene in a cross between tropical and temperate lines.
Given that genetic resistance most efficiently controls the disease, knowledge about the mode of resistance inheritance allows the breeder to define the best selection method, thus increasing the efficiency of selection and maximizing the genetic gains during this procedure. This work reports the mode of resistance to anthracnose stalk rot inheritance in generations of crosses between tropical maize inbred lines.
MATERIAL AND METHODS
Three inbred lines (Das21, Das64, and Das86) were used to develop two populations from the crosses between Das86 x Das21 and Das86 x Das64. Das21 is resistant to anthracnose stalk rot. It is derived from a synthetic population of tropical lines with a prevalence of Suwan DMR germplasm. Suwan DMR was developed in Thailand through selection of tropical genotypes containing Caribbean flint and Tuxpeño dent germplasm (Lanza et al. 1999, Coêlho et al. 2001, Rezende et al. 2004). Das64 and Das86 were derived from a synthetic population (narrow genetic base) formed by lines of Amarillo dent and Caribbean flint populations. Both lines have semi-hard orange grains. Das64 is short and resistant to anthracnose stalk rot while Das86 is intermediate in height and highly susceptible (Coêlho et al. 2001).
Field trials for resistance to stalk rot evaluation were comprised of parental (P1 and P2), F1, F2 and the two backcrosses (BC1 and BC2) generations of plants.
The pathogen was isolated from pieces of maize stalk collected in Iraí de Minas (Minas Gerais, Brazil). The stalk fragments were surface-disinfected (30 seconds in 70 % alcohol, 2 min in 1 % sodium hypochlorite, and 1 min in distilled water) and transferred to Pirexâ Petri dishes (9 cm diameter) containing oatmeal agar medium (40 g oatmeal, 17 g agar, and 1 L distilled water). Plates were kept inside a growth chamber at 22 ± 2 0C under fluorescent light (12-hr light and 12-hr dark) until the fungus sporulated (approximately 14 days).
Inoculum was prepared by adding 20 mL of distilled water to the Petri dishes and scrapping the colonies in order to dislodge the conidia. The suspension was then filtered through double layers of cheesecloth. Before inoculation, the concentration of the spore suspension was adjusted to 1.8 x 105 conidia mL-1.
Two trials were conducted in November (conventional planting season) and December (late planting season) 2001, in Indianópolis (Minas Gerais, Brazil) using a randomized complete block design. The trials were laid out in split plot design with three replications. Populations were assigned to main plots, and generations to subplots. The parental inbreds and the F1 generations were sown in single row plots; F2 generations, in four row plots, and the backcrosses (BC1 and BC2), in two row plots. Rows in each plot were 5m long, spaced 0.9cm apart, and contained approximately 20 plants.
Plants were inoculated at the flowering stage by injecting 1 mL of a 1.8 x 105 conidia mL-1 suspension into the first visible internode from above ground using a 50 mL veterinary syringe. Symptoms were evaluated 30 days after inoculation in all plants of the plots by removing leaves and ears and cutting the plants prior to longitudinally split them with an electric saw. Then, internal lesion length was measured (cm).
Analysis of variance (ANOVA) was performed for each trial as well as for the combined data using SAS (SAS Institute 2000) in order to detect differences between and within populations. Genetic effects were estimated through generation means analysis according to Mather and Jinks (1971). Initially, the genetic parameters were estimated by using a reduced genetic model comprised of additive and dominant effects as well as deviations from the model. In the presence of significant deviations, a more complete model was used, where additive x additive and additive x dominant epistatic interactions were included. Analysis of variance of the genetic parameters was performed according to Miranda Filho (1991).
Heterosis (H%) was calculated by the formula H% = (F1 - MP) x 100, where MP represents the mean trait value between the parental lines. Broad sense heritability (h2) was calculated by the formula: h2 , where
, where  is the phenotypic variance among individuals from the F2 generation and
is the phenotypic variance among individuals from the F2 generation and  is the environmental variance estimated by grouping the sum of squares and degrees of freedom from the parental lines and from the F1 generation (Hallauer and Miranda Filho 1988).
is the environmental variance estimated by grouping the sum of squares and degrees of freedom from the parental lines and from the F1 generation (Hallauer and Miranda Filho 1988).
RESULTS AND DISCUSSION
The experimental conditions in both trials were appropriate for the development of the disease, as indicated by the high values of internal lesion length observed in the susceptible hybrid Das86 x Das21. For evaluating the severity of this disease, grading scales related either to the number of discolored internodes (Badu-Apraku et al. 1987, Weldekidan and Hawk 1993, Frey et al. 2011, Muimba-Kankolongo and Bergstrom 2011) or to the internal lesioned area of the stalk (Pereira et al. 1989) are normally used. In this work, however, internal lesion length was measured instead because it is a direct and non-subjective method of assessing disease severity. This method was effective in distinguishing resistant from susceptible individuals within generations and the populations.
The combined ANOVA of the two trials indicated significant interaction between trials and generations within populations. Therefore, it was opted to present and discuss the results based on the individual ANOVA of each trial. Significant differences between and within populations for mean of internal lesion length of C. graminicola were found in both trials (Table 1) likewise Pereira et al. (1989) and Rezende et al. (2004). The coefficients of variation of two trials were small magnitude and suitable for trials involving interaction pathogen-host in agreement with Zuber et al. (1981), Paterniani et al. (2000) and Rezende et al. (2004).
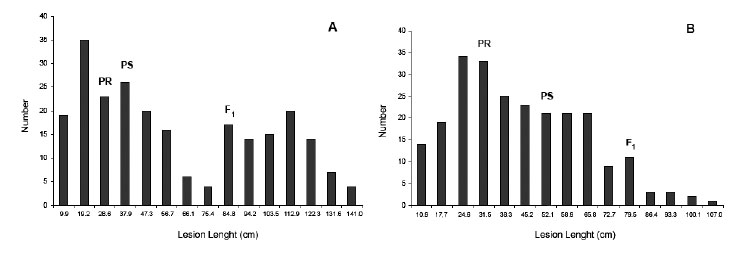
The susceptible line Das86 was the most susceptible while Das64 was the most resistant (Table 2). The F1 cross Das86 x Das21 was more susceptible than the susceptible parental line Das86. The distribution of lesion length in the F2 generation of this population was skewed towards susceptibility (Figure 1A, B). However, transgressive resistant individuals were found with approximately 29.6 and 16.2% of the individuals being more resistant than the resistant parent (Das21), in the two trials. On the other hand, the F1 cross Das86 x Das64 was as susceptible as the susceptible parent (Das86). However, 15 and 21.7% of F2 plants were more resistant (transgressive) than the resistant parental line (Das64) in trials 1 and 2, respectively (Figure 1C, D). Transgressive resistant individuals were observed as 15 and 21.7% of the F2 plants were more resistant than the resistant parental line in trials 1 and 2, respectively (Table 2).
In the population Das86 x Das64, the distribution of mean lesion length of the F2 generation was less skewed towards susceptibility (Figure 1C, D). The level of susceptibility of the F1 hybrids in both crosses suggests the presence of dominant alleles for susceptibility. The mean of backcross generations (BC1 and BC2) of both populations showed a typical behavior, that is, when the cross involved the susceptible genitor, the mean of internal lesion length of the generation was higher than when it involved the resistant one (Table 2). This corroborates earlier reports by Carson and Hooker (1981), Badu-Apraku et al. (1987), Callaway et al. (1990), as well as recent publication about this pathosystem by Frey et al. (2011) and Muimba-Kankolongo and Bergstrom (2011).
Genetic parameters were estimated by using the reduced model (additive-dominant) for Das86 x Das64 and the complete model for Das86 x Das21, due to the presence of significant deviations. Results indicated distinct modes of disease resistance inheritance between these populations. These results are consistent with those about anthracnose stalk rot, obtained by Lim and White (1978), Carson and Hooker (1981) and Pereira et al. (1989), as well as about anthracnose leaf blight, obtained by Badu-Apraku et al. (1987), Coêlho et al. (2001), Rezende et al. (2004). In Das86 x Das21, significant dominant and additive x dominant epistatic effects were detected in the first trial (Table 3). However, in the second trial additive, dominant, and both epistatic effects (additive x dominant and additive x additive) were significant. Notwithstanding, dominance effects were prevailed in both trials, accounting for 41.7 and 57.9% of the total genetic variance (Table 4). In the case of interactions, the additive x dominant explained 43.4% of genetic variance for the first trial, while on the second, the additive x additive interaction accounted for 23.4%. The other genetic effects estimated by the complete model were small.
On the other hand, in population Das86 x Das64 large additive effects, which accounted for more than 80% of the total genetic variation, were detected in both trials with only a small dominance effect being detected in the second trial. In this case, the additive-dominant reduced genetic model sufficed to explain the variations found between generations (Tables 3 and 4).
Analysis of generation means indicated a contrasting mode of inheritance between Das86 x Das21 and Das86 x Das64, which could be explained by differences in the genetic background of the resistant lines Das21 and Das64 (Badu-Apraku et al. 1987, Pereira et al. 1989, Lanza et al. 1999, Coêlho et al. 2001, Rezende et al. 2004). The prevalence of additive genetic action in the population Das86 x Das64 in both trials suggests that selection based on resistant individuals will result in genetic progress in this population. Carson and Hooker (1981) reported that the additive effects accounted for more than 90% of the total variation in crosses studied. The present results also showed that additive effects are an important source of variation (Das86 x Das64), especially when crossing lines contrasting for resistance expression. Weldekidan and Hawk (1993) found sum of squares for GCA 4-5 times larger than specific combining ability (SCA) indicating that additive effects were more important than nonadditive effects for ASR resistance.
In Das86 x Das21, however, most of the genetic variance was explained by dominance effects and digenic epistatic interactions. Even after adjusting the data to the complete model, deviations still remained significant, indicating that resistance is affected by higher order epistatic interactions involving more than two loci. These interactions were not estimated because it would require more generations. These results are in accordance with Badu-Apraku et al. (1987) and Rezende et al. (2004), who also reported dominance and epistatic effects in some crosses analyzed. The higher susceptibility observed in this hybrid could result from the combination of dominant alleles conferring susceptibility present in both parents at different loci. This conclusion is supported by the fact that Das21 presented intermediate levels of resistance, indicating that it could carry some susceptibility alleles.
Broad sense heritability estimates were of intermediate magnitude and varied according to the population and to the trial (Table 2). Heterosis estimates were positive (i.e. more susceptibility) and highly variable both between trials and between populations (Table 2). Likewise, Pereira et al. (1989) reported that the direction of dominance was not consistent among crosses, but indicated a tendency of heterosis toward higher susceptibility to C. graminicola.
Recently, Frey et al. (2011) reported that the presence of major resistance gene to ASR (Rcg1) reduced the overall yield impact incurred by infection in inoculated plots by almost three fold in comparison to hybrids that lack the gene. Studies indicate small utilization of locus Rcg1 in modern maize in the world. Frey et al. (2011) observed that, in a screen of inbred lines that represent greater than 90% of the genetic diversity in public inbred lines of maize, Rcg1 was found in 5 out of 93 maize inbreds.
The present results indicated distinct modes of resistance to anthracnose stalk rot inheritance between two populations derived from crossing three tropical maize inbreds. Thus, different selection methods may be necessary depending on the genetic background of the parental lines. However, genetic improvement is possible by selecting and recombining transgressive resistant individuals in both populations of tropical maize.
ACKNOWLEDGEMENTS
The authors thank DowAgrosciences Ltda. for the assistance in conducting field trials. They also thank Comissão de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES-PICDT) for sponsoring a scholarship for the first author and FAPESP for financial support through grant 01/02793-0.
Received 11 January 2012
Accepted 20 April 2012
- Badu-Apraku B, Gracen VE and Bergstrom GC (1987) A major gene for resistance to stalk rot in maize. Phytopathology 77:957-959.
- Balmer E and Pereira OAP (1987) Doenças de milho. In Paterniani E and Viégas GP (eds) Melhoramento e produção de milho Fundação Cargill, Campinas, p. 595-634.
- Bergstrom GC and Nicholson RL (1999) The biology of corn anthracnose. Plant Disease 83:596-608.
- Callaway MB, Smith ME and Coffman WR (1990) Diallel analysis of resistance to anthracnose stalk rot in maize inbreds. Crop Science 30:335-337.
- Carson ML and Hooker AL (1981) Inheritance of resistance to anthracnose leaf blight in five inbred lines of corn. Phytopathology 71:488-491.
- Coêlho RMS, Silva HP, Brunelli KR and Camargo LEA (2001) Controle genético da antracnose foliar em milho. Fitopatologia Brasileira 26:640-643.
- Costa RV, Silva DD, Cota LV, Parreira DF, Ferreira ASF and Casela CR (2010) Incidence of Colletotrichum graminicola in stalk from maize genotypes. Summa Phytopathologica 36:122-128.
- Dale JL (1963) Corn anthracnose. Plant Disease 47:245-249.
- Denti EA and Reis EM (2001) Efeito da rotação de culturas, da monocultura e da densidade de plantas na incidência das podridões da base do colmo e no rendimento de grãos do milho. Fitopatologia Brasileira 26:635-639.
- Denti EA and Reis EM (2003) Levantamento de fungos associados às podridões do colmo e quantificação de danos em lavouras de milho do planalto médio gaúcho e dos campos gerais do Paraná. Fitopatologia Brasileira 28:585-590.
- Frey TJ, Weldekidan T, Colbert T, Wolters PJCC and Hawk JA (2011) Fitness evaluation of Rcg1, a locus that confers resistance to Colletotrichum graminicola (Ces.) G. W. Wils. using near-isogenic maize hybrids. Crop Science 51:1551-1563.
- Gatch EW and Munkvold GP (2002) Fungal species composition in maize stalks in relation to European corn borer injury and transgenic insect protection. Plant Disease 86:1156-1162.
- Hallauer AR and Miranda Filho JB (1988) Quantitative genetics in maize breeding 2nd ed., Iowa State University Press, Ames, 468p.
- Jarvis JL, Clark RL, Guthrie WD, Berry EC and Russel WA (1984) The relationship between second generation European corn borers and stalk rot fungi in maize hybrids. Maydica 24:247-263.
- Lanza LLB, Souza Jr CL, Ottoboni LMM, Vieira MLC and Souza AP (1999) Genetic distance of inbred lines and prediction of maize single-cross performance using RAPD markers. Theoretical and Applied Genetics 94:1023-1030.
- Lim SM and White DG (1978) Estimates of heterosis and combining ability for resistance of maize to Colletotrichum graminicola Phytopathology 68:1336-1342.
- Mather K and Jinks JL (1971) Biometrical genetics Cornell University Press, New York, 382p.
- Miles JW, Dudley JW, White DG and Lambert RJ (1980) Improving corn population for grain yield and resistance to leaf blight and stalk rot. Crop Science 20:247-251.
- Miranda Filho JB (1991) Quantitative analysis of a cross between populations and their derived generations. Genetics and Molecular Biology 14:547-561.
- Muimba-Kankolongo A and Bergstrom GC (2011) Reduced anthracnose stalk rot in resistant maize is associate with restricted development of Colletotrichum graminicola in pith tissues. Journal of Phytopathology 159:329-341.
- Oestreich D (2005) Biotechnology: where is it coming from and where is it going. Proceedings 60th Annual Corn and Sorghum Research Conference. American Seed Trade Association, Washington, DC.
- Paterniani MEAGZ, Sawazaki E, Dudienas C, Duarte AP and Gallo PB (2000) Diallel crosses among maize lines with emphasis on resistance to foliar diseases. Genetics and Molecular Biology 23:381-385.
- Pereira OAP, Balmer E and Miranda Filho JB (1989) Inheritance of resistance to stalk rot, caused by Colletotrichum graminicola (Ces.) Wills, in maize (Zea mays L.). Genetics and Molecular Biology 12:53-65.
- Pereira OAP and Pereira WSP (1976) Estudo de Diplodia zeae (Shw) Lev. e Fusarium moniliforme Scheld. em colmo de milho. Summa Phytopathologica 2:165-171.
- Reis EM and Casa RT (1996) Manual de identificação e controle de doenças de milho Aldeia Norte, Passo Fundo, 78p.
- Rezende VF, Vencovsky R, Cárdenas FEN, Silva HP, Bearzoti E and Camargo LEA (2004) Mixed inheritance model for resistance to anthracnose leaf blight in maize. Crop Breeding and Applied Biotechnology 4:115-122.
- SAS Institute (2000) SAS /STAT user's guide: statistics Version 8.1., Cary, V.2.
- Shurtleff MC (1980) A compendium of corn diseases The American Phytopathological Society, Saint Paul, 105p.
- Toman JR and White DJ (1993) Inheritance of resistance to stalk rot of corn. Phytopathology 83:981-986.
- Weldekidan T and Hawk JA (1993) Inheritance of anthracnose stalk rot resistance in maize. Maydica 38:189-192.
- Zuber MS, Ainsworth TC, Blanco MH and Darrah LL (1981) Effect of anthracnose leaf blight on stalk rind strength and yield in F1 single crosses in maize. Plant Disease 65:719-722.
Publication Dates
-
Publication in this collection
25 Sept 2012 -
Date of issue
Sept 2012
History
-
Received
11 Jan 2012 -
Accepted
20 Apr 2012