ABSTRACT
Intergroup conflicts are energetically more costly than intra-group disputes, and thus typically occur in contexts in which the energetic returns are relatively high. In anthropogenic environments, provisioned resources tend to be rich in energy and highly agglomerated. While females are expected to defend provisioned resources, the adult males, in turn, are expected to defend the females. Based on this premise, the present study focused on an urban forest in the city of Goiânia (Goiás, Brazil), which is inhabited by two groups of bearded capuchins. Behavioral data were collected using instantaneous scan sampling and all-events records to document intergroup conflicts and define their context. We used a backward logistic regression and stepwise linear regression to analyze the participation of the individuals in the conflicts. Conflicts were more frequent in the context of provisioning, although the number of neither females nor males involved in the conflict varied significantly between contexts. The females did avoid participating in conflicts involving adult males, however, to minimize the risk of being attacked by them. The adult males participated more in the presence of other adult males, which is partly consistent with the hypothesis of the defense of reproductive partners. The conflicts were not more intense in the context of provisioned resources, which contrasted with expectations. The hierarchical relationship between the two study groups, and the fact that the groups were derived from the same social unit, may have contributed to a reduction in the intensity of the conflicts.
KEY WORDS:
Dear enemy; defense of reproductive partners; inter-group hierarchy; provisioned resources; risk avoidance; urban environment
INTRODUCTION
The home ranges of free-ranging primate groups often overlap, resulting in areas within which neighboring groups may encounter each other. These “intergroup encounters” often involve aggressive disputes which, from a socioecological pers pective (Wrangham 1980Wrangham RW (1980) An ecological model of female-bonded primate groups. Behaviour 75(3-4): 262-300. https://doi.org/10.1163/156853980X00447
https://doi.org/10.1163/156853980X00447...
), may ensure the access of the group to resources that are important for reproduction and survival, such as key sources of food or reproductive partners (Scarry 2016Scarry CJ (2016) Intergroup Encounters. In: Fuentes A (Ed.) The International Encyclopedia of Primatology. New Jersey, John Wiley & Sons, 1-6. https://doi.org/10.1002/9781119179313.wbprim0198
https://doi.org/10.1002/9781119179313.wb...
, Cooksey et al. 2020Cooksey K, Sanz C, Ebombi TF, Massamba JM, Teberd P, Magema E, Abea G, Peraleja JSO, Kienast I, Stephens C, Morgan D (2020) Socioecological factors influencing intergroup encounters in Western Lowland Gorillas (Gorilla gorilla gorilla). International Journal of Primatology 41(2): 181-202. https://doi.org/10.1007/s10764-020-00147-6
https://doi.org/10.1007/s10764-020-00147...
). Each individual will decide whether or not to participate in an intergroup dispute. In fact, each individual will decide whether or not to enter the “conflict zone”, which may be a large fruiting tree to threaten, chase or attack specific individuals, individuals will also choose whether to associate or not with other individuals already involved in the dispute (i.e., form coalitions). During disputes, opportunities may arise for males to approach females from other social groups, providing opportunities for extra-group copulations (Scarry 2016Scarry CJ (2016) Intergroup Encounters. In: Fuentes A (Ed.) The International Encyclopedia of Primatology. New Jersey, John Wiley & Sons, 1-6. https://doi.org/10.1002/9781119179313.wbprim0198
https://doi.org/10.1002/9781119179313.wb...
). In other words, although intergroup disputes affect the whole group, the decisions to participate (or otherwise) in the defense of a resource tend to be made individually (Majolo et al. 2020Majolo B, deBortoli Vizioli A, Martínez-Íñigo L, Lehmann J (2020) Effect of group size and individual characteristics on intergroup encounters in primates. International Journal of Primatology 41(2): 325-341. https://doi.org/10.1007/s10764-019-00119-5
https://doi.org/10.1007/s10764-019-00119...
).
The costs and benefits associated with disputes are different for males and females (Kitchen and Beehner 2007Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144(12): 1551-1581. https://doi.org/10.1163/156853907782512074
https://doi.org/10.1163/1568539077825120...
), so the strategies adopted during encounters are sex-specific. Female mammals have a high energetic investment in gestation, lactation, and infant caregiving, and require access to an adequate supply of nutrients (Trivers 1972Trivers RL (1972) Parental investment and sexual selection. In: Campbell BG (Ed.) Sexual selection and the descent of man: The Darwinian pivot. Chicago, Aldine Publishing Company, 136-179. https://psycnet.apa.org/record/1996-98307-040
https://psycnet.apa.org/record/1996-9830...
). On the other hand, females tend to be smaller than males in most species of mammals, which implies that engaging in aggressive interactions with males entails a high risk of injury (Koch et al. 2016Koch F, Signer J, Kappeler PM, Fichtel C (2016) Intergroup encounters in Verreaux”s sifakas (Propithecus verreauxi): who fights and why? Behavioral Ecology and Sociobiology 70(5): 797-808. https://doi.org/10.1007/s00265-016-2105-3
https://doi.org/10.1007/s00265-016-2105-...
, Mirville et al. 2018Mirville MO, Ridley AR, Samedi JP, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC (2018) Factors influencing individual participation during intergroup interactions in mountain gorillas. Animal Behaviour 144: 75-86. https://doi.org/10.1016/j.anbehav.2018.08.003
https://doi.org/10.1016/j.anbehav.2018.0...
). Males also need food to survive, but their evolutionary fitness is more dependent on the number of females they are able to copulate with. In this way, males should prioritize strategies that increase their access to reproductive females, while also restricting the access of other males to these females (Trivers 1972Trivers RL (1972) Parental investment and sexual selection. In: Campbell BG (Ed.) Sexual selection and the descent of man: The Darwinian pivot. Chicago, Aldine Publishing Company, 136-179. https://psycnet.apa.org/record/1996-98307-040
https://psycnet.apa.org/record/1996-9830...
, Georgiev et al. 2013Georgiev AV, Klimczuk AC, Traficonte DM, Maestripieri D (2013) When violence pays: A cost-benefit analysis of aggressive behavior in animals and humans. Evolutionary Psychology 11(3): 678-699. https://doi.org/10.1177/147470491301100313
https://doi.org/10.1177/1474704913011003...
). Dominant males are also expected to participate more in direct disputes than subordinate males, given that the potential return, in terms of reproductive success, are much greater for these males than for subordinate ones (Koch et al. 2016Koch F, Signer J, Kappeler PM, Fichtel C (2016) Intergroup encounters in Verreaux”s sifakas (Propithecus verreauxi): who fights and why? Behavioral Ecology and Sociobiology 70(5): 797-808. https://doi.org/10.1007/s00265-016-2105-3
https://doi.org/10.1007/s00265-016-2105-...
).
One other key factor directing decision-making is the magnitude of the benefit represented by the disputed resource. In anthropogenic environments, for example, provisioned resources may be extremely important for the survival of the population (Spehar 2018Spehar SN (2018) Anthropogenic landscapes. In: Trevathan W (Ed.) The International Encyclopedia of Biological Anthropology. New Jersey, John Wiley & Sons, 1-4. https://doi.org/10.1002/9781118584538.ieba0025
https://doi.org/10.1002/9781118584538.ie...
). These food sources are, in general, more palatable, digestible, and energy-rich than wild foods, being denser in simple sugars, saturated fats, and proteins (Milton 1999Milton K (1999) Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition 15(6): 488-498. https://doi.org/10.1016/S0899-9007(99)00078-7
https://doi.org/10.1016/S0899-9007(99)00...
). Provisioned resources tend to be available year-round and may be consumed more during periods when natural resources are scarce (Shochat et al. 2006Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology and Evolution 21(4): 186-191. https://doi.org/10.1016/j.tree.2005.11.019
https://doi.org/10.1016/j.tree.2005.11.0...
, Sha and Hanya 2013Sha JC, Hanya G (2013) Temporal Food resource correlates to the behavior and ecology of food-enhanced long-tailed macaques (Macaca fascicularis). Mammal Study 38(3): 163-175. https://doi.org/10.3106/041.038.0305
https://doi.org/10.3106/041.038.0305...
). This implies that the provisioned resources found in anthropogenic environments would be defended more intensively by the local social groups, principally the female members, than natural resources (Sterck 1999Sterck EHM (1999) Variation in langur social organization in relation to the socioecological model, human habitat alteration, and phylogenetic constraints. Primates 40(1): 199-213. https://doi.org/10.1007/BF02557711
https://doi.org/10.1007/BF02557711...
). In fact, the females should cooperate to guarantee access to the provisioned resources.
Robust capuchins (Sapajus spp.) are found throughout most of tropical South America and are dimorphic, with the males being heavier than the females (Fragazy et al. 2004Fragazy DM, Visalberghi E, Fedigan LM (2004) The complete capuchin: the biology of genus Cebus. Cambridge, Cambridge University Press, 339pp. https://doi.org/10.1007/s10329-005-0129-9
https://doi.org/10.1007/s10329-005-0129-...
). In the specific case of Sapajus libidinosus (Spix, 1823), the adult males (3.8-4.4 kg) are approximately one third heavier than the females, and the dominant males are around 20% heavier than subordinate males (Fragaszy et al. 2016Fragaszy DM, Izar P, Liu Q, Eshchar Y, Young LA, Visalberghi E (2016) Body mass in wild bearded capuchins, (Sapajus libidinosus): Ontogeny and sexual dimorphism. American Journal of Primatology 78(4): 473-484. https://doi.org/10.1002/ajp.22509
https://doi.org/10.1002/ajp.22509...
). The adult males are normally dominant over the females, although the alpha females tend to be dominant over the beta males (Fragazy et al. 2004Fragazy DM, Visalberghi E, Fedigan LM (2004) The complete capuchin: the biology of genus Cebus. Cambridge, Cambridge University Press, 339pp. https://doi.org/10.1007/s10329-005-0129-9
https://doi.org/10.1007/s10329-005-0129-...
). In Sapajus, the number of males involved in an intergroup conflict generally determines which group wins the conflict, with the males defending the feeding resources (Scarry 2013Scarry CJ (2013) Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Animal Behaviour 85(5): 931-939. https://doi.org/10.1016/j.anbehav.2013.02.013
https://doi.org/10.1016/j.anbehav.2013.0...
).
Given these theoretical expectations, we verified whether the availability of provisioned resources determines an increase in the frequency of agonistic intergroup encounters. We also verified the relative importance of the participation of adult males and females. For this, we tested two hypotheses: resource defense and risk avoidance. The resource defense hypothesis, derived from socioecological models (Wrangham 1980Wrangham RW (1980) An ecological model of female-bonded primate groups. Behaviour 75(3-4): 262-300. https://doi.org/10.1163/156853980X00447
https://doi.org/10.1163/156853980X00447...
), predicts that the females should defend feeding resources from the opposing group (Scarry 2016Scarry CJ (2016) Intergroup Encounters. In: Fuentes A (Ed.) The International Encyclopedia of Primatology. New Jersey, John Wiley & Sons, 1-6. https://doi.org/10.1002/9781119179313.wbprim0198
https://doi.org/10.1002/9781119179313.wb...
), particularly when the dispute involves resources of greater energetic value (Brown 1964Brown JL (1964) The evolution of diversity in avian territorial systems. The Wilson Bulletin 76(2): 160-169. https://www.jstor.org/stable/4159278
https://www.jstor.org/stable/4159278...
). On the other hand, demographic and sexual factors may determine differences in the strategies of males and females. According to the risk avoidance hypothesis (Koch et al. 2016Koch F, Signer J, Kappeler PM, Fichtel C (2016) Intergroup encounters in Verreaux”s sifakas (Propithecus verreauxi): who fights and why? Behavioral Ecology and Sociobiology 70(5): 797-808. https://doi.org/10.1007/s00265-016-2105-3
https://doi.org/10.1007/s00265-016-2105-...
, Mirville et al. 2018Mirville MO, Ridley AR, Samedi JP, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC (2018) Factors influencing individual participation during intergroup interactions in mountain gorillas. Animal Behaviour 144: 75-86. https://doi.org/10.1016/j.anbehav.2018.08.003
https://doi.org/10.1016/j.anbehav.2018.0...
), the females should avoid conflicts that involve males. In this case, the presence of adult males in the opposing group would inhibit the participation of the females in intergroup conflicts. The adult males may also defend the females from the males of other groups, based on the hypothesis of the defense reproductive partners (Kitchen and Beehner 2007Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144(12): 1551-1581. https://doi.org/10.1163/156853907782512074
https://doi.org/10.1163/1568539077825120...
).
MATERIAL AND METHODS
Study population and site
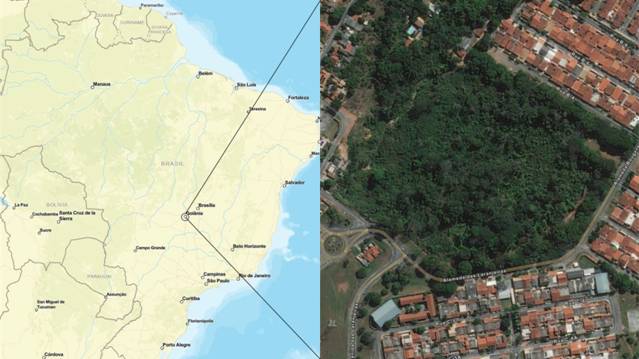
The present study was carried out in the Bosque Bougainville Padre Cesário Galvão, or Bosque Bougainville (-16.722443°; -49.228041°), a heavily forested urban park in the city of Goiânia, capital of the state of Goiás, Brazil, which has an area of 9 ha (Fig. 1). The park had negligible constructed infrastructure at the time of the study, which meant that the capuchins had little direct contact with the local residents from the neighborhood in which the park is located, and this type of interaction was observed very infrequently during the study period in 2009 and 2010. The contact of the capuchins with the provisions left regularly in the park was more frequent. The central region of Brazil has two climate seasons, one rainy and one dry, with the period between May and September being considered to be the dry season and the period between October to March comprising the rainy season (Costa et al. 2012Costa HC, Marcuzzo FF, Ferreira OM, Andrade LR (2012) Espacialização and Sazonalidade da Precipitação Pluviométrica do Estado de Goiás and Distrito Federal. Revista Brasileira de Geografia Física 1: 87-100. http://rigeo.cprm.gov.br/jspui/handle/doc/617
http://rigeo.cprm.gov.br/jspui/handle/do...
).
The study site is an isolated fragment of forest, surrounded by an urban matrix: Bosque Bougainville in Goiânia, Goiás, Brazil. Source: ArcGIS Data and Maps©.
The study site offers several distinct food resources for the local capuchins. On a specific part of the park, food is offered on platforms. On most days, except during heavy rain, a local resident puts 4-5 kg of bananas on a platform near the fence at around the same time each day, despite having been warned by the local authorities and researchers not to feed the monkeys. Personnel from the Goiânia Environment Agency (Agência Ambiental de Goiânia, or AMMA) place several types of fruits and vegetables on a platform at the center of the park regularly once a week, and irregularly on other days during the week. On a second platform in the center of the park, a variety of food is offered once a month as part of naturalistic experiments in the study area. In other parts of the park, the capuchins exploit domestic refuse from the plastic garbage bags left outside by local residents for collection. The capuchins rip open the bags to reach the leftover food. In the forest of the park, the capuchins spend more time feeding on wild fruit and insects.
The study population was formed by 31-35 capuchins during the study period. These individuals were divided into two stable social groups (see Supplementary Material - Tabs S1 and S2): the Dom Pedro group (two adult males, one of which disappeared at the beginning of the study, eight females, and 10 juveniles) and the Cicatriz group (one adult male, two subadult males, four females, and six juveniles). All the individuals were identifiable in the field.
Collection of behavioral data
Behavioral data were collected systematically between March and November 2009 and in January 2010. Aggressive behaviors included both active aggression (i.e.,threats, lunges, bites, and displacements) and submissive responses (i.e., avoid, flee, and weep), based on Vogel and Janson (2007Vogel ER, Janson CH (2007) Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. American Journal of Primatology 69(5): 533-550. https://doi.org/10.1002/ajp.20368
https://doi.org/10.1002/ajp.20368...
). An intergroup conflict was defined as any type of aggressive encounter between individuals of different groups, which were recorded using the all-events method (Altmann 1974Altmann J (1974) Observational Study of Behavior: Sampling Methods. Behaviour 49(3-4): 227-266. https://doi.org/10.1163/156853974X00534
https://doi.org/10.1163/156853974X00534...
).
For each intergroup conflict event, we recorded the identity of the individual that initiated the interaction with active aggression, the identity of individual(s) that reacted (usually with submissive responses), and whether a coalition was formed during the conflict. Coalitions were formed when at least two individuals directed a type of aggressive behavior simultaneously toward one or more individuals of the same species (following Gilby et al. 2013Gilby IC, Brent LJ, Wroblewski E, Rudicell RS, Hahn BH, Goodall J, Pusey A (2013) Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology 67(3): 373-381. https://doi.org/10.1007/s00265-012-1457-6
https://doi.org/10.1007/s00265-012-1457-...
). The initiator and respondent of each event were obligatorily members of different groups. We also classified the events according to their context - (i) non-feeding (no feeding resource involved), (ii) feeding on provisioned resources (food provided by humans, intentionally or otherwise), and (iii) feeding on natural resources (plant or animal foods available naturally within the forest).
The all-events data were complemented with scan sampling (5-minute scans at 15-minute intervals), which was used to determine the activity budgets of the two study groups (Altmann 1974Altmann J (1974) Observational Study of Behavior: Sampling Methods. Behaviour 49(3-4): 227-266. https://doi.org/10.1163/156853974X00534
https://doi.org/10.1163/156853974X00534...
). During each scan, we recorded the identity of each visible individual and its behavior (forage/feed, move, rest or socialize). When the individual was observed feeding, the type of food (wild or provisioned) was also recorded.
All applicable international, national, and institutional guidelines for the care and use of animals in research were followed during data collection.
Data analysis
All the individuals involved in a conflict were considered to have participated in this event. The individuals may either initiate or react to aggression, as well as form a coalition with individuals that had either initiated or reacted to the aggression. The total number of initiators in a conflict was defined by the individual that initiated the interaction, together with all the individuals that entered into the coalition with this capuchin. Similarly, the number of reactors was defined by the original reactor, together with all the individuals that formed the coalition. The total number of individuals involved in the conflict was used as a proxy for the intensity of the aggression.
The analyses were run in the WINKS SDA 7.0.7 statistical package (unlicensed, http://www.texasoft.com, 972-325-4415). We applied an Analysis of Variance (ANOVA) to evaluate the variation in the mean frequency of conflicts (dependent variable) per context (independent variable), that is, feeding (wild food or provisions) or non-feeding. Normally, the context was recorded in the field, but when no specific record was obtained, the context was defined by the feeding activity recorded immediately prior to and following the conflict. We used the number of scans during which members of the two groups were in close proximity to one another to determine which context was responsible for the highest conflict rate. We defined the context from the scan data using the same criterion used to define that of the conflicts (see above). To calculate the duration of the different contexts, we multiplied the number of scans in which each context was recorded by the 15-minute sampling interval, and then divided the number of conflicts recorded in each context by this time, to provide a rate of conflict per hour of activity.
We tested the hypotheses that the participation of the adult females and males of one group in a conflict will depend on the participation of the males and females of the opposing group and the context of the conflict using backward binary logistic regressions (backward LR). This approach was selected because it minimizes the probability of type II errors (Field 2009Field A (2009) Descobrindo a estatística usando o SPSS. Porto Alegre, Artmed, 687pp.). In this analysis, we used the total number of events according to the sex and age of the individuals that initiated the aggression and reacted to it as the variables. In the first model, the dependent variable (DV) was the participation or absence of females, while in the second model, the DV was the participation or absence of the adult males in the conflict. In the first model, the independent variables (IVs) were the number of adult males, the number of subadult males, the context (provisioning, wild food or non-feeding), and the climate season. In the second model, the IVs were the number of females, as well as the number of subadult males, the context, and the season.
We used two stepwise models of linear regression to test whether the number of adult females and males increases as a function of the presence or absence of males and females of the other group, or as a function of the availability of provisions and the climate season. In WINKS SDA 7.0.7, the stepwise approach combines forward and backward procedures, and removes the variables with little or no explanatory power that may arise in either case (George and Mallery 2016George D, Mallery P (2016) IBM SPSS Statistics 23 Step by Step: A simple guide and reference. New York, Taylor & Francis, 382 pp. https://doi.org/10.4324/9781315545899
https://doi.org/10.4324/9781315545899...
). In the first model of linear regression, we used the number of female initiators as the DV, while the IVs were the presence or absence of females reactors from the opposing group, adult male reactors from the opposing group, adult males from the initiating group that participated in a coalition, subadult males from the opposing group that reacted, the context of the conflict (provisioning, wild food ou non-feeding), and the climate season (rainy or dry). In the second model, the DV was the number of adult male from the initiating group, with the following IVs - the presence or absence of male reactors in the opposing group, the number of females reactors in the opposing group, females from the initiating group that participated in a coalition, and the subadult males from the opposing group involved in the intergroup interaction, in addition to the context and climate season.
RESULTS
Frequency of intergroup aggressions in different contexts
We recorded a total of 66 conflicts during 556 hours of observation (11.87 events per 100 hours of monitoring). On average, 2.70 (SE = 1.07, maximum = 6) individuals participated in each event. The non-feeding encounters involved the largest number of individuals, on average, although the difference among contexts was not significant (F = 0.85, df1 = 2, df2 = 63, p = ns). Overall, 359 (16.80%) of the 2137 scans conducted during the present study recorded at least one individual from each group. Although the majority of these scans did not involve feeding behavior, the frequency of conflicts in the context of provisioned food was greater than in both the other contexts combined. This is reflected in the much higher conflict rate (events per hour) recorded in the provisioned context, in comparison with the other two contexts (Table 1).
Number of conflicts between the two study groups recorded in the three different contexts, the encounter rates, and the mean number of individuals involved in the conflicts.
Participation in intergroup conflicts
The participation or absence of females in the conflicts was predicted by the reduction in the number of adult males (B = -1.76, WAID = 17.49, p < 0.001, R2 = 0.41), that is, more adult males were present in the aggression when females were absent in the event (average = 1.17) than when females participated in the event (average = 0.23). Similarly, the participation or absence of males was predicted by the reduction in the number of females in the conflict (B = -1.33, WAID = 12.29, p < 0.001, R2 = 0.32); more females participated in the aggression when adult males were absent (average = 1.51) than when they were present in the aggression (average = 0.52). Neither provisioning nor climate season were good predictors of the participation of the capuchins of either sex in the intergroup conflicts.
Number of participants in events of intergroup aggression
An increasing number of female participants in the group that initiated a conflict was predicted (R2 = 0.23) by the absence of adult males from the opposing group (Beta = -0.48, t = -4.37, p < 0.001). No conflicts were initiated by the females of one group when the adult male of the opposing group was present. By contrast, an increase in the number of adult males in the initiating group was predicted (R2 = 0.60) by the presence of adult males in the opposing group (Beta = 0.59, t = 7.28, p < 0.001), by the presence of females in the initiating group in coalition with the adult males (Beta = 0.50, t = 6.19, p < 0.001), and by the occurrence of the conflict in the rainy season (Beta = 0.24, t = 3.00, p < 0.001). Neither the context (provisioning) nor the dry season provided any insight into the number of adult females and males in the group that initiated the conflict.
DISCUSSION
Intergroup conflicts were more frequent in the context of provisioned food resources than in either wild food or non-feeding contexts, although the total number of participants did not vary significantly among these contexts. Our prediction that adult females would participate more in intergroup conflicts in the context of provisioned food resources was not supported. Neither feeding context nor climate season had any influence on the participation of the females. The adult males participated more in the intergroup interactions during the rainy season, which contradicts the resource defense hypothesis, given that resources are more abundant during this period (unpublished data).
The females participated more in the disputes as the presence of adult males declined, which is consistent with the hypothesis of risk avoidance. The males participated more in the conflicts as the participation of other adult males increased, which is consistent with the hypothesis of the defense of reproductive partners.
Defense of feeding resources: provisioning and seasonality
Feeding resources were expected to become scarcer during the dry season, which would have led to an increase in the participation of the females in the intergroup conflicts, although no such process was observed. The need for fewer individuals to defend the provisioned resources may be related to either intergroup dominance and/or the “dear enemy” effect (see below). Some of ours unpublished data showed that the Dom Pedro group wins most intergroup conflicts and has priority of access to the principal feeding resources. Established dominance may reduce the intensity conflicts between groups at important resources (Crofoot and Wrangham 2014Crofoot MC, Wrangham RW (2014) Intergroup aggression in primates and humans: the case for a unified theory. In: Kappeler P, Silk J (Eds) Mind the gap. Berlin, Springer, 171-196. https://doi.org/10.1007/9783642027253
https://doi.org/10.1007/9783642027253...
), given that the advantage of the dominant group has been pre-defined in previous interactions. This may have been the scenario during the dry season in the present study.
One other possible factor is the influence of the abundance and predictability of the provisioned resources, which accounted for half of the feeding time of the subjects in the present study (unpublished data). As the resource is relatively large, it may require less defense, given that all the individuals are able to satisfy their nutritional requirements (Hirsch 2007Hirsch BT (2007) Costs and benefits of within-group spatial position: a feeding competition model. The Quarterly Review of Biology 82(1): 9-27. https://doi.org/10.1086/511657
https://doi.org/10.1086/511657...
). The temporal and spatial predictability of most provisioned resources, which are replenished daily, facilitates the capacity of the animals to locate and defend these resources, and may, in turn, have reinforced the dominance relationship between the two groups (Grant 1993Grant JW (1993) Whether or not to defend? The influence of resource distribution. Marine Behaviour and Physiology 23(1-4): 137-153. https://doi.org10.1080/10236249309378862
https://doi.org10.1080/10236249309378862...
). The dominant Dom Pedro group controlled the access to the feeding platforms without necessarily investing energy in intergroup conflicts, given that, once the members of this group had satisfied their nutritional needs, they would leave the site free to be visited by the members of the subordinate Cicatriz group (unpublished data).
Previous studies have found that provisioned resources tend to result in an increase in the frequency of aggressive encounters in comparison with natural resources (Cooper et al. 2004Cooper MA, Aureli F, Singh M (2004) Between-group encounters among bonnet macaques (Macaca radiata). Behavioral Ecology and Sociobiology 56(3): 217-227. https://doi.org/10.1007/s00265-004-0779-4
https://doi.org/10.1007/s00265-004-0779-...
). In an experimental study using playback, Scarry (2017Scarry CJ (2017) Male resource defence during intergroup aggression among tufted capuchin monkeys. Animal Behaviour 123: 169-178. https://doi.org/10.1016/j.anbehav.2016.10.015
https://doi.org/10.1016/j.anbehav.2016.1...
) found that groups of Sapajus nigritus (Goldfuss, 1809) preferred to defend high-value provisioned resources rather than other types of resource, such as females in estrus. In the present study, we recorded an increase in the frequency of conflicts at provisioned resources, although these conflicts did not involve more individuals, nor the increased participation of adult males and females, in other words, they were not more intense.
In comparison with previous studies of free-ranging Sapajus, however, the rates of conflict observed in the present study were very high. Izar et al. (2012Izar P, Verderane MP, Peternelli-dos-Santos L, Mendonça-Furtado O, Presotto A, Tokuda M, Visalberghi E, Fragaszy D (2012) Flexible and conservative features of social systems in tufted capuchin monkeys: Comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology 74(4): 315-331. https://doi.org/10.1002/ajp.20968
https://doi.org/10.1002/ajp.20968...
) recorded a rate of 1.1 intergroup conflicts per 100 hours of observation of S. libidinosus on the Fazenda Boa Vista (FBV), in the Brazilian state of Piauí, and 1.4 events per 100 hours of observation of S. nigritus in the Carlos Botelho State Park (PECB), in São Paulo state. These rates are very much lower than the overall rate of 7.9 events along 100 hours recorded in the present study. While many different factors may have contributed to these differences, the availability of provisioned resources and the limited size of the forest in the Bosque Bougainville, which determines a high level of overlap between the two study groups, may have been decisive. However, to confirm this suspicion, more studies on populations that live in small urban forests are needed.
Intersexual aggression: sexual dimorphism and sexual selection
There was a reduction in the participation of the females in intergroup conflicts when adult males participated, and vice versa. This may be explained by the risk avoidance hypothesis, which predicts that the smaller sex will tend to avoid participating in encounters that involve members of the larger sex - adult males in this case (Kitchen and Beehner 2007Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144(12): 1551-1581. https://doi.org/10.1163/156853907782512074
https://doi.org/10.1163/1568539077825120...
). In the two capuchin genera (Cebus and Sapajus), females normally participate less in intergroup encounters than males (Perry 1996Perry S (1996) Intergroup encounters in wild white-faced capuchins (Cebus capucinus). International Journal of Primatology 17(3): 309-330. https://doi.org/10.1007/BF02736624
https://doi.org/10.1007/BF02736624...
, Scarry 2017Scarry CJ (2017) Male resource defence during intergroup aggression among tufted capuchin monkeys. Animal Behaviour 123: 169-178. https://doi.org/10.1016/j.anbehav.2016.10.015
https://doi.org/10.1016/j.anbehav.2016.1...
), with no relationship being found between the number of females in a group and the probability of its victory in a conflict (Scarry 2013Scarry CJ (2013) Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Animal Behaviour 85(5): 931-939. https://doi.org/10.1016/j.anbehav.2013.02.013
https://doi.org/10.1016/j.anbehav.2013.0...
).
Sexual dimorphism limits the participation of the females in intergroup conflicts that involve adult males, although it does not impede the participation of the females when the adult males are not present. The effects of sexual dimorphism were clear in the present study, because, while not significant, the subadult males were the target of more than half of the agonistic interactions that involved females (Table S1). As subadult males have little chance of copulating in their natal groups, they may take advantage of intergroup conflicts to approach adult females and attempt to copulate (Kitchen and Beehner 2007Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144(12): 1551-1581. https://doi.org/10.1163/156853907782512074
https://doi.org/10.1163/1568539077825120...
). According to the principles of intersexual selection, the females may depend on the protection of the dominant males (Koch et al. 2016Koch F, Signer J, Kappeler PM, Fichtel C (2016) Intergroup encounters in Verreaux”s sifakas (Propithecus verreauxi): who fights and why? Behavioral Ecology and Sociobiology 70(5): 797-808. https://doi.org/10.1007/s00265-016-2105-3
https://doi.org/10.1007/s00265-016-2105-...
) or establish coalitions to defend themselves from the subadult males (Watson-Capps 2009Watson-Capps JJ (2009) Evolution of sexual coercion with respect to sexual selection and sexual conflict theory. In: Muller MN, Wranghan RW (Eds) Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females. Cambridge, Harvard University Press, 23-41. https://doi.org/10.1086/jar.66.2.27820915
https://doi.org/10.1086/jar.66.2.2782091...
). This did not appear to be the case in the present study, however, as females did not appear to be protecting themselves from attempted matings. Rather, the females were simply competing for feeding resources with smaller-bodied males (i.e., reduced effect of sexual dimorphism).
Intra-sexual aggression: protection of the females?
The females did not target primarily other females during intergroup conflicts and their participation did not shift in relation to the context of the encounter. The adult males did increase their participation, however, in the presence of adult males in the opposing group in the conflict, which is consistent with the hypothesis of the defense of reproductive partners. One other important factor was the coalition between adult males. As the number of individuals participating in one of the groups involved in a conflict increases the probability of victory (Majolo et al. 2020Majolo B, deBortoli Vizioli A, Martínez-Íñigo L, Lehmann J (2020) Effect of group size and individual characteristics on intergroup encounters in primates. International Journal of Primatology 41(2): 325-341. https://doi.org/10.1007/s10764-019-00119-5
https://doi.org/10.1007/s10764-019-00119...
), the females in the present study may have established coalitions with adult males to reinforce the chance of victory during disputes for feeding resources. However, the adult males did not participate more in conflicts that involved the presence of subadult males in the opposing group, which contradicts the hypothesis of the defense of reproductive partners.
Although no significant relationship was observed, it is important to note that the adult males of the two study groups (DP and CI) established two coalitions between themselves in agonistic interactions with the subadult males. In this case, the adult males may have acted together to guarantee their preferential access to the females, in relation to the subadult males.
One other factor that may be relevant here is the relatively small number of males in the two study groups. The male to female ratio of the Dom Pedro group was 0.12, while that of the Cicatriz group was 0.6. Izar et al. (2012Izar P, Verderane MP, Peternelli-dos-Santos L, Mendonça-Furtado O, Presotto A, Tokuda M, Visalberghi E, Fragaszy D (2012) Flexible and conservative features of social systems in tufted capuchin monkeys: Comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. American Journal of Primatology 74(4): 315-331. https://doi.org/10.1002/ajp.20968
https://doi.org/10.1002/ajp.20968...
) recorded sex ratios of 0.58 in the group from the FBV and 0.65 in that from the PECB. The two study groups from the Bosque Bougainville thus appear to be single-male groups that are, essentially, harems. The subadult males of the Cicatriz group may not yet represent a challenge to the leadership of this group, given that they were clearly subordinate, despite the fact that one of these males was observed copulating with a subordinate female from the Dom Pedro group. This biased sex ratio, in particular in the Dom Pedro group, may be linked to the spatial isolation of the park, which prevents the immigration of new males.
Dear Enemy and inter-group dominance
The two study groups arose from the fission of a single social group following a forest fire, which occurred prior to the study period (Table S2). As all the study animals were originally members of the same group, they would have had long-term social relationships prior to the fission, and probably also a certain degree of genetic relatedness, and may thus be “dear enemies” (Temeles 1994Temeles EJ (1994) The role of neighbours in territorial systems: when are they “dear enemies”? Animal Behaviour 47(2): 339-350. https://doi.org/10.1006/anbe.1994.1047
https://doi.org/10.1006/anbe.1994.1047...
, Mirville et al. 2018Mirville MO, Ridley AR, Samedi JP, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC (2018) Factors influencing individual participation during intergroup interactions in mountain gorillas. Animal Behaviour 144: 75-86. https://doi.org/10.1016/j.anbehav.2018.08.003
https://doi.org/10.1016/j.anbehav.2018.0...
). The Dear Enemy effect refers to a scenario in which territorial groups interact with known neighbors, which may also be genetically related. These interactions tend to be less intense than those with unknown invaders.
The Dear Enemy effect is probably a factor in the Bosque Bougainville population. The Dom Pedro is dominant in the intergroup conflicts and the access to provisioned and natural resources (unpublished data). Despite having preferential access to the provisioned resources, the dominant Dom Pedro group does not appear to resort invariably to aggressiveness to gain access to these resources, given that the subordinate group appears to avoid the presence of the dominant group, principally by not visiting these resources during periods when the dominant group was present.
One other behavior pattern that supports the idea of the Dear Enemy effect is the fact that individuals from different groups formed coalitions, in particular, the two dominant adult males, which formed a coalition against the subordinate males of the Cicatriz group. This would appear to reflect a social relationship that predates the fission of the original group.
ACKNOWLEDGMENTS
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil - Finance Code 001) for the PhD scholarship and AMMA, the municipal environment agency of Goiânia, for permission to conduct the research in the park. We also thank all anonymous reviewers for their valuable comments and contributions to this manuscript.
LITERATURE CITED
- Altmann J (1974) Observational Study of Behavior: Sampling Methods. Behaviour 49(3-4): 227-266. https://doi.org/10.1163/156853974X00534
» https://doi.org/10.1163/156853974X00534 - Brown JL (1964) The evolution of diversity in avian territorial systems. The Wilson Bulletin 76(2): 160-169. https://www.jstor.org/stable/4159278
» https://www.jstor.org/stable/4159278 - Cooksey K, Sanz C, Ebombi TF, Massamba JM, Teberd P, Magema E, Abea G, Peraleja JSO, Kienast I, Stephens C, Morgan D (2020) Socioecological factors influencing intergroup encounters in Western Lowland Gorillas (Gorilla gorilla gorilla). International Journal of Primatology 41(2): 181-202. https://doi.org/10.1007/s10764-020-00147-6
» https://doi.org/10.1007/s10764-020-00147-6 - Cooper MA, Aureli F, Singh M (2004) Between-group encounters among bonnet macaques (Macaca radiata). Behavioral Ecology and Sociobiology 56(3): 217-227. https://doi.org/10.1007/s00265-004-0779-4
» https://doi.org/10.1007/s00265-004-0779-4 - Costa HC, Marcuzzo FF, Ferreira OM, Andrade LR (2012) Espacialização and Sazonalidade da Precipitação Pluviométrica do Estado de Goiás and Distrito Federal. Revista Brasileira de Geografia Física 1: 87-100. http://rigeo.cprm.gov.br/jspui/handle/doc/617
» http://rigeo.cprm.gov.br/jspui/handle/doc/617 - Crofoot MC, Wrangham RW (2014) Intergroup aggression in primates and humans: the case for a unified theory. In: Kappeler P, Silk J (Eds) Mind the gap. Berlin, Springer, 171-196. https://doi.org/10.1007/9783642027253
» https://doi.org/10.1007/9783642027253 - Field A (2009) Descobrindo a estatística usando o SPSS. Porto Alegre, Artmed, 687pp.
- Fragaszy DM, Izar P, Liu Q, Eshchar Y, Young LA, Visalberghi E (2016) Body mass in wild bearded capuchins, (Sapajus libidinosus): Ontogeny and sexual dimorphism. American Journal of Primatology 78(4): 473-484. https://doi.org/10.1002/ajp.22509
» https://doi.org/10.1002/ajp.22509 - Fragazy DM, Visalberghi E, Fedigan LM (2004) The complete capuchin: the biology of genus Cebus Cambridge, Cambridge University Press, 339pp. https://doi.org/10.1007/s10329-005-0129-9
» https://doi.org/10.1007/s10329-005-0129-9 - George D, Mallery P (2016) IBM SPSS Statistics 23 Step by Step: A simple guide and reference. New York, Taylor & Francis, 382 pp. https://doi.org/10.4324/9781315545899
» https://doi.org/10.4324/9781315545899 - Georgiev AV, Klimczuk AC, Traficonte DM, Maestripieri D (2013) When violence pays: A cost-benefit analysis of aggressive behavior in animals and humans. Evolutionary Psychology 11(3): 678-699. https://doi.org/10.1177/147470491301100313
» https://doi.org/10.1177/147470491301100313 - Gilby IC, Brent LJ, Wroblewski E, Rudicell RS, Hahn BH, Goodall J, Pusey A (2013) Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology 67(3): 373-381. https://doi.org/10.1007/s00265-012-1457-6
» https://doi.org/10.1007/s00265-012-1457-6 - Grant JW (1993) Whether or not to defend? The influence of resource distribution. Marine Behaviour and Physiology 23(1-4): 137-153. https://doi.org10.1080/10236249309378862
» https://doi.org10.1080/10236249309378862 - Hirsch BT (2007) Costs and benefits of within-group spatial position: a feeding competition model. The Quarterly Review of Biology 82(1): 9-27. https://doi.org/10.1086/511657
» https://doi.org/10.1086/511657 - Izar P, Verderane MP, Peternelli-dos-Santos L, Mendonça-Furtado O, Presotto A, Tokuda M, Visalberghi E, Fragaszy D (2012) Flexible and conservative features of social systems in tufted capuchin monkeys: Comparing the socioecology of Sapajus libidinosus and Sapajus nigritus American Journal of Primatology 74(4): 315-331. https://doi.org/10.1002/ajp.20968
» https://doi.org/10.1002/ajp.20968 - Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144(12): 1551-1581. https://doi.org/10.1163/156853907782512074
» https://doi.org/10.1163/156853907782512074 - Koch F, Signer J, Kappeler PM, Fichtel C (2016) Intergroup encounters in Verreaux”s sifakas (Propithecus verreauxi): who fights and why? Behavioral Ecology and Sociobiology 70(5): 797-808. https://doi.org/10.1007/s00265-016-2105-3
» https://doi.org/10.1007/s00265-016-2105-3 - Perry S (1996) Intergroup encounters in wild white-faced capuchins (Cebus capucinus). International Journal of Primatology 17(3): 309-330. https://doi.org/10.1007/BF02736624
» https://doi.org/10.1007/BF02736624 - Majolo B, deBortoli Vizioli A, Martínez-Íñigo L, Lehmann J (2020) Effect of group size and individual characteristics on intergroup encounters in primates. International Journal of Primatology 41(2): 325-341. https://doi.org/10.1007/s10764-019-00119-5
» https://doi.org/10.1007/s10764-019-00119-5 - Milton K (1999) Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition 15(6): 488-498. https://doi.org/10.1016/S0899-9007(99)00078-7
» https://doi.org/10.1016/S0899-9007(99)00078-7 - Mirville MO, Ridley AR, Samedi JP, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC (2018) Factors influencing individual participation during intergroup interactions in mountain gorillas. Animal Behaviour 144: 75-86. https://doi.org/10.1016/j.anbehav.2018.08.003
» https://doi.org/10.1016/j.anbehav.2018.08.003 - Scarry CJ (2013) Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Animal Behaviour 85(5): 931-939. https://doi.org/10.1016/j.anbehav.2013.02.013
» https://doi.org/10.1016/j.anbehav.2013.02.013 - Scarry CJ (2016) Intergroup Encounters. In: Fuentes A (Ed.) The International Encyclopedia of Primatology. New Jersey, John Wiley & Sons, 1-6. https://doi.org/10.1002/9781119179313.wbprim0198
» https://doi.org/10.1002/9781119179313.wbprim0198 - Scarry CJ (2017) Male resource defence during intergroup aggression among tufted capuchin monkeys. Animal Behaviour 123: 169-178. https://doi.org/10.1016/j.anbehav.2016.10.015
» https://doi.org/10.1016/j.anbehav.2016.10.015 - Sha JC, Hanya G (2013) Temporal Food resource correlates to the behavior and ecology of food-enhanced long-tailed macaques (Macaca fascicularis). Mammal Study 38(3): 163-175. https://doi.org/10.3106/041.038.0305
» https://doi.org/10.3106/041.038.0305 - Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology and Evolution 21(4): 186-191. https://doi.org/10.1016/j.tree.2005.11.019
» https://doi.org/10.1016/j.tree.2005.11.019 - Spehar SN (2018) Anthropogenic landscapes. In: Trevathan W (Ed.) The International Encyclopedia of Biological Anthropology. New Jersey, John Wiley & Sons, 1-4. https://doi.org/10.1002/9781118584538.ieba0025
» https://doi.org/10.1002/9781118584538.ieba0025 - Sterck EHM (1999) Variation in langur social organization in relation to the socioecological model, human habitat alteration, and phylogenetic constraints. Primates 40(1): 199-213. https://doi.org/10.1007/BF02557711
» https://doi.org/10.1007/BF02557711 - Temeles EJ (1994) The role of neighbours in territorial systems: when are they “dear enemies”? Animal Behaviour 47(2): 339-350. https://doi.org/10.1006/anbe.1994.1047
» https://doi.org/10.1006/anbe.1994.1047 - Trivers RL (1972) Parental investment and sexual selection. In: Campbell BG (Ed.) Sexual selection and the descent of man: The Darwinian pivot. Chicago, Aldine Publishing Company, 136-179. https://psycnet.apa.org/record/1996-98307-040
» https://psycnet.apa.org/record/1996-98307-040 - Vogel ER, Janson CH (2007) Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus), using a novel focal-tree method. American Journal of Primatology 69(5): 533-550. https://doi.org/10.1002/ajp.20368
» https://doi.org/10.1002/ajp.20368 - Watson-Capps JJ (2009) Evolution of sexual coercion with respect to sexual selection and sexual conflict theory. In: Muller MN, Wranghan RW (Eds) Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females. Cambridge, Harvard University Press, 23-41. https://doi.org/10.1086/jar.66.2.27820915
» https://doi.org/10.1086/jar.66.2.27820915 - Wrangham RW (1980) An ecological model of female-bonded primate groups. Behaviour 75(3-4): 262-300. https://doi.org/10.1163/156853980X00447
» https://doi.org/10.1163/156853980X00447
ADDITIONAL NOTES
-
Zoobank register
http://zoobank.org/AFFFDFC3-BD4D-4E48-BCEA-3F53E1A1298C -
How to cite this article
Lousa TC, Mendes FDC (2022) Inter-group conflicts involving adult female and male bearded capuchins, Sapajus libidinosus (Primates: Cebidae) in the context of provisioned resources: resource defense or sexual selection? Zoologia (Curitiba) 39: e21020. https://doi.org/10.1590/S1984-4689.v39.e21020 -
Published by
Sociedade Brasileira de Zoologia at Scientific Electronic Library Online (https://www.scielo.br/zool)
Supplementary Material 1
Table S1. Occurrences of intergroup aggression recorded during the study.
Authors: Túlio C. Lousa, Francisco D.C. Mendes
Data type: behavioral data.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Supplementary Material 2
Table S2. Individuals from social groups divided by sex and age.
Authors: Túlio C. Lousa, Francisco D.C. Mendes
Data type: individuals data.
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Edited by
Editorial responsibility
Publication Dates
-
Publication in this collection
21 Mar 2022 -
Date of issue
2022
History
-
Received
20 Aug 2021 -
Accepted
14 Dec 2021