Abstract
Serrasalmus maculatus is a species of piranha which, despite being abundant in a reservoir environment, has few studies related to its parasitological and diet aspects. Thus, we aimed to document the parasitic fauna and diet of the S. maculatus in a hydroelectric reservoir in Brazil. In addition, we perform two literature reviews for the Neotropical region, recording the parasitic fauna already associated with S. maculatus and the occurrence of parasite genera identified in this study parasitizing Characiformes from other aquatic systems. Thirty-one hosts were collected with gillnets, from August 2014 to September 2016. Serrasalmus maculatus had a piscivorous feeding habit and a low richness parasitic component community, including two taxa of monogeneans, Anacanthorus lepyrophallus and Mymarothecium sp.; no endohelminths were observed. Data from the literature review, together with the findings of the study, showed that S. maculatus in the Neotropical region harbors 25 helminth taxa, with the monogenean being the most prevalent parasitic group and Brazil is the country with the most reports of the parasitic genera. These findings provide information on the relationships between diet, social behavior, and parasitic fauna of S. maculatus and on the patterns of distribution and infection of the observed parasite rates.
Keywords:
Ectoparasites; Anacanthorus; Mymarothecium; freshwater fish; piranha
Resumo
Serrasalmus maculatus é uma espécie de piranha que, a despeito de ser abundante em ambiente de reservatório, possui poucas informações sobre seus aspectos parasitológicos e dieta. Assim, o presente estudo objetivou documentar a fauna parasitária e a dieta de S. maculatus em um reservatório brasileiro. Além disso, foram realizadas duas revisões literárias para a região Neotropical, registrando a fauna parasitária, já associada a S. maculatus e a ocorrência dos gêneros parasitários identificados neste estudo, registrados em outros peixes Characiformes em outros sistemas aquáticos. Foram coletados 31 hospedeiros com redes de espera entre agosto de 2014 e setembro de 2016. Serrasalmus maculatus apresentou hábito alimentar piscívoro e comunidade componente parasitária com baixa riqueza, incluindo dois táxons de monogenéticos, Anacanthorus lepyrophallus e Mymarothecium sp.; não foram observados endohelmintos. Dados da revisão da literatura, juntamente com os achados deste estudo, mostraram que S. maculatus, na região Neotropical, abriga 25 táxons de helmintos, sendo monogenéticos o grupo de parasitos mais prevalente, e o Brasil o país com mais relatos de parasitos. Estes resultados fornecem informações sobre as relações entre dieta, o comportamento social e a fauna parasitária de S. maculatus e sobre os padrões de distribuição e infecção das taxas de parasitos observadas.
Palavras-chave:
Ectoparasitos; Anacanthorus; Mymarothecium; peixe de água doce; piranha
Introduction
Parasites can influence local communities by affecting host physiology, morphology, reproduction, and behaviour, thereby affecting population, community, and ecosystem structures, and host behaviours (e.g., feeding habits and predator-prey relationships) in turn, can affect the structures of parasite communities (Timi & Poulin, 2020Timi JT, Poulin R. Why ignoring parasites in fish ecology is a mistake. Int J Parasitol 2020; 50(10-11): 755-761. http://dx.doi.org/10.1016/j.ijpara.2020.04.007. PMid:32592807.
http://dx.doi.org/10.1016/j.ijpara.2020....
). However, even though the ecological relevance of parasitism is widely recognised, many studies have neglected the effects of these organisms on their hosts (Timi & Poulin, 2020Timi JT, Poulin R. Why ignoring parasites in fish ecology is a mistake. Int J Parasitol 2020; 50(10-11): 755-761. http://dx.doi.org/10.1016/j.ijpara.2020.04.007. PMid:32592807.
http://dx.doi.org/10.1016/j.ijpara.2020....
). For example, even though Brazil harbours a megadiverse freshwater ichthyofauna (~3500 species) (Froese & Pauly, 2020aFroese R, Pauly D. List of Freshwater Fishes reported from Brazil [online]. FishBase; 2020a [cited 2020 Aug 26]. Available from: https://www.fishbase.de/Country/CountryChecklist.php?c_code=076&vhabitat=fresh⫏_code=&cpresence=present
https://www.fishbase.de/Country/CountryC...
), the parasitology of only 13% of the region’s species has been evaluated, of which the majority are economically important species. Nevertheles little is known about the parasitology of fish species with low commercial importance (Eiras et al., 2010Eiras JC, Takemoto RM, Pavanelli GC. Diversidade de peixes de água doce do Brasil. Maringa: Eduem; 2010., 2011Eiras JC, Takemoto RM, Pavanelli GC, Adriano EA. About the biodiversity of parasites of freshwater fish from Brazil. Bull Eur Assoc Fish Pathol 2011; 31(4): 161-168.).
The piranha, or pirambeba, Serrasalmus maculatus (Kner, 1858) is a medium-sized freshwater fish belonging to Characiformes, that is widely distributed in South America, throughout both the Amazon and Paraguay-Paraná River basins (Froese & Pauly, 2020bFroese R, Pauly D. Serrasalmus maculatus Kner, 1858. [online]. FishBase; 2020b [cited 2020 Aug 26]. Available from: https://www.fishbase.de/summary/serrasalmus-maculatus.html
https://www.fishbase.de/summary/serrasal...
). The species is piscivorous, preferentially consuming fish musculature, fins, and scales. Eventually, invertebrates are the speciesmost common prey (Agostinho & Marques, 2001Agostinho CS, Marques EE. Selection of netted prey by piranhas, Serrasalmus spilopleura and S. marginatus (Pisces, Serrasalmidae). Acta Sci Biol Sci 2001; 23(2): 461-464.; Agostinho et al., 2003Agostinho CS, Hahn NS, Marques EE. Patterns of food resource use by two congeneric species of piranhas (Serrasalmus) on the Upper Paraná River floodplain. Braz J Biol 2003; 63(2): 177-182. http://dx.doi.org/10.1590/S1519-69842003000200002. PMid:14509839.
http://dx.doi.org/10.1590/S1519-69842003...
; Villares et al., 2008Villares GA, Gomiero LM, Goitein R. Feeding of Serrasalmus maculatus (Kner, 1858) (Characiformes; Serrasalmidae) in the Sorocaba river, São Paulo State, Brazil. Acta Sci Biol Sci 2008; 30(3): 267-273. http://dx.doi.org/10.4025/actascibiolsci.v30i3.5011.
http://dx.doi.org/10.4025/actascibiolsci...
). It is also generally gregarious and, although has low economic importance, is one of the most abundant species in hydroelectric reservoirs, because readily adapts to artificial lentic environments (Sazima & Machado, 1990Sazima I, Machado FA. Underwater observations of piranhas in western Brazil. Environ Biol Fishes 1990; 28(1-4): 17-31. http://dx.doi.org/10.1007/BF00751026.
http://dx.doi.org/10.1007/BF00751026...
; Hoffmann et al., 2005Hoffmann AC, Orsi ML, Shibatta AO. Diversidade de peixes do reservatório da UHE Escola Engenharia Mackenzie (Capivara), Rio Paranapanema, bacia do alto rio Paraná, Brasil, e a importância dos grandes tributários na sua manutenção. Iheringia Ser Zool 2005; 95(3): 319-325. http://dx.doi.org/10.1590/S0073-47212005000300012.
http://dx.doi.org/10.1590/S0073-47212005...
; Behr & Signor, 2008Behr ER, Signor CA. Distribuição e alimentação de duas espécies simpátricas de piranhas Serrasalmus maculatus e Pygocentrus nattereri (Characidae, Serrasalminae) do rio Ibicuí, Rio Grande do Sul, Brasil. Iheringia Ser Zool 2008; 98(4): 501-507. http://dx.doi.org/10.1590/S0073-47212008000400014.
http://dx.doi.org/10.1590/S0073-47212008...
). Despite the abundance of S. maculatus in hydroelectric reservoirs, there are few studies on its parasitological aspects.
Most studies of the parasitology of S. maculatus have focused on populations in the Upper Paraná River floodplain region (Pavanelli et al., 1997Pavanelli GC, Machado MH, Takemoto RM. Fauna helmíntica de peixes do rio Paraná, região de Porto Rico, Paraná. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A Planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá: Eduem; 1997. p. 307-329., 2004Pavanelli GC, Machado MH, Takemoto RM, Guidelli GM, Lizama MAP. Helminth fauna of fishes: diversity and ecological aspects. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The Upper Paraná river and its Floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004. p. 309-329.; Takemoto et al., 2009Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, et al. Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 2009;69(2 Suppl 2): 691-705. http://dx.doi.org/10.1590/S1519-69842009000300023. PMid:19738975.
http://dx.doi.org/10.1590/S1519-69842009...
; Casali & Takemoto, 2016Casali GP, Takemoto RM. Endoparasitic fauna of Serrasalmus spp. (Characidae: Serrasalminae) in a neotropical floodplain. Acta Sci Biol Sci 2016; 38(1): 105-112. http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592.
http://dx.doi.org/10.4025/actascibiolsci...
; Moreira et al., 2019Moreira J, da Silva Carneiro J, Ruz EJH, Luque JL. New Species and Records of Anacanthorus (Monogenea: Dactylogyridae) Parasitizing serrasalmid fish (Characiformes) from Brazil, including molecular data. Acta Parasitol 2019; 64(3): 449-455. http://dx.doi.org/10.2478/s11686-019-00055-7. PMid:31020494.
http://dx.doi.org/10.2478/s11686-019-000...
), and few studies have examined this species ecology or parasitology in artificial environments. In addition, considering the diet is an important factor in host-parasite interactions and hosts with more diverse diets tend to be more susceptible to endoparasite infections (Lima et al., 2016Lima LB, Bellay S, Giacomini HC, Isaac A, Lima-Junior DP. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 2016; 143(3): 343-349. http://dx.doi.org/10.1017/S003118201500164X. PMid:26647725.
http://dx.doi.org/10.1017/S0031182015001...
), we aimed (i) document the parasitic fauna and (ii) characterize the diet of S. maculatus in a hydroelectric reservoir in Brazil. We targeted also (iii) to verify the parasite fauna already associated with S. maculatus in the Neotropical region; and (iv) the occurrence of parasite genera - identified in the present study - in characiform fishes from other aquatic systems (natural or artificial) in the Neotropical region.
Material and Methods
Study area
The Ilha Solteira hydroelectric reservoir is an accumulation basin that was formed in 1978 and is situated along the Upper Paraná River, between the states of São Paulo, Minas Gerais, and Mato Grosso do Sul, Brazil (Figure 1). With a mean depth of 17.6 m, maximum volume of21.06 × 109 m3, hydrographic basin area of 1195 km2, and residence time of 46.7 days, it is one of the largest artificial reservoirs in the neotropics (Garcia et al., 2014Garcia F, Kimpara JM, Valenti WC, Ambrosio LA. Emergy assessment of tilapia cage farming in a hydroelectric reservoir. Ecol Eng 2014; 68: 72-79. http://dx.doi.org/10.1016/j.ecoleng.2014.03.076.
http://dx.doi.org/10.1016/j.ecoleng.2014...
). For the present study, host sampling was conducted in the Can-Can arm in municipality of Santa Clara D’Oeste, São Paulo state, Brazil (50° 55ʹ 59.65″ W and 20° 02ʹ 30.54″ S).
Study area on Ilha Solteira hydroelectric reservoir, Upper Paraná River basin, São Paulo state, Brazil (Campos et al., 2020Campos DWJ, Manoel LO, Franceschini L, Veríssimo-Silveira R, Delariva RL, Ribeiro CS, et al. Occurrence of metacercariae of Austrodiplostomum compactum (Lutz, 1928) (Trematoda, Diplostomidae) in Pimelodus platicirris in the Ilha Solteira Reservoir, São Paulo, Brazil. An Acad Bras Cienc 2020;92(Suppl 2):e20180649. http://dx.doi.org/10.1590/0001-3765202020180649.
http://dx.doi.org/10.1590/0001-376520202... ).
Host sampling
Serrasalmus maculatus specimens were collected using gill nets (3, 4, 5, 6, 7, 8, 10, 12 and 14 cm between non-adjacent nodes) between August 2014 to September 2016 (authorization SISBio nº 42229-1). The collected specimens were euthanized (Authorization CEUA/FEIS nº 001/2014 and Certified SisGen A9038DB) and identified as described by Ota et al. (2018)Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol 2018; 16(2): e170094. http://dx.doi.org/10.1590/1982-0224-20170094.
http://dx.doi.org/10.1590/1982-0224-2017...
. The total weight (g, with viscera) and standard length (cm, from snout to last vertebra) of each specimen were recorded, and the fish were subsequently individually stored in plastic bags, frozen and sent to the laboratory for additional analyses. All measurements are expressed as the mean ± standard deviation followed by the range.
Parasitological procedures
The organs (skin, fins, nasal cavities, gills, eyes, heart, liver, gonads, intestines, swim bladder, spleen, gallbladder, and mesentery) were analysed for parasitological procedures, using a stereomicroscope, and parasites preserved in 70% ethanol or mounted on semipermanent slides using Gray and Wess medium. The parasite specimens were then subject to morphological analysis, using a computerised image analysis system with differential interference contrast (DIC) - LAS V3 (Leica Application Suite V3; Leica Microsystems, Wetzlar, Germany) and identified according to Kritsky et al. (1992)Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from Characoid Fishes of the Central Amazon. J Helminthol Soc Wash 1992; 59(1): 25-51. and Kritsky et al. (1996)Kritsky DC, Boeger WA, Jégu M. Neotropical Monogenoidea. 28. Ancyrocephalinae (Dactylogyridae) of piranha and their relatives (Teleostei, Serrasalmidae) from Brazil and French Guiana: Species of Notozothecium Boeger and Kritsky, 1988, and Mymarothecium gen. n. J Helminthol Soc Wash 1996; 63(1): 153-175.. Parasite prevalence (P, in percentage), mean intensity of infestation (MII), and mean abundance (MA) were then calculated according to Bush et al. (1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227. PMid:9267395.
http://dx.doi.org/10.2307/3284227...
. Mean intensity of infestation and mean abundance are expressed as the mean ± standard error followed by the range.
The host and parasite voucher specimens were deposited in the Fish Collection of São Paulo State University (UNESP), Campus of São José do Rio Preto, São Paulo state, Brazil (DZSJRP 21374), and the Helminthological Collection of the Institute of Bioscience, Section of Parasitology, UNESP, Campus of Botucatu, São Paulo state, Brazil, (Mymarothecium sp. - CHIBB 652 L‒655 L; Anacanthorus lepyrophallus - CHIBB 656 L‒663 L), respectively.
Literature review
Two literature reviews were conducted to verify the parasite fauna already associated with S. maculatus in the Neotropical region; and to verify the occurrence of parasite genera - identified in the present study - in characiform fishes from other aquatic systems (natural or artificial) in the Neotropical region. In the first review, we collected data on the helminth fauna previously reported for S. maculatus and its synonymy (= Serrasalmus spilopleura Kner, 1860) from the Neotropical region, from the first report in 1997 to 2021. In the second review, we collected data regarding the occurrence of monogenean species belonging to Anacanthorus and Mymarothecium genera in S. maculatus, as well as in other characiforms from the Neotropical region, from the first report of each genus (1965 to 2021 for Anacanthorus, and 1996 to 2021 for Mymarothecium).
The literature reviews were performed by searching relevant databases (SciELO, ISI, Scopus, Google Scholar, and WoRMS) for relevant terms: Serrasalmus, piranha, pirambeba, fish parasite, helminth, Monogenea, Dactylogyridae, Gyrodactylidae, Nematoda, Cestoda, Acanthocephala, Trematoda, Digenea, digenetic, digenean, monogenetic, monogenean, cestode, acanthocephalan, Anacanthorus, and Mymarothecium. All common names were searched using both singular and plural forms in English, Portuguese, and Spanish.
Diet analysis
The stomachs of the host specimens were removed, fixed in 4% formaldehyde, and preserved in 70% alcohol, and stomach contents were analysed using an optical stereomicroscope. Recovered food items were quantified using the volumetric method (displacement of each measured food item from stomach contents using a gridded Petri dish) (Hyslop, 1980Hyslop EJ. Stomach contents analysis – a review of methods and their application. J Fish Biol 1980; 17(4): 411-429. http://dx.doi.org/10.1111/j.1095-8649.1980.tb02775.x.
http://dx.doi.org/10.1111/j.1095-8649.19...
). Glass slides were used to compress food items to 1.0 mm in height, and the number of quadrants occupied by each food item was multiplied by 0.001 to calculate the volume in ml (Hellawell & Abel, 1971Hellawell JM, Abel R. A rapid volumetric method for the analysis of the food fishes. J Fish Biol 1971; 3(1): 20-37. http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x.
http://dx.doi.org/10.1111/j.1095-8649.19...
). All food items were identified to lowest possible taxonomic (Bicudo & Bicudo, 1970Bicudo CEM, Bicudo RMT. Algas de águas continentais brasileiras chave ilustrada para identificação de gêneros. São Paulo: Fundação Brasileira para o Desenvolvimento do Ensino de Ciências; 1970.; Mugnai et al., 2010Mugnai R, Nessemian JL, Baptista DF. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro. Rio de Janeiro: Technical Books Editora; 2010.; Ota et al., 2018Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol 2018; 16(2): e170094. http://dx.doi.org/10.1590/1982-0224-20170094.
http://dx.doi.org/10.1590/1982-0224-2017...
).
Results
The weight and standard length of the 31 S. maculatus specimens ranged from 32.24 to 650.40 g (139.95 ± 24.42 g) and from 9.5 to 24.0 cm (14.44 ± 0.53), respectively.
The richness of the S. maculatus component parasite community was low and included two monogenean ectoparasites from gills, belonging to Dactylogyridae: Anacanthorus lepyrophallus (P = 84.2%, MII = 7.51 ± 1.50 [1–35], MA = 6.54 ± 1.38 [0–35]) and Mymarothecium sp. (P = 10.5%, MII = 2.33 ± 1.33 [1–7], MA = 0.22 ± 0.92 [0–7]). A total of 210 specimens were collected, and the overall P, MII, and MA of the parasites were 87.09%, 7.78 ± 1.48 (1–35), and 6.77 ± 1.37 (0–35), respectively. No endohelminths were recorded.
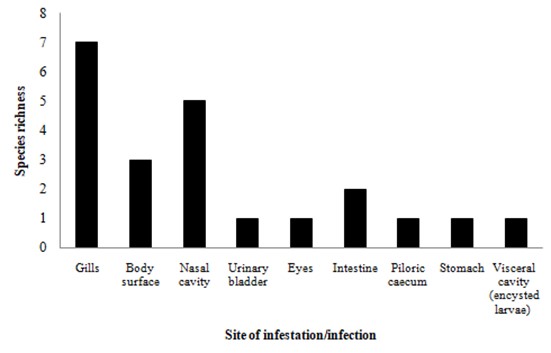
Data from the literature review jointly with data from the specimens evaluated here demonstrated that S. maculatus in the Neotropical region harbour 25 helminth taxa (Table 1). Of these 25 taxa, 10 are monogeneans, nine nematodes, three digeneans, two acanthocephalans, and one cestode (Figure 2). Monogeneans most commonly infect host gills, followed by the nasal cavities and body surface (mucus), whereas the endohelminth groups with higher richness, nematodes and acanthocephalans, most commonly infect host intestines (Table 1 and Figure 3). Furthermore, the majority (16/25) of parasite taxa were reported from the Upper Paraná River floodplain in Brazil.
Helminth parasites reported from the piranha Serrasalmus maculatus* * Parasitological reports from the Paraná River basin address the species Serrasalmus maculatus and Serrasalmus spilopleura Kner, 1860, as they were all synonymized with S. maculatus (Jégu & dos Santos, 2001; Rossin et al., 2019). However, for the Northern Brazil basin, the identification of S. spilopleura is still valid, so records of S. spilopleura in the northern basins were not included in the review. Furthermore, it is noteworthy that the occurrence of S. maculatus is recorded for the Amazon and Paraguay-Paraná River basins (Froese & Pauly, 2020b), while S. spilopleura is restricted to the basins of the Northern region of Brazil (Jégu & dos Santos, 2001). in Neotropical region.
Taxonomic distribution of parasitic fauna reported from the piranha Serrasalmus maculatus in Neotropical region.
Species richness of parasites reported in Serrasalmus maculatus from Neotropical region, according with their site of infection.
Monogenean species belonging to Anacanthorus and Mymarothecium in Neotropical hosts comprise 101 species (Table 2 and Figure 4). The genus Anacanthorus includes ~92 valid species (Table 2 and Figure 4), which are gill parasites of characiform fishes of the Serrasalmidae (41 species), Triportheidae (20 species), Bryconidae (19 species), Erythrinidae (eight species), and Characidae (four species). Brazil harbours the greatest number of Anacanthorus taxa (84 species). Meanwhile, the genus Mymarothecium includes nine species, which are also parasites of characiform fishes of the family Serrasalmidae, specifically of the genera Serrasalmus (four species), and Piaractus (two species), from Brazil, Peru, and Bolivia (Table 2 and Figure 4)
Checklist of valid species of monogeneans belonging to Anacanthorus and Mymarothecium (Dactylogyridae) reported in characiform fishes from Neotropical region.
Monogeneans belonging to Anacanthorus and Mymarothecium (Dactylogyridae) reported from Neotropical characiform fishes.
Stomach content analysis resulted in the identification of ten food items, which mostly included fish fragments (81.7%) but also included terrestrial plants and decapods (Macrobrachium sp.) (Table 3). Serrasalmus maculatus showed piscivorous food habits, due to the predominant consumption of fish fragments (81.7%).
Dietary components of piranha Serrasalmus maculatus specimens collected from the Ilha Solteira hydroelectric reservoir, Upper Paraná River basin, São Paulo state, Brazil.
Discussion
This is the first study to report the parasitic fauna of S. maculatus from the northwest region of the Upper Paraná River basin, São Paulo, Brazil. In addition, represents the first report of monogeneans belonging to Mymarothecium in this host species and first report of Anacanthorus lepyrophallus in the Ilha Solteira Reservoir. For monogeneans that parasitise fish gills, the phylogenetic relationships and evolutionary history between host orders are important factors for host-parasite interaction and distribution (Braga et al., 2014Braga MP, Araújo SBL, Boeger WA. Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitol Res 2014; 113(2): 481-490. http://dx.doi.org/10.1007/s00436-013-3677-8. PMid:24221891.
http://dx.doi.org/10.1007/s00436-013-367...
).
Previous studies have demonstrated that most monogeneans prefer to parasitise specific host lineages (Graça et al., 2018Graça RJ, Fabrin TMC, Gasques LS, Prioli SMAP, Balbuena JA, Prioli AJ, et al. Topological congruence between phylogenies of Anacanthorus spp. (Monogenea: Dactylogyridae) and their Characiformes (Actinopterygii) hosts: A case of host-parasite cospeciation. PLoS One 2018; 13(3): e0193408. http://dx.doi.org/10.1371/journal.pone.0193408. PMid:29538463.
http://dx.doi.org/10.1371/journal.pone.0...
; Moreira et al., 2019Moreira J, da Silva Carneiro J, Ruz EJH, Luque JL. New Species and Records of Anacanthorus (Monogenea: Dactylogyridae) Parasitizing serrasalmid fish (Characiformes) from Brazil, including molecular data. Acta Parasitol 2019; 64(3): 449-455. http://dx.doi.org/10.2478/s11686-019-00055-7. PMid:31020494.
http://dx.doi.org/10.2478/s11686-019-000...
) (e.g., Mymarothecium taxa parasitise members of the Serrasalmidae) (Braga et al., 2015Braga MP, Razzolini E, Boeger WA. Drivers of parasite sharing among Neotropical freshwater fishes. J Anim Ecol 2015; 84(2): 487-497. http://dx.doi.org/10.1111/1365-2656.12298. PMid:25283218.
http://dx.doi.org/10.1111/1365-2656.1229...
). However, in some cases, members of other monogenean families have been reported to colonize phylogenetically distant hosts. In both cases, host-parasite relationships result from a combination of factors, including cospeciation, host-switching, and ecological fitting (Janzen, 1985Janzen DH. On ecological fitting. Oikos 1985; 45(3): 308-310. http://dx.doi.org/10.2307/3565565.
http://dx.doi.org/10.2307/3565565...
; Brooks et al., 2006Brooks DR, León-Règagnon V, McLennan DA, Zelmer D. Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 2006;87(7 Suppl): S76-S85. http://dx.doi.org/10.1890/0012-9658(2006)87[76:EFAADO]2.0.CO;2. PMid:16922304.
http://dx.doi.org/10.1890/0012-9658(2006...
; Braga et al., 2014Braga MP, Araújo SBL, Boeger WA. Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitol Res 2014; 113(2): 481-490. http://dx.doi.org/10.1007/s00436-013-3677-8. PMid:24221891.
http://dx.doi.org/10.1007/s00436-013-367...
, 2015Braga MP, Razzolini E, Boeger WA. Drivers of parasite sharing among Neotropical freshwater fishes. J Anim Ecol 2015; 84(2): 487-497. http://dx.doi.org/10.1111/1365-2656.12298. PMid:25283218.
http://dx.doi.org/10.1111/1365-2656.1229...
). Considering the monophyly of the Characiformes and the diversification of the group only in the continental neotropics, the phylogenetic contiguity between the order’s families may indicate the sharing of a range of intrinsic resources (Braga et al., 2015Braga MP, Razzolini E, Boeger WA. Drivers of parasite sharing among Neotropical freshwater fishes. J Anim Ecol 2015; 84(2): 487-497. http://dx.doi.org/10.1111/1365-2656.12298. PMid:25283218.
http://dx.doi.org/10.1111/1365-2656.1229...
). Anacanthorus spp. are widely distributed in hosts of the five families of the order Characiformes (Figure 4). The sharing of resources (e.g., phylogenetic conservatism and phenotypic flexibility) may have favoured its occurrence within individuals of the same order and family (see Braga et al., 2014Braga MP, Araújo SBL, Boeger WA. Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitol Res 2014; 113(2): 481-490. http://dx.doi.org/10.1007/s00436-013-3677-8. PMid:24221891.
http://dx.doi.org/10.1007/s00436-013-367...
, 2015Braga MP, Razzolini E, Boeger WA. Drivers of parasite sharing among Neotropical freshwater fishes. J Anim Ecol 2015; 84(2): 487-497. http://dx.doi.org/10.1111/1365-2656.12298. PMid:25283218.
http://dx.doi.org/10.1111/1365-2656.1229...
and cited references).
The predominance of monogeneans in S. maculatus in Neotropical region could be associated with both the parasites’ monoxenous biology and host species’ gregarious habit (Sazima & Machado, 1990Sazima I, Machado FA. Underwater observations of piranhas in western Brazil. Environ Biol Fishes 1990; 28(1-4): 17-31. http://dx.doi.org/10.1007/BF00751026.
http://dx.doi.org/10.1007/BF00751026...
; Strona, 2015Strona G. The underrated importance of predation in transmission ecology of direct lifecycle parasites. Oikos 2015; 124(6): 685-690. http://dx.doi.org/10.1111/oik.01850.
http://dx.doi.org/10.1111/oik.01850...
). Indeed, the proximity of fish in shoals can facilitate monogenean transmission, which occurs through simple contact between hosts (Thatcher, 2006Thatcher VE. Amazon fish parasites. 2nd ed. Sofia: Pensoft Publishers; 2006.). Furthermore, gregarious behaviour also allows free-native larval forms (oncomiracidia) to locate hosts more easily (Thatcher, 2006Thatcher VE. Amazon fish parasites. 2nd ed. Sofia: Pensoft Publishers; 2006.), which would justify the results observed in the present study.
The low parasite richness and absence of endoparasites observed in the present study may be related to host behaviour and/or foraging. Several studies have reported that heteroxenous parasites are transmitted via food web interactions and that intermediate hosts are nearly always dietary components of the parasites’ definitive hosts (Luque & Poulin, 2008Luque JL, Poulin R. Linking ecology with parasite diversity in Neotropical fishes. J Fish Biol 2008; 72(1): 189-204. http://dx.doi.org/10.1111/j.1095-8649.2007.01695.x.
http://dx.doi.org/10.1111/j.1095-8649.20...
; Lima et al., 2016Lima LB, Bellay S, Giacomini HC, Isaac A, Lima-Junior DP. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 2016; 143(3): 343-349. http://dx.doi.org/10.1017/S003118201500164X. PMid:26647725.
http://dx.doi.org/10.1017/S0031182015001...
). Therefore, host diet is considered an important factor in host-parasite interactions, and hosts with more diverse diets tend to be more susceptible to endoparasite infections and, thus, usually harbour greater parasite richness (Lima et al., 2016Lima LB, Bellay S, Giacomini HC, Isaac A, Lima-Junior DP. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 2016; 143(3): 343-349. http://dx.doi.org/10.1017/S003118201500164X. PMid:26647725.
http://dx.doi.org/10.1017/S0031182015001...
).
The dietary components of S. maculatus identified in the present study were like the findings of previous studies in the Upper Paraná floodplain, including the Ibicuí River, Rio Grande do Sul state, and a lower stretch of the Sorocaba River basin, São Paulo state, Brazil (Agostinho & Marques, 2001Agostinho CS, Marques EE. Selection of netted prey by piranhas, Serrasalmus spilopleura and S. marginatus (Pisces, Serrasalmidae). Acta Sci Biol Sci 2001; 23(2): 461-464.; Agostinho et al., 2003Agostinho CS, Hahn NS, Marques EE. Patterns of food resource use by two congeneric species of piranhas (Serrasalmus) on the Upper Paraná River floodplain. Braz J Biol 2003; 63(2): 177-182. http://dx.doi.org/10.1590/S1519-69842003000200002. PMid:14509839.
http://dx.doi.org/10.1590/S1519-69842003...
; Behr & Signor, 2008Behr ER, Signor CA. Distribuição e alimentação de duas espécies simpátricas de piranhas Serrasalmus maculatus e Pygocentrus nattereri (Characidae, Serrasalminae) do rio Ibicuí, Rio Grande do Sul, Brasil. Iheringia Ser Zool 2008; 98(4): 501-507. http://dx.doi.org/10.1590/S0073-47212008000400014.
http://dx.doi.org/10.1590/S0073-47212008...
; Villares et al., 2008Villares GA, Gomiero LM, Goitein R. Feeding of Serrasalmus maculatus (Kner, 1858) (Characiformes; Serrasalmidae) in the Sorocaba river, São Paulo State, Brazil. Acta Sci Biol Sci 2008; 30(3): 267-273. http://dx.doi.org/10.4025/actascibiolsci.v30i3.5011.
http://dx.doi.org/10.4025/actascibiolsci...
). Serrasalmus maculatus is piscivorous, preferentially ingesting fish fragments (instead of ingesting the host's entire body), and its feeding behaviour includes the mutilation of prey scales, fins, and muscle tissue, which we infer can hinder the ingestion of endoparasites (Sazima & Pombal-Jr, 1988Sazima I, Pombal-Jr JP. Mutilação de nadadeiras em acarás, Geophagus brasiliensis, por piranhas, Serrasalmus spilopleura. Rev Bras Biol 1988; 48(3): 477-483.; Sazima & Machado, 1990Sazima I, Machado FA. Underwater observations of piranhas in western Brazil. Environ Biol Fishes 1990; 28(1-4): 17-31. http://dx.doi.org/10.1007/BF00751026.
http://dx.doi.org/10.1007/BF00751026...
; Casali & Takemoto, 2016Casali GP, Takemoto RM. Endoparasitic fauna of Serrasalmus spp. (Characidae: Serrasalminae) in a neotropical floodplain. Acta Sci Biol Sci 2016; 38(1): 105-112. http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592.
http://dx.doi.org/10.4025/actascibiolsci...
). In the present study, the dietary components of S. maculatus were fish fragments, terrestrial plants, and decapods (Macrobrachium sp.). However, even though Macrobrachium sp. is one of the most common of S. maculatus’ prey items, this genus of shrimp is native from Amazon basin (Collart & Moreira, 1993Collart OO, Moreira LC. Potencial pesqueiro de Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro): variação da abundância e do comprimento. Amazoniana 1993; 12(3/4): 399-413.), and was introduced in Paraná River basin (Bialetzki et al., 1997Bialetzki A, Nakatani K, Baumgartner G, Bond-Buckup G. Occurrence of Macrobrachium amazonicum (Heller) (Decapoda, Palaemonidae) in Leopoldo’s inlet (Ressaco do Leopoldo), upper Paraná River, Porto Rico, Paraná. Rev Bras Zool 1997; 14(2): 379-390. http://dx.doi.org/10.1590/S0101-81751997000200011.
http://dx.doi.org/10.1590/S0101-81751997...
). When a species is introduced to a new area, it may lose part of its natural parasite fauna (i.e., Enemy Release Hypothesis - Keane & Crawley, 2002Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 2002; 17(4): 164-170. http://dx.doi.org/10.1016/S0169-5347(02)02499-0.
http://dx.doi.org/10.1016/S0169-5347(02)...
; Tourchin et al., 2002Tourchin ME, Lafferty KD, Kuris AM. Parasites and marine invasions. Parasitology 2002;124(7 Suppl): 137-151. http://dx.doi.org/10.1017/S0031182002001506. PMid:12396221.
http://dx.doi.org/10.1017/S0031182002001...
; Mitchell & Power, 2003Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature 2003; 421(6923): 625-627. http://dx.doi.org/10.1038/nature01317. PMid:12571594.
http://dx.doi.org/10.1038/nature01317...
; Torchin et al., 2003Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature 2003; 421(6923): 628-630. http://dx.doi.org/10.1038/nature01346. PMid:12571595.
http://dx.doi.org/10.1038/nature01346...
) and, thereby, break the natural network of complex interactions between intermediate and definitive hosts, which alters the infection dynamics and enables the loss of parasite taxa (Madi & Ueta, 2009Madi RR, Ueta MT. O papel de Ancyrocephalinae (Monogenea: Dactylogyridae), parasito de Geophagus brasiliensis (Pisces: Cichlidae), como indicador ambiental. Rev Bras Parasitol Vet 2009; 18(2): 38-41. http://dx.doi.org/10.4322/rbpv.01802008. PMid:19602315.
http://dx.doi.org/10.4322/rbpv.01802008...
).
Several authors have reported rich endoparasite fauna for S. maculatus in the Upper Paraná River floodplain, whereas endoparasites were completely absent in the present study, and the richness of ectoparasites was low (Pavanelli et al., 1997Pavanelli GC, Machado MH, Takemoto RM. Fauna helmíntica de peixes do rio Paraná, região de Porto Rico, Paraná. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A Planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá: Eduem; 1997. p. 307-329.; Pavanelli et al., 2004Pavanelli GC, Machado MH, Takemoto RM, Guidelli GM, Lizama MAP. Helminth fauna of fishes: diversity and ecological aspects. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The Upper Paraná river and its Floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004. p. 309-329.; Takemoto et al., 2009Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, et al. Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 2009;69(2 Suppl 2): 691-705. http://dx.doi.org/10.1590/S1519-69842009000300023. PMid:19738975.
http://dx.doi.org/10.1590/S1519-69842009...
; Casali & Takemoto, 2016Casali GP, Takemoto RM. Endoparasitic fauna of Serrasalmus spp. (Characidae: Serrasalminae) in a neotropical floodplain. Acta Sci Biol Sci 2016; 38(1): 105-112. http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592.
http://dx.doi.org/10.4025/actascibiolsci...
‒ see Table 1). It is possible that the dynamics of parasitic infections are negatively affected by abiotic and biotic homogenisation in artificial habitats (Agostinho et al., 2007Agostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio HF Jr. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health Manage 2007; 10(2): 174-186. http://dx.doi.org/10.1080/14634980701341719.
http://dx.doi.org/10.1080/14634980701341...
), such as hydroelectric reservoirs, especially for endoparasites with heteroxenous life cycles.
Floodplains are highly dynamic and complex systems because they include a wide variety of aquatic habitats (e.g., rivers, lakes, and canals) (Junk, 1980Junk WJ. Áreas inundáveis: um desafio para limnologia. Acta Amaz 1980; 10(4): 775-795. http://dx.doi.org/10.1590/1809-43921980104775.
http://dx.doi.org/10.1590/1809-439219801...
; Power et al., 1995Power ME, Sun A, Parker G, Dietrich WE, Wootton JT. Hydraulic Food-chain Models: an approach to the study of food -web dynamics in large rivers. Bioscience 1995; 45(3): 159-167. http://dx.doi.org/10.2307/1312555.
http://dx.doi.org/10.2307/1312555...
), when compared to artificial reservoirs, since the hydrodynamics and biotic communities of such last environments are altered during the damming process. The conversion of lotic to lentic environments involves a series of negative biotic and abiotic impacts, including changes in flow and channel granulometry, increases in fish mortality, increased predation rates, simplification of trophic chains, interruption of fish migration, eutrophication, deterioration of water quality, reduction of benthic community stability, colonisation by macrophytes, invasion by non-native species, and simplification of habitats (Agostinho et al., 1992Agostinho AA, Júlio Júnior HF, Borghetti JR. Considerações sobre os impactos dos represamentos na ictiofauna e medidas para sua atenuação. Um estudo de caso: reservatório de Itaipu. Rev Unimar 1992; 14(Suppl): 89-107.; 2008Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 2008;68(4 Suppl): 1119-1132. http://dx.doi.org/10.1590/S1519-69842008000500019. PMid:19197482.
http://dx.doi.org/10.1590/S1519-69842008...
). Furthermore, these changes can ultimately reduce the abundance and richness of local biota, disrupt the dynamics of host-parasite relationships, and, consequently, alter the structure of parasitic communities (Morley, 2007Morley NJ. Anthropogenic effects of reservoir construction on the parasite fauna of aquatic wildlife. EcoHealth 2007; 4(4): 374-383. http://dx.doi.org/10.1007/s10393-007-0130-4.
http://dx.doi.org/10.1007/s10393-007-013...
), and these seem to be the drivers involved here regarding the low parasite richness observed for S. maculatus.
In summary, the richness of the component parasite community of S. maculatus in the Ilha Solteira hydroelectric reservoir in Brazil was low, in contrast to what has been previously reported in other water environments (Pavanelli et al., 1997Pavanelli GC, Machado MH, Takemoto RM. Fauna helmíntica de peixes do rio Paraná, região de Porto Rico, Paraná. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A Planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá: Eduem; 1997. p. 307-329., 2004Pavanelli GC, Machado MH, Takemoto RM, Guidelli GM, Lizama MAP. Helminth fauna of fishes: diversity and ecological aspects. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The Upper Paraná river and its Floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004. p. 309-329.; Takemoto et al., 2009Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, et al. Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 2009;69(2 Suppl 2): 691-705. http://dx.doi.org/10.1590/S1519-69842009000300023. PMid:19738975.
http://dx.doi.org/10.1590/S1519-69842009...
; Casali & Takemoto, 2016Casali GP, Takemoto RM. Endoparasitic fauna of Serrasalmus spp. (Characidae: Serrasalminae) in a neotropical floodplain. Acta Sci Biol Sci 2016; 38(1): 105-112. http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592.
http://dx.doi.org/10.4025/actascibiolsci...
). These findings provide insight into the relationships between S. maculatus diet, social behaviour, and parasite fauna and the distribution and infection patterns of the observed parasite taxa. The present study also illustrates the possible effects of habitat homogenisation on parasite infection dynamics in artificial reservoirs. However, additional multidisciplinary research is needed to elucidate the effects of biotic and abiotic factors on the structure and dynamics of component communities of fish parasites in natural and artificial habitats in the neotropics.
References
- Agostinho AA, Júlio Júnior HF, Borghetti JR. Considerações sobre os impactos dos represamentos na ictiofauna e medidas para sua atenuação. Um estudo de caso: reservatório de Itaipu. Rev Unimar 1992; 14(Suppl): 89-107.
- Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 2008;68(4 Suppl): 1119-1132. http://dx.doi.org/10.1590/S1519-69842008000500019 PMid:19197482.
» http://dx.doi.org/10.1590/S1519-69842008000500019 - Agostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio HF Jr. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health Manage 2007; 10(2): 174-186. http://dx.doi.org/10.1080/14634980701341719
» http://dx.doi.org/10.1080/14634980701341719 - Agostinho CS, Hahn NS, Marques EE. Patterns of food resource use by two congeneric species of piranhas (Serrasalmus) on the Upper Paraná River floodplain. Braz J Biol 2003; 63(2): 177-182. http://dx.doi.org/10.1590/S1519-69842003000200002 PMid:14509839.
» http://dx.doi.org/10.1590/S1519-69842003000200002 - Agostinho CS, Marques EE. Selection of netted prey by piranhas, Serrasalmus spilopleura and S. marginatus (Pisces, Serrasalmidae). Acta Sci Biol Sci 2001; 23(2): 461-464.
- Andrade SMS, Malta JCO. Parasite fauna monitoring of matrinxã Brycon amazonicus (Spix & Agassiz, 1829) raised in an intensive husbandry system a stream channel in the state of Amazonas, Brazil. Braz J Biol 2006; 66(4): 1123-1132. http://dx.doi.org/10.1590/S1519-69842006000600020 PMid:17299949.
» http://dx.doi.org/10.1590/S1519-69842006000600020 - Aragort W, Morales G, Leon E, Pino LA, Guillen A, Silva M. Patologías asociadas a monogeneos branquiales en cachama bajo cultivo. Vet Trop 2002; 27(2): 75-85.
- Azevedo RK, Abdallah VD, Luque JL. Biodiversity of fish parasites from Guandu river, Southeastern Brazil: an ecological approach. Neotrop Helminthol 2011; 5(2): 185-199.
- Behr ER, Signor CA. Distribuição e alimentação de duas espécies simpátricas de piranhas Serrasalmus maculatus e Pygocentrus nattereri (Characidae, Serrasalminae) do rio Ibicuí, Rio Grande do Sul, Brasil. Iheringia Ser Zool 2008; 98(4): 501-507. http://dx.doi.org/10.1590/S0073-47212008000400014
» http://dx.doi.org/10.1590/S0073-47212008000400014 - Bialetzki A, Nakatani K, Baumgartner G, Bond-Buckup G. Occurrence of Macrobrachium amazonicum (Heller) (Decapoda, Palaemonidae) in Leopoldo’s inlet (Ressaco do Leopoldo), upper Paraná River, Porto Rico, Paraná. Rev Bras Zool 1997; 14(2): 379-390. http://dx.doi.org/10.1590/S0101-81751997000200011
» http://dx.doi.org/10.1590/S0101-81751997000200011 - Bicudo CEM, Bicudo RMT. Algas de águas continentais brasileiras chave ilustrada para identificação de gêneros. São Paulo: Fundação Brasileira para o Desenvolvimento do Ensino de Ciências; 1970.
- Boeger WA, Husak WS, Martins ML. Neotropical monogenoidea. 25. Anacanthorus penilabiatus n. sp. (Dactylogyridae, Anacanthorinae) from Piaractus mesopotamicus (Osteichthyes, Serrasalmidae), cultivated in the State of São Paulo, Brazil. Mem Inst Oswaldo Cruz 1995; 90(6): 699-701. http://dx.doi.org/10.1590/S0074-02761995000600008
» http://dx.doi.org/10.1590/S0074-02761995000600008 - Boeger WA, Kritsky DC. Neotropical Monogenea. 12. Dactylogyridae from Serrasalmus nattereri (Cypriniformes, Serrasalmidae) and aspects of their morphologic variation and distribution in the Brazilian Amazon. Proc Helminthol Soc Wash 1988; 55(2): 188-213.
- Boeger WA, Tanaka LK, Pavanelli GC. Neotropical Monogenoidea. 39: a new species of Kritskyia (Dactylogyridae, Ancyrocephalinae) from the ureters and urinary bladder of Serrasalmus marginatus and S. spilopleura (Characiformes, Serrasalmidae) from southern Brazil with an emended generic diagnosis. Zoosystema 2001; 23(1): 5-10.
- Braga MP, Araújo SBL, Boeger WA. Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitol Res 2014; 113(2): 481-490. http://dx.doi.org/10.1007/s00436-013-3677-8 PMid:24221891.
» http://dx.doi.org/10.1007/s00436-013-3677-8 - Braga MP, Razzolini E, Boeger WA. Drivers of parasite sharing among Neotropical freshwater fishes. J Anim Ecol 2015; 84(2): 487-497. http://dx.doi.org/10.1111/1365-2656.12298 PMid:25283218.
» http://dx.doi.org/10.1111/1365-2656.12298 - Brandão H, Yamada FH, Toledo GM, Carvalho ED, da Silva RJ. Monogeneans (Dactylogyridae) parasitizing gills of Salminus hilarii from a Neotropical reservoir, Brazil. Rev Bras Parasitol Vet 2013; 22(4): 579-587. http://dx.doi.org/10.1590/S1984-29612013000400020 PMid:24473885.
» http://dx.doi.org/10.1590/S1984-29612013000400020 - Brito-Junior IA, Tavares-Dias M. Metazoários parasitos de quatro espécies de peixe da bacia Igarapé Fortaleza, estado do Amapá (Brasil). Biota Amazôn 2018; 8(2): 1-3. http://dx.doi.org/10.18561/2179-5746/biotamazonia.v8n2p1-3
» http://dx.doi.org/10.18561/2179-5746/biotamazonia.v8n2p1-3 - Brooks DR, León-Règagnon V, McLennan DA, Zelmer D. Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 2006;87(7 Suppl): S76-S85. http://dx.doi.org/10.1890/0012-9658(2006)87[76:EFAADO]2.0.CO;2 PMid:16922304.
» http://dx.doi.org/10.1890/0012-9658(2006)87[76:EFAADO]2.0.CO;2 - Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. http://dx.doi.org/10.2307/3284227 PMid:9267395.
» http://dx.doi.org/10.2307/3284227 - Campos DWJ, Manoel LO, Franceschini L, Veríssimo-Silveira R, Delariva RL, Ribeiro CS, et al. Occurrence of metacercariae of Austrodiplostomum compactum (Lutz, 1928) (Trematoda, Diplostomidae) in Pimelodus platicirris in the Ilha Solteira Reservoir, São Paulo, Brazil. An Acad Bras Cienc 2020;92(Suppl 2):e20180649. http://dx.doi.org/10.1590/0001-3765202020180649
» http://dx.doi.org/10.1590/0001-3765202020180649 - Casali GP, Takemoto RM. Endoparasitic fauna of Serrasalmus spp. (Characidae: Serrasalminae) in a neotropical floodplain. Acta Sci Biol Sci 2016; 38(1): 105-112. http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592
» http://dx.doi.org/10.4025/actascibiolsci.v38i1.28592 - Cayulla-Quispe D, Mondragón-Martínez A, Rojas-de-Los-Santos E, Garcia-Candela E, Babilonia-Medina J, Martínez-Rojas R. A New Species of Mymarothecium tantaliani n. sp (Monogenea: Dactylogiridae) in the Gills of Gamitana Colossoma macropomum (Cuvier) from Madre de Dios, Peru. Acta Parasitol 2021; 66(1): 34-38. http://dx.doi.org/10.1007/s11686-020-00248-5 PMid:32656730.
» http://dx.doi.org/10.1007/s11686-020-00248-5 - Centeno L, Silva-Acuna A, Silva-Acuna R, Perez JL. Fauna Ectoparasitaria Asociada a Colossoma macropomum y al Híbrido de C. macropomum x Piaractus brachypomus, Cultivados en el Estado Delta Amacuro, Venezuela. Bioagro- 2004; 16(2): 121-126.
- Chemes SB, Takemoto RM. Diversity of parasites from middle Paraná system freshwater fishes, Argentina. Int J Biodivers Conserv 2011; 3(7): 249-266.
- Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Parana River, State of Parana, Brazil. Zootaxa 2012; 3149(1): 57-68. http://dx.doi.org/10.11646/zootaxa.3149.1.3
» http://dx.doi.org/10.11646/zootaxa.3149.1.3 - Cohen SC, Kohn A. A new species of Mymarothecium and new host and geographical records for M. viatorum (Monogenea: Dactylogyridae), parasites of freshwater fishes in Brazil. Folia Parasitol (Praha) 2005; 52(4): 307-310. http://dx.doi.org/10.14411/fp.2005.042 PMid:16405294.
» http://dx.doi.org/10.14411/fp.2005.042 - Cohen SC, Kohn A. On Dactylogyridae (Monogenea) of four species of characid fishes from Brazil. Check List 2009; 5(2): 351-356. http://dx.doi.org/10.15560/5.2.351
» http://dx.doi.org/10.15560/5.2.351 - Collart OO, Moreira LC. Potencial pesqueiro de Macrobrachium amazonicum na Amazônia Central (Ilha do Careiro): variação da abundância e do comprimento. Amazoniana 1993; 12(3/4): 399-413.
- Córdova L, Pariselle A. Monogenoidea en Serrasalmus rhombeus (Linnaeus, 1766) de la Cuenca Amazónica Boliviana. Rev Peru Biol 2007; 14(1): 11-16. http://dx.doi.org/10.15381/rpb.v14i1.1748
» http://dx.doi.org/10.15381/rpb.v14i1.1748 - Dias KGA, Vieira DHMD, Camargo AA, Silva RJ, Azevedo RK, Abdallah VD. Diversity of monogeneans parasites from Characiformes fishes in the Batalha River and Peixe’s River, State of São Paulo, Brazil. Neotrop Helminthol 2017; 11: 317-330.
- Dias MKR, Neves LR, Marinho RGB, Pinheiro DA, Tavares-Dias M. Parasitismo em tambatinga (Colossoma macropomum x Piaractus brachypomus, Characidae) cultivados na Amazônia, Brasil. Acta Amazon 2015; 45(2): 231-238. http://dx.doi.org/10.1590/1809-4392201400974
» http://dx.doi.org/10.1590/1809-4392201400974 - Domingues MV, Boeger WA. Neotropical Monogenoidea. 47. Phylogeny and coevolution of species of Rhinoxenus (Platyhelminthes, Monogenoidea, Dactylogyridae) and their Characiformes hosts (Teleostei, Ostariophysi) with description of four new species. Zoosystema 2005; 27(3): 441-467.
- Eiras JC, Takemoto RM, Pavanelli GC, Adriano EA. About the biodiversity of parasites of freshwater fish from Brazil. Bull Eur Assoc Fish Pathol 2011; 31(4): 161-168.
- Eiras JC, Takemoto RM, Pavanelli GC. Diversidade de peixes de água doce do Brasil Maringa: Eduem; 2010.
- Fischer C, Malta JCO, Varella AMB. A fauna de parasitas do Tambaqui, Colossoma macropomum (Cuvier, 1818) (Characiformes: Characidae) do médio rio Solimões, estado do Amazonas (AM) e do baixo rio Amazonas, estado do Pará (PA), e seu potencial como indicadores biológicos. Acta Amazon 2003; 33(4): 651-662. http://dx.doi.org/10.1590/S0044-59672003000400012
» http://dx.doi.org/10.1590/S0044-59672003000400012 - Franceschini L, Zago AC, Schalch SH, Garcia F, Romera DM, da Silva RJ. Parasitic infections of Piaractus mesopotamicus and hybrid (P. mesopotamicus x Piaractus brachypomus) cultured in Brazil. Rev Bras Parasitol Vet 2013; 22(3): 407-414. http://dx.doi.org/10.1590/S1984-29612013000300015 PMid:24142174.
» http://dx.doi.org/10.1590/S1984-29612013000300015 - Froese R, Pauly D. Serrasalmus maculatus Kner, 1858. [online]. FishBase; 2020b [cited 2020 Aug 26]. Available from: https://www.fishbase.de/summary/serrasalmus-maculatus.html
» https://www.fishbase.de/summary/serrasalmus-maculatus.html - Froese R, Pauly D. List of Freshwater Fishes reported from Brazil [online]. FishBase; 2020a [cited 2020 Aug 26]. Available from: https://www.fishbase.de/Country/CountryChecklist.php?c_code=076&vhabitat=fresh⫏_code=&cpresence=present
» https://www.fishbase.de/Country/CountryChecklist.php?c_code=076&vhabitat=fresh⫏_code=&cpresence=present - Garcia F, Kimpara JM, Valenti WC, Ambrosio LA. Emergy assessment of tilapia cage farming in a hydroelectric reservoir. Ecol Eng 2014; 68: 72-79. http://dx.doi.org/10.1016/j.ecoleng.2014.03.076
» http://dx.doi.org/10.1016/j.ecoleng.2014.03.076 - Graça RJ, Fabrin TMC, Gasques LS, Prioli SMAP, Balbuena JA, Prioli AJ, et al. Topological congruence between phylogenies of Anacanthorus spp. (Monogenea: Dactylogyridae) and their Characiformes (Actinopterygii) hosts: A case of host-parasite cospeciation. PLoS One 2018; 13(3): e0193408. http://dx.doi.org/10.1371/journal.pone.0193408 PMid:29538463.
» http://dx.doi.org/10.1371/journal.pone.0193408 - Hamann MI. Aspectos ecológicos de la relación parasitaria entre larvas de Contracaecum sp. (Nematoda, Anisakidae) y Serrasalmus spilopleura Kner, 1860 (Pisces, Characidae) en poblaciones naturales del nordeste argentino. Bol Chil Parasitol 1999; 54(3-4): 74-82. http://dx.doi.org/10.4067/S0365-94021999000300007 PMid:10883494.
» http://dx.doi.org/10.4067/S0365-94021999000300007 - Hellawell JM, Abel R. A rapid volumetric method for the analysis of the food fishes. J Fish Biol 1971; 3(1): 20-37. http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x
» http://dx.doi.org/10.1111/j.1095-8649.1971.tb05903.x - Hoffmann AC, Orsi ML, Shibatta AO. Diversidade de peixes do reservatório da UHE Escola Engenharia Mackenzie (Capivara), Rio Paranapanema, bacia do alto rio Paraná, Brasil, e a importância dos grandes tributários na sua manutenção. Iheringia Ser Zool 2005; 95(3): 319-325. http://dx.doi.org/10.1590/S0073-47212005000300012
» http://dx.doi.org/10.1590/S0073-47212005000300012 - Hoshino MDFG, Tavares-Dias M. Ecology of parasites of Metynnis lippincottianus (Characiformes: Serrasalmidae) from the eastern Amazon region, Macapá, State of Amapá, Brazil. Acta Sci Biol Sci 2014; 36(2): 249-255. http://dx.doi.org/10.4025/actascibiolsci.v36i2.19876
» http://dx.doi.org/10.4025/actascibiolsci.v36i2.19876 - Hyslop EJ. Stomach contents analysis – a review of methods and their application. J Fish Biol 1980; 17(4): 411-429. http://dx.doi.org/10.1111/j.1095-8649.1980.tb02775.x
» http://dx.doi.org/10.1111/j.1095-8649.1980.tb02775.x - Iannacone JA, Luque JL. New records of helminths parasitic on Peruvian Amazonian fishes (Osteichthyes). Rev Biol Trop 1993; 41(2): 303-305.
- Janzen DH. On ecological fitting. Oikos 1985; 45(3): 308-310. http://dx.doi.org/10.2307/3565565
» http://dx.doi.org/10.2307/3565565 - Jégu M, dos Santos MG. Mise au point à propos de Serrasalmus spilopleura Kner, 1858 et réhabilitation de S. maculatus Kner, 1858 (Characidae: serrasalminae). Cybium (Paris) 2001; 25(2): 119-143.
- Junk WJ. Áreas inundáveis: um desafio para limnologia. Acta Amaz 1980; 10(4): 775-795. http://dx.doi.org/10.1590/1809-43921980104775
» http://dx.doi.org/10.1590/1809-43921980104775 - Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 2002; 17(4): 164-170. http://dx.doi.org/10.1016/S0169-5347(02)02499-0
» http://dx.doi.org/10.1016/S0169-5347(02)02499-0 - Kritsky DC, Boeger WA, Jégu M. Neotropical Monogenoidea. 28. Ancyrocephalinae (Dactylogyridae) of piranha and their relatives (Teleostei, Serrasalmidae) from Brazil and French Guiana: Species of Notozothecium Boeger and Kritsky, 1988, and Mymarothecium gen. n. J Helminthol Soc Wash 1996; 63(1): 153-175.
- Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from Characoid Fishes of the Central Amazon. J Helminthol Soc Wash 1992; 59(1): 25-51.
- Kritsky DC, Thatcher VE, Kayton RJ. Neotropical Monogenoidea. 2. The Anacanthorinae Price, 1967, with the proposal of four new species of Anacanthorus Mizelle & Price, 1965, from Amazonian fishes. Acta Amaz 1979; 9(2): 355-361. http://dx.doi.org/10.1590/1809-43921979092355
» http://dx.doi.org/10.1590/1809-43921979092355 - Kritsky DC, Thatcher VE. Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. J Helminthol 1974; 48(1): 59-66. http://dx.doi.org/10.1017/S0022149X00022604 PMid:4825435.
» http://dx.doi.org/10.1017/S0022149X00022604 - Leão MSL, Justo MCN, Bueno GW, Cohen SC, São Clemente SC. Parasitism by Monogenoidea in Piaractus mesopotamicus (Characiformes, Characidae) cultivated in Paraná River (Brazil). Braz J Biol 2017; 77(4): 787-793. http://dx.doi.org/10.1590/1519-6984.00916 PMid:28562776.
» http://dx.doi.org/10.1590/1519-6984.00916 - Leão MSL, São Clemente SC, Cohen S. Anacanthorus toledoensis n. sp. and Mymarothecium ianwhittingtoni n. sp. (Dactylogyridae: Monogenoidea) Parasitizing Cage-Reared Piaractus mesopotamicus (Characiformes, Characidae) in the State of Paraná, Brazil. Comp Parasitol 2015; 82(2): 269-274. http://dx.doi.org/10.1654/4759.1
» http://dx.doi.org/10.1654/4759.1 - Lima LB, Bellay S, Giacomini HC, Isaac A, Lima-Junior DP. Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology 2016; 143(3): 343-349. http://dx.doi.org/10.1017/S003118201500164X PMid:26647725.
» http://dx.doi.org/10.1017/S003118201500164X - Luque JL, Aguiar JC, Vieira FM, Gibson DI, Santos CP. Checklist of Nematoda associated with the fishes of Brazil. Zootaxa 2011; 3082(1): 1-88. http://dx.doi.org/10.11646/zootaxa.3082.1.1
» http://dx.doi.org/10.11646/zootaxa.3082.1.1 - Luque JL, Poulin R. Linking ecology with parasite diversity in Neotropical fishes. J Fish Biol 2008; 72(1): 189-204. http://dx.doi.org/10.1111/j.1095-8649.2007.01695.x
» http://dx.doi.org/10.1111/j.1095-8649.2007.01695.x - Madi RR, Ueta MT. O papel de Ancyrocephalinae (Monogenea: Dactylogyridae), parasito de Geophagus brasiliensis (Pisces: Cichlidae), como indicador ambiental. Rev Bras Parasitol Vet 2009; 18(2): 38-41. http://dx.doi.org/10.4322/rbpv.01802008 PMid:19602315.
» http://dx.doi.org/10.4322/rbpv.01802008 - Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature 2003; 421(6923): 625-627. http://dx.doi.org/10.1038/nature01317 PMid:12571594.
» http://dx.doi.org/10.1038/nature01317 - Mizelle JD, Kritsky DC, Crane JW. Studies on monogenetic trematodes. XXXVIII. Ancyrocephalinae from South America with the proposal of Jainus gen. n. Am Midl Nat 1969; 80(1): 186-198. http://dx.doi.org/10.2307/2423609
» http://dx.doi.org/10.2307/2423609 - Mizelle JD, Price CE. Studies on Monogenetic Trematodes. XXVIII. Gill Parasites of the Piranha with Proposal of Anacanthorus gen. n. J Parasitol 1965; 51(1): 30-36. http://dx.doi.org/10.2307/3275640 PMid:14259477.
» http://dx.doi.org/10.2307/3275640 - Monteiro CM, Cohen SC, Brasil-Sato MC. New species and reports of dactylogyrids (Monogenoidea) from Salminus franciscanus (Actinopterygii: Bryconidae) from the upper São Francisco River, Brazil. Zootaxa 2015; 3941(1): 137-143. http://dx.doi.org/10.11646/zootaxa.3941.1.9 PMid:25947500.
» http://dx.doi.org/10.11646/zootaxa.3941.1.9 - Monteiro CM, Kritsky DC, Brasil-Sato MC. Neotropical Monogenoidea. 56. New species of Anacanthorus (Dactylogyridae) from the gills of matrinchã, Brycon orthotaenia (Characiformes: Characidae), in the Rio São Francisco, Brazil. Folia Parasitol (Praha) 2010; 57(3): 164-168. http://dx.doi.org/10.14411/fp.2010.022 PMid:20941907.
» http://dx.doi.org/10.14411/fp.2010.022 - Morais AM, Malta JCO. Biodiversity of monogenoideans from red piranha Pygocentrus nattereri (Kner, 1958) (Characiformes: Serrasalmidae) in central Amazonia: Occurrence and taxonomy. Neotrop Helminthol 2015; 9(2): 265-276.
- Moreira ADC, Silva De Oliveira TT, Morey GAM, Malta JCO. Metazoários parasitas de Tripotheus angulatus (Spix & Agassiz, 1829) do lago Catalão, Rio Solimões, Amazonas, Brasil. Folia 2017; 26(1): 9-16. http://dx.doi.org/10.24841/fa.v26i1.415
» http://dx.doi.org/10.24841/fa.v26i1.415 - Moreira J, da Silva Carneiro J, Ruz EJH, Luque JL. New Species and Records of Anacanthorus (Monogenea: Dactylogyridae) Parasitizing serrasalmid fish (Characiformes) from Brazil, including molecular data. Acta Parasitol 2019; 64(3): 449-455. http://dx.doi.org/10.2478/s11686-019-00055-7 PMid:31020494.
» http://dx.doi.org/10.2478/s11686-019-00055-7 - Morey GAM, Aliano AMB, Grandez FAG.New species of Dactylogyridae Bychowsky, 1933 infecting the gills of Myloplus schomburgkii (Jardine) and Colossoma macropomum (Cuvier) in the Peruvian Amazon. Syst Parasitol 2019; 96(6): 511-519. http://dx.doi.org/10.1007/s11230-019-09865-9 PMid:31093872.
» http://dx.doi.org/10.1007/s11230-019-09865-9 - Morey GAM, Malta JCO. Metazoan parasites of Serrasalmus altispinis (Serrasalmidae) from floodplain lakes of the Brazilian Amazon. Neotrop Helminthol 2018; 12(2): 141-146.
- Morey GAM, Sol LGS, Cachique JCZ. New species and records of Anacanthorus (Monogenoidea: Dactylogyridae) from the gills of Brycon amazonicus (Characiformes: Bryconidae) in the Peruvian Amazon. Syst Parasitol 2021; 98(2): 85-97. http://dx.doi.org/10.1007/s11230-021-09962-8 PMid:33686564.
» http://dx.doi.org/10.1007/s11230-021-09962-8 - Morley NJ. Anthropogenic effects of reservoir construction on the parasite fauna of aquatic wildlife. EcoHealth 2007; 4(4): 374-383. http://dx.doi.org/10.1007/s10393-007-0130-4
» http://dx.doi.org/10.1007/s10393-007-0130-4 - Mugnai R, Nessemian JL, Baptista DF. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro Rio de Janeiro: Technical Books Editora; 2010.
- Neto JFS, Muriel-Cunha J, Domingues MV. New species of Anacanthorus (Dactylogyridae: Anacanthorinae) from the gills of Hoplerythrinus unitaeniatus and Erythrinus erythrinus (Characiformes: Erythrinidae) the coastal drainage in the Eastern Amazon, Brazil. Zootaxa 2019; 4615(2): 303-320. http://dx.doi.org/10.11646/zootaxa.4615.2.4
» http://dx.doi.org/10.11646/zootaxa.4615.2.4 - Neves LR, Negreiros LP, Silva LMA, Tavares-Dias M. Diversity of monogenean parasites on gills of fishes from the Matapi River, in the Brazilian Amazon. Rev Bras Parasitol Vet 2020; 29(4): e013520. http://dx.doi.org/10.1590/s1984-29612020081 PMid:33053058.
» http://dx.doi.org/10.1590/s1984-29612020081 - Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol 2018; 16(2): e170094. http://dx.doi.org/10.1590/1982-0224-20170094
» http://dx.doi.org/10.1590/1982-0224-20170094 - Pamplona-Basilio MC, Kohn A, Feitosa VA. New host records and description of the egg of Anacanthorus penilabiatus (Monogenea, Dactylogyridae). Mem Inst Oswaldo Cruz 2001; 96(5): 667-668. http://dx.doi.org/10.1590/S0074-02762001000500014 PMid:11500767.
» http://dx.doi.org/10.1590/S0074-02762001000500014 - Pavanelli GC, Machado MH, Takemoto RM. Fauna helmíntica de peixes do rio Paraná, região de Porto Rico, Paraná. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A Planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos Maringá: Eduem; 1997. p. 307-329.
- Pavanelli GC, Machado MH, Takemoto RM, Guidelli GM, Lizama MAP. Helminth fauna of fishes: diversity and ecological aspects. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The Upper Paraná river and its Floodplain: physical aspects, ecology and conservation Leiden: Backhuys Publishers; 2004. p. 309-329.
- Pereira FB, Mota MEBP, Paiva F, Tavares LE. Three new species of Anacanthorus Mizelle & Price, 1965 (Monogenea: Dactylogyridae) from Markiana nigripinnis Perugia (Actinopterygii: Characidae) in Pantanal wetlands, Brazil. Syst Parasitol 2020; 97(6): 661-667. http://dx.doi.org/10.1007/s11230-020-09935-3 PMid:32949340.
» http://dx.doi.org/10.1007/s11230-020-09935-3 - Power ME, Sun A, Parker G, Dietrich WE, Wootton JT. Hydraulic Food-chain Models: an approach to the study of food -web dynamics in large rivers. Bioscience 1995; 45(3): 159-167. http://dx.doi.org/10.2307/1312555
» http://dx.doi.org/10.2307/1312555 - Rossin MA, Francesco PN, Irigoitia MM, Scarabotti PA, Taglioretti V, Timi JT. Rhinoxenus (Dactylogyridae) parasitizing piranhas (Serrasalmidae) at its southernmost limit of distribution (Paraná River, Argentina), with the description of two new species. An Acad Bras Cienc 2019; 91(4): e20190711. http://dx.doi.org/10.1590/0001-3765201920190711 PMid:31800711.
» http://dx.doi.org/10.1590/0001-3765201920190711 - Sazima I, Machado FA. Underwater observations of piranhas in western Brazil. Environ Biol Fishes 1990; 28(1-4): 17-31. http://dx.doi.org/10.1007/BF00751026
» http://dx.doi.org/10.1007/BF00751026 - Sazima I, Pombal-Jr JP. Mutilação de nadadeiras em acarás, Geophagus brasiliensis, por piranhas, Serrasalmus spilopleura. Rev Bras Biol 1988; 48(3): 477-483.
- Silva EF, Tavares-Dias M. Infection by helminthes in Mylossoma duriventre Cuvier, 1817, a characid from the central Amazon, Brazil. Neotrop Helminthol 2012; 6(1): 67-73.
- Silva RM, Tavares-Dias M, Dias MWR, Dias MKR, Marinho RGB. Parasitic fauna in hybrid tambacu from fish farms. Pesq Agropec Bras 2013; 48(8): 1049-1057. http://dx.doi.org/10.1590/S0100-204X2013000800034
» http://dx.doi.org/10.1590/S0100-204X2013000800034 - Strona G. The underrated importance of predation in transmission ecology of direct lifecycle parasites. Oikos 2015; 124(6): 685-690. http://dx.doi.org/10.1111/oik.01850
» http://dx.doi.org/10.1111/oik.01850 - Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, et al. Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 2009;69(2 Suppl 2): 691-705. http://dx.doi.org/10.1590/S1519-69842009000300023 PMid:19738975.
» http://dx.doi.org/10.1590/S1519-69842009000300023 - Thatcher VE. Amazon fish parasites 2nd ed. Sofia: Pensoft Publishers; 2006.
- Timi JT, Poulin R. Why ignoring parasites in fish ecology is a mistake. Int J Parasitol 2020; 50(10-11): 755-761. http://dx.doi.org/10.1016/j.ijpara.2020.04.007 PMid:32592807.
» http://dx.doi.org/10.1016/j.ijpara.2020.04.007 - Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature 2003; 421(6923): 628-630. http://dx.doi.org/10.1038/nature01346 PMid:12571595.
» http://dx.doi.org/10.1038/nature01346 - Tourchin ME, Lafferty KD, Kuris AM. Parasites and marine invasions. Parasitology 2002;124(7 Suppl): 137-151. http://dx.doi.org/10.1017/S0031182002001506 PMid:12396221.
» http://dx.doi.org/10.1017/S0031182002001506 - Van Every LR, Kritsky DC. Neotropical Monogenoidea. 18. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) of piranha (Characoidea, Serrasalmidae) from the Central Amazon, their phylogeny, and aspects of host-parasite coevolution. J Helminthol Soc Wash 1992; 59(1): 52-75.
- Villares GA, Gomiero LM, Goitein R. Feeding of Serrasalmus maculatus (Kner, 1858) (Characiformes; Serrasalmidae) in the Sorocaba river, São Paulo State, Brazil. Acta Sci Biol Sci 2008; 30(3): 267-273. http://dx.doi.org/10.4025/actascibiolsci.v30i3.5011
» http://dx.doi.org/10.4025/actascibiolsci.v30i3.5011 - Yamada FH, Moreira LHA, Ceschini TL, Takemoto RM, Pavanelli GC. Novas ocorrências de metacercária de Austrodiplostomum compactum (Lutz, 1928) (Platyhelminthes: Digenea) parasito de olhos de peixes da Bacia do Rio Paraná. Rev Bras Parasitol Vet 2008; 17(3): 163-166. http://dx.doi.org/10.1590/S1984-29612008000300010 PMid:19245765.
» http://dx.doi.org/10.1590/S1984-29612008000300010
Publication Dates
-
Publication in this collection
23 Mar 2022 -
Date of issue
2022
History
-
Received
27 Oct 2021 -
Accepted
10 Feb 2022