Abstract
The aim of this study was to evaluate the presence of anti-Toxocara antibodies in naturally infected broiler chickens (n = 189) from the state of Paraná, southern Brazil. The chickens were reared in a semi-intensive system by small family farmers (n = 7). An enzyme-linked immunosorbent assay (ELISA) was performed to detect the presence of anti- Toxocara spp. IgY after serum adsorption with Ascaridia galli antigens. An overall seroprevalence of 67.7% (128/189; 95% CI = 61.1-74.4) was observed. The frequency of positive animals by farm ranged from 29.6% to 100%. The optical density and reactivity index values observed in ELISA test indicated the possible chronicity of infection of the evaluated chickens. Associations between the presence of antibodies and the area where the chickens were reared (p = 0.382) or the population density of dogs on the farm (p = 0.785) were not observed. This study shows a high prevalence of Toxocara spp. antibodies in broiler chickens reared in semi-intensive systems and provides evidence that chickens are a good indicator of environmental contamination by larva migrans agents. Further studies are necessary to assess the risk factors associated with poultry infection and the likelihood of toxocariasis transmission to humans via the ingestion of free-range chicken meat.
Keywords:
ELISA; Gallus gallus domesticus; seroprevalence; toxocariasis; zoonosis
Resumo
A finalidade do presente estudo foi avaliar a presença de anticorpos anti- Toxocara, em frangos de corte naturalmente infectados (n = 189), no Norte do Paraná, Sul do Brasil. Os frangos foram criados em sistema semi-intensivo, em pequenas propriedades rurais (n = 7). Os testes sorológicos foram realizados pela técnica de ELISA, para detecção de anticorpos IgY (IgG), com pré-adsorção do soro com antígenos de Ascaridia galli. Foi observada uma prevalência de 67,7% (128/189; IC 95% = 61,1-74,4). A frequência de animais soropositivos por propriedade variou de 29,6% a 100%. Os valores da Densidade Ótica e do Índice de Reatividade observados no teste de ELISA indicaram uma possível cronicidade de infecção dos frangos avaliados. Não foi observada correlação entre a positividade dos animais, quando comparada a área (p = 0,382) e a densidade populacional de cães por propriedade (p = 0,785). O presente estudo verificou uma alta prevalência de anticorpos anti-Toxocara em frangos de corte criados em sistema semi-intensivo e oferece dados que apontam esses animais como bons indicadores de contaminação ambiental por agentes de larva migrans . Estudos futuros são necessários para avaliar os fatores de risco associados e a possibilidade da transmissão de toxocaríase ao ser humano pela ingestão de carne de frango.
Palavras-chave:
ELISA; Gallus gallus domesticus; soroprevalência; toxocaríase; zoonose
Introduction
Poultry, one of the most important and richest sources of protein for humans, are reared both intensively in fully automated, environmentally controlled systems and semi-intensively in backyards ( FAO, 2014 Food and Agriculture Organization of the United Nations – FAO. Poultry and animal production [online]. Rome: FAO; 2014 [cited 2017 Apr 18]. Available from: http://www.fao.org/ag/againfo/themes/en/poultry/production.html
http://www.fao.org/ag/againfo/themes/en...
).
Backyard production methods imply low biosafety measures and a high risk of infectious diseases ( CONAN et al., 2012 Conan A, Goutard FL, Sorn S, Vong S. Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res 2012; 8(1): 240. http://dx.doi.org/10.1186/1746-6148-8-240. PMid:23216706.
http://dx.doi.org/10.1186/1746-6148-8-2...
; IBRAHIM et al., 2016 Ibrahim HM, Abdel-Ghaffar F, Osman GY, El-Shourbagy SH, Nishikawa Y, Khattab RA. Prevalence of Toxoplasma gondii in chicken samples from delta of Egypt using ELISA, histopathology and immunohistochemistry. J Parasit Dis 2016; 40(2): 485-490. http://dx.doi.org/10.1007/s12639-014-0530-7. PMid:27413325.
http://dx.doi.org/10.1007/s12639-014-05...
). Chickens reared in backyards, for instance, harbour a wide variety of geo-parasites, becoming highly infected during feeding ( BEN SLIMANE, 2016 Ben Slimane B. Prevalence of the gastro-intestinal parasites of domestic chicken Gallus domesticus Linnaeus, 1758 in Tunisia according to the agro-ecological zones. J Parasit Dis 2016; 40(3): 774-778. http://dx.doi.org/10.1007/s12639-014-0577-5. PMid:27605783.
http://dx.doi.org/10.1007/s12639-014-05...
; FERDUSHY et al., 2016 Ferdushy T, Hasan MT, Golam Kadir AK. Cross sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free range chickens in Narsingdi district, Bangladesh. J Parasit Dis 2016; 40(3): 818-822. http://dx.doi.org/10.1007/s12639-014-0585-5. PMid:27605790.
http://dx.doi.org/10.1007/s12639-014-05...
; JAVAREGOWDA et al., 2016 Javaregowda AK, Kavitha Rani B, Revanna SP, Udupa G. Prevalence of gastro-intestinal parasites of backyard chickens (Gallus domesticus) in and around Shimoga. J Parasit Dis 2016; 40(3): 986-990. http://dx.doi.org/10.1007/s12639-014-0620-6. PMid:27605824.
http://dx.doi.org/10.1007/s12639-014-06...
). In Bangladesh, chickens reared in backyards were significantly more susceptible to infections by parasites than those reared in intensive systems ( RABBI et al., 2006 Rabbi AKMA, Islam A, Majumder S, Anisuzzaman A, Rahman MH. Gastrointestinal helminths infection in different types of poultry. Bangl J Vet Med 2006; 4(1): 13-18. http://dx.doi.org/10.3329/bjvm.v4i1.1519.
http://dx.doi.org/10.3329/bjvm.v4i1.151...
).
Chickens have been considered indicators for environmental contamination by soil-borne parasitic diseases ( CARDOSO & YAMAMURA, 2004 Cardoso SP, Yamamura MH. Parasitas em produção de frangos no sistema de criação tipo colonial/caipira no Brasil. Semina: Ciênc Agrár 2004; 25(1): 63-74. http://dx.doi.org/10.5433/1679-0359.2004v25n1p63.
http://dx.doi.org/10.5433/1679-0359.200...
), especially toxoplasmosis, a zoonosis that may be transmitted to humans via the ingestion of undercooked, infected chicken meat ( DUBEY, 2010 Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 2010; 57(1): 60-73. http://dx.doi.org/10.1111/j.1863-2378.2009.01274.x. PMid:19744305.
http://dx.doi.org/10.1111/j.1863-2378.2...
).
Chickens are also considered an indicator for soil contamination by the eggs of dog/cat Toxocara spp. ( CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
; VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
), a nematode responsible for human toxocariasis, one of the most prevalent helminthiases in the world ( RUBINSKY-ELEFANT et al., 2010 Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010; 104(1): 3-23. http://dx.doi.org/10.1179/136485910X12607012373957. PMid:20149289.
http://dx.doi.org/10.1179/136485910X126...
). Toxocariasis is primarily transmitted to humans via the ingestion of soil containing embryonated eggs; however, the consumption of raw or undercooked meat from chickens may also represent an alternative route of transmission to humans ( NAGAKURA et al., 1989 Nagakura K, Tachibana H, Kaneda Y, Kato Y. Toxocariasis possibly caused by ingesting raw chicken. J Infect Dis 1989; 160(4): 735-736. http://dx.doi.org/10.1093/infdis/160.4.735. PMid:2794572.
http://dx.doi.org/10.1093/infdis/160.4....
; TAIRA et al., 2004 Taira K, Saeed I, Permin A, Kapel CMO. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol 2004; 121(1-2): 115-124. http://dx.doi.org/10.1016/j.vetpar.2004.01.018. PMid:15110409.
http://dx.doi.org/10.1016/j.vetpar.2004...
; MORIMATSU et al., 2006 Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg 2006; 75(2): 303-306. PMid:16896137. ).
Recent studies on natural infections in chickens reared in semi-intensive system/free-range chickens have shown a high prevalence of anti-Toxocara antibodies in poultry commercialized in street markets ( VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
) and from different origins ( CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
), along with the presence of Toxocara larvae (T. canis and T. cati) in the tissues of the animals ( ZIBAEI et al., 2017 Zibaei M, Sadjjadi SM, Maraghi S. The occurrence of Toxocara species in naturally infected broiler chickens revealed by molecular approaches. J Helminthol 2017; 91(5): 633-636. http://dx.doi.org/10.1017/S0022149X16000559. PMid:27571878.
http://dx.doi.org/10.1017/S0022149X1600...
).
Despite the high prevalence of anti-Toxocara antibodies in chickens, there is a lack of information regarding the factors associated with the infection.
In this study, we aimed to evaluate the presence of anti-Toxocara IgY antibodies in slaughtered broiler chickens, in addition to other factors associated with the infection.
Materials and Methods
Study area and farms
The serum samples of the chickens evaluated in this study were obtained in a slaughterhouse located in the municipality of Barra do Jacaré, state of Paraná, southern Brazil (23° 06’ 54” S; 50° 10’ 53” W).
The slaughterhouse was selected based on their management of an association of small farms (n = 7) that were located in the same municipality and were characterized by family maintenance, covered a small area (average = eight hectares), and produced broiler chickens as the major source of family income.
Animal rearing
In this association system, the slaughterhouse obtained, per week, approximately 1000 Label Rouge chicks at one day of age from a chick industry.
The chicks were maintained in a concrete shed base (250 m2) for a 30-day adaptive period and fed food based on corn (Zea mays) and water ad libitum .
Following the adaptive period, the chickens were then transferred to the seven associated farms (n = 100-150 chickens/farm). On the farms, the chickens were reared (cycles of 60-90 days) in a semi-intensive system (area from 1200 to 5000 m2) and given corn-based food (Zea mays) and water ad libitum.
All the information regarding the farm properties (number of dogs, total area of the farm) was provided by the slaughterhouse manager.
The study was approved by the Ethics Committee for Animal Use (CEUA) of the University of Western São Paulo (UNOESTE), Presidente Prudente, state of São Paulo, Brazil, under protocol 3114/2016.
Sample collection
Our study was carried out from May to July 2016 and was designed to randomly collect 27 samples of animals belonging to the same group of chicks (n = 100 animals/farm). Therefore, a total of 189 chickens, aged 60-90 days, were included in this study.
Following the slaughter of the chickens, blood samples were collected in vacuum tubes. The tubes were centrifuged (1,650 g, 10 min) to obtain the serum samples, which were transferred into microtubes and stored at -20 °C for processing.
Antigen preparation
Toxocara canis excretory-secretory L3 antigens (TES) were obtained according to a previously described method ( ELEFANT et al., 2006 Elefant GR, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal 2006; 20(4): 164-172. http://dx.doi.org/10.1002/jcla.20126. PMid:16874812.
http://dx.doi.org/10.1002/jcla.20126 ...
). Briefly, T. canis eggs were collected from the uteri of female T. canis adult worms and incubated (28°C) for a period of approximately one month in 2% formalin. Next, to induce the rupture of the mamilonated membrane of the embryonated eggs, the suspension was treated with a 5% sodium hypochlorite solution for 5 min at room temperature, and the second-stage larvae were recovered using a Baermann apparatus. The obtained larvae were maintained in serum-free Eagle’s medium (37 °C), and the culture supernatant containing the TES was transferred to sterile flasks each week. Then, the supernatant was treated with 200 mM phenyl-methyl-sulfonyl fluoride, a protease inhibitor (Sigma®, St. Louis, MO, USA), concentrated with Amicon Ultrafiltration units (Millipore®, Danvers, MA, USA), dialysed against distilled water, centrifuged (18,500 × g, 60 min, 4 °C), and filtered using 0.2-µM Millipore membranes.
Protein concentrations were measured using the Lowry method ( LOWRY et al., 1951 Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193(1): 265-275. PMid:14907713. ).
Ascaridia galli adult worm extract (AWE)
Birds living in direct contact with soil are susceptible to infection via the transmission of other soil-borne helminths, in addition to Toxocara spp. Before performing the enzyme-linked immunosorbent assay (ELISA), each chicken serum sample was treated with an A. galli antigen suspension to minimize the cross-reactivity with Toxocara antibodies and, consequently, to increase the specificity of the ELISA ( CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
; VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
).
To obtain the AWE, we followed a previously described protocol ( ROMASANTA et al., 2003 Romasanta A, Romero JL, Arias M, Sánchez-Andrade R, López C, Suárez JL, et al. Diagnosis of parasitic zoonoses by immunoenzymatic assays-analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis , and Ascaris suum. Immunol Invest 2003; 32(3): 131-142. http://dx.doi.org/10.1081/IMM-120022974. PMid:12916704.
http://dx.doi.org/10.1081/IMM-120022974...
; ELEFANT et al., 2006 Elefant GR, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal 2006; 20(4): 164-172. http://dx.doi.org/10.1002/jcla.20126. PMid:16874812.
http://dx.doi.org/10.1002/jcla.20126 ...
). First, adult A. galli were recovered from the bowel of a naturally infected chicken. The worms were then macerated in distilled water, and NaOH was added until a final concentration of 0.15 M was obtained. The material was then incubated (room temperature, 2 h) and neutralized with 6 M HCl. Thereafter, the extract was treated with ether to remove the lipids and centrifuged (18,500 g, 20 min, 4 °C). The aqueous phase was removed and filtered (0.22-µM Millipore membrane). The sera were pre-incubated at a final concentration of 25 µg/mL AWE in 0.01 M phosphate-buffered saline (PBS, pH 7.2) with 0.05% Tween 20 (PBS-T) (Sigma®, St. Louis) (37 °C, 30 min).
Indirect ELISA for anti-Toxocara IgY detection
An indirect ELISA was performed to evaluate the anti-Toxocara IgY (IgG) antibodies according to the previously described protocol ( SAVIGNY et al., 1979 Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol 1979; 32(3): 284-288. http://dx.doi.org/10.1136/jcp.32.3.284. PMid:372253.
http://dx.doi.org/10.1136/jcp.32.3.284 ...
) with some modifications ( ELEFANT et al., 2006 Elefant GR, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal 2006; 20(4): 164-172. http://dx.doi.org/10.1002/jcla.20126. PMid:16874812.
http://dx.doi.org/10.1002/jcla.20126 ...
). Briefly, 96-well polystyrene plates (Corning, Costar, New York, NY) were coated (100 µL/well) with TES diluted in 0.01 M PBS (pH 7.2) to a concentration of 1.9 μg/mL. Following incubation (37 °C, 2 h followed by 4 °C, 18 h), the wells were washed, and thereafter, each well was blocked with 200 µL of 1% bovine serum albumin (BSA, Sigma, St. Louis, USA) in PBS-T 20 (0.05%) with an incubation at 37 °C for 1 h.
After washing, the serum samples (100 µL/well), diluted 1:200, were incubated in duplicate (37 °C, 40 min). The plates were then washed and incubated (37 °C, 40 min) with peroxidase-conjugated anti-chicken IgY antibodies (IgG) produced in rabbit (A9046, Sigma, Birmingham, AL, USA) (100 µL/well), diluted 1:40,000 in 0.05% PBS-T. The material was washed, and tetramethylbenzidine (TMB-BD®, San Diego, CA, USA) was added as the chromogenic substrate (100 µL/well). Following incubation (37 °C, 6 min), the reaction was stopped using a 2 N H2SO4 solution (50 µL/well). The absorbance readings were performed at 450 nm (Titertek Multiskan MCC/340, Lab-System, Finland).
The positive and negative control sera used in our study were the same as those used in an experimental trial, in which three groups of chickens were infected with different infective doses of T. canis eggs (100, 1000, and 5000), and another group was employed as a negative control ( SILVA RAPOSO et al., 2016 Silva Raposo R, Santarém VA, Merigueti YF, Rubinsky-Elefant G, de Lima Cerazo LM, Pereira L, et al. Kinetic and avidity of IgY anti-Toxocara antibodies in experimentally infected chickens. Exp Parasitol 2016; 171: 33-41. http://dx.doi.org/10.1016/j.exppara.2016.09.009. PMid:27746165.
http://dx.doi.org/10.1016/j.exppara.201...
). According to these authors, an ELISA had 96.8% sensitivity and 100% specificity in the detection of IgY on the 60th day post-infection, independent of the infective dose.
All the samples were evaluated in duplicate, and the final OD was determined as the average of the duplicates. Antibody levels were expressed as reactivity indices (RIs) and were calculated from the ratio between the absorbance values of each tested sample and the cut-off value (RI = OD sample / OD cut-off). The samples with RIs greater than 1 were considered positive. The cut-off calculation was based on the OD value of the negative control (0.087) plus 4 SDs (4 × 0.0194 = 0.078), resulting in a value of 0.165.
Statistical analysis
The Pearson correlation test was performed to evaluate the relationship between the density of dogs per farm (m2) and the frequency of ELISA positivity. A 95% confidence interval (CI) was obtained using the Wilson method according to Sundar Dorai-Raj (2014) Dorai-Raj, S. binom: binomial confidence intervals for several parameterizations. R package version 1.1-1 [online]. Vienna: R Foundation for Statistical Computing; 2014 [cited 2017 May 22] Available from: https://CRAN.R-project.org/package=binom
https://CRAN.R-project.org/package=bino...
. All analyses were performed using R software ( R DEVELOPMENT CORE TEAM, 2014 R Development Core Team. R software: a language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing; 2014 [cited 2017 May 22]. Available from: http://www.r-project.org
http://www.r-project.org ...
), and the results were interpreted using a significance level of 0.05.
Results
Our screening obtained positive results for anti-Toxocara IgY antibodies in 67.7% (128/189; 95% CI = 61.1-74.4) of the free-range chickens according to the results of the indirect ELISA.
Table 1 summarizes the ODs, RIs, and frequencies of poultry that tested positive per farm. The ELISA positivity ranged from 29.6% to 100%.
Frequencies of anti-Toxocara antibodies (IgY) in free-range broiler chickens detected by indirect ELISA. State of Paraná, southern Brazil. 2017.
We compared the ODs of the positive animal samples with the ODs of the positive and negative controls. It was observed that 91.4% (n = 117) had ODs higher than the cut-off and lower than the positive control (PC), while in 8.6% (n = 11), the OD was equal to or higher than the PC.
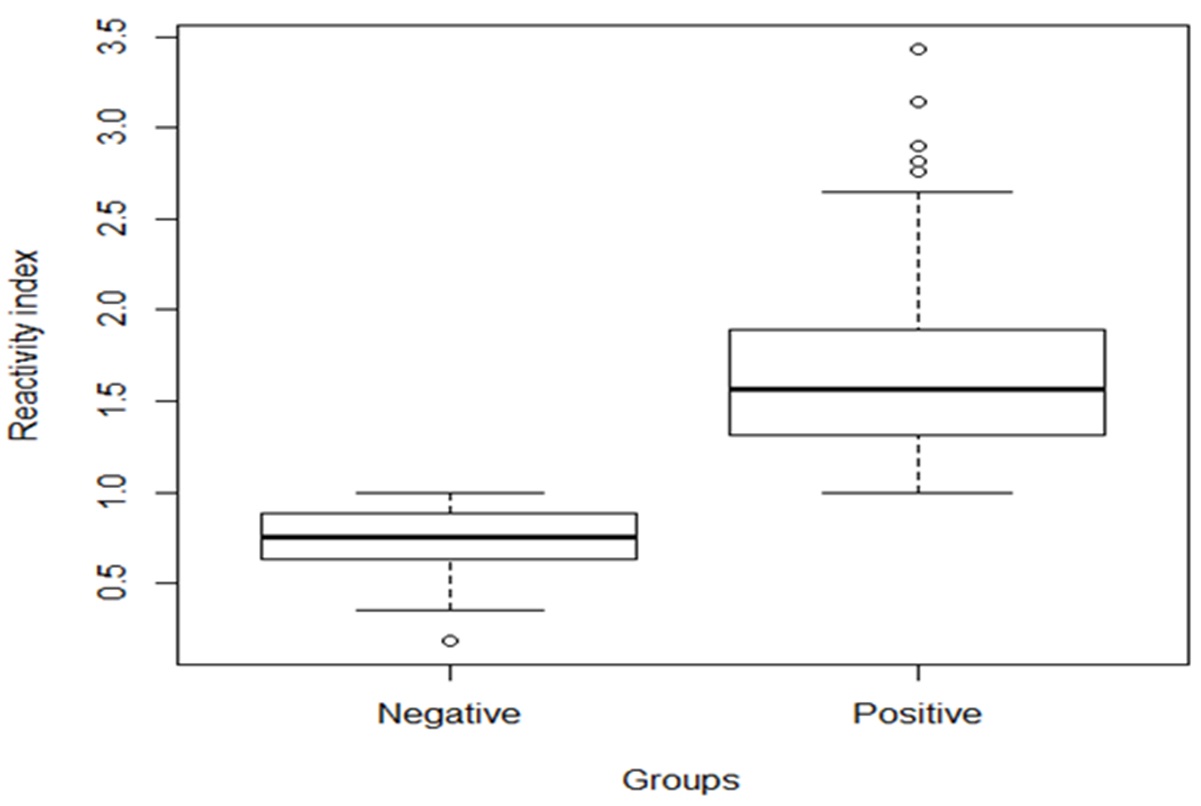
The RIs ranged from 0.187 to 0.993 and from 1.0 to 3.431, respectively, for the negative and positive samples (RI mean = 1.358). For chickens that tested positive, 44.5% had RIs between 1.0 and 1.49, while 47.7% had RIs from 1.500 to 2.499, and the RIs for 7.8% were higher than 2.500 ( Figure 1 ).
Box plot representing the distribution of the reactivity indexes of anti-Toxocara antibodies (IgY) in free-range broiler chickens detected by indirect ELISA. State of Paraná, southern Brazil - 2017.
The number of dogs by farm ranged from 1 to 12 (mean = 3.9), and the area where the chickens were reared varied from 1,200 to 5,000 m2 (mean = 2,293 m2). Considering the frequency of the positive ELISA results, no correlations were observed when the area (p value = 0.382) and the density of dogs (p value = 0.785) were compared.
Discussion
We screened for the presence of anti-Toxocara spp. antibodies in free-range chickens slaughtered in an abattoir in southern Brazil. Our data revealed a high seroprevalence of the antibodies (67.7%). In Brazil, a high frequency of anti-Toxocara IgY antibodies has also been detected by ELISA in the sera of free-range chickens of different origins ( CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
) and in broilers commercialized in street markets ( VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
), with positive results obtained in 58.5% and 89.9% of samples, respectively.
In this study, serum pre-absorption with A. galli antigen suspensions was performed to minimize cross-reactivity with Toxocara ( ELEFANT et al., 2006 Elefant GR, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal 2006; 20(4): 164-172. http://dx.doi.org/10.1002/jcla.20126. PMid:16874812.
http://dx.doi.org/10.1002/jcla.20126 ...
; CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
; VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
). These authors observed that the pretreatment of chicken serum with A. galli antigens reduced the OD values obtained in the ELISA by approximately 15%.
We evaluated samples of chickens that were 1) of the same breed, 2) exposed to the same rearing system, and 3) exposed during a defined period of time (60-90 days), thereby excluding certain confounding variables associated with the infection, particularly during the period of exposure. We observed that majority (91.4%) of the chicken samples had an OD higher than the cut-off value and lower than the PC. Campos-da-Silva et al. (2015) Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
considered samples from chickens with an OD higher than the cut-off and lower than the PC to be in three possible groups: infected, with past infection, or infected with another nematode (cross-infection). In this study, given the RI and OD values, the evaluated chickens could not have had past infections; due to the short lifespans of the evaluated chickens, the presence of antibodies was likely a consequence of chronic infection by Toxocara spp.
In this study, the frequencies of seropositive chickens ranged from 29.6% to 100%. This variation in the seropositivity for anti-Toxocara antibodies has been considered to be dependent on the origin of the chickens. Campos-da-Silva et al. (2015) Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
observed differences in the prevalence rates of antibodies in evaluated samples of chickens from small farms (78.6%), backyards (57.0%), and slaughterhouses (42.8%), and those necropsied at a university (42.5%). Another study ( VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
) reported similar findings (50.0% to 100%) and analysed samples of birds sold at street markets across eight different months.
Some risk factors have been associated with toxocariasis. In humans, toxocariasis tends to be more prevalent in rural settings, where the presence of dogs and high soil contamination ( RUBINSKY-ELEFANT et al., 2010 Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010; 104(1): 3-23. http://dx.doi.org/10.1179/136485910X12607012373957. PMid:20149289.
http://dx.doi.org/10.1179/136485910X126...
; MOREIRA et al., 2014 Moreira GM, Telmo PL, Mendonça M, Moreira AN, McBride AJ, Scaini CJ, et al. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol 2014; 30(9): 456-464. http://dx.doi.org/10.1016/j.pt.2014.07.003. PMid:25089038.
http://dx.doi.org/10.1016/j.pt.2014.07....
). Therefore, it is likely that free-range chickens reared in a rural environment are commonly exposed to Toxocara eggs.
The poultry evaluated in our study lived in environments that were cohabitated by pets, particularly dogs. However, the frequency of positive ELISA results was not associated with the density of dogs on the farm or with the area where the poultry were reared. Our study evaluated a small number of farms (n = 7), which likely limited the evaluation of risk factors associated with the infection.
Campos-da-Silva et al. (2015) Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
stated that free-range chickens may represent an indicator of soil contamination by Toxocara eggs, which may be reinforced by our results. These animals are also considered one of the best indicators of environmental contamination by Toxoplasma gondii oocysts ( DUBEY et al., 2006 Dubey JP, Gennari SM, Labruna MB, Camargo LM, Vianna MC, Marcet PL, et al. Characterization of Toxoplasma gondii isolates in free-range chickens from Amazon, Brazil. J Parasitol 2006; 92(1): 36-40. http://dx.doi.org/10.1645/GE-655R.1. PMid:16629312.
http://dx.doi.org/10.1645/GE-655R.1 ...
; DUBEY, 2010 Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 2010; 57(1): 60-73. http://dx.doi.org/10.1111/j.1863-2378.2009.01274.x. PMid:19744305.
http://dx.doi.org/10.1111/j.1863-2378.2...
).
In our study, it was not possible to evaluate the presence of larvae in the tissues of chickens. Zibaei et al. (2017) Zibaei M, Sadjjadi SM, Maraghi S. The occurrence of Toxocara species in naturally infected broiler chickens revealed by molecular approaches. J Helminthol 2017; 91(5): 633-636. http://dx.doi.org/10.1017/S0022149X16000559. PMid:27571878.
http://dx.doi.org/10.1017/S0022149X1600...
demonstrated the presence of Toxocara larvae (83.3% T. canis and 16.7% T. cati) in the tissue of broiler chickens by digesting the tissues in pepsin followed by a PCR assay. The authors concluded that broiler chickens can be natural paratenic hosts for the larvae of Toxocara spp., and in poultry, these larvae may be agents of human toxocariasis when humans consume raw, undercooked chicken meat, as observed by Nagakura et al. (1989) Nagakura K, Tachibana H, Kaneda Y, Kato Y. Toxocariasis possibly caused by ingesting raw chicken. J Infect Dis 1989; 160(4): 735-736. http://dx.doi.org/10.1093/infdis/160.4.735. PMid:2794572.
http://dx.doi.org/10.1093/infdis/160.4....
and Morimatsu et al. (2006) Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg 2006; 75(2): 303-306. PMid:16896137. .
Based on the conditions where chickens are reared and the high level of antibodies observed in our study and by others ( CAMPOS-DA-SILVA et al., 2015 Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
http://dx.doi.org/10.1007/s00436-015-46...
; VON SÖHSTEN et al., 2017 Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
http://dx.doi.org/10.1016/j.vprsr.2017....
), it is important to consider that the free-range environment may be a source of infection for humans and animals, and special attention should be given to farmers who are involved in free-range chicken-rearing activities.
In conclusion, the results of this study demonstrate that broiler chickens reared under semi-intensive conditions can be naturally infected by Toxocara species, thus serving as an indicator of soil contamination by Toxocara eggs. Further studies are necessary to evaluate the risk factors associated with the infection of chickens by Toxocara spp. and to determine the amount of cross-reactivity in ELISA.
Acknowledgements
A portion of our research funds was provided by Dr. Aristeu Vieira da Silva, supported by the National Council for Scientific and Technological Development (CNPq grant #477764/2012-6) and by the Research Productivity Grant #308093/2013-5 to Aristeu V. Silva, Grupo de Pesquisa em Zoonoses e Saúde Pública, Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana, Bahia, Brazil). Thanks to Dr. Rogério Giufrida (Unoeste) by statistical support.
References
- Ben Slimane B. Prevalence of the gastro-intestinal parasites of domestic chicken Gallus domesticus Linnaeus, 1758 in Tunisia according to the agro-ecological zones. J Parasit Dis 2016; 40(3): 774-778. http://dx.doi.org/10.1007/s12639-014-0577-5. PMid:27605783.
» http://dx.doi.org/10.1007/s12639-014-0577-5 - Campos-da-Silva DR, Paz JS, Fortunato VR, Beltrame MAV, Valli LCP, Pereira FEL. Natural infection of free-range chickens with the ascarid nematode Toxocara sp. Parasitol Res 2015; 114(11): 4289-4293. http://dx.doi.org/10.1007/s00436-015-4669-7. PMid:26319520.
» http://dx.doi.org/10.1007/s00436-015-4669-7 - Cardoso SP, Yamamura MH. Parasitas em produção de frangos no sistema de criação tipo colonial/caipira no Brasil. Semina: Ciênc Agrár 2004; 25(1): 63-74. http://dx.doi.org/10.5433/1679-0359.2004v25n1p63.
» http://dx.doi.org/10.5433/1679-0359.2004v25n1p63 - Conan A, Goutard FL, Sorn S, Vong S. Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res 2012; 8(1): 240. http://dx.doi.org/10.1186/1746-6148-8-240. PMid:23216706.
» http://dx.doi.org/10.1186/1746-6148-8-240 - Dorai-Raj, S. binom: binomial confidence intervals for several parameterizations. R package version 1.1-1 [online]. Vienna: R Foundation for Statistical Computing; 2014 [cited 2017 May 22] Available from: https://CRAN.R-project.org/package=binom
» https://CRAN.R-project.org/package=binom - Dubey JP, Gennari SM, Labruna MB, Camargo LM, Vianna MC, Marcet PL, et al. Characterization of Toxoplasma gondii isolates in free-range chickens from Amazon, Brazil. J Parasitol 2006; 92(1): 36-40. http://dx.doi.org/10.1645/GE-655R.1. PMid:16629312.
» http://dx.doi.org/10.1645/GE-655R.1 - Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 2010; 57(1): 60-73. http://dx.doi.org/10.1111/j.1863-2378.2009.01274.x. PMid:19744305.
» http://dx.doi.org/10.1111/j.1863-2378.2009.01274.x - Elefant GR, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal 2006; 20(4): 164-172. http://dx.doi.org/10.1002/jcla.20126. PMid:16874812.
» http://dx.doi.org/10.1002/jcla.20126 - Ferdushy T, Hasan MT, Golam Kadir AK. Cross sectional epidemiological investigation on the prevalence of gastrointestinal helminths in free range chickens in Narsingdi district, Bangladesh. J Parasit Dis 2016; 40(3): 818-822. http://dx.doi.org/10.1007/s12639-014-0585-5. PMid:27605790.
» http://dx.doi.org/10.1007/s12639-014-0585-5 - Food and Agriculture Organization of the United Nations – FAO. Poultry and animal production [online]. Rome: FAO; 2014 [cited 2017 Apr 18]. Available from: http://www.fao.org/ag/againfo/themes/en/poultry/production.html
» http://www.fao.org/ag/againfo/themes/en/poultry/production.html - Ibrahim HM, Abdel-Ghaffar F, Osman GY, El-Shourbagy SH, Nishikawa Y, Khattab RA. Prevalence of Toxoplasma gondii in chicken samples from delta of Egypt using ELISA, histopathology and immunohistochemistry. J Parasit Dis 2016; 40(2): 485-490. http://dx.doi.org/10.1007/s12639-014-0530-7. PMid:27413325.
» http://dx.doi.org/10.1007/s12639-014-0530-7 - Javaregowda AK, Kavitha Rani B, Revanna SP, Udupa G. Prevalence of gastro-intestinal parasites of backyard chickens (Gallus domesticus) in and around Shimoga. J Parasit Dis 2016; 40(3): 986-990. http://dx.doi.org/10.1007/s12639-014-0620-6. PMid:27605824.
» http://dx.doi.org/10.1007/s12639-014-0620-6 - Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193(1): 265-275. PMid:14907713.
- Moreira GM, Telmo PL, Mendonça M, Moreira AN, McBride AJ, Scaini CJ, et al. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol 2014; 30(9): 456-464. http://dx.doi.org/10.1016/j.pt.2014.07.003. PMid:25089038.
» http://dx.doi.org/10.1016/j.pt.2014.07.003 - Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg 2006; 75(2): 303-306. PMid:16896137.
- Nagakura K, Tachibana H, Kaneda Y, Kato Y. Toxocariasis possibly caused by ingesting raw chicken. J Infect Dis 1989; 160(4): 735-736. http://dx.doi.org/10.1093/infdis/160.4.735. PMid:2794572.
» http://dx.doi.org/10.1093/infdis/160.4.735 - R Development Core Team. R software: a language and environment for statistical computing [online]. Vienna: R Foundation for Statistical Computing; 2014 [cited 2017 May 22]. Available from: http://www.r-project.org
» http://www.r-project.org - Rabbi AKMA, Islam A, Majumder S, Anisuzzaman A, Rahman MH. Gastrointestinal helminths infection in different types of poultry. Bangl J Vet Med 2006; 4(1): 13-18. http://dx.doi.org/10.3329/bjvm.v4i1.1519.
» http://dx.doi.org/10.3329/bjvm.v4i1.1519 - Romasanta A, Romero JL, Arias M, Sánchez-Andrade R, López C, Suárez JL, et al. Diagnosis of parasitic zoonoses by immunoenzymatic assays-analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis , and Ascaris suum. Immunol Invest 2003; 32(3): 131-142. http://dx.doi.org/10.1081/IMM-120022974. PMid:12916704.
» http://dx.doi.org/10.1081/IMM-120022974 - Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010; 104(1): 3-23. http://dx.doi.org/10.1179/136485910X12607012373957. PMid:20149289.
» http://dx.doi.org/10.1179/136485910X12607012373957 - Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol 1979; 32(3): 284-288. http://dx.doi.org/10.1136/jcp.32.3.284. PMid:372253.
» http://dx.doi.org/10.1136/jcp.32.3.284 - Silva Raposo R, Santarém VA, Merigueti YF, Rubinsky-Elefant G, de Lima Cerazo LM, Pereira L, et al. Kinetic and avidity of IgY anti-Toxocara antibodies in experimentally infected chickens. Exp Parasitol 2016; 171: 33-41. http://dx.doi.org/10.1016/j.exppara.2016.09.009. PMid:27746165.
» http://dx.doi.org/10.1016/j.exppara.2016.09.009 - Taira K, Saeed I, Permin A, Kapel CMO. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol 2004; 121(1-2): 115-124. http://dx.doi.org/10.1016/j.vetpar.2004.01.018. PMid:15110409.
» http://dx.doi.org/10.1016/j.vetpar.2004.01.018 - Von Söhsten AL, Silva AV, Rubinsky-Elefant G, Macedo LMS, Guerra M. Anti- Toxocara spp. IgY antibodies in poultry sold in street markets from Feira de Santana, Bahia, Northeastern Brazil. Vet Parasitol: Reg Stud Rep 2017; 8: 86-89. http://dx.doi.org/10.1016/j.vprsr.2017.02.006.
» http://dx.doi.org/10.1016/j.vprsr.2017.02.006 - Zibaei M, Sadjjadi SM, Maraghi S. The occurrence of Toxocara species in naturally infected broiler chickens revealed by molecular approaches. J Helminthol 2017; 91(5): 633-636. http://dx.doi.org/10.1017/S0022149X16000559. PMid:27571878.
» http://dx.doi.org/10.1017/S0022149X16000559
Publication Dates
-
Publication in this collection
24 May 2018 -
Date of issue
Apr-Jun 2018
History
-
Received
21 Aug 2017 -
Accepted
14 Mar 2018