CASE REPORT
Hypoparathyroidism mimicking ankylosing spondylitis and myopathy: a case report
Thayana Ribeiro Kajitani; Renata Viana da Silva; Eloisa Bonfá; Rosa M. R. Pereira
Rheumatology Division, Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo/SP, Brazil
INTRODUCTION
Idiopathic hypoparathyroidism (HP) is an induced or inherited condition characterized by insufficient secretion of parathyroid hormone. The clinical manifestations of this condition are varied, and pain and stiffness affecting the back and hips resembling ankylosing spondylitis is an under-recognized symptom in these patients. Here, we report a case of delayed diagnosis of hypoparathyroidism referred to a rheumatologist due to progressive inflammatory back pain and myalgia.
Idiopathic hypoparathyroidism is an induced or inherited condition of unknown etiology and is characterized by insufficient secretion of parathyroid hormone due to atrophy or absence of parathyroid glands, which leads to hypocalcaemia and hyperphosphatemia.1
Hypoparathyroidism has multiple clinical manifestations, which primarily involve tissues of ectodermic origin.2 Pain and stiffness affecting the back and hips, limited movement and posture resembling that seen in patients with ankylosing spondylitis is an under-recognized feature in patients with this condition.1,3 Muscular complaints by these patients are common because the concentration of calcium ions is important for the maintenance and control of several biochemical processes during muscle contraction. Hypocalcaemia causes hyper-excitability of nerves and muscles, and a sporadic increase in muscular enzymes.4
Here, we report a case of delayed diagnosis of hypopar-athyroidism referred to a rheumatologist due to progressive inflammatory back pain and myalgia with concomitant elevation of inflammatory markers and muscle enzymes. The patient was initially erroneously diagnosed with ankylosing spondylitis (AS) and idiopathic myopathy. This case reinforces the relevance of investigating the underlying mineral metabolism disturbances in patients with inflammatory diseases.
CASE REPORT
A 40-year-old man was referred to the Rheumatology Outpatient Clinic with a fifteen-year history of progressive inflammatory back and neck pain and prolonged (two hours) morning stiffness. He also complained of diffuse myalgia, muscle cramps, and frequent changes in bowel habits characterized by chronic diarrhea (intermittent liquid diarrhea without abdominal pain, mucous or blood, usually occurring after meals). He reported a history of epilepsy since three months of age and cataracts by age 31. He had no history of surgical excision or damage to the parathyroid gland, radiation or neoplastic/granulomatosis infiltration. He also denied consanguinity, Arab ancestry, deafness or recurrent oral candidiasis.
Upon physical examination, the patient displayed a typical AS posture with decreased spinal mobility, a neck rotation of 10º, chest expansion of 1.5 cm, and a Schober test measurement of 10.5 cm. His hip movement was markedly restricted in all directions, particularly flexion and rotation. The Fabere test was positive. The patient fulfilled the modified New York criteria for ankylosing spondylitis.5 Assessment of the patient's muscle strength revealed Grade IV reduction in all four limbs. Trousseau's and Chvostek's signs also gave negative results. The patient had dental abnormalities and a cognitive deficit (two years' education and a Mini-Mental State Examination score of eight), but cleft palate, growth retardation, microcephaly, microphthalmia, and small hands and feet were not observed. Dental abnormalities on a panoramic X-Ray revealed few erupted teeth and the presence of multiple impacted teeth in the maxilla and mandible, producing a change in anatomical size and shape.
Laboratory investigation revealed hypocalcaemia (calcium 4mg/dL, ionized calcium 2.3 mg/dl), hyperpho-sphatemia (5.3 mg/dl), undetectable parathyroid hormone (<3 pg/ml) and vitamin D levels within the normal range (56.1 ng/ml). Inflammatory markers (CRP 29.9 mg/dl and ESR 31 mm) and muscle enzymes (CPK 2805 U/l, aldolase 8.6 U/l and lactic dehydrogenase 1397 U/l) were elevated (Table 1). The patient did not express HLA-B27.
Tests for anti-nuclear antibodies and rheumatoid factor were negative. The patient's thyroid hormones, serum, and urinary cortisol were normal. Blood counts and glucose, urea and creatinine levels were also within normal ranges. Serology for viral hepatitis, syphilis, HIV, and HTLV were negative. Echocardiogram and renal ultrasound were normal. A brain CT scan revealed calcifications in the basal ganglia, thalamus, and cerebellum. A mandibular panoramic radiograph confirmed hypoplasia and dental malformations. Electromyography was normal.
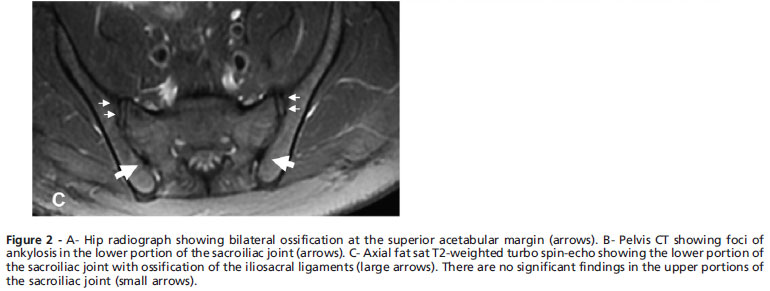
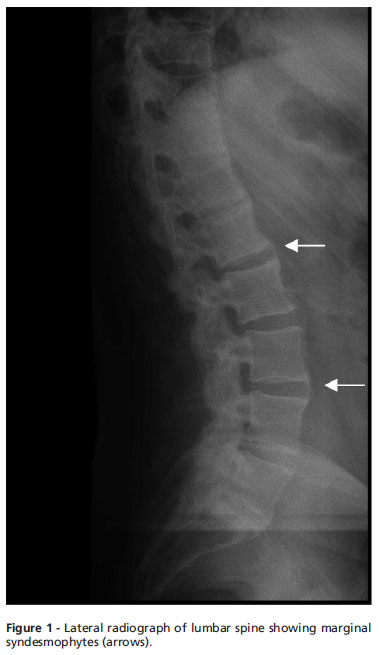
Bone fusions in the cervical spine, calcification of ligaments and syndesmophytes in the lumbar spine were observed through spine radiography (Figure 1), and Grade II (rights/Grade III (left) sacroiliitis was observed through sacroiliac joint radiography. Computed tomography (CT) revealed ankylosis foci at the sacroiliac joint (SI) and bilateral ossification of the iliolumbar ligament (Figure 2B). Similarly, magnetic resonance imaging demonstrated ossification of the iliosacral ligaments in the lower portions of the sacroiliac joints and no significant findings in the upper portions of the SI joint (Figure 2C).
Bone mineral density evaluated by DXA revealed a Z-score of +4.4 SD at the lumbar spine, -0.2 SD at the total femur and -1.0 SD at the femoral neck.
Treatment with 2 g/day calcium carbonate and 1 µg calcitriol led to evident clinical and laboratory improvement including normalization of the bowel habits. Increasing the calcium dose to 4 g/day was recommended. At the following visit, the patient had stopped taking the medicine, and the laboratory parameters consequently worsened, particularly inflammatory markers and muscle enzymes (Table 1). The importance of treatment adherence was reinforced, and calcium supplements were increased to 6 g/day, normalizing the serum calcium and muscle enzymes and reducing inflammatory markers. Concomitant improvement of neck and back pain, disappearance of the cramps and muscle pain and normalization of bowel habits were observed.
DISCUSSION
To our knowledge, this is the first case report of idiopathic HP simulating AS associated with idiopathic myopathy.
The diagnosis of HP was confirmed by hypocalcemia, hyperphosphatemia, and undetectable PTH. The chronic development of hypocalcemia may explain the absence of tetany in this case. In fact, the most prominent symptoms of tetany usually occur in acute forms of hypocalcemia and the development of tetany symptoms depends on the duration, severity, and rapidity of hypocalcemia.6
Developmental disorders are unlikely in this case because the patient did not exhibit the typical characteristics of DiGeorge Syndrome/Catch-22,7 hypoparathyroidism-sensorineural deafness-renal dysplasia syndrome,8 hypopa-rathyroidism-retardation-dysmorphism or Kenny-Caffey syndromes.9 Parathyroid gland destruction was excluded given that the patient did not have a history of surgical excision or radiation therapy. The patient had no family history or clinical features suggestive of autoimmune poly-endocrinopathy-candidiasis-ectodermal dystrophy.10 Autoimmune adrenal involvement was also excluded as the serum and urinary parameters were within normal ranges and clinical symptoms were absent. In addition, the classic triad of Kearn-Sayres syndrome (KSS) was not observed, and the muscle complaints began late in life.11 Based on the above evidence, the probable diagnosis is idiopathic HP, although other rare defects cannot be excluded because specific genetic analyses were not performed.
Occasionally, patients with HP may have clinical and radiologic characteristics resembling AS1 due to paravertebral calcification and ossification1 . The syndesmophyte in our HP patient, which exhibited the origination in the vertebral margin and preservation of disc space characteristic of AS, led to the misdiagnosis of AS. This finding is distinct from osteoarthritis in which osteophytes are horizontal and there is a significant reduction in disc space.13 The predominant localization in the lumbar region also suggested the diagnosis of AS, although any spinal location may be involved in HP.13 Diffuse idiopathic skeletal hyperostosis (DISH) was unlikely, considering the radiologic spine features, the early appearance of calcifications (before the fourth decade) and the absence of co-morbidities such as dyslipidemia and diabetes mellitus type .1
The radiographic findings in the sacroiliac joint were predominant in the lower portion with ossification of the iliolumbar ligament, whereas the involvement is more common in the upper region of the joint in AS. In addition, the evident ossification at the hip joint with preserved joint space is not characteristic of AS but has been reported in HP.1 Moreover, the lack of HLA-B27 expression may reduce the likelihood of the AS diagnosis as over 90% of AS patients are positive for this allele,13 although no data regarding the frequency of HLA-B27 in the healthy Brazilian population are available.
Of note, the extensive basal ganglia calcification in our case has been reported more often in HP patients with spine involvement than in those without this complication,13 suggesting a disseminated process of calcification associated with mineral metabolism disturbance. Reinforcing this possibility, long-term HP disease was more often associated with spinal involvement,13 which was also observed in the patient evaluated herein. In addition, there is much in vitro evidence that hyperphosphatemia may have a proinflam-matory role14 that could ultimately contribute to the calcification observed in our patient.
The novel description of concomitant association of HP and spondyloarthritis with myopathy was supported by the diminished muscle strength and marked muscle enzyme elevations. Chronic hypocalcemic myopathy secondary to HP and raised serum CK concentration has rarely been reported15 and is probably an under-recognized condition due to the patient's adaptation to longstanding hypocalcemia. The most likely explanation of this condition is that the increased permeability of the muscle membrane induced by hypocalcemia leads to CK release.16 The inverse correlation between CK and serum calcium concentrations as well as the normal electromyographic findings observed in this case support this possibility. Of note, rhabdomyolysis was excluded due to normal urine analysis, the absence of acute renal failure and the positive response to calcium treatment.
Idiopathic HP has been described as an unusual cause of diarrhea17 as observed in the present case. Reinforcing this possibility, the bowel habits were normalized after hypo-calcemia correction.
Importantly, the clinical and laboratory improvements achieved solely with calcium and calcitriol supplementation confirmed the diagnosis of HP and excluded the associations with ankylosing spondylitis and idiopathic myopathy, as inflammatory rheumatic diseases are unresponsive to this therapy.
This case emphasizes the importance of recognizing rheumatic manifestations of HP to preclude unnecessary treatments. HP should be considered in the differential diagnosis of spondyloarthropathies and myopathies, and calcium may be included in the diagnostic workup of these patients.
Email: rosamariarp@yahoo.com Tel.: 55 11 3061-7213
- 1. Korkmaz C, Yasan S. Hypoparathyroidism simulating ankylosing spondylitis. Joint Bone Spine. 2005;72:89-97, doi: 10.1016/j.jbspin.2004.08.004.
- 2. Nora DB, Fricke D, Becker J, Gomes G. Hypocalcemic myopathy without tetany due to idiopathic hypoparathyroidism. Arq Neuropsiquiatr. 2004;62:154-7, doi: 10.1590/S0004-282X2004000100028.
- 3. Yacoub YI, Rostom S, Hossouni NH. Uncommon case of ankylosing spondylitis associated with spontaneous occurring hypoparathyroidism. Rheumatol Int. 2011;31:681-3, doi: 10.1007/s00296-009-1220-0.
- 4. Van Offel JF, De Gendt CM, De Clerck LS, Stevens WJ. High bone mass and hypocalcaemic myopathy in a patient with idiophatic hypopar-athyroid. Clin Rheumatol. 2000;19:64-6.
- 5. Calin A. Comment on article by van der Linden et al. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1985;28:357-9, doi: 10.1002/art.1780280321.
- 6. Nora DB, Fricke D, Becker J, Gomes I. Hypocalcemic myopathy without tetany due to idiopathic hypoparathyroidism: case report. Arq Neuropsiquiatr. 2004;62:154-7, doi: 10.1590/S0004-282X2004000100028.
- 7. Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22qll.2.2 deletion syndrome. Lancet. 2007;370:1443-52, doi: 10.1016/S0140-6736(07)61601-8.
- 8. Ferraris S, Del Monaco AG, Garelli E, Carando A, De Vito B, Pappi P, et al. HDR syndrome: a novel "de novo" mutation in GATA3 gene. Am J Med Genet A. 2009;149A:770-5.
- 9. Naguib KK, Gouda SA, Elshafey A, Mohammed F, Bastaki L, Azab AS, et al. Sanjad-Sakati syndrome/Kenny-Caffey síndrome type 1: a study of 21 cases in Kuwait. East Mediterr Health J. 2009;15:345-52.
- 10. Ng WF, von Delwig A, Carmichael AJ, Arkwright PD, Abinun M, Cant AJ, et al. Impaired T(H)17 responses in patients with chronic mucocutaneous candidiasis with and without autoimmune polyendo-crinopathy-candidiasis- ectodermal dystrophy. J Allergy Clin Immunol. 2010;126:1006-15, doi: 10.1016/j.jaci.2010.08.027.
- 11. Ashizawa T, Subramony SH. What is Kearns-Sayre syndrome after all? Arch Neurol. 2001;58:1053-4, doi: 10.1001/archneur.58.7.1053.
- 12. Ünverdi S, Öztürk A, Inal S, Selek H, Goker B, Haznedaroglu S, et al. Idiopathic hypoparathyroidism mimicking diffuse idiopathic skeletal hyperostosis. J Clin Rheumatol. 2009;15:361-2, doi: 10.1097/RHÜ. 0b013e3181bb9865.
- 13. Goswami R, Ray D, Sharmat R, Tomar N, Gupta R, Gupta N, et al. Presence of spondyloarthropathy and its clinical profile in patients with hypoparathyroidism. Clinical Endocrinology. 2008;68:258-63.
- 14. Calò LA, Savica V, Piccoli A, Fusaro M, D'Angelo A, Davis PA. Reduction of hyperphosphatemia is related with the reduction of C-reactive protein in dialysis patients. Study in sevelamer-resistant dialysis patients treated with chitosan chewing gum as salivary phosphate binder. Ren Fail. 2011;33:11-4, doi: 10.3109/0886022X.2010.528116.
- 15. Barber J, Butler RC, Davie MWJ, Sewry CA. Hypoparathyroidism presenting as myopathy with raised creatine kinase. Rheumatology. 2001;40:1417-8, doi: 10.1093/rheumatology/40.12.1417.
- 16. Ishikawa T, Kanayama M, Oba T, Horie T. Hypocalcemic induced increase in creatine kinase in rats. Pediatr Neurol. 1998;18:326-30, doi: 10.1016/S0887-8994(97)00210-5.
- 17. Peracchi M, Bardella MT, Conte D. Late-onset idiopathic hypoparathyr-oidism as a cause of diarrhoea. Eur J Gastroenterol Hepatol. 1998;10:163-5, doi: 10.1097/00042737-199802000-00013.
Publication Dates
-
Publication in this collection
19 Aug 2011 -
Date of issue
2011