Abstract
We propose a revised classification of Doradidae based on phylogenetic analyses of sequence data for one nuclear (rag1) and two mitochondrial (co1, 16s) genes, and corroborated by caudal-fin morphology. The molecular dataset comprises 174 doradid specimens representing all 31 valid genera, 83 of the 96 valid extant species and 17 species-level taxa that remain undescribed or nominally unassigned. Parsimony and Bayesian analyses of molecular data support six major lineages of doradids assigned here to three nominal subfamilies (Astrodoradinae, Doradinae, Wertheimerinae) and three new ones (Acanthodoradinae, Agamyxinae, Rhinodoradinae). The maximum parsimony topology of Doradidae was sensitive to ingroup density and outgroup age. With the exceptions of Astrodoradinae and Doradinae, each subfamily is diagnosed by caudal-fin characteristics. The highest degree of fusion among skeletal elements supporting the caudal fin is observed in Acanthodoradinae and Aspredinidae, lineages that are sister to the remaining doradids and aspredinoids (i.e., Auchenipteridae + Doradidae), respectively. Fusion among caudal-fin elements tends to be higher in taxa with rounded, truncate or emarginate tails and such taxa typically occupy shallow, lentic habitats with ample structure. Caudal-fin elements are more separated in taxa with moderately to deeply forked tails that occupy lotic habitats in medium to large river channels.
Keywords:
Biogeography; Caudal fin; Osteology; Systematics; Taxonomy
Resumo
Propomos uma classificação revisada de Doradidae baseada na análise filogenética de dados moleculares dos genes rag1, co1 e 16s, e suportada pela morfologia da nadadeira caudal. A matriz molecular inclui 174 espécimes de doradídeos representando os 31 gêneros válidos, 83 das 96 espécies viventes e 17 táxons não descritos ou nominalmente não designados. As análises de parcimônia e bayesiana suportam seis linhagens principais de doradídeos atribuídas a três subfamílias nominais (Astrodoradinae, Doradinae, Wertheimerinae) e três novas subfamílias (Acanthodoradinae, Agamyxinae, Rhinodoradinae). A árvore de máxima parcimônia de Doradidae é sensível à densidade de grupo interno e a idade do grupo externo. Com exceção de Astrodoradinae e Doradinae, cada subfamília é diagnosticada por características da nadadeira caudal. Dentro da família Doradidae e da superfamília Aspredinioidea (Aspredinidae, Auchenipteridae e Doradidae), o maior grau de fusão entre os elementos da nadadeira caudal é observado nas linhagens mais antigas, Acanthodoradinae e Aspredinidae, respectivamente. A fusão entre os elementos da nadadeira caudal é maior em táxons com a caudal arredondada, truncada ou emarginada e esses táxons normalmente ocupam habitats lênticos rasos. Os elementos da nadadeira caudal são mais separados em táxons com a cauda bifurcada ocupando habitats lóticos em canais de rios médios a grandes.
Palavras-chave:
Biogeografia; Nadadeira caudal; Osteologia; Sistemática; Taxonomia.
INTRODUCTION
Thorny catfishes (Siluriformes: Doradidae) form a monophyletic group of about 96 valid extant and one fossil species endemic to freshwaters of South America on both sides of the Andes Mountains. Most doradids are easily distinguished from other catfishes by having a conspicuous midlateral row of bony scutes, each one with a central, caudally directed thorn (Fig. 1). Each midlateral scute is formed by dorsal and ventral aliform expansions of a lateral-line tubule. A single enlarged pore perforates the skin in the axil of each thorn. The infranuchal scute is exceptionally composed of both an expanded tubule and an ossified ligament that runs between the nuchal region of the cranium and the rib supported by the sixth vertebra, which is the first long-formed rib. As such, the infranuchal scute represents an unambiguous synapomorphy for Doradidae (Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
). Another synapomorphy for doradids is the presence of Sörensen’s ligament (Fig. 2), an unossified ligament between the anterolateral rim of the Müllerian disk and an ossified tubule or scute in the tympanic region (Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
).
Variation in scute morphology in cleared and stained specimens of Doradidae. A. Amblydoras nheco (ANSP 187416); B. Megalodoras uranoscopus (ANSP 178302); C. Hassar orestis (ANSP 181094); D. Leptodoras linnelli (ANSP 182791). Infranuchal scute (is), exceptionally composed of expanded lateral-line tubule and ossified ligament between nuchal region of skull and rib supported by 6th vertebra.
Sörensen’s ligament (sl), unossified ligament between anterolateral rim of Müllerian disk (md) and first ossified tubule or scute (not visible) in tympanic region; stained specimen of Oxydoras sifontesi (ANSP 181069, 149.5 mm SL). gb = gas bladder, is = infranuchal scute, pcp = posterior cleithral process, pnp = posterior nuchal plate, ptsc = posttemporal-supracleithrum.
Adult thorny catfishes vary in standard length from about 22 mm (Physopyxis ananas Sousa & Rapp Py-Daniel, 2005) to over one meter (Oxydoras spp.). Doradids generally occupy benthic habitats in lowland lakes and rivers, although a few taxa frequent pelagic habitats, such as Nemadoras hemipeltis (Eigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
) and Pterodoras Bleeker, 1862. Many of the smaller species are peculiar to floodplains and occupy sluggish streams and river margins during the low-water season. The larger species are restricted to the main channels of medium to large rivers. A few doradids (e.g., Rhinodoras Bleeker, 1862) often associate with large rocky rapids in rivers draining the Brazilian and Guiana shields. The propensity of thorny catfishes for large river channels and lowland floodplains, coupled with an absence from upland headwaters, makes Doradidae a prime candidate for investigating large scale shifts in Neotropical drainage patterns.
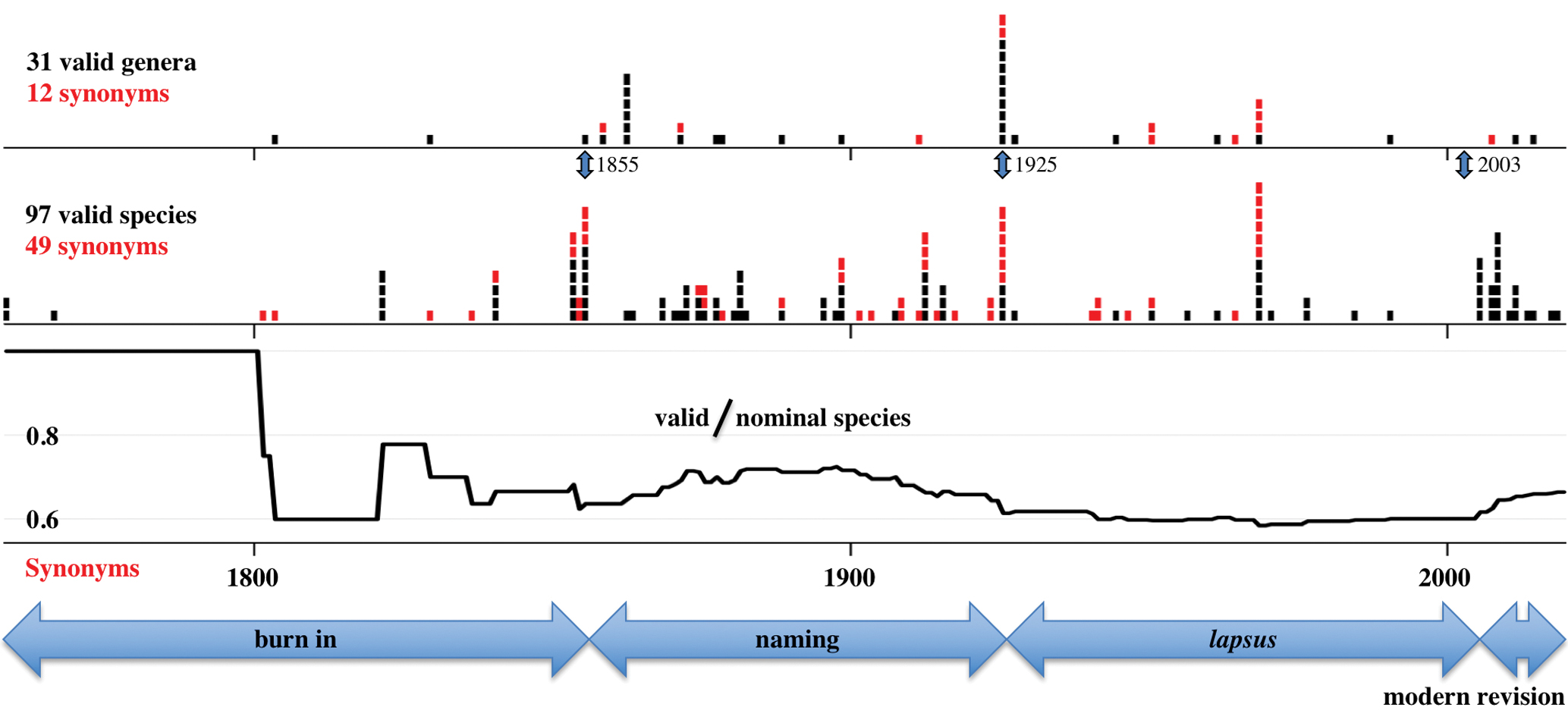
The taxonomic history of thorny catfishes includes 43 nominal genera and 146 nominal species (Fig. 3) dating back to the Linnaean (Linnaeus,1758) descriptions of Acanthodoras cataphractus and Platydoras costatus (Sabaj Pérez, 2014Sabaj Pérez MH. On the identity of Catesby’s fish in armour, “Cataphractus Americanus” (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2014; 163(1):119–32. https://doi.org/10.1635/053.163.0104
https://doi.org/10.1635/053.163.0104...
). Lacepède, (1803)Lacepède BGE. Histoire naturelle des poisons: Tomme Cinquieme. Paris; 1803. proposed the first genus (Doras), Bleeker, (1858)Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41. established the family-group name Doradidae (Van der Laan et al., 2014Van der Laan R, Eschmeyer WN, Fricke R. Family-group names of recent fishes. Zootaxa. 2014; 3882(1):1–230. https://doi.org/10.11646/zootaxa.3882.1.1
https://doi.org/10.11646/zootaxa.3882.1....
), and Higuchi et al., (2007)Higuchi H, Birindelli JLO, Sousa LM, Britski HA. Merodoras nheco, new genus and species from Rio Paraguay basin, Brazil (Siluriformes, Doradidae), and nomination of the new subfamily Astrodoradinae. Zootaxa. 2007; 1446(1):31–42. https://doi.org/10.11646/zootaxa.1446.1.3
https://doi.org/10.11646/zootaxa.1446.1....
described the first valid subfamily, Astrodoradinae. Kner (1853Kner R. Ueber einige sexual-unterschiede bei der gattung Callichthys und die schwimmblase bei Doras C. Val. Sitzungsber Akad Wiss Wien. 1853; 11:138–46., 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162.) published the first detailed descriptions of doradids in his treatment of 18 species including 13 proposed as new. Although Kner recognized only one genus (Doras), his species spanned 14 of the 31 genera considered valid in the family. Eigenmann, (1925)Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
compiled a comprehensive monograph on Doradidae that is rich with figures and acute observations that continue to inform modern studies. Sabaj, Ferraris (2003)Sabaj MH, Ferraris CJ Jr. Family Doradidae. In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.456–69. assembled an annotated checklist of doradids that clarified or highlighted a number of nomenclatural and taxonomic issues. The next fifteen years witnessed the descriptions of two new genera and 23 new species, nearly a quarter of the total species considered valid here. Although the classification of Doradidae is more or less complete to the genus level, taxonomic work remains at the species level for a number of genera, especially Acanthodoras Bleeker, 1862, Amblydoras Bleeker, 1862, AnadorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
, HemidorasBleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41., Platydoras Bleeker, 1862, and Pterodoras Bleeker, 1862.
Summary of taxonomic history of Doradidae. Each square represents a nominal valid taxon (black) or putative synonym (red) plotted against the year of its description (two nomina oblita and four replacement names not included). Continuous black line traces ratio of valid to nominal species through four time periods: burn in, naming, lapsus and modern revision. Monographs by Kner, (1855)Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162. and Eigenmann, (1925)Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464... mark ends of burn in and naming periods, respectively. Monograph by Sabaj, Ferraris (2003)Sabaj MH, Ferraris CJ Jr. Family Doradidae. In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.456–69. marks beginning of modern revision.
Cladistic studies of doradids began with Higuchi, (1992)Higuchi H. A phylogeny of the South American thorny catfishes (Osteichthyes, Siluriformes, Doradidae). [PhD Thesis]. Cambridge: Havard University; 1992. who used morphology to hypothesize relationships within the family inclusive of a previously contentious member, Wertheimeria maculata Steindachner, 1877. Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
provided robust support for alternative relationships based on phylogenetic analyses of molecular data. Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
assembled the most comprehensive morphological data set to date to investigate phylogenetic relationships among Doradidae and its sister family Auchenipteridae. Based on those results, Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
firmly diagnosed Doradidae and proposed a new subfamily, Wertheimerinae. Other recent studies have described variation in gas bladder morphology (Birindelli et al., 2009Birindelli JLO, Sousa LM, Sabaj Pérez MH. Morphology of the gas blader in thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2009; 158(1):261–96. https://doi.org/10.1635/053.158.0114
https://doi.org/10.1635/053.158.0114...
), sperm morphology (Quagio-Grassiotto et al., 2011Quagio-Grassiotto I, Ortiz RJ, Sabaj Pérez MH, Oliveira C. Sperm of Doradidae (Teleostei: Siluriformes). Tissue Cell. 2011; 43(1):8–23. http://dx.doi.org/10.1016/j.tice.2010.10.006
http://dx.doi.org/10.1016/j.tice.2010.10...
), bioacoustics (Kaatz, Stewart, 2012Kaatz IM, Stewart DJ. Bioacoustic variation of swimbladder disturbance sounds in Neotropical doradoid catfishes (Siluriformes: Doradidae, Auchenipteridae): Potential morphological correlates. Curr Zool. 2012; 58(1):171–88. https://doi.org/10.1093/czoolo/58.1.171
https://doi.org/10.1093/czoolo/58.1.171...
; Zebedin, Ladich, 2013Zebedin A, Ladich F. Does the hearing sensitivity in thorny catfishes depend on swim bladder morphology? PLoS ONE. 2013; 8(6):e67049. https://doi.org/10.1371/journal.pone.0067049
https://doi.org/10.1371/journal.pone.006...
; Knight, Ladich, 2014Knight L, Ladich F. Distress sounds of thorny catfishes emitted underwater and in air: Characteristics and potential significance. J Exp Biol. 2014; 217(22):4068–78. https://doi.org/10.1242/jeb.110957
https://doi.org/10.1242/jeb.110957...
), digestive tube morphology (de Melo Germano et al., 2014de Melo Germano R, Stabille SR, de Britto Mari R, Pereira JNB, Faglioni JRS, de Miranda Neto MH. Morphological characteristics of the Pterodoras granulosus digestive tube (Valenciennes, 1821) (Osteichthyes, Doradidae). Acta Zool. 2014; 95(2):166–75. https://doi.org/10.1111/azo.12016
https://doi.org/10.1111/azo.12016...
) musculature (Arce H., 2015Arce H. M. Mandibular, hyoid and pectoral musculature of thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2015; 164(1):229–77. https://doi.org/10.1635/053.164.0116
https://doi.org/10.1635/053.164.0116...
) and cytogenetics (Baumgärtner et al., 2018Baumgärtner L, Paiz LM, Takagui FH, Lui RL, Moreira-Filho O, Giuliano-Caetano L, Portela-Castro ALB, Margarido VP. Comparative cytogenetics analysis on five genera of thorny catfish (Siluriformes, Doradidae): Chromosome review in the family and inferences about chromosomal evolution integrated with phylogenetic proposals. Zebrafish. 2018; 15(3):270–78. https://doi.org/10.1089/zeb.2017.1554
https://doi.org/10.1089/zeb.2017.1554...
; Takagui et al., 2019Takagui FH, Baumgärtner L, Baldissera JN, Lui RL, Margarido VP, Fonteles SBA, Garcia C, Birindelli JO, Moreira-Filho O, Almeida FS, Giuliano-Caetano L. Chromosomal diversity of thorny catfishes (Siluriformes-Doradidae): A case of allopatric speciation among Wertheimerinae species of São Francisco and Brazilian eastern coastal drainages. Zebrafish. 2019; 16(5):477–85. https://doi.org/10.1089/zeb.2019.1769
https://doi.org/10.1089/zeb.2019.1769...
). Drawing heavily from variation in caudal-fin morphology, Birindelli, Sousa, (2018)Birindelli JLO, Sousa LM. Family Doradidae–Thorny Catfishes. In: van der Sleen P, Albert JS, editors. Field guide to the fishes of the Amazon, Orinoco & Guianas. Princeton: Princeton University Press; 2018. p.222–33. assembled a key to the 26 doradid genera inhabiting the Amazon, Orinoco and Guianas.
The primary goals of this study are to advance the classification and summarize the geographic distributions of thorny catfishes. We expanded the taxon sampling of the molecular data set analyzed by Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
and compiled comprehensive data on the caudal skeleton for all doradid taxa. Based on our analyses of those data, we propose a revised classification of Doradidae and comment on morphological trends observed in caudal-fin evolution among doradids and other catfishes.
MATERIAL AND METHODS
Molecular Data: markers and taxon sampling. Sequence data were assembled for one nuclear gene, recombination activating gene 1 (rag1), and two mitochondrial genes, cytochrome c oxidase subunit 1 (co1) and 16s ribosomal RNA (16s), from 218 specimens representing 37 outgroup taxa (44 specimens) and 100 ingroup taxa (174 specimens) (Tab. 1). The current analysis employed the same three markers used by Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
, but added 74 specimens (43 doradids and 31 outgroups) and 38 species-level taxa (14 doradids and 24 outgroups).
List of taxa, voucher specimens and DNA sequences analyzed. *Denotes individuals sequenced in Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02.... . Museum codes follow Sabaj, (2020)Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
https://doi.org/10.1643/ASIHCODONS2020... . a Sequence data published by Sullivan et al., (2006)Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
https://doi.org/10.1016/j.ympev.2006.05.... for voucher ANSP 180476. b Sequence data submitted to GenBank by Heok Hee Ng (2006) for voucher ANSP 180476 (tag 4515) from an unpublished study. c Sequence data published by Nakatani et al., (2011)Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. https://doi.org/10.1186/1471-2148-11-177
https://doi.org/10.1186/1471-2148-11-177... ; no voucher data. d Genus assignment based on Calegari et al., (2019)Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03... . e Questionably a junior synonym of Hemidoras boulengeri (Steindachner, 1915).
Outgroup taxa were selected on the basis of molecular studies (Sullivan et al., 2006Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
https://doi.org/10.1016/j.ympev.2006.05....
; Lundberg et al., 2007Lundberg JG, Sullivan JP, Rodiles-Hernández R, Hendrickson DA. Discovery of African roots for the Mesoamerican Chiapas catfish, Lacantunia enigmatica, requires an ancient intercontinental passage. Proc Acad Nat Sci Phila. 2007; 156(1):39–53. https://www.jstor.org/stable/27667759
https://www.jstor.org/stable/27667759...
; Nakatani et al., 2011Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. https://doi.org/10.1186/1471-2148-11-177
https://doi.org/10.1186/1471-2148-11-177...
; Arcila et al., 2017Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
https://doi.org/10.1038/s41559-016-0020...
; Betancur-R. et al., 2017Betancur-R. R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(1):162. https://doi.org/10.1186/s12862-017-0958-3
https://doi.org/10.1186/s12862-017-0958-...
; Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
) that support Diplomystidae as the sister group to Siluroidei, Cetopsidae as the sister group to all other siluroids, and Aspredinidae as sister to Auchenipteridae + Doradidae, with those three families comprising the superfamily Aspredinoidea Adams, 1854 (Van der Laan, 2019Van der Laan R. Freshwater fish list: 26th ed. Almere: Richard van der Laan; 2019.:121; see also Results). The ingroup taxa represented all 31 valid genera of Doradidae, 83 of the 96 extant valid species, and 17 taxa that are undescribed species or currently unassigned to nominal ones.
Molecular Data: DNA extraction, amplification and sequencing. Generally, tissues (e.g., fin, muscle or gill) were taken in the field and preserved in 95–100% ethanol; voucher specimens were fixed in 10% buffered formalin, then transferred to 70–75% ethanol for long-term museum storage. Ideally, the tissue sample is associated with a field tag number that is tied to the voucher specimen. Additional tissue samples were provided by generous colleagues (see Acknowledgments).
Total DNA was extracted using the Qiagen DNeasy blood and tissue kit. PCR was carried out in 20 μl reactions; primers for amplification and sequencing are listed inArce H. et al., (2013Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
:561, tab. 1). For co1 and 16s, the PCR reaction mixture consisted of 10 μl of Apex Taq DNA Polymerase Master Mix, 1.5 mM MgCl2 (Genesee Scientific), 0.5 μM of forward and reverse primer, 5–8 μl of distilled water and 1–4 μl of DNA template. Cycles of amplification were programmed accordingly: 95°C for 4 min (initial denaturation), 10 cycles of three steps, 50°C or 55°C for 30 sec (annealing, temperature decreased by 1°C after each cycle), 72°C for 2 min (extension) and 95°C for 1 min (denaturation); 30 cycles of three steps, 95°C for 1 min, 40°C or 44°C for 30 sec, and 72°C for 2 min; final extension step at 72°C for 10 min. Amplification of rag1 followed the protocol ofSullivan et al., (2006)Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
https://doi.org/10.1016/j.ympev.2006.05....
: 4 min at 95°C (initial denaturation), 35 cycles of three steps, 30 sec at either 50°C, 55°C or 59°C, 2 min at 72°C, and 30 sec at 95°C; final extension step for 4 min at 72°C. Amplifications were sent to Functional Biosciences, Inc. laboratories for purification and sequencing.
Molecular Data: sequence alignment and phylogenetic analyses. Sequences were edited and combined into contigs for each marker (rag1, co1, 16s) in Geneious 11.1.2 (Drummond et al., 2010Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A. Geneious v5.5. Biomatters; 2010. Available from: http://www.geneious.com
http://www.geneious.com...
). Complete gene sequences were aligned in MUSCLE 3.7 (Edgar, 2004Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5):1792–97. https://doi.org/10.1093/nar/gkh340
https://doi.org/10.1093/nar/gkh340...
) using default parameters. Alignments were refined manually, and sequences for the three markers were concatenated in Mesquite 3.40 (Maddison, Maddison, 2011Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Version 2.74 [Internet]. 2011. Available from: http://mesquiteproject.org
http://mesquiteproject.org...
). Translations of new sequences for co1 and rag1 were aligned in COBALT (Papadopoulos, Agarwala, 2007Papadopoulos JS, Agarwala R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007; 23(9):1073–79. https://doi.org/10.1093/bioinformatics/btm076
https://doi.org/10.1093/bioinformatics/b...
) to correct for frameshifts and to trim low-quality ends prior to DNA sequence alignment.
We analyzed combined nuclear and mitochondrial sequences using Maximum Parsimony (MP) and Bayesian Inference (BI), and employed the same parameters as Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
for comparability. Analyses were performed on the combined dataset with terminals restricted to those represented by at least two loci (i.e., 218 specimens; Tab. 1). For MP analysis, the trees were generated using the “new technologies search” implemented in TNT (Goloboff et al., 2008Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008; 24(5):774–86. https://doi.org/10.1111/j.1096-0031.2008.00217.x
https://doi.org/10.1111/j.1096-0031.2008...
) and performed in two steps. The first step used a combination of sectorial searches (RSS and CSS), 100 iterations of ratchet, 100 cycles of tree fusing, and 100 rounds of drift; driven was set to reach the minimum length 50 times. The second step used the trees produced in the first search to perform a traditional TBR search. Gaps were treated as missing data and all characters had equal weights. Godman-Bremer support (Goodman et al., 1982Goodman M, Olson CB, Beeber JE, Czelusniak J. New perspectives in the molecular biological analysis of mammalian phylogeny. Acta Zool Fenn. 1982; 169:19–35.; Bremer, 1988Bremer K. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988; 42(4):795–803. https://doi.org/10.2307/2408870
https://doi.org/10.2307/2408870...
, 1994Bremer K. Branch support and tree stability. Cladistics. 1994; 10(3):295–304. https://doi.org/10.1111/j.1096-0031.1994.tb00179.x
https://doi.org/10.1111/j.1096-0031.1994...
; Grant, Kluge, 2008Grant T, Kluge AG. Credit where credit is due: The Goodman-Bremer support metric. Mol Phylogenet Evol. 2008; 49(1):405–06. https://doi.org/10.1016/j.ympev.2008.04.023
https://doi.org/10.1016/j.ympev.2008.04....
) was calculated for each node and plotted on the consensus tree.
For Bayesian analyses, the concatenated gene matrix was divided into eight partitions: one for 16s, one for each nucleotide position per co1 codon, one for each nucleotide position per rag1 codon, and one for the rag1 intron. Bayesian analyses were conducted in MrBayes 3.1.6 (Huelsenbeck, Ronquist, 2001Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001; 17(8):754–55. https://doi.org/10.1093/bioinformatics/17.8.754
https://doi.org/10.1093/bioinformatics/1...
;Ronquist, Huelsenbeck, 2003Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19(12):1572–74. https://doi.org/10.1093/bioinformatics/btg180
https://doi.org/10.1093/bioinformatics/b...
) using the GTR + GAMMA model. We ran three heated chains and one cold chain for 60 million generations, sampling every 10,000th generation. To ensure sampling of the posterior distribution we discarded 0.25% of the trees.
Morphological Data. Specimens examined for morphological data were designated as alc (alcohol), sk (dry skeleton) or cs (cleared and stained following the methods of Taylor, Van Dyke, 1985Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19. Available from: https://sfi-cybium.fr/en/node/2423
https://sfi-cybium.fr/en/node/2423...
). Data on the caudal skeleton were taken from cleared and stained specimens and dry skeletons while immersed in 90% glycerin and 75% ethanol, respectively, and viewed under a Wild M3C stereomicroscope. Immersion facilitated the removal of residual muscle tissue and assessment of sutures. Midlateral scutes were removed from both sides to facilitate clear observations of the caudal skeleton. Observations were made on adult specimens and juveniles at stages where the caudal skeleton was already mostly ossified. In a few cases, ontogeny was used to hypothesize fusion between elements (e.g., procurrent caudal-fin rays in some astrodoradins). But for the most part, fusions between elements (e.g., hypurals, parhypural) was presumed and not directly observed via ontogenetic series (e.g., Vaz, Hilton, 2020Vaz DFB, Hilton EJ. The caudal skeleton of Batrachoidiformes (Teleostei: Percomorphacea): A study of morphological diversity, intraspecific variation, and phylogenetic inferences. Zool J Linn Soc. 2020; 189(1):228–86. https://doi.org/10.1093/zoolinnean/zlz094
https://doi.org/10.1093/zoolinnean/zlz09...
). Museum codes follow Sabaj, (2020)Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
https://doi.org/10.1643/ASIHCODONS2020...
.
For descriptions of the caudal skeleton, we employed the diural scheme which considers the last vertebra to be a compound caudal centrum formed by the fusion of the posteriormost preural centrum (PU1) plus anteriormost ural centrum (U1) (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
; Grande, Shardo, 2002Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
; de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
; Bird, Mabee, 2003Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn. 2003; 228(3):337–57. https://doi.org/10.1002/dvdy.10387
https://doi.org/10.1002/dvdy.10387...
; Bensimon-Brito et al., 2012Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
). In cases where a second ural centrum (U2) is visible, it is sometimes considered a fusion product of two or three originally distinct centra (Arratia, 2003Arratia G. The siluriform postcranial skeleton–An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.121–57.; de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
; Bensimon-Brito et al., 2010Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. J Appl Ichthyol. 2010; 26(2):235–38. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x
http://dx.doi.org/10.1111/j.1439-0426.20...
, 2012Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
). The compound caudal centrum (PU1+U1) supports the pleurostyle (PL), hypurals (HY) and parhypural (PH). We use the generic term pleurostyle for the elongate process that projects at an angle from the dorsal posterior corner of compound caudal centrum. Previous authors used the term uroneural (i.e., modified ural neural arch) for this process in catfishes (e.g.,Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
; Grande, Shardo, 2002Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
; de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
); however, the homology and evolution of this process remains uncertain among ostariophysans (Cumplido et al., 2020Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
https://doi.org/10.1186/s12983-020-00364...
). Hypurals are ventral bony elements separated into lower hypurals (HY1,2) and upper hypurals (HY3,4,5,6 in catfishes) by a diastema or gap for the passage of paired arterial and venous branches leading to and from the caudal fin (Desvignes et al., 2018Desvignes T, Carey A, Postlethwait JH. Evolution of caudal fin ray development and caudal fin hypural diastema complex in spotted gar, teleosts, and other neopterygian fishes. Dev Dyn. 2018; 247(6):832–53. https://doi.org/10.1002/dvdy.24630
https://doi.org/10.1002/dvdy.24630...
). The parhypural represents the last haemal arch and spine, and the hypurals are considered modified haemal spines of the ural centra (Arratia, Schultze, 1992Arratia G, Schultze H-P. Reevaluation of the caudal skeleton of certain actinopterygian fishes: III. Salmonidae. Homologization of caudal skeletal structures. J Morphol. 1992; 214(2):187–249. https://doi.org/10.1002/jmor.1052140209
https://doi.org/10.1002/jmor.1052140209...
; Schultze, Arratia, 2013Schultze H-P, Arratia G. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In: Arratia G, Schultze H-P, Wilson MVH, editors. Mesozoic Fishes 5 – Global diversity and evolution. München: Verlag Dr. Friedrich Pfeil; 2013. p.187–246.).
Lundberg, Baskin (1969)Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
introduced a formula for describing various patterns of fusion and/or loss among the elements supported by the compound caudal centrum (PU1+U1). They used a plus sign (+) between adjacent elements that are presumably completely fused (e.g., PH+HY1+2), and a semicolon (;) between adjacent elements that remain separated or at least distinguishable, often by a long and continuous suture (e.g., PH; HY1; 2). Although the parhypural and ventral hypurals may appear separate and scored as such, these three elements are tightly associated or fused (continuous) proximally near their fusion to the compound caudal centrum from early developmental stages to adulthood in catfishes (Grande, Shardo, 2002Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
; Adriaens, Vandewalle, 2003Adriaens D, Vandewalle P. Embryonic and larval development in catfishes. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.639–66.). When the sixth hypural was not distinguishable, it was presumed lost rather than fused, and thereby omitted from the formula.
For scoring individuals, we modified the formula of Lundberg, Baskin, (1969)Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
by using a hyphen (-) between elements that are only partially fused and retain features suggestive of independence such as distal or internal gaps and/or semitransparent windows of thin bone; figures in Grande, Shardo, (2002)Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
similarly employed hyphens. For scoring a taxon as a whole, a hyphen in the formula also might represent polymorphism where two elements may appear completely fused in some specimens, but separate in others. For completeness, we also included the pleurostyle (PL) and epural (EP) in the formula because those elements may fuse with each other or with the upper hypural plate in some taxa. Principal caudal-fin rays are reported as branched (Arabic numeral) or simple (lower case Roman numeral).
Character state mapping. For two characters associated with fusion patterns in the caudal skeleton, states were mapped on the Maximum Parsimony phylogeny generated in the current study for Aspredinidae, Auchenipteridae and Doradidae (i.e., Aspredinoidea). The first character was divided into two states: parahypural separate (1) or fused (2) with hypurals 1+2. The second character involved the upper hypurals (HY) and pleurostyle (PL) and exhibited three states treated as ordered: HY3+4; 5; PL (1), HY3+4+5; PL (2), and HY3+4+5-PL or HY3+4+5+PL (3). Next, each possible state was assigned to the common ancestor of the three families. Then, the number of transformations necessary to achieve the phylogenetic distribution of states in the terminal lineages was assessed by eye. The inferences from this exercise are presented in the Discussion.
RESULTS
Molecular Analyses. In our final analyses, 180 of the 218 specimens were represented by complete molecular data (all genes: rag1, co1, 16s; Tab. 1). Seven specimens were represented only by rag1 and co1 sequences, nine specimens were represented only by rag1 and 16s sequences, and 22 specimens were represented only by co1 and 16s sequences. The Maximum Parsimony (MP) analysis produced 144 most parsimonious trees of 9235 steps each. Under MP, the rag1 dataset consisted of 1861 total and 716 parsimony-informative base pairs for 196 specimens, the 16s dataset consisted of 583 total and 188 parsimony-informative base pairs for 211 specimens, and the co1 dataset consisted of 593 total and 246 parsimony-informative base pairs for 209 specimens. The combined dataset included 3037 total base pairs of which 1150 were parsimony informative for 218 terminals.
Trees produced by the Maximum Parsimony (MP) and Bayesian (BI) analyses were highly resolved and agreed on most intergeneric relationships (Figs. 4, S1, S2, S3) with a few notable exceptions. The largest disagreement between the MP and BI topologies involved the base of Doradidae. In the MP analysis, Acanthodoradinae was the first subfamily to diverge from the rest of Doradidae and Astrodoradinae was the second. BI reversed this topology with Astrodoradinae diverging first, followed by Acanthodoradinae. Relationships within Astrodoradinae also differed between the two analyses. Both identified AnadorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
as the first genus to diverge in Astrodoradinae. MP supported Physopyxis Cope, 1871 sister to Astrodoras + Hypodoras and Amblydoras Bleeker, 1862 sister to ScorpiodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
. BI placed Physopyxis sister to a clade composed of Scorpiodoras and Amblydoras (Astrodoras + Hypodoras).
Phylogenetic relationships among all genera and subfamilies of Doradidae inferred from Maximum Parsimony analysis of rag1, 16s and co1 DNA sequence data (strict consensus of 144 most parsimonious trees, each with 9235 steps).
Within the subfamily Doradinae, MP and BI differed in four major respects. In the parsimony analysis, Doraops + Pterodoras was the first group to diverge within Doradinae, followed by Oxydoras Kner, 1855. BI weakly supported (0.5 posterior probability) the reverse with Oxydoras as the first genus to split from the rest of Doradinae, followed by Doraops + Pterodoras. A second difference between MP and BI was placement of the clade Centrodoras (Lithodoras + Megalodoras). In the parsimony analysis, Centrodoras (Lithodoras + Megalodoras) was sister to the fimbriate-barbel doradids. Alternatively, BI supported a sister group relationship between Centrodoras (Lithodoras + Megalodoras) and Centrochir + Platydoras, and that clade was sister to the fimbriate-barbel doradids. Thirdly, MP supported the monophyly of Doras inclusive of Doras punctatusKner, 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162. a species formerly assigned to Ossancora (Birindelli, Sabaj Pérez, 2011Birindelli JLO, Sabaj Pérez MH. Ossancora, a new genus of thorny catfish (Teleostei: Siluriformes: Doradidae) with description of one new species. Proc Acad Nat Sci Phila. 2011; 161(1):117–52. https://doi.org/10.1635/053.161.0109
https://doi.org/10.1635/053.161.0109...
), and placed Doras sister to all other fimbriate-barbel doradids. In the BI analysis, Doras carinatus (Linnaeus, 1766; type species), D. micropoeus (Eigenmann, 1912), and D. higuchii Sabaj Pérez & Birindelli, 2008 formed a clade sister to all other fimbriate barbel taxa except D. phlyzakion Sabaj Pérez & Birindelli, 2008 and D. punctatus. Those two species, respectively, were successive sister taxa to the remaining fimbriate-barbel taxa. Finally, near the crown of the doradid tree, MP and BI disagreed on relationships within a clade composed of Hassar Eigenmann & Eigenmann, 1888, Nemadoras Eigenmann, 1925, TennellusBirindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
and Hemidoras + Ossancora. MP weakly supported two monophyletic clades, Nemadoras + Tennellus and Hassar (Hemidoras + Ossancora), each with a Godman-Bremer support value of 1 (Fig. S2). In the BI analysis, Nemadoras was the first genus to diverge and Tennellus + Hassar and Hemidoras + Ossancora formed reciprocally monophyletic clades (Fig. S1).
Our revised classification of Doradidae (Tab. 2; Fig. 4) is based on relationships supported by the Maximum Parsimony analysis of the DNA sequence data. The results of the Bayesian analysis are consistent with our classification except for the monophyly of Doras which is supported only by MP. Except for Astrodoradinae and Doradinae, each subfamily is diagnosed by caudal-fin or other characteristics.
Revised classification of Doradidae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41.. Nominal species that remain questionable as valid preceded by “?” and listed under possible senior synonym. Totals exclude species that are questionably valid, and species introduced to or questionably present in a given basin. Asterisk denotes species included in molecular phylogenetic analyses.
Caudal-fin shape. Most doradid caudal fins are separable into two shapes: evenly rounded vs. distinctly forked (Tab. 3; Fig. 4). An evenly rounded caudal fin is restricted to the monotypic Acanthodoradinae (Acanthodoras). Forked caudal fins are found in Anadoras (Astrodoradinae), Wertheimerinae and all members of the sister subfamilies Rhinodoradinae and Doradinae. In Anadoras, the caudal fin is shallowly to moderately forked with upper lobe often longer and lower lobe more broadly rounded. Among other doradids, caudal fins vary from shallowly forked with rounded or bluntly pointed lobes (e.g., Centrochir Agassiz, 1829, Platydoras, Oxydoras, Wertheimeria) to deeply forked with pointed lobes (Centrodoras Eigenmann, 1925, Doraops Schultz, 1944, Hassar, Hemidoras, Leptodoras Boulenger, 1898, Rhinodoras).
Caudal-fin shapes in the remaining doradids occupy a spectrum of conditions between evenly rounded and distinctly forked. The monotypic Agamyxinae (Agamyxis Cope, 1878) generally has a truncate to emarginate caudal fin, the latter with gently rounded lobes. Among astrodoradins apart from Anadoras, the branched rays of the upper lobe are often longer, imparting an unevenly emarginate or obliquely truncate distal margin, especially among species of Hypodoras and Physopyxis. Astrodoras is somewhat unusual in that the upper lobe is relatively narrow and pointed and the lower lobe is broadly rounded, often imparting an uneven S-shape to the distal margin of the caudal fin.
Caudal-fin rays. Like most other catfishes, doradid caudal-fin rays are divided into principal rays (segmented) and procurrent rays (anterior ones unsegmented, posterior ones distally segmented). Principal rays include all distally branched rays and typically two unbranched (simple) rays, one adjacent to the dorsalmost and ventralmost branched ray, respectively. The dorsalmost principal ray is supported by the dorsalmost hypural plate (HY5) and easily distinguished even in small juvenile specimens (15–20 mm SL). It is typically about twice as long as the adjacent procurrent ray, which is supported by the pleurostyle. The ventralmost principal ray typically articulates near the distal junction of the parhypural on the compound caudal centrum (PU1+U1) and the haemal spine on preural centrum two (PU2); it may be supported by either or both processes.
Precise determination of the ventralmost principal rays can be problematic in small juveniles of some doradids (especially Astrodoradinae) because those rays are the last ones to branch and the transition between principal and procurrent rays is more gradual than in the dorsal caudal-fin lobe. For example, based on the criterion of simple vs. distally branched, the fused ventral plate (PH+HY1+2) in small Amblydoras (Astrodoradinae) may support only four branched rays (vs. seven in adults) and up to three simple rays (vs. zero, simple principal ray usually supported by haemal spine on PU2 in adults). Although lower counts of branched ventral principal rays are restricted to juveniles in Amblydoras, this condition persists in adults of Physopyxis, another astrodoradin (Tab. 3). Adult Physopyxis have only four or five ventral branched principal rays that are typically supported by the fused ventral plate (PH+HY1+2) and sometimes the haemal spine on PU2. The ventralmost principal ray is usually supported by the haemal spine on PU2, and the posteriormost ventral procurrent ray is supported by the haemal spine on either PU2 or PU3. Physopyxis also mature at the smallest size among all doradids with adults not exceeding 35 mm SL. Therefore, the reduced count of principal caudal-fin rays in Physopyxis appears to be a paedomorphic condition.
The total number of principal caudal-fin rays in doradids varies from 13–17 (Tab. 3). Seventeen (i,7/8,i) is the most common count and synapomorphic for Rhinodoradinae + Doradinae, which includes 71 of the 96 valid extant species. The subfamily Wertheimerinae and four of the six genera of Astrodoradinae (Anadoras, Amblydoras, Astrodoras and Scorpiodoras) typically have 16 primary caudal-fin rays (i,7/7,i). A typical of count of 15 (i,6/7,i) is diagnostic of the monotypic subfamily Agamyxinae. Acanthodoradinae has 15 or 16 primary caudal-fin rays, eight in the dorsal half and seven or eight in the ventral half (i,7/6-7,i). The astrodoradin genera Hypodoras Eigenmann, 1925 and Physopyxis are respectively diagnosed by the lowest counts, 14 (i,6/6,i) and 13–14 (i,7/4–5,i) primary caudal-fin rays.
The number of procurrent caudal-fin rays varies considerably from 6–19 dorsally and 7–19 ventally among the doradids examined here. The monotypic Agamyxinae routinely exhibits the lowest number of procurrent rays, with 6–10 dorsally and 7–10 ventrally. Other doradids with relatively few procurrent rays include FranciscodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
(Wertheimerinae) with 8–11 dorsally and 9–11 ventrally, and a few members of the subfamilies Rhinodoradinae (Orinocodoras eigenmanni Myers, 1927) and Doradinae (Hemidoras stuebelii (Steindachner, 1882), Ossancora spp.) with 9–12 dorsally and 9–11 ventrally. The highest count of procurrent caudal-fin rays occurs in Rhynchodoras (Rhinodoradinae) with 19 dorsally and ventrally (this study) and up to 20 dorsally and 21 ventrally in R. castilloi Birindelli, Sabaj Pérez & Taphorn, 2007 according to Birindelli et al., (2007)Birindelli JLO, Sabaj MH, Taphorn DC. New species of Rhynchodoras from the Río Orinoco, Venezuela, with comments on the genus (Siluriformes: Doradidae). Copeia. 2007; 2007(3):672–84. https://doi.org/10.1643/0045-8511(2007)2007[672:NSORFT]2.0.CO;2
https://doi.org/10.1643/0045-8511(2007)2...
.
The total number of caudal-fin rays (principal + procurrent) varies from 26 to 55 among the doradids examined here. Agamyxinae usually has the fewest caudal-fin rays with a range of 28–34 (n = 19) and the modal count (31) is diagnostic of this monotypic subfamily. Only two genera exhibited ranges overlapping with that of Agamyxinae, the monotypic Franciscodoras (32–38) and Ossancora (34–40), members of Wertheimerinae and Doradinae, respectively. The highest counts (≥50) were recorded for individuals of Rhynchodoras (Rhinodoradinae) and the doradin genera Anduzedoras Fernández-Yépez, 1968, Leptodoras and Oxydoras.
Meristic data aside, doradids also varied in the morphology of the procurrent caudal-fin rays. In most doradids, the transverse width of the procurrent rays remains more or less consistent and the anteriormost ray originates well posterior to the base of adipose and anal fins, respectively (e.g., Figs. 1A,B, 5E). In several unrelated taxa, however, anterior procurrent rays become gradually wider, forming procumbent plates with the anteriormost one originating at or near the base of adipose and anal fins, respectively (Fig. 6). Plate-like procurrent rays are found in all doradid subfamilies except the monotypic Acanthodoradinae, specifically: Astrodoradinae (Anadoras, Hypodoras, some Astrodoras), Wertheimerinae (Franciscodoras), Rhinodoradinae (all members), Doradinae (Platydoras, some Ossancora) and the monotypic Agamyxinae (Agamyxis). In Agamyxis, Hypodoras and some species of Platydoras, the plate-like procurrent rays are especially robust and contact the dorsal and ventral wings of the midlateral scutes, thereby encasing the caudal peduncle in bony armor. In Anadoras, Franciscodoras, Rhinodoradinae and some Platydoras, the plate-like procurrent rays are similarly robust, but do not contact the midlateral scutes and thereby frame the caudal peduncle dorsally and ventrally. In Ossancora, the anteriormost procurrent rays may become procumbent and plate-like (i.e., elongate but relatively narrow) in larger adults.
Caudal-fin skeleton. The skeletal elements supporting the caudal fin are somewhat variable among doradids. Adults show little to no trace of ural centra beyond the terminal compound centrum (i.e., Type 1 of de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
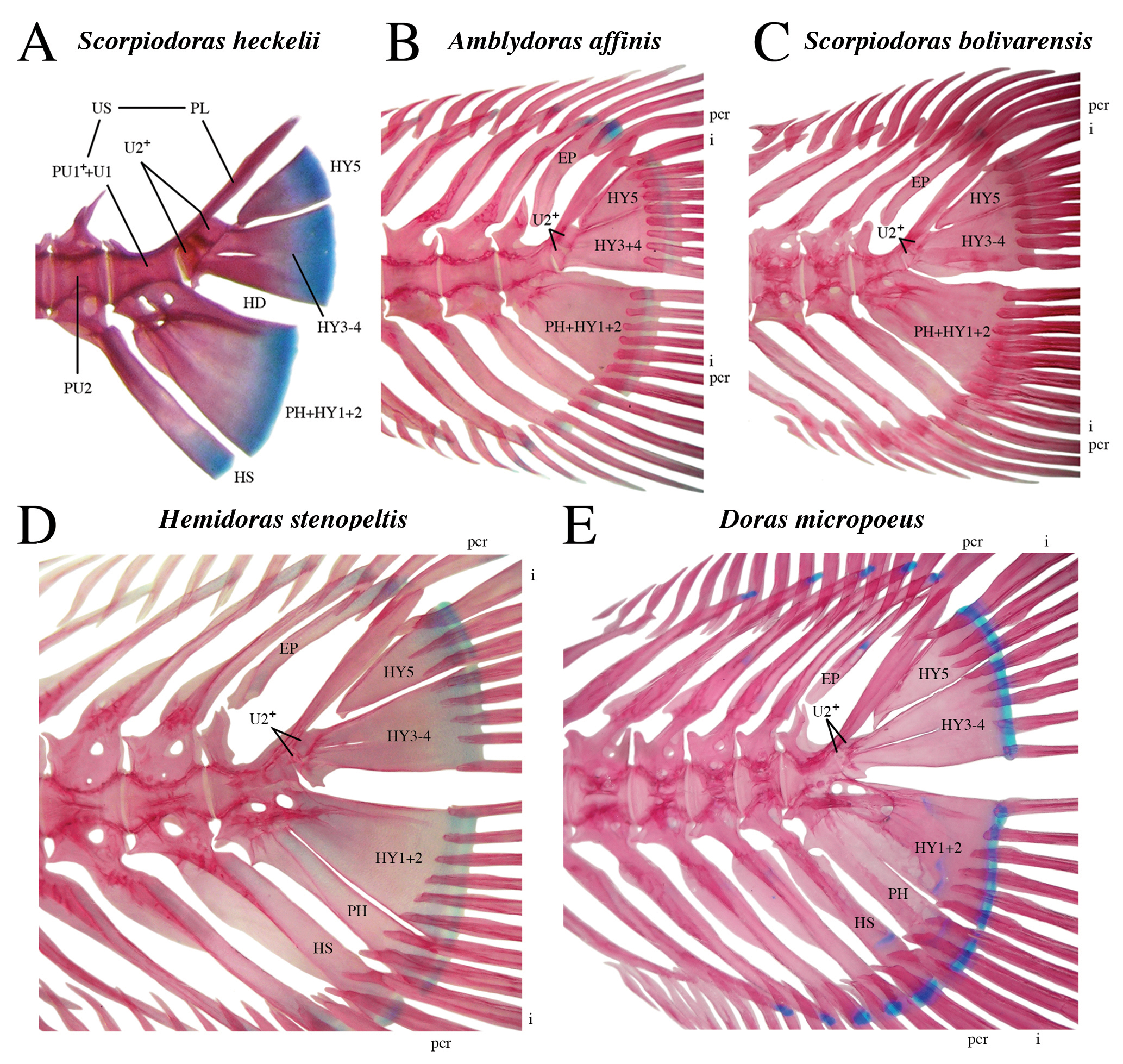
). However, an additional ural centrum (U2+) was observed in the smallest juveniles available for clearing and staining (Fig. 5), specifically Scorpiodoras bolivarensis (Fernández-Yépez, 1968) (21.3 and 24 mm SL), S. heckelii (Kner, 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162.) (14.9 mm SL), Amblydoras affinis (Kner, 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162.) (15.2–18.6 mm SL) and Hemidoras stenopeltis (Kner, 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162.) (22.7 mm SL) (see Discussion). There is only one epural and it remains detached from the compound caudal centrum.
The most common caudal-skeleton formula involves the least fusion among elements: PH; HY1+2; 3+4; 5; PL; EP (e.g., Fig. 9D). In this condition, the parhypural and hypural 1+2 are continuous proximally near their fusion to the compound caudal centrum, but distinguishable distally by a long continuous plane suture (i.e., butt joint). Hypural 3+4, hypural 5 and the pleurostyle are tightly associated, but distinguishable by complete plane sutures, and only the pleurostyle eventually fuses to the compound caudal centrum (see Discussion). This pattern is typical of Wertheimerinae and all members of Doradinae except Platydoras and Centrochir birindellii (Sousa, Chaves, Akama, Zuanon & Sabaj, 2018).
The second pattern is typical of Astrodoradinae, Agamyxinae, and doradins Platydoras and Centrochir birindellii: PH+HY1+2; 3+4; 5; PL; EP (e.g., Figs. 5A–C). In general, the parhypural appears completely fused to hypural 3+4. Alternatively, the fusion is partial, in that a suture is generally not visible between the parhypural and hypural 3+4, but the intervening bone has small gaps and/or is thin to the point of translucence in juveniles and sometimes adults (i.e., PH-HY1+2; see Figs. 7B, 8B,D).
The third pattern is unique to Acanthodoradinae and involves the highest degree of fusion: PH+HY1+2; 3+4+5-PL; EP (Figs. 7A, 8A). The parhypural and hypurals 1 and 2 are completely fused into a singular lower plate that typically supports seven principal caudal-fin rays including the ventralmost unbranched ray. Hypurals 3, 4 and 5 are likewise fused into a singular upper plate that supports eight primary caudal-fin rays including the uppermost unbranched ray. The upper hypural plate and pleurostyle may be separated by a suture, completely fused or partially fused with narrow distal gap. This gap marks the transition from the dorsalmost principal caudal-fin ray (supported by hypural 5) to the posteriormost dorsal procurrent ray (supported by the pleurostyle) of the evenly rounded caudal fin that is unique to Acanthodoradinae.
Caudal skeletons in cleared and stained juveniles of Astrodoradinae (A–C) and Doradinae (D–E) showing ontogenetic fate of ural centrum 2 (U2+). A. Scorpiodoras heckelii(ANSP 165801, 14.9 mm SL, PH+HY1+2; 3-4; 5; PL) with intervertebral joint evident between compound caudal centrum and U2+; B. Amblydoras affinis (ANSP 179798, 15.2 mm SL, PH+HY1+2; 3+4; 5; PL) with partial intervertebral joint evident between caudal centrum and U2+; one of three rays supported by HY5 is rudimentary (length half that of adjacent rays); C. Scorpiodoras bolivarensis (ANSP 165806, 21.3 mm SL, PH+HY1+2; 3-4; 5; PL) with narrow intervertebral joint evident between caudal centrum and U2+, and hairline suture evident between bases of hypurals 3 and 4; D. Hemidoras stenopeltis (ANSP 189444, 22.7 mm SL, PH; HY1+2; 3-4; 5; PL) with U2+ showing signs of fusion with base of hypurals 3 and 4; E. Doras micropoeus (ANSP 197119, 27.6 mm SL, PH; HY1+2; 3-4; 5; PL) with U2+ fused to bases of hypurals 3 and 4, and tightly associated with posterior end of compound caudal centrum. Abbreviations: EP = epural, HS = hemal spine, HY = hypurals fused partially (-) or completely (+), HD = hypural diastema, i = outermost primary caudal-fin ray, pcr = procurrent caudal-fin ray, PH = parhypural, PL = pleurostyle, PU = preural centrum, PU1++U1 = compound caudal centrum, U2+ = proximal and distal portions of ural centrum 2+, US = urostyle.
Caudal skeletons in cleared and stained Astrodoradinae having anteriormost procurrent caudal-fin rays flattened into plates. A. Anadoras grypus (INHS 43663, 68.4 mm SL, PH-HY1+2; 3+4; 5; PL) having anteriormost procurrent elements weakly plate-like; B. Anadoras weddellii (MCP 20940, 68.9 mm SL, PH-HY1+2; 3+4; 5; PL) having multiple procurrent elements forming distinct plates with anteriormost dorsal one (pcr10*) possibly formed by fusion of two rays and anteriormost ventral one (pcr10*) evidently formed by fusion of at least two rays (10+11); C. Anadoras sp. (AUM 45441, 71 mm SL, PH+HY1+2; 3+4; 5; PL) having anteriormost dorsal procurrent plate (pcr10*) evidently formed by fusion of at least two rays (10+11) and anteriormost ventral one (pcr9*) formed by fusion of at least three rays (9+10+11); D. Astrodoras asterifrons(ANSP 177996, 67.1 mm SL, PH+HY1+2; 3+4; 5; PL). E. Astrodoras sp. (ANSP 187490, 49.3 mm SL, PH+HY1+2; 3+4-5; PL) having enlarged anteriormost procurrent plate evidently formed by fusion of two rays (12+13 dorsally and ventrally); F. Hypodoras forficulatus (ANSP 179009, 55.5 mm SL, PH+HY1+2; 3+4; 5; PL) having enlarged anteriormost procurrent plate evidently formed by fusion of two rays (11+12 dorsally and 10+11 ventrally). Caudal vertebrae numbered beginning with compound caudal centrum (1); i = outermost primary caudal fin ray, pcr = procurrent caudal-fin ray (* fusion possible or evident).
Caudal skeletons in cleared and stained specimens of monotypic subfamilies Acanthodoradinae (A) and Agamyxinae (B, C). A. Acanthodoras sp. “shallow scute” (ANSP 161507, 57.6 mm SL) showing distal gap between partially fused hypural 5 and pleurostyle; B. Agamyxis albomaculatus (ANSP 134781, 37 mm SL) showing proximal gap in bony window between partially fused parhypural and hypurals 1+2; C. Agamyxis albomaculatus (INHS 30084, 72.4 mm SL) showing parhypural completely fused to hypurals 1+2.
Classification of Doradidae. Based on our phylogenetic analyses of DNA sequence data for three genes (rag1, co1, 16s) we recognize six major lineages of doradids assigned here to three nominal subfamilies (Astrodoradinae, Doradinae and Wertheimerinae) and three new ones (Acanthodoradinae, Agamyxinae and Rhinodoradinae). When possible, the morphology of the caudal fin is used to diagnose each subfamily.
This study also proposes a number of species-level taxonomic changes based on the results of the molecular analyses and/or examination of specimens. We transfer Platydoras birindellii Sousa, Santana, Akama, Zuanon & Sabaj, 2018 to genus Centrochir Agassiz, 1829. Doras punctatus and Oxydoras trimaculatus are removed from Ossancora and Tenellus, respectively. Doras punctatus is tentatively restored to the genus Doras, and Oxydoras trimaculatus is transferred to the genus Nemadoras. Furthermore, Doras helicophilus Günther, 1868 is considered valid in Platydoras, and Doras polygrammaKner, 1853Kner R. Ueber einige sexual-unterschiede bei der gattung Callichthys und die schwimmblase bei Doras C. Val. Sitzungsber Akad Wiss Wien. 1853; 11:138–46. is considered valid in Acanthodoras with A. spinosissimus (Eigenmann & Eigenmann, 1888) treated as a questionable synonym.
Aspredinoidea Adams, 1854
Included taxa: Aspredinidae Adams, 1854; Auchenipteridae Bleeker, 1862; Doradidae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41..
Diagnosis.Calegari et al., (2019)Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
diagnosed the clade Aspredinidae (Auchenipteridae + Doradidae) on the basis of 29 DNA sequence and four morphological synapomorphies: (1) anterior fontanel elliptic, (2) proximal extremity of pleural ribs twisted, (3) hyomandibula articulated to neurocranium via sphenotic and pterotic, and (4) compound centrum including up to fifth vertebra. Those authors noted that synapomorphies (1) and (2) are exclusive for the clade, but reversed in some members of Auchenipteridae + Doradidae; synapomorphy (3) is convergent in some Pimelodus (Pimelodidae); and synapomorphy (4) is highly homoplastic within the group. The clade Aspredinidae (Auchenipteridae + Doradidae) also is supported by other phylogenetic analyses of molecular sequence data (Sullivan et al., 2006Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
https://doi.org/10.1016/j.ympev.2006.05....
, 2008Sullivan JP, Peng Z, Lundberg JG, Peng J, He S. Molecular evidence for diphyly of the Asian catfish family Amblycipitidae (Teleostei: Siluriformes) and exclusion of the South American Aspredinidae from Sisoroidea. Proc Acad Nat Sci Phila. 2008; 157(1):51–65. https://doi.org/10.1635/0097-3157(2008)157[51:MEFDOT]2.0.CO;2
https://doi.org/10.1635/0097-3157(2008)1...
; Lundberg et al., 2007Lundberg JG, Sullivan JP, Rodiles-Hernández R, Hendrickson DA. Discovery of African roots for the Mesoamerican Chiapas catfish, Lacantunia enigmatica, requires an ancient intercontinental passage. Proc Acad Nat Sci Phila. 2007; 156(1):39–53. https://www.jstor.org/stable/27667759
https://www.jstor.org/stable/27667759...
; Arcila et al., 2017Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
https://doi.org/10.1038/s41559-016-0020...
; Betancur-R. et al., 2017Betancur-R. R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(1):162. https://doi.org/10.1186/s12862-017-0958-3
https://doi.org/10.1186/s12862-017-0958-...
).
Remarks. Auchenipteridae and Doradidae historically composed the superfamily Doradoidea (e.g., de Pinna, 1998de Pinna MCC. Phylogenetic relationships of neotropical Siluriformes (Teleostei: Ostariophysi): Historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279–330.; Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
; Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
) or informal “doradioids [sic]” (Mo, 1991Mo T. Anatomy and systematics of Bagridae (Teleostei), and Siluroid phylogeny. Theses Zoologicae, vol. 17. Koeltz Scientific Books; 1991.). In a broader sense, auchenipterids, doradids and the African family Mochokidae have been grouped together in the suborder Doradoidei (de Pinna, 1998de Pinna MCC. Phylogenetic relationships of neotropical Siluriformes (Teleostei: Ostariophysi): Historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279–330.), superfamily Doradoidea (Chardon, 1968Chardon M. Anatomie comparée de l’appareil de Weber et des structures connexes chez lez Siluriformes. Annalen. Reeks in 8 - Koninklijk Museum voor Midden-Afrika. Zoologische wetenschappen . 169. Tervuren: Musée Royal de l’Afrique Centrale; 1968.; Diogo, 2003Diogo R. Higher-level phylogeny of Siluriformes–An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.353–84.), or informal “doradoids” (Lundberg, 1993Lundberg JG. African-South American freshwater fish clades and continental drift: Problems with a paradigm. In: Goldblatt P, editor. Biological relationships between Africa and South America. Yale University Press; 1993. p.156–99.; Friel, 1994Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994.). Friel, (1994)Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994. also proposed a sister group relationship between his “doradoids” and Aspredinidae based on his phylogenetic analysis of morphological data. Calegari et al., (2019)Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
removed Mochokidae from the clade first proposed by Friel, (1994)Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994. and employed the subordinal name Doradoidei for Aspredinidae (Auchenipteridae + Doradidae).
We use the name Aspredinoidea at the superfamilial level for the clade Aspredinidae (Auchenipteridae + Doradidae) for four reasons: (1) it is premature to subdivide Siluriformes into more than the three commonly recognized suborders, Loricarioidei, Diplomystoidei and Siluroidei, (2) the composition of Doradoidea varies among recent authors (e.g., Diogo, 2003Diogo R. Higher-level phylogeny of Siluriformes–An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.353–84.vs.Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
and Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
), (3) a group composed exclusively of Aspredinidae, Auchenipteridae and Doradidae has not been proposed at the family-group level (i.e., no history of prevailing use as a superfamily), (4) Aspredinidae Adams, 1854 has priority over Auchenipteridae Bleeker, 1862 and Doradidae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41. in a family-group name exclusive to those taxa.
Doradidae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41.
Included taxa: Acanthodoradinae, new subfamily; Astrodoradinae Higuchi, Birindelli, Sousa & Britski, 2007; Wertheimerinae Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
; Agamyxinae, new subfamily; Rhinodoradinae, new subfamily; Doradinae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41..
Diagnosis.Birindelli (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
identified three synapomorphies unique to the family: midlateral scutes present; ligament present between Müllerian ramus and lateral line; and infranuchal ligament (between posterior nuchal plate and the first long-formed rib) ossified.
Distribution. Doradidae is endemic to South America where it occurs on both sides of the Andes Mountains, but is limited to systems draining into the Atlantic Ocean (Tab. 2). One subfamily, Wertheimerinae, contains three monotypic genera endemic to rivers draining the Atlantic Shield of eastern Brazil. The middle to lower reaches of the largest of those rivers, the São Francisco, may have recently included Oxydoras, a member of the subfamily Doradinae. Doras humboldti Spix, Agassiz 1829, currently a junior synonym of Oxydoras niger (Valenciennes 1821), was based on a specimen about 55.5 cm long reportedly from the rio São Francisco at or near Januária (Koerber, 2021Koerber S. On the type localities of some freshwater fishes collected by Spix and Martius in Brazil (1817–1820). Bull Fish Biol. 2021; 19:79–96. ). Furthermore, the Museu de História Natural Louis Jacques Brunet in Recife has a dry stuffed specimen of Oxydoras (MHN-LJB 0016) that is associated with other 19th Century specimens from the lower São Francisco (Flávio Bockmann, 2021, pers. comm.). Oxydoras, however, does not currently inhabit the São Francisco Basin and the historical records may have been based on specimens transported from the Amazonas Basin (Flávio Lima, 2021, pers. comm.).
Wertheimerinae aside, representatives of the other five subfamilies are preserved in the faunas of the Orinoco and Amazonas basins, although the latter is far more diverse with approximately 69 species (vs. 31 in the Orinoco). The doradid fauna of the Essequibo lacks the monotypic Agamyxinae, but includes an impressive 25 species distributed among four subfamilies. Compared to the other major cis-Andean river basins, the La Plata is relatively depauperate with only eight species representing three subfamilies (Astrodoradinae, Rhinodoradinae, Doradinae).
Three species representing two subfamilies (Doradinae + Rhinodoradinae) inhabit river systems draining into the southwestern Gulf of Mexico west of the Andean divide. Centrochir crocodili (Humboldt, 1821) (Doradinae) occurs in the Magdalena Valley between the Cordillera Central and Cordillera Oriental of the northern Andes. Doraops zuloagai Schultz, 1944 (Doradinae) and Rhinodoras thomersoni Taphorn & Lilyestrom, 1984 (Rhinodoradinae) occur in the smaller Catatumbo basin between the two major branches of the Cordillera Oriental, namely the Cordillera de Perijá to the west and Cordillera de Mérida to the east. These three species are not closely related to each other, but sister to other taxa widely distributed east of the Andes.
Rhinodoras thomersoni is the first species to diverge in its genus and the remaining ones are widely distributed in the Orinoco, Amazonas, Essequibo and Paraná basins. Rhinodoras also includes the only doradid species native to the upper Paraná basin above Iguaçu Falls. The monotypic Doraops zuloagai is sister to the genus Pterodoras which has a distribution pattern similar to cis-Andean species of Rhinodoras. Notable differences include the expansion of Pterodoras to coastal drainages east of the Essequibo (i.e., Corantijn-Nickerie) and its absence from the upper Paraná basin. The two lineages, Rhinodoras and Doraops + Pterodoras, are in separate, but sister subfamilies, Rhinodoradinae and Doradinae, respectively. It is not unreasonable to suspect that the same vicariant event, the uplift of the Mérida Andes beginning as early as Eocene-Early Miocene (Cediel, 2019Cediel F. Phanerozoic orogens of Northwestern South America: Cordilleran-Type orogens. Taphrogenic tectonics. The Maracaibo orogenic float. The Chocó-Panamá indenter. In: Cediel F, Shaw RP, editors. Geology and Tectonics of Northwestern South America: The Pacific-Caribbean-Andean Junction. Frontiers in Earth Sciences. Springer; 2019. p.3–95. https://doi.org/10.1007/978-3-319-76132-9_1
https://doi.org/10.1007/978-3-319-76132-...
), isolated Rhinodoras thomersoni and Doraops zuloagai from their respective, cis-Andean sister clades.
Centrochir was previously considered to be monotypic and sister to Platydoras (Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
), a cis-Andean genus that expands upon the Pterodoras-distribution pattern to include coastal drainages east of the Nickerie (i.e., Suriname, Maroni) and east of the Amazonas Delta (i.e., Mearim, Pindaré, Itapecuru, Parnaíba) (Piorski et al., 2008Piorski NM, Garavello JC, Arce H. M, Sabaj MH. Platydoras brachylecis, a new species of thorny catfish (Siluriformes: Doradidae) from northeastern Brazil. Neotrop Ichthyol. 2008; 6(3):481–94. https://doi.org/10.1590/S1679-62252008000300021
https://doi.org/10.1590/S1679-6225200800...
). Perhaps the biggest surprise of the current study was the discovery of a second, cis-Andean species of Centrochir. Centrochir birindellii was originally described in Platydoras (Sousa et al., 2018Sousa LM, Chaves MS, Akama A, Zuanon J, Sabaj MH. Platydoras birindellii, new species of striped raphael catfish (Siluriformes: Doradidae) from the Xingu Basin, Brazil. Proc Acad Nat Sci Phila. 2018; 166(1):1–13. https://doi.org/10.1635/053.166.0106
https://doi.org/10.1635/053.166.0106...
) and is endemic to the Xingu basin above the river’s departure from the Brazilian Shield where it co-occurs with a species of Platydoras. The occurrence of sister species in a major right bank tributary to the lower Amazonas (cis-Andean) and Magdalena Basin (trans-Andean), respectively, is mysterious.
Acanthodoradinae, new subfamily
urn:lsid:zoobank.org:act:A6A7B0AA-EB1D-412F-9566-2F11025B0C3C
Included taxa:Acanthodoras Bleeker 1862 [type genus] with three nominal valid species, Acanthodoras cataphractus (Linnaeus, 1758Linnaeus C. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis: Tomus I. Editio Decima, Reformata. Laurentius Salvius: Holmiae; 1758. Available from: https://www.biodiversitylibrary.org/page/726886
https://www.biodiversitylibrary.org/page...
), A. depressus (Steindachner, 1881) and A. polygrammus (Kner, 1853Kner R. Ueber einige sexual-unterschiede bei der gattung Callichthys und die schwimmblase bei Doras C. Val. Sitzungsber Akad Wiss Wien. 1853; 11:138–46.). The status of Acanthodoras spinosissimus(Eigenmann & Eigenmann 1888) as a valid species or a synonym of A. polygrammus remains uncertain.
Diagnosis. Acanthodoradinae is morphologically diagnosed by two characteristics that are unique among Doradidae: caudal fin symmetrically rounded (vs. unevenly rounded/emarginate, truncate or forked) and adults typically with a single upper hypural plate that incorporates fused hypurals 3–5 and usually the pleurostyle to some degree (vs. pleurostyle entirely separate from upper hypurals).
Remarks.Acanthodoras is the only doradid and the only member of the clade Doradidae + Auchenipteridae with an evenly rounded caudal fin (Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
; Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
; this study). The most closely related taxa with a similarly rounded caudal fin are found in Aspredinidae, the sister family to Doradidae + Auchenipteridae. Among doradids, Acanthodoras exhibits the highest degree of fusion among elements supporting the caudal fin (Figs. 7A, 8A). The three upper hypurals are always fused into a solid plate (HY3+4+5) that may be partially or completely fused to the pleurostyle or remain separated from the pleurostyle by a complete suture. Other doradids (Agamyxis, Hypodoras, Scorpiodoras) sometimes exhibit complete fusion of hypurals 3–5, but the pleurostyle remains separated by a complete suture. In Acanthodoras, the two lower hypurals and parhypural are always fused into a solid plate (PH+HY1+2). Fusion of hypurals 1+2 with the parhypural is common in Astrodoradinae and occasional in Agamyxinae.
Astrodoradinae Higuchi, Birindelli, Sousa & Britski, 2007
Included taxa:Amblydoras Bleeker, 1862 (synonyms Zathorax Cope, 1871, Merodoras Higuchi, Birindelli, Sousa & Britski, 2007), AnadorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
, Astrodoras Bleeker, 1862, HypodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
, Physopyxis Cope, 1871, and ScorpiodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
(synonym Autanadoras Fernández-Yépez, 1950).
Diagnosis. Astrodoradinae is not currently diagnosable by any morphological characters uniquely derived within Doradidae and unreversed within the subfamily (Higuchi et al., 2007Higuchi H, Birindelli JLO, Sousa LM, Britski HA. Merodoras nheco, new genus and species from Rio Paraguay basin, Brazil (Siluriformes, Doradidae), and nomination of the new subfamily Astrodoradinae. Zootaxa. 2007; 1446(1):31–42. https://doi.org/10.11646/zootaxa.1446.1.3
https://doi.org/10.11646/zootaxa.1446.1....
; Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
; this study). Nevertheless, the monophyly of this clade is strongly supported by phylogenetic analyses of DNA sequence data (Arce H. et al., 2013Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
; this study). Several spermatic characters may be uniquely diagnostic, but require evaluation for all astrodoradins. For example, Anadoras and Amblydoras are the only doradids in which spermatozoa have a bell-shaped nucleus and two flagella (Quagio-Grassiotto et al., 2011Quagio-Grassiotto I, Ortiz RJ, Sabaj Pérez MH, Oliveira C. Sperm of Doradidae (Teleostei: Siluriformes). Tissue Cell. 2011; 43(1):8–23. http://dx.doi.org/10.1016/j.tice.2010.10.006
http://dx.doi.org/10.1016/j.tice.2010.10...
). Both conditions are similarly found in Pseudobunocephalus amazonicus (Mees, 1989) (Aspredinidae) and the latter condition also occurs in the catfishes Nematogenys inermis (Guichenot, 1848) (Nematogenyidae), Malapterurus electricus (Gmelin, 1789) (Malapteruridae) and Cetopsis coecutiens (Lichtenstein, 1819) (Cetopsidae) (Quagio-Grassiotto et al., 2011Quagio-Grassiotto I, Ortiz RJ, Sabaj Pérez MH, Oliveira C. Sperm of Doradidae (Teleostei: Siluriformes). Tissue Cell. 2011; 43(1):8–23. http://dx.doi.org/10.1016/j.tice.2010.10.006
http://dx.doi.org/10.1016/j.tice.2010.10...
).
Remarks. Among doradid subfamilies, Astrodoradinae exhibits by far the most variation in the caudal fin. Its shape varies from forked (Anadoras) to truncate (Hypodoras) or unevenly rounded with upper lobe longer than lower (Physopyxis). Counts of principal rays vary from 13 or 14 (Physopyixs) to 14 (Hypodoras) or 16 (Amblydoras, Anadoras, Astrodoras and Scorpiodoras). Hypural 5 may be completely fused with hypural 3+4 (Hypodoras, sometimes Scorpiodoras) or partially so (sometimes Physopyxis). The parhypural is typically fused to hypurals 1 and 2 (PH+HY1+2), but separated by a lenticular window of thin bone in Anadoras (PH-HY1+2).
The procurrent caudal-fin rays grade anteriorly into procumbent plates in Anadoras, Astrodoras and Hypodoras (Figs. 6, 8B,C). In some species, the anteriormost procurrent elements become fused into an enlarged plate that looks singular from an external viewpoint. This fusion is inferred from ontogenetic changes and comparisons among congeners. For instance, the development of procurrent plates varies among species of Anadoras. Anadoras grypus (Cope, 1872) (Fig. 6A) exhibits a condition that is similar to most other doradids. The anteriormost procurrent element is only weakly plate-like and slightly larger than its neighbor. The anteriormost dorsal procurrent plate is supported by the neural spine on the 7th preural centrum (PU7) and has laterally paired ventral processes that occupy the gap between neural spines on PU6 and PU7. The anteriormost ventral procurrent plate lies below and just posterior to the distal end of the haemal spine on PU8 and has laterally paired dorsal processes that occupy the gap between haemal spines on PU7 and PU8. In Anadoras weddellii (Castelnau, 1855) (Fig. 6B), the procurrent caudal-fin elements form multiple distinct plates. The anteriormost dorsal plate is enlarged, supported by two neural spines on PU7 and PU8, respectively, and has a large ventral keel that occupies the gap between those two neural spines. Although displaced anteriorly by one centrum, the enlarged dorsal procurrent plate in A. weddellii resembles the overall shape and position of the two anteriormost procurrent elements in A. grypus. Also, procurrent elements typically articulate with only one neural spine. Since the enlarged dorsal procurrent plate in A. weddellii articulates with two neural spines, it likely represents a fusion of the two anteriormost plates.
The anteriormost ventral procurrent plate in A. weddellii also is enlarged, contacts the haemal spines on PU8 and PU9, respectively, and has a large middorsal crest that occupies two successive gaps between the haemal spines on PU7, PU8 and PU9, respectively. In this case, the shape and articulation of the enlarged plate resembles those of the 2–3 anteriormost plates in the unmodified condition of A. grypus. The fusion of 2–3 procurrent elements ventrally (vs. two fused dorsally) is corroborated by meristics. In A. grypus, there is typically one more procurrent element in the ventral series. In A. weddellii, the counts of dorsal and ventral procurrent elements are equal (or sometimes one greater in the dorsal series). Counts of procurrent caudal-fin elements are higher in A. grypus (12–14 dorsally and 13–15 ventrally) vs. A. weddellii (10–11 dorsally and 10 ventrally), again suggestive of plate fusion in A. weddellii.
Fusion of anteriormost dorsal and ventral procurrent elements also was observed in a cleared and stained adult of an undescribed species of Anadoras from the rio Tocantins Basin (Fig. 6C). The anteriormost dorsal plate is extremely enlarged and contacts three neural spines on PU6, PU7 and PU8, respectively. Its ventral keel appears weakly divided into three sections, one articulating with PU6 neural spine, the second articulating with PU7 neural spine and the third directed towards the gap between PU7 and PU8 neural spines. In this case, the anteriormost dorsal plate is almost certainly formed by the fusion of at least two procurrent elements. The anteriormost ventral plate has a large dorsal crest that articulates with three haemal spines on PU6, PU7 and PU8, respectively. Accordingly, the anteriormost ventral plate appears to be a fusion of at least three procurrent elements.
In some species of Astrodoras and its monotypic sister genus Hypodoras, the anteriormost procurrent plate is more than twice the size of its neighbor, with a noticeable increase in transverse width (vs. width of anteriormost procurrent plates more uniform in Anadoras). The dramatic enlargement of the anteriormost procurrent plate also provides evidence for fusion of procurrent elements. As in Anadoras grypus, the anteriormost procurrent element is only weakly plate-like in some species of Astrodoras (Fig. 6C). The anteriormost dorsal procurrent plate has laterally paired ventral processes that articulate with the distal margin of neural spine on PU6 (vs. PU7 in A. grypus). The anteriormost ventral procurrent plate has laterally paired processes that project dorsally and articulate with the ventroposterior end of the haemal spine on PU6. In other species of Astrodoras (Fig. 6E), the anteriormost dorsal procurrent plate is greatly enlarged and has a long ventral keel supported by two neural spines on PU7 and PU8, respectively. The supporting neural spines are shortened (PU7) or have a downwardly sloped distal margin (PU8) to accommodate the large keel. Likewise, the anteriormost ventral procurent plate is greatly enlarged and has a pronounced crest that articulates with similarly modified ends of haemal spines on PU7 and PU8, respectively. As in Anadoras, species of Astrodoras with enlarged procurrent plates have fewer procurrent elements than those lacking such plates. Therefore, the enlarged anterior plates in Astrodoras are likely due to the fusion of at least two procurrent elements.
Similar fusion seems to hold true for Hypodoras, a monotypic genus in which the anteriormost dorsal and ventral procurrent elements also are dramatically enlarged into a procumbent plate. In a small (26.4 mm SL) cleared and stained juvenile, the two anteriormost dorsal procurrent elements are plate-like and each one bears a shallow, ventral keel that articulates with the neural spine on PU7 and PU8, respectively. The two anteriormost ventral procurrent elements also are plate-like and articulate with the haemal spines on PU7 and PU8, respectively. In larger cleared and stained specimens (36 and 55.5 mm SL; Fig. 6F), a single enlarged plate spans the distal ends of the PU7 and PU8 neural spines, and has a shallow keel that ends opposite the distal margin of the more posterior spine. Likewise, the anteriormost ventral plate articulates with the expanded haemal spines on PU7 and PU8, respectively. Therefore, in adult Hypodoras the enlarged anteriormost procurrent plate appears to be a fusion of two procurrent elements.
Caudal skeletons typical of Acanthodoradinae (A), Astrodoradinae (B, C) and Wertheimerinae (D). A. Acanthodoras polygrammus (ANSP 179421, 121.5 mm SL) with pleurostyle fused to hypural 5; B. Anadoras grypus(ANSP 179170, 84 mm SL) with parhypural partially fused to hypural 1+2 via thin bony window (no suture); C. Hypodoras forficulatus (ANSP 182630, 87 mm SL) with parhypural fused to hypural 1+2 and hypural 3+4 fused to 5; D. Wertheimeria maculata (ANSP 189489, 123.5 mm SL) with hypurals 1 and 2 partially fused via thin bony window (no suture). Black dots indicate outermost primary caudal-fin rays. Scale bars = 1 cm.
Wertheimerinae
Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
Included taxa:FranciscodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
, Kalyptodoras Higuchi, Britski & Garavello, 1990, and Wertheimeria Steindachner, 1877 [type genus].
Diagnosis. Wertheimerinae can be diagnosed from all other doradid genera by the following unique combination: parhypural typically sutured to hypural 1+2 (vs. completely or partially fused with hypural 1+2) and caudal fin typically with i,7/7,i principal rays.
Remarks.Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
established the subfamily Wertheimerinae for two genera, Kalyptodoras and Wertheimeria, that shared a uniquely derived synapomorphy: hyomandibular crest well-developed for insertion of the m. levator arcus palatini (vs. absent, rudimentary or weakly developed in all other doradids). In Franciscodoras, Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
treated the hyomandibular crest as absent to rudimentary. Franciscodoras also differs by having procurrent rays grading anteriorly into procumbent plates (vs. all procurrent rays rod-like in Kalyptodoras and Wertheimeria). Nevertheless, molecular phylogenetic analyses support not only the inclusion of Franciscodoras in Wertheimerinae, but nests it in a sister group relationship with Kalyptodoras. Furthermore, cytogenetic data show a high similarity between Franciscodoras, Kalyptodoras and Wertheimeria (Takagui et al., 2019Takagui FH, Baumgärtner L, Baldissera JN, Lui RL, Margarido VP, Fonteles SBA, Garcia C, Birindelli JO, Moreira-Filho O, Almeida FS, Giuliano-Caetano L. Chromosomal diversity of thorny catfishes (Siluriformes-Doradidae): A case of allopatric speciation among Wertheimerinae species of São Francisco and Brazilian eastern coastal drainages. Zebrafish. 2019; 16(5):477–85. https://doi.org/10.1089/zeb.2019.1769
https://doi.org/10.1089/zeb.2019.1769...
). In Franciscodoras, Birindelli (2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
:533) noted that the parhypural and hypural elements may be distinct in small juveniles (e.g., 29.4 mm SL): PH; HY1; 2; 3; 4; 5 (vs. PH; HY1+2; 3+4; 5 in adults).
Agamyxinae, new subfamily
urn:lsid:zoobank.org:act:B6EAAB63-8F43-4763-9F26-D94861FACBD6
Included taxa:Agamyxis Cope, 1878 [type genus] with two nominal valid species, Agamyxis albomaculatus (Peters, 1877) and A. pectinifrons (Cope, 1870).
Diagnosis. Agamyxinae is diagnosed by two characters that are both unique within Doradidae: total number of caudal-fin rays extremely low, modally 31, range 28–34 (vs. 32–38 in Franciscodoras and 34–55 in all other doradids), and external bony surfaces and fin rays extremely spiny, ornamented with numerous small to large accessory spines (vs. accessory spines absent or present with limited distribution and entirely absent from anal and pelvic fins).
Remarks.Agamyxis is the thorniest of thorny catfishes. Conspicuous accessory spines occur on the posterior cleithral process, midlateral plates and the plate-like procurrent rays of the caudal fin. Much smaller spines occur along the free margins of bones of the nuchal shield and cranium including the infraorbitals. Accessory spines are also conspicuous along the lateral surfaces of the dorsal-fin spine and along the dorsal and ventral surfaces of the pectoral spine. Smaller spines occur along the rays of the anal and caudal fins, and minute spines are visible on the dorsal- and pelvic-fin rays (spines lacking from pectoral-fin rays). Accessory spines are present in other doradids, especially Acanthodoras and most members of Astrodoradinae, but consistently lacking from the anal and pelvic fins.
The caudal fin formula of Agamyxis is summarized as PH-HY1+2; 3+4; 5 due to variation in contact between parhypural and HY1+2. In most specimens, the distal portion of the parhypural and HY1+2 are fused (suture lacking), but the proximal portion retains evidence of a gap that may be partially open and/or occluded by a window of thin bone (Fig. 7B). Alternatively, parhypural and HY1+2 may be completely fused (PH+HY1+2; Fig. 7C) or separated by a complete suture (PH; HY1+2). The upper hypural plate invariably supports seven principal caudal-fin rays with HY5 supporting the upper unbranched ray as well as one branched ray. The lower hypural plate typically supports eight caudal-fin rays with one specimen having only seven. The lower unbranched principal ray is typically supported by the haemal spine on PU2 (or nearly so), and exceptionally supported by the parhypural in the one specimen with seven lower principal caudal-fin rays.
Rhinodoradinae, new subfamily
urn:lsid:zoobank.org:act:8CBB768E-912E-43F0-B299-B5BA54363E7E
“Rhinodoradini”.—Quagio-Grassiotto et al., 2011Quagio-Grassiotto I, Ortiz RJ, Sabaj Pérez MH, Oliveira C. Sperm of Doradidae (Teleostei: Siluriformes). Tissue Cell. 2011; 43(1):8–23. http://dx.doi.org/10.1016/j.tice.2010.10.006
http://dx.doi.org/10.1016/j.tice.2010.10...
:09 [unavailable; authors used in reference to “informally named tribe”].
Rhinodoradini.—Arce H. 2015Arce H. M. Mandibular, hyoid and pectoral musculature of thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2015; 164(1):229–77. https://doi.org/10.1635/053.164.0116
https://doi.org/10.1635/053.164.0116...
:244 [unavailable; author did not explicity indicate nominal taxon as intentionally new (see Van der Laan et al., 2014Van der Laan R, Eschmeyer WN, Fricke R. Family-group names of recent fishes. Zootaxa. 2014; 3882(1):1–230. https://doi.org/10.11646/zootaxa.3882.1.1
https://doi.org/10.11646/zootaxa.3882.1....
:08, Article 16.1)].
Included taxa.Orinocodoras Myers, 1927, RhinodorasBleeker, 1862 [type genus], and Rhynchodoras Klausewitz & Rössel, 1961.
Diagnosis. With respect to the caudal fin, Rhinodoradinae is diagnosed from all other doradids by the unique combination: principal caudal-fin rays i,7/8,i, procurrent rays grade anteriorly into procumbent plates and parhypural typically separated from hypural 1+2 by complete suture. Rhinodoradinae also is diagnosed by two characters unique within Doradidae as proposed by Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
for his “Rhinodoras clade”: posterior limit of autopalatine approximately at vertical through the middle of orbit (vs. finishing anterior to orbit), and hyomandibular with crest for attachment of portion of the m. adductor mandibulae (vs. crest absent). Unlike Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
, we do not consider the triangular posterior cleithral process to be a third exclusive character as suggested by the author. Arce H., (2015)Arce H. M. Mandibular, hyoid and pectoral musculature of thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2015; 164(1):229–77. https://doi.org/10.1635/053.164.0116
https://doi.org/10.1635/053.164.0116...
identified another character unique to Rhinodoradinae among doradids: portion of the m. extensor tentaculi inserting ventrally on the autopalatine (vs. insertion limited to dorsal surface of autopalatine). This may be associated with the autopalatine character described by Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
.
Remarks. Based on Birindelli’s (2014) phylogenetic analysis of morphology, members of Rhinodoradinae formed a monophyletic clade nested within his Doradinae as the sister group to Oxydoras + fimbriate-barbel doradids. Phylogenetic analyses of molecular data (Arce H. et al., 2013Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
; this study) support the removal of rhinodoradin taxa from Doradinae. In all species of Rhinodoradinae, the procurrent rays of the caudal fin grade anteriorly into 3–10 plates that frame the caudal peduncle dorsally and ventrally. This character, however, is homoplastic as it is also found in the subfamilies Astrodoradinae (some Astrodoras), Wertheimerinae (Franciscodoras), Agamyxinae (Agamyxis), and Doradinae (Platydoras).
Doradinae Bleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41.
Included taxa.AnduzedorasFernández-Yépez, 1968, CentrochirAgassiz, 1829, CentrodorasEigenmann, 1925, DoraopsSchultz, 1944, DorasLacepède, 1803 [type genus] (synonym Mormyrostoma Miranda Ribeiro, 1911), HassarEigenmann, Eigenmann, 1888, HemidorasBleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41. (synonym OpsodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
), LeptodorasBoulenger, 1898, LithodorasBleeker, 1862, MegalodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
(synonyms HoplodorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
, Deltadoras Fernández-Yépez, 1968), NemadorasEigenmann, 1925, OssancoraSabaj Pérez & Birindelli, 2011, OxydorasKner, 1855Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162. (synonyms PseudodorasBleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41., Hildadoras Fernández-Yépez, 1968), PlatydorasBleeker, 1862 (synonym Cataphractus Edwards, 1771 [unavailable]), PterodorasBleeker, 1862 (synonyms Apuredoras Fernández-Yépez, 1950, Parapterodoras Risso & Morra, 1964, Sachsdoras Fernández-Yépez, 1968), TenellusBirindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
, and TrachydorasEigenmann, 1925Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
https://doi.org/10.2307/1005464...
.
Diagnosis. As recognized here, Doradinae is not diagnosable by any unambiguous morphological synapomorphies. Molecular analyses (Arce H. et al., 2013Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
; this study) support the current composition of Doradinae divided into five subclades: Pterodoras + Doraops, Oxydoras, Centrochir + Platydoras, Centrodoras (Lithodoras + Megalodoras) and the fimbriate-barbel taxa (crown group). All doradins have i,7/8,i principal caudal-fin rays, a count shared with its sister subfamily Rhinodoradinae. Doradins typically have a caudal-fin formula of PH; HY1+2; 3+4; 5 (Figs. 5D,E, 9), a condition shared with Rhinodoradinae and Wertheimerinae (Fig. 8D). However, within the clade Centrochir + Platydoras the parhypural and hypural 1+2 are often completely fused in Platydoras (Fig. 9B), partially so in Centrochir birindellii (Fig. 9A) and rarely so in C. crocodili (see Tab. 2). In some large doradins (e.g., Doraops, Megalodoras, Pterodoras), the distal portions of parhypural and hypural 1+2 may appear secondarily fused (Fig. 9C). In nearly all doradins, the procurrent caudal-fin rays are typically rod-like, not flattened into plates as in Rhinodoradinae. Platydoras is the only doradin wherein the anteriormost procurrent caudal-fin rays become flattened into plates that either frame the caudal peduncle or encase it by contacting the midlateral scutes (Fig. 9B).
Caudal skeletons typical of Doradinae. A. Centrochir birindellii (ANSP 197107, 123.5 mm SL) with parhypural partially fused to hypural 1+2 via proximal bony window containing hairline suture; B. Platydoras hancockii (ANSP 180286, 296 mm SL) with parhypural completely fused to hypural 1+2; C. Pterodoras granulosus (ANSP 179244, 297 mm SL) with scarcely evident suture between parhypural and hypural 1+2; D. Lithodoras dorsalis (ANSP 187376, 212 mm SL) with clearly evident suture between parhypural and hypural 1+2. Black dots indicate outermost primary caudal-fin rays. Scale bars = 1 cm.
Remarks. The Doradinae of the current study is composed of the same taxa as in Birindelli, (2014)Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
minus members of Rhinodoradinae.
DISCUSSION
Impact of sampling on molecular topologies. There are two major differences between the molecular datasets analyzed here and previously so by Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
: density of ingroup sampling and age of outgroup. Both studies included representatives of all 31 valid doradid genera, and Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
analyzed 86 species-level taxa representing about 76% of the estimated species-level diversity (i.e., ~96 nominal valid species plus ~17 undescribed species-level taxa). The current study analyzed 100 species-level taxa or about 88% of the estimated total diversity. The current study also included 174 doradid individuals compared to 130 in Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
. With respect to outgroups, Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
included 10 species representing nine genera of Auchenipteridae and three species and genera of Aspredinidae based on other molecular studies that supported Aspredinidae sister to Auchenipteridae + Doradidae within the large catfish suborder Siluroidei. The current study expanded the number of auchenipterids to 25 species in 16 genera (sensuCalegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
) and aspredinids to nine species in eight genera. More importantly, this study included two species of Cetopsidae, the first family to diverge in Siluroidei, and one species of Diplomystidae, the sister group to Siluroidei (Sullivan et al., 2006Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
https://doi.org/10.1016/j.ympev.2006.05....
; Lundberg et al., 2007Lundberg JG, Sullivan JP, Rodiles-Hernández R, Hendrickson DA. Discovery of African roots for the Mesoamerican Chiapas catfish, Lacantunia enigmatica, requires an ancient intercontinental passage. Proc Acad Nat Sci Phila. 2007; 156(1):39–53. https://www.jstor.org/stable/27667759
https://www.jstor.org/stable/27667759...
; Nakatani et al., 2011Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. https://doi.org/10.1186/1471-2148-11-177
https://doi.org/10.1186/1471-2148-11-177...
; Arcila et al., 2017Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
https://doi.org/10.1038/s41559-016-0020...
; Betancur-R. et al., 2017Betancur-R. R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(1):162. https://doi.org/10.1186/s12862-017-0958-3
https://doi.org/10.1186/s12862-017-0958-...
).
To test the effects of the older outgroup, we performed a Maximum Parsimony (MP) analysis on the current (expanded) ingroup using the younger, more limited outgroup of Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
(Fig. S3, Tab. 4). The younger outgroup had the greatest impact on the deeper nodes. For example, Doradidae was non-monophyletic when the younger outgroup was used in Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
and the current study. However, whereas Astrodoradinae was sister to Auchenipteridae + remaining doradids in Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
, Acanthodoradinae + Auchenipteridae was sister to the remaining doradids in the MP analysis of the expanded ingroup with the restricted, younger outgroup. Therefore, increasing the age of outgroup in the current study established a deep and novel node supporting the monophyly of Doradidae (Godman-Bremer support 5). Also, Godman-Bremer support for the node joining subfamilies Wertheimerinae, Agamyxinae, Rhinodoradinae and Doradinae decreased from 13 (Arce H. et al., 2013Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
) to 11 with the younger outgroup (but expanded ingroup), but increased to 16 with the older outgroup. With respect to the monophyly of subfamilial nodes shared with Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
, Godman-Bremer support decreased for Acanthodoradinae, Wertheimerinae and Doradinae and increased for Astrodoradinae, Agamyxinae and Rhinodoradinae in the current study (increases greater for Astrodoradinae and Rhinodoradinae when using the older outgroup). Godman-Bremer support for the monophyly of genera more often increased in the current study and by similar amounts between the older and younger outgroups. In the most dramatic increases, Godman-Bremer support rose by five for both Oxydoras and Platydoras. The latter genus had the highest increase in species and individuals added to the current study, two and 16, respectively.
Comparison of taxon sampling and Godman-Bremer support values (GBS) for monophyly of genera and higher-level relationships based on Maximum Parsimony analyses performed in Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02.... and current study. With 2013 Outgroup: Aspredinidae (3 genera and species) and Auchenipteridae (9 genera, 10 species). With Current Outgroup: Diplomystidae (Diplomystes nahuelbutaensis), Cetopsidae (Cetopsis coecutiens, Helogenes marmoratus), Aspredinidae (8 genera, 9 species), Auchenipteridae (16 genera, 25 species, 32 individuals). Not supported (ns); not tested (nt); no change (–). *Centrochir including C. birindellii. **Doras including Doras punctatus. ***Ossancora minus Doras punctatus.
The addition of one ingroup taxon, Doras phlyzakion, to the current study had a significant effect on the placement of another taxon, Doras punctatus, in the Maximum Parsimony (MP) analysis. Birindelli, Sabaj Pérez, (2011)Birindelli JLO, Sabaj Pérez MH. Ossancora, a new genus of thorny catfish (Teleostei: Siluriformes: Doradidae) with description of one new species. Proc Acad Nat Sci Phila. 2011; 161(1):117–52. https://doi.org/10.1635/053.161.0109
https://doi.org/10.1635/053.161.0109...
included D. punctatus in their new genus Ossancora, its members sharing maxillary barbel long with smooth elongate fimbriae, teeth present on dentary and premaxilla and posterior coracoid and posterior cleithral processes finishing near the same vertical line. In the study by Arce H. et al., (2013)Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
https://doi.org/10.1016/j.ympev.2013.02....
, Bayesian and maximum likelihood analyses placed D. punctatus sister to a clade composed of three species of Doras, but the MP analysis placed D. punctatus sister to a clade of all remaining fimbriate-barbel doradids. The addition of Doras phlyzakion to the current study provided new support for placing D. punctatus in Doras (Godman-Bremer support 2 regardless of outgroup) and for a sister group relationship between the two species (Godman-Bremer support 5 regardless of outgroup). Oddly enough, this addition had the opposite effect on the Bayesian analysis. The new Bayesian topology did not support Doras as monophyletic, but had D. phlyzakion, D. punctatus and a clade of three other Doras species as successive sister groups to a clade composed of all other fimbriate-barbel doradids.
The phylogeny of Doradidae is clearly sensitive to ingroup density and outgroup age when Maximum Parsimony is used to analyze three genes, one nuclear (rag1) and two mitochondrial (co1, 16s). The question remains whether adding more genes and more ingroup species to the analysis will reinforce the nodes established in this study, or result in a new topology.
Caudal-fin shape in doradids. Caudal-fin shapes in Doradidae (evenly rounded, forked, evenly or unevenly truncate/emarginate) are loosely correlated with habitat and behavior. Doradids with caudal fins that are evenly rounded (Acanthodoradinae) or truncate/emarginate (Agamyxinae, Astrodoradinae except Anadoras) are generally benthic and occupy shallow, lentic habitats with ample structure such as woody debris, leaf litter and aquatic vegetation. Such habits are common to lakes, backwaters and river margins as well as floodplain creeks during the low-water season. During the day, Acanthodoras and Agamyxis are commonly found wedged in cavities in submerged logs. Many astrodoradins (e.g., Amblydoras, Hypodoras, Physopyxis) partially bury themselves in sand or mud.
Doradids with forked caudal fins occupy a wide variety of benthic and pelagic habitats in lakes, rivers and creeks. Anadoras is the only astrodoradin with a distinctly forked caudal fin (upper lobe often longer than lower). Like other astrodoradins, Anadoras occupies shallow lentic habitats with woody debris and may bury in loose substrates or wedge themselves in wood; but, species also frequent midwater habitats with aquatic vegetation (e.g., floating meadows). Benthic taxa that hide in cavities in rock or wood during the day (Centrochir birindellii, Platydoras) often have shallowly forked caudal fins. Benthic taxa that frequent open substrates of sand or silt in large rivers (e.g., Anduzedoras, Doras, Hassar, Hemidoras, Leptodoras) typically have deeply forked caudal fins with pointed lobes, as do benthic taxa associated with rocky rapids (Rhinodoras, Rhynchodoras). Benthic taxa that occupy more sluggish waters such as floodplain lakes (e.g., Megalodoras, Oxydoras) generally have moderately forked caudal fins with more rounded lobes. Midwater taxa that occupy large rivers and lakes (e.g., active swimmers Doraops zuloagai and Pterodoras) also have deeply forked caudal fins with pointed lobes.
Caudal-fin skeleton developmental morphology. Our interpretations for doradids stem from the developmental morphology of the caudal skeleton in the ictalurid catfish Ictalurus punctatus (Rafinesque, 1818) as described by Grande, Shardo, (2002)Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
and in the zebrafish as described by Bird, Mabee, (2003)Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn. 2003; 228(3):337–57. https://doi.org/10.1002/dvdy.10387
https://doi.org/10.1002/dvdy.10387...
, Bensimon-Brito et al., (2010Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. J Appl Ichthyol. 2010; 26(2):235–38. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x
http://dx.doi.org/10.1111/j.1439-0426.20...
, 2012Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
) and Desvignes et al., (2018)Desvignes T, Carey A, Postlethwait JH. Evolution of caudal fin ray development and caudal fin hypural diastema complex in spotted gar, teleosts, and other neopterygian fishes. Dev Dyn. 2018; 247(6):832–53. https://doi.org/10.1002/dvdy.24630
https://doi.org/10.1002/dvdy.24630...
. Those studies are summarized as follows with notes on contrasting views taken from Cumplido et al., (2020)Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
https://doi.org/10.1186/s12983-020-00364...
.
The compound caudal centrum is initally composed by the first preural centrum (PU1+) anteriorly and the first ural centrum (U1) posteriorly. PU1+ is thought to be formed by PU1 and an extra centrum (+) despite the absence of a physical separation between these centra (Bensimon-Brito et al., 2010Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. J Appl Ichthyol. 2010; 26(2):235–38. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x
http://dx.doi.org/10.1111/j.1439-0426.20...
). Both PU1+ and U1 begin as chordacentra, the products of direct mineralization from within the notochord sheath, generally in a ventral to dorsal direction. U1 appears first and PU1+ usually follows as a anteroventral extension of U1 due to a continuous and uniform mineralization process (i.e., no internal boundaries visible between PU1+ and U1). On rare occasions, PU1+ and U1 develop as two separate elements with subsequent fusion in a dorsal to ventral direction. As the fused chordacentra (PU1++U1) become fully formed, a perichordal layer of bone is deposited outside of the mineralized notochord sheath. Bone formation begins as two separate rings at the anterior and posterior edges of the compound centrum and progresses towards the central part of the centrum, thereby forming the autocentrum. In contrast, Cumplido et al., (2020)Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
https://doi.org/10.1186/s12983-020-00364...
considered the compound caudal centrum to be initially composed of ural centra 1 and 2 (U1+U2) because of their association with the proximal ends of hypurals 1 and 2, respectively, and the absence (loss) of the preural centrum 1 (PU1) near the base of the parhypural during early stages of development.
Shortly after the first ural chordacentrum (U1) becomes visible, the more posteriorly placed second ural centrum (U2+) forms separately within the notochord sheath. Similar to the first preural centrum (PU1+), U2+ is considered to be a compound structure formed by the fusion of multiple urals during the chordacentrum stage. Bensimon-Brito et al., (2012)Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
reported an inner dark line running perpedicular to the notochord and separating U2+ into anterior and posterior halves. The dividing line was clearly associated with mineralized notochord tissue (vs. notochord sheath). This internal zone of mineralization expands as U2+ eventually fuses with the compound caudal centrum (PU1++U1). Prior to that fusion, the narrow space between PU1++U1 and U2+ may appear as a partial intervertebral joint interrupted dorsally by bone. After fusion, PU1++U1+U2+ is collectively termed the urostyle (sensuBird, Mabee, 2003Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn. 2003; 228(3):337–57. https://doi.org/10.1002/dvdy.10387
https://doi.org/10.1002/dvdy.10387...
), a structure that also eventually incorporates the first pair of uroneurals (i.e., pleurostyle). In contrast, Cumplido et al., (2020)Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
https://doi.org/10.1186/s12983-020-00364...
treated the U2+ of Bensimon-Brito et al., (2012)Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
as the third ural (U3) which remains as a chordacentrum unfused to the compound caudal centrum.
The sequence of development of the vertebral centra is preceded by that of the modified neural and haemal arches and spines. The appearance of those epaxial (neural) and hypaxial (haemal) structures help to infer homologous landmarks along the notochord and incipient vertebral column. The haemal elements (parhypural and hypurals) appear first. The parhypural (PH) and hypural 1 (HY1) are closely associated with the ventral face of the compound caudal centrum (PU1++U1) in the region of the first preural centrum (PU1+). This association helps define PU1+ as a compound element formed by PU1 and an extra centrum (sensuBensimon-Brito et al., 2010Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. J Appl Ichthyol. 2010; 26(2):235–38. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x
http://dx.doi.org/10.1111/j.1439-0426.20...
, 2012Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
). Hypural 2 (HY2) is associated with the posterior portion of the compound caudal centrum in the region of the first ural centrum (U1). Subsequent hypurals (HY3–5 or 6) are associated with more distal urals (if present), presumably in a one-to-one ratio. A physical gap, or hypural diastema, occurs between HY2 and HY3.
The dorsal-lateral surface of the urostyle (PU1++U1+U2+ in zebrafish) is closely associated with uroneurals and epurals (epaxial elements). A uroneural is a modified neural arch of an ural vertebra (Schultze, Arratia, 2013Schultze H-P, Arratia G. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In: Arratia G, Schultze H-P, Wilson MVH, editors. Mesozoic Fishes 5 – Global diversity and evolution. München: Verlag Dr. Friedrich Pfeil; 2013. p.187–246.). A single uroneural may arise as a bilateral pair of bone slivers along the dorsal-lateral surface of the urostyle and extend from PU1+ to the tip of the notochord. This same uroneural eventually fuses to the urostyle to form the pleurostyle according to some authors (e.g., Grünbaum et al., 2003Grünbaum T, Cloutier R, Dumont P. Congruence between chondrification and ossification sequences during caudal skeleton development: A Moxostomatini case study. In: Browman HI, Skiftesvik AB, editors. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Bergen, Norway: Institute of Marine Research; 2003. p.161–76). Epurals develop slightly above the urostyle and are interpreted as neural spines detached from their neural arches. The anteriormost epural is sometimes interpreted as a neural spine detached from the neural arch vestige projecting dorsally from the PU1+ region of the urostyle.
In Doradidae, the compound caudal centrum appears to be composed of the first preural centrum plus an extra centrum (PU1+) and the first ural centrum (U1), as in zebrafish (setting aside the contrasting view by Cumplido et al., 2020Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
https://doi.org/10.1186/s12983-020-00364...
). Posterior to the compound caudal centrum, we observed an additional element interpreted as the second ural centrum (Fig. 5). This element seems to eventually fuse to base of hypurals 3 and 4 as similarly described by Lundberg, Baskin, (1969)Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
and supported (at least in part) by Grande, Shardo, (2002)Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
. Based on their developmental study of the ictalurid catfish Ictalurus punctatus, Grande, Shardo, (2002)Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
https://doi.org/10.5962/bhl.title.3555...
agreed that a structural unit does form between ural centrum 2 and hypurals 3 and 4, but noted that in rare cases U2 fuses with the compound caudal centrum as in zebrafish (sensuBird, Mabee, 2003Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn. 2003; 228(3):337–57. https://doi.org/10.1002/dvdy.10387
https://doi.org/10.1002/dvdy.10387...
; Bensimon-Brito et al., 2012Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
https://doi.org/10.1111/j.1525-142x.2011...
).
Our interpretations are based on observations of cleared and stained juveniles of doradins (Doras and Hemidoras) and astrodoradins (Amblydoras, Physopyxis, Scorpiodoras) including a growth series of Amblydoras affinis (Fig. 5). In the two smallest juveniles examined (14.9 and 15.2 mm SL for Scorpiodoras and A. affinis, respectively), the posterior end of the compound caudal centrum (PU1++U1) is followed by an elongated element that occupies the space and angle of the dorsally flexed terminus of the notochord. This element lies between and is in tight contact with the proximal end of the pleurostyle and the bases of hypurals 3 and 4, respectively. The elongated element is unevenly mineralized and longitudinally separable into proximal, middle and distal portions. The proximal portion has a conical articular surface (sensude Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
) separated from the posterior end of the compound caudal centrum (PU1++U1) by a distinct gap resembling an intervertebral joint. The distal portion resembles a longer tube. A similar element is clearly visible in the cleared and stained specimen of Physopyxis cristata Sousa & Rapp Py-Daniel, 2005 (11 mm SL) figured by Sousa, Rapp Py-Daniel, (2005Sousa LM, Rapp Py-Daniel LH. Description of two new species of Physopyxis and redescription of P. lyra (Siluriformes: Doradidae). Neotrop Ichthyol. 2005; 3(4):625–36. https://doi.org/10.1590/S1679-62252005000400019
https://doi.org/10.1590/S1679-6225200500...
:634, fig. 8a). We treat this element as ural centra 2+ because of its resemblance to the compound ural centrum (U2+) described by Bensimon-Brito et al., (2012:121, figs. 3D, G, H) in zebrafish.
In Amblydoras affinis, the proximal, conical portion second ural centrum (U2+) becomes smaller and more triangular in juveniles 17.1–17.7 mm SL, is scarcely visible by 18.6 mm SL, and indistinguishable at 23.7 mm SL as it fuses to the ventral base of hypural 3+4. The distal, tube-like portion of U2+ remains distinguishable at 23.7 mm SL. At 24.8 mm SL, the adult condition is nearly achieved wherein the second ural centrum (U2+) appears fully fused to the base of hypural 3+4. In adults, the proximal end of this structural unit (U2++HY3+4) becomes tightly wedged into the concavity formed in part by the posterior facet of the compound caudal centrum and fused pleurostyle (i.e., urostyle).
The juvenile doradids examined here were already at a stage that was too advanced to observe the ontogeny of the epaxial and hypaxial caudal elements. Paired vestiges of a neural arch project from the dorsal surface of the urostyle in the PU1+ region, and remain separate from their corresponding neural spine (epural). The proximal end of the uroneural appears to be fused to the compound caudal centrum (PU1++U1) at an early stage (thus forming the pleurostyle).
Caudal-fin skeleton in adult catfishes. Doradidae is a member of the suborder Siluroidei, one of the three major lineages of catfishes: Loricarioidei and Diplomystoidei + Siluroidei (Arcila et al., 2017Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
https://doi.org/10.1038/s41559-016-0020...
). Within the Loricarioidei, fusion is common among skeletal elements supporting the caudal fin, and at least two hypurals are fused (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
). The highest degree of fusion among loricarioids occurs in the families Loricariidae and Scoloplacidae. In most loricariids, the epural, pleurostyle and upper hypurals are fused into a single plate, the lower hypurals and parhypural are fused into a second plate, and a bony bridge between the two plates reduces the diastema to a shallow distal notch: PH+HY1+2; 3+4+5+PL+EP (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
:44, fig. 9). In the monotypic Scoloplacidae, fusion of the caudal skeleton is taken a step further as there is no demarcation between the upper and lower hypurals: PH+HY1+2+3+4+5+PL+EP (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
[as “Bunocephalus sp.”]; Schaefer, 1990Schaefer SA. Anatomy and relationships of the scoloplacid catfishes. Proc Acad Nat Sci Phila. 1990; 142:167–210. Available from: https://www.jstor.org/stable/4064976
https://www.jstor.org/stable/4064976...
:191, fig. 17). At the opposite extreme within Loricarioidei is the monotypic Nematogenyidae, the first family to diverge within the suborder. In Nematogenys Girard, 1855, only hypurals 1 and 2 are fused, hypurals 3, 4 and 5 remain separate from each other and the pleurostyle, and the parhypural remains separate from the lower hypurals: PH; HY1+2; 3; 4; 5; PL (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
:43, fig. 8). The epural also persists and is fully developed in Nematogenys vs. lost in some Loricarioidei, the family Trichomycteridae in particular (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
).
The caudal skeleton in the monotypic suborder Diplomystoidei represents the most dissociated condition in catfishes. In DiplomystesBleeker, 1858Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41., the hypurals are represented by six separate elements, the upper hypurals are unfused to the compound caudal centrum (PU1++U1) and separated from the lower hypurals by a distinct diastema, the pleurostyle and parhypural remain separated from hypurals, and the epural is fully developed and detached from its neural arch: PH; HY1; 2; 3; 4; 5; 6; PL; EP (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
). A few members of Siluroidei share this extremely dissociated condition, including most species of Cetopsidae (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
; de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
), the oldest extant family in the suborder (Arcila et al., 2017Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
https://doi.org/10.1038/s41559-016-0020...
). A few siluroids also have an extremely consolidated caudal skeleton such as Chacidae and some plotosids wherein the pleurostyle, hypurals and parhypural are completely fused into a singular plate that lacks a diastema: PH+HY1+2+3+4+5+PL (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
).
Within the Siluroidei, the caudal skeleton in Aspredinoidea [Aspredinidae (Auchenipteridae + Doradidae)] exhibits close to the full range of conditions known for catfishes. All members of Aspredinoidea have five hypurals (vs. six in some siluroids). Furthermore, hypurals 1 and 2 and hypurals 3 and 4, respectively, are almost always fused. Within Aspredinoidea, the highest degree of fusion occurs in Aspredinidae, the oldest of the three families. In Aspredinidae, the parhypural is fused to hypurals 1 and 2 (PH+HY1+2) and the three dorsal hypurals are fused into a solid plate (HY3+4+5) (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
; Friel, 1994Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994.; de Pinna, Ng, 2004de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
https://doi.org/10.1206/0003-0082(2004)4...
; Friel, Carvalho, 2016Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay river Basin. Zootaxa. 2016; 4088(4):531–46. https://doi.org/10.11646/zootaxa.4088.4.4
https://doi.org/10.11646/zootaxa.4088.4....
; MHS, pers. obs.). In two genera, Hoplomyzon Myers, 1942 and Pseudobunocephalus Friel, 2008, the pleurostyle and epural are incorporated into the upper plate: PH+HY1+2; 3+4+5+PL+EP (Friel, 1994Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994.; MHS, pers. obs.). Hoplomyzon also has a bony bridge between the upper and lower plates that reduces the diastema to a distal notch, as in some loricariids. In Auchenipteridae, the caudal skeleton is relatively conserved. Nearly all auchenipterids share the pattern PH+HY1+2; 3+4; 5; PL (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
; Birindelli, 2014Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
https://doi.org/10.1590/1982-0224-201200...
; Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
; MHS, pers. obs.). In at least two species, Pseudauchenipterus jequitinhonhae (Steindachner, 1877) and Tatia strigata Soares-Porto, 1995, the three upper hypurals are fused into a singular plate (Calegari et al., 2019Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
https://doi.org/10.1093/zoolinnean/zlz03...
).
In Doradidae, the skeletal elements supporting the caudal fin generally fall into one of three patterns. In the most common one, three hypural plates are distinguishable (HY1+2, 3+4, 5) and the parhypural is separate, though tightly associated with HY1+2. In the second pattern, the fusion between the parhypural is complete (PH+HY1+2) or partial (PH-HY1+2). The third pattern involves the highest degree of fusion and is unique to the monotypic Acanthodoradinae. The parhypural and hypurals 1 and 2 are fused into a solid ventral plate and hypurals 3–5 are fused into a solid dorsal plate that generally incorporates the pleurostyle to some degree (HY3+4+5-PL).
Caudal-fin skeleton evolution in Aspredinoidea. Among catfishes, Lundberg, Baskin (1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
:45) noticed a “trend from the primitive condition of six separate hypurals to the most advanced condition of complete fusion of caudal elements”. This trend is consistent with conditions observed in the first lineages to diverge within each of the two major clades of catfishes, Loricarioidei and Diplomystoidei + Siluroidei. The first lineage to diverge within Loricarioidei, Nematogenys, exhibits the least degree of fusion among hypural elements (HY1+2). The highest degree of fusion among caudal elements in Loricarioidei is found in the crown clade Scoloplacidae (Astroblepidae + Loricariidae). In Astroblepidae, the parhypural is fused to hypurals 1 and 2 and the upper hypurals are fused to the pleurostyle (PH+HY1+2; 3+4+5+PL). In Loricariidae, the upper plate fuses to the epural but remains distinguishable from the lower plate (PH+HY1+2; 3+4+5+PL+EP), and in Scoloplacidae the upper and lower plates are indistinguishable (PH+HY1+2+3+4+5+PL). As mentioned above, diplomystids have the plesiomorphic formula with six separate hypurals. This formula similarly occurs in first family to diverge among siluroids, Cetopsidae, but is restricted therein to the subfamily Cetopsinae. In the other cetopsid subfamily, Helogeninae, fusions occur between hypurals 1 and 2 (HY1+2) and 3 and 4 (HY3+4), respectively, and the sixth hypural (HY6) is lost (Lundberg, Baskin, 1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
http://hdl.handle.net/2246/2608...
:42, fig. 7).
Scenarios for evolution of fusion patterns observed in ventral (A) and dorsal (B) elements of the caudal-fin skeleton in Aspredinoidea mapped onto Maximum Parsimony phylogeny generated in this study. Character states (ordered): 1 = PH; HY1+2 (ventral) or HY3+4; 5; PL (dorsal); 2 = PH+HY1+2 (ventral) or HY3+4+5; PL (dorsal); 3 = HY3+4+5-PL or HY3+4+5+PL (dorsal only). Transformation series distinguished by state assumed for common ancestor: 1 (triangles), 2 (squares), 3 (circles); each symbol represents one transformation (except for bolded symbols representing common ancestor). Illustrations adapted from Lundberg, Baskin (1969:42, fig. 7A).
The evolutionary trend from hypural elements separate (ancestral) to fused (derived) is not observed in the superfamily Aspredinoidea based on the optimization of transformations for two caudal-skeleton characters mapped onto the Maximum Parsimony tree generated in this study (Fig. 10). With respect to the ventral elements, the most parsimonius traversal of the tree begins with a common ancestor having a greater degree of fusion, PH+HY1+2 (vs. PH; HY1+2). From this starting point, it takes only four transformations to account for the phylogenetic distribution of character states among extant Aspredinoidea. If the parhypural is treated as separate in the common ancestor of Aspredinoidea, five steps are needed to achieve the same distribution for extant Aspredinoidea. With respect to the dorsal hypural plate, the condition for the common ancestor of Aspredinoidea is ambiguous. Six transformations are needed to achieve the diversity of fusion patterns among extant Aspredinoidea whether their common ancestor exhibited the least, most or an intermediate degree of fusion among the dorsal hypurals and pleurostyle.
Generally speaking, aspredinoid catfishes with rounded, truncate and emarginate caudal fins tend to exhibit greater degrees of fusion among the caudal supporting elements (parhypural, hypurals, pleurostyle) and occupy relatively shallow, lentic habitats with ample structure (e.g., floodplain creeks and lakes, quiet backwaters and margins of rivers). Skeletal elements supporting the caudal fin are more separated in taxa with moderately to deeply forked tails that occupy lotic habitats in medium to large river channels.
ACKNOWLEDGEMENTS
Thanks to reviewers Bárbara Calegari and Eric Hilton for providing thoughtful comments and suggestions that improved the manuscript. For helpful discussions on doradid osteology, taxonomy and systematics, we thank in particular José L.O. Birindelli, Flávio Bockmann, Kole Kubicek, Flávio C.T. Lima, John Lundberg, Leandro Sousa and Richard van der Laan. For sharing observations on live doradids, thanks to John Crooks, Charles Godfrey, Steven Grant, Allan James Sr., Michael Kirkham, Flávio C.T. Lima, Oliver Lucanus and Alexey Malyshev. Among the countless individuals who have facilitated our fieldwork, museum and molecular studies on doradids over the years, we thank in particular: B. Brown (AMNH), K. Luckenbill, J. Lundberg, W. Saul (ANSP), J. Armbruster, N. Lujan, D. Werneke (AUM), O. Crimmen, A. Gill, J. Maclaine (BMNH), D. Catania, W. Eschmeyer, J. Fong, T. Iwamoto (CAS), Amanda Cramer, Devon Donahue, Matthew Miller, Tyler Narsingh (Drexel University), M.A. Rogers, K. Swagel, P. Willink (FMNH), J.D. Bogotá, C. DoNascimiento (IAvH), C. Gama (IEPA), K.S Cummings, C. Mayer, L.M. Page, C.A. Phillips, C.A. Taylor, J. Tiemann (INHS), C. Cox-Fernandes, M. Ito, R. de Oliveira, R. Ota, L. Rapp Py-Daniel, M. Rocha, I. Soares (INPA), R. Feeney (LACM), C. Oliveira, V. Tagliacollo (LBP), A. Gonçalves, L. Sousa (LIA), M. Araújo, F.A. Bockmann, P. Pereira Rizzato (LIRP), F. Provenzano (MBUCV), O. Castillo, O. León Mata, D. Taphorn (MCNG), T. Carvalho, C. Lucena, M. Lucena, R. Reis (MCP), K. Hartel (MCZ), R. Covain, S. Fisch–Muller (MHNG), A. Akama, I. Maschio, M. Mendonça, A.L. Netto-Ferreira, W. Wosiacki (MPEG), M. Bruna Inverniei, M. Luisa Cattaneo, G. Primo, B. Enrico (MSNG), E.V. Correa, H. Ortega (MUSM), J.L.O. Birindelli, O. Shibatta (MZUEL), O. Oyakawa (MZUSP), E. Holm, H. López–Fernández (ROM), E. Mikschi, H. Wellendorf, A. Palandacic (NMW), S. Kullander, Erik Åhlander (NRM), B. Burr, K. Elkin, J. Stewart (SIUC), D. Nelson (UMMZ), S. Jewett, L. Palmer, S. Raredon, C.D. Santana, R. Vari, J. Williams (USNM), C.J. Ferraris, Jr., and especially M. Hardman. For help in the field we are especially thankful to: J.W. Armbruster, J.L.O. Birindelli, S.A. Bullard, T. Carvalho, E.V. Correa, C. DoNascimiento, A. Gonçalves, M. Hardman, O. León Mata, M. Littmann, A. López, N.R. Lovejoy, N.K. Lujan, A.L. Netto-Ferreira, C.A. Phillips, L. Rapp Py-Daniel, B. Rengifo, N. Salcedo, B. Sidlauskas, L.M. Sousa, J. Tiemann, D. Werneke. Research supported in part by All Catfish Species Inventory (NSF DEB–0315963) and iXingu Project (NSF DEB–1257813, PI MHS).
REFERENCES
- Adriaens D, Vandewalle P. Embryonic and larval development in catfishes. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.639–66.
- Arce H. M. Mandibular, hyoid and pectoral musculature of thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2015; 164(1):229–77. https://doi.org/10.1635/053.164.0116
» https://doi.org/10.1635/053.164.0116 - Arce H. M, Reis RE, Geneva AJ, Sabaj Pérez MH. Molecular phylogeny of thorny catfishes (Siluriformes: Doradidae). Mol Phylogenet Evol. 2013; 67(3):560–77. https://doi.org/10.1016/j.ympev.2013.02.021
» https://doi.org/10.1016/j.ympev.2013.02.021 - Arcila D, Ortí G, Vari R, Armbruster JW, Stiassny MLJ, Ko KD, Sabaj MH, Lundberg J, Revell LJ, Betancur-R. R. Genome-wide interrogation advances resolution of recalcitrant groups in the Tree of Life. Nat Ecol Evol. 2017; 1:0020. https://doi.org/10.1038/s41559-016-0020
» https://doi.org/10.1038/s41559-016-0020 - Arratia G. The siluriform postcranial skeleton–An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.121–57.
- Arratia G, Schultze H-P. Reevaluation of the caudal skeleton of certain actinopterygian fishes: III. Salmonidae. Homologization of caudal skeletal structures. J Morphol. 1992; 214(2):187–249. https://doi.org/10.1002/jmor.1052140209
» https://doi.org/10.1002/jmor.1052140209 - Baumgärtner L, Paiz LM, Takagui FH, Lui RL, Moreira-Filho O, Giuliano-Caetano L, Portela-Castro ALB, Margarido VP. Comparative cytogenetics analysis on five genera of thorny catfish (Siluriformes, Doradidae): Chromosome review in the family and inferences about chromosomal evolution integrated with phylogenetic proposals. Zebrafish. 2018; 15(3):270–78. https://doi.org/10.1089/zeb.2017.1554
» https://doi.org/10.1089/zeb.2017.1554 - Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. The zebrafish (Danio rerio) caudal complex – a model to study vertebral body fusion. J Appl Ichthyol. 2010; 26(2):235–38. http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x
» http://dx.doi.org/10.1111/j.1439-0426.2010.01412.x - Bensimon-Brito A, Cancela ML, Huysseune A, Witten PE. Vestiges, rudiments and fusion events: The zebrafish caudal fin endoskeleton in an evo-devo perspective. Evol Dev. 2012; 14(1):116–27. https://doi.org/10.1111/j.1525-142x.2011.00526.x
» https://doi.org/10.1111/j.1525-142x.2011.00526.x - Betancur-R. R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(1):162. https://doi.org/10.1186/s12862-017-0958-3
» https://doi.org/10.1186/s12862-017-0958-3 - Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn. 2003; 228(3):337–57. https://doi.org/10.1002/dvdy.10387
» https://doi.org/10.1002/dvdy.10387 - Birindelli JLO. Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotrop Ichthyol. 2014; 12(3):451–564. https://doi.org/10.1590/1982-0224-20120027
» https://doi.org/10.1590/1982-0224-20120027 - Birindelli JLO, Sabaj MH, Taphorn DC. New species of Rhynchodoras from the Río Orinoco, Venezuela, with comments on the genus (Siluriformes: Doradidae). Copeia. 2007; 2007(3):672–84. https://doi.org/10.1643/0045-8511(2007)2007[672:NSORFT]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2007)2007[672:NSORFT]2.0.CO;2 - Birindelli JLO, Sabaj Pérez MH Ossancora, a new genus of thorny catfish (Teleostei: Siluriformes: Doradidae) with description of one new species. Proc Acad Nat Sci Phila. 2011; 161(1):117–52. https://doi.org/10.1635/053.161.0109
» https://doi.org/10.1635/053.161.0109 - Birindelli JLO, Sousa LM. Family Doradidae–Thorny Catfishes. In: van der Sleen P, Albert JS, editors. Field guide to the fishes of the Amazon, Orinoco & Guianas. Princeton: Princeton University Press; 2018. p.222–33.
- Birindelli JLO, Sousa LM, Sabaj Pérez MH. Morphology of the gas blader in thorny catfishes (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2009; 158(1):261–96. https://doi.org/10.1635/053.158.0114
» https://doi.org/10.1635/053.158.0114 - Bleeker P. De heer Bleeker brengt nog ter tafel het eerste deel van eene ichthyologiae Archipelagi Indici Prodromus... Natuurk Tijdschr Ned-Indië. 1858; 16:38–41.
- Bremer K. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988; 42(4):795–803. https://doi.org/10.2307/2408870
» https://doi.org/10.2307/2408870 - Bremer K. Branch support and tree stability. Cladistics. 1994; 10(3):295–304. https://doi.org/10.1111/j.1096-0031.1994.tb00179.x
» https://doi.org/10.1111/j.1096-0031.1994.tb00179.x - Calegari BB, Vari RP, Reis RE. Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): A combined morphological and molecular analysis. Zool J Linn Soc. 2019; 187(3):661–773. https://doi.org/10.1093/zoolinnean/zlz036
» https://doi.org/10.1093/zoolinnean/zlz036 - Cediel F. Phanerozoic orogens of Northwestern South America: Cordilleran-Type orogens. Taphrogenic tectonics. The Maracaibo orogenic float. The Chocó-Panamá indenter. In: Cediel F, Shaw RP, editors. Geology and Tectonics of Northwestern South America: The Pacific-Caribbean-Andean Junction. Frontiers in Earth Sciences. Springer; 2019. p.3–95. https://doi.org/10.1007/978-3-319-76132-9_1
» https://doi.org/10.1007/978-3-319-76132-9_1 - Chardon M. Anatomie comparée de l’appareil de Weber et des structures connexes chez lez Siluriformes. Annalen. Reeks in 8 - Koninklijk Museum voor Midden-Afrika. Zoologische wetenschappen . 169. Tervuren: Musée Royal de l’Afrique Centrale; 1968.
- Cumplido N, Allende ML, Arratia G. From Devo to Evo: Patterning, fusion and evolution of the zebrafish terminal vertebra. Front Zool. 2020; 17:18. https://doi.org/10.1186/s12983-020-00364-y
» https://doi.org/10.1186/s12983-020-00364-y - Desvignes T, Carey A, Postlethwait JH. Evolution of caudal fin ray development and caudal fin hypural diastema complex in spotted gar, teleosts, and other neopterygian fishes. Dev Dyn. 2018; 247(6):832–53. https://doi.org/10.1002/dvdy.24630
» https://doi.org/10.1002/dvdy.24630 - Diogo R. Higher-level phylogeny of Siluriformes–An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers, Inc.; 2003. p.353–84.
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A. Geneious v5.5. Biomatters; 2010. Available from: http://www.geneious.com
» http://www.geneious.com - Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32(5):1792–97. https://doi.org/10.1093/nar/gkh340
» https://doi.org/10.1093/nar/gkh340 - Eigenmann CH. A review of the Doradidae, a family of South American Nematognathi, or catfishes. Trans Am Philos Soc. 1925; 22(5):279–365. https://doi.org/10.2307/1005464
» https://doi.org/10.2307/1005464 - Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham: Duke University; 1994.
- Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay river Basin. Zootaxa. 2016; 4088(4):531–46. https://doi.org/10.11646/zootaxa.4088.4.4
» https://doi.org/10.11646/zootaxa.4088.4.4 - Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008; 24(5):774–86. https://doi.org/10.1111/j.1096-0031.2008.00217.x
» https://doi.org/10.1111/j.1096-0031.2008.00217.x - Goodman M, Olson CB, Beeber JE, Czelusniak J. New perspectives in the molecular biological analysis of mammalian phylogeny. Acta Zool Fenn. 1982; 169:19–35.
- Grande T, Shardo JD. Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Fieldiana, Zool. 2002; 99:1518. https://doi.org/10.5962/bhl.title.3555
» https://doi.org/10.5962/bhl.title.3555 - Grant T, Kluge AG. Credit where credit is due: The Goodman-Bremer support metric. Mol Phylogenet Evol. 2008; 49(1):405–06. https://doi.org/10.1016/j.ympev.2008.04.023
» https://doi.org/10.1016/j.ympev.2008.04.023 - Grünbaum T, Cloutier R, Dumont P. Congruence between chondrification and ossification sequences during caudal skeleton development: A Moxostomatini case study. In: Browman HI, Skiftesvik AB, editors. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Bergen, Norway: Institute of Marine Research; 2003. p.161–76
- Higuchi H. A phylogeny of the South American thorny catfishes (Osteichthyes, Siluriformes, Doradidae). [PhD Thesis]. Cambridge: Havard University; 1992.
- Higuchi H, Birindelli JLO, Sousa LM, Britski HA Merodoras nheco, new genus and species from Rio Paraguay basin, Brazil (Siluriformes, Doradidae), and nomination of the new subfamily Astrodoradinae. Zootaxa. 2007; 1446(1):31–42. https://doi.org/10.11646/zootaxa.1446.1.3
» https://doi.org/10.11646/zootaxa.1446.1.3 - Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001; 17(8):754–55. https://doi.org/10.1093/bioinformatics/17.8.754
» https://doi.org/10.1093/bioinformatics/17.8.754 - Kaatz IM, Stewart DJ. Bioacoustic variation of swimbladder disturbance sounds in Neotropical doradoid catfishes (Siluriformes: Doradidae, Auchenipteridae): Potential morphological correlates. Curr Zool. 2012; 58(1):171–88. https://doi.org/10.1093/czoolo/58.1.171
» https://doi.org/10.1093/czoolo/58.1.171 - Kner R. Ueber einige sexual-unterschiede bei der gattung Callichthys und die schwimmblase bei Doras C. Val. Sitzungsber Akad Wiss Wien. 1853; 11:138–46.
- Kner R. Ichthyologische Beuträge [Subtitles I-III]. Sitzungsber Akad Wiss Wien. 1855; 17:92–162.
- Knight L, Ladich F. Distress sounds of thorny catfishes emitted underwater and in air: Characteristics and potential significance. J Exp Biol. 2014; 217(22):4068–78. https://doi.org/10.1242/jeb.110957
» https://doi.org/10.1242/jeb.110957 - Koerber S. On the type localities of some freshwater fishes collected by Spix and Martius in Brazil (1817–1820). Bull Fish Biol. 2021; 19:79–96.
- Lacepède BGE. Histoire naturelle des poisons: Tomme Cinquieme. Paris; 1803.
- Linnaeus C. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis: Tomus I. Editio Decima, Reformata. Laurentius Salvius: Holmiae; 1758. Available from: https://www.biodiversitylibrary.org/page/726886
» https://www.biodiversitylibrary.org/page/726886 - Lundberg JG. African-South American freshwater fish clades and continental drift: Problems with a paradigm. In: Goldblatt P, editor. Biological relationships between Africa and South America. Yale University Press; 1993. p.156–99.
- Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1–49. Available from: http://hdl.handle.net/2246/2608
» http://hdl.handle.net/2246/2608 - Lundberg JG, Sullivan JP, Rodiles-Hernández R, Hendrickson DA. Discovery of African roots for the Mesoamerican Chiapas catfish, Lacantunia enigmatica, requires an ancient intercontinental passage. Proc Acad Nat Sci Phila. 2007; 156(1):39–53. https://www.jstor.org/stable/27667759
» https://www.jstor.org/stable/27667759 - Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. Version 2.74 [Internet]. 2011. Available from: http://mesquiteproject.org
» http://mesquiteproject.org - de Melo Germano R, Stabille SR, de Britto Mari R, Pereira JNB, Faglioni JRS, de Miranda Neto MH. Morphological characteristics of the Pterodoras granulosus digestive tube (Valenciennes, 1821) (Osteichthyes, Doradidae). Acta Zool. 2014; 95(2):166–75. https://doi.org/10.1111/azo.12016
» https://doi.org/10.1111/azo.12016 - Mo T. Anatomy and systematics of Bagridae (Teleostei), and Siluroid phylogeny. Theses Zoologicae, vol. 17. Koeltz Scientific Books; 1991.
- Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11:177. https://doi.org/10.1186/1471-2148-11-177
» https://doi.org/10.1186/1471-2148-11-177 - Papadopoulos JS, Agarwala R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007; 23(9):1073–79. https://doi.org/10.1093/bioinformatics/btm076
» https://doi.org/10.1093/bioinformatics/btm076 - de Pinna MCC. Phylogenetic relationships of neotropical Siluriformes (Teleostei: Ostariophysi): Historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279–330.
- de Pinna MCC, Ng HH. The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). Am Mus Novit. 2004; 2004(3437):1–23. https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2
» https://doi.org/10.1206/0003-0082(2004)437%3C0001:TSUCIS%3E2.0.CO;2 - Piorski NM, Garavello JC, Arce H. M, Sabaj MH Platydoras brachylecis, a new species of thorny catfish (Siluriformes: Doradidae) from northeastern Brazil. Neotrop Ichthyol. 2008; 6(3):481–94. https://doi.org/10.1590/S1679-62252008000300021
» https://doi.org/10.1590/S1679-62252008000300021 - Quagio-Grassiotto I, Ortiz RJ, Sabaj Pérez MH, Oliveira C. Sperm of Doradidae (Teleostei: Siluriformes). Tissue Cell. 2011; 43(1):8–23. http://dx.doi.org/10.1016/j.tice.2010.10.006
» http://dx.doi.org/10.1016/j.tice.2010.10.006 - Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19(12):1572–74. https://doi.org/10.1093/bioinformatics/btg180
» https://doi.org/10.1093/bioinformatics/btg180 - Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
» https://doi.org/10.1643/ASIHCODONS2020 - Sabaj MH, Ferraris CJ Jr. Family Doradidae. In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.456–69.
- Sabaj Pérez MH. On the identity of Catesby’s fish in armour, “Cataphractus Americanus” (Siluriformes: Doradidae). Proc Acad Nat Sci Phila. 2014; 163(1):119–32. https://doi.org/10.1635/053.163.0104
» https://doi.org/10.1635/053.163.0104 - Schaefer SA. Anatomy and relationships of the scoloplacid catfishes. Proc Acad Nat Sci Phila. 1990; 142:167–210. Available from: https://www.jstor.org/stable/4064976
» https://www.jstor.org/stable/4064976 - Schultze H-P, Arratia G. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In: Arratia G, Schultze H-P, Wilson MVH, editors. Mesozoic Fishes 5 – Global diversity and evolution. München: Verlag Dr. Friedrich Pfeil; 2013. p.187–246.
- Sousa LM, Chaves MS, Akama A, Zuanon J, Sabaj MH Platydoras birindellii, new species of striped raphael catfish (Siluriformes: Doradidae) from the Xingu Basin, Brazil. Proc Acad Nat Sci Phila. 2018; 166(1):1–13. https://doi.org/10.1635/053.166.0106
» https://doi.org/10.1635/053.166.0106 - Sousa LM, Rapp Py-Daniel LH. Description of two new species of Physopyxis and redescription of P. lyra (Siluriformes: Doradidae). Neotrop Ichthyol. 2005; 3(4):625–36. https://doi.org/10.1590/S1679-62252005000400019
» https://doi.org/10.1590/S1679-62252005000400019 - Sullivan JP, Lundberg JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
» https://doi.org/10.1016/j.ympev.2006.05.044 - Sullivan JP, Peng Z, Lundberg JG, Peng J, He S. Molecular evidence for diphyly of the Asian catfish family Amblycipitidae (Teleostei: Siluriformes) and exclusion of the South American Aspredinidae from Sisoroidea. Proc Acad Nat Sci Phila. 2008; 157(1):51–65. https://doi.org/10.1635/0097-3157(2008)157[51:MEFDOT]2.0.CO;2
» https://doi.org/10.1635/0097-3157(2008)157[51:MEFDOT]2.0.CO;2 - Takagui FH, Baumgärtner L, Baldissera JN, Lui RL, Margarido VP, Fonteles SBA, Garcia C, Birindelli JO, Moreira-Filho O, Almeida FS, Giuliano-Caetano L. Chromosomal diversity of thorny catfishes (Siluriformes-Doradidae): A case of allopatric speciation among Wertheimerinae species of São Francisco and Brazilian eastern coastal drainages. Zebrafish. 2019; 16(5):477–85. https://doi.org/10.1089/zeb.2019.1769
» https://doi.org/10.1089/zeb.2019.1769 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19. Available from: https://sfi-cybium.fr/en/node/2423
» https://sfi-cybium.fr/en/node/2423 - Van der Laan R. Freshwater fish list: 26th ed. Almere: Richard van der Laan; 2019.
- Van der Laan R, Eschmeyer WN, Fricke R. Family-group names of recent fishes. Zootaxa. 2014; 3882(1):1–230. https://doi.org/10.11646/zootaxa.3882.1.1
» https://doi.org/10.11646/zootaxa.3882.1.1 - Vaz DFB, Hilton EJ. The caudal skeleton of Batrachoidiformes (Teleostei: Percomorphacea): A study of morphological diversity, intraspecific variation, and phylogenetic inferences. Zool J Linn Soc. 2020; 189(1):228–86. https://doi.org/10.1093/zoolinnean/zlz094
» https://doi.org/10.1093/zoolinnean/zlz094 - Zebedin A, Ladich F. Does the hearing sensitivity in thorny catfishes depend on swim bladder morphology? PLoS ONE. 2013; 8(6):e67049. https://doi.org/10.1371/journal.pone.0067049
» https://doi.org/10.1371/journal.pone.0067049
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Sabaj MH, Arce H. M. Towards a complete classification of the Neotropical thorny catfishes (Siluriformes: Doradidae). Neotrop Ichthyol. 2021; 19(4):e210064. https://doi.org/10.1590/1982-0224-2021-0064
Edited-by
Publication Dates
-
Publication in this collection
10 Dec 2021 -
Date of issue
2021
History
-
Received
14 Mar 2021 -
Accepted
17 Aug 2021