Abstract
Reproductive tactics and strategies contribute to the persistence and maintenance of long-term populations in fish species. Members of the subfamily Goodeinae are a group of small-bodied freshwater fish with specialized reproduction (viviparity-matrotrophy). They are found in the highlands of central Mexico, most of them endemic. The aim of this study was to conduct a comprehensive investigation to evaluate the annual reproductive cycle of seven species of goodeines (splitfins). We carried out our study in the subtropical Lake Zacapu, Mexico, with bi-monthly sampling from May 2019 to March 2020. We obtain the fertility, size at first maturity (L50), sex ratio, and gonadosomatic index. Our result shows that populations of goodeines have high fertility compared to other populations of the same species in other aquatic systems and also to other species of goodein. We found that males mature at smaller sizes than females, the observed proportion of females was greater than males in all the goodeines. Lake Zacapu goodeines have two reproductive peaks, one in spring (April to June) and another in fall (September to November). These tactics (fertility rates, sex ratio, reproductive period) and strategies (viviparity-matrotrophy) favor reproductive success in this environmentally stable subtropical lake in the highlands of Mexico.
Keywords:
Fertility; First sexual maturity; Goodeines; Lake Zacapu; Reproduction
Resumen
Las tácticas y estrategias reproductivas contribuyen a la persistencia y el mantenimiento de las poblaciones a largo plazo en especies de peces. Miembros de la subfamilia Goodeinae son un grupo de peces de agua dulce con reproducción especializada (viviparidad-matrotrofia). Se encuentran en el centro de México, la mayoría de ellos endémicos. El objetivo de este estudio fue realizar una investigación integral para evaluar el ciclo reproductivo anual de siete especies de goodeines o mexclapiques. Realizamos nuestro estudio en el lago subtropical de Zacapu, México, con muestreo bimestral de mayo de 2019 a marzo de 2020. Nuestros resultados muestran que las poblaciones de goodeines tienen una alta fertilidad en comparación con otras poblaciones de la misma especie en otros sistemas acuáticos y también con otras especies de goodeines. Encontramos que los machos maduran en tamaños más pequeños que las hembras, la proporción observada de hembras fue mayor que los machos en todas las goodeines. Los goodeines del lago Zacapu tienen dos picos reproductivos, uno en primavera (abril a junio) y otro en otoño (septiembre a noviembre). Estas tácticas (fertilidad, proporción de sexos, período reproductivo) y estrategias (viviparidad-matrotrofia) favorecen el éxito reproductivo en este lago subtropical ambientalmente estable en el altiplano de México.
Palabras clave:
Fertilidad; Goodeines; Lago Zacapu; Primera madurez sexual; Reproducción
INTRODUCTION
Reproductive strategies and tactics are important components in the life history of a species (Nikolsky, 1963Nikolsky GV. The ecology of fishes. London: Academic Press; 1963. ; Balon, 1984Balon EK. Patterns in the evolution of reproductive styles in fishes. In: Potts GW, Wootton RJ, editors. Fish reproduction: Strategies and tactics. New York: Academic Press; 1984. p.35–53.; Snelson, , 1989Snelson FF Jr. Social and environmental control of life history traits in poeciliid fishes. In: Meffe GK, Snelson FF Jr, editors. Ecology and evolution of livebearing fishes (Poeciliidae). New Jersey: Prentice Hall; 1989. p.149–61.; Stearns, 1992Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992.). Reproductive strategies are specific adaptations such as distinct breeding systems (oviparity, viviparity, and ovoviviparity), sex-specific reproductive behaviors, and number of partners. Alternatively, reproductive tactics are linked life history traits that develop as adaptations towards environmental conditions and ecological niches (Murua, Saborido-Rey, 2003Murua H, Saborido-Rey F. Female reproductive strategies of marine fish species of the North Atlantic. J Northwest Atl Fish Sci. 2003; 33:23–31.). Fishes exhibit a wide range of reproductive tactics such as variations in age of sexual maturity, fertility, and reproductive period (Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ). The variety in reproductive characteristics that fishes exhibit has been argued as an evolutionary advantage in allowing exploitation of a huge range of niches, leading to fish being the most diverse group of vertebrates in the world (Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ; Pérez-Rodríguez et al., 2015Pérez-Rodríguez R, Domínguez-Domínguez O, Doadrio I, Cuevas-García E, Pérez-Ponce de León G. Comparative historical biogeography of three groups of Nearctic freshwater fishes across central Mexico. J Fish Biol. 2015; 86(3):993–1015. https://doi.org/10.1111/jfb.12611
https://doi.org/10.1111/jfb.12611...
; Nelson et al., 2016Nelson JS, Grande TC, Wilson MVH. Fishes of the world. New York: John Wiley & Sons; 2016. https://doi.org/10.1002/9781119174844
https://doi.org/10.1002/9781119174844...
).
The reproductive success is mainly related to age of sexual maturity, fertility, and sex ratio, and influence the population’s growth and dynamics. A population with successful reproductive tactics exhibits features such as a clear size structure (organisms of different sizes, from juveniles to mature adults), reproductive seasonality for best offspring survival, and individuals with a healthy body condition factor (Caddy, Agnew, 2004Caddy JF, Agnew DJ. An overview of recent global experience with recovery plans for depleted marine resources and suggested guidelines for recovery planning. Rev Fish Biol Fish. 2004; 14(43):43–112. https://doi.org/10.1007/s11160-004-3770-2
https://doi.org/10.1007/s11160-004-3770-...
; Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ). Reproductive tactics can help us understand when, where, and how the reproductive cycle of a species functions and determines how spatial and temporal environmental factors affect recruitment success and, consequently, population persistence (Balon, 1984Balon EK. Patterns in the evolution of reproductive styles in fishes. In: Potts GW, Wootton RJ, editors. Fish reproduction: Strategies and tactics. New York: Academic Press; 1984. p.35–53.; Pecquerie et al., 2009Pecquerie L, Petitgas P, Kooijman SALM. Modeling fish growth and reproduction in the context of the dynamic energy budget theory to predict environmental impact on anchovy spawning duration. J Sea Res. 2009; 62(2–3):93–105. https://doi.org/10.1016/j.seares.2009.06.002
https://doi.org/10.1016/j.seares.2009.06...
).
Among the most reproductively specialized groups of fishes, it is the subfamily Goodeinae (i.e., teleost fish endemic to Mexico known as splitfins) (Uribe et al., 2018Uribe MC, Grier HJ, Ávila-Zúñiga SA, García-Alarcón A. Change of lecithotrophic to matrotrophic nutrition during gestation in the viviparous teleost Xenotoca eiseni (Goodeidae). J Morphol. 2018; 279(9):1336–45. https://doi.org/10.1002/jmor.20874
https://doi.org/10.1002/jmor.20874...
). Specializations in this group include: modification of the anal fin (the gonopodium) in males, permitting internal fertilization (Turner et al., 1962Turner CL, Mendoza G, Reiter R. Development and comparative morphology of the gonopodium of goodeid fishes. Proc Iowa Acad Sci. 1962; 69(1):571–86. Available from: https://scholarworks.uni.edu/pias/vol69/iss1/87
https://scholarworks.uni.edu/pias/vol69/...
); strong sexual selection, female choice related to male color patterns, fin size, courtship display, and body shape (Macías-García et al., 1994Macías-García CM, Jiménez G, Contreras B. Correlational evidence of a sexually-selected handicap. Behav Ecol Sociobiol. 1994; 35(4):253–59. https://doi.org/10.1007/BF00170706
https://doi.org/10.1007/BF00170706...
; Macías-García, Ramírez, 2005Macías-García C, Ramírez E. Evidence that sensory traps can involve into honest signals. Nature. 2005; 434:501–05. https://doi.org/10.1038/nature03363
https://doi.org/10.1038/nature03363...
) and male choice influenced by female belly area, hue, and size (Méndez-Janovitz, Macías-García, 2017Méndez-Janovitz M, Macías-García C. Do male fish prefer them big and colourful? Non-random male courtship effort in a viviparous fish with negligible paternal investment. Behav Ecol Sociobiol. 2017; 71(160):1–12. https://doi.org/10.1007/s00265-017-2385-2
https://doi.org/10.1007/s00265-017-2385-...
); and specialized embryonic development in the ovary whereby embryos develop a complex ribbon-like tissue, known as atrophotaenia, through which the interchange of nutrients and gases between the female and embryo takes place (Uribe et al., 2014Uribe MC, Rosa-Cruz G, García-Alarcón A. Branchial placenta in the viviparous teleost Ilyodon whitei (Goodeidae). J Morphol. 2014; 275(12):1406–17. https://doi.org/10.1002/jmor.20315
https://doi.org/10.1002/jmor.20315...
; 2018Uribe MC, Grier HJ, Ávila-Zúñiga SA, García-Alarcón A. Change of lecithotrophic to matrotrophic nutrition during gestation in the viviparous teleost Xenotoca eiseni (Goodeidae). J Morphol. 2018; 279(9):1336–45. https://doi.org/10.1002/jmor.20874
https://doi.org/10.1002/jmor.20874...
). The Goodeinae includes around 40 species of small-bodied freshwater fishes found in the central Mexican highlands, most of them endemic or microendemic to a specific water body or spring (Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
; Lyons et al., 2019Lyons J, Piller KP, Artigas-Azas JM, Domínguez-Domínguez O, Gesundheit P, Köck M, Medina-Nava M, Mercado-Silva N, Ramírez-García A, Findley KM. Distribution and current conservation status of the Mexican Goodeidae (Actinopterygii, Cyprinodontiformes). Zookey. 2019; 885:115–58. https://doi.org/10.3897/zookeys.885.38152
https://doi.org/10.3897/zookeys.885.3815...
).
Within the freshwater ecosystems of central Mexico, Lake Zacapu is considered a hotspot of biodiversity, hosting two introduced and 11 native species of fish in its small area (15 ha) (Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
; personal obs.). The introduced species (Ctenopharyngodon idella (Valenciennes, 1844) and Cyprinus carpio Linnaeus, 1758)) have been largely identified as a threat to native fish in other aquatic systems (Lowe et al., 2000Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species database. Auckland: The Invasive Species Specialist Group (ISSG); 2000. ; Cudmore et al., 2017Cudmore B, Jones LA, Mandrak NE, Dettmers JM, Chapman DC, Kolar CS, Conover G. Ecological risk assessment of grass carp (Ctenopharyngodon idella) in the Great Lakes Basin. Ottawa: Fisheries and Oceans Canada, Canada Science Advisory Secretariat; 2017. ; Gibson-Reinemer et al., 2017Gibson-Reinemer DK, Chick JH, VanMiddlesworth TD, VanMiddlesworth M, Casper AF. Widerspread and enduring demographic collapse of invasive common carp (Cyprinus carpio) in the Upper Mississippi River System. Biol Invasions. 2017; 19:1905–16. https://doi.org/10.1007/s10530-017-1405-5
https://doi.org/10.1007/s10530-017-1405-...
). Among the native species, there are seven goodeines: Catarina Allotoca (Allotoca zacapuensis Meyer, Radda & Domínguez-Domínguez, 2001), Bulldog Goodeid (Alloophorus robustus (Bean, 1892)), Blackfin Goodea (Goodea atripinnis Jordan, 1880), Olive Skiffia (Skiffia lermae Meek, 1902), Jeweled Splitfin (Xenotoca variata (Bean, 1887)), Picotee Splitfin (Zoogoneticus quitzeoensis (Bean, 1898)), and Highland Splitfin (Hubbsina turneri (de Buen, 1940)). Both A. zacapuensis and G. turneri are microendemic to the lake (Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
). According to the International Union for Conservation of Nature and Mexican Federal laws, A. zacapuensis and G. turneri are Critically Endangered (CR), whilst the remaining five species are of conservation concern (NOM-059-SEMARNAT-2019NORMA Oficial Mexicana (NOM-059-SEMARNAT-2010). Lista de especies en riesgo de la Norma Oficial Mexicana. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo [Internet]. Diário Oficial de la Federación; 2019. Available from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019
https://www.dof.gob.mx/nota_detalle.php?...
; Lyons et al., 2019Lyons J, Piller KP, Artigas-Azas JM, Domínguez-Domínguez O, Gesundheit P, Köck M, Medina-Nava M, Mercado-Silva N, Ramírez-García A, Findley KM. Distribution and current conservation status of the Mexican Goodeidae (Actinopterygii, Cyprinodontiformes). Zookey. 2019; 885:115–58. https://doi.org/10.3897/zookeys.885.38152
https://doi.org/10.3897/zookeys.885.3815...
; IUCN, 2020International Union for Conservation of the Nature (IUCN). The IUCN Red List of Threatened Species; 2020. Available from: https://www.iucnredlist.org/
https://www.iucnredlist.org/...
). Despite the status the fishes have been poorly studied. Only the general biology of single species (Moncayo-Estrada, 2012Moncayo-Estrada R. Análisis histórico de la biología de la cherehuita (Hubbsina turneri) (Pisces: Goodeidae), especie endémica y en peligro de extinción de México. Rev Chapingo Ser Cienc For Ambiente. 2012; 18(1):101–10. https://doi.org/10.5154/r.rchscfa.2011.02.020
https://doi.org/10.5154/r.rchscfa.2011.0...
), taxonomic studies of fish parasites (Martínez-Aquino et al., 2012Martínez-Aquino A, Pérez-Rodríguez R, Hernández-Mena DI, Garrido-Olvera L, Aguilar-Aguilar R, Pérez-Ponce de León G. Endohelminth parasites of seven goodein species (Cyprinodontiformes: Goodeidae) from Lake Zacapu, Michoacán, Central Mexico Plateau. Hidrobiologica. 2012; 22(1):89–93. ), and biological integrity at a sub-basin level (Ramírez-Herrejón et al., 2012Ramírez-Herrejón JP, Mercado-Silva N, Medina-Nava M, Domínguez-Domínguez O. Validación de dos índices biológicos de integridad (IBI) en la subcuenca del río Angulo en el centro de México. Rev Biol Trop. 2012; 60(4):1669–85. https://doi.org/10.15517/rbt.v60i4.2160
https://doi.org/10.15517/rbt.v60i4.2160...
).
In addition, although Lake Zacapu is considered an environmentally stable water body because several springs feed the system, water is extracted for urban use, and most of its shoreline is occupied by the town of Zacapu. As such, the ecosystems of the lake are under threat due to pollution and a drop in water level. This is of particular concern as the lake acts as a refuge for several species that have disappeared from other water bodies (Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
).
This study aims to evaluate the reproductive cycle and to describe the annual variation of the sex ratio, size at first maturity, gonadosomatic index, fertility and condition factor in seven species of goodeines or splitfins inhabiting Lake Zacapu. According to the limnological characteristics prevailing at Lake Zacapu, we hypothesize that the native species present a combination of life-history traits (early maturity, high fertility rates, good condition) producing high reproductive success. The results of this study have important conservation implications and can be used to support specific conservation actions and management to maintain biological diversity in the lake and other small sub-tropical lake ecosystems in Mexico.
MATERIAL AND METHODS
Study area. Lake Zacapu is a small sub-tropical lake (ca. 15 ha) located in the State of Michoacán, central-western Mexico, at 1980 m above sea level and is part of the Lerma-Chapala River basin. It is considered a monomictic ecosystem with low turbidity. The lake is maintained by the contribution of 12 large springs, presenting a high hydraulic renewal (approx. five days) and high buffering capacity (Ayala-Ramírez et al., 2007Ayala-Ramírez G, Ruiz-Sevilla G, Chacón-Torres A. La Laguna de Zacapu, Michoacán. In: Lanza G, editor. Las aguas interiores de México: Conceptos y casos. Distrito Federal: AGT Editor; 2007. p.268–84.; Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
; Valencia-Vargas, Escalera-Vázquez, 2021Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
https://doi.org/10.22201/ib.20078706e.20...
). The lake is one of the most important areas in central Mexico for aquatic fauna conservation, it is also considered homogeneous, with good water quality, and low variations in physical and chemical conditions spatially and temporarily (Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
; Ramírez-Herrejón, 2008Ramírez-Herrejón JP. Análisis temporal de la calidad ambiental de los ecosistemas acuáticos en la subcuenca del río Angulo, cuenca Lerma-Chapala. [Master Thesis]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo; 2008. ; Ramírez-Herrejón et al., 2012Ramírez-Herrejón JP, Mercado-Silva N, Medina-Nava M, Domínguez-Domínguez O. Validación de dos índices biológicos de integridad (IBI) en la subcuenca del río Angulo en el centro de México. Rev Biol Trop. 2012; 60(4):1669–85. https://doi.org/10.15517/rbt.v60i4.2160
https://doi.org/10.15517/rbt.v60i4.2160...
; Ramírez-García, Domínguez-Domínguez, 2019Ramírez-García A, Domínguez-Domínguez O. Zacapu Lake, a hot spot of native fishes. American Currents. 2019; 44(3):8–10. Available from: http://www.nanfa.org/ac/zacapu-lake-mexico.pdf
http://www.nanfa.org/ac/zacapu-lake-mexi...
; Valencia-Vargas, Escalera-Vázquez, 2021Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
https://doi.org/10.22201/ib.20078706e.20...
).
Field samples. We conducted sampling across an annual cycle with bimonthly sampling from May 2019 to March 2020 in four locations. Sampling locations covered different habitat characteristics that have been argued to affect the distribution of fish species in Lake Zacapu (Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
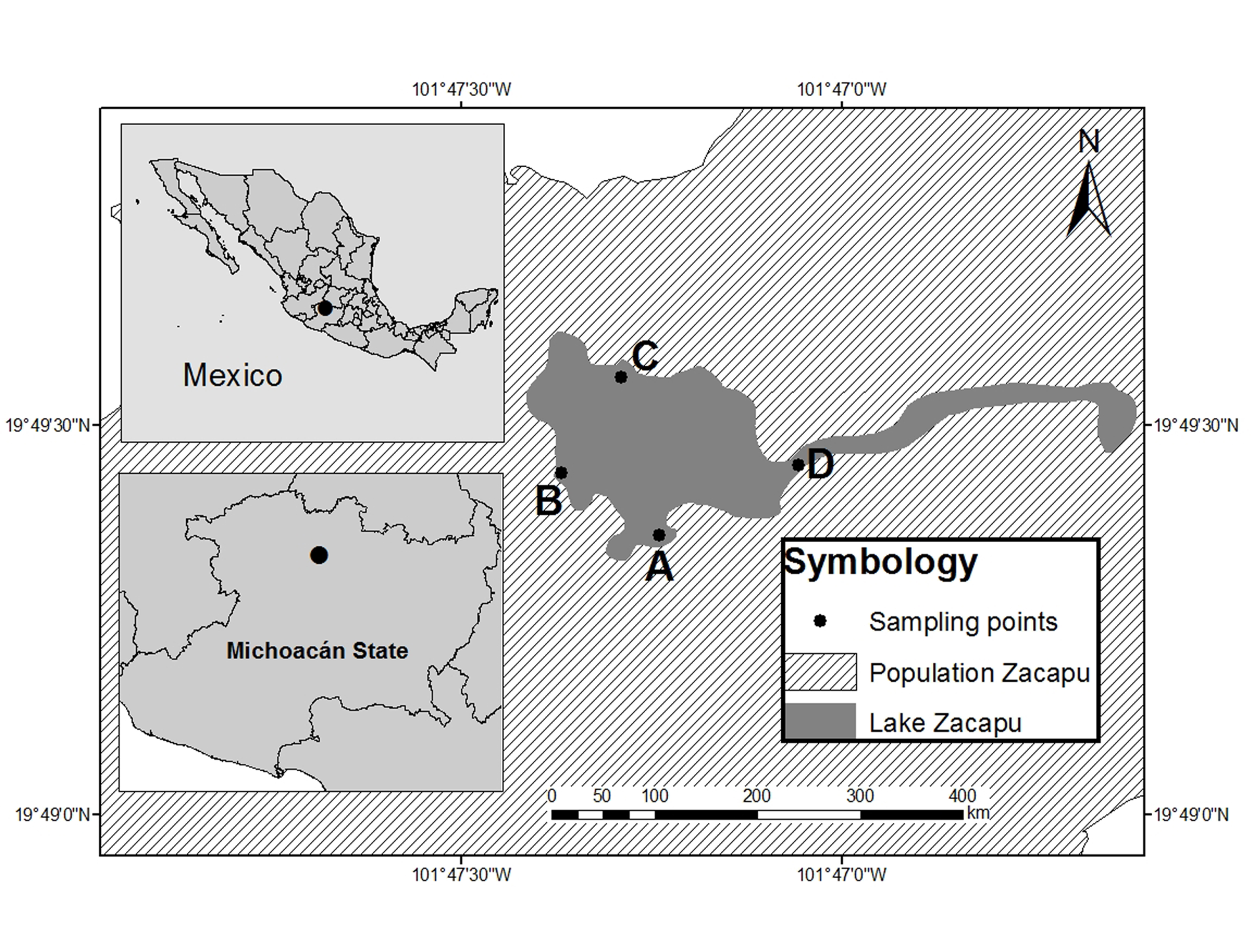
), such as difference in the type of substrate, depth, location of tributaries and effluents, presence of vegetation, and human influence (Fig. 1).
We used a seine net (25 m length, 1.8 m height, and 5 mm mesh size) and five minnow traps set for one hour per site (stainless steel, stretch mesh 0.5 cm, cylindrical, 42 cm long, and 19 cm in diameter, with two 2.5 cm holes with inverted cone inlets). The fish captured were preserved in 4% formaldehyde, then they were identified, quantified, measured (SL, 0.01 mm), and weighed (WW, 0.001 g). Voucher specimens were incorporated to the fish collection at the Universidad Michoacana de San Nicolas de Hidalgo in Mexico (CPUM, registration key: MICH.-PEC-227-07-09). Catalogue numbers: A. zacapuensis (14887); A. robustus (14888), G. atripinnis (14889), S. lermae (14890), X. variata (14891), Z. quitzeoensis (14892), and G. turneri (14893).
Data analysis. Size structure was monthly analyzed, grouping the data into length ranges (Sturges, 1926Sturges HA. The choice of a class interval. J Am Stat Assoc. 1926; 21(153):65–66. https://doi.org/10.1080/01621459.1926.10502161
https://doi.org/10.1080/01621459.1926.10...
). We applied Kruskal Wallis non-parametric analysis of variance, and Dunn (as a posteriori) tests to identify significant differences in size between months and species. Additionally, we used Mann-Whitney U tests (α = 0.05) to identify size differences between sexes for each species. These statistics were chosen because in all cases data were no-normal (Anderson-Darling and Shapiro-Wilk tests). We used the libraries MVN (Korkmaz et al., 2014Korkmaz S, Goksuluk D, Zararsiz G. MVN: An R package for assessing multivariate normality. R J. 2014; 6(2):151–62.) and Dunn.test (Dinno, 2017Dinno A. Dunn’s Test of Multiple Comparisons Using Rank Sums [Internet]. CRAN R-Project; 2017. Available from: http://cran.stat.unipd.it/web/packages/dunn.test/dunn.test.pdf
http://cran.stat.unipd.it/web/packages/d...
) in the program R (R Development Core Team, 2021R Development Core Team. R: a language and environment for statistical computing [Internet]. Vienna: R foundation for statistical computing; 2021. Available from: https://www.r-project.org/
https://www.r-project.org/...
, version 1.4.1106).
Gonad maturity. Estimated per month with the modification of the criteria proposed by Ramírez-Herrejón et al., (2007)Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71. (Tab.1).
Gonadal maturity stages of viviparous fishes from Lake Zacapu, Mexico, modified from Ramírez-Herrejón et al., (2007)Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71.
Sex ratio. was described per site and season following the criteria of Sparre, Venema, (1997)Sparre P, Venema SC. Introducción a la evaluación de recursos pesqueros tropicales. Roma: FAO; 1997. . We established the statistical significance by fitting to a Chi-squared test (χ2, α = 0.05).
Size at first maturity (L50). Related to the standard length using the logistic regression model to fit sigmoid curves, according to the following equation M(L) = 1/(1 + e (–aL + b)), where M(L) is the probability of an individual of being mature at a determinate L length, b is the intercept, and a is the slope. We used the sizeMat package in R to estimate the size and confidence limits derived by Bayesian inference based on stochastic simulation (Torrejon-Magallanes, 2020Torrejon-Magallanes J. Package ‘sizeMat’. Estimate Size at Sexual Maturity [Internet]. CRAN R-Project; 2020. Available from: https://cran.r-project.org/web/packages/sizeMat/sizeMat.pdf
https://cran.r-project.org/web/packages/...
).
Gonadosomatic index (GSI). Calculated per sampling season and sex by dividing the gonad mass by total body mass (values in grams) × 100 (Zeyl et al., 2014Zeyl JN, Love OP, Higgs DM. Evaluating gonadosomatic index as an estimator of reproductive condition in the invasive round goby, Neogobius melanostomus. J Great Lakes Res. 2014; 40(1):164–71. https://doi.org/10.1016/j.jglr.2013.12.004
https://doi.org/10.1016/j.jglr.2013.12.0...
). We analyzed the differences between months and sites with Kruskal Wallis and Dunn tests.
Fertility. We dissected the ovaries of each female and quantified embryonated eggs and embryos. We derived a fertility (F) model and adjusted it to the potential model (Holden, Raitt, 1975Holden MJ, Raitt DFS. Manual de ciencia pesquera Parte 2 - Métodos para investigar los recursos y su aplicación. Roma: FAO; 1975.; Schoenherr, 1977Schoenherr AA. Density dependent and density independent regulation of reproduction in the Gila Topminnow, Poeciliopsis occidentalis (Baird and Girard). Ecology. 1977; 58(2):438–44. https://doi.org/10.2307/1935619
https://doi.org/10.2307/1935619...
): F = a Lb, where a and b are constants in the potential model. We applied a correlation analysis (Pearson’s coefficient) to determine the relationship between size (standard length) and fertility (number of embryos).
Condition factor. Assessed with Fulton’s condition factor (K) (Froese, 2006Froese R. Cube law, condition factor and weight–length relationships: History, meta- analysis and recommendations. J Appl Ichthyol. 2006; 22(4):241–53. https://doi.org/10.1111/j.1439-0426.2006.00805.x
https://doi.org/10.1111/j.1439-0426.2006...
): K = 100 (W/L3), where W is the body wet weight (g), and L is the length (cm), and the value of 100 is used to bring K close to unity. As in the case of the gonadosomatic index, we analyzed the differences among months and sites with Kruskal Wallis and Dunn tests.
Physicochemical parameter. We measure conductivity (µs/cm), water temperature (ºC), dissolved oxygen (O2 mg/l), reduction oxide potential (mv), total dissolved solids (TDS mg/L), and hydrogen potential (pH), per month and sampling sites with a multiparameter probe (YSI EXO2; YSI Inc., Yellow Springs, OH, U.S.A). We applied Kruskal Wallis tests to describe differences among sites and months. We measured the relationship between the environment and reproductive features (GSI and K) with correlation tests (Spearman correlation, 0.05). We built Linear Models to identify which environmental measures (predictor variables) best explained the fish reproduction (response variables). We used the library Hmisc (Harrell, 2021Harrell FE. Harrell Miscellaneous, Packeges ‘Hmisc’ [Internet]. CRAN R-Project; 2021. Available from: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf
https://cran.r-project.org/web/packages/...
) and the function lm in the R program to obtain the correlation and multiple regression, respectively.
RESULTS
In this study, we examined 112 specimens of Hubbsina turneri (72 females and 40 males), 113 of Allotoca zacapuensis (75 females and 38 males), 188 of Alloophorus robustus (138 females and 50 males), 278 of Xenotoca variata (303 females and 76 males), 283 of Skiffia lermae (159 females and 124 males), 531 of Goodea atripinnis (415 females and 116 males), and 972 of Zoogoneticus quitzeoensis (553 females and 419 males).
Size structure.Goodea atripinnis presented the largest range of size structure, 15.1 to 173.0 mm in SL for females and 15.0 to 121.2 mm in males. The smallest range of size structure was found in G. turneri: 12.6 mm to 45.9 mm of SL in females and 17.7 mm to 40.1 mm in males (Tab. 2). There were not significant differences in sizes among sexes in all species. Alloophorus robustus (w = 328, p = 0.403), A. zacapuensis (w = 288, p = 1), G. turneri (w = 337, p = 0.296), S. lermae (w = 350, p = 0.204), Z. quitzeoensis (w = 301, p = 0.798), G. atripinnis (w = 280, p = 0.877), and X. variata (w = 330, p = 0.391).
Reproductive biology information for species of goodeines or splitfins from Lake Zacapu, Mexico. *Significative Pearson coefficient. SD = Standard deviation.
There were significant differences in sizes by months and sites in Z. quitzeoensis (χ2 = 18.2 months, p = 0.002; χ2 = 9.2 sites, p = 0.025) and A. robustus (χ2 = 13.4 months, p = 0.019; χ2 = 18.3 sites, p = 0.0003). There were significant differences by sites in A. zacapuensis (χ2 = 45.7, p = 6.447e-10) and G. turneri (χ2 = 0.6, p = 0.001) and by months in S. lermae (χ2 = 11.5, p = 0.04), X. variata (χ2 = 13.3, p = 0.020) and G. atripinnis (χ2 = 35.7, p=1.071e-06). There were not significant differences in sizes by sites in S. lermae (χ2 = 4.2, p = 0.23), X. variata (χ2 = 1.7, p = 0.63) and G. atripinnis (χ2 = 0.3, p = 0.941) and by moths in A. zacapuensis (χ2 = 0.2, p = 0.99), and G. turneri (χ2 = 9.1, p = 0.102).
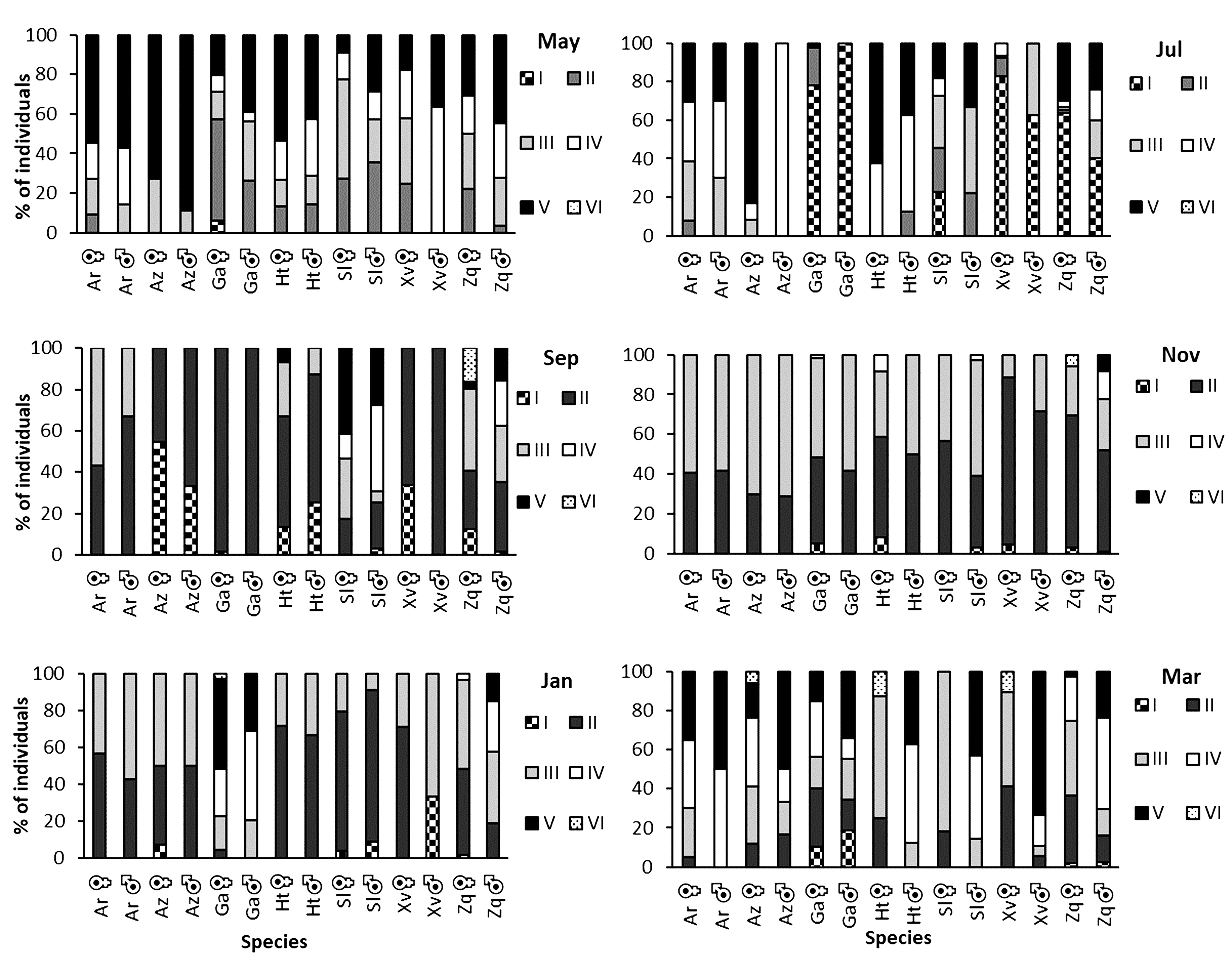
Gonad maturity stage. March and May showed the highest percentage of mature individuals (stages IV and V) for all species. In general, July, November, and January showed the highest percentage of juveniles (stages I, II, and III) (Fig. 2).
Gonadic maturity stages of species of goodeines in percentage (Ar = Alloophorus robustus, Az = Allotoca zacapuensis, Ga = Goodea atripinnis, Ht = Hubbsina turneri, Sl = Skiffia lermae, Xv = Xenotoca variata and Zq = Zoogoneticus quitzeoensis) for each month in Lake Zacapu, Mexico.
Sex ratio. Overall female:male ratio was significantly different from expected 1:1 ratio in all species: A. robustus (3:1, χ2 = 41.19, p = 1.38e-10), A. zacapuensis (2:1, χ2 = 11.11, p = 0.0005), X. variata (4:1, χ2 = 135.12, p < 2.2e-16), G. turneri (2:1, χ2 = 9.14, p = 0.002), G. atripinnis (4:1, χ2 = 168.36, p < 2.2e-16), S. lermae (1:1, χ2 = 4.328, p = 0.037) and Z. quitzeoensis (1:1, χ2 = 17.74, p = 2.524e-05).
Size at first maturity. Males mature at smaller sizes than females in all the species. In S. lermae reproduction was found to begin at the smallest size for both sexes (29.5 ± 5.7 mm SL in females and 25.2 ± 5.4 mm SL in males). In A. robustus reproduction was found to begin at the largest size for both sexes (87.4 ± 24.0 mm SL for females and 69.0 ± 17.5 mm SL for males) (Tab. 2).
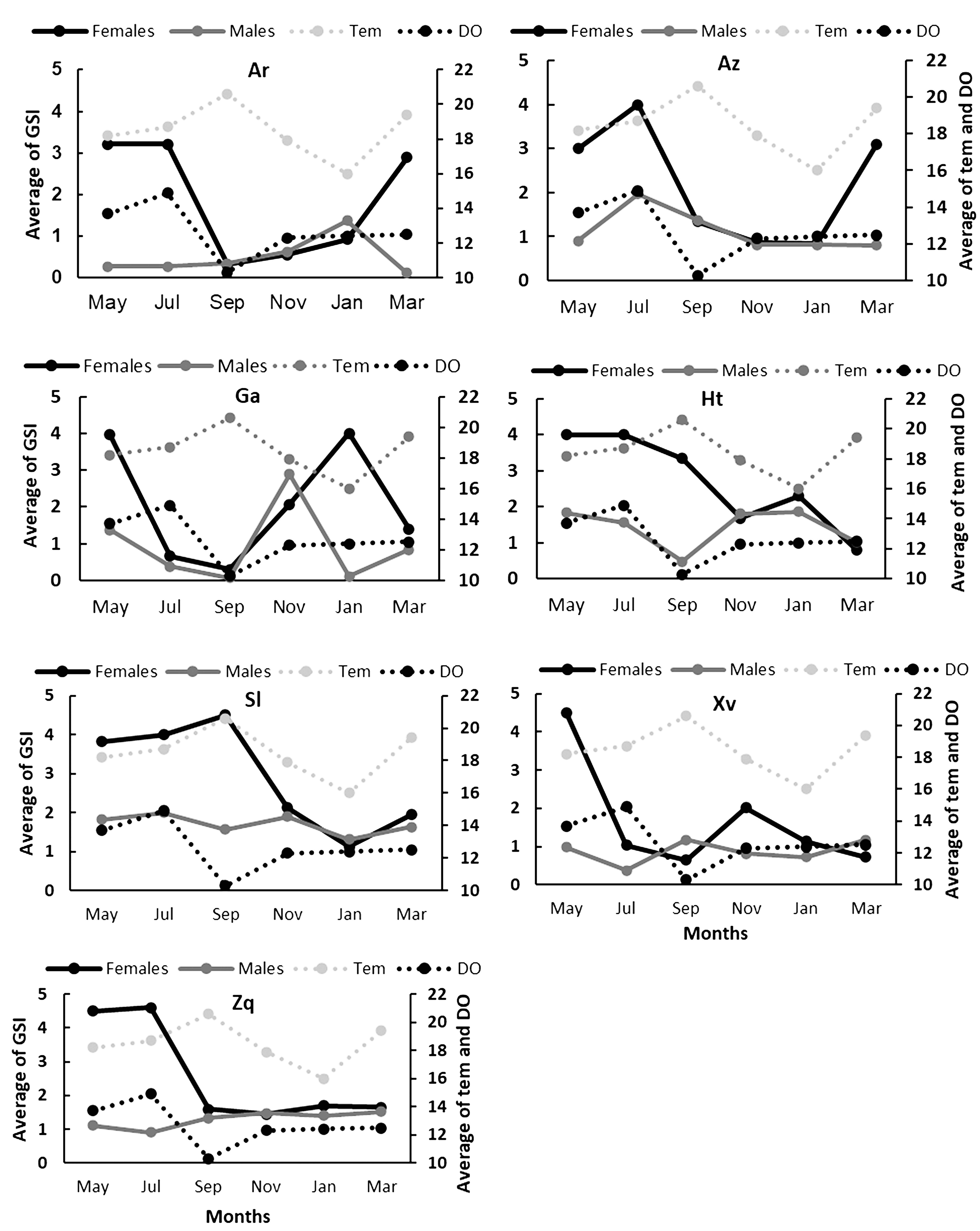
Gonadosomatic index (GSI). The values of the GSI show a reproductive peak from May to July for all the species and both sexes. Skiffia lermae and G. turneri extend their reproductive peak until September. Xenotoca variata shows a second reproductive peak in November for females and March for males. Goodea atripinnis shows a second reproductive peak in November for males and in January for females. Allotoca zacapuensis and A. robustus show a second reproductive peak in March for females and January for males (Fig. 3). There were no significant differences between GSI values by months (Tab. 3), only in G. atripinnis did Dunn tests shows that September is different from other months (alpha = 0.05, p < 0.02). There were significant differences by sites in G. turneri and A. zacapuensis (Tab. 3).
Bimonthly variation in the average of the GSI values and temperature (tem, C°) and dissolved oxygen (DO, O2 mg/L), the average for each sampling month for females (black line) and males (gray lines) of all goodein species (Ar = Alloophorus robustus, Az = Allotoca zacapuensis, Ga = Goodea atripinnis, Ht = Hubbsina turneri, Sl = Skiffia lermae, Xv = Xenotoca variata and Zq = Zoogoneticus quitzeoensis) in Lake Zacapu, Mexico.
Kruskal Wallis Test for GSI (gonadosomatic index) and K (Fulton’s condition factor) for each goodein species from Lake Zacapu, Mexico. χ2 = Chi-square value, *= significant differences.
Fertility.Skiffia lermae possess the lowest fertility value (range 6 to 23, average of 12 ± 4.5 embryos per female). The highest fertility was found in G. atripinnis (range 35 to 198, average 88 ± 40.9 embryos per female). We found a significant influence of standard length of females on the number of embryos in the species X. variata (r = 0.77; P = 0.0001), Z. quitzeoensis (r = 0.78; P = 0.0001) and A. robustus (r = 0.68; P = 0.0001) (Tab. 2). Correlation was marginally significant in the species G. atripinnis (r = 0.43; P = 0.0001), G. turneri (r = 0.23; P = 0.31), and S. lermae (r = 0.25; P = 0.15). Whereas A. zacapuensis showed a marginally inverse correlation (r = -0.13; P = 0.5).
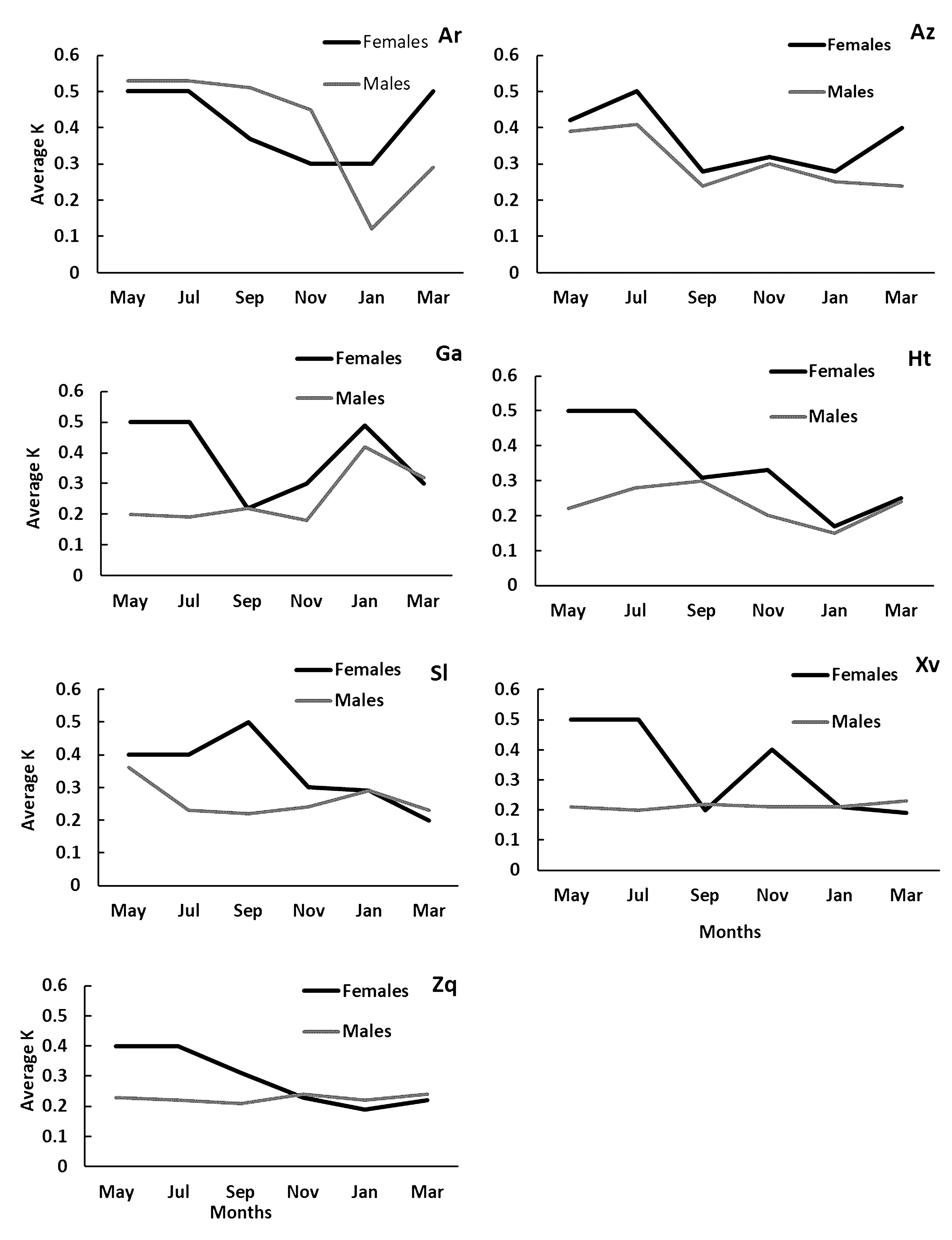
Condition factor (K). Condition factors showed a similar tendency as the reproductive peaks of GSI values in both sexes for all the species. All the species showed the highest average of K during May (Fig. 4). There were no significant differences between the Fulton factor values by months (Tab. 3), only in G. atripinnis did Dunn tests shows significant differences by months (p < 0.0001). There were significant differences (Dunn test: alpha = 0.005, p < 0.001) by sites in G. turneri, and A. zacapuensis (Tab. 3).
Physicochemical water conditions. The temperature ranged from 16.0 ± 0.2 ºC (in January) to 20.6 ± 1.6 ºC (in September). The pH (8-9) indicated slightly basic water, with moderate electrical conductivity in a range from 135.9 ± 1.7 µs/cm in January to 155.2 ± 9.8 µs/cm in September. Total dissolved solids showed a range from 105.8 ± 1.9 mg/L in May to 109.9 ± 3.5 mg/L in September. Dissolved oxygen concentrations ranged between 10.3 ± 2.0 mg/L in September to 14.9 ± 3.1 mg/L in July (Tab. 4).
Bimonthly variation in the average of K values for each sampling month for females (black line) and males (gray lines) of all goodein species (Ar = Alloophorus robustus, Az = Allotoca zacapuensis, Ga = Goodea atripinnis, Ht = Hubbsina turneri, Sl = Skiffia lermae, Xv = Xenotoca variata and Zq = Zoogoneticus quitzeoensis) in Lake Zacapu, Mexico.
Physical and chemical water characteristics in Lake Zacapu, Michoacán, Mexico. Tem = water temperature (°C), DO = dissolved oxygen (O2 mg/L), pH = potential for hydrogen, TDS = total dissolved solids (mg/L), Cond = conductivity (µs/cm). SD = Standard deviation.
There were not significant differences between sampling sites in water temperature (χ2 = 0.3, p = 0.28), dissolved oxygen (χ2 = 4.5, p = 0.206), total dissolved solids (χ2 = 4.9, p = 0.1788), and pH (χ2 = 0.2, p = 0.96). The Spearman correlation showed a significant relationship in females between GSI and the total dissolved solids in S. lermae, Z. quitzeoensis, and A. robustus (R2 = -0.5, p = 0.011; R2 = -0.4, p = 0.02; R2 = -0.5, p = 0.004, respectively) and a significant relationship between the GSI and temperature in G. atripinnis (R2 = -0.4, p = 0.02). For the males only A. robustus showed a significant relationship between the GSI and temperature (R2 = -0.5, p = 0.012).
The linear models between the environmental variables and the GSI recovered significant relationships in G. atripinnis:(temperature t-value= -2.7, p = 0.013; dissolved oxygen t-value= 2.3, p = 0.029; total dissolved solids t-value= 3.1, p = 0.005; conductivity t-value= -2.9, p = 0.008). In S. lermae GSI was recovered with a significant relationship with pH (t-value= -2.1, p = 0.045). In the case of the males no relationship was found in all species in the linear models.
DISCUSSION
The present study documents the reproductive cycle of seven native splitfin species of the Goodeinae subfamily in a sub-tropical lake in Central Mexico. The favorable and stable environmental condition that Lake Zacapu provides to the species of splitfins (shallow, monomythic, fed by springs, subtropical ecosystem, high food availability; Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
; Ayala-Ramírez et al., 2007Ayala-Ramírez G, Ruiz-Sevilla G, Chacón-Torres A. La Laguna de Zacapu, Michoacán. In: Lanza G, editor. Las aguas interiores de México: Conceptos y casos. Distrito Federal: AGT Editor; 2007. p.268–84.; Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
; Valencia-Vargas, Escalera-Vázquez, 2021Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
https://doi.org/10.22201/ib.20078706e.20...
) could be related to high reproductive success. These environmental features, contrast to those experienced by the same species or other species of the same subfamily in other water bodies in Central Mexico (Tab. 2) (Mendoza, 1962Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi. Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
https://doi.org/10.2307/1539280...
; Ramírez-Herrejón et al., 2007Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71.; Cruz-Gómez et al., 2013Cruz-Gómez A, Rodríguez-Varela AC, Vázquez-López H. Reproductive aspects of yellow fish Girardinichthys multiradiatus (Meek, 1904) (Pisces: Goodeidae) in the Huapango Reservoir, State of México, México. Am J Life Sci. 2013; 1(5):189–94. https://doi.org/10.11648/j.ajls.20130105.11
https://doi.org/10.11648/j.ajls.20130105...
; Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
).
Xenotoca variata and G. atripinnis show size dimorphism, with X. variata the most dimorphic (males are smaller than females) and the species with the brightest coloration. This sexual dimorphism in size has been shown in other species of goodeines (i.e., splitfins) (Ritchie et al., 2007Ritchie MG, Hamill RM, Graves JA, Magurran AE, Webb SA, Macías-García C. Sex and differentiation: Population genetic divergence and sexual dimorphism in Mexican goodeid fish. J Evol Biol. 2007; 20(5):2048–55. https://doi.org/10.1111/j.1420-9101.2007.01357.x
https://doi.org/10.1111/j.1420-9101.2007...
). Several hypotheses have tried to explain these differences: Firstly, relating to the energetics, a greater investment in testes and sperm production leading to smaller males (Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ). Secondly, the loss of feeding opportunities and extra-spend energy in species with active courtship in males leading to smaller males, as demonstrated in Darkedged Splitfin (Girardinichthys multiradiatus (Meek, 1904)) (Macías-García, Valero, 2010Macías-García C, Valero A. Sexual conflict and sexual selection in the Goodeinae, a clade of viviparous fish with effective female mate choice. In Macedo R, editor. Behavioral ecology of tropical animals. Advances in the study of behavior, vol. 42. Massachusetts: Academic Press; 2010. p.1–54. https://doi.org/10.1016/S0065-3454(10)42001-X
https://doi.org/10.1016/S0065-3454(10)42...
). Thirdly, ontogeny considerations (developmental constraints hypothesis), selection should favor prolonged development, but if juvenile mortality exceeds adults, selection for survival should favor rapid development and maturation at a smaller size. If reproductive success is not positively correlated with body size in males, this selection for early maturation will dominate among males, leading to protandry and female-biased size dimorphism (Fairbairn, 1990Fairbairn DJ. Factors influencing sexual size dimorphism in temperate waterstriders. Am Nat. 1990; 136(1):61–86. https://doi.org/10.1086/285082
https://doi.org/10.1086/285082...
). Fourth, an indirect consequence of the mating systems (Magurran, Macías-García, 2000Magurran AE, Macías-García C. Sex differences in behavior as an indirect consequence of mating system. J Fish Biol. 2000; 57(4):839–57. https://doi.org/10.1111/j.1095-8649.2000.tb02196.x
https://doi.org/10.1111/j.1095-8649.2000...
), for example, males maneuver better at the smaller size when trying to copulate (Clutton-Brock, Parker, 1995Clutton-Brock TH, Parker GA. Sexual coercion in animal societies. Anim Behav. 1995; 49(5):1345–65. https://doi.org/10.1006/anbe.1995.0166
https://doi.org/10.1006/anbe.1995.0166...
). Fifth, predation has also been related to size dimorphism. Laboratory experiments in X. variata demonstrate that specimens with more marked secondary sexual traits, such as a conspicuous distal edge yellow band in males, may increase predation risk (Moyaho et al., 2004Moyaho A, Macías-García C, Manjarrez J. Predation risk is associated with the geographic variation of a sexually selected trait in a viviparous fish, Xenotoca variata. J Zool. 2004; 262(3):265–70. https://doi.org/10.1017/S095283690300459X
https://doi.org/10.1017/S095283690300459...
). Species with the bright yellow band suffer more damage in this area, and consequently, as more tissues must be regenerated, they gain mass more slowly (Macías-García, Ramírez, 2005Macías-García C, Ramírez E. Evidence that sensory traps can involve into honest signals. Nature. 2005; 434:501–05. https://doi.org/10.1038/nature03363
https://doi.org/10.1038/nature03363...
).
The observed proportion of females was greater for most studied species from Lake Zacapu. Sex ratio generally favors females in wild populations to ensure the offspring (Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ). These sexual differences have frequently been attributed to foraging behavior and male-biased high predation mortality (Rodd, Reznick, 1997Rodd FH, Reznick DN. Variation in the demography of guppy populations: The importance of predation and life histories. Ecology. 1997; 78(2):405–18. https://doi.org/10.2307/2266017
https://doi.org/10.2307/2266017...
). In goodeines, which show strong sexual dimorphism in appearance (males more colorful or/and ornamented than females) and behaviour (with the males undertaking an elaborate courtship to persuade the female to copulate), males are more prone to predation (Macías-García et al., 1994Macías-García CM, Jiménez G, Contreras B. Correlational evidence of a sexually-selected handicap. Behav Ecol Sociobiol. 1994; 35(4):253–59. https://doi.org/10.1007/BF00170706
https://doi.org/10.1007/BF00170706...
; Macías-García, Valero, 2010Macías-García C, Valero A. Sexual conflict and sexual selection in the Goodeinae, a clade of viviparous fish with effective female mate choice. In Macedo R, editor. Behavioral ecology of tropical animals. Advances in the study of behavior, vol. 42. Massachusetts: Academic Press; 2010. p.1–54. https://doi.org/10.1016/S0065-3454(10)42001-X
https://doi.org/10.1016/S0065-3454(10)42...
), all these features are similar to poeciliids (Magalhães, Jacobi, 2017Magalhães ALB, Jacobi CM. Colorful invasion in permissive Neotropical ecosystems: Establishment of ornamental non-native poeciliids of the genera Poecilia/Xiphophorus (Cyprinodontiformes: Poeciliidae) and management alternatives. Neotrop Ichthyol. 2017; 15(1):e160094. https://doi.org/10.1590/1982-0224-20160094
https://doi.org/10.1590/1982-0224-201600...
). It has been demonstrated that individuals with more secondary sexual traits may be at an increased risk of predation, especially in clear waters where prey fish can be easily identified by predatory fish, reptiles, and wading birds (Macías-García et al., 1994Macías-García CM, Jiménez G, Contreras B. Correlational evidence of a sexually-selected handicap. Behav Ecol Sociobiol. 1994; 35(4):253–59. https://doi.org/10.1007/BF00170706
https://doi.org/10.1007/BF00170706...
; Moyaho et al., 2004Moyaho A, Macías-García C, Manjarrez J. Predation risk is associated with the geographic variation of a sexually selected trait in a viviparous fish, Xenotoca variata. J Zool. 2004; 262(3):265–70. https://doi.org/10.1017/S095283690300459X
https://doi.org/10.1017/S095283690300459...
). The sexual dimorphism and courtship behavior of goodeines in combination with the high-water transparency of Lake Zacapu (> 120 cm), could promote male-biased predation and consequently observed sex ratio biased to females in the seven studied species, as have been previously explained for Girardinichthys multiradiatus (Macías-García et al., 1998Macías-García C, Saborío E, Berea C. Does male-biased predation lead to male scarcity in viviparous fish? J Fish Biol. 1998; 53(sA):104–17. https://doi.org/10.1111/j.1095-8649.1998.tb01021.x
https://doi.org/10.1111/j.1095-8649.1998...
), and poeciliid species, such as the guppy Poecilia reticulata, black molly Poecilia sphenops, Yucatan molly Poecilia velifera, green swordtail Xiphophorus hellerii, southern platyfish Xiphophorus maculatus, variable platyfish Xiphophorus variatus (Rodd, Reznick, 1997Rodd FH, Reznick DN. Variation in the demography of guppy populations: The importance of predation and life histories. Ecology. 1997; 78(2):405–18. https://doi.org/10.2307/2266017
https://doi.org/10.2307/2266017...
; Magalhães, Jacobi, 2017Magalhães ALB, Jacobi CM. Colorful invasion in permissive Neotropical ecosystems: Establishment of ornamental non-native poeciliids of the genera Poecilia/Xiphophorus (Cyprinodontiformes: Poeciliidae) and management alternatives. Neotrop Ichthyol. 2017; 15(1):e160094. https://doi.org/10.1590/1982-0224-20160094
https://doi.org/10.1590/1982-0224-201600...
) and Gambusia holbrooki (Kahn et al., 2013Kahn AT, Kokko H, Jennions MD. Adaptive sex allocation in anticipation of changes in offspring mating opportunities. Nat Commun. 2013; 4(1603):1–07. https://doi.org/10.1038/ncomms2634
https://doi.org/10.1038/ncomms2634...
).
An important factor that could be related to reproductive success is food availability. If food availability is limited or environmental conditions are unfavorable for reproduction, females animals have lower fertility rates than predicted and/or reproduce at smaller sizes (Lombardi, Wourms, 1988Lombardi J, Wourms J. Embryonic growth and trophotaenial development in goodeid fishes (Teleostei: Atheriniformes). J Morphol. 1988; 197(2):193–208. https://doi.org/10.1002/jmor.1051970206
https://doi.org/10.1002/jmor.1051970206...
; Hollenberg, Wourms, 1994Hollenberg F, Wourms JP. Ultrastructure and protein uptake of the embryonic trophotaeniae of four species of goodeid fish (Teleostei: Atheriniformes). J Morphol. 1994; 219(2):105–29. https://doi.org/10.1002/jmor.1052190202
https://doi.org/10.1002/jmor.1052190202...
; Wootton, Smith, 2014Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014. ; Magalhães, Jacobi, 2017Magalhães ALB, Jacobi CM. Colorful invasion in permissive Neotropical ecosystems: Establishment of ornamental non-native poeciliids of the genera Poecilia/Xiphophorus (Cyprinodontiformes: Poeciliidae) and management alternatives. Neotrop Ichthyol. 2017; 15(1):e160094. https://doi.org/10.1590/1982-0224-20160094
https://doi.org/10.1590/1982-0224-201600...
). Food availability is especially important in viviparous fishes (Trexler, DeAngelis, 2003Trexler JC, DeAngelis DL. Resource allocation in offspring provisioning: An evaluation of the conditions favoring the evolution of matrotrophy. Am Nat. 2003; 162(5):574–85. https://doi.org/10.1086/378822
https://doi.org/10.1086/378822...
; Tobler, Culumber, 2018Tobler M, Culumber Z. Ecology and diversification of reproductive strategies in viviparous fishes. bioRxiv. 2018; 442830. https://doi.org/10.1101/442830
https://doi.org/10.1101/442830...
). Goodeines are matrotrophic, with the embryos growing up to 38,700% after yolk absorption (Hollenberg, Wourms, 1995Hollenberg F, Wourms JP. Embryonic growth and maternal nutrient sources in goodeid fishes (Teleostei: Cyprinodontiformes). J Exp Zool. 1995; 271(5):379–94. https://doi.org/10.1002/jez.1402710508
https://doi.org/10.1002/jez.1402710508...
), nourished through a placenta-like structure connecting the embryo lower gut to the mother’s ovarian cavity (Macías-García, Valero, 2010Macías-García C, Valero A. Sexual conflict and sexual selection in the Goodeinae, a clade of viviparous fish with effective female mate choice. In Macedo R, editor. Behavioral ecology of tropical animals. Advances in the study of behavior, vol. 42. Massachusetts: Academic Press; 2010. p.1–54. https://doi.org/10.1016/S0065-3454(10)42001-X
https://doi.org/10.1016/S0065-3454(10)42...
). Therefore, the nutrition condition of the female (related to food availability; Reznick et al., 1996Reznick DN, Callahan H, Llauredo R. Maternal effects on offspring quality in Poeciliid fishes. Am Zool. 1996; 36(2):147–56. https://doi.org/10.1093/icb/36.2.147
https://doi.org/10.1093/icb/36.2.147...
; Wourms, 2005Wourms JP. Functional morphology, development, and evolution of trophotaeniae. In: Uribe MC, Grier HJ, editors. Viviparous fishes. Homestead: New Life Publications; 2005. p.237–62.; Trexler, DeAngelis, 2003Trexler JC, DeAngelis DL. Resource allocation in offspring provisioning: An evaluation of the conditions favoring the evolution of matrotrophy. Am Nat. 2003; 162(5):574–85. https://doi.org/10.1086/378822
https://doi.org/10.1086/378822...
), is closely linked to the quality and quantity of embryos (Blackburn, 2005Blackburn DG. Evolutionary origins of viviparity in fishes. In: Uribe MC, Grier H, editors: Viviparity in fishes. Homestead: New Life Publications; 2005. p.303–17. ; Uribe et al., 2018Uribe MC, Grier HJ, Ávila-Zúñiga SA, García-Alarcón A. Change of lecithotrophic to matrotrophic nutrition during gestation in the viviparous teleost Xenotoca eiseni (Goodeidae). J Morphol. 2018; 279(9):1336–45. https://doi.org/10.1002/jmor.20874
https://doi.org/10.1002/jmor.20874...
). An indicator of the nutrition condition of a species is the Fulton factor (Hutchings, Gerber, 2002Hutchings JA, Gerber L. Sex-biased dispersal in a salmonid fish. Proc R Soc Lond B Biol Sci. 2002; 269(1508):2487–93. https://doi.org/10.1098/rspb.2002.2176
https://doi.org/10.1098/rspb.2002.2176...
). We found that developing embryos increased more in mass and volume in the most robust individuals (highest Fulton factor), leading to an increase in the female reproductive allocation (Fig. 4). In Lake Zacapu, fishes reach sexual maturity at larger sizes and have higher fertility rates compared to fish in other aquatic systems (Mendoza, 1962Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi. Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
https://doi.org/10.2307/1539280...
; Navarrete-Salgado et al., 2007Navarrete-Salgado NA, Cedillo-Díaz BE, Contreras-Rivero G, Elías-Fernández G. Crecimiento, reproducción y supervivencia de Girardinichthys multiradiatus (Pisces: Goodeidae) en el embalse San Miguel Arco, Estado de México. Rev Chapingo Ser Cienc For Ambiente. 2007; 13(1):15–21.; Ramírez-Herrejón et al., 2007Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71.; Cruz-Gómez et al., 2011Cruz-Gómez A, Rodríguez-Varela AC, Vázquez-López H. Reproductive aspects of Girardinichthys multiradiatus, Meek 1904 (Pisces: Goodeidae). Biocyt. 2011; 4(13–16):215–28. https://doi.org/10.22201/fesi.20072082.2011.4.75942
https://doi.org/10.22201/fesi.20072082.2...
; Gómez-Márquez et al., 2013Gómez-Márquez JL, Mendoza BP, Guzmán-Santiago JL. Occurrence of the fish Girardinichthtys viviparus (Cyprinodontiformes: Goodeidae) in an urban lake at Mexico City. UNED Res J. 2013; 5(1):89–95. https://doi.org/10.1007/s10641-006-0039-8
https://doi.org/10.1007/s10641-006-0039-...
; Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
). The higher food availability and stable habitat conditions in Lake Zacapu (Valencia-Vargas, Escalera-Vázquez, 2021Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
https://doi.org/10.22201/ib.20078706e.20...
) may lead to an increased rate of development of individuals, compared to the members of the same species in other habitats. For example, Lake Patzcuaro is a eutrophic lake with low transparency, high wastewater discharges, and low food availability (Mendoza, 1962Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi. Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
https://doi.org/10.2307/1539280...
; Ramírez-Herrejón et al., 2014Ramírez-Herrejón JP, Zambrano L, Mercado-Silva N, Torres-Téllez A, Pineda-García F, Caraveo-Patiño J, Balart EF. Long term changes in the fish fauna of Lago de Pátzcuaro in Central México. Lat Am J Aquat Res, 2014; 42(1):137–49. ), and Teuchitlán river, is a system with high dominance of exotic species, completely modified by human settlements, highly polluted and with low availability of food (Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
; Hernández-Morales et al., 2020Hernández-Morales R, Medina-Nava M, Tafolla-Venegas D, Herrerías-Diego Y, Escalante-Jiménez L, Escalera-Vázquez LH, Hernández-Valencia F, Domínguez-Domínguez O. Reintroducción de Zoogoneticus tequila en los manantiales de Teuchitlán, Jalisco: primera fase [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, Informe final SNIB-CONABIO, Proyecto No. NE002; 2020. Available from: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfNE002.pdf
http://www.conabio.gob.mx/institucion/pr...
; Mar-Silva et al., 2021Mar-Silva V, Herrerías-Diego Y, Medina-Nava M, Ramírez-Herrejón JP, Mendoza-Cuenca LF, Hernández-Morales R, Domínguez-Domínguez O. Spatial and temporal variation of fish assemblage structure in a Neotropical Mexican River. Rev Mex Biodivers. 2021; 92:e923433. http://dx.doi.org/10.22201/ib.20078706e.2021.92.3433
http://dx.doi.org/10.22201/ib.20078706e....
). In Lake Zacapu relative to in other habitats, goodeines could use more energy for growth and offspring nutrition (Wootton, 1998Wootton RJ. Ecology of Teleost Fishes. Dordrecht: Kluwer Academic Publisher; 1998.), resulting in higher fertility rates. Previous studies in the Teuchitlan River, a system with high anthropic pressures (e.g., pollution, introduction of non-native species and habitat modification), and low food availability, (Herrerías-Diego et al., 2018Herrerías-Diego Y, Domínguez-Domínguez O, Medina-Nava M, Ávila O, Mar-Silva V. Comparación de la composición y abundancia de la comunidad íctica del río Teuchitlán, Jalisco, México, empleando tres artes de pesca. In: Ornelas-García CP, Álvarez F, Wegier A, editors. Antropización: Primer Análisis Integral. Ciudad de Mexico: CONACYT-IBUNAM; 2018. p.265–82. https://doi.org/10.22201/ib.9786073020305e.2019.c14
https://doi.org/10.22201/ib.978607302030...
; Hernández-Morales et al., 2020Hernández-Morales R, Medina-Nava M, Tafolla-Venegas D, Herrerías-Diego Y, Escalante-Jiménez L, Escalera-Vázquez LH, Hernández-Valencia F, Domínguez-Domínguez O. Reintroducción de Zoogoneticus tequila en los manantiales de Teuchitlán, Jalisco: primera fase [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, Informe final SNIB-CONABIO, Proyecto No. NE002; 2020. Available from: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfNE002.pdf
http://www.conabio.gob.mx/institucion/pr...
; Mar-Silva et al., 2021Mar-Silva V, Herrerías-Diego Y, Medina-Nava M, Ramírez-Herrejón JP, Mendoza-Cuenca LF, Hernández-Morales R, Domínguez-Domínguez O. Spatial and temporal variation of fish assemblage structure in a Neotropical Mexican River. Rev Mex Biodivers. 2021; 92:e923433. http://dx.doi.org/10.22201/ib.20078706e.2021.92.3433
http://dx.doi.org/10.22201/ib.20078706e....
) show that species of goodeines, including G. atripinnis and Z. purhepechus, mature at lower size and present lower fertility rate relative to Lake Zacapu (Ramírez-García et al., 2018Ramírez-García A, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. J Appl Ichthyol. 2018; 34(1):81–90. https://doi.org/10.1111/jai.13543
https://doi.org/10.1111/jai.13543...
, 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
). Consequently, the unfavorable conditions of Teuchitlan River could be causing the species to produce offspring early in life but in low numbers (Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
).
It has been frequently found that hydrologic variability and environmental variables affect reproduction in fish in different aquatic ecosystems (Mims, Olden, 2012Mims MC, Olden JD. Life history theory predicts fish assemblage response to hydrologic regimes. Ecology. 2012; 93(1):35–45. https://doi.org/10.1890/11-0370.1
https://doi.org/10.1890/11-0370.1...
). Temperature, pH, and dissolved oxygen are closely linked to reproductive periods in different species of viviparous fishes, such as poeciliids (Gómez-Márquez et al., 1999Gómez-Márquez JL, Guzmán-Santiago JL, Olvera-Soto A. Reproducción y crecimiento de Heterandria bimaculata (Cyprinodontiformes: Poeciliidae) en la Laguna “El Rodeo”, Morelos, México. Rev Biol Trop. 1999; 47(3):581–92., 2008Gómez-Márquez JL, Peña-Mendoza B, Salgado-Ugarte IH, Sánchez-Herrera AK, Sastré-Baez L. Reproduction of the fish Poeciliopsis gracilis (Cyprinodontiformes: Poeciliidae) in Coatetelco, a tropical shallow lake in Mexico. Rev Biol Trop. 2008; 56(4):1801–12. https://doi.org/10.15517/rbt.v56i4.5760
https://doi.org/10.15517/rbt.v56i4.5760...
, 2016Gómez-Márquez JL, Peña-Mendoza B, Guzmán-Santiago JL. Reproductive biology of Poecilia sphenops Valenciennes, 1846 (Cyprinidontiformes: Poeciliidae) at the Emiliano Zapata Reservoir in Morelos. Mexico. Neotrop Ichthyol. 2016; 14(2):e140127. https://doi.org/10.1590/1982-0224-20140127
https://doi.org/10.1590/1982-0224-201401...
; Ramírez-García et al., 2018Ramírez-García A, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. J Appl Ichthyol. 2018; 34(1):81–90. https://doi.org/10.1111/jai.13543
https://doi.org/10.1111/jai.13543...
) and goodeines (Mendoza, 1962Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi. Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
https://doi.org/10.2307/1539280...
; Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
, 2012Moncayo-Estrada R. Análisis histórico de la biología de la cherehuita (Hubbsina turneri) (Pisces: Goodeidae), especie endémica y en peligro de extinción de México. Rev Chapingo Ser Cienc For Ambiente. 2012; 18(1):101–10. https://doi.org/10.5154/r.rchscfa.2011.02.020
https://doi.org/10.5154/r.rchscfa.2011.0...
; Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
). Although in the species studied, we found the same two reproductive peaks, one in spring (April to June) and other in fall (September to November) as have been found for other goodein species (Ramírez-Herrejón et al., 2007Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71.; Cruz-Gómez et al., 2013Cruz-Gómez A, Rodríguez-Varela AC, Vázquez-López H. Reproductive aspects of yellow fish Girardinichthys multiradiatus (Meek, 1904) (Pisces: Goodeidae) in the Huapango Reservoir, State of México, México. Am J Life Sci. 2013; 1(5):189–94. https://doi.org/10.11648/j.ajls.20130105.11
https://doi.org/10.11648/j.ajls.20130105...
; Silva-Santos et al., 2016Silva-Santos JR, Martínez-Saldaña MC, Rico-Martínez R, Gómez-Márquez JL, Arredondo-Figueroa JL. Reproductive biology of Goodea atripinnis (Jordan, 1880) (Cyprinodontiformes: Goodeidae) under controlled conditions. J Exp Biol Agric Sci. 2016; 4(2):180–93. ; Ramírez-García et al., 2020Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
http://dx.doi.org/10.3750%2FAIEP%2F02513...
), in general, our results did not find a relationship between the reproductive peaks and environmental variables, except for G. atripinnis and A. robustus (Fig. 3). The lack of correlation between environmental variables and reproduction could be explained to a variety of factors: In the past, the wider Zacapu region (Fig. 1) was a swamp area of 15,000 ha fed by several springs. At the beginning of the 20th century Zacapu was dried out for agricultural purposes, and currently the remaining wetland constitutes 15 ha of lake (Lake Zacapu) fed by 12 springs, which promote high water turnover (Zubieta-Rojas et al., 2005Zubieta-Rojas T, Alvarado-Villanueva R, Ortega-Murillo MR, Medina-Nava M, Sánchez-Heredia JD. Plan de Manejo del área natural protegida “Laguna de Zacapu y su ribera” [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, y Secretaria de Urbanismo y Medio Ambiente (SUMA); 2005. Available from: https://vdocumento.com/universidad-michoacana-de-habitantes-de-las-colonias-colindantes-con-la-laguna-y.html
https://vdocumento.com/universidad-micho...
) which give to the waterbody more stable and homogenous physicochemical conditions in space and time than other aquatic ecosystems (Tab. 4) (Moncayo-Estrada, 1996Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
https://www.repositoriodigital.ipn.mx/ha...
; Ayala-Ramírez et al., 2007Ayala-Ramírez G, Ruiz-Sevilla G, Chacón-Torres A. La Laguna de Zacapu, Michoacán. In: Lanza G, editor. Las aguas interiores de México: Conceptos y casos. Distrito Federal: AGT Editor; 2007. p.268–84.; Domínguez-Domínguez et al., 2008Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
http://www.scielo.org.mx/scielo.php?scri...
; Valencia-Vargas, Escalera-Vázquez, 2021Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
https://doi.org/10.22201/ib.20078706e.20...
). In this case, there is the possibility of reproductive resilience of the fish species (Lowerre-Barbieri et al., 2015Lowerre-Barbieri S, Crabtree L, Switzer T, Burnsed SW, Guenther C. Assessing reproductive resilience: an example with South Atlantic red snapper Lutjanus campechanus. Mar Ecol Prog Ser. 2015; 526:125–41. https://doi.org/10.3354/meps11212
https://doi.org/10.3354/meps11212...
), to adapt to changing conditions to maintain the level of reproductive success needed to result in long-term population stability in abundance but not changing its key traits, such as reproductive periods, despite the desiccation of the wider Zacapu wetland and the expected homogenization of the physicochemical parameters given the high water exchange resulting from the contribution of the 12 springs that feed the lake. Another possibility to explain the lack of correlation between environmental variables and reproduction may be linked to viviparity, a common reproductive strategy of the studied species. Since eggs and embryos develop inside the female’s body, they are less influenced by several external environmental conditions. Also, the physiological and energetic cost associated with the gestation period (Wourms, 1981Wourms JP. Viviparity: The maternal-fetal relationship in fishes. Am Zool. 1981; 21:473–515.; Lombardi, Wourms, 1988Lombardi J, Wourms J. Embryonic growth and trophotaenial development in goodeid fishes (Teleostei: Atheriniformes). J Morphol. 1988; 197(2):193–208. https://doi.org/10.1002/jmor.1051970206
https://doi.org/10.1002/jmor.1051970206...
) could force females to undertake a period of rest or recovery before becoming pregnant again, leading to the appearance of two reproductive peaks. This hypothesis is supported by controlled experiments, whereby two reproductive periods with a consistent time between them have been found in matrotrophic species in captivity (Mendoza, 1962Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi. Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
https://doi.org/10.2307/1539280...
; Silva-Santos et al., 2016Silva-Santos JR, Martínez-Saldaña MC, Rico-Martínez R, Gómez-Márquez JL, Arredondo-Figueroa JL. Reproductive biology of Goodea atripinnis (Jordan, 1880) (Cyprinodontiformes: Goodeidae) under controlled conditions. J Exp Biol Agric Sci. 2016; 4(2):180–93. ). One final possibility is that we simply have not included in our study the environmental variables that may be related to the reproductive periods of the studied species.
Goodeines or splitfins from Lake Zacapu show high reproductive effort, and favorable reproductive tactics: Female biased sex ratios, early maturity at small sizes, and females reach larger sizes than males, thus increasing their fertility with a large number of embryos. Additionally, the reproductive strategy (viviparity-matrotrophy) of the goodein species helps to promote this reproductive success together with the favorable conditions that Lake Zacapu offers to the species. Although Zacapu Lake is a protected area (Zubieta-Rojas et al., 2005Zubieta-Rojas T, Alvarado-Villanueva R, Ortega-Murillo MR, Medina-Nava M, Sánchez-Heredia JD. Plan de Manejo del área natural protegida “Laguna de Zacapu y su ribera” [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, y Secretaria de Urbanismo y Medio Ambiente (SUMA); 2005. Available from: https://vdocumento.com/universidad-michoacana-de-habitantes-de-las-colonias-colindantes-con-la-laguna-y.html
https://vdocumento.com/universidad-micho...
), the conservation strategies included in the management plan must be implemented. We recommend some conservation management strategies to protect aquatic biota in Lake Zacapu: regulate the water extraction from the lake and recharge basin; urban development plan on the shore of the lake that prioritizes the conservation of the lake system, lakeshore restoration program, cessation of water pollution in Lake Zacapu, and through environmental restoration, increase the wetland areas in Lake Zacapu, cessation of the reintroduction of non-native species, including translocation from neighbor areas.
ACKNOWLEDGEMENTS
We thank to the Unión de Pescadores of Zacapu Lake, the government of Zacapu, and Centro Regional de Investigación Pesquera, CRIAP Pátzcuaro for facilities and support. Field assistance was provided by I. A. Vargas-Figueroa, I. Betancourt-Resendes, E. Marin-Gallardo, D. L. Nuñez-Zavala, R. Valencia-Vargas, H. G. Flores-Vera, J. M. González-Flores, A. Morales-Corral, C. A. Ayala-Negron. Funding was provided by Chester Zoo, The Rufford Fundation Small Grants, the Goodeid Working Group, the American Livebearer Association and CIC-UMSNH. We sincerely acknowledge Tom Jameson of the University of Cambridge for reviewing the English of the manuscript. We also thank the anonymous reviewers for their valuable comments. Arely Ramírez-García receive a CONACYT fellowship grant for PhD studies. Rodrigo Moncayo-Estrada is Instituto Politécnico Nacional COFAA and EDI fellow.
REFERENCES
- Ayala-Ramírez G, Ruiz-Sevilla G, Chacón-Torres A. La Laguna de Zacapu, Michoacán. In: Lanza G, editor. Las aguas interiores de México: Conceptos y casos. Distrito Federal: AGT Editor; 2007. p.268–84.
- Balon EK. Patterns in the evolution of reproductive styles in fishes. In: Potts GW, Wootton RJ, editors. Fish reproduction: Strategies and tactics. New York: Academic Press; 1984. p.35–53.
- Blackburn DG. Evolutionary origins of viviparity in fishes. In: Uribe MC, Grier H, editors: Viviparity in fishes. Homestead: New Life Publications; 2005. p.303–17.
- Caddy JF, Agnew DJ. An overview of recent global experience with recovery plans for depleted marine resources and suggested guidelines for recovery planning. Rev Fish Biol Fish. 2004; 14(43):43–112. https://doi.org/10.1007/s11160-004-3770-2
» https://doi.org/10.1007/s11160-004-3770-2 - Clutton-Brock TH, Parker GA. Sexual coercion in animal societies. Anim Behav. 1995; 49(5):1345–65. https://doi.org/10.1006/anbe.1995.0166
» https://doi.org/10.1006/anbe.1995.0166 - Cruz-Gómez A, Rodríguez-Varela AC, Vázquez-López H. Reproductive aspects of Girardinichthys multiradiatus, Meek 1904 (Pisces: Goodeidae). Biocyt. 2011; 4(13–16):215–28. https://doi.org/10.22201/fesi.20072082.2011.4.75942
» https://doi.org/10.22201/fesi.20072082.2011.4.75942 - Cruz-Gómez A, Rodríguez-Varela AC, Vázquez-López H. Reproductive aspects of yellow fish Girardinichthys multiradiatus (Meek, 1904) (Pisces: Goodeidae) in the Huapango Reservoir, State of México, México. Am J Life Sci. 2013; 1(5):189–94. https://doi.org/10.11648/j.ajls.20130105.11
» https://doi.org/10.11648/j.ajls.20130105.11 - Cudmore B, Jones LA, Mandrak NE, Dettmers JM, Chapman DC, Kolar CS, Conover G. Ecological risk assessment of grass carp (Ctenopharyngodon idella) in the Great Lakes Basin. Ottawa: Fisheries and Oceans Canada, Canada Science Advisory Secretariat; 2017.
- Dinno A. Dunn’s Test of Multiple Comparisons Using Rank Sums [Internet]. CRAN R-Project; 2017. Available from: http://cran.stat.unipd.it/web/packages/dunn.test/dunn.test.pdf
» http://cran.stat.unipd.it/web/packages/dunn.test/dunn.test.pdf - Domínguez-Domínguez O, Zambrano L, Escalera-Vázquez LH, Pérez-Rodríguez R, Pérez-Ponce de León G. Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en la cuencas hidrológicas del centro de Mexico. Rev Mex Biodiv. 2008; 79(2):501–12. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023
» http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-34532008000200023 - Fairbairn DJ. Factors influencing sexual size dimorphism in temperate waterstriders. Am Nat. 1990; 136(1):61–86. https://doi.org/10.1086/285082
» https://doi.org/10.1086/285082 - Froese R. Cube law, condition factor and weight–length relationships: History, meta- analysis and recommendations. J Appl Ichthyol. 2006; 22(4):241–53. https://doi.org/10.1111/j.1439-0426.2006.00805.x
» https://doi.org/10.1111/j.1439-0426.2006.00805.x - Gibson-Reinemer DK, Chick JH, VanMiddlesworth TD, VanMiddlesworth M, Casper AF. Widerspread and enduring demographic collapse of invasive common carp (Cyprinus carpio) in the Upper Mississippi River System. Biol Invasions. 2017; 19:1905–16. https://doi.org/10.1007/s10530-017-1405-5
» https://doi.org/10.1007/s10530-017-1405-5 - Gómez-Márquez JL, Guzmán-Santiago JL, Olvera-Soto A. Reproducción y crecimiento de Heterandria bimaculata (Cyprinodontiformes: Poeciliidae) en la Laguna “El Rodeo”, Morelos, México. Rev Biol Trop. 1999; 47(3):581–92.
- Gómez-Márquez JL, Mendoza BP, Guzmán-Santiago JL. Occurrence of the fish Girardinichthtys viviparus (Cyprinodontiformes: Goodeidae) in an urban lake at Mexico City. UNED Res J. 2013; 5(1):89–95. https://doi.org/10.1007/s10641-006-0039-8
» https://doi.org/10.1007/s10641-006-0039-8 - Gómez-Márquez JL, Peña-Mendoza B, Guzmán-Santiago JL. Reproductive biology of Poecilia sphenops Valenciennes, 1846 (Cyprinidontiformes: Poeciliidae) at the Emiliano Zapata Reservoir in Morelos. Mexico. Neotrop Ichthyol. 2016; 14(2):e140127. https://doi.org/10.1590/1982-0224-20140127
» https://doi.org/10.1590/1982-0224-20140127 - Gómez-Márquez JL, Peña-Mendoza B, Salgado-Ugarte IH, Sánchez-Herrera AK, Sastré-Baez L. Reproduction of the fish Poeciliopsis gracilis (Cyprinodontiformes: Poeciliidae) in Coatetelco, a tropical shallow lake in Mexico. Rev Biol Trop. 2008; 56(4):1801–12. https://doi.org/10.15517/rbt.v56i4.5760
» https://doi.org/10.15517/rbt.v56i4.5760 - Harrell FE. Harrell Miscellaneous, Packeges ‘Hmisc’ [Internet]. CRAN R-Project; 2021. Available from: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf
» https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf - Hernández-Morales R, Medina-Nava M, Tafolla-Venegas D, Herrerías-Diego Y, Escalante-Jiménez L, Escalera-Vázquez LH, Hernández-Valencia F, Domínguez-Domínguez O. Reintroducción de Zoogoneticus tequila en los manantiales de Teuchitlán, Jalisco: primera fase [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, Informe final SNIB-CONABIO, Proyecto No. NE002; 2020. Available from: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfNE002.pdf
» http://www.conabio.gob.mx/institucion/proyectos/resultados/InfNE002.pdf - Herrerías-Diego Y, Domínguez-Domínguez O, Medina-Nava M, Ávila O, Mar-Silva V. Comparación de la composición y abundancia de la comunidad íctica del río Teuchitlán, Jalisco, México, empleando tres artes de pesca. In: Ornelas-García CP, Álvarez F, Wegier A, editors. Antropización: Primer Análisis Integral. Ciudad de Mexico: CONACYT-IBUNAM; 2018. p.265–82. https://doi.org/10.22201/ib.9786073020305e.2019.c14
» https://doi.org/10.22201/ib.9786073020305e.2019.c14 - Holden MJ, Raitt DFS. Manual de ciencia pesquera Parte 2 - Métodos para investigar los recursos y su aplicación. Roma: FAO; 1975.
- Hollenberg F, Wourms JP. Ultrastructure and protein uptake of the embryonic trophotaeniae of four species of goodeid fish (Teleostei: Atheriniformes). J Morphol. 1994; 219(2):105–29. https://doi.org/10.1002/jmor.1052190202
» https://doi.org/10.1002/jmor.1052190202 - Hollenberg F, Wourms JP. Embryonic growth and maternal nutrient sources in goodeid fishes (Teleostei: Cyprinodontiformes). J Exp Zool. 1995; 271(5):379–94. https://doi.org/10.1002/jez.1402710508
» https://doi.org/10.1002/jez.1402710508 - Hutchings JA, Gerber L. Sex-biased dispersal in a salmonid fish. Proc R Soc Lond B Biol Sci. 2002; 269(1508):2487–93. https://doi.org/10.1098/rspb.2002.2176
» https://doi.org/10.1098/rspb.2002.2176 - International Union for Conservation of the Nature (IUCN). The IUCN Red List of Threatened Species; 2020. Available from: https://www.iucnredlist.org/
» https://www.iucnredlist.org/ - Kahn AT, Kokko H, Jennions MD. Adaptive sex allocation in anticipation of changes in offspring mating opportunities. Nat Commun. 2013; 4(1603):1–07. https://doi.org/10.1038/ncomms2634
» https://doi.org/10.1038/ncomms2634 - Korkmaz S, Goksuluk D, Zararsiz G. MVN: An R package for assessing multivariate normality. R J. 2014; 6(2):151–62.
- Lombardi J, Wourms J. Embryonic growth and trophotaenial development in goodeid fishes (Teleostei: Atheriniformes). J Morphol. 1988; 197(2):193–208. https://doi.org/10.1002/jmor.1051970206
» https://doi.org/10.1002/jmor.1051970206 - Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species database. Auckland: The Invasive Species Specialist Group (ISSG); 2000.
- Lowerre-Barbieri S, Crabtree L, Switzer T, Burnsed SW, Guenther C. Assessing reproductive resilience: an example with South Atlantic red snapper Lutjanus campechanus Mar Ecol Prog Ser. 2015; 526:125–41. https://doi.org/10.3354/meps11212
» https://doi.org/10.3354/meps11212 - Lyons J, Piller KP, Artigas-Azas JM, Domínguez-Domínguez O, Gesundheit P, Köck M, Medina-Nava M, Mercado-Silva N, Ramírez-García A, Findley KM. Distribution and current conservation status of the Mexican Goodeidae (Actinopterygii, Cyprinodontiformes). Zookey. 2019; 885:115–58. https://doi.org/10.3897/zookeys.885.38152
» https://doi.org/10.3897/zookeys.885.38152 - Macías-García C, Ramírez E. Evidence that sensory traps can involve into honest signals. Nature. 2005; 434:501–05. https://doi.org/10.1038/nature03363
» https://doi.org/10.1038/nature03363 - Macías-García C, Saborío E, Berea C. Does male-biased predation lead to male scarcity in viviparous fish? J Fish Biol. 1998; 53(sA):104–17. https://doi.org/10.1111/j.1095-8649.1998.tb01021.x
» https://doi.org/10.1111/j.1095-8649.1998.tb01021.x - Macías-García CM, Jiménez G, Contreras B. Correlational evidence of a sexually-selected handicap. Behav Ecol Sociobiol. 1994; 35(4):253–59. https://doi.org/10.1007/BF00170706
» https://doi.org/10.1007/BF00170706 - Macías-García C, Valero A. Sexual conflict and sexual selection in the Goodeinae, a clade of viviparous fish with effective female mate choice. In Macedo R, editor. Behavioral ecology of tropical animals. Advances in the study of behavior, vol. 42. Massachusetts: Academic Press; 2010. p.1–54. https://doi.org/10.1016/S0065-3454(10)42001-X
» https://doi.org/10.1016/S0065-3454(10)42001-X - Magalhães ALB, Jacobi CM. Colorful invasion in permissive Neotropical ecosystems: Establishment of ornamental non-native poeciliids of the genera Poecilia/Xiphophorus (Cyprinodontiformes: Poeciliidae) and management alternatives. Neotrop Ichthyol. 2017; 15(1):e160094. https://doi.org/10.1590/1982-0224-20160094
» https://doi.org/10.1590/1982-0224-20160094 - Magurran AE, Macías-García C. Sex differences in behavior as an indirect consequence of mating system. J Fish Biol. 2000; 57(4):839–57. https://doi.org/10.1111/j.1095-8649.2000.tb02196.x
» https://doi.org/10.1111/j.1095-8649.2000.tb02196.x - Mar-Silva V, Herrerías-Diego Y, Medina-Nava M, Ramírez-Herrejón JP, Mendoza-Cuenca LF, Hernández-Morales R, Domínguez-Domínguez O. Spatial and temporal variation of fish assemblage structure in a Neotropical Mexican River. Rev Mex Biodivers. 2021; 92:e923433. http://dx.doi.org/10.22201/ib.20078706e.2021.92.3433
» http://dx.doi.org/10.22201/ib.20078706e.2021.92.3433 - Martínez-Aquino A, Pérez-Rodríguez R, Hernández-Mena DI, Garrido-Olvera L, Aguilar-Aguilar R, Pérez-Ponce de León G. Endohelminth parasites of seven goodein species (Cyprinodontiformes: Goodeidae) from Lake Zacapu, Michoacán, Central Mexico Plateau. Hidrobiologica. 2012; 22(1):89–93.
- Méndez-Janovitz M, Macías-García C. Do male fish prefer them big and colourful? Non-random male courtship effort in a viviparous fish with negligible paternal investment. Behav Ecol Sociobiol. 2017; 71(160):1–12. https://doi.org/10.1007/s00265-017-2385-2
» https://doi.org/10.1007/s00265-017-2385-2 - Mendoza G. The reproductive cycles of three viviparous teleosts Alloophorus robustus, Goodea luitpoldi and Neoophorus diazi Biol Bull. 1962; 123(2):351–65. https://doi.org/10.2307/1539280
» https://doi.org/10.2307/1539280 - Mims MC, Olden JD. Life history theory predicts fish assemblage response to hydrologic regimes. Ecology. 2012; 93(1):35–45. https://doi.org/10.1890/11-0370.1
» https://doi.org/10.1890/11-0370.1 - Moncayo-Estrada R. Estructura y función de la comunidad de peces de la Laguna de Zacapu, Michoacán, México. [Master Thesis]. Zacapu: Instituto Politécnico Nacional; 1996. Available from: https://www.repositoriodigital.ipn.mx/handle/123456789/15234
» https://www.repositoriodigital.ipn.mx/handle/123456789/15234 - Moncayo-Estrada R. Análisis histórico de la biología de la cherehuita (Hubbsina turneri) (Pisces: Goodeidae), especie endémica y en peligro de extinción de México. Rev Chapingo Ser Cienc For Ambiente. 2012; 18(1):101–10. https://doi.org/10.5154/r.rchscfa.2011.02.020
» https://doi.org/10.5154/r.rchscfa.2011.02.020 - Moyaho A, Macías-García C, Manjarrez J. Predation risk is associated with the geographic variation of a sexually selected trait in a viviparous fish, Xenotoca variata J Zool. 2004; 262(3):265–70. https://doi.org/10.1017/S095283690300459X
» https://doi.org/10.1017/S095283690300459X - Murua H, Saborido-Rey F. Female reproductive strategies of marine fish species of the North Atlantic. J Northwest Atl Fish Sci. 2003; 33:23–31.
- Navarrete-Salgado NA, Cedillo-Díaz BE, Contreras-Rivero G, Elías-Fernández G. Crecimiento, reproducción y supervivencia de Girardinichthys multiradiatus (Pisces: Goodeidae) en el embalse San Miguel Arco, Estado de México. Rev Chapingo Ser Cienc For Ambiente. 2007; 13(1):15–21.
- Nelson JS, Grande TC, Wilson MVH. Fishes of the world. New York: John Wiley & Sons; 2016. https://doi.org/10.1002/9781119174844
» https://doi.org/10.1002/9781119174844 - Nikolsky GV. The ecology of fishes. London: Academic Press; 1963.
- NORMA Oficial Mexicana (NOM-059-SEMARNAT-2010). Lista de especies en riesgo de la Norma Oficial Mexicana. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo [Internet]. Diário Oficial de la Federación; 2019. Available from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019
» https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019 - Pecquerie L, Petitgas P, Kooijman SALM. Modeling fish growth and reproduction in the context of the dynamic energy budget theory to predict environmental impact on anchovy spawning duration. J Sea Res. 2009; 62(2–3):93–105. https://doi.org/10.1016/j.seares.2009.06.002
» https://doi.org/10.1016/j.seares.2009.06.002 - Pérez-Rodríguez R, Domínguez-Domínguez O, Doadrio I, Cuevas-García E, Pérez-Ponce de León G. Comparative historical biogeography of three groups of Nearctic freshwater fishes across central Mexico. J Fish Biol. 2015; 86(3):993–1015. https://doi.org/10.1111/jfb.12611
» https://doi.org/10.1111/jfb.12611 - R Development Core Team. R: a language and environment for statistical computing [Internet]. Vienna: R foundation for statistical computing; 2021. Available from: https://www.r-project.org/
» https://www.r-project.org/ - Ramírez-García A, Domínguez-Domínguez O. Zacapu Lake, a hot spot of native fishes. American Currents. 2019; 44(3):8–10. Available from: http://www.nanfa.org/ac/zacapu-lake-mexico.pdf
» http://www.nanfa.org/ac/zacapu-lake-mexico.pdf - Ramírez-García A, Piller K, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of three native livebearer fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in the Teuchitlán River, Mexico. Acta Ichthyol Piscat. 2020; 50(1):1–12. http://dx.doi.org/10.3750%2FAIEP%2F02513
» http://dx.doi.org/10.3750%2FAIEP%2F02513 - Ramírez-García A, Ramírez-Herrejón JP, Medina-Nava M, Hernández-Morales R, Domínguez-Domínguez O. Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. J Appl Ichthyol. 2018; 34(1):81–90. https://doi.org/10.1111/jai.13543
» https://doi.org/10.1111/jai.13543 - Ramírez-Herrejón JP. Análisis temporal de la calidad ambiental de los ecosistemas acuáticos en la subcuenca del río Angulo, cuenca Lerma-Chapala. [Master Thesis]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo; 2008.
- Ramírez-Herrejón JP, Medina-Nava M, Salazar-Tinoco CI, Zubieta TLE. Algunos aspectos reproductivos de Zoogoneticus quitzeoensis Hubbs y Turner (1939) (Osteichtyes-Goodeidae) en la represa La Mintzita Morelia, Michoacán, México. Biológicas. 2007; 9:63–71.
- Ramírez-Herrejón JP, Mercado-Silva N, Medina-Nava M, Domínguez-Domínguez O. Validación de dos índices biológicos de integridad (IBI) en la subcuenca del río Angulo en el centro de México. Rev Biol Trop. 2012; 60(4):1669–85. https://doi.org/10.15517/rbt.v60i4.2160
» https://doi.org/10.15517/rbt.v60i4.2160 - Ramírez-Herrejón JP, Zambrano L, Mercado-Silva N, Torres-Téllez A, Pineda-García F, Caraveo-Patiño J, Balart EF. Long term changes in the fish fauna of Lago de Pátzcuaro in Central México. Lat Am J Aquat Res, 2014; 42(1):137–49.
- Reznick DN, Callahan H, Llauredo R. Maternal effects on offspring quality in Poeciliid fishes. Am Zool. 1996; 36(2):147–56. https://doi.org/10.1093/icb/36.2.147
» https://doi.org/10.1093/icb/36.2.147 - Ritchie MG, Hamill RM, Graves JA, Magurran AE, Webb SA, Macías-García C. Sex and differentiation: Population genetic divergence and sexual dimorphism in Mexican goodeid fish. J Evol Biol. 2007; 20(5):2048–55. https://doi.org/10.1111/j.1420-9101.2007.01357.x
» https://doi.org/10.1111/j.1420-9101.2007.01357.x - Rodd FH, Reznick DN. Variation in the demography of guppy populations: The importance of predation and life histories. Ecology. 1997; 78(2):405–18. https://doi.org/10.2307/2266017
» https://doi.org/10.2307/2266017 - Schoenherr AA. Density dependent and density independent regulation of reproduction in the Gila Topminnow, Poeciliopsis occidentalis (Baird and Girard). Ecology. 1977; 58(2):438–44. https://doi.org/10.2307/1935619
» https://doi.org/10.2307/1935619 - Silva-Santos JR, Martínez-Saldaña MC, Rico-Martínez R, Gómez-Márquez JL, Arredondo-Figueroa JL. Reproductive biology of Goodea atripinnis (Jordan, 1880) (Cyprinodontiformes: Goodeidae) under controlled conditions. J Exp Biol Agric Sci. 2016; 4(2):180–93.
- Snelson FF Jr. Social and environmental control of life history traits in poeciliid fishes. In: Meffe GK, Snelson FF Jr, editors. Ecology and evolution of livebearing fishes (Poeciliidae). New Jersey: Prentice Hall; 1989. p.149–61.
- Sparre P, Venema SC. Introducción a la evaluación de recursos pesqueros tropicales. Roma: FAO; 1997.
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992.
- Sturges HA. The choice of a class interval. J Am Stat Assoc. 1926; 21(153):65–66. https://doi.org/10.1080/01621459.1926.10502161
» https://doi.org/10.1080/01621459.1926.10502161 - Tobler M, Culumber Z. Ecology and diversification of reproductive strategies in viviparous fishes. bioRxiv. 2018; 442830. https://doi.org/10.1101/442830
» https://doi.org/10.1101/442830 - Torrejon-Magallanes J Package ‘sizeMat’ Estimate Size at Sexual Maturity [Internet]. CRAN R-Project; 2020. Available from: https://cran.r-project.org/web/packages/sizeMat/sizeMat.pdf
» https://cran.r-project.org/web/packages/sizeMat/sizeMat.pdf - Trexler JC, DeAngelis DL. Resource allocation in offspring provisioning: An evaluation of the conditions favoring the evolution of matrotrophy. Am Nat. 2003; 162(5):574–85. https://doi.org/10.1086/378822
» https://doi.org/10.1086/378822 - Turner CL, Mendoza G, Reiter R. Development and comparative morphology of the gonopodium of goodeid fishes. Proc Iowa Acad Sci. 1962; 69(1):571–86. Available from: https://scholarworks.uni.edu/pias/vol69/iss1/87
» https://scholarworks.uni.edu/pias/vol69/iss1/87 - Uribe MC, Rosa-Cruz G, García-Alarcón A. Branchial placenta in the viviparous teleost Ilyodon whitei (Goodeidae). J Morphol. 2014; 275(12):1406–17. https://doi.org/10.1002/jmor.20315
» https://doi.org/10.1002/jmor.20315 - Uribe MC, Grier HJ, Ávila-Zúñiga SA, García-Alarcón A. Change of lecithotrophic to matrotrophic nutrition during gestation in the viviparous teleost Xenotoca eiseni (Goodeidae). J Morphol. 2018; 279(9):1336–45. https://doi.org/10.1002/jmor.20874
» https://doi.org/10.1002/jmor.20874 - Valencia-Vargas R, Escalera-Vázquez LH. Abundancia de la salamandra Ambystoma andersoni con relación a la dinámica estacional y heterogeneidad espacial en el lago de Zacapu, Michoacán, México. Rev Mex Biodivers. 2021; e923283. https://doi.org/10.22201/ib.20078706e.2021.92.3283
» https://doi.org/10.22201/ib.20078706e.2021.92.3283 - Wootton RJ. Ecology of Teleost Fishes. Dordrecht: Kluwer Academic Publisher; 1998.
- Wootton R, Smith C. Reproductive Biology of Teleost Fishes. Hoboken: John Wiley & Sons Ltd; 2014.
- Wourms JP. Viviparity: The maternal-fetal relationship in fishes. Am Zool. 1981; 21:473–515.
- Wourms JP. Functional morphology, development, and evolution of trophotaeniae. In: Uribe MC, Grier HJ, editors. Viviparous fishes. Homestead: New Life Publications; 2005. p.237–62.
- Zeyl JN, Love OP, Higgs DM. Evaluating gonadosomatic index as an estimator of reproductive condition in the invasive round goby, Neogobius melanostomus. J Great Lakes Res. 2014; 40(1):164–71. https://doi.org/10.1016/j.jglr.2013.12.004
» https://doi.org/10.1016/j.jglr.2013.12.004 - Zubieta-Rojas T, Alvarado-Villanueva R, Ortega-Murillo MR, Medina-Nava M, Sánchez-Heredia JD. Plan de Manejo del área natural protegida “Laguna de Zacapu y su ribera” [Internet]. Morelia: Universidad Michoacana de San Nicolás de Hidalgo, y Secretaria de Urbanismo y Medio Ambiente (SUMA); 2005. Available from: https://vdocumento.com/universidad-michoacana-de-habitantes-de-las-colonias-colindantes-con-la-laguna-y.html
» https://vdocumento.com/universidad-michoacana-de-habitantes-de-las-colonias-colindantes-con-la-laguna-y.html
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Ramírez-García A, Moncayo-Estrada R, González-Cárdenas JJ, Domínguez-Domínguez O. Reproductive cycle of native viviparous fish species (Actinopterygii: Cyprinodontiformes: Goodeidae) in a subtropical Mexican lake. Neotrop Ichthyol. 2021; 19(4):e210105. https://doi.org/10.1590/1982-0224-2021-0105
Edited-by
Publication Dates
-
Publication in this collection
01 Dec 2021 -
Date of issue
2021
History
-
Received
16 June 2021 -
Accepted
20 Sept 2021