Abstract
Despite Amazonia possessing the highest freshwater biodiversity on Earth, urban landing data show how huge fishing pressure is placed on only a dozen species. However, truly characterising the fishery and understanding the drivers of species selectivity is challenging, given the neglect of artisanal fishing activity, who may catch most of the Amazon’s fish. We register the catch of 824 fishing trips by interviewing artisanal fishers in their rural riverside communities. We use these data to characterise the artisanal fishery of the Rio Purus, the main fish source sub-system for the Amazon’s largest city (Manaus), and investigate the factors determining catch composition. Fishers caught 80 fish species, yet just four species made up over half of the harvested biomass. Urban markets appear to drive greater selectivity, with a significantly lower species diversity in commercial compared to subsistence catches. Fish catch composition varied significantly both seasonally and with geographical remoteness from Manaus. The spatial turnover in catch composition appears to be driven by urban access, with more commercially important species dominating where Manaus-based fish-buyers frequent. Our data may partially explain observed overfishing in some commercially important species, particularly as most Amazonians now live in urban areas.
Keywords:
Flood pulse; Overfishing; Species diversity; Urbanization
Resumo
Apesar da Amazônia possuir a maior biodiversidade de água doce do mundo, dados de desembarques urbanos mostram como a grande pressão da pesca é focada em apenas uma dúzia de espécies. Entretanto, caracterizar a pesca e entender as causas da seletividade das espécies é desafiador pela pesca artesanal, que pode capturar a maioria dos peixes da Amazônia, ser negligenciada. Registramos as capturas de 824 pescarias entrevistando pescadores artesanais em suas comunidades ribeirinhas. Usamos esses dados para caracterizar a pesca artesanal do rio Purus, o principal subsistema de origem do pescado para a maior cidade da Amazônia (Manaus), e investigamos os fatores que determinam a composição das capturas. Os pescadores capturaram 80 espécies de peixes, e apenas quatro representaram mais da metade da biomassa. Os mercados urbanos parecem direcionar maior seletividade, com diversidade de espécies significativamente menor nas capturas comerciais que nas de subsistência. A composição do pescado variou significativamente tanto sazonalmente quanto com o afastamento geográfico de Manaus. A substituição espacial na composição das capturas parece ser direcionada pelo acesso urbano, com espécies comercialmente importantes dominando onde os compradores de peixes de Manaus frequentam. Nossos dados podem explicar parcialmente a sobrepesca observada de algumas espécies comercialmente importantes, particularmente porque a maioria da população da Amazônia vive atualmente em áreas urbanas.
Palavras-chave:
Diversidade de espécies; Pulso de inundação; Sobrepesca; Urbanização
INTRODUCTION
Millions of Amazonians rely on fishing for their livelihood and food security, with fish constituting the main source of protein (Dufour et al., 2016Dufour DL, Piperata BA, Murrieta RSS, Wilson WM, Williams DD. Amazonian foods and implications for human biology. Ann Hum Biol. 2016; 43(4):330–48. https://doi.org/10.1080/03014460.2016.1196245
https://doi.org/10.1080/03014460.2016.11...
) and earned income (Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
) for much of the rural population. With over 2,400 native freshwater fish species already registered and an estimated 1,000 left to discover or describe scientifically, the Amazon Basin contains the highest freshwater biodiversity on Earth (Jézéquel et al., 2020Jézéquel C, Tedesco PA, Bigorne R, Maldonado-Ocampo JA, Ortega H, Hidalgo M et al. A database of freshwater fish species of the Amazon Basin. Sci Data. 2020; 7(96):1–09. https://doi.org/10.1038/s41597-020-0436-4
https://doi.org/10.1038/s41597-020-0436-...
). Despite this, fishing is highly selective (Hallwass, Silvano, 2016Hallwass G, Silvano RAM. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. J Environ Plan Manag. 2016; 59(9):1537–59. https://doi.org/10.1080/09640568.2015.1081587
https://doi.org/10.1080/09640568.2015.10...
), and it is estimated that around 100 fish species are commercially exploited in the Central Amazon, and that around 90% of the catch is made up of just a dozen species (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.). This puts severe pressures on these selected species, and there is evidence that this selective overharvesting has led to some of them becoming overfished or endangered (Castello et al., 2013Castello L, McGrath DG, Hess LL, Coe MT, Lefebvre PA, Petry P et al. The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217–29. https://doi.org/10.1111/conl.12008
https://doi.org/10.1111/conl.12008...
).
Amazonians are among the world’s greatest fish consumers (per capita), and although this figure is higher among rural-dwellers (Isaac, Almeida, 2011Isaac VJ, Almeida MC. El Consumo del pescado en la Amazonía brasileña. Rome: 2011. Available from: http://www.fao.org/3/i2408s/i2408s.pdf
http://www.fao.org/3/i2408s/i2408s.pdf...
; Isaac et al., 2015Isaac VJ, Almeida MC, Giarrizzo T, Deus CP, Vale R, Klein G et al. Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An Acad Bras Cienc. 2015; 87(4):2229–42. https://doi.org/10.1590/0001-3765201520140250
https://doi.org/10.1590/0001-37652015201...
), consumption remains globally high in towns and cities where over three-quarters of the region’s population now lives (IBGE, 2010IBGE. Censo Demográfico Rio de Janeiro 2010 [Internet]. Rio de Janeiro; 2010. Available from: https://cidades.ibge.gov.br/brasil/rj/rio-de-janeiro/panorama
https://cidades.ibge.gov.br/brasil/rj/ri...
). Yet most of the fish landed there are thought to be caught by rural artisanal fishers, and subsequently purchased by fish-buyers who sell to urban markets (Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.). The rural catch is partly dictated by these fish buyers, and hence the urban market demand and access, which is highly selective for certain species and often larger body-sizes. This urban demand has been blamed for the overfishing, and therefore the decline, of fish stocks, including large-bodied species near to urban areas (Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
; Keppeler et al., 2018Keppeler FW, Souza AC, Hallwass G, Almeida MC, Azevedo R, Silvano M et al. Ecological influences of human population size and distance to urban centres on fish communities in tropical lakes. Aquat Conserv. 2018; 28(5):1030–43. https://doi.org/10.1002/aqc.2910
https://doi.org/10.1002/aqc.2910...
).
Current understanding of Amazonia’s fisheries comes largely from urban landing data (Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.). The remoteness of artisanal fishers and their “invisibility” to wider society (Pontual, 2014Pontual FB. The last move of the ribeirinho: Indigenous sovereignty and servitude in the Middle and Lower Rio Negro Basin, Brazilian Amazonia. [PhD Thesis]. Berkeley: University of California; 2014. Available from: https://www.proquest.com/openview/a273ee6fe262f31d9daf644b1906daae/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/a273ee...
) has resulted in a paucity of artisanal fishing data and studies, thereby excluding many catches used for subsistence and local sale from official estimates, even though they could account for the majority of the region’s overall fish catch (Lopes et al., 2019Lopes PFM, Hallwass G, Begossi A, Isaac VJ, Almeida M, Silvano RAM. The challenge of managing Amazonian small-scale fisheries in Brazil. In: Salas S, Barragán-Paladines M, Chuenpagdee R, editors. Viability and sustainability of small-scale fisheries in Latin America and the Caribbean. MARE Publication Series, vol 19. Cham: Springer; 2019. p.219–41. https://doi.org/10.1007/978-3-319-76078-0_10
https://doi.org/10.1007/978-3-319-76078-...
). Where artisanal fish catch has been studied, previous work shows the predominance of a limited number of species, and significant seasonal differences (Batista et al., 1998Batista VDS, Inhamuns AJ, Freitas CEDC, Freire-Brasil D. Characterization of the fishery in the river communities in the low-Solimões/high-Amazon region. Fish Manag Ecol. 1998; 5(5):419–35. https://doi.org/10.1046/j.1365-2400.1998.550419.x
https://doi.org/10.1046/j.1365-2400.1998...
; Isaac, 2000Isaac VJ. Fish catches among riverside communities around Lago Grande de Monte Alegre, Lower Amazon, Brazil. Fish Manag Ecol. 2000; 7:355–74.). However, while market access is a well-established major determinant of fishing activities in marine systems (e.g., Brewer et al., 2012Brewer TD, Cinner JE, Fisher R, Green A, Wilson SK. Market access, population density, and socioeconomic development explain diversity and functional group biomass of coral reef fish assemblages. Glob Environ Chang. 2012; 22(2):399–406. https://doi.org/10.1016/j.gloenvcha.2012.01.006
https://doi.org/10.1016/j.gloenvcha.2012...
), studies in an Amazonian context are yet to disentangle market access from other ecological and social determinants of fish catch. As most fish is thought to be caught by remote fishing communities (Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.) with limited refrigeration capacity and connected only by rivers (Parry et al., 2018Parry L, Davies G, Almeida O, Frausin G, Moraés A, Rivero S et al. Social vulnerability to climatic shocks is shaped by urban accessibility. Ann Am Assoc Geogr. 2018; 108(1):125–43. https://doi.org/10.1080/24694452.2017.1325726
https://doi.org/10.1080/24694452.2017.13...
), a community’s ability to sell their fish to the urban market can be strongly determined by their ability to preserve (often in ice) and transport (by boat) their catch along rivers to urban centres.
In this study we characterise the artisanal fishery of the lowest ~1,300 km section of the Rio Purus, which is the main fishing river to the Amazon’s largest city (Manaus), through detailed interviews with fishers in their communities. In doing so we ask, what drives the variation in catch composition and diversity? We do so by investigating the factors that determine dissimilarities in species catch composition, and how species diversity varies between subsistence and commercial catches. We hypothesise that catch composition will be determined by distance to urban market centres (Brewer et al., 2013Brewer TD, Cinner JE, Green A, Pressey RL. Effects of human population density and proximity to markets on coral reef fishes vulnerable to extinction by fishing. Conserv Biol. 2013; 27(3):443–52. https://doi.org/10.1111/j.1523-1739.2012.01963.x
https://doi.org/10.1111/j.1523-1739.2012...
), but also by hydrological seasons (Isaac et al., 2016Isaac VJ, Castello L, Santos PRB, Ruffino ML. Seasonal and interannual dynamics of river-floodplain multispecies fisheries in relation to flood pulses in the Lower Amazon. Fish Res. 2016; 183:352–59. https://doi.org/10.1016/j.fishres.2016.06.017
https://doi.org/10.1016/j.fishres.2016.0...
), flooded forest cover (Arantes et al., 2018Arantes CC, Winemiller KO, Petrere M, Castello L, Hess LL, Freitas CEC. Relationships between forest cover and fish diversity in the Amazon River floodplain. J Appl Ecol. 2018; 55(1):386–95. https://doi.org/10.1111/1365-2664.12967
https://doi.org/10.1111/1365-2664.12967...
, 2019Arantes CC, Winemiller KO, Asher A, Castello L, Hess LL, Petrere M et al. Floodplain land cover affects biomass distribution of fish functional diversity in the Amazon River. Sci Rep. 2019; 9(16684):1–13. https://doi.org/10.1038/s41598-019-52243-0
https://doi.org/10.1038/s41598-019-52243...
), and population density (Brewer et al., 2013Brewer TD, Cinner JE, Green A, Pressey RL. Effects of human population density and proximity to markets on coral reef fishes vulnerable to extinction by fishing. Conserv Biol. 2013; 27(3):443–52. https://doi.org/10.1111/j.1523-1739.2012.01963.x
https://doi.org/10.1111/j.1523-1739.2012...
).
In our study system of remote roadless rural communities, access to the Manaus market is largely determined by Manaus-based fish-buyer boats, who visit the communities nearer to Manaus (< 600 km fluvial travel distance) regularly to deposit ice (normally the only refrigeration) and purchase fish. Hence to investigate market access we investigate how the fish assemblages caught varies with distance from Manaus (and other towns), and then investigate the species that dominate in those communities with better and worse market access (that receive regular visits from fish-buyer boats and those that do not). We predict a domination of more commercially valuable species, such as Colossoma macropomum (Cuvier, 1816) and Arapaima gigas (Schinz, 1822), in those communities with better access to the city’s market. We then compare the diversity of catches intended for consumption and for sale, predicting a lower diversity in commercial catches.
MATERIAL AND METHODS
Study area. This in situ study of artisanal fishing activity was carried out in rural riverside communities along the mid-lower Rio Purus in the Brazilian Amazon (Fig. 1). Communities were situated in and around várzea forest, which is a floodplain forest seasonally inundated by whitewater rivers. The Rio Purus supplies more fish to the Amazon’s largest city, Manaus (population 2.2 million people; IBGE, 2020IBGE. Censo Demográfico Manaus 2020 [Internet]. Manaus; 2020. Available from: https://cidades.ibge.gov.br/brasil/am/manaus/panorama
https://cidades.ibge.gov.br/brasil/am/ma...
) than any other Amazonian sub-system (Batista, Petrere Júnior, 2003Batista VDS, Petrere Júnior M. Characterization of the commercial fish production landed at Manaus, Amazonas State, Brazil. Acta Amaz. 2003; 33(1):53–66. https://doi.org/10.1590/1809-4392200331066
https://doi.org/10.1590/1809-43922003310...
; Cardoso et al., 2004Cardoso RS, Batista VDS, Henry C, Júnior F, Martins WR. Aspectos econômicos e operacionais das viagens da frota pesqueira de Manaus, Amazônia Central. Acta Amaz. 2004; 34(2):301–07. https://doi.org/10.1590/S0044-59672004000200016
https://doi.org/10.1590/S0044-5967200400...
). Demand from Manaus has been attributed to causing overfishing of the commercially important Colossoma macropomum in the river (Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
). However, apart from overfishing, the mid-lower Purus does not suffer significantly from the other major threats to Amazonian freshwater degradation: deforestation, pollution and dam construction (Castello et al., 2013Castello L, McGrath DG, Hess LL, Coe MT, Lefebvre PA, Petry P et al. The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217–29. https://doi.org/10.1111/conl.12008
https://doi.org/10.1111/conl.12008...
). The mid-lower Rio Purus catchment has high remaining forest cover, and low human population densities (in Tab. S1). It is one of three Amazonian tributaries with an entirely undammed watershed (Winemiller et al., 2016Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016; 351(6269):128–29. https://doi.org/10.1126/science.aac7082
https://doi.org/10.1126/science.aac7082...
). The Purus therefore presents a unique opportunity to assess fishing activity while minimising the contribution of other major confounding factors that are known to impact fish populations.
The Rio Purus sees some of the highest seasonal amplitudes (~15 m) in river levels in the Amazon Basin (Castello, Macedo, 2016Castello L, Macedo MN. Large-scale degradation of Amazonian freshwater ecosystems. Glob Chang Biol. 2016; 22(3):990–1007. https://doi.org/10.1111/gcb.13173
https://doi.org/10.1111/gcb.13173...
), transforming much of the catchment into flooded forest. The seasonal flood pulse has an enormous impact on aquatic and terrestrial ecology and the activities of the local people in the Amazonian floodplain (Junk et al., 1989Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci. 1989; 106(1):110–27. Available from: https://ftp.cs.ru.nl/toinesmits/Recommended_readings_IWRM_2009/Water_Ecomorphological_principles/1989JunkThe%20flood%20pulse%20concept%20in.pdf
https://ftp.cs.ru.nl/toinesmits/Recommen...
; Endo et al., 2016Endo W, Peres CA, Haugaasen T. Flood pulse dynamics affects exploitation of both aquatic and terrestrial prey by Amazonian floodplain settlements. Biol Conserv. 2016; 201:129–36. https://doi.org/10.1016/j.biocon.2016.07.006
https://doi.org/10.1016/j.biocon.2016.07...
). Data were collected during high-water (April – July 2014) and low-water (August – November 2014) field seasons. This also avoided working during the fishing closed period (Corrêa et al., 2014Corrêa MAA, Kahn JR, Freitas CEDC. Perverse incentives in fishery management: The case of the defeso in the Brazilian Amazon. Ecol Econ. 2014; 106:186–94. https://doi.org/10.1016/j.ecolecon.2014.07.023
https://doi.org/10.1016/j.ecolecon.2014....
), thereby avoiding another level of variation in fishing activity or the reporting of. The timing of our two descents of the Rio Purus were intended to accompany the fluctuating water levels, aiming to visit each sampled community at approximately the peak of both the high and low-water seasons. In order to achieve this, we planned the timing of the journeys on the river-level calendar based on long-term averages (Coe et al., 2002Coe MT, Costa MH, Botta A, Birkett C. Long-term simulations of discharge and floods in the Amazon Basin. J Geophys Res Atmos. 2002; 107(D20):1–17. https://doi.org/10.1029/2001JD000740
https://doi.org/10.1029/2001JD000740...
).
Sampling. We worked in the Amazonas state, downstream of the town of Lábrea and upstream from the confluence with the Rio Solimões, visiting 22 communities. Travelling downstream, our protocol was to stop at the first community we came to that (a) had 10–30 occupied residences, (b) had fishing grounds relatively independent of other sampled communities (mean = 61 km fluvial travel distance between communities, minimum = 13 km), and (c) avoiding communities in the federally designated Abufari Biological Reserve. These communities were spread along a distance gradient of 205–1,483 km fluvial travel distance from Manaus, as calculated using the travel network function in ArcGIS 10.2.2 (ArcMAP, 2014ArcMAP ESRI. 10.2.2 ESRI (Environmental Systems Research Institute). Redlands; 2014.).
We visited a maximum of 20 households per community (mean = 13.2 per community visit). Where a community had more than 20 households, we would ask the village president (or another representative where absent) for the name of the head of each household, which they would then select randomly in a lottery system, aiming to achieve a representative sample of the community. Within each household we interviewed every resident of 16 years of age or older that had been fishing in the past 30 days. Interviews were used to collect data concerning fishing activities, including a recall of all the fish that they had caught in recent days (past 72 h).
Fishing data. All data concerning fishing catch was obtained via interviews with fishers within their communities. Conservation scientists are increasingly utilising interviews with resource-users to recall catches in order to estimate harvest levels and species composition, which has been shown to be similarly accurate and precise to ecological methods (Johnson, Van Densen, 2007Johnson TR, Van Densen WLT. Benefits and organization of cooperative research for fisheries management. ICES J Mar Sci. 2007; 64(4):834–40. https://doi.org/10.1093/icesjms/fsm014
https://doi.org/10.1093/icesjms/fsm014...
; Thurstan et al., 2015Thurstan RH, Buckley SM, Ortiz JC, Pandolfi JM. Setting the record straight: Assessing the reliability of retrospective accounts of change. Conserv Lett. 2015; 9(2):98–105. https://doi.org/10.1111/conl.12184
https://doi.org/10.1111/conl.12184...
). All surveyed fishers were asked in detail about the species catch composition of every fishing trip that they had undertaken in the 72 h prior to the interview. To keep response variables spatially associated with the community’s location, we restricted information to fishing trips that had occurred within 2 h journey by motorised canoe (“rabeta”) from the fisher’s home in the community. Insights from a pilot study indicated that two hours of travel time was a measure to which local people could accurately relate, equating to a standard distance of around 18 km fluvial travel distance from the community (Parry, Peres, 2015Parry L, Peres CA. Evaluating the use of local ecological knowledge to monitor hunted tropical-forest wildlife over large spatial scales. Ecol Soc. 2015; 20(3):15. https://doi.org/10.5751/ES-07601-200315
https://doi.org/10.5751/ES-07601-200315...
). Regarding catch, respondents were asked to identify every fish caught (to species level where possible), and approximately how many individuals of that taxa that had been caught. They were also asked whether the catch from each fishing trip was for consumption, sale, or both.
Our pilot study confirmed previous work (Pinho et al., 2012Pinho PF, Orlove B, Lubell M. Overcoming barriers to collective action in community-based fisheries management in the Amazon. Hum Organ. 2012; 71(1):99–109. https://doi.org/10.17730/humo.71.1.c34057171x0w8g5p
https://doi.org/10.17730/humo.71.1.c3405...
) that states that where fish are sold directly by the fisher per unit weight, the fisher can estimate biomass of these species accurately. In our study system this was the case for larger species (those that commonly weighed > 1 kg), namely the large catfish (Pseudoplatystoma punctifer (Castelnau, 1855), Phractocephalus hemioliopterus (Bloch & Schneider, 1801), Pseudoplatystoma tigrinum (Valenciennes, 1840), Brachyplatystoma filamentosum (Lichtenstein, 1819), and Zungaro zungaro (Humboldt, 1821)), and Arapaima gigas, Colossoma macropomum, Osteoglossum bicirrhosum (Cuvier, 1829), and Piaractus brachypomus (Cuvier, 1818)), which were commonly sold species, and priced per kilogram. In the few cases that these estimates were missing (rarely, fishers felt unable to estimate) a mean of all other estimates of the relevant species’ weight was used. Smaller species (e.g., Mylossoma albiscopum (Cope, 1872) (formerly M. duriventre) were however commonly fished for subsistence or sold per individual, and fishers found estimating biomass of these species more challenging during the pilot study, so we therefore did not rely on fisher biomass estimates for these species, and instead calculated catches using average species weights as landed by Purus fishers.
While maximum species weights can be derived from FishBase (Froese, Pauly, 2015Froese R, Pauly D, editors. FishBase [Internet]. Stockholm, Sweden; 2015. Available from: www.fishbase.org), we wanted to accurately represent average landed species weights. This was challenging to obtain in the field because we required the average weight that was caught and landed by a local fisher as uninfluenced by the researcher, and hence we were unable to weigh fish caught by ourselves, or fish that we asked a fisher to catch for us. We therefore opportunistically weighed 1,515 individual fishes of 42 species (two of which were identified only to genus level) that were caught and landed by local fishers. A mean weight per species was used in analyses for those weighed. However, for certain rarer species (e.g., Acestrorhynchus falcirostris (Cuvier, 1819)) where we lacked locally measured weights, we calculated mean landed weight. We first calculated the maximum species weight by inputting maximum lengths and relevant coefficients from FishBase (Froese, Pauly, 2015Froese R, Pauly D, editors. FishBase [Internet]. Stockholm, Sweden; 2015. Available from: www.fishbase.org) into the fish weight-length equation. We then calculated how much smaller the fish landed by Purus fishers were than maximum sized fish from the literature (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.; Froese, Pauly, 2015Froese R, Pauly D, editors. FishBase [Internet]. Stockholm, Sweden; 2015. Available from: www.fishbase.org), finding that the mean landed fish was 60–89% (reduction factor) of the maximum species weight. Reducing the maximum species weight by the calculated reduction factor of the closest related species possible where FishBase data was available, gave us our estimates of mean landed weights for each species. The overall weight of fish caught in each harvesting trip (past 72 h) was calculated by multiplying the number of individuals caught of a particular fish species by the average tabulated weight (in Tab. S2) for that species.
Statistical analysis. Statistical analyses were undertaken to investigate which factors determine the composition and diversity of artisanal fish catch, and namely test the hypothesis that it is determined by urban market access. As urban market access per se. cannot be measured, we statistically compare two distinct indicators of it. Firstly, we investigated how caught fish assemblages differ between communities of different levels of urban access; a distance based linear model (DistLM) compared species assemblages along a continuous measure of distance to Manaus, while a similarity percentage (SIMPER) analysis specifically compared those communities that do or do not receive regular visits from Manaus-based fish-buyers. Due to the low species richness of individual fishing trips, fish community assemblages were analysed at the level of community (made up of multiple fishers and fishing trips) by season (n = 43). Secondly, we compared the species diversity of individual fishing trips (n = 824) between catches that were for consumption or sale.
DistLMs were used to assess the relationship between a multivariate species resemblance matrix describing similarities between species biomass and predictor variables. DistLM is used in studies that look to explain species compositional differences within ecological communities (e.g., Moore et al., 2010Moore CH, Harvey ES, Van Niel K. The application of predicted habitat models to investigate the spatial ecology of demersal fish assemblages. Mar Biol. 2010; 157(12):2717–29. https://doi.org/10.1007/s00227-010-1531-4
https://doi.org/10.1007/s00227-010-1531-...
). In essence, DistLM tells us which variables best explain the (dis)similarities in species biomass. The variables used in this analysis were seasons (high- and low-water), percentage flooded forest cover (within a 5 km radius of the community), municipality population density, and fluvial travel distance to Manaus and to the nearest town. A resemblance matrix was made for the biomass of the 20 fish species with the greatest total biomass that were caught in the 72 h prior to interview. The Bray-Curtis similarity index was used to analyse these data. All data were aggregated to the level of community by season (n = 43, as 22 communities were visited during the high and low-water seasons, apart from one that was abandoned on revisit in the low-water season).
We used SIMPER analysis to identify the fish species that most contributed to the dissimilarity in fish assemblages (e.g., Hallwass, Silvano, 2016Hallwass G, Silvano RAM. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. J Environ Plan Manag. 2016; 59(9):1537–59. https://doi.org/10.1080/09640568.2015.1081587
https://doi.org/10.1080/09640568.2015.10...
) among variables in the significant models. The software Primer+Permanova (Clarke, Gorley, 2006Clarke KR, Gorley RN. PRIMER v6: User manual/Tutorial. PRIMER-E Ltd, Plymouth; 2006.) was used to perform DistLM and SIMPER analyses. SIMPER analyses compare two categories, which we used as seasons (high- and low-water), and access to Manaus (highly or poorly accessible). The latter is a discrete categorisation, distinct to the continuous kilometre scaling used in the DistLM tests. It distinguishes communities with good access to the Manaus market that receive regular (at least weekly) visits from Manaus-based boats that deposit ice and sell fish, from those that do not. Due to the activity of the fish-buyer boat, those communities with good market access are also less-remote (205–577 km fluvial travel distance from Manaus) compared with those poorly accessible communities (779–1,483 km fluvial travel distance from Manaus). While irregular and sometimes community-owned fish-transportation boats did exist, they were particularly rare in the more-remote and less-accessible communities, probably due to the huge travel distances to Manaus, with consequent high transport costs and poorer ability to keep fresh fish preserved.
Non-metric Multidimensional Scaling (NMDS) graphs were made to compare fish assemblages between hydrological seasons and urban access. The NMDS analyses do not provide variance explained by the two first NMDS axes, but the stress. Stress is the goodness-of-fit statistic that MDS tries to minimise. It consists of the square root of the normalized squared discrepancies between interpoint distances in the MDS plot and the smoothed distances predicted from the dissimilarities. Stress varies between 0 and 1, with values near 0 indicating better fit. Lower than 0.05, the graph gives an excellent representation of the high-dimensional data. A stress value between 0.2 and 0.3 is considered a poor but still useful representation. However, acceptable values of stress depend on the quality of the distance matrix and the number of objects in that matrix.
Species diversity calculations and statistical analyses were undertaken using R statistical software version 4.02 (R Core Team, 2020R Core Team. R: A language and environment for statistical computing; 2020.), and were analysed at the level of fishing trip. Species diversity was calculated using the Shannon’s diversity index in the vegan package. Due to the high number of fishing trips where species richness was 1, and hence the diversity score was zero, we used a zero-inflated GLMM (general linear mixed-effect model) with fisher I.D. as a random factor to test for trends in the glmmADMB package. Model diagnostic plots were subsequently inspected. We compared diversity between fishing trips just for consumption, for consumption and sale, and just for sale using Tukey post-hoc tests.
RESULTS
During the high and low-water seasons in 2014 we recorded the recent catch (past 72 h) of 374 different fishers from 824 fishing trips (once fishing trips yielding no catch were excluded). Local fishers reported catching almost 26,000 individuals of 80 species of fish, only four of which were not identified to species level. These four fish types were known locally as cará-açu, consisting of Astronotus crassipinnis (Heckel, 1840) and Astronotus ocellatus (Agassiz, 1831), piranha branca consisting of Serrasalmus striolatus Steindachner, 1908 and Serrasalmus gouldingi Fink & Machado-Allison, 1992, bodó consisting of the Pterygoplichthys genus (previously Liposarcus); mainly P. pardalis (Castelnau, 1855) (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.), and sarapó, which are Gymnotiformes (Tab. S2). They were grouped together because many local people were often unable to distinguish between them.
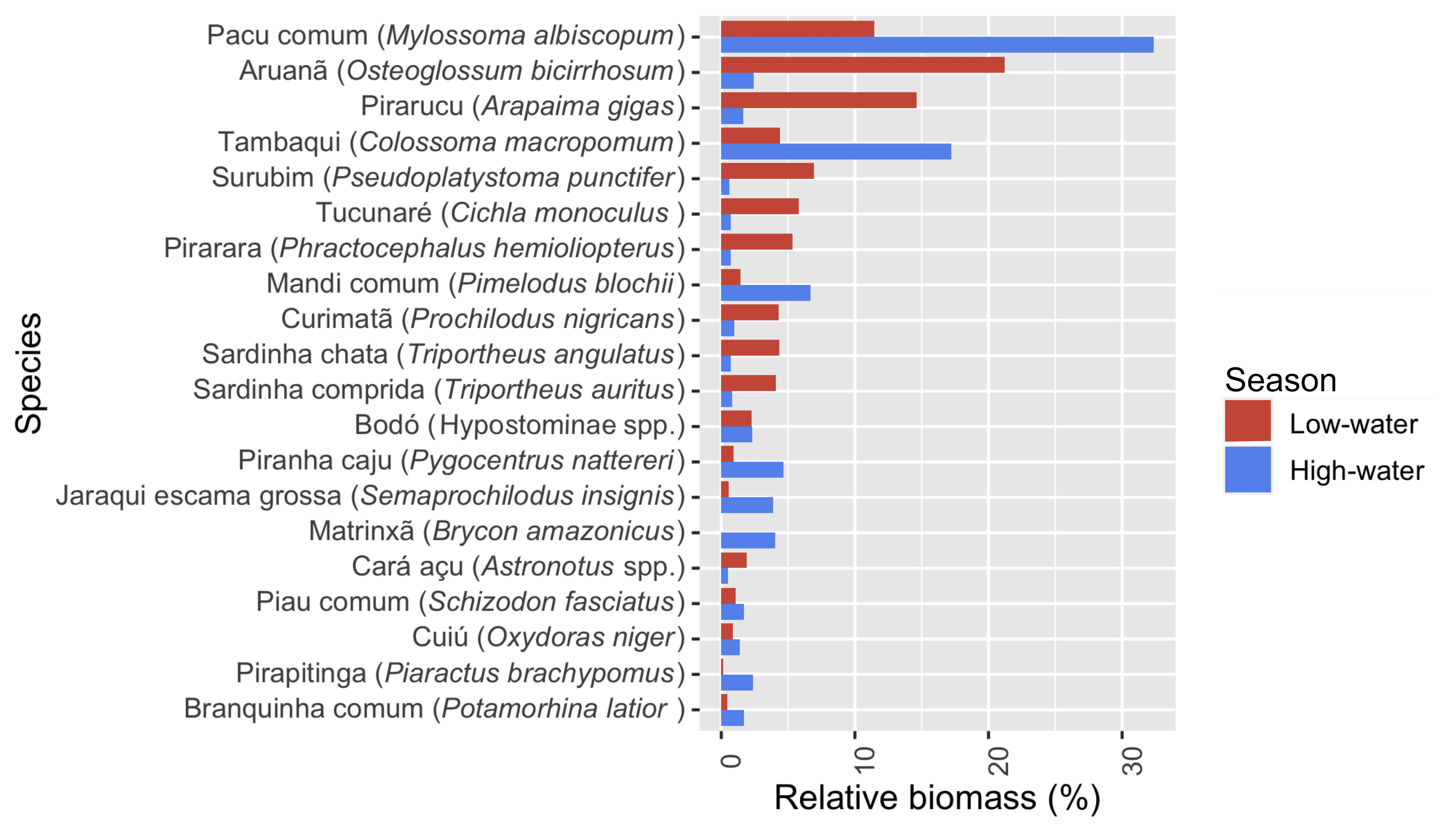
Despite the high species richness of fish captured overall, a fraction of the species made up the vast majority of the total biomass of fish landed both in high- and low-water seasons (Fig. 2). For example, over half of the biomass caught came from just four species: Mylossoma albiscopum, O. bicirrhosum, C. macropomum, and A. gigas, while 20 species made up 90% of the biomass (Fig. 2; Fig. S3; Tab. S2).
The most important 20 fish species by total biomass caught, in descending order, split by season. These species represent 90% of the total biomass caught.
What determine dissimilarities in fish catch composition?
DistLM analyses support our prediction that distance from Manaus and season explain much of the difference in fish catch composition (Tab. 1), both of which were highly significant (P < 0.001). The other factors included in the model, distance to the nearest town, population density and percentage várzea cover, were not found to be significant.
Results of DistLM analyses showing factors explaining dissimilarities in fish catch composition. These are marginal tests for each variable, applying the Bray Curtis similarity index. Significant variables (Distance to Manaus and Season) are highlighted in bold.
Highly distinct differences in seasonal catch composition can be seen through NMDS (Non-metric Multidimensional Scaling) graphs (Fig. 3). High-water catch was dominated by M. albiscopum and C. macropomum. Along with Pimelodus blochii (Valenciennes, 1840), which was also mainly caught in the high-water (Fig. 2), these three species were identified by SIMPER analysis to contribute the most to seasonal dissimilarity in catch composition, together contributing to 35% of this variation (Tab. 2). Catch in the low-water season was more diverse, and O. bicirrhosum, A. gigas, and M. albiscopum made up the greatest biomass of fish caught (Fig. 2). However, SIMPER analyses show that Triportheus angulatus (Spix & Agassiz, 1829), O. bicirrhosum, P. punctifer (formerly P. fasciatum) and A. gigas, which each contribute 5–6% to seasonal catch dissimilarity, contribute most to seasonal differences in favour of the low-water (Tab. S4).
Fish assemblages vary markedly by (A) hydrological season and (B) geographical remoteness, shown by NMDS (Non-metric Multidimensional Scaling) graphs. Based on similarity analysis of seasonal fish assemblages. Less-remote communities are those < 600 km fluvial travel distance from Manaus, which receive visits by Manaus-based boats that purchase fish and deposit ice at least weekly, while more-remote communities are those > 600 km fluvial travel distance from Manaus that do not.
The fish species that contribute most to the variation in catch biomass between seasons and levels of urban access (with the city of Manaus) – SIMPER analyses. Access to the Manaus market is largely defined by the visitation of fish-buyer boats from Manaus, as most fishers without this are unable to gain regular access to the Manaus market. Refer to Tabs. S4 and S5 for full results.
Spatially, we investigated differences in the catch composition of communities based on their degree of access to the Manaus market. For many fishers this is largely determined by the regular visitation of fish-buyer boats from Manaus, as most fishers without this are unable to gain regular access to the Manaus market. Hence in this study, those communities defined as having good-access to Manaus received a weekly visit by a fish-buyer boat, while those defined as having poorly-access did not. Three commercially important species (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.), M. albiscopum, C. macropomum, and A. gigas, were mainly caught in communities with good access to the Manaus market, making up 39% of the spatial variation in biomass catch composition (Tab. S5). Species with established trade in salted fish, O. bicirrhosum, P. punctifer, and Phractocephalus hemioliopterus, were mainly caught in communities with poorer access to the Manaus market, together contributing 20% of the spatial catch composition variation.
Consumption or sale
Whether a catch was for consumption or sale (as defined by the fisher) was our second indicator of urban market influence on catch. Most fishing trips were reported to be for consumption only (n = 552), followed by both consumption and sale (n = 216), and with less only for sale (n = 56). Despite this, most of the total fish biomass was caught during trips for consumption and sale (58.4%), as the mean biomass caught during fishing trips for consumption (5.0 kg) was much lower than those for consumption and sale (25.8 kg) or sale (21.9 kg). Most fishing trips in communities with poorer urban market access (not receiving regular fish-buyers) were for consumption only (80%), while this was less than half (47%) in those communities with better access (Tab. S6). Species caught in trips only for sale were dominated by M. albiscopum and C. macropomum in the high-water (Fig. 4A), and A. gigas in the low-water season (Fig. 4B).
Fish species rank curve (by percentage biomass), during the (A) high-water and (B) low-water seasons. (A) Mylossoma albiscopum and Colossoma macropomum dominate high water catches, both for sale only (red) and for both sale and consumption (black). These species are also both important in catches for consumption only (green), but Pimelodus blochii is the most important species in this category. (B) Arapaima gigas dominates low-water season commercial fish catches (red), Osteoglossum bicirrhosum in catches for both consumption and sale (black), and Mylossoma albiscopum (=M. duriventre) and Triportheus angulatus in catches for consumption only.
Fish species diversity was significantly greater in the low-water season (mean Shannon’s diversity index of 0.49) compared to the high-water season (0.38; p = 0.0146), and varied significantly among fishing trip type (p < 0.001; Fig. 5). Post-hoc testing showed no significant difference between fishing trips for consumption only (0.49) and those for both consumption and sale (0.36; p = 0.060). However, fishing trips only for sale (0.14) were significantly less diverse from both trips for consumption only (p = 0.006) and those for both consumption and sale (p = 0.017).
Fish species diversity caught by rural artisanal fishers on the Rio Purus. Fishing catches to be sold were less diverse than those to be consumed. Trips were classified based on whether the stated intention by the fisher was to sell, for household consumption or both. The Shannon’s diversity index, calculated per fishing trip, is shown.
DISCUSSION
In this paper we analyse the fishery of the lower Rio Purus, which is the main fishing river for the Amazon’s largest city, through the catch composition of the artisanal fishers that rely on it for their subsistence and livelihoods. To understand the fishery, we looked at differences in fish catch between hydrological seasons, levels of urban market access, and between subsistence and commercial catches. The seasons impacted catch composition. Our results also emphasise a concentration of demand on a limited number of species, and how urban market access appears to drive a different and more selective catch composition, as this pattern was seen in our two analyses of urban market access. Firstly, distance to the urban market of Manaus was a significant determinant of differences in fish assemblages, and a greater focus on several commercially important species in communities with better access to the city via fish-buyers contributed to much of this difference. Secondly, the species diversity of the catch was significantly lower when it was for sale and not for consumption, suggesting that fishing is more selective when intended for the urban market.
Concentrated demand on few species
A main finding was that the artisanal fishery is concentrated on few species, with over half of the catch in biomass being made up of just four species. Given the enormous richness of thousands of fish species in Amazonia (Jézéquel et al., 2020Jézéquel C, Tedesco PA, Bigorne R, Maldonado-Ocampo JA, Ortega H, Hidalgo M et al. A database of freshwater fish species of the Amazon Basin. Sci Data. 2020; 7(96):1–09. https://doi.org/10.1038/s41597-020-0436-4
https://doi.org/10.1038/s41597-020-0436-...
), even a seemingly rich catch of 80 fish species caught during over 800 fishing trips emphasises how fishers are selective on a relatively small proportion of the whole fish assemblage. This supports studies from other parts of the Amazon, for example where Isaac, (2000)Isaac VJ. Fish catches among riverside communities around Lago Grande de Monte Alegre, Lower Amazon, Brazil. Fish Manag Ecol. 2000; 7:355–74. found that 10 taxa comprised 75% of the artisanal fish catch of 70 species in communities in the eastern Amazon. Moreover, analyses of the Manaus fishery estimate that around 100 fish species are commercially exploited, 37 are regularly offered in the city’s market, and just 10 make up 80% of its city’s catch (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.; Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.). By incorporating fisher knowledge which determines whether a catch was for consumption or sale, we expand on the well-established conclusion that Amazonian fish catch is highly selective, by showing how it is significantly more selective (lower catch diversity) when destined for sale (see Urban market access section).
The literature offers several explanations for why fish catch is highly selective, relating to ecological and cultural factors including catchability, cultural preferences, and market demand (Tsikliras, Polymeros, 2014Tsikliras AC, Polymeros K. Fish market prices drive overfishing of the ‘big ones.’ PeerJ. 2014; 2:e638. https://doi.org/10.7717/peerj.638
https://doi.org/10.7717/peerj.638...
). There is a general preference in fisheries for large-bodied species (Allan et al., 2005Allan JD, Abell R, Hogan Z, Revenga C. Overfishing of inland waters. BioScience. 2005; 55(12):1041–51.), and this is reflected in the commercial focus towards A. gigas, C. macropomum and large catfish (e.g., P. punctifer), in our data. Moreover, C. macropomum is also renowned for its good taste, and is by far the preferred species among local Purus fishers (Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
), and the most consumed among urban Manaus dwellers (Pesquisa365, 2015Pesquisa365. Consumo365 [Internet]. Manaus: 2015. p.14-15. Available from: https://www.slideshare.net/pesquisa365/consumo365
https://www.slideshare.net/pesquisa365/c...
).
Urban market access
While artisanal fishers only exploit a small part of the fish assemblage, our data showing even lower diversity in commercial catches suggests that urban market access can drive artisanal fishers to be even more selective. Our catch data demonstrate concentration of fishing pressure on a few species raising the prospect of overfishing. This possibility is supported by evidence that these catches are dominated by several commercially important valuable (R$/kg) species (Fig. S3), classified as Nearly Threatened (NT) (A. gigas) (ICMBio, 2018ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Volume VI – Peixes. Brasília, DF. 2018. ) or overfished (C. macropomum and P. punctifer) (Castello et al., 2013Castello L, McGrath DG, Hess LL, Coe MT, Lefebvre PA, Petry P et al. The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217–29. https://doi.org/10.1111/conl.12008
https://doi.org/10.1111/conl.12008...
). This highly selective fishing pressure on vulnerable stocks is likely to explain spatial declines (defaunation ‘shadows’ around urban areas) and temporal declines in fish catch-per-unit-effort, body size or urban landings (Batista, Petrere Júnior, 2003Batista VDS, Petrere Júnior M. Characterization of the commercial fish production landed at Manaus, Amazonas State, Brazil. Acta Amaz. 2003; 33(1):53–66. https://doi.org/10.1590/1809-4392200331066
https://doi.org/10.1590/1809-43922003310...
; Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
).
Species composition was influenced by distance to Manaus, and urban-based fish-buyers appear to have played an important role. As hypothesised, commercially important species that are sold on ice in the metropolitan centre of Manaus, including C. macropomum, A. gigas, and M. albiscopum (Santos et al., 2006Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.), were more important in those communities with better access to the Manaus market that receive regular (at least weekly) visits from city-based boats that buy fish and deposit ice. Given the tropical climate and large travel distances from our studied communities of up to ~1,500 km to Manaus and ~250 km to the nearest urban area, some form of refrigeration is essential for the sale of fresh fish to the urban market. The availability and access to ice is regarded as an important determinant of fishing capacity in tropical fisheries in which refrigeration is otherwise not possible (Almeida, 2004Almeida O. Fisheries management in the Brazilian Amazon. [PhD Thesis]. London: University of London; 2004. Available from: https://ipam.org.br/wp-content/uploads/2004/03/fisheries_management_in_the_brazilian_am2.pdf
https://ipam.org.br/wp-content/uploads/2...
; Brewer et al., 2013Brewer TD, Cinner JE, Green A, Pressey RL. Effects of human population density and proximity to markets on coral reef fishes vulnerable to extinction by fishing. Conserv Biol. 2013; 27(3):443–52. https://doi.org/10.1111/j.1523-1739.2012.01963.x
https://doi.org/10.1111/j.1523-1739.2012...
). Our data supports and furthers this idea, suggesting that ice availability is an important driver not only of fishing capacity, but also of species composition. These trends can perhaps partially explain why certain commercially important species in our study system (Tregidgo et al., 2017Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
https://doi.org/10.1073/pnas.1614499114...
), as well as in marine (Brewer et al., 2009Brewer TD, Cinner JE, Green A, Pandolfi JM. Thresholds and multiple scale interaction of environment, resource use, and market proximity on reef fishery resources in the Solomon Islands. Biol Conserv. 2009; 142(8):1797–807. https://doi.org/10.1016/j.biocon.2009.03.021
https://doi.org/10.1016/j.biocon.2009.03...
) and terrestrial (Parry, Peres, 2015Parry L, Peres CA. Evaluating the use of local ecological knowledge to monitor hunted tropical-forest wildlife over large spatial scales. Ecol Soc. 2015; 20(3):15. https://doi.org/10.5751/ES-07601-200315
https://doi.org/10.5751/ES-07601-200315...
) wildlife appear predominantly defaunated nearer to centres of urban demand. These results therefore demonstrate the importance of urban markets and specifically urban access in shaping the catch of artisanal fishers.
The relative importance of fresh fish has increased compared to salted and dried fish throughout the Amazon over recent decades (Garcia et al., 2009Garcia A, Tello S, Vargas G, Duponchelle F. Patterns of commercial fish landings in the Loreto region (Peruvian Amazon) between 1984 and 2006. Fish Physiol Biochem. 2009; 35(1):53–67. https://doi.org/10.1007/s10695-008-9212-7
https://doi.org/10.1007/s10695-008-9212-...
). Salting fish is a more traditional form of preservation in Amazonia (McGrath et al., 1993McGrath DG, Castro F, Futemma C, Amaral BD, Calabria J. Fisheries and the evolution of resource management on the lower Amazon floodplain. Hum Ecol. 1993; 21(2):167–95. https://doi.org/10.1007/BF00889358
https://doi.org/10.1007/BF00889358...
), and is still widely practiced for certain species and in areas without reliable refrigeration (Ferreira et al., 2020Ferreira G, Marcovitch J, Val AL. A systematic review of the production chain of the Arapaima gigas, the giant fish of the Amazon. Manag Environ Qual Int J. 2020; 31(2):349–63. https://doi.org/10.1108/MEQ-11-2019-0238
https://doi.org/10.1108/MEQ-11-2019-0238...
; Guzmán Maldonado et al., 2017Guzmán Maldonado A, Macedo Lopes PF, Rodríguez Fernández CA, Lasso Alcala CA, Sumalia UR. Transboundary fisheries management in the Amazon: Assessing current policies for the management of the ornamental silver arawana (Osteoglossum bicirrhosum). Mar Policy. 2017; 76(September 2016):192–99. https://doi.org/10.1016/j.marpol.2016.11.021
https://doi.org/10.1016/j.marpol.2016.11...
; DT, pers. obs.). Three species that explained a lot of the spatial variation in catch (Tab. S5), O. bicirrhosum, P. punctifer, and P. hemioliopterus, were mainly caught around more-remote rural communities not regularly provisioned with ice, and are species with an established trade in being sold salted, which could explain this.
Seasons
Hydrological seasonality was a significant factor determining catch composition, albeit explaining just 11% of the variation of the catches in biomass when considered alone. This was expected due to the huge importance of the flood pulse in local ecology, and as a strong seasonal component has been observed in the catch composition in other rural fishing studies (Batista et al., 1998Batista VDS, Inhamuns AJ, Freitas CEDC, Freire-Brasil D. Characterization of the fishery in the river communities in the low-Solimões/high-Amazon region. Fish Manag Ecol. 1998; 5(5):419–35. https://doi.org/10.1046/j.1365-2400.1998.550419.x
https://doi.org/10.1046/j.1365-2400.1998...
) and of urban landings (Batista, Petrere Júnior, 2003Batista VDS, Petrere Júnior M. Characterization of the commercial fish production landed at Manaus, Amazonas State, Brazil. Acta Amaz. 2003; 33(1):53–66. https://doi.org/10.1590/1809-4392200331066
https://doi.org/10.1590/1809-43922003310...
; Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.). Many Amazonian fish species are migratory, and are only locally abundant during certain periods of the year (Araújo-Lima, Ruffino, 2003Araújo-Lima CARM, Ruffino ML. Migratory fishes of the Brazilian Amazon. In: Carolsfeld J, Harvey B, Ross C, Baer A, editors. Migr. Fishes South Am Biol Fish Conserv Status. Victoria: World Fisheries Trust, The World Bank and The International Development Research Centre; 2003. p.233–301.). The disproportionate contribution of A. gigas and O. bicirrhosum to the low-water season catch can likely be explained by easier capture of when they are concentrated in floodplain lakes (Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.), which are inundated during the high-water season. We do however note one limitation of our study design, which captured the high and low-water season catch, but missed other distinct periods such as the rising and falling waters, in which other species are likely to make a more important contribution (Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.).
The annual flood pulse inundates large areas of forest, providing a distinct seasonal fishing ground, as fish are temporarily dispersed throughout the forest. While most fishing among Purus fishers is undertaken in rivers and lakes during low-water, almost all fishing during the high-water season occurs in the flooded forest (Tregidgo et al., 2020Tregidgo D, Barlow J, Pompeu PS, Parry L. Tough fishing and severe seasonal food insecurity in Amazonian flooded forests. People Nat. 2020; 2(2):468–82. https://doi.org/10.1002/pan3.10086
https://doi.org/10.1002/pan3.10086...
). During this period, fruits from floodplain forests dominate the diets of many Amazonian frugivorous fish (Lucas, 2008Lucas CM. Within flood season variation in fruit consumption and seed dispersal by two characin fishes of the Amazon. Biotropica. 2008; 40(5):581–89. https://doi.org/10.1111/j.1744-7429.2008.00415.x
https://doi.org/10.1111/j.1744-7429.2008...
), and local fishers take advantage of this by targeting fruit-consuming fish (e.g., P. blochii and M. albiscopum) under fruit trees, using fruits as bait, and/or through acoustic imitation of fruit falling into the water via a gaponga or flicking the water’s surface (DT, pers. obs.; Goulding, 1981Goulding M. Man and fisheries on an Amazon frontier. Cham: Springer; 1981. Available from: https://link.springer.com/book/10.1007/978-94-017-2161-5
https://link.springer.com/book/10.1007/9...
). Given the essential ecosystem service of seed dispersal performed by frugivorous fish species, their overfishing can have important cascading ecosystem effects (Correa et al., 2015Correa SB, Costa-Pereira R, Fleming T, Goulding M, Anderson JT. Neotropical fish-fruit interactions: eco-evolutionary dynamics and conservation. Biol Rev. 2015; 90(4):1263–78. https://doi.org/10.1111/brv.12153
https://doi.org/10.1111/brv.12153...
; Costa-Pereira et al., 2018Costa-Pereira R, Lucas C, Crossa M, Anderson JT, Albuquerque BW, Dary EP et al. Defaunation shadow on mutualistic interactions. Proc Natl Acad Sci U S A. 2018; 115(12):E2673–75. https://doi.org/10.1073/pnas.1801106115
https://doi.org/10.1073/pnas.1801106115...
).
In consuming large quantities of fruit, frugivorous fish accumulate larger stores of fat in high-water periods (Junk, 1985Junk WJ. Temporary fat storage, an adaptation of some fish species to the water level fluctuations and related environmental changes of the Amazon River. Amazoniana. 1985; 9(3):315–51.). Frugivorous fish may be relatively important as a fat source given that some Amazonian fish species have significantly lower fat content during the high-water period (Petenuci et al., 2016Petenuci ME, Rocha INA, Sousa SC, Schneider VVA, Costa LAMA, Visentainer JV. Seasonal variations in lipid content, fatty acid composition and nutritional profiles of five freshwater fish from the Amazon basin. J Am Oil Chem Soc. 2016; 93(10):1373–81. https://doi.org/10.1007/s11746-016-2884-8
https://doi.org/10.1007/s11746-016-2884-...
). Due to the severe food insecurity identified in floodplain populations during the high-water season (Tregidgo et al., 2020Tregidgo D, Barlow J, Pompeu PS, Parry L. Tough fishing and severe seasonal food insecurity in Amazonian flooded forests. People Nat. 2020; 2(2):468–82. https://doi.org/10.1002/pan3.10086
https://doi.org/10.1002/pan3.10086...
), and that fat is generally in more scarce supply than protein in many tropical forest-dwellers (Sirén, Machoa, 2008Sirén AH, Machoa J. Fish, wildlife, and human nutrition in tropical forests: a fat gap? Interciencia. 2008; 33(3):186–93. Available from: https://www.redalyc.org/pdf/339/33933306.pdf
https://www.redalyc.org/pdf/339/33933306...
), frugivorous fish may play a particularly important role in nutrition of riverine populations during this lean season.
Challenges for management and final remarks
Our data suggests that much of the pressure on fish populations comes from the highly selective demand for relatively few species, particularly from the metropolitan centre of Manaus. Diversifying the fish species consumed in urban areas could potentially alleviate some of the pressure on the populations of target species (Hallwass, Silvano, 2016Hallwass G, Silvano RAM. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. J Environ Plan Manag. 2016; 59(9):1537–59. https://doi.org/10.1080/09640568.2015.1081587
https://doi.org/10.1080/09640568.2015.10...
; Soares et al., 2020Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.). However, this is likely to be challenging in practice, given the taste and cultural preferences for certain species. Alternative sourcing, particularly of large slow-reproducing species (e.g., A. gigas and C. macropomum), through community-based management (Castello et al., 2009Castello L, Viana JP, Watkins G, Pinedo-Vasquez M, Luzadis VA. Lessons from integrating fishers of arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ Manage. 2009; 43(2):197–209. https://doi.org/10.1007/s00267-008-9220-5
https://doi.org/10.1007/s00267-008-9220-...
; De Mattos Vieira et al., 2015De Mattos Vieira MAR, Von Muhlen EM, Shepard Jr GH. Participatory monitoring and management of subsistence hunting in the Piagaçu-Purus Reserve, Brazil. Conserv Soc. 2015; 13(3):254–64. https://www.jstor.org/stable/26393204
https://www.jstor.org/stable/26393204...
; Petersen et al., 2016Petersen TA, Brum SM, Rossoni F, Silveira GFV, Castello L. Recovery of Arapaima sp. populations by community-based management in floodplains of the Purus River, Amazon. J Fish Biol. 2016; 89(1):241–48. https://doi.org/10.1111/jfb.12968
https://doi.org/10.1111/jfb.12968...
) or fish farming (Mattos et al., 2021Mattos BO, Pantoja-Lima J, Oliveira AT, Aride PHR. Aquicultura na Amazônia: Estudos técnico-científicos e difusão de tecnologias. Atena Editora; 2021. https://doi.org/10.22533/at.ed.042211503
https://doi.org/10.22533/at.ed.042211503...
) also hopes to alleviate pressures on wild stocks.
However, this study demonstrates how these species remain a target for local fishers, probably in a large part due to the high price paid for them by fish buyers (Fig. S7).
Amazonian fisheries policy is largely based on restrictions (Corrêa et al., 2014Corrêa MAA, Kahn JR, Freitas CEDC. Perverse incentives in fishery management: The case of the defeso in the Brazilian Amazon. Ecol Econ. 2014; 106:186–94. https://doi.org/10.1016/j.ecolecon.2014.07.023
https://doi.org/10.1016/j.ecolecon.2014....
), and fish farming has been suggested as a method to reduce fishing pressure on wild stocks (Montenegro, Souza, 2016Montenegro LS, Souza LA. Produção pesqueira e sua relação com as oscilações do ciclo hidrológico e o crescimento demográfico da cidade de Manaus-Am. Sci Amaz. 2016; 5(2):14–23. Available from: http://scientia-amazonia.org/wp-content/uploads/2016/09/v5-n2-14-23-2016.pdf
http://scientia-amazonia.org/wp-content/...
). Yet the clear importance of the vulnerable C. macropomum in the high-water season catch shows how the region’s most important farmed fish species (IBGE, 2018IBGE. Produção da Pecuária Municipal 2018. Rio de Janeiro; 2018. 46:1–08. Available from: https://biblioteca.ibge.gov.br/visualizacao/periodicos/84/ppm_2018_v46_br_informativo.pdf
https://biblioteca.ibge.gov.br/visualiza...
) is still heavily exploited in the wild, and how important it appears to be to the food and livelihood security of local people during this season of tough fishing (Tregidgo et al., 2020Tregidgo D, Barlow J, Pompeu PS, Parry L. Tough fishing and severe seasonal food insecurity in Amazonian flooded forests. People Nat. 2020; 2(2):468–82. https://doi.org/10.1002/pan3.10086
https://doi.org/10.1002/pan3.10086...
). While high selectivity in this fishery may threaten those most captured, further restrictions on them should be considered with caution, as restricting wild resource use can have a comparable detrimental impact on fishers (and hunters) to biological population declines (Adams, 2004Adams WM. Biodiversity conservation and the eradication of poverty. Science. 2004; 306(5699):1146–49. https://doi.org/10.1126/science.1097920
https://doi.org/10.1126/science.1097920...
; Wilkie et al., 2006Wilkie DS, Morelli GA, Demmer J, Starkey M, Telfer P, Steil M. Parks and people: Assessing the human welfare effects of establishing protected areas for biodiversity conservation. Conserv Biol. 2006; 20(1):247–49. https://doi.org/10.1111/j.1523-1739.2005.00291.x
https://doi.org/10.1111/j.1523-1739.2005...
; Sodhi, 2008Sodhi NS. Tropical biodiversity loss and people – A brief review. Basic Appl Ecol. 2008; 9(2):93–99. https://doi.org/10.1016/j.baae.2007.11.001
https://doi.org/10.1016/j.baae.2007.11.0...
; Antunes et al., 2019Antunes AP, Rebêlo GH, Pezzuti JCB, Vieira MARM, Constantino PAL, Campos-Silva JV et al. A conspiracy of silence: Subsistence hunting rights in the Brazilian Amazon. Land use policy. 2019; 84(July 2018):1–11. https://doi.org/10.1016/j.landusepol.2019.02.045
https://doi.org/10.1016/j.landusepol.201...
).
Fish populations and fishers can prosper through the sustainable harvest and community co-management of commercially important species like A. gigas (Gonçalves et al., 2018Gonçalves ACT, Cunha JBC, Batista JS. The Amazonian giant: Sustainable management of Arapaima (Pirarucu). Tefé: IDSM; 2018. Available from: https://www.mamiraua.org.br/documentos/4163f5aaff5d05e1a9e1804bb5e06307.pdf
https://www.mamiraua.org.br/documentos/4...
). However, as this is limited to certain highly managed areas, and as it is most successful with non-migratory species (Petersen et al., 2016Petersen TA, Brum SM, Rossoni F, Silveira GFV, Castello L. Recovery of Arapaima sp. populations by community-based management in floodplains of the Purus River, Amazon. J Fish Biol. 2016; 89(1):241–48. https://doi.org/10.1111/jfb.12968
https://doi.org/10.1111/jfb.12968...
), it is only part of the solution in the Amazon where management resources are highly limited, and many species are migratory. Commercially important catfish are known to migrate hundreds or even many thousands of kilometres, and rely on an unobstructed passage to complete their life cycles (Araújo-Lima, Ruffino, 2003Araújo-Lima CARM, Ruffino ML. Migratory fishes of the Brazilian Amazon. In: Carolsfeld J, Harvey B, Ross C, Baer A, editors. Migr. Fishes South Am Biol Fish Conserv Status. Victoria: World Fisheries Trust, The World Bank and The International Development Research Centre; 2003. p.233–301.; Barthem et al., 2017Barthem RB, Goulding M, Leite RG, Cañas C, Forsberg B, Venticinque E et al. Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci Rep. 2017; 7(41784):1–13. https://doi.org/10.1038/srep41784
https://doi.org/10.1038/srep41784...
). They are therefore particularly vulnerable to widespread dam construction (Duponchelle et al., 2016Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno J-F, Panfili J et al. Trans-Amazonian natal homing in giant catfish. J Appl Ecol. 2016; 53(5):1511–20. https://doi.org/10.1111/1365-2664.12665
https://doi.org/10.1111/1365-2664.12665...
; Vasconcelos et al., 2021Vasconcelos LP, Alves DC, Câmara LF, Hahn L. Dams in the Amazon: The importance of maintaining free-flowing tributaries for fish reproduction. Aquat Conserv Mar Freshw Ecosyst. 2021; 31(5):1106–16. https://doi.org/10.1002/aqc.3465
https://doi.org/10.1002/aqc.3465...
), which inhibits the passage of migratory fish (Carolsfeld et al., 2003Carolsfeld J, Harvey B, Ross C, Baer A. Migratory fishes of South America: Biology, social importance and conservation status. Victoria: World fisheries trust, the world bank and the international development research centre; 2003. Available from: https://www.idrc.ca/en/book/migratory-fishes-south-america-biology-fisheries-and-conservation-status
https://www.idrc.ca/en/book/migratory-fi...
; Winemiller et al., 2016Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016; 351(6269):128–29. https://doi.org/10.1126/science.aac7082
https://doi.org/10.1126/science.aac7082...
).
The large presence of migratory fish species in the catch of remote communities may reflect the study river, as the Rio Purus is undammed. Our data shows how migratory species like Phractocephalus hemioliopterus and P. punctifer, which have an established market in their salted form, make an important contribution to the commercial catch in remote Purus communities with poor access to the more lucrative fresh fish trade, due to lack of regular fish-buyers and ice supply. Communities elsewhere in Amazonia may therefore be vulnerable to the depletion of migratory catfish populations due to dam construction, which will leave only three free-flowing Amazonian tributaries if all planned dams are constructed (Castello, Macedo, 2016Castello L, Macedo MN. Large-scale degradation of Amazonian freshwater ecosystems. Glob Chang Biol. 2016; 22(3):990–1007. https://doi.org/10.1111/gcb.13173
https://doi.org/10.1111/gcb.13173...
).
This paper responds to a lack of data and study of Amazonian artisanal fish catch composition, by characterising the Rio Purus artisanal fishery, and exploring variation in the catch profile. We demonstrate that most fishing pressure is placed on relatively few species, and even fewer when destined for the main urban market in the region. Given the important nutritional (Heilpern et al., 2021Heilpern SA, DeFries R, Fiorella K, Flecker A, Sethi SA, Uriarte M et al. Declining diversity of wild-caught species puts dietary nutrient supplies at risk. Sci Adv. 2021; 7(22):eabf9967. https://doi.org/10.1126/sciadv.abf9967
https://doi.org/10.1126/sciadv.abf9967...
) and ecological (Costa-Pereira et al., 2018Costa-Pereira R, Lucas C, Crossa M, Anderson JT, Albuquerque BW, Dary EP et al. Defaunation shadow on mutualistic interactions. Proc Natl Acad Sci U S A. 2018; 115(12):E2673–75. https://doi.org/10.1073/pnas.1801106115
https://doi.org/10.1073/pnas.1801106115...
) roles of many of these targeted species, the potential implications of selective overfishing go beyond the fishery itself. While reducing major threats to Amazonian freshwaters such as overfishing, mining, landcover change and dam construction is essential, a multifaceted approach is required to reduce selective pressures on targeted fish species, incorporating a variety of stakeholders, from rural fishers to urban consumers.
ACKNOWLEDGEMENTS
We offer our profound thanks to Mayana de Almeida Rocha for her dedicated assistance in the field, and the people of the Rio Purus for their time and kind hospitality. PSP received a research fellowship from the CNPq (303548/2017-7).
REFERENCES
- Adams WM. Biodiversity conservation and the eradication of poverty. Science. 2004; 306(5699):1146–49. https://doi.org/10.1126/science.1097920
» https://doi.org/10.1126/science.1097920 - Allan JD, Abell R, Hogan Z, Revenga C. Overfishing of inland waters. BioScience. 2005; 55(12):1041–51.
- Almeida O. Fisheries management in the Brazilian Amazon. [PhD Thesis]. London: University of London; 2004. Available from: https://ipam.org.br/wp-content/uploads/2004/03/fisheries_management_in_the_brazilian_am2.pdf
» https://ipam.org.br/wp-content/uploads/2004/03/fisheries_management_in_the_brazilian_am2.pdf - Antunes AP, Rebêlo GH, Pezzuti JCB, Vieira MARM, Constantino PAL, Campos-Silva JV et al A conspiracy of silence: Subsistence hunting rights in the Brazilian Amazon. Land use policy. 2019; 84(July 2018):1–11. https://doi.org/10.1016/j.landusepol.2019.02.045
» https://doi.org/10.1016/j.landusepol.2019.02.045 - Arantes CC, Winemiller KO, Asher A, Castello L, Hess LL, Petrere M et al Floodplain land cover affects biomass distribution of fish functional diversity in the Amazon River. Sci Rep. 2019; 9(16684):1–13. https://doi.org/10.1038/s41598-019-52243-0
» https://doi.org/10.1038/s41598-019-52243-0 - Arantes CC, Winemiller KO, Petrere M, Castello L, Hess LL, Freitas CEC. Relationships between forest cover and fish diversity in the Amazon River floodplain. J Appl Ecol. 2018; 55(1):386–95. https://doi.org/10.1111/1365-2664.12967
» https://doi.org/10.1111/1365-2664.12967 - ArcMAP ESRI. 10.2.2 ESRI (Environmental Systems Research Institute). Redlands; 2014.
- Araújo-Lima CARM, Ruffino ML. Migratory fishes of the Brazilian Amazon. In: Carolsfeld J, Harvey B, Ross C, Baer A, editors. Migr. Fishes South Am Biol Fish Conserv Status. Victoria: World Fisheries Trust, The World Bank and The International Development Research Centre; 2003. p.233–301.
- Barthem RB, Goulding M, Leite RG, Cañas C, Forsberg B, Venticinque E et al Goliath catfish spawning in the far western Amazon confirmed by the distribution of mature adults, drifting larvae and migrating juveniles. Sci Rep. 2017; 7(41784):1–13. https://doi.org/10.1038/srep41784
» https://doi.org/10.1038/srep41784 - Batista VDS, Inhamuns AJ, Freitas CEDC, Freire-Brasil D. Characterization of the fishery in the river communities in the low-Solimões/high-Amazon region. Fish Manag Ecol. 1998; 5(5):419–35. https://doi.org/10.1046/j.1365-2400.1998.550419.x
» https://doi.org/10.1046/j.1365-2400.1998.550419.x - Batista VDS, Petrere Júnior M. Characterization of the commercial fish production landed at Manaus, Amazonas State, Brazil. Acta Amaz. 2003; 33(1):53–66. https://doi.org/10.1590/1809-4392200331066
» https://doi.org/10.1590/1809-4392200331066 - Brewer TD, Cinner JE, Fisher R, Green A, Wilson SK. Market access, population density, and socioeconomic development explain diversity and functional group biomass of coral reef fish assemblages. Glob Environ Chang. 2012; 22(2):399–406. https://doi.org/10.1016/j.gloenvcha.2012.01.006
» https://doi.org/10.1016/j.gloenvcha.2012.01.006 - Brewer TD, Cinner JE, Green A, Pandolfi JM. Thresholds and multiple scale interaction of environment, resource use, and market proximity on reef fishery resources in the Solomon Islands. Biol Conserv. 2009; 142(8):1797–807. https://doi.org/10.1016/j.biocon.2009.03.021
» https://doi.org/10.1016/j.biocon.2009.03.021 - Brewer TD, Cinner JE, Green A, Pressey RL. Effects of human population density and proximity to markets on coral reef fishes vulnerable to extinction by fishing. Conserv Biol. 2013; 27(3):443–52. https://doi.org/10.1111/j.1523-1739.2012.01963.x
» https://doi.org/10.1111/j.1523-1739.2012.01963.x - Cardoso RS, Batista VDS, Henry C, Júnior F, Martins WR. Aspectos econômicos e operacionais das viagens da frota pesqueira de Manaus, Amazônia Central. Acta Amaz. 2004; 34(2):301–07. https://doi.org/10.1590/S0044-59672004000200016
» https://doi.org/10.1590/S0044-59672004000200016 - Carolsfeld J, Harvey B, Ross C, Baer A. Migratory fishes of South America: Biology, social importance and conservation status. Victoria: World fisheries trust, the world bank and the international development research centre; 2003. Available from: https://www.idrc.ca/en/book/migratory-fishes-south-america-biology-fisheries-and-conservation-status
» https://www.idrc.ca/en/book/migratory-fishes-south-america-biology-fisheries-and-conservation-status - Castello L, Macedo MN. Large-scale degradation of Amazonian freshwater ecosystems. Glob Chang Biol. 2016; 22(3):990–1007. https://doi.org/10.1111/gcb.13173
» https://doi.org/10.1111/gcb.13173 - Castello L, McGrath DG, Hess LL, Coe MT, Lefebvre PA, Petry P et al The vulnerability of Amazon freshwater ecosystems. Conserv Lett. 2013; 6(4):217–29. https://doi.org/10.1111/conl.12008
» https://doi.org/10.1111/conl.12008 - Castello L, Viana JP, Watkins G, Pinedo-Vasquez M, Luzadis VA. Lessons from integrating fishers of arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ Manage. 2009; 43(2):197–209. https://doi.org/10.1007/s00267-008-9220-5
» https://doi.org/10.1007/s00267-008-9220-5 - Clarke KR, Gorley RN. PRIMER v6: User manual/Tutorial. PRIMER-E Ltd, Plymouth; 2006.
- Coe MT, Costa MH, Botta A, Birkett C. Long-term simulations of discharge and floods in the Amazon Basin. J Geophys Res Atmos. 2002; 107(D20):1–17. https://doi.org/10.1029/2001JD000740
» https://doi.org/10.1029/2001JD000740 - Correa SB, Costa-Pereira R, Fleming T, Goulding M, Anderson JT. Neotropical fish-fruit interactions: eco-evolutionary dynamics and conservation. Biol Rev. 2015; 90(4):1263–78. https://doi.org/10.1111/brv.12153
» https://doi.org/10.1111/brv.12153 - Corrêa MAA, Kahn JR, Freitas CEDC. Perverse incentives in fishery management: The case of the defeso in the Brazilian Amazon. Ecol Econ. 2014; 106:186–94. https://doi.org/10.1016/j.ecolecon.2014.07.023
» https://doi.org/10.1016/j.ecolecon.2014.07.023 - Costa-Pereira R, Lucas C, Crossa M, Anderson JT, Albuquerque BW, Dary EP et al Defaunation shadow on mutualistic interactions. Proc Natl Acad Sci U S A. 2018; 115(12):E2673–75. https://doi.org/10.1073/pnas.1801106115
» https://doi.org/10.1073/pnas.1801106115 - Dufour DL, Piperata BA, Murrieta RSS, Wilson WM, Williams DD. Amazonian foods and implications for human biology. Ann Hum Biol. 2016; 43(4):330–48. https://doi.org/10.1080/03014460.2016.1196245
» https://doi.org/10.1080/03014460.2016.1196245 - Duponchelle F, Pouilly M, Pécheyran C, Hauser M, Renno J-F, Panfili J et al Trans-Amazonian natal homing in giant catfish. J Appl Ecol. 2016; 53(5):1511–20. https://doi.org/10.1111/1365-2664.12665
» https://doi.org/10.1111/1365-2664.12665 - Endo W, Peres CA, Haugaasen T. Flood pulse dynamics affects exploitation of both aquatic and terrestrial prey by Amazonian floodplain settlements. Biol Conserv. 2016; 201:129–36. https://doi.org/10.1016/j.biocon.2016.07.006
» https://doi.org/10.1016/j.biocon.2016.07.006 - Ferreira G, Marcovitch J, Val AL. A systematic review of the production chain of the Arapaima gigas, the giant fish of the Amazon. Manag Environ Qual Int J. 2020; 31(2):349–63. https://doi.org/10.1108/MEQ-11-2019-0238
» https://doi.org/10.1108/MEQ-11-2019-0238 - Froese R, Pauly D, editors. FishBase [Internet]. Stockholm, Sweden; 2015. Available from: www.fishbase.org
- Garcia A, Tello S, Vargas G, Duponchelle F. Patterns of commercial fish landings in the Loreto region (Peruvian Amazon) between 1984 and 2006. Fish Physiol Biochem. 2009; 35(1):53–67. https://doi.org/10.1007/s10695-008-9212-7
» https://doi.org/10.1007/s10695-008-9212-7 - Gonçalves ACT, Cunha JBC, Batista JS. The Amazonian giant: Sustainable management of Arapaima (Pirarucu). Tefé: IDSM; 2018. Available from: https://www.mamiraua.org.br/documentos/4163f5aaff5d05e1a9e1804bb5e06307.pdf
» https://www.mamiraua.org.br/documentos/4163f5aaff5d05e1a9e1804bb5e06307.pdf - Goulding M. Man and fisheries on an Amazon frontier. Cham: Springer; 1981. Available from: https://link.springer.com/book/10.1007/978-94-017-2161-5
» https://link.springer.com/book/10.1007/978-94-017-2161-5 - Guzmán Maldonado A, Macedo Lopes PF, Rodríguez Fernández CA, Lasso Alcala CA, Sumalia UR. Transboundary fisheries management in the Amazon: Assessing current policies for the management of the ornamental silver arawana (Osteoglossum bicirrhosum). Mar Policy. 2017; 76(September 2016):192–99. https://doi.org/10.1016/j.marpol.2016.11.021
» https://doi.org/10.1016/j.marpol.2016.11.021 - Hallwass G, Silvano RAM. Patterns of selectiveness in the Amazonian freshwater fisheries: implications for management. J Environ Plan Manag. 2016; 59(9):1537–59. https://doi.org/10.1080/09640568.2015.1081587
» https://doi.org/10.1080/09640568.2015.1081587 - Heilpern SA, DeFries R, Fiorella K, Flecker A, Sethi SA, Uriarte M et al Declining diversity of wild-caught species puts dietary nutrient supplies at risk. Sci Adv. 2021; 7(22):eabf9967. https://doi.org/10.1126/sciadv.abf9967
» https://doi.org/10.1126/sciadv.abf9967 - IBGE. Censo Demográfico Manaus 2020 [Internet]. Manaus; 2020. Available from: https://cidades.ibge.gov.br/brasil/am/manaus/panorama
» https://cidades.ibge.gov.br/brasil/am/manaus/panorama - IBGE Censo Demográfico Rio de Janeiro 2010 [Internet]. Rio de Janeiro; 2010. Available from: https://cidades.ibge.gov.br/brasil/rj/rio-de-janeiro/panorama
» https://cidades.ibge.gov.br/brasil/rj/rio-de-janeiro/panorama - IBGE. Produção da Pecuária Municipal 2018. Rio de Janeiro; 2018. 46:1–08. Available from: https://biblioteca.ibge.gov.br/visualizacao/periodicos/84/ppm_2018_v46_br_informativo.pdf
» https://biblioteca.ibge.gov.br/visualizacao/periodicos/84/ppm_2018_v46_br_informativo.pdf - ICMBio. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Volume VI – Peixes. Brasília, DF. 2018.
- Isaac VJ. Fish catches among riverside communities around Lago Grande de Monte Alegre, Lower Amazon, Brazil. Fish Manag Ecol. 2000; 7:355–74.
- Isaac VJ, Almeida MC. El Consumo del pescado en la Amazonía brasileña. Rome: 2011. Available from: http://www.fao.org/3/i2408s/i2408s.pdf
» http://www.fao.org/3/i2408s/i2408s.pdf - Isaac VJ, Almeida MC, Giarrizzo T, Deus CP, Vale R, Klein G et al Food consumption as an indicator of the conservation of natural resources in riverine communities of the Brazilian Amazon. An Acad Bras Cienc. 2015; 87(4):2229–42. https://doi.org/10.1590/0001-3765201520140250
» https://doi.org/10.1590/0001-3765201520140250 - Isaac VJ, Castello L, Santos PRB, Ruffino ML. Seasonal and interannual dynamics of river-floodplain multispecies fisheries in relation to flood pulses in the Lower Amazon. Fish Res. 2016; 183:352–59. https://doi.org/10.1016/j.fishres.2016.06.017
» https://doi.org/10.1016/j.fishres.2016.06.017 - Jézéquel C, Tedesco PA, Bigorne R, Maldonado-Ocampo JA, Ortega H, Hidalgo M et al A database of freshwater fish species of the Amazon Basin. Sci Data. 2020; 7(96):1–09. https://doi.org/10.1038/s41597-020-0436-4
» https://doi.org/10.1038/s41597-020-0436-4 - Johnson TR, Van Densen WLT. Benefits and organization of cooperative research for fisheries management. ICES J Mar Sci. 2007; 64(4):834–40. https://doi.org/10.1093/icesjms/fsm014
» https://doi.org/10.1093/icesjms/fsm014 - Junk WJ. Temporary fat storage, an adaptation of some fish species to the water level fluctuations and related environmental changes of the Amazon River. Amazoniana. 1985; 9(3):315–51.
- Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci. 1989; 106(1):110–27. Available from: https://ftp.cs.ru.nl/toinesmits/Recommended_readings_IWRM_2009/Water_Ecomorphological_principles/1989JunkThe%20flood%20pulse%20concept%20in.pdf
» https://ftp.cs.ru.nl/toinesmits/Recommended_readings_IWRM_2009/Water_Ecomorphological_principles/1989JunkThe%20flood%20pulse%20concept%20in.pdf - Keppeler FW, Souza AC, Hallwass G, Almeida MC, Azevedo R, Silvano M et al Ecological influences of human population size and distance to urban centres on fish communities in tropical lakes. Aquat Conserv. 2018; 28(5):1030–43. https://doi.org/10.1002/aqc.2910
» https://doi.org/10.1002/aqc.2910 - Lopes PFM, Hallwass G, Begossi A, Isaac VJ, Almeida M, Silvano RAM. The challenge of managing Amazonian small-scale fisheries in Brazil. In: Salas S, Barragán-Paladines M, Chuenpagdee R, editors. Viability and sustainability of small-scale fisheries in Latin America and the Caribbean. MARE Publication Series, vol 19. Cham: Springer; 2019. p.219–41. https://doi.org/10.1007/978-3-319-76078-0_10
» https://doi.org/10.1007/978-3-319-76078-0_10 - Lucas CM. Within flood season variation in fruit consumption and seed dispersal by two characin fishes of the Amazon. Biotropica. 2008; 40(5):581–89. https://doi.org/10.1111/j.1744-7429.2008.00415.x
» https://doi.org/10.1111/j.1744-7429.2008.00415.x - Mattos BO, Pantoja-Lima J, Oliveira AT, Aride PHR. Aquicultura na Amazônia: Estudos técnico-científicos e difusão de tecnologias. Atena Editora; 2021. https://doi.org/10.22533/at.ed.042211503
» https://doi.org/10.22533/at.ed.042211503 - De Mattos Vieira MAR, Von Muhlen EM, Shepard Jr GH. Participatory monitoring and management of subsistence hunting in the Piagaçu-Purus Reserve, Brazil. Conserv Soc. 2015; 13(3):254–64. https://www.jstor.org/stable/26393204
» https://www.jstor.org/stable/26393204 - McGrath DG, Castro F, Futemma C, Amaral BD, Calabria J. Fisheries and the evolution of resource management on the lower Amazon floodplain. Hum Ecol. 1993; 21(2):167–95. https://doi.org/10.1007/BF00889358
» https://doi.org/10.1007/BF00889358 - Montenegro LS, Souza LA. Produção pesqueira e sua relação com as oscilações do ciclo hidrológico e o crescimento demográfico da cidade de Manaus-Am. Sci Amaz. 2016; 5(2):14–23. Available from: http://scientia-amazonia.org/wp-content/uploads/2016/09/v5-n2-14-23-2016.pdf
» http://scientia-amazonia.org/wp-content/uploads/2016/09/v5-n2-14-23-2016.pdf - Moore CH, Harvey ES, Van Niel K. The application of predicted habitat models to investigate the spatial ecology of demersal fish assemblages. Mar Biol. 2010; 157(12):2717–29. https://doi.org/10.1007/s00227-010-1531-4
» https://doi.org/10.1007/s00227-010-1531-4 - Parry L, Davies G, Almeida O, Frausin G, Moraés A, Rivero S et al Social vulnerability to climatic shocks is shaped by urban accessibility. Ann Am Assoc Geogr. 2018; 108(1):125–43. https://doi.org/10.1080/24694452.2017.1325726
» https://doi.org/10.1080/24694452.2017.1325726 - Parry L, Peres CA. Evaluating the use of local ecological knowledge to monitor hunted tropical-forest wildlife over large spatial scales. Ecol Soc. 2015; 20(3):15. https://doi.org/10.5751/ES-07601-200315
» https://doi.org/10.5751/ES-07601-200315 - Pesquisa365. Consumo365 [Internet]. Manaus: 2015. p.14-15. Available from: https://www.slideshare.net/pesquisa365/consumo365
» https://www.slideshare.net/pesquisa365/consumo365 - Petenuci ME, Rocha INA, Sousa SC, Schneider VVA, Costa LAMA, Visentainer JV. Seasonal variations in lipid content, fatty acid composition and nutritional profiles of five freshwater fish from the Amazon basin. J Am Oil Chem Soc. 2016; 93(10):1373–81. https://doi.org/10.1007/s11746-016-2884-8
» https://doi.org/10.1007/s11746-016-2884-8 - Petersen TA, Brum SM, Rossoni F, Silveira GFV, Castello L Recovery of Arapaima sp. populations by community-based management in floodplains of the Purus River, Amazon. J Fish Biol. 2016; 89(1):241–48. https://doi.org/10.1111/jfb.12968
» https://doi.org/10.1111/jfb.12968 - Pinho PF, Orlove B, Lubell M. Overcoming barriers to collective action in community-based fisheries management in the Amazon. Hum Organ. 2012; 71(1):99–109. https://doi.org/10.17730/humo.71.1.c34057171x0w8g5p
» https://doi.org/10.17730/humo.71.1.c34057171x0w8g5p - Pontual FB. The last move of the ribeirinho: Indigenous sovereignty and servitude in the Middle and Lower Rio Negro Basin, Brazilian Amazonia. [PhD Thesis]. Berkeley: University of California; 2014. Available from: https://www.proquest.com/openview/a273ee6fe262f31d9daf644b1906daae/1?pq-origsite=gscholar&cbl=18750
» https://www.proquest.com/openview/a273ee6fe262f31d9daf644b1906daae/1?pq-origsite=gscholar&cbl=18750 - R Core Team. R: A language and environment for statistical computing; 2020.
- Santos GM, Ferreira EJ, Zuanon JA. Peixes comerciais de Manaus. Manaus: ProVárzea; 2006.
- Sirén AH, Machoa J. Fish, wildlife, and human nutrition in tropical forests: a fat gap? Interciencia. 2008; 33(3):186–93. Available from: https://www.redalyc.org/pdf/339/33933306.pdf
» https://www.redalyc.org/pdf/339/33933306.pdf - Soares MGM, Junk WJ, Freitas CEC, Oliveira AM. Pesca comercial e piscicultura do Estado do Amazonas: Estado atual e perspectivas. In: Junk WJ, Piedade MTF, Wittmann F, Schöngart J, editors. Várzeas Amaz. Desafios para um Manejo Sustentável. Manaus: Editora do INPA; 2020. p.207–25.
- Sodhi NS. Tropical biodiversity loss and people – A brief review. Basic Appl Ecol. 2008; 9(2):93–99. https://doi.org/10.1016/j.baae.2007.11.001
» https://doi.org/10.1016/j.baae.2007.11.001 - Thurstan RH, Buckley SM, Ortiz JC, Pandolfi JM. Setting the record straight: Assessing the reliability of retrospective accounts of change. Conserv Lett. 2015; 9(2):98–105. https://doi.org/10.1111/conl.12184
» https://doi.org/10.1111/conl.12184 - Tregidgo D, Barlow J, Pompeu PS, Almeida M, Parry L. Rainforest metropolis casts 1,000-km defaunation shadow. Proc Natl Acad Sci U S A. 2017; 114(32):8655–59. https://doi.org/10.1073/pnas.1614499114
» https://doi.org/10.1073/pnas.1614499114 - Tregidgo D, Barlow J, Pompeu PS, Parry L. Tough fishing and severe seasonal food insecurity in Amazonian flooded forests. People Nat. 2020; 2(2):468–82. https://doi.org/10.1002/pan3.10086
» https://doi.org/10.1002/pan3.10086 - Tsikliras AC, Polymeros K. Fish market prices drive overfishing of the ‘big ones.’ PeerJ. 2014; 2:e638. https://doi.org/10.7717/peerj.638
» https://doi.org/10.7717/peerj.638 - Vasconcelos LP, Alves DC, Câmara LF, Hahn L. Dams in the Amazon: The importance of maintaining free-flowing tributaries for fish reproduction. Aquat Conserv Mar Freshw Ecosyst. 2021; 31(5):1106–16. https://doi.org/10.1002/aqc.3465
» https://doi.org/10.1002/aqc.3465 - Wilkie DS, Morelli GA, Demmer J, Starkey M, Telfer P, Steil M. Parks and people: Assessing the human welfare effects of establishing protected areas for biodiversity conservation. Conserv Biol. 2006; 20(1):247–49. https://doi.org/10.1111/j.1523-1739.2005.00291.x
» https://doi.org/10.1111/j.1523-1739.2005.00291.x - Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016; 351(6269):128–29. https://doi.org/10.1126/science.aac7082
» https://doi.org/10.1126/science.aac7082
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Tregidgo D, Parry L, Barlow J, Pompeu PS. Urban market amplifies strong species selectivity in Amazonian artisanal fisheries. Neotrop Ichthyol. 2021; 19(3):e200097. https://doi.org/10.1590/1982-0224-2020-0097
Edited-by
Publication Dates
-
Publication in this collection
11 Oct 2021 -
Date of issue
2021
History
-
Received
25 Nov 2020 -
Accepted
26 July 2021