Abstract
Cadmium and Mercury induced varying responses in Abelmoschus esculentus L. in relation to enzymes (ascorbate peroxidase (APX, 1.11.1.11), catalase (CAT, 1.11.1.6), glutathione reductase (GR, 1.6.4.2) and superoxide dismutase (SOD, 1.15.1.1) which are most related to the levels of Hg and Cd applied and concentrations of thiol groups already present or induced upon treatment. In the present investigation varying concentrations of CdCl2 and HgCl2 (0, 0.05, 0.10, 0.50, 1 and 2mM respectively) applied to plant in the soil shows a significant increase in ascorbate peroxidase, glutathione reductase and superoxide dismutase activities, and the respective metal accumulation. It reveals a mechanism for constant detoxification of H2O2 that have to be associated with adaptations and ultimate survival of this plant species during stress conditions. A reduction of catalase activities was observed on exposure to these metals, which is supposedly due to the inhibition of enzyme synthesis. Root length, shoot length, number of leaves showed an enhancement with 0.05 mM CdCl2 dose then a gradual decline with the increase in concentrations. The results indicate that A. esculentus is tolerant to high concentrations of these metals, a property related to a differential activation of its enzymatic antioxidant system, and also reveal that this species has a higher capacity of Cd absorption.
antioxidative enzymes; metals detoxification; reactive oxygen species
RESEARCH ARTICLE
Differential activation of the enzymatic antioxidant system of Abelmoschus esculentus L. under CdCl2 and HgCl2 exposure
Asiya Hameed; Tabasum N. Qadri, Mahmooduzzafar; T.O. Siddiqi and M. Iqbal
Department of Botany, Hamdard University Hamdard Nagar, N. Delhi-110062, India
ABSTRACT
Cadmium and Mercury induced varying responses in Abelmoschus esculentus L. in relation to enzymes (ascorbate peroxidase (APX, 1.11.1.11), catalase (CAT, 1.11.1.6), glutathione reductase (GR, 1.6.4.2) and superoxide dismutase (SOD, 1.15.1.1) which are most related to the levels of Hg and Cd applied and concentrations of thiol groups already present or induced upon treatment. In the present investigation varying concentrations of CdCl2 and HgCl2 (0, 0.05, 0.10, 0.50, 1 and 2mM respectively) applied to plant in the soil shows a significant increase in ascorbate peroxidase, glutathione reductase and superoxide dismutase activities, and the respective metal accumulation. It reveals a mechanism for constant detoxification of H2O2 that have to be associated with adaptations and ultimate survival of this plant species during stress conditions. A reduction of catalase activities was observed on exposure to these metals, which is supposedly due to the inhibition of enzyme synthesis. Root length, shoot length, number of leaves showed an enhancement with 0.05 mM CdCl2 dose then a gradual decline with the increase in concentrations. The results indicate that A. esculentus is tolerant to high concentrations of these metals, a property related to a differential activation of its enzymatic antioxidant system, and also reveal that this species has a higher capacity of Cd absorption.
Key words: antioxidative enzymes, metals detoxification, reactive oxygen species
INTRODUCTION
Anthropogenic activities such as mining and smelting and the respective metal pollution are becoming a major risk to many ecosystems. Among the pollution producing metals, cadmium and mercury have attracted much attention since both have been proved to be very toxic to plants and animals, and are extremely persistent in the environment (Salt et al., 1995). In plants, heavy metal toxicity, including that promoted by Cd and Hg, varies from slight injury to lethality or crop failure. The general symptoms are stunting growth, chlorosis and alteration of anatomical, morphological, physiological and biochemical properties of leaf, stem and roots (Liu et al., 2000; Rubio et al., 1994; Godbold and Huttermann, 1985).
Contamination of soils and water with metals is becoming a major environmental problem, leading to considerable losses in plant productivity and hazardous health effects. Exposure to toxic metals can intensify the production of reactive oxygen species (ROS), which are continuously produced in both unstressed and stressed plant cells. Higher plants have evolved various protective mechanisms to eliminate or reduce the excessive cellular ROS accumulation and the related oxidative injury (Asada, 1999). One of them is the activation of enzymatic antioxidant systems, including the enzymes APX, CAT, GR and SOD. Each of these enzymes has physiological functions under non-stressed conditions, but their activities are increased under many environmental stresses, which induce a variety of ROS such as superoxide anion radical (O2-), hydrogen peroxide (H2O2), hydroxyl radicals (OH-) and singlet oxygen (1O2) (Elstner, 1982; Jung, 2004). Moreover, in addition to the antioxidant enzymes, plants also can activate non-enzymatic systems such as the production of tocopherol, carotene, Glutathione-S-Transferase (GSH) and ascorbate, which are involved in the scavenging of reactive oxygen species against heavy metal toxicity (Rosen, 2002).
Heavy metals once accumulated at toxic levels inside plant tissues can inhibit most physiological and metabolic processes. However, the extent of the induced inhibition on plant growth, photosynthesis, ions and water uptake, and nutrients assimilation is greatly dependent on the concentration of the metal ions, and on the thresholds of toxicity characteristic of the plant species. Therefore, the metal concentration and the level of susceptibility of the germplasm are always key factors to be analyzed towards interpreting the heavy metal effects on the physiology of a yet unexplored or underexplored plant species.
Heavy metal contamination of soil resulting from wastewater irrigation is a cause of serious concern particularly in poor tropical regions mainly due to the potential health impacts by consuming contaminated vegetables. The present study analyses the effect of different concentrations of cadmium (Cd2+) and mercury (Hg2+) on physiological aspects of bhindi plants (Abelmoschus esculentus L.) with special reference to oxidative stress to further explore their contrasting inhibitory and stimulatory effects on productivity of this still underexplored plant species.
MATERIALS AND METHODS
Plant material: Abelmoschus esculentus was selected for present study for its economic importance, mainly for the tropical zone of developing and non-developed countries. It is cultivated especially in Africa, Brazil and India, where it is also commonly known as Okra, Quiabo and lady's finger, respectively. The tender pods are used as vegetable, ripe seeds, which are rich in protein (18-26%) are roasted and can be used as substitute for coffee. Immature pods are emollient, demulcent and diuretic and are employed in the form of decoction in catarrhal affections, dysuria and gonorrhea. Seeds are stimulant, cordial and antispasmodic. Fatty fraction of fresh watery extract of seeds impaired cancerous cell growth in vitro (CSIR, 1985).
Experimental setup: Healthy and authentic seeds of A. esculentus were obtained from Indian Agricultural Research Institute, New Delhi. New Delhi is situated 28.38 ` N latitude and 77.11' E longitude at an altitude of 228 m above the mean sea level. The soil is formally loam and clayey loam with pH 6.8-7.2. It has a semi arid and sub-tropical climate with extreme of hot weather in summer and cold in winter. The maximum rainfall, 80-100 cm, is observed in July and August, winter showers are accompanied with high velocity winds and hale storms. The relative humidity increases from 45% in June to 81% in July and August. Wind velocity is 2 m sec-1. The temperature exceeds up to 45ºC.
Experiments were conducted under natural conditions in twelve inch earthen pots containing 10 kg of soil per pot. The pots were arranged in randomized design for a possible uniform light condition. Four to five seeds were sown in each pot at 2.5 cm depth, and after germination, the seedlings were thinned to three plants per pot. The treatments were given to the healthy plants 15-20 days after germination. The metals applied to the soil were cadmium, mercury and their combinations in the form of CdCl2, HgCl2 and CdCl2+HgCl2. These salts were added to the soil of the experimental pots individually in the following doses: 0.05 mM, 0.10 mM, 0.50 mM, 1 mM and 2 mM for CdCl2, HgCl2 and their combinations i.e., (CdCl2+HgCl2) respectively. The background concentrations of cadmium and mercury in the experimental soil were 0.03 mM and 0.02 mM respectively. Soil pH at all treatments was almost similar at the beginning of the experiment. In the control plants no metal treatment was given. Few gm green manure was given to all experimental plants, treated as well as control, for a better growth. These plants were suitably irrigated with de-ionized water to provide a possible uniform soil moisture conditions.
The experiments were repeated during three successive seasons. The sowing for the plant was done in the first week of March and harvested in the last week of May. The sampling was done for laboratory analysis at three developmental stages, i.e., at pre-flowering, flowering and post-flowering stages.
Root length and shoot length was recorded with the help of a centimeter scale and expressed as centimeter per scale. Cadmium and mercury in the leaves of A. esculentus L. was determined by digestion of dried plant material in concentrated HNO3-HClO4 (3:1). Metal ion concentrations were determined by atomic absorption spectrophotometer.
Ascorbate peroxidase (APX) activity was determined as previously described (Nokano and Asada, 1981) by the decrease in absorbance of ascorbate at 290 nm due to its enzymatic breakdown on Uv-vis spectrophotometer (Model DU 640, Beckman, USA). 1.0 mL of the reaction buffer contained 0.5 mM ascorbate, 0.1 mM H2O2, 0.1 mM EDTA and enzyme extract. The reaction was allowed to run for 3/5 minutes at 250C. APX activity was calculated by using extinction coefficient (e) 2.8 mM-1 cm-1 and expressed in Enzyme Units (EU) mg-1 protein. One unit of enzyme determines the amount necessary to decompose 1 mmol of substrate consumed per minutes at 250C.
1.0 g of the fresh material was ground in 4 ml of extraction buffer and centrifuged at 10,000 x g for 10 minutes at 40C. The supernatant was collected and used for the assay immediately or kept under deep freeze conditions.
Catalase (CAT) activity was determined as previously described (Aebi, 1984), by monitoring the disappearance of H2O2 by measuring a decrease in absorbance at 240 nm on Uv-vis spectrophotometer (Model DU 640, Beckman, USA). Reaction was carried in a final volume of 2 ml of reaction mixture containing reaction buffer with 0.1 mL 3 mM EDTA, 0.1 mL of enzyme extract and 0.1 mL of 3 mM H2O2.The reaction was allowed to run for 5 minutes. Activity was calculated by using e = 0.036 mM -1 cm-1 and expressed in Enzyme Units (mg-1 protein). One unit of enzyme determines the amount necessary to decompose 1 mmol of H2O2 per minutes at 25 0C. One gram of the fresh material was ground in 4 mL of extraction buffer and centrifuged at 10,000 x g for 10 minutes at 40C. The supernatant was collected and used for the assay immediately or kept under deep freeze conditions.
Glutathione Reductase (GR) activity was determined as previously described (Foyer and Halliwell, 1976; modified by Rao, 1992), by monitoring the glutathione-dependent oxidation of NADPH at its absorption maxima of the wavelength 340 nm on Uv-vis spectrophotometer (Model DU 640, Beckman, USA). 1.0 mL reaction mixture contained 0.2 mM NADPH, 0.5 mM GSSG and 50 µL of enzyme extract. The reaction was allowed to run for 5 minutes at 250C. Corrections were made for any GSSG oxidation in the absence of NADPH. The activity was calculated by using e = 6.2 mM-1 cm-1 and expressed in Enzyme Units mg-1 protein. One unit of enzyme determines the amount necessary to decompose 1 mmol of NADPH per minutes at 250C. 0.5 g of the fresh material was ground in 2 mL of extraction buffer and centrifuged at 10,000 x g for 10 minutes. The supernatant was collected and used for the assay immediately or stored in deep freeze condition.
Superoxide dismutase (SOD) activity was determined as previously described (Dhindsa et al., 1981), in the supernatant, by its ability to inhibit the photochemical reduction. The assay mixture, consisting of 1.5 mL reaction buffer, 0.2 mL of methionine, 0.1 mL enzyme extract with equal amount of 1 M Na2CO3, 2.25 mM NBT solution, 3 mM EDTA, riboflavin and 1.0 ml of DDW was taken in testube which were incubated under the light of 15 W influorescent lamp for 10 minutes at 280C. Blank A, containing all the above substances of the reaction mixture, along with the enzyme extract was placed in the dark. Blank B, containing all the above substances of reaction mixture except enzyme was placed in light along with the sample. The reaction was terminated by switching off the light and the tubes were covered with a black cloth. The non-irradiated reaction mixture containing enzyme extract did not develop light blue color. Absorbance of samples along with blank B was read at 560 nm against the blank A. One gram of fresh material was homogenized in 2.0 mL of extraction mixture with the help of mortar and pestle. The process was carried out under cold condition (40C). The mortar and pestle was kept in ice during the course of homogenization. The homogenate was transferred to centrifuge tubes and centrifuged at 40C.
The difference of percentage of reduction in the color between blank B and the sample was then calculated. Fifty percent reduction in the color was considered as one unit of enzyme activity and the activity was expressed in Enzyme Unit (EU) mg-1 protein h-1. Statistical analysis were carried out by two-way classification of Anova to evaluate whether the means were significantly different (p ≤ 0.05) as previously described (Cochran and Cox, 1957).
RESULTS
The efficiency of the metal treatments was characterized by their effects on Abelmoschus esculentus growth. In agreement with literature data, an increase in root and shoot length was verified, and number of leaves induced by the lower (0.05 mM) CdCl2 concentration. Thereafter, a gradual decline was observed with the increase of the metal concentration. In case of HgCl2, no stimulatory effect could be found, only a gradual decrease in both root and shoot growth was detected (not shown).
All the enzymes studied in non treated plants exhibited maximum activities at the flowering stage (Tables 1-8). However, the ascorbate peroxidase activity increased with the increase in heavy metal concentrations at all stages of development studied. The maximum stimulatory effect on this enzyme reached values higher than 7000 % with 2 mM HgCl2 and 1400 % with 2 mM CdCl2 at post-flowering stage of growth (Tables 1 and 2).
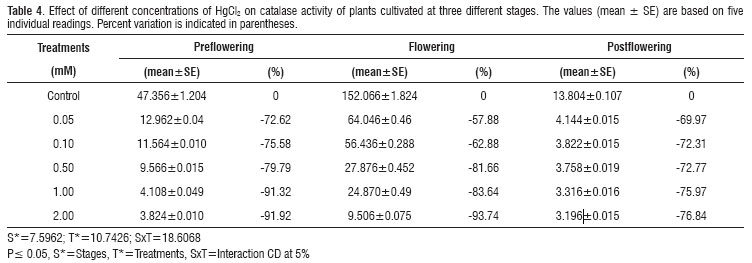
Catalase activity decreased markedly with respect to the non-treated control at all the stages of growth and in the treatments with both CdCl2 and HgCl2. Maximum inhibition of about 97 % was observed with 2 mM CdCl2 and of about 94 % with 2 mM HgCl2 at the flowering stage (Tables 3 and 4).
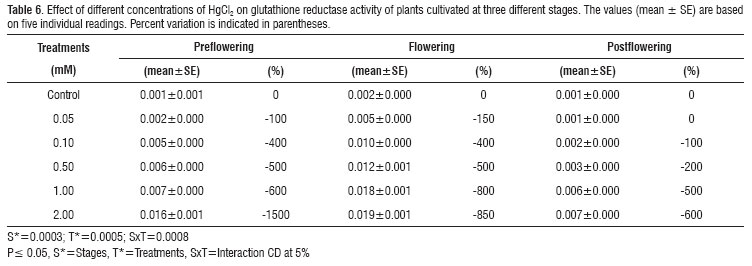
Glutathione reductase activity increased considerably with the application of both heavy metals in A. esculentus. Maximum stimulus was observed to be 2250 % with 2 mM CdCl2 and 850 % with 2 mM HgCl2 at the flowering stage (Tables 5 and 6).
Superoxide dismutase (SOD) is a key enzyme of antioxidant system responsible for detoxification of superoxide anion and therefore its activity was also measured to assess the magnitude of CdCl2 and HgCl2 induced oxidative stress as well as the antioxidant potential of A. esculentus. The SOD activities increased during all stages and in both treatments (Tables 7 and 8).
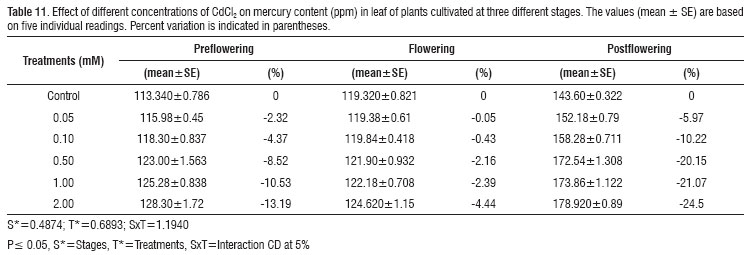
Cadmium and mercury contents in leaves of the non-treated controls increased with the plants age, reflecting the capacity of A. esculentus to capture and accumulate heavy metals present in the environment. In treated plants, Cd content increased in the leaf tissues with the increase in the metal concentration, reaching a maximum stimulation of about 700 % with 2 mM CdCl2 and 450 % with 2 mM HgCl2 at the flowering stage (Table 9 and 10). However, the maximum value for the Hg content in leaves was observed with 2 mM CdCl2 and was about 25 % higher at post-flowering stage of growth (Table 11), while the maximum value obtained with 2 mM HgCl2 was found to be 90 % higher at post-flowering stage of growth (Table 12).
DISCUSSION
In the present study, Cd and Hg were found to adversely affect plant growth and metabolism in A. esculentus except at 0.05mM of CdCl2 which showed a positive effect. Root length, shoot length and number of leaves decreased with Cd and Hg treatments except at 0.05mM of CdCl2 as previously described not only to Cd, but also to Cu and Al treatments (Barnabas et al., 2000; Horvath et al., 1996). The obtained data are also in line with the notion that the symptoms of Cd and Hg toxicity include stunted growth, leaf chlorosis and alteration in the activity of different enzymes of various metabolic pathways (Godbold and Huttermann, 1985; Arduini et al., 1996).
Heavy metal concentrations varied with species and the parts considered for analysis. This is probably due to variable capabilities of plants to absorb and accumulate heavy metals. Furthermore, variations in growth period and growth rates (Moseholm et al., 1992) as well as physical and chemical properties of soil also affect the heavy metal uptake (verloo and Eeckhout, 1990). Plants have a high capacity to take nutrients and trace elements available in the air and in the soil environment, and specifically the root uptake of Cd has been shown to be strongly related to its chemical form and solubility when present in the soil solution (Pandey et al., 2009).
The protective mechanisms developed by plants to scavenge free radicals and peroxides over-produced in response to heavy metals exposure include several antioxidative enzymes such as APX, CAT, GR and SOD. In fact, these enzymes are key components in preventing the oxidative stress in plants as the activity of one or more of these enzymes is generally increased in plants when exposed to stressful conditions (Malekzadeh et al., 2007b).
A Cd-induced increase in antioxidant enzyme activities was also reported by Shah et al. (2001) and Lannelli et al. (2002). The enhanced activity with Cd treatment was also reported in barley seedlings (Hegedus et al., 2001). It appears that Cd-induced increase in antioxidant enzyme activities is a consequence of active oxygen species overproduction (Thompson et al., 1987).
The present study indicated that Hg-exposure resulted in an increase in H2O2 content in plants. Although the mechanism of Hg induced H2O2 formation is not presently known, heavy metals are known to be involved in production of active oxygen species in many ways (Luna et al., 1994). The H2O2 accumulation after Hg-exposure may be produced in a manner similar to H2O2 in cold-stressed plants (Prasad et al., 1994). The susceptibility to oxidative stress is a function of the overall balance between the factors that increase oxidant generation and those substances that exhibit antioxidant capability (De vos et al., 1991; Foyer et al., 1994).
Cadmium and mercury enhanced the A. esculentus APX activity. It was also studied by Schutzendubel et al. (2001), when pine seedlings treated with Cd, H2O2 accumulation was followed within few hours and significant increase in APX activity was observed. Therefore, the present study suggests its role in constant detoxification of H2O2 in A. esculentus. It may also be attributed to adaptations and ultimate survival of the plant during the period of stress.
In the present study a decrease in catalase (CAT) activity was observed in both Cd and Hg treatments. This result coincides with experiments in Phaseolus aureus (Shaw, 1995); Pisum sativum (Dulurzo et al., 1997) and Amaranthus levidus (Battacharjee, 1998) following the application of Cd to growth medium. Decreased CAT activity has also been observed in Phaseolus vulgaris (Chaoui et al., 1997; Somashekariah et al., 1992) and pea (Sandalio et al., 2001). Shah et al (2001) and vaglio and Landriscina (1999) also described a general reduction of CAT activities upon Cd exposure. The data are also in line with The results obtained by Moussa (2005) where CdCl2 treated faba beans showed a concentration-dependent oxidative stress situation in the leaves, characterized by an accumulation of H2O2, as a result of the inhibition of the CAT. It is also regarded as a general response to many stresses and is supposedly due to the inhibition of enzyme synthesis or change in assembly of enzyme subunits (Mac-Rac and Fergusson, 1985). Catalase is mainly present in peroxisomes and mitochondria, which often decreased following exposure to elevated Cd concentrations (Fornazier et al., 2002; Shim et al., 2003). The decrease may also be associated with degradation caused by induced peroxisomal proteases or may be due to photoinactivation of enzyme.
The increase in APX activity induced by Hg was reported in seedlings of Phaseolus aureus (Shaw, 1995). The APX stimulation has also been verified in several plants subjected to Cd, zn, Cu, Pb and Fe treatment (Patra and Panda, 1998; Prasad et al., 1999). Therefore, increased activities of SOD and APX and other antioxidative enzymes under heavy metals treatments may be considered as circumstantial evidence for tolerance mechanism evolved by the plant species.
The present investigation demonstrated an enhanced activity of Glutathione Reductase in response to increasing concentrations of HgCl2, a result which can often be exacerbated by the addition of GSSG and ameliorated by NADPH (Halliwell and Foyer, 1978; Serrano et al., 1984). Activation of the ascorbate-glutathione cycle has been found to be essential in stressed plants to combat oxidative damage (Alscher et al., 1997). Although ascorbate, an oxidant and a major metabolite in plants and enzymes involved in its metabolism were not monitored, the increase in APX and GR activates in Cd-exposed A. esculentus maintains ascorbate and glutathione turnover and activation of the H2O2 scavenging ascorbate-glutathione cycle. Also the similar RESULTSwith Cd-exposure were noticed in Phaseolus vulgaris and Alyssum (Chaoui et al., 1997; Schickler and Caspi, 1999). The increased activity of GR could be explained by transcriptional or translational modification to keep an adequate GR level to protect plant against Cd stress (Romero-Puertas et al., 2002; Xiang and Oliver, 1998). The participation of the GR pathway activated upon Hg stress has also been observed in our study, is the best documented role for this enzyme (Chaoui et al., 1997; Stroinski et al., 1999).
Superoxide Dismutase (SOD) represents a key element of the enzymatic system that protects the plant cell against deleterious peroxidation reactions (Monk et al., 1998). Leaves of A. esculentus show an enhancement in SOD activity upon Cd and Hg stress. Increases in SOD activity is often attributed to de-novo synthesis of the enzyme and shown to confer increased protection from oxidative damage in transgenic plants (Allen et al., 1997). Similar SOD enhancement under a variety of stressful conditions including Cu, Al, Mn, Fe and zn toxicities have been observed in different plant species by Prasad (1997) as well as Cakmak and Horst (1991). It is postulated that the activation of SOD enzymes exerts a pivotal role during metal stress resistance and for the maintenance of overall defense system of plants subjected to this kind of oxidative damage (Slooten et al., 1995).
In the present study the increase in H2O2 was concentration dependent in the leaves of A. esculentus for Hg as well as Cd treated plants. Our RESULTSare also in agreement with the results of Schickler and Caspi. (1999) in Alyssum which may be attributed to the increased production of ROS (Somashekariah et al., 1992) and also related to an increased expression of genes encoding SOD (Bowler et al., 1992). Increased SOD activities were also found in Cdexposed fungi and marine microalgae (Jacob et al, 2001; Collen et al., 2003; Guelfi et al., 2003) and in a number of Cd/Hg-exposed plant species (Chaoui et al., 1997; Elstner et al., 1988; Schickler and Caspi, 1999).
In conclusion, the higher amount of Cd and Hg accumulation in leaves of A. esculentus reveal that the tolerance of the species seems to be genetically determined by the capability to transport those heavy metals from roots to shoots where they are compartmentalized in the cells of leaves, with minimal effects on the growth and yield of the plant. The data also revealed a coordinated increase in the activities of SOD, APX and GR in the leaves upon Cd and Hg stress, suggesting a key role for those enzymes in Cd and Hg tolerance of A. esculentus. The results suggest that A. esculentus has a potential to be used as a phytoremediator in areas contaminated with Cd due to its higher antioxidant potential and lesser susceptibility to Cdstress. Further studies under field conditions are needed to evaluate the level of Cd accumulation in the A. esculentus seeds and fruits towards a responsible recommendation for the commercialization of the products derived from plants grown in polluted areas.
Acknowledgement: The authors would like to thank Mr. Sharma Senior Assistant of central instrumental facility (CIF) as well as Mr. Saleem and Mr. Irshad (Lab. Assistants) in the Department of Botany for there timely help and support.
Received: 11 December 2009
Accepted: 22 February 2011
- Aebi H (1984) Catalase in vitro. Methods Enzymol. 105: 121-126.
- Allen RD, Webb RP, Schake SA (1997) Use of transgenic plants to study antioxidant defenses. Free Radical, Biol. Med. 23: 472-479.
- Alscher RG, Dooahoe JL, Cramer Cl (1997) Reactive oxygen species and antioxidants: relationships in green cells. Plant Physiol. 100: 224-233.
- Arduini I, Goldbild DL, Onnis A (1996) Cadmium and copper uptake and distribution in Mediterranean tree seedlings. Plant Physiol. 155: 111-117.
- Asada, K (1999) The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annu. Rev. Plant physiol. Plant Mol Biol. 50: 601.
- Barnabas B, Kovacs G, Hegedus A, Erdei S, Horvath G (2000) Regeneration of double haploid plants from in vitro selected microspores to improve aluminium tolerance in wheat. Plant Physiol. 156: 217-222.
- Battacharjee S (1998) Membrane lipid peroxidation, free radical scavengers and ethylene evolution in Amaranthus as affected by lead and cadmium. Biol. Plant. 40: 131-135.
- Bowler C, van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 43: 83-116.
- Cakmak I, Horst JW (1991) Effect of aluminium on lipid peroxidation , SOD, CAT, POX activities in root tips of Glycine max. Plant Physiol. 83: 463-468.
- Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127:139-147.
- Cochran WG, Cox GM (1957) Experimentl Designs. pp. 127-131.
- Collen J, Pinto E, Pedersen M, Colepicolo P (2003) Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Arch Environ Contam Toxicol. 45: 337-342.
- CSIR (1985) The wealth of India. Counsel of Scientific and Industrial Research. New Delhi. vol I: 1-13.
- De vos CHR, Schat H, De Waal MAM, voojis R, Ernst WHO (1991) Increased resistance to copper-induced damage of the root cell plasmalemma in copper-tolerant Silene cucubalus Physiol Plant. 82: 523-528.
- Dhindsa RH, Dhindsa P, Thorpe TA (1981) Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased level of SOD and CAT. J Exp Bot. 32: 93-101.
- Dalurzo HC, Sandalio LM, Gomez M, Rio del LA (1997) Cadmium infilteration of detached pea leaves: Effect on its activated oxygen metabolism. Phyton Ann Rei Bot. 37: 59-64.
- Elstner EF, Wagner GA, Schultz W (1988) Activated oxygen in green plants in relation to stress situations. Curr Top Plant Biochem Physiol. 7: 159-187.
- Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 33:73-96.
- Fornazier RF, Ferreira RR, vitoria AP, Molina SMG, Lea PJ, Azevedo RA (2002) Effects of cadmium on antioxidant enzymes in sugar cane. Biol.Plantarum. 45: 91-97.
- Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta. 133: 21-25.
- Foyer CH, Lelandais M, Kunert JK (1994) Photooxidative stress in plants. Plant Physiol. 92:696-717.
- Godbold DL, Huttermann A (1985) Effect of zinc, cadmium and mercury on root elongation of P. abies (Karst) seedlings and the significance to forest dieback. Environ. Pollut. 38: 375-381.
- Halliwell B, Foyer CH (1978) Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 139: 9-17.
- Hegedûs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 160:1085-1093.
- Horvath G, Droppa M, Oraveez A, Raskin vI, Marder JB (1996) Formation of the photosynthetic apparatus during greening of cadmium-poisoned barley leaves. Planta. 199: 238-243.
- Jacob C, Courbot M, Brun A, Steinman HM, Jaquot JP, Botton B, Chalot M (2001) Molecular cloning, characterizing and regulation by cadmium of a superoxide dismutase from the ectomycorrhizal fungus Paxillus involutus Eur. J. Biochem. 268, 3223-3232.
- Jung S (2004) variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Sci. 166: 459-466.
- Lannelli MA, Pietrni F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites anstrals plants. Plant Physiol Biochem. 40: 977-982.
- Liu J, Reid RJ, Smith FA (2000) The mechanism of cobalt toxicity in mung beans. Plant Physiol. 110: 104-110.
- Luna CM, Gonzalez CA, Trippi vS (1994) Oxidative damage caused by excess of copper in oat leaves. Plant Cell Physiol. 35: 11-15.
- MacRae EA, Fergusam IB (1985) Changes in catalase activity and H2O2 concentration in plants in response to low temp. Plant Physiol. 65: 51-56.
- Malekzadeh P, Khara J, Farshian S, Jamal-Abad AK, Rahmatzadeh S (2007b) Cadmium toxicity in maize seedlings: Change in antioxidant enzyme activities and root growth. Pak. J. Biol. Sci. 10: 127-131.
- Monk LS, Fogerstedt Kv, RMM (1998) Oxygen toxicity and SOD as an antioxidant in physiological stress. Plant Physiol. 76: 456-459.
- Moseholm L, Larsen E H, Andersen B, Nielsen MM (1992). Atmospheric deposition of trace elements around point sources and human health risk assessment. I: impact zones near a source of lead emissions. Sci.Total Environ, 126, 243-262.
- Moussa HR ( 2005) Effect of cadmium on growth and oxidative metabolism of faba bean plants. Acta Agron. Hung 269-276
- Nakano y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867-880.
- Nakano y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbatespecific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22: 867-880.
- Pandey J, Pandey U (2009) Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environ Monit Assess. 148:61-74.
- Patra J, Panda BB (1998) A comparison of biochemical responses to oxidative and metal stress in seedlings of barley Hordeum vulgare L. Environ Pollut. 101: 99-105.
- Prasad KvSK, Saradhi PP, Sharmila P (1999) Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea Environ. Exp. Bot. 42: 1-10.
- Prasad MNv (1997) Trace metals In: Prasad MNv (Ed) Plant Ecophysiol, wiley, New york, 207-249.
- Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 6: 65-74.
- Rao Mv (1992) Cellular detoxification mechanisms to determine age dependent injury in tropical plant exposed to SO2. J Plant Physiol. 140: 733740.
- Romero-Puertas MC, Palma JM, Gomez M, del Rio LA, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 25: 677-686.Rosen BP (2002). Biochemistry of arsenic detoxification. FEBS. Lett. 076 529: 86-92.
- Rubio MI, Escrig I, Martinez Cortina C, Lopez-Benet FJ, Sanz A (1994) Cadmium and nickel accumulation in rice plants: effect on mineral nutrition and possible interaction of abscisic and gibberelic acids. Plant Growth Regul. 14: 151-157.
- Salt DE, Blaylock M, Kumar NPBA, Dushenkov v, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnol. 13: 468-474.
- Sandalio LM, Dalura HC, Gomez M, Romero-Puertas MC, del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot. 52: 2115-2126.
- Schickler H, Caspi H (1999) Response of antioxidant enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum Plant Physiol. 105: 39-44.
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, H2O2 content and differentiation in pine (Pinus sylvestris) roots. Plant Physiol. 127: 887-892.
- Serrano A, Rivas J, Losada M (1984) Purification and properties of glutathione reductase from the cyanobacterium Anabaena sp. Strain 7119. J. Bacteriol. 158: 317-324.
- Shah K, Kumar RG, verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedling. Plant Sci. 161: 1135-1144.
- Shaw BP (1995) Effects of Hg and Cd on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol. Plant. 37: 587-596.
- Shaw BP (1995) Effects of mercury and cadmium on the activities of antioxidative enzymes in seedlings of Phaseolus aureus. Biol. Plantarum. 37: 587-596.
- Shim IS, Momose y, yamamoto A, Kim DW, Usui K (2003) Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul. 39: 285-292.
- Slooten L, Capiau K, van Montago W, Sybesma MC, Inze D (1995) Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco over expressing Mn-SOD in the chloroplast. Plant Physiol. 107: 737-750.
- Somashekaraiah Sv, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): Involvement of lipid peroxidation in chlorophyll degradation. Plant Physiol. 85: 85-89.
- Stroinski A, Kubis, J, zielezinska M (1999) Effect of cadmium on glutathione reductase in potato tubers. Acta Physiol Plant. 21: 201-207.
- Thompson JE, Legge RL, Barber RF (1987) The role of free radical in senescence and wounding. New Phytol. 105: 317-344.
- Vaglio A Landriscina C (1999) Changes in liver enzyme activity in the teleost Sparus aurata in response to cadmium intoxication .Ecotoxicol. Environ. Safety. 43: 111-116.
- Verloo M, Eeckhout M (1990). Metals species transportation in soil: an analytical approach. Int. J.Environ. Anal.Chem, 39, 179-186.
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes co-ordinately respond to heavy metals and jasmonic acid in Arabidopsis Plant Cell. 10: 1539-1550.
Publication Dates
-
Publication in this collection
16 Aug 2011 -
Date of issue
2011
History
-
Accepted
22 Feb 2011 -
Received
11 Dec 2009