Abstract
The human respiratory syncytial virus (hRSV) is the most common cause of severe lower respiratory tract diseases in young children worldwide, leading to a high number of hospitalizations and significant expenditures for health systems. Neutrophils are massively recruited to the lung tissue of patients with acute respiratory diseases. At the infection site, they release neutrophil extracellular traps (NETs) that can capture and/or inactivate different types of microorganisms, including viruses. Evidence has shown that the accumulation of NETs results in direct cytotoxic effects on endothelial and epithelial cells. Neutrophils stimulated by the hRSV-F protein generate NETs that are able to capture hRSV particles, thus reducing their transmission. However, the massive production of NETs obstructs the airways and increases disease severity. Therefore, further knowledge about the effects of NETs during hRSV infections is essential for the development of new specific and effective treatments. This study evaluated the effects of NETs on the previous or posterior contact with hRSV-infected Hep-2 cells. Hep-2 cells were infected with different hRSV multiplicity of infection (MOI 0.5 or 1.0), either before or after incubation with NETs (0.5–16 μg/mL). Infected and untreated cells showed decreased cellular viability and intense staining with trypan blue, which was accompanied by the formation of many large syncytia. Previous contact between NETs and cells did not result in a protective effect. Cells in monolayers showed a reduced number and area of syncytia, but cell death was similar in infected and non-treated cells. The addition of NETs to infected tissues maintained a similar virus-induced cell death rate and an increased syncytial area, indicating cytotoxic and deleterious damages. Our results corroborate previously reported findings that NETs contribute to the immunopathology developed by patients infected with hRSV.
Keywords:
viral infection; innate immunity; human respiratory syncytial virus; neutrophils; NETs
Resumo
O vírus sincicial respiratório humano (hRSV) é a causa mais comum de doenças graves do trato respiratório inferior em crianças pequenas em todo o mundo, resultando em grande número de hospitalizações e gastos significativos para os sistemas de saúde. Neutrófilos são recrutados em massa para o tecido pulmonar de pacientes com doenças respiratórias agudas. No local da infecção, eles liberam armadilhas extracelulares de neutrófilos (NETs) que podem capturar e/ou inativar diferentes tipos de microrganismos, incluindo vírus. Evidências demonstraram que o acúmulo de NETs resulta em efeitos citotóxicos diretos nas células endoteliais e epiteliais. Os neutrófilos estimulados pela proteína F do vírus sincicial respiratório (hRSV-F) geram NETs que são capazes de capturar partículas virais, reduzindo assim sua transmissão. No entanto, a produção maciça de NETs obstrui as vias aéreas e aumenta a gravidade da doença. Assim, um maior conhecimento sobre os efeitos das NETs durante as infecções por hRSV é essencial para o desenvolvimento de novos tratamentos específicos e eficazes. Este estudo avaliou os efeitos das NETs no contato prévio ou posterior à infecção de células Hep-2 com hRSV. As células Hep-2 foram infectadas com diferentes quantidades de hRSV (multiplicidade de infecção ou MOI 0,5 ou 1,0), antes ou após a incubação com NETs (0,5–16 μg/mL). Células infectadas e não tratadas mostraram redução da viabilidade celular e intensa coloração com azul de tripano, que foi acompanhada pela formação de sincícios numerosos e grandes. O contato prévio entre as NETs e as células não resultou em efeito protetor. As células em monocamadas mostraram um número e área de sincícios reduzidos, mas a morte celular foi semelhante àquela apresentada por células infectadas e não tratadas. A adição de NETs aos tecidos infectados manteve taxa de morte celular e formação de sincícios semelhantes àqueles induzidos pelo vírus em células não tratadas, indicando danos citotóxicos e deletérios. Nossos resultados corroboram achados relatados anteriormente de que as NETs contribuem para a imunopatologia desenvolvida por pacientes infectados com hRSV.
Palavras-chave:
infecção viral; imunidade inata; vírus sincicial respiratório humano; neutrófilos; NETs
1. Introduction
The human respiratory syncytial virus (hRSV) was first described in 1957 as the agent responsible for bronchiolitis in children, especially those under five years of age (Hall, 2001HALL, C.B., 2001. Respiratory syncytial virus and parainfluenza virus. The New England Journal of Medicine, vol. 344, no. 25, pp. 1917-1928. http://dx.doi.org/10.1056/NEJM200106213442507. PMid:11419430.
http://dx.doi.org/10.1056/NEJM2001062134...
). hRSV infections affect people of all ages, but mostly children and the elderly. Co-infections with respiratory viruses, including hRSV, result in high mortality and morbidity (Bawage et al., 2013BAWAGE, S.S., TIWARI, P.M., PILLAI, S., DENNIS, V. and SINGH, S.R., 2013. Recent advances in diagnosis, prevention, and treatment of human respiratory syncytial virus. Advances in Virology, vol. 2013, pp. 595768. http://dx.doi.org/10.1155/2013/595768. PMid:24382964.
http://dx.doi.org/10.1155/2013/595768...
). To date, treatments for hRSV are only palliative and there is no vaccine available.
The inflammatory process induced by hRSV in the airways of infants with bronchiolitis is dominated by an intense influx of neutrophils (Everard et al., 1994EVERARD, M.L., SWARBRICK, A., WRIGHTHAM, M., MCINTYRE, J., DUNKLEY, C., JAMES, P.D., SEWELL, H.F. and MILNER, A.D., 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Archives of Disease in Childhood, vol. 71, no. 5, pp. 428-432. http://dx.doi.org/10.1136/adc.71.5.428. PMid:7826113.
http://dx.doi.org/10.1136/adc.71.5.428...
; Smith et al., 2001SMITH, P.K., WANG, S.Z., DOWLING, K.D. and FORSYTH, K.D., 2001. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. Journal of Paediatrics and Child Health, vol. 37, no. 2, pp. 146-151. http://dx.doi.org/10.1046/j.1440-1754.2001.00618.x. PMid:11328469.
http://dx.doi.org/10.1046/j.1440-1754.20...
). The F-protein, located on the hRSV surface, activates the release of neutrophil extracellular traps (NETs) from neutrophils in the infected tissue (Funchal et al., 2015FUNCHAL, G.A., JAEGER, N., CZEPIELEWSKI, R.S., MACHADO, M.S., MURARO, S.P., STEIN, R.T., BONORINO, C.B.C. and PORTO, B.N., 2015. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One, vol. 10, no. 4, pp. e0124082. http://dx.doi.org/10.1371/journal.pone.0124082. PMid:25856628.
http://dx.doi.org/10.1371/journal.pone.0...
). NETs were first described in 2004; their release is stimulated by several inflammatory mediators and pathogens (Brinkmann et al., 2004BRINKMANN, V., REICHARD, U., GOOSMANN, C., FAULER, B., UHLEMANN, Y., WEISS, D.S., WEINRAUCH, Y. and ZYCHLINSKY, A., 2004. Neutrophil extracellular traps kill bacteria. Science, vol. 303, no. 5663, pp. 1532-1535. http://dx.doi.org/10.1126/science.1092385. PMid:15001782.
http://dx.doi.org/10.1126/science.109238...
). These traps are composed of nuclear/mitochondrial DNA and proteins from the nucleus, cytoplasm, and cytoplasmic granules (Fuchs et al., 2007FUCHS, T.A., ABED, U., GOOSMANN, C., HURWITZ, R., SCHULZE, I., WAHN, V., WEINRAUCH, Y., BRINKMANN, V. and ZYCHLINSKY, A., 2007. Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology, vol. 176, no. 2, pp. 231-241. http://dx.doi.org/10.1083/jcb.200606027. PMid:17210947.
http://dx.doi.org/10.1083/jcb.200606027...
; Urban et al., 2009URBAN, C.F., ERMERT, D., SCHMID, M., ABU-ABED, U., GOOSMANN, C., NACKEN, W., BRINKMANN, V., JUNGBLUT, P.R. and ZYCHLINSKY, A., 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathogens, vol. 5, no. 10, pp. e1000639. http://dx.doi.org/10.1371/journal.ppat.1000639. PMid:19876394.
http://dx.doi.org/10.1371/journal.ppat.1...
), and have a microbicidal and viricidal role (Jenne et al., 2013JENNE, C.N., WONG, C.H.Y., ZEMP, F.J., MCDONALD, B., RAHMAN, M.M., FORSYTH, P.A., MCFADDEN, G. and KUBES, P., 2013. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host & Microbe, vol. 13, no. 2, pp. 169-180. http://dx.doi.org/10.1016/j.chom.2013.01.005. PMid:23414757.
http://dx.doi.org/10.1016/j.chom.2013.01...
; Saitoh et al., 2012SAITOH, T., KOMANO, J., SAITOH, Y., MISAWA, T., TAKAHAMA, M., KOZAKI, T., UEHATA, T., IWASAKI, H., OMORI, H., YAMAOKA, S., YAMAMOTO, N. and AKIRA, S., 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host & Microbe, vol. 12, no. 1, pp. 109-116. http://dx.doi.org/10.1016/j.chom.2012.05.015. PMid:22817992.
http://dx.doi.org/10.1016/j.chom.2012.05...
; Brinkmann et al., 2004BRINKMANN, V., REICHARD, U., GOOSMANN, C., FAULER, B., UHLEMANN, Y., WEISS, D.S., WEINRAUCH, Y. and ZYCHLINSKY, A., 2004. Neutrophil extracellular traps kill bacteria. Science, vol. 303, no. 5663, pp. 1532-1535. http://dx.doi.org/10.1126/science.1092385. PMid:15001782.
http://dx.doi.org/10.1126/science.109238...
). In vitro, NETs induce death in pulmonary epithelial cells and endothelial cell lines (Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, pp. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
), suggesting that they have a toxic effect on the alveolar-capillary barrier. In some chronic respiratory diseases, such as cystic fibrosis (CF), asthma, and chronic obstructive pulmonary disease (COPD), airway obstruction by a dense mass of DNA and protein-rich mucus is a pathological marker (Dubois et al., 2012DUBOIS, A.V., GAUTHIER, A., BRÉA, D., VARAIGNE, F., DIOT, P., GAUTHIER, F. and ATTUCCI, S., 2012. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. American Journal of Respiratory Cell and Molecular Biology, vol. 47, no. 1, pp. 80-86. http://dx.doi.org/10.1165/rcmb.2011-0380OC. PMid:22343221.
http://dx.doi.org/10.1165/rcmb.2011-0380...
; Manzenreiter et al., 2012MANZENREITER, R., KIENBERGER, F., MARCOS, V., SCHILCHER, K., KRAUTGARTNER, W.D., OBERMAYER, A., HUML, M., STOIBER, W., HECTOR, A., GRIESE, M., HANNIG, M., STUDNICKA, M., VITKOV, L. and HARTL, D., 2012. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society, vol. 11, no. 2, pp. 84-92. http://dx.doi.org/10.1016/j.jcf.2011.09.008. PMid:21996135.
http://dx.doi.org/10.1016/j.jcf.2011.09....
; Papayannopoulos et al., 2011PAPAYANNOPOULOS, V., STAAB, D. and ZYCHLINSKY, A., 2011. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving Dnase therapy. PLoS One, vol. 6, no. 12, pp. e28526. http://dx.doi.org/10.1371/journal.pone.0028526. PMid:22174830.
http://dx.doi.org/10.1371/journal.pone.0...
; Wright et al., 2016WRIGHT, T.K., GIBSON, P.G., SIMPSON, J.L., MCDONALD, V.M., WOOD, L.G. and BAINES, K.J., 2016. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology, vol. 21, no. 3, pp. 467-475. http://dx.doi.org/10.1111/resp.12730. PMid:26804470.
http://dx.doi.org/10.1111/resp.12730...
).
During hRSV infections, NETs trap hRSV particles, thereby limiting the spread of the virus (Cortjens et al., 2016CORTJENS, B., BOER, O.J., JONG, R., ANTONIS, A.F., PIÑEROS, Y.S.S., LUTTER, R., VAN WOENSEL, J.B. and BEM, R.A., 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. The Journal of Pathology, vol. 238, no. 3, pp. 401-411. http://dx.doi.org/10.1002/path.4660. PMid:26468056.
http://dx.doi.org/10.1002/path.4660...
). On the other hand, the dense mass of NETs found in the airways and lungs of children with severe hRSV infection impairs their respiratory capacity and contributes to the aggravation of the disease (Cortjens et al., 2016CORTJENS, B., BOER, O.J., JONG, R., ANTONIS, A.F., PIÑEROS, Y.S.S., LUTTER, R., VAN WOENSEL, J.B. and BEM, R.A., 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. The Journal of Pathology, vol. 238, no. 3, pp. 401-411. http://dx.doi.org/10.1002/path.4660. PMid:26468056.
http://dx.doi.org/10.1002/path.4660...
). Although several studies have proposed the adverse effects of neutrophil responses in hRSV infections (Stokes et al., 2011STOKES, C.A., ISMAIL, S., DICK, E.P., BENNETT, J.A., JOHNSTON, S.L., EDWARDS, M.R., SABROE, I. and PARKER, L.C., 2011. Role of interleukin-1 and MyD88-dependent signaling in rhinovirus infection. Journal of Virology, vol. 85, no. 15, pp. 7912-7921. http://dx.doi.org/10.1128/JVI.02649-10. PMid:21593174.
http://dx.doi.org/10.1128/JVI.02649-10...
; Stokes et al., 2013STOKES, K.L., CURRIER, M.G., SAKAMOTO, K., LEE, S., COLLINS, P.L., PLEMPER, R.K. and MOORE, M.L., 2013. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. Journal of Virology, vol. 87, no. 18, pp. 10070-10082. http://dx.doi.org/10.1128/JVI.01347-13. PMid:23843644.
http://dx.doi.org/10.1128/JVI.01347-13...
; Stoppelenburg et al., 2014STOPPELENBURG, A.J., ROOCK, S., HENNUS, M.P., BONT, L. and BOES, M., 2014. Elevated Th17 response in infants undergoing respiratory viral infection. American Journal of Pathology, vol. 184, no. 5, pp. 1274-1279. http://dx.doi.org/10.1016/j.ajpath.2014.01.033. PMid:24650560.
http://dx.doi.org/10.1016/j.ajpath.2014....
), whether the presence of NETs during hRSV infections is more beneficial or detrimental for the host has yet to be determined.
Studies on the microbicidal role of NETs in bacteria are common and continue to increase (Papayannopoulos, 2018PAPAYANNOPOULOS, V., 2018. Neutrophil extracellular traps in immunity and disease. Nature Reviews. Immunology, vol. 18, no. 2, pp. 134-147. http://dx.doi.org/10.1038/nri.2017.105. PMid:28990587.
http://dx.doi.org/10.1038/nri.2017.105...
). However, there is a lack of knowledge regarding the effects of NETs on viruses, and more research is necessary to elucidate the benefits or drawbacks of interactions between NETs and virus. Here, we evaluated the effects of NETs during the course of an hRSV infection in vitro. For this purpose, epithelial cells were pre- and post-treated with NETs and infected with hRSV. They were then evaluated for cell viability and the presence of syncytial formation, a morphologic characteristic of replicating hRSV.
2. Material and Methods
2.1. Ethics statement
The experimental procedures involving human blood were approved by the Local Ethics Committee on Human Research of the Faculdade de Ciências e Letras de Assis – FCL UNESP (42048315.5.0000.540), Assis, São Paulo state (SP), Brazil. Written informed consent, which was suggested and approved by the Committee, was obtained from each participant before beginning the investigation procedures.
2.2. Cellular culture
Hep-2 cells (oropharyngeal carcinoma) were purchased from the Bank of Cells of Rio de Janeiro (BCRJ, Rio de Janeiro, Brazil) and expanded in 75 cm2 culture flasks (Corning Glass Works, New York, NY, USA). They were grown in a humidified incubator at 37 °C and 5% CO2 in DMEM-F12 medium (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil), antibiotics, and antimycotics (Gibco, Life Technologies, Gaithersburg, MD, USA).
2.3. Viral stock
The hRSV long strain, provided by Prof. Dr. Eurico de Arruda Neto (Medical School of Ribeirão Preto, USP, Ribeirão Preto, SP, Brazil), was amplified in Hep-2 cells. Viral stocks were prepared in serum-free DMEM-F12 medium, and aliquots were subsequently frozen at -80°C in DMEM supplemented with 10% trehalose (Gupta et al., 1996GUPTA, C.K., LESZCZYNSKI, J., GUPTA, R.K. and SIBER, G.R., 1996. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine, vol. 14, no. 15, pp. 1417-1420. http://dx.doi.org/10.1016/S0264-410X(96)00096-5. PMid:8994316.
http://dx.doi.org/10.1016/S0264-410X(96)...
). The viral titer was established through a plaque assay, as previously described (McKimm-Breschkin, 2004MCKIMM-BRESCHKIN, J.L., 2004. A simplified plaque assay for respiratory syncytial virus - direct visualization of plaques without immunostaining. Journal of Virological Methods, vol. 120, no. 1, pp. 113-117. http://dx.doi.org/10.1016/j.jviromet.2004.02.020. PMid:15234816.
http://dx.doi.org/10.1016/j.jviromet.200...
), and confirmed using the TCID50 method (Rasmussen et al., 2011RASMUSSEN, L., MADDOX, C., MOORE, B.P., SEVERSON, W. and WHITE, E.L., 2011. A high-throughput screening strategy to overcome virus instability. Assay and Drug Development Technologies, vol. 9, no. 2, pp. 184-190. http://dx.doi.org/10.1089/adt.2010.0298. PMid:21050067.
http://dx.doi.org/10.1089/adt.2010.0298...
).
2.4. Isolation and stimulus of human neutrophils for NET production
We collected 10 mL of peripheral blood from healthy volunteers, both female and male individuals (> 18 years old) were included and individuals using anti-inflammatory drugs were excluded. The blood was placed it in BD Vacutainer® tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing sodium citrate and was processed in a double density gradient (Histopaque 1119 and 1077, Sigma-Aldrich) according to the manufacturer’s instructions. NETs were generated following the protocol described by Brinkmann et al. (2010)BRINKMANN, V., LAUBE, B., ABED, U.A., GOOSMANN, C. and ZYCHLINSKY, A., 2010. Neutrophil extracellular traps: how to generate and visualize them. Journal of Visualized Experiments, no. 36, pp. 1-3. http://dx.doi.org/10.3791/1724. PMid:20182410.
http://dx.doi.org/10.3791/1724...
, with modifications (Najmeh et al., 2015NAJMEH, S., COOLS-LARTIGUE, J., GIANNIAS, B., SPICER, J. and FERRI, L.E., 2015. Simplified human neutrophil extracellular traps (NETs) isolation and handling. Journal of Visualized Experiments, no. 98, pp. 353025. http://dx.doi.org/10.3791/52687. PMid:25938591.
http://dx.doi.org/10.3791/52687...
; Saffarzadeh et al., 2012SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, pp. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
). Briefly, the isolated neutrophils were diluted in serum-free RPMI medium supplemented with 25 nM phorbol myristate acetate (PMA), and seeded in a 6-well plate (1.8×106 cells/well). The medium was discarded after incubation (3 h, 37 °C, 5% CO2) and the NETs were collected by washing with serum-free RPMI and incubated in a water bath (24 h at 40 °C) (Souza et al., 2018SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J. SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: Possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77.). Next, samples were centrifuged (800×g, 5 min) and the supernatant was stored at -20 °C in a freezer. The DNA concentration of the samples was measured with a Qubit® 2.0 Fluorometer using the PicoGreen dsDNA Assay Kit (Invitrogen, Inc., Grand Island, NY, USA).
2.5. Evaluation of the anti-hRSV effect of NETs
The anti-hRSV properties of human PMA-induced NETs were assayed for their pre- and post-treatment effects on hRSV-infected Hep-2 cells as described by Bettega et al. (2004)BETTEGA, J.M., TEIXEIRA, H., BASSANI, V.L., BARARDI, C.R. and SIMÕES, C.M., 2004. Evaluation of the antiherpetic activity of standardized extracts of Achyrocline satureioides. Phytotherapy Research, vol. 18, no. 10, pp. 819-823. http://dx.doi.org/10.1002/ptr.1568. PMid:15551398.
http://dx.doi.org/10.1002/ptr.1568...
. Cell viability was estimated using a colorimetric MTT [1-(4,5-Dimethylthiazol-2-yl)-3,5-Diphenylformazan thiazolyl blue formazan] assay and trypan blue dye incorporation assay. Multinucleated cells (syncytia) were identified and counted, and their areas were calculated as described below.
Pre-treatment assay: Confluent Hep-2 monolayers (5×104 cells/well) were incubated with NETs (0.5-16 µg/mL) for 2 h at 37 °C in a 96-well plate. Next, the NET supernatant was replaced with a viral inoculum with different multiplicity of infection (MOI 0.5 or 1.0). Following infection (2 h, 37 °C), the viral suspension was replaced with serum-free DMEM-F12 medium. The cells were incubated for three days at 37 °C and 5% CO2. Cellular control (CC, untreated and uninfected cells) and viral control (VC, untreated and infected cells) were included in the assay. Analyses were performed on the third day after infection.
Post-treatment assay: Confluent Hep-2 monolayers (5×104 cells/well) were infected with viral inoculum (MOI 0.5 or 1.0) for 2 h at 37 °C in a 96-well plate. The viral suspension was replaced with NETs (0.5–16 μg/mL) diluted in serum-free DMEM-F12 medium. Cells were incubated for three days at 37 °C and 5% CO2. CC and VC were also included in this assay. Analyses were performed on the third day after infection.
2.6. Syncytium evaluation
Three days after infection, cells were analyzed microscopically for the presence of syncytia. The number of syncytia was counted through microscopic observation using the well diameter as a parameter (from top to base). After counting, images from each well were captured using a camera attached to a Nikon eclipse TS 100 microscope (Nikon, Tokyo, Japan). These images were processed using an algorithm developed and carried out using the MATLAB® software (Pasieka et al., 2003PASIEKA, T.J., WOOLSON, R.F. and GROSE, C., 2003. Viral induced fusion and syncytium formation: measurement by the Kolmogorov-Smirnov statistical test. Journal of Virological Methods, vol. 111, no. 2, pp. 157-161. http://dx.doi.org/10.1016/S0166-0934(03)00152-6. PMid:12880931.
http://dx.doi.org/10.1016/S0166-0934(03)...
; Matkowskyj et al., 2000MATKOWSKYJ, K.A., SCHONFELD, D. and BENYA, R.V., 2000. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software Photoshop and Matlab. The Journal of Histochemistry and Cytochemistry, vol. 48, no. 2, pp. 303-312. http://dx.doi.org/10.1177/002215540004800216. PMid:10639497.
http://dx.doi.org/10.1177/00221554000480...
; Larsen, 1998LARSEN, J.O., 1998. Stereology of nerve cross sections. Journal of Neuroscience Methods, vol. 85, no. 1, pp. 107-118. http://dx.doi.org/10.1016/S0165-0270(98)00129-0. PMid:9874147.
http://dx.doi.org/10.1016/S0165-0270(98)...
). In brief, the image files were segmented to isolate the syncytium from the background. A binary matrix was obtained in which the number zero (0) represented the background pixels, and the number one (1) represented syncytium pixels (object). The syncytial area was estimated by converting the pixels to mm2 using an area limit of 0.01 mm2.
2.7. MTT assay (cellular viability)
The protective effect of NETs against infected cells, pre- or post-treatment, was indirectly assessed using a colorimetric MTT assay following the manufacturer’s recommendations (Sigma-Aldrich). This colorimetric assay evaluates the cell viability based on the ability of cells to reduce the MTT salt to formazan crystals using the succinic dehydrogenase enzyme. The metabolism of the salt is directly proportional to the metabolic rate, meaning that cellular viability can be calculated from the measured absorbance. Following treatment (3-day incubation), we replaced the culture medium with the MTT solution (0.5 mg/mL) and incubated the cells for 2 h at 37 °C. Subsequently, the MTT solution was replaced with 50 μL/well dimethylsulfoxide (DMSO, Sigma-Aldrich) to dissolve the formazan crystals. Absorbance at 560 nm was recorded using a Multiskan™ FC Microplate Photometer (Thermo Fisher Scientific, Waltham, MA, USA) and the values were converted to the percentage of viable cells. Cellular control (untreated and uninfected cells) and viral control (untreated and infected cells) represented 100% and 0% cellular viability, respectively. The cellular viability percentage was obtained for each tested condition using a protection equation (Equation 1) as follows:
where:
OD is the optical density at 560 nm;
ODT-ODVC is the optical density of the treatment – optical density of the viral control;
ODCC - ODVC is the optical density of the cell control – optical density of the viral control.
2.8. Trypan blue staining
Cell viability was also evaluated based on trypan blue incorporation. At the end of each treatment, we administered a 0.4% trypan blue solution to each well for 30 s and then carefully washed it with phosphate buffered saline. Blue stains indicate regions of cell death, as evaluated using visual analysis.
2.9. Statistical analysis
All assays were repeated three or five times and were performed using triplicate samples. Experimental data were processed using Microsoft Excel (version 16.33) or GraphPad Prism 6 and evaluated using Wilcoxon test. A level of significance of p ≤ 0.05 was used.
3. Results
3.1. Effects of pre-treatment with NETs on the hRSV-infected Hep-2 cells
Hep-2 cells were incubated with different concentrations of NETs (0.5–16 μg/mL) for 2 h and then infected with the virus at different MOIs (0.5 or 1.0). The following parameters were observed after infection: cell viability, the presence/absence of syncytia, and the number and area of syncytia.
The results of the treatment with viral inoculum at a MOI of 0.5 demonstrated that previous contact of the cells with NETs did not result in total protection from hRSV infection, although it resulted in a reduction in the number and area of syncytia (Figure 1). We observed that only NET concentrations of 8 and 16 μg/mL showed trend to reducing number of syncytia, but that was not statistically significant compared with non-treated cells (Figure 1A, left). Meanwhile, the cells treated with NETs showed a decreasing syncytial area in a dose-dependent manner (Figure 1A, center). The area of the syncytium was smaller than the detection limit of the algorithm used in the calculation for NET concentrations of 8 and 16 μg/mL. Cell viability was also evaluated (Figure 1A, right). Data from the MTT assay did not reveal any protective effects of NETs, as the viability of the treated cells was similar to that viral control (VC). These observations were complemented and corroborated by the trypan blue assay results (Figure 1B, bottom panel). Trypan blue is not a permeable dye; as such, the incorporation of trypan blue into cells occurs when there is cell membrane damage, resulting in dead cells being stained blue. Trypan blue staining revealed intense cell damage in all infected cells, both treated and non-treated (Figure 1B; bottom panel). The morphology of cells (Figure 1B, top panel) confirmed the presence of syncytia and elongated cells among the VC following infection. Infected cells that were treated with NETs showed an elongated morphology (at 2 and 4 μg/mL) and detachment (at 8-16 μg/mL). Together, these results demonstrate that pre-treatment of hRSV-infected cells with NETs reduces the presence of cellular syncytia but does not protect the tissue from cell damage.
Effects of pre-treatment with NETs on hRSV-infected cells (MOI 0.5). Hep-2 cells were pre-treated with different concentrations of NETs (0.5-16 μg/mL) and infected with hRSV (MOI 0.5). On the third day of culture, cells were evaluated for (A) syncytium number and area and cellular viability via the MTT assay, and (B) morphology and cell viability through trypan blue incorporation (TB incorporation). Graphs and images are representative of three independent experiments performed in triplicate. They show the mean of obtained values ± standard deviation. Human respiratory syncytial virus (hRSV); neutrophil extracellular traps (NETs)
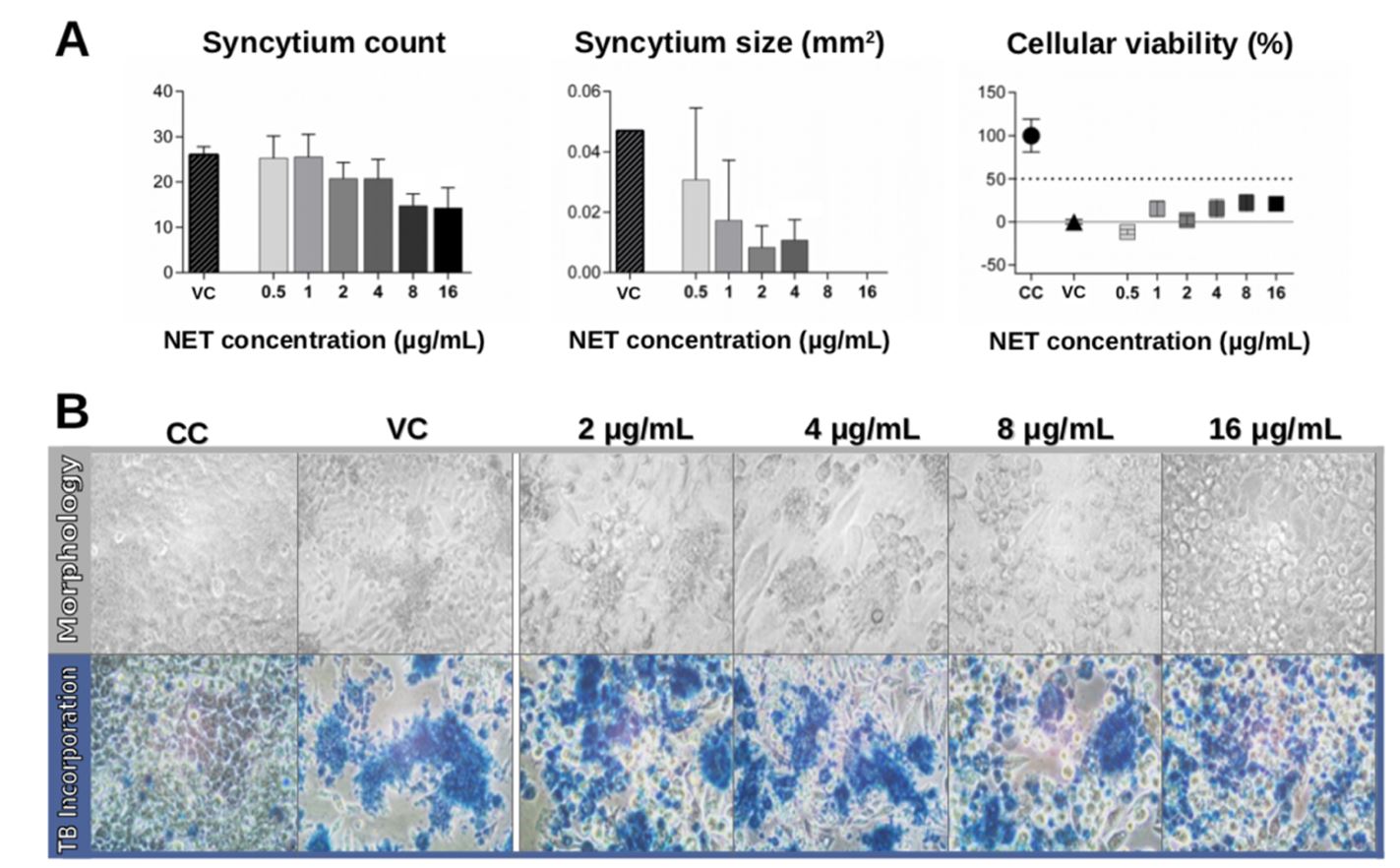
Treatment with the inoculum of higher MOI (1.0) showed similar results to those obtained using an inoculum with a MOI of 0.5, with none of the tested NET concentrations inducing significant levels of protection (Figure 2). Prior contact with NETs (8 and 16 μg/mL) resulted in an apparent decrease in the syncytium count (Figure 2A, left), and all NET concentrations resulted in a reduction in syncytium area (Figure 2A, center). Despite this reduction in syncytium number and area, cell viability remained low, as determined by the MTT assay. The cell viability of infected and treated cells was close to that of the VC (Figure 2A, on the right). The incorporation of trypan blue was intense in infected cells, treated with or without NETs (Figure 2B, bottom panel). Cell morphology analysis confirmed the presence of syncytia in infected cells (Figure 2B, top panel). The cells treated with NETs showed an elongated morphology (2-8 μg/mL) and detachment from the culture vessel (16 μg/mL). The results obtained with MOI 1.0 repeated the findings from the MOI 0.5 replicates, that prior contact with NETs did not protect the tissue from damage.
Effects of pre-treatment with NETs on hRSV-infected cells (MOI 1.0). Hep-2 cells were pre-treated with different concentrations of NETs (0.5-16 μg/mL) and infected with hRSV (MOI 1.0). On the third day of culture, cells were evaluated for (A) syncytium number and area and cellular viability via the MTT assay, and (B) cell morphology and viability through trypan blue incorporation (TB incorporation). Graphs and images are representative of three independent experiments performed in triplicate. They show the mean of obtained values ± standard deviation. Human respiratory syncytial virus (hRSV); neutrophil extracellular traps (NETs)
3.2. NET post-treatment effects on hRSV-Hep-2 infected cells
Hep-2 cells were incubated with the virus at different MOIs (0.5 or 1.0) for 2 h. After incubation, the cell monolayers were maintained for three days in the presence of different concentrations of NETs (0.5–16 μg/mL).
hRSV infection at MOI 0.5 induced syncytium formation and cellular death (Figure 3). The syncytium count was reduced when the cells were post-treated with NETs, particularly at higher concentrations (8 and 16 μg/mL; Figure 3A, left). We also analyzed the area of the syncytia (Figure 3A, center), which remained at similar levels to that of the control at NET concentrations of 0.5–2 μg/mL, but was reduced at higher NET concentrations (4–16 μg/mL). Morphological data confirmed the presence of syncytia and the cells were distant from each other (Figure 3B, top panel). Cells post-treated with NETs showed a similar morphology to infected control cells, including a reduction in the number of attached cells. The data showed that NETs did not provide a protective effect at concentrations of 0.5–2 μg/mL (Figure 3A, right). There was a trend toward improved results for treatment with NET concentrations of 4–16 µg/mL, although this was not statistically significant (p > 0.05). The presence of cells dying was confirmed through the trypan blue assays (Figure 3B, bottom panel). All infected cells, even those that were treated with NETs, showed large blue-colored regions, indicating cellular death.
Effects of post-treatment with NETs on hRSV-infected cells (MOI 0.5). Hep-2 cells were infected with hRSV (MOI 0.5) and subsequently cultured in the presence of different NET concentrations (0.5–16 μg/mL). On the third day of culture, cells were evaluated for (A) syncytium number and area and cellular viability via the MTT assay, and (B) cell morphology and viability through trypan blue incorporation (TB incorporation). Graphs and images are representative of three independent experiments performed in triplicate. They show the mean of obtained values ± standard deviation. (*p < 0.001, **p < 0.0005 compared to the viral control, VC). Human respiratory syncytial virus (hRSV); neutrophil extracellular traps (NETs)
Hep-2 cells were also post-treated with NETs after being infected with a higher viral inoculum (MOI 1.0) (Figure 4). All NET concentrations tested resulted in a reduction in the syncytium count in the infected cells (Figure 4A, left). On the other hand, NET concentrations of 0.5–4 μg/mL induced an increase in the area of the syncytia, while the cells treated with NETs at 8 and 16 μg/mL showed a reduced syncytium area (Figure 4A, center). These morphological changes can be seen in the images captured from the cell monolayer (Figure 4B, top panel). The CC showed cellular confluency. The presence of syncytia gradually decreased with increasing NET concentrations. It is important to note that there was a reduced number of attached cells after treatment with NETs at concentrations of 8–16 μg/mL. Cellular viability results did not improve with NET treatment (Figure 4A, right). Trypan blue was incorporated in both treated and non-treated infected cells (Figure 4B, bottom panel), indicating cell damage. The lowest number of attached cells was observed in the wells treated with 16 μg/mL NETs. Our data demonstrate that the post-infection contact of hRSV-infected cells with NETs does not protect them from cellular damage.
Effects of post-treatment with NETs on hRSV-infected cells (MOI 1.0). Hep-2 cells were infected with hRSV (MOI 1.0) and subsequently cultured in the presence of different NET concentrations (0.5-16 μg/mL). On the third day of culture, cells were evaluated for (A) syncytium number and area and cellular viability via the MTT assay, and (B) cell morphology and viability through trypan blue incorporation (TB incorporation). Graphs and images are representative of three independent experiments performed in triplicate. They show the mean of obtained values ± standard deviation. (**p < 0.01, ***p < 0.001, ****p<0.0001 compared to viral control, VC). Human respiratory syncytial virus (hRSV); neutrophil extracellular traps (NETs)
4. Discussion
In hRSV-infected lung tissue, leukocyte recruitment occurs after the pathogens arrive in the tissue. Neutrophils are attracted by inflammatory mediators secreted by infected cells and other resident leukocytes (Nuriev and Johansson, 2019NURIEV, R. and JOHANSSON, C., 2019. Chemokine regulation of inflammation during respiratory syncytial virus infection.F1000Research, vol. 8, pp. 1837.). Once they arrive at the site of infection, they are exposed to an environment with infected and non-infected cells, as well as extracellular viral particles.
Neutrophil accumulation in the respiratory tract tissues of patients infected with hRSV is well-documented (Smith et al., 2001SMITH, P.K., WANG, S.Z., DOWLING, K.D. and FORSYTH, K.D., 2001. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. Journal of Paediatrics and Child Health, vol. 37, no. 2, pp. 146-151. http://dx.doi.org/10.1046/j.1440-1754.2001.00618.x. PMid:11328469.
http://dx.doi.org/10.1046/j.1440-1754.20...
; Everard et al., 1994EVERARD, M.L., SWARBRICK, A., WRIGHTHAM, M., MCINTYRE, J., DUNKLEY, C., JAMES, P.D., SEWELL, H.F. and MILNER, A.D., 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Archives of Disease in Childhood, vol. 71, no. 5, pp. 428-432. http://dx.doi.org/10.1136/adc.71.5.428. PMid:7826113.
http://dx.doi.org/10.1136/adc.71.5.428...
). The contact between neutrophils and hRSV leads to several inflammatory responses, including the release of NETs via Toll-like receptors (TLRs) (Funchal et al., 2015FUNCHAL, G.A., JAEGER, N., CZEPIELEWSKI, R.S., MACHADO, M.S., MURARO, S.P., STEIN, R.T., BONORINO, C.B.C. and PORTO, B.N., 2015. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One, vol. 10, no. 4, pp. e0124082. http://dx.doi.org/10.1371/journal.pone.0124082. PMid:25856628.
http://dx.doi.org/10.1371/journal.pone.0...
). Although NETs have a microbicidal effect, their presence in tissues has also been reported to be associated with deleterious effects in various infections (Massberg et al., 2010MASSBERG, S., GRAHL, L., VON BRUEHL, M.-L., MANUKYAN, D., PFEILER, S., GOOSMANN, C., BRINKMANN, V., LORENZ, M., BIDZHEKOV, K., KHANDAGALE, A.B., KONRAD, I., KENNERKNECHT, E., REGES, K., HOLDENRIEDER, S., BRAUN, S., REINHARDT, C., SPANNAGL, M., PREISSNER, K.T. and ENGELMANN, B., 2010. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature Medicine, vol. 16, no. 8, pp. 887-896. http://dx.doi.org/10.1038/nm.2184. PMid:20676107.
http://dx.doi.org/10.1038/nm.2184...
; Narasaraju et al., 2011NARASARAJU, T., YANG, E., SAMY, R.P., NG, H.H., POH, W.P., LIEW, A.A., PHOON, M.C., VAN ROOIJEN, N. and CHOW, V.T., 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. American Journal of Pathology, vol. 179, no. 1, pp. 199-210. http://dx.doi.org/10.1016/j.ajpath.2011.03.013. PMid:21703402.
http://dx.doi.org/10.1016/j.ajpath.2011....
; Papayannopoulos et al., 2011PAPAYANNOPOULOS, V., STAAB, D. and ZYCHLINSKY, A., 2011. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving Dnase therapy. PLoS One, vol. 6, no. 12, pp. e28526. http://dx.doi.org/10.1371/journal.pone.0028526. PMid:22174830.
http://dx.doi.org/10.1371/journal.pone.0...
). Thus, we aimed to investigate the role of NETs during hRSV infections in both infected and non-infected cells, using pre- and post-treatment protocols.
The NETs generated in this study were stimulated by PMA in the absence of FBS, since the latter contains DNAse that could break down traps (Zimmer et al., 2001ZIMMER, G., BUDZ, L. and HERRLER, G., 2001. Proteolytic activation of respiratory syncytial virus fusion protein. Cleavage at two furin consensus sequences. The Journal of Biological Chemistry, vol. 276, no. 34, pp. 31642-31650. http://dx.doi.org/10.1074/jbc.M102633200. PMid:11418598.
http://dx.doi.org/10.1074/jbc.M102633200...
). We analyzed the role of NETs during hRSV infection using two different protocols, pre- and post-treatment, in which cells were treated with NETs before or after a viral infection, respectively. In both treatments, NETs did not provide any protective effects. In the pre-treatment trials, there was reduction in the number and area of syncytia, but the rate of cell death was maintained. Post-treatment trials, in which Hep-2 cells were first infected and then underwent cultivation with NETs, showed a general trend of NETs aggravating the course of infection, mainly when they were treated with a viral inoculum of MOI 1.0.
Both pre- and post-treatments with NETs interfered with the number and size of syncytia in cells infected with hRSV. The term “respiratory syncytial virus” is based on the fact that infections generate syncytia as a result of the fusion of infected cells, leading to the formation of multinucleated cells. Studies indicate that a syncytium promotes an efficient and rapid viral cell-cell dissemination mechanism (Dutch et al., 2000DUTCH, R.E., JARDETZKY, T.S. and LAMB, R.A., 2000. Virus membrane fusion proteins: biological machines that undergo a metamorphosis. Bioscience Reports, vol. 20, no. 6, pp. 597-612. http://dx.doi.org/10.1023/A:1010467106305. PMid:11426696.
http://dx.doi.org/10.1023/A:101046710630...
; Pastey et al., 2000PASTEY, M.K., GOWER, T.L., SPEARMAN, P.W., CROWE JUNIOR, J.E. and GRAHAM, B.S., 2000. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nature Medicine, vol. 6, no. 1, pp. 35-40. http://dx.doi.org/10.1038/71503. PMid:10613821.
http://dx.doi.org/10.1038/71503...
). We have not found any previous work that reported the interference of NETs with the size and number of syncytia, but it is known that the F-protein is a central mediator for the optimal induction of syncytia formation among viral proteins (Techaarpornkul et al., 2001TECHAARPORNKUL, S., BARRETTO, N. and PEEPLES, M.E., 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. Journal of Virology, vol. 75, no. 15, pp. 6825-6834. http://dx.doi.org/10.1128/JVI.75.15.6825-6834.2001. PMid:11435561.
http://dx.doi.org/10.1128/JVI.75.15.6825...
). Although there is no correlation between the relative quantity of F-protein mRNA and the number or size of the syncytia (Gagliardi et al., 2017GAGLIARDI, T.B., CRIADO, M.F., PROENÇA-MÓDENA, J.L., SARANZO, A.M., IWAMOTO, M.A., PAULA, F.E., CARDOSO, R.C., DELCARO, L.S., SILVA, M.L., CÂMARA, A.A. and ARRUDA, E., 2017. Syncytia induction by clinical isolates of human respiratory syncytial virus A. Intervirology, vol. 60, no. 1-2, pp. 56-60. http://dx.doi.org/10.1159/000480014. PMid:28869960.
http://dx.doi.org/10.1159/000480014...
), it is interesting to note that there are some correlations between NETs and F-protein. F-protein stimulates neutrophils to produce NETs (Funchal et al., 2015FUNCHAL, G.A., JAEGER, N., CZEPIELEWSKI, R.S., MACHADO, M.S., MURARO, S.P., STEIN, R.T., BONORINO, C.B.C. and PORTO, B.N., 2015. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One, vol. 10, no. 4, pp. e0124082. http://dx.doi.org/10.1371/journal.pone.0124082. PMid:25856628.
http://dx.doi.org/10.1371/journal.pone.0...
), and in silico assays suggests that NETs can interact with F-protein (Souza et al., 2018SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J. SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: Possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77.). Future studies may answer whether the reduction in the number and size of syncytia induced by cellular treatment with NETs interferes with viral dissemination in tissue.
Kotelkin et al. (2003)KOTELKIN, A., PRIKHOD’KO, E.A., COHEN, J.I., COLLINS, P.L. and BUKREYEV, A., 2003. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. Journal of Virology, vol. 77, no. 17, pp. 9156-9172. http://dx.doi.org/10.1128/JVI.77.17.9156-9172.2003. PMid:12915532.
http://dx.doi.org/10.1128/JVI.77.17.9156...
demonstrated that hRSV infections sensitize cells to apoptotic cell death by modulating the expression of caspases and pro-apoptotic factors of the Bcl-2 family, as well as anti-apoptotic factors, such as Mcl-1. Saffarzadeh et al. (2012)SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, pp. e32366. http://dx.doi.org/10.1371/journal.pone.0032366. PMid:22389696.
http://dx.doi.org/10.1371/journal.pone.0...
observed cell death after in vitro incubation of endothelial and epithelial cells with NETs. The authors described that the cytotoxic effects of NETs were mediated by histones and myeloperoxidase. Souza et al. (2018)SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J. SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: Possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77. reported the antiviral effects of NET, where virus particles trapped by NETs were not able to infected and to kill the cells. Complementary, we demonstrated that the previous or posterior contact of cells with NETs did not protect against death induced by free virus particules. We conclude that NETs have a beneficial effect in capturing and reducing the number of infected cells, but they do not interfere with the cytopathic effect induced by the free viral particles that were able to infect the tissue.”
In vivo strategies to inhibit or degrade NETs have yielded positive results (Caudrillier et al., 2012CAUDRILLIER, A., KESSENBROCK, K., GILLISS, B.M., NGUYEN, J.X., MARQUES, M.B., MONESTIER, M., TOY, P., WERB, Z. and LOONEY, M.R., 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. The Journal of Clinical Investigation, vol. 122, no. 7, pp. 2661-2671. http://dx.doi.org/10.1172/JCI61303. PMid:22684106.
http://dx.doi.org/10.1172/JCI61303...
; Rossaint et al., 2014ROSSAINT, J., HERTER, J.M., VAN AKEN, H., NAPIREI, M., DÖRING, Y., WEBER, C., SOEHNLEIN, O. and ZARBOCK, A., 2014. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood, vol. 123, no. 16, pp. 2573-2584. http://dx.doi.org/10.1182/blood-2013-07-516484. PMid:24335230.
http://dx.doi.org/10.1182/blood-2013-07-...
; Thomas et al., 2012THOMAS, G.M., CARBO, C., CURTIS, B.R., MARTINOD, K., MAZO, I.B., SCHATZBERG, D., CIFUNI, S.M., FUCHS, T.A., VON ANDRIAN, U.H., HARTWIG, J.H., ASTER, R.H. and WAGNER, D.D., 2012. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood, vol. 119, no. 26, pp. 6335-6343. http://dx.doi.org/10.1182/blood-2012-01-405183. PMid:22596262.
http://dx.doi.org/10.1182/blood-2012-01-...
). In these studies, DNAse or histone-blocking antibodies were used to reduce lung endothelial injury and protect mice from acute lung injury.
Here, we demonstrated that the contact of NETs with cells in two different stages of in vitro hRSV infection interferes with the number and size of syncytia while maintaining a constant rate of cell death. Our results reinforce the delicate and balanced action of NETs during hRSV infection when viral dissemination mechanisms, as syncytia, can be reduced in the presence of NETs, but the damage tissue is maintained in parallel. These aspects are important, and they need to be considered when designing new and specific pharmacological strategies against hRSV infection.
Acknowledgements
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Master’s scholarship) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; Grant 2016/13867-3) for funding this study and the UNESP for institutional support.
References
- BAWAGE, S.S., TIWARI, P.M., PILLAI, S., DENNIS, V. and SINGH, S.R., 2013. Recent advances in diagnosis, prevention, and treatment of human respiratory syncytial virus. Advances in Virology, vol. 2013, pp. 595768. http://dx.doi.org/10.1155/2013/595768 PMid:24382964.
» http://dx.doi.org/10.1155/2013/595768 - BETTEGA, J.M., TEIXEIRA, H., BASSANI, V.L., BARARDI, C.R. and SIMÕES, C.M., 2004. Evaluation of the antiherpetic activity of standardized extracts of Achyrocline satureioides Phytotherapy Research, vol. 18, no. 10, pp. 819-823. http://dx.doi.org/10.1002/ptr.1568 PMid:15551398.
» http://dx.doi.org/10.1002/ptr.1568 - BRINKMANN, V., LAUBE, B., ABED, U.A., GOOSMANN, C. and ZYCHLINSKY, A., 2010. Neutrophil extracellular traps: how to generate and visualize them. Journal of Visualized Experiments, no. 36, pp. 1-3. http://dx.doi.org/10.3791/1724 PMid:20182410.
» http://dx.doi.org/10.3791/1724 - BRINKMANN, V., REICHARD, U., GOOSMANN, C., FAULER, B., UHLEMANN, Y., WEISS, D.S., WEINRAUCH, Y. and ZYCHLINSKY, A., 2004. Neutrophil extracellular traps kill bacteria. Science, vol. 303, no. 5663, pp. 1532-1535. http://dx.doi.org/10.1126/science.1092385 PMid:15001782.
» http://dx.doi.org/10.1126/science.1092385 - CAUDRILLIER, A., KESSENBROCK, K., GILLISS, B.M., NGUYEN, J.X., MARQUES, M.B., MONESTIER, M., TOY, P., WERB, Z. and LOONEY, M.R., 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. The Journal of Clinical Investigation, vol. 122, no. 7, pp. 2661-2671. http://dx.doi.org/10.1172/JCI61303 PMid:22684106.
» http://dx.doi.org/10.1172/JCI61303 - CORTJENS, B., BOER, O.J., JONG, R., ANTONIS, A.F., PIÑEROS, Y.S.S., LUTTER, R., VAN WOENSEL, J.B. and BEM, R.A., 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. The Journal of Pathology, vol. 238, no. 3, pp. 401-411. http://dx.doi.org/10.1002/path.4660 PMid:26468056.
» http://dx.doi.org/10.1002/path.4660 - DUBOIS, A.V., GAUTHIER, A., BRÉA, D., VARAIGNE, F., DIOT, P., GAUTHIER, F. and ATTUCCI, S., 2012. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. American Journal of Respiratory Cell and Molecular Biology, vol. 47, no. 1, pp. 80-86. http://dx.doi.org/10.1165/rcmb.2011-0380OC PMid:22343221.
» http://dx.doi.org/10.1165/rcmb.2011-0380OC - DUTCH, R.E., JARDETZKY, T.S. and LAMB, R.A., 2000. Virus membrane fusion proteins: biological machines that undergo a metamorphosis. Bioscience Reports, vol. 20, no. 6, pp. 597-612. http://dx.doi.org/10.1023/A:1010467106305 PMid:11426696.
» http://dx.doi.org/10.1023/A:1010467106305 - EVERARD, M.L., SWARBRICK, A., WRIGHTHAM, M., MCINTYRE, J., DUNKLEY, C., JAMES, P.D., SEWELL, H.F. and MILNER, A.D., 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Archives of Disease in Childhood, vol. 71, no. 5, pp. 428-432. http://dx.doi.org/10.1136/adc.71.5.428 PMid:7826113.
» http://dx.doi.org/10.1136/adc.71.5.428 - FUCHS, T.A., ABED, U., GOOSMANN, C., HURWITZ, R., SCHULZE, I., WAHN, V., WEINRAUCH, Y., BRINKMANN, V. and ZYCHLINSKY, A., 2007. Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology, vol. 176, no. 2, pp. 231-241. http://dx.doi.org/10.1083/jcb.200606027 PMid:17210947.
» http://dx.doi.org/10.1083/jcb.200606027 - FUNCHAL, G.A., JAEGER, N., CZEPIELEWSKI, R.S., MACHADO, M.S., MURARO, S.P., STEIN, R.T., BONORINO, C.B.C. and PORTO, B.N., 2015. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One, vol. 10, no. 4, pp. e0124082. http://dx.doi.org/10.1371/journal.pone.0124082 PMid:25856628.
» http://dx.doi.org/10.1371/journal.pone.0124082 - GAGLIARDI, T.B., CRIADO, M.F., PROENÇA-MÓDENA, J.L., SARANZO, A.M., IWAMOTO, M.A., PAULA, F.E., CARDOSO, R.C., DELCARO, L.S., SILVA, M.L., CÂMARA, A.A. and ARRUDA, E., 2017. Syncytia induction by clinical isolates of human respiratory syncytial virus A. Intervirology, vol. 60, no. 1-2, pp. 56-60. http://dx.doi.org/10.1159/000480014 PMid:28869960.
» http://dx.doi.org/10.1159/000480014 - GUPTA, C.K., LESZCZYNSKI, J., GUPTA, R.K. and SIBER, G.R., 1996. Stabilization of respiratory syncytial virus (RSV) against thermal inactivation and freeze-thaw cycles for development and control of RSV vaccines and immune globulin. Vaccine, vol. 14, no. 15, pp. 1417-1420. http://dx.doi.org/10.1016/S0264-410X(96)00096-5 PMid:8994316.
» http://dx.doi.org/10.1016/S0264-410X(96)00096-5 - HALL, C.B., 2001. Respiratory syncytial virus and parainfluenza virus. The New England Journal of Medicine, vol. 344, no. 25, pp. 1917-1928. http://dx.doi.org/10.1056/NEJM200106213442507 PMid:11419430.
» http://dx.doi.org/10.1056/NEJM200106213442507 - JENNE, C.N., WONG, C.H.Y., ZEMP, F.J., MCDONALD, B., RAHMAN, M.M., FORSYTH, P.A., MCFADDEN, G. and KUBES, P., 2013. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host & Microbe, vol. 13, no. 2, pp. 169-180. http://dx.doi.org/10.1016/j.chom.2013.01.005 PMid:23414757.
» http://dx.doi.org/10.1016/j.chom.2013.01.005 - KOTELKIN, A., PRIKHOD’KO, E.A., COHEN, J.I., COLLINS, P.L. and BUKREYEV, A., 2003. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. Journal of Virology, vol. 77, no. 17, pp. 9156-9172. http://dx.doi.org/10.1128/JVI.77.17.9156-9172.2003 PMid:12915532.
» http://dx.doi.org/10.1128/JVI.77.17.9156-9172.2003 - LARSEN, J.O., 1998. Stereology of nerve cross sections. Journal of Neuroscience Methods, vol. 85, no. 1, pp. 107-118. http://dx.doi.org/10.1016/S0165-0270(98)00129-0 PMid:9874147.
» http://dx.doi.org/10.1016/S0165-0270(98)00129-0 - MANZENREITER, R., KIENBERGER, F., MARCOS, V., SCHILCHER, K., KRAUTGARTNER, W.D., OBERMAYER, A., HUML, M., STOIBER, W., HECTOR, A., GRIESE, M., HANNIG, M., STUDNICKA, M., VITKOV, L. and HARTL, D., 2012. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society, vol. 11, no. 2, pp. 84-92. http://dx.doi.org/10.1016/j.jcf.2011.09.008 PMid:21996135.
» http://dx.doi.org/10.1016/j.jcf.2011.09.008 - MASSBERG, S., GRAHL, L., VON BRUEHL, M.-L., MANUKYAN, D., PFEILER, S., GOOSMANN, C., BRINKMANN, V., LORENZ, M., BIDZHEKOV, K., KHANDAGALE, A.B., KONRAD, I., KENNERKNECHT, E., REGES, K., HOLDENRIEDER, S., BRAUN, S., REINHARDT, C., SPANNAGL, M., PREISSNER, K.T. and ENGELMANN, B., 2010. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nature Medicine, vol. 16, no. 8, pp. 887-896. http://dx.doi.org/10.1038/nm.2184 PMid:20676107.
» http://dx.doi.org/10.1038/nm.2184 - MATKOWSKYJ, K.A., SCHONFELD, D. and BENYA, R.V., 2000. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software Photoshop and Matlab. The Journal of Histochemistry and Cytochemistry, vol. 48, no. 2, pp. 303-312. http://dx.doi.org/10.1177/002215540004800216 PMid:10639497.
» http://dx.doi.org/10.1177/002215540004800216 - MCKIMM-BRESCHKIN, J.L., 2004. A simplified plaque assay for respiratory syncytial virus - direct visualization of plaques without immunostaining. Journal of Virological Methods, vol. 120, no. 1, pp. 113-117. http://dx.doi.org/10.1016/j.jviromet.2004.02.020 PMid:15234816.
» http://dx.doi.org/10.1016/j.jviromet.2004.02.020 - NAJMEH, S., COOLS-LARTIGUE, J., GIANNIAS, B., SPICER, J. and FERRI, L.E., 2015. Simplified human neutrophil extracellular traps (NETs) isolation and handling. Journal of Visualized Experiments, no. 98, pp. 353025. http://dx.doi.org/10.3791/52687 PMid:25938591.
» http://dx.doi.org/10.3791/52687 - NARASARAJU, T., YANG, E., SAMY, R.P., NG, H.H., POH, W.P., LIEW, A.A., PHOON, M.C., VAN ROOIJEN, N. and CHOW, V.T., 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. American Journal of Pathology, vol. 179, no. 1, pp. 199-210. http://dx.doi.org/10.1016/j.ajpath.2011.03.013 PMid:21703402.
» http://dx.doi.org/10.1016/j.ajpath.2011.03.013 - NURIEV, R. and JOHANSSON, C., 2019. Chemokine regulation of inflammation during respiratory syncytial virus infection.F1000Research, vol. 8, pp. 1837.
- PAPAYANNOPOULOS, V., 2018. Neutrophil extracellular traps in immunity and disease. Nature Reviews. Immunology, vol. 18, no. 2, pp. 134-147. http://dx.doi.org/10.1038/nri.2017.105 PMid:28990587.
» http://dx.doi.org/10.1038/nri.2017.105 - PAPAYANNOPOULOS, V., STAAB, D. and ZYCHLINSKY, A., 2011. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving Dnase therapy. PLoS One, vol. 6, no. 12, pp. e28526. http://dx.doi.org/10.1371/journal.pone.0028526 PMid:22174830.
» http://dx.doi.org/10.1371/journal.pone.0028526 - PASIEKA, T.J., WOOLSON, R.F. and GROSE, C., 2003. Viral induced fusion and syncytium formation: measurement by the Kolmogorov-Smirnov statistical test. Journal of Virological Methods, vol. 111, no. 2, pp. 157-161. http://dx.doi.org/10.1016/S0166-0934(03)00152-6 PMid:12880931.
» http://dx.doi.org/10.1016/S0166-0934(03)00152-6 - PASTEY, M.K., GOWER, T.L., SPEARMAN, P.W., CROWE JUNIOR, J.E. and GRAHAM, B.S., 2000. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nature Medicine, vol. 6, no. 1, pp. 35-40. http://dx.doi.org/10.1038/71503 PMid:10613821.
» http://dx.doi.org/10.1038/71503 - RASMUSSEN, L., MADDOX, C., MOORE, B.P., SEVERSON, W. and WHITE, E.L., 2011. A high-throughput screening strategy to overcome virus instability. Assay and Drug Development Technologies, vol. 9, no. 2, pp. 184-190. http://dx.doi.org/10.1089/adt.2010.0298 PMid:21050067.
» http://dx.doi.org/10.1089/adt.2010.0298 - ROSSAINT, J., HERTER, J.M., VAN AKEN, H., NAPIREI, M., DÖRING, Y., WEBER, C., SOEHNLEIN, O. and ZARBOCK, A., 2014. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood, vol. 123, no. 16, pp. 2573-2584. http://dx.doi.org/10.1182/blood-2013-07-516484 PMid:24335230.
» http://dx.doi.org/10.1182/blood-2013-07-516484 - SAFFARZADEH, M., JUENEMANN, C., QUEISSER, M.A., LOCHNIT, G., BARRETO, G., GALUSKA, S.P., LOHMEYER, J. and PREISSNER, K.T., 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One, vol. 7, no. 2, pp. e32366. http://dx.doi.org/10.1371/journal.pone.0032366 PMid:22389696.
» http://dx.doi.org/10.1371/journal.pone.0032366 - SAITOH, T., KOMANO, J., SAITOH, Y., MISAWA, T., TAKAHAMA, M., KOZAKI, T., UEHATA, T., IWASAKI, H., OMORI, H., YAMAOKA, S., YAMAMOTO, N. and AKIRA, S., 2012. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host & Microbe, vol. 12, no. 1, pp. 109-116. http://dx.doi.org/10.1016/j.chom.2012.05.015 PMid:22817992.
» http://dx.doi.org/10.1016/j.chom.2012.05.015 - SMITH, P.K., WANG, S.Z., DOWLING, K.D. and FORSYTH, K.D., 2001. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. Journal of Paediatrics and Child Health, vol. 37, no. 2, pp. 146-151. http://dx.doi.org/10.1046/j.1440-1754.2001.00618.x PMid:11328469.
» http://dx.doi.org/10.1046/j.1440-1754.2001.00618.x - SOUZA, P.S.S., BARBOSA, L.V., DINIZ, L.F.A., SILVA, G.S., LOPES, B.R.P., SOUZA, P.M.R., ARAUJO, G.C., PESSOA, D., OLIVEIRA, J. SOUZA, F.P. and TOLEDO, K.A., 2018. Neutrophil extracellular traps possess anti-human respiratory syncytial virus activity: Possible interaction with the viral F protein. Virus Research, vol. 251, pp. 68-77.
- STOKES, C.A., ISMAIL, S., DICK, E.P., BENNETT, J.A., JOHNSTON, S.L., EDWARDS, M.R., SABROE, I. and PARKER, L.C., 2011. Role of interleukin-1 and MyD88-dependent signaling in rhinovirus infection. Journal of Virology, vol. 85, no. 15, pp. 7912-7921. http://dx.doi.org/10.1128/JVI.02649-10 PMid:21593174.
» http://dx.doi.org/10.1128/JVI.02649-10 - STOKES, K.L., CURRIER, M.G., SAKAMOTO, K., LEE, S., COLLINS, P.L., PLEMPER, R.K. and MOORE, M.L., 2013. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. Journal of Virology, vol. 87, no. 18, pp. 10070-10082. http://dx.doi.org/10.1128/JVI.01347-13 PMid:23843644.
» http://dx.doi.org/10.1128/JVI.01347-13 - STOPPELENBURG, A.J., ROOCK, S., HENNUS, M.P., BONT, L. and BOES, M., 2014. Elevated Th17 response in infants undergoing respiratory viral infection. American Journal of Pathology, vol. 184, no. 5, pp. 1274-1279. http://dx.doi.org/10.1016/j.ajpath.2014.01.033 PMid:24650560.
» http://dx.doi.org/10.1016/j.ajpath.2014.01.033 - TECHAARPORNKUL, S., BARRETTO, N. and PEEPLES, M.E., 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. Journal of Virology, vol. 75, no. 15, pp. 6825-6834. http://dx.doi.org/10.1128/JVI.75.15.6825-6834.2001 PMid:11435561.
» http://dx.doi.org/10.1128/JVI.75.15.6825-6834.2001 - THOMAS, G.M., CARBO, C., CURTIS, B.R., MARTINOD, K., MAZO, I.B., SCHATZBERG, D., CIFUNI, S.M., FUCHS, T.A., VON ANDRIAN, U.H., HARTWIG, J.H., ASTER, R.H. and WAGNER, D.D., 2012. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood, vol. 119, no. 26, pp. 6335-6343. http://dx.doi.org/10.1182/blood-2012-01-405183 PMid:22596262.
» http://dx.doi.org/10.1182/blood-2012-01-405183 - URBAN, C.F., ERMERT, D., SCHMID, M., ABU-ABED, U., GOOSMANN, C., NACKEN, W., BRINKMANN, V., JUNGBLUT, P.R. and ZYCHLINSKY, A., 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathogens, vol. 5, no. 10, pp. e1000639. http://dx.doi.org/10.1371/journal.ppat.1000639 PMid:19876394.
» http://dx.doi.org/10.1371/journal.ppat.1000639 - WRIGHT, T.K., GIBSON, P.G., SIMPSON, J.L., MCDONALD, V.M., WOOD, L.G. and BAINES, K.J., 2016. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology, vol. 21, no. 3, pp. 467-475. http://dx.doi.org/10.1111/resp.12730 PMid:26804470.
» http://dx.doi.org/10.1111/resp.12730 - ZIMMER, G., BUDZ, L. and HERRLER, G., 2001. Proteolytic activation of respiratory syncytial virus fusion protein. Cleavage at two furin consensus sequences. The Journal of Biological Chemistry, vol. 276, no. 34, pp. 31642-31650. http://dx.doi.org/10.1074/jbc.M102633200 PMid:11418598.
» http://dx.doi.org/10.1074/jbc.M102633200
Publication Dates
-
Publication in this collection
11 Oct 2021 -
Date of issue
2023
History
-
Received
07 Apr 2021 -
Accepted
17 June 2021