Abstract
Background
The brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals.
Aim
The study examined the expression of Neuroglobin (Ngb) and Hypoxia-inducible factor-1α (Hif-1α) in adult and young yak brain tissues, and provided researchers with meaningful insight into the anatomy, physiology, and biochemistry of this mammal.
Method
The study employed immunohistochemistry (IHC), quantitative real-time PCR (qRT-PCR), and Western blot (WB) to obtain the results.
Results
Ngb and Hif-1α were significantly (P<0.05) expressed in the cerebellar cortex, piriform lobe, medulla, and corpus callosum of the adult yak while in the young yak brain tissues, the protein expressions were significantly found in the white matter of the cerebellum, pineal gland, corpus callosum, and cerebellar cortex. The Ngb and Hif-1α expression showed similarities and differences. This may have resulted from similar animal species, source of nutrition, age factors, brain size, emotional activities, and communication. The findings documented that Ngb and Hif-1α are commonly expressed in various adult and young yak brain tissues. Multiple roles in the brain tissues of the adult and young yaks are involved in the expression and distribution and are proposed to play a significant role in the adaptation of the yak to the high altitude environment.
Conclusion
This study provides meaningful data to understand the adaptive mechanism to hypoxia and recommended researchers to expand on the adaptive mechanism and brain tissues that are not recorded.
Keywords:
Neuroglobin; hypoxia-inducible factor; yak; brain; oxygen; adaptation
Resumo
Contexto
O cérebro é um órgão que funciona como o centro do sistema nervoso em todos os animais vertebrados e na maioria dos invertebrados.
Objetivo
O estudo examinou a expressão de neuroglobina (Ngb) e fator-1α indutível por hipóxia (Hif-1α) em tecidos cerebrais de iaques adultos e jovens e forneceu aos pesquisadores uma visão significativa da anatomia, fisiologia e bioquímica desse mamífero.
Método
O estudo utilizou imuno-histoquímica (IHC), PCR quantitativo em tempo real (qRT-PCR) e western blot (WB) para a obtenção dos resultados.
Resultados
Ngb e Hif-1α foram significativamente (P < 0,05) expressos no córtex cerebelar, lobo piriforme, medula e corpo caloso do iaque adulto, enquanto nos tecidos cerebrais do iaque jovem as expressões proteicas foram encontradas significativamente na substância branca do cerebelo, glândula pineal, corpo caloso e córtex cerebelar. A expressão de Ngb e Hif-1α apresentou semelhanças e diferenças. Isso pode ter resultado de espécies animais semelhantes, fonte de nutrição, fatores de idade, tamanho do cérebro, atividades emocionais e comunicação. Os resultados documentaram que o Ngb e o Hif-1α são comumente expressos em vários tecidos cerebrais de iaques adultos e jovens. Múltiplos papéis nos tecidos cerebrais de iaques adultos e jovens estão envolvidos na expressão e distribuição e são propostos para desempenhar um papel significativo na adaptação do iaque ao ambiente de alta altitude.
Conclusão
Este estudo fornece dados significativos para compreender o mecanismo adaptativo à hipóxia e recomendou que os pesquisadores expandissem o mecanismo adaptativo e os tecidos cerebrais que não foram registrados.
Palavras-chave:
Neuroglobina; fator induzível por hipóxia; iaque; cérebro; oxigênio; adaptação
1. Introduction
Decreased oxygen availability and decreased temperature make life at such altitudes challenging, though many species have managed to successfully adapt via considerable physiological changes. There are several factors (low temperature, hypoxia, strong ultraviolet light, and dryness) at high altitudes that affect the survival of mammals especially deoxygenated atmosphere which leads to inadequate oxygen reaching the animal's body. Although the brain has 2-5% of the total body weight, however, it is one of the largest and most complex organs in the animal's body and controls functions of the entire body. Therefore; limited oxygen supply to the brain may have an enormous effect on other body parts. Ngb is a neuronal hemeprotein that shares its capability for oxygen binding while Hif-1α is a transcription factor that responds to decreases in available oxygen in the cellular environment or hypoxia. In early 2000, Burmester et al revealed that Ngb is expressed in the vertebrate nervous system especially occupying the central and peripheral nervous system (CNS and PNS), (Burmester et al., 2000BURMESTER, T., WEICH, B., REINHARDT, S. and HANKELN, T., 2000. A vertebrate globin expressed in the brain. Nature, vol. 407, no. 6803, pp. 520-523. http://dx.doi.org/10.1038/35035093. PMid:11029004.
http://dx.doi.org/10.1038/35035093...
). Other researchers reported that Ngb is also found in some endocrine, auditory tissues and brain tissues of humans, mice, turtle, and pig (Reuss et al., 2002REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5. PMid:12435404.
http://dx.doi.org/10.1016/S0306-4522(02)...
; Wystub et al., 2003WYSTUB, S., LAUFS, T., SCHMIDT, M., BURMESTER, T., MAAS, U., SAALER-REINHARDT, S., HANKELN, T. and REUSS, S., 2003. Localization of neuroglobin protein in the mouse brain. Neuroscience Letters, vol. 346, no. 1-2, pp. 114-116. http://dx.doi.org/10.1016/S0304-3940(03)00563-9. PMid:12850561.
http://dx.doi.org/10.1016/S0304-3940(03)...
; Laufs et al., 2004LAUFS, T., WYSTUB, S., REUSS, S., BURMESTER, T., SAALER-REINHARDT, S. and HANKELN, T., 2004. Neurons specific expression of Neuroglobin in mammals. Neuroscience Letters, vol. 362, no. 2, pp. 83-86. http://dx.doi.org/10.1016/j.neulet.2004.02.072. PMid:15193759.
http://dx.doi.org/10.1016/j.neulet.2004....
; Reuss et al., 2016REUSS, S., BANICA, O., ELGURT, M., MITZ, S., DISQUE-KAISER, U., RIEMANN, R., HILL, M., JAQUISH, D.V., KOEHRN, F.J., BURMESTER, T., HANKELN, T. and WOOLF, N.K., 2016. Neuroglobin Expression in the Mammalian Auditory System. Molecular Neurobiology, vol. 53, no. 3, pp. 1461-1477. http://dx.doi.org/10.1007/s12035-014-9082-1. PMid:25636685.
http://dx.doi.org/10.1007/s12035-014-908...
; Fabrizius et al., 2016FABRIZIUS, A., ANDRE, D., LAUFS, T., BICKER, A., REUSS, S., BURMESTER, T., and HANKELN T., 2016. A critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience, vol. 337, no. 16, pp. 339-354. http://dx.doi.org/10.1016/j.neuroscience.2016.07.042.
https://doi.org/10.1016/j.neuroscience.2...
). In subsequent time, studies revealed that the strongest Ngb transcription was observed in the human Hypothalamus (Fabrizius et al., 2016FABRIZIUS, A., ANDRE, D., LAUFS, T., BICKER, A., REUSS, S., BURMESTER, T., and HANKELN T., 2016. A critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience, vol. 337, no. 16, pp. 339-354. http://dx.doi.org/10.1016/j.neuroscience.2016.07.042.
https://doi.org/10.1016/j.neuroscience.2...
; Hundahl et al., 2013HUNDAHL, C.A., KELSEN, J. and HAY-SCHMIDT, A., 2013. Neuroglobin and Cytoglobin expression in the human brain. Brain Structure & Function, vol. 218, no. 2, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-0480-8. PMid:23160832.
http://dx.doi.org/10.1007/s00429-012-048...
). In mammals and other non-vertebrate species, the primary transcriptional factor that response to hypoxic stress is mediated by a dimeric protein called the Hif-1α. Burmester et al. reported that Hif-1α regulation in response to hypoxia occurs primarily on the level of protein stability due to posttranslational hydroxylation and proteasomal degradation (Burmester et al., 2000BURMESTER, T., WEICH, B., REINHARDT, S. and HANKELN, T., 2000. A vertebrate globin expressed in the brain. Nature, vol. 407, no. 6803, pp. 520-523. http://dx.doi.org/10.1038/35035093. PMid:11029004.
http://dx.doi.org/10.1038/35035093...
). Despite these reported results and several years of research, the exact function, pattern, and quantities of expression and mechanism of Ngb is still a debate among scientists, and a large variety of alternative and scientific analysis has been documented but attention has not been given to the Ngb and Hif-1α expression in the adult and young yak brain. Therefore; the current study sought to provide detailed references about Ngb and Hif-1α expression in the brain tissues of the adult and young yak. The yak is critical for the economic and social activities of people on the vast and inhospitable Qinghai-Tibetan plateau and in the surrounding mountainous areas. The present study provides important morphological, physiological, and biochemical data which contributes to the advancement of Ngb and Hif-1α.
2. Materials and Methods
2.1. Animals and setting
All experimental procedures performed in this study were reviewed and approved by the Animal Ethics and Welfare Committee of Gansu Agricultural University in October of 2019 (AEWC-GAU-2019-039). All animals were housed at the cooperative city of Gannan Tibetan Autonomous Prefecture in Gansu Province in China. Six (6) healthy adult yak (3 years old) and young yak (3 months old) were purchased from the center. The animals were housed and monitored by trained personnel and fed on grasses and sedges, such as Carex, Stipa, and Kobresia. In the plateau environment of Gannan Tibetan Autonomous Prefecture, the altitude was 3000m. Experiments were carried out using adult and young yak weighing 550-720 kg and 350–585 kg, respectively. The animals were maintained at a temperature between -7 ºC and -8 ºC and had free access to food and water. Every effort was applied to reduce the number of animals used and minimize animal suffering during the sampling process.
2.1.1. Treatment and Specimen Sampling
Animals were retrieved one at a time from their living areas and minimally immobilized to facilitate sacrificing and then extraction of the brain. Per the guidance of resident veterinarians, this practice was undertaken to reduce harm and pain to the animals. Upon sacrificing each animal, the whole brain was quickly extracted by craniotomy. Subsequently, other brain tissues were extracted. Tissue samples prepared for immunohistochemistry were fixed in 4% paraformaldehyde (PH 7.4, w/v) and samples for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western-blotting were stored at -80 °C.
2.1.2. Reagents and instrumentations
Quantitative real-time polymerase chain reaction (qRT-PCR) reagents and supplies are: AG RNAex Pro RNA kit, SYBR Green Pro Taq HS kit, Evo M-MLV reverse-transcription kit (removal gDNA reagent), and Rox were purchased from Accurate Biotechnology (Hunan) Co. Ltd. P.R. China. Western-blotting reagents and supplies are: Rabbit Anti-Ngb, Polyclonal Antibody (bs-1859R), Rabbit Anti-HIF-1, Alpha Polyclonal Antibody (bs-0737R), Rabbit Anti-beta-Actin (Loading Control), Polyclonal antibody (bs-0737R), and goat anti-rabbit IgG/HRP(bs-0295G-HRP) were purchased from Bioss Co. Ltd. P.R. China. RIPA tissue or cell rapid lysate was purchased from Bio topped and 0.22μm PVDF membranes, 4 × protein loading buffer (DTT), Rainbow 245 broad-spectrum protein marker (11-245KD), and ECL hypersensitivity luminescent solution were purchased from Solarbio Co. Ltd. P.R. China. Immunohistochemical reagents and supplies are: Immunohistochemical staining kit and HRP-DAB kit were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd. P.R. China.
2.1.3. Total RNA isolation and qRT-PCR
Total RNA was isolated using the TRIzol reagent (Accurate Biotechnology, China). Eight hundred nanograms of total RNA was reverse transcribed using the Evo M-MLV cDNA synthesis kit (Accurate Biotechnology, China). Real-time PCR was performed using Quant Studio 5. The qRT-PCR primer sequences and accession numbers are shown in Table 1. Reaction mixtures (20 μL) consisted of 10 μL SYBR Green Pro Taq (Accurate Biotechnology, China), 0.8 μL forward and reverse primers (0.2 μmol/mL), 0.4 μl Rox, 2 μl cDNA, 6 μl ddH2O. The thermocycler was set to 50 °C 2min, 95 °C 2min, 40 cycles at 95 °C 10s, annealing for 34s (annealing temperatures are shown in Table 1), with melting temperatures examined from 65 °C to 95 °C, increments of 0.5 °C every 5s. The 2-ΔΔCt method was used to analyze the expression of Ngb and Hif-1α mRNA relative to β-actin mRNA expression according to the system-generated Ct value.
2.1.4. Western-blotting
Frozen tissue samples for Western blotting analysis were weighed from different areas (Song et al., 2018SONG, L.L., CUI, Y., YU, S.J., LIU, P.G., LIU, J., YANG, X., HE, J.F. and ZHANG, Q., 2018. Expression characteristics of BMP2, BMPR-IA, and Noggin in different stages of the hair follicle in yak skin. General and Comparative Endocrinology, vol. 260, no. 1, pp. 18-24. http://dx.doi.org/10.1016/j.ygcen.2017.11.016. PMid:29174869.
http://dx.doi.org/10.1016/j.ygcen.2017.1...
). After that, the tissues were homogenized using a glass rod in a lysis buffer at ice-cold temperature (1ml RIPA + 10μl PMSF), shaken for 2 hours in an ice bath (120r / min), and centrifuged to absorb the supernatant for 10 minutes at 12,000 rpm at 4 °C. The protein was subjected to SDS polyacrylamide gel electrophoresis (PAGE). Separated proteins were transferred via a transfer apparatus to the polyvinylidene difluoride filter (PVDF) membrane at 110V for 60 minutes. The membranes were then blocked by 5% milk/PBST overnight at 4 °C and then incubated with primary antibodies called Alpha Polyclonal Antibody (bs-0737R) against Ngb, Hif-1 alpha, and β-actin at room temperature for 3 hours. The antibody concentrations (v/v) of Ngb, Hif-1α and β-actin were 1:800, 1:500, 1:3000. The membranes were washed thrice (10 min each) with PBST and incubated with secondary antibody (HRP-conjugated goat anti-rabbit IgG, 1:4000) for 1 h at room temperature. The concentrations of Ngb, Hif-1 alp and β-actin antibodies (v/v) were 1:800, 1:500 and 1:3000. The signals were analyzed using Image J software to determine the relative expression levels of Ngb and Hif-1 alpha (NIH, Bethesda, MD, USA).
2.1.5. Immunohistochemical staining
Tissue samples were fixed (4 percent paraformaldehyde) and trimmed (2 cm x 2 cm) from various regions of the young and adult yak brains. The tissue samples were processed through traditional gradient alcohol dehydration, rendered paraffin-embedded tissue wax blocks, cut tissues with serial parts (4 μm thickness), showing, patching, baking sheet sorting, regular staining of hematoxylin-eosin (HE), microscopy. The parts of the paraffin-embedded tissue were deparaffinized in xylene and rehydrated in graded alcohol. PBS (0.01mol/L, pH = 7.2) was rinsed 3 times, 5 minutes at a time. 0.125 percent of trypsin antigen was repaired for 30 minutes and 2 times rinsed in PBS. By incubating the parts for 10 minutes in a 30 mL/L hydrogen peroxide blocking solution, the endogenous peroxidase activity was blocked, and rinsed three times with PBS for 5 minutes each time to reduce the first antibody's unspecific binding. For blocking purposes, regular sheep serum was added and incubated at room temperature for 15 minutes. In the parts, the corresponding primary antibody was inserted, incubated for 2 hours at 37 ° C and rinsed 3 times in PBS. After removal from the PBS, the required secondary antibody was added and incubated at 37 ° C for 15 minutes. In the pieces, streptomyces avidin-peroxidase solution was applied, incubated at 37 °C for 15 minutes and rinsed with PBS 3 times, 5 minutes each time. After eliminating PBS, the immuno-peroxidase color reaction was produced with the HRP-DAB substrate chromogen solution. The reaction was stopped by distilled water and the parts were lightly counterstained with hematoxylin, dehydrated, cleared and coated with mounting medium and cover slips (at 4 °C) at increasing ethanol concentrations.
2.1.6. Anesthesia or Euthanasia Procedures
Under the legislation of Gansu Agricultural University's Animal Ethics and Protection Committee, all animals involved in the study were placed separately until they It has been confirmed to be safe. The animals were observed for two weeks before further procedures were conducted according to the committee regulations. Although under observation, the animals were free and the observation indicated that the animals were free of any infectious diseases which may have an adverse effect on the experimental procedures. The diet of the animals was given during observation and no shortage of food. To stop pain or mitigate it, the animals were treated calmly by trained personnel. Dealing with these large animals requires more personnel, so additional trained personnel were employed for assistance during the sacrifice. The animals were spoken to by the personnel and loud sounds were avoided to avoid the animals escaping. More food was simultaneously given to the animals to enable interaction between the animals and the personnel and supported a developing relationship with the personnel. The animals were made to lie on their side by scratching their back and flanks by the personnel. While in a calm state, the injections were administrated and scarification took place.
2.1.7. Animals housing conditions
In Hezuo town, Gansu Province, the People's Republic of China, the Hezuo Xingfa Yak and sheep breeding collaboration is located. Hezuo has a subarctic alpine climate at nearly 3,000 meters (9,800 ft) in altitude, with winters that are long, very cold, dry, and short, mild summers. In January, the coldest month, the monthly standard everyday temperature is -9.3 ° C (15.3 °F), while in July, the warmest month, the same figure is 13.3 °C (55.9 °F); the annual average is 2.82 °C (37.1 °F). The bulk of annual precipitation is distributed from May to September. The town receives 2,370 hours of bright sunshine annually, with monthly percent of potential sunshine ranging from 44 percent in June and September to 71 percent in December.
2.1.8. Data analysis
Statistical analyses were performed using SPSS version 22 (SPSS, Inc., Chicago, IL, USA). The data for Ngb and Hif-1α protein levels were subjected to analysis of variance (ANOVA), and the treatment means were separated by Duncan's multiple range test at (p<0.05) using SPSS 22.0 version. Data were presented as mean and standard deviation (SD). Statistical significance was defined as P<0.05. The expressions intensity was analyzed using image J software and calculated according to the software standard value.
3. Results
3.1. Determination of results
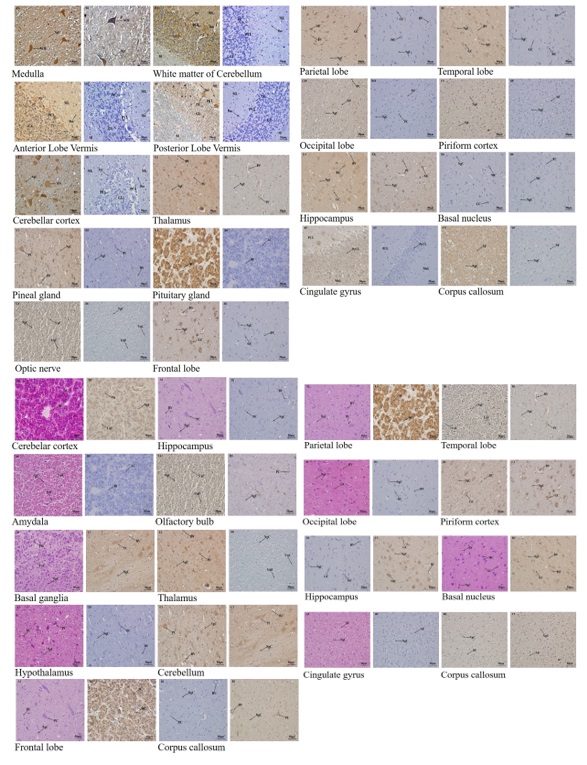
Immunohistochemical (IHC), Real-time PCR (qRT-PCR), and Western Blot (WB)
An immunohistochemical analysis was conducted to determine the precise position of Ngb and Hif-1α between the adult and young yak brain tissues. Real-time PCR for Ngb and Hif-1α mRNA levels, quantification of adult and young yak brain tissue Ngb levels. Tables 2 and 3, mean ± standard deviation, minimum and maximum level, percentage, and relevant level, present the study. Western blot was performed to detect and confirm the protein levels of Ngb and Hif-1α in the adult and young yak brain tissues.
3.1.1. Results description
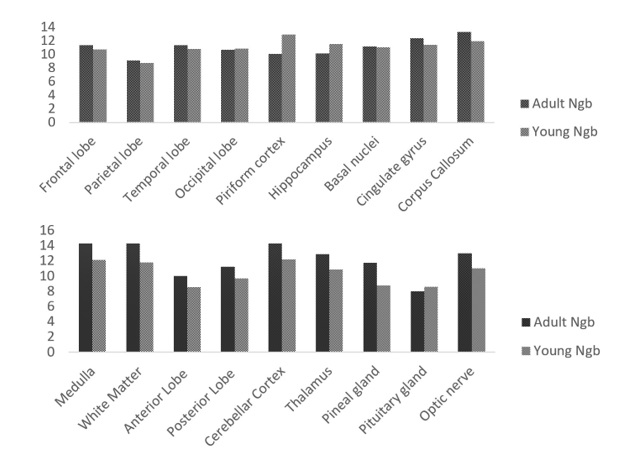
The descriptive statistics for the expression of Ngb and Hif-1α in the adult and young yak are presented into tables and graphs. Immunohistochemical outcomes are displayed in photos. The primary sequence is shown in Table 1, while the complete analysis of the expression of Ngb and Hif-1α in the adult yak brain tissue is shown in Table 2, and the analysis of the expression of Ngb and Hif-1α in the young yak brain is shown in Table 3. The results indicated that both adult and young yak Ngb and Hif-1α were significantly expressed in some brain tissues, while other tissues were higher, but not statistically significant. In the adaptation of the yak to the high altitude climate, the significant and higher expressions play an important role. A comparison of the mean and standard deviation between Ngb and Hif-1α expression in the adult yak base is shown in Figure 1 (I-III), while Ngb expression in both adult and young yak brain tissues is shown in Figure 2 (I-II), and Ngb and Hif-1α expression in young yak brain tissues is shown in detail in Table 3. There was significant expression in some tissues, close to the adult yak tissues, while others displayed higher expression but no significant difference. Explanations of the mean expression and standard deviation of Ngb and Hif-1α in the young yak base can be found in Figure 3. (I-III). The comparison of Hif-1α means and standard deviation in adult and young yak neuronal tissues is shown in Figure 4 (I-III), while Immunohistochemical results in all brains tissues are mentioned in Figure 5 (I-II). The western blot results are correctly positioned.
I. The expression of Ngb and Hif-1α in the brain tissues of adult yak. II. The expression of Ngb and Hif-1α in various brain tissues of adult yak. III. The expression of Ngb and Hif-1α in the brain tissues of adult yak.
I. Ngb expression in the brain tissues of adult and young yak. II. Ngb expression in the brain tissues of adult and young yak.
I. The expression of Ngb and Hif-1α in the brain tissues of young yak. II. The expression of Ngb and Hif-1α in the brain tissues of young yak. III. The expression of Ngb and Hif-1α in the brain tissues of young yak.
I. Hif-1α expression in the brain tissues of adult and young yak. II. Hif-1α expression in the brain tissues of adult and young yak.
I. Ngb and Hif-1α expression in different tissues of the adult yak brain. II. Ngb and Hif-1α expression in different tissues of the young yak brain.
Ngb and a Hif-1α were significantly expressed in the cerebellar cortex, piriform lobe, and medulla while other regions demonstrated fewer expressions.
The comparison shows that Ngb and Hif-1α are highly found in the Cerebellar cortex and Medulla tissues.
Ngb and Hif-1α expressions show higher in the optic nerve, frontal lobe, and temporal lobe as compare to other regions.
The expression rate of Ngb and Hif-1α was recorded higher in the piriform cortex, corpus callosum tissues of the adult yak brain.
The expression rate of Ngb and Hif-1α was recorded higher in the piriform cortex, corpus callosum tissues of the adult yak brain.
The pattern of Ngb expression in the medulla, white matter, and cerebellar cortex was higher in adult yak than young yak but the pituitary gland showed higher in the young yak as compared to the adult yak.
The trend of Ngb expression was recorded higher in the corpus callosum, cingulate gyrus of the adult yak while the piriform cortex, hippocampus showed higher in the young yak.
The Ngb and Hif-1α are highly expressed in the white matter, pineal gland, corpus callosum, and cerebellar cortex while other regions recorded middle and lower expressions.
The comparison shows that Ngb and Hif-1α are highly found in the white matter of the cerebellum, cerebellar cortex, and medulla tissues.
Ngb and Hif-1α expressions are higher in the optic nerve, pineal gland, and temporal lobe than other regions.
The expression rate of Ngb and Hif-1α was recorded higher in the corpus callosum and cingulate gyrus tissues of the young yak brain.
The trend of Hif-1α expression was recorded higher in the medulla and posterior lobe of the adult yak while the optic nerve, thalamus, and white matter showed higher in the young yak.
The pattern of Hif-1α expression in the piriform cortex, hippocampus, and cingulate gyrus was higher in adult yak than young yak and equal expression levels of Hif-1α were observed in the corpus callosum.
4. Discussion
4.1. Medulla
The expression of Ngb and Hif-1α in this neuron tissue plays a significant role in the adaptation of the yak to the high altitude environment. Previous studies have shown that in the rat medulla oblongata (Christian et al., 2008CHRISTIAN, A.H., JESPER, K. and HAY-SCHMIDT, A., 2008. Regulation of Neuroglobin and Cytoglobin throughout the human brain. Brain Structure & Function, vol. 218, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-04808.
https://doi.org/10.1007/s00429-012-04808...
; Manotham et al., 2005MANOTHAM, K., TETSUHIRO, T., TAKAMOTO, O., ICHIRO, K., TOSHIO, M., REIKO, I., HIROTOSHI, T., RYOJI, S., TOSHIRO, F. and MASAOMI, N., 2005. A Biologic Role of HIF-1 in the Renal Medulla. Kidney International, vol. 67, no. 4, pp. 1428-1439. http://dx.doi.org/10.1111/j.1523-1755.2005.00220.x. PMid:15780095.
http://dx.doi.org/10.1111/j.1523-1755.20...
), Ngb and Hif-1α are present. Christian et al. examined Ngb expression in the postrema region (AP) and the commensurate part of the tractus solitary nucleus, primarily in the medial part, while Manotham et al. observed the Hif-1α in the inner stripe and the inner medulla of normal rats (Christian et al., 2008CHRISTIAN, A.H., JESPER, K. and HAY-SCHMIDT, A., 2008. Regulation of Neuroglobin and Cytoglobin throughout the human brain. Brain Structure & Function, vol. 218, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-04808.
https://doi.org/10.1007/s00429-012-04808...
; Manotham et al., 2005MANOTHAM, K., TETSUHIRO, T., TAKAMOTO, O., ICHIRO, K., TOSHIO, M., REIKO, I., HIROTOSHI, T., RYOJI, S., TOSHIRO, F. and MASAOMI, N., 2005. A Biologic Role of HIF-1 in the Renal Medulla. Kidney International, vol. 67, no. 4, pp. 1428-1439. http://dx.doi.org/10.1111/j.1523-1755.2005.00220.x. PMid:15780095.
http://dx.doi.org/10.1111/j.1523-1755.20...
). Similarly, the present research has detected the expression and distribution in the medulla of both adult and young yak proteins. In the adult yak medulla, the pattern of Ngb and Hif-1α expression showed no significant difference as compared to the young yak medulla. This may have resulted from the mode of nutrition (grazing/eat grass) that both animals consume. The adult and young yak accumulate a layer of subcutaneous fat before winter which helps heat conservation and provides an energy reserve. The Ngb and Hif-1α expression in the adult and young yak medulla is also suggested to aid in respiration, heart rate, and other hematological factors (a declining trend) during the yak movement in the high altitude environment.
4.2. White Matter of Cerebellum
The expression strength in adult and young yaks of Ngb and Hif-1α was substantially consistent. The consistency may be involved in the neuroprotection of information channels between neuron tissues of the CNS in adult and young white matter. The skin thickness might be another factor for the similarity. The adult and young yak skin are highly pigmented and the predominant hair color is black. These attributes help to resist the effects of solar radiation. The expression of Ngb and Hif-1α in the rodent and other mammalian brains was discovered in previous studies (Reuss et al., 2002REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5. PMid:12435404.
http://dx.doi.org/10.1016/S0306-4522(02)...
; Yuen et al., 2014YUEN, T.J., JOHN, C.S., AMELIE, G., SANDRA, M.C., RICHARD, D., STEPHEN, P.J.F., HENGAMEH, Z., EMIN, M. and DAVID, H.R., 2014. Oligodendrocyte-encoded HIF function couples’ postnatal myelination and white matter angiogenesis. Cell, vol. 158, no. 2, pp. 383-396. http://dx.doi.org/10.1016/j.cell.2014.04.052. PMid:25018103.
http://dx.doi.org/10.1016/j.cell.2014.04...
). The Ngb expression in the rodent brain's white matter indicates that Ngb mRNA is transported to provides protein synthesis in axons and dendrites in distal regions in nerve cell processes (Job and Eberwine, 2001JOB, C. and EBERWINE, J., 2001. Localization, and translation of mRNA in dendrites and axons. Nature Reviews. Neuroscience, vol. 2, no. 12, pp. 889-898. http://dx.doi.org/10.1038/35104069. PMid:11733796.
http://dx.doi.org/10.1038/35104069...
). An example of Ngb mRNA expression in varicosities, synaptic terminals, and dendritic spines can also be this. An active metabolic mechanism that possibly needs a massive amount of oxygen for synaptic plasticity, the distribution and expression of Ngb transcripts may have important functional implications. Whereas in the white matter couples, postnatal white matter angiogenesis, the Hif-1α expression, the integrity of axon, and the beginning of myelination in mammalians' forebrain.
4.3. Anterior and Posterior Lobe Vermis
Since there are few references to the expression of Ngb and Hif-1α in many mammalian neuronal tissues, this research is the first study of adult and young yak anterior and posterior lobe vermis to document the expression of Ngb and Hif-1 alpha. The Ngb and Hif-1α expression trends were recorded differently. The Ngb and Hif-1α expression intensity in the adult yak anterior and posterior lobe vermis showed highly significance than the young yak. The different trends of expression may have resulted from the emotional activities (such as movement and defense against predators) in the adult yak than the young yak. The body structure of the adult yak is more mature than the young yak and this enables the adult yak to be involved in several external activities than the young yak. Additionally, the adult yak consumed more oxygen when the yak is covering long distances in the high altitudes environment and in the defense of the anterior and posterior lobe vermis during locomotion, Ngb and Hif-1α played an important role.
4.4. Cerebellar Cortex
Reuss et al., stated that Ngb is expressed predominantly in the cerebellar cortex of the rodent brain. The extent of Ngb mRNA expression (Reuss et al., 2002REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5. PMid:12435404.
http://dx.doi.org/10.1016/S0306-4522(02)...
) was also shown by Purkinje cells of the cerebellar cortex. Similarly, a substantial degree of Ngb and Hif-1α expression in the adult and young yak cerebellar cortex has been documented in current research. However; the Ngb and Hif-1α expression in the structure of the cerebellar cortex differ between adult and young yak. The expression trend in the adult cerebellar cortex showed higher as compared to the young yak cerebellar cortex and this may have resulted from the flow of information received from other neuronal regions and how the adult yak response is more rapid than the young yak. The response to information by the adult and young have a function in control movement and influences many other functions within the cerebellar cortex. Ngb and Hif-1α expression aid in a protective role in the information collected from other body regions to the cerebellar cortex and mitochondrial functions. A research conducted by Christian et al. also confirmed the substantial expression of Ngb in the adult mouse brain cerebellar cortex, however Fabrizius et al. Interestingly, during fetal development of the mouse brain, a lower Ngb expression in the cerebellar cortex was revealed and continues to increase as the mouse reaches adulthood. In the human brain, a low Ngb expression was also reported (Hundahl et al., 2012HUNDAHL, C.A., FAHRENKRUG, J., LUUK, H., HAY-SCHMIDT, A. and HANNIBAL, J., 2012. Restricted expression of Neuroglobin in the mouse retina and co-localization with Melanopsin and Tyrosine Hydroxylase. Biochemical and Biophysical Research Communications, vol. 425, no. 1, pp. 100-106. http://dx.doi.org/10.1016/j.bbrc.2012.07.061. PMid:22820193.
http://dx.doi.org/10.1016/j.bbrc.2012.07...
). Meanwhile, Hif-1α expression was recorded significantly in the current study. However; Huquing et al. reported that Hif-1α expression increased with increasing age in the cerebellar cortex between 3 to 18 months (Huqing et al., 2012HUQING, W., HAIQIN, W., HENA, G., GUILIAN, Z., RU, Z. and SHUQIN, Z., 2012. Increased hypoxia-inducible factor 1alpha expression in rat brain tissues in response to aging. Neural Regeneration Research, vol. 7, no. 10, pp. 778-782. http://dx.doi.org/10.3969/j.issn.1673-5374.2012.10.010. PMid:25737702.
https://doi.org/10.3969/j.issn.1673-5374...
).
4.5. Thalamus
The present results reported that Ngb and Hif-1α expression in the adult yak thalamus showed a significant rate of expression than the young yak thalamus. The different expressions may have resulted from the stress that the adult yak undergoes as compared to the young yak. Activities such as responding to predators can be stressful. Adult yaks respond to a predator's approach by huddling closely together, lowering their horns as if ready to strike, with the yaks on the outside of the circle. By charging and defending her calves, Yak will also try to scare predators away. Reproduction is one of the most stressful behaviors of all adult animals, and this may be one of the factors that contribute to the adult and young yak thalamus' various patterns of Ngb and Hif-1α. During delivery, the adult female yak displays a highly protective maternal instinct and doesn’t need aid from the herdsmen. Researchers' observations revealed that the degree of Ngb expression in the thalamus of the human brain is similar to what is found in the thalamus of rats and mice, particularly in the regions heavily involved in stressful activities (Dellavalle et al., 2010DELLAVALLE, B., HEMPEL, C., KURTZHALS, J.A., and PENKOWA, M., 2010. In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia, vol. 58, 10, pp. 1220-1227. http://dx.doi.org/10.1002/glia.21002.
https://doi.org/10.1002/glia.21002...
; Hundahl et al., 2012HUNDAHL, C.A., FAHRENKRUG, J., LUUK, H., HAY-SCHMIDT, A. and HANNIBAL, J., 2012. Restricted expression of Neuroglobin in the mouse retina and co-localization with Melanopsin and Tyrosine Hydroxylase. Biochemical and Biophysical Research Communications, vol. 425, no. 1, pp. 100-106. http://dx.doi.org/10.1016/j.bbrc.2012.07.061. PMid:22820193.
http://dx.doi.org/10.1016/j.bbrc.2012.07...
; Laufs et al., 2004LAUFS, T., WYSTUB, S., REUSS, S., BURMESTER, T., SAALER-REINHARDT, S. and HANKELN, T., 2004. Neurons specific expression of Neuroglobin in mammals. Neuroscience Letters, vol. 362, no. 2, pp. 83-86. http://dx.doi.org/10.1016/j.neulet.2004.02.072. PMid:15193759.
http://dx.doi.org/10.1016/j.neulet.2004....
; Schubert et al., 2011SCHUBERT, G.A., SEIZ, M., HEGEWALD, A.A., MANVILLE, J. and THOME, C., 2011. Hypoperfusion in the acute phase of subarachnoid hemorrhage. Acta Neurochirurgica. Supplementum, vol. 110, no. Pt 1, pp. 35-38. http://dx.doi.org/10.1007/978-3-7091-0353-1_6. PMid:21116911.
http://dx.doi.org/10.1007/978-3-7091-035...
). Although researches have a focus on Ngb expression in the thalamus, however, there still exist limited references detailing the Hif-1α expression in the thalamus.
4.6. Pineal and Pituitary gland
Results reported by Reuss et al. showed that the Ngb pattern of expression was limited to the anterior lobe of the pineal and pituitary gland, where the endocrine adenohypophysis cells showed a relatively strong signal (Reuss et al., 2002REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5. PMid:12435404.
http://dx.doi.org/10.1016/S0306-4522(02)...
). Christian et al. study also confirmed Ngb expression in the pituitary gland of humans and its expression is at a normal level (Christian et al., 2013CHRISTIAN, A.H., JESPER, K. and HAY-SCHMIDT, A., 2013. Neuroglobin and Cytoglobin expression in the human brain. Brain Structure & Function, vol. 218, no. 2, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-0480-8. PMid:23160832.
http://dx.doi.org/10.1007/s00429-012-048...
). The present study showed a highly significant degree of expression of Ngb and Hif-1α in the adult and young yak pineal and pituitary glands, and the expression pattern was consistently read. The significant level of consistency may have resulted from the hormones produced and regulated by the pineal gland which is involved with hair growth on the adult and young yak body. The long-coated hair on the species body aid the animals to sleep comfortably on the snow. Another factor showing the similarity is that the Hif-1α expressions might be associated with neuroprotection during the blood flow while the yak is at sleep during the winter. Kouroupi et al. stated that Hif-1α reactivity is associated with pituitary gland VEGF expression, indicating that under hypoxic conditions, the Hif-1α protein pathway can regulate vascular density and blood flow in the gland (Kouroupi et al., 2018KOUROUPI, M., SIVRIDIS, E., PAPAZOGLOU, D., KOUKOURAKIS, M.I. and GIATROMANOLAKI, A., 2018. Hypoxia-Inducible Factor Expression and Angiogenesis – Analysis in the pituitary gland and patterns of death. In Vivo (Athens, Greece), vol. 32, no. 1, pp. 185-190. http://dx.doi.org/10.21873/invivo.11223. PMid:29275318.
https://doi.org/10.21873/invivo.11223...
).
4.7. Optic nerve
Ngb knockdown caused deleterious effects on retinal structure and function has been documented by a review. The optic nerves showed a major defect in complex I and III respiratory chain activity (Lechauve et al., 2012LECHAUVE, C., SÉBASTIEN, A., HÉLÈNE, C.T., AÏCHA, B., VALÉRIE, F., CHANTAL, C., PIERRE, R., MICHAEL, C.M., JOSÉ, A.S. and MARISOL, C.D., 2012. Neuroglobin involvement in respiratory chain function and retinal ganglion cell integrity. Biochimica et Biophysica Acta, vol. 1823, no. 12, pp. 2261-2273. http://dx.doi.org/10.1016/j.bbamcr.2012.09.009. PMid:23036890.
http://dx.doi.org/10.1016/j.bbamcr.2012....
). Our research stated, however, that the expression of Ngb and Hif-1α was important in adult and young yak optic nerves, but the expression pattern was higher in the young yak than in the adult yak. The higher expression of Ngb and Hif-1α in the young yak's optic nerve showed that the demand for oxygen in the young yak's retina is higher relative to that of the adult yak. As Dloman and Quigley have documented (Quigley at al., 1989QUIGLEY, H.A., DUNKELBERGER, G.R. and GREEN, W.R., 1989. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. American Journal of Ophthalmology, vol. 107, no. 5, pp. 453-464. http://dx.doi.org/10.1016/0002-9394(89)90488-1. PMid:2712129.
http://dx.doi.org/10.1016/0002-9394(89)9...
), as a result of decreased retinal ganglion cells and nerve fibers, aging or optic nerve aging can result in decreased visual sensitivity or loss of vision. In the optic nerve of the adult mouse retina, generating central optic axons exhibiting outgrowth is lacking (Kayo et al., 2017KAYO, S., YOSHIKI, K., MAYUKO, S., KUNIZO, A., KAZUHIRO, O. and WAKASUGI, K., 2017. A novel function of neuroglobin for neuroregeneration in mice after optic nerve injury. Biochemical and Biophysical Research Communications, vol. 493, no. 3, pp. 1254-1259. http://dx.doi.org/10.1016/j.bbrc.2017.09.127. PMid:28951213.
http://dx.doi.org/10.1016/j.bbrc.2017.09...
). The outgrowths are shown to enhanced Ngb expression in the mouse retina. Hif-1α stabilization in neuronal cells is important for cell survival in the hypoxic brain (Chen at al., 2008CHEN, W., JADHAV, V., TANG, J. and ZHANG, J.H., 2008. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiology of Disease, vol. 31, no. 3, pp. 433-441. http://dx.doi.org/10.1016/j.nbd.2008.05.020. PMid:18602008.
http://dx.doi.org/10.1016/j.nbd.2008.05....
). Analysis by Cheng et al. found that Hif-1α was observed in humans in the retina and optic nerve. There is a level of Hif-1α expression in the adult and young yak optic nerve, as reported. This protein was involved in shielding nerve fibers from hypoxia in the optic nerve of the adult yak brain (Cheng et al., 2017CHENG, L., HONGHUA, Y., NAIHONG, Y., KUNBEI, L. and MENGQING, X., 2017. Hypoxia-Inducible Factor-1α Target Genes Contribute to Retinal Neuroprotection. Frontiers in Cellular Neuroscience, vol. 11, pp. 11-20. http://dx.doi.org/10.3389/fncel.2017.00020. PMid:28289375.
http://dx.doi.org/10.3389/fncel.2017.000...
).
4.8. Frontal lobe
Ngb expression in the frontal lobe was observed in the brain of a rat and its expression was measured and recognized by the enzyme-linked immunosorbent assay (ELISA) and Western blot (Liu et al., 2009LIU, C., SUN, S.Q., YU, J.B., WANG, K.J., XU, Q., CHEN, H. and LI, J., 2009. Expression of neuroglobin in rats with brain injury induced by LPS. Zhonghua Shao Shang Za Zhi, vol. 25, no. 3, pp. 222-226. PMid:19842562.). In the frontal lobe of the human brain, the expression of Ngb was also found (Fabrizius et al., 2016FABRIZIUS, A., ANDRE, D., LAUFS, T., BICKER, A., REUSS, S., BURMESTER, T., and HANKELN T., 2016. A critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience, vol. 337, no. 16, pp. 339-354. http://dx.doi.org/10.1016/j.neuroscience.2016.07.042.
https://doi.org/10.1016/j.neuroscience.2...
), Fabrizius et al. reported. Similarly, the current researchers reported a substantial level of Ngb and Hif-1α expression in the frontal lobe of adult and young yak, but the distribution pattern in the frontal lobe of adult yak was higher than that of the frontal lobe of young yak. The difference in expression may be derived from the body size of the adult yak which generates more heat during movement and longer frost-free periods. The sweat glands are distributed in the skin over the whole body of adult and young yak but fully mature in the adult yak than young yak. The highly neuroprotective maternal effect the female exhibit during delivery is observed to be another factor causing the differences. This research also indicated that the expression of Ngb and Hif-1α could increase the supply of O2 to the metabolically active neurons' mitochondria. The existence of these proteins in metabolically active cells and subcellular compartments supports this hypothesis, arguing that Ngb may supply O2 to the neuronal respiratory chain, especially the frontal lobe, which is the center of attraction (Liu et al., 2009LIU, C., SUN, S.Q., YU, J.B., WANG, K.J., XU, Q., CHEN, H. and LI, J., 2009. Expression of neuroglobin in rats with brain injury induced by LPS. Zhonghua Shao Shang Za Zhi, vol. 25, no. 3, pp. 222-226. PMid:19842562.). In the research reported ny Chen et al., Hif-1α is expressed in the frontal lobe of the rat model and apoptosis in the frontal cortex. The pattern of expression is shown to protect against cognitive impairment caused by chronic cerebral ischemic injury by an anti-apoptotic mechanism (Chen et al., 2013CHEN, H., AIXUAN, W., JINTING, H., MING, Y., JING, M. and ZHONGXIN, X., 2013. Changes of hypoxia-inducible factor-1 signaling and the effect of cilostazol in chronic cerebral ischemia. Neural Regeneration Research, vol. 8, no. 19, pp. 1803-1813. http://dx.doi.org/10.3969/j.issn.1673-5374.2013.19.008. PMid:25206477.
https://doi.org/10.3969/j.issn.1673-5374...
).
4.9. Parietal lobe
The expressions of Ngb and Hif-1α are substantially found in the parietal lobe of the parietal lobe of the adult and young yak, but the expression is greater in the parietal lobe of the adult than in the young yak. The difference in expression may be linked to the adaptability of the adult yak to a harsh temperature of 5,500 m in the lower ranges of the Himalayas in the Tibetan region, while the young yak may struggle to adapt to the high-altitude climate over a long period. The expression of Ngb and Hif-1α in the parietal lobe may also be involved in adapting the adult and young yak to the precipitous terrain and detecting the possibility of predators. According to a study conducted by Hu et al., Ngb expression is highly expressed in 39years old adult human and lower in 33 years old adult human (Hu et al., 2017HU, J., XIYUE, C., DEJIANG, P., QIHUI, L., YUANFENG, Z., BIN, F., LIXIA, L., ZHENGLI, C. and CHAO, H., 2017. Tumor grade related expression of neuroglobin is negatively regulated by PPARγ and confers antioxidant activity in glioma progression. Redox Biology, vol. 12, no. 12, pp. 682-689. http://dx.doi.org/10.1016/j.redox.2017.03.023. PMid:28410531.
http://dx.doi.org/10.1016/j.redox.2017.0...
). The enzyme-linked immunosorbent assay (ELISA) and Western blot were used by Yang et al. to test the levels of Hif-1α in the parietal lobe of the epileptic rats (Yang et al., 2016YANG, J., HE, F., MENG, Q., SUN, Y., WANG, W. and WANG, C., 2016. Inhibiting HIF-1α Decreases Expression of TNF-α and Caspase-3 in Specific Brain Regions Exposed Kainic Acid-Induced Status Epilepticus. Cellular Physiology and Biochemistry, vol. 38, no. 1, pp. 75-82. http://dx.doi.org/10.1159/000438610. PMid:26741705.
http://dx.doi.org/10.1159/000438610...
). The study revealed that after induction of status epilepticus (SE) Hif-1α significantly increased in the parietal lobe and decreased when the amplified TNF-α expression is evoked by the epileptic status. However, in the current study, a major expression of Hif-1α in the parietal cortex was reported.
4.10. Temporal lobe
A previous study reported that Ngb expression in the 55 years old female (human) recorded higher than in the 45 years old female (human), and 48 years old male (human). This demonstrates that the age factor has an influence on Ngb expression in the temporal lobe (Hu et al., 2017HU, J., XIYUE, C., DEJIANG, P., QIHUI, L., YUANFENG, Z., BIN, F., LIXIA, L., ZHENGLI, C. and CHAO, H., 2017. Tumor grade related expression of neuroglobin is negatively regulated by PPARγ and confers antioxidant activity in glioma progression. Redox Biology, vol. 12, no. 12, pp. 682-689. http://dx.doi.org/10.1016/j.redox.2017.03.023. PMid:28410531.
http://dx.doi.org/10.1016/j.redox.2017.0...
). The present results showed that the Ngb expressions and Hif-1α were significantly expressed as opposed to young yak in the temporal lobe of the adult yak and showed greater but no significance. A previous study has reported that temporal lobe memory, especially when temporal interference is strong, begins to decline in adulthood (Lindsay et al., 2015LINDSAY, J.R., CATHERINE, A.S., EMILY, J.V.E., EVA, P.T., JERLYN, C.T. and PAUL, E.G., 2015. Differences in temporal order memory among young, middle-aged, and older adults may depend on the level of interference. Frontiers in Aging Neuroscience, vol. 7, pp. 28. http://dx.doi.org/10.3389/fnagi.2015.00028. PMid:25852544.
https://doi.org/10.3389/fnagi.2015.00028...
). Ngb and Hif-1α expression play an important function in the neuroprotection as the temporal lobe decline in the adult yak as compare to the young yak. Wei-De et al. reported that the levels of Ngb protein significantly increased in the temporal cortex in Subarachnoid hemorrhage (SAH) groups and peaked at 24 h after SAH (Yaohua et al., 2016YAOHUA, L., CHENG, H., PEIMIN, F., YANPING, J., WEI, W., DONG, Z. and LEI, C., 2016. Aberrant expression of miR-153 is associated with overexpression of hypoxia-inducible factor-1α in refractory epilepsy. Scientific Reports, vol. 6, pp. 32-91.). In the temporal lobe of humans (Yaohua et al., 2016YAOHUA, L., CHENG, H., PEIMIN, F., YANPING, J., WEI, W., DONG, Z. and LEI, C., 2016. Aberrant expression of miR-153 is associated with overexpression of hypoxia-inducible factor-1α in refractory epilepsy. Scientific Reports, vol. 6, pp. 32-91.), aberrant expression of Hif-1α has been expressed. In the adult yak temporal lobe, a moderate level of Hif-1α and higher expression in the young yak were, however, reported in the current result, but not significant. The level of expression of this protein in the temporal lobe depends solely on age-related variables, and current researchers also propose that Ngb and Hif-1α have a role in the yak retina during hypoxia.
4.11. Occipital lobe
A study showing the expression of Ngb in the occipital lobe showed that Ngb is involved in E2 treatment (Guglielmotto et al., 2016GUGLIELMOTTO, M., REINERI, S., IANNELLO, A., FERRERO, G., VANZAN, L., MIANO, V., RICCI, L., TAMAGNO, E., DE BORTOLI, M. and CUTRUPI, S., 2016. E2 Regulates Epigenetic Signature on Neuroglobin Enhancer-Promoter in Neuronal Cells. Frontiers in Cellular Neuroscience, vol. 10, pp. 147. http://dx.doi.org/10.3389/fncel.2016.00147. PMid:27313512.
http://dx.doi.org/10.3389/fncel.2016.001...
). The study also hypothesized that E2 may mediate the neuroprotective action of Ngb against neurotoxic stimuli, opening the way for aging hormone therapy. A significant level of Ngb and Hif-1α expression in the occipital lobe of the adult yak and a sufficient pattern of expression in the young yak have been revealed in current research, but not significant. The significant expression of Ngb and Hif-1α might aid in protecting the adult yak retina from neuron diseases. Burmester et al. confirmed that the expression of Ngb is 70% in adult humans' occipital lobe (Burmester et al., 2000BURMESTER, T., WEICH, B., REINHARDT, S. and HANKELN, T., 2000. A vertebrate globin expressed in the brain. Nature, vol. 407, no. 6803, pp. 520-523. http://dx.doi.org/10.1038/35035093. PMid:11029004.
http://dx.doi.org/10.1038/35035093...
), while Juan et al. stated that Hif-1α is expressed in the rat brain's occipital lobe (Chávez et al., 2000CHÁVEZ, J.C., AGANI, F., PICHIULE, P. and LAMANNA, J.C., 2000. Expression of hypoxia-inducible factor-1α in the brain of rats during chronic hypoxia. Journal of Applied Physiology, vol. 89, no. 5, pp. 1937-1942. http://dx.doi.org/10.1152/jappl.2000.89.5.1937. PMid:11053346.
http://dx.doi.org/10.1152/jappl.2000.89....
). It has been shown that the rate of expression regulates hypoxic brain tissues, thus restoring neural impairment. In the occipital lobe of the adult and young yak occipital lobe, the present study found a higher degree of Hif-1α expression, but the expression pattern between adult and young yak differs. In addition to protecting the retina from neuron diseases, the Hif-1α may be involved in repairing neural tissues during hypoxia.
4.12. Piriform lobe
The present results showed high degree of Ngb expression and Hif-1α in the adult and young yak piriform lobe, but not important. The trend of expression has shown similar because of the two proteins which help to respond to the insufficient oxygen supply between neurons of the piriform lobe. In the piriform lobe of the rat brain, Ngb expression was observed throughout the piriform lobe in layer 2 (Hundahl et al., 2012HUNDAHL, C.A., FAHRENKRUG, J., LUUK, H., HAY-SCHMIDT, A. and HANNIBAL, J., 2012. Restricted expression of Neuroglobin in the mouse retina and co-localization with Melanopsin and Tyrosine Hydroxylase. Biochemical and Biophysical Research Communications, vol. 425, no. 1, pp. 100-106. http://dx.doi.org/10.1016/j.bbrc.2012.07.061. PMid:22820193.
http://dx.doi.org/10.1016/j.bbrc.2012.07...
). Immunostaining was applied by Avivi et al. and confirmed that Ngb was strongly expressed in the Spalax rat piriform lobe (Avivi et al., 2010AVIVI, A., GERLACH, F., JOEL, A., REUSS, S., BURMESTER, T., NEVO, E. and HANKELN, T., 2010. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 50, pp. 21570-21575. http://dx.doi.org/10.1073/pnas.1015379107. PMid:21115824.
http://dx.doi.org/10.1073/pnas.101537910...
). In the piriform cortex of the aged rat, Hif-1α is highly expressed and the expression is involved in mediating the adaptive response of mammalian cells and tissues to changes in tissue oxygenation (Ndubuizu et al., 2009NDUBUIZU, O.I., CHAVEZ, J.C. and LAMANNA, J.C., 2009. Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain. The American Journal of Physiology, vol. 297, no. 1, pp. R158-R165. http://dx.doi.org/10.1152/ajpregu.90829.2008. PMid:19420289.
https://doi.org/10.1152/ajpregu.90829.20...
). The pattern of Hif-1α in the piriform lobe of the adult and young yak is indicated to have a significant role in the response to hypoxia during respiration in the high-altitude environment.
4.13. Hippocampus
Burmester et al. reported that in the vertebrae globin expression study, Ngb is expressed at 11 percent in the hippocampus of the human brain, while Reuss et al. suggested that the positive expression of Ngb identified is involved in the development of the rodent hippocampus (Reuss et al., 2002REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5. PMid:12435404.
http://dx.doi.org/10.1016/S0306-4522(02)...
). A substantial level of Ngb and Hif-1α in the adult and young yak hippocampus has been recorded in the current findings. The trend of expressions is similar which may have originated from the same shape and volume of the suprachiasmatic nucleus of the hypothalamus that is involved with circadian rhythms. It is suggested that Ngb and Hif-1α may also have a function in the circadian rhythms of yak. Additionally, the current finding also hypothesizes that Ngb and Hif-1α regulate the total 20% of the oxygen the adult and young yak need during rest. Hif-1α was expressed in the rat hippocampus and the administration of rAAV-Hif-1α also induced robust and prolonged Hif-1α production in the rat hippocampus (Chai et al., 2014CHAI, X., KONG, W., LIU, L., YU, W., ZHANG, Z. and SUN, Y., 2014. A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis. Neural Regeneration Research, vol. 9, no. 11, pp. 1145-1153. http://dx.doi.org/10.4103/1673-5374.135317. PMid:25206774.
http://dx.doi.org/10.4103/1673-5374.1353...
). The substantial level of Hif-1α in the adult and young yak hippocampus may have the capacity during apoptosis to attenuate hippocampal neurons.
4.14. Basal nuclei
In a 26 years old male, Ngb is highly expressed in the basal nuclei while a female of 42 years showed low expression (Hu et al., 2017HU, J., XIYUE, C., DEJIANG, P., QIHUI, L., YUANFENG, Z., BIN, F., LIXIA, L., ZHENGLI, C. and CHAO, H., 2017. Tumor grade related expression of neuroglobin is negatively regulated by PPARγ and confers antioxidant activity in glioma progression. Redox Biology, vol. 12, no. 12, pp. 682-689. http://dx.doi.org/10.1016/j.redox.2017.03.023. PMid:28410531.
http://dx.doi.org/10.1016/j.redox.2017.0...
). Age factors may have an influence on the expression of Ngb in neuronal tissues especially the basal nuclei. A substantial expression of Ngb and Hif-1α in the basal nuclei of the adult and young yak was identified in the current findings and a similar expression pattern was shown. The expression pattern may participate in protecting neuron tissues during transportation or movement in the high altitude environment. During transportation, the breath rate of yak often increases and oxygen is paramount in this process. With the significant expression of Ngb in the basal ganglia, the movement and coordination are conducted and neuronal tissues are protective. The expression of Hif-1α protects the basal nuclei from hypoxic or ischemic conditions, probably reducing brain damage. Studies have reported that heavy Hif-1α expression in basal nuclei and other tissues of the neuron reacts against tumors (Hawa et al., 2016HAWA, N., LAVINA, A., JARLE, B., ARNES, H.W. and LARS, A.A., 2016. High Hypoxia-Inducible Factor-1 alpha (HIF-1 alpha) expression is associated with Axl expression and African Breast Cancer Aggressive Tumor Characteristics. PLoS One, vol. 1, no. 1, pp. e0146823. http://dx.doi.org/10.1371/journal.pone.0146823.
https://doi.org/10.1371/journal.pone.014...
).
4.15. Cingulate gyrus
According to Raida et al. (Raida et al., 2013RAIDA, Z., CHRISTIAN, A., JENS, H., NYENGAARD, R. and HAY-SCHMIDT, A., 2013. Neuroglobin Over Expressing Mice: Expression Pattern and Effect on Brain Ischemic Infarct Size. PLoS One, vol. 8, no. 10, pp. e7656. http://dx.doi.org/10.1371/journal.pone.0076565. PMid:24098534.
http://dx.doi.org/10.1371/journal.pone.0...
), Ngb is over-expressed in the cingulate gyrus of the brain of mice. A substantial decrease in the amount of infarction 24 hours after ischemia is involved in the expression. In the meantime, a study suddenly found that Ngb expression in the brain of the Gerbil showed different consequences of intranasal transmission for ischaemic insults in the Gerbil (Raida et al., 2013RAIDA, Z., CHRISTIAN, A., JENS, H., NYENGAARD, R. and HAY-SCHMIDT, A., 2013. Neuroglobin Over Expressing Mice: Expression Pattern and Effect on Brain Ischemic Infarct Size. PLoS One, vol. 8, no. 10, pp. e7656. http://dx.doi.org/10.1371/journal.pone.0076565. PMid:24098534.
http://dx.doi.org/10.1371/journal.pone.0...
; Yan et al., 2011YAN, G., YUNEIDIS, M., YAMILA, R.C., ADRIANA, M., ILIANA, S.T., JORGE, D.G., YONGHONG, W., JULIO, C., GARCÍA, R. and CHENGGANG, Z., 2011. Different Expression Patterns of Ngb and EPOR in the cerebral cortex and hippocampus revealed distinctive therapeutic effects of intranasal delivery of Neuro-EPO for Ischemic Insults to the Gerbil Brain. The Journal of Histochemistry and Cytochemistry, vol. 59, no. 2, pp. 214-227. http://dx.doi.org/10.1369/0022155410390323. PMid:21339183.
http://dx.doi.org/10.1369/00221554103903...
). Current results, however, have recorded a substantial level of Ngb and Hif-1α in the adult yak cingulate gyrus and a high level of expression in the young yak, but without significance. The trend of expression in the adult yak cingulate gyrus differs from the young yak because the adult yak is more sensitive to stress response than the young yak. Responding to stress is difficult for young yak. The Ngb and Hif-1α expression in the cingulate gyrus of the adult yak also function to assist oxygen transport to the body tissues. One common adaptation is the modulate blood flow to make oxygen more attracted to the hemoglobin molecule within the blood. Ngb presents in the cingulate tissue aid the yak in adapting to the high altitude environment by lowering its pressure value making it easier to obtain oxygen from a low-pressure environment. The significant expression of Hif-1α in the adult and young yak increased affinity for oxygen due to lowered concentrations at high altitude.
4.16. Corpus Callosum
A substantial amount of Ngb and Hif-1α was intensively expressed in both adult and young yak corpus callosum in the current study. The pattern of Ngb and Hif-1α read similarly and this may have resulted from the similarity of the signaling transmission of the adult and young yak corpus callosum between the left and right hemispheres. The Ngb and Hif-1α expression might have relevant neuron protective functions in the signal transmission across the hemispheres. An earlier study by Avivi et al. stated that Ngb was observed in the rat corpus callosum (Spalax) subterranean mole (Avivi et al., 2010AVIVI, A., GERLACH, F., JOEL, A., REUSS, S., BURMESTER, T., NEVO, E. and HANKELN, T., 2010. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 50, pp. 21570-21575. http://dx.doi.org/10.1073/pnas.1015379107. PMid:21115824.
http://dx.doi.org/10.1073/pnas.101537910...
), while extreme Ngb-IR was expressed in the transgenic mouse corpus callosum as reported by Raida et al. (Raida et al., 2013RAIDA, Z., CHRISTIAN, A., JENS, H., NYENGAARD, R. and HAY-SCHMIDT, A., 2013. Neuroglobin Over Expressing Mice: Expression Pattern and Effect on Brain Ischemic Infarct Size. PLoS One, vol. 8, no. 10, pp. e7656. http://dx.doi.org/10.1371/journal.pone.0076565. PMid:24098534.
http://dx.doi.org/10.1371/journal.pone.0...
). The Hif-1α expression is documented significantly in the current study and the researchers have suggested that its expression in the corpus callosum protects the brain from oxidative stress and severe injury.
5. Conclusion
The researchers’ data argue against a single role of Ngb and Hif-1α and revealed that age factors play a significant role in the expression of Ngb and Hif-1α in mammals. The results also displayed a similar and different expression pattern of Ngb and Hif-1α in the brain tissues of the adult and young yak and explained the factors causing the changes. An interesting finding ascertained by this study is that Ngb and Hif-1α distribution and expression are suggested to influence the adaptive mechanism of the adult and young yak to the high altitude environment and protect neuron tissues against any factor that could cause apoptosis. The neuroprotective mechanism in yak may be related to its structural features. Because Ngb and Hif-1α have a high affinity for oxygen, it is hypothesized that these expressions in the brain of adult and young yak make it easier for adaptation to the high altitude environment. The overall expression of Ngb in the brain tissues showed higher than Hif-1α and have significant functions in the yak adaptation to high altitude. The results provide basic data for further studies on the mechanism of hypoxic adaptation of yak brain tissue at high altitude.
Acknowledgements
The researchers extend heartfelt thanks and appreciation to the Gannan Tibetan Autonomous Prefecture for the facility. This work could not have been possible without assistance rendered by the groups aforementioned. The research project was funded by the National Natural Science Foundation of China (Grant No. 31760305). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- AVIVI, A., GERLACH, F., JOEL, A., REUSS, S., BURMESTER, T., NEVO, E. and HANKELN, T., 2010. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 50, pp. 21570-21575. http://dx.doi.org/10.1073/pnas.1015379107 PMid:21115824.
» http://dx.doi.org/10.1073/pnas.1015379107 - BURMESTER, T., WEICH, B., REINHARDT, S. and HANKELN, T., 2000. A vertebrate globin expressed in the brain. Nature, vol. 407, no. 6803, pp. 520-523. http://dx.doi.org/10.1038/35035093 PMid:11029004.
» http://dx.doi.org/10.1038/35035093 - CHAI, X., KONG, W., LIU, L., YU, W., ZHANG, Z. and SUN, Y., 2014. A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis. Neural Regeneration Research, vol. 9, no. 11, pp. 1145-1153. http://dx.doi.org/10.4103/1673-5374.135317 PMid:25206774.
» http://dx.doi.org/10.4103/1673-5374.135317 - CHÁVEZ, J.C., AGANI, F., PICHIULE, P. and LAMANNA, J.C., 2000. Expression of hypoxia-inducible factor-1α in the brain of rats during chronic hypoxia. Journal of Applied Physiology, vol. 89, no. 5, pp. 1937-1942. http://dx.doi.org/10.1152/jappl.2000.89.5.1937 PMid:11053346.
» http://dx.doi.org/10.1152/jappl.2000.89.5.1937 - CHEN, H., AIXUAN, W., JINTING, H., MING, Y., JING, M. and ZHONGXIN, X., 2013. Changes of hypoxia-inducible factor-1 signaling and the effect of cilostazol in chronic cerebral ischemia. Neural Regeneration Research, vol. 8, no. 19, pp. 1803-1813. http://dx.doi.org/10.3969/j.issn.1673-5374.2013.19.008. PMid:25206477.
» https://doi.org/10.3969/j.issn.1673-5374.2013.19.008 - CHEN, W., JADHAV, V., TANG, J. and ZHANG, J.H., 2008. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiology of Disease, vol. 31, no. 3, pp. 433-441. http://dx.doi.org/10.1016/j.nbd.2008.05.020 PMid:18602008.
» http://dx.doi.org/10.1016/j.nbd.2008.05.020 - CHENG, L., HONGHUA, Y., NAIHONG, Y., KUNBEI, L. and MENGQING, X., 2017. Hypoxia-Inducible Factor-1α Target Genes Contribute to Retinal Neuroprotection. Frontiers in Cellular Neuroscience, vol. 11, pp. 11-20. http://dx.doi.org/10.3389/fncel.2017.00020 PMid:28289375.
» http://dx.doi.org/10.3389/fncel.2017.00020 - CHRISTIAN, A.H., JESPER, K. and HAY-SCHMIDT, A., 2008. Regulation of Neuroglobin and Cytoglobin throughout the human brain. Brain Structure & Function, vol. 218, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-04808.
» https://doi.org/10.1007/s00429-012-04808 - CHRISTIAN, A.H., JESPER, K. and HAY-SCHMIDT, A., 2013. Neuroglobin and Cytoglobin expression in the human brain. Brain Structure & Function, vol. 218, no. 2, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-0480-8 PMid:23160832.
» http://dx.doi.org/10.1007/s00429-012-0480-8 - DELLAVALLE, B., HEMPEL, C., KURTZHALS, J.A., and PENKOWA, M., 2010. In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis. Glia, vol. 58, 10, pp. 1220-1227. http://dx.doi.org/10.1002/glia.21002.
» https://doi.org/10.1002/glia.21002 - FABRIZIUS, A., ANDRE, D., LAUFS, T., BICKER, A., REUSS, S., BURMESTER, T., and HANKELN T., 2016. A critical re-evaluation of neuroglobin expression reveals conserved patterns among mammals. Neuroscience, vol. 337, no. 16, pp. 339-354. http://dx.doi.org/10.1016/j.neuroscience.2016.07.042.
» https://doi.org/10.1016/j.neuroscience.2016.07.042 - GUGLIELMOTTO, M., REINERI, S., IANNELLO, A., FERRERO, G., VANZAN, L., MIANO, V., RICCI, L., TAMAGNO, E., DE BORTOLI, M. and CUTRUPI, S., 2016. E2 Regulates Epigenetic Signature on Neuroglobin Enhancer-Promoter in Neuronal Cells. Frontiers in Cellular Neuroscience, vol. 10, pp. 147. http://dx.doi.org/10.3389/fncel.2016.00147 PMid:27313512.
» http://dx.doi.org/10.3389/fncel.2016.00147 - HAWA, N., LAVINA, A., JARLE, B., ARNES, H.W. and LARS, A.A., 2016. High Hypoxia-Inducible Factor-1 alpha (HIF-1 alpha) expression is associated with Axl expression and African Breast Cancer Aggressive Tumor Characteristics. PLoS One, vol. 1, no. 1, pp. e0146823. http://dx.doi.org/10.1371/journal.pone.0146823.
» https://doi.org/10.1371/journal.pone.0146823 - HU, J., XIYUE, C., DEJIANG, P., QIHUI, L., YUANFENG, Z., BIN, F., LIXIA, L., ZHENGLI, C. and CHAO, H., 2017. Tumor grade related expression of neuroglobin is negatively regulated by PPARγ and confers antioxidant activity in glioma progression. Redox Biology, vol. 12, no. 12, pp. 682-689. http://dx.doi.org/10.1016/j.redox.2017.03.023 PMid:28410531.
» http://dx.doi.org/10.1016/j.redox.2017.03.023 - HUNDAHL, C.A., FAHRENKRUG, J., LUUK, H., HAY-SCHMIDT, A. and HANNIBAL, J., 2012. Restricted expression of Neuroglobin in the mouse retina and co-localization with Melanopsin and Tyrosine Hydroxylase. Biochemical and Biophysical Research Communications, vol. 425, no. 1, pp. 100-106. http://dx.doi.org/10.1016/j.bbrc.2012.07.061 PMid:22820193.
» http://dx.doi.org/10.1016/j.bbrc.2012.07.061 - HUNDAHL, C.A., KELSEN, J. and HAY-SCHMIDT, A., 2013. Neuroglobin and Cytoglobin expression in the human brain. Brain Structure & Function, vol. 218, no. 2, pp. 603-609. http://dx.doi.org/10.1007/s00429-012-0480-8 PMid:23160832.
» http://dx.doi.org/10.1007/s00429-012-0480-8 - HUQING, W., HAIQIN, W., HENA, G., GUILIAN, Z., RU, Z. and SHUQIN, Z., 2012. Increased hypoxia-inducible factor 1alpha expression in rat brain tissues in response to aging. Neural Regeneration Research, vol. 7, no. 10, pp. 778-782. http://dx.doi.org/10.3969/j.issn.1673-5374.2012.10.010. PMid:25737702.
» https://doi.org/10.3969/j.issn.1673-5374.2012.10.010 - JOB, C. and EBERWINE, J., 2001. Localization, and translation of mRNA in dendrites and axons. Nature Reviews. Neuroscience, vol. 2, no. 12, pp. 889-898. http://dx.doi.org/10.1038/35104069 PMid:11733796.
» http://dx.doi.org/10.1038/35104069 - KAYO, S., YOSHIKI, K., MAYUKO, S., KUNIZO, A., KAZUHIRO, O. and WAKASUGI, K., 2017. A novel function of neuroglobin for neuroregeneration in mice after optic nerve injury. Biochemical and Biophysical Research Communications, vol. 493, no. 3, pp. 1254-1259. http://dx.doi.org/10.1016/j.bbrc.2017.09.127 PMid:28951213.
» http://dx.doi.org/10.1016/j.bbrc.2017.09.127 - KOUROUPI, M., SIVRIDIS, E., PAPAZOGLOU, D., KOUKOURAKIS, M.I. and GIATROMANOLAKI, A., 2018. Hypoxia-Inducible Factor Expression and Angiogenesis – Analysis in the pituitary gland and patterns of death. In Vivo (Athens, Greece), vol. 32, no. 1, pp. 185-190. http://dx.doi.org/10.21873/invivo.11223. PMid:29275318.
» https://doi.org/10.21873/invivo.11223 - LAUFS, T., WYSTUB, S., REUSS, S., BURMESTER, T., SAALER-REINHARDT, S. and HANKELN, T., 2004. Neurons specific expression of Neuroglobin in mammals. Neuroscience Letters, vol. 362, no. 2, pp. 83-86. http://dx.doi.org/10.1016/j.neulet.2004.02.072 PMid:15193759.
» http://dx.doi.org/10.1016/j.neulet.2004.02.072 - LECHAUVE, C., SÉBASTIEN, A., HÉLÈNE, C.T., AÏCHA, B., VALÉRIE, F., CHANTAL, C., PIERRE, R., MICHAEL, C.M., JOSÉ, A.S. and MARISOL, C.D., 2012. Neuroglobin involvement in respiratory chain function and retinal ganglion cell integrity. Biochimica et Biophysica Acta, vol. 1823, no. 12, pp. 2261-2273. http://dx.doi.org/10.1016/j.bbamcr.2012.09.009 PMid:23036890.
» http://dx.doi.org/10.1016/j.bbamcr.2012.09.009 - LINDSAY, J.R., CATHERINE, A.S., EMILY, J.V.E., EVA, P.T., JERLYN, C.T. and PAUL, E.G., 2015. Differences in temporal order memory among young, middle-aged, and older adults may depend on the level of interference. Frontiers in Aging Neuroscience, vol. 7, pp. 28. http://dx.doi.org/10.3389/fnagi.2015.00028. PMid:25852544.
» https://doi.org/10.3389/fnagi.2015.00028 - LIU, C., SUN, S.Q., YU, J.B., WANG, K.J., XU, Q., CHEN, H. and LI, J., 2009. Expression of neuroglobin in rats with brain injury induced by LPS. Zhonghua Shao Shang Za Zhi, vol. 25, no. 3, pp. 222-226. PMid:19842562.
- MANOTHAM, K., TETSUHIRO, T., TAKAMOTO, O., ICHIRO, K., TOSHIO, M., REIKO, I., HIROTOSHI, T., RYOJI, S., TOSHIRO, F. and MASAOMI, N., 2005. A Biologic Role of HIF-1 in the Renal Medulla. Kidney International, vol. 67, no. 4, pp. 1428-1439. http://dx.doi.org/10.1111/j.1523-1755.2005.00220.x PMid:15780095.
» http://dx.doi.org/10.1111/j.1523-1755.2005.00220.x - NDUBUIZU, O.I., CHAVEZ, J.C. and LAMANNA, J.C., 2009. Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain. The American Journal of Physiology, vol. 297, no. 1, pp. R158-R165. http://dx.doi.org/10.1152/ajpregu.90829.2008. PMid:19420289.
» https://doi.org/10.1152/ajpregu.90829.2008 - QUIGLEY, H.A., DUNKELBERGER, G.R. and GREEN, W.R., 1989. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. American Journal of Ophthalmology, vol. 107, no. 5, pp. 453-464. http://dx.doi.org/10.1016/0002-9394(89)90488-1 PMid:2712129.
» http://dx.doi.org/10.1016/0002-9394(89)90488-1 - RAIDA, Z., CHRISTIAN, A., JENS, H., NYENGAARD, R. and HAY-SCHMIDT, A., 2013. Neuroglobin Over Expressing Mice: Expression Pattern and Effect on Brain Ischemic Infarct Size. PLoS One, vol. 8, no. 10, pp. e7656. http://dx.doi.org/10.1371/journal.pone.0076565 PMid:24098534.
» http://dx.doi.org/10.1371/journal.pone.0076565 - REUSS, S., BANICA, O., ELGURT, M., MITZ, S., DISQUE-KAISER, U., RIEMANN, R., HILL, M., JAQUISH, D.V., KOEHRN, F.J., BURMESTER, T., HANKELN, T. and WOOLF, N.K., 2016. Neuroglobin Expression in the Mammalian Auditory System. Molecular Neurobiology, vol. 53, no. 3, pp. 1461-1477. http://dx.doi.org/10.1007/s12035-014-9082-1 PMid:25636685.
» http://dx.doi.org/10.1007/s12035-014-9082-1 - REUSS, S., SAALER-REINHARDT, A.S., WEICH, B., WYSTUB, S., REUSS, M.H., BURMESTER, T. and HANKELN, T., 2002. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience, vol. 115, no. 3, pp. 645-656. http://dx.doi.org/10.1016/S0306-4522(02)00536-5 PMid:12435404.
» http://dx.doi.org/10.1016/S0306-4522(02)00536-5 - SCHUBERT, G.A., SEIZ, M., HEGEWALD, A.A., MANVILLE, J. and THOME, C., 2011. Hypoperfusion in the acute phase of subarachnoid hemorrhage. Acta Neurochirurgica. Supplementum, vol. 110, no. Pt 1, pp. 35-38. http://dx.doi.org/10.1007/978-3-7091-0353-1_6 PMid:21116911.
» http://dx.doi.org/10.1007/978-3-7091-0353-1_6 - SONG, L.L., CUI, Y., YU, S.J., LIU, P.G., LIU, J., YANG, X., HE, J.F. and ZHANG, Q., 2018. Expression characteristics of BMP2, BMPR-IA, and Noggin in different stages of the hair follicle in yak skin. General and Comparative Endocrinology, vol. 260, no. 1, pp. 18-24. http://dx.doi.org/10.1016/j.ygcen.2017.11.016 PMid:29174869.
» http://dx.doi.org/10.1016/j.ygcen.2017.11.016 - WYSTUB, S., LAUFS, T., SCHMIDT, M., BURMESTER, T., MAAS, U., SAALER-REINHARDT, S., HANKELN, T. and REUSS, S., 2003. Localization of neuroglobin protein in the mouse brain. Neuroscience Letters, vol. 346, no. 1-2, pp. 114-116. http://dx.doi.org/10.1016/S0304-3940(03)00563-9 PMid:12850561.
» http://dx.doi.org/10.1016/S0304-3940(03)00563-9 - YAN, G., YUNEIDIS, M., YAMILA, R.C., ADRIANA, M., ILIANA, S.T., JORGE, D.G., YONGHONG, W., JULIO, C., GARCÍA, R. and CHENGGANG, Z., 2011. Different Expression Patterns of Ngb and EPOR in the cerebral cortex and hippocampus revealed distinctive therapeutic effects of intranasal delivery of Neuro-EPO for Ischemic Insults to the Gerbil Brain. The Journal of Histochemistry and Cytochemistry, vol. 59, no. 2, pp. 214-227. http://dx.doi.org/10.1369/0022155410390323 PMid:21339183.
» http://dx.doi.org/10.1369/0022155410390323 - YANG, J., HE, F., MENG, Q., SUN, Y., WANG, W. and WANG, C., 2016. Inhibiting HIF-1α Decreases Expression of TNF-α and Caspase-3 in Specific Brain Regions Exposed Kainic Acid-Induced Status Epilepticus. Cellular Physiology and Biochemistry, vol. 38, no. 1, pp. 75-82. http://dx.doi.org/10.1159/000438610 PMid:26741705.
» http://dx.doi.org/10.1159/000438610 - YAOHUA, L., CHENG, H., PEIMIN, F., YANPING, J., WEI, W., DONG, Z. and LEI, C., 2016. Aberrant expression of miR-153 is associated with overexpression of hypoxia-inducible factor-1α in refractory epilepsy. Scientific Reports, vol. 6, pp. 32-91.
- YUEN, T.J., JOHN, C.S., AMELIE, G., SANDRA, M.C., RICHARD, D., STEPHEN, P.J.F., HENGAMEH, Z., EMIN, M. and DAVID, H.R., 2014. Oligodendrocyte-encoded HIF function couples’ postnatal myelination and white matter angiogenesis. Cell, vol. 158, no. 2, pp. 383-396. http://dx.doi.org/10.1016/j.cell.2014.04.052 PMid:25018103.
» http://dx.doi.org/10.1016/j.cell.2014.04.052
Publication Dates
-
Publication in this collection
03 Sept 2021 -
Date of issue
2023
History
-
Received
06 Nov 2020 -
Accepted
15 Feb 2021