Abstract
The poultry sector in Pakistan is contributing mainly in bridging gap between demand and supply for protein. Mycoplasma gallisepticum is an emerging bacterium causing serious problems in poultry industry of Pakistan. A cross-sectional study was conducted to evaluate the M. gallisepticum load in poultry populated regions of Pakistan. Total 600 serum and 600 swab samples were collected, 200 from each broiler, layers and breeders poultry in Rawalpindi and Abbottabad districts. Serum samples were analyzed through ELISA for seroprevalence. Swabs were cultured on Frey’s medium followed by PCR and partial mgc2 gene sequencing. Results of seroprevalence of M. gallisepticum showed that layers (75%, n=150) are more positive as compared to breeders (70%, n=140) and broilers (50%, n=100). Typical colonies of the M. gallisepticum were observed in breeder (26.5%), followed by layer (21%) and broilers (9%). A total of 37.1% (n=42) samples were identified positive through PCR out of total 113 cultured based positive samples. A total of six M. gallisepticum isolates of current study showed 98-99 percent similarity with previously reported isolates on the basis of mgc2 gene partial sequencing. The M. gallisepticum was found highly prevalent in different poultry breads. Results of this study would add into basic data and provide a direction for livestock sector to strengthen a control strategy for mycoplasmosis in poultry farms.
Keywords:
mycoplasmosis; ELISA; mgc2 gene; PCR; poultry
Resumo
O setor avícola do Paquistão está contribuindo principalmente para preencher a lacuna entre a demanda e a oferta de proteína. Mycoplasma gallisepticum é uma bactéria emergente que causa sérios problemas na indústria avícola do Paquistão. Um estudo transversal foi conduzido para avaliar a carga de M. gallisepticum em regiões de avicultura do Paquistão. Um total de 600 amostras de soro e 600 amostras de esfregaço foi coletado, 200 de cada frango de corte, poedeiras e aves reprodutoras nos distritos de Rawalpindi e Abbottabad. Amostras de soro foram analisadas por ELISA para soroprevalência. As zaragatoas foram cultivadas em meio Frey, seguido de PCR e sequenciação parcial do gene mgc2. Os resultados da soroprevalência de M. gallisepticum mostraram que as poedeiras (75%, n = 150) são mais positivas em comparação com matrizes (70%, n = 140) e frangos de corte (50%, n = 100). Colônias típicas de M. gallisepticum foram observadas em reprodutoras (26,5%), seguidas de poedeiras (21%) e frangos de corte (9%). Um total de 37,1% (n = 42) das amostras foi identificado como positivas por PCR de um total de 113 amostras positivas baseadas em cultura. Um total de seis isolados de M. gallisepticum do estudo atual mostrou 98-99% de similaridade com isolados relatados anteriormente com base no sequenciamento parcial do gene mgc2. O M. gallisepticum foi encontrado com alta prevalência em diferentes pães de aves. Os resultados deste estudo acrescentariam dados básicos e forneceriam orientação para o setor pecuário fortalecer uma estratégia de controle da micoplasmose em granjas avícolas.
Palavras-chave:
micoplasmose; ELISA; gene mgc2; PCR; aves
1. Introduction
Mycoplasma gallisepticum (M. gallisepticum) is causing mycoplasmosis and is considered as Chronic Respiratory Disease (CRD) in both chicken and turkeys (Osman et al., 2009OSMAN, K., ALY, M., AMIN, Z. and HASAN, B., 2009. Mycoplasma gallisepticum: an emerging challenge to the poultry industry in Egypt. Revue Scientifique et Technique, vol. 28, no. 3, pp. 1015-1023. http://dx.doi.org/10.20506/rst.28.3.1940. PMid:20462158.
http://dx.doi.org/10.20506/rst.28.3.1940...
). Clinical manifestation of the disease includes nasal and ocular discharge, coughing, abnormal feathers, poor productivity and moderate mortality (McMullin, 2004MCMULLIN, P., 2004. A pocket guide to poultry health and disease. Sheffield: 5M Enterprises Ltd.; Nascimento et al., 2005NASCIMENTO, E.R., PEREIRA, V., NASCIMENTO, M. and BARRETO, M., 2005. Avian mycoplasmosis update. Brazilian Journal of Poultry Science, vol. 7, no. 1, pp. 1-9. http://dx.doi.org/10.1590/S1516-635X2005000100001.
http://dx.doi.org/10.1590/S1516-635X2005...
). Breaking the chain of transmission of M. gallisepticum further in poultry flocks is possible with implementing the strict biosecurity measures at individual farm level. It will help in controlling and preventing the CRD in poultry industry (Ferguson et al., 2005FERGUSON, N.M., HEPP, D., SUN, S., IKUTA, N., LEVISOHN, S., KLEVEN, S.H. and GARCÍA, M., 2005. Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies. Microbiology, vol. 151, no. 6, pp. 1883-1893. http://dx.doi.org/10.1099/mic.0.27642-0. PMid:15941996.
http://dx.doi.org/10.1099/mic.0.27642-0...
). Regional surveillance M. gallisepticum is very necessary because the data from genome sequencing of M. gallisepticum revealed that with passage of time new strain are evolving in the field which may hamper the success of vaccine and controlling the infection as well as detection in detection (Delaney et al., 2012DELANEY, N.F., BALENGER, S., BONNEAUD, C., MARX, C.J., HILL, G.E., FERGUSON-NOEL, N., TSAI, P., RODRIGO, A. and EDWARDS, S.V., 2012. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLOS Genetics, vol. 8, no. 2, e1002511. http://dx.doi.org/10.1371/journal.pgen.1002511. PMid:22346765.
http://dx.doi.org/10.1371/journal.pgen.1...
; Ferguson et al., 2005FERGUSON, N.M., HEPP, D., SUN, S., IKUTA, N., LEVISOHN, S., KLEVEN, S.H. and GARCÍA, M., 2005. Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies. Microbiology, vol. 151, no. 6, pp. 1883-1893. http://dx.doi.org/10.1099/mic.0.27642-0. PMid:15941996.
http://dx.doi.org/10.1099/mic.0.27642-0...
). Further, there is need to develop vaccine and diagnostic antigens based on local isolates for effective management and eradication of mycoplasmosis in the poultry at country level. (Silva et al., 2021SILVA, R., SILVA, I., JESUS, M., FERNANDES, M., OLIVEIRA, F. and EVÊNCIO-NETO, J., 2021. Co-relationship between Escherichia coli in broiler cellulitis and liver lesions. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 3, pp. 714-718. http://dx.doi.org/10.1590/1519-6984.230243.
http://dx.doi.org/10.1590/1519-6984.2302...
). In Pakistan, CRD is big issue and one of major reason of huge economic loss in poultry industry. The financial constrains facing by the farmers, only a few of them are using a vaccine to protect their poultry from M. gallisepticum with compromised results. The remaining are using only antibiotics for treatment of the infection which can ultimately develop antibiotic resistance in the field. Current study was designed to find the burden of local M. gallisepticum using ELISA, culturing based method and PCR in all breeds of poultry in two major districts Abbottabad and Rawalpindi of Pakistan.
2. Materials and Methods
A cross-sectional study was conducted in one of the most poultry populated districts of Pakistan, where large number of commercial farms are located. A conventional isolation method followed by PCR and ELISA method was used for comparative analysis and confirmation of M. gallisepticum. A partial sequencing of targeted mgc2 gene from local isolates was carried out to measure the similarity in the species. Total 600 serum and 600 swab samples were collected, 200 from each broiler, layers and breeders poultry in Rawalpindi and Abbottabad.
2.1. ELISA test and culturing
The serum samples were processed through ELISA (ProFLOK® Synbiotics, USA) to estimate the prevalence of M. gallisepticum antibodies. The traches swab samples were processed on commercially available Frey’s medium (Part One: Mycoplasma broth base 5.62 gm, Thallium acetate 0.074 gm,, phenol red 0.0074 gm, yeast extracts 1.25 gm, distilled water, 200 mL; Part Two: Cysteine HCL 0.025 gm, Penicillin G 0.156 gm, horse serum 30 mL, glucose 0.074 gm, Nicotinamide Adenine Dinucleotide 0.025 gm, distilled water 20 mL). Part one ingredients were adjusted to 250 mL with distilled and pH 7.8 and autoclaved at 121 °C temperature for 15 min at 15 psi pressure. The part two containing temperature labile ingredients which were filtered from 0.2 µm syringe filter and was added to part at part one at low temperature.
2.2. Molecular detection
All suspected positive samples showing fried egg-shaped colonies were processed though PCR for further confirmation. A DNA was extracted from samples with typical mycoplasma colonies using commercially available genome extraction QIAGEN kit (USA). A specie specific primers for identification of M. gallisepticum were used for amplification of partial gene of mgc2 (Forward mgc2- 5’-GGTCCTAATCCCCAACAAAGAAT-3’; Reverse mgc2-5’-CTTGGTTGGTTCATA-TTAGGCATTT-3’ (Grodio et al., 2008GRODIO, J.L., DHONDT, K.V., O’CONNELL, P.H. and SCHAT, K.A., 2008. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathology, vol. 37, no. 4, pp. 385-391. http://dx.doi.org/10.1080/03079450802216629. PMid:18622854.
http://dx.doi.org/10.1080/03079450802216...
). Amplification of the gene was carried out in Thermocycler (BioRed CFX96, USA) with ready to use fermentas master mix (Thermo Scientific, USA) as per instructions of manufacturer. The thermocycler was set up on PCR condition as initial denaturation at 95 °C for 5 mints, 35 cycles of 94 °C for 30 sec, 55 °C for 30 sec and 72 °C for 30 sec and then final extension at 72 °C for 5 minutes. Amplified PCR products were run on 2 percent agarose gel using Tris Boric acid EDTA buffer in gel electrophoresis assembly. A QIAquick Gel Extraction Kit (QIAGEN, USA) was used for extraction of positive PCR products for further sequencing and analysis. Two samples of PCR amplified products from each layer, broiler and breeder were selected for sequence analysis through ABI 3130XL Automated Sequencer (Applied Biosystem Inc, Foster city, CA). Sequence similarity was found using BLASTN analysis and mgc2 gene partial sequences of local isolates were submitted to NCBI for accession number. All partial mgc2 gene sequences of local isolates were phylogenetically analyzed with globally reported mgc2 gene sequences through MEGA5 software using Bootstrap method and statistical test of Neighbor-joining with 1000-boostrap replicate (Tamura et al., 2011TAMURA, K., PETERSON, D., PETERSON, N., STECHER, G., NEI, M. and KUMAR, S., 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, vol. 28, no. 10, pp. 2731-2739. http://dx.doi.org/10.1093/molbev/msr121. PMid:21546353.
http://dx.doi.org/10.1093/molbev/msr121...
).
2.3. Ethical consideration
The Ethical Research Board in The University of Veterinary and Animal Sciences, Lahore approved the blood samples collected from poultry for analysis. All samples were collected using guidelines of International Animal Care and Use Committee (IACUC) with prior consent from farm’s owner.
2.4. Statistical analysis
Results obtained from ELISA, cultured based test and PCR were analyzed using χ2 square test through Statistical Packages for the Social Sciences (SPSS) software version 26.0 (Priya and Madhavan, 2002PRIYA, K. and MADHAVAN, H., 2002. Diagnostic value of enzyme linked immuno-sorbent assay for cytomegalovirus disease. Journal of Postgraduate Medicine, vol. 48, no. 3, pp. 176-178. PMid:12432189.).
3. Results
Out of total 600 serum collected, 200 from each broiler, layers and breeders, a reasonable number showed detectable level of serum anti-M. gallisepticum ELISA antibodies. The ELISA results showed that breeder breeds 75% (n=150) were more positive as compared to layers 70% (n=140) and broilers 50% (n=100) broiler as shown in Table 1. In the tracheal swabs streaked on Frey’s agar showed fried egg-shaped colonies which were suspected as Mycoplasma species. Samples 18.8% (n=113) were found positive for M. gallisepticum on the basis of colony morphology. Typical colonies of the M. gallisepticum were observed in breeder (26.5%), followed by layer (21%) and broilers (9%) as shown in Table 1. A total of 37.1% (n=42) samples were identified positive through PCR out of total 113 cultured based positive samples (Figure 1). Identification of M. gallisepticum through PCR using primers against mgc2 gene showed that broiler flocks (50%) were more positive as compared to breeder (37.7%) and layer (30.9%) as shown in Table 1. The sequences of partial mgc2 gene of M. gallisepticum isolates in current study were compared with already reported and available sequences of the same gene on NCBI GenBank using nucleotide BLAST analysis. All sequences were processed through MEGA5 software using statistical test of Neighbor-Joining with 1000-boostrap replicates. The Phylogenetic tree represented that the sequences of M. gallisepticum from this study clustered with previously reported sequences of mgc2 gene of this region as shown in Figure 2. A pairwise analysis of nucleotide sequences of currently characterized isolates showed 98 to 100 percent similarity with worldwide M. gallisepticum isolates and 98 to 99 percent similarity with previously reported isolated reported from Pakistan (Table 2). All sequences of isolated from this study are available at NCBI GenBank with accession numbers: KF874283, KF874282, KF874281, KF874280, KF874279, and KF874278.
Results of three different diagnostic techniques (ELISA, Culturing and PCR) for Mycoplasma gallisepticum.
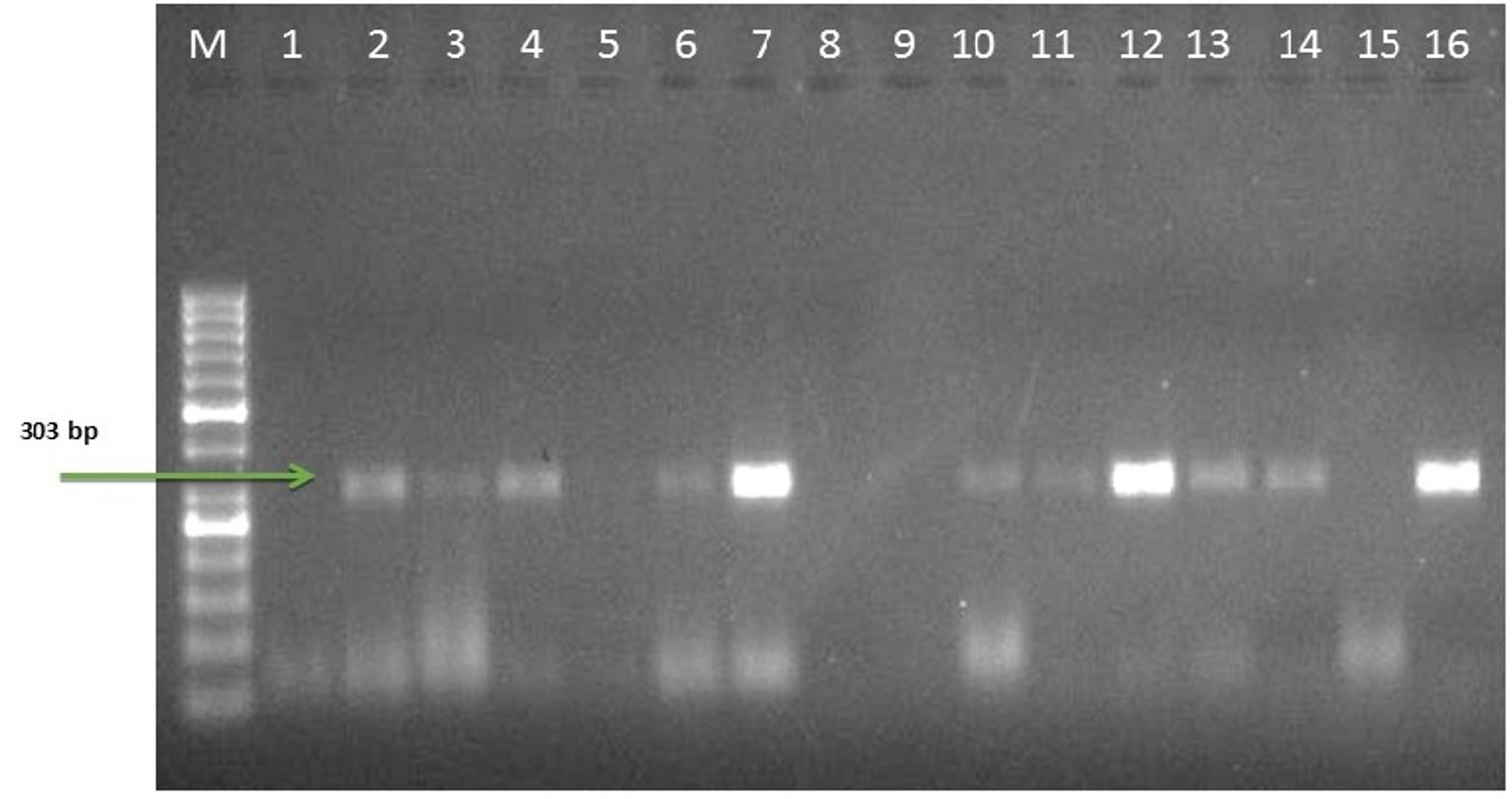
Agarose gel electrophoresis of PCR products of Mycoplasma gallisepticum (mgc2 gene); Lane M: DNA marker 50 bp, Lane 1: Negative control, Lane 2: Positive control (303bp), Lane 3, 4, 6, 7, 10-14, and 16: Mycoplasma gallisepticum positive isolates (303bp), Lane 5, 8, 9 and 15: Mycoplasma gallisepticum negative sample.
Partial mgc2 gene sequence analysis of Mycoplasma gallisepticum isolates from Pakistan by the Neighbor-joining method with 1000-Bootstrap replicates using MEGA5 software.
Percentage identity of local isolates of Mycoplasma gallisepticum from Pakistan with reported isolates globally.
4. Discussion
Previous studies showed that every test has its own specificity and sensitivity and results of all tests such as conventional isolation methods, spot agglutination test, ELISA, Haemagglutination inhibition test, and PCR provide variations (Feberwee et al., 2005FEBERWEE, A., MEKKES, D., DE WIT, J., HARTMAN, E. and PIJPERS, A., 2005. Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections. Avian Diseases, vol. 49, no. 2, pp. 260-268. http://dx.doi.org/10.1637/7274-090804R. PMid:16094832.
http://dx.doi.org/10.1637/7274-090804R...
; Luciano et al., 2011LUCIANO, R., CARDOSO, A., STOPPA, G., KANASHIRO, A., DE CASTRO, A. and TESSARI, E., 2011. Comparative study of serological tests for Mycoplasma synoviae diagnosis in commercial poultry breeders. Veterinary Medicine International, vol. 2011, pp. 2011. http://dx.doi.org/10.4061/2011/304349. PMid:21547263.
http://dx.doi.org/10.4061/2011/304349...
). These variations may be due to cross reactivity with other mycoplasma species, samples collected from birds with different age and improper sample transportation (Buchala et al., 2006BUCHALA, F., ISHIZUKA, M., MATHIAS, L., BERCHIERI JÚNIOR, A., CASTRO, A., CARDOSO, A. and KANASHIRO, A., 2006. Detecção de resposta sorológica contra Mycoplasma em aves de criatórios de “fundo de quintal” próximos a explorações comerciais do estado de São Paulo. Arquivos do Instituto Biológico, vol. 73, no. 2, pp. 143-148.). Diagnosis of Mycoplasma through conventional method of culturing is a “Gold Standard” but this technique is very laborious, expensive, time consuming due to slow growth and require very skilled staff. These things may lead to variations in results of conventional methods (Gharaibeh and Al Roussan, 2007GHARAIBEH, S. and AL ROUSSAN, D., 2007. The use of molecular techniques in isolation and characterization of Mycoplasma gallisepticum from commercial chickens in Jordan. International Journal of Poultry Science, vol. 7, no. 1, pp. 28-35. http://dx.doi.org/10.3923/ijps.2008.28.35.
http://dx.doi.org/10.3923/ijps.2008.28.3...
; Khalifa et al., 2013KHALIFA, K.A., SIDAHMED ABDELRAHIM, E., BADWI, M. and MOHAMED, A.M., 2013. Isolation and Molecular Characterization of Mycoplasma gallisepticum and Mycoplasma synoviae in Chickens in Sudan. Journal of Veterinary Medicine, vol. 2013, pp. 2013. http://dx.doi.org/10.1155/2013/208026. PMid:26464902.
http://dx.doi.org/10.1155/2013/208026...
). Results of current study from same samples showed similar variations in three tests (ELSA, culture, and PCR). Seroprevalence of anti- M. gallisepticum in all breeders, layers and broilers were found 75%. While tracheal swabs from same birds showed 37.1% positivity through conventional method in terms of mycoplasma typical fried egg-shaped colonies. The swab tested through PCR showed 26.5% positivity for M. gallisepticum. High number of positivity through culturing as compared to PCR is due to growth of other mycoplasma species such as M. synoviae. For diagnosis of M. gallisepticum, ELISA is the most common technique, but chances of non-specific reaction give false positive results and it might be the reason of high positivity in our results (Kleven, 2008KLEVEN, S., 2008. Control of avian mycoplasma infections in commercial poultry. Avian Diseases, vol. 52, no. 3, pp. 367-374. http://dx.doi.org/10.1637/8323-041808-Review.1. PMid:18939621.
http://dx.doi.org/10.1637/8323-041808-Re...
; Much et al., 2002MUCH, P., WINNER, F., STIPKOVITS, L., ROSENGARTEN, R. and CITTI, C., 2002. Mycoplasma gallisepticum: influence of cell invasiveness on the outcome of experimental infection in chickens. FEMS Immunology and Medical Microbiology, vol. 34, no. 3, pp. 181-186. http://dx.doi.org/10.1111/j.1574-695X.2002.tb00622.x. PMid:12423769.
http://dx.doi.org/10.1111/j.1574-695X.20...
). During comparison between ELISA and culturing, breeder showed the highest positivity 75%, 26.5% followed by layers 70%, 21% and broiler 50%, 9%, respectively as shown in Table 1. Diagnosis and control of M. gallisepticum on the basis of sero-conversion is not sufficient (Waites and Talkington, 2004WAITES, K.B. and TALKINGTON, D.F., 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clinical Microbiology Reviews, vol. 17, no. 4, pp. 697-728. http://dx.doi.org/10.1128/CMR.17.4.697-728.2004. PMid:15489344.
http://dx.doi.org/10.1128/CMR.17.4.697-7...
). As per World Organization for Animal Health (OIE) standard, an alternative to culturing, the PCR is a test of choice for diagnosis of M. gallisepticum in poultry industry due to its high sensitivity and rapid diagnosis. Identification of M. gallisepticum through PCR using primers against mgc2 gene showed that broiler flocks (50%) were more positive as compared to breeder (37.7%) and layer (30.9%). Similar results were found in previous studies (Marois et al., 2001MAROIS, C., DUFOUR‐GESBERT, F. and KEMPF, I., 2001. Molecular differentiation of Mycoplasma gallisepticum and Mycoplasma imitans strains by pulsed‐field gel electrophoresis and random amplified polymorphic DNA. Journal of Veterinary Medicine, Series B, vol. 48, no. 9, pp. 695-703. http://dx.doi.org/10.1046/j.1439-0450.2001.00496.x. PMid:11765805.
http://dx.doi.org/10.1046/j.1439-0450.20...
). Mycoplasmosis is majorly present in breeder (27%) which plays a significant role in vertical transmission through eggs from parents to progeny (Buim et al., 2009BUIM, M.R., METTIFOGO, E., TIMENETSKY, J., KLEVEN, S. and FERREIRA, A.J.P., 2009. Epidemiological survey on Mycoplasma gallisepticum and M. synoviae by multiplex PCR in commercial poultry. Pesquisa Veterinária Brasileira, vol. 29, no. 7, pp. 552-556. http://dx.doi.org/10.1590/S0100-736X2009000700009.
http://dx.doi.org/10.1590/S0100-736X2009...
). In present study, high level of positivity in broiler (50%) showed alarming situation because in broilers, the transmission is mainly horizontally due to transmission through aerosol and contaminated water, feed and environment. It might be also due to the ability of Mycoplasma to survive for long time on different fomites and remain major source of infection in new flock in absence of hygienic environment in the farm. Absence of good hygienic practices may be one of major reason high prevalence of M. gallisepticum in studied districts as compared to previous study (21.84%) conducted in Pakistan (Rauf et al., 2013RAUF, M., CHAUDHARY, Z., YOUNUS, M., ANJUM, A., ALI, M., AHMAD, A. and KHAN, M., 2013. Identification of Mycoplasma gallisepticum by polymerase chain reaction and conventional diagnostics from white leghorn layer flocks. JAPS. Journal of Animal and Plant Sciences, vol. 23, no. 2, pp. 393-397.). Isolates and strains vary in their pathogenicity pattern with differentiation in phenotypic and genotypic characteristics (García et al., 2005GARCÍA, M., IKUTA, N., LEVISOHN, S. and KLEVEN, S., 2005. Evaluation and comparison of various PCR methods for detection of Mycoplasma gallisepticum infection in chickens. Avian Diseases, vol. 49, no. 1, pp. 125-132. http://dx.doi.org/10.1637/7261-0812204R1. PMid:15839425.
http://dx.doi.org/10.1637/7261-0812204R1...
). For in depth study of M. gallisepticum in a specific region to understand the epidemiological niche, transmission of diseases and finding of new track of outbreaks, there is need to adopt a most reliable technique for differentiation the specie in more detail (Loolmani et al., 2014LOOLMANI, F., POURBAKHSH, S., BANANI, M. and CHARKHKAR, S., 2014. Phylogenetic analysis of mgc2 gene of Mycoplasma gallisepticum isolates from broiler breeder flocks in Tehran province, Iran. European Journal of Zoological Research, vol. 3, no. 2, pp. 37-42.). The mgc2 gene targeted in this study is most specific, and sensitive and can be used as reference only for molecular based identification of M. gallisepticum (Youni et al., 2018YOUNI, G., ABDELGAWAD, R.H., ELKENANY, R. and GLAL, A., 2018. Molecular identification and sequencing of Mycoplasma gallisepticum recovered from broilers in Egypt. Pakistan Journal of Biological Sciences, vol. 21, no. 5, pp. 253-261. http://dx.doi.org/10.3923/pjbs.2018.253.261.
http://dx.doi.org/10.3923/pjbs.2018.253....
; Emam et al., 2020EMAM, M., HASHEM, Y.M., EL-HARIRI, M. and EL-JAKEE, J., 2020. Detection and antibiotic resistance of Mycoplasma gallisepticum and Mycoplasma synoviae among chicken flocks in Egypt. Veterinary World, vol. 13, no. 7, pp. 1410-1416. http://dx.doi.org/10.14202/vetworld.2020.1410-1416. PMid:32848318.
http://dx.doi.org/10.14202/vetworld.2020...
). The particle sequencing and analysis of mgc2 gene showed a 100 percent similarity of local isolates from this study with isolated reported from India. Further 98 to 99 percent similarity was found with isolates already reported from Pakistan, Panama, USA, Spain, and Egypt. Minor variation was found between isolates used in this study which showed heterogenetically different isolates are prevailing in Pakistan and same scenario is prevails in India. Mycoplasma gallisepticum isolates from Breeder showed 100% similarity with isolates from USA and Spain. Sequence analysis on the basis of partial mgc2 gene has differentiated many genotypes prevailing in Pakistan. These minor variations found in this study in mgc2 gene in same region has also been reported by previous studies (Armour et al., 2013ARMOUR, N.K., LAIBINIS, V.A., COLLETT, S.R. and FERGUSON-NOEL, N., 2013. The development and application of a Mycoplasma gallisepticum sequence database. Avian Pathology, vol. 42, no. 5, pp. 408-415. http://dx.doi.org/10.1080/03079457.2013.819486. PMid:23889487.
http://dx.doi.org/10.1080/03079457.2013....
). Similar reports have also been published from USA, Israel, and Australia (Ferguson et al., 2005FERGUSON, N.M., HEPP, D., SUN, S., IKUTA, N., LEVISOHN, S., KLEVEN, S.H. and GARCÍA, M., 2005. Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies. Microbiology, vol. 151, no. 6, pp. 1883-1893. http://dx.doi.org/10.1099/mic.0.27642-0. PMid:15941996.
http://dx.doi.org/10.1099/mic.0.27642-0...
). Mycoplasma gallisepticum isolates studied in current study showed 96 to 98% similarity to ts-11 vaccinal strain (KC247898) of M. gallisepticum while F strain (KC247897) has 93 to 94% similarity. A whole genome sequencing is required for detail study of nature of existing wild type strain in Pakistan. It will help us to understand that why the infection due to M. gallisepticum is prevailing in the field because the vaccine against Mycoplasmosis has been using from several decades in Pakistan. Dissimilarity of local wild type isolates from F strain (vaccine strain) also suggests unsuccessful replacement of wild type stain with vaccine strain in field which may be due to improper vaccination or storage.
5. Conclusion
To sum up this study, prevalence of M. gallisepticum is high in both Rawalpindi and Abbottabad districts of Pakistan in all breeds of poultry. This study suggests the involvement of M. gallisepticum in CRD infections in poultry. To control infection of M. gallisepticum in poultry industry, a strict biosecurity measures should be taken in all poultry farms and a hygienic environment should be maintained in the farms. Further, a countrywide surveillance should be conducted which will help to control CRD in poultry and economic losses in the industry.
Acknowledgements
Thanks to PAK-USA Science and Technology Cooperation program International Collaborative Research Project # 335 “Characterization of M. gallisepticum isolates in Pakistan and their use in production of indigenous diagnostic antigen and bacterin” funded by National Science Academies, USA through HEC, Islamabad, and Department of Pathobiology and Veterinary Science, University of Connecticut, Storrs, CT, USA.
References
- ARMOUR, N.K., LAIBINIS, V.A., COLLETT, S.R. and FERGUSON-NOEL, N., 2013. The development and application of a Mycoplasma gallisepticum sequence database. Avian Pathology, vol. 42, no. 5, pp. 408-415. http://dx.doi.org/10.1080/03079457.2013.819486 PMid:23889487.
» http://dx.doi.org/10.1080/03079457.2013.819486 - BUCHALA, F., ISHIZUKA, M., MATHIAS, L., BERCHIERI JÚNIOR, A., CASTRO, A., CARDOSO, A. and KANASHIRO, A., 2006. Detecção de resposta sorológica contra Mycoplasma em aves de criatórios de “fundo de quintal” próximos a explorações comerciais do estado de São Paulo. Arquivos do Instituto Biológico, vol. 73, no. 2, pp. 143-148.
- BUIM, M.R., METTIFOGO, E., TIMENETSKY, J., KLEVEN, S. and FERREIRA, A.J.P., 2009. Epidemiological survey on Mycoplasma gallisepticum and M. synoviae by multiplex PCR in commercial poultry. Pesquisa Veterinária Brasileira, vol. 29, no. 7, pp. 552-556. http://dx.doi.org/10.1590/S0100-736X2009000700009
» http://dx.doi.org/10.1590/S0100-736X2009000700009 - DELANEY, N.F., BALENGER, S., BONNEAUD, C., MARX, C.J., HILL, G.E., FERGUSON-NOEL, N., TSAI, P., RODRIGO, A. and EDWARDS, S.V., 2012. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum PLOS Genetics, vol. 8, no. 2, e1002511. http://dx.doi.org/10.1371/journal.pgen.1002511 PMid:22346765.
» http://dx.doi.org/10.1371/journal.pgen.1002511 - EMAM, M., HASHEM, Y.M., EL-HARIRI, M. and EL-JAKEE, J., 2020. Detection and antibiotic resistance of Mycoplasma gallisepticum and Mycoplasma synoviae among chicken flocks in Egypt. Veterinary World, vol. 13, no. 7, pp. 1410-1416. http://dx.doi.org/10.14202/vetworld.2020.1410-1416 PMid:32848318.
» http://dx.doi.org/10.14202/vetworld.2020.1410-1416 - FEBERWEE, A., MEKKES, D., DE WIT, J., HARTMAN, E. and PIJPERS, A., 2005. Comparison of culture, PCR, and different serologic tests for detection of Mycoplasma gallisepticum and Mycoplasma synoviae infections. Avian Diseases, vol. 49, no. 2, pp. 260-268. http://dx.doi.org/10.1637/7274-090804R PMid:16094832.
» http://dx.doi.org/10.1637/7274-090804R - FERGUSON, N.M., HEPP, D., SUN, S., IKUTA, N., LEVISOHN, S., KLEVEN, S.H. and GARCÍA, M., 2005. Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies. Microbiology, vol. 151, no. 6, pp. 1883-1893. http://dx.doi.org/10.1099/mic.0.27642-0 PMid:15941996.
» http://dx.doi.org/10.1099/mic.0.27642-0 - GARCÍA, M., IKUTA, N., LEVISOHN, S. and KLEVEN, S., 2005. Evaluation and comparison of various PCR methods for detection of Mycoplasma gallisepticum infection in chickens. Avian Diseases, vol. 49, no. 1, pp. 125-132. http://dx.doi.org/10.1637/7261-0812204R1 PMid:15839425.
» http://dx.doi.org/10.1637/7261-0812204R1 - GHARAIBEH, S. and AL ROUSSAN, D., 2007. The use of molecular techniques in isolation and characterization of Mycoplasma gallisepticum from commercial chickens in Jordan. International Journal of Poultry Science, vol. 7, no. 1, pp. 28-35. http://dx.doi.org/10.3923/ijps.2008.28.35
» http://dx.doi.org/10.3923/ijps.2008.28.35 - GRODIO, J.L., DHONDT, K.V., O’CONNELL, P.H. and SCHAT, K.A., 2008. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathology, vol. 37, no. 4, pp. 385-391. http://dx.doi.org/10.1080/03079450802216629 PMid:18622854.
» http://dx.doi.org/10.1080/03079450802216629 - KHALIFA, K.A., SIDAHMED ABDELRAHIM, E., BADWI, M. and MOHAMED, A.M., 2013. Isolation and Molecular Characterization of Mycoplasma gallisepticum and Mycoplasma synoviae in Chickens in Sudan. Journal of Veterinary Medicine, vol. 2013, pp. 2013. http://dx.doi.org/10.1155/2013/208026 PMid:26464902.
» http://dx.doi.org/10.1155/2013/208026 - KLEVEN, S., 2008. Control of avian mycoplasma infections in commercial poultry. Avian Diseases, vol. 52, no. 3, pp. 367-374. http://dx.doi.org/10.1637/8323-041808-Review.1 PMid:18939621.
» http://dx.doi.org/10.1637/8323-041808-Review.1 - LOOLMANI, F., POURBAKHSH, S., BANANI, M. and CHARKHKAR, S., 2014. Phylogenetic analysis of mgc2 gene of Mycoplasma gallisepticum isolates from broiler breeder flocks in Tehran province, Iran. European Journal of Zoological Research, vol. 3, no. 2, pp. 37-42.
- LUCIANO, R., CARDOSO, A., STOPPA, G., KANASHIRO, A., DE CASTRO, A. and TESSARI, E., 2011. Comparative study of serological tests for Mycoplasma synoviae diagnosis in commercial poultry breeders. Veterinary Medicine International, vol. 2011, pp. 2011. http://dx.doi.org/10.4061/2011/304349 PMid:21547263.
» http://dx.doi.org/10.4061/2011/304349 - MAROIS, C., DUFOUR‐GESBERT, F. and KEMPF, I., 2001. Molecular differentiation of Mycoplasma gallisepticum and Mycoplasma imitans strains by pulsed‐field gel electrophoresis and random amplified polymorphic DNA. Journal of Veterinary Medicine, Series B, vol. 48, no. 9, pp. 695-703. http://dx.doi.org/10.1046/j.1439-0450.2001.00496.x PMid:11765805.
» http://dx.doi.org/10.1046/j.1439-0450.2001.00496.x - MCMULLIN, P., 2004. A pocket guide to poultry health and disease Sheffield: 5M Enterprises Ltd.
- MUCH, P., WINNER, F., STIPKOVITS, L., ROSENGARTEN, R. and CITTI, C., 2002. Mycoplasma gallisepticum: influence of cell invasiveness on the outcome of experimental infection in chickens. FEMS Immunology and Medical Microbiology, vol. 34, no. 3, pp. 181-186. http://dx.doi.org/10.1111/j.1574-695X.2002.tb00622.x PMid:12423769.
» http://dx.doi.org/10.1111/j.1574-695X.2002.tb00622.x - NASCIMENTO, E.R., PEREIRA, V., NASCIMENTO, M. and BARRETO, M., 2005. Avian mycoplasmosis update. Brazilian Journal of Poultry Science, vol. 7, no. 1, pp. 1-9. http://dx.doi.org/10.1590/S1516-635X2005000100001
» http://dx.doi.org/10.1590/S1516-635X2005000100001 - OSMAN, K., ALY, M., AMIN, Z. and HASAN, B., 2009. Mycoplasma gallisepticum: an emerging challenge to the poultry industry in Egypt. Revue Scientifique et Technique, vol. 28, no. 3, pp. 1015-1023. http://dx.doi.org/10.20506/rst.28.3.1940 PMid:20462158.
» http://dx.doi.org/10.20506/rst.28.3.1940 - PRIYA, K. and MADHAVAN, H., 2002. Diagnostic value of enzyme linked immuno-sorbent assay for cytomegalovirus disease. Journal of Postgraduate Medicine, vol. 48, no. 3, pp. 176-178. PMid:12432189.
- RAUF, M., CHAUDHARY, Z., YOUNUS, M., ANJUM, A., ALI, M., AHMAD, A. and KHAN, M., 2013. Identification of Mycoplasma gallisepticum by polymerase chain reaction and conventional diagnostics from white leghorn layer flocks. JAPS Journal of Animal and Plant Sciences, vol. 23, no. 2, pp. 393-397.
- SILVA, R., SILVA, I., JESUS, M., FERNANDES, M., OLIVEIRA, F. and EVÊNCIO-NETO, J., 2021. Co-relationship between Escherichia coli in broiler cellulitis and liver lesions. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 3, pp. 714-718. http://dx.doi.org/10.1590/1519-6984.230243
» http://dx.doi.org/10.1590/1519-6984.230243 - TAMURA, K., PETERSON, D., PETERSON, N., STECHER, G., NEI, M. and KUMAR, S., 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, vol. 28, no. 10, pp. 2731-2739. http://dx.doi.org/10.1093/molbev/msr121 PMid:21546353.
» http://dx.doi.org/10.1093/molbev/msr121 - WAITES, K.B. and TALKINGTON, D.F., 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clinical Microbiology Reviews, vol. 17, no. 4, pp. 697-728. http://dx.doi.org/10.1128/CMR.17.4.697-728.2004 PMid:15489344.
» http://dx.doi.org/10.1128/CMR.17.4.697-728.2004 - YOUNI, G., ABDELGAWAD, R.H., ELKENANY, R. and GLAL, A., 2018. Molecular identification and sequencing of Mycoplasma gallisepticum recovered from broilers in Egypt. Pakistan Journal of Biological Sciences, vol. 21, no. 5, pp. 253-261. http://dx.doi.org/10.3923/pjbs.2018.253.261
» http://dx.doi.org/10.3923/pjbs.2018.253.261
Publication Dates
-
Publication in this collection
09 Aug 2021 -
Date of issue
2023
History
-
Received
12 Dec 2020 -
Accepted
27 Jan 2021