Abstract
Poultry industry is expanding rapidly and producing million tons of feather waste annually. Massive production of keratinaceous byproducts in the form of industrial wastes throughout the world necessitates its justified utilization. Chemical treatment of keratin waste is proclaimed as an eco-destructive approach by various researchers since it generates secondary pollutants. Keratinase released by a variety of microbes (bacteria and fungi) can be used for the effective treatment of keratin waste. Microbial degradation of keratin waste is an emerging and eco-friendly approach and offers dual benefits, i.e., treatment of recalcitrant pollutant (keratin) and procurement of a commercially important enzyme (keratinase). This study involves the isolation, characterization, and potential utility of fungal species for the degradation of chicken-feather waste through submerged and solid-state fermentation. The isolated fungus was identified and characterized as Aspergillus (A.) flavus. In a trial of 30 days, it was appeared that 74 and 8% feather weight was reduced through sub-merged and solid-state fermentation, respectively by A. flavus. The pH of the growth media in submerged fermentation was changed from 4.8 to 8.35. The exploited application of keratinolytic microbes is, therefore, recommended for the treatment of keratinaceous wastes to achieve dual benefits of remediation.
Keywords:
Aspergillus flavus; biodegradation; bioremediation; economical bioremediation; keratinase; poultry industry
Resumo
A indústria avícola está se expandindo rapidamente e produzindo milhões de toneladas de resíduos de penas anualmente. A produção massiva de subprodutos queratinosos na forma de resíduos agrícolas e industriais em todo o mundo exige sua utilização justificada. O tratamento químico de resíduos de queratina é proclamado como uma abordagem ecodestrutiva por vários pesquisadores, uma vez que gera poluentes secundários. A queratinase liberada por uma variedade de micróbios (bactérias e fungos) pode ser usada para o tratamento eficaz de resíduos de queratina. A degradação microbiana de resíduos de queratina é uma abordagem emergente e ecológica e oferece benefícios duplos, ou seja, tratamento de poluente recalcitrante (queratina) e obtenção de uma enzima comercialmente importante (queratinase). Este estudo envolve o isolamento, caracterização e utilidade potencial de espécies de fungos para a degradação de resíduos de penas de frango por meio da fermentação submersa e em estado sólido. O fungo isolado foi identificado e caracterizado como Aspergillus (A.) flavus. Em um ensaio de 30 dias, constatou-se que 74% e 8% do peso das penas foram reduzidos por A. flavus, respectivamente, por meio da fermentação submersa e em estado sólido. O pH do meio de crescimento em fermentação submersa foi alterado de 4,8 para 8,35. A aplicação explorada de micróbios queratinolíticos é, portanto, recomendada para o tratamento de resíduos ceratinosos para obter benefícios duplos de remediação.
Palavras-chave:
Aspergillus flavus; biodegradação; biorremediação; biorremediação econômica; queratinase; indústria avícola
1. Introduction
Keratin is an insoluble, fibrous, and structural protein which is recalcitrant in nature due to abundance of hydrogen and disulfide bonds. A variety of vertebrates like fishes, reptiles, birds, and mammals have compact keratin in their integuments and due to abundance in nature it occupies third number after chitin and cellulose (Kreplak et al., 2004KREPLAK, L., DOUCET, J., DUMAS, P. and BRIKI, F., 2004. New aspects of the α-helix to β-sheet transition in stretched hard α-keratin fibers. Biophysical Journal, vol. 87, no. 1, pp. 640-647. http://dx.doi.org/10.1529/biophysj.103.036749. PMid:15240497.
http://dx.doi.org/10.1529/biophysj.103.0...
; Bragulla and Homberger, 2009BRAGULLA, H.H. and HOMBERGER, D.G., 2009. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. Journal of Anatomy, vol. 214, no. 4, pp. 516-559. http://dx.doi.org/10.1111/j.1469-7580.2009.01066.x. PMid:19422428.
http://dx.doi.org/10.1111/j.1469-7580.20...
; Mckittrick et al., 2012MCKITTRICK, J., CHEN, P.Y., BODDE, S.G., YANG, W., NOVITSKAYA, E.E. and MEYERS, M.A., 2012. The structure, functions, and mechanical properties of keratin. Journal of Management, vol. 64, pp. 449-468. http://dx.doi.org/10.1007/s11837-012-0302-8.
http://dx.doi.org/10.1007/s11837-012-030...
). Keratins are divided into two types, i.e., α-keratin and β-keratin (Meyers et al., 2008MEYERS, M.A., CHEN, P., LIN, A.Y. and SEKI, Y., 2008. Biological materials: structure and mechanical properties. Progress in Materials Science, vol. 53, no. 1, pp. 1-206. http://dx.doi.org/10.1016/j.pmatsci.2007.05.002.
http://dx.doi.org/10.1016/j.pmatsci.2007...
; Lange et al., 2014LANGE, L., BUSK, P.K. and HUANG, Y., 2014. Use of a microbial composition for the degradation of keratinaceous materials. Denmark. Patent WO 2014/169920 A2, 23-10-2014.; Huang et al., 2015HUANG, Y., BUSK, P.K., HERBST, F.A. and LANGE, L., 2015. Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvine. Applied Microbiology and Biotechnology, vol. 99, no. 22, pp. 9635-9649. http://dx.doi.org/10.1007/s00253-015-6805-9. PMid:26177915.
http://dx.doi.org/10.1007/s00253-015-680...
). Keratin proteins are resistant to chemical and/or mechanical breakdown due to presence of several disulfide (S-S) cross-linkages (Korniłłowicz-Kowalska, 1997bKORNIŁŁOWICZ-KOWALSKA, T., 1997b. Studies on the decomposition of keratin wastes by saprotrophic microfungi. P. II. Sulphur and nitrogen balance. Acta Mycologica, vol. 32, no. 1, pp. 81-93. http://dx.doi.org/10.5586/am.1997.007.
http://dx.doi.org/10.5586/am.1997.007...
). Only keratinolytic microbes and some insects like moths can efficiently degrade keratin by secreting keratinolytic enzymes (keratinases) that can degrade complex cross-linked bonds of keratin in coordination with other enzymes (Lange et al., 2016LANGE, L., HUANG, Y. and BUSK, P.K., 2016. Microbial decomposition of keratin in nature–a new hypothesis of industrial relevance. Applied Microbiology and Biotechnology, vol. 100, no. 5, pp. 2083-2096. http://dx.doi.org/10.1007/s00253-015-7262-1. PMid:26754820.
http://dx.doi.org/10.1007/s00253-015-726...
; Jin et al., 2017JIN, H.S., PARK, S.Y., KIM, K., LEE, Y.J., NAM, G.W., KANG, N.J. and LEE, D.W., 2017. Development of a keratinase activity assay using recombinant chicken feather keratin substrates. PLoS One, vol. 12, no. 2, pp. e0172712. http://dx.doi.org/10.1371/journal.pone.0172712. PMid:28231319.
http://dx.doi.org/10.1371/journal.pone.0...
).
More than 25 thousand chicken farms are efficiently working to meet protein needs of people in Pakistan. A large amount of poultry waste is produced as byproduct of poultry processing plants (Abedullah and Bukhsh, 2007ABEDULLAH, A. and BUKHSH, K., 2007. Issues and economics of poultry production: a case study of Faisalabad, Pakistan. Pakistan Veterinary Journal, vol. 27, pp. 25-28.; Hussain et al., 2015HUSSAIN, J., RABBANI, I., ASLAM, S. and AHMAD, H., 2015. An overview of poultry industry in Pakistan. World’s Poultry Science Journal, vol. 71, no. 4, pp. 689-700. http://dx.doi.org/10.1017/S0043933915002366. PMid:26696690.
http://dx.doi.org/10.1017/S0043933915002...
; Khan et al., 2015KHAN, M.B., ASAR, A.U. and NAWAZ, A., 2015. Reducing capital cost and providing electricity to grid by power generation from poultry farms. International Journal of Computers and Applications, vol. 123, no. 2, pp. 5-8. http://dx.doi.org/10.5120/ijca2015905233.
http://dx.doi.org/10.5120/ijca2015905233...
). Four million tons of feathers are collected as poultry slaughtering waste annually (Onifade et al., 1998ONIFADE, A.A., AL-SANE, N.A., AL-MUSALLAM, A.A. and AL-ZARBAN, S., 1998. A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresource Technology, vol. 66, no. 1, pp. 1-11. http://dx.doi.org/10.1016/S0960-8524(98)00033-9.
http://dx.doi.org/10.1016/S0960-8524(98)...
; Gousterova et al., 2005GOUSTEROVA, A., BRAIKOVA, D., GOSHEV, I., CHRISTOV, P., TISHINOV, K., VASILEVA-TONKOVA, E., HAERTLÉ, T. and NEDKOV, P., 2005. Degradation of keratin and collagen containing wastes by newly isolated thermoactinomycetes or by alkaline hydrolysis. Letters in Applied Microbiology, vol. 40, no. 5, pp. 335-340. http://dx.doi.org/10.1111/j.1472-765X.2005.01692.x. PMid:15836735.
http://dx.doi.org/10.1111/j.1472-765X.20...
). However, it must be treated because a variety of pathogens are associated with this waste. The poultry waste is generally heated or dumped in soil (Suzuki et al., 2006SUZUKI, Y., TSUJIMOTO, Y., MATSUI, H. and WATANABE, K., 2006. Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. Journal of Bioscience and Bioengineering, vol. 102, no. 2, pp. 73-81. http://dx.doi.org/10.1263/jbb.102.73. PMid:17027867.
http://dx.doi.org/10.1263/jbb.102.73...
; Ghaffar et al., 2018GHAFFAR, I., IMTIAZ, A., HUSSAIN, A., JAVID, A., JABEEN, F., AKMAL, M. and QAZI, J.I., 2018. Microbial production and industrial applications of keratinases: an overview. International Microbiology, vol. 21, no. 4, pp. 163-174. http://dx.doi.org/10.1007/s10123-018-0022-1. PMid:30810899.
http://dx.doi.org/10.1007/s10123-018-002...
). Production of keratinase by bacteria is focused mainly, however, only few studies presented fungal keratin degradation (Gupta and Ramnani, 2006GUPTA, R. and RAMNANI, P., 2006. Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology, vol. 70, no. 1, pp. 21-33. http://dx.doi.org/10.1007/s00253-005-0239-8. PMid:16391926.
http://dx.doi.org/10.1007/s00253-005-023...
; Brandelli et al., 2010BRANDELLI, A., DAROIT, D.J. and RIFFEL, A., 2010. Biochemical features of microbial keratinases and their production and applications. Applied Microbiology and Biotechnology, vol. 85, no. 6, pp. 1735-1750. http://dx.doi.org/10.1007/s00253-009-2398-5. PMid:20039036.
http://dx.doi.org/10.1007/s00253-009-239...
; Korniłłowicz-Kowalska and Bohacz, 2011KORNIŁŁOWICZ-KOWALSKA, T. and BOHACZ, J., 2011. Biodegradation of keratin waste: theory and practical aspects. Waste Management (New York, N.Y.), vol. 31, no. 8, pp. 1689-1701. http://dx.doi.org/10.1016/j.wasman.2011.03.024. PMid:21550224.
http://dx.doi.org/10.1016/j.wasman.2011....
; Gupta et al., 2013GUPTA, R., SHARMA, R. and BEG, Q.K., 2013. Revisiting microbial keratinases: next generation proteases for sustainable biotechnology. Critical Reviews in Biotechnology, vol. 33, no. 2, pp. 216-228. http://dx.doi.org/10.3109/07388551.2012.685051. PMid:22642703.
http://dx.doi.org/10.3109/07388551.2012....
; Sahni et al., 2015SAHNI, N., SAHOTA, P.P. and PHUTELA, U.G., 2015. Bacterial keratinases and their prospective applications: a review. International Journal of Current Microbiology and Applied Sciences, vol. 4, pp. 768-783.). Fungal keratinases may play a crucial role in the economical and environment-friendly treatment of keratin waste (Gradisar et al., 2005GRADISAR, H., FRIEDRICH, J., KRIZAJ, I. and JERALA, R., 2005. Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Applied and Environmental Microbiology, vol. 71, no. 7, pp. 3420-3426. http://dx.doi.org/10.1128/AEM.71.7.3420-3426.2005. PMid:16000744.
http://dx.doi.org/10.1128/AEM.71.7.3420-...
; Huang et al., 2015HUANG, Y., BUSK, P.K., HERBST, F.A. and LANGE, L., 2015. Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvine. Applied Microbiology and Biotechnology, vol. 99, no. 22, pp. 9635-9649. http://dx.doi.org/10.1007/s00253-015-6805-9. PMid:26177915.
http://dx.doi.org/10.1007/s00253-015-680...
).
Filamentous fungi produce keratinases by using two ways of fermentation. These fermentation processes include solid state (SSF) and submerged fermentation (SmF) (Battaglino et al., 1991BATTAGLINO, R.A., HUERGO, M., PILOSOF, A.M.R. and BARTHOLOMAI, G.B., 1991. Culture requirements for the production of protease by Aspergillus oryzae in solid state fermentation. Applied Microbiology and Biotechnology, vol. 35, no. 3, pp. 292-296. http://dx.doi.org/10.1007/BF00172714. PMid:22622927.
http://dx.doi.org/10.1007/BF00172714...
; Krishna, 2005KRISHNA, S., 2005. Quantum dots-in-a-well infrared photodetectors. Journal of Physics. D, Applied Physics, vol. 38, no. 13, pp. 2142-2150. http://dx.doi.org/10.1088/0022-3727/38/13/010.
http://dx.doi.org/10.1088/0022-3727/38/1...
). SmF plays a major role in production of enzymes at industrial scale and contributes more than 75% of the overall enzymatic production (Subramaniyam and Vimala, 2012SUBRAMANIYAM, R. and VIMALA, R., 2012. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. International Journal of Science and Nature, vol. 3, pp. 480-486.). SmF process is more suitable for that microbes which need high moisture contents for their growth (Subramaniyam and Vimala, 2012SUBRAMANIYAM, R. and VIMALA, R., 2012. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. International Journal of Science and Nature, vol. 3, pp. 480-486.). SSF is a process in which microbes are cultured on solid and moist medium (Pandey, 2003PANDEY, A., 2003. Solid-state fermentation. Biochemical Engineering Journal, vol. 13, no. 2-3, pp. 81-84. http://dx.doi.org/10.1016/S1369-703X(02)00121-3.
http://dx.doi.org/10.1016/S1369-703X(02)...
; Hölker and Lenz, 2005HÖLKER, U. and LENZ, J., 2005. Solid-state fermentation: are there any biotechnological advantages? Current Opinion in Microbiology, vol. 8, no. 3, pp. 301-306. http://dx.doi.org/10.1016/j.mib.2005.04.006. PMid:15939353.
http://dx.doi.org/10.1016/j.mib.2005.04....
; Mitchell et al., 2006MITCHELL, D.A., LUZ, L.F.L., KRIEGER, N. and BEROVIC, M., 2006. Bioreactors for solid-state fermentation. Heidelberg: Springer. http://dx.doi.org/10.1007/3-540-31286-2. ). Therefore, keeping in view the notable remedial properties of fungi, the present study was designed to isolate, characterize and employ keratinolytic fungi for the treatment of poultry (keratin) waste following SmF and SSF processes.
2. Materials and methods
2.1. Sample collection and isolation of pure culture of keratinolytic fungal strain
For the isolation of keratinolytic fungus, decaying feathers were collected under hygienic conditions from feather-dumping sites in district Kasur. The collected samples were then transported to Applied and Environmental Microbiology Laboratory, Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore (Ravi campus, Pattoki), Pakistan for further processing. Chicken feathers were thoroughly washed with distilled water and soaked in sterile water for 2−3 h. Then 0.5 mL of the feather-suspended water was spread over skim milk agar (CM0681, Oxoid) and incubated for 3 days at 30 oC. The isolated fungal strain was then pure cultured by streak-plate method.
2.2. Phenotypic characterization
Fungal pure culture was morphologically identified based on macroscopic and microscopic features. Macroscopic features like shape, size, color, and texture of the fungal colony were examined. Microscopic features like septation of hyphae, metulae, phialides, vesicle and conidiophore were observed by staining the fungus with lactophenol-cotton blue.
2.3. Molecular identification of the fungal isolate
The pure cultured keratinolytic fungal isolate was then identified at the molecular level by 18S rRNA gene sequencing (Jing et al., 2015JING, L., ZHAO, S., XUE, J.L., ZHANG, Z., YANG, Q., XIAN, L. and FENG, J.X., 2015. Isolation and characterization of a novel Penicillium oxalicum strain Z1-3 with enhanced cellobiohydrolase production using cellulase-hydrolyzed sugarcane bagasse as carbon source. Industrial Crops and Products, vol. 77, pp. 666-675. http://dx.doi.org/10.1016/j.indcrop.2015.09.052.
http://dx.doi.org/10.1016/j.indcrop.2015...
; Saini et al., 2015SAINI, R., SAINI, J.K., ADSUL, M., PATEL, A.K., MATHUR, A., TULI, D. and SINGHANIA, R.R., 2015. Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresource Technology, vol. 188, pp. 240-246. http://dx.doi.org/10.1016/j.biortech.2015.01.048. PMid:25661515.
http://dx.doi.org/10.1016/j.biortech.201...
). Required sequence was created from forward and reverse sequences and Bio-Edit sequence alignment editor version 7.0.9.0 was used for sequence edition (Tom Hall, Ibis Biosciences, Carlsbad, California). Initial analysis of Pakistani sequences was carried out through BLAST (Basic Local Alignment Search Tool). The Pakistani sequences from GenBank were arrayed accompanied by most resembled retrieved sequence using Clustal W program of Molecular Evolutionary Genetics Analysis (MEGA 6) software (Tamura et al., 2013TAMURA, K., STECHER, G., PETERSON, D., FILIPSKI, A. and KUMAR, S., 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, vol. 30, no. 12, pp. 2725-2729. http://dx.doi.org/10.1093/molbev/mst197. PMid:24132122.
http://dx.doi.org/10.1093/molbev/mst197...
). Neighbor-Joining method was used for deducing the evolutionary history (Saitou and Nei, 1987SAITOU, N. and NEI, M., 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, vol. 4, no. 4, pp. 406-425. PMid:3447015.). Next to the branches, replicate trees percentages were combined with closely related taxa in bootstrap test (100 replicates) (Felsenstein, 1985FELSENSTEIN, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution; International Journal of Organic Evolution, vol. 39, no. 4, pp. 783-791. http://dx.doi.org/10.1111/j.1558-5646.1985.tb00420.x. PMid:28561359.
http://dx.doi.org/10.1111/j.1558-5646.19...
). Branch lengths of the tree drawn on scale were in the same units so as evolutionary distances used to deduce phylogenetic tree. The calculated evolutionary distances were in the units of the number of base substitutions per site as explained by Tamura and Nei (1993)TAMURA, K. and NEI, M., 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, vol. 10, no. 3, pp. 512-526. PMid:8336541.. The rate difference between sites was shown with gamma distribution (shape parameter = 1). In the final dataset, the total positions were 734 and gaps referred as data.
2.4. Optimization of growth conditions of the fungal isolate
After molecular analysis, the fungal isolate was inoculated on malt extract agar (MEA) with one loop full of fungal spores and incubated at 30 oC for 3 days. Different parameters like temperature, pH, inoculum size and incubation period were then optimized. For the optimization of temperature, fungal spores were incubated at 20, 30 and 40 oC on petri plates containing MEA for 3 days and different number of colonies was shown at different temperatures. For pH optimization, petri plates were incubated at 30 oC having pH 5, 7 and 9 for 3 days and number of colonies was noted on each plate. Inoculum size was optimized by inoculating petri plates with 0.1, 0.2, 0.3, 0.4 and 0.5 mL of fungal spores and incubated at 30 oC for 3 days and colonies were counted. Inoculated petri plates were incubated at 30 oC and number of colonies was counted on daily basis for consecutive 5 days.
2.5. Determination of feather-degrading potential of the fungal isolate in SmF and SSF
Feather-degrading potential of the fungal isolate in SmF and SSF was determined by using basal medium [composition (gL−1): K2HPO4, 1.5; NaCl, 0.01; MgCl2.7H2O, 0.05; H2O, 1000 mL] and dried feather meal (2% w/v) as growth substrate. Inoculum was prepared by using basal medium and feather meal as a source of carbon and energy. Medium was autoclaved and 100 mL of the medium was poured into sterile 250 mL Erlenmeyer flask; 1 g of feather meal was used. Fungal spores were transferred into medium through sterile inoculating loop. Flask was incubated at 29 oC and pH was maintained at 4.8. Maximum growth was observed after 15 days. This culture was used as fungal inoculum for all further experiments.
Feathers were washed thoroughly with distilled water and then dried at 170 oC for 4 h for complete removal of moisture. Experiment was conducted in sterile 250 mL Erlenmeyer flasks containing 1 g of sterile feathers and 100 mL of basal medium. Flasks containing medium and feathers were autoclaved and then inoculated with fungal spores upon cooling. Flasks containing medium were inoculated with 0.5 mL of the fungal spores and incubated at 29 oC and pH of 4.8 (six sets of flasks). All the experiments were conducted in triplicates. The control flasks were kept un-inoculated. After every 5 days, feathers were washed with distilled water, dried at 80 oC for 4 h to remove moisture contents and weighed to determine degree of degradation of feathers by the isolated fungal strain in SmF up to 30 days. It was observed that weight was varied in each set of flasks.
For checking degradation potential of the isolated fungal strain in SSF, feathers were washed thoroughly with distilled water and then dried at 170 oC for 4 h for complete removal of moisture. Experiment was conducted in 250 mL Erlenmeyer flasks containing 5 g of sterile feathers and the moisture contents of basal medium was adjusted up to 60% (w/v). Flasks containing feathers with specific moisture contents were autoclaved and then inoculated with fungal spores upon cooling. Flasks were inoculated with 0.5 mL of the fungal spores, incubated at 29 oC and pH was maintained at 4.8 (six sets of flasks). After every 5 days, feathers were washed with distilled water, dried at 170 oC for 4 h to remove moisture contents and weighed to determine the degree of degradation of feathers by the isolated fungal strain in SSF up to 30 days. It was observed that weight was varied in each set. All the experiments were conducted in triplicates. One flask was kept as control (uninoculated) under the same experimental conditions.
2.6. Statistical analysis
The data were analyzed according to Completely Randomized Design (CRD) under factorial arrangement using General Linear Model (GLM) procedures. Means were separated out using Duncan’s Multiple Range (DMR) test with the help of SAS 9.1 for windows (SAS Institute Inc., 2002SAS Institute Inc. Statistics Version 901. Cary, N.C.: SAS Institute Inc., 2002.). Differences between means were considered significant at P < 0.05.
3. Results
3.1. Isolation of pure culture of keratinolytic fungal strain
The present study was conducted to isolate keratinolytic fungal strain from feather waste. The isolated strain was pure cultured and further identified phenotypically and genotypically.
3.2. Phenotypic characterization
The isolated fungal strain depicted maximum growth in 3 days on MEA medium. Colony color was olive green with white edges and shape of the colony was circular having powdery texture. By staining of fungal hyphae rough spiny conidiophore bearing vesicles were observed. Hyphae were non-septate, conidia were terminal and vesicles were globose and loosely radiated uniseriate phialides were present all over the vesicles. Metulae was absent and conidia were directly attached with vesicles.
3.3. Molecular identification of the fungal isolate
BLAST search of 18S rDNA nucleotide sequence of the fungal isolate revealed that the fungal isolate belonged to genus Aspergillus having 98% similarity with Aspergillus flavus (ATCC 16883).
3.4. Optimization of growth conditions of the fungal isolate
At pH 5, maximum number of colonies was shown (Figures 1 and 2). The fungal strain showed different growth on different degrees of temperature, pH, inoculum size and incubation period. The fungal strain showed maximum growth at 30 oC (Figure 3). Temperature was then optimized by incubating the fungal strain at 28, 29, 31 and 32 oC. The maximum number of colonies was shown at 29 oC (Figure 4). Inoculum size was optimized by using previously optimized parameters and at maximum inoculum size maximum growth was observed (Figure 5). Fungal colonies showed maximum number of colonies in 3 days of incubation period at optimized temperature, pH and inoculum size.
3.5. Determination of feather-degrading potential of the fungal isolate in SmF and SSF
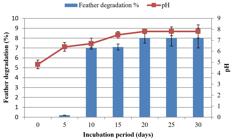
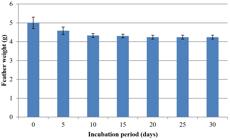
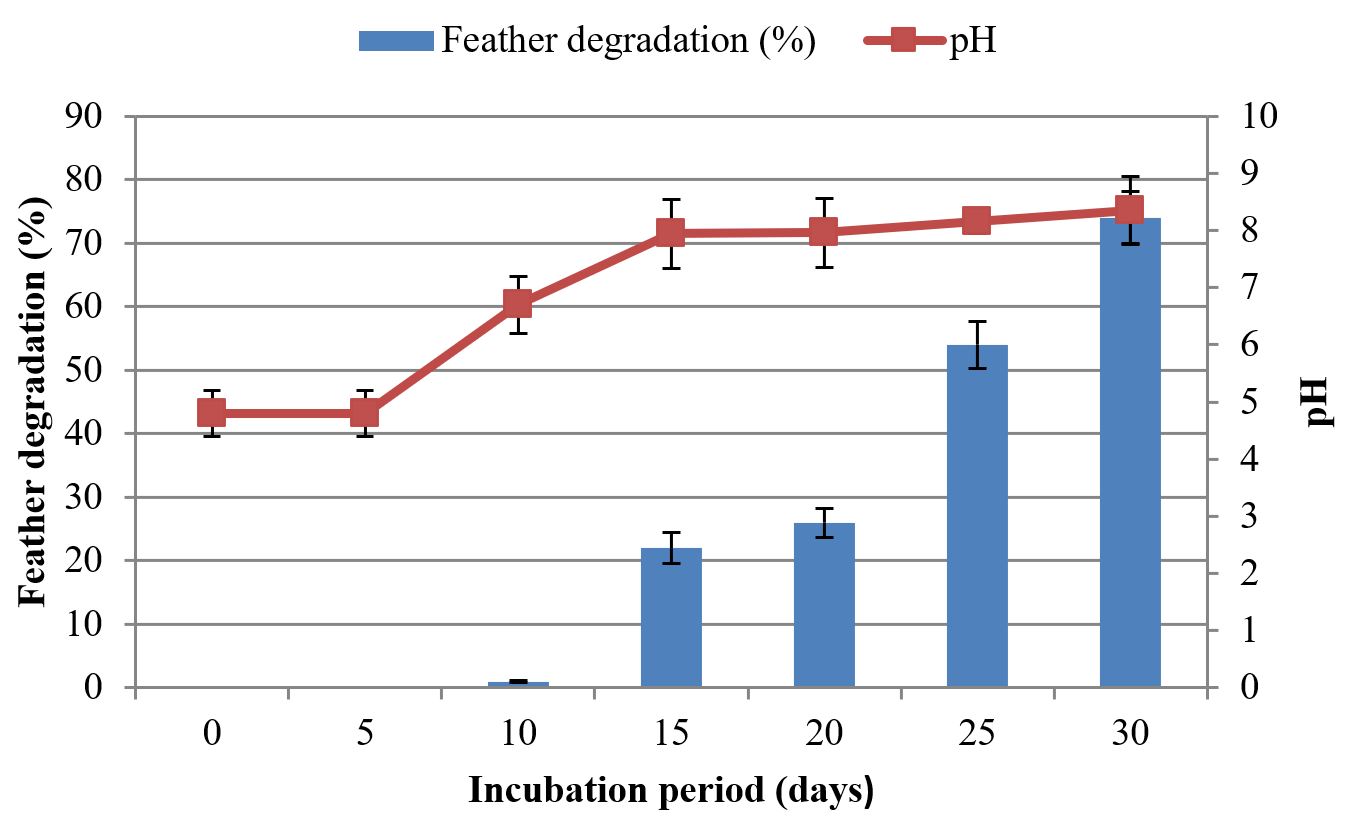
The utilization of keratin as substrate is an important factor for assessment of keratinolytic abilities of microorganisms. Ability of keratinolytic fungus to degrade chicken feathers was assessed in this study. With the passage of time, weight of feathers was reduced, and keratin degrading activity was increased. Different amounts of feathers were degraded among consecutive days in both fermentation processes. SmF and SSF showed drastic variation in feather loss. During SmF, initial amount of feathers was 1 g. Reduction of feathers was started after 5 days of incubation period. In the first 5 days no reduction was observed. The maximum amount of feather reduction was observed at 30th day of incubation period, i.e., 74%. Maximum reduction in weight of feathers was 0.74 g. Results showed keratinolytic activity was increased with the passage of time:
3.6. 30 days > 25 days > 20 days > 15 days > 10 days > 5 days
During the first 5 days of SSF, 0.2% reduction was observed. The maximum amount of feather reduction was shown at 20th day of incubation period, i.e., 8%. After 20 days, the feather reduction remained constant till 30th day of incubation period. Maximum reduction in weight of feathers was 8% on 20th day. Results showed that keratinolytic activity was increased with the passage of time:
3.7. 20 days > 15 days > 10 days > 5 days
During keratin degradation in SmF and SSF by A. flavus, pH changed from acidic to alkaline. As degradation of keratin releases S, N, O ions and several other compounds which cause pH to increase. In SmF, a remarkable increase in pH was observed on the day at which maximum feather reduction was shown (30th day), i.e., 8.35 (control pH = 4.8). In SSF, maximum pH was observed on the day at which maximum feather reduction was shown (20th day), i.e., 7.81 (control pH = 4.8). Results showed that pH increased with the increase of keratinolytic activity (Figures 6 to 9).
4. Discussion
The current study was conducted to evaluate the keratinolytic potential of A. flavus in SmF and SSF. A keratinolytic fungal species was isolated from chicken feather waste to check its potential for degrading chicken feathers (keratin) in both conditions (SmF and SSF). The isolated strain was morphologically identified as A. flavus and molecular characterization showed closer resemblance with A. flavus. The isolated fungal strain had following morphological features, i.e., green colored fungus with uniseriate phialides and conidia were globose. These features were almost same with the results of Hedayati et al. (2007)HEDAYATI, M.T., PASQUALOTTO, A.C., WARN, P.A., BOWYER, P. and DENNING, D.W., 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology, vol. 153, no. Pt 6, pp. 1677-1692. http://dx.doi.org/10.1099/mic.0.2007/007641-0. PMid:17526826.
http://dx.doi.org/10.1099/mic.0.2007/007...
who reported that A. flavus is either uniseriate or biseriate and yellow to green in color. Conidia are globose to sub-globose.
This isolated fungal strain showed maximum growth at 29 oC temperature and pH 4.8 and its activity was inhibited at 40 oC. The optimum temperature for the isolated fungus was in accordance with temperature range for A. flavus as reported by Samapundo et al. (2007)SAMAPUNDO, S., DEVLIEGHERE, F., GEERAERD, A.H., DE MEULENAER, B., VAN IMPE, J.F. and DEBEVERE, J., 2007. Modelling of the individual and combined effects of water activity and temperature on the radial growth of Aspergillus flavus and A. parasiticus on corn. Food Microbiology, vol. 24, no. 5, pp. 517-529. http://dx.doi.org/10.1016/j.fm.2006.07.021. PMid:17367685.
http://dx.doi.org/10.1016/j.fm.2006.07.0...
. Cai and Zheng (2009)CAI, C. and ZHENG, X.D., 2009. Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. Journal of Industrial Microbiology & Biotechnology, vol. 36, no. 7, pp. 875-883. http://dx.doi.org/10.1007/s10295-009-0565-4. PMid:19350297.
http://dx.doi.org/10.1007/s10295-009-056...
also reported that maximum keratinolytic activity was observed at 28 oC. Kote et al. (2009)KOTE, N.V., PATIL, A.G.G. and MULIMANI, V.H., 2009. Optimization of the production of thermostable endo-β-1,4 mannanase from a newly isolated Aspergillus niger gr and Aspergillus flavus gr. Applied Biochemistry and Biotechnology, vol. 152, no. 2, pp. 213-223. http://dx.doi.org/10.1007/s12010-008-8250-z. PMid:18597050.
http://dx.doi.org/10.1007/s12010-008-825...
reported that A. flavus showed maximum activity at pH 5. A. flavus is considered as an outstanding fungal producer of keratinase as compared to other fungal species (Friedrich et al., 1999FRIEDRICH, J., GRADISAR, H., MANDIN, D. and CHAUMONT, J.P., 1999. Screening fungi for synthesis of keratinolytic enzymes. Letters in Applied Microbiology, vol. 28, no. 2, pp. 127-130. http://dx.doi.org/10.1046/j.1365-2672.1999.00485.x.
http://dx.doi.org/10.1046/j.1365-2672.19...
). Mostly SmF is used for keratinase production (shaking conditions for bacteria and static conditions for fungi) (Riessen and Antranikian, 2001RIESSEN, S. and ANTRANIKIAN, G., 2001. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles, vol. 5, no. 6, pp. 399-408. http://dx.doi.org/10.1007/s007920100209. PMid:11778841.
http://dx.doi.org/10.1007/s007920100209...
; Nam et al., 2002NAM, G.W., LEE, D.W., LEE, H.S., LEE, N.J., KIM, B.C., CHOE, E.A., HWANG, J.K., SUHARTONO, M.T. and PYUN, Y.R., 2002. Native feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Archives of Microbiology, vol. 178, no. 6, pp. 538-547. http://dx.doi.org/10.1007/s00203-002-0489-0. PMid:12420177.
http://dx.doi.org/10.1007/s00203-002-048...
). Only a few reports are concerned with solid-state production of keratinase (De Azeredo et al., 2006DE AZEREDO, L.A.I., DE LIMA, M.B., COELHO, R.R.R. and FREIRE, D.M.G., 2006. Thermophilic protease production by Streptomyces sp. 594 in submerged and solid-state fermentations using feather meal. Journal of Applied Microbiology, vol. 100, no. 4, pp. 641-647. http://dx.doi.org/10.1111/j.1365-2672.2005.02791.x. PMid:16553718.
http://dx.doi.org/10.1111/j.1365-2672.20...
; Esawy, 2007ESAWY, M.A., 2007. Isolation and partial characterization of extracellular keratinase from a novel mesophilic Streptomyces albus AZA. Research Journal of Agriculture and Biological Sciences, vol. 3, pp. 808-817.). The keratinolytic potential in sub-merged and solid-state fermentation was assessed in a trial of 30 days. There are a few reports on keratin degradation by A. flavus in SmF and fungal keratin degradation in SSF is also scarcely reported. Results showed that in SmF, A. flavus exhibited 9 times greater keratin degradation than that of SSF. In most of the studies, it has been reported that in SSF, microbes show more degradation than in SmF. Mazotto et al. (2013)MAZOTTO, A.M., COURI, S., DAMASO, M.C.T. and VERMELHO, A.B., 2013. Degradation of feather waste by Aspergillus niger keratinases: comparison of submerged and solid-state fermentation. International Biodeterioration & Biodegradation, vol. 85, pp. 189-195. http://dx.doi.org/10.1016/j.ibiod.2013.07.003.
http://dx.doi.org/10.1016/j.ibiod.2013.0...
reported 7 times greater keratinolytic activity of A. niger in SSF than in SmF. However, in the present study, results were completely opposite. In SmF, the keratinolytic activity started after 5 days of incubation period and increased with time up to 4 weeks of incubation period. The highest degradation (74%) of chicken feathers was shown at 30th day of incubation period. Korniłłowicz-Kowalska (1997a)KORNIŁŁOWICZ-KOWALSKA, T., 1997a. Studies on the decomposition of keratin wastes by saprotrophic microfungi. I. criteria for evaluating keratinolytic activity. Acta Mycologica, vol. 32, no. 1, pp. 51-79. http://dx.doi.org/10.5586/am.1997.006.
http://dx.doi.org/10.5586/am.1997.006...
reported that loss of substrate is a clear sign of keratinolytic activity. The keratinolytic activity of the A. flavus on chicken feathers in SmF in the current study was almost double than the activity of A. flavus in the study of Muhsin and Hadi (2002)MUHSIN, T.M. and HADI, R.B., 2002. Degradation of keratin substrates by fungi isolated from sewage sludge. Mycopathologia, vol. 154, no. 4, pp. 185-189. http://dx.doi.org/10.1023/A:1016335623534. PMid:12206319.
http://dx.doi.org/10.1023/A:101633562353...
. Based on this percentage, the strain used in the present study can be defined as strongly keratinolytic. As reported by Kunert (2000)KUNERT, J., 2000. Physiology of keratinophilic fungi. In: R.K.S. KUSHWAHA and J. GUARRO, eds. Biology of dermatophytes and other keratinophilic fungi. Bilbao: Revista Iberoamericana de Micologia, pp. 77-85., the microbes which can degrade keratin more than 40% within 60 days in submerged conditions are strongly keratinolytic. Bohacz (2017)BOHACZ, J., 2017. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World Journal of Microbiology & Biotechnology, vol. 33, no. 1, pp. 13. http://dx.doi.org/10.1007/s11274-016-2177-2. PMid:27885567.
http://dx.doi.org/10.1007/s11274-016-217...
reported that strain of Chrysosporium articulatum showed 63.7% of feather loss after 42 days in liquid culture conditions. This degradation is also less than the present results of A. flavus. A strain of Chrysosporium keratinophilum showed weaker keratinolytic activity, i.e., 35%,hile in SSF, A. flavus showed a weaker keratinolytic activity, i.e., 8%. The maximum degradation activity was exhibited on 20th day, afterwards it remained constant. This was the lowest keratin degradation in SSF reported till now.
Change in pH is an important characteristic of keratin degradation. Apparently, a remarkable increase in pH (4.8−8.3) was also observed in post-liquid culture medium. pH was also more inclined towards alkalinity in solid-state keratin fermentation. There was a direct relation between pH changes and keratinolytic activity. As keratinolytic activity increased, pH also became alkaline. In initial days of SmF, pH was low, i.e., 6.7 and a less keratinolytic activity was observed at this pH. Cai and Zheng (2009)CAI, C. and ZHENG, X.D., 2009. Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. Journal of Industrial Microbiology & Biotechnology, vol. 36, no. 7, pp. 875-883. http://dx.doi.org/10.1007/s10295-009-0565-4. PMid:19350297.
http://dx.doi.org/10.1007/s10295-009-056...
also reported that at low pH less keratinolytic activity was occurred. Maximum pH of 8.3 was recorded on the day of maximum keratinolytic activity in liquid culture. Cai et al. (2008)CAI, C.G., LOU, B.G. and ZHENG, X.D., 2008. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University. Science. B., vol. 9, no. 1, pp. 60-67. http://dx.doi.org/10.1631/jzus.B061620. PMid:18196614.
http://dx.doi.org/10.1631/jzus.B061620...
observed that pH level of the medium increased up to 8.5 during keratinase production. The change of pH towards alkalinity is due to degradation of keratin, release of keratinase and significant amount of ammonia, sulfur, and other compounds (Saparrat et al., 2007SAPARRAT, M.C.N., ARAMBARRI, A.M. and BALATTI, P.A., 2007. Growth response and extracellular enzyme activity of Ulocladium botrytis LPSC 813 cultured on carboxy-methylcellulose under a pH-range. Biology and Fertility of Soils, vol. 44, no. 2, pp. 383-386. http://dx.doi.org/10.1007/s00374-007-0217-7.
http://dx.doi.org/10.1007/s00374-007-021...
). It has been reported that fungi with more keratinolytic activity tend the post-culture medium more alkaline than the fungi with less keratinolytic activity (Kaul and Sumbali, 1999KAUL, S. and SUMBALI, G., 1999. Production of extracellular keratinases by keratinophilic fungal species inhabiting feathers of living poultry birds (Gallus domesticus): a comparison. Mycopathologia, vol. 146, no. 1, pp. 19-24. http://dx.doi.org/10.1023/A:1007086720237.
http://dx.doi.org/10.1023/A:100708672023...
). Similar changes in pH have also been reported by Hasiia et al. (1990)HASIIA, S.K., MALVIYA, H.K. and RAJAK, R.C., 1990. Keratinolytic ability of some fungi isolated from gelatin factory campus, Jabalpur (MP). Proceedings of the National Academy Science India, vol. 60, pp. 305-309. and Elíades et al. (2010)ELÍADES, L., CABELLO, M., VOGET, C., GALARZA, B. and SAPARRAT, M., 2010. Screening for alkaline keratinolytic activity in fungi isolated from soils of the biosphere reserve “Parque Costero del Sur” (Argentina). World Journal of Microbiology & Biotechnology, vol. 26, no. 11, pp. 2105-2111. http://dx.doi.org/10.1007/s11274-010-0389-4.
http://dx.doi.org/10.1007/s11274-010-038...
.
It is concluded that A. flavus can be efficiently employed for the degradation of feather waste and production of keratinase which is a valuable industrial enzyme. The present study was conducted at lab scale, however, future studies are required to explore remedial process kinetics at pilot and commercial scales for the viable implication of keratinolytic fungi.
References
- ABEDULLAH, A. and BUKHSH, K., 2007. Issues and economics of poultry production: a case study of Faisalabad, Pakistan. Pakistan Veterinary Journal, vol. 27, pp. 25-28.
- BATTAGLINO, R.A., HUERGO, M., PILOSOF, A.M.R. and BARTHOLOMAI, G.B., 1991. Culture requirements for the production of protease by Aspergillus oryzae in solid state fermentation. Applied Microbiology and Biotechnology, vol. 35, no. 3, pp. 292-296. http://dx.doi.org/10.1007/BF00172714 PMid:22622927.
» http://dx.doi.org/10.1007/BF00172714 - BOHACZ, J., 2017. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World Journal of Microbiology & Biotechnology, vol. 33, no. 1, pp. 13. http://dx.doi.org/10.1007/s11274-016-2177-2 PMid:27885567.
» http://dx.doi.org/10.1007/s11274-016-2177-2 - BRAGULLA, H.H. and HOMBERGER, D.G., 2009. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. Journal of Anatomy, vol. 214, no. 4, pp. 516-559. http://dx.doi.org/10.1111/j.1469-7580.2009.01066.x PMid:19422428.
» http://dx.doi.org/10.1111/j.1469-7580.2009.01066.x - BRANDELLI, A., DAROIT, D.J. and RIFFEL, A., 2010. Biochemical features of microbial keratinases and their production and applications. Applied Microbiology and Biotechnology, vol. 85, no. 6, pp. 1735-1750. http://dx.doi.org/10.1007/s00253-009-2398-5 PMid:20039036.
» http://dx.doi.org/10.1007/s00253-009-2398-5 - CAI, C. and ZHENG, X.D., 2009. Medium optimization for keratinase production in hair substrate by a new Bacillus subtilis KD-N2 using response surface methodology. Journal of Industrial Microbiology & Biotechnology, vol. 36, no. 7, pp. 875-883. http://dx.doi.org/10.1007/s10295-009-0565-4 PMid:19350297.
» http://dx.doi.org/10.1007/s10295-009-0565-4 - CAI, C.G., LOU, B.G. and ZHENG, X.D., 2008. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University. Science. B., vol. 9, no. 1, pp. 60-67. http://dx.doi.org/10.1631/jzus.B061620 PMid:18196614.
» http://dx.doi.org/10.1631/jzus.B061620 - DE AZEREDO, L.A.I., DE LIMA, M.B., COELHO, R.R.R. and FREIRE, D.M.G., 2006. Thermophilic protease production by Streptomyces sp. 594 in submerged and solid-state fermentations using feather meal. Journal of Applied Microbiology, vol. 100, no. 4, pp. 641-647. http://dx.doi.org/10.1111/j.1365-2672.2005.02791.x PMid:16553718.
» http://dx.doi.org/10.1111/j.1365-2672.2005.02791.x - ELÍADES, L., CABELLO, M., VOGET, C., GALARZA, B. and SAPARRAT, M., 2010. Screening for alkaline keratinolytic activity in fungi isolated from soils of the biosphere reserve “Parque Costero del Sur” (Argentina). World Journal of Microbiology & Biotechnology, vol. 26, no. 11, pp. 2105-2111. http://dx.doi.org/10.1007/s11274-010-0389-4
» http://dx.doi.org/10.1007/s11274-010-0389-4 - ESAWY, M.A., 2007. Isolation and partial characterization of extracellular keratinase from a novel mesophilic Streptomyces albus AZA. Research Journal of Agriculture and Biological Sciences, vol. 3, pp. 808-817.
- FELSENSTEIN, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution; International Journal of Organic Evolution, vol. 39, no. 4, pp. 783-791. http://dx.doi.org/10.1111/j.1558-5646.1985.tb00420.x PMid:28561359.
» http://dx.doi.org/10.1111/j.1558-5646.1985.tb00420.x - FRIEDRICH, J., GRADISAR, H., MANDIN, D. and CHAUMONT, J.P., 1999. Screening fungi for synthesis of keratinolytic enzymes. Letters in Applied Microbiology, vol. 28, no. 2, pp. 127-130. http://dx.doi.org/10.1046/j.1365-2672.1999.00485.x
» http://dx.doi.org/10.1046/j.1365-2672.1999.00485.x - GHAFFAR, I., IMTIAZ, A., HUSSAIN, A., JAVID, A., JABEEN, F., AKMAL, M. and QAZI, J.I., 2018. Microbial production and industrial applications of keratinases: an overview. International Microbiology, vol. 21, no. 4, pp. 163-174. http://dx.doi.org/10.1007/s10123-018-0022-1 PMid:30810899.
» http://dx.doi.org/10.1007/s10123-018-0022-1 - GOUSTEROVA, A., BRAIKOVA, D., GOSHEV, I., CHRISTOV, P., TISHINOV, K., VASILEVA-TONKOVA, E., HAERTLÉ, T. and NEDKOV, P., 2005. Degradation of keratin and collagen containing wastes by newly isolated thermoactinomycetes or by alkaline hydrolysis. Letters in Applied Microbiology, vol. 40, no. 5, pp. 335-340. http://dx.doi.org/10.1111/j.1472-765X.2005.01692.x PMid:15836735.
» http://dx.doi.org/10.1111/j.1472-765X.2005.01692.x - GRADISAR, H., FRIEDRICH, J., KRIZAJ, I. and JERALA, R., 2005. Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Applied and Environmental Microbiology, vol. 71, no. 7, pp. 3420-3426. http://dx.doi.org/10.1128/AEM.71.7.3420-3426.2005 PMid:16000744.
» http://dx.doi.org/10.1128/AEM.71.7.3420-3426.2005 - GUPTA, R. and RAMNANI, P., 2006. Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology, vol. 70, no. 1, pp. 21-33. http://dx.doi.org/10.1007/s00253-005-0239-8 PMid:16391926.
» http://dx.doi.org/10.1007/s00253-005-0239-8 - GUPTA, R., SHARMA, R. and BEG, Q.K., 2013. Revisiting microbial keratinases: next generation proteases for sustainable biotechnology. Critical Reviews in Biotechnology, vol. 33, no. 2, pp. 216-228. http://dx.doi.org/10.3109/07388551.2012.685051 PMid:22642703.
» http://dx.doi.org/10.3109/07388551.2012.685051 - HASIIA, S.K., MALVIYA, H.K. and RAJAK, R.C., 1990. Keratinolytic ability of some fungi isolated from gelatin factory campus, Jabalpur (MP). Proceedings of the National Academy Science India, vol. 60, pp. 305-309.
- HEDAYATI, M.T., PASQUALOTTO, A.C., WARN, P.A., BOWYER, P. and DENNING, D.W., 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology, vol. 153, no. Pt 6, pp. 1677-1692. http://dx.doi.org/10.1099/mic.0.2007/007641-0 PMid:17526826.
» http://dx.doi.org/10.1099/mic.0.2007/007641-0 - HÖLKER, U. and LENZ, J., 2005. Solid-state fermentation: are there any biotechnological advantages? Current Opinion in Microbiology, vol. 8, no. 3, pp. 301-306. http://dx.doi.org/10.1016/j.mib.2005.04.006 PMid:15939353.
» http://dx.doi.org/10.1016/j.mib.2005.04.006 - HUANG, Y., BUSK, P.K., HERBST, F.A. and LANGE, L., 2015. Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvine. Applied Microbiology and Biotechnology, vol. 99, no. 22, pp. 9635-9649. http://dx.doi.org/10.1007/s00253-015-6805-9 PMid:26177915.
» http://dx.doi.org/10.1007/s00253-015-6805-9 - HUSSAIN, J., RABBANI, I., ASLAM, S. and AHMAD, H., 2015. An overview of poultry industry in Pakistan. World’s Poultry Science Journal, vol. 71, no. 4, pp. 689-700. http://dx.doi.org/10.1017/S0043933915002366 PMid:26696690.
» http://dx.doi.org/10.1017/S0043933915002366 - JIN, H.S., PARK, S.Y., KIM, K., LEE, Y.J., NAM, G.W., KANG, N.J. and LEE, D.W., 2017. Development of a keratinase activity assay using recombinant chicken feather keratin substrates. PLoS One, vol. 12, no. 2, pp. e0172712. http://dx.doi.org/10.1371/journal.pone.0172712 PMid:28231319.
» http://dx.doi.org/10.1371/journal.pone.0172712 - JING, L., ZHAO, S., XUE, J.L., ZHANG, Z., YANG, Q., XIAN, L. and FENG, J.X., 2015. Isolation and characterization of a novel Penicillium oxalicum strain Z1-3 with enhanced cellobiohydrolase production using cellulase-hydrolyzed sugarcane bagasse as carbon source. Industrial Crops and Products, vol. 77, pp. 666-675. http://dx.doi.org/10.1016/j.indcrop.2015.09.052
» http://dx.doi.org/10.1016/j.indcrop.2015.09.052 - KAUL, S. and SUMBALI, G., 1999. Production of extracellular keratinases by keratinophilic fungal species inhabiting feathers of living poultry birds (Gallus domesticus): a comparison. Mycopathologia, vol. 146, no. 1, pp. 19-24. http://dx.doi.org/10.1023/A:1007086720237
» http://dx.doi.org/10.1023/A:1007086720237 - KHAN, M.B., ASAR, A.U. and NAWAZ, A., 2015. Reducing capital cost and providing electricity to grid by power generation from poultry farms. International Journal of Computers and Applications, vol. 123, no. 2, pp. 5-8. http://dx.doi.org/10.5120/ijca2015905233
» http://dx.doi.org/10.5120/ijca2015905233 - KORNIŁŁOWICZ-KOWALSKA, T. and BOHACZ, J., 2011. Biodegradation of keratin waste: theory and practical aspects. Waste Management (New York, N.Y.), vol. 31, no. 8, pp. 1689-1701. http://dx.doi.org/10.1016/j.wasman.2011.03.024 PMid:21550224.
» http://dx.doi.org/10.1016/j.wasman.2011.03.024 - KORNIŁŁOWICZ-KOWALSKA, T., 1997a. Studies on the decomposition of keratin wastes by saprotrophic microfungi. I. criteria for evaluating keratinolytic activity. Acta Mycologica, vol. 32, no. 1, pp. 51-79. http://dx.doi.org/10.5586/am.1997.006
» http://dx.doi.org/10.5586/am.1997.006 - KORNIŁŁOWICZ-KOWALSKA, T., 1997b. Studies on the decomposition of keratin wastes by saprotrophic microfungi. P. II. Sulphur and nitrogen balance. Acta Mycologica, vol. 32, no. 1, pp. 81-93. http://dx.doi.org/10.5586/am.1997.007
» http://dx.doi.org/10.5586/am.1997.007 - KOTE, N.V., PATIL, A.G.G. and MULIMANI, V.H., 2009. Optimization of the production of thermostable endo-β-1,4 mannanase from a newly isolated Aspergillus niger gr and Aspergillus flavus gr. Applied Biochemistry and Biotechnology, vol. 152, no. 2, pp. 213-223. http://dx.doi.org/10.1007/s12010-008-8250-z PMid:18597050.
» http://dx.doi.org/10.1007/s12010-008-8250-z - KREPLAK, L., DOUCET, J., DUMAS, P. and BRIKI, F., 2004. New aspects of the α-helix to β-sheet transition in stretched hard α-keratin fibers. Biophysical Journal, vol. 87, no. 1, pp. 640-647. http://dx.doi.org/10.1529/biophysj.103.036749 PMid:15240497.
» http://dx.doi.org/10.1529/biophysj.103.036749 - KRISHNA, S., 2005. Quantum dots-in-a-well infrared photodetectors. Journal of Physics. D, Applied Physics, vol. 38, no. 13, pp. 2142-2150. http://dx.doi.org/10.1088/0022-3727/38/13/010
» http://dx.doi.org/10.1088/0022-3727/38/13/010 - KUNERT, J., 2000. Physiology of keratinophilic fungi In: R.K.S. KUSHWAHA and J. GUARRO, eds. Biology of dermatophytes and other keratinophilic fungi Bilbao: Revista Iberoamericana de Micologia, pp. 77-85.
- LANGE, L., BUSK, P.K. and HUANG, Y., 2014. Use of a microbial composition for the degradation of keratinaceous materials Denmark. Patent WO 2014/169920 A2, 23-10-2014.
- LANGE, L., HUANG, Y. and BUSK, P.K., 2016. Microbial decomposition of keratin in nature–a new hypothesis of industrial relevance. Applied Microbiology and Biotechnology, vol. 100, no. 5, pp. 2083-2096. http://dx.doi.org/10.1007/s00253-015-7262-1 PMid:26754820.
» http://dx.doi.org/10.1007/s00253-015-7262-1 - MAZOTTO, A.M., COURI, S., DAMASO, M.C.T. and VERMELHO, A.B., 2013. Degradation of feather waste by Aspergillus niger keratinases: comparison of submerged and solid-state fermentation. International Biodeterioration & Biodegradation, vol. 85, pp. 189-195. http://dx.doi.org/10.1016/j.ibiod.2013.07.003
» http://dx.doi.org/10.1016/j.ibiod.2013.07.003 - MCKITTRICK, J., CHEN, P.Y., BODDE, S.G., YANG, W., NOVITSKAYA, E.E. and MEYERS, M.A., 2012. The structure, functions, and mechanical properties of keratin. Journal of Management, vol. 64, pp. 449-468. http://dx.doi.org/10.1007/s11837-012-0302-8
» http://dx.doi.org/10.1007/s11837-012-0302-8 - MEYERS, M.A., CHEN, P., LIN, A.Y. and SEKI, Y., 2008. Biological materials: structure and mechanical properties. Progress in Materials Science, vol. 53, no. 1, pp. 1-206. http://dx.doi.org/10.1016/j.pmatsci.2007.05.002
» http://dx.doi.org/10.1016/j.pmatsci.2007.05.002 - MITCHELL, D.A., LUZ, L.F.L., KRIEGER, N. and BEROVIC, M., 2006. Bioreactors for solid-state fermentation Heidelberg: Springer. http://dx.doi.org/10.1007/3-540-31286-2.
- MUHSIN, T.M. and HADI, R.B., 2002. Degradation of keratin substrates by fungi isolated from sewage sludge. Mycopathologia, vol. 154, no. 4, pp. 185-189. http://dx.doi.org/10.1023/A:1016335623534 PMid:12206319.
» http://dx.doi.org/10.1023/A:1016335623534 - NAM, G.W., LEE, D.W., LEE, H.S., LEE, N.J., KIM, B.C., CHOE, E.A., HWANG, J.K., SUHARTONO, M.T. and PYUN, Y.R., 2002. Native feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Archives of Microbiology, vol. 178, no. 6, pp. 538-547. http://dx.doi.org/10.1007/s00203-002-0489-0 PMid:12420177.
» http://dx.doi.org/10.1007/s00203-002-0489-0 - ONIFADE, A.A., AL-SANE, N.A., AL-MUSALLAM, A.A. and AL-ZARBAN, S., 1998. A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresource Technology, vol. 66, no. 1, pp. 1-11. http://dx.doi.org/10.1016/S0960-8524(98)00033-9
» http://dx.doi.org/10.1016/S0960-8524(98)00033-9 - PANDEY, A., 2003. Solid-state fermentation. Biochemical Engineering Journal, vol. 13, no. 2-3, pp. 81-84. http://dx.doi.org/10.1016/S1369-703X(02)00121-3
» http://dx.doi.org/10.1016/S1369-703X(02)00121-3 - RIESSEN, S. and ANTRANIKIAN, G., 2001. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles, vol. 5, no. 6, pp. 399-408. http://dx.doi.org/10.1007/s007920100209 PMid:11778841.
» http://dx.doi.org/10.1007/s007920100209 - SAHNI, N., SAHOTA, P.P. and PHUTELA, U.G., 2015. Bacterial keratinases and their prospective applications: a review. International Journal of Current Microbiology and Applied Sciences, vol. 4, pp. 768-783.
- SAINI, R., SAINI, J.K., ADSUL, M., PATEL, A.K., MATHUR, A., TULI, D. and SINGHANIA, R.R., 2015. Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresource Technology, vol. 188, pp. 240-246. http://dx.doi.org/10.1016/j.biortech.2015.01.048 PMid:25661515.
» http://dx.doi.org/10.1016/j.biortech.2015.01.048 - SAITOU, N. and NEI, M., 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, vol. 4, no. 4, pp. 406-425. PMid:3447015.
- SAMAPUNDO, S., DEVLIEGHERE, F., GEERAERD, A.H., DE MEULENAER, B., VAN IMPE, J.F. and DEBEVERE, J., 2007. Modelling of the individual and combined effects of water activity and temperature on the radial growth of Aspergillus flavus and A. parasiticus on corn. Food Microbiology, vol. 24, no. 5, pp. 517-529. http://dx.doi.org/10.1016/j.fm.2006.07.021 PMid:17367685.
» http://dx.doi.org/10.1016/j.fm.2006.07.021 - SAPARRAT, M.C.N., ARAMBARRI, A.M. and BALATTI, P.A., 2007. Growth response and extracellular enzyme activity of Ulocladium botrytis LPSC 813 cultured on carboxy-methylcellulose under a pH-range. Biology and Fertility of Soils, vol. 44, no. 2, pp. 383-386. http://dx.doi.org/10.1007/s00374-007-0217-7
» http://dx.doi.org/10.1007/s00374-007-0217-7 - SAS Institute Inc. Statistics Version 901 Cary, N.C.: SAS Institute Inc., 2002.
- SUBRAMANIYAM, R. and VIMALA, R., 2012. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. International Journal of Science and Nature, vol. 3, pp. 480-486.
- SUZUKI, Y., TSUJIMOTO, Y., MATSUI, H. and WATANABE, K., 2006. Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. Journal of Bioscience and Bioengineering, vol. 102, no. 2, pp. 73-81. http://dx.doi.org/10.1263/jbb.102.73 PMid:17027867.
» http://dx.doi.org/10.1263/jbb.102.73 - TAMURA, K. and NEI, M., 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, vol. 10, no. 3, pp. 512-526. PMid:8336541.
- TAMURA, K., STECHER, G., PETERSON, D., FILIPSKI, A. and KUMAR, S., 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, vol. 30, no. 12, pp. 2725-2729. http://dx.doi.org/10.1093/molbev/mst197 PMid:24132122.
» http://dx.doi.org/10.1093/molbev/mst197
Publication Dates
-
Publication in this collection
26 July 2021 -
Date of issue
2023
History
-
Received
09 Dec 2020 -
Accepted
20 Jan 2021