Abstract
Zinc is an essential micronutrient that is required for optimum plant growth. It is present in soil in insoluble forms. Bacterial solubilization of soil unavailable form of Zn into available form, is an emerging approach to alleviate the Zn deficiency for plants and human beings. Zinc solubilizing bacteria (ZSB) could be a substitute for chemical Zn fertilizer. The present study aimed to isolate and characterize bacterial species from the contaminated soil and evaluate their Zn solubilizing potential. Zn resistant bacteria were isolated and evaluated for their MIC against Zn. Among the 13 isolated bacterial strains ZSB13 showed maximum MIC value upto 30mM/L. The bacterial strain with the highest resistance against Zn was selected for further analysis. Molecular characterization of ZSB13 was performed by 16S rRNA gene amplification which confirmed it as Pseudomonas oleovorans. Zn solubilization was determined through plate assay and broth medium. Four insoluble salts (zinc oxide (ZnO), zinc carbonate (ZnCO3), zinc sulphite (ZnS) and zinc phosphate (Zn3(PO4)2) were used for solubilization assay. Our results shows 11 mm clear halo zone on agar plates amended with ZnO. Likewise, ZSB13 showed significant release of Zn in broth amended with ZnCO3 (17 and 16.8 ppm) and ZnO (18.2 ppm). Furthermore, Zn resistance genes czcD was also enriched in ZSB13. In our study, bacterial strain comprising Zn solubilization potential has been isolated that could be further used for the growth enhancement of crops.
Keywords:
zinc solubilization; Pseudomonas oleovorans; zinc resistance bacteria; biofertilizers
Resumo
O zinco é um micronutriente essencial necessário para o crescimento ideal das plantas. Ele está presente no solo em formas insolúveis. A solubilização bacteriana da forma indisponível de Zn no solo para a forma disponível é uma abordagem emergente para aliviar a deficiência de Zn em plantas e seres humanos. Bactérias solubilizadoras de zinco (ZSB) podem ser um substituto para fertilizantes químicos de Zn. O presente estudo teve como objetivo isolar e caracterizar espécies bacterianas de solo contaminado e avaliar seu potencial de solubilização de Zn. Bactérias resistentes ao Zn foram isoladas e avaliadas quanto ao seu MIC contra o Zn. Entre as 13 cepas bacterianas isoladas, ZSB13 apresentou valor máximo de MIC de até 30 mM/L. A cepa bacteriana com maior resistência ao Zn foi selecionada para análise posterior. A caracterização molecular de ZSB13 foi realizada por amplificação do gene 16S rRNA que o confirmou como Pseudomonas oleovorans. A solubilização do Zn foi determinada através de ensaio em placa e meio caldo. Quatro sais insolúveis (óxido de zinco (ZnO), carbonato de zinco (ZnCO3), sulfito de zinco (ZnS) e fosfato de zinco (Zn3 (PO4) 2) foram usados para o ensaio de solubilização. Nossos resultados mostram uma zona de halo clara de 11 mm em placas de ágar corrigidas com ZnO. Da mesma forma, ZSB13 mostrou liberação significativa de Zn em caldo alterado com ZnCO3 (17 e 16,8 ppm) e ZnO (18,2 ppm). Além disso, os genes de resistência ao Zn czcD também foram enriquecidos em ZSB13. Em nosso estudo, a cepa bacteriana compreendendo potencial de solubilização de Zn foi isolada e poderia ser usada posteriormente para o aumento do crescimento de safras.
Palavras-chave:
solubilização de zinco; Pseudomonas oleovorans; bactéria resistente ao zinco; biofertilizantes
1. Introduction
Zinc is one of the key micronutrients that is required in small concentrations both for plants and animals for healthy growth (Saravanan et al., 2011SARAVANAN, V.S., KUMAR, M.R. and SA, T.M., 2011. Microbial zinc solubilization and their role on plants. In: D.K. MAHESHWARI, ed. Bacteria in agrobiology: plant nutrient management. Berlin: Springer, pp. 47-63. http://dx.doi.org/10.1007/978-3-642-21061-7_3.
http://dx.doi.org/10.1007/978-3-642-2106...
). It is a necessary constituent involved in many physiological and metabolic actions in plants, humans and microorganisms (Greany, 2005GREANY, K.M. 2005.An assessment of heavy metal contamination in the marine sediments of Las Perlas Archipelago, Gulf of Panama. Edinburgh, Scotland: School of Life Sciences Heriot-Watt University, M.S. thesis.). Although, it plays vital roles in membrane integrity, photosynthesis, pollen formation, immune system and protein synthesis (Hrynkiewicz and Baum, 2014HRYNKIEWICZ, K. and BAUM, C., 2014. Application of microorganisms in bioremediation of environment from heavy metals. In: A. MALIK, E. GROHMANN and R. AKHTAR, eds. Environmental deterioration and human health. Dordrecht: Springer, pp. 215-227. http://dx.doi.org/10.1007/978-94-007-7890-0_9.
http://dx.doi.org/10.1007/978-94-007-789...
; Turpeinen et al., 2002TURPEINEN, R., KAIRESALO, T. and HAGGBLOM, M., 2002. Microbial activity community structure in arsenic, chromium and copper contaminated soils. Journal of Environmental Microbiology, vol. 35, pp. 998-1002.), but on the other side, increase of Zn in soil and environment to a certain limit is toxic. For human, it is toxic if Zn level is 100-500 mg per day (Manivasagaperumal et al., 2011MANIVASAGAPERUMAL, R., BALAMURUGAN, S., THIYAGARAJAN, G. and SEKAR, J., 2011. Effect of zinc on germination, seedling growth and biochemical content of cluster bean (Cyamopsis tetragonoloba (L.) Taub). Current Biotica, vol. 2, no. 5, pp. 11-15.).
In Pakistan, Zn concentration ranges from >0.1 to 1193mg/kg in soil except the polluted area where the maximum concentration of Zn in soil/dust is considered to be 29755mg/kg. (Radojevic and Bashkin, 2006RADOJEVIC, M. and BASHKIN, V.N., 2006. Practical environmental analysis. London, UK: RSC Publishing.; Muhammad et al., 2011MUHAMMAD, S., SHAH, M.T. and KHAN, S., 2011. Heavy metal concentrations in soil and wild plants growing around Pb–Zn sulfide terrain in the Kohistan region, northern Pakistan. Microchemical Journal, vol. 99, no. 1, pp. 67-75. http://dx.doi.org/10.1016/j.microc.2011.03.012.
http://dx.doi.org/10.1016/j.microc.2011....
). In general, Zn metal cannot be degraded to soluble form and persist in the environment. Its extensive use and low melting temperature caused contamination of environment, soil and freshwater due to divalent cation (Khalid et al., 2017KHALID, S., SHAHID, M., NIAZI, N.K., MURTAZA, B., BIBI, I. and DUMAT, C., 2017. A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, vol. 182, pp. 247-268. http://dx.doi.org/10.1016/j.gexplo.2016.11.021.
http://dx.doi.org/10.1016/j.gexplo.2016....
). Notably, metal resistance has been reported in many microorganisms. Czc determinant encodes a multi-protein complex that is associated with resistance to cadmium, zinc and cobalt in bacteria. CzcA is one of the proteins characterized in several metal and Zn resistance bacteria such as Cupriavidus metallidurans CH34, Pseudomonas putida CD2 and Gluconacetobacter diazotrophicus PAl 5 (Intorne et al., 2012INTORNE, A.C., OLIVEIRA, M.V., PEREIRA, L. and SOUZA FILHO, G.A., 2012. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. International Microbiology, vol. 15, no. 2, pp. 69-78. PMid:22847268.).
It is evident that excessive presence of Zn in soil have adverse effect but on the other way its deficiency also responsible for impaired plant and human growth (Abaid-Ullah et al., 2015ABAID-ULLAH, M., NADEEM, M., HASSAN, M., GANTER, J., MUHAMMAD, B., NAWAZ, K., SHAH, A.S. and HAFEEZ, F.Y., 2015. Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). International Journal of Agriculture and Biology, vol. 17, no. 1, pp. 51-60.). It has been reported that worldwide occurrence of Zn deficiency in crops is not due to scarcity of total Zn but it is due to low solubility of Zn in soils (Cakmak, 2008CAKMAK, I., 2008. Enrichment of cereal grains with zinc: agronomic or genetic biofortification. Plant and Soil, vol. 302, no. 1-2, pp. 1-17. http://dx.doi.org/10.1007/s11104-007-9466-3.
http://dx.doi.org/10.1007/s11104-007-946...
). Normally, zinc is found in soil in the forms such as smithsonite (ZnCO3), sphalerite (ZnS), zincite (ZnO), franklinite (ZnFe2O4), wellemite (Zn2SiO4), and hopeite (Zn3(PO4)2·4H2O) that are insoluble forms of Zn and could not be used by the plants. Low availability of Zn not only effect the crop growth but also lowers Zn level in seeds and grains, so affecting the nutritional quality that ultimately leads to Zn deficieny in human beings (Cakmak and Hoffland, 2012CAKMAK, I. and HOFFLAND, E., 2012. Zinc for the improvement of crop production and human health. Plant and Soil, vol. 361, no. 1-2, pp. 1-2. http://dx.doi.org/10.1007/s11104-012-1504-0.
http://dx.doi.org/10.1007/s11104-012-150...
).
To overcome the Zn deficiency in soil for plant growth, normally chemical fertilizers are applied that causing environmental problems. Therefore, feasible alternative could be the use of soil microorganisms having Zn solubilization capacity. Certain bacteria including Pseudomonas sp, Bacillus sp., Burkholderia cenocepacia have been reported to transform insoluble forms of Zn to soluble form for enhanced availability and uptake by plants (Fasim et al., 2002FASIM, F., AHMED, N., PARSONS, R. and GADD, G.M., 2002. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiology Letters, vol. 213, no. 1, pp. 1-6. http://dx.doi.org/10.1111/j.1574-6968.2002.tb11277.x. PMid:12127480.
http://dx.doi.org/10.1111/j.1574-6968.20...
; Abaid-Ullah et al., 2015ABAID-ULLAH, M., NADEEM, M., HASSAN, M., GANTER, J., MUHAMMAD, B., NAWAZ, K., SHAH, A.S. and HAFEEZ, F.Y., 2015. Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). International Journal of Agriculture and Biology, vol. 17, no. 1, pp. 51-60.; Pawar et al., 2015PAWAR, A., ISMAIL, S., MUNDHE, S. and PATIL, V.D., 2015. Solubilization of insoluble zinc compounds by different microbial isolates in vitro condition. International Journal of Tropical Agriculture, vol. 33, no. 2, pp. 865-869.; Khande et al., 2017KHANDE, R., SHARMA, S.K., RAMESH, A. and SHARMA, M.P., 2017. Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere, vol. 4, pp. 126-138. http://dx.doi.org/10.1016/j.rhisph.2017.09.002.
http://dx.doi.org/10.1016/j.rhisph.2017....
).
Zn solubilizing bacteria can solubilize Zn through various mechanisms like acidification, production of siderophores and oxidoreductive systems on cell membranes (Chang et al., 2005CHANG, H.B., LIN, C.-W. and HUANG, H.-J., 2005. Zinc-induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regulation, vol. 46, no. 3, pp. 261-266. http://dx.doi.org/10.1007/s10725-005-0162-0.
http://dx.doi.org/10.1007/s10725-005-016...
; Saravanan et al., 2011SARAVANAN, V.S., KUMAR, M.R. and SA, T.M., 2011. Microbial zinc solubilization and their role on plants. In: D.K. MAHESHWARI, ed. Bacteria in agrobiology: plant nutrient management. Berlin: Springer, pp. 47-63. http://dx.doi.org/10.1007/978-3-642-21061-7_3.
http://dx.doi.org/10.1007/978-3-642-2106...
). These bacteria produce organic acids in soil which sequester the zinc cations and can also chelate zinc and enhance zinc solubility (Jones and Darrah, 1994JONES, D.L. and DARRAH, P.R., 1994. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant and Soil, vol. 166, no. 2, pp. 247-257. http://dx.doi.org/10.1007/BF00008338.
http://dx.doi.org/10.1007/BF00008338...
). This study has been designed to identify and characterize Zn solubilizing bacteria and to investigate presence of Zn resistant genes.
2. Materials and Methods
2.1. Sample collection and metal analysis of samples
Thirty five soil samples were collected from Paharang drain, Bawachak, Faisalabad (31°25’0”N/73°5’0”E). The major source of contamination is that industries discharge their waste in to Chenab river through Paharang drain originating from Chak Jhumra. This drain passes through Faisalabad with these industrial effluents. Soil samples were collected in sterile plastic containers at the depth of 10 cm at 600 meters distance intervals. Total 35 samples were collected in triplicate and transported to Laboratory for further use and stored at 4 °C. After collection, heavy metals were determined in the samples using the method previously described by Zeiner et al. (2007)ZEINER, M., REZIC, I. and STEFFAN, I., 2007. Analytical methods for the determination of heavy metals in the textile industry. Kemija U Industriji: Časopis Kemičara I Kemijskih Inženjera Hrvatske, vol. 56, no. 11, pp. 587 595.. Cadmium (Cd), nickel (Ni), zinc (Zn) and lead (Pb) were included for analysis. Samples were digested and metal analysis was performed using Atomic Absorption Spectrophotometer (AAS) (Zeeman Atomic Absorption Spectrophotometer, ZA3000 Series).
2.2. Isolation of zinc resistant bacteria
Soil samples were taken in sterile water and to isolate bacteria from soil, samples were shaken at 150 rpm for 2 hr. After that, samples were settled for 5 min and one mL of the suspension was serially diluted upto 10−6. Each dilution was spread onto an LB plate supplemented with 1 mM Zn and incubated at 30 °C. The growing colonies were observed and again inoculated onto LB plates containing Zn to get pure culture.
2.3. Determination of Minimum Inhibitory Concentration (MIC)
Minimum Inhibitory Concentration of Zn for selected isolated bacteria (based on their morphology) was determined by the plate dilution method (Ansari and Malik, 2007ANSARI, M.I. and MALIK, A., 2007. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresource Technology, vol. 98, no. 16, pp. 3149-3153.). The zinc salt ZnSO4.7H2O in varying concentrations ranging from 1 mM to 35 mM was used to determine MIC. Petri plate containing nutrient agar with varying concentrations of Zn was prepared and inoculated with 0.5 mL of overnight grown culture of bacterial isolate and incubated at 28-30 °C for 24h. The lowest concentration of the metal, where the growth of the microorganism was inhibited, considered as the MIC of the zinc against the bacterial isolates (Haroun et al., 2017HAROUN, A.A., KAMALUDDEEN, K.K., ALHAJI, I., MAGAJI, Y. and OAIKHENA, E.E., 2017. Evaluation of heavy metal tolerance level (MIC) and bioremediation potential of Pseudomonas aeruginosa isolated from Makera-Kakuri industrial drain in Kaduna, Nigeria. European Journal of Experimental Biology, vol. 7, no. 5, pp. 28.).
2.4. Identification of bacterial isolate
After selecting Zn resistant bacterial isolate, identification was done on the basis of cultural characteristics, Gram’s staining and biochemical characteristics including oxidase reaction, catalase reaction, starch hydrolysis, fermentation tests and glucose utilization.
2.5. Bacterial genomic DNA Isolation and 16S ribosomal RNA (rRNA) sequencing
After biochemical identification, molecular characterization of bacterial isolate on the basis of 16S rRNA gene sequencing was performed. Bacterial genomic DNA was isolated by ZR/Bacterial DNA Extraction Kit. From isolated DNA, amplification of 16S rRNA gene was performed by using Universal primer, 27 F: 5’-AGATTGATCTGGCTAGGGA-3’ and 1492 R: 5’-TACGGTACCTTGTTACGCTT-3’ (1500 bp product) (Vasas et al., 2013VASAS, G., FARKAS, O., BORICS, G., FELFÖLDI, T., SRAMKÓ, G., BATTA, G. and GONDA, S., 2013. Appearance of Planktothrix rubescens bloom with [D-Asp3, Mdha7] MC–RR in gravel pit pond of a shallow lake-dominated area. Toxins, vol. 5, no. 12, pp. 2434-2455.). The PCR was carried out with following conditions: initial denaturation for 5 min at 94 °C, followed by 35 cycles of denaturation at 94 °C, 30 sec of annealing at 52 °C, and 40 sec of elongation at 72 °C. The last step was a final extension at 72 °C for 10 min. For ribotyping, commercial sequencing of PCR product was obtained from Macrogen (Korea). The sequences obtained were checked for base calling using FinchTV and contigs made using NCBI BLAST (two sequence alignments). Using NCBI blast analysis by the sequence of the 16S rRNA gene was submitted to the database of GenBank and compared with similar sequences. The phylogenetic tree of partial 16S rRNA was constructed using maximum likelihood method by the software MEGA version 6.0.
2.6. Zinc solubilization assay
To determine the solubilization potential of selected bacterial isolate, four insoluble zinc salts like zinc carbonate (ZnCO3.4H2O), zinc sulphide (ZnS), zinc oxide (ZnO) and zinc phosphate (Zn (PO4)2. H2O) were used.
For qualitative solubilization, Mineral Salt medium (MSM) was prepared. The media was prepared by adding NaCl 1 g, CaCl2 0.1 g, MgSO4 0.5 g, KH2PO4 1 g, K2HPO4 1 g, yeast extract 4 g and agar 16-18 g in one litre dH2O and pH was maintained at 7.2. MS media was prepared containing 0.1% conc. of each insoluble zinc salt. Petri plates containing MS media were inoculated by pour plate method with bacterial strain and incubated at 30 °C for 48 hrs (Binder, Germany). After that, clear zone around the colonies was observed (Khanghahi et al., 2018KHANGHAHI, M.Y., RICCIUTI, P., ALLEGRETTA, I., TERZANO, R. and CRECCHIO, C., 2018. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environmental Science and Pollution Research International, vol. 25, no. 26, pp. 25862-25868. http://dx.doi.org/10.1007/s11356-018-2638-2. PMid:29959742.
http://dx.doi.org/10.1007/s11356-018-263...
).
For quantitative solubilization of zinc, MS broth was prepared with 0.1% zinc concentration for each zinc salts at pH 7. 1 mL of bacterial culture was inoculated into each flask containing MS Broth. The flasks were incubated at 30 °C for 72 hrs on shaking at 150 rpm (Thermo Scientific™, UK). After 72 hrs incubation, culture broth was centrifuged at 10,000 rpm for 10min. Concentration of Zn in supernatant was measured with atomic absorption spectrophotometer (Shimatzu AAS-7000). After 72 hrs incubation, pH of the medium was also measured.
2.7. Detection of resistant genes in strain ZSB13
Analysis of Zn resistance encoding genes were performed with primers encoding for Zn (CzcA, CzcB and CzcD). Primers used for czcA F 5-GTTTGAACGTATCATTAGTTTC-3, R 5-GTAGCCATCCGAAATATTCG-3 with 1885 bp. czcD F 5-CAGGTCACTGACACGACCAT-3, R 5-CATGCTGATGAGATTGATGATC-3 with 1000 bp. czcB F 5-CTATTTCGAACAAACAAAAGG-3, R 5-CTTCAGAACAAAACTGTTGG-3 with 1520 bp. The PCR conditions used were initial denaturation at 95 °C for 5 min followed by 35 cycles at denaturation: 95 °C for 30 sec, annealing: 55 °C for 30 sec, amplification: 72 °C for 2 min and final extension at 72 °C for 7 min (Babalola and Ayangbenro, 2019BABALOLA, O.O. and AYANGBENRO, A.S., 2019. Draft genome sequence of Pseudomonas koreensis strain AB36, isolated from gold mining soil. Microbiology Resource Announcements, vol. 8, no. 20, e00175-19. http://dx.doi.org/10.1128/MRA.00175-19. PMid:31097496.
http://dx.doi.org/10.1128/MRA.00175-19...
). Electrophoresis was done with amplified PCR products on 1.5% gel.
3. Results
3.1. Metal analysis in samples and isolation of zinc resistant bacteria
Heavy metal analysis of soil samples through atomic absorption spectrophotometer revealed the presence of different heavy metals in the samples. Concentrations of different heavy metals are: Zn 129.7 ± 0.30, Pb 54. ± 0.07, Cd 11.0 ± 0.03 and Ni 36.5 ± 0.09. Out of 35 samples, only 13 samples exhibited positive growth on medium containing Zn. 13 isolated bacteria using spread plate procedure labeled as ZSB1 to ZSB13 exhibited resistance to Zn.
3.2. Determination of Minimum Inhibitory Concentration (MIC)
13 bacterial isolates were used to determine the MIC. Isolates were inoculated on the nutrient agar plates having zinc 0.5, 1, 2, 5, 6, 8, 10, 12, 15, 20, 25, 30, 35mM. With increasing concentration of zinc, the growth of bacterial isolates decreased. The maximum zinc level where bacteria showed growth was 30mM. Isolate ZSB13 showing MIC upto 30 mM.
3.3. Identification of bacterial isolate
Based on the morphological and biochemical characteristics ZSB13 was found to be gram negative, rod shaped, non-spore forming, non-capsulated and non-motile (Table 1).
3.4. Molecular identification of bacterial isolate based on 16S ribosomal RNA (rRNA) sequencing
The 16S rRNA sequence of the strain ZSB13 was amplified by PCR. Ribotyping confirmed bacterial strain ZSB13 as Pseudomonas oleovorans and was submitted to the database of GenBank with the accession number (GenBank accession no.: MN396696.1).
The strain displayed highest level of similarity 99.30% with Pseudomonas mendocina strain Y12 (Accession number KP324955.1). In order to determine the relationship between strain ZSB13 and the other Pseudomonas species, the phylogenetic tree based on 16S rRNA sequence was constructed. So, based on the results of morphological, biochemical characteristics and 16S rRNA sequence analysis, strain ZSB13 was identified as Pseudomonas oleovorans ZSB13 shown in (Figure 1).
Neighbour-Joining phylogenetic tree of 16S rRNA gene sequences of Pseudomonas oleovorans strain ZSB13 isolated from contaminated soil.
3.5. Zinc solubilization assay
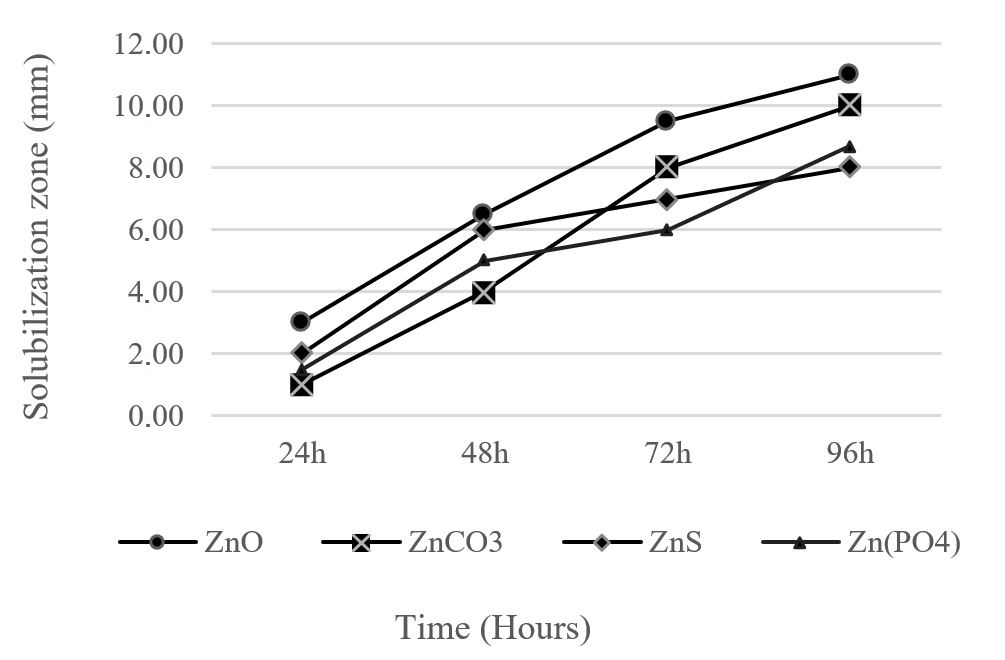
Zn solubilization potential of bacterial strain ZSB13 was evaluated by clear zone against four insoluble zinc salts (ZnCO3.4H2O, ZnS, ZnO and (Zn(PO4)2. H2O). Maximum Zn solubilization zone observed on ZnO medium was 11 mm followed by 10 mm for ZnCO3 as shown in Figure 2.
Quantitative assay for zinc solubilization revealed that strain ZSB was able to dissolve in18.2, 15.5, 17.3 and 13.8 ppm from ZnO, ZnCO3, Zn(PO4)2 and ZnS respectively in liquid medium as shown in Figure 3. However, maximum solubilization was observed in ZnO in liquid assay.
Available zinc (ppm) released by ZB13 in broth medium containing different zinc salts after 96 hrs.
3.6. Determination of resistant genes in strain ZSB13
Zn resistant genes were detected in ZSB13 and our results showed the presence of only czcD while others Zn resistance genes i.e., czcA and czcB were not observed in Pseudomonas oleovorans ZSB13 (Figure 4).
PCR products of czcA, czcB and czcD genes: M, gene ruler, cont: negative control (czcD 1000bp).
4. Discussion
In the present study efforts were made to isolate Zn resistant bacterial strains. In the present investigation, bacterial strains isolated from Paharang drain, Bawachak, Faisalabad showed a promising solubilizing efficiency for ZnO. After initial screening, 13 bacterial isolates were recovered on nutrient agar supplemented with 1 mM concentration of zinc from 35 soil samples. Morphological characterization of isolates showed as Gram-negative species. Gram negative bacteria have two layer of cell membrane enabling them to resist and grow at higher metal concentration than gram positive bacteria (Khanghahi et al., 2018KHANGHAHI, M.Y., RICCIUTI, P., ALLEGRETTA, I., TERZANO, R. and CRECCHIO, C., 2018. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environmental Science and Pollution Research International, vol. 25, no. 26, pp. 25862-25868. http://dx.doi.org/10.1007/s11356-018-2638-2. PMid:29959742.
http://dx.doi.org/10.1007/s11356-018-263...
). MIC of selected bacterial strains were evaluated and one strain ZSB13 showed maximum growth on nutrient agar incorporated with 30 mM of Zn. Lee et al. (2009)LEE, S., JEON, U.S., LEE, S.J., KIM, Y.K., PERSSON, D.P., HUSTED, S., SCHJORRING, J.K., KAKEI, Y., MASUDA, H., NISHIZAWA, N.K. and AN, G., 2009. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proceedings of the National Academy of Sciences of the United States of America, vol. 106, no. 51, pp. 22014-22019. http://dx.doi.org/10.1073/pnas.0910950106. PMid:20080803.
http://dx.doi.org/10.1073/pnas.091095010...
evaluated the MIC value upto 11.5 mmol/L of zinc for Pseudomonas. putida strain 06909. Muzammil et al. (2021)MUZAMMIL, S., SIDDIQUE, M.H., MUREED, F., ANDLEEB, R., JABEEN, F., WASEEM, M., ZAFAR, S., REHMAN, H.F., ALI, T. and ASHRAF, A., 2021. Assessment of cadmium tolerance and biosorptive potential of Bacillus Cereus GCFSD01 isolated from cadmium contaminated soil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 2, pp. 398-405. http://dx.doi.org/10.1590/1519-6984.227200.
http://dx.doi.org/10.1590/1519-6984.2272...
also isolated cadmium resistant bacteria (Bacillus cereus GCFSD01) from Paharang drain.
Morphological and biochemical characteristics showed the ZSB13 was gram negative, rod shaped and non-spore forming bacteria. Molecular characterization confirmed the strain as Pseudomonas oleovorans ZSB13. It has been reported in literature that most of the Zinc solubilizing bacteria belong to different genera like Pseudomonas, Bacillus, Enterobacter, Xanthomonas, Stenotrophomonas, and Acinetobacter etc (Hussain et al., 2015HUSSAIN, A., ARSHAD, M., ZAHIR, Z.A. and ASGHAR, M., 2015. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pakistan Journal of Agricultural Sciences, vol. 52, no. 4, pp. 915-922.; Gandhi and Muralidharan, 2016GANDHI, A. and MURALIDHARAN, G., 2016. Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. European Journal of Soil Biology, vol. 76, pp. 1-8. http://dx.doi.org/10.1016/j.ejsobi.2016.06.006.
http://dx.doi.org/10.1016/j.ejsobi.2016....
; Sunithakumari et al., 2016SUNITHAKUMARI, K., PADMA DEVI, S.N. and VASANDHA, S., 2016. Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore. Current Science, vol. 110, no. 2, pp. 196-205. http://dx.doi.org/10.18520/cs/v110/i2/196-205.
http://dx.doi.org/10.18520/cs/v110/i2/19...
). Saravanan et al., (2003)SARAVANAN, V.S., SUBRAMONIAM, S.R. and RAJ, S.A., 2003. Assessing in vitro solubilization potential of different zinc solubilizing bacteria (ZSB) isolates. Brazilian Journal of Microbiology, vol. 34, no. 1, pp. 121-125. studied Bacillus sp. and Pseudomonas sp. for their Zn solubilizing potential in broth assays and found that solubilization potential vary in different bacterial isolates.
In our study, the selected strain was identified as Pseudomonas oleovorans that can transform unavailable forms of Zn salts into available forms through solubilization as PGPB strains (Scagliola et al., 2016SCAGLIOLA, M., PII, Y., MIMMO, T., CESCO, S., RICCIUTI, P. and CRECCHIO, C., 2016. Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiology and Biochemistry, vol. 107, pp. 187-196. http://dx.doi.org/10.1016/j.plaphy.2016.06.002. PMid:27295343.
http://dx.doi.org/10.1016/j.plaphy.2016....
). In literature, many species of Pseudomonas like P. putida, P. fluorescens, P. aeruginosa have been studied for their Zn solubilizing potential (Goteti et al., 2013GOTETI, P.K., EMMANUEL, L.D.A., DESAI, S. and SHAIK, M.H.A., 2013. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). International Journal of Microbiology. http://dx.doi.org/10.1155/2013/869697.
https://doi.org/10.1155/2013/869697...
) but according to our knowledge, this is the first report on zinc solubilization potential of Pseudomonas oleovorans. The selected zinc solubilizing bacterial strain was screened for solubilization of insoluble zinc compounds and in our study, we used four different insoluble sources of Zinc like ZnO, ZnCO3, ZnS and Zn3(PO4)2.
In our study, it has been observed that Pseudomonas oleovorans. ZSB13 showed maximum solubilization potential for ZnO. These finding are in accordance with previous studies in which ZSB strains showed the highest solubilizing efficiency for ZnO compared to ZnCO3 and Zn3(PO4)2 (Gandhi and Muralidharan, 2016GANDHI, A. and MURALIDHARAN, G., 2016. Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. European Journal of Soil Biology, vol. 76, pp. 1-8. http://dx.doi.org/10.1016/j.ejsobi.2016.06.006.
http://dx.doi.org/10.1016/j.ejsobi.2016....
; Khanghahi et al., 2018KHANGHAHI, M.Y., RICCIUTI, P., ALLEGRETTA, I., TERZANO, R. and CRECCHIO, C., 2018. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environmental Science and Pollution Research International, vol. 25, no. 26, pp. 25862-25868. http://dx.doi.org/10.1007/s11356-018-2638-2. PMid:29959742.
http://dx.doi.org/10.1007/s11356-018-263...
). The zone of solubilization is ranged from 16 mm to 6 mm for insoluble compounds at 96 hrs. The halo zone showing the solubilization could be due to decrease of medium pH. In contrast to our results Sharma et al. (2014)SHARMA, P., KUNAWAT, K.C., KAUR, S. and KAUR, N., 2014. Assessment of zinc solubilization by endophytic bacteria in legume rhizosphere. Indian Journal of Applied Research, vol. 4, no. 6, pp. 439-441. http://dx.doi.org/10.15373/2249555X/June2014/137.
http://dx.doi.org/10.15373/2249555X/June...
found greater solubilization zone for Zn3(PO4)2) and Vidyashree (2016)VIDYASHREE, N.D., 2016. Isolation and characterization of zinc solubilizing bacteria from stone quarry dust powder. International Journal of Current Microbiology and Applied Sciences, vol. 8, no. 6, pp. 1248-1258. found it for ZnCO3. However, size of the solubilization zone could vary with the carbon source provided during the assay (Saravanan et al., 2008SARAVANAN, V.S., MADHAIYAN, M., OSBORNE, J., THANGARAJU, M. and SA, T.M., 2008. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen-fixing Acetobacteraceae members: their possible role in plant growth promotion. Microbial Ecology, vol. 55, no. 1, pp. 130. PMid:17574542.) and it could be altered with strains used.
The selected Pseudomonas strain ZSB13 was also evaluated for qualitative liquid assay containing insoluble Zn compounds. The broth assay also confirmed the ability of Pseudomonas ZSB13 to solubilize the Zn compound and here again we observed maximum solubization for ZnO. Different mechanisms could be involved in solubilisation of zinc that include excretion of organic acids, proton extrusion and production of inorganic acids such as sulphuric acid, gluconic acid and nitric acid (Desai et al., 2012DESAI, S., KUMAR, G.P., SULTANA, U., PINISETTY, S., AHMED, S.K.M.H., AMALRAJ, E.L.D. and REDDY, G., 2012. Potential microbial candidate strains for management of nutrient requirements of crops. African Journal of Microbiological Research, vol. 6, no. 17, pp. 3924-3931.). Di Simine investigated the Zn phosphate solubilization by Pseudomonas fluorescens and identified gluconic acids producedion in medium. The solubilization potential may be vary for different Zn salts as already reported in literature (Abaid-Ullah et al., 2015ABAID-ULLAH, M., NADEEM, M., HASSAN, M., GANTER, J., MUHAMMAD, B., NAWAZ, K., SHAH, A.S. and HAFEEZ, F.Y., 2015. Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). International Journal of Agriculture and Biology, vol. 17, no. 1, pp. 51-60.). In the present study, we found decrease in pH of the medium in all cases of the insoluble salt that gave the idea that solubilization could be due to production of organic acids. Although significant decrease in pH has been observed for ZnS but highest solubilization was for ZnO. No significant relation between pH and solubilization has been established. Although in our study, production of acids like gluconic acid has not been evaluated but its relation with the decrease in pH is evident (Desai et al., 2012DESAI, S., KUMAR, G.P., SULTANA, U., PINISETTY, S., AHMED, S.K.M.H., AMALRAJ, E.L.D. and REDDY, G., 2012. Potential microbial candidate strains for management of nutrient requirements of crops. African Journal of Microbiological Research, vol. 6, no. 17, pp. 3924-3931.). Pseudomonas oleovorans ZSB13 capable of solubilizing zinc. are also needed to evaluate for further use of these bacteria for bioremediation. It is therefore likely to express genes that confer metal resistance. In the present study, czc determinants (czcA, czcB and czcD) were used for analysis of metal-resistant determinant of ZSB13. It is also evident from literature that some metal resistance bacteria could harbor also antibiotics resistance genes with metal resistance genes (Rave et al., 2019RAVE, A.F.G., KUSS, A.V., PEIL, G.H.S., LADEIRA, S.R., VILLARREAL, J.P.V. and NASCENTE, P.S., 2019. Biochemical identification techniques and antibiotic susceptibility profile of lipolytic ambiental bacteria from effluents. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 4, pp. 555-565. http://dx.doi.org/10.1590/1519-6984.05616. PMid:30484476.
http://dx.doi.org/10.1590/1519-6984.0561...
). Therefore, with the evaluation of metal resistance genes, some antiobiotics resistance genes. The resistance ability of Pseudomonas oleovorans ZSB13 for zinc was first evaluated in an MIC assay. The results revealed that ZSB13 was tolerant to Zn.
5. Conclusions
On the basis of this study, it was concluded that native bacterial strain ZSB13 Pseudomonas oleovorans isolated from soil possessed Zn solubilization potential. However, further investigation need to use of this strain for plant growth promotion and to detoxify contaminated soil, but it may be a potential candidate as bioremediation agent development and as a substitute to synthetic fertilizers.
References
- ABAID-ULLAH, M., NADEEM, M., HASSAN, M., GANTER, J., MUHAMMAD, B., NAWAZ, K., SHAH, A.S. and HAFEEZ, F.Y., 2015. Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). International Journal of Agriculture and Biology, vol. 17, no. 1, pp. 51-60.
- ANSARI, M.I. and MALIK, A., 2007. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresource Technology, vol. 98, no. 16, pp. 3149-3153.
- BABALOLA, O.O. and AYANGBENRO, A.S., 2019. Draft genome sequence of Pseudomonas koreensis strain AB36, isolated from gold mining soil. Microbiology Resource Announcements, vol. 8, no. 20, e00175-19. http://dx.doi.org/10.1128/MRA.00175-19 PMid:31097496.
» http://dx.doi.org/10.1128/MRA.00175-19 - CAKMAK, I. and HOFFLAND, E., 2012. Zinc for the improvement of crop production and human health. Plant and Soil, vol. 361, no. 1-2, pp. 1-2. http://dx.doi.org/10.1007/s11104-012-1504-0
» http://dx.doi.org/10.1007/s11104-012-1504-0 - CAKMAK, I., 2008. Enrichment of cereal grains with zinc: agronomic or genetic biofortification. Plant and Soil, vol. 302, no. 1-2, pp. 1-17. http://dx.doi.org/10.1007/s11104-007-9466-3
» http://dx.doi.org/10.1007/s11104-007-9466-3 - CHANG, H.B., LIN, C.-W. and HUANG, H.-J., 2005. Zinc-induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regulation, vol. 46, no. 3, pp. 261-266. http://dx.doi.org/10.1007/s10725-005-0162-0
» http://dx.doi.org/10.1007/s10725-005-0162-0 - DESAI, S., KUMAR, G.P., SULTANA, U., PINISETTY, S., AHMED, S.K.M.H., AMALRAJ, E.L.D. and REDDY, G., 2012. Potential microbial candidate strains for management of nutrient requirements of crops. African Journal of Microbiological Research, vol. 6, no. 17, pp. 3924-3931.
- FASIM, F., AHMED, N., PARSONS, R. and GADD, G.M., 2002. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiology Letters, vol. 213, no. 1, pp. 1-6. http://dx.doi.org/10.1111/j.1574-6968.2002.tb11277.x PMid:12127480.
» http://dx.doi.org/10.1111/j.1574-6968.2002.tb11277.x - GANDHI, A. and MURALIDHARAN, G., 2016. Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. European Journal of Soil Biology, vol. 76, pp. 1-8. http://dx.doi.org/10.1016/j.ejsobi.2016.06.006
» http://dx.doi.org/10.1016/j.ejsobi.2016.06.006 - GOTETI, P.K., EMMANUEL, L.D.A., DESAI, S. and SHAIK, M.H.A., 2013. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). International Journal of Microbiology http://dx.doi.org/10.1155/2013/869697.
» https://doi.org/10.1155/2013/869697 - GREANY, K.M. 2005.An assessment of heavy metal contamination in the marine sediments of Las Perlas Archipelago, Gulf of Panama Edinburgh, Scotland: School of Life Sciences Heriot-Watt University, M.S. thesis.
- HAROUN, A.A., KAMALUDDEEN, K.K., ALHAJI, I., MAGAJI, Y. and OAIKHENA, E.E., 2017. Evaluation of heavy metal tolerance level (MIC) and bioremediation potential of Pseudomonas aeruginosa isolated from Makera-Kakuri industrial drain in Kaduna, Nigeria. European Journal of Experimental Biology, vol. 7, no. 5, pp. 28.
- HRYNKIEWICZ, K. and BAUM, C., 2014. Application of microorganisms in bioremediation of environment from heavy metals. In: A. MALIK, E. GROHMANN and R. AKHTAR, eds. Environmental deterioration and human health Dordrecht: Springer, pp. 215-227. http://dx.doi.org/10.1007/978-94-007-7890-0_9
» http://dx.doi.org/10.1007/978-94-007-7890-0_9 - HUSSAIN, A., ARSHAD, M., ZAHIR, Z.A. and ASGHAR, M., 2015. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pakistan Journal of Agricultural Sciences, vol. 52, no. 4, pp. 915-922.
- INTORNE, A.C., OLIVEIRA, M.V., PEREIRA, L. and SOUZA FILHO, G.A., 2012. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. International Microbiology, vol. 15, no. 2, pp. 69-78. PMid:22847268.
- JONES, D.L. and DARRAH, P.R., 1994. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant and Soil, vol. 166, no. 2, pp. 247-257. http://dx.doi.org/10.1007/BF00008338
» http://dx.doi.org/10.1007/BF00008338 - KHALID, S., SHAHID, M., NIAZI, N.K., MURTAZA, B., BIBI, I. and DUMAT, C., 2017. A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, vol. 182, pp. 247-268. http://dx.doi.org/10.1016/j.gexplo.2016.11.021
» http://dx.doi.org/10.1016/j.gexplo.2016.11.021 - KHANDE, R., SHARMA, S.K., RAMESH, A. and SHARMA, M.P., 2017. Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere, vol. 4, pp. 126-138. http://dx.doi.org/10.1016/j.rhisph.2017.09.002
» http://dx.doi.org/10.1016/j.rhisph.2017.09.002 - KHANGHAHI, M.Y., RICCIUTI, P., ALLEGRETTA, I., TERZANO, R. and CRECCHIO, C., 2018. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environmental Science and Pollution Research International, vol. 25, no. 26, pp. 25862-25868. http://dx.doi.org/10.1007/s11356-018-2638-2 PMid:29959742.
» http://dx.doi.org/10.1007/s11356-018-2638-2 - LEE, S., JEON, U.S., LEE, S.J., KIM, Y.K., PERSSON, D.P., HUSTED, S., SCHJORRING, J.K., KAKEI, Y., MASUDA, H., NISHIZAWA, N.K. and AN, G., 2009. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proceedings of the National Academy of Sciences of the United States of America, vol. 106, no. 51, pp. 22014-22019. http://dx.doi.org/10.1073/pnas.0910950106 PMid:20080803.
» http://dx.doi.org/10.1073/pnas.0910950106 - MANIVASAGAPERUMAL, R., BALAMURUGAN, S., THIYAGARAJAN, G. and SEKAR, J., 2011. Effect of zinc on germination, seedling growth and biochemical content of cluster bean (Cyamopsis tetragonoloba (L.) Taub). Current Biotica, vol. 2, no. 5, pp. 11-15.
- MUHAMMAD, S., SHAH, M.T. and KHAN, S., 2011. Heavy metal concentrations in soil and wild plants growing around Pb–Zn sulfide terrain in the Kohistan region, northern Pakistan. Microchemical Journal, vol. 99, no. 1, pp. 67-75. http://dx.doi.org/10.1016/j.microc.2011.03.012
» http://dx.doi.org/10.1016/j.microc.2011.03.012 - MUZAMMIL, S., SIDDIQUE, M.H., MUREED, F., ANDLEEB, R., JABEEN, F., WASEEM, M., ZAFAR, S., REHMAN, H.F., ALI, T. and ASHRAF, A., 2021. Assessment of cadmium tolerance and biosorptive potential of Bacillus Cereus GCFSD01 isolated from cadmium contaminated soil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 81, no. 2, pp. 398-405. http://dx.doi.org/10.1590/1519-6984.227200
» http://dx.doi.org/10.1590/1519-6984.227200 - PAWAR, A., ISMAIL, S., MUNDHE, S. and PATIL, V.D., 2015. Solubilization of insoluble zinc compounds by different microbial isolates in vitro condition. International Journal of Tropical Agriculture, vol. 33, no. 2, pp. 865-869.
- RADOJEVIC, M. and BASHKIN, V.N., 2006. Practical environmental analysis London, UK: RSC Publishing.
- RAMESH, A., SHARMA, S.K., SHARMA, M.P., YADAV, N. and JOSHI, O.P., 2014. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Applied Soil Ecology, vol. 73, pp. 87-96.
- RAVE, A.F.G., KUSS, A.V., PEIL, G.H.S., LADEIRA, S.R., VILLARREAL, J.P.V. and NASCENTE, P.S., 2019. Biochemical identification techniques and antibiotic susceptibility profile of lipolytic ambiental bacteria from effluents. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 79, no. 4, pp. 555-565. http://dx.doi.org/10.1590/1519-6984.05616 PMid:30484476.
» http://dx.doi.org/10.1590/1519-6984.05616 - SARAVANAN, V.S., KUMAR, M.R. and SA, T.M., 2011. Microbial zinc solubilization and their role on plants. In: D.K. MAHESHWARI, ed. Bacteria in agrobiology: plant nutrient management. Berlin: Springer, pp. 47-63. http://dx.doi.org/10.1007/978-3-642-21061-7_3
» http://dx.doi.org/10.1007/978-3-642-21061-7_3 - SARAVANAN, V.S., MADHAIYAN, M., OSBORNE, J., THANGARAJU, M. and SA, T.M., 2008. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen-fixing Acetobacteraceae members: their possible role in plant growth promotion. Microbial Ecology, vol. 55, no. 1, pp. 130. PMid:17574542.
- SARAVANAN, V.S., SUBRAMONIAM, S.R. and RAJ, S.A., 2003. Assessing in vitro solubilization potential of different zinc solubilizing bacteria (ZSB) isolates. Brazilian Journal of Microbiology, vol. 34, no. 1, pp. 121-125.
- SCAGLIOLA, M., PII, Y., MIMMO, T., CESCO, S., RICCIUTI, P. and CRECCHIO, C., 2016. Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiology and Biochemistry, vol. 107, pp. 187-196. http://dx.doi.org/10.1016/j.plaphy.2016.06.002 PMid:27295343.
» http://dx.doi.org/10.1016/j.plaphy.2016.06.002 - SHARMA, P., KUNAWAT, K.C., KAUR, S. and KAUR, N., 2014. Assessment of zinc solubilization by endophytic bacteria in legume rhizosphere. Indian Journal of Applied Research, vol. 4, no. 6, pp. 439-441. http://dx.doi.org/10.15373/2249555X/June2014/137
» http://dx.doi.org/10.15373/2249555X/June2014/137 - SUNITHAKUMARI, K., PADMA DEVI, S.N. and VASANDHA, S., 2016. Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore. Current Science, vol. 110, no. 2, pp. 196-205. http://dx.doi.org/10.18520/cs/v110/i2/196-205
» http://dx.doi.org/10.18520/cs/v110/i2/196-205 - TURPEINEN, R., KAIRESALO, T. and HAGGBLOM, M., 2002. Microbial activity community structure in arsenic, chromium and copper contaminated soils. Journal of Environmental Microbiology, vol. 35, pp. 998-1002.
- VASAS, G., FARKAS, O., BORICS, G., FELFÖLDI, T., SRAMKÓ, G., BATTA, G. and GONDA, S., 2013. Appearance of Planktothrix rubescens bloom with [D-Asp3, Mdha7] MC–RR in gravel pit pond of a shallow lake-dominated area. Toxins, vol. 5, no. 12, pp. 2434-2455.
- VIDYASHREE, N.D., 2016. Isolation and characterization of zinc solubilizing bacteria from stone quarry dust powder. International Journal of Current Microbiology and Applied Sciences, vol. 8, no. 6, pp. 1248-1258.
- ZEINER, M., REZIC, I. and STEFFAN, I., 2007. Analytical methods for the determination of heavy metals in the textile industry. Kemija U Industriji: Časopis Kemičara I Kemijskih Inženjera Hrvatske, vol. 56, no. 11, pp. 587 595.
Publication Dates
-
Publication in this collection
26 July 2021 -
Date of issue
2023
History
-
Received
23 June 2020 -
Accepted
01 Feb 2021