Abstract
The work aims were to describe the histological and histochemical structure of the gastroesophageal tube of Iguana iguana and verify the occurrence and distribution of immunoreactive serotonin (5-HT) and somatostatin (SS) cells. Fragments of the gastrointestinal tract (GIT) of five iguanas were which underwent standard histological and immunohistochemistry technique. Immunoreactive cells for 5-HT and SS were quantified using the STEPanizer. The oesophagus has ciliated columnar pseudostratified epithelium with staining Alcian blue (AB) + and goblet cells highly reactive to periodic acid Schiff (PAS). In the cervical oesophagus, the numerical density of 5-HT cells per unit area (QA [5-HT cells]/µm2) was 4.6x10-2 ± 2.0 and celomatic oesophagus presented QA = 4.0x10-2 ± 1.0. The epithelium of the stomach is simple columnar, PAS and AB +. The cranial and middle regions of the stomach presented (QA [5-HT cells]/µm2) = 6.18x10-2 ± 3.2 and the caudal region, QA = 0.6x10-2 ± 0.2. The SS cells were only observed in the caudal stomach, with numerical density (QA [SS cells]/µm2) = 1.4x10-2 ± 0.9 In I. iguana, variation was observed in terms of the distribution of mucus secretions and the pattern of occurrence of serotonin and somatostatin-secreting enteroendocrine cells in the TGI, which possibly will result in an interspecific adaptive response.
Keywords:
serotonin; somatostatin; stomach-oesophagus; morphology; reptilian
Resumo
Os objetivos do trabalho foram descrever a estrutura histológica e histoquímica do tubo gastroesofágico da Iguana iguana e verificar a ocorrência e distribuição de células serotonina (5-HT) e somatostatina (SS) imunorreativas. Fragmentos do trato gastrointestinal (TGI) de cinco iguanas foram submetidos à técnica histológica e imunohistoquímica padrão. As células imunorreativas para 5-HT e SS foram quantificadas usando o STEPanizer. O esôfago apresenta epitélio pseudoestratificado colunar ciliado Alcian blue (AB) positivo, com células caliciformes altamente reativas ao ácido periódico de Schiff (PAS). No esôfago cervical, a densidade numérica de células 5-HT por unidade de área (QA [células 5-HT] / µm2) foi de 4.6x10-2 ± 2.0 e o esôfago celomático apresentou QA = 4.0x10-2 ± 1.0. O epitélio do estômago é colunar simples, PAS e AB positivo. As regiões cranial e média do estômago apresentaram (QA [células 5-HT] / µm2) = 6.18x10-2 ± 3.2 e a região caudal, QA = 0.6x10-2 ± 0.2. As células SS foram observadas apenas no estômago caudal, com densidade numérica (QA [células SS] / µm2) = 1.4x10-2 ± 0.9. Em I. iguana, foi observada variações em termos da distribuição das secreções de muco e padrão de ocorrência das células enteroendócrinas secretoras de serotonina e somatostatina no TGI, o que possivelmente reflete uma resposta adaptativa interespecifica.
Palavras-chave:
serotonina; somatostatina; estômago-esôfago; morfologia; réptil
1. Introduction
The green iguana belongs to the order of Squamata, which is considered one of the most diverse among vertebrates, with 10,857 species (Uetz et al., 2020UETZ, P., FREED, P. and HOŠEK, J., eds. 2020 [viewed 19 May 2020]. The Reptile Database [online]. Available from: http://www.reptile-database.org
http://www.reptile-database.org...
). Its Iguanidae family holds more than 45 species (Uetz et al., 2020UETZ, P., FREED, P. and HOŠEK, J., eds. 2020 [viewed 19 May 2020]. The Reptile Database [online]. Available from: http://www.reptile-database.org
http://www.reptile-database.org...
), but the genus Iguana is comprised of only three species: Iguana iguana (Linnaeus, 1758), widely distributed throughout Latin America, Iguana delicatissima (Laurenti, 1768), restricted to the Lesser Antilles, and the one recently described Iguana melanoderma (Breuil et al., 2020BREUIL, M., SCHIKORSKI, D., VUILLAUME, B., KRAUSS, U., MORTON, M.N., CORRY, E., BECH, N., JELIĆ, M. and GRANDJEAN, F., 2020. Painted black: Iguana melanoderma (Reptilia, Squamata, Iguanidae) a new melanistic endemic species from Saba and Montserrat islands (Lesser Antilles). ZooKeys, vol. 926, pp. 95-131. http://dx.doi.org/10.3897/zookeys.926.48679. PMid:32336922.
http://dx.doi.org/10.3897/zookeys.926.48...
), endemic to the islands of Saba and Montserrat. The common green iguana is heliothermal arboreal and primarily herbivorous and consumes animal protein in the form of snails and insects that occur, probably incidentally, on the surrounding vegetation, thus they are considered a polyspecific herbivore (Hirth, 1963HIRTH, H.F., 1963. Some apects of the natural history of Iguana iguana on a tropical strand. Ecology, vol. 44, no. 3, pp. 613-615. http://dx.doi.org/10.2307/1932553.
http://dx.doi.org/10.2307/1932553...
; Lara-López and Gonzáles-Romero, 2002; Townsend et al., 2005TOWNSEND, J.H., SLAPCINSKY, J., KRYSKO, K.L., DONLAN, E.M. and GOLDEN, E.A., 2005. Predation of a Tree Snail Drymaeus multilineatus (Gastropoda: Bulimulidae) by Iguana iguana (Reptilia: Iguanidae) on Key Biscayne, Florida. Southeastern Naturalist, vol. 4, no. 2, pp. 361-364. http://dx.doi.org/10.1656/1528-7092(2005)004[0361:POATSD]2.0.CO;2.
http://dx.doi.org/10.1656/1528-7092(2005...
; Teles et al., 2017TELES, D.A., BRITO, S.V., TEIXEIRA, A.A.M., RIBEIRO, S.C., ARAUJO-FILHO, J.A., LIMA, V.F., PEREIRA, A.M.A. and ALMEIDA, W.O., 2017. Nematodes associated with Iguana iguana (Linnaeus, 1758) (Squamata, Iguanidae) in Semi-arid areas of Northeastern Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 77, no. 3, pp. 514-518. http://dx.doi.org/10.1590/1519-6984.17615. PMid:27683813.
http://dx.doi.org/10.1590/1519-6984.1761...
).
The green iguanas are originated from central and south America. They are considered an invasive species due to inadvertent movement of wild individuals or the release of captive animals (Falcón et al., 2013FALCÓN, W., ACKERMAN, J.D., RECART, W. and DAEHLER, C.C., 2013. Biology and Impacts of Pacific Island Invasive Species. 10. Iguana iguana, the Green Iguana (Squamata: iguanidae). Pacific Science, vol. 67, no. 2, pp. 157-186. http://dx.doi.org/10.2984/67.2.2.
http://dx.doi.org/10.2984/67.2.2...
; Kubiak, 2019KUBIAK, M., 2019. Veterinary care of green iguanas (Iguana iguana) part 1: husbandry. Companion Animal, vol. 24, no. 7, pp. 386-389. http://dx.doi.org/10.12968/coan.2019.0023.
http://dx.doi.org/10.12968/coan.2019.002...
; Spinner, 2018SPINNER, L., 2018 [viewed 5 August 2020] Caring for the green iguana. Reptiles Magazine [online]. Available from: https://www.reptilesmagazine.com/caring-for-the-green-iguana/
https://www.reptilesmagazine.com/caring-...
). In Brazil, consumption of meat and eggs of the green iguana is a possible source of food for a few rural communities, which together with the illegal capture of wild populations for the illicit supply of the international domestic animal market can be a great threat to the species. It is worth mentioning that all Iguana spp. are listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), resulting in restrictions on trade of wild individuals (Kubiak, 2019KUBIAK, M., 2019. Veterinary care of green iguanas (Iguana iguana) part 1: husbandry. Companion Animal, vol. 24, no. 7, pp. 386-389. http://dx.doi.org/10.12968/coan.2019.0023.
http://dx.doi.org/10.12968/coan.2019.002...
; Spinner, 2018SPINNER, L., 2018 [viewed 5 August 2020] Caring for the green iguana. Reptiles Magazine [online]. Available from: https://www.reptilesmagazine.com/caring-for-the-green-iguana/
https://www.reptilesmagazine.com/caring-...
). The green iguana is already endangered in Costa Rica and Panamá (Andrade, 2009ANDRADE, C.A.F., 2009. Iguana verde (Iguana iguana). Bicho da Vez, no. 6, pp. 1-3.).
Morphological studies on the structure of the digestive tract are essential to understand the vertebrate lifestyle as well as physiological aspects. Studies on the reptilian gastrointestinal tract (GIT) have been performed using histochemical techniques showing histological peculiarities in the oesophagus and stomach (Loo and Wong, 1975LOO, S.K. and WONG, W.C., 1975. Histochemical observations on the mucins of the gastrointestinal tract in the toad (Bufo melanostictus). Cells, Tissues, Organs, vol. 91, no. 1, pp. 97-103. http://dx.doi.org/10.1159/000144374. PMid:1136708.
http://dx.doi.org/10.1159/000144374...
; Rovira et al., 1993ROVIRA, J., VILLARO, A.C., BODEGAS, M.E., VALVERDE, E. and SESMA, P., 1993. Structural study of the frog Rana temporaria larval stomach. Tissue & Cell, vol. 25, no. 5, pp. 695-707. http://dx.doi.org/10.1016/0040-8166(93)90051-L. PMid:8296308.
http://dx.doi.org/10.1016/0040-8166(93)9...
; Bani et al., 1992BANI, G., FORMIGLI, L. and CECCHI, R., 1992. Morphological observations on the glands of the oesophagus and stomach of adult Rana esculenta and Bombina variegata. Italian Journal of Anatomy and Embryology, vol. 97, no. 2, pp. 75-87. PMid:1285678.).
About 3% of the lizards are herbivores (King, 1996KING, G., 1996. Reptiles and herbivory. London: Chapman & Hall, pp. 29-42.) belonging mainly to the families Iguanidae (e.g, Green iguana) and Agamidae (e.g, Uromastyx) (O’Malley, 2005O’MALLEY, B., 2005. Clinical anatomy and physiology of exotic species. New York: Elsevier, pp. 57-75. https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7.
https://doi.org/10.1016/B978-0-7020-2782...
). The oesophagus of a lizard has thin walls that enters the stomach through the left antimere of the abdomen. Unlike that observed in other reptiles, such as snakes, this is short and has a temporary food storage capacity (Zug et al., 2001ZUG, G.R., VITT, L.J. and CALDWELL, J.P., 2001. Herpetology: an introductory biology of amphibians and reptiles. 2nd ed. San Diego: Academic Press, pp. 123-146.; O’Malley, 2005O’MALLEY, B., 2005. Clinical anatomy and physiology of exotic species. New York: Elsevier, pp. 57-75. https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7.
https://doi.org/10.1016/B978-0-7020-2782...
).
The herbivores reptiles generally have larger stomach than carnivores (Pianka and Vitt, 2003PIANKA, E.R. and VITT, L.J., 2003. Lizards: windows to the evolution of diversity. Berkeley: University of California Press, pp. 141-170.) however, the digestive efficiency is lower (King, 1996KING, G., 1996. Reptiles and herbivory. London: Chapman & Hall, pp. 29-42.). This is due to the fact that foods of plant origin are digested with more difficulty than those of animal origin (Ricklefs, 2003RICKLEFS, R.E., 2003. A economia da natureza. Rio de Janeiro: Guanabara-Koogan, 503 p.), and also, because reptiles do not ferment food during night cooling, since low temperatures slow down gastrointestinal motility and secretion of digestive juices (Troyer, 1984TROYER, K., 1984. Structure and function of the digestive tract of a herbivorous lizard Iguana iguana. Physiological Zoology, vol. 57, no. 1, pp. 1-8. http://dx.doi.org/10.1086/physzool.57.1.30155960.
http://dx.doi.org/10.1086/physzool.57.1....
; O’Malley, 2005O’MALLEY, B., 2005. Clinical anatomy and physiology of exotic species. New York: Elsevier, pp. 57-75. https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7.
https://doi.org/10.1016/B978-0-7020-2782...
). Therefore, the stomach can function as a reservoir or fermentation chamber, allowing the absorption of digestible proteins and nutrients (O’Malley, 2005O’MALLEY, B., 2005. Clinical anatomy and physiology of exotic species. New York: Elsevier, pp. 57-75. https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7.
https://doi.org/10.1016/B978-0-7020-2782...
).
Enteroendocrine cells (EECs) represent the largest population of endocrine cells in the body. Many EECs subtypes have been described in vertebrates, each characterized by the secretion of a distinct hormone (Schonhoff et al., 2004SCHONHOFF, S.E., GIEL-MOLONEY, M. and LEITER, A.B., 2004. Minireview: development and differentiation of gut endocrine cells. Endocrinology, vol. 145, no. 6, pp. 2639-2644. http://dx.doi.org/10.1210/en.2004-0051. PMid:15044355.
http://dx.doi.org/10.1210/en.2004-0051...
; Santos et al., 2008SANTOS, C.M., NASCIMENTO, A.A., PERACCHI, A.L., SALES, A., MIKALAUSKAS, J.S. and GOUVEIA, S.F., 2008. Immunocytochemical study of gastrintestinal endocrine cells in insectivorous bats (Mammalia: chiroptera). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 68, no. 3, pp. 663-669. http://dx.doi.org/10.1590/S1519-69842008000300026. PMid:18833490.
http://dx.doi.org/10.1590/S1519-69842008...
; Ryu et al., 2018RYU, G.R., LEE, E., KIM, J.J., MOON, S.D., KO, S.H., AHN, Y.B. and SONG, K.H., 2018. Comparison of enteroendocrine cells and pancreatic β-cells using gene expression profiling and insulin gene methylation. PLoS One, vol. 13, no. 10, pp. e0206401. http://dx.doi.org/10.1371/journal.pone.0206401. PMid:30379923.
http://dx.doi.org/10.1371/journal.pone.0...
). EECs that produce peptides that regulate motility such as serotonin and somatostatin are more frequent in the GIT than endocrine cells that produce peptides regulating digestion (Solcia et al., 1975SOLCIA, E., CAPELLA, C., VASSALLO, G. and BUFFA, R., 1975. Endocrine cells of the gastric mucosa. International Review of Cytology, vol. 42, pp. 223-286. http://dx.doi.org/10.1016/S0074-7696(08)60982-1. PMid:53215.
http://dx.doi.org/10.1016/S0074-7696(08)...
). Lee and Ku, 2004, demonstrated that more serotonin- and somatostatin-positive cells were found in the GIT of the grass lizard than cells positive for digestion-regulating peptides. Beside this, EECs have been reported in the GIT of Squamata’s species: the Grass lizard, Mabuya quinquetaeniata (El-Salhy and Grimelius, 1981EL-SALHY, M. and GRIMELIUS, L., 1981. The endocrine cells of the gastrointestinal mucosa of a squamate reptile, the grass lizard (Mabuya quinquetaeniata). A histological and immunohistochemical study. Biomedical Research, vol. 2, no. 6, pp. 639-658. http://dx.doi.org/10.2220/biomedres.2.639.
http://dx.doi.org/10.2220/biomedres.2.63...
), King's skink, Egernia kingii (Arena et al., 1990ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.), Ocellated skink, Chalcides ocellatus (Virgilio et al., 2004VIRGILIO, F., SCIARRILLO, R., DE FALCO, M., LAFORGIA, V., CAVAGNUOLO, A. and VARANO, L., 2004. Seasonality in thyroid function in chalcides ocellatus (reptilia, scincidae). The Italian Journal of Zoology, vol. 71, no. 2, pp. 53-57. http://dx.doi.org/10.1080/11250000409356606.
http://dx.doi.org/10.1080/11250000409356...
) and the Tiger keelback snake, Rhabdophis tigrinus tigrinus (Lee et al., 1999LEE, J.H., KU, S.K. and LEE, H.S., 1999. An immunohistochemical study of endocrine cells in the alimentary tract of the snake, Rhabdophis tigrinus tigrinus. Korean Journal of Veterinary Research, vol. 39, no. 4, pp. 689-697.). More than fifteen regulatory peptides were found in the GIT of these lizards. Because of the strategic evolutionary position, information on the EECs of the reptilian digestive tract is important.
As there are few studies of the biology, habitat requirements and of the Iguana iguana utilization in Brazil, histological knowledge about the GIT of reptiles is an essential tool for an understanding of their biology as well as a prerequisite for the implementation of conservation and management projects. In light of this, the work aims were to describe the histological and histochemical structure of the gastroesophageal tube in I. iguana and verify the occurrence and distribution of serotonin and somatostatin secretory cells through a specific immunohistochemical method, providing data on the enteroendocrine cells of this species for comparative purposes with other reptiles.
2. Material and Methods
2.1. Tissue preparation and staining
Five green iguanas, three males and two female free-living were collected in Barão de Grajaú-MA, caatinga region, and they were deposited in the Natural History Collection of the Federal University of Piauí with authorization SISBIO 54501-2. The GIT fragments were donated to the Laboratory of Teaching and Research in Histology and Compared Embryology at UFF. The GIT Fragments were fixed in 10% buffered formaldehyde and were processed according to the standard histological techniques for paraffin embedding. Five micrometers thick histological serial sections were deparaffinized in xylene, after gradually hydrated through graded alcohols followed by distilled water and stained with hematoxylin-eosin (HE) and Gomori’s trichrome method (GT) (Lillie and Fullmer, 1976LILLIE, R.D. and FULLMER, H.M., 1976. Histopathologic technic and practical histochemistry. 4th ed. New York: McGraw-Hill, pp. 559-610.). Staining with Alcian blue (AB) 8GX at pH 2.5 (Kiernan, 1990KIERNAN, J.A. 1990. Histological & histochemical methods: theory and practice. 2nd ed. Frankfurt: Pergamon Press, 433 p.) was employed for the demonstration of sulfated and carboxylated acidic glycoconjugates. The periodic acid Schiff (PAS) staining was employed to detect neutral glycoconjugates.
2.2. Immunohistochemistry
After deparaffinization and hydration, the sections were incubated by 3% hydrogen peroxide in phosphate buffered saline (PBS, pH 7.2) for 30 minutes to block endogenous peroxidase. Antigen retrieval was performed by immersing the sections in citrate buffer at pH 6.0, in a water bath at 96 °C for 45min. Sections were incubated with goat serum 10% (EP-12-20531- Easypath) in humid chamber during 10 minutes at room temperature for Blocking of the non-specific reaction. They were then incubated overnight at 4◦C with rabbit polyclonal anti-serotonin (S 5545 - Sigma-Aldrich, inc.) 1:8000 and anti-somatostatin (A0566, Dako) diluted to 1:300. Afterwards, rinsing in a PBS, the sections were incubated with polymer Envision DuoFLEX Doublestain System (SK11021) for 30 minutes. Subsequently, the reaction was revealed by with 3, 3'-diaminobenzidine tetrahydrochloride (DAB) (Dakocytomation 003222) and sections were counterstained with Harris hematoxylin.
Digital images of the histological sections were obtained at 40 and 20x magnification using Aperio CS™ scanner (Leica Biosystems, USA).
2.2.1. 5-HT and SS quantification
Ten random microscopic fields were analyzed per animal on a light microscope DM 500 and a Leica ICC50 HD camera. Digital images were taken in 40X objective magnification. The numerical density per area of 5-HT and SS cells was evaluated considering the number of cells into a frame of known area produced with the STEPanizer webbased system (www.stepanizer.com) (Tschanz et al., 2011TSCHANZ, S.A., BURRI, P.H. and WEIBEL, E.R., 2011. A simple tool for stereological assessment of digital images: the STEPanizer. Journal of Microscopy, vol. 243, no. 1, pp. 47-59. http://dx.doi.org/10.1111/j.1365-2818.2010.03481.x. PMid:21375529.
http://dx.doi.org/10.1111/j.1365-2818.20...
), totaling and area of ≈3.39x102 µm2/animal. The 5-HT and SS cells were counted into the frame when they did not hit the “forbidden lines” or its extensions (Gundersen, 1977GUNDERSEN, H.J.G., 1977. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. Journal of Microscopy, vol. 111, no. 2, pp. 219-223. http://dx.doi.org/10.1111/j.1365-2818.1977.tb00062.x.
http://dx.doi.org/10.1111/j.1365-2818.19...
). The frequency of occurrence is expressed as mean +/- SD (standard deviation) per unit area (1 µm2).
3. Results
3.1. Light microscopy
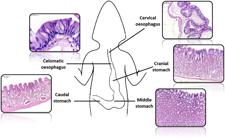
The oesophagus of the I. iguana presents a tubular form and is divided into two regions: cervical and celomatic, evidenced by the increase in folds in the region close to the stomach (Figure 1). The stomach is divided into three: cranial, middle and caudal (Figure 1). The esophageal mucosa protrudes into the lumen through longitudinal folds that vary in size. In the stomach there are also longitudinal folds that vary in size depending on the degree of repletion of the organ.
Histological sections of the mucosa layer of I. iguana in different regions of the oesophagus and stomach. HE. Bars = 100 μm. Caudal eosophagus 20 μm.
In the oesophagus the lining is composed of ciliated columnar pseudostratified epithelium with AB positive goblet cells (Figure 2b), and moderate PAS for columnar cells and strongly PAS positive for goblet cells (Figure 2a). The lamina propria consists of loose connective tissue and no mucous glands were observed (Figure 3). Below the, lamina propria is a muscularis mucosa, consisted of bundles of smooth muscle fibers in longitudinal direction in the cervical oesophagus (Figure 3), but circular in the celomatic region.
Histological sections of the (a,b) oesophagus, (c) cranial, (d) middle and (e-f) caudal regions of stomach of I. iguana. (a) After Periodic Acid Schiff (PAS) staining the glycoconjugates are identified in magenta in the goblet cells (arrow) in oesophagus; (b) The ephitelium (EP) of oesophagus has positive histochemical reaction to Alcian Blue (AB, pH 2.5); (c-f) The epithelium lining of stomach is simple columnar mucous with positive histochemical reactions to PAS (c,e) and AB (d,f). (c) Note the mucous cells PAS-positive along the glands (arrowhead) in cranial region of stomach; (e) In caudal region gastric glands (G) are positive only for PAS reaction.
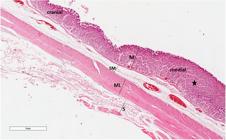
Histological sections of cervical oesophagus of I. iguana. Observe longitudinal folds. Absence of glands in the lamina propria (LP) and submucosa (SM), and evident muscular layer (ML) and adventitia (A). Bars = 3 mm. Detail of mucous layer with epithelium (EP), lamina propria (LP) and muscularis mucosa (MM). Gomori`s tricrome staining.
The submucosa was formed by collagenous fibers without glands (Figure 3).
In the cervical oesophagus, the muscular layer has bundles of smooth muscle fibers in two directions: inner circular and outer longitudinal (Figure 3), which becomes outer circular and inner longitudinal in the celomatic region.
The adventitial layer is formed by loose connective tissue with the presence of blood vessels and nerves (Figure 3).
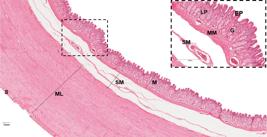
The mucosa layer throughout the entire stomach has a simple columnar mucous epithelium (Figure 4 and 5 – detail) with strong PAS (Figure 2c, e) and AB positivity (Figure 2d, f). This epithelium invaginates to form the gastric pits (Figure 4 and 5).
Histological sections of cranial and middle region in stomach. Note the gastric glands are more developed in the middle region, occupying a large part of the lamina propria (star). M: mucosa, SM: submucosa, ML: muscular layer and S: serosa. HE staining.
Histological sections of caudal region in stomach. The epithelium lining (EP) is simple columnar with gastric glands (G) in the lamina propria (LP). M: mucosa. Note the thickening of the muscular layer (ML) and the presence of serosa (S). Detail of mucous layer with epithelium (EP), lamina propria (LP), gastric glans (G), muscularis mucosa (MM) and submucosa (SM). HE staining. Bars = 300 μm.
The lamina propria, a region formed of loose connective tissue and blood vessels, has an abundance of simple tubular gastric glands (Figure 5). When comparing the cranial and middle regions, similarity was observed, except for the presence of more abundant connective tissue in the cranial region and more developed glands in the middle region, occupying a large part of the lamina propria (Figure 4). These glands have a predominance of oxynticopeptic cells that were AB and PAS negative. PAS-positive mucous cells are also noticed along the gland (Figure 2c). The caudal portion presents a decrease in the size of these glands, which show predominantly mucous cells, PAS-positive (Figure 2e), but they are less reactive when compared to those of the lining epithelium, and weakly stained in AB (Figure 2f).
The muscularis mucosa, which is below the lamina propria, consists of bundles of smooth muscle fibers in two directions: internal circular and external longitudinal (Figure 4 and 5).
The submucosa layer is composed of loose connective tissue richly vascularized (Figure 4 and 5).
The cranial and middle stomach muscular layer has bundles of smooth muscle fibers in two directions: internal circular and external longitudinal (Figure 4), while the caudal region consists of only one layer of smooth fibers oriented circularly and notably thicker (Figure 5).
The stomach is covered by a serous membrane which is quite evident in the caudal region (Figure 5).
3.2. Immunohistochemistry
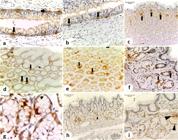
5-HT cells were observed in all portions of the oesophagus and stomach of the I. iguana (Figure 6). In the oesophagus, 5-HT positive cells were located at the base of the epithelium (Figure 6a, b). In the cervical oesophagus the numerical density of 5-HT cells per unit area (QA [5-HT cells]/µm2) was 4.6x10-2 ± 2 (Graph 1) and celomatic oesophagus QA= 4.0x10-2 ± 1.0 (Graph 1). While in the stomach, they are also found in the gastric glands beyond the epithelium, mainly in the apex in the cranial (Figure 6c) and middle (Figure 5d, e) regions of the stomach: QA [5-HT cells]/µm2 = 6.18x10-2 ± 3.2 (Graph 1). In the caudal part of the stomach, the marking occurs along the entire gland without a specific pattern (Figure 6f, g), with QA= 0.6x10-2 ± 0.2 (Graph 1). The 5-HT cells observed were the “closed type” ones without visible cytoplasmic process. The markings found on the connective tissue of the lamina propria are possibly mast cells.
Immunoreactivity for Serotonin (5-HT) a-g and Somatostatin (SS) i-h in histological sections of the oesophagus (a,b), cranial stomach (c), middle stomach (d,e) and caudal stomach (f-i) of I. iguana. a-g Serotonin-positive cells (arrow). h,i Somatostatin-positive cells (arrowhead) in the caudal region of the stomach.
Number of 5-HT and SS-cells/µm2 per oesophageal and gastric region in I. iguana. Values are averages with their standard deviatin shown by vertical bars (p = 0,05). Cervical oesophagus (CER): 4.6x10-2 ± 2.0 [5-HT cells]/µm2. Celomatic oesophagus (CEL): 4.0x10-2 ± 1.0 [5-HT cells]/µm2. Cranial and middle regions of the stomach (C/MS): 6.18x10-2 ± 3.2 [5-HT cells]/µm2. Caudal Stomach (CS): 0.6x10-2 ± 0.2 [5-HT cells]/µm2 and 1.4x 10-2 ± 0.9 [SS-cells]/µm2.
Somatostatin secretory cells were only observed in the caudal region near the intestine (Figure 6h, i). These cells occupy the glandular area and were not seen in the lining epithelium, being the QA [SS cells]/µm2 = 1.4x10-2 ± 0.9 (Graph 1). They are “closed type”, with no evident cytoplasmic process.
4. Discussion
The oesophagus is a slender tube that expands easily to accommodate even a large bolus of food (Kardong, 2018KARDONG, K.V., 2018. Vertebrates: comparative anatomy, function, evolution. 8th ed. New York: McGraw-Hill Education, pp. 504-545.). The oesophagus of the I. iguana were divided into cervical and celomatic, evidenced by the increase in folds in the region close to the stomach.
According to George and Castro (1998)GEORGE, L.L. and CASTRO, R.R.L., 1998. Histologia comparada. 2. ed. São Paulo: Roca, 286 p. and Elliott (2007)ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles. In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25., the esophageal mucosa of reptiles, in general, is lined by epithelium with one or two layers of columnar cells. In Squamata’s, of different genus as I. iguana, Hemidactylus mabouia (Rodrigues-Sartori et al., 2011) and E. kingii (Arena et al., 1990ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.), the esophageal epithelium is usually ciliated and pseudostratified with goblet cells, although a simple columnar epithelium has been observed in other genus such as snake Enhydris enhydris (Masyitha et al., 2020MASYITHA, D., MAULIDAR, L., ZAINUDDIN, Z., SALIM, M.N., ALIZA, D., GANI, F.A. and RUSLI, R., 2020. Histology of watersnake (Enhydris Enhydris) digestive system. E3S Web of Conferences, vol. 151, 01052. http://dx.doi.org/10.1051/e3sconf/202015101052.
http://dx.doi.org/10.1051/e3sconf/202015...
), in the chameleon Chamaeleon africanus (Hamdi et al., 2014HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395.
http://dx.doi.org/10.5829/idosi.wasj.201...
) and in the lizards Laudakia stellio (Koca and Gürcü, 2011KOCA, Y.B. and GÜRCÜ, B., 2011. Morphological and histochemical investigations of esophagogastric tract of a lizard, Laudakia stellio (Agamidae, Linnaeus 1758). Acta Biologica Hungarica, vol. 62, no. 4, pp. 376-387. http://dx.doi.org/10.1556/ABiol.62.2011.4.4. PMid:22119867.
http://dx.doi.org/10.1556/ABiol.62.2011....
) and Varanus niloticus (Ahmed et al., 2009AHMED, Y.A., EL-HAFEZ, A.A.E. and ZAYED, A.E., 2009. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. Journal of Veterinary Anatomy, vol. 2, no. 1, pp. 35-48. http://dx.doi.org/10.21608/jva.2009.45136.
http://dx.doi.org/10.21608/jva.2009.4513...
). Thus, there is no phylogenetic relationship that explains the change in the type of esophageal epithelium between different species, which may be better associated with variations in eating habits.
The ciliary propulsion can help gather small crumbs from the meal and move these along to the stomach, facilizing swallowing as well as cleaning the esophageal covering. Moreover, the ciliated cells it controls the flow of lubricating mucus around the food to aid in the passage (Kardong, 2018KARDONG, K.V., 2018. Vertebrates: comparative anatomy, function, evolution. 8th ed. New York: McGraw-Hill Education, pp. 504-545.).
The absence of oesophageal glands in the lamina propria and submucosa layer, described in I. iguana and Uromastyx aegyptiaca (herbivouous) (Zaher et al., 2012ZAHER, M., EL-GHAREEB, A.W., HAMDI, H., ESSA, A. and LAHSIK, S., 2012. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Science Journal, vol. 9, pp. 84-104.), H. mabouia (feeds on a wide variety of arthropods) (Rodrigues-Sartori et al., 2015RODRIGUES-SARTORI, S.S., NOGUEIRA, K.P.O.C., ARAÚJO, V.A. and NEVES, C.A., 2015. Functional morphology of the esophagus of the tropical house gecko Hemidactylus mabouia (Squamata: gekkonidae). Animal Biology, vol. 65, no. 2, pp. 177-191. http://dx.doi.org/10.1163/15707563-00002469.
http://dx.doi.org/10.1163/15707563-00002...
) and Caiman latirostris (carnivorous) (Machado-Santos et al., 2011MACHADO-SANTOS, C., ZECA, S.G., ABIDU-FIGUEIREDO, M., RIBEIRO, I. and SALES, A., 2011. The esophagus of the crocodilian Caiman latirostris (Reptilia, Crocodylia): histological, histochemical and immunohistochemical study. Journal of Morphological Sciences, vol. 28, no. 2, pp. 113-119.), can be correlated with the rapid transit of food to the stomach, with no involvement of the oesophagus in the digestion. However, when the oesophageal glands are present, these, play some role in digestion. Such as the branched tubular glands in L. stellio (Koca and Gürcü, 2011KOCA, Y.B. and GÜRCÜ, B., 2011. Morphological and histochemical investigations of esophagogastric tract of a lizard, Laudakia stellio (Agamidae, Linnaeus 1758). Acta Biologica Hungarica, vol. 62, no. 4, pp. 376-387. http://dx.doi.org/10.1556/ABiol.62.2011.4.4. PMid:22119867.
http://dx.doi.org/10.1556/ABiol.62.2011....
) that secrete mucins neutrals and acids, especially acidic mucins, which are important for lubrication of the mucosa and allow the passage of food particles, similar to what occurs in C. africanus (Hamdi et al., 2014HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395.
http://dx.doi.org/10.5829/idosi.wasj.201...
), since are both insectivorous. So, there is no relationship between the systematic position of the animal and the presence or absence of such glands.
The muscularis mucosa of the oesophagus is absent in many species of reptiles (Elliott, 2007ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles. In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25.). However, it is present in the species of suborder Iguania, e.g: I. iguana (in this study), C. africanus (Hamdi et al., 2014HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395.
http://dx.doi.org/10.5829/idosi.wasj.201...
), U. aegyptiaca (Zaher et al., 2012ZAHER, M., EL-GHAREEB, A.W., HAMDI, H., ESSA, A. and LAHSIK, S., 2012. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Science Journal, vol. 9, pp. 84-104.) and L. stellio (Koca and Gürcü, 2011KOCA, Y.B. and GÜRCÜ, B., 2011. Morphological and histochemical investigations of esophagogastric tract of a lizard, Laudakia stellio (Agamidae, Linnaeus 1758). Acta Biologica Hungarica, vol. 62, no. 4, pp. 376-387. http://dx.doi.org/10.1556/ABiol.62.2011.4.4. PMid:22119867.
http://dx.doi.org/10.1556/ABiol.62.2011....
). The movement of the muscularis mucosa is related to the secretion and absorption of the esophageal glands, however, as these glands are absent in I. iguana, their presence can be related to the secretion of the epithelial secretory cells.
The oesophagus of the green iguana presented a well-developed muscularis wall formed of smooth muscle fibers in two directions: inner circular and outer longitudinal in the cervical oesophagus, and inner longitudinal and outer circular in the celomatic region. Which may help in performing the function of mechanical conveyance and in food swallowing. However, the amount of muscle in the esophageal wall varies among the main groups of reptiles (Elliott, 2007ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles. In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25.).
In reptiles the stomach varies in shape (Elliott, 2007ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles. In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25.). In the Squamata order, the anatomy of the stomach can be of saccular type as in distant families of lizards: Ophisops elegans (Çakici and Akat, 2013ÇAKICI, Ö. and AKAT, E., 2013. Some histomorphological and histochemical characteristics of the digestive tract of the snake-eyed lizard, Ophisops elegans Menetries, 1832 (Squamata: lacertidae). North-Western Journal of Zoology, vol. 9, no. 2, pp. 257-263.) and V. niloticus (Ahmed et al., 2009AHMED, Y.A., EL-HAFEZ, A.A.E. and ZAYED, A.E., 2009. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. Journal of Veterinary Anatomy, vol. 2, no. 1, pp. 35-48. http://dx.doi.org/10.21608/jva.2009.45136.
http://dx.doi.org/10.21608/jva.2009.4513...
), or tubular in the shape of a ‘J’ in the lizards of the Gekkonidae family H. mabouia (Rodrigues-Sartori et al., 2011) and Cyrtodactylus peguensis (Thongboon et al., 2019THONGBOON, L., SENARAT, S., KETTRATAD, J., JIRAUNGKOORSKUL, W., WANGKULANGKUL, S., POOLPRASERT, P., PARA, C., KANEKO, G. and PENGSAKU, T., 2019. Gastrointestinal tract and accessory organs in the spotted bent-toed gecko, Cyrtodactylus peguensis (boulenger, 1893): a histological and histochemical study. Journal of Morphological Sciences, vol. 36, no. 4, pp. 223-230. http://dx.doi.org/10.1055/s-0039-1693021.
http://dx.doi.org/10.1055/s-0039-1693021...
), considered carnivores, or can present a ‘U’-shaped stomach as seen in I. iguana, a mainly herbivorous animal.
Usually, the stomach of the reptiles can be distinguished into two regions: fundus and pylorus, according to the characteristics of the present glands (Luppa, 1977LUPPA, H., 1977. Histology of the digestive tract. In: C. GANS and T.S. PARSONS, eds. Biology of the Reptilia. New York: Academic Press, vol. 6, pp. 225-313.). However, the stomach of I. iguana was distinguished into three regions: cranial, middle and caudal as previously described by Smith and collaborators in their study using radiography (Smith et al., 2001SMITH, D., DOBSON, H. and SPENCE, E., 2001. Gastrointestinal studies in the green iguana: technique and reference values. Veterinary Radiology & Ultrasound, vol. 42, no. 6, pp. 515-520. http://dx.doi.org/10.1111/j.1740-8261.2001.tb00979.x. PMid:11768518.
http://dx.doi.org/10.1111/j.1740-8261.20...
).
The simple columnar mucous epithelium is indicative of the stomach, as soon as in all reptiles. This mucus secretion plays important roles, including the protection of underlying epithelium from mechanical and chemical stress, electrolyte absorption, increasing digestive efficiency and lubrication of tract (Gupta, 1989GUPTA, B.L., 1989. The relationship of mucoid substances and ion and water transport, with new data on intestinal goblet cells and a model for gastric secretion. Symposia of the Society for Experimental Biology, vol. 43, pp. 81-110. PMid:2701492.; Allen and Snary, 1972ALLEN, A. and SNARY, D., 1972. The structure and function of gastric mucus. Gut, vol. 13, no. 8, pp. 666-672. http://dx.doi.org/10.1136/gut.13.8.666. PMid:4562023.
http://dx.doi.org/10.1136/gut.13.8.666...
).
In some groups of reptiles, the glandular region contains two cell types: oxynticopeptic cells and mucous cells (Kardong, 2018KARDONG, K.V., 2018. Vertebrates: comparative anatomy, function, evolution. 8th ed. New York: McGraw-Hill Education, pp. 504-545.). The ratios of the two cell types vary among stomach regions and among species (George and Castro, 1998GEORGE, L.L. and CASTRO, R.R.L., 1998. Histologia comparada. 2. ed. São Paulo: Roca, 286 p.; Arena et al., 1990ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.; Ferri et al., 1999FERRI, D., LIQUORI, G.E. and SCILLITANI, G., 1999. Morphological and histochemical variations of mucous and oxynticopeptic cells in the stomach of the seps, Chalcides chalcides. Journal of Anatomy, vol. 194, no. 1, pp. 71-77. http://dx.doi.org/10.1046/j.1469-7580.1999.19410071.x. PMid:10227668.
http://dx.doi.org/10.1046/j.1469-7580.19...
; Kardong, 2018KARDONG, K.V., 2018. Vertebrates: comparative anatomy, function, evolution. 8th ed. New York: McGraw-Hill Education, pp. 504-545.). In the cranial and middle stomach of I. iguana, oxynticopeptics cells are the predominant. These cells produce both hydrochloric acid (HCl) and pepsinogen (Arena et al., 1990ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.; Ferri et al., 1999FERRI, D., LIQUORI, G.E. and SCILLITANI, G., 1999. Morphological and histochemical variations of mucous and oxynticopeptic cells in the stomach of the seps, Chalcides chalcides. Journal of Anatomy, vol. 194, no. 1, pp. 71-77. http://dx.doi.org/10.1046/j.1469-7580.1999.19410071.x. PMid:10227668.
http://dx.doi.org/10.1046/j.1469-7580.19...
). In the lizard Chalcides chalcides the oxynticopeptics cells show different cytological features. In the oral fundic mucosa these cells, show a cytoplasm filled with granules, characteristic of protein secreting cells and in the aboral fundic region, the oxynticopeptic cells, are typical of the mammalian parietal acid-secreting cells, suggesting a secretion gradient of proteolytic enzymes and perhaps hydrochloric acid along the proximodistal axis of the stomach (Ferri et al.,1999FERRI, D., LIQUORI, G.E. and SCILLITANI, G., 1999. Morphological and histochemical variations of mucous and oxynticopeptic cells in the stomach of the seps, Chalcides chalcides. Journal of Anatomy, vol. 194, no. 1, pp. 71-77. http://dx.doi.org/10.1046/j.1469-7580.1999.19410071.x. PMid:10227668.
http://dx.doi.org/10.1046/j.1469-7580.19...
).
The neutral glycoconjugates shown by the PAS method, have a protective function against mechanical injuries, pathogens and pepsin (Ferri et al., 2001FERRI, D., LIQUORI, G.E., NATALE, L., SANTARELLI, G. and SCILLITANI, G., 2001. Mucin histochemistry of the digestive tract of the red-legged frog Rana aurora aurora. Acta Histochemica, vol. 103, no. 2, pp. 225-237. http://dx.doi.org/10.1078/0065-1281-00582. PMid:11368102.
http://dx.doi.org/10.1078/0065-1281-0058...
). In the oesophagus of I. iguana, as well as the H. mabouia (Rodrigues-Sartori et al., 2015RODRIGUES-SARTORI, S.S., NOGUEIRA, K.P.O.C., ARAÚJO, V.A. and NEVES, C.A., 2015. Functional morphology of the esophagus of the tropical house gecko Hemidactylus mabouia (Squamata: gekkonidae). Animal Biology, vol. 65, no. 2, pp. 177-191. http://dx.doi.org/10.1163/15707563-00002469.
http://dx.doi.org/10.1163/15707563-00002...
) the goblet cells were AB and PAS-positive. And in the stomach the gastric glands exhibit a positive reaction only with PAS, as also observed in the lizards C. peguensis (Thongboon et al., 2019THONGBOON, L., SENARAT, S., KETTRATAD, J., JIRAUNGKOORSKUL, W., WANGKULANGKUL, S., POOLPRASERT, P., PARA, C., KANEKO, G. and PENGSAKU, T., 2019. Gastrointestinal tract and accessory organs in the spotted bent-toed gecko, Cyrtodactylus peguensis (boulenger, 1893): a histological and histochemical study. Journal of Morphological Sciences, vol. 36, no. 4, pp. 223-230. http://dx.doi.org/10.1055/s-0039-1693021.
http://dx.doi.org/10.1055/s-0039-1693021...
), C. africanus (Hamdi et al., 2014HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395.
http://dx.doi.org/10.5829/idosi.wasj.201...
), O. elegans (Çakici and Akat, 2013ÇAKICI, Ö. and AKAT, E., 2013. Some histomorphological and histochemical characteristics of the digestive tract of the snake-eyed lizard, Ophisops elegans Menetries, 1832 (Squamata: lacertidae). North-Western Journal of Zoology, vol. 9, no. 2, pp. 257-263.).
However, the gastric epithelium of I. iguana revealed strong reactivity to AB, similar to that observed in the lizards U. aegyptiaca (Zaher et al., 2012ZAHER, M., EL-GHAREEB, A.W., HAMDI, H., ESSA, A. and LAHSIK, S., 2012. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Science Journal, vol. 9, pp. 84-104.) and L. stellio (Koca and Gürcü, 2011KOCA, Y.B. and GÜRCÜ, B., 2011. Morphological and histochemical investigations of esophagogastric tract of a lizard, Laudakia stellio (Agamidae, Linnaeus 1758). Acta Biologica Hungarica, vol. 62, no. 4, pp. 376-387. http://dx.doi.org/10.1556/ABiol.62.2011.4.4. PMid:22119867.
http://dx.doi.org/10.1556/ABiol.62.2011....
), all belonging to suborder Iguania. Bearing in mind that the acid glycoconjugates shown by the AB method can be responsible for an increasing viscosity of the gastric secretions (Díaz et al., 2008DÍAZ, A.O., GARCÍA, A.M. and GOLDEMBERG, A.L., 2008. Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: A histochemical study. Acta Histochemica, vol. 110, no. 1, pp. 76-85. http://dx.doi.org/10.1016/j.acthis.2007.08.002. PMid:17945334.
http://dx.doi.org/10.1016/j.acthis.2007....
), facilitating the movement of the bolus through the stomach (Ahmed et al., 2009AHMED, Y.A., EL-HAFEZ, A.A.E. and ZAYED, A.E., 2009. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. Journal of Veterinary Anatomy, vol. 2, no. 1, pp. 35-48. http://dx.doi.org/10.21608/jva.2009.45136.
http://dx.doi.org/10.21608/jva.2009.4513...
). In addition, Dehlawi and Zaher (1985)DEHLAWI, G.Y. and ZAHER, M.M., 1985. Histological studies on the mucosal epithelium of the alimentary canal of the lizard Acanthodactylus boskianus (Family Lacertidae). Proceedings of the Zoological Society A.R. Egypt, vol. 9, pp. 67-90., in their studies with Acanthodactylus Boskianus, suggested that the gastric glands of this lizard might be the source of acid mucopolysaccharides.
The presence of a relatively thick gastric muscularis layer, as described in I. iguana, initiate the first major digestive breakdown of food through strong muscular contraction (Elliott, 2007ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles. In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25.). Besides that, the gastric transit time is influenced by a variety of intrinsic and extrinsic factors and can vary from 3 to 6 days in carnivorous lizards, compared to 15-30 days in herbivorous lizards (Smith et al., 2001SMITH, D., DOBSON, H. and SPENCE, E., 2001. Gastrointestinal studies in the green iguana: technique and reference values. Veterinary Radiology & Ultrasound, vol. 42, no. 6, pp. 515-520. http://dx.doi.org/10.1111/j.1740-8261.2001.tb00979.x. PMid:11768518.
http://dx.doi.org/10.1111/j.1740-8261.20...
). Thus, according to the variation in eating habits among the lizards, it was possible to observe a difference in the thickness of muscle fibers in the muscle layer (Dehlawi and Zaher, 1989DEHLAWI, G.Y. and ZAHER, M., 1989. Histochemical localization of carbohydrates in the mucosal epithelium of the alimentary tract of the skink Mabuya brevicollis. Journal of King Abdul Aziz University, vol. 1, no. 1, pp. 113-124. http://dx.doi.org/10.4197/Sci.1-1.10.
http://dx.doi.org/10.4197/Sci.1-1.10...
; Hamdi et al., 2014HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395.
http://dx.doi.org/10.5829/idosi.wasj.201...
; Rodrigues-Sartori et al., 2011; Ahmed et al., 2009AHMED, Y.A., EL-HAFEZ, A.A.E. and ZAYED, A.E., 2009. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus. Journal of Veterinary Anatomy, vol. 2, no. 1, pp. 35-48. http://dx.doi.org/10.21608/jva.2009.45136.
http://dx.doi.org/10.21608/jva.2009.4513...
; Zaher et al., 2012ZAHER, M., EL-GHAREEB, A.W., HAMDI, H., ESSA, A. and LAHSIK, S., 2012. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Science Journal, vol. 9, pp. 84-104.).
Serotonin secreting cells (5-HT) are considered one of the major chemosensory cells of the GIT (Cheng et al., 2019CHENG, X., VOSS, U. and EKBLAD, E., 2019. A novel serotonin-containing tuft cell subpopulation in mouse intestine. Cell and Tissue Research, vol. 376, no. 2, pp. 189-197. http://dx.doi.org/10.1007/s00441-018-02988-3. PMid:30666535.
http://dx.doi.org/10.1007/s00441-018-029...
). In the amphibian and reptile oesophagus these cells can be observed distributed along their epithelium, however in birds and mammals these are not reported, due to the epithelial change, from ciliated columnar pseudostratified epithelium to stratified squamous epithelium. In I. iguana was possible to observe the presence of 5-HT cells in the epithelium of the entire oesophagus like observed in all studies in reptiles, indicating that the presence of these cells does not depend on food habit.
In the gastric glands in the stomach, the 5HT cells, may have a role in stimulating gastrointestinal mucosa secretion, expansion of blood vessels and smooth muscle contraction, therefore accelerating the movement of the digestive tract (Wang et al., 2007WANG, S.H., DONG, L., LUO, J.Y., GONG, J., LI, L., LU, X.L. and HAN, S.P., 2007. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum inpatients with irritable bowel syndrome. World Journal of Gastroenterology, vol. 13, no. 45, pp. 6041-6047. http://dx.doi.org/10.3748/wjg.v13.45.6041. PMid:18023097.
http://dx.doi.org/10.3748/wjg.v13.45.604...
). Despite being widely distributed in the stomach, the relative frequency may be different among reptiles. In I. iguana these were more frequent among the most developed glands in the cranial and medial parts of the stomach, as in Gekko japonicus (Huang and Wu, 2005HUANG, X.G. and WU, X.B., 2005. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World Journal of Gastroenterology, vol. 11, no. 35, pp. 5498-5505. http://dx.doi.org/10.3748/wjg.v11.i35.5498. PMid:16222743.
http://dx.doi.org/10.3748/wjg.v11.i35.54...
). However, in Eumeces chinensis, Sphenomorphus indicus and Eumeces elegans (Huang and Wu, 2005HUANG, X.G. and WU, X.B., 2005. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World Journal of Gastroenterology, vol. 11, no. 35, pp. 5498-5505. http://dx.doi.org/10.3748/wjg.v11.i35.5498. PMid:16222743.
http://dx.doi.org/10.3748/wjg.v11.i35.54...
) and Takydromus wolteri (Lee and Ku, 2004LEE, H.S. and KU, S.K., 2004. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae). Acta Histochemica, vol. 106, no. 2, pp. 171-178. http://dx.doi.org/10.1016/j.acthis.2003.10.008. PMid:15147638.
http://dx.doi.org/10.1016/j.acthis.2003....
) these were more abundant in the caudal part. These data can be related to a phylogenetic approach, since the Lacertidae and Scincidae are closer to each other when compared to Iguanidae and Gekkonidae.
Many studies suggest that the distribution pattern of SS-IR cells varies greatly in reptiles. SS has an inhibitory effect on the release of gastrin, thereby reducing stomach acid secretion (Friis-Hansen, 2007FRIIS-HANSEN, L., 2007. Lessons from the gastrin knockout mice. Regulatory Peptides, vol. 139, no. 1-3, pp. 5-2. http://dx.doi.org/10.1016/j.regpep.2006.12.008. PMid:17234279.
http://dx.doi.org/10.1016/j.regpep.2006....
). Consequently, somatostatin exerts a potent suppressive effect on gastric emptying (Van Op den bosch et al., 2009VAN OP DEN BOSCH, J., ADRIAENSEN, D., VAN NASSAUW, L. and TIMMERMANS, J.P., 2009. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regulatory Peptides, vol. 156, no. 1-3, pp. 1-8. http://dx.doi.org/10.1016/j.regpep.2009.04.003. PMid:19362110.
http://dx.doi.org/10.1016/j.regpep.2009....
). In the oesophagus of the green iguana, as well as the T. wolteri (Lee and Ku, 2004LEE, H.S. and KU, S.K., 2004. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae). Acta Histochemica, vol. 106, no. 2, pp. 171-178. http://dx.doi.org/10.1016/j.acthis.2003.10.008. PMid:15147638.
http://dx.doi.org/10.1016/j.acthis.2003....
) and E. kingii (Arena et al., 1990ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.), SS-IR cells were not detected. However, in M. quinquetaeniata (El-Salhy and Grimelius, 1981EL-SALHY, M. and GRIMELIUS, L., 1981. The endocrine cells of the gastrointestinal mucosa of a squamate reptile, the grass lizard (Mabuya quinquetaeniata). A histological and immunohistochemical study. Biomedical Research, vol. 2, no. 6, pp. 639-658. http://dx.doi.org/10.2220/biomedres.2.639.
http://dx.doi.org/10.2220/biomedres.2.63...
) SS-IR cells were observed in the distal region of the oesophagus. In the stomach, SS-IR cells are predominant in the pylorus region in T. wolteri (Lee and Ku, 2004LEE, H.S. and KU, S.K., 2004. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae). Acta Histochemica, vol. 106, no. 2, pp. 171-178. http://dx.doi.org/10.1016/j.acthis.2003.10.008. PMid:15147638.
http://dx.doi.org/10.1016/j.acthis.2003....
), E. chinensis, S. indicus and E. elegans (Huang and Wu, 2005HUANG, X.G. and WU, X.B., 2005. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World Journal of Gastroenterology, vol. 11, no. 35, pp. 5498-5505. http://dx.doi.org/10.3748/wjg.v11.i35.5498. PMid:16222743.
http://dx.doi.org/10.3748/wjg.v11.i35.54...
). These results are in accordance with those seen in I. iguana, whose SS-IR cells occupy the stomach glandular region near the intestine. It indicates an important function of SS-IR cells in gastric emptying.
5. Conclusion
In I. iguana, variation was observed in terms of the distribution of mucus secretions and the pattern of occurrence of serotonin and somatostatin-secreting enteroendocrine cells in the TGI, which possibly will result in an interspecific adaptive response.
Acknowledgements
We thank Unidade Integrada de Patologia Especializada (UnIPE) for allowing us to use the Aperio CS™ scanner to obtain the digital imagens present in this article. We thank Amanda Ribeiro Ricardo Brito for assistance with the laboratory techniques and article translation, besides great encouragement. We thank Beatriz Gouvea de Luca for help in the laboratory and encouragement in the construction of this article.
References
- AHMED, Y.A., EL-HAFEZ, A.A.E. and ZAYED, A.E., 2009. Histological and histochemical studies on the esophagus, stomach and small intestines of Varanus niloticus Journal of Veterinary Anatomy, vol. 2, no. 1, pp. 35-48. http://dx.doi.org/10.21608/jva.2009.45136
» http://dx.doi.org/10.21608/jva.2009.45136 - ALLEN, A. and SNARY, D., 1972. The structure and function of gastric mucus. Gut, vol. 13, no. 8, pp. 666-672. http://dx.doi.org/10.1136/gut.13.8.666 PMid:4562023.
» http://dx.doi.org/10.1136/gut.13.8.666 - ANDRADE, C.A.F., 2009. Iguana verde (Iguana iguana). Bicho da Vez, no. 6, pp. 1-3.
- ARENA, P.C., RICHARDSON, K.C. and YAMADA, J., 1990. An immunohistochemical study of endocrine cells of the alimentary tract of the King’s skink (Egernia kingii). Journal of Anatomy, vol. 170, pp. 73-85. PMid:2254171.
- BANI, G., FORMIGLI, L. and CECCHI, R., 1992. Morphological observations on the glands of the oesophagus and stomach of adult Rana esculenta and Bombina variegata. Italian Journal of Anatomy and Embryology, vol. 97, no. 2, pp. 75-87. PMid:1285678.
- BREUIL, M., SCHIKORSKI, D., VUILLAUME, B., KRAUSS, U., MORTON, M.N., CORRY, E., BECH, N., JELIĆ, M. and GRANDJEAN, F., 2020. Painted black: Iguana melanoderma (Reptilia, Squamata, Iguanidae) a new melanistic endemic species from Saba and Montserrat islands (Lesser Antilles). ZooKeys, vol. 926, pp. 95-131. http://dx.doi.org/10.3897/zookeys.926.48679 PMid:32336922.
» http://dx.doi.org/10.3897/zookeys.926.48679 - ÇAKICI, Ö. and AKAT, E., 2013. Some histomorphological and histochemical characteristics of the digestive tract of the snake-eyed lizard, Ophisops elegans Menetries, 1832 (Squamata: lacertidae). North-Western Journal of Zoology, vol. 9, no. 2, pp. 257-263.
- CHENG, X., VOSS, U. and EKBLAD, E., 2019. A novel serotonin-containing tuft cell subpopulation in mouse intestine. Cell and Tissue Research, vol. 376, no. 2, pp. 189-197. http://dx.doi.org/10.1007/s00441-018-02988-3 PMid:30666535.
» http://dx.doi.org/10.1007/s00441-018-02988-3 - DEHLAWI, G.Y. and ZAHER, M., 1989. Histochemical localization of carbohydrates in the mucosal epithelium of the alimentary tract of the skink Mabuya brevicollis. Journal of King Abdul Aziz University, vol. 1, no. 1, pp. 113-124. http://dx.doi.org/10.4197/Sci.1-1.10
» http://dx.doi.org/10.4197/Sci.1-1.10 - DEHLAWI, G.Y. and ZAHER, M.M., 1985. Histological studies on the mucosal epithelium of the alimentary canal of the lizard Acanthodactylus boskianus (Family Lacertidae). Proceedings of the Zoological Society A.R. Egypt, vol. 9, pp. 67-90.
- DÍAZ, A.O., GARCÍA, A.M. and GOLDEMBERG, A.L., 2008. Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: A histochemical study. Acta Histochemica, vol. 110, no. 1, pp. 76-85. http://dx.doi.org/10.1016/j.acthis.2007.08.002 PMid:17945334.
» http://dx.doi.org/10.1016/j.acthis.2007.08.002 - ELLIOTT, J.R. 2007. Overview of reptile biology, anatomy, and histology. infectious diseases and pathology of reptiles In: J.R. ELLIOTT, ed. New York: Taylor & Francis Group, pp. 1-25.
- EL-SALHY, M. and GRIMELIUS, L., 1981. The endocrine cells of the gastrointestinal mucosa of a squamate reptile, the grass lizard (Mabuya quinquetaeniata). A histological and immunohistochemical study. Biomedical Research, vol. 2, no. 6, pp. 639-658. http://dx.doi.org/10.2220/biomedres.2.639
» http://dx.doi.org/10.2220/biomedres.2.639 - FALCÓN, W., ACKERMAN, J.D., RECART, W. and DAEHLER, C.C., 2013. Biology and Impacts of Pacific Island Invasive Species. 10. Iguana iguana, the Green Iguana (Squamata: iguanidae). Pacific Science, vol. 67, no. 2, pp. 157-186. http://dx.doi.org/10.2984/67.2.2
» http://dx.doi.org/10.2984/67.2.2 - FERRI, D., LIQUORI, G.E. and SCILLITANI, G., 1999. Morphological and histochemical variations of mucous and oxynticopeptic cells in the stomach of the seps, Chalcides chalcides. Journal of Anatomy, vol. 194, no. 1, pp. 71-77. http://dx.doi.org/10.1046/j.1469-7580.1999.19410071.x PMid:10227668.
» http://dx.doi.org/10.1046/j.1469-7580.1999.19410071.x - FERRI, D., LIQUORI, G.E., NATALE, L., SANTARELLI, G. and SCILLITANI, G., 2001. Mucin histochemistry of the digestive tract of the red-legged frog Rana aurora aurora. Acta Histochemica, vol. 103, no. 2, pp. 225-237. http://dx.doi.org/10.1078/0065-1281-00582 PMid:11368102.
» http://dx.doi.org/10.1078/0065-1281-00582 - FRIIS-HANSEN, L., 2007. Lessons from the gastrin knockout mice. Regulatory Peptides, vol. 139, no. 1-3, pp. 5-2. http://dx.doi.org/10.1016/j.regpep.2006.12.008 PMid:17234279.
» http://dx.doi.org/10.1016/j.regpep.2006.12.008 - GEORGE, L.L. and CASTRO, R.R.L., 1998. Histologia comparada 2. ed. São Paulo: Roca, 286 p.
- GUNDERSEN, H.J.G., 1977. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. Journal of Microscopy, vol. 111, no. 2, pp. 219-223. http://dx.doi.org/10.1111/j.1365-2818.1977.tb00062.x
» http://dx.doi.org/10.1111/j.1365-2818.1977.tb00062.x - GUPTA, B.L., 1989. The relationship of mucoid substances and ion and water transport, with new data on intestinal goblet cells and a model for gastric secretion. Symposia of the Society for Experimental Biology, vol. 43, pp. 81-110. PMid:2701492.
- HAMDI, H., EL-GHAREEB, A.W., ZAHER, M., ESSA, A. and LAHSIK, S., 2014. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: II-Chamaeleon africanus. World Applied Sciences Journal, vol. 30, no. 10, pp. 1306-1316. http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395
» http://dx.doi.org/10.5829/idosi.wasj.2014.30.10.82395 - HIRTH, H.F., 1963. Some apects of the natural history of Iguana iguana on a tropical strand. Ecology, vol. 44, no. 3, pp. 613-615. http://dx.doi.org/10.2307/1932553
» http://dx.doi.org/10.2307/1932553 - HUANG, X.G. and WU, X.B., 2005. Immunohistochemical study on gastrointestinal endocrine cells of four reptiles. World Journal of Gastroenterology, vol. 11, no. 35, pp. 5498-5505. http://dx.doi.org/10.3748/wjg.v11.i35.5498 PMid:16222743.
» http://dx.doi.org/10.3748/wjg.v11.i35.5498 - KARDONG, K.V., 2018. Vertebrates: comparative anatomy, function, evolution 8th ed. New York: McGraw-Hill Education, pp. 504-545.
- KIERNAN, J.A. 1990. Histological & histochemical methods: theory and practice 2nd ed. Frankfurt: Pergamon Press, 433 p.
- KING, G., 1996. Reptiles and herbivory London: Chapman & Hall, pp. 29-42.
- KOCA, Y.B. and GÜRCÜ, B., 2011. Morphological and histochemical investigations of esophagogastric tract of a lizard, Laudakia stellio (Agamidae, Linnaeus 1758). Acta Biologica Hungarica, vol. 62, no. 4, pp. 376-387. http://dx.doi.org/10.1556/ABiol.62.2011.4.4 PMid:22119867.
» http://dx.doi.org/10.1556/ABiol.62.2011.4.4 - KUBIAK, M., 2019. Veterinary care of green iguanas (Iguana iguana) part 1: husbandry. Companion Animal, vol. 24, no. 7, pp. 386-389. http://dx.doi.org/10.12968/coan.2019.0023
» http://dx.doi.org/10.12968/coan.2019.0023 - LARA-LÓPEZ, M.D.S. and GONZÁLEZ-ROMERO, A., 2002 [viewed 15 September 2020]. Alimentación de la iguana verde Iguana iguana (Squamata: Iguanidae) en La Mancha, Veracruz, México. Acta Zoológica Mexicana [online], vol. 85, pp. 139-152. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372002000100009&lng=es&nrm=iso
» http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372002000100009&lng=es&nrm=iso - LEE, H.S. and KU, S.K., 2004. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae). Acta Histochemica, vol. 106, no. 2, pp. 171-178. http://dx.doi.org/10.1016/j.acthis.2003.10.008 PMid:15147638.
» http://dx.doi.org/10.1016/j.acthis.2003.10.008 - LEE, J.H., KU, S.K. and LEE, H.S., 1999. An immunohistochemical study of endocrine cells in the alimentary tract of the snake, Rhabdophis tigrinus tigrinus. Korean Journal of Veterinary Research, vol. 39, no. 4, pp. 689-697.
- LILLIE, R.D. and FULLMER, H.M., 1976. Histopathologic technic and practical histochemistry 4th ed. New York: McGraw-Hill, pp. 559-610.
- LOO, S.K. and WONG, W.C., 1975. Histochemical observations on the mucins of the gastrointestinal tract in the toad (Bufo melanostictus). Cells, Tissues, Organs, vol. 91, no. 1, pp. 97-103. http://dx.doi.org/10.1159/000144374 PMid:1136708.
» http://dx.doi.org/10.1159/000144374 - LUPPA, H., 1977. Histology of the digestive tract. In: C. GANS and T.S. PARSONS, eds. Biology of the Reptilia New York: Academic Press, vol. 6, pp. 225-313.
- MACHADO-SANTOS, C., ZECA, S.G., ABIDU-FIGUEIREDO, M., RIBEIRO, I. and SALES, A., 2011. The esophagus of the crocodilian Caiman latirostris (Reptilia, Crocodylia): histological, histochemical and immunohistochemical study. Journal of Morphological Sciences, vol. 28, no. 2, pp. 113-119.
- MASYITHA, D., MAULIDAR, L., ZAINUDDIN, Z., SALIM, M.N., ALIZA, D., GANI, F.A. and RUSLI, R., 2020. Histology of watersnake (Enhydris Enhydris) digestive system. E3S Web of Conferences, vol. 151, 01052. http://dx.doi.org/10.1051/e3sconf/202015101052
» http://dx.doi.org/10.1051/e3sconf/202015101052 - O’MALLEY, B., 2005. Clinical anatomy and physiology of exotic species New York: Elsevier, pp. 57-75. https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7
» https://doi.org/10.1016/B978-0-7020-2782-6.X5001-7 - PIANKA, E.R. and VITT, L.J., 2003. Lizards: windows to the evolution of diversity Berkeley: University of California Press, pp. 141-170.
- RICKLEFS, R.E., 2003. A economia da natureza Rio de Janeiro: Guanabara-Koogan, 503 p.
- RODRIGUES SARTORI, S.S., NOGUEIRA, K.O.P.C., ROCHA, A.S. and NEVES, C.A., 2011. Morphology of the stomach of the tropical house gecko Hemidactylus mabouia (Squamata: gekkonidae). Acta Zoologica, vol. 92, no. 2, pp. 179-186. http://dx.doi.org/10.1111/j.1463-6395.2010.00451.x
» http://dx.doi.org/10.1111/j.1463-6395.2010.00451.x - RODRIGUES-SARTORI, S.S., NOGUEIRA, K.P.O.C., ARAÚJO, V.A. and NEVES, C.A., 2015. Functional morphology of the esophagus of the tropical house gecko Hemidactylus mabouia (Squamata: gekkonidae). Animal Biology, vol. 65, no. 2, pp. 177-191. http://dx.doi.org/10.1163/15707563-00002469
» http://dx.doi.org/10.1163/15707563-00002469 - ROVIRA, J., VILLARO, A.C., BODEGAS, M.E., VALVERDE, E. and SESMA, P., 1993. Structural study of the frog Rana temporaria larval stomach. Tissue & Cell, vol. 25, no. 5, pp. 695-707. http://dx.doi.org/10.1016/0040-8166(93)90051-L PMid:8296308.
» http://dx.doi.org/10.1016/0040-8166(93)90051-L - RYU, G.R., LEE, E., KIM, J.J., MOON, S.D., KO, S.H., AHN, Y.B. and SONG, K.H., 2018. Comparison of enteroendocrine cells and pancreatic β-cells using gene expression profiling and insulin gene methylation. PLoS One, vol. 13, no. 10, pp. e0206401. http://dx.doi.org/10.1371/journal.pone.0206401 PMid:30379923.
» http://dx.doi.org/10.1371/journal.pone.0206401 - SANTOS, C.M., NASCIMENTO, A.A., PERACCHI, A.L., SALES, A., MIKALAUSKAS, J.S. and GOUVEIA, S.F., 2008. Immunocytochemical study of gastrintestinal endocrine cells in insectivorous bats (Mammalia: chiroptera). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 68, no. 3, pp. 663-669. http://dx.doi.org/10.1590/S1519-69842008000300026 PMid:18833490.
» http://dx.doi.org/10.1590/S1519-69842008000300026 - SCHONHOFF, S.E., GIEL-MOLONEY, M. and LEITER, A.B., 2004. Minireview: development and differentiation of gut endocrine cells. Endocrinology, vol. 145, no. 6, pp. 2639-2644. http://dx.doi.org/10.1210/en.2004-0051 PMid:15044355.
» http://dx.doi.org/10.1210/en.2004-0051 - SMITH, D., DOBSON, H. and SPENCE, E., 2001. Gastrointestinal studies in the green iguana: technique and reference values. Veterinary Radiology & Ultrasound, vol. 42, no. 6, pp. 515-520. http://dx.doi.org/10.1111/j.1740-8261.2001.tb00979.x PMid:11768518.
» http://dx.doi.org/10.1111/j.1740-8261.2001.tb00979.x - SOLCIA, E., CAPELLA, C., VASSALLO, G. and BUFFA, R., 1975. Endocrine cells of the gastric mucosa. International Review of Cytology, vol. 42, pp. 223-286. http://dx.doi.org/10.1016/S0074-7696(08)60982-1 PMid:53215.
» http://dx.doi.org/10.1016/S0074-7696(08)60982-1 - SPINNER, L., 2018 [viewed 5 August 2020] Caring for the green iguana. Reptiles Magazine [online]. Available from: https://www.reptilesmagazine.com/caring-for-the-green-iguana/
» https://www.reptilesmagazine.com/caring-for-the-green-iguana/ - TELES, D.A., BRITO, S.V., TEIXEIRA, A.A.M., RIBEIRO, S.C., ARAUJO-FILHO, J.A., LIMA, V.F., PEREIRA, A.M.A. and ALMEIDA, W.O., 2017. Nematodes associated with Iguana iguana (Linnaeus, 1758) (Squamata, Iguanidae) in Semi-arid areas of Northeastern Brazil. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 77, no. 3, pp. 514-518. http://dx.doi.org/10.1590/1519-6984.17615 PMid:27683813.
» http://dx.doi.org/10.1590/1519-6984.17615 - THONGBOON, L., SENARAT, S., KETTRATAD, J., JIRAUNGKOORSKUL, W., WANGKULANGKUL, S., POOLPRASERT, P., PARA, C., KANEKO, G. and PENGSAKU, T., 2019. Gastrointestinal tract and accessory organs in the spotted bent-toed gecko, Cyrtodactylus peguensis (boulenger, 1893): a histological and histochemical study. Journal of Morphological Sciences, vol. 36, no. 4, pp. 223-230. http://dx.doi.org/10.1055/s-0039-1693021
» http://dx.doi.org/10.1055/s-0039-1693021 - TOWNSEND, J.H., SLAPCINSKY, J., KRYSKO, K.L., DONLAN, E.M. and GOLDEN, E.A., 2005. Predation of a Tree Snail Drymaeus multilineatus (Gastropoda: Bulimulidae) by Iguana iguana (Reptilia: Iguanidae) on Key Biscayne, Florida. Southeastern Naturalist, vol. 4, no. 2, pp. 361-364. http://dx.doi.org/10.1656/1528-7092(2005)004[0361:POATSD]2.0.CO;2
» http://dx.doi.org/10.1656/1528-7092(2005)004[0361:POATSD]2.0.CO;2 - TROYER, K., 1984. Structure and function of the digestive tract of a herbivorous lizard Iguana iguana Physiological Zoology, vol. 57, no. 1, pp. 1-8. http://dx.doi.org/10.1086/physzool.57.1.30155960
» http://dx.doi.org/10.1086/physzool.57.1.30155960 - TSCHANZ, S.A., BURRI, P.H. and WEIBEL, E.R., 2011. A simple tool for stereological assessment of digital images: the STEPanizer. Journal of Microscopy, vol. 243, no. 1, pp. 47-59. http://dx.doi.org/10.1111/j.1365-2818.2010.03481.x PMid:21375529.
» http://dx.doi.org/10.1111/j.1365-2818.2010.03481.x - UETZ, P., FREED, P. and HOŠEK, J., eds. 2020 [viewed 19 May 2020]. The Reptile Database [online]. Available from: http://www.reptile-database.org
» http://www.reptile-database.org - VAN OP DEN BOSCH, J., ADRIAENSEN, D., VAN NASSAUW, L. and TIMMERMANS, J.P., 2009. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regulatory Peptides, vol. 156, no. 1-3, pp. 1-8. http://dx.doi.org/10.1016/j.regpep.2009.04.003 PMid:19362110.
» http://dx.doi.org/10.1016/j.regpep.2009.04.003 - VIRGILIO, F., SCIARRILLO, R., DE FALCO, M., LAFORGIA, V., CAVAGNUOLO, A. and VARANO, L., 2004. Seasonality in thyroid function in chalcides ocellatus (reptilia, scincidae). The Italian Journal of Zoology, vol. 71, no. 2, pp. 53-57. http://dx.doi.org/10.1080/11250000409356606
» http://dx.doi.org/10.1080/11250000409356606 - WANG, S.H., DONG, L., LUO, J.Y., GONG, J., LI, L., LU, X.L. and HAN, S.P., 2007. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum inpatients with irritable bowel syndrome. World Journal of Gastroenterology, vol. 13, no. 45, pp. 6041-6047. http://dx.doi.org/10.3748/wjg.v13.45.6041 PMid:18023097.
» http://dx.doi.org/10.3748/wjg.v13.45.6041 - ZAHER, M., EL-GHAREEB, A.W., HAMDI, H., ESSA, A. and LAHSIK, S., 2012. Anatomical, histological and histochemical adaptations of the reptilian alimentary canal to their food habits: I. Uromastyx aegyptiaca. Life Science Journal, vol. 9, pp. 84-104.
- ZUG, G.R., VITT, L.J. and CALDWELL, J.P., 2001. Herpetology: an introductory biology of amphibians and reptiles 2nd ed. San Diego: Academic Press, pp. 123-146.
Publication Dates
-
Publication in this collection
21 June 2021 -
Date of issue
2023
History
-
Received
15 Sept 2020 -
Accepted
12 Jan 2021