Abstract
Cadmium (Cd) is one of the major toxicants, which affects human health through occupational and environmental exposure. In the current study, we evaluated the protective effects of morel mushrooms against Cd-induced reproductive damages in rats. For this purpose, 30 male rats were divided into 6 groups (n=5/group), the first group served as the control group, second group was treated with an intraperitoneal (i.p) injection of 1 mg/kg/day of Cd. Third and fourth groups were co-treated with 1 mg/kg/day of Cd (i.p) and 10 and 20 mg/kg/day of morel mushroom extract (orally) respectively. The final 2 groups received oral gavage of 10 and 20 mg/kg/day of morel mushroom extract alone. After treatment for 17 days, the animals were euthanized, and testes and epididymis were dissected out. One testis and epididymis of each animal were processed for histology, while the other testis and epididymis were used for daily sperm production (DSP) and comet assay. Our results showed that Cd and morel mushrooms have no effect on animal weight, but Cd significantly decreases the DSP count and damages the heritable DNA which is reversed in co-treatment groups. Similarly, the histopathological results of testes and epididymis show that morel mushrooms control the damage to these tissues. Whereas the morel mushroom extract alone could enhance the production of testosterone. These results conclude that morel mushrooms not only control the damage done by Cd, but it could also be used as a protection mechanism for heritable DNA damage.
Keywords:
cadmium toxicity; Morchella esculenta; herbal medicine; DNA damage; spermatogenesis
Resumo
O cádmio (Cd) é um dos principais tóxicos, que afeta a saúde humana por meio da exposição ocupacional e ambiental. No presente estudo, avaliamos os efeitos protetores dos cogumelos morel contra os danos reprodutivos induzidos pelo Cd em ratos. Para tanto, 30 ratos machos foram divididos em 6 grupos (n = 5 / grupo); o primeiro grupo serviu de controle, o segundo grupo foi tratado com injeção intraperitoneal (i.p) de 1 mg / kg / dia de Cd. O terceiro e o quarto grupos foram cotratados com 1 mg / kg / dia de Cd (i.p) e 10 e 20 mg / kg / dia de extrato de cogumelo morel (por via oral), respectivamente. Os dois grupos finais receberam gavagem oral de 10 e 20 mg / kg / dia de extrato de cogumelo morel sozinho. Após o tratamento por 17 dias, os animais foram sacrificados e os testículos e o epidídimo foram dissecados. Um testículo e epidídimo de cada animal foram processados para histologia, enquanto o outro testículo e epidídimo foram usados para produção diária de esperma (DSP) e ensaio cometa. Nossos resultados mostraram que os cogumelos Cd e morel não têm efeito sobre o peso do animal, mas o Cd diminui significativamente a contagem de DSP e danifica o DNA hereditário, que é revertido em grupos de cotratamento. Da mesma forma, os resultados histopatológicos dos testículos e do epidídimo mostram que os cogumelos morel controlam os danos a esses tecidos. Considerando que o extrato de cogumelo morel sozinho pode aumentar a produção de testosterona. Esses resultados concluem que os cogumelos morel não apenas controlam os danos causados pelo Cd, mas também podem ser usados como um mecanismo de proteção para danos hereditários ao DNA.
Palavras-chave:
toxicidade de cádmio; Morchella esculenta; fitoterapia; dano ao DNA; espermatogênese
1. Introduction
Heavy metal pollution is a challenging and ill-posed problem affecting the cellular physiology of living organisms (Cheng et al., 2019CHENG, Y., ZHANG, J., WU, T., JIANG, X., JIA, H., QING, S., AN, Q., ZHANG, Y. and SU, J., 2019. Reproductive toxicity of acute Cd exposure in mouse: resulting in oocyte defects and decreased female fertility. Toxicology and Applied Pharmacology, vol. 379, pp. 114684. http://dx.doi.org/10.1016/j.taap.2019.114684. PMid:31325558.
http://dx.doi.org/10.1016/j.taap.2019.11...
; Islam et al., 2018ISLAM, M.A., ROMIĆ, D., AKBER, M.A. and ROMIĆ, M., 2018. Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environmental Geochemistry and Health, vol. 40, no. 1, pp. 59-85. http://dx.doi.org/10.1007/s10653-017-9907-8. PMid:28101717.
http://dx.doi.org/10.1007/s10653-017-990...
; Qing et al., 2015QING, X., YUTONG, Z. and SHENGGAO, L., 2015. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicology and Environmental Safety, vol. 120, pp. 377-385. http://dx.doi.org/10.1016/j.ecoenv.2015.06.019. PMid:26114257.
http://dx.doi.org/10.1016/j.ecoenv.2015....
; Zhuang et al., 2009ZHUANG, P., MCBRIDE, M.B., XIA, H., LI, N. and LI, Z., 2009. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine. The Science of the Total Environment, vol. 407, no. 5, pp. 1551-1561. http://dx.doi.org/10.1016/j.scitotenv.2008.10.061. PMid:19068266.
http://dx.doi.org/10.1016/j.scitotenv.20...
). The modern world depends on industrialization to meet the demand for different daily used products, which results in the production of different toxicants as by-products. Mostly these by-product toxicants are disposed to environment. Among theses One important by-product toxicant is Cadmium (Cd), featured by its solubility in water, transferability, persistence and durability, universality, and severe toxicity (Li et al., 2015LI, P., LIN, C., CHENG, H., DUAN, X. and LEI, K., 2015. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicology and Environmental Safety, vol. 113, pp. 391-399. http://dx.doi.org/10.1016/j.ecoenv.2014.12.025. PMid:25540851.
http://dx.doi.org/10.1016/j.ecoenv.2014....
). Because of these properties, Cd is reported as the sixth most dangerous chemical for living things by the Agency of Toxic substances and Disease Registry (ATSDR) (Li et al., 2018LI, X., LIU, J., WU, S., ZHENG, W., LI, H., BAO, S., CHEN, Y., GUO, X., ZHANG, L. and GE, R.S., 2018. In utero single low-dose exposure of cadmium induces rat fetal Leydig cell dysfunction. Chemosphere, vol. 194, pp. 57-66. http://dx.doi.org/10.1016/j.chemosphere.2017.11.159. PMid:29197250.
http://dx.doi.org/10.1016/j.chemosphere....
; Ramelli et al., 2009RAMELLI, G.P., TADDEO, I., HERRMANN, U. and WEBER, P., 2009. V13 Poster location 013 Paroxysmal tonic upgaze of infancy: 5 additional cases. European Journal of Paediatric Neurology, vol. 13, suppl. 1, pp. S10. http://dx.doi.org/10.1016/S1090-3798(09)70033-9.
http://dx.doi.org/10.1016/S1090-3798(09)...
; Zhang et al., 2017ZHANG, W., WU, T., ZHANG, C., LUO, L., XIE, M. and HUANG, H., 2017. Cadmium exposure in newborn rats ovary induces developmental disorders of primordial follicles and the differential expression of SCF/c-kit gene. Toxicology Letters, vol. 280, pp. 20-28. http://dx.doi.org/10.1016/j.toxlet.2017.08.004. PMid:28801138.
http://dx.doi.org/10.1016/j.toxlet.2017....
). Despite these hazardous features of Cd and warning of ATSDR, the world health organization (WHO) reported that the quantity of Cd is increasing with human activities and accumulate in the air, soil, and water (WHO, 1960WORLD HEALTH ORGANIZATION – WHO, 1960. Exposure to cadmium: a major public health concern. Geneva: WHO, pp. 3-6.; Krzyzanowski and Cohen, 2008KRZYZANOWSKI, M. and COHEN, A., 2008. Update of WHO air quality guidelines. Air Quality, Atmosphere & Health, vol. 1, no. 1, pp. 7-13. http://dx.doi.org/10.1007/s11869-008-0008-9.
http://dx.doi.org/10.1007/s11869-008-000...
; Ramelli et al., 2009RAMELLI, G.P., TADDEO, I., HERRMANN, U. and WEBER, P., 2009. V13 Poster location 013 Paroxysmal tonic upgaze of infancy: 5 additional cases. European Journal of Paediatric Neurology, vol. 13, suppl. 1, pp. S10. http://dx.doi.org/10.1016/S1090-3798(09)70033-9.
http://dx.doi.org/10.1016/S1090-3798(09)...
; Trejo et al., 2016TREJO, N., MATUS, I., DEL POZO, A., WALTER, I. and HIRZEL, J., 2016. Cadmium phytoextraction capacity of white lupine (Lupinus albus L.) and narrow-leafed lupine (Lupinus angustifolius L.) in three contrasting agroclimatic conditions of Chile. Chilean Journal of Agricultural Research, vol. 76, no. 2, pp. 228-235. http://dx.doi.org/10.4067/S0718-58392016000200013.
http://dx.doi.org/10.4067/S0718-58392016...
) resulting in its adsorption by plants, which is diet source for human and animals (Rafati Rahimzadeh et al., 2017RAFATI RAHIMZADEH, M., RAFATI RAHIMZADEH, M., KAZEMI, S., and MOGHADAMNIA, A.A., 2017. Cadmium toxicity and treatment: an update. Caspian Journal of Internal Medicine, vol. 8, no. 3, pp. 135-145. http://dx.doi.org/10.22088/cjim.8.3.135. PMid:28932363.
http://dx.doi.org/10.22088/cjim.8.3.135...
; Faroon et al., 2012FAROON, O., ASHIZAWA, A., WRIGHT, S., TUCKER, P., JENKINS, K., INGERMAN, L. and RUDISILL, C., 2012. Toxicological profile for cadmium, Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles. Atlanta: Agency for Toxic Substances and Disease Registry.; Järup and Åkesson, 2009JÄRUP, L. and ÅKESSON, A., 2009. Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology, vol. 238, no. 3, pp. 201-208. http://dx.doi.org/10.1016/j.taap.2009.04.020. PMid:19409405.
http://dx.doi.org/10.1016/j.taap.2009.04...
; Ramelli et al., 2009RAMELLI, G.P., TADDEO, I., HERRMANN, U. and WEBER, P., 2009. V13 Poster location 013 Paroxysmal tonic upgaze of infancy: 5 additional cases. European Journal of Paediatric Neurology, vol. 13, suppl. 1, pp. S10. http://dx.doi.org/10.1016/S1090-3798(09)70033-9.
http://dx.doi.org/10.1016/S1090-3798(09)...
). With respect to food intake, in plant food sources Cd is found in cereals (wheat and rice), root vegetables (potato, celeriac, carrot), and green leafy vegetables, while in animals it is found in cephalopods, crabs, molluscs, crustaceans, and offal products of old animals. It is reported that food from plant sources has a higher concentration of Cd than dairy and poultry products (Järup and Åkesson, 2009JÄRUP, L. and ÅKESSON, A., 2009. Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology, vol. 238, no. 3, pp. 201-208. http://dx.doi.org/10.1016/j.taap.2009.04.020. PMid:19409405.
http://dx.doi.org/10.1016/j.taap.2009.04...
).
Recent studies have reported diverse toxic effects of Cd, including teratogenicity, oncogenicity, renal dysfunction, endocrine disruption, and reproductive toxicity (Bernard, 2008BERNARD, A., 2008. Cadmium & its adverse effects on human health. The Indian Journal of Medical Research, vol. 128, no. 4, pp. 557-564. PMid:19106447.; Zhang et al., 2017ZHANG, W., WU, T., ZHANG, C., LUO, L., XIE, M. and HUANG, H., 2017. Cadmium exposure in newborn rats ovary induces developmental disorders of primordial follicles and the differential expression of SCF/c-kit gene. Toxicology Letters, vol. 280, pp. 20-28. http://dx.doi.org/10.1016/j.toxlet.2017.08.004. PMid:28801138.
http://dx.doi.org/10.1016/j.toxlet.2017....
). According to some epidemiological studies, Cd and lead (Pb) have an equivocal effect on hormones concentration, sperm parameters, and male infertility (Benoff et al., 2000BENOFF, S., JACOB, A. and HURLEY, I.R., 2000. Male infertility and environmental exposure to lead and cadmium. Human Reproduction Update, vol. 6, no. 2, pp. 107-121. http://dx.doi.org/10.1093/humupd/6.2.107. PMid:10782569.
http://dx.doi.org/10.1093/humupd/6.2.107...
). In addition to environmental toxicants, climatic seasons and geographical locations greatly affect reproductive physiology (Becker and Berhane, 1997BECKER, S. and BERHANE, K., 1997. A meta-analysis of 61 sperm count studies revisited. Fertility and Sterility, vol. 67, no. 6, pp. 1103-1108. http://dx.doi.org/10.1016/S0015-0282(97)81446-X. PMid:9176451.
http://dx.doi.org/10.1016/S0015-0282(97)...
; Fisch and Goluboff, 1996FISCH, H. and GOLUBOFF, E.T., 1996. Geographic variations in sperm counts: a potential cause of bias in studies of semen quality. Fertility and Sterility, vol. 65, no. 5, pp. 1044-1046. http://dx.doi.org/10.1016/S0015-0282(16)58284-3. PMid:8612832.
http://dx.doi.org/10.1016/S0015-0282(16)...
; Paulsen et al., 1996PAULSEN, C.A., BERMAN, N.G. and WANG, C., 1996. Data from men in greater Seattle area reveals no downward trend in semen quality: further evidence that deterioration of semen quality is not geographically uniform. Fertility and Sterility, vol. 65, no. 5, pp. 1015-1020. http://dx.doi.org/10.1016/s0015-0282(16)58279-x. PMid:8612827.
http://dx.doi.org/10.1016/s0015-0282(16)...
; Šrám et al., 1996ŠRÁM, R.J., BENEŠ, I., BINKOVÁ, B., DEJMEK, J., HORSTMAN, D., KOTĚŠOVEC, F., OTTO, D., PERREAULT, S.D., RUBEŠ, J., SELEVAN, S.G., SKALÍK, I., STEVENS, R.K. and LEWTAS, J., 1996. Teplice Program - The impact of air pollution on human health. Environmental Health Perspectives, vol. 104, suppl. 4, pp. 699-714. http://dx.doi.org/10.1289/ehp.104-1469669. PMid:8879999.
http://dx.doi.org/10.1289/ehp.104-146966...
). But enough data is available which demonstrate that high concentration of heavy metals like Cd in the environment are associated with low semen quality (Danielsson et al., 1984DANIELSSON, B.R.G., DENCKER, L., LINDGREN, A. and TJÄLVE, H., 1984. Accumulation of toxic metals in male reproduction organs. In: P.L. CHAMBERS, P. PREZIOSI and C.M. CHAMBERS, eds. Disease, metabolism and reproduction in the toxic response to drugs and other chemicals. archives of toxicology. Berlin, Heidelberg: Springer, pp. 177-180.; Oldereid et al., 1993OLDEREID, N.B., THOMASSEN, Y., ATTRAMADAL, A., OLAISEN, B. and PURVIS, K., 1993. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. Journal of Reproduction and Fertility, vol. 99, no. 2, pp. 421-425. http://dx.doi.org/10.1530/jrf.0.0990421. PMid:8107024.
http://dx.doi.org/10.1530/jrf.0.0990421...
; Ragan and Mast, 1990RAGAN, H.A. and MAST, T.J., 1990. Cadmium inhalation and male reproductive toxicity. In: G.W. WARE, ed. Reviews of environmental contamination and toxicology. USA: Springer, pp. 1-22. http://dx.doi.org/10.1007/978-1-4612-3368-8_1.
http://dx.doi.org/10.1007/978-1-4612-336...
). The exact mechanism of Cd toxicity is not understood completely till now, but in the past few decades, different studies have reported major cellular toxicities including oxidative stress (Casalino et al., 2002CASALINO, E., CALZARETTI, G., SBLANO, C. and LANDRISCINA, C., 2002. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology, vol. 179, no. 1-2, pp. 37-50. http://dx.doi.org/10.1016/S0300-483X(02)00245-7. PMid:12204541.
http://dx.doi.org/10.1016/S0300-483X(02)...
; Hussain et al., 1987HUSSAIN, T., SHUKLA, G.S. and CHANDRA, S.V., 1987. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacology & Toxicology, vol. 60, no. 5, pp. 355-358. http://dx.doi.org/10.1111/j.1600-0773.1987.tb01526.x. PMid:3615346.
http://dx.doi.org/10.1111/j.1600-0773.19...
; Shukla et al., 1987SHUKLA, G.S., HUSSAIN, T. and CHANDRA, S.V., 1987. Possible Role of Regional Superoxide Dismutase Activity and Lipid. Life Sciences, vol. 41, no. 19, pp. 2215-2221. http://dx.doi.org/10.1016/0024-3205(87)90518-2. PMid:3669920.
http://dx.doi.org/10.1016/0024-3205(87)9...
), variation in thiol proteins (Chan and Cherian, 1992CHAN, H.M. and CHERIAN, M.G., 1992. Protective roles of metallothionein and glutathione in hepatotoxicity of cadmium. Toxicology, vol. 72, no. 3, pp. 281-290. http://dx.doi.org/10.1016/0300-483X(92)90179-I. PMid:1585382.
http://dx.doi.org/10.1016/0300-483X(92)9...
; Li et al., 1993LI, W., ZHAO, Y. and CHOU, I.N.. 1993. Alterations in cytoskeletal protein sulfhydryls and cellular glutathione in cultured cells exposed to cadmium and nickel ions. Toxicology, vol. 77, no. 1-2, pp. 65-79. http://dx.doi.org/10.1016/0300-483X(93)90138-I. PMid:8442019.
http://dx.doi.org/10.1016/0300-483X(93)9...
), inhibition of mitochondrial activity (Müller, 1986MÜLLER, L., 1986. Consequences of cadmium toxicity in rat hepatocytes: mitochondrial dysfunction and lipid peroxidation. Toxicology, vol. 40, no. 3, pp. 285-295. http://dx.doi.org/10.1016/0300-483X(86)90061-2. PMid:3750329.
http://dx.doi.org/10.1016/0300-483X(86)9...
), variation in membrane structure and function (Müller, 1986MÜLLER, L., 1986. Consequences of cadmium toxicity in rat hepatocytes: mitochondrial dysfunction and lipid peroxidation. Toxicology, vol. 40, no. 3, pp. 285-295. http://dx.doi.org/10.1016/0300-483X(86)90061-2. PMid:3750329.
http://dx.doi.org/10.1016/0300-483X(86)9...
; Shukla et al., 1987SHUKLA, G.S., HUSSAIN, T. and CHANDRA, S.V., 1987. Possible Role of Regional Superoxide Dismutase Activity and Lipid. Life Sciences, vol. 41, no. 19, pp. 2215-2221. http://dx.doi.org/10.1016/0024-3205(87)90518-2. PMid:3669920.
http://dx.doi.org/10.1016/0024-3205(87)9...
), damage to DNA structure (Coogan et al., 1992COOGAN, T.P., BARE, R.M. and WAALKES, M.P., 1992. Cadmium-induced DNA strand damage in cultured liver cells: reduction in cadmium genotoxicity following zinc pretreatment. Toxicology and Applied Pharmacology, vol. 113, no. 2, pp. 227-233. http://dx.doi.org/10.1016/0041-008X(92)90118-C. PMid:1561631.
http://dx.doi.org/10.1016/0041-008X(92)9...
), expression of stress gene (Goering et al., 1993GOERING, P.L., FISHER, B.R. and KISH, C.L., 1993. Stress protein synthesis induced in rat liver by cadmium precedes hepatotoxicity. Toxicology and Applied Pharmacology, vol. 122, no. 1, pp. 139-148. http://dx.doi.org/10.1006/taap.1993.1181. PMid:8378928.
http://dx.doi.org/10.1006/taap.1993.1181...
; Wang and Templeton, 1998WANG, Z. and TEMPLETON, D.M., 1998. Induction of c-fos proto-oncogene in mesangial cells by cadmium. The Journal of Biological Chemistry, vol. 273, no. 1, pp. 73-79. http://dx.doi.org/10.1074/jbc.273.1.73. PMid:9417049.
http://dx.doi.org/10.1074/jbc.273.1.73...
) and variation in some enzymatic activities (Casalino et al., 2002CASALINO, E., CALZARETTI, G., SBLANO, C. and LANDRISCINA, C., 2002. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology, vol. 179, no. 1-2, pp. 37-50. http://dx.doi.org/10.1016/S0300-483X(02)00245-7. PMid:12204541.
http://dx.doi.org/10.1016/S0300-483X(02)...
, 2000CASALINO, E., CALZARETTI, G., SBLANO, C. and LANDRISCINA, C., 2000. Cadmium-dependent enzyme activity alteration is not imputable to lipid peroxidation. Archives of Biochemistry and Biophysics, vol. 383, no. 2, pp. 288-295. http://dx.doi.org/10.1006/abbi.2000.2056. PMid:11185565.
http://dx.doi.org/10.1006/abbi.2000.2056...
, 1997CASALINO, E., SBLANO, C. and LANDRISCINA, C., 1997. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Archives of Biochemistry and Biophysics, vol. 346, no. 2, pp. 171-179. https://doi.org/10.1006/abbi.1997.0197.
https://doi.org/10.1006/abbi.1997.0197...
; Jay et al., 1991JAY, D., ZAMORANO, R., MUÑOZ, E., GLEASON, R. and BOLDU, J.L., 1991. Study of the interaction of cadmium with membrane-bound succinate dehydrogenase. Journal of Bioenergetics and Biomembranes, vol. 23, no. 2, pp. 381-389. http://dx.doi.org/10.1007/BF00762229. PMid:2050657.
http://dx.doi.org/10.1007/BF00762229...
; Manca et al., 1991MANCA, D., RICARD, A.C., TROTTIER, B. and CHEVALIER, G., 1991. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology, vol. 67, no. 3, pp. 303-323. http://dx.doi.org/10.1016/0300-483X(91)90030-5. PMid:1828634.
http://dx.doi.org/10.1016/0300-483X(91)9...
; Wätjen et al., 2001WÄTJEN, W., BENTERS, J., HAASE, H., SCHWEDE, F., JASTORFF, B. and BEYERSMANN, D., 2001. Zn2+ and Cd2+ increase the cyclic GMP level in PC12 cells by inhibition of the cyclic nucleotide phosphodiesterase. Toxicology, vol. 157, no. 3, pp. 167-175. http://dx.doi.org/10.1016/S0300-483X(00)00370-X. PMid:11164982.
http://dx.doi.org/10.1016/S0300-483X(00)...
)
For centuries Morchella Esculenta (ME) along with other species of Morchella has been used in Traditional Chinese Medicine (TCM) because of its active pharmacological constituents (Baati et al., 2011BAATI, T., HORCAJADA, P., GREF, R., COUVREUR, P. and SERRE, C., 2011. Quantification of fumaric acid in liver, spleen and urine by high-performance liquid chromatography coupled to photodiode-array detection. Journal of Pharmaceutical and Biomedical Analysis, vol. 56, no. 4, pp. 758-762. http://dx.doi.org/10.1016/j.jpba.2011.07.011. PMid:21820831.
http://dx.doi.org/10.1016/j.jpba.2011.07...
; Duncan et al., 2002DUNCAN, C.J.G., PUGH, N., PASCO, D.S. and ROSS, S.A., 2002. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. Journal of Agricultural and Food Chemistry, vol. 50, no. 20, pp. 5683-5685. http://dx.doi.org/10.1021/jf020267c. PMid:12236698.
http://dx.doi.org/10.1021/jf020267c...
; Halliwell, 2012HALLIWELL, B., 2012. Free radicals and antioxidants: updating a personal view. Nutrition Reviews, vol. 70, no. 5, pp. 257-265. http://dx.doi.org/10.1111/j.1753-4887.2012.00476.x. PMid:22537212.
http://dx.doi.org/10.1111/j.1753-4887.20...
, 2011HALLIWELL, B., 2011. Free radicals and antioxidants - Quo vadis? Trends in Pharmacological Sciences, vol. 32, no. 3, pp. 125-130. http://dx.doi.org/10.1016/j.tips.2010.12.002. PMid:21216018.
http://dx.doi.org/10.1016/j.tips.2010.12...
; Heleno et al., 2013HELENO, S.A., STOJKOVIĆ, D., BARROS, L., GLAMOČLIJA, J., SOKOVIĆ, M., MARTINS, A., QUEIROZ, M.J.R.P. and FERREIRA, I.C.F.R., 2013. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Research International, vol. 51, no. 1, pp. 236-243. http://dx.doi.org/10.1016/j.foodres.2012.12.020.
http://dx.doi.org/10.1016/j.foodres.2012...
; Meng et al., 2010MENG, F., ZHOU, B., LIN, R., JIA, L., LIU, X., DENG, P., FAN, K., WANG, G., WANG, L. and ZHANG, J., 2010. Extraction optimization and in vivo antioxidant activities of exopolysaccharide by Morchella esculenta SO-01. Bioresource Technology, vol. 101, no. 12, pp. 4564-4569. http://dx.doi.org/10.1016/j.biortech.2010.01.113. PMid:20153962.
http://dx.doi.org/10.1016/j.biortech.201...
; Raman, 2018RAMAN, V.K., 2018. Morchella Esculenta: a herbal boon to pharmacology. International Journal of Development Research, vol. 8, no. 3, pp. 19660-19665.). It is also used as medicine in Japan, Malaysia, India, and Pakistan for its aphrodisiac properties (Gewali, 2009GEWALI, M.B., 2009. Studies on the most traded medicinal plants from the Dolpa District of Nepal. Toyama: University of Toyama Repository, pp. 1-28.; Raman, 2018RAMAN, V.K., 2018. Morchella Esculenta: a herbal boon to pharmacology. International Journal of Development Research, vol. 8, no. 3, pp. 19660-19665.; Sud and Sud, 2017SUD, V.S. and SUD, V.K.S., 2017. A review of toxic effects and aphrodisiac action of Morchella Esculenta (Wild Morel- Guchhi Mushroom) - a Himalayan Delight. European Journal of Pharmaceutical and Medical Research, vol. 4, no. 8, pp. 726-730.). It is reported that the fruiting body of ME contains profound antitumor, anti-inflammatory, and antioxidant activity (Elmastas et al., 2006ELMASTAS, M., TURKEKUL, I., OZTURK, L., GULCIN, I., ISILDAK, O. and ABOUL-ENEIN, H., 2006. Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Combinatorial Chemistry & High Throughput Screening, vol. 9, no. 6, pp. 443-448. http://dx.doi.org/10.2174/138620706777698544. PMid:16842225.
http://dx.doi.org/10.2174/13862070677769...
; Nitha et al., 2010NITHA, B., DE, S., ADHIKARI, S.K., DEVASAGAYAM, T.P.A. and JANARDHANAN, K.K., 2010. Evaluation of free radical scavenging activity of morel mushroom, Morchella esculenta mycelia: A potential source of therapeutically useful antioxidants. Pharmaceutical Biology, vol. 48, no. 4, pp. 453-460. http://dx.doi.org/10.3109/13880200903170789. PMid:20645726.
http://dx.doi.org/10.3109/13880200903170...
; Nitha and Janardhanan, 2008NITHA, B. and JANARDHANAN, K.K., 2008. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food and Chemical Toxicology, vol. 46, no. 9, pp. 3193-3199. http://dx.doi.org/10.1016/j.fct.2008.07.007. PMid:18692113.
http://dx.doi.org/10.1016/j.fct.2008.07....
). To the best of our knowledge, no scientific data regarding its role in reproductive physiology is known.
Based on damages caused by Cd, and the presence of bioactive elements in ME, which could be used in controlling the oxidative in living organisms, the current study is designed to check the effects of Cadmium Chloride (CdCl2) administration on reproductive parameters of male rats and whether ME can reverse these effects.
2. Materials and Methods
2.1. Animals
Thirty adult male Sprague Dawley (SD) rats, having an average weight of 260 ± 45 grams, were obtained from animal house of College of Animal Sciences and Veterinary Medicine Jilin University Changchun, and were kept in stainless steel cages according to standard guidelines at controlled temperature (24 ± 2oC) and humidity (50-60%) for one week to acclimatize to lab environment. All the animals were kept at 12h/12h dark/light cycle and were fed with standard feed and had free access to water ad libitum. The whole experiment was approved by the ethical committee of Jilin University Changchun China (Permit Number SY201909012)
2.2. Chemicals
Cadmium chloride (CdCl2) was purchased from BDH Chemicals Ltd (pool, England)
2.3. Collection of ME and preparation of its extract
Morel Mushrooms (Marchella esculenta) (wild) was obtained from different fields of District Swat Pakistan. It was verified as Morel Mushrooms at the department of Plant sciences Quaid-i-Azam University Islamabad. After verification, the mushrooms were dried under shade and stored in humid free environment.
Ten days before the animal trials started the ME was weighted and mixed with ethanol. The ratio of dried ME and ethanol was roughly 1:3 in bottle. This mixture was on magnetic stirrer for one week.After one week this mixture was filtered using filter paper . The solvent was evaporated, and the remaining extract was weighed and used for further study.
2.4. Experimental design
Adult male rats were divided into six groups (n=5/group). The first group served as Control group and received intraperitoneal (i.p) injection of saline, three groups were treated with an i.p. injection of 1 mg/kg/day of Cd using CdCl2 solution. Among these 3 groups, 2 groups were co-treated with oral gavage of ME extract (one group was co-treated with 10mg/kg/day and the other with 20mg/kg/day of ME extract along with Cd). The remaining 2 groups received 10 and 20 mg/kg of ME extract alone respectively using an oral gavage (Figure 1). The exposure of Cd was for 17 days according to ATSDR, Cilenk, and our previous work (Çilenk et al., 2016ÇILENK, K.T., ÖZTÜRK, İ. and SÖNMEZ, M.F., 2016. Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Experimental and Molecular Pathology, vol. 101, no. 2, pp. 207-213. http://dx.doi.org/10.1016/j.yexmp.2016.08.004. PMid:27587086.
http://dx.doi.org/10.1016/j.yexmp.2016.0...
; Iqbal et al., 2021IQBAL, T., CAO, M., ZHAO, Z., ZHAO, Y., CHEN, L., CHEN, T., LI, C. and ZHOU, X., 2021. Damage to the testicular structure of rats by acute oral exposure of cadmium. International Journal of Environmental Research and Public Health, vol. 18, no. 11, pp. 6038. http://dx.doi.org/10.3390/ijerph18116038. PMid:34199704.
http://dx.doi.org/10.3390/ijerph18116038...
; Ramelli et al., 2009RAMELLI, G.P., TADDEO, I., HERRMANN, U. and WEBER, P., 2009. V13 Poster location 013 Paroxysmal tonic upgaze of infancy: 5 additional cases. European Journal of Paediatric Neurology, vol. 13, suppl. 1, pp. S10. http://dx.doi.org/10.1016/S1090-3798(09)70033-9.
http://dx.doi.org/10.1016/S1090-3798(09)...
). On day 18 (roughly 24 hours after last dose administration) the animals were anesthetized and euthanized according to guidelines of Jilin University. Blood plasma was collected for hormonal analysis, while reproductive organs (Testes and Epididymis) were dissected out and processed for tissue and sperm analysis. One of the testis and epididymis of each animal were fixed in 10% Formaldehyde for histological analysis, while the others were stored at -70oC until further analysis.
experimental design showing that the control group is treated with ip dosage of saline, 3 groups are treated with ip dosage of Cd, in which 2 received oral dosage of ME, and final 2 groups received oral gavage of ME alone (ME= Morchella esculenta).
Before storing or processing the organs, the volume of testes was measured in measuring cylinder using saline (37oC) as measuring liquid.
2.5. Daily sperm production (DSP)
The testes stored at -70oC were defrosted and used to calculate DSP according to Robb et al (Robb et al., 1978ROBB, G.W., AMANN, R.P. and KILLIAN, G.J., 1978. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Journal of Reproduction and Fertility, vol. 54, no. 1, pp. 103-107. http://dx.doi.org/10.1530/jrf.0.0540103. PMid:712697.
http://dx.doi.org/10.1530/jrf.0.0540103...
) with some modifications. Briefly, testes were weighted, tunica albuginea were removed and 90 mg of tissue was homogenized in 2ml of saline and diluted according to Jahan et al (Jahan et al., 2016JAHAN, S., REHMAN, S., ULLAH, H., MUNAWAR, A., AIN, Q.U. and IQBAL, T., 2016. Ameliorative effect of quercetin against arsenic-induced sperm DNA damage and daily sperm production in adult male rats. Drug and Chemical Toxicology, vol. 39, no. 3, pp. 290-296. http://dx.doi.org/10.3109/01480545.2015.1101772. PMid:26524343.
http://dx.doi.org/10.3109/01480545.2015....
), then 5.5µl of the sample was taken on Neubauer chamber (haemoctmeter), covered with a small coverslip, and late sperm cells were counted under a microscope at 40X magnification.. The DSP was counted using Formula 1
Formula for DSP
Where Y is the total number of spermatids, x is the number of spermatids counted on hemocytometer. 16 is the total number of squares observed, 100 is the total number of squares, 5 is dilution made, 5.5 µl was loaded on the haemocytometer, while 1000 is to convert µl into ml.
2.6. Histology
One testis and epididymis of each animal were fixed in 10% formaldehyde and dehydrated using different concentrations of ethanol before embedding in paraffin wax. Then 5-7µm thick sections were cut from the prepared paraffin blocks using a microtome. Sections were affixed on glass slides, deparaffinized, and stained with hematoxylin and eosin (H&E) stains. These slides were examined using microscope equipped with the micro-photographic system. Different parameters (diameter of seminiferous tubules, interstitial space, diameter of tubular lumen, tunica albuginea, and height of the epithelium) of seminiferous tubules were measured in the slides using image J software.
2.7. Quantitative determination of Testosterone concentration
Testosterone concentration in blood plasma was determined using Enzyme Linked Immuno Sorbant Assay (ELISA) kit. The ELISA kit was purchased from Amgenix, Burlingame, CA, USA. All samples were quantified in duplicate in a single assay.
2.8. Comet assay (single-cell gel electrophoresis (SCGE))
The DNA damage in sperm cells was determined using comet assay with some modifications (Ahmad et al., 2007AHMAD, L., JALALI, S., SHAMI, S.A. and AKRAM, Z., 2007. Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Archives of Andrology, vol. 53, no. 6, pp. 325-338. http://dx.doi.org/10.1080/01485010701730963. PMid:18357962.
http://dx.doi.org/10.1080/01485010701730...
). Briefly 100 µl of 1% regular melting point agarose was placed on glass slides and allowed to solidify. After that 20 µl of homogenate of cauda epididymis and 65 µl of low melting point- agarose were mixed and spread on the agarose-coated slides. After solidification, slides were submerged in lysing solution (100 mM EDTA Na2, 10mM Tris, 2.5M NaCl, pH 10, 1% triton X 100) overnight in dark (so that direct light doesn’t produce more DNA damage). In the final step of processing a horizontal gel electrophoresis tank was filled with electrophoresis solution (300mM NaOH, and 1mM EDTA, pH 12.5), and slides were placed in it with agarose end facing toward the positive terminal. The DNA fragments were separated by electrophoresis for 10 min at 300 mA and 25 V.
For fluorescent microscopy, 100-200 ml of 20mg/ml acridine orange solution was overlayed by using a coverslip. The comets were analyzed using Casplab_1.2.3b2.
2.9. Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey’s test was applied to all the experimental data for comparison of different groups by using Graphpad prism software. All the results are shown as mean ± SEM. The significance level was set at p < 0.05.
3. Results
3.1. Final weight of animals and reproductive organs
The mean final weight of animals in the control and all experimental groups, and the weight of testis, epididymis and volume of testes showed no significant variation (Table 1)
Mean ± SEM of I initial and final weight of rats, its testes and epididymis, volume of testis, and daily sperm production after treatment.
3.2. Daily Sperm Production (DSP/T x 106)
The mean value of daily sperm production in all the experimental groups showed significant change. The number of sperm in Cd treated group was significantly reduced (p <0.001), while non-significant increase was seen in co-treatment groups as compared to Cd group (Table 1). This indicates that ME plays a role in preventing (controlling) the damage caused by Cd to DSP. The number of sperm in the ME treated animals are significantly higher than the Cd treated group, but interestingly the results shows a slight decrease (non-significant) in DSP of ME treated group as compared to the Control. (Table 1)
3.3. Protective effect of Morel Mushrooms against Cd induced Damage to heritable DNA
Anomalies in the DNA of cauda epididymal sperms were measured by comet assay, the mean value of comet head length in Cd treated group showed significant (p <0,01) reduction when compared to the control group while the remaining parameters showed no significant variations from the control group (table 2, figure 2b)in both the cotreatment groups, no significant variations were noticed in head length of comet and percentage of DNA in head or tail (table 2). But the tail length was reduced significantly in both groups as compared to the control group (p <0.05 for Cd + 10 mg/kg extract and p < 0.01 for Cd + 20 mg/kg extract) (table 2, figure 2C &D). the 10 and 20mg/kg extract treated groups showed significant variation from the remaining groups. The head length in 10 mg extract alone group was significantly increased from Cd treated (p<0.001) and Cd +10mg extract treated (p<0.05) groups (table 2). The tail length in both the extract alone treated groups was significantly (p<0.01) reduced than that in the control group, while the quantity of DNA in head was significantly increased in both these groups as compared to the control and Cd treated groups, while the tail moment was also significantly reduced in both these groups when compared to the control group (figure 1, table 2)
DNA damage as expressed by different parameters in control, Cadmium, Cd + 10 mg of extract, Cd +20 mg extract, 10 mg extract and 20 mg extract treated groups.
Fluorescent photomicrograph of sperm DNA, using comet assay, stained with acridine orange. (A) control with more intact DNA; (B) cadmium group with comets; (C) Cd + 10 mg extract group showing intact DNA with a very short tail length; (D) Cd +20 mg extract group having tail of long length and intact DNA; (E) 10 mg extract alone group in which a tail could be notice but very lesser DNA damage was found; (F) 20 mg extract alone group having short tails and intact DNA.
3.4. Protective Effect of Morel Mushroom Against Cd induced Histopathological Changes
3.4.1. Testicular tissue
Parameters studied in testicular histology include the mean length of interstitial space, height of tunica albuginea, height of epithelium and diameter of seminiferous tubules and luminal diameter. All the tubules (in all 6 groups) could be devided into different categories based on there morphological appearance. We broadly devided all the tubules into 2 categories, first showing the first half (stage 1to 8) of spermatogenesis, and 2nd showing final half of spermatogenesis (stage 9-14). Table 3 is representing the average values of all the tubules, where we observed significant variation in all 5 parameters when we compared the Cd treated group, to control group, the interstitial space and lumen of the tubules was increased significantly (p<0.001), while the remaining three parameters showed significant reduction (Table 3, figure 3b). In both co-treatment groups, the interstitial space was similar to the control group, but significantly (p<0.001) increased from the Cd treated group. similarly, the thickness of tunica albuginea and diameter of the seminiferous tubules in co-treated groups were decreased (p<0.001) than the control group, but significantly (p<0.001) increased than the Cd treated group. The change in histological parameters of the extract treated groups were similar to the co-treatment groups (figure 3 E & F, Table 3).
Mean ± SEM of interstitial space (µm), tunica albugenia height (µm), seminiferous tubule diameter (µm), epithelial height (µm) and tubular lumen (µm) of rat’s testis in control, Cadmium, Cd + 10 mg of extract, Cd +20 mg extract, 10 mg extract and 20 mg extract treated groups.
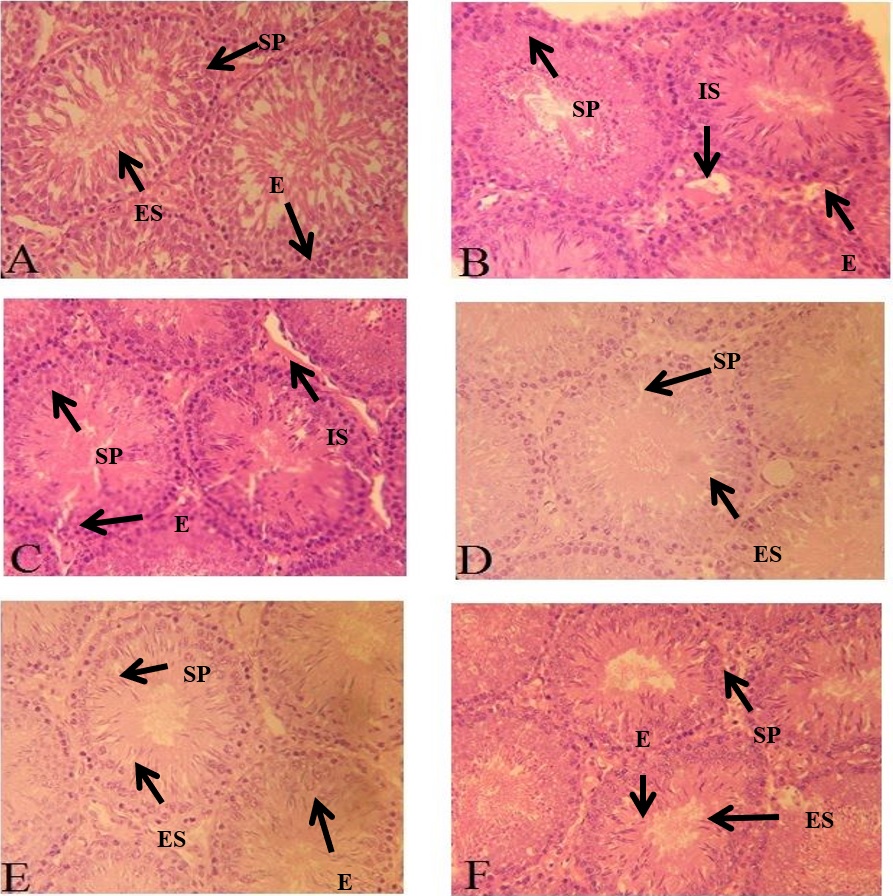
Photomicrograph of seminiferous tubules. (A) Control; showing compact tubules, filled lumen with spermatid, normal germ cell proliferation along epithelium; (B) Cd treated group; showing tubules with empty lumen and degenerated epithelial layer with increased interstitial space; (C) Cd+10 mg extract treated group & (D) Cd+ 20 mg extract treated group; showing minimal damage to epithelium, lumen filled with spermatid and less interstitial space; (E) 10 mg extract alone group and (F) 20 mg extract alone group; showing narrow lumen, increased epithelial height and compact tubules with less interstitial space. Magnification x40. Spermatogonia (SP), Elongated spermatids (ES), Interstitial space (IS), Epithelium (E).
3.4.2. Epididymis
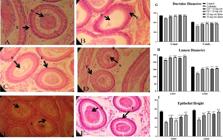
The histological analysis of caput and cauda epididymis showed that the ductular diameter along with its lumen diameter and height of the epithelium in the Cd treated group was significantly reduced as compared to all the remaining groups (figure 4). The co-treatment groups showed reduction in diameter of ducts and its lumen in comparison to the control, but non-significantly increase than the Cd treated group. (figure 4)
photomicrograph of cross section of epididymis (cauda) of rats (H&E, 40X) from: (A) Control group; showing normal morphology of cauda epididymis showing compactly arranged tubules with thick epithelium, lumen filled with sperm; (B) Cadmium group; showing marked changes in structure of tubule with decreased concentration of sperm; (C) Cd+10 mg extract group & (D) Cd+ 20 mg extract group; showing regular arrangement of tubules surrounded by stroma, lumen filled with spermatozoa; (E) 10 mg extract alone group and (F) 20 mg extract alone group ; showing increase in epithelium an lumen sperm concentration. Spermatozoa (S), Epithelium (E), Stroma (St). G, H and I summarizes the variations in tubule and lumen diameter and height of epithelium.
3.5 Effect of Cd and ME on blood plasma Testosterone level
The plasma testosterone level was reduced significantly (p<0.001) in Cd treated group as compared to the Control Group. Whereas in co-treatment groups, a decrease was noticed but only Cd + 20 mg group showed significant p<0.05) change when comparison was made with control. The testosterone level in both co-treated groups was significantly (p<0.001) higher that Cd group. While the testosterone level in both the extract alone groups was slightly higher that the control group, but the difference was not significant (Figure 5).
Mean ± SEM of plasma testosterone (ng/ml) concentration in rats of control, Cadmium, Cd + 10 mg of extract, Cd +20 mg extract, 10 mg extract and 20 mg extract group. (All values are expressed as Mean ± SEM) (*= P<0.05, ** P<0.01, *** P < 0.001, a=control, b= Cadmium, c= Cd+10 mg extract).
4. Discussion
Edible Mushrooms are the source of food and medicine for centuries (Wasser, 2002WASSER, S.P., 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, vol. 60, no. 3, pp. 258-274. http://dx.doi.org/10.1007/s00253-002-1076-7. PMid:12436306.
http://dx.doi.org/10.1007/s00253-002-107...
). These are considered as rich source of proteins and carbohydrates (Yun and Hall, 2004YUN, W. and HALL, I.R., 2004. Edible ectomycorrhizal mushrooms: challenges and achievements. Canadian Journal of Botany, vol. 82, no. 8, pp. 1063-1073. http://dx.doi.org/10.1139/b04-051.
http://dx.doi.org/10.1139/b04-051...
). In preterit past, the water-soluble contents in mushrooms were used as medicine. Specifically, the polysaccharides in mushrooms are reported to have immunomodulating and antitumor features (Borchers et al., 1999BORCHERS, A.T., STERN, J.S., HACKMAN, R.M., KEEN, C.L. and GERSHWIN, M.E., 1999. Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biology and Medicine, vol. 221, no. 4, pp. 281-293.; Wasser, 2002WASSER, S.P., 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, vol. 60, no. 3, pp. 258-274. http://dx.doi.org/10.1007/s00253-002-1076-7. PMid:12436306.
http://dx.doi.org/10.1007/s00253-002-107...
). The fruiting body of ME is rich in carbohydrates, protein, vitamins (B, A, C and D) and minerals, while low in calories and fat content (Mattila et al., 2001MATTILA, P., KÖNKÖ, K., EUROLA, M., PIHLAVA, J.M., ASTOLA, J., VAHTERISTO, L., HIETANIEMI, V., KUMPULAINEN, J., VALTONEN, M. and PIIRONEN, V., 2001. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. Journal of Agricultural and Food Chemistry, vol. 49, no. 5, pp. 2343-2348. http://dx.doi.org/10.1021/jf001525d. PMid:11368601.
http://dx.doi.org/10.1021/jf001525d...
; Negi, 2006NEGI, C., 2006. Morels (Morchella spp.) in Kumaun Himalaya. Indian Journal of Natural Products and Resources, vol. 5, no. 4, pp. 306-310.). The ME along with its dietary and pharmacological properties is also reported to be widely used for its aphrodisiac properties (Gewali, 2009GEWALI, M.B., 2009. Studies on the most traded medicinal plants from the Dolpa District of Nepal. Toyama: University of Toyama Repository, pp. 1-28.; Sud and Sud, 2017SUD, V.S. and SUD, V.K.S., 2017. A review of toxic effects and aphrodisiac action of Morchella Esculenta (Wild Morel- Guchhi Mushroom) - a Himalayan Delight. European Journal of Pharmaceutical and Medical Research, vol. 4, no. 8, pp. 726-730.). On the other side Cd is reported with opposite properties. In remote past It is suggested that different cells and pathways, are interfered by Cd particularly the pituitary-hypothalamus-gonadal pathway (Bertin and Averbeck, 2006BERTIN, G. and AVERBECK, D., 2006. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie, vol. 88, no. 11, pp. 1549-1559. http://dx.doi.org/10.1016/j.biochi.2006.10.001. PMid:17070979.
http://dx.doi.org/10.1016/j.biochi.2006....
; Cheng et al., 2019CHENG, Y., ZHANG, J., WU, T., JIANG, X., JIA, H., QING, S., AN, Q., ZHANG, Y. and SU, J., 2019. Reproductive toxicity of acute Cd exposure in mouse: resulting in oocyte defects and decreased female fertility. Toxicology and Applied Pharmacology, vol. 379, pp. 114684. http://dx.doi.org/10.1016/j.taap.2019.114684. PMid:31325558.
http://dx.doi.org/10.1016/j.taap.2019.11...
; Waisberg et al., 2003WAISBERG, M., JOSEPH, P., HALE, B. and BEYERSMANN, D., 2003. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, vol. 192, no. 2-3, pp. 95-117. http://dx.doi.org/10.1016/S0300-483X(03)00305-6. PMid:14580780.
http://dx.doi.org/10.1016/S0300-483X(03)...
). The cell proliferation, cell cycle progression, differentiation, the process of DNA repair and replication and the apoptotic pathways are all impaired (Cheng et al., 2019CHENG, Y., ZHANG, J., WU, T., JIANG, X., JIA, H., QING, S., AN, Q., ZHANG, Y. and SU, J., 2019. Reproductive toxicity of acute Cd exposure in mouse: resulting in oocyte defects and decreased female fertility. Toxicology and Applied Pharmacology, vol. 379, pp. 114684. http://dx.doi.org/10.1016/j.taap.2019.114684. PMid:31325558.
http://dx.doi.org/10.1016/j.taap.2019.11...
; Fang et al., 2002FANG, M., MAR, W. and CHO, M., 2002. Cadmium affects genes involved in growth regulation during two-stage transformation of Balb / 3T3 cells 177. Toxicology, vol. 177, no. 2-3, pp. 253-265. https://doi.org/10.1016/s0300-483x(02)00229-9.
https://doi.org/10.1016/s0300-483x(02)00...
; Oh and Lim, 2006OH, S. and LIM, S., 2006. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N -acetylcysteine-mediated catalase upregulation. Toxicology and Applied Pharmacology, vol. 212, no. 3, pp. 212-223. https://doi.org/10.1016/j.taap.2005.07.018.
https://doi.org/10.1016/j.taap.2005.07.0...
; Yang et al., 2004YANG, P.M., CHIU, S.J., LIN, K.A. and LIN, L.Y., 2004. Effect of cadmium on cell cycle progression in chinese hamster ovary cells. Chemico-Biological Interactions, vol. 149, no. 2-3, pp. 125-136. https://doi.org/10.1016/j.cbi.2004.08.001.
https://doi.org/10.1016/j.cbi.2004.08.00...
).
In previous literature, contradictory results have been reported about the effect of Cd on weight of organisms, where some articles had reported that Cd has negative effect on weight gain of organisms (Lynch et al., 1976LYNCH, G.P., SMITH, D.F.I., FISHER, M., PIKE, T.L. and WEINLAND, B.T., 1976. Physiological responses of calves to cadmium and lead. Journal of Animal Science, vol. 42, no. 2, pp. 410-421. http://dx.doi.org/10.2527/jas1976.422410x.
http://dx.doi.org/10.2527/jas1976.422410...
; Ren et al., 2019REN, Y., SHAO, W., ZUO, L., ZHAO, W., QIN, H., HUA, Y., LU, D., MI, C., ZENG, S. and ZU, L., 2019. Mechanism of cadmium poisoning on testicular injury in mice. Ondology Letters, vol. 18, no. 2, pp. 1035-1042. https://doi.org/10.3892/ol.2019.10418.
https://doi.org/10.3892/ol.2019.10418...
; Asagba et al., 2007ASAGBA, S.O., ADAIKPOH, M.A., KADIRI, H. and OBI, F.O., 2007. Influence of aqueous extract of Hibiscus sabdariffa L. petal on cadmium toxicity in rats. Biological Trace Element Research, vol. 115, no. 1, pp. 47-57. PMid:17406073.), but our findings are in accordance with Bebe and Panemangalore (1996)BEBE, F.N. and PANEMANGALORE, M., 1996. Modulation of tissue trace metal concentrations in weanling rats fed different levels of zinc and exposed to oral lead and cadmium. Nutrition Research, vol. 16, no. 8, pp. 1369-1380. https://doi.org/10.1016/j.fct.2004.05.001.
https://doi.org/10.1016/j.fct.2004.05.00...
, as there was no significant difference observed in weight gain of animals treated with Cd and the control group (Table 1). While looking at the nutritious components of ME (Halliwell, 2012HALLIWELL, B., 2012. Free radicals and antioxidants: updating a personal view. Nutrition Reviews, vol. 70, no. 5, pp. 257-265. http://dx.doi.org/10.1111/j.1753-4887.2012.00476.x. PMid:22537212.
http://dx.doi.org/10.1111/j.1753-4887.20...
; Raman, 2018RAMAN, V.K., 2018. Morchella Esculenta: a herbal boon to pharmacology. International Journal of Development Research, vol. 8, no. 3, pp. 19660-19665.) low calories and fat content, we observed no significant difference in weight gain of all ME extract treated groups from the control group.
In current study, a vital Cd generated reproductive damage in Cd treated group was observed, but the weight of testis, epididymis and volume of testis was almost similar in all the groups. In some previous studies (El-demerdash et al., 2004El-DEMERDASH, F.M., YOUSEF, M.I., KEDWANY, F.S. and BAGHDADI, H.H., 2004. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food and Chemical Toxicology, vol. 42, no. 10, pp. 1563-1571. https://doi.org/10.1016/j.fct.2004.05.001.
https://doi.org/10.1016/j.fct.2004.05.00...
; Asagba et al., 2007ASAGBA, S.O., ADAIKPOH, M.A., KADIRI, H. and OBI, F.O., 2007. Influence of aqueous extract of Hibiscus sabdariffa L. petal on cadmium toxicity in rats. Biological Trace Element Research, vol. 115, no. 1, pp. 47-57. PMid:17406073.; Santos et al., 2006SANTOS, F.W., GRAC, D.L., ZENI, G., WEIS, S.N., FAVERO, A.M. and NOGUEIRA, C.W., 2006. Sub-chronic administration of diphenyl diselenide potentiates cadmium-induced testicular damage in mice. Reproductive Toxicology, vol. 22, no. 3, pp. 546-550. https://doi.org/10.1016/j.reprotox.2005.12.009.
https://doi.org/10.1016/j.reprotox.2005....
) a huge impact of Cd is reported on reproductive and accessory sex organs along with weight of animals, although some studies suggest no or very less change on accessory organs (Predes et al., 2010PREDES, F.D.S., DIAMANTE, M.A.S. and DOLDER, H., 2010. Testis response to low doses of cadmium in Wistar rats. International Journal of Experimental Pathology, vol. 91, no. 2, pp. 125-131. https://doi.org/10.1111/j.1365-2613.2009.00692.x.
https://doi.org/10.1111/j.1365-2613.2009...
; Wade et al., 2002WADE, M.G., FOSTER, W.G., YOUNGLAI, E.V., MCMAHON, A., LEINGARTNER, K., YAGMINAS, A., BLAKEY, D., FOURNIER, M., DESAULNIERS, D. and HUGHES, C.L., 2002. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: systemic, immune, and reproductive effects. Toxicological Sciences: An Official Journal of the Society of Toxicology, vol. 67, no. 1, pp. 131-143. http://dx.doi.org/10.1093/toxsci/67.1.131.
http://dx.doi.org/10.1093/toxsci/67.1.13...
). These structural discrepancies are directly related to physiological problems(Sinha Hikim et al., 1988SINHA HIKIM, A.P., BARTKE, A. and RUSSELL, L.D., 1988. Morphometric studies on hamster testes in gonadally active and inactive states: light microscope findings. Biology of Reproduction, vol. 39, no. 5, pp. 1225-1237. http://dx.doi.org/10.1095/biolreprod39.5.1225. PMid:3219392.
http://dx.doi.org/10.1095/biolreprod39.5...
). To determine the protective effect of ME against the morphological damage caused by Cd, histopathology of testis and epididymis was performed and clear deformities in the seminiferous tubules of Cd treated animals were noted. Similar results were reported previously in case of reproductive toxicity caused by Cd (Adamkovicova et al., 2014ADAMKOVICOVA, M., TOMAN, R., CABAJ, M., MASSANYI, P., MARTINIAKOVA, M., OMELKA, R., KRAJCOVICOVA, V. and DURANOVA, H., 2014. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. TheScientificWorldJournal, vol. 2014, pp. 632581. http://dx.doi.org/10.1155/2014/632581. PMid:25548789.
http://dx.doi.org/10.1155/2014/632581...
; Afsar et al., 2018AFSAR, T., RAZAK, S., ALMAJWAL, A. and KHAN, M.R., 2018. Acacia hydaspica R. Parker ameliorates cisplatin induced oxidative stress, DNA damage and morphological alterations in rat pulmonary tissue. BMC Complementary and Alternative Medicine, vol. 18, no. 1, pp. 1-13. http://dx.doi.org/10.1186/s12906-018-2113-0. PMid:29394892.
http://dx.doi.org/10.1186/s12906-018-211...
; Sakr and Nooh, 2013SAKR, S.A. and NOOH, H.Z., 2013. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anatomy & Cell Biology, vol. 46, no. 2, pp. 122-130. http://dx.doi.org/10.5115/acb.2013.46.2.122. PMid:23869259.
http://dx.doi.org/10.5115/acb.2013.46.2....
; Wang et al., 2019WANG, H., ZHANG, R., SONG, Y., LI, T. and GE, M., 2019. Protective Effect of Ganoderma Triterpenoids on Cadmium-Induced Testicular Toxicity in Chickens. Biological Trace Element Research, vol. 187, no. 1, pp. 281-290. http://dx.doi.org/10.1007/s12011-018-1364-4. PMid:29717433.
http://dx.doi.org/10.1007/s12011-018-136...
). The co-treatment and extract alone treated groups showed interesting results as ME is never used before as treatment for heavy metal toxicity or improving the process of spermatogenesis. The use of medicinal herb and its extracts are repeatedly reported in Ayurvedic medicine (India), Unani medicine (Pakistan) and Chinese Traditional Medicine (TCM) for different purposes, (Mishra and Singh, 2016MISHRA, R.K. and SINGH, S.K., 2016. Biphasic effect of Syzygium aromaticum flower bud on reproductive physiology of male mice. Andrologia, vol. 48, no. 9, pp. 923-932. http://dx.doi.org/10.1111/and.12533. PMid:26840772.
http://dx.doi.org/10.1111/and.12533...
). Cd group showed expected results on DSP as discussed in earlier studies that morphological changes are directly related to physiological changes(Pires et al., 2013PIRES, V.C., GOLLÜCKE, A.P.B., RIBEIRO, D.A., LUNGATO, L., D’ALMEIDA, V. and AGUIAR JUNIOR, O., 2013. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: a chronic assay. The British Journal of Nutrition, vol. 110, no. 11, pp. 2020-2029. http://dx.doi.org/10.1017/S0007114513001360. PMid:23656754.
http://dx.doi.org/10.1017/S0007114513001...
). Additionally, Cd induced oxidative stress which is the vital reason of decrease in DSP (Wong and Cheng, 2011WONG, E.W.P. and CHENG, C.Y., 2011. Impacts of environmental toxicants on male reproductive dysfunction. Trends in Pharmacological Sciences, vol. 32, no. 5, pp. 290-299. http://dx.doi.org/10.1016/j.tips.2011.01.001. PMid:21324536.
http://dx.doi.org/10.1016/j.tips.2011.01...
). But in Co-treatment groups and ME extract alone group the sperm count is increased significantly as compared to the Cd group. According to Pires and his team, the low level of testosterone is related to injurious effects of Cd (Pires et al., 2013PIRES, V.C., GOLLÜCKE, A.P.B., RIBEIRO, D.A., LUNGATO, L., D’ALMEIDA, V. and AGUIAR JUNIOR, O., 2013. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: a chronic assay. The British Journal of Nutrition, vol. 110, no. 11, pp. 2020-2029. http://dx.doi.org/10.1017/S0007114513001360. PMid:23656754.
http://dx.doi.org/10.1017/S0007114513001...
). Results of current study showed that ME not only restored the damage done by CD, but also enhances the production of testosterone. And this might explain why in past people used ME as an aphrodisiac medicine.
5. Conclusion
The current study provides an evidence that ME control the damages caused by acute exposure of Cd to testicular tissue. But further study is require to select proper dose to completely control the damage. In past ME is used for its aphrodisiac properties, in current study the results of testosterone level could explain the aphrodisiac properties of ME. But further study are required in this regard.The results of this study explain the potential prospects of ME for the treatment of heavy metal toxicity.
6. Future Prospects
Based on findings of this article, the effects of ME on different reproductive system pathways including pituitary-hypothalamus-gonadal axis should be studied. This study provides basis for herbal treatment of reproductive impairments caused by heavy metals. Further studies are required to find underlying metabolic pathways and molecular mechanisms adopted by ME extract affecting reproduction.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31772596, 31872983 and 31672417); Jilin Key Program for Science and Technology Development (201903011008NY).
References
- ADAMKOVICOVA, M., TOMAN, R., CABAJ, M., MASSANYI, P., MARTINIAKOVA, M., OMELKA, R., KRAJCOVICOVA, V. and DURANOVA, H., 2014. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. TheScientificWorldJournal, vol. 2014, pp. 632581. http://dx.doi.org/10.1155/2014/632581 PMid:25548789.
» http://dx.doi.org/10.1155/2014/632581 - AFSAR, T., RAZAK, S., ALMAJWAL, A. and KHAN, M.R., 2018. Acacia hydaspica R. Parker ameliorates cisplatin induced oxidative stress, DNA damage and morphological alterations in rat pulmonary tissue. BMC Complementary and Alternative Medicine, vol. 18, no. 1, pp. 1-13. http://dx.doi.org/10.1186/s12906-018-2113-0 PMid:29394892.
» http://dx.doi.org/10.1186/s12906-018-2113-0 - AHMAD, L., JALALI, S., SHAMI, S.A. and AKRAM, Z., 2007. Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Archives of Andrology, vol. 53, no. 6, pp. 325-338. http://dx.doi.org/10.1080/01485010701730963 PMid:18357962.
» http://dx.doi.org/10.1080/01485010701730963 - ASAGBA, S.O., ADAIKPOH, M.A., KADIRI, H. and OBI, F.O., 2007. Influence of aqueous extract of Hibiscus sabdariffa L. petal on cadmium toxicity in rats. Biological Trace Element Research, vol. 115, no. 1, pp. 47-57. PMid:17406073.
- BAATI, T., HORCAJADA, P., GREF, R., COUVREUR, P. and SERRE, C., 2011. Quantification of fumaric acid in liver, spleen and urine by high-performance liquid chromatography coupled to photodiode-array detection. Journal of Pharmaceutical and Biomedical Analysis, vol. 56, no. 4, pp. 758-762. http://dx.doi.org/10.1016/j.jpba.2011.07.011 PMid:21820831.
» http://dx.doi.org/10.1016/j.jpba.2011.07.011 - BEBE, F.N. and PANEMANGALORE, M., 1996. Modulation of tissue trace metal concentrations in weanling rats fed different levels of zinc and exposed to oral lead and cadmium. Nutrition Research, vol. 16, no. 8, pp. 1369-1380. https://doi.org/10.1016/j.fct.2004.05.001
» https://doi.org/10.1016/j.fct.2004.05.001 - BECKER, S. and BERHANE, K., 1997. A meta-analysis of 61 sperm count studies revisited. Fertility and Sterility, vol. 67, no. 6, pp. 1103-1108. http://dx.doi.org/10.1016/S0015-0282(97)81446-X PMid:9176451.
» http://dx.doi.org/10.1016/S0015-0282(97)81446-X - BENOFF, S., JACOB, A. and HURLEY, I.R., 2000. Male infertility and environmental exposure to lead and cadmium. Human Reproduction Update, vol. 6, no. 2, pp. 107-121. http://dx.doi.org/10.1093/humupd/6.2.107 PMid:10782569.
» http://dx.doi.org/10.1093/humupd/6.2.107 - BERNARD, A., 2008. Cadmium & its adverse effects on human health. The Indian Journal of Medical Research, vol. 128, no. 4, pp. 557-564. PMid:19106447.
- BERTIN, G. and AVERBECK, D., 2006. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie, vol. 88, no. 11, pp. 1549-1559. http://dx.doi.org/10.1016/j.biochi.2006.10.001 PMid:17070979.
» http://dx.doi.org/10.1016/j.biochi.2006.10.001 - BORCHERS, A.T., STERN, J.S., HACKMAN, R.M., KEEN, C.L. and GERSHWIN, M.E., 1999. Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biology and Medicine, vol. 221, no. 4, pp. 281-293.
- CASALINO, E., SBLANO, C. and LANDRISCINA, C., 1997. Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Archives of Biochemistry and Biophysics, vol. 346, no. 2, pp. 171-179. https://doi.org/10.1006/abbi.1997.0197
» https://doi.org/10.1006/abbi.1997.0197 - CASALINO, E., CALZARETTI, G., SBLANO, C. and LANDRISCINA, C., 2000. Cadmium-dependent enzyme activity alteration is not imputable to lipid peroxidation. Archives of Biochemistry and Biophysics, vol. 383, no. 2, pp. 288-295. http://dx.doi.org/10.1006/abbi.2000.2056 PMid:11185565.
» http://dx.doi.org/10.1006/abbi.2000.2056 - CASALINO, E., CALZARETTI, G., SBLANO, C. and LANDRISCINA, C., 2002. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology, vol. 179, no. 1-2, pp. 37-50. http://dx.doi.org/10.1016/S0300-483X(02)00245-7 PMid:12204541.
» http://dx.doi.org/10.1016/S0300-483X(02)00245-7 - CHAN, H.M. and CHERIAN, M.G., 1992. Protective roles of metallothionein and glutathione in hepatotoxicity of cadmium. Toxicology, vol. 72, no. 3, pp. 281-290. http://dx.doi.org/10.1016/0300-483X(92)90179-I PMid:1585382.
» http://dx.doi.org/10.1016/0300-483X(92)90179-I - CHENG, Y., ZHANG, J., WU, T., JIANG, X., JIA, H., QING, S., AN, Q., ZHANG, Y. and SU, J., 2019. Reproductive toxicity of acute Cd exposure in mouse: resulting in oocyte defects and decreased female fertility. Toxicology and Applied Pharmacology, vol. 379, pp. 114684. http://dx.doi.org/10.1016/j.taap.2019.114684 PMid:31325558.
» http://dx.doi.org/10.1016/j.taap.2019.114684 - ÇILENK, K.T., ÖZTÜRK, İ. and SÖNMEZ, M.F., 2016. Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Experimental and Molecular Pathology, vol. 101, no. 2, pp. 207-213. http://dx.doi.org/10.1016/j.yexmp.2016.08.004 PMid:27587086.
» http://dx.doi.org/10.1016/j.yexmp.2016.08.004 - COOGAN, T.P., BARE, R.M. and WAALKES, M.P., 1992. Cadmium-induced DNA strand damage in cultured liver cells: reduction in cadmium genotoxicity following zinc pretreatment. Toxicology and Applied Pharmacology, vol. 113, no. 2, pp. 227-233. http://dx.doi.org/10.1016/0041-008X(92)90118-C PMid:1561631.
» http://dx.doi.org/10.1016/0041-008X(92)90118-C - DANIELSSON, B.R.G., DENCKER, L., LINDGREN, A. and TJÄLVE, H., 1984. Accumulation of toxic metals in male reproduction organs. In: P.L. CHAMBERS, P. PREZIOSI and C.M. CHAMBERS, eds. Disease, metabolism and reproduction in the toxic response to drugs and other chemicals. archives of toxicology Berlin, Heidelberg: Springer, pp. 177-180.
- DUNCAN, C.J.G., PUGH, N., PASCO, D.S. and ROSS, S.A., 2002. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. Journal of Agricultural and Food Chemistry, vol. 50, no. 20, pp. 5683-5685. http://dx.doi.org/10.1021/jf020267c PMid:12236698.
» http://dx.doi.org/10.1021/jf020267c - El-DEMERDASH, F.M., YOUSEF, M.I., KEDWANY, F.S. and BAGHDADI, H.H., 2004. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food and Chemical Toxicology, vol. 42, no. 10, pp. 1563-1571. https://doi.org/10.1016/j.fct.2004.05.001
» https://doi.org/10.1016/j.fct.2004.05.001 - ELMASTAS, M., TURKEKUL, I., OZTURK, L., GULCIN, I., ISILDAK, O. and ABOUL-ENEIN, H., 2006. Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Combinatorial Chemistry & High Throughput Screening, vol. 9, no. 6, pp. 443-448. http://dx.doi.org/10.2174/138620706777698544 PMid:16842225.
» http://dx.doi.org/10.2174/138620706777698544 - FANG, M., MAR, W. and CHO, M., 2002. Cadmium affects genes involved in growth regulation during two-stage transformation of Balb / 3T3 cells 177. Toxicology, vol. 177, no. 2-3, pp. 253-265. https://doi.org/10.1016/s0300-483x(02)00229-9
» https://doi.org/10.1016/s0300-483x(02)00229-9 - FAROON, O., ASHIZAWA, A., WRIGHT, S., TUCKER, P., JENKINS, K., INGERMAN, L. and RUDISILL, C., 2012. Toxicological profile for cadmium, Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles Atlanta: Agency for Toxic Substances and Disease Registry.
- FISCH, H. and GOLUBOFF, E.T., 1996. Geographic variations in sperm counts: a potential cause of bias in studies of semen quality. Fertility and Sterility, vol. 65, no. 5, pp. 1044-1046. http://dx.doi.org/10.1016/S0015-0282(16)58284-3 PMid:8612832.
» http://dx.doi.org/10.1016/S0015-0282(16)58284-3 - GEWALI, M.B., 2009. Studies on the most traded medicinal plants from the Dolpa District of Nepal Toyama: University of Toyama Repository, pp. 1-28.
- GOERING, P.L., FISHER, B.R. and KISH, C.L., 1993. Stress protein synthesis induced in rat liver by cadmium precedes hepatotoxicity. Toxicology and Applied Pharmacology, vol. 122, no. 1, pp. 139-148. http://dx.doi.org/10.1006/taap.1993.1181 PMid:8378928.
» http://dx.doi.org/10.1006/taap.1993.1181 - HALLIWELL, B., 2011. Free radicals and antioxidants - Quo vadis? Trends in Pharmacological Sciences, vol. 32, no. 3, pp. 125-130. http://dx.doi.org/10.1016/j.tips.2010.12.002 PMid:21216018.
» http://dx.doi.org/10.1016/j.tips.2010.12.002 - HALLIWELL, B., 2012. Free radicals and antioxidants: updating a personal view. Nutrition Reviews, vol. 70, no. 5, pp. 257-265. http://dx.doi.org/10.1111/j.1753-4887.2012.00476.x PMid:22537212.
» http://dx.doi.org/10.1111/j.1753-4887.2012.00476.x - HELENO, S.A., STOJKOVIĆ, D., BARROS, L., GLAMOČLIJA, J., SOKOVIĆ, M., MARTINS, A., QUEIROZ, M.J.R.P. and FERREIRA, I.C.F.R., 2013. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Research International, vol. 51, no. 1, pp. 236-243. http://dx.doi.org/10.1016/j.foodres.2012.12.020
» http://dx.doi.org/10.1016/j.foodres.2012.12.020 - HUSSAIN, T., SHUKLA, G.S. and CHANDRA, S.V., 1987. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacology & Toxicology, vol. 60, no. 5, pp. 355-358. http://dx.doi.org/10.1111/j.1600-0773.1987.tb01526.x PMid:3615346.
» http://dx.doi.org/10.1111/j.1600-0773.1987.tb01526.x - IQBAL, T., CAO, M., ZHAO, Z., ZHAO, Y., CHEN, L., CHEN, T., LI, C. and ZHOU, X., 2021. Damage to the testicular structure of rats by acute oral exposure of cadmium. International Journal of Environmental Research and Public Health, vol. 18, no. 11, pp. 6038. http://dx.doi.org/10.3390/ijerph18116038 PMid:34199704.
» http://dx.doi.org/10.3390/ijerph18116038 - ISLAM, M.A., ROMIĆ, D., AKBER, M.A. and ROMIĆ, M., 2018. Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environmental Geochemistry and Health, vol. 40, no. 1, pp. 59-85. http://dx.doi.org/10.1007/s10653-017-9907-8 PMid:28101717.
» http://dx.doi.org/10.1007/s10653-017-9907-8 - JAHAN, S., REHMAN, S., ULLAH, H., MUNAWAR, A., AIN, Q.U. and IQBAL, T., 2016. Ameliorative effect of quercetin against arsenic-induced sperm DNA damage and daily sperm production in adult male rats. Drug and Chemical Toxicology, vol. 39, no. 3, pp. 290-296. http://dx.doi.org/10.3109/01480545.2015.1101772 PMid:26524343.
» http://dx.doi.org/10.3109/01480545.2015.1101772 - JÄRUP, L. and ÅKESSON, A., 2009. Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology, vol. 238, no. 3, pp. 201-208. http://dx.doi.org/10.1016/j.taap.2009.04.020 PMid:19409405.
» http://dx.doi.org/10.1016/j.taap.2009.04.020 - JAY, D., ZAMORANO, R., MUÑOZ, E., GLEASON, R. and BOLDU, J.L., 1991. Study of the interaction of cadmium with membrane-bound succinate dehydrogenase. Journal of Bioenergetics and Biomembranes, vol. 23, no. 2, pp. 381-389. http://dx.doi.org/10.1007/BF00762229 PMid:2050657.
» http://dx.doi.org/10.1007/BF00762229 - KRZYZANOWSKI, M. and COHEN, A., 2008. Update of WHO air quality guidelines. Air Quality, Atmosphere & Health, vol. 1, no. 1, pp. 7-13. http://dx.doi.org/10.1007/s11869-008-0008-9
» http://dx.doi.org/10.1007/s11869-008-0008-9 - LI, P., LIN, C., CHENG, H., DUAN, X. and LEI, K., 2015. Contamination and health risks of soil heavy metals around a lead/zinc smelter in southwestern China. Ecotoxicology and Environmental Safety, vol. 113, pp. 391-399. http://dx.doi.org/10.1016/j.ecoenv.2014.12.025 PMid:25540851.
» http://dx.doi.org/10.1016/j.ecoenv.2014.12.025 - LI, W., ZHAO, Y. and CHOU, I.N.. 1993. Alterations in cytoskeletal protein sulfhydryls and cellular glutathione in cultured cells exposed to cadmium and nickel ions. Toxicology, vol. 77, no. 1-2, pp. 65-79. http://dx.doi.org/10.1016/0300-483X(93)90138-I PMid:8442019.
» http://dx.doi.org/10.1016/0300-483X(93)90138-I - LI, X., LIU, J., WU, S., ZHENG, W., LI, H., BAO, S., CHEN, Y., GUO, X., ZHANG, L. and GE, R.S., 2018. In utero single low-dose exposure of cadmium induces rat fetal Leydig cell dysfunction. Chemosphere, vol. 194, pp. 57-66. http://dx.doi.org/10.1016/j.chemosphere.2017.11.159 PMid:29197250.
» http://dx.doi.org/10.1016/j.chemosphere.2017.11.159 - LYNCH, G.P., SMITH, D.F.I., FISHER, M., PIKE, T.L. and WEINLAND, B.T., 1976. Physiological responses of calves to cadmium and lead. Journal of Animal Science, vol. 42, no. 2, pp. 410-421. http://dx.doi.org/10.2527/jas1976.422410x
» http://dx.doi.org/10.2527/jas1976.422410x - MANCA, D., RICARD, A.C., TROTTIER, B. and CHEVALIER, G., 1991. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology, vol. 67, no. 3, pp. 303-323. http://dx.doi.org/10.1016/0300-483X(91)90030-5 PMid:1828634.
» http://dx.doi.org/10.1016/0300-483X(91)90030-5 - MATTILA, P., KÖNKÖ, K., EUROLA, M., PIHLAVA, J.M., ASTOLA, J., VAHTERISTO, L., HIETANIEMI, V., KUMPULAINEN, J., VALTONEN, M. and PIIRONEN, V., 2001. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. Journal of Agricultural and Food Chemistry, vol. 49, no. 5, pp. 2343-2348. http://dx.doi.org/10.1021/jf001525d PMid:11368601.
» http://dx.doi.org/10.1021/jf001525d - MENG, F., ZHOU, B., LIN, R., JIA, L., LIU, X., DENG, P., FAN, K., WANG, G., WANG, L. and ZHANG, J., 2010. Extraction optimization and in vivo antioxidant activities of exopolysaccharide by Morchella esculenta SO-01. Bioresource Technology, vol. 101, no. 12, pp. 4564-4569. http://dx.doi.org/10.1016/j.biortech.2010.01.113 PMid:20153962.
» http://dx.doi.org/10.1016/j.biortech.2010.01.113 - MISHRA, R.K. and SINGH, S.K., 2016. Biphasic effect of Syzygium aromaticum flower bud on reproductive physiology of male mice. Andrologia, vol. 48, no. 9, pp. 923-932. http://dx.doi.org/10.1111/and.12533 PMid:26840772.
» http://dx.doi.org/10.1111/and.12533 - MÜLLER, L., 1986. Consequences of cadmium toxicity in rat hepatocytes: mitochondrial dysfunction and lipid peroxidation. Toxicology, vol. 40, no. 3, pp. 285-295. http://dx.doi.org/10.1016/0300-483X(86)90061-2 PMid:3750329.
» http://dx.doi.org/10.1016/0300-483X(86)90061-2 - NEGI, C., 2006. Morels (Morchella spp.) in Kumaun Himalaya. Indian Journal of Natural Products and Resources, vol. 5, no. 4, pp. 306-310.
- NITHA, B. and JANARDHANAN, K.K., 2008. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food and Chemical Toxicology, vol. 46, no. 9, pp. 3193-3199. http://dx.doi.org/10.1016/j.fct.2008.07.007 PMid:18692113.
» http://dx.doi.org/10.1016/j.fct.2008.07.007 - NITHA, B., DE, S., ADHIKARI, S.K., DEVASAGAYAM, T.P.A. and JANARDHANAN, K.K., 2010. Evaluation of free radical scavenging activity of morel mushroom, Morchella esculenta mycelia: A potential source of therapeutically useful antioxidants. Pharmaceutical Biology, vol. 48, no. 4, pp. 453-460. http://dx.doi.org/10.3109/13880200903170789 PMid:20645726.
» http://dx.doi.org/10.3109/13880200903170789 - OH, S. and LIM, S., 2006. A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N -acetylcysteine-mediated catalase upregulation. Toxicology and Applied Pharmacology, vol. 212, no. 3, pp. 212-223. https://doi.org/10.1016/j.taap.2005.07.018
» https://doi.org/10.1016/j.taap.2005.07.018 - OLDEREID, N.B., THOMASSEN, Y., ATTRAMADAL, A., OLAISEN, B. and PURVIS, K., 1993. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. Journal of Reproduction and Fertility, vol. 99, no. 2, pp. 421-425. http://dx.doi.org/10.1530/jrf.0.0990421 PMid:8107024.
» http://dx.doi.org/10.1530/jrf.0.0990421 - PAULSEN, C.A., BERMAN, N.G. and WANG, C., 1996. Data from men in greater Seattle area reveals no downward trend in semen quality: further evidence that deterioration of semen quality is not geographically uniform. Fertility and Sterility, vol. 65, no. 5, pp. 1015-1020. http://dx.doi.org/10.1016/s0015-0282(16)58279-x PMid:8612827.
» http://dx.doi.org/10.1016/s0015-0282(16)58279-x - PIRES, V.C., GOLLÜCKE, A.P.B., RIBEIRO, D.A., LUNGATO, L., D’ALMEIDA, V. and AGUIAR JUNIOR, O., 2013. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: a chronic assay. The British Journal of Nutrition, vol. 110, no. 11, pp. 2020-2029. http://dx.doi.org/10.1017/S0007114513001360 PMid:23656754.
» http://dx.doi.org/10.1017/S0007114513001360 - PREDES, F.D.S., DIAMANTE, M.A.S. and DOLDER, H., 2010. Testis response to low doses of cadmium in Wistar rats. International Journal of Experimental Pathology, vol. 91, no. 2, pp. 125-131. https://doi.org/10.1111/j.1365-2613.2009.00692.x
» https://doi.org/10.1111/j.1365-2613.2009.00692.x - QING, X., YUTONG, Z. and SHENGGAO, L., 2015. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicology and Environmental Safety, vol. 120, pp. 377-385. http://dx.doi.org/10.1016/j.ecoenv.2015.06.019 PMid:26114257.
» http://dx.doi.org/10.1016/j.ecoenv.2015.06.019 - RAFATI RAHIMZADEH, M., RAFATI RAHIMZADEH, M., KAZEMI, S., and MOGHADAMNIA, A.A., 2017. Cadmium toxicity and treatment: an update. Caspian Journal of Internal Medicine, vol. 8, no. 3, pp. 135-145. http://dx.doi.org/10.22088/cjim.8.3.135 PMid:28932363.
» http://dx.doi.org/10.22088/cjim.8.3.135 - RAGAN, H.A. and MAST, T.J., 1990. Cadmium inhalation and male reproductive toxicity. In: G.W. WARE, ed. Reviews of environmental contamination and toxicology USA: Springer, pp. 1-22. http://dx.doi.org/10.1007/978-1-4612-3368-8_1
» http://dx.doi.org/10.1007/978-1-4612-3368-8_1 - RAMAN, V.K., 2018. Morchella Esculenta: a herbal boon to pharmacology. International Journal of Development Research, vol. 8, no. 3, pp. 19660-19665.
- RAMELLI, G.P., TADDEO, I., HERRMANN, U. and WEBER, P., 2009. V13 Poster location 013 Paroxysmal tonic upgaze of infancy: 5 additional cases. European Journal of Paediatric Neurology, vol. 13, suppl. 1, pp. S10. http://dx.doi.org/10.1016/S1090-3798(09)70033-9
» http://dx.doi.org/10.1016/S1090-3798(09)70033-9 - REN, Y., SHAO, W., ZUO, L., ZHAO, W., QIN, H., HUA, Y., LU, D., MI, C., ZENG, S. and ZU, L., 2019. Mechanism of cadmium poisoning on testicular injury in mice. Ondology Letters, vol. 18, no. 2, pp. 1035-1042. https://doi.org/10.3892/ol.2019.10418
» https://doi.org/10.3892/ol.2019.10418 - ROBB, G.W., AMANN, R.P. and KILLIAN, G.J., 1978. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Journal of Reproduction and Fertility, vol. 54, no. 1, pp. 103-107. http://dx.doi.org/10.1530/jrf.0.0540103 PMid:712697.
» http://dx.doi.org/10.1530/jrf.0.0540103 - SAKR, S.A. and NOOH, H.Z., 2013. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anatomy & Cell Biology, vol. 46, no. 2, pp. 122-130. http://dx.doi.org/10.5115/acb.2013.46.2.122 PMid:23869259.
» http://dx.doi.org/10.5115/acb.2013.46.2.122 - SANTOS, F.W., GRAC, D.L., ZENI, G., WEIS, S.N., FAVERO, A.M. and NOGUEIRA, C.W., 2006. Sub-chronic administration of diphenyl diselenide potentiates cadmium-induced testicular damage in mice. Reproductive Toxicology, vol. 22, no. 3, pp. 546-550. https://doi.org/10.1016/j.reprotox.2005.12.009
» https://doi.org/10.1016/j.reprotox.2005.12.009 - SHUKLA, G.S., HUSSAIN, T. and CHANDRA, S.V., 1987. Possible Role of Regional Superoxide Dismutase Activity and Lipid. Life Sciences, vol. 41, no. 19, pp. 2215-2221. http://dx.doi.org/10.1016/0024-3205(87)90518-2 PMid:3669920.
» http://dx.doi.org/10.1016/0024-3205(87)90518-2 - SINHA HIKIM, A.P., BARTKE, A. and RUSSELL, L.D., 1988. Morphometric studies on hamster testes in gonadally active and inactive states: light microscope findings. Biology of Reproduction, vol. 39, no. 5, pp. 1225-1237. http://dx.doi.org/10.1095/biolreprod39.5.1225 PMid:3219392.
» http://dx.doi.org/10.1095/biolreprod39.5.1225 - ŠRÁM, R.J., BENEŠ, I., BINKOVÁ, B., DEJMEK, J., HORSTMAN, D., KOTĚŠOVEC, F., OTTO, D., PERREAULT, S.D., RUBEŠ, J., SELEVAN, S.G., SKALÍK, I., STEVENS, R.K. and LEWTAS, J., 1996. Teplice Program - The impact of air pollution on human health. Environmental Health Perspectives, vol. 104, suppl. 4, pp. 699-714. http://dx.doi.org/10.1289/ehp.104-1469669 PMid:8879999.
» http://dx.doi.org/10.1289/ehp.104-1469669 - SUD, V.S. and SUD, V.K.S., 2017. A review of toxic effects and aphrodisiac action of Morchella Esculenta (Wild Morel- Guchhi Mushroom) - a Himalayan Delight. European Journal of Pharmaceutical and Medical Research, vol. 4, no. 8, pp. 726-730.
- TREJO, N., MATUS, I., DEL POZO, A., WALTER, I. and HIRZEL, J., 2016. Cadmium phytoextraction capacity of white lupine (Lupinus albus L.) and narrow-leafed lupine (Lupinus angustifolius L.) in three contrasting agroclimatic conditions of Chile. Chilean Journal of Agricultural Research, vol. 76, no. 2, pp. 228-235. http://dx.doi.org/10.4067/S0718-58392016000200013
» http://dx.doi.org/10.4067/S0718-58392016000200013 - WADE, M.G., FOSTER, W.G., YOUNGLAI, E.V., MCMAHON, A., LEINGARTNER, K., YAGMINAS, A., BLAKEY, D., FOURNIER, M., DESAULNIERS, D. and HUGHES, C.L., 2002. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: systemic, immune, and reproductive effects. Toxicological Sciences: An Official Journal of the Society of Toxicology, vol. 67, no. 1, pp. 131-143. http://dx.doi.org/10.1093/toxsci/67.1.131
» http://dx.doi.org/10.1093/toxsci/67.1.131 - WAISBERG, M., JOSEPH, P., HALE, B. and BEYERSMANN, D., 2003. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, vol. 192, no. 2-3, pp. 95-117. http://dx.doi.org/10.1016/S0300-483X(03)00305-6 PMid:14580780.
» http://dx.doi.org/10.1016/S0300-483X(03)00305-6 - WANG, H., ZHANG, R., SONG, Y., LI, T. and GE, M., 2019. Protective Effect of Ganoderma Triterpenoids on Cadmium-Induced Testicular Toxicity in Chickens. Biological Trace Element Research, vol. 187, no. 1, pp. 281-290. http://dx.doi.org/10.1007/s12011-018-1364-4 PMid:29717433.
» http://dx.doi.org/10.1007/s12011-018-1364-4 - WANG, Z. and TEMPLETON, D.M., 1998. Induction of c-fos proto-oncogene in mesangial cells by cadmium. The Journal of Biological Chemistry, vol. 273, no. 1, pp. 73-79. http://dx.doi.org/10.1074/jbc.273.1.73 PMid:9417049.
» http://dx.doi.org/10.1074/jbc.273.1.73 - WASSER, S.P., 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, vol. 60, no. 3, pp. 258-274. http://dx.doi.org/10.1007/s00253-002-1076-7 PMid:12436306.
» http://dx.doi.org/10.1007/s00253-002-1076-7 - WÄTJEN, W., BENTERS, J., HAASE, H., SCHWEDE, F., JASTORFF, B. and BEYERSMANN, D., 2001. Zn2+ and Cd2+ increase the cyclic GMP level in PC12 cells by inhibition of the cyclic nucleotide phosphodiesterase. Toxicology, vol. 157, no. 3, pp. 167-175. http://dx.doi.org/10.1016/S0300-483X(00)00370-X PMid:11164982.
» http://dx.doi.org/10.1016/S0300-483X(00)00370-X - WONG, E.W.P. and CHENG, C.Y., 2011. Impacts of environmental toxicants on male reproductive dysfunction. Trends in Pharmacological Sciences, vol. 32, no. 5, pp. 290-299. http://dx.doi.org/10.1016/j.tips.2011.01.001 PMid:21324536.
» http://dx.doi.org/10.1016/j.tips.2011.01.001 - WORLD HEALTH ORGANIZATION – WHO, 1960. Exposure to cadmium: a major public health concern Geneva: WHO, pp. 3-6.
- YANG, P.M., CHIU, S.J., LIN, K.A. and LIN, L.Y., 2004. Effect of cadmium on cell cycle progression in chinese hamster ovary cells. Chemico-Biological Interactions, vol. 149, no. 2-3, pp. 125-136. https://doi.org/10.1016/j.cbi.2004.08.001
» https://doi.org/10.1016/j.cbi.2004.08.001 - YUN, W. and HALL, I.R., 2004. Edible ectomycorrhizal mushrooms: challenges and achievements. Canadian Journal of Botany, vol. 82, no. 8, pp. 1063-1073. http://dx.doi.org/10.1139/b04-051
» http://dx.doi.org/10.1139/b04-051 - ZHANG, W., WU, T., ZHANG, C., LUO, L., XIE, M. and HUANG, H., 2017. Cadmium exposure in newborn rats ovary induces developmental disorders of primordial follicles and the differential expression of SCF/c-kit gene. Toxicology Letters, vol. 280, pp. 20-28. http://dx.doi.org/10.1016/j.toxlet.2017.08.004 PMid:28801138.
» http://dx.doi.org/10.1016/j.toxlet.2017.08.004 - ZHUANG, P., MCBRIDE, M.B., XIA, H., LI, N. and LI, Z., 2009. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine. The Science of the Total Environment, vol. 407, no. 5, pp. 1551-1561. http://dx.doi.org/10.1016/j.scitotenv.2008.10.061 PMid:19068266.
» http://dx.doi.org/10.1016/j.scitotenv.2008.10.061
Publication Dates
-
Publication in this collection
09 Aug 2021 -
Date of issue
2022
History
-
Received
11 May 2021 -
Accepted
30 June 2021