Abstract
The influence of pharmaceutical residues and heavy metals on living organisms has received global attention. The present study assessed the interactive effect of antibiotic residues and heavy metals in soil, as contaminated food with cadmium (Cd) and oxytetracycline (OTC) on the isopod Porcellio leavis. It was fed on fresh plant leaves contaminated with different concentrations of cadmium, Cd+OTC1000 ppm, Cd+OTC2000 ppm and Cd+OTC3000 ppm for 4 weeks. The changes in the feeding patterns, protein, lipid peroxidation (LPO), catalase activity (CAT), and total free amino acids (TFAA) were recorded. There were significant differences in the obtained results where Cd reduced the egestion ratio (ER) however, OTC enhanced this ratio. Biochemical analysis illustrated that combination between OTC and Cd inhibits the toxic effects of Cd at low concentration (1000 ppm), while at high concentration (3000 ppm) raise the toxicity. Detailed studies are required for further understanding of the interaction between OTC and heavy metals, and also its impact on soil animals and for improving soil risk evaluation.
Keywords:
isopoda; pharmaceutical residues; heavy metals; feeding patterns; biochemical responses
Resumo
A influência de resíduos farmacêuticos e metais pesados em organismos vivos tem recebido atenção global. O presente estudo avaliou o efeito de resíduos de antibióticos e metais pesados no solo, como alimentos contaminados com cádmio (Cd) e oxitetraciclina (OTC), sobre o isópode Porcellio laevis, o qual foi alimentado com folhas frescas de plantas contaminadas com diferentes concentrações de cádmio, Cd + OTC1000 ppm, Cd + OTC2000 ppm e Cd + OTC3000 ppm, por quatro semanas. As mudanças nos padrões de alimentação, proteína, peroxidação lipídica (LPO), atividade da catalase (CAT) e aminoácidos livres totais (TFAA) foram registradas. Houve diferenças significativas nos resultados obtidos, em que o Cd reduziu a taxa de excreção (ER), no entanto o OTC aumentou essa proporção. A análise bioquímica mostrou que a combinação entre OTC e Cd inibe os efeitos tóxicos do Cd em baixa concentração (1.000 ppm), enquanto, em alta concentração (3.000 ppm), aumenta a toxicidade. Estudos detalhados são necessários para uma maior compreensão da interação entre OTC e metais pesados, e seu impacto sobre os animais do solo, bem como para melhorar a avaliação de risco do solo.
Palavras-chave:
isópode; resíduos farmacêuticos; metais pesados; padrões de alimentação; respostas bioquímicas
1. Introduction
Pharmaceutical residues are chemical compounds, reaches water ways and soil, influencing human health and non-target animals causing severe problems (Volcão et al., 2020VOLCÃO, L.M., FRAGA, L.S., DE LIMA BRUM, R., DE MOURA, R.R., BERNARDI, E., RAMOS, D.F. and DA SILVA JÚNIOR, F.M.R., 2020. Toxicity of Biocide Formulations in the Soil to the Gut Community inBalloniscus selowiiBrandt, 1983 (Crustacea: Isopoda: Oniscidea). Water, Air, and Soil Pollution, vol. 231, no. 6, pp. 306. http://dx.doi.org/10.1007/s11270-020-04689-6.
http://dx.doi.org/10.1007/s11270-020-046...
). Additionally, some plants can transfer these chemicals from the soil to both human and animals through food chains (Tiwari et al., 2017TIWARI, B., SELLAMUTHU, B., OUARDA, Y., DROGUI, P., TYAGI, R.D. and BUELNA, G., 2017. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresource Technology, vol. 224, pp. 1-12. http://dx.doi.org/10.1016/j.biortech.2016.11.042. PMid:27889353.
http://dx.doi.org/10.1016/j.biortech.201...
; Nasr et al., 2020NASR, E.E., KHATER, Z.Z., ZELENAKOVA, M., VRANAYOVA, Z. and ABU-HASHIM, M., 2020. Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution. Sustainability, vol. 12, no. 12, pp. 4805. http://dx.doi.org/10.3390/su12124805.
http://dx.doi.org/10.3390/su12124805...
). Veterinary drugs can be introduced by various means to the ecosystem (Bártíková et al., 2016BÁRTÍKOVÁ, H., PODLIPNÁ, R. and SKÁLOVÁ, L., 2016. Veterinary drugs in the environment and their toxicity to plants. Chemosphere, vol. 144, pp. 2290-2301. http://dx.doi.org/10.1016/j.chemosphere.2015.10.137. PMid:26606183.
http://dx.doi.org/10.1016/j.chemosphere....
), such as soil fertilization using manure of animals.
Oxytetracycline (OTC) is a major one of the tetracycline, extensively used worldwide in veterinary medicines and feed additives. It cannot be completely absorbed in their intestine and up to 75% of OTC administered to animals will enter the environment by excretion and pollute the soil (Bao et al., 2013BAO, Y.Y., WAN, Y., ZHOU, Q.X., LI, W. and LIU, Y., 2013. Competitive adsorption and desorption of oxy-tetracycline and cadmium with different input loadings on cinnamon soil. Journal of Soils and Sediments, vol. 13, no. 2, pp. 364-374. http://dx.doi.org/10.1007/s11368-012-0600-3.
http://dx.doi.org/10.1007/s11368-012-060...
; Leston et al., 2014LESTON, S., NUNES, M., VIEGAS, I., NEBOT, C., CEPEDA, A., PARDAL, M.A. and RAMOS, F., 2014. The influence of sulfathiazole on the macroalgae Ulva lactuca. Chemosphere, vol. 100, pp. 105-110. http://dx.doi.org/10.1016/j.chemosphere.2013.12.038. PMid:24393561.
http://dx.doi.org/10.1016/j.chemosphere....
; Li et al., 2015LI, C., CHEN, J., WANG, J., MA, Z., HAN, P., LUAN, Y., LU, A., 2015. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Science of the Total Environment, vol. 521-522, n. 19, pp. 101-107. https://doi.org/110.1016/j.scitotenv.2015.1003.1070.
https://doi.org/110.1016/j.scitotenv.201...
). With the growth of intensive animal and poultry breeding programs and the large use of manure fertilizers, increasing the ecotoxicological effects of antibiotics on the surrounding environment (Thiele-Bruhn, 2005THIELE-BRUHN, S., 2005. Microbial inhibition by pharmaceutical antibiotics in different soils--dose-response relations determined with the iron (III) reduction test.Environmental toxicology and chemistry, vol. 24, no. 4, pp. 869–876. https://doi.org/10.1897/04-166r.1.
https://doi.org/10.1897/04-166r.1...
; Hammesfahr et al., 2008HAMMESFAHR, U., HEUER, H., MANZKE, B., SMALLA, K. and THIELE-BRUHN, S., 2008. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biology & Biochemistry, vol. 40, no. 7, pp. 1583-1591. http://dx.doi.org/10.1016/j.soilbio.2008.01.010.
http://dx.doi.org/10.1016/j.soilbio.2008...
; Liu et al., 2009LIU, F., YING, G.G., TAO, R., ZHAO, J.L., YANG, J.F. and ZHAO, L.F., 2009. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environmental Pollution, vol. 157, no. 5, pp. 1636-1642. http://dx.doi.org/10.1016/j.envpol.2008.12.021. PMid:19157661.
http://dx.doi.org/10.1016/j.envpol.2008....
).
OTC inhibits bacteria, actinomycetes, and total microorganism populations (Wang and Zhang, 2009WANG, L.P. and ZHANG, M.K., 2009. Effects of oxytetracycline pollution on soil biological properties. Nongye Huanjing Kexue Xuebao, vol. 28, no. 7, pp. 1434-1438.); decreases urease, sucrose phosphatase, as well as hydrogen peroxidase activity (Yao et al., 2010YAO, J.H., NIU, D.K., LI, Z.J., LIANG, Y.C. and ZHANG, S.Q., 2010. Effects of antibiotics oxytetracycline on soil enzyme activities and microbial biomass in wheat rhizosphere. Zhongguo Nong Ye Ke Xue, vol. 43, no. 4, pp. 721-728.); reduces root and shoot elongation in wheat and chinese cabbage (Jin et al., 2009JIN, C.X., LIU, J.J. and CHEN, Q.Y., 2009. Toxicological effects of veterinary drugs in soil on the inhibition of root elongation of wheat and Chinese cabbages. Nongye Huanjing Kexue Xuebao, vol. 28, no. 7, pp. 1358-1362.); and causes genotoxicity to earthworms (Dong et al., 2012DONG, L., GAO, J., XIE, X.J. and ZHOU, Q.X., 2012. DNA damage and biochemical toxicity of antibiotics in soil on the earthworm (Eisenia fetida). Chemosphere, vol. 89, no. 1, pp. 44-51. http://dx.doi.org/10.1016/j.chemosphere.2012.04.010. PMid:22647195.
http://dx.doi.org/10.1016/j.chemosphere....
).
Cadmium is known for its non-corrosive nature and one of the non-essential metals. Cd may antagonist essential trace elements and causes competition with nutrient elements by blocking binding sites of transport and storage proteins (Wright and Frain, 1981WRIGHT, D.A. and FRAIN, J.W., 1981. The effect of calcium on cadmium toxicity in the freshwater amphipod, Gammarus pulex (L.). Archives of Environmental Contamination and Toxicology, vol. 10, no. 3, pp. 321-328. http://dx.doi.org/10.1007/BF01055633. PMid:7259300.
http://dx.doi.org/10.1007/BF01055633...
). It is an extremely toxic trace element to the ecosystem that mainly comes from industrial processes and phosphate fertilizers (Liu et al., 2007LIU, Y., WANG, X., ZENG, G., QU, D., GU, J., ZHOU, M. and CHAI, L., 2007. Cadmium induced oxidative stress and response of the ascorbate–glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere, vol. 69, no. 1, pp. 99-107. http://dx.doi.org/10.1016/j.chemosphere.2007.04.040. PMid:17532363.
http://dx.doi.org/10.1016/j.chemosphere....
, 2013LIU, A.J., LIU, M., LI, M.H., MA, X.X., SUN, X.J. and WANG, H.H., 2013. Collaborative effects of Cu and antibiotic on soil microbial activities. Shengtai Huanjing Xuebao, vol. 22, no. 11, pp. 1825-1829.) and animal feeds are generally contain higher heavy metals (Kumar et al., 2013KUMAR, R.R., PARK, B.J. and CHO, J.Y., 2013. Application and environmental risks of livestock manure. Journal of the Korean Society for Applied Biological Chemistry, vol. 56, no. 5, pp. 497-503. http://dx.doi.org/10.1007/s13765-013-3184-8.
http://dx.doi.org/10.1007/s13765-013-318...
; Wang and Wei, 2013WANG, R. and WEI, Y.S., 2013. Pollution and control of tetracyclines and heavy metals residues in animal manure. Nongye Huanjing Kexue Xuebao, vol. 32, no. 9, pp. 1705-1719. and Adamse et al., 2017ADAMSE, P., VAN DER FELS-KLERX, H.J.I. and DE JONG, J., 2017. Cadmium, lead, mercury and arsenic in animal feed and feed materials–trend analysis of monitoring results. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, vol. 34, no. 8, pp. 1298-1311. http://dx.doi.org/10.1080/19440049.2017.1300686. PMid:28278122.
http://dx.doi.org/10.1080/19440049.2017....
). Thus, excessive levels of heavy metals are often passed to the soil and environment in feces. It reaches high levels in agricultural soil, and is easily absorbed by plants. These metals cannot be degenerated chemically or biologically; hence they form serious threats to the surrounding environment, food safety, and consequently human health (Olawoyin et al., 2012OLAWOYIN, R., OYEWOLE, S.A. and GRAYSON, R.L., 2012. Potential risk effect from elevated levels of soil heavy metals on human health in the Niger delta. Ecotoxicology and Environmental Safety, vol. 85, pp. 120-130. http://dx.doi.org/10.1016/j.ecoenv.2012.08.004. PMid:22921257.
http://dx.doi.org/10.1016/j.ecoenv.2012....
).
Terrestrial isopods (woodlice) are important detritivores in soil macrofauna (Schultz, 1982SCHULTZ, G.A., 1982. Four species of Alloniscus Dana, 1854, from the West coast of North America and Hawaii (Isopoda, Oniscoidea). Crustaceana, vol. 47, no. 2, pp. 149-168. http://dx.doi.org/10.1163/156854084X00388.
http://dx.doi.org/10.1163/156854084X0038...
). They greatly contribute to the breakdown of leaf litter and are an intrinsic part of the decomposition process of organic matter, which recycles essential nutrients for the soil and maintains its fertility (Wieser, 1979WIESER, W., 1979. The flow of copper through a terrestrial food web. In: J.O. NRIAGU, ed. Copper in the Environment. New York: John Wiley and Sons Inc., Part 1, pp. 325-335.; Hassall et al., 1987HASSALL, M., TURNER, T.G. and RANDS, M.R., 1987. Effects of terrestrial isopods on the decomposition of woodland leaf litter. Oecologia, vol. 72, no. 4, pp. 597-604. http://dx.doi.org/10.1007/BF00378988. PMid:28312524.
http://dx.doi.org/10.1007/BF00378988...
). The terrestrial isopods might be useful for monitoring bio-accumulation of such contaminants and can serve as bio-indicators of heavy metal pollution (Paoletti and Hassall, 1999PAOLETTI, M.G. and HASSALL, M., 1999. Woodlice (Isopoda: Oniscidea): their potential for assessing sustainability and use as bioindicators. Agriculture, Ecosystems & Environment, vol. 74, no. 1-3, pp. 157-165. http://dx.doi.org/10.1016/S0167-8809(99)00035-3.
http://dx.doi.org/10.1016/S0167-8809(99)...
). Pollution of soil in which these animals live and feed may decrease their number that consequently influences the flow of matter and energy through the food webs (Drobne, 1997DROBNE, D., 1997. Terrestrial isopods: a good choice for toxicity testing of pollutants in the terrestrial environment. Environmental Toxicology and Chemistry. International Journal (Toronto, Ont.), vol. 16, no. 6, pp. 1159-1164.). Additionally, presence of antibiotic and heavy metals in low concentrations for long periods results metal and antibiotic resistant bacteria (Zhang et al., 2005ZHANG, S.Q., ZHANG, F.D., LIU, X.M., WANG, Y.J., ZOU, S.W. and HE, X.S., 2005. Determination and analysis on main harmful composition in excrement of scale livestock and poultry feedlots. Zhiwu Yingyang Yu Feiliao Xuebao, vol. 11, no. 6, pp. 822-829.; De Souza et al., 2006DE SOUZA, M.J., NAIR, S., LOKA BHARATHI, P.A. and CHANDRAMOHAN, D., 2006. Metal and antibiotic-resistance in psychrotrophic bacteria from Antarctic Marine waters. Ecotoxicology (London, England), vol. 15, no. 4, pp. 379-384. http://dx.doi.org/10.1007/s10646-006-0068-2. PMid:16703457.
http://dx.doi.org/10.1007/s10646-006-006...
; Park et al., 2009PARK, K., BANG, H.W., PARK, J. and KWAK, I.S., 2009. Ecotoxicological multilevel-evaluation of the effects of fenbendazole exposure to Chironomus riparius larvae. Chemosphere, vol. 77, no. 3, pp. 359-367. http://dx.doi.org/10.1016/j.chemosphere.2009.07.019. PMid:19683327.
http://dx.doi.org/10.1016/j.chemosphere....
; Gao et al., 2015GAO, M., QI, Y., SONG, W. and ZHOU, Q., 2015. Biomarker analysis of combined oxytetracycline and zinc pollution in earthworms (Eisenia fetida). Chemosphere, vol. 139, pp. 229-234. http://dx.doi.org/10.1016/j.chemosphere.2015.06.059. PMid:26134676.
http://dx.doi.org/10.1016/j.chemosphere....
).
The combined effects of antibiotics and heavy metals on the feeding patterns need more investigations. Therefore, the current study aimed to assess the combined toxicity of OTC and Cd on Porcellio leavis in laboratory.
2. Materials and Methods
2.1. Sampling sites
Specimens of Porcellio leavis were collected randomly from mango orchard at Assiut University farm, Egypt, during November 2017.
2.2. Experimental design
The collected isopods were maintained in plastic containers for one week in the laboratory; they were fed on mango leaves. Animals were starved for 48 hours before experiment to allow the contents of the gut to be evacuated. They were checked for ovigerous females at the beginning of the experiment but it was not possible to know if females will give future juvenile or not. All isopods in the experiment were within the same size and weight range, so it was assumed to be the same age.
Cadmium solution 80 ppm and Oxytetracycline concentrations, 1000, 2000 and 3000 ppm were prepared and kept in the fridge at 4ºc. Every 15 isopods were placed in plastic boxes and divided into five groups. The experiment consists of control, cadmium treatment, T1 (Cd +1000 ppm OTC), T2 (Cd +2000 ppm OTC), and T3 (Cd +3000 ppm OTC) groups. The plastic boxes were supplied with a plaster substrate to maintain humidity necessary for the animal life. There were three replicates of control and each treatment.
Dry leaves of mango were fragmented into small pieces (1 cm2), every week; the fragmented leaves (2±1 g) were sprayed with the treatment solutions (9 ml), which prepared from 80 ppm Cd mixed with OTC solutions (1000, 2000 and 3000 ppm). The leaves supplied to control groups were sprayed with 9 ml of distilled water.
The experiment lasted 4 weeks. Dead individuals were removed daily along the experiment period. Every week, the animals were weighed and transferred to clean boxes and supplied with freshly sprayed food with treatment concentrations. The remaining food and fecal pellets were separated and weighed. Feces were collected, weighed weekly and stored in plastic bags to avoid coprophagy.
2.3. Feeding parameters calculations
At the end of the experiment, food (mango leave), animals and fecal pellets weights were used to calculate feeding parameters such as, food consumption, assimilation and egestion ratios and assimilation efficiency. These parameters were calculated weekly by the Equations (1, 2, 3, and 4) according to Abd El-Wakeil (2001ABD EL-WAKEIL, K.F., 2001. Effects of ecological factors and heavy metals on the distribution of soil Isopoda (Arthropoda, Crustacea) in Assiut, Egypt. Egypt: Assiut University, 150 p. M.Sc. Thesis., 2005ABD EL-WAKEIL, K.F., 2005. Ecotoxicolgical studies on terrestrial isopods (Crustacea) in Assiut, Egypt. Egypt: Assiut University, 271 p. Ph. D. Thesis.) as follows:
Where, CR: consumption rate, AR: assimilation rate, AE: assimilation efficiency, ER: egestion rate, Wf.wt: food weight, Wr.f.wt: remaining food weight, W isop: animal weight and Fp.wt: fecal pellets weight.
2.4. Biochemical analysis
The randomly chosen gut and heptopancreas of four woodlice from each experimental group were weighed and homogenized in ice-cold 50 mM K-phosphate buffer pH 7.4 to get 0.1% homogenate solution, centrifuged at (8000 g for 20 min) and supernatant of homogenates were used directly for the evaluation of biochemical parameters. Total protein concentration was determined using assay kits supplied by (spectrum diagnostics, Egypt) and expressed as mg/ml. The activity of catalase (CAT) was determined based on its ability to decompose H2O2 to H2O and O2 according to (Beers Junior and Sizer, 1952BEERS JUNIOR, R.F. and SIZER, I.W., 1952. A spectrophotometric methods for measuring the breakdown of hydrogen peroxide by catalase. The Journal of Biological Chemistry, vol. 195, no. 1, pp. 133-140. http://dx.doi.org/10.1016/S0021-9258(19)50881-X. PMid:14938361.
http://dx.doi.org/10.1016/S0021-9258(19)...
). Lipid peroxidation was indirectly measured according to the method of Ohkawa et al. (1979)OHKAWA, H., OHISHI, N. and YAGI, K., 1979. Assay for lipid peroxides in animal tissue by thiobarbaturic acid reaction. Analytical Biochemistry, vol. 95, no. 2, pp. 351-358. http://dx.doi.org/10.1016/0003-2697(79)90738-3. PMid:36810.
http://dx.doi.org/10.1016/0003-2697(79)9...
(thiobarbituric acid reactive substance, TBARS test). Lipid peroxidation (LPO) was done by the reaction of lipid radicals and oxygen to form peroxyl radicals (Powell, 2000POWELL, S.R., 2000. The antioxidant properties of zinc. The Journal of Nutrition, vol. 130, no. 5S, suppl., pp. 1447s-1454S. http://dx.doi.org/10.1093/jn/130.5.1447S. PMid:10801958.
http://dx.doi.org/10.1093/jn/130.5.1447S...
). Total free amino acids also are known as Ninhydrin positive substances were estimated by the Ninhydrin method using leucine as standard (Moor and stein, 1954).
2.5. Data analysis
The obtained data were statistically analyzed using SPSS software (Version 20) and Excel (Office 2010). After data was checked for normality, analysis of Variance (ANOVA) was used to test the differences between means of experimental groups followed by Duncan test to detect the distinct variances between means. While for the non-normality of the data, the non-parametric Kruskal-Wallis test was used to test the differences. Repeated measures analysis of variance was used to analyze the weekly changes in feeding parameters.
3. Results
3.1. Impact of Cd and OTC on mortality during the experiment
The average mortality of the isopod per week was represented in Table 1. Statistical analysis did not show significant differences in mortality among different groups (χ2 =2.98, P=0.561) and also among weeks (χ2 =1.119, P=0.772).
3.2. Impact of Cd and OTC on the feeding parameters in different treatments
Feeding behavior of P. leavis was influenced by concentration of pollutants. Feeding parameters of isopods in different groups was not significantly differs except egestion ratio was high significantly affected. Highly significant differences were noticed in all feeding parameters among weeks of study period. Consumption ratio showed highly significant difference in the interaction among treatments and weeks (Table 2).
Two-way multivariate analysis of variance (MANOVA) for feeding parameters of P. leavis treatment groups (P<0.05 is significant; P<0.01 is highly significant).
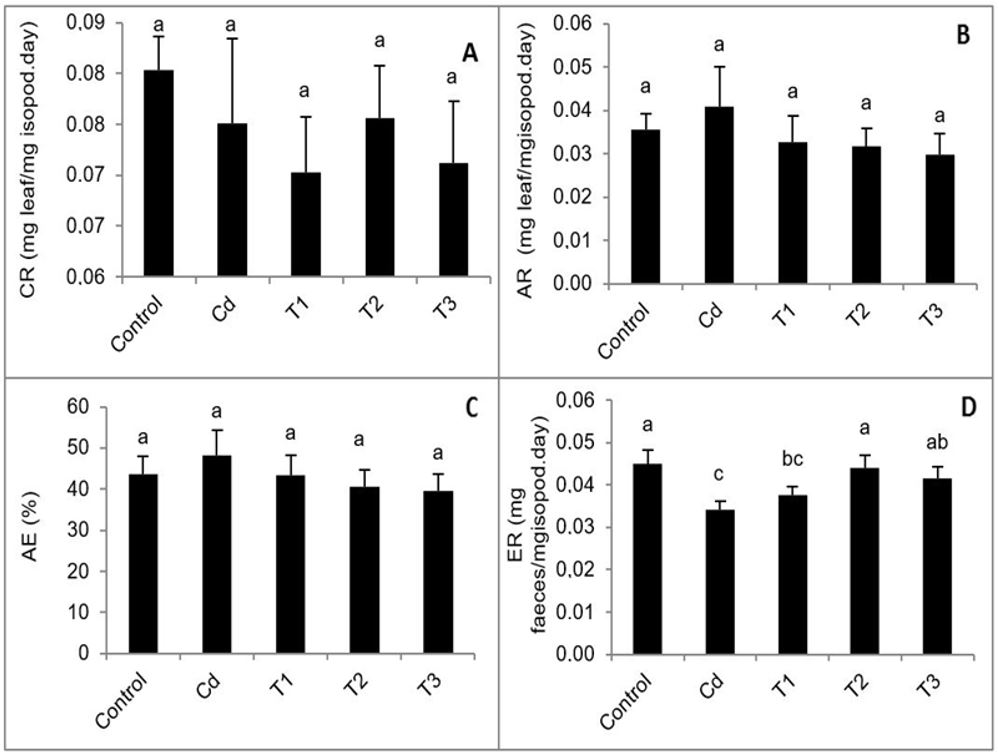
Obviously, the highest consumption ratio was observed in the control animal group. Non-significant decrease was observed in ingested Cd or Cd combined with different concentrations of OTC (Figure 1A). In contrast, the assimilation ratio recorded non-significant increase in animals fed on Cd-treated leaves then decreased in treatment combination with different concentrations of OTC compared to control (Figure 1B). Also assimilation efficient showed, non-significant increase in animals fed on Cd and decreased gradually with high concentrations of OTC compared to the control (Figure 1C). Similarly, the control animal group recorded the highest egestion ratio whereas there was a statistically significant decrease in animals fed on Cd, and combined Cd with 1000 ppm OTC (Figure 1D).
Difference between the means of feeding parameters; (A) consumption rate (CR), (B) assimilation rate (AR), (C) assimilation efficiency (AE), (D) egestion rate (ER) of control and treated isopod groups. Bars show mean ± SD (similar characters mean no significant difference).
3.3. Impact of Cd and OTC on the feeding parameters of P. leavis
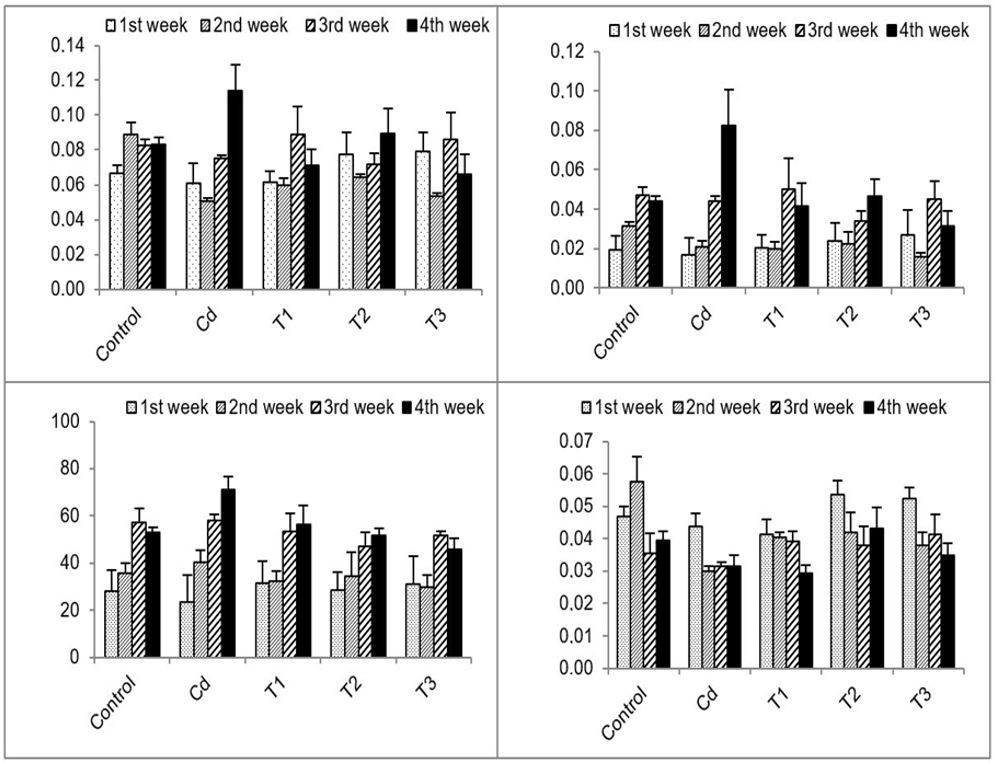
There was increase in CR, AR and AE, in the control group along the period of the experiment. The CR of the Cd treatment was fluctuating during the first three weeks then sharply increased in the fourth week, while each AR and AE in the Cd group gradually increased with time of exposure. The CR and AR of the T1 group recorded the highest increment in the third week and slightly decreased in the fourth week, while AE increased by time of exposure. In T2 group, all feeding parameters (CR, AR, and AE) decreased in the first weeks, and then upsurge occurred in the fourth week. In T3 group all parameters CR, AR, and AE attained the highest values in the third week and then decreased in the fourth week. ER in the control group showed the highest value in the second week and decreased with time. In the treated groups (Cd, T1, T2, and T3), ER showed high beak in the first week then recorded significant decrease in Cd, T2, and T3 and a non-significant decrease in T1 (Figure 2).
Histogram showing mean ± SD of Consumption Ratio (CR) (mg leaf/mg isopod. Day), AR (mg leaf/mg isopod. Day), AE % and ER (mg faeces/mg isopod. Day) of isopod Porcellio leavis in different weeks of study period.
3.4. Impact of Cd and OTC on the biochemical parameters
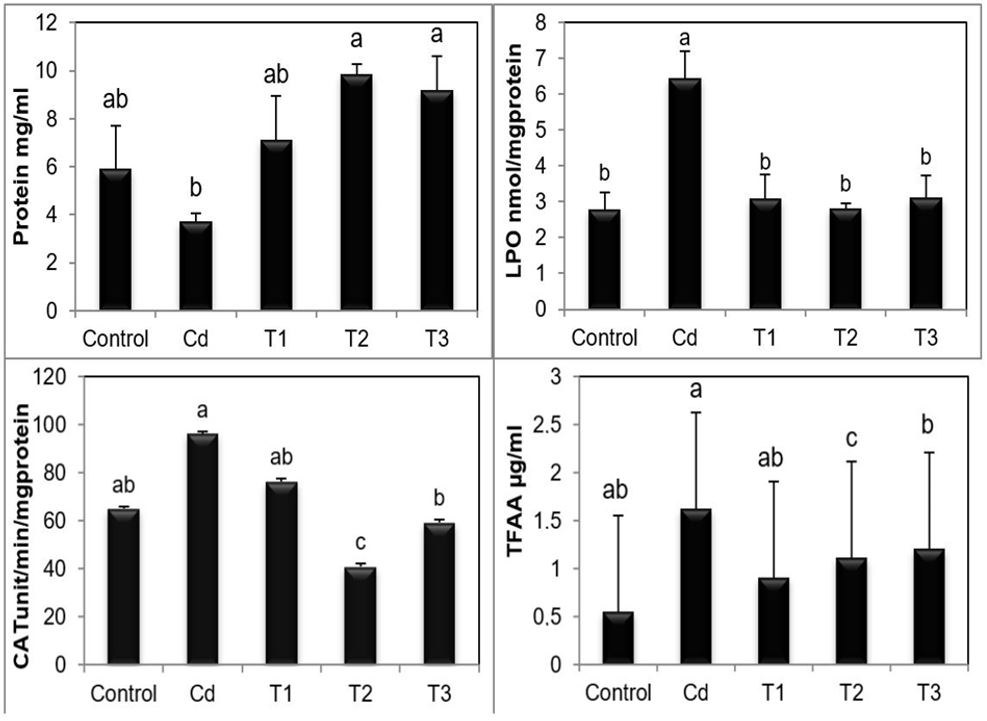
A significant decrease was noticed in the concentration of total protein in animals fed on Cd sprayed leaves, while no significant increase was found in T1 group and there was a significant increase in T2 and T3 groups compared with the control. Lipid peroxidation showed high significant increment in animals fed on Cd sprayed leaves; moreover, there was no significant difference recorded change in lipid peroxidation in T1, T2, and T3 groups compared with control. Catalase activity showed a high significant increase in the Cd group comparing to control, while no significant decrease was noticed in the T2 group and no significant differences were observed for catalase activity in T1 and T3 groups. A significant increase in total free amino acids was noticed in Cd, T2, and T3; however, no significant difference was found in T1 compared to control group (Figure 3).
Concentration of biochemical parameters: Total protein, Lipid peroxidation (LPO), Catalase activity (CAT) and Total free amino acids (TFAA) in P. leavis in different treated groups control, Cd, T1, T2, and T3. Values are the mean of three replicates (n=3) ± SE. The significant difference between the groups were analysed by one-way analysis of variance. Mean values of different subscript letters (a,b,c,d) were significantly different (p<0.001).
4. Discussion
The present study indicated that neither single nor combined OTC with heavy metal (Cd) caused mortality for isopods during the experiments. Remains of the dead animals were observed that, led to the suggestion that the main cause of death was due to the cannibalism not according to toxicity. This could be due to accumulation of Cd in cells of mid-gut and digestive gland (Nasr et al., 2020NASR, E.E., KHATER, Z.Z., ZELENAKOVA, M., VRANAYOVA, Z. and ABU-HASHIM, M., 2020. Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution. Sustainability, vol. 12, no. 12, pp. 4805. http://dx.doi.org/10.3390/su12124805.
http://dx.doi.org/10.3390/su12124805...
) for short-term exposure (Rost-Roszkowska et al., 2020ROST-ROSZKOWSKA, M., POPRAWA, I., CHAJEC, Ł., CHACHULSKA-ŻYMEŁKA, A., WILCZEK, G., WILCZEK, P., STUDENT, S., SKOWRONEK, M., NADGÓRSKA-SOCHA, A. and LEŚNIEWSKA, M., 2020. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal, vol. 87, no. 1, pp. 242-262. http://dx.doi.org/10.1080/24750263.2020.1757168.
http://dx.doi.org/10.1080/24750263.2020....
).
The single and combined impacts of Cd and OTC induced an irregular decrease in consumption ratio of food for P. leavis, where food consumption is indicative of the overall condition of an organism (De Coen and Janssen, 2003DE COEN, W. M., and JANSSEN, C. R., 2003. The missing biomarker link: relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics.Environmental toxicology and chemistry, vol. 22, no. 7, pp. 1632–1641.). This result may be due to the toxic effect of Cd that led P. leavis to reduce its consumption of food where Cd may influence the gut epithelium (Rost-Roszkowska et al., 2020ROST-ROSZKOWSKA, M., POPRAWA, I., CHAJEC, Ł., CHACHULSKA-ŻYMEŁKA, A., WILCZEK, G., WILCZEK, P., STUDENT, S., SKOWRONEK, M., NADGÓRSKA-SOCHA, A. and LEŚNIEWSKA, M., 2020. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal, vol. 87, no. 1, pp. 242-262. http://dx.doi.org/10.1080/24750263.2020.1757168.
http://dx.doi.org/10.1080/24750263.2020....
). The observed excessive decrease in food consumption ratio in T1 group may be due to P. leavis reduced consumption of contaminated food; where declined feeding rate was recorded after exposure of isopods to metals, biocides or veterinary drugs in a dose-dependent manner (Novak et al., 2012NOVAK, S., DROBNE, D. and MENARD, A., 2012. Prolonged feeding of terrestrial isopod (Porcellio scaber, Isopoda, Crustacea) on TiO2 nanoparicles. Absence of toxic effect. ZooKeys, vol. 176, no. 176, pp. 261-273. http://dx.doi.org/10.3897/zookeys.176.2463. PMid:22536113.
http://dx.doi.org/10.3897/zookeys.176.24...
, 2013NOVAK, S., DROBNE, D., GOLOBIC, M., ZUPANC, J., ROMIH, T., GIANONCELLI, A., KISKINOVA, M., KAULICH, B., PELICON, P., VAVPETIČ, P., JEROMEL, L., OGRINC, N. and MAKOVEC, D., 2013. Cellular internalization of dissolved cobalt ions from ingested CoFe2O4 nanoparticles: In Vivo Experimental Evidence. Environmental Science & Technology, vol. 47, no. 10, pp. 5400-5408. http://dx.doi.org/10.1021/es305132g. PMid:23578201.
http://dx.doi.org/10.1021/es305132g...
; Volcão et al., 2020VOLCÃO, L.M., FRAGA, L.S., DE LIMA BRUM, R., DE MOURA, R.R., BERNARDI, E., RAMOS, D.F. and DA SILVA JÚNIOR, F.M.R., 2020. Toxicity of Biocide Formulations in the Soil to the Gut Community inBalloniscus selowiiBrandt, 1983 (Crustacea: Isopoda: Oniscidea). Water, Air, and Soil Pollution, vol. 231, no. 6, pp. 306. http://dx.doi.org/10.1007/s11270-020-04689-6.
http://dx.doi.org/10.1007/s11270-020-046...
).
Decreased assimilation ratio and assimilation efficiency in Cd combined with different concentrations of OTC because P. leavis consumed less contaminated leaves in all treatments. The increment of assimilation efficiency might be explicated by the slower passage of food material through the animal’s gut, which consequently decreased fecal production. As the longer time the food stays in the gut the higher the assimilation of food and this might be associated with food characters, like leaf hardness and nutrients availability. Previous studies showed that terrestrial isopods displayed high assimilation efficiencies in animals fed on leaves with high levels of contamination more than animals fed on uncontaminated leaves (Drobne and Hopkin, 1995DROBNE, D. and HOPKIN, S.P., 1995. The Toxicity of Zinc to Terrestrial Isopods in a Standard Laboratory Test. Ecotoxicology and Environmental Safety, vol. 31, no. 1, pp. 1-6. http://dx.doi.org/10.1006/eesa.1995.1037. PMid:7544260.
http://dx.doi.org/10.1006/eesa.1995.1037...
; Loureiro et al., 2006LOUREIRO, S., SAMPAIO, A., BRANDÃO, A., NOGUEIRA, A.J. and SOARES, A.M., 2006. Feeding behaviour of the terrestrial isopod Porcellionides pruinosus Brand, 1833 (Crustacea, Isopoda) in response to changes in food quality and contamination. The Science of the Total Environment, vol. 369, no. 1–3, pp. 119-128. http://dx.doi.org/10.1016/j.scitotenv.2006.05.023. PMid:16842839.
http://dx.doi.org/10.1016/j.scitotenv.20...
). The upsurge of consumption rate, assimilation rate and assimilation efficiency in isopod, P. leavis fed on a diet with Cd only (conc. 80 ppm) makes the animal more obese than the control and may affect its vitality and movement. This means that the pollution of Cd may increase obesity in soil isopods.
Antagonistic effect of OTC on heavy metal was observed through increasing OTC concentrations; this was more visible in assimilation ratio and assimilation efficiency of food. OTC may chelate heavy metal, and consequently can decrease the toxicity of metal (Kong et al., 2006KONG, W.D., ZHU, Y.G., FU, B.J., MARSCHNER, P. and HE, J.Z., 2006. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environmental Pollution, vol. 143, no. 1, pp. 129-137. http://dx.doi.org/10.1016/j.envpol.2005.11.003. PMid:16413090.
http://dx.doi.org/10.1016/j.envpol.2005....
). A similar result was obtained from earthworms treated with oxytetracycline and Pb (Gao et al., 2014GAO, M., ZHOU, Q., SONG, W. and MA, X., 2014. Combined effects of oxytetracycline and Pb on earthworm Eisenia fetida. Environmental Toxicology and Pharmacology, vol. 37, no. 2, pp. 689-696. http://dx.doi.org/10.1016/j.etap.2014.02.004. PMid:24607684.
http://dx.doi.org/10.1016/j.etap.2014.02...
). In this study, the significance antagonistic effect of OTC and Cd appears after 2 weeks of exposure. Zhu et al. (2006)ZHU, J., ZHAO, Z. and LU, Y., 2006. Evaluation of genotoxicity of combined soil pollution by cadmium and phenanthrene on earthworm. Journal of Environmental Sciences (China), vol. 18, no. 6, pp. 1210-1215. http://dx.doi.org/10.1016/S1001-0742(06)60064-8. PMid:17294967.
http://dx.doi.org/10.1016/S1001-0742(06)...
stated that combined toxicity might be similar, stronger, or weaker than single toxicity; it depends on various factors such as concentrations, exposure times and the constituents of the mixtures.
Egestion ratio is an ecologically related parameter because fecal production occurs as a direct outcome of litter fragmentation, included in the primary step in the leaf decomposition process furthermore, it was grinded and digested to be easily decayed by fungi and bacteria than fallen decay leaves, thereby accelerating leaves decomposition. In this study egestion ratio was decreased in animals fed on contaminated food with Cd then increased till reached the control value in Cd combined with OTC. The decrease of ER may be attributed to the movement of the gut which could be affected by the toxicity of Cd furthermore the increase in ER may a result of the antagonistic effect of OTC on Cd.
The highly significant decrease in total protein concentrations of animals fed on contaminated food with Cd; may be attributed to the reduction in general metabolic activity (Obuid-allah et al., 2015OBUID-ALLAH, Z., EL-BAKARY, A., WAKEIL, K. F. and EL MOHAMMAD, W. A., 2015. Effects of UV-A radiation on some antioxidant biomarkers in the freshwater zooplankter Simocephalus vetulus (Schoedler, 1858) (Crustacean, Cladocera). International Journal of Advanced Research, vol. 3, no. 6, pp. 354-361.). The increase of total protein concentrations in animals fed on Cd combined with OTC (1000 and 2000 ppm) and began to retreat in Cd combined with 3000ppm OTC. This result may explain that adding OTC to food contaminated with Cd may antagonists the toxic effect of Cd and increase the metabolic activity of the animal, while high concentrations of OTC may influence the gut micro flora (Ihnen and Zimmer, 2008IHNEN, K. and ZIMMER, M., 2008. Selective consumption and digestion of litter microbes by Porcellio scaber (Isopoda: oniscidea). Pedobiologia, vol. 51, no. 5-6, pp. 335-342. http://dx.doi.org/10.1016/j.pedobi.2007.06.001.
http://dx.doi.org/10.1016/j.pedobi.2007....
; Volcão et al., 2020VOLCÃO, L.M., FRAGA, L.S., DE LIMA BRUM, R., DE MOURA, R.R., BERNARDI, E., RAMOS, D.F. and DA SILVA JÚNIOR, F.M.R., 2020. Toxicity of Biocide Formulations in the Soil to the Gut Community inBalloniscus selowiiBrandt, 1983 (Crustacea: Isopoda: Oniscidea). Water, Air, and Soil Pollution, vol. 231, no. 6, pp. 306. http://dx.doi.org/10.1007/s11270-020-04689-6.
http://dx.doi.org/10.1007/s11270-020-046...
).
In the present study, a significant increase in total free amino acids in animals fed on Cd contaminated diet only, this can be presumed to be an indication of the acute effect of the used concentration of cadmium on those tissues. The decline in total protein content and the simultaneous increase in free amino acid in the digestive tract of P. leavis may indicate the activation of protein catabolism to counteract the toxic effects of cadmium (Reddy and Bhagyalakshmi, 1994REDDY, P.S. and BHAGYALAKSHMI, A., 1994. Changes in oxidative metabolism in selected tissues of the crab (Scylla serrata) in response to cadmium toxicity. Ecotoxicology and Environmental Safety, vol. 29, no. 3, pp. 255-264. http://dx.doi.org/10.1016/0147-6513(94)90002-7. PMid:7534686.
http://dx.doi.org/10.1016/0147-6513(94)9...
); where the breakdown of protein is a functional response to deal with the extra energy requirements to cope with Cd stress. De Smet and Blust (2001)DE SMET, H. and BLUST, R., 2001. Stress responses and changes in protein metabolism in carp Cyprinus carpio during cadmium exposure. Ecotoxicology and Environmental Safety, vol. 48, no. 3, pp. 255-262. http://dx.doi.org/10.1006/eesa.2000.2011. PMid:11222034.
http://dx.doi.org/10.1006/eesa.2000.2011...
, illustrated that proteolysis is a sign of using protein in energy production during cadmium stress. Furthermore, Rost-Roszkowska et al. (2020)ROST-ROSZKOWSKA, M., POPRAWA, I., CHAJEC, Ł., CHACHULSKA-ŻYMEŁKA, A., WILCZEK, G., WILCZEK, P., STUDENT, S., SKOWRONEK, M., NADGÓRSKA-SOCHA, A. and LEŚNIEWSKA, M., 2020. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal, vol. 87, no. 1, pp. 242-262. http://dx.doi.org/10.1080/24750263.2020.1757168.
http://dx.doi.org/10.1080/24750263.2020....
reported that Cd induces cell lysis.
The significant increase of TBARS content, in the tissue of gut and hepatopancreas of P. leavis was in animals fed on Cd-sprayed leaves, may indicate that cadmium toxicity was linked to lipid peroxidation because Cd produces excessive reactive oxygen species including superoxide radicals (Obuid-allah et al., 2015OBUID-ALLAH, Z., EL-BAKARY, A., WAKEIL, K. F. and EL MOHAMMAD, W. A., 2015. Effects of UV-A radiation on some antioxidant biomarkers in the freshwater zooplankter Simocephalus vetulus (Schoedler, 1858) (Crustacean, Cladocera). International Journal of Advanced Research, vol. 3, no. 6, pp. 354-361.) Rijstenbil (2000)RIJSTENBIL, J.W., 2000. Effects of periodic, low UVA radiation on cell characteristics and oxidative stress in the marine planktonic diatom Ditylum brightwellii. European Journal of Phycology, vol. 36, no. 1, pp. 1-8. http://dx.doi.org/10.1080/09670260110001735138.
http://dx.doi.org/10.1080/09670260110001...
observed increase of LPO caused by UV-A radiation in marine invertebrate Ditylum brightwellii. On the other side, the non-significant differences in groups fed on Cd combined with OTC, may be due to OTC antagonists the toxic effect of Cd preventing the loss of membrane integrity and cell degeneration.
Catalase (CAT) is an anti-oxidative enzyme, which has been involved in protection against H2O2. High significant increase was noticed in CAT activity in animals fed on Cd alone. This increment could be considered as a kind of defense mechanism of the cells in order to offset the oxidative stress induced by increased H2O2. This happened in many invertebrate species as response to pollution (Stephensen et al., 2000STEPHENSEN, E., SVAVARSSON, J., STURVE, J., ERICSON, G., ADOLFSSON-ERICI, M. and FÖRLIN, L., 2000. Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquatic Toxicology (Amsterdam, Netherlands), vol. 48, no. 4, pp. 431-442. http://dx.doi.org/10.1016/S0166-445X(99)00062-4. PMid:10794829.
http://dx.doi.org/10.1016/S0166-445X(99)...
). Previous studies reported that Cd had adverse effects on cellular/subcellular levels of earthworms (Panzarino et al., 2016PANZARINO, O., HYRSL, P., DOBES, P., VOJTEK, L., VERNILE, P., BARI, G., TERZANO, R., SPAGNUOLO, M. and DE LILLO, E., 2016. Rank-based biomarker index to assess cadmium ecotoxicity on the earthworm Eisenia andrei. Chemosphere, vol. 145, pp. 480-486. http://dx.doi.org/10.1016/j.chemosphere.2015.11.077. PMid:26694799.
http://dx.doi.org/10.1016/j.chemosphere....
; Yang et al., 2012YANG, X., SONG, Y., ACKLAND, M.L., LIU, Y. and CAO, X., 2012. Biochemical responses of earthworm Eisenia fetida exposed to cadmium-contaminated soil with long duration. Bulletin of Environmental Contamination and Toxicology, vol. 89, no. 6, pp. 1148-1153. http://dx.doi.org/10.1007/s00128-012-0837-y. PMid:23052576.
http://dx.doi.org/10.1007/s00128-012-083...
; Zhou et al., 2016ZHOU, D., NING, Y., WANG, B., WANG, G., SU, Y., LI, L. and WANG, Y., 2016. Study on the influential factors of Cd(II) on the earthworm Eisenia fetida in oxidative stress based on factor analysis approach. Chemosphere, vol. 157, pp. 181-189. http://dx.doi.org/10.1016/j.chemosphere.2016.05.045. PMid:27219294.
http://dx.doi.org/10.1016/j.chemosphere....
).
In conclusion, decreasing of CR, AR, and AE observed in P. leavis fed on Cd combined with OTC at high concentrations. For a long period of exposure (4 weeks) the moderate concentration of OTC (2000 ppm) may antagonist the toxic effect of Cd enhancing the above feeding parameters. Treatment of woodlice only with Cd containing diet inhibited the concentration of total protein, increased LPO, CAT activity and TFAA. In contrast combination between Cd and OTC in the diet increased the concentration of the total protein and decreased LPO followed by inhibition of CAT activity. This combination of Cd and OTC increased the amount of TFAA in gut and hepatopancreas tissues. More investigations are required to indicate the effect of antibiotics and heavy metals pollution and their effects on soil animals.
Acknowledgements
The authors of this work deeply appreciate the deanship of scientific research for funding this work by researchers supporting initiative Project number (TURSP-2020/119), Taif University, Saudi Arabia.
-
#excluir The authors of this work deeply appreciate the deanship of scientific research, Taif University, Saudi Arabia, for funding this work by researchers supporting initiative Project number (TURSP-2020/119).
References
- ABD EL-WAKEIL, K.F., 2001. Effects of ecological factors and heavy metals on the distribution of soil Isopoda (Arthropoda, Crustacea) in Assiut, Egypt. Egypt: Assiut University, 150 p. M.Sc. Thesis.
- ABD EL-WAKEIL, K.F., 2005. Ecotoxicolgical studies on terrestrial isopods (Crustacea) in Assiut, Egypt Egypt: Assiut University, 271 p. Ph. D. Thesis.
- ADAMSE, P., VAN DER FELS-KLERX, H.J.I. and DE JONG, J., 2017. Cadmium, lead, mercury and arsenic in animal feed and feed materials–trend analysis of monitoring results. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, vol. 34, no. 8, pp. 1298-1311. http://dx.doi.org/10.1080/19440049.2017.1300686 PMid:28278122.
» http://dx.doi.org/10.1080/19440049.2017.1300686 - BAO, Y.Y., WAN, Y., ZHOU, Q.X., LI, W. and LIU, Y., 2013. Competitive adsorption and desorption of oxy-tetracycline and cadmium with different input loadings on cinnamon soil. Journal of Soils and Sediments, vol. 13, no. 2, pp. 364-374. http://dx.doi.org/10.1007/s11368-012-0600-3
» http://dx.doi.org/10.1007/s11368-012-0600-3 - BÁRTÍKOVÁ, H., PODLIPNÁ, R. and SKÁLOVÁ, L., 2016. Veterinary drugs in the environment and their toxicity to plants. Chemosphere, vol. 144, pp. 2290-2301. http://dx.doi.org/10.1016/j.chemosphere.2015.10.137 PMid:26606183.
» http://dx.doi.org/10.1016/j.chemosphere.2015.10.137 - BEERS JUNIOR, R.F. and SIZER, I.W., 1952. A spectrophotometric methods for measuring the breakdown of hydrogen peroxide by catalase. The Journal of Biological Chemistry, vol. 195, no. 1, pp. 133-140. http://dx.doi.org/10.1016/S0021-9258(19)50881-X PMid:14938361.
» http://dx.doi.org/10.1016/S0021-9258(19)50881-X - DE COEN, W. M., and JANSSEN, C. R., 2003. The missing biomarker link: relationships between effects on the cellular energy allocation biomarker of toxicant-stressed Daphnia magna and corresponding population characteristics.Environmental toxicology and chemistry, vol. 22, no. 7, pp. 1632–1641.
- DE SMET, H. and BLUST, R., 2001. Stress responses and changes in protein metabolism in carp Cyprinus carpio during cadmium exposure. Ecotoxicology and Environmental Safety, vol. 48, no. 3, pp. 255-262. http://dx.doi.org/10.1006/eesa.2000.2011 PMid:11222034.
» http://dx.doi.org/10.1006/eesa.2000.2011 - DE SOUZA, M.J., NAIR, S., LOKA BHARATHI, P.A. and CHANDRAMOHAN, D., 2006. Metal and antibiotic-resistance in psychrotrophic bacteria from Antarctic Marine waters. Ecotoxicology (London, England), vol. 15, no. 4, pp. 379-384. http://dx.doi.org/10.1007/s10646-006-0068-2 PMid:16703457.
» http://dx.doi.org/10.1007/s10646-006-0068-2 - DONG, L., GAO, J., XIE, X.J. and ZHOU, Q.X., 2012. DNA damage and biochemical toxicity of antibiotics in soil on the earthworm (Eisenia fetida). Chemosphere, vol. 89, no. 1, pp. 44-51. http://dx.doi.org/10.1016/j.chemosphere.2012.04.010 PMid:22647195.
» http://dx.doi.org/10.1016/j.chemosphere.2012.04.010 - DROBNE, D. and HOPKIN, S.P., 1995. The Toxicity of Zinc to Terrestrial Isopods in a Standard Laboratory Test. Ecotoxicology and Environmental Safety, vol. 31, no. 1, pp. 1-6. http://dx.doi.org/10.1006/eesa.1995.1037 PMid:7544260.
» http://dx.doi.org/10.1006/eesa.1995.1037 - DROBNE, D., 1997. Terrestrial isopods: a good choice for toxicity testing of pollutants in the terrestrial environment. Environmental Toxicology and Chemistry International Journal (Toronto, Ont.), vol. 16, no. 6, pp. 1159-1164.
- GAO, M., QI, Y., SONG, W. and ZHOU, Q., 2015. Biomarker analysis of combined oxytetracycline and zinc pollution in earthworms (Eisenia fetida). Chemosphere, vol. 139, pp. 229-234. http://dx.doi.org/10.1016/j.chemosphere.2015.06.059 PMid:26134676.
» http://dx.doi.org/10.1016/j.chemosphere.2015.06.059 - GAO, M., ZHOU, Q., SONG, W. and MA, X., 2014. Combined effects of oxytetracycline and Pb on earthworm Eisenia fetida. Environmental Toxicology and Pharmacology, vol. 37, no. 2, pp. 689-696. http://dx.doi.org/10.1016/j.etap.2014.02.004 PMid:24607684.
» http://dx.doi.org/10.1016/j.etap.2014.02.004 - HAMMESFAHR, U., HEUER, H., MANZKE, B., SMALLA, K. and THIELE-BRUHN, S., 2008. Impact of the antibiotic sulfadiazine and pig manure on the microbial community structure in agricultural soils. Soil Biology & Biochemistry, vol. 40, no. 7, pp. 1583-1591. http://dx.doi.org/10.1016/j.soilbio.2008.01.010
» http://dx.doi.org/10.1016/j.soilbio.2008.01.010 - HASSALL, M., TURNER, T.G. and RANDS, M.R., 1987. Effects of terrestrial isopods on the decomposition of woodland leaf litter. Oecologia, vol. 72, no. 4, pp. 597-604. http://dx.doi.org/10.1007/BF00378988 PMid:28312524.
» http://dx.doi.org/10.1007/BF00378988 - IHNEN, K. and ZIMMER, M., 2008. Selective consumption and digestion of litter microbes by Porcellio scaber (Isopoda: oniscidea). Pedobiologia, vol. 51, no. 5-6, pp. 335-342. http://dx.doi.org/10.1016/j.pedobi.2007.06.001
» http://dx.doi.org/10.1016/j.pedobi.2007.06.001 - JIN, C.X., LIU, J.J. and CHEN, Q.Y., 2009. Toxicological effects of veterinary drugs in soil on the inhibition of root elongation of wheat and Chinese cabbages. Nongye Huanjing Kexue Xuebao, vol. 28, no. 7, pp. 1358-1362.
- KONG, W.D., ZHU, Y.G., FU, B.J., MARSCHNER, P. and HE, J.Z., 2006. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environmental Pollution, vol. 143, no. 1, pp. 129-137. http://dx.doi.org/10.1016/j.envpol.2005.11.003 PMid:16413090.
» http://dx.doi.org/10.1016/j.envpol.2005.11.003 - KUMAR, R.R., PARK, B.J. and CHO, J.Y., 2013. Application and environmental risks of livestock manure. Journal of the Korean Society for Applied Biological Chemistry, vol. 56, no. 5, pp. 497-503. http://dx.doi.org/10.1007/s13765-013-3184-8
» http://dx.doi.org/10.1007/s13765-013-3184-8 - LESTON, S., NUNES, M., VIEGAS, I., NEBOT, C., CEPEDA, A., PARDAL, M.A. and RAMOS, F., 2014. The influence of sulfathiazole on the macroalgae Ulva lactuca. Chemosphere, vol. 100, pp. 105-110. http://dx.doi.org/10.1016/j.chemosphere.2013.12.038 PMid:24393561.
» http://dx.doi.org/10.1016/j.chemosphere.2013.12.038 - LESTON, S., NUNES, M., VIEGAS, I., NEBOT, C., CEPEDA, A., PARDAL, M.A. and RAMOS, F., 2014. The influence of sulfathiazole on the macroalgae Ulva lactuca. Chemosphere, vol. 100, pp. 105-110. http://dx.doi.org/10.1016/j.chemosphere.2013.12.038 PMid:24393561.
» http://dx.doi.org/10.1016/j.chemosphere.2013.12.038 - LI, C., CHEN, J., WANG, J., MA, Z., HAN, P., LUAN, Y., LU, A., 2015. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Science of the Total Environment, vol. 521-522, n. 19, pp. 101-107. https://doi.org/110.1016/j.scitotenv.2015.1003.1070
» https://doi.org/110.1016/j.scitotenv.2015.1003.1070 - LIU, A.J., LIU, M., LI, M.H., MA, X.X., SUN, X.J. and WANG, H.H., 2013. Collaborative effects of Cu and antibiotic on soil microbial activities. Shengtai Huanjing Xuebao, vol. 22, no. 11, pp. 1825-1829.
- LIU, F., YING, G.G., TAO, R., ZHAO, J.L., YANG, J.F. and ZHAO, L.F., 2009. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environmental Pollution, vol. 157, no. 5, pp. 1636-1642. http://dx.doi.org/10.1016/j.envpol.2008.12.021 PMid:19157661.
» http://dx.doi.org/10.1016/j.envpol.2008.12.021 - LIU, Y., WANG, X., ZENG, G., QU, D., GU, J., ZHOU, M. and CHAI, L., 2007. Cadmium induced oxidative stress and response of the ascorbate–glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere, vol. 69, no. 1, pp. 99-107. http://dx.doi.org/10.1016/j.chemosphere.2007.04.040 PMid:17532363.
» http://dx.doi.org/10.1016/j.chemosphere.2007.04.040 - LOUREIRO, S., SAMPAIO, A., BRANDÃO, A., NOGUEIRA, A.J. and SOARES, A.M., 2006. Feeding behaviour of the terrestrial isopod Porcellionides pruinosus Brand, 1833 (Crustacea, Isopoda) in response to changes in food quality and contamination. The Science of the Total Environment, vol. 369, no. 1–3, pp. 119-128. http://dx.doi.org/10.1016/j.scitotenv.2006.05.023 PMid:16842839.
» http://dx.doi.org/10.1016/j.scitotenv.2006.05.023 - NASR, E.E., KHATER, Z.Z., ZELENAKOVA, M., VRANAYOVA, Z. and ABU-HASHIM, M., 2020. Soil Physicochemical Properties, Metal Deposition, and Ultrastructural Midgut Changes in Ground Beetles, Calosoma chlorostictum, under Agricultural Pollution. Sustainability, vol. 12, no. 12, pp. 4805. http://dx.doi.org/10.3390/su12124805
» http://dx.doi.org/10.3390/su12124805 - NOVAK, S., DROBNE, D. and MENARD, A., 2012. Prolonged feeding of terrestrial isopod (Porcellio scaber, Isopoda, Crustacea) on TiO2 nanoparicles. Absence of toxic effect. ZooKeys, vol. 176, no. 176, pp. 261-273. http://dx.doi.org/10.3897/zookeys.176.2463 PMid:22536113.
» http://dx.doi.org/10.3897/zookeys.176.2463 - NOVAK, S., DROBNE, D., GOLOBIC, M., ZUPANC, J., ROMIH, T., GIANONCELLI, A., KISKINOVA, M., KAULICH, B., PELICON, P., VAVPETIČ, P., JEROMEL, L., OGRINC, N. and MAKOVEC, D., 2013. Cellular internalization of dissolved cobalt ions from ingested CoFe2O4 nanoparticles: In Vivo Experimental Evidence. Environmental Science & Technology, vol. 47, no. 10, pp. 5400-5408. http://dx.doi.org/10.1021/es305132g PMid:23578201.
» http://dx.doi.org/10.1021/es305132g - OBUID-ALLAH, Z., EL-BAKARY, A., WAKEIL, K. F. and EL MOHAMMAD, W. A., 2015. Effects of UV-A radiation on some antioxidant biomarkers in the freshwater zooplankter Simocephalus vetulus (Schoedler, 1858) (Crustacean, Cladocera). International Journal of Advanced Research, vol. 3, no. 6, pp. 354-361.
- OHKAWA, H., OHISHI, N. and YAGI, K., 1979. Assay for lipid peroxides in animal tissue by thiobarbaturic acid reaction. Analytical Biochemistry, vol. 95, no. 2, pp. 351-358. http://dx.doi.org/10.1016/0003-2697(79)90738-3 PMid:36810.
» http://dx.doi.org/10.1016/0003-2697(79)90738-3 - OLAWOYIN, R., OYEWOLE, S.A. and GRAYSON, R.L., 2012. Potential risk effect from elevated levels of soil heavy metals on human health in the Niger delta. Ecotoxicology and Environmental Safety, vol. 85, pp. 120-130. http://dx.doi.org/10.1016/j.ecoenv.2012.08.004 PMid:22921257.
» http://dx.doi.org/10.1016/j.ecoenv.2012.08.004 - PANZARINO, O., HYRSL, P., DOBES, P., VOJTEK, L., VERNILE, P., BARI, G., TERZANO, R., SPAGNUOLO, M. and DE LILLO, E., 2016. Rank-based biomarker index to assess cadmium ecotoxicity on the earthworm Eisenia andrei. Chemosphere, vol. 145, pp. 480-486. http://dx.doi.org/10.1016/j.chemosphere.2015.11.077 PMid:26694799.
» http://dx.doi.org/10.1016/j.chemosphere.2015.11.077 - PAOLETTI, M.G. and HASSALL, M., 1999. Woodlice (Isopoda: Oniscidea): their potential for assessing sustainability and use as bioindicators. Agriculture, Ecosystems & Environment, vol. 74, no. 1-3, pp. 157-165. http://dx.doi.org/10.1016/S0167-8809(99)00035-3
» http://dx.doi.org/10.1016/S0167-8809(99)00035-3 - PARK, K., BANG, H.W., PARK, J. and KWAK, I.S., 2009. Ecotoxicological multilevel-evaluation of the effects of fenbendazole exposure to Chironomus riparius larvae. Chemosphere, vol. 77, no. 3, pp. 359-367. http://dx.doi.org/10.1016/j.chemosphere.2009.07.019 PMid:19683327.
» http://dx.doi.org/10.1016/j.chemosphere.2009.07.019 - POWELL, S.R., 2000. The antioxidant properties of zinc. The Journal of Nutrition, vol. 130, no. 5S, suppl., pp. 1447s-1454S. http://dx.doi.org/10.1093/jn/130.5.1447S PMid:10801958.
» http://dx.doi.org/10.1093/jn/130.5.1447S - REDDY, P.S. and BHAGYALAKSHMI, A., 1994. Changes in oxidative metabolism in selected tissues of the crab (Scylla serrata) in response to cadmium toxicity. Ecotoxicology and Environmental Safety, vol. 29, no. 3, pp. 255-264. http://dx.doi.org/10.1016/0147-6513(94)90002-7 PMid:7534686.
» http://dx.doi.org/10.1016/0147-6513(94)90002-7 - RIJSTENBIL, J.W., 2000. Effects of periodic, low UVA radiation on cell characteristics and oxidative stress in the marine planktonic diatom Ditylum brightwellii. European Journal of Phycology, vol. 36, no. 1, pp. 1-8. http://dx.doi.org/10.1080/09670260110001735138
» http://dx.doi.org/10.1080/09670260110001735138 - ROST-ROSZKOWSKA, M., POPRAWA, I., CHAJEC, Ł., CHACHULSKA-ŻYMEŁKA, A., WILCZEK, G., WILCZEK, P., STUDENT, S., SKOWRONEK, M., NADGÓRSKA-SOCHA, A. and LEŚNIEWSKA, M., 2020. Influence of soil contaminated with cadmium on cell death in the digestive epithelium of soil centipede Lithobius forficatus (Myriapoda, Chilopoda). The European Zoological Journal, vol. 87, no. 1, pp. 242-262. http://dx.doi.org/10.1080/24750263.2020.1757168
» http://dx.doi.org/10.1080/24750263.2020.1757168 - SCHULTZ, G.A., 1982. Four species of Alloniscus Dana, 1854, from the West coast of North America and Hawaii (Isopoda, Oniscoidea). Crustaceana, vol. 47, no. 2, pp. 149-168. http://dx.doi.org/10.1163/156854084X00388
» http://dx.doi.org/10.1163/156854084X00388 - STEPHENSEN, E., SVAVARSSON, J., STURVE, J., ERICSON, G., ADOLFSSON-ERICI, M. and FÖRLIN, L., 2000. Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquatic Toxicology (Amsterdam, Netherlands), vol. 48, no. 4, pp. 431-442. http://dx.doi.org/10.1016/S0166-445X(99)00062-4 PMid:10794829.
» http://dx.doi.org/10.1016/S0166-445X(99)00062-4 - THIELE-BRUHN, S., 2005. Microbial inhibition by pharmaceutical antibiotics in different soils--dose-response relations determined with the iron (III) reduction test.Environmental toxicology and chemistry, vol. 24, no. 4, pp. 869–876. https://doi.org/10.1897/04-166r.1
» https://doi.org/10.1897/04-166r.1 - TIWARI, B., SELLAMUTHU, B., OUARDA, Y., DROGUI, P., TYAGI, R.D. and BUELNA, G., 2017. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresource Technology, vol. 224, pp. 1-12. http://dx.doi.org/10.1016/j.biortech.2016.11.042 PMid:27889353.
» http://dx.doi.org/10.1016/j.biortech.2016.11.042 - VOLCÃO, L.M., FRAGA, L.S., DE LIMA BRUM, R., DE MOURA, R.R., BERNARDI, E., RAMOS, D.F. and DA SILVA JÚNIOR, F.M.R., 2020. Toxicity of Biocide Formulations in the Soil to the Gut Community inBalloniscus selowiiBrandt, 1983 (Crustacea: Isopoda: Oniscidea). Water, Air, and Soil Pollution, vol. 231, no. 6, pp. 306. http://dx.doi.org/10.1007/s11270-020-04689-6
» http://dx.doi.org/10.1007/s11270-020-04689-6 - WANG, L.P. and ZHANG, M.K., 2009. Effects of oxytetracycline pollution on soil biological properties. Nongye Huanjing Kexue Xuebao, vol. 28, no. 7, pp. 1434-1438.
- WANG, R. and WEI, Y.S., 2013. Pollution and control of tetracyclines and heavy metals residues in animal manure. Nongye Huanjing Kexue Xuebao, vol. 32, no. 9, pp. 1705-1719.
- WIESER, W., 1979. The flow of copper through a terrestrial food web. In: J.O. NRIAGU, ed. Copper in the Environment New York: John Wiley and Sons Inc., Part 1, pp. 325-335.
- WRIGHT, D.A. and FRAIN, J.W., 1981. The effect of calcium on cadmium toxicity in the freshwater amphipod, Gammarus pulex (L.). Archives of Environmental Contamination and Toxicology, vol. 10, no. 3, pp. 321-328. http://dx.doi.org/10.1007/BF01055633 PMid:7259300.
» http://dx.doi.org/10.1007/BF01055633 - YANG, X., SONG, Y., ACKLAND, M.L., LIU, Y. and CAO, X., 2012. Biochemical responses of earthworm Eisenia fetida exposed to cadmium-contaminated soil with long duration. Bulletin of Environmental Contamination and Toxicology, vol. 89, no. 6, pp. 1148-1153. http://dx.doi.org/10.1007/s00128-012-0837-y PMid:23052576.
» http://dx.doi.org/10.1007/s00128-012-0837-y - YAO, J.H., NIU, D.K., LI, Z.J., LIANG, Y.C. and ZHANG, S.Q., 2010. Effects of antibiotics oxytetracycline on soil enzyme activities and microbial biomass in wheat rhizosphere. Zhongguo Nong Ye Ke Xue, vol. 43, no. 4, pp. 721-728.
- ZHANG, S.Q., ZHANG, F.D., LIU, X.M., WANG, Y.J., ZOU, S.W. and HE, X.S., 2005. Determination and analysis on main harmful composition in excrement of scale livestock and poultry feedlots. Zhiwu Yingyang Yu Feiliao Xuebao, vol. 11, no. 6, pp. 822-829.
- ZHOU, D., NING, Y., WANG, B., WANG, G., SU, Y., LI, L. and WANG, Y., 2016. Study on the influential factors of Cd(II) on the earthworm Eisenia fetida in oxidative stress based on factor analysis approach. Chemosphere, vol. 157, pp. 181-189. http://dx.doi.org/10.1016/j.chemosphere.2016.05.045 PMid:27219294.
» http://dx.doi.org/10.1016/j.chemosphere.2016.05.045 - ZHU, J., ZHAO, Z. and LU, Y., 2006. Evaluation of genotoxicity of combined soil pollution by cadmium and phenanthrene on earthworm. Journal of Environmental Sciences (China), vol. 18, no. 6, pp. 1210-1215. http://dx.doi.org/10.1016/S1001-0742(06)60064-8 PMid:17294967.
» http://dx.doi.org/10.1016/S1001-0742(06)60064-8
Publication Dates
-
Publication in this collection
11 June 2021 -
Date of issue
2022
History
-
Received
24 Dec 2020 -
Accepted
01 Feb 2021