Abstract
Metals and agrochemicals are among the main aquatic contaminants, being able to trigger oxidative stress in exposed organisms. The objective of this work was to evaluate the correlation between the level of oxidative stress biomarkers in Aegla crabs (Crustacea, Anomura) with (i) the set of metals present in the streams sediment and (ii) with land uses of three hydrographic basins. The study was carried out in streams (≤ 2nd order) of hydrographic basins in southern Brazil (Basins of Rio Suzana, Rio Ligeirinho-Leãozinho and Rio Dourado). In these streams were quantified the land uses and Cu, Cr, Cd, Fe, Mn and Zn concentrations in the sediment. The enzymes Catalase (CAT) and Glutathione Reductase (GR), as well as the level of membrane lipid peroxidation (TBARS), were analyzed in adult females. The PCA analysis showed that the distribution of metals was different between the basins. Cd, Cr and Fe were correlated positively with CAT and negatively with TBARS and GR. The Dourado basin had the lowest concentrations of these three metals and the highest levels of TBARS. However, in Dourado basin there is predominance of agriculture land use, and TBARS was positively correlated with agricultural land use. Besides in Dourado basin, GR activity was higher than in the others basins, indicating a compensatory response in relation to CAT inhibition. The basins of Suzana and Ligeirinho-Leãozinho rivers had lower TBARS values, which may be due to the induction of CAT in response to metals accumulated in sediment. In summary, this work indicates that in the basins with a higher concentration of toxic metals there is an adaptive response of CAT induction, which reduces TBARS in Aegla. On the other hand, in the basin with lower metallic contamination, TBARS occurrence was primarily influenced by agricultural land use.
Keywords:
watershed; catalase; TBARS; gluthatione redutase; biomonitoring
Resumo
Os metais e agroquímicos estão entre os principais contaminantes aquáticos, podendo desencadear estresse oxidativo em organismos expostos. O objetivo deste trabalho foi avaliar uma possível correlação entre o nível de biomarcadores de estresse oxidativo em Aegla (Crustacea, Anomura) com (i) o conjunto de metais presentes no sedimento e (ii) com os usos da terra, em três bacias hidrográficas distintas. O estudo foi realizado em riachos (≤ 2ª ordem) de bacias hidrográficas do Sul do Brasil (Bacias do Rio Suzana, do Rio Ligeirinho-Leãozinho e do Rio Dourado), as quais foram caracterizadas em função do percentual de usos da terra e do nível de Cu, Cr, Cd, Fe, Mn e Zn no sedimento. As enzimas Catalase (CAT) e Glutationa Redutase (GR), bem como o nível de peroxidação lipídica das membranas (TBARS), foram analisadas em fêmeas adultas. Uma análise de PCA mostrou que a distribuição de metais foi distinta entre as bacias. Cd, Cr e Fe no sedimento correlacionaram positivamente com a CAT e negativamente com TBARS e GR. Entretanto, a bacia do Dourado apresentou os menores níveis destes três metais e os maiores níveis de TBARS, o que pode ser justificado pelo predomínio da agricultura nesta bacia, já que o TBARS correlacionou positivamente com o percentual de uso agrícola. Nesta bacia, a atividade da GR foi mais alta do que nas outras, indicando uma resposta compensatória em relação a inibição da CAT. As bacias do rio Suzana e rio Ligeirinho-Leãozinho apresentaram valores menores de TBARS, o que pode decorrer da indução da CAT em função dos metais acumulados no sedimento. Em síntese, este trabalho indica que nas bacias com maior concentração de metais tóxicos ocorre uma resposta adaptativa de indução da CAT, o que reduz os níveis de TBARS em Aegla. Por outro lado, na bacia com menor contaminação metálica os níveis de TBARS foram primariamente influenciados pelo uso agrícola.
Palavras-chave:
bacia hidrográfica; catalase; TBARS; glutationa redutase; biomonitoramento
1. Introduction
The combination of stressors as habitat and water flow alterations, sudden changes in physical-chemical parameters and, especially, contamination by metals and pesticides, have deleterious effects for many aquatic organisms and ecosystems (Romero, 2004ROMERO, L.M., 2004. Physiological stress in ecology: lessons from biomedical research. Trends in Ecology & Evolution, vol. 19, no. 5, pp. 249-255. http://dx.doi.org/10.1016/j.tree.2004.03.008. PMid:16701264.
http://dx.doi.org/10.1016/j.tree.2004.03...
; Magalhães et al., 2015MAGALHÃES, D.P., MARQUES, M.R.C., BAPTISTA, D.F. and BUSS, D.F., 2015. Metal bioavaliability and toxicity in freshwaters. Environmental Chemistry Letters, vol. 13, no. 1, pp. 69-87. http://dx.doi.org/10.1007/s10311-015-0491-9.
http://dx.doi.org/10.1007/s10311-015-049...
). Metals are among the main aquatic contaminants, being common that a same aquatic environment to be contaminated by a range of different metals (La Colla et al., 2017LA COLLA, N.S., BOTTÉ, S.E., OLIVA, A.L. and MARCOVECCHIO, J.E., 2017. Tracing Cr, Pb, Fe and Mn occurrence in the Bahía Blanca estuary through commercial fish species. Chemosphere, vol. 175, pp. 286-293. http://dx.doi.org/10.1016/j.chemosphere.2017.02.002. PMid:28232139.
http://dx.doi.org/10.1016/j.chemosphere....
; Qu et al., 2017QU, X., REN, Z., ZHANG, M., LIU, X. and PENG, W., 2017. Sediment heavy metals and benthic diversities in Hun-Tai River, northeast of China. Environmental Science and Pollution Research International, vol. 24, no. 11, pp. 10662-10673. http://dx.doi.org/10.1007/s11356-017-8642-0. PMid:28283976.
http://dx.doi.org/10.1007/s11356-017-864...
).
Besides metals, agrochemicals and pesticides are also stressors frequently encountered as pollutants of surface water specially in cultivated areas (Moreno-González et al., 2013MORENO-GONZÁLEZ, R., CAMPILLO, J.A. and LEÓN, V.M., 2013. Influence of an intensive agricultural drainage basin on the seasonal distribution of organic pollutants in seawater from a Mediterranean coastal lagoon (Mar Menor, SE Spain). Marine Pollution Bulletin, vol. 77, no. 1-2, pp. 400-411. http://dx.doi.org/10.1016/j.marpolbul.2013.09.040. PMid:24139646.
http://dx.doi.org/10.1016/j.marpolbul.20...
). Agricultural activity requires major changes in the environment for its development, including the removal of riparian forest and the intensive use of agrochemicals and fertilizers (Callisto et al., 2004CALLISTO, M. and GONÇALVES JÚNIOR, J.F. and MORENO, P., 2004. Invertebrados aquáticos como bioindicadores. In: E.M.A. GOULART, ed. Navegando o Rio das Velhas das Minas aos Gerais. Belo Horizonte: UFMG, pp. 1-12.; Campos, 2008CAMPOS, V.D., 2008. Dinâmica de uso e ocupação da terra na bacia hidrográfica do Arroio dos Pereiras em Irati – PR e sua influência na qualidade das águas superficiais. Ponta Grossa: Universidade Estadual de Ponta Grossa, 112 p. Dissertação de Mestrado em Gestão do Território.). These chemical compounds can accumulate in the environment as well in the tissues of living organisms (Paulo and Serra, 2015PAULO, R.L. and SERRA, J.C.V., 2015. Estudo de caso envolvendo uma indústria de fertilizantes na cidade de Porto Nacional/TO. Sistemas & Gestão, vol. 10, no. 2, pp. 316-323. http://dx.doi.org/10.7177/sg.2015.v10.n2.a8.
http://dx.doi.org/10.7177/sg.2015.v10.n2...
).
The pattern of aquatic accumulation of metals and pesticides is influenced by human activities (Wang et al., 2016WANG, A., KAWSER, A., XU, Y., YE, X., RANI, S. and CHEN, K., 2016. Heavy metal accumulation during the last 30 years in the Karnaphuli River estuary, Chittagong, Bangladesh. SpringerPlus, vol. 5, pp. 2079. http://dx.doi.org/10.1186/s40064-016-3749-1. PMid:28018787.
http://dx.doi.org/10.1186/s40064-016-374...
; Sundararajan et al., 2017SUNDARARAJAN, S., KHADANGA, M.K., KUMAR, J.P., RAGHUMARAN, S., VIJAYA, R. and JENA, B.K., 2017. Ecological risk assessment of trace metal accumulation in sediments of Veraval Harbor, Gujarat, Arabian Sea. Marine Pollution Bulletin, vol. 114, no. 1, pp. 592-601. http://dx.doi.org/10.1016/j.marpolbul.2016.09.016. PMid:27817889.
http://dx.doi.org/10.1016/j.marpolbul.20...
) and land uses in the drainage areas (Bunzel et al., 2014BUNZEL, K., LIESS, M. and KATTWINKEL, M., 2014. Landscape parameters driving aquatic pesticide exposure and effects. Environmental Pollution, vol. 186, pp. 90-97. http://dx.doi.org/10.1016/j.envpol.2013.11.021. PMid:24365537.
http://dx.doi.org/10.1016/j.envpol.2013....
). So, hydrographic basins are an important scale in the studies of environment impacts in aquatic habitats. Hydrographic basins with intensive agricultural land use are subjected to great inputs of pesticides (Moreno-González et al., 2013MORENO-GONZÁLEZ, R., CAMPILLO, J.A. and LEÓN, V.M., 2013. Influence of an intensive agricultural drainage basin on the seasonal distribution of organic pollutants in seawater from a Mediterranean coastal lagoon (Mar Menor, SE Spain). Marine Pollution Bulletin, vol. 77, no. 1-2, pp. 400-411. http://dx.doi.org/10.1016/j.marpolbul.2013.09.040. PMid:24139646.
http://dx.doi.org/10.1016/j.marpolbul.20...
) and metals on water bodies (Naveedullah et al., 2013NAVEEDULLAH., HASHMI, M.Z., YU, C., SHEN, H., DUAN, D., SHEN, C., LOU, L. and CHEN, Y., 2013. Risk Assessment of Heavy Metals Pollution in Agricultural Soils of Siling Reservoir Watershed in Zhejiang Province, China. BioMed Research International, vol. 2013, pp. 590306. http://dx.doi.org/10.1155/2013/590306. PMid:24151611.
http://dx.doi.org/10.1155/2013/590306...
).
In this context, it is essential to understand how aforementioned pollutants interfere with the health of aquatic environments as well of the species that inhabit them, especially in basins affected by anthropogenic uses. Biomarkers are an excellent tool to assess effects of multiple stressors on living organisms (Damásio et al., 2011aDAMÁSIO, J., BARCELÓ, D., BRIX, R., POSTIGO, C., GROS, M., PETROVIC, M., SABATER, S., GUASCH, H., DE ALDA, M.L. and BARATA, C., 2011a. Are pharmaceuticals more harmful than other pollutants to aquatic invertebrate species: a hypothesis tested using multi-biomarker and multi-species responses in field collected and transplanted organisms. Chemosphere, vol. 85, no. 10, pp. 1548-1554. http://dx.doi.org/10.1016/j.chemosphere.2011.07.058. PMid:21925701.
http://dx.doi.org/10.1016/j.chemosphere....
; Colin et al., 2016COLIN, N., PORTE, C., FERNANDES, D., BARATA, C., PADRÓS, F., CARRASSÓN, M., MONROY, M., CANO-ROCABAYERA, O., SOSTOA, A., PIÑA, B. and MACEDA-VEIGA, A., 2016. Ecological relevance of biomarkers in monitoring studies of macro-invertebrates and fish in Mediterranean rivers. The Science of the Total Environment, vol. 540, pp. 307-323. http://dx.doi.org/10.1016/j.scitotenv.2015.06.099. PMid:26148426.
http://dx.doi.org/10.1016/j.scitotenv.20...
), because they provide early warning signals at individual level to predict outcomes at population or community (Chapman et al., 2013CHAPMAN, P.M., WANG, F. and CAEIRO, S.S., 2013. Assessing and managing sediment contamination in transitional waters. Environment International, vol. 55, pp. 71-91. http://dx.doi.org/10.1016/j.envint.2013.02.009. PMid:23528483.
http://dx.doi.org/10.1016/j.envint.2013....
).
Oxidative stress is one of the biological conditions that can be induced by exposure to metals (Kaur et al., 2014KAUR, R., KAUR, J., MAHAJAN, J., KUMAR, R. and ARORA, S., 2014. Oxidative stress-implications, source and its prevention. Environmental Science and Pollution Research International, vol. 21, no. 3, pp. 1599-1613. http://dx.doi.org/10.1007/s11356-013-2251-3. PMid:24170504.
http://dx.doi.org/10.1007/s11356-013-225...
) and pesticides (Ndonwi et al., 2019NDONWI, E.N., ATOGHO-TIEDEU, B., LONTCHI-YIMAGOU, E., SHINKAFI, T.S., NANFA, D., BALTI, E.V., INDUSMITA, R., MAHMOOD, A., KATTE, J.-C., ARMMBANYA., MATSHA, T., MBANYA, J.C., SHAKIR, A. and SOBNGWI, E., 2019. Gestational exposure to pesticides induces oxidative stress and lipid peroxidation in offspring that persist at adult age in an animal model. Toxicological Research, vol. 35, no. 3, pp. 241-248. http://dx.doi.org/10.5487/TR.2019.35.3.241. PMid:31341553.
http://dx.doi.org/10.5487/TR.2019.35.3.2...
). An organism in oxidative stress has impairment of redox homeostasis and use different defenses mechanisms to deal with this condition. The antioxidant defense can occur with help of the enzymes such as catalase (CAT), which is present in virtually all aerobic organisms (Halliwell and Gutteridge, 2007HALLIWELL, B. and GUTTERIDGE, J.M.C., 2007. Free radicals in biology and medicine. 4th ed. New York: Oxford University Press.). Another important line of defense involves the glutathione system, within which enzyme Glutathione Reductase (GR) plays a key role in the recycling of reduced glutathione molecule (Halliwell and Gutteridge, 2007HALLIWELL, B. and GUTTERIDGE, J.M.C., 2007. Free radicals in biology and medicine. 4th ed. New York: Oxford University Press.). If the antioxidant defense systems are not sufficiently efficient to cope with the stress, the body may be damaged at different cellular targets including oxidation of membrane lipids. This oxidation generates organic peroxides that can be detected by analysis of thiobarbituric acid reactive substances (TBARS) (Regoli and Winston, 1999REGOLI, F. and WINSTON, G.W., 1999. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicology and Applied Pharmacology, vol. 156, no. 2, pp. 96-105. http://dx.doi.org/10.1006/taap.1999.8637. PMid:10198274.
http://dx.doi.org/10.1006/taap.1999.8637...
). CAT, GR and TBARS are employed in environmental quality assessments because they are robust indicators with easy experimental evaluation (Damásio et al., 2008 DAMÁSIO, J., TAULER, R., TEIXIDÓ, E., RIERADEVALL, M., PRAT, N., RIVA, M.C., SOARES, A.M. and BARATA, C., 2008. Combined use of Daphnia magna in situ bioassays, biomarkers and biological indices to diagnose and identify environmental pressures on invertebrate communities in two Mediterranean urbanized and industrialized rivers (NE Spain). Aquatic Toxicology, vol. 87, no. 4, pp. 310-320. http://dx.doi.org/10.1016/j.aquatox.2008.02.016. PMid:18420289.
http://dx.doi.org/10.1016/j.aquatox.2008...
, 2011bDAMÁSIO, J., FERNÁNDEZ-SANJUAN, M., SÁNCHEZ-AVILA, J., LACORTE, S., PRAT, N., RIERADEVALL, M., SOARES, A.M.V.M. and BARATA, C., 2011b. Multi-biochemical responses of benthic macroinvertebrate species as a complementary tool to diagnose the cause of community impairment in polluted rivers. Water Research, vol. 45, no. 12, pp. 3599-3613. http://dx.doi.org/10.1016/j.watres.2011.04.006. PMid:21571352.
http://dx.doi.org/10.1016/j.watres.2011....
). In addition, to being responsive to changes in land use and contamination by metals, pesticides and other pollutants (Damásio et al., 2011bDAMÁSIO, J., FERNÁNDEZ-SANJUAN, M., SÁNCHEZ-AVILA, J., LACORTE, S., PRAT, N., RIERADEVALL, M., SOARES, A.M.V.M. and BARATA, C., 2011b. Multi-biochemical responses of benthic macroinvertebrate species as a complementary tool to diagnose the cause of community impairment in polluted rivers. Water Research, vol. 45, no. 12, pp. 3599-3613. http://dx.doi.org/10.1016/j.watres.2011.04.006. PMid:21571352.
http://dx.doi.org/10.1016/j.watres.2011....
).
In this work, freshwater crustaceans of Aegla genus Leach (1820) were used as bioindicadors for oxidative stress ecotoxicological analysis. Invertebrates of this genus are links in food chain (Bueno and Bond-Buckup, 2004BUENO, A.A.P. and BOND-BUCKUP, G., 2004. Natural diet of Aegla platensis Schmitt and Aegla ligulata. Acta Limnologica Brasiliensia, vol. 16, pp. 115-127.; Cogo and Santos, 2013COGO, G.B. and SANTOS, S., 2013. The role of aeglids in shredding organic matter in Neotropical streams. Journal of Crustacean Biology, vol. 33, no. 4, pp. 519-526. http://dx.doi.org/10.1163/1937240X-00002165.
http://dx.doi.org/10.1163/1937240X-00002...
) as well are good models for in situ analyses of oxidative stress biomarkers (Borges et al., 2018BORGES, A.C.P., PIASSÃO, J.F.G., PAULA, M.O., SEPP, S., BEZ, C.F.S., HEPP, L.U., VALDUGA, A.T., PEREIRA, A.A.M. and CANSIAN, R.L., 2018. Characterization of oxidative stress biomarkers in a freshwater anomuran crab. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 61-67. http://dx.doi.org/10.1590/1519-6984.04816. PMid:28614422.
http://dx.doi.org/10.1590/1519-6984.0481...
). Thus, our objective was characterized three hydrographic basins in relation to the metals set in their sediments and in relation to the land uses, and so correlated these data with oxidative stress biomarkers in Aegla crabs. The main hypothesis of this work is that GR, CAT and TBARS, will response to the metals set and agricultural use at hydrographic basin scale.
2. Material and Methods
2.1. Characterization of study area and sampling period
The study was performed in 14 streams (≤ 2nd order) within 3 basins of Southern Brazil (Suzana River basin, Ligeirinho-Leãozinho River basin and Dourado River basin) (Figure 1). The climate belongs to category Cfb (Köppen), mean annual temperature is 17.6 °C and rainfall annual mean of 1912.3 mm (Alvares et al., 2013ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., GONÇALVES, J.L. and SPAROVEK, G., 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, vol. 22, no. 6, pp. 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507.
http://dx.doi.org/10.1127/0941-2948/2013...
). The rainfall mean along the sampling period was about 151 mm (INMET, 2019INSTITUTO NACIONAL DE METEREOLOGIA – INMET, 2019 [viewed 2 May 2020]. Boletins climáticos para o Rio Grande do Sul: ano 2019 [online]. Available from: http://www.inmet.gov.br/portal/index.php?r=clima/boletimRioGrandeDoSul
http://www.inmet.gov.br/portal/index.php...
) and the water temperature was 16.8 ± 2.5 °C (our data).
Map of the sampling sites. (A) Suzana River basin (between 27°35’38” and 27°36’16”S; 52°11’11” and 52°13’41”W); (B) Ligeirinho-Leãozinho River basin (between 27°40’15” and 27°36’16”S; 52°16’03” and 52°13’41”W); (C) Dourado River basin (between 27°33’59” and 27°37’13”S; 52°17’46” and 52°19’36”W). The circles (•) indicate the sampled streams. The numbered boxes indicate the areas of land uses analysis.
The rivers sampled inside each basin were characterized in relation to drainage area land uses. For this purpose, the perimeter of analyses was defined around the 2 streams (upstream and its respective downstream stretch) in the same main river, resulting in 7 sampling points (Figure 1 – numbered boxes). The land uses classes were percentage of water blade, exposed soil, natural vegetation, agriculture, pasture and urbanized area, obtained with Mapinfo 8.5 software.
The sampling of biological material and sediment were carried out between March and June 2014 (fall season of south hemisphere). This period was defined to avoid the capture of juvenile crabs (Bueno and Bond-Buckup, 2004BUENO, A.A.P. and BOND-BUCKUP, G., 2004. Natural diet of Aegla platensis Schmitt and Aegla ligulata. Acta Limnologica Brasiliensia, vol. 16, pp. 115-127.; Noro and Buckup, 2002NORO, C.K. and BUCKUP, L., 2002. Biologia reprodutiva e ecologia de Aegla leptoaectyla Buckup & Rossi (Crustacea, Anomura, Aeglidae). Revista Brasileira de Zoologia, vol. 19, no. 4, pp. 1063-1079. http://dx.doi.org/10.1590/S0101-81752002000400011.
http://dx.doi.org/10.1590/S0101-81752002...
). In all the cases, the sampling of Aegla and sediment from streams was made at same day.
2.2. Metals analysis in sediment
Concentrations of Cu (copper), Cr (chromium), Cd (cadmium), Fe (iron), Mn (manganese) and Zn (zinc) were analyzed in the potentially bioavailable fraction of the sediment. In each stream were collected three samples of sediment in the first 10 cm depth, using a sampler of 70 mm diameter. In the laboratory, they were dried at 45 °C/24h and sieved on sieve ≤ 62 µm mesh. The resulting powder was diluted in 10 mL of HCl 0.1 mol L-1 (150 rpm, 20 °C, 12h), filtered with 25 μm filter and keep at 4 °C until analyses by flame atomic absorption spectrophotometry (Varian, SpectraAA55). For each metal, were generated standard curves used to calculate the metal concentration in the samples. The results are presented as mean ± standard error.
2.3. Organisms and biological extracts
The crabs were collected using dip nets with a 30 x 50 cm mouth, a depth 60 cm and 1.0 mm mesh. The study was made only with non ovigerous female of Aegla, all that being adults (with at least 15 mm carapace length). Females have no seasonal variation in oxidative stress biomarkers, in opposite to the observed in males (Borges et al., 2018BORGES, A.C.P., PIASSÃO, J.F.G., PAULA, M.O., SEPP, S., BEZ, C.F.S., HEPP, L.U., VALDUGA, A.T., PEREIRA, A.A.M. and CANSIAN, R.L., 2018. Characterization of oxidative stress biomarkers in a freshwater anomuran crab. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 61-67. http://dx.doi.org/10.1590/1519-6984.04816. PMid:28614422.
http://dx.doi.org/10.1590/1519-6984.0481...
). Sex and gender identification were done in field according to Melo (2003)MELO, G.A.S., 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo: Edições Loyola.. Were collected 3 organisms per sampling site, totalizing 42 organisms (18 organisms from Suzana River basin; 12 from Ligeirinho-Leãozinho River basin; 12 from Dourado River basin). The organisms were kept inside of ice boxes and transported alive to the laboratory in flasks containing water from the own sampling site. The time interval between collection and arrival at the laboratory did not exceed 20 minutes. Once in the laboratory, each crab was used to the preparation of an individual biological extract (each crab considered as a sample unit) as described following. For the development of a robust assessment, in preparation of biological extracts we chose to use whole organisms, without separation of organs or tissues (except exoskeleton).
2.4. Oxidative stress biomarkers analyses
Biological extracts were obtained from individual crabs (Borges et al., 2018BORGES, A.C.P., PIASSÃO, J.F.G., PAULA, M.O., SEPP, S., BEZ, C.F.S., HEPP, L.U., VALDUGA, A.T., PEREIRA, A.A.M. and CANSIAN, R.L., 2018. Characterization of oxidative stress biomarkers in a freshwater anomuran crab. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 61-67. http://dx.doi.org/10.1590/1519-6984.04816. PMid:28614422.
http://dx.doi.org/10.1590/1519-6984.0481...
). Protein determination was assayed according to Bradford (1976)BRADFORD, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, vol. 72, no. 1-2, pp. 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3. PMid:942051.
http://dx.doi.org/10.1016/0003-2697(76)9...
. CAT (EC 1.11.1.6) activity was measured by rate of H2O2 degradation rate at 240 nm (Bertholdo-Vargas et al., 2009BERTHOLDO-VARGAS, L.R., MARTINS, J.N., BORDIN, D., SALVADOR, M., SCHAFER, A.L., BARROS, N.M., BARBIERI, L., STIRPE, F. and CARLINI, C.R., 2009. Type 1 ribosome-inactivating proteins - Entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: noctuidae). Journal of Insect Physiology, vol. 55, no. 1, pp. 51-58. http://dx.doi.org/10.1016/j.jinsphys.2008.10.004. PMid:19000694.
http://dx.doi.org/10.1016/j.jinsphys.200...
) and was expressed in international units (U), which is defined as the amount of enzyme that catalyzes the degradation of 1μmol H2O2 min-1 mg-1 protein. GR (EC 1.6.4.2) activity was measured following NADPH consumption at 340 nm (Ramos-Vasconcelos and Hermes-Lima, 2003RAMOS-VASCONCELOS, G.R. and HERMES-LIMA, M., 2003. Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. The Journal of Experimental Biology, vol. 206, no. Pt 4, pp. 675-685. http://dx.doi.org/10.1242/jeb.00124. PMid:12517985.
http://dx.doi.org/10.1242/jeb.00124...
). Were performed kinetic reactions of 6 minutes, with data acquisition at each 30 seconds, being that in the first 3 minutes was made the reading of blank (buffer, NADPH and biological samples) and then the reaction was initiated by GSSG addition. GR activity was expressed in international units (U), defined as the amount of enzyme that catalyzes the consumption of 1μmol NADPH min-1 mg-1 protein. TBARS levels were quantified in according to Esterbauer and Cheeseman (1990)ESTERBAUER, H. and CHEESEMAN, K.H., 1990. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology, vol. 186, pp. 407-421. http://dx.doi.org/10.1016/0076-6879(90)86134-H. PMid:2233308.
http://dx.doi.org/10.1016/0076-6879(90)8...
and were expressed as nmol MDA mg-1 protein. The data are presenting as mean ± standard error. Biochemical analyses were performed at least in triplicate. Biomarkers general means were obtained from the average of replications of each individual organism (each crab was considering one sample unit).
2.5. Data analysis
The normality of data was tested by Anderson-Darling test. Only TBARS not fit in normal curve and was then normalized by logarithmic conversion using box-cox approach. The metal content in sediment as well biomarkers in Aegla between three basins were compared by one way ANOVA and Tukey post hoc test. A principal component analysis (PCA) was performed to evaluate the metal distribution in sediment of the three basins. The metal sediment matrix was standardized in 0-1 values using the ‘range’ function of “vegan” package (Oksanen et al., 2010OKSANEN, J., BLANCHET, F.G., KINDT, R., LEGENDRE, P., O’HARA, R.B., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H. and WAGNER, H. 2010 [viewed 2 May 2020]. Multivariate analysis of ecological communities [online]. Vienna: R Foundation for Statistical Computing. Available from: https://vegan.r-forge.r-project.org/
https://vegan.r-forge.r-project.org/...
). This standardization considers the maximum values within each data set variables. Pearson correlation test was used to analyze the correlation between the metal concentration and biomarker levels, and Spearman correlation was employed to analyze the correlation between biomarkers and land uses. The analyses were performed using “vegan” package (Oksanen et al., 2010OKSANEN, J., BLANCHET, F.G., KINDT, R., LEGENDRE, P., O’HARA, R.B., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H. and WAGNER, H. 2010 [viewed 2 May 2020]. Multivariate analysis of ecological communities [online]. Vienna: R Foundation for Statistical Computing. Available from: https://vegan.r-forge.r-project.org/
https://vegan.r-forge.r-project.org/...
) software R (R Development Core Team, 2010R DEVELOPMENT CORE TEAM, 2010. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from: http://www.R-project.org
http://www.R-project.org...
) and p < 0.05 were considered statistically significant.
3. Results
3.1. Hydrographic basins land uses
The rivers of all basins studied presented high percentage of anthropogenic land uses, with lower value observed inside Suzana River basin (58.8%) and the upper value on Ligeirinho-Leãozinho basin (87.5%) (Table 1). In these two basins, the main anthropogenic influence was exposed soil, while agriculture and pasture predominates in Dourado River basin.
3.2. Metal concentration in sediment
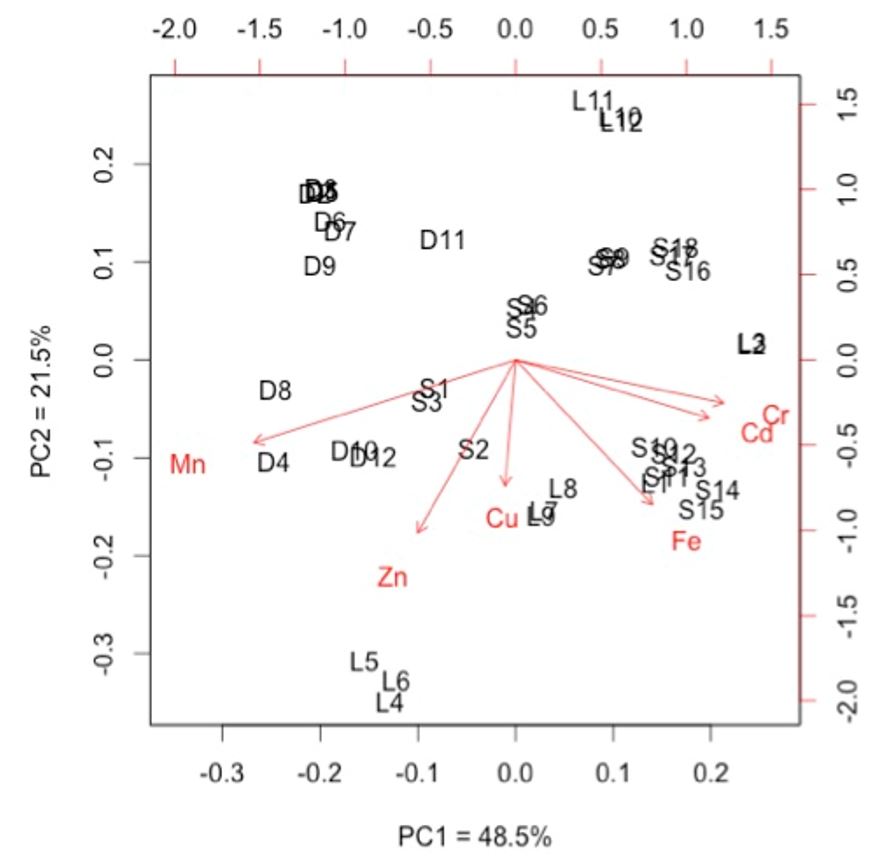
Metal content varied between the hydrographic basins (Table 2). Dourado River basin presented the lower concentrations of Cd, Cr, Fe and the highest level of Mn, distinguished itself from the two other basins. Suzana River basin and Ligeirinho-Leãozinho River basin presented similar metal composition in the sediment, except for Fe and Zn (Table 2). In agreement, PCA also showed that metals were distributed in relation to hydrographic basin. The first two principal components (PC) explained 70% of the variation, with PC1 explained 48.5% and PC2 explained 21.5% of data variation (Figure 2). For Dourado River basin, Mn concentration ordered the study sites. In Ligeirinho-Leãozinho River basin, the main ordination factor was Zn concentration. For Suzana River basin, the concentration of Fe, Cr and Cd ordered studied sites and presented partial superposition with the Ligeirinho-Leãozinho River basin (Figure 2).
Mean ± standard error of metal concentration in bioavailable fraction of the sediment in the studied hydrographic basins (Suzana River basin, Ligeirinho-Leãozinho River basin, Dourado River basin).
Biplot of PCA ordination for the metal concentration in sediment of the three studied basins. Suzana River basin (S); Ligeirinho-Leãozinho River basin (L); Dourado River basin (D).
3.3. Oxidative stress biomarkers
TBARS, CAT and GR showed variation between the studied hydrographic basins (Figure 3). CAT and TBARS had a significant negative correlation at basin scale (r=-0.98, p=0.039). TBARS levels was approximately 60% higher in the organisms from Dourado River basin as compared to Suzana River basin (0.75 and 0.47 nmol MDA mg-1 protein, respectivelly). CAT showed opposite pattern, highest enzymatic activity was observed in organisms from Suzana River basin (11.48 U), whereas lower enzymatic activity occurred in organisms from Dourado River basin (7.27 U) (Figure 3). GR activity was also higher in Dourado river basin (72.6 U), reaching values 28% and 53% upper in relation to Ligeirinho-Leãozinho and Suzana Rivers basins (56.76 and 47.34 U), respectively. However, GR activity no have significant correlation with the two other biomarkers.
Biomarkers grouped by hydrographic basin (Suzana River basin, Ligeirinho-Leãozinho River basin, Dourado River basin). Different letters indicate significant differences (p < 0.05), as compared by one-way ANOVA plus Tukey post-test (between basins).
3.4. Metal concentration, land uses and oxidative stress biomarkers
Several analyzed metals showed correlation with studied biomarkers (Table 3). CAT presented positive correlation with Cd, Cr and Fe, while was negatively related to Mn concentration. The concentrations of Cd, Cr and Fe in sediment were also correlated to TBARS and GR biomarkers; however, in these cases the correlations were negative. In relation to the land uses, TBARS was correlated positively with agricultural land use. The remain land uses no presented any significant correlation with biomarkers (Table 4).
Correlation (r) between sediment metal concentration and oxidative stress biomarkers (TBARS, CAT and GR).
4. Discussion
In this work, we quantify the concentrations of metals in stream sediments in three watersheds and evaluate the relationship with oxidative stress biomarkers in Aegla crabs. We observed that the relationships between metals and biomarkers were different and that agricultural use was related to oxidative stress (TBARS) of studied organisms.
Metals are common elements on lotic environments in which the metals variety and magnitude will dependent to the natural sources and contamination by external inputs as well of land uses (Thévenot et al., 2007THÉVENOT, D.R., MOILLERON, R., LESTEL, L., GROMAIRE, M.C., ROCHER, V., CAMBIER, P., BONTÉ, P., COLIN, J.L., DE PONTEVÈS, C. and MEYBECK, M., 2007. Critical budget of metal sources and pathways in the Seine River basin (1994-2003) for Cd, Cr, Cu, Hg, Ni, Pb and Zn. The Science of the Total Environment, vol. 375, no. 1-3, pp. 180-203. http://dx.doi.org/10.1016/j.scitotenv.2006.12.008. PMid:17267024.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Proshad et al., 2019PROSHAD, R., KORMOKER, T. and ISLAM, S., 2019. Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa River, Bangladesh. Toxin Reviews, pp. 1-25. http://dx.doi.org/10.1080/15569543.2018.1564143.
http://dx.doi.org/10.1080/15569543.2018....
). In this study, the three analyzed hydrographic basins presented singularities related to the set of metal in their sediments. This result reflects the diffuse nature of water contaminations in rural areas (Turunen et al., 2019TURUNEN, J., MARKKULA, J., RAJAKALLIO, M. and AROVIITA, J., 2019. Riparian forests mitigate harmful ecological effects of agricultural diffuse pollution in medium-sized streams. The Science of the Total Environment, vol. 649, pp. 495-503. http://dx.doi.org/10.1016/j.scitotenv.2018.08.427. PMid:30176461.
http://dx.doi.org/10.1016/j.scitotenv.20...
), which are affected by variables as uncontrolled management of fertilizers (Smith and Siciliano, 2015SMITH, L.E.D. and SICILIANO, G.A., 2015. Comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agriculture, Ecosystems & Environment, vol. 209, no. 1, pp. 15-25. http://dx.doi.org/10.1016/j.agee.2015.02.016.
http://dx.doi.org/10.1016/j.agee.2015.02...
), rainfall rate (Schulz, 2001SCHULZ, R., 2001. Rainfall-induced sediment and pesticide input from orchards into the Lourens river, western cape, South Africa: importance of a single event. Water Research, vol. 35, no. 8, pp. 1869-1876. http://dx.doi.org/10.1016/S0043-1354(00)00458-9. PMid:11337831.
http://dx.doi.org/10.1016/S0043-1354(00)...
), soil erosion (Thévenot et al., 2007THÉVENOT, D.R., MOILLERON, R., LESTEL, L., GROMAIRE, M.C., ROCHER, V., CAMBIER, P., BONTÉ, P., COLIN, J.L., DE PONTEVÈS, C. and MEYBECK, M., 2007. Critical budget of metal sources and pathways in the Seine River basin (1994-2003) for Cd, Cr, Cu, Hg, Ni, Pb and Zn. The Science of the Total Environment, vol. 375, no. 1-3, pp. 180-203. http://dx.doi.org/10.1016/j.scitotenv.2006.12.008. PMid:17267024.
http://dx.doi.org/10.1016/j.scitotenv.20...
) and topography of the watershed (Ye et al., 2009YE, L., CAI, Q., LIU, R. and CAO, M., 2009. The influence of topography and land use on water quality of Xiangxi River in Three Gorges Reservoir region. Environmental Geology, vol. 58, no. 5, pp. 937-942. http://dx.doi.org/10.1007/s00254-008-1573-9.
http://dx.doi.org/10.1007/s00254-008-157...
).
The concentration of several metals at the rivers sediment showed correlation with the levels of oxidative stress biomarkers in Aegla. Sediments are sites for long-term metals storage and consequently their profile is an important indicator of environmental quality (Qu et al., 2017QU, X., REN, Z., ZHANG, M., LIU, X. and PENG, W., 2017. Sediment heavy metals and benthic diversities in Hun-Tai River, northeast of China. Environmental Science and Pollution Research International, vol. 24, no. 11, pp. 10662-10673. http://dx.doi.org/10.1007/s11356-017-8642-0. PMid:28283976.
http://dx.doi.org/10.1007/s11356-017-864...
). Metals in sediments are able to bioaccumulate in aquatic organisms and this process is influenced by the feeding strategy and agricultural activity around streams (Loureiro et al., 2018LOUREIRO, R.C., MENEGAT, M., RESTELLO, R.M. and HEPP, L.U., 2018. Incorporation of zinc and copper by insects of different functional feeding groups in agricultural streams. Environmental Science and Pollution Research International, vol. 25, no. 18, pp. 17402-17408. http://dx.doi.org/10.1007/s11356-018-1971-9. PMid:29654465.
http://dx.doi.org/10.1007/s11356-018-197...
). Aegla crab is a filter macroinvertebrate and feeds on lower invertebrates and leaf debris (Cogo and Santos, 2013COGO, G.B. and SANTOS, S., 2013. The role of aeglids in shredding organic matter in Neotropical streams. Journal of Crustacean Biology, vol. 33, no. 4, pp. 519-526. http://dx.doi.org/10.1163/1937240X-00002165.
http://dx.doi.org/10.1163/1937240X-00002...
), which facilities the access to sediment content. The bioaccumulation of Cd in Aegla is negatively correlated with the Cd presence in the stream sediments (Piassão et al., 2019PIASSÃO, J.F.G., GASPARIN, B.G., MARTINS, M.C., DECIAN, V.S., CANSIAN, R.L., RESTELLO, R.M. and MIELNICZKI-PEREIRA, A.A., 2019. Analysis of metals bioaccumulation and oxidative stress biomarkers in crustaceans of Aegla genus (Crustacea, Anomura). Perspectiva, vol. 43, no. 161, pp. 111-122.) which could suggest an easily of this metal transfer into the crab body.
The Dourado River basin was the most differentiated in terms of metal composition, showing the lower levels of Cd, Cr and Fe. Those last are metals with high potential to generate oxidative stress (Valko et al., 2005VALKO, M., MORRIS, H. and CRONIN, M.T.D., 2005. Metals, toxicity and oxidative stress. Current Medicinal Chemistry, vol. 12, no. 10, pp. 1161-1208. http://dx.doi.org/10.2174/0929867053764635. PMid:15892631.
http://dx.doi.org/10.2174/09298670537646...
), so it’s surprising that samples of Aegla from Dourado River basin had presented the highest values of TBARS. On the other hand, the Dourado River basin has a predominance of agricultural land use. This is related to the increase in lipid peroxidation in Aegla, since the TBARS showed a strong correlation with agriculture in the river basins. Agriculture is an important economic activity in the study region, with crop rotation during the year (Rovani et al., 2020ROVANI, I.L., DECIAN, V.S., ZANIN, E.M., BRANDALISE, M., QUADROS, F.R. and HEPP, L.H., 2020. Socioeconomic changes and land use and land cover of the Northern Region of Rio Grande do Sul, Brazil. Floresta e Ambiente, vol. 27, no. 3, e20180258. http://dx.doi.org/10.1590/2179-8087.025818.
http://dx.doi.org/10.1590/2179-8087.0258...
), which promotes a continuous use of agrochemicals and pesticides (Lima et al., 2020LIMA, J.A.M.C., LABANOWSKI, J., BASTOS, M.C., ZANELLA, R., PRESTES, O.D., VARGAS, J.P.R., MONDAMERT, L., GRANADO, E., TIECHER, T., ZAFAR, M., TROJAN, A., LE GUET, T. and SANTOS, D.R., 2020. “Modern agriculture” transfers many pesticides to watercourses: a case study of a representative rural catchment of southern Brazil. Environmental Science and Pollution Research International, vol. 27, no. 10, pp. 10581-10598. http://dx.doi.org/10.1007/s11356-019-06550-8. PMid:31942716.
http://dx.doi.org/10.1007/s11356-019-065...
). These latter can contaminate water bodies by spraying, leaching, erosion processes, among others (Peters et al., 2013PETERS, K., BUNDSCHUH, M. and SCHÄFER, R.B., 2013. Review on the effects of toxicants on freshwater ecosystem functions. Environmental Pollution, vol. 180, pp. 324-329. http://dx.doi.org/10.1016/j.envpol.2013.05.025. PMid:23725857.
http://dx.doi.org/10.1016/j.envpol.2013....
). Beside metals, several pesticides also have a strong ability to generate oxidative stress and, in particular, increasing lipid peroxidation (Clasen et al., 2018CLASEN, B., LORO, V.L., MURUSSI, C.R., TIECHER, T.L., MORAES, B. and ZANELLA, R., 2018. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. The Science of the Total Environment, vol. 626, pp. 737-743. http://dx.doi.org/10.1016/j.scitotenv.2018.01.154. PMid:29358144.
http://dx.doi.org/10.1016/j.scitotenv.20...
; Ndonwi et al., 2019NDONWI, E.N., ATOGHO-TIEDEU, B., LONTCHI-YIMAGOU, E., SHINKAFI, T.S., NANFA, D., BALTI, E.V., INDUSMITA, R., MAHMOOD, A., KATTE, J.-C., ARMMBANYA., MATSHA, T., MBANYA, J.C., SHAKIR, A. and SOBNGWI, E., 2019. Gestational exposure to pesticides induces oxidative stress and lipid peroxidation in offspring that persist at adult age in an animal model. Toxicological Research, vol. 35, no. 3, pp. 241-248. http://dx.doi.org/10.5487/TR.2019.35.3.241. PMid:31341553.
http://dx.doi.org/10.5487/TR.2019.35.3.2...
).
Reduction of CAT activity can occur by in vivo exposure to pesticides and herbicides (Toni et al., 2010TONI, C., MENEZES, C.C., LORO, V.L., CLASEN, B.E., CATTANEO, R., SANTI, A., PRETTO, A., ZANELLA, R. and LEITEMPERGER, J., 2010. Oxidative stress biomarkers in Cyprinus carpio exposed to commercial herbicide bispyribac‐sodium. Journal of Applied Toxicology, vol. 30, no. 6, pp. 590-595. http://dx.doi.org/10.1002/jat.1530. PMid:20809548.
http://dx.doi.org/10.1002/jat.1530...
; Ndonwi et al., 2019NDONWI, E.N., ATOGHO-TIEDEU, B., LONTCHI-YIMAGOU, E., SHINKAFI, T.S., NANFA, D., BALTI, E.V., INDUSMITA, R., MAHMOOD, A., KATTE, J.-C., ARMMBANYA., MATSHA, T., MBANYA, J.C., SHAKIR, A. and SOBNGWI, E., 2019. Gestational exposure to pesticides induces oxidative stress and lipid peroxidation in offspring that persist at adult age in an animal model. Toxicological Research, vol. 35, no. 3, pp. 241-248. http://dx.doi.org/10.5487/TR.2019.35.3.241. PMid:31341553.
http://dx.doi.org/10.5487/TR.2019.35.3.2...
). In the present work, the lower levels of CAT were measured in organism from Dourado River basin, in which agricultural activity was predominant. Besides, our data showed an inverse correlation between TBARS and CAT activity in Aegla. So, it is possible infer that agricultural land uses, and consequently, their contaminants, could be inhibiting CAT in Aegla from Dourado River basin. As a consequence of this, there may be a favoring of lipid peroxidation in these organisms. The inverse profile of CAT and lipid peroxidation in response to agrochemicals was described for Eriocheir sinensis crab exposed to subletal concentrations of avermectin (Huang et al., 2019HUANG, Y., HONG, Y., HUANG, Z., ZHANG, J. and HUANG, Q., 2019. Avermectin induces the oxidative stress, genotoxicity, and immunological responses in the Chinese Mitten Crab, Eriocheir sinensis. PLoS One, vol. 14, no. 11, pp. e0225171. http://dx.doi.org/10.1371/journal.pone.0225171. PMid:31765405.
http://dx.doi.org/10.1371/journal.pone.0...
) and to synthetic pyrethroid deltamethrin (Hong et al., 2019HONG, Y., HUANG, Y., YAN, G. and HUANG, Z., 2019. Effects of deltamethrin on the antioxidant defense and heat shock protein expression in Chinese mitten crab, Eriocheir sinensis. Environmental Toxicology and Pharmacology, vol. 66, pp. 1-6. http://dx.doi.org/10.1016/j.etap.2018.12.012. PMid:30584970.
http://dx.doi.org/10.1016/j.etap.2018.12...
). The increased GR activity in Dourado River basin can represents a response of the Aegla to cope with stress using defenses linked to the glutathione antioxidant system, as well for xenobiotics detoxification by Gluthatione-S-Transferase. This behavior has already been observed in crabs exposed to pesticides avermectin, trichlorphon and β-cypermethrin (Zhang et al., 2020ZHANG, X., SHEN, G., WANG, Y., HUANG, P., AME, K.H., ZANG, Y. and SHEN, H., 2020. Molecular characterization, expression and enzyme activity of three glutathione S-transferase genes from Eriocheir sinensis under pesticide stresses. Comparative Biochemistry and Physiology. Part C, vol. 230, pp. 108700. http://dx.doi.org/10.1016/j.cbpc.2019.108700. PMid:31899308.
http://dx.doi.org/10.1016/j.cbpc.2019.10...
).
The three analyzed biomarkers were correlate with a common group of metals (Cd, Cr and Fe). CAT activity can be influenced differently depending on the nature and magnitude of contact with metallic elements. For example, Cd is known for its ability to inhibit catalase in vivo and in vitro (Wang et al., 2015WANG, J., ZHANG, H., ZHANG, T., ZHANG, R., LIU, R. and CHEN, Y., 2015. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. International Journal of Biological Macromolecules, vol. 77, pp. 59-67. http://dx.doi.org/10.1016/j.ijbiomac.2015.02.037. PMid:25795390.
http://dx.doi.org/10.1016/j.ijbiomac.201...
). However, in Sinopotamon henanense freshwater crab, the treatment with Cd can both stimulates or inhibit CAT, depending on the concentration (Wu et al., 2013WU, H., XUAN, R., LI, Y., ZHANG, X., WANG, Q. and WANG, L., 2013. Effects of cadmium exposure on digestive enzymes, antioxidant enzymes, and lipid peroxidation in the freshwater crab Sinopotamon henanense. Environmental Science and Pollution Research International, vol. 20, no. 6, pp. 4085-4092. http://dx.doi.org/10.1007/s11356-012-1362-6. PMid:23224505.
http://dx.doi.org/10.1007/s11356-012-136...
). In Tubifex tubifex (a freshwater worm) the simultaneous treatment with Cd and Fe increase CAT in vivo, being that certain concentrations of Fe appears to protect against Cd toxicity (Chen et al., 1994CHEN, T., FURST, A. and CHIEN, P.K., 1994. The effects of cadmium and iron on catalase activities in tubifex. International Journal of Toxicology, vol. 13, no. 2, pp. 112-120. http://dx.doi.org/10.3109/10915819409140992.
http://dx.doi.org/10.3109/10915819409140...
). Cr are also associated with increase of CAT activity in vivo (Elia et al., 2007ELIA, A.C., GALARINI, R., MARTIN DÖRR, A.J. and TATICCHI, M.I., 2007. Heavy metal contamination and antioxidant response of a freshwater bryozoan (Lophopus crystallinus Pall., Phylactolaemata). Ecotoxicology and Environmental Safety, vol. 66, no. 2, pp. 188-194. http://dx.doi.org/10.1016/j.ecoenv.2005.12.004. PMid:16469378.
http://dx.doi.org/10.1016/j.ecoenv.2005....
). Our results indicated that Cd, Cr and Fe concentrations in sediment of the Suzana and Ligeirinho-Leãozinho Rivers basins, having the effect of CAT induction and, consequently, containing the lipid peroxidation in Aegla from these hydrographic basins.
In summary, this work showed that both agriculture land use and the concentration of metals in sediments affect oxidative biomarkers in Aegla. Basins with higher content of poisoning metals leads to an adaptive response mediate by increase in CAT activity, which reduces TBARS levels. On the other hand, the hydrographic basin with minor metals contamination (Dourado River basin) was primarily influenced by the agricultural land use. In these case, the agrochemicals toxicity overcomes the effect of metals, resulting in CAT inhibition and adjustment of GR levels, which does not seem to be enough to contain oxidative stress, as TBARS levels remain high.
Acknowledgements
The authors thank National Council for Scientific and Technological Development - CNPq (Grants n. 473648/2013-0), Foundation for Research Support of the State of Rio Grande do Sul – FAPERGS, all them from Brazil, for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES). LUH receives CNPq (421632/2016-0) and CNPq grants (305203/2017-7).
References
- ALVARES, C.A., STAPE, J.L., SENTELHAS, P.C., GONÇALVES, J.L. and SPAROVEK, G., 2013. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift, vol. 22, no. 6, pp. 711-728. http://dx.doi.org/10.1127/0941-2948/2013/0507
» http://dx.doi.org/10.1127/0941-2948/2013/0507 - BERTHOLDO-VARGAS, L.R., MARTINS, J.N., BORDIN, D., SALVADOR, M., SCHAFER, A.L., BARROS, N.M., BARBIERI, L., STIRPE, F. and CARLINI, C.R., 2009. Type 1 ribosome-inactivating proteins - Entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: noctuidae). Journal of Insect Physiology, vol. 55, no. 1, pp. 51-58. http://dx.doi.org/10.1016/j.jinsphys.2008.10.004 PMid:19000694.
» http://dx.doi.org/10.1016/j.jinsphys.2008.10.004 - BORGES, A.C.P., PIASSÃO, J.F.G., PAULA, M.O., SEPP, S., BEZ, C.F.S., HEPP, L.U., VALDUGA, A.T., PEREIRA, A.A.M. and CANSIAN, R.L., 2018. Characterization of oxidative stress biomarkers in a freshwater anomuran crab. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 1, pp. 61-67. http://dx.doi.org/10.1590/1519-6984.04816 PMid:28614422.
» http://dx.doi.org/10.1590/1519-6984.04816 - BRADFORD, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, vol. 72, no. 1-2, pp. 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3 PMid:942051.
» http://dx.doi.org/10.1016/0003-2697(76)90527-3 - BUENO, A.A.P. and BOND-BUCKUP, G., 2004. Natural diet of Aegla platensis Schmitt and Aegla ligulata. Acta Limnologica Brasiliensia, vol. 16, pp. 115-127.
- BUNZEL, K., LIESS, M. and KATTWINKEL, M., 2014. Landscape parameters driving aquatic pesticide exposure and effects. Environmental Pollution, vol. 186, pp. 90-97. http://dx.doi.org/10.1016/j.envpol.2013.11.021 PMid:24365537.
» http://dx.doi.org/10.1016/j.envpol.2013.11.021 - CALLISTO, M. and GONÇALVES JÚNIOR, J.F. and MORENO, P., 2004. Invertebrados aquáticos como bioindicadores. In: E.M.A. GOULART, ed. Navegando o Rio das Velhas das Minas aos Gerais Belo Horizonte: UFMG, pp. 1-12.
- CAMPOS, V.D., 2008. Dinâmica de uso e ocupação da terra na bacia hidrográfica do Arroio dos Pereiras em Irati – PR e sua influência na qualidade das águas superficiais Ponta Grossa: Universidade Estadual de Ponta Grossa, 112 p. Dissertação de Mestrado em Gestão do Território.
- CHAPMAN, P.M., WANG, F. and CAEIRO, S.S., 2013. Assessing and managing sediment contamination in transitional waters. Environment International, vol. 55, pp. 71-91. http://dx.doi.org/10.1016/j.envint.2013.02.009 PMid:23528483.
» http://dx.doi.org/10.1016/j.envint.2013.02.009 - CHEN, T., FURST, A. and CHIEN, P.K., 1994. The effects of cadmium and iron on catalase activities in tubifex. International Journal of Toxicology, vol. 13, no. 2, pp. 112-120. http://dx.doi.org/10.3109/10915819409140992
» http://dx.doi.org/10.3109/10915819409140992 - CLASEN, B., LORO, V.L., MURUSSI, C.R., TIECHER, T.L., MORAES, B. and ZANELLA, R., 2018. Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. The Science of the Total Environment, vol. 626, pp. 737-743. http://dx.doi.org/10.1016/j.scitotenv.2018.01.154 PMid:29358144.
» http://dx.doi.org/10.1016/j.scitotenv.2018.01.154 - COGO, G.B. and SANTOS, S., 2013. The role of aeglids in shredding organic matter in Neotropical streams. Journal of Crustacean Biology, vol. 33, no. 4, pp. 519-526. http://dx.doi.org/10.1163/1937240X-00002165
» http://dx.doi.org/10.1163/1937240X-00002165 - COLIN, N., PORTE, C., FERNANDES, D., BARATA, C., PADRÓS, F., CARRASSÓN, M., MONROY, M., CANO-ROCABAYERA, O., SOSTOA, A., PIÑA, B. and MACEDA-VEIGA, A., 2016. Ecological relevance of biomarkers in monitoring studies of macro-invertebrates and fish in Mediterranean rivers. The Science of the Total Environment, vol. 540, pp. 307-323. http://dx.doi.org/10.1016/j.scitotenv.2015.06.099 PMid:26148426.
» http://dx.doi.org/10.1016/j.scitotenv.2015.06.099 - DAMÁSIO, J., BARCELÓ, D., BRIX, R., POSTIGO, C., GROS, M., PETROVIC, M., SABATER, S., GUASCH, H., DE ALDA, M.L. and BARATA, C., 2011a. Are pharmaceuticals more harmful than other pollutants to aquatic invertebrate species: a hypothesis tested using multi-biomarker and multi-species responses in field collected and transplanted organisms. Chemosphere, vol. 85, no. 10, pp. 1548-1554. http://dx.doi.org/10.1016/j.chemosphere.2011.07.058 PMid:21925701.
» http://dx.doi.org/10.1016/j.chemosphere.2011.07.058 - DAMÁSIO, J., FERNÁNDEZ-SANJUAN, M., SÁNCHEZ-AVILA, J., LACORTE, S., PRAT, N., RIERADEVALL, M., SOARES, A.M.V.M. and BARATA, C., 2011b. Multi-biochemical responses of benthic macroinvertebrate species as a complementary tool to diagnose the cause of community impairment in polluted rivers. Water Research, vol. 45, no. 12, pp. 3599-3613. http://dx.doi.org/10.1016/j.watres.2011.04.006 PMid:21571352.
» http://dx.doi.org/10.1016/j.watres.2011.04.006 - DAMÁSIO, J., TAULER, R., TEIXIDÓ, E., RIERADEVALL, M., PRAT, N., RIVA, M.C., SOARES, A.M. and BARATA, C., 2008. Combined use of Daphnia magna in situ bioassays, biomarkers and biological indices to diagnose and identify environmental pressures on invertebrate communities in two Mediterranean urbanized and industrialized rivers (NE Spain). Aquatic Toxicology, vol. 87, no. 4, pp. 310-320. http://dx.doi.org/10.1016/j.aquatox.2008.02.016 PMid:18420289.
» http://dx.doi.org/10.1016/j.aquatox.2008.02.016 - ELIA, A.C., GALARINI, R., MARTIN DÖRR, A.J. and TATICCHI, M.I., 2007. Heavy metal contamination and antioxidant response of a freshwater bryozoan (Lophopus crystallinus Pall., Phylactolaemata). Ecotoxicology and Environmental Safety, vol. 66, no. 2, pp. 188-194. http://dx.doi.org/10.1016/j.ecoenv.2005.12.004 PMid:16469378.
» http://dx.doi.org/10.1016/j.ecoenv.2005.12.004 - ESTERBAUER, H. and CHEESEMAN, K.H., 1990. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology, vol. 186, pp. 407-421. http://dx.doi.org/10.1016/0076-6879(90)86134-H PMid:2233308.
» http://dx.doi.org/10.1016/0076-6879(90)86134-H - HALLIWELL, B. and GUTTERIDGE, J.M.C., 2007. Free radicals in biology and medicine 4th ed. New York: Oxford University Press.
- HONG, Y., HUANG, Y., YAN, G. and HUANG, Z., 2019. Effects of deltamethrin on the antioxidant defense and heat shock protein expression in Chinese mitten crab, Eriocheir sinensis. Environmental Toxicology and Pharmacology, vol. 66, pp. 1-6. http://dx.doi.org/10.1016/j.etap.2018.12.012 PMid:30584970.
» http://dx.doi.org/10.1016/j.etap.2018.12.012 - HUANG, Y., HONG, Y., HUANG, Z., ZHANG, J. and HUANG, Q., 2019. Avermectin induces the oxidative stress, genotoxicity, and immunological responses in the Chinese Mitten Crab, Eriocheir sinensis. PLoS One, vol. 14, no. 11, pp. e0225171. http://dx.doi.org/10.1371/journal.pone.0225171 PMid:31765405.
» http://dx.doi.org/10.1371/journal.pone.0225171 - INSTITUTO NACIONAL DE METEREOLOGIA – INMET, 2019 [viewed 2 May 2020]. Boletins climáticos para o Rio Grande do Sul: ano 2019 [online]. Available from: http://www.inmet.gov.br/portal/index.php?r=clima/boletimRioGrandeDoSul
» http://www.inmet.gov.br/portal/index.php?r=clima/boletimRioGrandeDoSul - KAUR, R., KAUR, J., MAHAJAN, J., KUMAR, R. and ARORA, S., 2014. Oxidative stress-implications, source and its prevention. Environmental Science and Pollution Research International, vol. 21, no. 3, pp. 1599-1613. http://dx.doi.org/10.1007/s11356-013-2251-3 PMid:24170504.
» http://dx.doi.org/10.1007/s11356-013-2251-3 - LA COLLA, N.S., BOTTÉ, S.E., OLIVA, A.L. and MARCOVECCHIO, J.E., 2017. Tracing Cr, Pb, Fe and Mn occurrence in the Bahía Blanca estuary through commercial fish species. Chemosphere, vol. 175, pp. 286-293. http://dx.doi.org/10.1016/j.chemosphere.2017.02.002 PMid:28232139.
» http://dx.doi.org/10.1016/j.chemosphere.2017.02.002 - LIMA, J.A.M.C., LABANOWSKI, J., BASTOS, M.C., ZANELLA, R., PRESTES, O.D., VARGAS, J.P.R., MONDAMERT, L., GRANADO, E., TIECHER, T., ZAFAR, M., TROJAN, A., LE GUET, T. and SANTOS, D.R., 2020. “Modern agriculture” transfers many pesticides to watercourses: a case study of a representative rural catchment of southern Brazil. Environmental Science and Pollution Research International, vol. 27, no. 10, pp. 10581-10598. http://dx.doi.org/10.1007/s11356-019-06550-8 PMid:31942716.
» http://dx.doi.org/10.1007/s11356-019-06550-8 - LOUREIRO, R.C., MENEGAT, M., RESTELLO, R.M. and HEPP, L.U., 2018. Incorporation of zinc and copper by insects of different functional feeding groups in agricultural streams. Environmental Science and Pollution Research International, vol. 25, no. 18, pp. 17402-17408. http://dx.doi.org/10.1007/s11356-018-1971-9 PMid:29654465.
» http://dx.doi.org/10.1007/s11356-018-1971-9 - MAGALHÃES, D.P., MARQUES, M.R.C., BAPTISTA, D.F. and BUSS, D.F., 2015. Metal bioavaliability and toxicity in freshwaters. Environmental Chemistry Letters, vol. 13, no. 1, pp. 69-87. http://dx.doi.org/10.1007/s10311-015-0491-9
» http://dx.doi.org/10.1007/s10311-015-0491-9 - MELO, G.A.S., 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil São Paulo: Edições Loyola.
- MORENO-GONZÁLEZ, R., CAMPILLO, J.A. and LEÓN, V.M., 2013. Influence of an intensive agricultural drainage basin on the seasonal distribution of organic pollutants in seawater from a Mediterranean coastal lagoon (Mar Menor, SE Spain). Marine Pollution Bulletin, vol. 77, no. 1-2, pp. 400-411. http://dx.doi.org/10.1016/j.marpolbul.2013.09.040 PMid:24139646.
» http://dx.doi.org/10.1016/j.marpolbul.2013.09.040 - NAVEEDULLAH., HASHMI, M.Z., YU, C., SHEN, H., DUAN, D., SHEN, C., LOU, L. and CHEN, Y., 2013. Risk Assessment of Heavy Metals Pollution in Agricultural Soils of Siling Reservoir Watershed in Zhejiang Province, China. BioMed Research International, vol. 2013, pp. 590306. http://dx.doi.org/10.1155/2013/590306 PMid:24151611.
» http://dx.doi.org/10.1155/2013/590306 - NDONWI, E.N., ATOGHO-TIEDEU, B., LONTCHI-YIMAGOU, E., SHINKAFI, T.S., NANFA, D., BALTI, E.V., INDUSMITA, R., MAHMOOD, A., KATTE, J.-C., ARMMBANYA., MATSHA, T., MBANYA, J.C., SHAKIR, A. and SOBNGWI, E., 2019. Gestational exposure to pesticides induces oxidative stress and lipid peroxidation in offspring that persist at adult age in an animal model. Toxicological Research, vol. 35, no. 3, pp. 241-248. http://dx.doi.org/10.5487/TR.2019.35.3.241 PMid:31341553.
» http://dx.doi.org/10.5487/TR.2019.35.3.241 - NORO, C.K. and BUCKUP, L., 2002. Biologia reprodutiva e ecologia de Aegla leptoaectyla Buckup & Rossi (Crustacea, Anomura, Aeglidae). Revista Brasileira de Zoologia, vol. 19, no. 4, pp. 1063-1079. http://dx.doi.org/10.1590/S0101-81752002000400011
» http://dx.doi.org/10.1590/S0101-81752002000400011 - OKSANEN, J., BLANCHET, F.G., KINDT, R., LEGENDRE, P., O’HARA, R.B., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H. and WAGNER, H. 2010 [viewed 2 May 2020]. Multivariate analysis of ecological communities [online]. Vienna: R Foundation for Statistical Computing. Available from: https://vegan.r-forge.r-project.org/
» https://vegan.r-forge.r-project.org/ - PAULO, R.L. and SERRA, J.C.V., 2015. Estudo de caso envolvendo uma indústria de fertilizantes na cidade de Porto Nacional/TO. Sistemas & Gestão, vol. 10, no. 2, pp. 316-323. http://dx.doi.org/10.7177/sg.2015.v10.n2.a8
» http://dx.doi.org/10.7177/sg.2015.v10.n2.a8 - PETERS, K., BUNDSCHUH, M. and SCHÄFER, R.B., 2013. Review on the effects of toxicants on freshwater ecosystem functions. Environmental Pollution, vol. 180, pp. 324-329. http://dx.doi.org/10.1016/j.envpol.2013.05.025 PMid:23725857.
» http://dx.doi.org/10.1016/j.envpol.2013.05.025 - PIASSÃO, J.F.G., GASPARIN, B.G., MARTINS, M.C., DECIAN, V.S., CANSIAN, R.L., RESTELLO, R.M. and MIELNICZKI-PEREIRA, A.A., 2019. Analysis of metals bioaccumulation and oxidative stress biomarkers in crustaceans of Aegla genus (Crustacea, Anomura). Perspectiva, vol. 43, no. 161, pp. 111-122.
- PROSHAD, R., KORMOKER, T. and ISLAM, S., 2019. Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa River, Bangladesh. Toxin Reviews, pp. 1-25. http://dx.doi.org/10.1080/15569543.2018.1564143
» http://dx.doi.org/10.1080/15569543.2018.1564143 - QU, X., REN, Z., ZHANG, M., LIU, X. and PENG, W., 2017. Sediment heavy metals and benthic diversities in Hun-Tai River, northeast of China. Environmental Science and Pollution Research International, vol. 24, no. 11, pp. 10662-10673. http://dx.doi.org/10.1007/s11356-017-8642-0 PMid:28283976.
» http://dx.doi.org/10.1007/s11356-017-8642-0 - R DEVELOPMENT CORE TEAM, 2010. R: a language and environment for statistical computing Vienna: R Foundation for Statistical Computing. Available from: http://www.R-project.org
» http://www.R-project.org - RAMOS-VASCONCELOS, G.R. and HERMES-LIMA, M., 2003. Hypometabolism, antioxidant defenses and free radical metabolism in the pulmonate land snail Helix aspersa. The Journal of Experimental Biology, vol. 206, no. Pt 4, pp. 675-685. http://dx.doi.org/10.1242/jeb.00124 PMid:12517985.
» http://dx.doi.org/10.1242/jeb.00124 - REGOLI, F. and WINSTON, G.W., 1999. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicology and Applied Pharmacology, vol. 156, no. 2, pp. 96-105. http://dx.doi.org/10.1006/taap.1999.8637 PMid:10198274.
» http://dx.doi.org/10.1006/taap.1999.8637 - ROMERO, L.M., 2004. Physiological stress in ecology: lessons from biomedical research. Trends in Ecology & Evolution, vol. 19, no. 5, pp. 249-255. http://dx.doi.org/10.1016/j.tree.2004.03.008 PMid:16701264.
» http://dx.doi.org/10.1016/j.tree.2004.03.008 - ROVANI, I.L., DECIAN, V.S., ZANIN, E.M., BRANDALISE, M., QUADROS, F.R. and HEPP, L.H., 2020. Socioeconomic changes and land use and land cover of the Northern Region of Rio Grande do Sul, Brazil. Floresta e Ambiente, vol. 27, no. 3, e20180258. http://dx.doi.org/10.1590/2179-8087.025818
» http://dx.doi.org/10.1590/2179-8087.025818 - SCHULZ, R., 2001. Rainfall-induced sediment and pesticide input from orchards into the Lourens river, western cape, South Africa: importance of a single event. Water Research, vol. 35, no. 8, pp. 1869-1876. http://dx.doi.org/10.1016/S0043-1354(00)00458-9 PMid:11337831.
» http://dx.doi.org/10.1016/S0043-1354(00)00458-9 - SMITH, L.E.D. and SICILIANO, G.A., 2015. Comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agriculture, Ecosystems & Environment, vol. 209, no. 1, pp. 15-25. http://dx.doi.org/10.1016/j.agee.2015.02.016
» http://dx.doi.org/10.1016/j.agee.2015.02.016 - SUNDARARAJAN, S., KHADANGA, M.K., KUMAR, J.P., RAGHUMARAN, S., VIJAYA, R. and JENA, B.K., 2017. Ecological risk assessment of trace metal accumulation in sediments of Veraval Harbor, Gujarat, Arabian Sea. Marine Pollution Bulletin, vol. 114, no. 1, pp. 592-601. http://dx.doi.org/10.1016/j.marpolbul.2016.09.016 PMid:27817889.
» http://dx.doi.org/10.1016/j.marpolbul.2016.09.016 - THÉVENOT, D.R., MOILLERON, R., LESTEL, L., GROMAIRE, M.C., ROCHER, V., CAMBIER, P., BONTÉ, P., COLIN, J.L., DE PONTEVÈS, C. and MEYBECK, M., 2007. Critical budget of metal sources and pathways in the Seine River basin (1994-2003) for Cd, Cr, Cu, Hg, Ni, Pb and Zn. The Science of the Total Environment, vol. 375, no. 1-3, pp. 180-203. http://dx.doi.org/10.1016/j.scitotenv.2006.12.008 PMid:17267024.
» http://dx.doi.org/10.1016/j.scitotenv.2006.12.008 - TONI, C., MENEZES, C.C., LORO, V.L., CLASEN, B.E., CATTANEO, R., SANTI, A., PRETTO, A., ZANELLA, R. and LEITEMPERGER, J., 2010. Oxidative stress biomarkers in Cyprinus carpio exposed to commercial herbicide bispyribac‐sodium. Journal of Applied Toxicology, vol. 30, no. 6, pp. 590-595. http://dx.doi.org/10.1002/jat.1530 PMid:20809548.
» http://dx.doi.org/10.1002/jat.1530 - TURUNEN, J., MARKKULA, J., RAJAKALLIO, M. and AROVIITA, J., 2019. Riparian forests mitigate harmful ecological effects of agricultural diffuse pollution in medium-sized streams. The Science of the Total Environment, vol. 649, pp. 495-503. http://dx.doi.org/10.1016/j.scitotenv.2018.08.427 PMid:30176461.
» http://dx.doi.org/10.1016/j.scitotenv.2018.08.427 - VALKO, M., MORRIS, H. and CRONIN, M.T.D., 2005. Metals, toxicity and oxidative stress. Current Medicinal Chemistry, vol. 12, no. 10, pp. 1161-1208. http://dx.doi.org/10.2174/0929867053764635 PMid:15892631.
» http://dx.doi.org/10.2174/0929867053764635 - WANG, A., KAWSER, A., XU, Y., YE, X., RANI, S. and CHEN, K., 2016. Heavy metal accumulation during the last 30 years in the Karnaphuli River estuary, Chittagong, Bangladesh. SpringerPlus, vol. 5, pp. 2079. http://dx.doi.org/10.1186/s40064-016-3749-1 PMid:28018787.
» http://dx.doi.org/10.1186/s40064-016-3749-1 - WANG, J., ZHANG, H., ZHANG, T., ZHANG, R., LIU, R. and CHEN, Y., 2015. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. International Journal of Biological Macromolecules, vol. 77, pp. 59-67. http://dx.doi.org/10.1016/j.ijbiomac.2015.02.037 PMid:25795390.
» http://dx.doi.org/10.1016/j.ijbiomac.2015.02.037 - WU, H., XUAN, R., LI, Y., ZHANG, X., WANG, Q. and WANG, L., 2013. Effects of cadmium exposure on digestive enzymes, antioxidant enzymes, and lipid peroxidation in the freshwater crab Sinopotamon henanense. Environmental Science and Pollution Research International, vol. 20, no. 6, pp. 4085-4092. http://dx.doi.org/10.1007/s11356-012-1362-6 PMid:23224505.
» http://dx.doi.org/10.1007/s11356-012-1362-6 - YE, L., CAI, Q., LIU, R. and CAO, M., 2009. The influence of topography and land use on water quality of Xiangxi River in Three Gorges Reservoir region. Environmental Geology, vol. 58, no. 5, pp. 937-942. http://dx.doi.org/10.1007/s00254-008-1573-9
» http://dx.doi.org/10.1007/s00254-008-1573-9 - ZHANG, X., SHEN, G., WANG, Y., HUANG, P., AME, K.H., ZANG, Y. and SHEN, H., 2020. Molecular characterization, expression and enzyme activity of three glutathione S-transferase genes from Eriocheir sinensis under pesticide stresses. Comparative Biochemistry and Physiology. Part C, vol. 230, pp. 108700. http://dx.doi.org/10.1016/j.cbpc.2019.108700 PMid:31899308.
» http://dx.doi.org/10.1016/j.cbpc.2019.108700
Publication Dates
-
Publication in this collection
15 Mar 2021 -
Date of issue
2022
History
-
Received
18 Oct 2019 -
Accepted
18 June 2020