Abstract
Triatoma lecticularia (Hemiptera: Reduviidae) (Stal, 1859) is a potential vector of Chagas’s disease and the comprehension of its reproductive biology is an important tool to control this insect. In the reproductive tract of female insects, the spermatheca plays a crucial role storing male spermatozoa after mating. Whithin insects the spermatheca shows a wide morphological diversity and the analysis of this characteristic can contribute to understand the reproductive biology of the species. This study describes the histology and histochemistry of the spermatheca of T. lecticularia. Females have a pair of elongated spermathecal reservoirs without associated accessory gland. The reservoir opens into the common oviduct via a narrow muscular duct. The reservoir epithelium has single layer of columnar secretory cells. The control of the release of spermatozoa from the spermatheca occurs via the muscular duct. The anatomical features of the spermatheca of T. lecticularia resemble those described of other Reduviidae. However, the histological and histochemical features of spermatheca observed in T. lecticularia were important to explain the maintenance of the viability of the spermatozoa stored.
Keywords:
Triatominae; morphology; secretion; spermatozoa
Resumo
Triatoma lecticularia (Hemiptera: Reduviidae) (Stal, 1859) é um potencial vetor da doença de Chagas e a compreensão de sua biologia reprodutiva é um importante fator para seu controle populacional. No aparelho reprodutor feminino dos insetos, a espermateca desempenha a importante funcão de armazenar os espermatozoides após cópula. Nos insetos, a espermateca apresenta uma ampla diversidade morfológica e a análise destas características pode contribuir com o entendimento da biologia reprodutiva das espécies. Este estudo descreve histológica e histoquimicamente a espermateca de T. lecticularia. As fêmeas tem um par de espermatecas alongadas sem glândulas acessórias associadas. O reservatório conecta-se ao oviduto comum através de um ducto muscular curto que controla a liberação dos espermatozoides. O epitélio do reservatório possui uma camada de células secretoras colunares. As características anatômicas da espermateca de T. lecticularia são semelhantes às encontradas em outros Reduviidae. Entretanto, as características histológicas e histoquímicas observadas na espermateca são importantes para explicar a manutenção da viabilidade dos espermatozoides armazenados.
Palavras-chave:
Triatominae; morfologia; secreção; espermatozoides
1. Introduction
The Triatominae (Hemiptera: Reduviidae) are obligate hematophagous insects feeding on different hosts. Many representatives of Triatominae are potential vectors of Trypanosoma cruzi (Chagas, 1909), the etiological agent of Chagas’s disease (Lent and Wygodzinsky, 1979LENT, H. and WYGODZINSKY, P., 1979. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bulletin of the American Museum of Natural History, vol. 163, pp. 123-520.).
Triatoma lecticularia (Hemiptera: Reduviidae) (Stal, 1859) is a forest dwelling species found in the United States and Mexico (Lent and Wygodzinsky, 1979LENT, H. and WYGODZINSKY, P., 1979. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bulletin of the American Museum of Natural History, vol. 163, pp. 123-520.; Jurberg and Costa 1989JURBERG, J. and COSTA, J.M., 1989. Estudos sobre a resistência ao jejum e aspectos nutricionais de Triatoma lecticularia (Stal, 1859)(Hemiptera, Reduviidae, Triatominae). Memorias do Instituto Oswaldo Cruz, vol. 84, no. 3, pp. 393-399. http://dx.doi.org/10.1590/S0074-02761989000300016. PMid:2520831.
http://dx.doi.org/10.1590/S0074-02761989...
; Silva et al., 1993SILVA, I.G., SANTOS, L.G.P. and NAKANO, R., 1993. Ciclo evolutivo de Triatoma lecticularia (Stal, 1859) (Hemiptera, Reduviidae). Revista de Patologia Tropical, vol. 22, pp. 259-263.; Galvão et al., 2003GALVÃO, C., CARCAVALLO, R., ROCHA, D.D.S. and JURBERG, J., 2003. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa, vol. 202, no. 1, pp. 1-36. http://dx.doi.org/10.11646/zootaxa.202.1.1.
http://dx.doi.org/10.11646/zootaxa.202.1...
) and is generally found infected with T. cruzi, being important for the maintenance of the wild cycle of this pathogen at North America (Silva et al., 1993SILVA, I.G., SANTOS, L.G.P. and NAKANO, R., 1993. Ciclo evolutivo de Triatoma lecticularia (Stal, 1859) (Hemiptera, Reduviidae). Revista de Patologia Tropical, vol. 22, pp. 259-263.; Bern et al., 2011BERN, C., KJOS, S., YABSLEY, M.J. and MONTGOMERY, S.P., 2011. Trypanossoma cruzi and Chagas’ disease in the United States. Clinical Microbiology Reviews, vol. 24, no. 4, pp. 655-681. http://dx.doi.org/10.1128/CMR.00005-11. PMid:21976603.
http://dx.doi.org/10.1128/CMR.00005-11...
).
The reproductive female tract of Hemiptera is formed by ovaries, lateral oviducts, a common oviduct, accessory glands and spermatheca (Jahnke et al., 2006JAHNKE, S.M., REDAELLI, L.R. and DIEFENBACH, L.M.G., 2006. Internal reproductive organs of Cosmoclopius nigroannulatus (Hemiptera; Reduviidae). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 66, no. 2A, pp. 509-512. http://dx.doi.org/10.1590/S1519-69842006000300017. PMid:16862306.
http://dx.doi.org/10.1590/S1519-69842006...
; Chiang et al., 2012CHIANG, R.G., CHIANG, J.A., SARQUIS, O. and LIMA, M.M., 2012. Morphology of reproductive accessory glands in eight species of blood-feeding Hemiptera (Hemiptera, Reduviidae) insect vectors of Chagas disease. Acta Tropica, vol. 122, no. 2, pp. 196-204. http://dx.doi.org/10.1016/j.actatropica.2012.01.011. PMid:22314031.
http://dx.doi.org/10.1016/j.actatropica....
; Chapman, 2013CHAPMAN, R.F. 2013. The insects: structure and function. 5th ed. Cambridge: University Press, 929 p.). During mating the spermatozoa are transferred together with the secretion of the male accessory glands to the spermatheca (Chapman, 2013CHAPMAN, R.F. 2013. The insects: structure and function. 5th ed. Cambridge: University Press, 929 p.). The spermatheca is an organ of the female reproductive tract, responsible for storing and maintaining the viability of spermatozoa from mating until oocyte fecundation, reducing or eliminating the need for multiple matings and, consequently, the probability of the female being preyed upon (Thornhill and Alcock 1983THORNHILL, R. and ALCOCK, J., 1983. The evolution of insect mating systems. Cambridge: Harvard University Press.
Cambridge...
; Souza et al., 2008SOUZA, E.A., CAMPOS, L.A.O., NEVES, C.A., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Effect of delayed mating on spermathecal activation in Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) queens. Apidologie, vol. 39, no. 3, pp. 293-301. http://dx.doi.org/10.1051/apido:2008008.
http://dx.doi.org/10.1051/apido:2008008...
, 2016SOUZA, E.A., LISBOA, L.C.O., ARAÚJO, V.A. and SERRÃO, J.E., 2016. Morphology of the spermathecae of Leptoglossus zonatus (Heteroptera: Coreidae). Annals of the Entomological Society of America, vol. 109, no. 1, pp. 106-111. http://dx.doi.org/10.1093/aesa/sav097.
http://dx.doi.org/10.1093/aesa/sav097...
).
In the spermatheca, the spermatozoa are protected from physical damage as well as from contact with hemolymph content and free radicals (Collins et al., 2004COLLINS, A.M., WILLIAMS, V. and EVANS, J.D., 2004. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Molecular Biology, vol. 13, no. 2, pp. 141-146. http://dx.doi.org/10.1111/j.0962-1075.2004.00469.x. PMid:15056361.
http://dx.doi.org/10.1111/j.0962-1075.20...
; Al-Lawati et al., 2009AL-LAWATI, H., KAMP, G. and BIENEFELD, K., 2009. Characteristics of the spermathecal contents of old and young honeybee queens. Journal of Insect Physiology, vol. 55, no. 2, pp. 116-121. http://dx.doi.org/10.1016/j.jinsphys.2008.10.010. PMid:19027748.
http://dx.doi.org/10.1016/j.jinsphys.200...
; King et al., 2011KING, M., EUBEL, H., MILLAR, A.H. and BAER, B., 2011. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. Journal of Insect Physiology, vol. 57, no. 3, pp. 409-414. http://dx.doi.org/10.1016/j.jinsphys.2010.12.011. PMid:21192944.
http://dx.doi.org/10.1016/j.jinsphys.201...
; Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
). Generally, the spermatozoa are stored in a spermathecal bulb that is associated with a muscular duct, which controls spermatozoa release. The reservoir is lined by a layer epithelial cells in bees (Dallai, 1975DALLAI, R., 1975. Fine structure of the spermatheca of Apis mellifera. Journal of Insect Physiology, vol. 21, no. 1, pp. 89-109. http://dx.doi.org/10.1016/0022-1910(75)90072-4.
http://dx.doi.org/10.1016/0022-1910(75)9...
; Martins and Serrão, 2002MARTINS, G.F. and SERRÃO, J.E., 2002. A comparative study of the spermatheca in bees (Hymenoptera; Apoidea). Sociobiology, vol. 40, pp. 711-720.; Souza et al., 2008SOUZA, E.A., CAMPOS, L.A.O., NEVES, C.A., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Effect of delayed mating on spermathecal activation in Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) queens. Apidologie, vol. 39, no. 3, pp. 293-301. http://dx.doi.org/10.1051/apido:2008008.
http://dx.doi.org/10.1051/apido:2008008...
) or multiple layers with different cell types in Hemiptera and Diptera (Souza et al., 2016SOUZA, E.A., LISBOA, L.C.O., ARAÚJO, V.A. and SERRÃO, J.E., 2016. Morphology of the spermathecae of Leptoglossus zonatus (Heteroptera: Coreidae). Annals of the Entomological Society of America, vol. 109, no. 1, pp. 106-111. http://dx.doi.org/10.1093/aesa/sav097.
http://dx.doi.org/10.1093/aesa/sav097...
; Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
). The reservoir epithelium seems to contribute to the transportation of the hemolymph molecules to the spermathecal lumen (Gobin et al., 2006GOBIN, B., ITO, F., PEETERS, C. and BILLEN, J., 2006. Queen-worker differences in spermatheca reservoir of phylogenetically basal ants. Cell and Tissue Research, vol. 326, no. 1, pp. 169-178. http://dx.doi.org/10.1007/s00441-006-0232-2. PMid:16773314.
http://dx.doi.org/10.1007/s00441-006-023...
; Martins et al., 2008MARTINS, G.F., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Spermatheca morphology of the social wasp Polistes erythrocephalus. Bulletin of Insectology, vol. 61, pp. 37-41.). In some insects, there is a spermathecal gland containing a secretion rich in glycogen, glycoproteins, phospholipids and mucopolysaccharides (Bhatnagar and Musgrave, 1971BHATNAGAR, R.D. and MUSGRAVE, A., 1971. Aspects of histophysiology of the spermathecal gland of Sitophilus granarius (L.)(Coleoptera). Canadian Journal of Zoology, vol. 49, no. 2, pp. 275-277. http://dx.doi.org/10.1139/z71-038. PMid:5101971.
http://dx.doi.org/10.1139/z71-038...
; Wolfner, 2011WOLFNER, M.F., 2011. Precious essences : female secretions promote sperm storage in Drosophila. PLoS Biology, vol. 9, no. 11, pp. e1001191. http://dx.doi.org/10.1371/journal.pbio.1001191. PMid:22087072.
http://dx.doi.org/10.1371/journal.pbio.1...
; Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
), which is released in the reservoir lumen to contribute to the maintenance of the spermatozoa viability.
The spermatheca is present in all orders of insect and generally, there is one spermatheca per individual, although in Rhodnius prolixus (Hemiptera, Reduviidae) and representatives of Culicidae and Caliphoridae (Diptera) have been reported multiple spermathecae with different morphologies (Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
).
The control of the spermatozoa release from the spermatheca during oocytes fecundation is carried out by the muscular duct (King et al., 2011KING, M., EUBEL, H., MILLAR, A.H. and BAER, B., 2011. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. Journal of Insect Physiology, vol. 57, no. 3, pp. 409-414. http://dx.doi.org/10.1016/j.jinsphys.2010.12.011. PMid:21192944.
http://dx.doi.org/10.1016/j.jinsphys.201...
; Chapman, 2013CHAPMAN, R.F. 2013. The insects: structure and function. 5th ed. Cambridge: University Press, 929 p.), which can also show morphological variation according to species (Pendergrast, 1957PENDERGRAST, J.G., 1957. Studies on the reproductive organs of the Heteroptera with a consideration of their bearing on classification. Ecological Entomology, vol. 109, pp. 1-63.; Kocorek and Danielczok-Demska, 2002KOCOREK, A. and DANIELCZOK-DEMSKA, T., 2002. Comparative morphology of the spermatheca within the family Dinidoridae (Hemiptera: Heteroptera). European Journal of Entomology, vol. 99, no. 1, pp. 91-98. http://dx.doi.org/10.14411/eje.2002.016.
http://dx.doi.org/10.14411/eje.2002.016...
).
Considering the importance of the spermatheca in the reproductive biology of insects, studies that provide morphological and physiological data regarding this organ are important for future research into population management of insects that are disease vectors. This study described the histology and histochemistry of the spermatheca of T. lecticularia, an insect directly related to the maintenance of the forest cycle of T. cruzi.
2. Material and Methods
Mated Triatoma lecticularia females in oviposition were obtained from colonies in the Insectary of the Parasitology Department at the Universidade Federal do Triângulo Mineiro (UFTM), Uberaba, Minas Gerais, Brazil (19°44’21” S, 47°57’26” W). The insects were maintained in acrylic cylindrical vials containing a cardboard strip with accordion folds to increase surface area, providing space and permitting access for food. The vials were sealed with fine cottonwool balls to allow the blood meal of the insects which was realized every seven days, using chickens (Mendes, 2014MENDES, M.T., 2014. Avaliação do efeito da saliva de triatomíneos (Heteroptera: Reduviidae) sobre a biologia de células dendríticas murinas. Uberaba: Universidade Federal do Triângulo Mineiro, 99 p. Dissertação de Mestrado em Medicina Tropical e Infectologia.).
2.1. Histology and histochemistry
Six mated T. lecticularia females were cryo-anesthetized at 0 °C and dissected in presence of 125 mM NaCl. The spermatheca were transferred to Zamboni fixative solution (Stefanini et al., 1967STEFANINI, M., DE MARTINO, C. and ZAMBONI, L., 1967. Fixation of ejaculated spermatozoa for electron microscopy. Nature, vol. 216, no. 5111, pp. 173-174. http://dx.doi.org/10.1038/216173a0. PMid:4862079.
http://dx.doi.org/10.1038/216173a0...
) for four hours, dehydrated in a graded ethanol series (70, 80, 90 and 95%), and embedded in historesin (Leica). Slices 3µm thick were stained with toluidine blue and analyzed under a light microscope.
Some sections were submitted for histochemical testing with mercury bromophenol to detect total proteins and Periodic Acid-Schiff (P.A.S) to detect neutral polysaccharides and glycoconjugates (Bancroft and Gamble, 2008BANCROFT, J.D. and GAMBLE, M., 2008. Theory and practice of histological techniques. 6th ed. London: Livingstone Churchill, 744 p.).
3. Results
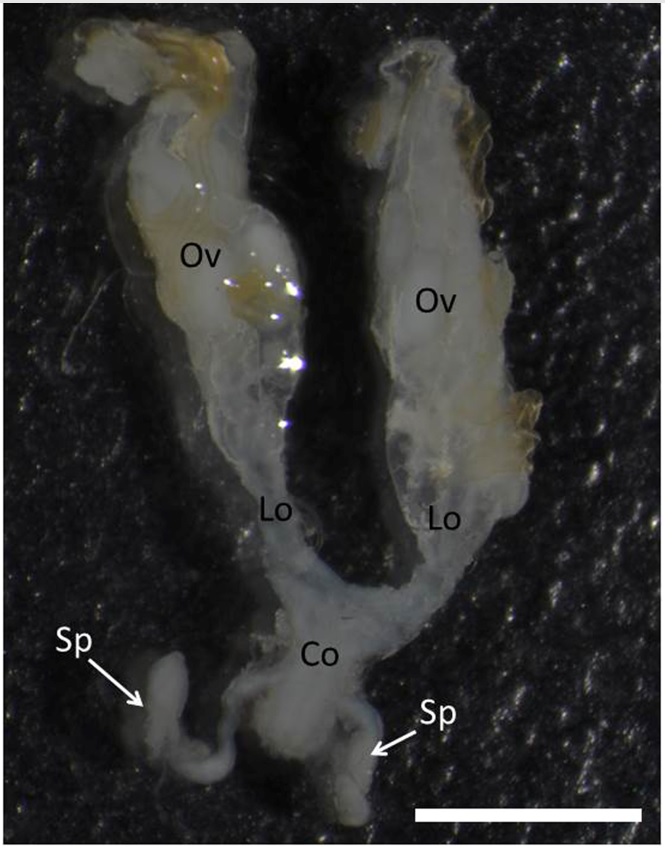
The female reproductive tract of T. lecticularia is formed by a pair of ovaries connected to lateral oviducts, a common oviduct and a pair of spermatheca (Figure 1).
Female reproductive tract of Triatoma lecticularia showing ovaries (OV), lateral oviducts (LO), common oviduct (CO) and two spermathecae (Sp). Bar: 3mm.
Spermathecae are irregular shaped tubes with c.a. 3 mm width connected at the common oviduct by a narrow duct (Figure 2A). Glands associated with the spermatheca were no found in the T. lecticularia reproductive tract.
Histology section of the spermathecae of Triatoma lecticularia. A: General view of the spermatheca showing its elongated shape and the single layered epithelium (Ep) lining the lumen (L). Bar: 200µm; B: Columnar epithelial cells (Ep) with basophil cytoplasm (*) and nucleus with predominate decondensed chromatin. Note lumen (L) filled with spermathozoa. Bar: 20µm; C: Spermatheca submitted to the P.A.S test with positive reaction into the lumen (L) and epithelial cells (Ep) with homogenously positive cytoplasm. Bar: 20µm; D: Spermatheca submitted to the mercury bromophenol test for proteins showing positive reaction into the lumen (L) and epithelium (Ep). Bar: 20µm; E: Muscular duct (md) opening in the common oviduct (Co). Bar: 200µm; F: Detail of the muscle layers (m) of the spermathecal duct. Bar: 50 µm. n: nucleus.
The lumen of the spermathecal reservoir was lined by a single layered columnar epithelium (Figure 2A, B) with the nucleus placed in the center of the cell with a predominance of decondensed chromatin (Figure 2B). The perinuclear and apical regions of the columnar cells were strongly basophil in comparison with the basal cell region.
The columnar epithelial cells were weakly positive for the P.A.S test, with homogenous distribution of glycoconjugates in whole cytoplasm (Figure 2C). The secretion in the spermathecal lumen was positive for the P.A.S (Figure 2C). The mercury bromophenol histochemical test showed strong reaction in the apical cell region (Figure 2D) as well as the secretion of the spermathecal lumen.
The connection of the spermathecal reservoir to the common oviduct is made via a short muscular duct with circular muscles (Figure 2E, F).
4. Discussion
The presence of the two tubular spermathecae without associated glands in T. lecticularia is similar to what has been reported in other Triatominae (Davey, 1958DAVEY, K.G., 1958. The migration of spermatozoa in the femanle of Rhodnius prolixus Stal. The Journal of Experimental Biology, vol. 35, pp. 694-701.; Chiang et al., 2012CHIANG, R.G., CHIANG, J.A., SARQUIS, O. and LIMA, M.M., 2012. Morphology of reproductive accessory glands in eight species of blood-feeding Hemiptera (Hemiptera, Reduviidae) insect vectors of Chagas disease. Acta Tropica, vol. 122, no. 2, pp. 196-204. http://dx.doi.org/10.1016/j.actatropica.2012.01.011. PMid:22314031.
http://dx.doi.org/10.1016/j.actatropica....
; Nascimento, 2015NASCIMENTO, J.D., 2015. Estudo histológico das espermatecas e traqueias de seis espécies de Triatominae (Hemiptera, Reduviidae). Campinas: Universidade Estadual de Campinas, 60 p. Dissertação de Mestrado em Biologia Animal.), except in Triatoma infestans which has oval espermatheca (Nascimento, 2015NASCIMENTO, J.D., 2015. Estudo histológico das espermatecas e traqueias de seis espécies de Triatominae (Hemiptera, Reduviidae). Campinas: Universidade Estadual de Campinas, 60 p. Dissertação de Mestrado em Biologia Animal.).
In T. lecticularia, the spermatheca are sinuous with the reservoir connected to the common oviduct with a narrower duct in comparison with the duct portion near to the reservoir, as found in the triatomines Panstrongylus megistus, P. lignarius and T. tibiamaculata (Nascimento, 2015NASCIMENTO, J.D., 2015. Estudo histológico das espermatecas e traqueias de seis espécies de Triatominae (Hemiptera, Reduviidae). Campinas: Universidade Estadual de Campinas, 60 p. Dissertação de Mestrado em Biologia Animal.).
The spermatheca of T. lecticularia has a single layer of columnar cells lining a lumen that stores the spermatozoa. These cells are basophils in the apical region, indicating that they can assume a secretory function, releasing molecules into the spermathecal lumen contributing to spermatozoa maintenance.
To reinforce the secretory role of the spermathecal epithelium of T. lecticularia, the histochemical tests show that both the cell cytoplasm and the lumen content are positive for the P.A.S test, suggesting that the epithelial cells release polysaccharides and/or glycoconjugates to the spermathecal lumen or transport them from the hemolymph. The transportation of molecules from the hemolymph to the spermathecal lumen has been reported in Hymenoptera, due to the basal region of the epithelial cells of the reservoir rich in invaginations associated with mitochondria, which characterizes the active transportation of molecules (Dallai, 1975DALLAI, R., 1975. Fine structure of the spermatheca of Apis mellifera. Journal of Insect Physiology, vol. 21, no. 1, pp. 89-109. http://dx.doi.org/10.1016/0022-1910(75)90072-4.
http://dx.doi.org/10.1016/0022-1910(75)9...
; Wheeler and Krutzsch, 1994WHEELER, D.E. and KRUTZSCH, P., 1994. Ultrastructure of the spermatheca and its associated gland in the ant Crematogaster opuntiae (Hymenoptera, Formicidae). Zoomorphology, vol. 114, no. 4, pp. 203-212. http://dx.doi.org/10.1007/BF00416859.
http://dx.doi.org/10.1007/BF00416859...
; Martins et al., 2008MARTINS, G.F., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Spermatheca morphology of the social wasp Polistes erythrocephalus. Bulletin of Insectology, vol. 61, pp. 37-41.; Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
).
The accumulation of total proteins in the apical cell cytoplasm of the T. lecticularia spermatheca suggests that the proteins synthesized by these cells may be released into the lumen of the organ. According to Souza et al. (2008)SOUZA, E.A., CAMPOS, L.A.O., NEVES, C.A., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Effect of delayed mating on spermathecal activation in Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) queens. Apidologie, vol. 39, no. 3, pp. 293-301. http://dx.doi.org/10.1051/apido:2008008.
http://dx.doi.org/10.1051/apido:2008008...
, the epithelium of the spermatheca of stingless bee Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) has a single layered epithelium and apical basophil indicating a secretory function. However, M. quadrifasciata anthidioides and other Hymenoptera have a pair of glands for the spermatheca that take on the main secretory role (Dallai, 1975DALLAI, R., 1975. Fine structure of the spermatheca of Apis mellifera. Journal of Insect Physiology, vol. 21, no. 1, pp. 89-109. http://dx.doi.org/10.1016/0022-1910(75)90072-4.
http://dx.doi.org/10.1016/0022-1910(75)9...
; Martins and Serrão, 2002MARTINS, G.F. and SERRÃO, J.E., 2002. A comparative study of the spermatheca in bees (Hymenoptera; Apoidea). Sociobiology, vol. 40, pp. 711-720.; Gobin et al., 2006GOBIN, B., ITO, F., PEETERS, C. and BILLEN, J., 2006. Queen-worker differences in spermatheca reservoir of phylogenetically basal ants. Cell and Tissue Research, vol. 326, no. 1, pp. 169-178. http://dx.doi.org/10.1007/s00441-006-0232-2. PMid:16773314.
http://dx.doi.org/10.1007/s00441-006-023...
; Souza et al., 2008SOUZA, E.A., CAMPOS, L.A.O., NEVES, C.A., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Effect of delayed mating on spermathecal activation in Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) queens. Apidologie, vol. 39, no. 3, pp. 293-301. http://dx.doi.org/10.1051/apido:2008008.
http://dx.doi.org/10.1051/apido:2008008...
; Gotoh et al., 2009GOTOH, A., BILLEN, J., HASHIM, R. and ITO, F., 2009. Evolution of specialized spermatheca morphology in ant queens: Insight from comparative developmental biology between ants and polistine wasps. Arthropod Structure & Development, vol. 38, no. 6, pp. 521-525. http://dx.doi.org/10.1016/j.asd.2009.08.003. PMid:19720157.
http://dx.doi.org/10.1016/j.asd.2009.08....
), structures absent in T. lecticularia.
In Heteroptera, the spermatheca has morphology distinct from that found in Triatoma spp. (Kocorek and Danielczok-Demska, 2002KOCOREK, A. and DANIELCZOK-DEMSKA, T., 2002. Comparative morphology of the spermatheca within the family Dinidoridae (Hemiptera: Heteroptera). European Journal of Entomology, vol. 99, no. 1, pp. 91-98. http://dx.doi.org/10.14411/eje.2002.016.
http://dx.doi.org/10.14411/eje.2002.016...
; Stacconi and Romani, 2011STACCONI, M. and ROMANI, R., 2011. Ultrastructural and functional aspects of the spermatheca in the American Harlequin Bug, Murgantia histrionica (Hemiptera: Pentatomidae). Neotropical Entomology, vol. 40, no. 2, pp. 222-230. http://dx.doi.org/10.1590/S1519-566X2011000200011. PMid:21584404.
http://dx.doi.org/10.1590/S1519-566X2011...
; Candan et al., 2014CANDAN, S., ERBEY, M., ÖZYURT, N. and SULUDERE, Z., 2014. Spermathecae morphology in Four Species of Eurydema Laporte, 1833 (Heteroptera: Pentatomidae) from Turkey: a scanning electron microscope study. Journal of Entomology and Zoology Studies, vol. 2, pp. 206-213.; Souza et al., 2016SOUZA, E.A., LISBOA, L.C.O., ARAÚJO, V.A. and SERRÃO, J.E., 2016. Morphology of the spermathecae of Leptoglossus zonatus (Heteroptera: Coreidae). Annals of the Entomological Society of America, vol. 109, no. 1, pp. 106-111. http://dx.doi.org/10.1093/aesa/sav097.
http://dx.doi.org/10.1093/aesa/sav097...
), with only one spermatheca. In these species, the reservoir shows three types of cells: i) class III secretory cells, characterized by the presence of intracellular canaliculi, ii) cells that form ducts for the transportation of secretions to the lumen of the spermatheca and iii) epithelial cells associated with the cuticle (Noirot and Quennedey, 1991NOIROT, C. and QUENNEDEY, A., 1991. Glands, gland cell, glandular units: some comments on terminology and classification. Annales de la Société Entomologique de France, vol. 23, pp. 123-128.; Stacconi and Romani, 2011STACCONI, M. and ROMANI, R., 2011. Ultrastructural and functional aspects of the spermatheca in the American Harlequin Bug, Murgantia histrionica (Hemiptera: Pentatomidae). Neotropical Entomology, vol. 40, no. 2, pp. 222-230. http://dx.doi.org/10.1590/S1519-566X2011000200011. PMid:21584404.
http://dx.doi.org/10.1590/S1519-566X2011...
; Souza et al., 2016SOUZA, E.A., LISBOA, L.C.O., ARAÚJO, V.A. and SERRÃO, J.E., 2016. Morphology of the spermathecae of Leptoglossus zonatus (Heteroptera: Coreidae). Annals of the Entomological Society of America, vol. 109, no. 1, pp. 106-111. http://dx.doi.org/10.1093/aesa/sav097.
http://dx.doi.org/10.1093/aesa/sav097...
; Pascini and Martins 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
).
A significant feature of the spermatheca of insects is the presence of the cuticle, due to its ectodermal origin (Chapman, 2013CHAPMAN, R.F. 2013. The insects: structure and function. 5th ed. Cambridge: University Press, 929 p.; Pascini and Martins, 2017PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001. PMid:28089345.
http://dx.doi.org/10.1016/j.zool.2016.12...
). However, the cuticle was not observed in the spermatheca of T. lecticularia. The spermathecal epithelium in T. lecticularia assumes the secretory function and the absence of the cuticle indicates that the secretion is quickly transported into the lumen.
In conclusion, the spermatheca of T. lecticularia is similar to the spermathecae of other Reduviidae. In addition, the histological data reveal the importance of reservoir epithelium for the spermatozoa maintenance stored in this organ.
Acknowledgements
This research was supported by Minas Gerais State Research Agency (FAPEMIG).
-
(With 2 figures)
References
- AL-LAWATI, H., KAMP, G. and BIENEFELD, K., 2009. Characteristics of the spermathecal contents of old and young honeybee queens. Journal of Insect Physiology, vol. 55, no. 2, pp. 116-121. http://dx.doi.org/10.1016/j.jinsphys.2008.10.010 PMid:19027748.
» http://dx.doi.org/10.1016/j.jinsphys.2008.10.010 - BANCROFT, J.D. and GAMBLE, M., 2008. Theory and practice of histological techniques 6th ed. London: Livingstone Churchill, 744 p.
- BERN, C., KJOS, S., YABSLEY, M.J. and MONTGOMERY, S.P., 2011. Trypanossoma cruzi and Chagas’ disease in the United States. Clinical Microbiology Reviews, vol. 24, no. 4, pp. 655-681. http://dx.doi.org/10.1128/CMR.00005-11 PMid:21976603.
» http://dx.doi.org/10.1128/CMR.00005-11 - BHATNAGAR, R.D. and MUSGRAVE, A., 1971. Aspects of histophysiology of the spermathecal gland of Sitophilus granarius (L.)(Coleoptera). Canadian Journal of Zoology, vol. 49, no. 2, pp. 275-277. http://dx.doi.org/10.1139/z71-038 PMid:5101971.
» http://dx.doi.org/10.1139/z71-038 - CANDAN, S., ERBEY, M., ÖZYURT, N. and SULUDERE, Z., 2014. Spermathecae morphology in Four Species of Eurydema Laporte, 1833 (Heteroptera: Pentatomidae) from Turkey: a scanning electron microscope study. Journal of Entomology and Zoology Studies, vol. 2, pp. 206-213.
- CHAPMAN, R.F. 2013. The insects: structure and function 5th ed. Cambridge: University Press, 929 p.
- CHIANG, R.G., CHIANG, J.A., SARQUIS, O. and LIMA, M.M., 2012. Morphology of reproductive accessory glands in eight species of blood-feeding Hemiptera (Hemiptera, Reduviidae) insect vectors of Chagas disease. Acta Tropica, vol. 122, no. 2, pp. 196-204. http://dx.doi.org/10.1016/j.actatropica.2012.01.011 PMid:22314031.
» http://dx.doi.org/10.1016/j.actatropica.2012.01.011 - COLLINS, A.M., WILLIAMS, V. and EVANS, J.D., 2004. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Molecular Biology, vol. 13, no. 2, pp. 141-146. http://dx.doi.org/10.1111/j.0962-1075.2004.00469.x PMid:15056361.
» http://dx.doi.org/10.1111/j.0962-1075.2004.00469.x - DALLAI, R., 1975. Fine structure of the spermatheca of Apis mellifera. Journal of Insect Physiology, vol. 21, no. 1, pp. 89-109. http://dx.doi.org/10.1016/0022-1910(75)90072-4
» http://dx.doi.org/10.1016/0022-1910(75)90072-4 - DAVEY, K.G., 1958. The migration of spermatozoa in the femanle of Rhodnius prolixus Stal. The Journal of Experimental Biology, vol. 35, pp. 694-701.
- GALVÃO, C., CARCAVALLO, R., ROCHA, D.D.S. and JURBERG, J., 2003. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa, vol. 202, no. 1, pp. 1-36. http://dx.doi.org/10.11646/zootaxa.202.1.1
» http://dx.doi.org/10.11646/zootaxa.202.1.1 - GOBIN, B., ITO, F., PEETERS, C. and BILLEN, J., 2006. Queen-worker differences in spermatheca reservoir of phylogenetically basal ants. Cell and Tissue Research, vol. 326, no. 1, pp. 169-178. http://dx.doi.org/10.1007/s00441-006-0232-2 PMid:16773314.
» http://dx.doi.org/10.1007/s00441-006-0232-2 - GOTOH, A., BILLEN, J., HASHIM, R. and ITO, F., 2009. Evolution of specialized spermatheca morphology in ant queens: Insight from comparative developmental biology between ants and polistine wasps. Arthropod Structure & Development, vol. 38, no. 6, pp. 521-525. http://dx.doi.org/10.1016/j.asd.2009.08.003 PMid:19720157.
» http://dx.doi.org/10.1016/j.asd.2009.08.003 - JAHNKE, S.M., REDAELLI, L.R. and DIEFENBACH, L.M.G., 2006. Internal reproductive organs of Cosmoclopius nigroannulatus (Hemiptera; Reduviidae). Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 66, no. 2A, pp. 509-512. http://dx.doi.org/10.1590/S1519-69842006000300017 PMid:16862306.
» http://dx.doi.org/10.1590/S1519-69842006000300017 - JURBERG, J. and COSTA, J.M., 1989. Estudos sobre a resistência ao jejum e aspectos nutricionais de Triatoma lecticularia (Stal, 1859)(Hemiptera, Reduviidae, Triatominae). Memorias do Instituto Oswaldo Cruz, vol. 84, no. 3, pp. 393-399. http://dx.doi.org/10.1590/S0074-02761989000300016 PMid:2520831.
» http://dx.doi.org/10.1590/S0074-02761989000300016 - KING, M., EUBEL, H., MILLAR, A.H. and BAER, B., 2011. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. Journal of Insect Physiology, vol. 57, no. 3, pp. 409-414. http://dx.doi.org/10.1016/j.jinsphys.2010.12.011 PMid:21192944.
» http://dx.doi.org/10.1016/j.jinsphys.2010.12.011 - KOCOREK, A. and DANIELCZOK-DEMSKA, T., 2002. Comparative morphology of the spermatheca within the family Dinidoridae (Hemiptera: Heteroptera). European Journal of Entomology, vol. 99, no. 1, pp. 91-98. http://dx.doi.org/10.14411/eje.2002.016
» http://dx.doi.org/10.14411/eje.2002.016 - LENT, H. and WYGODZINSKY, P., 1979. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bulletin of the American Museum of Natural History, vol. 163, pp. 123-520.
- MARTINS, G.F. and SERRÃO, J.E., 2002. A comparative study of the spermatheca in bees (Hymenoptera; Apoidea). Sociobiology, vol. 40, pp. 711-720.
- MARTINS, G.F., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Spermatheca morphology of the social wasp Polistes erythrocephalus. Bulletin of Insectology, vol. 61, pp. 37-41.
- MENDES, M.T., 2014. Avaliação do efeito da saliva de triatomíneos (Heteroptera: Reduviidae) sobre a biologia de células dendríticas murinas Uberaba: Universidade Federal do Triângulo Mineiro, 99 p. Dissertação de Mestrado em Medicina Tropical e Infectologia.
- NASCIMENTO, J.D., 2015. Estudo histológico das espermatecas e traqueias de seis espécies de Triatominae (Hemiptera, Reduviidae) Campinas: Universidade Estadual de Campinas, 60 p. Dissertação de Mestrado em Biologia Animal.
- NOIROT, C. and QUENNEDEY, A., 1991. Glands, gland cell, glandular units: some comments on terminology and classification. Annales de la Société Entomologique de France, vol. 23, pp. 123-128.
- PASCINI, T.V. and MARTINS, G.F., 2017. The insect spermatheca: an overview. Zoology, vol. 121, pp. 56-71. http://dx.doi.org/10.1016/j.zool.2016.12.001 PMid:28089345.
» http://dx.doi.org/10.1016/j.zool.2016.12.001 - PENDERGRAST, J.G., 1957. Studies on the reproductive organs of the Heteroptera with a consideration of their bearing on classification. Ecological Entomology, vol. 109, pp. 1-63.
- SILVA, I.G., SANTOS, L.G.P. and NAKANO, R., 1993. Ciclo evolutivo de Triatoma lecticularia (Stal, 1859) (Hemiptera, Reduviidae). Revista de Patologia Tropical, vol. 22, pp. 259-263.
- SOUZA, E.A., CAMPOS, L.A.O., NEVES, C.A., ZANUNCIO, J.C. and SERRÃO, J.E., 2008. Effect of delayed mating on spermathecal activation in Melipona quadrifasciata anthidioides (Hymenoptera, Apidae) queens. Apidologie, vol. 39, no. 3, pp. 293-301. http://dx.doi.org/10.1051/apido:2008008
» http://dx.doi.org/10.1051/apido:2008008 - SOUZA, E.A., LISBOA, L.C.O., ARAÚJO, V.A. and SERRÃO, J.E., 2016. Morphology of the spermathecae of Leptoglossus zonatus (Heteroptera: Coreidae). Annals of the Entomological Society of America, vol. 109, no. 1, pp. 106-111. http://dx.doi.org/10.1093/aesa/sav097
» http://dx.doi.org/10.1093/aesa/sav097 - STACCONI, M. and ROMANI, R., 2011. Ultrastructural and functional aspects of the spermatheca in the American Harlequin Bug, Murgantia histrionica (Hemiptera: Pentatomidae). Neotropical Entomology, vol. 40, no. 2, pp. 222-230. http://dx.doi.org/10.1590/S1519-566X2011000200011 PMid:21584404.
» http://dx.doi.org/10.1590/S1519-566X2011000200011 - STEFANINI, M., DE MARTINO, C. and ZAMBONI, L., 1967. Fixation of ejaculated spermatozoa for electron microscopy. Nature, vol. 216, no. 5111, pp. 173-174. http://dx.doi.org/10.1038/216173a0 PMid:4862079.
» http://dx.doi.org/10.1038/216173a0 - THORNHILL, R. and ALCOCK, J., 1983. The evolution of insect mating systems Cambridge: Harvard University Press.
» Cambridge - WHEELER, D.E. and KRUTZSCH, P., 1994. Ultrastructure of the spermatheca and its associated gland in the ant Crematogaster opuntiae (Hymenoptera, Formicidae). Zoomorphology, vol. 114, no. 4, pp. 203-212. http://dx.doi.org/10.1007/BF00416859
» http://dx.doi.org/10.1007/BF00416859 - WOLFNER, M.F., 2011. Precious essences : female secretions promote sperm storage in Drosophila. PLoS Biology, vol. 9, no. 11, pp. e1001191. http://dx.doi.org/10.1371/journal.pbio.1001191 PMid:22087072.
» http://dx.doi.org/10.1371/journal.pbio.1001191
Publication Dates
-
Publication in this collection
22 Mar 2018 -
Date of issue
Jan-Mar 2019
History
-
Received
02 June 2017 -
Accepted
22 Aug 2017