Abstracts
We identified the avian assembly that consumes Miconia ligustroides (Melastomataceae) fruit and described its phenophases in a fragment of cerrado vegetation located in southeastern Brazil. The fruiting period occurred between March and June, a period of food shortage. In 2005 and 2008, we registered eighteen bird species consuming fruits, during 156 hours of observations. Species of the families Thraupidae and Tyrannidae were the most representative in the number of visits and fruit consumption. Short visits (less than three minutes) and low incidence of defecation apparently indicate that seeds may be released far from the parent-plant, suggesting dispersal efficiency by the studied assembly. Owing to its characteristics, we believe Miconia ligustroides may be useful in the restoration of degraded areas.
cerrado; frugivory; Miconia; ornitochory; phenology
Identificamos a assembleia de aves que consome frutos de Miconia ligustroides (Melastomataceae) e descrevemos a fenologia dessa planta, em um fragmento de cerrado no sudeste do Brasil. O período de frutificação ocorreu entre março e junho, um período de escassez de alimento. Em 2005 e 2008, foram registradas 18 espécies de aves consumindo frutos, ao longo de 156 horas de observação. Espécies das famílias Thraupidae e Tyrannidae foram as mais representativas em número de visitas e consumo de frutos. Visitas curtas (menos de três minutos) e a baixa incidência de defecação, aparentemente, indicam que as sementes devem ser liberadas longe da planta-mãe, o que sugere eficiência na dispersão pela assembleia estudada. Em função de suas características, acreditamos que Miconia ligustroides pode ser útil na restauração de áreas degradadas.
cerrado; frugivoria; Miconia; ornitocoria; fenologia
ECOLOGY
Phenology and frugivory by birds on Miconia ligustroides (MELASTOMATACEAE) in a fragment of cerrado, southeastern Brazil
Fenologia e frugivoria por aves em Miconia ligustroides (MELASTOMATACEAE) em um fragmento de cerrado, sudeste do Brasil
Allenspach, N.* * e-mail: naty_allenspach@yahoo.com.br ; Telles, M.; Dias, MM.
Departamento de Ecologia e Biologia Evolutiva DEBE, Universidade Federal de São Carlos UFSCar, Rod. Washington Luiz, Km 235, CP 676, CEP 13565-905, São Carlos, SP, Brazil
ABSTRACT
We identified the avian assembly that consumes Miconia ligustroides (Melastomataceae) fruit and described its phenophases in a fragment of cerrado vegetation located in southeastern Brazil. The fruiting period occurred between March and June, a period of food shortage. In 2005 and 2008, we registered eighteen bird species consuming fruits, during 156 hours of observations. Species of the families Thraupidae and Tyrannidae were the most representative in the number of visits and fruit consumption. Short visits (less than three minutes) and low incidence of defecation apparently indicate that seeds may be released far from the parent-plant, suggesting dispersal efficiency by the studied assembly. Owing to its characteristics, we believe Miconia ligustroides may be useful in the restoration of degraded areas.
Keywords: cerrado, frugivory, Miconia, ornitochory, phenology.
RESUMO
Identificamos a assembleia de aves que consome frutos de Miconia ligustroides (Melastomataceae) e descrevemos a fenologia dessa planta, em um fragmento de cerrado no sudeste do Brasil. O período de frutificação ocorreu entre março e junho, um período de escassez de alimento. Em 2005 e 2008, foram registradas 18 espécies de aves consumindo frutos, ao longo de 156 horas de observação. Espécies das famílias Thraupidae e Tyrannidae foram as mais representativas em número de visitas e consumo de frutos. Visitas curtas (menos de três minutos) e a baixa incidência de defecação, aparentemente, indicam que as sementes devem ser liberadas longe da planta-mãe, o que sugere eficiência na dispersão pela assembleia estudada. Em função de suas características, acreditamos que Miconia ligustroides pode ser útil na restauração de áreas degradadas.
Palavras-chave: cerrado, frugivoria, Miconia, ornitocoria, fenologia.
1. Introduction
Seed dispersal is a key step in the life cycle of Angiosperms (Regal, 1977). In this process, fruit is usually eaten by animals, which defecate and/or regurgitate seeds far from the parent plant (Van der Pijl, 1972). Thus, animals obtain food, while plants have an opportunity to increase their seed germination (Snow, 1981; Van der Pijl, 1972; Galetti et al., 2004). In the cerrado, approximately 30% of all vegetation is dispersed by animals, which represents more than 60% of the woody species (Batalha and Martins, 2004).
Birds are the main seed dispersers in tropical environments (Howe, 1980). They probably play an important role in the evolution of small, colorful and juicy fruit, easy to be detached from the parent plant (Van der Pijl, 1972). According to Howe and Steven (1979), birds are also responsible for setting plant phenology, which is an important factor in fruiting timing.
Miconia ligustroides (Naudin) is a Melastomataceae that reaches up to eight meters height. It produces a large amount of small fruit, purple and juicy, bearing a large number of small seeds (Gottsberger and Silberbauer-Gottsberger, 1983; Martins et al., 1996). According to Snow (1985), these characteristics make Miconia a genus whose fruits are dispersed by generalistic birds, which also include insects and other items in their diets. Miconia ligustroides, as well as various species of the genus, colonizes edges and constitutes secondary vegetation (Snow, 1981).
As frugivory and seed dispersal are fundamental for forest management (Wunderle Junior, 1997) and forest restoration (Silva et al., 2010), the main aim of this study was to describe the phenology of M. ligustroides and identify its potential seed dispersers in a fragment of cerrado within São Paulo State.
2. Material and Methods
We conducted the study in a 124,68 ha cerrado fragment (Paese, 1997) inside the campus of the Federal University of São Carlos, Southeastern Brazil (21° 58' S and 47° 52' W). The regional climate is Cwa.i Awi, according to Köppen's system. The vegetation is compromised by exotic grass, mainly Brachiaria sp. and Melinis sp. (Poaceae). Despite all the anthropogenic effects, 274 bird species can be found in the area (Dias, Branco and Francisco, In Press), of which approximately 33% consume fruit (Francisco and Galetti, 2001).
Miconia ligustroides is a common, but not abundant species in this area (Oliveira and Batalha, 2005). Its phenophases (see Figure 1) were accompanied in 30 random selected individuals, every two weeks, between March, 2008 and February, 2009. We used the Fournier intensity percentage (Fournier, 1974) and the activity index (percentage of individuals that show the phenophase) to summarize monthly field data.
Frugivory by birds was observed using the focal tree metodology between March-May 2005 (five trees, 60 hours) and February-May 2008 (eight trees, 96 hours), always during fruiting peaks. We observed each individual from a minimum distance of 8-10 meters, with 7 × 35 binoculars, from 06:00 to 18:00 hours, and therefore each time interval was equally sampled in every tree. We sampled: visiting bird species, time of the visits, amount of consumed fruit, foraging behavior, and occurrence of agonistic encounters, defecation and regurgitation. In 2008, we also registered the duration of each bird's visit to the plants.
Visits were disconsidered when it was not possible to count the amount of fruit consumed. We applied the Spearman coefficient to verify correlations between bird's body mass, fruit consumption and visit duration, as well as tree height, number of visits and available fruit. Data concerning body masses and predominant diets were obtained from the literature (Motta-Junior, 1990; Marini et al., 1997; Sick, 1997; Piratelli and Pereira, 2002; Antunes, 2005). We obtained meteorological data from Embrapa Pecuária Sudeste (Embrapa, 2009), located about 3.5 km from the study area.
We used a few captive birds from the Ecological Park in São Carlos to evaluate seed retention time in the bird's digestive tract. The species observed were: Turdus amaurochalinus (n = 3), Mimus saturninus (n = 2) and Pitangus sulphuratus (n = 5). M. ligustroides fruit was given to the birds early in the morning, before providing their usual food. Using a chronometer, we measured the time between the first fruit consumption and defecation.
3. Results
We found flowers and flower buds throughout the whole year, in a small number, but a flowering peak was observed in January (activity index, see Figure 2). The fruiting period occurred between March and June, when we observed a peak in the activity index of ripe fruits. Individuals showed high synchrony, fruiting along the transition from the wet season to the dry season. A low amount of ripe fruit was recorded because it was readily eaten by birds, and also many of them fell during heavy rains in March.
Miconia ligustroides fruits (n = 50) presented a mean diameter of 5.63 (±1.028) mm and average weight of 0.129 (±0.057) g. We found a mean of 16.44 (±6.447) seeds per fruit, with an average weight of 5.31 mg each (n = 1000). The seeds correspond to approximately 6.77% of the fruit weight.
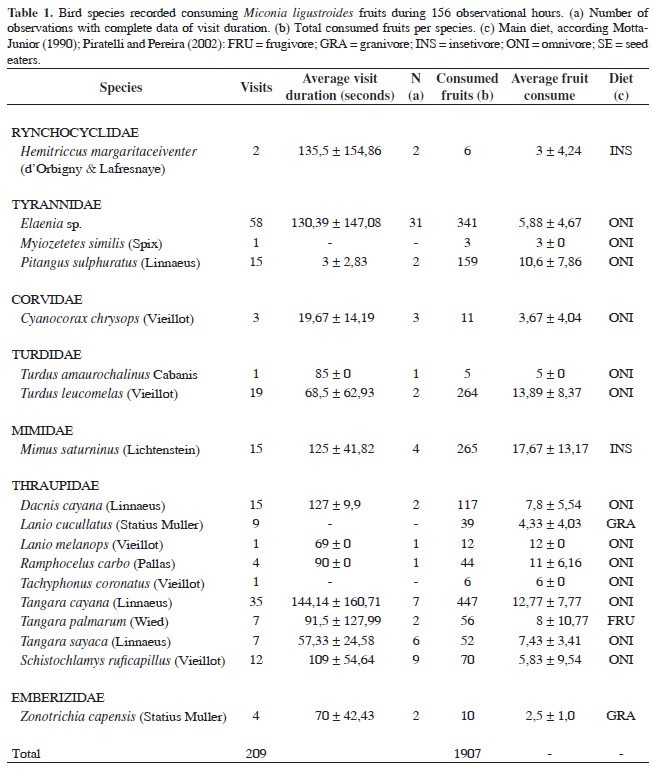
We sampled a total of 209 visits with fruit consumption by birds, performed by eighteen species, from seven families (Table 1). The average frequency was 1.34 ± 2.24 visits/hour and there were no significant frequency changes throughout the daytime.
The species of the family Thraupidae was the most representative in number of visits (43.50%) and fruit consumption (44.17%), followed by Tyrannidae (respectively 35.37% and 26.36%). All species were observed swallowing the whole fruits. On a few occasions, we observed Schistochlamys ruficapillus, Tangara cayana, Tangara sayaca and Zonotrichia capensis mandibulating fruits, but swallowing them totally.
Defecation, regurgitation and agonistic encounters were rarely observed. The average fruit consumption per visit varied from 2.5 fruits/visit (Zonotrichia capensis) to 17.67 (Mimus saturninus). We found no correlation between body mass and average fruit consumption per visit, nor between the average fruit consumption per visit and average visit duration.
The height of the trees used for focal observations varied from 1.5 to 4.0 m, holding from 1,500 to 15,000 fruit. We found no significant correlations between the tree height and number of visits, nor between the available fruit and number of visits.
Seed retention observations made with captive birds showed the following results: 8.03 (±5.9) minutes for T. amaurochalinus, 8.91 (±4.28) minutes for M. saturninus, and 4.98 (±1.53) minutes for P. sulphuratus. We also observed two regurgitations (P. sulphuratus) approximately 15 minutes after fruit consumption. In all cases, retention time was much longer than the average visit duration that varied from three seconds (P. sulphuratus) to 2.4 minutes (Tangara cayana).
4. Discussion
Miconia ligustroides individuals showed highlighted and synchronized phenophases. According to Augspurger (1981), high synchrony of fruit production may draw the attention of frugivores and could be a possible strategy to increase seed dispersal. The fruiting period occurs during the transition between wet and dry seasons, when few fruit is available in the studied area (pers. obs.). There is a mismatching between the number of registered flowers and immature fruit. It probably happened because M. ligustroides flowers remain open only for a few days, so we were unable to register them all in the biweekly observations.
Fruit from M. ligustroides were eaten by birds with generalistic diets and may be of great importance as an energy source for these opportunistic frugivores in a period of scarcity. A study on four Miconia species, all typical from the Cerrado domain, reports their fruit is rich in water and carbohydrates (Maruyama et al., 2007). Furthermore, dark fruit may be preferentially chosen by birds due to their high anthocyanin content, a class of pigments with antioxidant properties (Schaefer, 2011).
Short visits (less than three minutes) and low defecation rates suggests seeds may be carried far away from the parent-plant (Pratt and Stilles, 1983). Long seed retention observed in M. saturninus, T. amaurochalinus and P. sulphuratus may increase the distance of seed deposition. Based on the average values of fruit consumption and number of seeds per fruit, M. saturninus ingests and transports about 290 seeds after a single visit to a M. ligustroides tree. However, contributions from smaller birds such as T. cayana cannot be underestimated as they carry approximately 209 M. ligustroides seeds after one visit. This species alone is responsible for 23.44% of fruit consumption.
Snow (1981) characterized fruit produced by the Miconia species as a good example of those consumed preferentially by opportunistic birds as the fruit is small and juicy and bears many small seeds. Our results confirm a general dispersal system for M. ligustroides, while a similar study conducted on M. albicans, in the same study area, registered nineteen opportunistic bird species consuming its fruit (Allenspach and Dias, 2012). The guilds consuming M. albicans and M. ligustroides fruit are similar (Jaccard Index = 0.67) and the dissimilarities can possibly be explained by different fruiting seasons, migrant bird species and the sites of the trees in the studied area.
Being dispersed by common birds in fragments and being able to establish in disturbed areas, we suggest M. ligustroides may be helpful in the restoration of degraded areas. Its large fruit production is an energy source for birds during the scarcity period, probably helping to maintain local avifauna. Characterized by a generalistic dispersal system, its seeds may be dispersed even where large and/or specialized frugivores are absent. However, further studies should be carried out to observe M. ligustroides germination and establishment capabilities.
Acknowledgements We would like to thank Dra. Maria Inês Salgueiro Lima for identifying the plants, to the biologist Julia Ramos Estêvão for helping during field studies, and to Ecological Park in São Carlos for allowing the tests. N. Allenspach received a Master's scholarship from CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Received October 26, 2011
Accepted February 06, 2012
Distributed November 30, 2012
- ALLENSPACH, N. and DIAS, MM., 2012. Frugivory by birds on Miconia albicans (MELASTOMATACEAE), in a fragment of cerrado in São Carlos, southeastern Brazil. Brazilian Journal of Biology, vol. 72, no. 2.
- ANTUNES, AZ., 2005. Alteração na composição da comunidade de aves ao longo do tempo em um fragmento florestal no sudeste do Brasil. Ararajuba, vol. 13, no. 1, p. 47-61.
- BATALHA, MA. and MARTINS, FR., 2004. Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Australian Journal of Botany, vol. 52, no. 2, p. 149-161. http://dx.doi.org/10.1071/BT03098
- DIAS, MM., BRANCO, MBC. and FRANCISCO, MR. In Press. A avifauna da UFSCar, Campus São Carlos, Estado de São Paulo: 34 anos de levantamentos. In LIMA, MIS. and PENTEADO-DIAS MA. Ambientes terrestres do Campus da UFSCar, São Carlos.
- EMBRAPA. Pecuária Sudeste, 2009. Serviços - dados meteorológicos Available from: <http://www.cppse.embrapa.br>. Acess in: 10 mar. 2009.
- FOURNIER, LA., 1974. Un método cuantitativo para la medición de características fenológicas en arboles. Turrialba, vol. 24, no. 4, p. 422-423.
- FRANCISCO, MR. and GALETTI, M., 2001. Frugivoria e dispersão de sementes de Rapanea lancifolia (Myrcinaceae) por aves numa área de cerrado do Estado de São Paulo, Sudeste do Brasil. Ararajuba, vol. 9, no. 1, p. 13-19.
- GALETTI, M., PIZO, MA. and MORELLATO, PC., 2004. Fenologia, frugivoria e dispersão de sementes. In CULLEN JUNIOR, L.; RUDRAN, R. and VALLADARES-PÁDUA, C. Métodos de estudos em biologia da conservação e maneja da vida silvestre Curitiba: UFPR. p. 395-422.
- GOTTSBERGER, G. and SILBERBAUER-GOTTSBERGER, I., 1983. Dispersal and disptribution in the cerrado vegetation of Brazil. Sonderbände des Naturwissenschaftlichen Vereins in Hamburg, vol. 7, p. 315-352.
- HOWE, HF., 1980. Dispersal of a neotropical nutmeg (Virola subifera) by birds. The Auk, vol. 98, p.89-98.
- HOWE, HF. and STEVEN, D., 1979. Fruit production, migrant bird visitation, and seed dispersal of Guarea glabra in Panama. Oecologia, vol. 39, no. 2, p. 185-196. http://dx.doi.org/10.1007/BF00348067
- MARINI, MA., MOTTA-JÚNIOR, JC., VASCONCELLOS, LAS. and CAVALCANTI, RB., 1997. Avian body masses from the cerrado region of central Brazil. Ornitologia Neotropical, vol. 8, p. 93-99.
- MARTINS, AB., SENIR, J., GOLDENBERG,R. and MARTINS, E., 1996. O gênero Miconia Ruiz & Pav. (Melastomataceae) no estado de São Paulo. Acta Botanica Brasilica, vol. 10, no. 2, p. 267-316.
- MARUYAMA, PK., ALVES-SILVA, E. and MELO, C., 2007. Oferta qualitativa e quantitativa de frutos em espécies ornitocóricas do gênero Miconia (Melastomataceae). Revista Brasileira de Biociências, vol. 5, no. 1, p. 672-674.
- MOTTA-JUNIOR, JC., 1990. Estrutura trófica e composição das avifaunas de três hábitats terrestres na região central do estado de São Paulo. Ararajuba, vol. 1, p. 65-71.
- OLIVEIRA, FF. and BATALHA, MA., 2005. Lognormal abundance distribution of woody species in a cerrado fragment (São Carlos, Southeasthern Brazil). Revista Brasileira de Botânica, vol. 28, no. 1, p. 39-45.
- PAESE, A., 1997. Caracterização e análise ambiental do campus da Universidade Federal de São Carlos (UFSCar), São Carlos, Brasil São Carlos: Universidade Federal de São Carlos. Dissertação de Mestrado em Ecologia e Recursos Naturais.
- PIRATELLI, A. and PEREIRA, MR., 2002. Dieta de aves na região leste do Mato Grosso do Sul, Brasil. Ararajuba, vol. 10, no. 2, p. 131-139.
- PRATT, TK. and STILES, EW., 1983. How long fruit-eating birds stay in the plants where they feed: implications for seed dispersal. The American Naturalist, vol. 122, no. 6, p. 797-805. http://dx.doi.org/10.1086/284172
- REGAL, PJ., 1977. Ecology and evolution of flowering plant dominance. Science, vol. 196, p. 622-629. PMid:17760038. http://dx.doi.org/10.1126/science.196.4290.622
- SCHAEFER, HM., 2011. Why fruits go to the dark side. Acta Oecologica, vol. 30, p. 1-7.
- SICK, H., 1997. Ornitologia brasileira Rio de Janeiro: Nova Fronteira. 912 p.
- SILVA, WR., PIZO, MA. and GABRIEL, VA., 2010. A avifauna como promotora da restauração ecológica. In VON MATTER, S., STRAUBE, FC., ACCORDI, IA., PIACENTINI, VQ. and CÂNDIDO JUNIOR, JF. (Orgs.). Ornitologia e Conservação. Rio de Janeiro: Technical Books. p. 507-516.
- SNOW, DW., 1981. Tropical frugivorous birds and their food plants: a world survey. Biotropica, vol. 13, no. 1, p. 1-14. http://dx.doi.org/10.2307/2387865
- -, 1985. The web of adaptation – Bird studies in the American tropics Ithaca: Cornell University Press. 176 p.
- VAN DER PIJL, L., 1972. Principles of dispersal in higher plants New York: Spring-Verlag. 162 p.
- WUNDERLE JUNIOR, JM., 1997. The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. Forestry Ecology and Management, vol. 99, p. 223-235.
Publication Dates
-
Publication in this collection
03 Jan 2013 -
Date of issue
Nov 2012
History
-
Received
26 Oct 2011 -
Accepted
02 June 2012