NOTES AND COMMENTS
Abundance of MyoD and myostatin transcripts in chicken embryos submitted to distinct incubation temperatures and timing exposures
Gabriel, JE.I,* * e-mail: jane.gabriel@univasf.edu.br ; Alves, HJ.II; Do Rosário, MF.II; Secatto, A.III; Coutinho, LL.II; Macari, M.III
ICentro de Ciências Agrárias, Universidade Federal do Vale do São Francisco UNIVASF, Rod. BR 407, Km 12, Lote 543, Projeto de Irrigação Senador Nilo Coelho, C1, s/n, CEP 56300-000, Petrolina, PE, Brazil
IIDepartamento de Produção Animal, Escola Superior de Agricultura "Luiz de Queiroz" ESALQ USP, Av. Pádua Dias, 11, CEP 13418-900, Piracicaba, SP, Brazil
IIIDepartamento de Anatomia e Fisiologia Animal, Universidade Estadual Paulista UNESP, Via de Acesso Prof. Paulo Donato Castellane, s/n, CEP 14870-000, Jaboticabal, SP, Brazil
The formation of skeletal muscle tissue is directly modulated by intricate expressing the distinct signalling molecules orchestrated by the myogenic regulatory factors, such as the myogenic determination factor MyoD, that activates the transcription of muscle-specific genes by binding to consensus DNA sites found in the regulatory sequences of these genes, differentiate and fuse the myoblasts in order to form the muscle fibres (Buckingham, 2006). Its expression is detected at an earlier stage of myogenesis when quiescent stem cells rapidly expand in number to generate the myoblasts needed to repair tissue damage (Glass, 2010) and several studies have demonstrated that MyoD is sufficient and necessary for the formation or survival of skeletal myoblasts (Emerson, 1993; Buckingham, 2006). In this context, it was already well characterised that myostatin is a secreted growth and differentiation factor belonging to the transforming growth factor (TGF)-beta superfamily that exert an important modulator role of body composition in experimental animals by controlling the proliferation of myoblasts, followed by inhibiting the differentiation of these cells (Rodgers and Garikipati, 2008; Glass, 2010).
Few reports in the literature have investigated how the abundance of myogenic regulatory genes is altered in chicken embryos exposed to distinct adverse incubation temperatures and timing exposures (Gabriel et al., 2003, 2006). Based on this evidence, the purpose of the present study was to quantify the levels of MyoD and myostatin transcripts in chicken embryos exposed to low and high incubation temperatures for distinct timing exposure using real time PCR assays.
Fertilised chicken eggs (Gallus gallus domesticus, Linnaeus, 1758) from the Cobb line were maintained in a humidified atmosphere in an incubator (Premium Ecológica, model IP120). After a four-day incubation period at 37 ± 0.5 °C (control incubation condition) and 60% relative humidity, some eggs were transferred to other incubators registering low (33 ± 0.5 °C) and high (44 ± 0.5 °C) incubation temperatures. These eggs remained for one and two hours under these conditions, and at the end of each hour, six embryos per treatment were then collected for further experiments. The collection of embryos on the fourth day of incubation was based on the evidence that chicken embryos at the developmental stage HH24 present accentuated expression of specific myogenesis genes, such as MyoD and myostatin, in comparison to basal levels detected in preceding stages (Emerson, 1993). During the procedure described above, embryos from eggs submitted to control incubation conditions were also collected.
The extraction of total RNA and the reverse transcription reactions in order to obtain the single-strand cDNA synthesis from samples were performed according to Marchesin (2008). The primers used in this experimental approach were initially designed and tested by Gabriel et al. (2003). The quantification of levels of MyoD and myostatin transcripts was established by real time PCR assays using the Taq Platinum DNA polimerase (Invitrogen) and SYBR Green I/Nucleic Acid Gel Stain (Roche Applied Science) in the LightCycler PCR System (Roche Applied Science), as optimised by Marchesin (2008). In order to normalise the values generated, the same experimental approach was carried out using specific primers for amplification of β-actin transcripts. Each sample was tested in duplicate, and the mean of the two values was shown as the copy number of the sample.
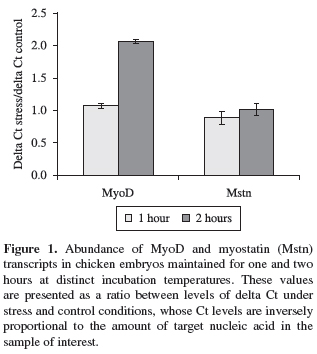
In a real time PCR assay a positive reaction is detected by accumulation of a fluorescent signal. The Ct (cycle threshold) is defined as the number of cycles required for the fluorescent signal to cross the threshold (i.e. exceeds background level). Ct levels are inversely proportional to the amount of target nucleic acid in the sample (i.e. the lower the Ct level indicates the greater amount of target nucleic acid in the sample). These data were initially normalised in order to calculate the ∆Ct values (∆Ct = Ct gene of interest Ct β-actin gene). Additionally, rations between ∆Ct levels in stress treatment and under control conditions were established and such results were statistically analysed using the software SAS® (Statistical Analysis System).
Under these experimental conditions, no variations in MyoD transcript levels were detected in embryos maintained at cold or heat incubation temperatures for one hour (Figure 1) (p > 0.05). In contrast, the MyoD gene expression was significantly reduced by 50% in animals submitted to hot incubation temperatures for two hour exposure compared to control animals (Figure 1) (p < 0.05). No significant differences in the abundance of myostatin transcripts were found in cold and heat stressed embryos, irrespective of timing exposure analysed (p > 0.05) (Figure 1).
Additionally, the internal embryonic temperature in each treatment was measured prior to collection of embryos by using an infrared thermometer (Horiba, model IT-330). Interestingly, expressive increases in internal temperatures were only detected in chicken embryos exposed to hot incubation temperature for two hours (p < 0.05), whereas no significant variations were detected in the other treatments (data not shown). Based on these results describing alterations in MyoD transcript levels and variations in internal embryonic temperature in avian embryo development, it can be speculated that the high incubation temperature could inhibit the distinct signalling pathways involved in mechanisms of activation of myogenesis by arresting of functional transcriptional factors. Alternatively, the transcripts of the MyoD factor could be rapidly denatured as a response to the effect of high incubation temperature on biological stability changes in steady-state cellular mRNA.
Recently, Hennebry et al. (2009) have demonstrated that the myostatin gene negatively regulates the MyoD expression, affecting the fibre-type composition in the murine muscle. Since the myostatin gene is implicated in controlling G1-to-S progression of myoblasts, MyoD could be triggering myoblast withdrawal from the cell cycle by regulating the myostatin gene expression (Hennebry et al., 2010). Additionally, Engelbrecht et al. (2010) investigated the effects of physiological stress on gene expression associated with muscle atrophy using a rat model of restraint stress in adult male animals. Daily brief restraint stress resulted in a significant increase in myostatin levels, whereas the MyoD expression was significantly attenuated under their experimental conditions (Engelbrecht et al., 2010). Therefore, the expression of myogenic factors, such as MyoD or myogenin, has not been affected by myostatin in mouse pectoralis muscle tissue, as observed by Rachagani et al. (2010).
Thus, the recent findings described above suggest that the molecular events and signalling pathways responsible by activating and regulating the expression of MyoD and myostatin genes, seem to trigger a wide spectrum of cellular responses in vertebrates. In conclusion, the results presented in this study provide new evidence at a molecular level how adverse incubation temperatures affect the expression of essential regulators of skeletal muscle lineage determination in chicken embryogenesis.
Acknowledgements The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) for financial support.
References
BUCKINGHAM, M., 2006. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Current Opinion on Genetic and Development, vol. 16, p. 525-532. doi:10.1016/j.gde.2006.08.008
EMERSON, CP. JR., 1993. Embryonic signals for skeletal myogenesis: arriving at the beginning. Current Opinion on Cell Biology, vol. 5, p. 1057-1064. doi:10.1016/0955-0674(93)90092-5
ENGELBRECHT, AM., SMITH, C., NEETHLING, I., THOMAS, M., ELLIS, B., MATTHEYSE, M. and MYBURGH KH., 2010. Daily brief restraint stress alters signaling pathways and induces atrophy and apoptosis in rat skeletal muscle. Stress, vol. 13, p. 132-141. PMid:19929313. doi:10.3109/10253890903089834
GABRIEL, JE., ALVARES, LE., GOBET, MC., DE PAZ, CCP., PACKER, IU., MACARI, M. and COUTINHO, LL., 2003. Expression of MyoD, myogenin, myostatin and Hsp70 transcripts in chicken embryos submitted to mild cold or heat. Journal of Thermal Biology, vol. 28, p. 261269. doi:10.1016/S0306-4565(02)00085-2
GABRIEL, JE., ALVARES, LE., PAZ, CCP., PACKER, IU. and COUTINHO, LL., 2006. Temporal expression pattern of myostatin transcripts during chicken embryogenesis. Arquivos Brasileiros de Medicina Veterinária e Zootecnia, vol. 58, p. 940-943.
GLASS, DJ., 2010. Signaling pathways perturbing muscle mass. Current Opinion on Clinical Nutrition and Metabolical Care, vol. 13, p. 225-229. doi:10.1097/MCO.0b013e32833862df
HENNEBRY, A., BERRY, C., SIRIETT, V., O'CALLAGHAN, P., CHAU, L., WATSON, T., SHARMA, M. and KAMBADUR, R., 2009. Myostatin regulates fiber type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. American Journal of Physiology and Cell Physiology, vol. 296, p. C525-C534. PMid:19129464. doi:10.1152/ajpcell.00259.2007
MARCHESIN, ML., 2008. Análise da expressão gênica de MyoD, MRF4, Miogenina e Miostatina nos músculos Bíceps femoris e Gastrocnemius lateralis em duas linhagens de Gallus gallus (corte e postura). Rio Claro: Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista. 86 p. Dissertação de Mestrado em Biologia celular e molecular.
RACHAGANI, S., CHENG, Y. and REECY, JM., 2010. Myostatin genotype regulates muscle-specific miRNA expression in mouse pectoralis muscle. BMC Research Notes, vol. 11, p. 293-297.
RODGERS, BD. and GARIKIPATI, DK., 2008. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocrinology Review, vol. 29, p. 513-534. PMid:18591260. PMCid:2528853. doi:10.1210/er.2008-0003
Received October 18, 2010
Accepted January 21, 2011
Distributed May 31, 2011
- BUCKINGHAM, M., 2006. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Current Opinion on Genetic and Development, vol. 16, p. 525-532. doi:10.1016/j.gde.2006.08.008
- EMERSON, CP. JR., 1993. Embryonic signals for skeletal myogenesis: arriving at the beginning. Current Opinion on Cell Biology, vol. 5, p. 1057-1064. doi:10.1016/0955-0674(93)90092-5
- ENGELBRECHT, AM., SMITH, C., NEETHLING, I., THOMAS, M., ELLIS, B., MATTHEYSE, M. and MYBURGH KH., 2010. Daily brief restraint stress alters signaling pathways and induces atrophy and apoptosis in rat skeletal muscle. Stress, vol. 13, p. 132-141. PMid:19929313. doi:10.3109/10253890903089834
- GABRIEL, JE., ALVARES, LE., GOBET, MC., DE PAZ, CCP., PACKER, IU., MACARI, M. and COUTINHO, LL., 2003. Expression of MyoD, myogenin, myostatin and Hsp70 transcripts in chicken embryos submitted to mild cold or heat. Journal of Thermal Biology, vol. 28, p. 261269. doi:10.1016/S0306-4565(02)00085-2
- GABRIEL, JE., ALVARES, LE., PAZ, CCP., PACKER, IU. and COUTINHO, LL., 2006. Temporal expression pattern of myostatin transcripts during chicken embryogenesis. Arquivos Brasileiros de Medicina Veterinária e Zootecnia, vol. 58, p. 940-943.
- GLASS, DJ., 2010. Signaling pathways perturbing muscle mass. Current Opinion on Clinical Nutrition and Metabolical Care, vol. 13, p. 225-229. doi:10.1097/MCO.0b013e32833862df
- HENNEBRY, A., BERRY, C., SIRIETT, V., O'CALLAGHAN, P., CHAU, L., WATSON, T., SHARMA, M. and KAMBADUR, R., 2009. Myostatin regulates fiber type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. American Journal of Physiology and Cell Physiology, vol. 296, p. C525-C534. PMid:19129464. doi:10.1152/ajpcell.00259.2007
- MARCHESIN, ML., 2008. Análise da expressão gênica de MyoD, MRF4, Miogenina e Miostatina nos músculos Bíceps femoris e Gastrocnemius lateralis em duas linhagens de Gallus gallus (corte e postura) Rio Claro: Instituto de Biociências do Campus de Rio Claro, Universidade Estadual Paulista. 86 p. Dissertação de Mestrado em Biologia celular e molecular.
- RACHAGANI, S., CHENG, Y. and REECY, JM., 2010. Myostatin genotype regulates muscle-specific miRNA expression in mouse pectoralis muscle. BMC Research Notes, vol. 11, p. 293-297.
- RODGERS, BD. and GARIKIPATI, DK., 2008. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocrinology Review, vol. 29, p. 513-534. PMid:18591260. PMCid:2528853. doi:10.1210/er.2008-0003
Publication Dates
-
Publication in this collection
15 July 2011 -
Date of issue
May 2011