Abstracts
Decomposition of aquatic plants is influenced by several biotic and abiotic factors. Among them, temperature plays an important role. Despite the increasing number of studies describing the effects of temperature on the decomposition of aquatic macrophytes, little attention has been given to the decay of submerged macrophytes. In this paper, we assessed the effect of temperature on weight loss and chemical composition of detritus of the submerged aquatic macrophyte Egeria najas Planchon (Hydrocharitaceae). Fresh plant material was maintained at 17ºC and 27ºC, in the dark, in incubation chambers. The overall decay process was best described by a linear model, with rates of 0.014 day-1 (R² = 94%) and 0.045 day-1 (R² = 96%) obtained at 17ºC and 27ºC, respectively. The analysis of covariance (ANCOVA) indicated a significant difference between the decomposition rates at the two temperatures. The rapid breakdown of E. najas detritus, indicated by the decay coefficient, may be explained by its low content of resistant compounds such as cellulose and lignin. The variables analyzed in this study (pH, electrical conductivity, dissolved oxygen in the water and organic matter, total nitrogen and total phosphorus concentration in detritus) showed accentuated responses at 27ºC. It is likely that the higher temperature increased microbial activity and, therefore, oxygen consumption in the water, consequently affecting the pH and the rate of ion and nutrient liberation into the aquatic ecosystem. Due to the rapid decomposition of E. najas at high temperatures, a small exportation is expected of this species from its stands to distant regions in tropical reservoirs, where it is considered a potential nuisance species.
decomposition; temperature; Egeria najas
Inúmeros fatores bióticos e abióticos influenciam a decomposição de plantas aquáticas, destacando-se, entre eles, a temperatura. Apesar do grande número de estudos descrevendo os efeitos da temperatura sobre a decomposição de macrófitas aquáticas, pouca atenção tem sido dada à decomposição de espécies submersas. O objetivo do presente trabalho foi avaliar o efeito da temperatura sobre a perda de peso e composição química do detrito da macrófita aquática submersa Egeria najas Planchon (Hydrocharitaceae). O material vegetal fresco foi mantido em temperaturas constantes de 17ºC e 27ºC, no escuro, em uma câmara de incubação. O modelo que melhor descreveu o processo de decomposição foi o modelo linear, com taxas de 0,014 dia-1 (R² = 94%) e 0,045 dia-1 (R² = 96%) obtidas às temperaturas de 17ºC e 27ºC, respectivamente. A análise de covariância (ANCOVA) indicou diferença significativa entre as taxas de decomposição nas duas temperaturas estudadas. É possível que o baixo conteúdo de compostos de resistência presentes na planta, como celulose e lignina, seja o responsável pela alta taxa de decomposição de E. najas, indicada pelo coeficiente de decomposição. As variáveis analisadas (pH, condutividade elétrica, oxigênio dissolvido na água, concentração de matéria orgânica, nitrogênio total e fósforo total no detrito) demonstraram resposta acentuada a 27ºC. Provavelmente, as maiores temperaturas aumentam a atividade microbiana, aumentando, assim, o consumo de oxigênio na água e, conseqüentemente, afetando o pH e a taxa de liberação de íons e nutrientes para o ecossistema aquático. Em razão da rápida decomposição de E. najas em altas temperaturas, espera-se uma baixa taxa de exportação dessa espécie de seus estandes para regiões distantes em reservatórios tropicais, onde é considerada uma espécie potencialmente daninha.
decomposição; temperatura; Egeria najas
Effects of temperature on decomposition of a potential nuisance species: the submerged aquatic macrophyte Egeria najas planchom (Hydrocharitaceae)
Efeitos da temperatura sobre a decomposição de uma espécie potencialmente daninha: a macrófita aquática submersa Egeria najas Planchon (Hydrocharitaceae)
Carvalho, PI, II; Thomaz, S. M.II; Bini, L. M.I
IUniversidade Federal de Goiás, ICB, DBG, C.P. 131, CEP 74001-970, Goiânia, GO, Brazil
IIUniversidade Estadual de Maringá, Departamento de Biologia-Nupélia, CEP 87020-900, Maringá, PR, Brazil
Correspondence to Correspondence to Luis M. Bini Universidade Federal de Goiás ICB, DBG C.P. 131, CEP 74001-970, Goiânia, GO, Brazil E-mail: bini@icb.ufg.br
ABSTRACT
Decomposition of aquatic plants is influenced by several biotic and abiotic factors. Among them, temperature plays an important role. Despite the increasing number of studies describing the effects of temperature on the decomposition of aquatic macrophytes, little attention has been given to the decay of submerged macrophytes. In this paper, we assessed the effect of temperature on weight loss and chemical composition of detritus of the submerged aquatic macrophyte Egeria najas Planchon (Hydrocharitaceae). Fresh plant material was maintained at 17ºC and 27ºC, in the dark, in incubation chambers. The overall decay process was best described by a linear model, with rates of 0.014 day-1 (R2 = 94%) and 0.045 day-1 (R2 = 96%) obtained at 17ºC and 27ºC, respectively. The analysis of covariance (ANCOVA) indicated a significant difference between the decomposition rates at the two temperatures. The rapid breakdown of E. najas detritus, indicated by the decay coefficient, may be explained by its low content of resistant compounds such as cellulose and lignin. The variables analyzed in this study (pH, electrical conductivity, dissolved oxygen in the water and organic matter, total nitrogen and total phosphorus concentration in detritus) showed accentuated responses at 27ºC. It is likely that the higher temperature increased microbial activity and, therefore, oxygen consumption in the water, consequently affecting the pH and the rate of ion and nutrient liberation into the aquatic ecosystem. Due to the rapid decomposition of E. najas at high temperatures, a small exportation is expected of this species from its stands to distant regions in tropical reservoirs, where it is considered a potential nuisance species.
Key words: decomposition, temperature, Egeria najas.
RESUMO
Inúmeros fatores bióticos e abióticos influenciam a decomposição de plantas aquáticas, destacando-se, entre eles, a temperatura. Apesar do grande número de estudos descrevendo os efeitos da temperatura sobre a decomposição de macrófitas aquáticas, pouca atenção tem sido dada à decomposição de espécies submersas. O objetivo do presente trabalho foi avaliar o efeito da temperatura sobre a perda de peso e composição química do detrito da macrófita aquática submersa Egeria najas Planchon (Hydrocharitaceae). O material vegetal fresco foi mantido em temperaturas constantes de 17ºC e 27ºC, no escuro, em uma câmara de incubação. O modelo que melhor descreveu o processo de decomposição foi o modelo linear, com taxas de 0,014 dia-1 (R2 = 94%) e 0,045 dia-1 (R2 = 96%) obtidas às temperaturas de 17ºC e 27ºC, respectivamente. A análise de covariância (ANCOVA) indicou diferença significativa entre as taxas de decomposição nas duas temperaturas estudadas. É possível que o baixo conteúdo de compostos de resistência presentes na planta, como celulose e lignina, seja o responsável pela alta taxa de decomposição de E. najas, indicada pelo coeficiente de decomposição. As variáveis analisadas (pH, condutividade elétrica, oxigênio dissolvido na água, concentração de matéria orgânica, nitrogênio total e fósforo total no detrito) demonstraram resposta acentuada a 27ºC. Provavelmente, as maiores temperaturas aumentam a atividade microbiana, aumentando, assim, o consumo de oxigênio na água e, conseqüentemente, afetando o pH e a taxa de liberação de íons e nutrientes para o ecossistema aquático. Em razão da rápida decomposição de E. najas em altas temperaturas, espera-se uma baixa taxa de exportação dessa espécie de seus estandes para regiões distantes em reservatórios tropicais, onde é considerada uma espécie potencialmente daninha.
Palavras-chave: decomposição, temperatura, Egeria najas.
INTRODUCTION
The importance of aquatic macrophytes in the structure and functioning of aquatic ecosystems is associated, among several other factors, with their capacity to incorporate nutrients from the sediment or water into their biomass and to release them by excretion or through decomposition (Esteves & Barbieri, 1983). Furthermore, the detrital food web probably represents the dominant energy-transfer pathway in most aquatic ecosystems, processing ca. 90% of the energy (Odum, 1985; Wetzel, 1990).
Most studies on decomposition of aquatic macrophytes, especially in the case of emergent and floating ones, have focused on weight loss and changes in the chemical composition of coarse particulate detritus over time (Esteves & Barbieri, 1983; Ayyappan et al., 1986; Bianchini Jr. et al., 1988; Findlay et al., 1990; Pagioro & Thomaz, 1999a). However, a few studies have examined the decay of submerged macrophytes (Carpenter & Adams, 1979; Rublee & Roman, 1982; Ferreira & Esteves, 1992; Gessner, 2001).
Aquatic macrophyte decomposition is influenced by several biotic factors such as bacterial and fungal action, and abiotic factors such as physical abrasion, pH, nutrient levels, and water temperature. Temperature has been cited as an important environmental factor (Hynes & Kaushik, 1969; Carpenter & Adams, 1979). In general, decomposition is expected to occur more rapidly during warmer periods. Refinements could be made to VanT Hoff's rule by including a temperature dependent function following leaching where an elevation of 10ºC could increase biological reactions by two or three times during decomposition (Flanagan & Bunnel, 1976).
In the Itaipu Reservoir, Brazil, the submerged macrophyte Egeria najas is a widely distributed, potential nuisance species (Thomaz et al., 1999). In the subtropical climate of Itaipu, conditions are suitable for this species to grow year round. As a consequence, dead material decomposition occurs under different abiotic conditions prevailing during different periods of the year. Water temperature, for example, may reach values close to 17ºC in winter and 30ºC in summer. In view of the important influence of this variable on detritus decay, we conducted laboratory experiments to assess its effects on changes in weight and chemical composition of detritus of the submersed aquatic macrophyte Egeria najas Planchon (Hydrocharitaceae). We hypothesized that at higher temperatures, detritus of a submerged aquatic macrophyte (1) incurs increased mass-loss rates, (2) demonstrates faster declines in phosphorus and nitrogen content, and (3) causes stronger changes in the water quality.
MATERIALS AND METHODS
Fresh E. najas tissue was harvested during one day from different arms of the Itaipu Reservoir, Brazil,. It was important to use freshly harvested material for the experiment because leaf drying fractures membranes and alters the cuticle, rendering the leaf more susceptible to attack by microbes and increasing the loss of soluble compounds (Boulton & Boon, 1991).
The plant material was washed in tap water to remove adhering matter. The laboratory experiment was conducted by placing 13 g of plant material in beakers containing 1,000 ml of filtered water. The beakers were maintained in the dark in an incubator at either 17ºC or 27ºC, representing the minimum and maximum water temperatures found in Itaipu Reservoir.
After periods of 0, 5, 10, 15, 20, 25, 35, 45, and 55 days, the plant material was collected and oven dried at 70ºC to constant weight. At the same time, the pH, electrical conductivity, and dissolved oxygen concentration of the water was measured with portable digital potentiometers. The plant material was ground in preparation for determining nitrogen concentration (Kjeldahl digestion) and total phosphorus concentration (spectrophotometry) (Allen et al., 1974). The organic matter content was determined by incineration of plant samples at 550ºC (Wetzel & Likens, 1991). Organic-matter values were multiplied by 0.465 to obtain carbon concentrations (Westlake, 1965). Leaf quality was assessed by the C:N ratio, calculated as percentage C divided by percentage N of the dry mass.
Statistical analysis
Two models (linear and exponential) were applied to describe mass-loss over time at the two temperatures. The model that best described macrophyte decay, following residual analysis, was the linear equation:
Wt = Wo - kt
where Wo is the initial weight; Wt, the weight at time t; k, the decay constant; and t, time in days.
Analysis of covariance (ANCOVA) was performed to test for the homogeneity hypothesis, i.e., whether the decomposition rates at the two temperatures were significantly different from each other (Crawley, 1993). In this analysis, time was considered the covariate and temperature was considered a fixed factor with two levels (17ºC and 27ºC) (Tonhasca Jr., 1999) representing the thermal extremes observed in Itaipu Reservoir.
RESULTS
The overall decay process of E. najas was best described by a linear model, and decay constants of 0.014 day-1 (R2 = 94%) and 0.045 day-1 (R2 = 96%) were obtained at 17ºC and 27ºC, respectively (Fig. 1a). The residual analysis indicated that this model was adequate for describing the weight loss at the two temperatures studied (Fig. 1b).
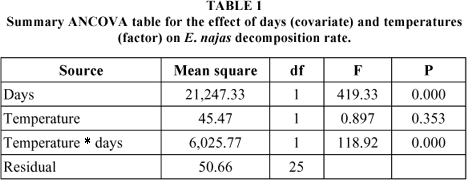
The ANCOVA indicated a significant difference between decomposition rates at the two temperatures studied. The interaction between temperature and time (days) was also significant (Table 1).
Water pH, electrical conductivity, and dissolved oxygen differed slightly between the two temperatures at the start of the experiment (Table 2). In order to exclude this effect (different initial values), the final values (effect of decomposition) were subtracted from the initial value of each abiotic variable. The graphs of pH, electrical conductivity, and dissolved oxygen show, therefore, the effect of decomposition.
The pH values showed a decrease during the first five days at both temperatures. A subsequent increase was observed, particularly when the plant material was incubated at 27ºC (Fig. 2a). An electrical conductivity increase of the water was observed at both temperatures (Fig. 2b). Maximum values observed were 374 µS/cm and 430 µS/cm, when the plant material was incubated at 27ºC and 17ºC, respectively. Dissolved oxygen concentration of the water decreased throughout the experiment (Fig. 2c). The increase on the last day could be associated with atmospheric diffusion.
In general, it was clear that the abiotic variables of the water showed a faster and more pronounced response to decomposition when the plant material was incubated at the warmer temperature (27ºC).
In the first days of decomposition, a small decrease in the percentage of ash-free organic matter was observed, particularly in the lower-temperature treatment (Fig. 3a). A substantial increase (mean of 6% of the initial percentage of ash-free organic matter) was observed after 20 days of decomposition at the higher-temperature treatment.
Detritus N concentration increased throughout the experiment (Fig. 3b). At the warmer temperature, nitrogen increased to a mean of 170% of the original value. At 17ºC, nitrogen concentration in the detritus was almost constant. The C:N ratio, which had an initial mean of 21.2, fell to values of 8 at 27ºC, and 13 at 17ºC (Fig. 3c).
During the first ten days, the detritus P concentration showed a pronounced increase at 27ºC (140% of the initial P content) (Fig. 3d), following which phosphorus was then lost at relatively high rates. Continuous enrichment occurred at 17ºC with a maximum value 90% higher than the initial P content measured on the 45th day.
DISCUSSION
Most studies of decomposition carried out with aquatic plants in freshwater ecosystems have used the negative exponential model originally developed by Olson (1963). This model has two distinct phases: the initial, of marked leaching of soluble organic compounds (soluble carbohydrates, lipids, and polyphenols), followed by a second, with a slower decrease in mass, when colonization by microorganisms, degradation via invertebrate feeding, and physical abrasion of structural compounds such as cellulose and lignin become more important (Esteves & Barbieri, 1983; Boulton & Boon, 1991). However, the model that best described our data was the linear model (Wt = W0 - kt), which can therefore be regarded as a convenient way of describing the overall decay process in E. najas in the laboratory. Good fit of linear models for the decomposition process has also been reported elsewhere (Findlay et al., 1990; Royer & Minshall, 1997).
The analysis of covariance (ANCOVA) showed a significant temperature effect. Moreover, the angular coefficient value was three times greater at 27ºC (-4.52 ± 0.29) than at 17ºC (-1.378 ± 0.080). Thus the decomposition rate was significantly greater at the higher temperature. Several studies conducted in freshwater have demonstrated seasonal variation in aquatic macrophyte decomposition rates, with faster breakdown occurring during warmer periods (Kaushik & Hynes, 1971; Iversen, 1975; Godshalk & Wetzel, 1978b; Carpenter & Adams, 1979; Wetzel & Corners, 1979). Howard-Williams & Davies (1979) showed the temperature effect on decomposition rates through the Q10 coefficient. They observed that a 10ºC increase could cause a two- to three-fold increase in bacterial activity, a finding corroborated by this study. Of all the potential factors, the high temperatures found in tropical and subtropical aquatic ecosystems are probably responsible for rapid detritus breakdown and macrophyte biomass turnover (Esteves & Barbieri, 1983).
Carpenter & Adams (1979) also found an increase in the decay coefficient (k) of the submersed aquatic macrophyte Myriophyllum spicatum following an increase in temperature to 28ºC. This was succeeded by a decay coefficient decline, probably due to decreased heterotrophic activity at higher temperatures. In fact, several studies have emphasized the higher aquatic macrophyte decay coefficients found in tropical regions as opposed to those of temperate ones (Ayyappan et al., 1986; Royer & Minshall, 1997).
Differences in fiber content may also explain differences observed in the decomposition of emergent, floating, and submerged aquatic plants. Rapid decomposition of the submerged macrophyte E. najas, as indicated by the high decay coefficient (T1/2 = 27 and 9 days at 17ºC and 27ºC, respectively), may be explained by low content of resistant compounds such as cellulose and lignin in this species, as has been shown in other investigations (Esteves, 1998).
Water quality changed rapidly during decomposition. Regarding pH values, the results of this study corroborate those of other studies (Camargo et al., 1983; Gaur et al., 1992; Pagioro & Thomaz, 1999b). Initial pH decrease can be associated with rapid leaching of acids during the initial phase and rapid dissolved oxygen depletion through microbial respiration and consequent CO2 liberation (Godshalk & Wetzel, 1978a). The subsequent increase can be attributed to Ca2+ and HCO3_ leaching from detritus. In addition, electrical conductivity of the water increased throughout the experiment.
The dissolved oxygen concentration decrease throughout the experiment was probably due to microbial activity and, according to Esteves (1998), the combination of organic matter and high temperatures are responsible for water desoxygenation.
All of the variables showed much more accentuated responses at 27ºC. Thus, the higher temperature increased the microbial activity, which increased oxygen consumption in the water, consequently affecting the pH and the rate of ion and nutrient liberation to the aquatic ecosystem. In general, we suggest that water quality is highly affected inside the macrophyte stands in Itaipu, especially during the summer months when water temperature may reach 27ºC-30ºC. In fact, significant differences in limnological features between samples collected inside and outside stands of E. najas have been found in situ (Bini, 2001).
Chemical composition of the detritus changed considerably during decomposition. Despite being measured in static chambers in the laboratory, the results resembled those obtained in situ in other investigations. Initial leaching of soluble inorganic compounds observed in our experiment may be responsible for percentage decrease of ash-free organic matter (Rublee & Roman, 1982; Esteves & Barbieri, 1983; Ferreira & Esteves, 1992; Pagioro & Thomaz, 1999a). Subsequent increase in organic matter in the detritus may be the result of the microbial colonization that plays important roles in both the early and later decomposition stages.
Nitrogen plays a role in several critical metabolism processes in the littoral zone. One very common observation is nitrogen increase in detritus during decomposition (Carpenter & Adams, 1979; Godshalk & Wetzel, 1978b; Camargo et al., 1983; Roland et al., 1990; Pagioro & Thomaz, 1999a), a finding corroborated by our study. There are three possible reasons for this nitrogen concentration increase during decomposition: (1) nitrogen content, as microbial protein, increases with growing populations of attached decomposers; (2) extracellular protein, largely as exoenzymes secreted by decomposing microorganisms, is complexed into particulate detrital material; and (3) inorganic nitrogen may be adsorbed onto the detritus surface (Wetzel & Manny, 1972; Godshalk & Wetzel, 1978b; Helbing et al., 1986; Pagioro & Thomaz, 1999a). Microbial protein associated with detritus is usually considered a significant aquatic detritivore food source (Godshalk & Wetzel, 1978b).
The C:N ratio reduction may be attributed to nitrogen immobilization by microorganisms and/or to carbon decrease due to respiration. Since the % AFDW remained approximately constant (see Fig. 3a), the above processes may explain the decrease of C:N quotients in E. najas detritus. According to Anderson (1973), the C:N ratio may indicate the nutritional value of a decomposing plant. Thus, C:N ratio decrease and nitrogen increase during decomposition (discussed above) denote detritus enrichment for aquatic detritivores (Godshalk & Wetzel, 1978b) and, in fact, stable isotope analyses indicate that aquatic plant detritus has been used by detritivorous fishes in Itaipu Reservoir (Lopes, 2001). According to preliminary analyses, isotopic composition values found for Prochilodus lineatus (a detritivorous species) are close to those found for E. najas (Lopes, personal communication).
Phosphorus increase in the detritus during decomposition has also been observed in other studies (Howard-Williams & Davies, 1979; Poi de Neiff & Neiff, 1988; Pagioro & Thomaz, 1999a; Villar et al., 2001). This increase may have occurred because macrophytes were in closed bottles in which part of the phosphorus released to the water became available to the attached microorganisms (Pagioro & Thomaz, 1999a). However, at 27ºC a marked decline in phosphorus concentration in the detritus was observed after 10 days, which according to Helbing et al. (1986) and Ferreira & Esteves (1992) could be due to cytoplasmic phosphorus being rapidly lost from cells and not replaced.
The use of litterbags to study decomposition is widespread, although methods requiring them have been criticized for a variety of reasons (Boulton & Boon, 1991; Schnitzer & Neely, 2000), including microbial activity reduction, invertebrate access reduction, and alteration of flow regimes and light intensity. While experiments in vitro have likewise been criticized due to the impossibility of duplicating actual field situations, they have the advantage of allowing control of variables of interest (Webster & Benfield, 1986; Schnitzer & Neely, 2000), e.g., temperature. But, interestingly enough, both methodologies sometimes yield equivalent results (Rublee & Roman, 1982).
So as to compare field and laboratory incubations, we used data obtained from in-situ experiments (S. M. Boschilia, unpublished data). In that study, E. najas detritus was initially dried and then left to decompose in litterbags inside stands of E. najas located in two arms of the Itaipu Reservoir. The average temperatures (ca. 26.5ºC) were similar to that of our high temperature treatment (27ºC). Using a linear model, decay coefficients of 0.019 day-1 (R2 = 75%) and 0.024 day-1 in each arm (R2 = 78%) were estimated (Fig. 4). These results show that the decomposition rate of E. najas obtained in the field using a completely different approach (initial drying and containment in litterbags) was close to the rate obtained using fresh material in static chambers in the laboratory. This similarity suggests a great potential for extrapolating the results obtained with aquatic plants incubated in the laboratory to field conditions.
Taking into account that this plant grows in sheltered conditions with little water current, the rapid decomposition rate of E. najas at both temperatures (17ºC and 27ºC) suggests that the decomposition of this species occurs close to macrophyte stands. Therefore, it is predicted that very little E. najas detritus is exported to distant regions of the reservoir, particularly during summer when the water temperature usually exceeds 25ºC.
Acknowledgements The authors thank Raul Ribeiro and Maria do Carmo Roberto for their excellent assistance in fieldwork and laboratory analyses, Solana Boschilia for providing data on E. najas decomposition in litterbags in the Itaipu Reservoir, and Judith May Milne for english correction. The study was sponsored by the Brazilian Council of Research (CNPq) and Itaipu Binacional.
Received July 1, 2003 - Accepted September 9, 2003 - Distributed February 28, 2005
- ALLEN, S. E., GRINSHAH, H. M., PARKINSON, J. A. & QUARMDY, C., 1974, Chemical analysis of ecological material Blackwell Scientific Publications, Oxford, 565p.
- ANDERSON, J. M., 1973, The breakdown and decomposition of sweet chestnut (Castanea sativa Mill.) and beech (Fagus silvatica L.) leaf litter in two deciduous woodland soils II. Changes in the carbon, hydrogen and polyphenol content. Oecologia, 12: 275-288.
- AYYAPPAN, S., OLÁH, J., RAGHAVAN, S. L. & SINHA, V. R. P., 1986, Macrophyte decomposition in two tropical lakes. Arch. Hydrobiol., 106: 219-231.
- BIANCHINI, Jr., I., ROCHA, M. G. B. & TOLEDO, A. P. P., 1988, Estudo do fluxo de detritos a partir da decomposição de macrófitas aquáticas na represa do Lobo (Broa) - Nymphoides indica. In: J. G. Tundisi (ed.), Limnologia e manejo de represas Série: Monografias em Limnologia, EESC/CRHEA, Universidade de São Paulo.
- BINI, L. M., 2001, Dinâmica populacional de Egeria najas Planchon (Hydrocaritaceae) em braços túrbidos de um grande reservatório subtropical (reservatório de Itaipu Binacional, Brasil-Paraguai) Ph.D. Thesis, Universidade Estadual de Maringá, Maringá, 41p.
- BOULTON, A. J. & BOON, P. I., 1991, A review of methodology used to measure leaf litter decomposition in lotic environments: time to turn over an old leaf? Aust. J. Mar. Fresh. Res., 42: 1-43.
- CAMARGO, A. F. M., ISHII, I. H. & ESTEVES, F. A., 1983, Liberação de compostos orgânicos e inorgânicos para a coluna d'água durante o processo de decomposição de duas espécies de macrófitas aquáticas tropicais. In: Anais do III Seminário Regional de Ecologia São Carlos, SP.
- CARPENTER, S. R. & ADAMS, M. S., 1979, Effects of nutrients and temperature on decomposition of Myriophyllum spicatum L. in a hardwater eutrophic lake. Limnol. Oceanogr., 24: 520-528.
- CRAWLEY, M. J., 1993, Glim for ecologists Blackwell Scientific Publications, Oxford, 379p.
- ESTEVES, F. A. & BARBIERI, R., 1983, Dry weight and chemical changes during decomposition of tropical macrophytes in Lobo Reservoir - São Paulo, Brazil. Aquat. Bot., 16: 285-295.
- ESTEVES, F. A., 1998, Fundamentos de limnologia Interciência/FINEP, Rio de Janeiro, 575p.
- FERREIRA, C. M. L. & ESTEVES, F. A., 1992, Decomposition of Potamogeton stenostachys K. Schum. and evaluation of its detritus as a potential energy source in a tropical coastal lagoon. Inter. J. Ecol. Environ. Sci., 18: 47-54.
- FINDLAY, S., HOWE, K. & AUSTIN, H. K., 1990, Comparison of detritus dynamics in two tidal freshwater wetlands. Ecology, 71(1): 288-295.
- FLANAGAN, P. W. & BRUNNEL, F. L., 1976, Decomposition models based on climatic variables, substrate variables, microbial respiration and production. In: J. M. Anderson & A. Macfadyen (eds.), The role of terrestrial and aquatic organisms in decomposition processes Blackwell Scientific Publications, Oxford.
- GAUR, S., SINGHAL, P. K. & HASIJA, S. K., 1992, Relative contributions of bacteria and fungi to water hyacinth decomposition. Aquat. Bot., 43: 1-15.
- GESSNER, M. O., 2001, Mass loss, fungal colonization and nutrient dynamics of Phragmites australis leaves during senescence and early aerial decay. Aquat. Bot., 69: 325-339.
- GODSHALK, G. L. & WETZEL, R. G., 1978a, Decomposition of aquatic angiosperms. I. Dissolved components. Aquat. Bot., 5: 281-300.
- GODSHALK, G. L. & WETZEL, R. G., 1978b, Decomposition of aquatic angiosperms. II. Particulate components. Aquat. Bot., 5: 301-327.
- HELBING, U. W., ESTEVES, F. A., TILZER, M. M. & STABEL, H. H., 1986, Influência dos produtos de decomposição da macrófita aquática Nymphoides indica (L.) O. Kuntze, na composição química da água da Represa do Lobo (Broa) - São Paulo. Acta Limnol. Bras., 1: 611-637.
- HOWARD-WILLIAMS, C. & DAVIES, B. R., 1979, The rates of dry matter and nutrient loss from decomposing Potamogeton pectinatus in a brackish southtemperate coastal lake. Freshwater Biol., 9: 13-21.
- HYNES, H. B. N. & KAUSHIK, N. K., 1969, The relationship between dissolved nutrient salts and protein production in submerged autumnal leaves. Verh. Internat. Verein. Limnol., 17: 95-103.
- IVERSEN, T. M., 1975, Disappearance of autumn shed beech leaves placed in bags in small streams. Verh. Internat. Verein. Limnol., 19: 1687-1692.
- KAUSHIK, N. K. & HYNES, H. B. N., 1971, The fate of the dead leaves that fall into streams. Arch. Hydrobiol., 68: 465-515.
- LOPES, C. A., 2001, Variabilidade de d13C e de d15N em fontes alóctones e autóctones e suas contribuições energéticas para o Prochilodus lineatus (Prochilodontidae, Characiformes) na bacia do rio Paraná, entre a foz dos rios Paranapanema e Iguaçu Dissertação de Mestrado, Universidade Estadual de Maringá, Maringá.
- ODUM, E. P., 1985, Ecologia Interamericana, Rio de Janeiro, 434p.
- OLSON, J. S., 1963, Energy storage and the balance of decomposers in ecological systems. Ecology, 44: 322-332.
- PAGIORO, T. A. & THOMAZ, S. M., 1999a, Decomposition of Eichhornia azurea from limnologically different environments of the Upper Paraná river floodplain. Hydrobiologia, 411: 45-51.
- PAGIORO, T. A. & THOMAZ, S. M., 1999b, Influence of the decomposition of Eichhornia azurea on selected abiotic limnological variables of different environments of the floodplain of the high Paraná River. Acta Limnol. Bras., 11(2): 157-171.
- POI DE NEIFF, A. & NEIFF, J. J., 1988, Decomposition of Eichhornia crassipes Solms in a pond of Paraná river valley and colonization by invertebrates. Trop. Ecol., 29(2): 79-85.
- ROLAND, F., ESTEVES, F. A. & SANTOS, J. E., 1990, Decomposição da macrófita aquática Eichhornia azurea (Kunth), com ênfase na colonização por bactérias epifíticas. Acta Limnol. Bras., 3(2): 653-673.
- ROYER, T. V. & MINSHALL, G. W., 1997, Rapid breakdown of allochthonous and autochthonous plant material in a eutrophic river. Hydrobiologia, 344: 81-86.
- RUBLEE, P. A. & ROMAN, M. R., 1982, Decomposition of turtlegrass (Thalassia testudinum Konig) in flowing sea-water tanks and litterbags: compositional changes and comparison with natural particulate matter. J. Exp. Mar. Biol. Ecol., 58: 47-58.
- SCHNITZER, S. A. & NEELY, R. K., 2000, Criticism of the litterbag technique for the study of aquatic plant decay: suppression of epiphytic algal biomass. Arch. Hydrobiol., 148(3): 433-440.
- THOMAZ, S. M., BINI, L. M., SOUZA, M. C., KITA, K. K. & CAMARGO, A. F. M., 1999, Aquatic macrophytes of Itaipu reservoir, Brazil: survey of species and ecological considerations. Braz. Arch. Biol. Technol., 42: 15-22.
- TONHASCA Jr., A., 1999, Factorial experiments - principles and guidelines for their use. Ciência e Cultura, 5(2): 88-91.
- VILLAR, C. A., CABO, L. DE, VAITHIYANATHAN, P. & BONETTO, C., 2001, Litter decomposition of emergent macrophytes in a floodplain marsh of the Lower Paraná river. Aquat. Bot., 70: 105-116.
- WEBSTER, J. R. & BENFIELD, E. F., 1986, Vascular plant breakdown in freshwater ecosystems. Ann. Rev. Ecol. Syst., 17: 567-594.
- WESTLAKE, D. F., 1965, Some basic data for investigations of the productivity of aquatic macrophytes. Men. Ist. Ital. Idrobiol., 18: 229-248.
- WETZEL, R. G., 1990, Detritus, macrophytes and nutrient cycling in lakes. Mem. Ist. Ital. Idrobiol., 47: 233-249.
- WETZEL, R. G. & MANNY, B. A., 1972, Secretion of dissolved organic carbon and nitrogen by aquatic macrophytes. Int. Ver. Theor. Angew. Limnol. Verh., 18: 162-170.
- WETZEL, R. G. & CORNERS, H., 1979, The role of the littoral zone and detritus in lake metabolism. Arch. Hydrobiol. Beih. Ergebn. Limnol., 13: 145-161.
- WETZEL, R. G. & LIKENS, G. E., 1991, Limnological analysis Springer-Verlag, New York, 391p.
Publication Dates
-
Publication in this collection
16 Nov 2005 -
Date of issue
Feb 2005
History
-
Accepted
09 Sept 2003 -
Received
01 July 2003