Abstracts
A study of the diatoms Eupodiscus radiatus Bailey and Fryxelliella floridana Prasad, mainly focussing on the mantle and cingulum, provided new morphological information. In E. radiatus dendritic structures and two types of a palisade-like structure fixed to silica rings were found on the lower valve mantle. Cingulum presented 1-3 bands with areolae arranged in decussate rows. Furthermore, the pars interior of the valvocopula is fimbriate; and the external openings of the rimoportulae are located along the rim of the scalloped extension. The valvocopula of F. floridana is open and its copula is ligulate. Both bands possess poroid areolae similar in size to the cribral pores on the valve face. The genus Eupodiscus is compared to Fryxelliella, based on material sampled in estuaries of Southern Brazil.
Eupodiscus; Fryxelliella; taxonomy; diatom; Bacillariophyta; Southern Brazil
Um estudo morfológico sob microscopia eletrônica das diatomáceas Eupodiscus radiatus e Fryxelliella floridana (Triceratiaceae) foi realizado, enfocando principalmente o manto e o cíngulo. Ornamentações não registradas previamente são descritas. Em E. radiatus, estruturas dendríticas semelhantes a estrelas foram encontradas sobre o manto, logo abaixo das ondulações marginais. Dois tipos de paliçada silicosa provida de grânulos e fixada a anéis silicosos também foram observados, todo o conjunto envolvendo o manto. O cíngulo é provido de 1-3 bandas com aréolas ordenadas perpendicularmente ao eixo pervalvar, tendo ''fimbrias'' nas bordas superiores da valvocópula (pars interior) e encaixadas na parte interna da extremidade do manto. A abertura externa da rimopórtula localiza-se nas margens das ondulações do manto. F. floridana possui duas faixas silicosas no manto e um cíngulo ligulado com aréolas semelhantes às da superfície valvar. Adicionalmente, o gênero Eupodiscus é comparado à Fryxelliella a partir de observações de frústulas de F. floridana e E. radiatus coletadas em estuários do sul do Brasil.
Eupodiscus; Fryxelliella; taxonomia; diatomácea; estuário; sul do Brasil
New observations on frustule morphology of Eupodiscus radiatus Bailey and Fryxelliella floridana Prasad
Observações inéditas sobre a morfologia das frústulas de Eupodiscus radiatus Bailey e Fryxelliella floridana Prasad
Fernandes, L. F.
Universidade Federal do Paraná, Departamento de Botânica, Setor de Ciências Biológicas, Centro Politécnico, C.P. 19031, Jardim das Américas, CEP 81531-970, Curitiba, Paraná, Brazil
Correspondence Correspondence to Luciano F. Fernandes Universidade Federal do Paraná, Departamento de Botânica, Setor de Ciências Biológicas, Centro Politécnico, C.P. 19031, Jardim das Américas CEP 81531-970, Curitiba, Paraná, Brazil e-mail: lff@ufpr.br
ABSTRACT
A study of the diatoms Eupodiscus radiatus Bailey and Fryxelliella floridana Prasad, mainly focussing on the mantle and cingulum, provided new morphological information. In E. radiatus dendritic structures and two types of a palisade-like structure fixed to silica rings were found on the lower valve mantle. Cingulum presented 1-3 bands with areolae arranged in decussate rows. Furthermore, the pars interior of the valvocopula is fimbriate; and the external openings of the rimoportulae are located along the rim of the scalloped extension. The valvocopula of F. floridana is open and its copula is ligulate. Both bands possess poroid areolae similar in size to the cribral pores on the valve face. The genus Eupodiscus is compared to Fryxelliella, based on material sampled in estuaries of Southern Brazil.
Key words:Eupodiscus, Fryxelliella, taxonomy, diatom, Bacillariophyta, Southern Brazil.
RESUMO
Um estudo morfológico sob microscopia eletrônica das diatomáceas Eupodiscus radiatus e Fryxelliella floridana (Triceratiaceae) foi realizado, enfocando principalmente o manto e o cíngulo. Ornamentações não registradas previamente são descritas. Em E. radiatus, estruturas dendríticas semelhantes a estrelas foram encontradas sobre o manto, logo abaixo das ondulações marginais. Dois tipos de paliçada silicosa provida de grânulos e fixada a anéis silicosos também foram observados, todo o conjunto envolvendo o manto. O cíngulo é provido de 1-3 bandas com aréolas ordenadas perpendicularmente ao eixo pervalvar, tendo ''fimbrias'' nas bordas superiores da valvocópula (pars interior) e encaixadas na parte interna da extremidade do manto. A abertura externa da rimopórtula localiza-se nas margens das ondulações do manto. F. floridana possui duas faixas silicosas no manto e um cíngulo ligulado com aréolas semelhantes às da superfície valvar. Adicionalmente, o gênero Eupodiscus é comparado à Fryxelliella a partir de observações de frústulas de F. floridana e E. radiatus coletadas em estuários do sul do Brasil.
Palavras-chave:Eupodiscus, Fryxelliella, taxonomia, diatomácea, estuário, sul do Brasil.
INTRODUCTION
The Genus Eupodiscus J. W. Bailey (Triceratiaceae) is readily recognised by the presence of marginal ocelli, rimoportulae intercalated to ocelli, loculate areolae and a scalloped wing-like extension of the valve margin. VanLandinghan (1969) listed 21 taxa of Eupodiscus, and Sullivan & Porguen (1990) recently described the new species E. paracaënsis Sullivan & Porguen. Sullivan (1988) elucidated some taxonomic problems concerning Eupodiscus, and designated a lectotype for the generitype, E. radiatus Bailey. All species in the genus are fossil, except for E. radiatus, which is found in modern phytoplankton or benthic communities (Prasad & Nienow, 1988; Fernandes et al., 1990; Fernandes et al., 1999).
Studies on Eupodiscus taxa using electron microscope techniques have examined the general morphology of the valve (Ross & Sims, 1973; Navarro, 1982; Prasad & Nienow, 1988; Sullivan, 1988; Sullivan & Porguen, 1990; Round et al., 1990), but the mantle and the cingulum received little attention.
Recently, Prasad et al. (1997) established the genus Fryxelliella Prasad, which is distinguished from other related genera by the presence of a circumferential marginal tube, loculate areolae with a circular pattern of external cribra, a variable number of ocelli, and rimoportulae bearing an external tube, opening to the inner side by means of a labiate fissure. Two species were placed in the genus: F. floridana Prasad and F. inconspicua (Rattray) Prasad (= Eupodiscus inconspicuous Rattray). Although the genus Fryxelliella is closely related to Eupodiscus, Prasad et al. (1997) considered the occurrence of a marginal tube to be a diagnostic character, absent in other diatom taxa.
In this work we have studied the frustular morphology of the generitypes Eupodiscus radiatus and Fryxelliella floridana, but have mainly focussed on the mantle and cingulum. We describe previously unrecorded structures on the valve of these two species. In addition, we discuss the evolutionary relationships between the two genera in light of our new observations.
MATERIAL AND METHODS
Material for study was obtained from two estuaries of Southern Brazil: Paranaguá Bay (25°25'S-25°35'S and 48°20'W-48°45'W), Paraná State, an estuarine complex with an area of 612 km2 and average depth of 2.5 meters; and Ratones river estuary (27°15'S-27°29'S and 48°30'W-48°40'W), Santa Catarina State, with an area of 17 km2 and average depth of 1.8 meter. Both estuaries are influenced by the subtropical climate with two well-defined seasons: rainy in summer and dry in winter. The average annual rainfall is 1,988 mm. Salinity varies from 12 to 34, and the average annual range of the semidiurnal tides is 2.2 m. Mangrove forests, Spartina spp. marshes and tidal flats are the main habitats bordering the estuaries.
Plankton samples were collected using a standard plankton net with a 25 mm mesh. Samples were preserved with buffered formaldehyde (2%) and prepared for light and electron microscopy according to Hasle & Fryxell (1970). An aliquot of each sample was washed with distilled water, and selected specimens were picked out and transferred to electron microscope aluminium stubs. Permanent slides were prepared using Naphrax as a mounting medium. Light microscopy (LM) was performed using an Olympus BX40 microscope equipped with a 100x oil immersion objective. For scanning electron microscopy (SEM), samples were air-dried onto coverslips, which were mounted on aluminium stubs with conductive paint, coated with 16-20 nm gold and examined at an accelerating voltage of 15-30 kV in a Phillips Model XL30 scanning electron microscope. Slides and preserved field material used in this investigation were deposited in the FLOR Herbarium at the Federal University of Santa Catarina (UFSC).
The descriptive terminology follows that of Ross et al. (1979) and Round et al. (1990).
OBSERVATIONS
Eupodiscus radiatus J. W. Bailey (Figs. 1-15)
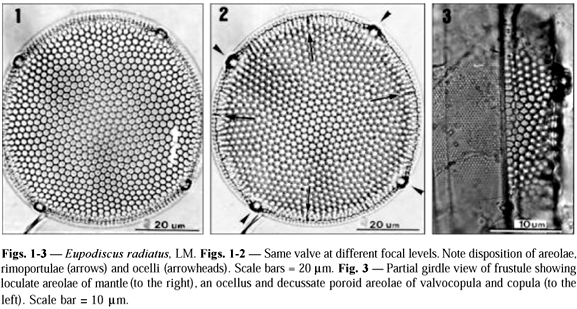
Valve surface. The morphology of the valve surface has already been described in detail by Sullivan (1988); thus only a brief description is furnished here. Living cells contained several ellipsoidal chloroplasts, parietal. Frustules are drum-shaped, strongly silicified, with a range in diameter of 48-133 mm (n = 43). The valve surface is flat or slightly concave, and covered by conspicuous hexagonal areolae (Figs. 1, 2, 6). Each areola bears an external cribra with cribral pores (Fig. 6) arranged in an isostoic pattern (sensu Sullivan &Porguen, 1990). The areolae open internally via foramina with slightly raised rims (Figs. 14, 15). Also present are larger cribral pores with a thickened rim (Fig. 6), irregularly scattered on the valve surface and mantle, that is, though some of them are aligned to the row of pores. In the marginal region, there is an undulated projection (Figs. 5, 7-9) in parallel with the valve surface, termed a scalloped wing-like extension by Sullivan (1988). This structure is reinforced by many silica ribs in either the top and the bottom portions (Figs. 8, 9). The margin of the extension presents a delicate reticulation (Fig. 9). The mantle is also areolated (Fig. 3), but these areolae are smaller than those on the valve face.
Rimoportulae. Rimoportulae are located in the transition region between the valve surface and mantle (Fig. 2), located approximately midway between two adjacent ocelli. Externally, each rimoportula opens via a circular aperture placed in a small cavity on the scalloped extension (Figs. 8-9). In this area, the rim of the scalloped extension is slightly thickened. Internally, the rimoportula opens via a radially oriented slit flanked by two labia, and located on a hemispherical protuberance (Fig. 14; see also Fig. 10 of Sullivan, 1988).
Ocelli. We found 2-6, equally spaced, ocelli along the margin, (Fig. 2). Smaller valves possessed fewer ocelli (2-3). Each ocellus bears a thickened, hyaline rim surrounding the porelli at the centre (Fig. 7).
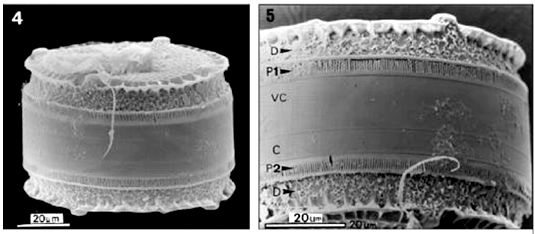
Mantle. At the base of the scalloped extension there is a region composed of dendritic structures with irregular ramifications bearing (or not) a central aperture, occupying two thirds of mantle surface (Figs. 5, 10). Following the region of dendritic structures, the base of the mantle in each valve is occupied by a fence-like band (Fig. 5), which we have termed a ''palisade'' (Latin vallum). Two types of palisade are present in the same frustule. The Type 1 palisade is comb-like with the teeth (termed flange from here on) of the comb curving inward and contacting the mantle along its lower one-third (Figs. 5, 10, 11). The base of the comb is flushed with the valvocopula and bears uniseriate rows of small areolae (Fig. 11). The combs also have small granules. The Type 2 palisade appears as two strips of silica encircling the lower one-third of the mantle, with somewhat wavy struts of silica, bearing abundant knob-like structures (or bars) arranged in parallel occurring between the strips (Figs. 5, 12, 13). However, these strips are only connected to the lower strip of silica, which is connected to the valvocopula (Figs. 12, 13). The bars are covered by refringent granules; and some of them show small branching and their distal apices are frequently folded (Fig. 13).
Cingulum. The cingulum consists of 2 to 4 open bands, and only the valvocopula was studied in our material. In LM, one can observe the decussate pattern of its areolae, which are much smaller than those of the valve (Fig. 3). Areolae are hexagonal and arranged in transverse rows (Fig. 3), though scanning electron microscopy just shows pores on the external surface (Fig. 11). The areola likely opens to the inner side by means of a small foramen (Fig. 15). The pars interior of the valvocopula possesses delicate fimbriae (to use a term coined by Johnson & Rosowski, 1992), which are flush with the lowermost portion of the internal valve mantle (Figs. 14-15).
Fryxelliella floridana Prasad (Figs. 16-28)
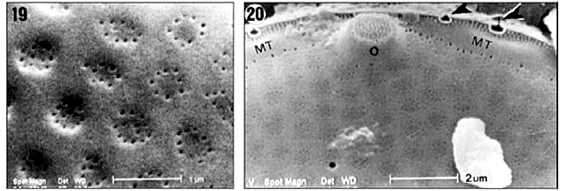
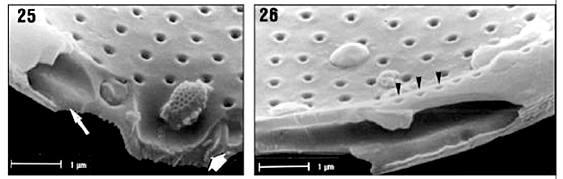
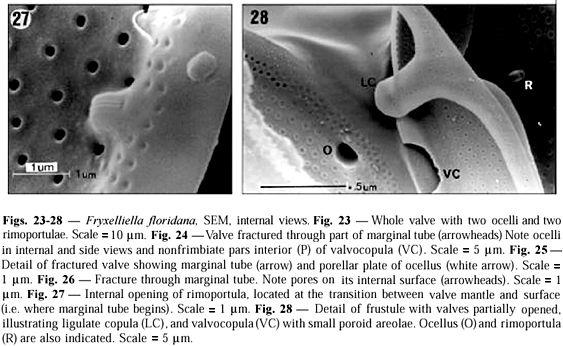
Valvar surface. Valves are circular. The valve diameter ranges from 20 to 40 mm (n = 70), but is usually less than 32 mm. The valve surface is convex and covered by hexagonal loculate areolae arranged in radial and concentric rows (Figs. 16-18). In the central region, the areolae are larger and more widely spaced than elsewhere on the valve. The areolae are slightly sunken on the valve surface. The external cribrum of each the areola is perforated by 7-13 cribral pores (Fig. 19), which are mainly arranged in a circular pattern, although some pores may be present at the centre of an areola. Internally, the areolae open via foramina with slightly raised rims (Figs. 26, 27). The valvar margin has a ring of refringent granules encircling the external tube of the rimoportulae but not the ocelli (Figs. 20, 21). Between the marginal granules and the valve face areolae a hyaline area is present (indicated by MT in Fig. 20), which is interrupted only by the ocelli and rimoportulae. At the edge of the valvar margin are numerous triangular to elliptical holes (or apertures) encircled by silica rims (Figs. 20-22). Each hole continues down the uppermost part of the mantle as a fissure (Fig. 21).
Rimoportulae. Three, sometimes two, rimoportulae are intercalated to the ocelli, and located on the valvar margin (Fig. 16). Each rimoportula has a short external tube (Fig. 22), and opens internally via a radially directed slit flanked by two labia (Fig. 27).
Ocelli. Three, sometimes two, marginal ocelli are equally spaced around the valve perimeter (Figs. 16-18). Each ocellus bears concentric rows of porelli, surrounded by a thickened hyaline rim (Figs. 20-21). The Fig. 25 illustrates a fracture through an ocellus in an internal view showing the unoccluded, small channels of the porelli.
Mantle. The valvar mantle is divided in two well-defined regions (Figs. 21-22), separated by a sulcus: (i) an upper hyaline strip interrupted by pervalvarly directed fissures of marginal triangular holes slightly elevated above the margin of the valve surface and (ii) a lower series of juxtaposed quadrangular plates bearing fine granules. A marginal circumferential tube is present along the outer edge of the valve (Figs. 20, 26) that is, occupying the internal structure of the mantle. Externally, the marginal tube opens through the triangular holes (Figs. 20-22). The marginal tube is interrupted in the ocelli (Figs. 23, 24). Internally, the marginal tube is perforated by small pores, which are radially arranged (Figs. 26-27).
Cingulum. Some interpretations of the cingulum structure were made based on Figs. 24 and 28. We only observed frustules with two bands. A wide valvocopula is connected to the mantle by its pars interior, which underlaps the marginal tube (Fig. 24). Fimbriae are absent. The areolae of the valvocopulae are poroid and arranged in transverse rows (Fig. 22); pores are disposed in small circles of 5 to 6 pores (Figs. 22, 28). The copula is narrow and ligulate. Ligula is rounded (Fig. 28).
DISCUSSION
Taxonomic studies on diatoms are traditionally based on frustule morphology as viewed in the light and electron microscopes. The usual methodology for preparing material involves cleaning of cells through harsh chemical reactions, generally using potassium permanganate and strong acids. Although this technique makes valvar structures evident, it may preclude important observations concerning the cingulum and mantle. For instance, the bands of the cingulum may be dissociated during the cleaning process. Our work illustrates well the disadvantages of using harsh cleaning methods. The new structures (palisade and dendritic structures) found in this work were well preserved because an aliquot of the material was not submitted to acid attack. On the other hand, judging from material described and photographed by other authors and our personal observations, those structures disappear when valves are prepared by conventional procedures using strong acids.
For instance, in illustrations of Eupodiscus radiatus furnished by Sullivan (1988), Round et al. (1990) and Navarro (1982) one may only observe vestiges of the thickened marginal strips that sustain the palisade, as well as the refringent points that could be the basis of the dendritic structures. On the other hand, the scutella of E. paracaënsis endured the harsh cleaning methods used by Sullivan & Porguen (1990).
In E. radiatus we found, for the first time, the external openings of rimoportulae located on the outer edge of the scalloped extension. Prasad & Nienow (1988) and Prasad et al. (1997) commented that these external openings were flushed with the valve surface and could not be viewed, or they did not exist. The location of the external opening of the rimoportulae is interesting from a phylogenetic viewpoint. They could have surfaced as an independent external tube before the scalloped extension evolved, to which it later fused to the tube. If we assume that rimoportulae in Eupodiscus are older than a scalloped extension, and the hypothesis is true, then E. radiatus would be closely related to other Eupodiscus taxa with rimoportula bearing external tubes. To date, the rimoportula of E. paracaënsis has a conspicuous external tube located on the mantle, just below the scalloped extension (Sullivan & Porguen, 1990). In E. oculatus Greville, the external tubes are short, with the same location as in E. paracaënsis (Ross & Sims, 1973). Sullivan & Porguen (1990) also suggested that E. radiatus was a more recent species than E. paracaënsis and E. oculatus, based on the eventual loss of the external tube in E. radiatus. Our findings indicate that this view should be changed, as the external tube in E. radiatus was not lost but fused to the scalloped extension.
The two types of palisade were observed in all frustules analysed, to which we used gentle methods of valve cleaning. When samples were prepared with harsh methods, only traces of the palisade and dendritic structures remained on the mantle. Round et al. (1990) recorded ''dendritic structures'' in E. radiatus, and they should correspond to the ornamentations found in our material. In E. paracaënsis, which is closely related to E. radiatus, there is a series of plates (scutella) on the mantle base which encircle the valve (Sullivan & Porguen, 1990). Moreover, E. paracaënsis shows a marginal silica strip on the lower one-third of the mantle similar to that observed in E. radiatus. This structure could be the support for other ornamentations, as we observed for E. radiatus.
In Cerataulus Ehrenberg, which also belongs to Triceratiaceae, there are structures on the mantle edge very similar to that in the palisade of E. radiatus, and they are also sustained by silica strips. Furthermore, in figure ''g'' of Round et al. (1990, p. 234) for Cerataulus one can discern that the bars of the palisade appear eroded, their former presence suggested by a row of granules on the mantle base.
As for Eupodiscus, Pleurosira laevis (Ehrenberg) Compère bears projecting flanges with spines on the mantle, which partially cover the valvocopula (Johnson & Rosowski, 1992, Fig. 3). These authors suggested the flanges (and the mucilage recovering the cells) might function as adhesion mechanisms between valve and cingulum. The palisade of E. radiatus could carry out the same function. Examples of associations between mantle and valvocopula occur in other diatom genera. Flanges at the base of the mantle and a fimbriate pars interior of the valvocopula also appear in Melosira Agardh and Odontella Agardh (Crawford, 1975; Round et al., 1990). In the pennate genera Gomphonema Ehrenberg and Luticola D. G. Mann, the valvocopula bears undulated projections which underlap the valve mantle (Round et al., 1990).
If mantle characteristics are taken into account, two groups may be distinguished in the Family Triceratiaceae sensu Round et al. (1990): (i) pseudoloculi present on the mantle and siliceous strips plus accompanying structures absent (Amphitetras Ehrenberg, Sheshuskovia Glezer, Triceratium Ehrenberg partim), and (ii) siliceous strip present bearing specific structures such as scutella, flanges, a palisade and granules; with pesudoloculi absent (Auliscus Ehrenberg (?), Cerataulus Ehrenberg, Eupodiscus, Fryxelliella, Pleurosira (Meneghini) Trevisan, Pseudoauliscus A. Schmidt, Triceratium partim). The genus Lampriscus A. Schmidt is problematic since it possesses pseudocellus and linking spines, and shares some features with Isthmia Agardh of the Biddulphiaceae. Therefore, its placement in Triceratiaceae sensu Round et al. (1990) is questionable.
The mantle and cingulum of Fryxelliella floridana showed structures not previously recorded (Prasad et al., 1997). Two silica strips separated by a sulcus were observed on the mantle edge, resembling the ornamentations found in Cerataulus (Round et al., 1990). In the latter, there is only one single silica strip, but it also possesses granular plates, as in F. floridana. Prasad et al. (1997) also observed the silica strip bearing granules, but not the smooth strip, perhaps because their Figs. 34 and 35 masked it due to the valve positioning in the stub. The bands of the cingulum in F. floridana have poroid areolae, similar in size to the cribral pores of the loculate areolae on the valve surface. In the Family Triceratiaceae, such complex feature is shared with Amphitetras (Round et al., 1990) and Eupodiscus (this work); in other genera we generally found simple poroid areolae or rotae.
It is useful to compare Eupodiscus and Fryxelliella in view of similarities between the two genera pointed out by Prasad et al. (1997) and of the evolutionary relationships among the ''eupodiscoid'' group. Both genera have marginal ocelli, loculate areolae, rimoportulae between the ocelli, and girdle bands bearing complex poroid areolae. All these characteristics lead to the inclusion of these genera in the Triceratiaceae. Fryxelliella is distinguished from Eupodiscus by the existence of a marginal circumferential tube along the valve edge. Furthermore, cribral pores arranged in circles in contrast to parallel rows in Eupodiscus, and the presence of triangular apertures on the valve margin of Fryxelliella are further features claimed by Prasad et al. (1997) to distinguish the two genera.
Comparing Fryxelliella to Triceratium, on the mantle edge of Triceratium favus Ehrenberg there is a 'circumferential canal' with small pores opening to the inside (Miller & Collier, 1978) very similar in structure to the marginal circumferential tube found in Fryxelliella. If the two structures are homologous, then Triceratium and Fryxelliella exhibit more affinity to each another than previously believed. At least, the species T. favus and Fryxelliella spp. could represent an evolutionary line apart from the other genera of Triceratiaceae.
Although Prasad et al. (1997) maintained that a marginal tube did not occur in Eupodiscus, the authors did not provide any illustration (or evidence from the literature) proving that the structure definitely does not exist in Eupodiscus. Despite our efforts, we also were not able to determine its presence in valves of E. radiatus studied in the present work. The best illustration we have obtained (Fig. 15) hampers a reliable conclusion, but the figure shows a chamber that could correspond to an areola or a marginal tube. In this way, the absence of evidences pointing out the presence of marginal tube in Eupodiscus cast doubts on the validity of Fryxelliella, though other characters such as triangular apertures, rimoportula positioning and morphology of marginal tube would support the validation of the genus.
In spite of differences in opinion concerning the choice of criteria to circumscribe taxonomic categories, both genera seem to constitute an evolutionary group closely related. However, choosing phylogenetic criteria will better help the resolution of taxonomic problems into the large Family Triceratiaceae towards a more natural system of classification. Proposed earlier by Simonsen (1979), this phylogenetic viewpoint was recently advocated by Kociolek (1998), who invited diatomists to adopt criteria based on a more evolutionary perspective, especially when proposing new taxa or diatom systems.
Acknowledgments Dr. Roseli M. Souza-Mosimann (Horto Botânico/Universidade Federal de Santa Catarina) loaned us some pictures of F. floridana. We wish acknowledge Dr. Maurício P. Cantão (Cooperativa Paranaense de Energia Elétrica/LAC) and Dr. Daura R. Stofella (Electron Microscopy Centre/Universidade Federal do Paraná) for SEM operational support. Dr. James J. Roper (Department of Botany/UFPR) kindly reviewed the English version of the manuscript.
Received November 12, 2001
Accepted February 5, 2002
Distributed August 31, 2003
- CRAWFORD, R. M., 1975, The taxonomy and classification of the diatom genus Melosira C. Ag. I. The type species M. nummuloides C. Ag.. Br. Phycol. J., 10: 323-338.
- FERNANDES, L. F., SOUZA-MOSIMANN, R. M. & FELÍCIO-FERNANDES, G., 1990, Diatomáceas (Bacillariophyceae) do Rio Ratones, Florianópolis, Santa Catarina, Brasil. I. Baixo curso e estuário. Insula, 20: 11-112.
- FERNANDES, L. F., BRANDINI, F. P., GUTSEIT, K. S., FONSECA, A. L. & PELLIZZARI, F. M., 1999, Benthic diatoms growing on glass slides in the Paranaguá Bay, Southern Brazil: taxonomic structure and seasonal variation. Insula, 28: 53-100.
- HASLE, G. R. & FRYXELL, G. A., 1970, Diatoms: cleaning and mounting for light and electron microscopy. Trans. Am. Microscop. Soc, 89: 469-474.
- JOHNSON, L. M. & ROSOWSKI, J. R., 1992, Valve and band morphology of some freshwater diatoms. V. Variations in the cingulum of Pleurosira laevis (Bacillariophyceae). J. Phycol, 28: 247-259.
- KOCIOLEK, J. P., 1998, Does each genus of diatoms have at least one unique feature? a reply to Round. Diatom Res, 13: 177-179.
- MILLER, W. I. & COLLIER, A., 1978, Ultrastructure of the frustule of Triceratium favus (Bacillariophyceae). J. Phycol, 14: 56-62.
- NAVARRO, J. N., 1982, Marine diatoms associated with mangrove prop roots in the Indian river, Florida, U.S.A. Bibl. Phycol, 61: 1-151.
- PRASAD, A. K. S. K. & NIENOW, J. A., 1988, Rimoportulae in Eupodiscus radiatus (Bacillariophyceae) from the Northeast Gulf of Mexico. J. Phycol, 24: 120-123.
- PRASAD, A. K. S. K., RIDDLE, K. A. & LIVINGSTON, R. J., 1997, Fine structure, taxonomy, and systematics of the centric diatom Fryxelliella gen. nov. (Eupodiscaceae, Bacillariophyta) having a new valve structure, the circumferential marginal tube, with descriptions of F. floridana sp. nov. and F. inconspicua (Rattray) comb. nov.. Phycologia, 36: 305-323.
- ROSS, R., COX, E. J., KARAYEVA, N. I., MANN, D. G., PADDOCK, T. B. B., SIMONSEN, R. & SIMS, P. A., 1979, An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia, Beih., 64: 513-533.
- ROSS, R. & SIMS, P. A., 1973, Observations on family and generic limits in the Centrales. Nova Hedwigia, Beih., 45: 97-128.
- ROUND, F. E., CRAWFORD, R. M. & MANN, D. G., 1990, The diatoms. Biology and morphology of the Genera Cambridge University Press, Cambridge, 747p.
- SIMONSEN, R., 1979, The diatom system: ideas on phylogeny. Bacillaria, 2: 9-71.
- SULLIVAN, M. J., 1988, A light and scanning electron microscope study of Eupodiscus radiatus Bailey (Eupodiscaceae). In: M. Ricard (ed.), Proceedings of the Eighth International Diatom Symposium, 1986 O. Koeltz, Koenigstein, pp. 113-123.
- SULLIVAN, M. J. & PORGUEN, V., 1990, A new species of Eupodiscus closely related to the generitype E. radiatus (Eupodiscaceae). In: H. Simola (ed.), Proceedings of the Tenth International Diatom Symposium, 1988 O. Koeltz, Koenigstein, p. 117-126.
- VANLANDINGHAN, S. L., 1969, Catalogue of the fossil and recent genera and species of diatoms and their synonyms Part III. Coscinosphaena through Fibula J. Cramer Verlag, Vaduz, pp. 1087-1756.
Publication Dates
-
Publication in this collection
20 Jan 2004 -
Date of issue
Aug 2003
History
-
Accepted
05 Feb 2002 -
Received
12 Nov 2001